Abstract
Background & Aims:
Mathematical modeling of viral kinetics has been shown to identify patients with chronic hepatitis C virus (HCV) infection who could be cured with a shorter duration of direct-acting antiviral (DAA) treatment. However, modeling therapy duration has yet to be evaluated in recently infected individuals. The aim of this study was to retrospectively examine whether modeling can predict outcomes of six-week sofosbuvir (SOF) and weight-based ribavirin (R) therapy in individuals with recent HCV infection.
Methods:
Modeling was used to estimate viral host parameters and to predict time to cure for 12 adults with recent HCV infection (<12 months of infection) who received six weeks of treatment with SOF+R.
Results:
Modeling results yielded a 100% negative predictive value for SOF+R treatment failure in nine participants and suggested that a median of 13 [interquartile range: 8–16] weeks of therapy would be required for these patients to achieve cure. Modeling predicted cure after 5 weeks of therapy in the only modeled participant who achieved a sustained virological response. However, cure was also predicted for two participants who relapsed following treatment.
Conclusions:
The modeling results confirm that longer than 6 weeks of SOF+R is needed to reach cure in individuals with recent HCV infection. Prospective real-time modeling under current potent DAA regimens is needed to validate the potential of response-guided therapy in the management of recent HCV infection.
Keywords: hepatitis c virus, recent infection, direct-acting antivirals, mathematical modeling
1. Introduction
Progress toward eliminating hepatitis C virus (HCV) infection was accelerated by the introduction of all-oral direct-acting antivirals (DAAs) in 2014. The development of potent DAA regimens has resulted in cure rates exceeding 90% after 8–12 weeks of treatment. While DAAs have transformed the management of chronic HCV, there is still much to learn about optimizing DAA treatment for patients with recently acquired infections. Observational and modeling studies have suggested the potential for a treatment-as-prevention effect with expanded access and high uptake of DAA therapy (Boerekamps et al., 2018; Echevarria et al., 2019; Iversen et al., 2019; Martin et al., 2016; Martinello et al., 2019b; Salazar-Vizcaya et al., 2016). A recent modeling study showed that diagnosis and treatment of people with acute HCV infection (duration of infection <6 months) led to better health and economic outcomes (Bethea et al., 2018). While it has been well-documented that interferon-α therapy (IFN) was much more effective if treatment was administered early after infection (Martinello et al., 2015), pilot studies of DAA therapy for acute (<6 months) and recent (<12 months) HCV infection have demonstrated variable efficacy (sustained virologic response, SVR, 32%−100%), with higher efficacy seen with more potent dual- and triple-class DAA regimens (Martinello et al., 2018).
We have shown that retrospective modeling of early HCV treatment response in people with chronic infection receiving DAA therapy can be used to predict the time until the virus is eliminated from the extracellular fluid (i.e. time to cure) (Canini et al., 2017; Dahari et al., 2016; Gambato et al., 2019). These analyses suggest the possibility of reducing treatment duration in a sizeable proportion, thereby cutting costs. More recently, we successfully implemented a prospective, real-time modeling-based approach to identify the optimal length of treatment in patients with chronic HCV infection (Etzion et al., 2018). However, modeling has yet to be evaluated for subjects with recent HCV infections. These individuals are often asymptomatic and infrequently identified (Shteyer et al., 2019).
Our prior DARE C II study of six weeks of sofosbuvir (SOF) and weight-based ribavirin (R) in adults with recent (estimated duration < 12 months) HCV infection demonstrated that this combination was safe and well tolerated, but that efficacy was suboptimal (Martinello et al., 2016). Of the 18 subjects who completed the scheduled course of therapy, only six (33%) achieved a sustained virologic response at post-treatment week 12 (SVR). Two patients (11%) had virus levels above the limit of quantification at the end of treatment (one of whom achieved spontaneous clearance more than 12 weeks post treatment), nine (50%) participants experienced relapse, and a single patient (6%) was re-infected.
The aim of this study was to examine whether mathematical modeling of the viral kinetics of the participants from the DARE C II study could retrospectively predict treatment outcomes under short-duration SOF+R therapy.
2. Methods
2.1. Participants
Nineteen participants (≥18 years) with recent HCV infection were enrolled in the DAA-Based Therapy for Recent HCV II (DARE-C II) study between October 2014 and May 2015 in hospitals in Australia (n=4) and New Zealand (n=1). Eligibility criteria were reported previously (Martinello et al., 2016). Study participants were predominantly male (n=17, 89%), Caucasian (n=14, 74%), and men who have sex with men (n=16, 84%). The distribution of genotypes included genotype 1 (n=13, 68%) and genotype 3 (n=5, 26%). Fourteen participants (74%) were co-infected with human immunodeficiency virus (HIV), all of whom had HIV prior to their diagnosis of HCV, and 12 were on anti-retroviral therapy. Participants acquired HCV through injection drug use (n=10, 53%) or sexual exposure (n=9, 47%). The median HCV RNA level at baseline was 5.4 (4.5–6.7) log10 IU/ml. Eighteen participants (95%) completed the full 6 weeks of SOF+R therapy and one participant discontinued treatment after two weeks. As explained in Results, only 12 patients had sufficient viral kinetic data for modeling (Table 1).
