Abstract
Background
Atrial fibrillation is a common post‐operative complication of cardiac surgery and is associated with an increased risk of post‐operative stroke, increased length of intensive care unit and hospital stays, healthcare costs and mortality. Numerous trials have evaluated various pharmacological and non‐pharmacological prophylactic interventions for their efficacy in preventing post‐operative atrial fibrillation. We conducted an update to a 2004 Cochrane systematic review and meta‐analysis of the literature to gain a better understanding of the effectiveness of these interventions.
Objectives
The primary objective was to assess the effects of pharmacological and non‐pharmacological interventions for preventing post‐operative atrial fibrillation or supraventricular tachycardia after cardiac surgery. Secondary objectives were to determine the effects on post‐operative stroke or cerebrovascular accident, mortality, cardiovascular mortality, length of hospital stay and cost of treatment during the hospital stay.
Search methods
We searched the Cochrane Central Register of ControlLed Trials (CENTRAL) (Issue 8, 2011), MEDLINE (from 1946 to July 2011), EMBASE (from 1974 to July 2011) and CINAHL (from 1981 to July 2011).
Selection criteria
We selected randomized controlled trials (RCTs) that included adult patients undergoing cardiac surgery who were allocated to pharmacological or non‐pharmacological interventions for the prevention of post‐operative atrial fibrillation or supraventricular tachycardia, except digoxin, potassium (K+), or steroids.
Data collection and analysis
Two review authors independently abstracted study data and assessed trial quality.
Main results
One hundred and eighteen studies with 138 treatment groups and 17,364 participants were included in this review. Fifty‐seven of these studies were included in the original version of this review while 61 were added, including 27 on interventions that were not considered in the original version. Interventions included amiodarone, beta‐blockers, sotalol, magnesium, atrial pacing and posterior pericardiotomy. Each of the studied interventions significantly reduced the rate of post‐operative atrial fibrillation after cardiac surgery compared with a control. Beta‐blockers (odds ratio (OR) 0.33; 95% confidence interval) CI 0.26 to 0.43; I2 = 55%) and sotalol (OR 0.34; 95% CI 0.26 to 0.43; I2 = 3%) appear to have similar efficacy while magnesium's efficacy (OR 0.55; 95% CI 0.41 to 0.73; I2 = 51%) may be slightly less. Amiodarone (OR 0.43; 95% CI 0.34 to 0.54; I2 = 63%), atrial pacing (OR 0.47; 95% CI 0.36 to 0.61; I2 = 50%) and posterior pericardiotomy (OR 0.35; 95% CI 0.18 to 0.67; I2 = 66%) were all found to be effective. Prophylactic intervention decreased the hospital length of stay by approximately two‐thirds of a day and decreased the cost of hospital treatment by roughly $1250 US. Intervention was also found to reduce the odds of post‐operative stroke, though this reduction did not reach statistical significance (OR 0.69; 95% CI 0.47 to 1.01; I2 = 0%). No significant effect on all‐cause or cardiovascular mortality was demonstrated.
Authors' conclusions
Prophylaxis to prevent atrial fibrillation after cardiac surgery with any of the studied pharmacological or non‐pharmacological interventions may be favored because of its reduction in the rate of atrial fibrillation, decrease in the length of stay and cost of hospital treatment and a possible decrease in the rate of stroke. However, this review is limited by the quality of the available data and heterogeneity between the included studies. Selection of appropriate interventions may depend on the individual patient situation and should take into consideration adverse effects and the cost associated with each approach.
Plain language summary
Intervention is favored in the prevention of post‐operative atrial fibrillation after heart surgery
Atrial fibrillation after heart surgery is a common complication that has been associated with poor outcomes. We reviewed the literature to better understand the role of preventative interventions for this condition. By combining the results of 118 studies with 17,364 participants, we are able to gain a better understanding of the evidence behind each of these interventions. All of the interventions studied were effective in reducing the occurrence of atrial fibrillation, length of hospital stay, cost of hospital treatment and may be effective in reducing the risk of stroke. The interventions did not have an effect on death after heart surgery. It was not possible to analyze the adverse events associated with the medications studied in this review, but these should be considered by clinicians when choosing an appropriate intervention for their patients. Furthermore, differences in the design between the studies combined in this review may complicate interpretation of these results.
