Abstract
A range of scalp electroencephalogram (EEG) abnormalities correlate with the core symptoms of autism spectrum disorder (ASD). Among these are alterations of brain oscillations in the gamma-frequency EEG band in adults and children with ASD, whose origin has been linked to dysfunctions of inhibitory interneuron signaling. While therapeutic interventions aimed to modulate gamma oscillations are being tested for neuropsychiatric disorders such as schizophrenia, Alzheimer’s disease and frontotemporal dementia, the prospects for therapeutic gamma modulation in ASD have not been extensively studied. Accordingly, we discuss gamma-related alterations in the setting of ASD pathophysiology, as well as potential interventions that can enhance gamma oscillations in patients with ASD. Ultimately, we argue that transcranial electrical stimulation (tES) modalities capable of entraining gamma oscillations, and thereby potentially modulating inhibitory interneuron circuitry, are promising methods to study and mitigate gamma alterations in ASD.
Keywords: autism spectrum disorder (ASD), gamma, transcranial electrical stimulation (tES), transcranial alternating current stimulation (tACS), transcranial direct current stimulation (tDCS)
Lay Summary
Brain functions are mediated by various oscillatory waves of neuronal activity, ranging in amplitude and frequency. In certain neuropsychiatric disorders, such as schizophrenia and Alzheimer’s disease, reduced high-frequency oscillations in the “gamma” band have been observed, and therapeutic interventions to enhance such activity are being explored. Here, we review and comment on evidence of reduced gamma activity in ASD, arguing that modalities used in other disorders may benefit individuals with ASD as well.
Introduction
Autism spectrum disorder (ASD) is a broad clinical term used to describe a group of complex neurodevelopmental disorders defined by two core behavioral symptoms, according to the DSM-5: (1) persistent deficiencies in social communication and social interaction, and (2) limited interest, repetitive behavior, and activity (Association, 2013). ASD and its common co-morbid disorders, which include anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and obsessive compulsive disorder (OCD), often lead to significant impairments in activities of daily living (ADLs), both in children and adults (Haertl, Callahan, Markovics, & Sheppard, 2013). According to a 2018 Autism and Developmental Disabilities Monitoring (ADDM) Network report, the United States prevalence of ASD in 2012 was 14.5 per 1,000 children aged 8 years, with prevalence estimates ranging among states from 8.2 to 24.6 per 1,000 (Christensen et al., 2018). The report urged research initiatives to respond to this growing health issue and described the need in particular to address the biological substrates leading to ASD’s core symptoms (Christensen et al., 2018).
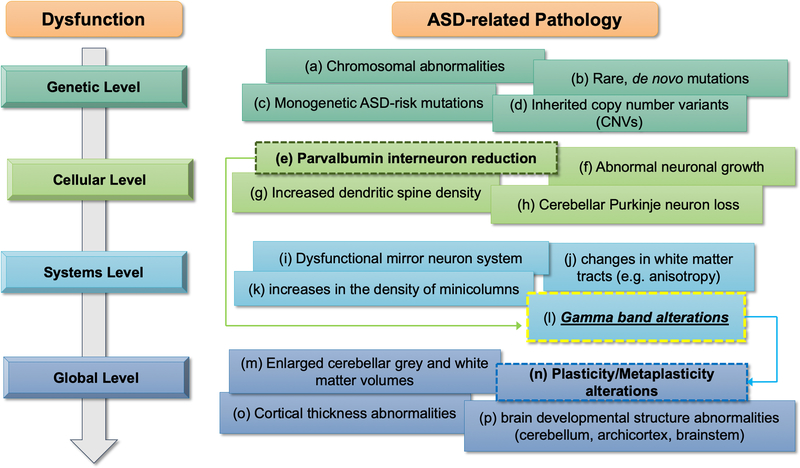
Recent research into ASD pathophysiology, combined with active development of practical ASD biomarkers (e.g., Jannati et al., in press), has led to identification of numerous genetic, physiologic and anatomic variants that are reliably found in the ASD population. Among these is growing list of gene mutations that contribute to ASD, and (even in cases where a genetic abnormality is not evident) aberrant synaptic plasticity, and interneuron deficits (Figure 1). From the physiologic perspective, most EEG studies in ASD indicate deficient neural oscillatory patterns in the gamma frequency band (Maxwell et al., 2015; Rojas & Wilson, 2014; Stroganova et al., 2015; Sun et al., 2012; van Diessen, Senders, Jansen, Boersma, & Bruining, 2015). Notably, gamma-band EEG findings in ASD share similar characteristics with those in Alzheimer’s disease (AD), frontotemporal Dementia (FTD), and schizophrenia (SCZ), where novel therapies aimed at restoring gamma activity are currently being tested (Gandal, Edgar, Klook, & Siegel, 2012a; Iaccarino et al., 2016; Poza, Hornero, Abasolo, Fernandez, & Escudero, 2007; Sami et al., 2018; Spencer et al., 2003a; Suazo et al., 2012; Woo, Spencer, & McCarley, 2010). Thus, in ASD, as in other central nervous system (CNS) disorders, gamma band EEG metrics may provide both a disease biomarker and a measure of therapeutic target engagement.
Figure 1. Framework for ASD-related alterations.
ASD pathophysiology spans from genetic to global levels, with gamma abnormalities possibly playing a crucial role in linking cellular-level alterations involving inhibitory interneurons to E/I imbalance in ASD. Sources: (a) Depienne et al., 2009; (b) Iossifov et al., 2015; (c) Hulbert et al., 2016; (d) Devlin & Scherer, 2012; (e) Hashemi et al., 2016; (f) Wegiel et al., 2014; (g) Hutsler & Zhang, 2010; (h) Courchesne et al., 1997; (i) Dapretto et al., 2006; (j) Jou et al., 2011; (k) Casanova et al., 2010; (l) Brown et al., 2005; (m) Courchesne et al., 2001; (n) Oberman et al., 2016; (o) Zielinski et al., 2014; (p) Wegiel et al., 2010.
In the present commentary we will: (1) examine how gamma EEG band alterations may inform ASD symptomatology and (2) discuss plausible interventions to target gamma abnormalities in patients with ASD by transcranial electrical stimulation (tES), which we argue is a safe and feasible methodology to entrain and modulate gamma activity in the human brain.
The role of gamma EEG activity in the healthy and pathological brain
Endogenous brain oscillatory activity represents synchronized activity of neuronal ensembles. In humans, fast oscillatory EEG activity takes place in the 30–120 Hz frequency range, known as the “gamma” frequency band (Jia & Kohn, 2011). Recent studies have further identified gamma frequency sub-bands, characterized as slow/low gamma (30–50 Hz), mid-frequency gamma (50–90 Hz) or fast/high gamma (>90 Hz), although these frequency sub-band ranges vary between studies (Buzsáki & Wang, 2012a). Most notably, the discrete 40 Hz point has served as a neurophysiological frequency of interest, as it may be integral in supporting cortical arousal and sensory processing (McDermott et al., n.d.); thus, 40 Hz has served as a common EEG frequency for studies in gamma modulation and targeting.
Electrophysiological and behavioral evidence suggests that gamma activity mediates a range of essential neural functions, including sensory-motor integration, perceptual binding, working memory, attention-dependent stimulus selection, network synchronization, and higher-order cognition (Tallon-Baudry, 2009, 1999; Jia & Kohn, 2011; Buzsáki & Wang, 2012b; Hermes, Miller, Wandell, & Winawer, 2015; Uhlhaas & Singer, 2006). As measured by EEG and magnetoencephalography (MEG), cortical gamma activity is present during resting-state conditions as “spontaneous gamma” (Rojas & Wilson, 2014). Stimulus-related gamma perturbations can also be measured as “evoked” or “induced” gamma modulation (Amo, de Santiago, Barea, López-Dorado, & Boquete, 2017; Rojas & Wilson, 2014). Evoked responses are both time- and phase-locked to the onset of the stimulus, whereas induced responses are time-locked, but not phase-locked, to the stimulus onset (Cohen, 2014).
Gamma activity is thought to arise from populations of fast-spiking, parvalbumin (PV)-expressing interneurons, a family of gamma aminobutyric acid- (GABA-)ergic inhibitory neurons distributed throughout the cerebral cortex (Sohal, Zhang, Yizhar, & Deisseroth, 2009; Tamás, Buhl, Lörincz, & Somogyi, 2000; Whittington, Traub, & Jefferys, 1995; Ylinen et al., 1995). PV interneurons interact with local excitatory pyramidal neurons (PN) and other inhibitory cells to drive the excitation/inhibition (E/I) ratio in cortical and subcortical networks (Bartos, Vida, & Jonas, 2007; Hu, Vogt, Sandberg, & Rubenstein, 2017; Markram et al., 2004; Yizhar et al., 2011). PV interneurons generate feedforward and feedback inhibitory circuits and, notably, induce gamma rhythms as an emergent property of this interneuronal synchronization (Ferguson & Gao, 2018; Tamás et al., 2000; Whittington et al., 1995). Loss of PV density and disrupted PV-PN network interactions are associated with alterations of inhibitory control and correspond to abnormalities in gamma activity (Ferguson & Gao, 2018; Volman, Behrens, & Sejnowski, 2011). Although studies have generally reported reduced gamma power associated with aberrant PV interneuron dynamics (Sohal et al., 2009), there is likely a rich interplay of PV-PN connections, PV-PV autoconnections, and cortical state-dependent conditions involved in the network dynamics that predict how PV abnormalities affect gamma rhythms in disease states (Deleuze et al., 2019; Gandal et al., 2012a; Gonzalez-Burgos, Fish, & Lewis, 2011; Lodge, Behrens, & Grace, 2009).
In recent years, gamma activity has gained attention as a potential biomarker for AD, FTD and SCZ (Gandal et al., 2012a; Sami et al., 2018). While the nature of alterations in gamma activity varies among these disease states, the commonality of abnormal gamma activity indicates its physiological significance across neuropsychiatric disorders. For instance, AD and FTD patients exhibit loss of power/connectivity in the gamma band (Guillon et al., 2017; Koenig et al., 2005; Ribary et al., 1991; Stam et al., 2002), a finding that has been replicated in mouse models of AD (Gillespie et al., 2016; Iaccarino et al., 2016; Kurudenkandy et al., 2014; Verret et al., 2012). In contrast, SCZ patients exhibit irregular rhythmicity and elevated power in the gamma band (Gandal, Edgar, Klook, & Siegel, 2012b; Spencer et al., 2003b). A study on SCZ patients found the degree of abnormal elevation of gamma power to be correlated with the severity of negative symptoms in SCZ (Suazo et al., 2012), even though pathological studies indicate loss of PV+ interneuron function in SCZ (Do, Cuenod, & Hensch, 2015).
Because gamma EEG abnormalities have been reliably detected across these neuropsychiatric disorders, therapeutic applications aimed at normalizing aberrant gamma activity are being investigated in both animal models and exploratory clinical studies. In a seminal study on a mouse model of AD, optogenetic activation of hippocampal PV-interneurons at 40 Hz reduced levels of hippocampal amyloid-β (Aβ) isoforms (Iaccarino et al., 2016). Moreover, a noninvasive, 40-Hz light-flicker treatment targeting primary visual cortex (V1) significantly reduced Aβ isoforms and microglia response in V1 (Iaccarino et al., 2016). In a recent study on mouse models of neurodegeneration, 40-Hz light-flicker stimulation markedly preserved synaptic density, improved spatial learning, and enhanced neuroprotective factors in the visual cortex, hippocampus and prefrontal cortex (Adaikkan et al., 2019).
While gamma modulation for therapeutic purposes in AD remains a novel approach, preliminary studies using multisensory and noninvasive stimulation show promising results. For example, an exploratory study reported marked behavioral improvement following 40-Hz somatosensory stimulation in patients with mild to moderate AD (Clements-Cortes, Ahonen, Evans, Freedman, & Bartel, 2016). Similar gamma-modulating approaches in animal models and pilot clinical studies of SCZ have been investigated. For example, Lee and colleagues, (H. Lee, Dvorak, & Fenton, 2014), found applying the anticonvulsant ethosuximide normalized task-associated gamma activity in rats with neonatal ventral hippocampus lesions. In another study, repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex normalized gamma hyperactivity and improved cognitive performance among SCZ patients (Farzan, Barr, Sun, Fitzgerald, & Daskalakis, 2012). Overall, converging lines of evidence suggest gamma-band abnormalities in AD, FTD, and SCZ may serve as valuable therapeutic targets and could lead to advances in treatment of neuropsychiatric patients.