Table 1:
Baseline Characteristics.
| Participant Number | Gender | Age | Weight (kg) | Height (cm) | Ethnicity | HCV Genotype | Mode of Acquiring HCV | HIV(Antiretro viral Therapy) | HCV Treatment Outcome* |
|---|---|---|---|---|---|---|---|---|---|
| 2 | Female | 23 | 59.0 | 166 | Caucasian | 3a | IDU | No | SVR |
| 4 | Male | 47 | 76.0 | 180.5 | Caucasian | 1a | IDU | No | Relapse |
| 8 | Male | 31 | 61.1 | 170 | Asian | 1a | IDU | Yes (nevirapine, TDF, FTC) | Relapse |
| 9 | Male | 73 | 68.1 | 178 | Caucasian | 1a | SE, MSM | Yes (etravirine, lamivudine, raltegravir) | Relapse |
| 10 | Male | 39 | 64.0 | 171 | Caucasian | 1a | IDU | Yes (raltegravir, TDF, FTC) | Relapse |
| 12 | Male | 31 | 94.6 | 186 | Caucasian | 1a | SE, MSM | Yes (rilpivirine, TDF, FTC) | Relapse |
| 13 | Male | 44 | 68.0 | 179 | Asian | 1a | IDU | Yes (efarivirenz, TDF, FTC) | Relapse |
| 14 | Male | 49 | 77.3 | 185 | Caucasian | 1a | SE, MSM | Yes (TDF, FTC) | Relapse |
| 15 | Male | 54 | 91.1 | 181 | Caucasian | 1a | SE, MSM | Yes (efavirenz, TDF, FTC) | Relapse |
| 17 | Male | 53 | 94.3 | 171 | Hispanic | 3a | SE, MSM | Yes (rilpivirine, TDF, FTC) | Non-response‡ |
| 18 | Male | 30 | 79.4 | 190 | Caucasian | 1a | IDU | Yes (TDF, FTC) | Non-response |
| 19 | Male | 45 | 90.5 | 186 | Caucasian | 3a | IDU | No | Relapse |
Non-response, signifies that HCV RNA was quantifiable at end of treatment (EOT); Relapse, signifies quantifiable HCV RNA, confirmed as homologous virus, at post-treatment week 12 despite unquantifiable HCV RNA at EOT;
Spontaneous clearance of virus more than 12 weeks post treatment observed; IDU, injecting drug use; MSM, men who have sex with men. HIV, human immunodeficiency virus; SVR, sustained virological response; SE, sexual exposure; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine.
2.2. HCV RNA Measurements
HCV RNA levels were measured at baseline, hour 4, day 2, and weeks 1, 2, 4, and 6 after initiation of SOF+R along with post-treatment weeks 4, 12, and 24 (Martinello et al., 2016). Central HCV RNA testing was performed using COBAS TaqMan assay v2.0 (lower limit of quantification [LLoQ] 25 IU/mL; lower limit of detection 15 IU/mL). SVR was defined as HCV RNA target not detected (TND) 12 weeks after cessation of SOF+R. In performing kinetic analysis and model fitting, we defined detectable but not quantifiable HCV RNA measurements to be 20 IU/mL (i.e., the average of lower limit of detection and LLoQ), and we took undetectable measurements to be 1 IU/ml.
2.3. Mathematical Modeling
HCV viral kinetic response to therapy was assumed to follow the standard biphasic model (Neumann et al., 1998):
| (Eq.1) |
where V, is the viral load in blood. Virus, V, infects target cells with rate constant β, generating productively-infected cells, I, which produce new virions at rate p per infected cell. Infected cells are lost at a rate δ per infected cell and virions are assumed to be cleared from blood at rate c per virion. Effect ε is defined as the therapy effectiveness 0≤ε≤1 in preventing viral production/secretion. We assume that therapy was effective after a pharmacological delay, t0. Similar to previous modeling efforts, we assume the target cell level remained constant during therapy at pre-treatment level T0=cδ/βp (Dahari et al., 2015).
Solving Eq. 1 predicts
| (Eq.2) |
where
2.4. Parameter Estimation
For each patient, we fit our model to the measured viral kinetic data until the first TND was reached. Viral host parameter estimates of t0, ε and δ (Eq. 2) were obtained using a Constrained Optimization by Linear Approximation (COBYLA) algorithm (Powell, 1994; Reinharz et al., 2019). Initial viral load, V0, was set during fitting based on each participant’s measured baseline HCV RNA, and the serum virus clearance rate c was fixed at 2 d−1 for all participants, as explained in Results.