Background
Description of the condition
Atrial fibrillation is a common post‐operative complication of cardiac surgery, occurring in 17% to 47% of patients (Almassi 1997; Chung 2000b; Frost 1992; Leitch 1990; Mathew 1996) with the incidence being greater in older patients (Leitch 1990). Besides directly causing patient discomfort and leading to hemodynamic compromise, several studies have demonstrated that post‐operative atrial fibrillation is associated with an increased risk of post‐operative stroke (Almassi 1997; Creswell 1993; Reed 1998) and mortality (Almassi 1997), longer intensive care unit and hospital stays (Almassi 1997; Aranki 1996; Creswell 1993; Loubani 2000; Mathew 1996) and greater costs of treatment (Kowey 1992; Taylor 1990). Atrial fibrillation, both paroxysmal and chronic, has been shown to significantly increase the risk of stroke, especially in older patients (Wolf 1991).The main mechanism of stroke in atrial fibrillation is believed to be intracardiac embolism. Blood stasis within the atrial chamber likely plays a role, but continuing research has identified multiple other factors that lead to thrombus formation (Whitlock 2009), including: atherosclerosis (SPAF Investigators Committee 1998), a pro‐inflammatory state, and endothelial dysfunction (Guazzi 2009), as well as platelet activation (Müller 2002) that leads to a hypercoagulable state (Watson 2009). With the low rate and multifactorial etiology of post‐operative stroke after cardiac surgery, it is difficult for any single trial to definitively demonstrate a benefit from atrial fibrillation prevention. Prophylaxis for post‐operative atrial fibrillation is a common practice in the cardiac surgery population and aims to prevent the complications and outcomes discussed above.
Description of the intervention
Numerous trials have studied various interventions for their efficacy in preventing post‐operative atrial fibrillation after cardiac surgery (Chung 2000b). These interventions fall into one of two categories: pharmacological or non‐pharmacological. The most commonly studied prophylactic interventions to prevent atrial fibrillation after cardiac surgery are beta‐blockers, including atenolol, metoprolol, propranolol and timolol, among others. This class of drugs works by blocking the effects of catecholamines on β1‐adrenergic receptors, thus decreasing the effects of the sympathetic nervous system on the heart. Amiodarone, a class III antiarrhythmic, has also been studied extensively in this setting. This agent primarily prolongs the repolarization phase of the cardiac cycle by blocking potassium channels. However, it also has other effect similar to those of antiarrhythmic classes Ia, II, and IV. Sotalol demonstrates properties of both Class III antiarrhythmics and beta‐blockers. Finally, magnesium has been proposed as a prophylactic measure for atrial fibrillation after cardiac surgery and is thought to work through its effects on transmembrane ion gradients and catecholamines. Studies investigating non‐pharmacological interventions for preventing post‐operative atrial fibrillation have largely concentrated on atrial pacing, which controls the heart rate via electrical stimulation. However, posterior pericardiotomy, an intraoperative procedure that involves a 4 cm longitudinal incision in the pericardium posterior and parallel to the phrenic nerve, has also been evaluated. This technique is thought to work by allowing post‐operative drainage of fluid and blood out of the pericardium, thereby preventing pericardial effusion, a condition known to be associated with the development of atrial fibrillation (Angelini 1987; Bryan 1990). Each of these interventions are associated with adverse effects that range from abnormal laboratory tests to hemodynamic instability. Knowledge of these possible adverse effects and the methods to address them are important skills for the clinician offering post‐operative atrial fibrillation prophylaxis, as they may have important clinical consequences for this vulnerable patient population. While an in‐depth discussion of the safety and monitoring of each of these interventions is beyond the scope of this review, the most common and most serious potential adverse events for each of these interventions are outlined in Table 1.