Abnormal Gamma Activity in ASD
Recent studies indicate gamma-band activity significantly differs between healthy controls and individuals with ASD, in both pediatric and adult populations. These studies vary in methodology and gamma sub-bands of interest, as some measure spontaneous gamma activity while others assess evoked or induced gamma activity during sensory or cognitive tasks (Table 1). While the majority suggest alterations in low- and mid-frequency gamma in ASD, future research should establish a consensus regarding a taxonomy of methodologies for measuring and modulating gamma oscillations in ASD.
Table 1.
Evidence for Gamma-Related Alterations in ASD.
| Paper | Sample Size | Age | Frequency of Interest | Gamma Type | Condition | Measured by | Gamma Findings in ASD compared to HC | Region/Activity of Gamma Alterations | Other Findings |
|---|---|---|---|---|---|---|---|---|---|
| Orekhova, 2007 | n = 40 ASD (male); n= 40 HC | 3–8 y/o | Gamma; Beta | Spontaneous Gamma | Sustained Visual Attention Task | EEG | + Gamma | Midline, central and parietal electrodes | N/A |
| Orekhova, 2008 | n=21 ASD; n=21 HC | 3–8 y/o | Gamma; P50 supression | Spontaneous Gamma | Paired-click Paradigm | EEG | + Gamma | Frontal, central and parietal electrodes | − P50 supression |
| Machado, 2015 | n=11 ASD, n=14 HC | 3–8 y/o | Gamma; Alpha; Delta; Beta | Spontaneous Gamma | Basal versus Visual+/−Audio | qEEG | + Gamma | in Visual–Audio versus Visual with muted audio band, Right hemisphere | − Alpha; + Delta; +Beta |
| Cornew, 2013 | n= 27 ASD, n= 23 HC | 6–15 y/o | Gamma; Delta; Aplha | Spontaneous Gamma | Eyes-closed resting state | MEG | +Gamma (low, high and fast) | Anterior temporal, posterior temporal and occipital sites | +Alpha; +Delta |
| Sheikhani, 2009 | n=15 ASD, n=11 HC | 6–11 y/o | Gamma; Beta; Alpha | Spontaneous Gamma | Eyes-open resting state | qEEG | − Gamma | Temporal and frontal electrodes | − Alpha; +Beta |
| Maxwell, 2015 | n= 15 ASD (all male), n=18 HC | 9–18 y/o | Gamma | Spontaneous Gamma | Eyes-open resting state | EEG | − Gamma | Right lateral electrodes | N/A |
| Sheikhani, 2012 | n=17 ASD, n=11 HC | 6–11 y/o | Gamma; Beta; Alpha | Spontaneous Gamma | Eyes-open resting state | qEEG | − Gamma | Temporal and frontal electrodes | − Alpha; +Beta |
| Milne, 2009 | n= 20 ASD, n=20 HC | 8–18 y/o | Gamma; Alpha | Visually evoked gamma | Gabor patches | EEG | − Gamma | During Gabor patch visual perpection, Posterior cortical regions | − Low Alpha |
| Snijders, 2013 | n= 12 ASD, n=12 HC | 21–25 y/o | Gamma | Visually induced gamma | Gabor patches | EEG | − Gamma | During Gabor patch visual perpection, Posterior cortical regions | N/A |
| Grice, 2001 | n=8 ASD, n= 8 WS, n=8 HC | 30–36 y/o (mean ages) | Gamma | Visually evoked and induced gamma | Mooney face | EEG | − Induced Gamma | In response to upright Mooney faces relative to inverted Mooney faces | N/A |
| Sun, 2012 | n= 13 ASD, n=16 HC | ~30 y/o (mean age) | Gamma | Visually evoked and induced gamma | Mooney face | MEG | − Induced Gamma and phase-locking | To upright Mooney versus inverted Mooney; occipitoparietal sensors | N/A |
| Brown, 2005 | n= 16 ASD, 8=HC | 12–16 y/o (mean) | Gamma | Visually induced gamma | Prescence vs Absence of Kanizsa figures | EEG | + Gamma | In response to Kanizsa-shape trials, parietal electrode sites | N/A |
| Stroganova, 2012 | n=23 ASD (all male); n=23 HC (all male) | 3–7 y/o | Gamma; Beta | Visually evoked gamma | Kanizsa squares versus non-Kanizsa squares | EEG | − Gamma | In response to Kanizsa-shape trials, V1 electrodes | − Beta |
| Rojas, 2008 | n= 16 parents of ASD children; n = 11 ASD adults; n= 16 HC | 20–50 y/o | Gamma | Auditory gamma-band responses | 1 kHz sine-wave stimuli | MEG | + Induced Gamma; − Evoked Gamma | In response to 1 kHz sine-wave stimulus | Parents of ASD children had same response to 1 kHz as ASD group |
| Gandal, 2010 | n=25 ASD children, n=17 HC | 8–12 y/o | Gamma | Auditory gamma-band responses | 200, 300, 500, 1000 Hz | MEG | − Gamma-band phase-locking | To pure-tone stimulus presentations | Valproic acid (VPA) ASD mouse showed reduced gamma frequency phase-locking |
| Edgar, 2015 | n=105 ASD children, n=36 HC | 6–16 y/o | Gamma | Auditory gamma-band responses | 200, 300, 500, 1000 Hz | MEG | + Total Gamma Power (Induced+ Evoked) | In all frequency bands, left and right superior temporal gyrus | N/A |
Note. Spontaneous, induced, and evoked gamma activity is generally reduced in ASD. However, some studies have obtained contradicting evidence, likely due to variability in experimental design, age group, and gamma-estimation techniques.
In one of the first studies on spontaneous gamma activity in ASD, young boys with ASD showed abnormally increased activity in the beta/gamma range (24.4–44.0 Hz) in midline, central and parietal EEG electrodes, during a sustained visual attention task (Orekhova et al., 2007), and the amount of beta/gamma activity was positively correlated with the degree of developmental delay. Because both PV- and somatostatin- (SOM-)expressing GABAergic interneurons contribute to the balance between gamma- and beta-band activity (Kuki et al., 2015), these results may indicate abnormal activity in both PV- and SOM-expressing interneurons in ASD. In a follow-up study that reproduced elevated spontaneous gamma activity in a different pediatric ASD cohort, the authors hypothesized this excess of high-frequency activity recorded in frontal, central and parietal EEG electrodes may reflect an underlying imbalance in cortical E/I tone (Orekhova et al., 2008). Similar results were obtained by Machado et al., (Machado et al., 2015), who found an abnormally increased activity in the 25–55 Hz range in the right hemisphere of children with ASD. In an MEG study, Cornew and colleagues, (Cornew, Roberts, Blaskey, & Edgar, 2012), found abnormally elevated spontaneous gamma power in temporal and occipital regions in high-functioning children with ASD. However, considering the critical role of PV interneurons in generating endogenous gamma oscillations (Cardin et al., 2009; Sohal et al., 2009) and the evidence for loss of PV-interneuron expression/function in ASD (Filice, Vörckel, Sungur, Wöhr, & Schwaller, 2016; Hashemi, Ariza, Rogers, Noctor, & Martínez-Cerdeño, 2016; Saunders et al., 2013; Wöhr et al., 2015), the gamma activity in ASD is expected to be abnormally reduced. Consistent with this hypothesis, several studies in ASD have found reduced spontaneous gamma activity in frontal, temporal, and right-lateral regions (Maxwell et al., 2015; Sheikhani, Behnam, Mohammadi, Noroozian, & Mohammadi, 2012; Sheikhani, Behnam, Noroozian, Mohammadi, & Mohammadi, 2009), reduced left-hemispheric MEG steady-state gamma responses (Wilson, Rojas, Reite, Teale, & Rogers, 2007), and reduced task-related gamma power and reduced long- and short-range gamma connectivity (Khan et al., 2013; Sun et al., 2012).
Abnormalities in evoked and induced gamma activity by sensory tasks have also been reported in ASD populations. In the visual domain, evoked gamma activity in both low-level sensory and high-level cognitive tasks has been found to be abnormally reduced in adolescents and adults with ASD (Milne, Scope, Pascalis, Buckley, & Makeig, 2009; Snijders, Milivojevic, & Kemner, 2013). Induced gamma activity during visual-perception tasks also has been found to be reduced in adults and adolescents with ASD (Grice et al., 2001; Stroganova et al., 2012; Sun et al., 2012). Similarly, studies in the auditory domain have found reduced evoked and induced gamma activity in both adults and children with ASD (Edgar et al., 2015; Gandal et al., 2010; Rojas, Maharajh, Teale, & Rogers, 2008).
While a majority of studies have found reduced evoked and induced gamma activity in individuals with ASD, some studies have obtained contradicting results in terms of localization, magnitude and direction of altered gamma activity (Rojas & Wilson, 2014)(see Table 1). Moreover, due to considerable variability in age range, method of gamma estimation, and stimulus type across studies (Rojas & Wilson, 2014), more robust and generalizable findings on gamma abnormalities may require establishing a consensus on techniques for quantifying spontaneous and induced/evoked gamma activity among ASD populations.
Gamma Activity and ASD Pathophysiology
In light of these lines of evidence, we argue that abnormal activity in the gamma range –either increased or decreased— should be considered part of the broad pathophysiological framework of ASD (Figure 1). Conventional models of ASD, based on decades of animal research and clinical studies, span from genetic to multi-system, global levels in an effort to better inform ASD etiology and pathophysiological mechanisms (Varghese et al., 2017). The genetic level of ASD pathophysiology points to mutations and genetic risk factors including inherited chromosomal abnormalities, rare genetic variants, and de novo mutations (Depienne et al., 2009; Devlin & Scherer, 2012; Grove et al., 2019; Hulbert & Jiang, 2016; Iossifov et al., 2014; Ramaswami & Geschwind, 2018). The cellular level focuses on abnormalities in neuronal morphology, growth, development, pruning, and cell-type densities (Courchesne, 1997; Hashemi et al., 2016; Hutsler & Zhang, 2010; Marchetto et al., 2017; Peter et al., 2016; Wegiel et al., 2014; Yip, Soghomonian, & Blatt, 2007). The framework then expands to systems and circuit-based alterations, commonly highlighted by regional cytoarchitecture and connectivity alterations (Bachevalier & Loveland, 2006; Casanova, El-Baz, Vanbogaert, Narahari, & Switala, 2010; Dapretto et al., 2006; Eigsti & Shapiro, 2003; Gotts et al., 2012; Jou et al., 2011; Just, Keller, Malave, Kana, & Varma, 2012; Marsh & Hamilton, 2011; Minshew, Luna, & Sweeney, 1999; Takarae, Minshew, Luna, & Sweeney, 2007; Zikopoulos & Barbas, 2013); of note, this level also captures the mirror neuron system (MNS), which has come under scrutiny in recent years as various studies and meta-analyses now argue against MNS dysfunction in ASD (Dapretto et al., 2006; Hamilton, 2013; Hamilton, Brindley, & Frith, 2007; Schulte-Rüther et al., 2017). It is at this systems level that we propose abnormal gamma activity be integrated into the framework of ASD pathophysiology. This could serve as a relevant, informative model as gamma alterations could play a role in linking cellular-level alterations involving inhibitory interneurons to E/I imbalances reported in ASD (Brown, Gruber, Boucher, Rippon, & Brock, 2005; Gogolla et al., 2009; Nelson & Valakh, 2015; Rubenstein & Merzenich, 2003). Finally, global-level characteristics, such as brain morphology, development and activity, serve to bridge higher-level dysfunction and behavioral deficits seen in core symptoms of ASD (Brambilla et al., 2003; Courchesne et al., 2001; Jannati et al., 2020; McAlonan et al., 2005; Oberman et al., 2016; Sparks et al., 2002; Wegiel et al., 2010; Zielinski et al., 2014).