2.5. Cure Boundary
The time to cure was defined as the time to reach less than one HCV particle in the entire extracellular body fluid (blood, interstitial and trans cellular) as previously done (Canini et al., 2017; Dahari et al., 2016; Gambato et al., 2019). The extracellular fluid volume is approximately 15 L, corresponding to a value of 7×10−5 for V (IU/ml) for the cure threshold. A sensitivity analysis was performed assuming 5 L to 20 L of extracellular body fluid volume, corresponding to cure threshold values of 2×10−4 and 5×10−5 IU/mL, respectively. Based on an assumed number of infected hepatocytes in the liver, a more speculative time to cure of less than one virus and infected hepatocyte in the body was previously analyzed and was found to overestimate the time to cure (Dahari et al., 2016; Gambato et al., 2019). Therefore, this speculative time to cure was not analyzed in the current study.
2.6. Statistical Analysis
Associations between participants’ baseline characteristics, their viral kinetics, fitted model parameters, and predicted cure times were evaluated with non-parametric tests. Fisher’s exact test was used for checking associations between categorical variables, and the Wilcoxon rank-sum test was used when one of the considered variables was continuous. For all analyses, a P-value, P≤0.05 was considered statistically significant. Data analyses were performed using R 3.5.0.
3. Results
3.1. Viral Kinetics
Because modeling and viral kinetic analysis requires sufficient data from both the 1st and the 2nd decline phases, only 12 of 19 DARE-C II participants were included in the modelling analysis. Of the seven participants excluded from the modeling analysis, reasons for exclusion included no data at week 1 (n=2), achieved TND at day 2 (n=2), or achieved TND at week 1 (n=3). Of these seven participants, 5 achieved SVR, 1 became re-infected, and 1 was lost to follow-up. As we previously reported, viral load below LLoQ by week 1 correlated strongly with treatment outcomes (p=0.006) (Martinello et al., 2016), and since early unquantifiable data presented an obstacle to modeling the data, there was a strong association between treatment failure and inclusion in our modeling analysis (p=0.001).
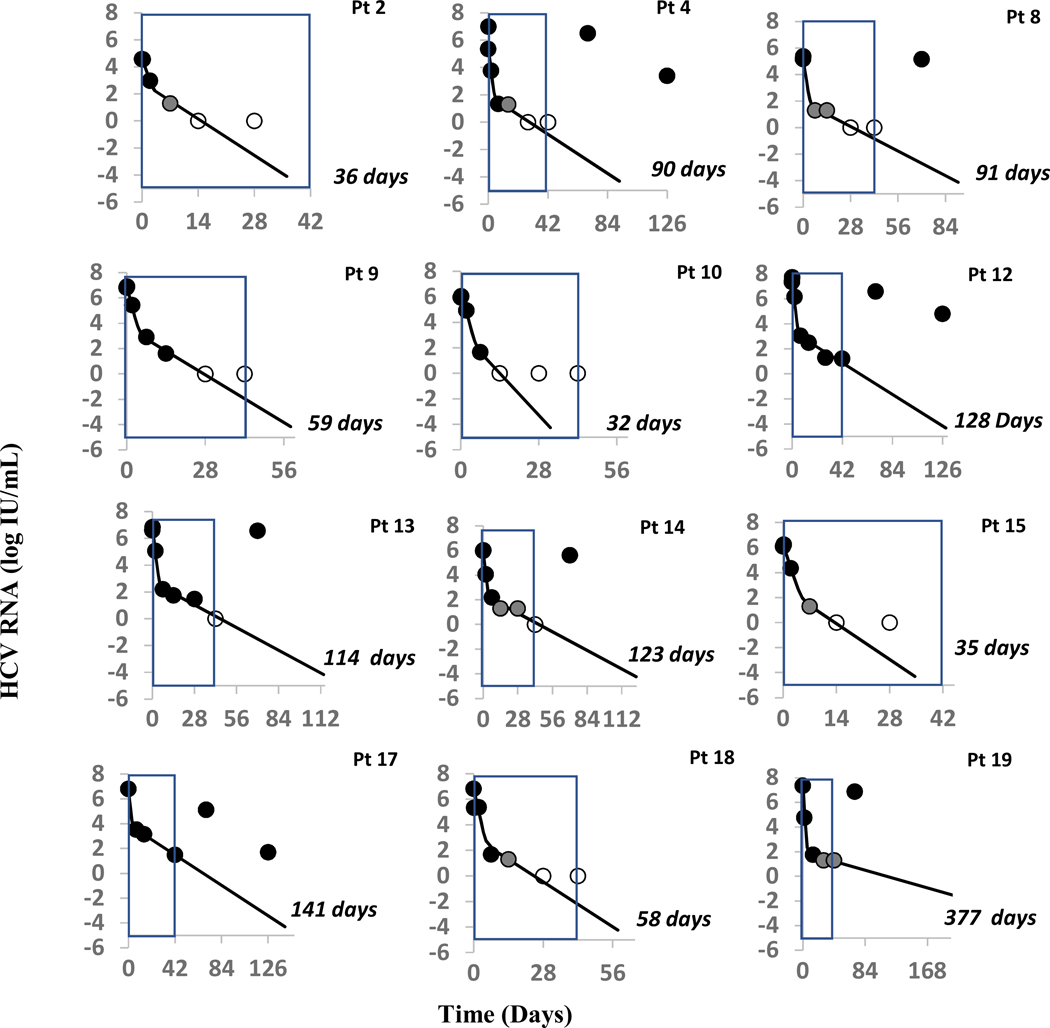
Of the 12 patients for whom sufficient data was available for modeling, only 1 achieved SVR. Clinical, demographic, and baseline viral characteristics of these 12 participants are detailed in Table 1. Median baseline HCV RNA was 6.36 (interquartile range, IQR: 5.81–6.82) log10 IU/ml. HCV RNA 4 hours after initiation of SOF+R was 6.25 (5.68–6.95) log10 IU/ml (with a single modeled patient missing data for this time point) and was not significantly different (p=0.054) from pre-treatment levels. Thereafter HCV RNA levels declined in a biphasic manner consisting of a rapid first phase (median slope −4.54 log/week corresponding to a HCV half-life of 11 hours) that lasted for the first week, followed by a slower second phase (slope −0.52 log/week) (Figure 1). There was no association between baseline characteristics and viral kinetics.