1. Potential adverse events associated with interventions for prevention of post‐operative atrial fibrillation.
| Intervention | Potential Adverse Events (% incidence) |
| Pharmacological Interventions | |
| Amiodarone | ‐ Serum creatinine increase (93%) ‐ Hypotension (16%) ‐ Phlebitis of administration site (not defined) ‐ Ventricular arrhythmias (2%‐5%) ‐ Hepatotoxicity (3%‐20%) |
| Beta‐blockers | ‐ Bradycardia (3%) ‐ Hypotension (1%) ‐ Exacerbation of decompensated congestive heart failure (< 1%) ‐ Bronchospasm (rare) |
| Sotalol | ‐ Dyspnea (21%) ‐ Bradycardia (16%) ‐ Hypotension (6%) ‐ Torsades de pointes or new ventricular arrhythmia (4% in patients with supraventricular arrhythmia) |
| Magnesium | ‐ Hypotension (rare) |
| Non‐Pharmacological Interventions | |
| Atrial Pacing | ‐ Atrial irritability (not defined) |
| Posterior Pericardiotomy | ‐ Not defined |
Why it is important to do this review
Many studies have investigated various proposed prophylactic interventions for their efficacy in preventing post‐operative atrial fibrillation after cardiac surgery. However, few of these trials have been sufficiently powered to definitively determine the usefulness of these treatments in preventing this arrhythmia. Furthermore, no studies have had sufficient power to reliably estimate the effects of these treatments on the rates of clinically relevant outcomes such as stroke, mortality and the length and cost of hospital stay. This is an update of a Cochrane review originally published in 2004 (Crystal 2004). Since that date, a number of studies have been published that further evaluate the major interventions considered and re‐explore interventions that were not included in the original review. We updated this systematic review and meta‐analysis to determine the efficacy of various interventions for preventing post‐operative atrial fibrillation after cardiac surgery and for their effects on stroke, mortality, cardiovascular mortality, length of hospital stay and cost of treatment.
Objectives
The primary objective was to assess the effects of pharmacological and non‐pharmacological interventions for preventing post‐operative atrial fibrillation or supraventricular tachycardia after cardiac surgery. Secondary objectives were to determine the effects on post‐operative stroke or cerebrovascular accident, mortality, cardiovascular mortality, length of hospital stay and cost of treatment during the hospital stay.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) in which patients undergoing cardiac surgery, including coronary artery bypass graft (CABG), valvular and combined procedures with or without cardiopulmonary bypass (CPB), were randomized to pharmacological or non‐pharmacological interventions for the prevention of post‐operative atrial fibrillation or supraventricular tachycardia.
Types of participants
Adult (≥ 18 years old) undergoing CABG, valvular surgery or combined CABG and valvular surgery with or without CPB with no history of chronic atrial fibrillation.
Types of interventions
Any pharmacological or non‐pharmacological intervention aimed at preventing post‐operative atrial fibrillation except digoxin, potassium (K+), or steroids. Digoxin is mainly used for its rate‐control properties and therefore was not deemed to be important in this analysis of rhythm‐control prophylaxis. While hypokalemia is an important factor in the generation of atrial fibrillation, its use is guided mainly by plasma levels and clinical decision‐making rather than specific use for post‐operative prophylaxis. Interest in steroids in the cardiac surgery setting has increased in recent years. We decided not to include steroids in this review because there have been several extensive reviews of this topic (Cappabianca 2011; Marik 2009; Whitlock 2008) and our literature search did not reveal any studies that would significantly add to the established review literature. We pooled the results of studies evaluating amiodarone, beta‐blockers, sotalol, magnesium, atrial pacing and posterior pericardiotomy.
Types of outcome measures
Primary outcomes
Incidence of atrial fibrillation or supraventricular tachycardia.
Secondary outcomes
Incidence of stroke or cerebrovascular accident
Mortality rate
Cardiovascular mortality rate
Length of hospital stay
Cost of treatment during hospital stay
Adverse events associated with the interventions studied were not reported in a standardized format and the included trials were not powered to analyze these events. Therefore, data on adverse events were not collected.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 8, 2011), MEDLINE (from 1946 to July 2011), EMBASE (from 1974 to July 2011) and CINAHL (from 1981 to July 2011). The initial search was based on the following terms: "atrial fibrillation", "atrial flutter", or “atrial arrhythmia” and “heart surgery”, “cardiac surgery”, "CABG" or "valve surgery".
The search strategies used can be found in Appendix 1; Appendix 2; Appendix 3; Appendix 4. A standard RCT filter was used for MEDLINE (Dickersin 1994) and EMBASE (Lefebvre 2008).
Data collection and analysis
Selection of studies
Study eligibility was assessed in two stages. First, two review authors independently screened the titles and abstracts of each citation identified in our search. All articles identified as having any possibility of fulfilling the above eligibility criteria in the screening process were retrieved to undergo full text evaluation.
Two review authors independently evaluated each full text article selected during the screening stage. In cases of disagreement, the review authors discussed the reasoning for their decisions and came to a consensus. If disagreements were not resolved during this process, an independent third adjudicator assessed the paper in question and made a final decision. Non‐English studies were assessed by third‐party translators.