While the potential pathophysiological role of gamma activity in ASD continues to gain attention, there is no robust initiative focused on gamma abnormalities in ASD to understand their etiology and to investigate therapeutic approaches to mitigate or normalize them. Thus, for the remainder of the paper, we will discuss the gamma-modulatory capabilities of transcranial electrical stimulation (tES) and its theoretical and practical considerations in ASD-related gamma dysfunction.
Transcranial Electrical Stimulation (tES)
tES is a broad term referring to a subset of noninvasive brain stimulation (NIBS) techniques, which deliver weak electric currents (typically 1–2 mA) transcranially via scalp electrodes (M. A. Nitsche, Liebetanz, Tergau, & Paulus, 2002). This electric current modulates spontaneous neuronal firing using subthreshold changes in resting-membrane potentials without generating action potentials (Brunoni et al., 2012). Through this mechanism, tES influences the ongoing neural activity and induces long-term modulatory effects leading to reproducible facilitatory or inhibitory plastic changes in the targeted neural systems (Yavari, Jamil, Mosayebi Samani, Vidor, & Nitsche, 2018; Yavari, Nitsche, & Ekhtiari, 2017). A notable feature of tES is its ability to apply electrical currents in various forms (e.g., direct current, random noise, alternating current) to almost any cortical region and concurrently influence a behavior of interest, yielding short-term and long-term behavioral outcomes (Reed & Cohen Kadosh, 2018; Yavari et al., 2018). Because of their valuable applications in experimental and clinical neuroscience research, tES techniques have gained popularity as affordable, low-risk, and user-friendly tools, particularly in double-blind, sham-controlled studies (Yavari et al., 2018).
tES modalities include transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS), and transcranial alternating current stimulation (tACS) (Figure 2A) (Santarnecchi et al., 2015; Yavari et al., 2018). tDCS uses a constant weak electrical current to induce bidirectional, polarity-dependent changes in cortical regions, enabling measuring effects at neuropsychological, physiological, and motor levels (Brunoni et al., 2012; Michael A Nitsche & Paulus, 2000). Because tDCS is well-tolerated and is associated with mild adverse effects in humans, it has been employed in clinical research to investigate major depressive disorder, addiction, chronic neuropathy, Parkinsonism, stroke, and traumatic spinal cord injury (Brunoni et al., 2012). In comparison, tRNS delivers a low-intensity, randomly alternating biphasic current directly to the scalp, with frequencies ranging from 0.1–640 Hz (Antal & Herrmann, 2016). The use of tRNS has been particularly advantageous in studying cortical excitability in motor learning and perceptual processing (Antal & Herrmann, 2016). Finally, tACS administers sinusoidal or biphasic currents at a chosen frequency to interact with the brain’s endogenous oscillatory activity, aimed at entraining large populations of neurons (Tavakoli & Yun, 2017). tACS has emerged as a promising neuromodulatory tool in the setting of cognition, neuroplasticity, and network dynamics (Ali, Sellers, & Fröhlich, 2013; Helfrich et al., 2014; Santarnecchi et al., 2016; Violante et al., 2017; Vossen, Gross, & Thut, 2015).
Figure 2. tES modalities and potential applications.
(A) The three major types of tES: tDCS, tRNS and tACS. Note, electrical current parameters, mechanism of action, and neural effects all differ across the three modalities, and in the case of tDCS stimulation can be used to either increase or decrease cortical excitability and/or specific brain oscillations. An example of a tACS montage aimed at synchronizing two brain networks (e.g. default-mode network – blue) is shown to highlight the potential of using multichannel tES to tap into network-level dynamics. (B) Some of the potential tES targets in ASD: the right temporoparietal junction (TPJ) and posterior superior temporal sulcus (pSTS) involved in Theory of Mind, social comprehension, and attention (Krall et al., 2015; Redcay, 2008; Santiesteban et al., 2012; Young et al., 2010); the right inferior frontal gyrus (IFG) involved in social impairments and communicative deficits (Bastiaansen et al., 2011; Dapretto et al., 2006; Lee et al., 2008; Shamay-Tsoory et al., 2009); and the dorsolateral prefrontal cortex (DLFPC) involved in comorbid depressive disorder and executive dysfunction (Just et al., 2004, 2007; Silk et al., 2006). (C-E) Three potential applications of inducing or modulating gamma oscillations. Specifically, stimulation can be used to modulate (C) spontaneous or (D) evoked gamma activity, measuring resting EEG before and after tES at gamma frequency (e.g., 40 Hz) delivered during resting state or a gamma-evoking task, respectively. The latter approach benefits from pre-activation of neural structures relevant to generating gamma oscillations, thus potentially amplifying the effect of tACS. Finally, (D) tES is delivered at gamma frequency concurrently with a gamma-inducing perceptual/cognitive task. (E) The same setup is used, but tACS is constantly informed by a closed-loop EEG-tES system triggered by, e.g., the phase of theta oscillations or spontaneous gamma bursts.
Of note, the induction effects of tES modalities are highly variable, with intra-individual and inter-individual responses varying widely in some studies (Chew, Ho, & Loo, 2015; Jacobson, Koslowsky, & Lavidor, 2012; Li, Uehara, & Hanakawa, 2015). As mentioned by Li and colleagues, (Li et al., 2015), it is likely that interindividual factors predicting tES induction effects, specifically tDCS, include baseline neurological state, neuroanatomy, age, and neuropathological states. To address these measured variabilities, multiple models have been proposed, including establishing a heuristic spatio-mechanistic framework (Yavari et al., 2017) and optimization and design of tES stimulation protocols (Opitz et al., 2016). Likely future investigations should investigate the value of personalized electrode montages, such as closed-loop tES/EEG stimulation paradigms.
While tDCS and tRNS have been effective in modulating cortical excitability and plasticity mechanisms, only tACS has been directly associated to frequency-specific modulation of oscillatory dynamics, with animal and human evidence of its effect on gamma activity. Therefore, while we consider the potential of each of the three major tES modalities for targeting gamma alterations in ASD, we note that tACS offers the greatest potential for neural oscillatory entrainment and modulation in humans to date. Thus, we will delve deeper into the theoretical and practical applications of tACS neuromodulatory approaches and how tACS can be utilized to target gamma-related ASD pathophysiology.
Transcranial Alternating Current Stimulation (tACS)
The development of non-invasive electrical stimulation techniques in the late 20th century led to a subsequent interest in generating electric patterns capable of shaping network activity. Seminal in vitro work by Fröhlich and McCormick, (Fröhlich & McCormick, 2010), was among the first to demonstrate that active neocortical networks can be modulated by positive and negative electric feedback fields tuned at a specific frequency, suggesting a robust feed-back loop between neural activity and endogenous electric fields. These findings support the utility of tACS for delivering weak sinusoidal currents at biologically relevant frequencies that interact with the brain’s endogenous oscillations (Herrmann, Rach, Neuling, & Strüber, 2013; Vossen et al., 2015). tACS acts by synchronizing spiking activity to different driving frequencies, essentially entraining neuronal firing to the electrically applied field and exerting robust neuromodulatory effects at the systems level (Herrmann et al., 2013; Vossen et al., 2015). For these reasons, tACS has broad applications in human neuroscience research, with recent studies demonstrating the value of closed-loop tACS systems (Ketz, Jones, Bryant, Clark, & Pilly, 2018; Raco, Bauer, Tharsan, & Gharabaghi, 2016; Wilde, Bruder, Binder, Marshall, & Schweikard, 2015). For example, closed-loop techniques have been used to detect the onset of slow-wave sleep and to deliver tACS matching the frequency and the phase of the dominant oscillation during physiologically-relevant temporal windows (Ketz et al., 2018). Thus, closed-loop tACS systems could serve as a valuable electrophysiologic tool for measuring a desired frequency range and triggering stimulation matching the desired phase and frequency of the oscillations. Considering the heterogeneity of symptoms, differences in their severity, and, presumably, the inter-individual variability of their associated alterations in gamma activity among individuals with ASD, closed-loop tACS systems have the potential to individually tailor the gamma entrainment based on the paradigm (resting/active), task modality (visual/auditory/etc., if applicable), and the region/symptom of interest in certain ASD subpopulations (Figure 2C–2E).
Moreover, recent tACS applications have explored the possibility of modulating not just one oscillatory pattern but rather the coupling between multiple oscillations, e.g., cross-frequency coupling (CFC). CFC has been shown to be a key neural mechanism supporting complex brain functioning (Canolty et al., 2006; Canolty & Knight, 2010) and cognition (Aru et al., 2015; Chaieb et al., 2015; Gągol et al., 2018), suggesting the potential of tES techniques for modulating such interplay. Studies have shown the feasibility of simultaneous targeting of two frequencies both at rest and during a cognitive task, showing an advantage for combining multiple oscillatory targets (Alekseichuk, Turi, Amador de Lara, Antal, & Paulus, 2016). A form of CFC known as phase-amplitude coupling (PAC) indicates the modulation of amplitude of a higher-frequency signal by the phase of a concomitant lower-frequency neural signal, representing the integration of fast local circuits with slower long-range inputs. Both task-induced and resting-state alpha-to-gamma PAC are abnormally increased in ASD (Berman et al., 2015; Khan et al., 2013; Seymour, Rippon, Gooding-Williams, Schoffelen, & Kessler, 2019). Moreover, gamma-band PAC in animal models requires proper PV+ interneuron function (Port et al., 2019). Thus, tES modulation of gamma-related PAC may be an additional target of therapeutic investigation in ASD.
Gamma Induction and Modulation in Humans using tES
The gamma-frequency band has become a recent target of interest in tES modulation, with tACS being the most suitable modality for gamma-specific entrainment (Feurra et al., 2011; Kanai, Chaieb, Antal, Walsh, & Paulus, 2008; Reed & Cohen Kadosh, 2018; Santarnecchi et al., 2015). While the exact mechanisms of tACS-induced gamma entrainment remain unclear, its robust effects are thought to be mediated by spike timing-dependent plasticity (Dan & Poo, 2006) and driving the activity of local PV interneuron networks (Witkowski et al., 2016).
Studies investigating the human motor system were among the first to provide robust evidence for tACS-induced gamma modulation. Moisa and colleagues found gamma-tACS, but not beta-tACS or sham stimulation, of the primary motor cortex (M1) improved the velocity and acceleration of visually triggered movements (Moisa, Polania, Grueschow, & Ruff, 2016a). These gamma tACS-induced enhancements were significantly correlated with the changed blood oxygenation level-dependent (BOLD) activity in the stimulated M1 region (Moisa, Polania, Grueschow, & Ruff, 2016b). Guerra and colleagues, (Guerra, Bologna, et al., 2018), applied gamma and beta tACS stimulation to M1 in healthy controls during a repetitive finger tapping behavioral paradigm; gamma tACS (70 Hz) of M1 led to motor evoked potential (MEP) amplitude increment (i.e., progressive increase in amplitude) across the first ten movements of the finger tapping motor sequence, while beta tACS (20 Hz) led to amplitude decrement, with no significant effect on other movement parameters, nor any changes in MEPs (Guerra, Bologna, et al., 2018). Additionally, tACS targeting of M1 has been found to enhance force generation and motor reactivity in a visually guided movement-initiation task (Joundi, Jenkinson, Brittain, Aziz, & Brown, 2012). Miyaguchi et al., (Miyaguchi et al., 2018), demonstrated that M1-cerebellum gamma tACS applied during an isometric force task with a visuomotor control of the right index finger improved motor performance in healthy controls with poor motor performance at baseline. Similarly, enhanced motor performance during a visuo-motor coordination task has been demonstrated during high-gamma (80 Hz) stimulation (Santarnecchi et al., 2017).
tACS gamma-entrainment techniques also have been used to target higher-order behavioral processes. For example, Hoy and colleagues (Hoy, Bailey, Arnold, Windsor, et al., 2015) found selective improvement in working-memory performance following gamma-tACS, compared to tDCS and sham. Additionally, gamma-tACS applied to the right temporal lobe increased accuracy in a verbal insight task, as represented as a Eureka! moment (Santarnecchi et al., 2016), and was effective in improving abstract reasoning when applied over the left DLPFC (Emiliano Santarnecchi et al., 2013; Emiliano Santarnecchi, Rossi, & Rossi, 2015).These findings support the utility of tACS gamma-entrainment for improving cognitive and behavioral outcomes in humans.