Figure 1:
Viral Kinetics Modeling Results
Fig. 1: Pt 2* achieved SVR. Observed viral kinetics and model predicted curves in eleven subjects, who did not achieve SVR. Black circles: quantifiable HCV; grey circles: detected, below LLoQ; open circles: below lower limit of detection; solid lines: biphasic model best fit curve, ending when cure threshold is reached; blue box represents the six weeks of therapy received by each patient. Model curves end when predicted time to cure is reached. Orange transparent box indicates the cure boundary predicted by the modeling. During modeling, the initial virus clearance, c, value was set to 2.0 d−1 for all 11 patients. Patient 19 only shows the curve till 300 days as model predicted time to cure that is too long to be graphed.
3.2. Parameter Estimation
Since viral kinetic data was not frequent enough to estimate all model parameters t0, c, ε and δ simultaneously (i.e., identifiability issue), the serum virus clearance rate c was fixed at 2/d based on the calculated median half-life of 11 hours thus allowing the other parameters to be estimated. Modeling results indicate a median pharmacological delay t0 of 2.6 (IQR 1.4–3.5) hours. After the delay, the biphasic model described the data well (Figure 1), and viral kinetic parameters for each participant were estimated (Table 3). The median treatment efficacy in blocking viral infection was ε=0.9995 d−1, and the median death/loss rate of infected cells δ was 0.223 (IQR 0.139–0.424) d-1. There was no association between participants’ baseline characteristics and viral-host parameters.
Table 3: Individual model parameter estimates.
V0, pre-treatment measured HCV RNA; N, number of HCV RNA measurements used for modeling; d, days; ε, therapy effectiveness; t0: pharmacological delay (h = hours); δ, infected-cell loss rate; viral clearance was fixed c=2 d−1; [ ], min-max estimate of time to cure based on 5L and 20L of extracellular body fluid; Δ,difference between model-predicted time to cure and length of administered therapy (negative number indicates model recommends therapy longer than that administered);
| Participant Number | N | V0 (log IU/ml) | t0 (h) | ε | δ (d−1) | Predicted time to cure (d) | Δ (d) |
|---|---|---|---|---|---|---|---|
| 2* | 5 | 4.58 | 2.6 | 0.9941 | 0.420 | 36 [33–37] | 6 |
| 4 | 6 | 5.36 | 4.1 | 0.9996 | 0.158 | 90 [83–92] | −48 |
| 8 | 5 | 5.18 | 3.4 | 0.9995 | 0.153 | 91 [84–94] | −49 |
| 9 | 6 | 6.82 | 4.8 | 0.9995 | 0.311 | 59 [54–60] | −17 |
| 10 | 5 | 5.97 | 14.6 | 0.9992 | 0.545 | 32 [29–33] | 10 |
| 12 | 7 | 7.37 | 1.2 | 0.9999 | 0.138 | 128 [122–134] | −86 |
| 13 | 7 | 6.60 | 1.4 | 0.9998 | 0.139 | 114 [106–117] | −72 |
| 14 | 7 | 5.96 | 2.4 | 0.9998 | 0.124 | 123 [114–126] | −81 |
| 15 | 5 | 6.11 | 2.6 | 0.9997 | 0.475 | 35 [33–37] | 7 |
| 17 | 4 | 6.80 | 2.9 | 0.9988 | 0.135 | 141 [133–144] | −99 |
| 18 | 6 | 6.84 | 0 | 0.9999 | 0.288 | 58 [54–66] | −16 |
| 19 | 5 | 7.02 | 0 | 0.9999 | 0.038 | 377 [349–385] | −335 |
, Participants who achieved SVR.