Data extraction and management
Two review authors independently abstracted the following descriptive data from eligible studies: year of publication, country of origin, interventions, treatment schema and doses, number of participants in each group, patient characteristics, concurrent antiarrhythmic medications, type of surgery, type of monitoring for outcomes, length of follow‐up, definition of primary outcome and end points of atrial fibrillation, stroke, mortality, cardiovascular mortality, length of stay and cost. Data from the two review authors were compared and any discrepancies were resolved. We converted non‐US currencies into 2011 US dollars using the Bank of Canada rates for June 21, 2011. Studies that did not present data on individual secondary outcomes were not included in those analyses. We did not contact trial authors for this missing data.
Trials that evaluated more than one dosage of a medication, more than one atrial pacing site or more than one intervention were entered into the analysis as multiple single trials, leading to control groups for these trials being counted twice in pooled analyses. Numbers of participants presented in the Effects of interventions section include these duplicated control groups.
Assessment of risk of bias in included studies
Risk of bias in included studies was assessed by two review authors using the criteria and technique described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Each study was assigned a level of risk of bias (high risk, unclear risk, low risk) for each of seven categories.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Intention‐to‐treat analysis
The use of intention‐to‐treat analysis was evaluated in addition to the categories suggested in the Cochrane Handbook for Systematic Reviews of Interventions because it was felt that this analysis technique is important for, and specific to, the quality of randomized trials.
Using the primary outcome of incidence of atrial fibrillation or supraventricular tachycardia, studies were grouped by the assigned risk of bias level for each of the seven bias categories. Pharmacological and non‐pharmacological interventions were separated for the performance bias and detection bias categories due to the technical difficulties of blinding in a non‐pharmacological study.
Measures of treatment effect
Statistical analyses were performed using the statistical package provided by The Cochrane Collaboration (RevMan 5.1). We analyzed dichotomous outcomes using odds ratios within a Mantel‐Haenszel random‐effects model. We analyzed continuous variables using mean difference within an inverse variance random‐effects model.
Unit of analysis issues
All of the trials included in this review were of a simple parallel‐group design, with individuals randomized to one intervention group and a single measurement for each outcome was collected from each participant.
Assessment of heterogeneity
Subgroup differences were assessed using the χ2 test. Sensitivity analyses were undertaken for 'Risk of bias' categories that demonstrated significant subgroup differences, defined as P < 0.10. Sensitivity analyses compared all studies to studies that were at low risk of bias.
Heterogeneity was assessed using the I2 statistic (Higgins 2002) due to its consistency for meta‐analyses that include a large number of studies (Higgins 2003). An I2 value greater than or equal to 50% was considered to be substantial in this analysis. When heterogeneity was identified, we explored individual trial characteristics within each intervention to determine possible sources.
Results
Description of studies
Results of the search
Figure 1 displays a PRISMA diagram of our study selection process. Of the 3864 studies identified in the literature search and an additional three studies included from 'Studies awaiting classification' in the original review (Gerstenfeld 2001; Matsuura 2001; White 2002), 432 were reviewed in full text format and 170 met the inclusion criteria. Interventions included in fewer than four studies were not appropriate for pooling in our meta‐analysis and therefore were not analyzed in this review. A total of 118 studies with 138 treatment groups and 17364 participants were considered in this review. Fifty‐seven of these studies were included in the original version of this review (Crystal 2004) while 61 were added, including 27 on interventions that were not considered in the original version. The Kurz 1999 study was excluded from the original version of this review because the trial was prematurely aborted. We decided to include the preliminary results of this trial. The Tokmakoglu 2002 study was also excluded due to a lack of a control group. This trial was designed with three randomized groups: one that received amiodarone, one that received metoprolol and digoxin and one that received no prophylaxis. It is possible that the triple‐group setup of this trial led to an error in its eligibility assessment in the original version of this review. Upon further assessment of this reference, it was included in our review. Twenty‐three additional studies from the period covered by the literature search of the original review were identified (Arbatli 2003; Asimakopoulos 1997; Casthely 1994; Crystal 2003; Dagdelen 2002; England 1992; Fanning 1991; Farsak 2002; Gerstenfeld 2001; Jensen 1997; Kaplan 2003; Karmy‐Jones 1995; Kuralay 1999; Matsuura 2001; Mulay 1995; Nurözler 1996; Parikka 1993; Speziale 2000; Toraman 2001; White 2002; White 2003; Wilkes 2002; Yeatman 2002) of which 13 studied the effects of magnesium (Casthely 1994; Dagdelen 2002; England 1992; Fanning 1991; Jensen 1997; Kaplan 2003; Karmy‐Jones 1995; Nurözler 1996; Parikka 1993; Speziale 2000; Toraman 2001; Wilkes 2002; Yeatman 2002) and five evaluated posterior pericardiotomy (Arbatli 2003; Asimakopoulos 1997; Farsak 2002; Kuralay 1999; Mulay 1995), interventions that were not analyzed in the original version of this review.