There is strong evidence for the role of PV interneurons in endogenous gamma oscillations (Cardin et al., 2009; Sohal et al., 2009). Moreover, gamma activity has been found to drive plasticity throughout critical periods of development (Hensch & Bilimoria, 2012). Thus, enhancing the power and duration of endogenous gamma activity by tACS may enhance and strengthen these plasticity dynamics.
Recent studies have shown the specificity of gamma-tACS in modulating plasticity mechanisms in humans. For instance, Guerra et al., found tACS in the gamma band (but not in other frequency bands) enhanced the plasticity effects induced by intermittent theta-burst stimulation (iTBS) over the primary motor cortex, suggesting a possible link between gamma oscillations, interneuron activity, and long-term potentiation- (LTP) like plasticity (Guerra, Suppa, et al., 2018), a finding relevant in the context of LTP deficiency described in ASD models (Jung et al., 2013; Martin, Lassalle, Brown, & Manzoni, 2016). In a follow-up study, when continuous theta burst stimulation (cTBS) was paired with tACS to M1, cTBS-coupled with gamma tACS converted the expected after-effects of cTBS from inhibition to facilitation, while beta-tACS did not modify cTBS aftereffects (Guerra et al., 2019). Interestingly, this reversal of cTBS-induced plasticity effects by gamma-tACS suggests that gamma band activity may mediate long-term depression (LTD)-like plasticity in the human motor system (Guerra et al., 2019), which has been found to be abnormal in both children and adults with ASD (Jannati et al., 2020; Oberman et al., 2016). Additionally, Nowak and colleagues, (Nowak et al., 2017), utilized a combined tACS-TMS approach to drive gamma frequency oscillations, which resulted in an attenuation of GABAA inhibition in the human motor cortex.
While the majority of tES gamma-entrainment studies have used the tACS modality, several studies suggest tDCS can also modulate gamma activity. In an MEG study, Wilson and colleagues, (Wilson, McDermott, Mills, Coolidge, & Heinrichs-Graham, 2018), found anodal tDCS applied to the occipital cortices enhanced gamma activity during a subsequent visual task in the right occipital, prefrontal and superior parietal cortices, compared to sham stimulation. Additionally, 2-mA tDCS applied to the prefrontal cortex was found to significantly increase gamma power and improve working memory in patients with SCZ compared to sham (Hoy, Bailey, Arnold, & Fitzgerald, 2015). While there is still a lack of consensus regarding optimal gamma-entrainment parameters for inducing robust and reproducible physiological and behavioral effects, the existing preliminary evidence suggests the utility of gamma-tACS, and possibly tDCS, for modulation of gamma-mediated functions and outcomes. The quest for detecting changes in gamma activity and their neurophysiological substrates should leverage multimodal imaging techniques, including, e.g., magnetic resonance spectroscopy (MRS) to look at GABAergic and glutamatergic function and assess E/I (Puts & Edden, 2012) or combined TMS-EEG for fine characterization of evoked responses indexing GABAA and GABAB activity (Farzan et al., 2010), or to understand the impact of tES on local and distributed dynamics (Romero Lauro et al., 2014).
tES and ASD: Opportunities and Challenges
Although current clinical interventions help alleviate various behavioral deficits of ASD, they are limited in their ability to affect the underlying pathophysiological mechanisms that give rise to the core symptoms of ASD. Therefore, targeting gamma band abnormalities in patients with ASD could serve as a non-invasive, non-pharmacological approach to address these shortcomings. Due to the heterogeneity of ASD populations, it is likely that tES gamma-modulation should target specific symptoms or characteristics of ASD patients rather than simply any individual with an ASD diagnosis. tES modalities, particularly frequency-specific tACS paradigms, can be investigated as therapeutic approaches to modulating gamma oscillations and restoring neurotypical gamma activity in ASD populations. While several pilot studies have explored the therapeutic potential of tES by means of tDCS, there is no robust initiative to explore tACS for gamma modulation in ASD (Kang et al., 2018; Rothärmel et al., 2019; Wilson et al., 2018). As such, tACS gamma-modulation techniques are a worthy area for future research and may serve as an integral part of a multi-modal clinical approach to ASD treatment.
Studies on gamma dysfunction in ASD tend to show reduced gamma power and frequency phase-locking across age groups, neural states, and sensory/perceptual tasks (Rojas & Wilson, 2014). While evidence suggests these patterns of gamma alterations in ASD are complex in nature (Rojas & Wilson, 2014), tACS gamma-modulation paradigms have the potential to target the relevant cortical areas through the use of appropriate stimulation paradigms. Previous studies have identified neuroanatomical correlates of ASD symptoms, which have largely focused on cortical regions implicated in social and executive functioning: (1) inferior frontal gyrus (IFG) mediating social impairments and communicative deficits including understanding emotion in others and mirror-neuron dysfunction (Bastiaansen et al., 2011; Dapretto et al., 2006; P. S. Lee et al., 2008; Shamay-Tsoory et al., 2009); (2) the right posterior superior temporal sulcus (pSTS) and temporoparietal junction (TPJ) mediating Theory of Mind, social comprehension, and attention (Krall et al., 2015; Redcay, 2008; Santiesteban et al., 2012; Young et al., 2010), indicating abnormality in the default-mode network (Padmanabhan et al., 2017), attributing intention to others (Costa et al., 2008), distinguishing ‘self-relevant’ from ‘other-relevant’ information (Heinisch et al., 2012), and predicting the behaviors of other individuals in social contexts (Carter et al., 2012); and (3) dorsolateral prefrontal cortex (DLFPC) mediating comorbid depressive disorder and executive dysfunction (Just et al., 2007, 2004; Silk, Rinehart, Bradshaw D Sc, et al., 2006). Moreover, additional evidence related to alterations in network-level functioning, including atypical white matter connectivity, most prominently characterized by impaired development of limbic connectivity and altered maturation of short-distance tracts (Ameis & Catani, 2015; Shukla et al., 2011), and aberrant cerebellar connectivity, which have been implicated in language development deficits and restrictive/repetitive behaviors (Stoodley et al., 2017; Verly et al., 2014), have been proposed in ASD populations. Finally, functional MRI network abnormalities in ASD populations have been identified as well, typically involving frontostriatal networks (Silk, Rinehart, Bradshaw, et al., 2006), the default-mode network (Cherkassky et al., 2006; Kennedy & Courchesne, 2008) and dorsal and ventral attention networks (Farrant & Uddin, 2016; Fitzgerald et al., 2015).
Based on these results, potential stimulation targets that can be explored include frontal (IFG, DLPFC), temporal, and parietal (pSTS, TPJ) regions to investigate improvements in social/communicative deficits, executive dysfunction and comorbid depressive disorder, as well as Theory of Mind, social comprehension, and attention, respectively (Figure 2B). These regions have shown generally consistent reproducible modulatory effects across spontaneous, induced, and evoked gamma activity (Table 1). Additionally, since the majority of studies find an overall reduction of gamma activity in ASD, applying gamma-tACS to these regions might be able to entrain and restore, at least partially, neurotypical gamma activity in the ASD population (Table 1). However, future studies must establish consensus tACS stimulation paradigms, including electrode montages, current amplitude, and perturbation-based/resting-state gamma protocols (Brennan, Peck, & Holodny, 2016; Chen, 2001).
If gamma entrainment is a valid objective in tES therapeutic strategies in ASD, then the implementation of tES in clinical settings will require prospective studies to demonstrate tACS is capable of modulating gamma in a heterogeneous patient population. Additionally, in a stepwise approach to tES deployment for management of core ASD symptoms, pilot studies can test whether isolated behavioral measures may improve with gamma entrainment. For example, future investigations could access the neuromodulatory effects of gamma tACS on motor learning in ASD, which is typically impaired in ASD rodent models and in ASD patients (Izadi-Najafabadi, Mirzakhani-Araghi, Miri-Lavasani, Nejati, & Pashazadeh-Azari, 2015; Marko et al., 2015; Piochon et al., 2014). Gamma oscillations may play an integral role in mediating motor learning mechanisms and gamma-tACS significantly enhances these learning processes (Guerra, Bologna, et al., 2018; Miyaguchi et al., 2018). In neurotypical subjects, 70 Hz tACS significantly increased the capacity for motor learning, compared to 10 Hz, 20 Hz, and sham stimulation (Sugata et al., 2018). Similar findings in healthy participants suggest low gamma-tACS applied to M1 improves implicit performance on a motor learning task (Giustiniani et al., 2019) and gamma-tACS improves acceleration of the practiced movement during motor task training (Bologna et al., 2019; E. Santarnecchi et al., 2017b). Interestingly, theta-gamma coupled tACS to right M1 in healthy participants enhanced motor skill acquisition, compared to sham and an active stimulation control, in a ballistic thumb abduction task of the left hand (Akkad et al., 2019). These data suggest gamma tACS can also be applied to M1 and measured in motor learning task performance in ASD populations, to probe for the extent of responsivity to these neuromodulatory effects in motor learning and motor control.
From a biomarker perspective, Nowak and colleagues, (Nowak et al., 2017), found the neurophysiological response to application of gamma-tACS to M1 in heathy controls is closely related to the ability to learn a motor task. This data suggests that probing M1 with gamma-tACS can serve as a tool to predict “responders” from “non-responders” in motor learning paradigms, although these robust findings in healthy participants would have to be studied and reproduced in ASD populations. Importantly, personalization of stimulation protocols/treatment is key, especially when targeting brain oscillatory activity which can differ due to state- and trait-dependent variability in individuals with ASD who also exhibit intrinsic individual variability due to developmental trajectories and clinical phenotypes. In this regard, our team has recently promoted the validation of so-called perturbation-based biomarkers (PBB), leveraging the combination of EEG recording and brain stimulation techniques (TMS, tES) to quantify brain responses to perturbation. These biomarkers have demonstrated high reliability and also a potential for “fingerprinting”, i.e. characterize each individual brain on the basis of patterns of activity propagation after, e.g., a TMS pulse (Ozdemir et al., 2020). Even more interestingly, this approach has been tested for targeting specific brain resting-state networks (e.g. default-mode network, dorsal attention network), showing the ability to capture network-specific responses that could be indeed used to tailor personalized interventions in patients with ASD. The same approach could be used to combine tES and EEG, leading to a portable and cheaper equivalent of TMS-EEG potentially deployable at home. All these methods could be used to characterize fine brain dynamics in the frequency domain, including gamma activity which is captured by TMS-EEG as the earliest local GABAergic evoked response after a TMS pulse (Ferrarelli et al., 2008). Overall, and beyond PBB, improved operational definition and understanding of gamma activity in ASD is a prerequisite for further biomarker discovery or therapeutic trials.
In the current commentary, we have argued for the application of gamma tACS entrainment in ASD. However, we acknowledge that gamma is not the only endogenous oscillatory frequency band that could serve as a suitable target for tACS neuromodulation in ASD. Disrupted oscillatory activity and synchronization across various frequency bands is recognized as a neurophysiological characteristic of ASD, most notably relating to sensory and perceptual functioning (Simon & Wallace, 2016); these altered frequencies include alpha, beta and theta activity. Preliminary studies investigating sensory processing in ASD suggest abnormalities in stimulus induced alpha activity (Spaak, de Lange, & Jensen, 2014), as well as reduction in the ability to phase lock alpha and beta oscillations to repetitive sensory stimulation (V. V. Lazarev, Pontes, Mitrofanov, & deAzevedo, 2010; Vladimir V. Lazarev, Pontes, & deAzevedo, 2009a). Additionally, Jochaut and colleagues utilized an EEG and fMRI study assessing cortical speech processing, demonstrating that gamma and theta cortical activity do not engage synergistically in response to speech in ASD subjects, compared to healthy controls (Jochaut et al., 2015). Future investigations should consider possible applications of targeting other frequency bands in ASD.