3.3. Predicting time to cure
Using the individual model fits, we calculated the time for each participant to achieve cure (Table 3 and Figure 1). Median time to cure for all modeled participants was 10.6 (IQR 6.9–14.0) weeks. Modeling correctly predicted non-SVR in 9 individuals with six weeks of treatment and suggested that a median of 13.0 (IQR 8.4–16.3) weeks of therapy would be required in these cases. The negative predictive value of our model was thus 100%, as each participant who received a model-predicted cure-time exceeding six weeks failed to achieve SVR (relapse, n=7; non-response, n=2). The model correctly predicted cure after 5.1 weeks of therapy in the only modeled participant who achieved SVR (Participant 2) but incorrectly predicted cure after 4.6 and 5.0 weeks in two individuals (Participants 10 and 15) who relapsed post-treatment. There was no association between baseline characteristics, anti-retroviral therapy, and predicted time to cure.
4. Discussion
This study serves as a proof-of-concept for modeling the viral kinetics of people who receive DAA therapy for recent HCV infection. With treatment failure in 13 (68%) cases, the results of the DARE-C II study (Martinello et al., 2016) indicated that six weeks of SOF+R therapy was sub-optimal for people with recent HCV. Our retrospective analysis of 12 of the study participants, including 9 individuals with chronic HIV infection, indicate that modeling would have provided a negative predictive value of 100%. In order to assess positive predictive value, efforts should be undertaken to model the viral kinetics of individuals with recent HCV infection who are treated with a more potent DAA regimen.
Prior to the availability of DAA therapy, higher efficacy was seen with shortened duration interferon-based regimens in recent HCV, as compared with standard duration in chronic HCV infection (Martinello et al., 2015). It was speculated that innate and adaptive immune responses were more robust in recent HCV compared to chronic HCV. The efficacy of various DAA regimens in recent HCV infection, including shortened treatment duration, have been evaluated. DARE-C II, which included frequent HCV RNA testing at early time points, was the first pilot study of a pan-genotypic interferon-free DAA regimen in recent HCV, and as such, the current study provides the first detailed HCV kinetic analysis and modeling of individuals with recent HCV treated with DAA therapy.
Historically, the standard biphasic model was used to explain the biphasic decline seen in chronic HCV-infected patients who were treated with daily IFN (Neumann et al, 1988). We showed that if the major effect of IFN treatment is to block the production or release of virions by infected cells (0<ε<1), the viral decline will be biphasic, with the initial slope of the first phase governed by the clearance rate of free virions c and by the efficacy, ε. The subsequent second-phase decline was predicted to mainly reflect the death/loss rate of productively infected cells. In the era of IFN-free all oral DAA therapy, the typical HCV decline is also biphasic (Dahari et al. 2011 and 2016). In the current study, it is not possible to identify all viral kinetic parameters (t0, c, ε and δ due to lack of frequent viral sampling during the first phase of decline. Thus, we fixed the HCV clearance rate to c=2/d based on the direct measurement of HCV half-life using linear regression. Interestingly, a previous modeling study (Osinusi et al., 2013) in chronic HCV-infected patients treated with SOF+R estimated a large range of c (1.88–7.15 d−1), so it is not known whether the HCV half-life differs between individuals with chronic versus acute HCV infections.