1.
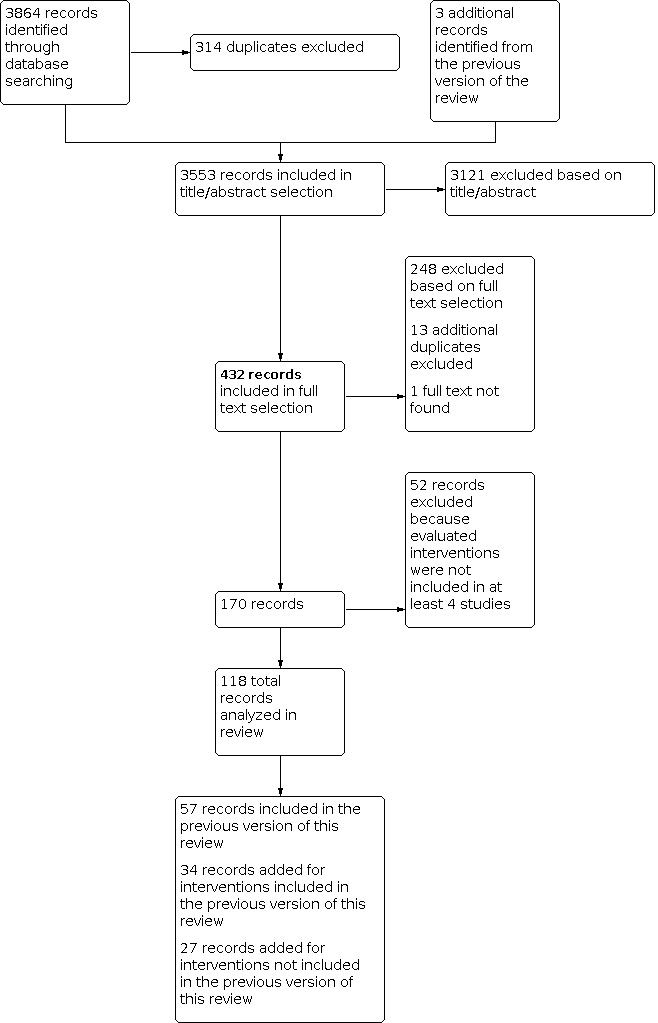
PRISMA diagram of study selection process
Included studies
See: Characteristics of included studies
We identified studies with the following pharmacological interventions.
Amiodarone
Beta‐blockers
Sotalol
Magnesium
In the studies included in this review, beta‐blockers included acebutolol, atenolol, landiolol, metoprolol, nadolol, propranolol and timolol.
We identified studies with the following non‐pharmacological interventions.
Atrial pacing
Posterior pericardiotomy
In the studies included in this review, atrial pacing included Bachmann's bundle pacing, biatrial pacing, left atrial pacing, right atrial pacing and triple‐site pacing.
Studies that included multiple intervention arms are marked in the analyses.
There were 17,364 participants in the 118 studies included in this review. The weighted mean age of trial participants was 60.2 years old. A weighted mean of 76.9% of participants were men. The trials were undertaken in various countries as outlined in Table 2.
2. Country of origin of included trials.
| Country | Number of Trials |
| Austria | 1 |
| Belgium | 4 |
| Brazil | 7 |
| Canada | 7 |
| China | 2 |
| Denmark | 4 |
| Finland | 2 |
| France | 1 |
| Germany | 6 |
| India | 2 |
| Iran | 6 |
| Israel | 1 |
| Italy | 3 |
| Japan | 2 |
| Jordan | 1 |
| Lebanon | 1 |
| Netherlands | 2 |
| New Zealand | 1 |
| Norway | 1 |
| Pakistan | 1 |
| Serbia | 2 |
| Sweden | 1 |
| Switzerland | 5 |
| Taiwan | 1 |
| Turkey | 15 |
| UK | 9 |
| USA | 29 |
| Yugoslavia | 1 |
Only three (2.5%) of the included studies described multicentre trials. The median length of follow‐up was five days (interquartile range: three to seven). The length of treatment was not specifically reported in the majority of included studies, but was generally at least the duration of the follow‐up period.