While tES-induced gamma modulation could serve as a useful therapeutic approach for ASD, this emerging technology faces various challenges. First, patients with ASD exhibit a wide range of capabilities in language expression, physical abilities, and intellectual functioning. Because many patients with ASD are typically dependent on care providers, any novel technique should be carefully assessed to ensure the well-being and safety of patients is upheld and prioritized. Therefore, it is important that investigations involving tES and ASD participants be aware of the special accommodations of the ASD community, as well the neuroethical issues this technology may present (Sarrett, 2016). While tES techniques have undergone robust developments in their current delivery and temporal precision, progress in refining the technology is still needed, especially with the closed-loop systems that deliver stimulation upon detection of set oscillatory thresholds. Of note, oscillatory gamma-band measurements in resting and active states are challenging and often criticized; EEG-recorded gamma activity is often contaminated by electromyographic activity and eye-movement artifacts (Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008), making it difficult to isolate neural gamma activity from noise. However, this issue could potentially be circumvented by taking advantage of studies on patients with ASD and epilepsy who undergo evaluation for epilepsy surgery and have intracranial electrodes in place (Kokoszka et al., 2017). Another method to optimize investigating pathophysiological signatures of gamma activity would be to employ MEG, in which cerebral gamma activity can be more clearly and robustly identified (Mandal, Banerjee, Tripathi, & Sharma, 2018). Future investigations aimed at improving the temporal and spatial parameters of stimulation paradigms, as well as capturing the physiological effects of the stimulation, are needed for further development of this novel therapeutic application.
Conclusion
In summary, we present evidence suggesting that gamma-frequency alterations can serve as a potential biomarker of ASD pathophysiology and as a target for noninvasive neuromodulatory interventions currently under investigation for other neurological and psychiatric conditions. In particular, we argue that tACS, among other tES techniques, could be utilized to modulate and restore neurotypical gamma-band activity in patients with ASD.
Acknowledgments
Dr. Santarnecchi was supported in part by Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014-13121700007, Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) Award 2017, the Defence Advanced Research Projects Agency (DARPA) via HR001117S0030, and the National Institutes of Health (NIH P01 AG031720-06A1, R01 MH117063-01, R01 AG060981-01). Dr. Jannati was supported in part by a fellowship from the Canadian Institutes of Health Research (CIHR 41791). Dr. Rotenberg was supported in part by the NIH (R01 NS088583), The Boston Children’s Hospital Translational Research Program, Autism Speaks, Massachusetts Life Sciences, The Assimon Family, Brainsway, CRE Medical, Eisai, Neuroelectrics, Roche, Sage Therapeutics, and Takeda Medical. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views, policies or endorsements, either expressed or implied, of ODNI, IARPA, the U.S. Government, Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or any of the other listed granting agencies.
Footnotes
Conflicts of Interest Statement
Dr. Rotenberg is a founder and advisor for Neuromotion, serves on the medical advisory board or has consulted for Cavion, Epihunter, Gamify, NeuroRex, Roche, Otsuka, and is listed as inventor on a patent related to integration of TMS and EEG. Dr. Santarnecchi serves as a consultant for EBNeuro, Neuroelectrics, Neurocare, and is listed as an inventor on patents related to the application of brain stimulation in brain tumors and dementia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Adaikkan C, Middleton SJ, Marco A, Pao P-C, Mathys H, Kim DN-W, … Tsai L-H (2019). Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron, 102(5), 929–943.e8. doi: 10.1016/j.neuron.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkad H, Dupont-Hadwen J, Frese A, Tetkovic I, Barrett L, Bestmann S, & Stagg CJ (2019). Increasing motor skill acquisition by driving theta-gamma coupling. BioRxiv, 2019.12.20.883926. doi: 10.1101/2019.12.20.883926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseichuk I, Turi Z, de Amador, Lara G, Antal A, & Paulus W (2016). Spatial Working Memory in Humans Depends on Theta and High Gamma Synchronization in the Prefrontal Cortex. Current Biology, 26(12), 1513–1521. doi: 10.1016/j.cub.2016.04.035 [DOI] [PubMed] [Google Scholar]
- Ali MM, Sellers KK, & Fröhlich F (2013). Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(27), 11262–11275. doi: 10.1523/JNEUROSCI.5867-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis SH, & Catani M (2015). Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex, 62, 158–181. doi: 10.1016/j.cortex.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Amo C, de Santiago L, Barea R, López-Dorado A, & Boquete L (2017). Analysis of Gamma-Band Activity from Human EEG Using Empirical Mode Decomposition. Sensors (Basel, Switzerland), 17(5). doi: 10.3390/s17050989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, & Herrmann CS (2016). Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plasticity, 2016, 1–12. doi: 10.1155/2016/3616807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, … Vicente R (2015). Untangling cross-frequency coupling in neuroscience. Current Opinion in Neurobiology, 31, 51–61. [DOI] [PubMed] [Google Scholar]
- Association AP (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, & Loveland KA (2006). The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews, 30(1), 97–117. doi: 10.1016/j.neubiorev.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, & Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews. Neuroscience, 8(1), 45–56. doi: 10.1038/nrn2044 [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, van der Gaag C, Ketelaars C, Minderaa R, & Keysers C (2011). Age-related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biological Psychiatry, 69(9), 832–838. [DOI] [PubMed] [Google Scholar]
- Berman JI, Liu S, Bloy L, Blaskey L, Roberts TPL, & Edgar JC (2015). Alpha-to-gamma phase-amplitude coupling methods and application to autism spectrum disorder. Brain Connectivity, 5(2), 80–90. doi: 10.1089/brain.2014.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna M, Guerra A, Paparella G, Colella D, Borrelli A, Suppa A, … Berardelli A (2019). Transcranial Alternating Current Stimulation Has Frequency-Dependent Effects on Motor Learning in Healthy Humans. Neuroscience, 411, 130–139. doi: 10.1016/j.neuroscience.2019.05.041 [DOI] [PubMed] [Google Scholar]
- Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, & Barale F (2003). Brain anatomy and development in autism: Review of structural MRI studies. Brain Research Bulletin, 61(6), 557–569. doi: 10.1016/j.brainresbull.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Brennan NP, Peck KK, & Holodny A (2016). Language Mapping Using fMRI and Direct Cortical Stimulation for Brain Tumor Surgery: The Good, the Bad, and the Questionable. Topics in Magnetic Resonance Imaging: TMRI, 25(1), 1–10. doi: 10.1097/RMR.0000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Gruber T, Boucher J, Rippon G, & Brock J (2005). Gamma Abnormalities During Perception of Illusory Figures in Autism. Cortex, 41(3), 364–376. doi: 10.1016/S0010-9452(08)70273-9 [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, … Fregni F (2012). Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimulation, 5(3), 175–195. doi: 10.1016/j.brs.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, & Wang X-J (2012a). Mechanisms of Gamma Oscillations. Annual Review of Neuroscience, 35(1), 203–225. doi: 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, & Wang X-J (2012b). Mechanisms of Gamma Oscillations. Annual Review of Neuroscience, 35(1), 203–225. doi: 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, … Knight RT (2006). High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science, 313(5793), 1626–1628. doi: 10.1126/science.1128115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, & Knight RT (2010). The functional role of cross-frequency coupling. Trends in Cognitive Sciences, 14(11), 506–515. doi: 10.1016/j.tics.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, … Moore CI (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459(7247), 663–667. doi: 10.1038/nature08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Bowling DL, Reeck C, & Huettel SA (2012). A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science (New York, N.Y.), 337(6090), 109–111. doi: 10.1126/science.1219681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Vanbogaert E, Narahari P, & Switala A (2010). A Topographic Study of Minicolumnar Core Width by Lamina Comparison between Autistic Subjects and Controls: Possible Minicolumnar Disruption due to an Anatomical Element In-Common to Multiple Laminae. Brain Pathology, 20(2), 451–458. doi: 10.1111/j.1750-3639.2009.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaieb L, Leszczynski M, Axmacher N, Höhne M, Elger CE, & Fell J (2015). Theta-gamma phase-phase coupling during working memory maintenance in the human hippocampus. Cognitive Neuroscience, 6(4), 149–157. doi: 10.1080/17588928.2015.1058254 [DOI] [PubMed] [Google Scholar]
- Chen AC (2001). New perspectives in EEG/MEG brain mapping and PET/fMRI neuroimaging of human pain. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 42(2), 147–159. doi: 10.1016/s0167-8760(01)00163-5 [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, & Just MA (2006). Functional connectivity in a baseline resting-state network in autism. Neuroreport, 17(16), 1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c [DOI] [PubMed] [Google Scholar]
- Chew T, Ho K-A, & Loo CK (2015). Inter- and Intra-individual Variability in Response to Transcranial Direct Current Stimulation (tDCS) at Varying Current Intensities. Brain Stimulation, 8(6), 1130–1137. doi: 10.1016/j.brs.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN, … Yeargin-Allsopp M (2018). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR. Surveillance Summaries, 65(13), 1–23. doi: 10.15585/mmwr.ss6513a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Cortes A, Ahonen H, Evans M, Freedman M, & Bartel L (2016). Short-Term Effects of Rhythmic Sensory Stimulation in Alzheimer’s Disease: An Exploratory Pilot Study. Journal of Alzheimer’s Disease, 52(2), 651–660. doi: 10.3233/JAD-160081 [DOI] [PubMed] [Google Scholar]
- Cohen MX (2014). Analyzing neural time series data: Theory and practice. MIT press. [Google Scholar]
- Cornew L, Roberts TPL, Blaskey L, & Edgar JC (2012). Resting-State Oscillatory Activity in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 42(9), 1884–1894. doi: 10.1007/s10803-011-1431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Torriero S, Oliveri M, & Caltagirone C (2008). Prefrontal and temporo-parietal involvement in taking others’ perspective: TMS evidence. Behavioural Neurology, 19(1–2), 71–74. doi: 10.1155/2008/694632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E (1997). Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Current Opinion in Neurobiology, 7(2), 269–278. doi: 10.1016/S0959-4388(97)80016-5 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, … Courchesne RY (2001). Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology, 57(2), 245–254. doi: 10.1212/wnl.57.2.245 [DOI] [PubMed] [Google Scholar]
- Dan Y, & Poo M-M (2006). Spike timing-dependent plasticity: From synapse to perception. Physiological Reviews, 86(3), 1033–1048. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, & Iacoboni M (2006). Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience, 9(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis M, & Wagner KD (2016). Treatment of Autism Spectrum Disorder in Children and Adolescents. Psychopharmacology Bulletin, 46(2), 18–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, Bhumbra GS, Pazienti A, Lourenço J, Mailhes C, Aguirre A, … Bacci A (2019). Strong preference for autaptic self-connectivity of neocortical PV interneurons facilitates their tuning to γ-oscillations. PLOS Biology, 17(9), e3000419. doi: 10.1371/journal.pbio.3000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Moreno-De-Luca D, Heron D, Bouteiller D, Gennetier A, Delorme R, … Betancur C (2009). Screening for Genomic Rearrangements and Methylation Abnormalities of the 15q11-q13 Region in Autism Spectrum Disorders. Biological Psychiatry, 66(4), 349–359. doi: 10.1016/j.biopsych.2009.01.025 [DOI] [PubMed] [Google Scholar]
- Devlin B, & Scherer SW (2012). Genetic architecture in autism spectrum disorder. Current Opinion in Genetics & Development, 22(3), 229–237. doi: 10.1016/j.gde.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Do KQ, Cuenod M, & Hensch TK (2015). Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophrenia Bulletin, 41(4), 835–846. doi: 10.1093/schbul/sbv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, … Roberts TPL (2015). Neuromagnetic Oscillations Predict Evoked-Response Latency Delays and Core Language Deficits in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 45(2), 395–405. doi: 10.1007/s10803-013-1904-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti I-M, & Shapiro T (2003). A systems neuroscience approach to autism: Biological, cognitive, and clinical perspectives. Mental Retardation and Developmental Disabilities Research Reviews, 9(3), 205–215. doi: 10.1002/mrdd.10081 [DOI] [PubMed] [Google Scholar]