Sofosbuvir plus ribavirin for 12–24 weeks was previously evaluated as a treatment for chronic genotype 1–3 HCV infection, with variable SVR rates which were high in genotype 2 [SVR at 12 weeks (SVR12): 88%−97%] and more modest in genotype 1 [SVR12: 68%−85%] and genotype 3 [SVR12: 56%−89%] infected patients. (Gane et al., 2013; Zeuzem et al., 2014; Molina et al., 2015; Sulkowski et al., 2014). However, since HCV kinetic analysis (or modeling) was not performed in these studies, the reason(s) for the different SVR rates under SOF+R is not known. In the current modeling study, most (n=9) participants were genotype 1 with only three participants infected with genotype 3, precluding us from drawing conclusions as to whether viral kinetic parameters differ across genotype in recent HCV infections treated with SOF+R. Another major limitation of our study is that only one of the modeled individuals achieved SVR, and we were thus precluded from drawing conclusions about the model’s positive predictive value for response to recent HCV infection under SOF+R therapy. Future studies of the treatment of patients with recent HCV infection with more potent DAAs will be needed to establish the positive predictive value of the model. For example, a pilot study of people with recent HCV infection treated with glecaprevir-pibrentasvir (GLE-PIB) for six weeks (TARGET3D Cohort 2, clinical trial number 02634008) demonstrated high efficacy (per-protocol SVR 96%; relapse, n=1) (Martinello et al., 2019a). The high SVR suggested that some of these participants could have achieved cure in less than six weeks. Indeed, in a recent retrospective modeling of a cohort of chronically-infected patients (composed of treatment-naïve, treatment-experienced, cirrhotic, and non-cirrhotic participants) who received eight or 12 weeks of GLE-PIB, we found that 64% (28/44) might have been cured with less than seven weeks of the therapy, 50% in less than six weeks, and 16% in less than four weeks (Dasgupta et al., 2019). The findings suggest that modeling provides a means to identify chronically infected patients who would respond to a shorter duration therapy with GLE-PIB. Modeling could provide a similar advantage for recently infected participants treated with sofosbuvir/velpatasvir (Matthews et al., 2020).
In conclusion, modeling could be relevant to advancing a more individualized, response-guided approach to HCV treatment. Such an approach using early viral kinetic data (baseline, day 2, week 1 and/or 2 and week 4) has recently been implemented for the first time in a clinical setting for chronically-infected patients (Etzion et al., 2018). In the setting of recent HCV infection, where DAA regimens have yet to be standardized, response-guided therapy could provide great value.
Table 2: Viral Kinetics.
VBL: Viral load at baseline; Vh4: Viral load four hours after treatment initiation; Vw1: Viral load at the end of week 1; N: Number of measurements used to calculate slope for phase II; NA: Viral load measurement not available;
| Phase | Delay | I | II | ||
|---|---|---|---|---|---|
| Participant ID | VBL − Vh4 (log IU/ml) | VBL − Vw1 (log IU/ml) | Slope [log/week] | N | Slope [log/week] |
| 2 | 0.027 | 3.28 | −3.13 | 2 | −1.30 |
| 4 | −1.64 | 4.02 | −5.29 | 3 | −0.48 |
| 8 | −0.19 | 3.88 | −4.17 | 3 | −0.46 |
| 9 | −0.074 | 3.91 | −3.96 | 3 | −0.95 |
| 10 | −0.07 | 1.04 | −4.49 | 2 | −1.67 |
| 12 | −0.36 | 4.30 | −4.68 | 4 | −0.39 |
| 13 | −0.22 | 4.40 | −4.59 | 4 | −0.41 |
| 14 | −0.038 | 3.78 | −3.65 | 4 | −0.38 |
| 15 | −0.14 | 4.81 | −4.90 | 2 | −1.30 |
| 17* | NA | 3.28 | −3.28 | 3 | −0.41 |
| 18 | 1.50 | 5.15 | −5.16 | 3 | −0.58 |
| 19** | −0.34 | NA | −9.89 | 4 | −0.55 |
Vh4 was missing;
Viral load at end of week 1 missing, so phase 1 slope was computed using viral load at the end of day 2.
Highlights.
Modeling identifies chronic hepatitis C patients who were cured with a reduced duration of direct-acting antivirals (DAA)
Duration of DAA treatment has not been previously modeled in persons with recent (<12 months) hepatitis C infection
We retrospectively modeled ultra-short (6-week) sofosbuvir+ribavirin therapy in recently infected persons
Modeling might help identify persons with recent hepatitis C who are appropriate for ultra-short duration of DAA therapy
Acknowledgments
Financial support: The study was supported in part by U.S. NIH grants R01AI078881 and R01GM121600. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The clinical research reported in the previous DARE C II publication was supported by Gilead Sciences Inc. as an investigator-initiated study. The funders had no role in current study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Meeting procedures: The study was presented at the 70th Annual Meeting of the American Association for the study of Liver Diseases. Boston, MA, USA, Nov 8–12, 2019