- Farrant K, & Uddin LQ (2016). Atypical developmental of dorsal and ventral attention networks in autism. Developmental Science, 19(4), 550–563. doi: 10.1111/desc.12359 [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, & Daskalakis ZJ (2010). Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain, 133(5), 1505–1514. doi: 10.1093/brain/awq046 [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Sun Y, Fitzgerald PB, & Daskalakis ZJ (2012). Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia: Gamma oscillations and schizophrenia. Annals of the New York Academy of Sciences, 1265(1), 25–35. doi: 10.1111/j.1749-6632.2012.06543.x [DOI] [PubMed] [Google Scholar]
- Ferguson BR, & Gao W-J (2018). PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Frontiers in Neural Circuits, 12, 37. doi: 10.3389/fncir.2018.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, … Tononi G (2008). Reduced Evoked Gamma Oscillations in the Frontal Cortex in Schizophrenia Patients: A TMS/EEG Study. American Journal of Psychiatry, 165(8), 996–1005. doi: 10.1176/appi.ajp.2008.07111733 [DOI] [PubMed] [Google Scholar]
- Feurra M, Bianco G, Santarnecchi E, Del Testa M, Rossi A, & Rossi S (2011). Frequency-Dependent Tuning of the Human Motor System Induced by Transcranial Oscillatory Potentials. Journal of Neuroscience, 31(34), 12165–12170. doi: 10.1523/JNEUROSCI.0978-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice F, Vörckel KJ, Sungur AÖ, Wöhr M, & Schwaller B (2016). Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Molecular Brain, 9, 10. doi: 10.1186/s13041-016-0192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J, Johnson K, Kehoe E, Bokde ALW, Garavan H, Gallagher L, & McGrath J (2015). Disrupted functional connectivity in dorsal and ventral attention networks during attention orienting in autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research, 8(2), 136–152. doi: 10.1002/aur.1430 [DOI] [PubMed] [Google Scholar]
- Fröhlich F, & McCormick DA (2010). Endogenous Electric Fields May Guide Neocortical Network Activity. Neuron, 67(1), 129–143. doi: 10.1016/j.neuron.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gągol A, Magnuski M, Kroczek B, Kałamała P, Ociepka M, Santarnecchi E, & Chuderski A (2018). Delta-gamma coupling as a potential neurophysiological mechanism of fluid intelligence. Intelligence, 66, 54–63. doi: 10.1016/j.intell.2017.11.003 [DOI] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, & Siegel SJ (2010). Validating γ Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biological Psychiatry, 68(12), 1100–1106. doi: 10.1016/j.biopsych.2010.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, & Siegel SJ (2012a). Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology, 62(3), 1504–1518. doi: 10.1016/j.neuropharm.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, & Siegel SJ (2012b). Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology, 62(3), 1504–1518. doi: 10.1016/j.neuropharm.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie AK, Jones EA, Lin Y-H, Karlsson MP, Kay K, Yoon SY, … Huang Y (2016). Apolipoprotein E4 Causes Age-Dependent Disruption of Slow Gamma Oscillations during Hippocampal Sharp-Wave Ripples. Neuron, 90(4), 740–751. doi: 10.1016/j.neuron.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustiniani A, Tarantino V, Bonaventura RE, Smirni D, Turriziani P, & Oliveri M (2019). Effects of low-gamma tACS on primary motor cortex in implicit motor learning. Behavioural Brain Research, 376, 112170. doi: 10.1016/j.bbr.2019.112170 [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, & Hensch TK (2009). Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of Neurodevelopmental Disorders, 1(2), 172–181. doi: 10.1007/s11689-009-9023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, & Lewis DA (2011). GABA Neuron Alterations, Cortical Circuit Dysfunction and Cognitive Deficits in Schizophrenia. Neural Plasticity, 2011, 1–24. doi: 10.1155/2011/723184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, & Martin A (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain: A Journal of Neurology, 135(Pt 9), 2711–2725. doi: 10.1093/brain/aws160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, & Johnson MH (2001). Disordered visual processing and oscillatory brain activity in autism and Williams Syndrome: Neuroreport, 12(12), 2697–2700. doi: 10.1097/00001756-200108280-00021 [DOI] [PubMed] [Google Scholar]
- Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, … Børglum AD (2019). Identification of common genetic risk variants for autism spectrum disorder. Nature Genetics, 51(3), 431–444. doi: 10.1038/s41588-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, Bologna M, Paparella G, Suppa A, Colella D, Di Lazzaro V, … Berardelli A (2018). Effects of Transcranial Alternating Current Stimulation on Repetitive Finger Movements in Healthy Humans. Neural Plasticity, 2018. doi: 10.1155/2018/4593095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, Suppa A, Asci F, De Marco G, D’Onofrio V, Bologna M, … Berardelli A (2019). LTD-like plasticity of the human primary motor cortex can be reversed by γ-tACS. Brain Stimulation, 12(6), 1490–1499. doi: 10.1016/j.brs.2019.06.029 [DOI] [PubMed] [Google Scholar]
- Guerra A, Suppa A, Bologna M, D’Onofrio V, Bianchini E, Brown P, … Berardelli A (2018). Boosting the LTP-like plasticity effect of intermittent theta-burst stimulation using gamma transcranial alternating current stimulation. Brain Stimulation, 11(4), 734–742. doi: 10.1016/j.brs.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon J, Attal Y, Colliot O, La Corte V, Dubois B, Schwartz D, … De Vico Fallani F (2017). Loss of brain inter-frequency hubs in Alzheimer’s disease. Scientific Reports, 7(1), 10879. doi: 10.1038/s41598-017-07846-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertl K, Callahan D, Markovics J, & Sheppard SS (2013). Perspectives of Adults Living With Autism Spectrum Disorder: Psychosocial and Occupational Implications. Occupational Therapy in Mental Health, 29(1), 27–41. doi: 10.1080/0164212X.2012.760303 [DOI] [Google Scholar]
- Hamilton AF de C. (2013). Reflecting on the mirror neuron system in autism: A systematic review of current theories. Developmental Cognitive Neuroscience, 3, 91–105. doi: 10.1016/j.dcn.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, de C, Brindley RM, & Frith U (2007). Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia, 45(8), 1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022 [DOI] [PubMed] [Google Scholar]
- Hashemi E, Ariza J, Rogers H, Noctor SC, & Martínez-Cerdeño V (2016). The Number of Parvalbumin-Expressing Interneurons Is Decreased in the Medial Prefrontal Cortex in Autism. Cerebral Cortex, bhw021. doi: 10.1093/cercor/bhw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch C, Krüger MC, & Brüne M (2012). Repetitive transcranial magnetic stimulation over the temporoparietal junction influences distinction of self from famous but not unfamiliar others. Behavioral Neuroscience, 126(6), 792–796. doi: 10.1037/a0030581 [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Knepper H, Nolte G, Strüber D, Rach S, Herrmann CS, … Engel AK (2014). Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biology, 12(12), e1002031. doi: 10.1371/journal.pbio.1002031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, & Bilimoria PM (2012). Re-opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum: The Dana Forum on Brain Science, 2012, 11. [PMC free article] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Wandell BA, & Winawer J (2015). Stimulus Dependence of Gamma Oscillations in Human Visual Cortex. Cerebral Cortex, 25(9), 2951–2959. doi: 10.1093/cercor/bhu091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, & Strüber D (2013). Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Frontiers in Human Neuroscience, 7. doi: 10.3389/fnhum.2013.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy KE, Bailey N, Arnold S, Windsor K, John J, Daskalakis ZJ, & Fitzgerald PB (2015). The effect of γ-tACS on working memory performance in healthy controls. Brain and Cognition, 101, 51–56. doi: 10.1016/j.bandc.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Hoy KE, Bailey NW, Arnold SL, & Fitzgerald PB (2015). The effect of transcranial Direct Current Stimulation on gamma activity and working memory in schizophrenia. Psychiatry Research, 228(2), 191–196. doi: 10.1016/j.psychres.2015.04.032 [DOI] [PubMed] [Google Scholar]
- Hu JS, Vogt D, Sandberg M, & Rubenstein JL (2017). Cortical interneuron development: A tale of time and space. Development, 144(21), 3867–3878. doi: 10.1242/dev.132852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert SW, & Jiang Y-H (2016). Monogenic mouse models of autism spectrum disorders: Common mechanisms and missing links. Neuroscience, 321, 3–23. doi: 10.1016/j.neuroscience.2015.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, & Zhang H (2010). Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Research, 1309, 83–94. doi: 10.1016/j.brainres.2009.09.120 [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, … Tsai L-H (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature, 540(7632), 230–235. doi: 10.1038/nature20587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, … Wigler M (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515(7526), 216–221. doi: 10.1038/nature13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi-Najafabadi S, Mirzakhani-Araghi N, Miri-Lavasani N, Nejati V, & Pashazadeh-Azari Z (2015). Implicit and explicit motor learning: Application to children with Autism Spectrum Disorder (ASD). Research in Developmental Disabilities, 47, 284–296. doi: 10.1016/j.ridd.2015.09.020 [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, & Lavidor M (2012). tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Experimental Brain Research, 216(1), 1–10. doi: 10.1007/s00221-011-2891-9 [DOI] [PubMed] [Google Scholar]
- Jannati A, Block G, Ryan MA, Kaye HL, Kayarian FB, Bashir S, … Rotenberg A (2020). Continuous Theta-Burst Stimulation in Children With High-Functioning Autism Spec- trum Disorder and Typically Developing Children. Frontiers in Integrative Neuroscience, 14 10.3389/fnint.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannati A, Ryan MA, Kaye HL, Tsuboyoma M, & Rotenberg A (in press). Biomarkers obtained by transcranial magnetic stimulation in neurodevelopmental disorders. Journal of Clinical Neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, & Kohn A (2011). Gamma Rhythms in the Brain. PLoS Biology, 9(4), e1001045. doi: 10.1371/journal.pbio.1001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochaut D, Lehongre K, Saitovitch A, Devauchelle A-D, Olasagasti I, Chabane N, … Giraud A-L (2015). Atypical coordination of cortical oscillations in response to speech in autism. Frontiers in Human Neuroscience, 9. doi: 10.3389/fnhum.2015.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, & Pelphrey KA (2011). Structural neural phenotype of autism: Preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. AJNR. American Journal of Neuroradiology, 32(9), 1607–1613. doi: 10.3174/ajnr.A2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Jenkinson N, Brittain J-S, Aziz TZ, & Brown P (2012). Driving Oscillatory Activity in the Human Cortex Enhances Motor Performance. Current Biology, 22(5), 403–407. doi: 10.1016/j.cub.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung NH, Janzarik WG, Delvendahl I, Münchau A, Biscaldi M, Mainberger F, … Mall V (2013). Impaired induction of long-term potentiation-like plasticity in patients with high-functioning autism and Asperger syndrome. Developmental Medicine and Child Neurology, 55(1), 83–89. doi: 10.1111/dmcn.12012 [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, & Minshew NJ (2007). Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex (New York, N.Y.: 1991), 17(4), 951–961. doi: 10.1093/cercor/bhl006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, & Minshew NJ (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain, 127(8), 1811–1821. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, & Varma S (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36(4), 1292–1313. doi: 10.1016/j.neubiorev.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Chaieb L, Antal A, Walsh V, & Paulus W (2008). Frequency-Dependent Electrical Stimulation of the Visual Cortex. Current Biology, 18(23), 1839–1843. doi: 10.1016/j.cub.2008.10.027 [DOI] [PubMed] [Google Scholar]
- Kang J, Cai E, Han J, Tong Z, Li X, Sokhadze EM, … Li X (2018). Transcranial Direct Current Stimulation (tDCS) Can Modulate EEG Complexity of Children With Autism Spectrum Disorder. Frontiers in Neuroscience, 12, 201. doi: 10.3389/fnins.2018.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, & Courchesne E (2008). Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognitive and Affective Neuroscience, 3(2), 177–190. doi: 10.1093/scan/nsn011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, & Pilly PK (2018). Closed-loop slow-wave tACS improves sleep-dependent long-term memory generalization by modulating endogenous oscillations. Journal of Neuroscience, 38(33), 7314–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, … Kenet T (2013). Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America, 110(8), 3107–3112. doi: 10.1073/pnas.1214533110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, & Jelic V (2005). Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiology of Aging, 26(2), 165–171. doi: 10.1016/j.neurobiolaging.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Kokoszka MA, McGoldrick PE, La Vega-Talbott M, Raynes H, Palmese CA, Wolf SM, … Ghatan S (2017). Epilepsy surgery in patients with autism. Journal of Neurosurgery: Pediatrics, 19(2), 196–207. doi: 10.3171/2016.7.PEDS1651 [DOI] [PubMed] [Google Scholar]