Abbreviations:
- HCV
hepatitis C virus
- SOF
sofosbuvir
- R
ribavirin
- GLE-PIB
glecaprevir-pibrentasvir
- DAA
direct-acting antiviral
- SVR
sustained virological response
- DARE C II
DAA-Based Therapy for Recent HCV II
- HIV
human immunodeficiency virus
- IQR
interquartile range
Footnotes
Conflicts of interest: GVM advises, is on the speakers’ bureau, and received grants from Gilead. She is on the speakers’ bureau and received grants from AbbVie and Bristol-Myers Squibb. She received grants from Janssen. HD has consulted for CoCrystal Inc. None of the other authors has any financial interest or conflict of interest related to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bethea ED, Chen Q, Hur C, Chung RT, Chhatwal J, 2018. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology 67, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerekamps A, van den Berk GE, Lauw FN, Leyten EM, van Kasteren ME, van Eeden A, Posthouwer D, Claassen MA, Dofferhoff AS, Verhagen DWM, Bierman WF, Lettinga KD, Kroon FP, Delsing CE, Groeneveld PH, Soetekouw R, Peters EJ, Hullegie SJ, Popping S, van de Vijver D, Boucher CA, Arends JE, Rijnders BJ, 2018. Declining Hepatitis C Virus (HCV) Incidence in Dutch Human Immunodeficiency Virus-Positive Men Who Have Sex With Men After Unrestricted Access to HCV Therapy. Clin Infect Dis 66, 1360–1365. [DOI] [PubMed] [Google Scholar]
- Canini L, Imamura M, Kawakami Y, Uprichard SL, Cotler SJ, Dahari H, Chayama K, 2017. HCV kinetic and modeling analyses project shorter durations to cure under combined therapy with daclatasvir and asunaprevir in chronic HCV-infected patients. PLoS One 12, e0187409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahari H, Guedj J, Perelson AS, Layden TJ, 2011. Hepatitis C Viral Kinetics in the Era of Direct Acting Antiviral Agents and Interleukin-28B. Current Hepatitis Reports 10, 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahari H, Canini L, Graw F, Uprichard SL, Araujo ES, Penaranda G, Coquet E, Chiche L, Riso A, Renou C, Bourliere M, Cotle SJ, Halfon P, 2016. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol 64, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahari H, Shteingart S, Gafanovich I, Cotler SJ, D’Amato M, Pohl RT, Weiss G, Ashkenazi YJ, Tichler T, Goldin E, Lurie Y, 2015. Sustained virological response with intravenous silibinin: individualized IFN-free therapy via real-time modelling of HCV kinetics. Liver Int 35, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Imamura M, Gorstein E, Nakahara T, Tsuge M, Churkin A, Yardeni D, Etzion O, Uprichard SL, Barash D, Cotler SJ, Dahari H, Chayama K, 2020. Modeling-based response-guided therapy for chronic hepatitis C under glecaprevir/pibrentasvir may identify patients for ultra-short treatment duration. J. Infect. Dis. doi: 10.1093/infdis/jiaa219.32363394 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria D, Gutfraind A, Boodram B, Layden J, Ozik J, Page K, Cotler SJ, Major M, Dahari H, 2019. Modeling indicates efficient vaccine-based interventions for the elimination of hepatitis C virus among persons who inject drugs in metropolitan Chicago. Vaccine 37, 2608–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzion O, Dahari H, Yardeni D, Issachar A, Nevo-Shor A, Cohen-Naftaly M, al., e., 2018. Response‐guided therapy with DAA shortens treatment duration in 50% of HCV treated patients. Hepatology 68, 1468A-1469A. [Google Scholar]
- Gambato M, Canini L, Lens S, Graw F, Perpinan E, Londono MC, Uprichard SL, Marino Z, Reverter E, Bartres C, Gonzalez P, Pla A, Costa J, Burra P, Cotler SJ, Forns X, Dahari H, 2019. Early HCV viral kinetics under DAAs may optimize duration of therapy in patients with compensated cirrhosis. Liver Int 39, 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368:34–44. [DOI] [PubMed] [Google Scholar]
- Iversen J, Dore GJ, Catlett B, Cunningham P, Grebely J, Maher L, 2019. Association between rapid utilisation of direct hepatitis C antivirals and decline in the prevalence of viremia among people who inject drugs in Australia. J Hepatol 70, 33–39. [DOI] [PubMed] [Google Scholar]
- Martin NK, Thornton A, Hickman M, Sabin C, Nelson M, Cooke GS, Martin TCS, Delpech V, Ruf M, Price H, Azad Y, Thomson EC, Vickerman P, 2016. Can Hepatitis C Virus (HCV) Direct-Acting Antiviral Treatment as Prevention Reverse the HCV Epidemic Among Men Who Have Sex With Men in the United Kingdom? Epidemiological and Modeling Insights. Clin Infect Dis 62, 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinello M, Matthews GV, 2015. Enhancing the detection and management of acute hepatitis C virus infection. International Journal of Drug Policy 26, 899–910. [DOI] [PubMed] [Google Scholar]