- Krall S, Rottschy C, Oberwelland E, Bzdok D, Fox P, Eickhoff S, … Konrad K (2015). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Structure and Function, 220(2), 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuki T, Fujihara K, Miwa H, Tamamaki N, Yanagawa Y, & Mushiake H (2015). Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Frontiers in Neural Circuits, 9, 6. doi: 10.3389/fncir.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurudenkandy FR, Zilberter M, Biverstål H, Presto J, Honcharenko D, Strömberg R, … Fisahn A (2014). Amyloid-β-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(34), 11416–11425. doi: 10.1523/JNEUROSCI.1195-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarev VV, Pontes A, Mitrofanov AA, & deAzevedo LC (2010). Interhemispheric asymmetry in EEG photic driving coherence in childhood autism. Clinical Neurophysiology, 121(2), 145–152. doi: 10.1016/j.clinph.2009.10.010 [DOI] [PubMed] [Google Scholar]
- Lazarev Vladimir V., Pontes A, & deAzevedo LC (2009). EEG photic driving: Right-hemisphere reactivity deficit in childhood autism. A pilot study. International Journal of Psychophysiology, 71(2), 177–183. doi: 10.1016/j.ijpsycho.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Lee H, Dvorak D, & Fenton AA (2014). Targeting Neural Synchrony Deficits is Sufficient to Improve Cognition in a Schizophrenia-Related Neurodevelopmental Model. Frontiers in Psychiatry, 5. doi: 10.3389/fpsyt.2014.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, … Kenworthy LE (2008). Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: A fcMRI study of response inhibition. Cerebral Cortex, 19(8), 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LM, Uehara K, & Hanakawa T (2015). The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Frontiers in Cellular Neuroscience, 9. doi: 10.3389/fncel.2015.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, & Grace AA (2009). A Loss of Parvalbumin-Containing Interneurons Is Associated with Diminished Oscillatory Activity in an Animal Model of Schizophrenia. Journal of Neuroscience, 29(8), 2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C, Estévez M, Leisman G, Melillo R, Rodríguez R, DeFina P, … Beltrán C (2015). QEEG Spectral and Coherence Assessment of Autistic Children in Three Different Experimental Conditions. Journal of Autism and Developmental Disorders, 45(2), 406–424. doi: 10.1007/s10803-013-1909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Banerjee A, Tripathi M, & Sharma A (2018). A Comprehensive Review of Magnetoencephalography (MEG) Studies for Brain Functionality in Healthy Aging and Alzheimer’s Disease (AD). Frontiers in Computational Neuroscience, 12, 60. doi: 10.3389/fncom.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria K, … Muotri AR (2017). Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Molecular Psychiatry, 22(6), 820–835. doi: 10.1038/mp.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, & Mostofsky SH (2015). Behavioural and neural basis of anomalous motor learning in children with autism. Brain, 138(3), 784–797. doi: 10.1093/brain/awu394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, & Wu C (2004). Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience, 5(10), 793–807. doi: 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- Marsh LE, & Hamilton AF, de C. (2011). Dissociation of mirroring and mentalising systems in autism. NeuroImage, 56(3), 1511–1519. doi: 10.1016/j.neuroimage.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Martin HGS, Lassalle O, Brown JT, & Manzoni OJ (2016). Age-Dependent Long-Term Potentiation Deficits in the Prefrontal Cortex of the Fmr1 Knockout Mouse Model of Fragile X Syndrome. Cerebral Cortex, 26(5), 2084–2092. doi: 10.1093/cercor/bhv031 [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Villalobos ME, Schultz RT, Herpertz-Dahlmann B, Konrad K, & Kohls G (2015). Atypical Laterality of Resting Gamma Oscillations in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 45(2), 292–297. doi: 10.1007/s10803-013-1842-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, … Chua SE (2005). Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain: A Journal of Neurology, 128(Pt 2), 268–276. doi: 10.1093/brain/awh332 [DOI] [PubMed] [Google Scholar]
- McDermott B, Porter E, Hughes D, McGinley B, Lang M, O’Halloran M, & Jones M (n.d.). Gamma Band Neural Stimulation in Humans and the Promise of a New Modality to Prevent and Treat Alzheimer’s Disease. Journal of Alzheimer’s Disease, 65(2), 363–392. doi: 10.3233/JAD-180391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E, Scope A, Pascalis O, Buckley D, & Makeig S (2009). Independent Component Analysis Reveals Atypical Electroencephalographic Activity During Visual Perception in Individuals with Autism. Biological Psychiatry, 65(1), 22–30. doi: 10.1016/j.biopsych.2008.07.017 [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, & Sweeney JA (1999). Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology, 52(5), 917–922. doi: 10.1212/wnl.52.5.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaguchi S, Otsuru N, Kojima S, Saito K, Inukai Y, Masaki M, & Onishi H (2018). Transcranial Alternating Current Stimulation With Gamma Oscillations Over the Primary Motor Cortex and Cerebellar Hemisphere Improved Visuomotor Performance. Frontiers in Behavioral Neuroscience, 12, 132. doi: 10.3389/fnbeh.2018.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisa M, Polania R, Grueschow M, & Ruff CC (2016a). Brain Network Mechanisms Underlying Motor Enhancement by Transcranial Entrainment of Gamma Oscillations. The Journal of Neuroscience, 36(47), 12053–12065. doi: 10.1523/JNEUROSCI.2044-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisa M, Polania R, Grueschow M, & Ruff CC (2016b). Brain Network Mechanisms Underlying Motor Enhancement by Transcranial Entrainment of Gamma Oscillations. Journal of Neuroscience, 36(47), 12053–12065. doi: 10.1523/JNEUROSCI.2044-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, & Valakh V (2015). Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron, 87(4), 684–698. doi: 10.1016/j.neuron.2015.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Tergau F, & Paulus W (2002). [Modulation of cortical excitability by transcranial direct current stimulation]. Der Nervenarzt, 73(4), 332–335. doi: 10.1007/s00115-002-1272-9 [DOI] [PubMed] [Google Scholar]
- Nitsche Michael A, & Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M, Hinson E, van Ede F, Pogosyan A, Guerra A, Quinn A, … Stagg CJ (2017). Driving Human Motor Cortical Oscillations Leads to Behaviorally Relevant Changes in Local GABAA Inhibition: A tACS-TMS Study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(17), 4481–4492. doi: 10.1523/JNEUROSCI.0098-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ifert-Miller F, Najib U, Bashir S, Gonzalez-Heydrich J, Picker J, … Pascual-Leone A (2016). Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome. Journal of Child and Adolescent Psychopharmacology, 26(7), 617–624. doi: 10.1089/cap.2015.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz A, Falchier A, Yan C-G, Yeagle EM, Linn GS, Megevand P, … Schroeder CE (2016). Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Scientific Reports, 6(1), 1–11. doi: 10.1038/srep31236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, & Elam M (2007). Excess of High Frequency Electroencephalogram Oscillations in Boys with Autism. Biological Psychiatry, 62(9), 1022–1029. doi: 10.1016/j.biopsych.2006.12.029 [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, & Elam M (2008). Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters, 434(2), 218–223. doi: 10.1016/j.neulet.2008.01.066 [DOI] [PubMed] [Google Scholar]
- Ozdemir RA, Tadayon E, Boucher P, Momi D, Karakhanyan KA, Fox MD, … Santarnecchi E (2020). Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1911240117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Lynch CJ, Schaer M, & Menon V (2017). The Default Mode Network in Autism. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 2(6), 476–486. doi: 10.1016/j.bpsc.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S, Ten Brinke MM, Stedehouder J, Reinelt CM, Wu B, Zhou H, … De Zeeuw CI (2016). Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nature Communications, 7, 12627. doi: 10.1038/ncomms12627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Kloth AD, Grasselli G, Titley HK, Nakayama H, Hashimoto K, … Hansel C (2014). Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nature Communications, 5(1), 1–13. doi: 10.1038/ncomms6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port RG, Berman JI, Liu S, Featherstone RE, Roberts TPL, & Siegel SJ (2019). Parvalbumin Cell Ablation of NMDA-R1 Leads to Altered Phase, But Not Amplitude, of Gamma-Band Cross-Frequency Coupling. Brain Connectivity, 9(3), 263–272. doi: 10.1089/brain.2018.0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poza J, Hornero R, Abasolo D, Fernandez A, & Escudero J (2007). Analysis of Spontaneous MEG Activity in Patients with Alzheimer’s Disease using Spectral Entropies. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 6179–6182. doi: 10.1109/IEMBS.2007.4353766 [DOI] [PubMed] [Google Scholar]
- Puts NAJ, & Edden RAE (2012). In vivo magnetic resonance spectroscopy of GABA: A methodological review. Progress in Nuclear Magnetic Resonance Spectroscopy, 60, 29–41. doi: 10.1016/j.pnmrs.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raco V, Bauer R, Tharsan S, & Gharabaghi A (2016). Combining TMS and tACS for closed-loop phase-dependent modulation of corticospinal excitability: A feasibility study. Frontiers in Cellular Neuroscience, 10, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G, & Geschwind DH (2018). Genetics of autism spectrum disorder. In Handbook of Clinical Neurology (Vol. 147, pp. 321–329). doi: 10.1016/B978-0-444-63233-3.00021-X [DOI] [PubMed] [Google Scholar]
- Redcay E (2008). The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neuroscience & Biobehavioral Reviews, 32(1), 123–142. [DOI] [PubMed] [Google Scholar]
- Reed T, & Cohen Kadosh R (2018). Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. Journal of Inherited Metabolic Disease, 41(6), 1123–1130. doi: 10.1007/s10545-018-0181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, … Llinas R (1991). Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proceedings of the National Academy of Sciences, 88(24), 11037–11041. doi: 10.1073/pnas.88.24.11037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, & Rogers SJ (2008). Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry, 8(1), 66. doi: 10.1186/1471-244X-8-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, & Wilson LB (2014). γ-band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine, 8(3), 353–368. doi: 10.2217/bmm.14.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Lauro LJ, Rosanova M, Mattavelli G, Convento S, Pisoni A, Opitz A, … Vallar G (2014). TDCS increases cortical excitability: Direct evidence from TMS-EEG. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 58, 99–111. doi: 10.1016/j.cortex.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Rothärmel M, Moulier V, Vasse M, Isaac C, Faerber M, Bendib B, … Guillin O (2019). A Prospective Open-Label Pilot Study of Transcranial Direct Current Stimulation in High-Functioning Autistic Patients with a Dysexecutive Syndrome. Neuropsychobiology, 78(4), 189–199. doi: 10.1159/000501025 [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, & Merzenich MM (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior, 2(5), 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S, Williams N, Hughes LE, Cope TE, Rittman T, Coyle-Gilchrist ITS, … Rowe JB (2018). Neurophysiological signatures of Alzheimer’s disease and frontotemporal lobar degeneration: Pathology versus phenotype. Brain, 141(8), 2500–2510. doi: 10.1093/brain/awy180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Biasella A, Tatti E, Rossi A, Prattichizzo D, & Rossi S (2017a). High-gamma oscillations in the motor cortex during visuo-motor coordination: A tACS interferential study. Brain Research Bulletin, 131, 47–54. doi: 10.1016/j.brainresbull.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, Biasella A, Tatti E, Rossi A, Prattichizzo D, & Rossi S (2017b). High-gamma oscillations in the motor cortex during visuo-motor coordination: A tACS interferential study. Brain Research Bulletin, 131, 47–54. doi: 10.1016/j.brainresbull.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, Brem A-K, Levenbaum E, Thompson T, Kadosh RC, & Pascual-Leone A (2015). Enhancing cognition using transcranial electrical stimulation. Current Opinion in Behavioral Sciences, 4, 171–178. doi: 10.1016/j.cobeha.2015.06.003 [DOI] [Google Scholar]
- Santarnecchi E, Muller T, Rossi S, Sarkar A, Polizzotto NR, Rossi A, & Cohen Kadosh R (2016). Individual differences and specificity of prefrontal gamma frequency-tACS on fluid intelligence capabilities. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 75, 33–43. doi: 10.1016/j.cortex.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Santarnecchi Emiliano, Polizzotto NR, Godone M, Giovannelli F, Feurra M, Matzen L, … Rossi S (2013). Frequency-Dependent Enhancement of Fluid Intelligence Induced by Transcranial Oscillatory Potentials. Current Biology, 23(15), 1449–1453. doi: 10.1016/j.cub.2013.06.022 [DOI] [PubMed] [Google Scholar]
- Santarnecchi Emiliano, Rossi S, & Rossi A (2015). The smarter, the stronger: Intelligence level correlates with brain resilience to systematic insults. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 64, 293–309. doi: 10.1016/j.cortex.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Santiesteban I, Banissy MJ, Catmur C, & Bird G (2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22(23), 2274–2277. [DOI] [PubMed] [Google Scholar]
- Sarrett JC (2016). Biocertification and Neurodiversity: The Role and Implications of Self-Diagnosis in Autistic Communities. Neuroethics, 9(1), 23–36. doi: 10.1007/s12152-016-9247-x [DOI] [Google Scholar]
- Saunders JA, Tatard-Leitman VM, Suh J, Billingslea EN, Roberts TP, & Siegel SJ (2013). Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Research: Official Journal of the International Society for Autism Research, 6(2), 69–77. doi: 10.1002/aur.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Otte E, Adigüzel K, Firk C, Herpertz-Dahlmann B, Koch I, & Konrad K (2017). Intact mirror mechanisms for automatic facial emotions in children and adolescents with autism spectrum disorder. Autism Research, 10(2), 298–310. doi: 10.1002/aur.1654 [DOI] [PubMed] [Google Scholar]
- Seymour RA, Rippon G, Gooding-Williams G, Schoffelen JM, & Kessler K (2019). Dysregulated oscillatory connectivity in the visual system in autism spectrum disorder. Brain: A Journal of Neurology, 142(10), 3294–3305. doi: 10.1093/brain/awz214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, & Perry D (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. [DOI] [PubMed] [Google Scholar]
- Sheikhani A, Behnam H, Mohammadi MR, Noroozian M, & Mohammadi M (2012). Detection of Abnormalities for Diagnosing of Children with Autism Disorders Using of Quantitative Electroencephalography Analysis. Journal of Medical Systems, 36(2), 957–963. doi: 10.1007/s10916-010-9560-6 [DOI] [PubMed] [Google Scholar]
- Sheikhani A, Behnam H, Noroozian M, Mohammadi MR, & Mohammadi M (2009). Abnormalities of quantitative electroencephalography in children with Asperger disorder in various conditions. Research in Autism Spectrum Disorders, 3(2), 538–546. doi: 10.1016/j.rasd.2008.11.002 [DOI] [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, & Müller R-A (2011). Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia, 49(5), 1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw D Sc JL, Tonge B, Egan G, O’Boyle MW, & Cunnington R (2006). Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: A functional MRI study. American Journal of Psychiatry, 163(8), 1440–1443. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O’Boyle MW, & Cunnington R (2006). Visuospatial Processing and the Function of Prefrontal-Parietal Networks in Autism Spectrum Disorders: A Functional MRI Study. American Journal of Psychiatry, 163(8), 1440–1443. doi: 10.1176/ajp.2006.163.8.1440 [DOI] [PubMed] [Google Scholar]
- Simon DM, & Wallace MT (2016). Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neuroscience & Biobehavioral Reviews, 68, 848–861. doi: 10.1016/j.neubiorev.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TM, Milivojevic B, & Kemner C (2013). Atypical excitation–inhibition balance in autism captured by the gamma response to contextual modulation. NeuroImage: Clinical, 3, 65–72. doi: 10.1016/j.nicl.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, & Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature, 459(7247), 698–702. doi: 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak E, de Lange FP, & Jensen O (2014). Local entrainment of α oscillations by visual stimuli causes cyclic modulation of perception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(10), 3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, … Dager SR (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology, 59(2), 184–192. doi: 10.1212/wnl.59.2.184 [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, & McCarley RW (2003a). Abnormal neural synchrony in schizophrenia. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(19), 7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, & McCarley RW (2003b). Abnormal Neural Synchrony in Schizophrenia. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 23(19), 7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, van Cappellen van Walsum AM, Pijnenburg YAL, Berendse HW, de Munck JC, Scheltens P, & van Dijk BW (2002). Generalized synchronization of MEG recordings in Alzheimer’s Disease: Evidence for involvement of the gamma band. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 19(6), 562–574. doi: 10.1097/00004691-200212000-00010 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, … Tsai PT (2017). Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nature Neuroscience, 20(12), 1744–1751. doi: 10.1038/s41593-017-0004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Butorina AV, Sysoeva OV, Prokofyev AO, Nikolaeva A, Yu, Tsetlin MM, & Orekhova EV (2015). Altered modulation of gamma oscillation frequency by speed of visual motion in children with autism spectrum disorders. Journal of Neurodevelopmental Disorders, 7(1), 21. doi: 10.1186/s11689-015-9121-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Prokofyev AO, Tsetlin MM, Gratchev VV, Morozov AA, & Obukhov YV (2012). High-frequency oscillatory response to illusory contour in typically developing boys and boys with autism spectrum disorders. Cortex, 48(6), 701–717. doi: 10.1016/j.cortex.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Suazo V, Díez Á, Martín C, Ballesteros A, Casado P, Martín-Loeches M, & Molina V (2012). Elevated noise power in gamma band related to negative symptoms and memory deficit in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 38(2), 270–275. doi: 10.1016/j.pnpbp.2012.04.010 [DOI] [PubMed] [Google Scholar]
- Sugata H, Yagi K, Yazawa S, Nagase Y, Tsuruta K, Ikeda T, … Kawakami K (2018). Modulation of Motor Learning Capacity by Transcranial Alternating Current Stimulation. Neuroscience, 391, 131–139. doi: 10.1016/j.neuroscience.2018.09.013 [DOI] [PubMed] [Google Scholar]
- Sun L, Grutzner C, Bolte S, Wibral M, Tozman T, Schlitt S, … Uhlhaas PJ (2012). Impaired Gamma-Band Activity during Perceptual Organization in Adults with Autism Spectrum Disorders: Evidence for Dysfunctional Network Activity in Frontal-Posterior Cortices. Journal of Neuroscience, 32(28), 9563–9573. doi: 10.1523/JNEUROSCI.1073-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, & Sweeney JA (2007). Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research, 156(2), 117–127. doi: 10.1016/j.pscychresns.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C (1999). Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences, 3(4), 151–162. doi: 10.1016/S1364-6613(99)01299-1 [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C (2009). The roles of gamma-band oscillatory synchrony in human visual cognition. Frontiers in Bioscience (Landmark Edition), 14, 321–332. doi: 10.2741/3246 [DOI] [PubMed] [Google Scholar]
- Tamás G, Buhl EH, Lörincz A, & Somogyi P (2000). Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nature Neuroscience, 3(4), 366–371. doi: 10.1038/73936 [DOI] [PubMed] [Google Scholar]
- Tavakoli AV, & Yun K (2017). Transcranial Alternating Current Stimulation (tACS) Mechanisms and Protocols. Frontiers in Cellular Neuroscience, 11, 214. doi: 10.3389/fncel.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, & Singer W (2006). Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron, 52(1), 155–168. doi: 10.1016/j.neuron.2006.09.020 [DOI] [PubMed] [Google Scholar]
- van Diessen E, Senders J, Jansen FE, Boersma M, & Bruining H (2015). Increased power of resting-state gamma oscillations in autism spectrum disorder detected by routine electroencephalography. European Archives of Psychiatry and Clinical Neuroscience, 265(6), 537–540. doi: 10.1007/s00406-014-0527-3 [DOI] [PubMed] [Google Scholar]
- Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL, … Hof PR (2017). Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathologica, 134(4), 537–566. doi: 10.1007/s00401-017-1736-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, … Sunaert S (2014). Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. NeuroImage: Clinical, 4, 374–382. doi: 10.1016/j.nicl.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, … Palop JJ (2012). Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell, 149(3), 708–721. doi: 10.1016/j.cell.2012.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violante IR, Li LM, Carmichael DW, Lorenz R, Leech R, Hampshire A, … Sharp DJ (2017). Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. ELife, 6. doi: 10.7554/eLife.22001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, Behrens MM, & Sejnowski TJ (2011). Downregulation of Parvalbumin at Cortical GABA Synapses Reduces Network Gamma Oscillatory Activity. Journal of Neuroscience, 31(49), 18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen A, Gross J, & Thut G (2015). Alpha Power Increase After Transcranial Alternating Current Stimulation at Alpha Frequency (α-tACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimulation, 8(3), 499–508. doi: 10.1016/j.brs.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Flory M, Kuchna I, Nowicki K, Ma SY, Imaki H, … Wisniewski T (2014). Brain-region–specific alterations of the trajectories of neuronal volume growth throughout the lifespan in autism. Acta Neuropathologica Communications, 2(1), 28. doi: 10.1186/2051-5960-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, … Wisniewski T (2010). The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathologica, 119(6), 755–770. doi: 10.1007/s00401-010-0655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, & Jefferys JGR (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature, 373(6515), 612–615. doi: 10.1038/373612a0 [DOI] [PubMed] [Google Scholar]
- Wilde C, Bruder R, Binder S, Marshall L, & Schweikard A (2015). Closed-loop transcranial alternating current stimulation of slow oscillations. Current Directions in Biomedical Engineering, 1(1), 85–88. [Google Scholar]
- Wilson TW, McDermott TJ, Mills MS, Coolidge NM, & Heinrichs-Graham E (2018). tDCS Modulates Visual Gamma Oscillations and Basal Alpha Activity in Occipital Cortices: Evidence from MEG. Cerebral Cortex (New York, N.Y.: 1991), 28(5), 1597–1609. doi: 10.1093/cercor/bhx055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, & Rogers SJ (2007). Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biological Psychiatry, 62(3), 192–197. doi: 10.1016/j.biopsych.2006.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski M, Garcia-Cossio E, Chander BS, Braun C, Birbaumer N, Robinson SE, & Soekadar SR (2016). Mapping entrained brain oscillations during transcranial alternating current stimulation (tACS). NeuroImage, 140, 89–98. doi: 10.1016/j.neuroimage.2015.10.024 [DOI] [PubMed] [Google Scholar]
- Wöhr M, Orduz D, Gregory P, Moreno H, Khan U, Vörckel KJ, … Schwaller B (2015). Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Translational Psychiatry, 5, e525. doi: 10.1038/tp.2015.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo T-UW, Spencer K, & McCarley RW (2010). Gamma oscillation deficits and the onset and early progression of schizophrenia. Harvard Review of Psychiatry, 18(3), 173–189. doi: 10.3109/10673221003747609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari F, Jamil A, Mosayebi Samani M, Vidor LP, & Nitsche MA (2018). Basic and functional effects of transcranial Electrical Stimulation (tES)—An introduction. Neuroscience & Biobehavioral Reviews, 85, 81–92. doi: 10.1016/j.neubiorev.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Yavari F, Nitsche MA, & Ekhtiari H (2017). Transcranial Electric Stimulation for Precision Medicine: A Spatiomechanistic Framework. Frontiers in Human Neuroscience, 11. doi: 10.3389/fnhum.2017.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian J-J, & Blatt GJ (2007). Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: Pathophysiological implications. Acta Neuropathologica, 113(5), 559–568. doi: 10.1007/s00401-006-0176-3 [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, … Deisseroth K (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature, 477(7363), 171–178. doi: 10.1038/nature10360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Soltész I, Bragin A, Penttonen M, Sik A, & Buzsáki G (1995). Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus, 5(1), 78–90. doi: 10.1002/hipo.450050110 [DOI] [PubMed] [Google Scholar]
- Young L, Camprodon JA, Hauser M, Pascual-Leone A, & Saxe R (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences, 107(15), 6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, & Deouell LY (2008). Transient Induced Gamma-Band Response in EEG as a Manifestation of Miniature Saccades. Neuron, 58(3), 429–441. doi: 10.1016/j.neuron.2008.03.027 [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, … Lainhart JE (2014). Longitudinal changes in cortical thickness in autism and typical development. Brain, 137(6), 1799–1812. doi: 10.1093/brain/awu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, & Barbas H (2013). Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Frontiers in Human Neuroscience, 7, 609. doi: 10.3389/fnhum.2013.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]