- Martinello M, Gane E, Hellard M, Sasadeusz J, Shaw D, Petoumenos K, Applegate T, Grebely J, Maire L, Marks P, Dore GJ, Matthews GV, 2016. Sofosbuvir and ribavirin for 6 weeks is not effective among people with recent hepatitis C virus infection: The DARE-C II study. Hepatology 64, 1911–1921. [DOI] [PubMed] [Google Scholar]
- Martinello M, Hajarizadeh B, Grebely J, Dore GJ, Matthews GV, 2018. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat Rev Gastroenterol Hepatol 15, 412–424. [DOI] [PubMed] [Google Scholar]
- Martinello M, Orkin C, Cooke G, Bhagani S, Gane E, Kulasegaram R, Shaw D, Tu E, Petoumenos K, Marks P, Grebely J, Dore GJ, Nelson M, Matthews GV, 2019a. Short duration pan-genotypic therapy with glecaprevir-pibrentasvir for six weeks among people with recent HCV infection. Hepatology. In press [DOI] [PubMed] [Google Scholar]
- Martinello M, Yee J, Bartlett SR, Read P, Baker D, Post JJ, Finlayson R, Bloch M, Doyle J, Shaw D, Hellard M, Petoumenos K, Lin L, Marks P, Applegate T, Dore GJ, Matthews GV, team C.s., 2019b. Moving towards hepatitis C micro- elimination among people living with HIV in Australia: the CEASE study. Clin Infect Dis. In press [DOI] [PubMed] [Google Scholar]
- Matthews GV, Bhagani S, Van der Valk M, Rockstroh JK, Kim A, Thurnheer C, Feld JJ, Bruneau J, Gane E, Hellard M, Grebely J, Applegate T, Marks P, Martinello M, Petoumenos K, Dore GJ, Inferiority of short duration sofosbuvir-velpatasvir for recent HCV (REACT Study). Conference on Retroviruses and Opportunistic Infections (CROI) March 8–11, 2020 Boston. Abstract 121. [Google Scholar]
- Molina JM, Orkin C, Iser DM, Zamora F-X, Nelson M, Stephan C, Massetto B, Gaggar A, Ni L, Svarovskaia E, Brainard D, Subramanian GM, McHutchison JG, Puoti M, Rockstroh JK, 2015. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. The Lancet 385(9973), 1098–106. [DOI] [PubMed] [Google Scholar]
- Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS, 1998. Hepatitis C Viral Dynamics in Vivo and the Antiviral Efficacy of Interferon-Therapy. Science 282, 103–107. doi: 10.1126/science.282.5386.103 [DOI] [PubMed] [Google Scholar]
- Osinusi A, Meissner EG, Lee Y-J, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M, Herrmann E, Shivakumar B, Gu W, Kwan R, Teferi G, Talwani R, Silk R, Kotb C, Wroblewski S, Fishbein D, Dewar R, Highbarger H, Zhang X, Kleiner D, Wood BJ, Chavez J, Symonds WT, Subramanian M, Mchutchison J, Polis MA, Fauci AS, Masur H, Kottilil S, 2013. Sofosbuvir and Ribavirin for Hepatitis C Genotype 1 in Patients With Unfavorable Treatment Characteristics. Jama 310, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MJD, 1994. A direct search optimization method that models the objective and constraint functions by linear interpolation, in: Gomez S, Hennart J-P (Eds.), Advances in Optimization and Numerical Analysis. Kluwer Academic; (Dordrecht: ), pp. 51–67. [Google Scholar]
- Reinharz V, Churkin A, Lewkiewicz S, Dahari H, Barash D, 2019. A Parameter Estimation Method for Multiscale Models of Hepatitis C Virus Dynamics. Bull Math Biol 81, 3675–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Vizcaya L, Kouyos RD, Zahnd C, Wandeler G, Battegay M, Darling KE, Bernasconi E, Calmy A, Vernazza P, Furrer H, Egger M, Keiser O, Rauch A, Swiss HIVCS, 2016. Hepatitis C virus transmission among human immunodeficiency virus-infected men who have sex with men: Modeling the effect of behavioral and treatment interventions. Hepatology 64, 1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski M, Gardiner D, Rodriguez-Torres M, Reddy K, Hassanein T, Jacobson I, Lawitz E, Lok A, Hinestrosa F, Thuluvath P, Schwartz H, Nelson D, Everson T, Eley T, Wind-Rotolo M, Huang S, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela D, 2014. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. New England Journal of Medicine 370(15), 1469–1469. [DOI] [PubMed] [Google Scholar]
- Shteyer E, Shekhtman L, Zinger T, Harari S, Gafanovich I, Wolf D, Ivgi H, Barsuk R, Dery I, Armoni D, Rivkin M, Pipalia R, Cohen Eliav M, Skorochod Y, Breuer GS, Tur-Kaspa R, Weil Wiener Y, Stern A, Cotler SJ, Dahari H, Lurie Y, 2019. Modeling suggests that microliter volumes of contaminated blood caused an outbreak of hepatitis C during computerized tomography. PLoS One 14, e0210173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370:1993–2001. [DOI] [PubMed] [Google Scholar]