Excluded studies
Interventions included in fewer than four studies were not appropriate for pooling in our meta‐analysis and therefore were not analyzed in this review.
Risk of bias in included studies
See: Characteristics of included studies
Combining the results from all seven of the categories considered in our study quality assessment, 41.5% of studies were at low risk for bias and 17.6% were at high risk. In 40.9% of studies, the risk of bias in the categories considered was unclear from the publication. There were 44 studies that were not at high risk for bias in any of the seven categories. Three of these (Auer 2004; Mitchell 2005; Zangrillo 2005) were deemed to be low risk in all of the categories. The results of the risk of bias assessments are displayed in Figure 2. Figure 3 displays a funnel plot of the results of all included studies for the primary outcome. While there were some outliers, the funnel plot was overall symmetrical and did not raise the concern of significant publication bias in this review.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Funnel plot of comparison: 1 Atrial fibrillation or Supraventricular tachycardia, outcome: 1.1 All Treatments.
Allocation
See: Analysis 7.1; Analysis 7.2
7.1. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 1: All Treatments ‐ Randomization sequence generation
7.2. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 2: All Treatments ‐ Allocation concealment
As the inclusion criteria for this review required a study to be a randomized trial, the risk of selection bias was expected to be low. Unfortunately, the majority of studies did not provide sufficient information to judge the level of risk from inappropriate randomization sequence generation (61.0%) or allocation concealment (70.3%). Approximately one third (32.3%) of studies were deemed to be low risk in the randomization sequence generation category while approximately a quarter (24.6%) were at low risk for inappropriate allocation concealment (Figure 2).
When the studies were pooled according to the level of risk of bias, no significant difference between the subgroups was found for randomization sequence generation but significant differences were found in the allocation concealment analysis between high and low risk subgroups (P = 0.02). When only studies at low risk for this type of bias were considered, amiodarone (odds ratio (OR) 0.52; 95% confidence interval (CI) 0.38 to 0.70), beta‐blockers (OR 0.44; 95% CI 0.30 to 0.66) and magnesium (OR 0.64; 95% CI 0.50 to 0.82) demonstrated summary odds ratios for post‐operative atrial fibrillation closer to 1 while sotalol (OR 0.30; 95% CI 0.19 to 0.49), atrial pacing (OR 0.23; 95% CI 0.09 to 0.60) and posterior pericardiotomy (OR 0.19; 95% CI 0.09 to 0.39) had summary odds ratios further from 1. Each of these analyses, especially those of the non‐pharmacological interventions, contained few studies.
Blinding
See: Analysis 7.3; Analysis 7.4; Analysis 7.5; Analysis 7.6
7.3. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 3: Pharmacological Treatments ‐ Blinding of participants and personnel
7.4. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 4: Non‐Pharmacological Treatments ‐ Blinding of participants and personnel
7.5. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 5: Pharmacological Treatments ‐ Blinding of outcome assessment
7.6. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 6: Non‐Pharmacological Treatments ‐ Blinding of outcome assessment
The majority of studies in this review were determined to be at low risk for performance (51.7%) or detection (51.7%) bias, as defined by appropriate blinding of the participants and healthcare providers or outcome assessors. Approximately one quarter of studies were at high risk for each bias (27.1% and 24.6%, respectively). Since a placebo cannot be used in non‐pharmacological studies, blinding in these studies was rare. Only seven atrial pacing studies (Da Silva 2008; Eslami 2005; Fan 2000; Gerstenfeld 1999; Greenberg 2000; Levy 2000; Schweikert 1998) and one posterior pericardiotomy (Farsak 2002) utilized at least single‐blinding; the majority blinded the outcome assessor to the treatment allocation (Figure 2).
Considering both performance and detection bias, pharmacological treatments demonstrated a trend towards differences between high and low risk subgroups (P = 0.12 and P = 0.11, respectively). This difference was not seen for non‐pharmacological interventions (P = 0.84 and P = 0.81, respectively). Sensitivity analyses did not reveal any important change in the summary estimate for amiodarone, beta‐blockers, sotalol or magnesium.
Incomplete outcome data
See: Analysis 7.7
7.7. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 7: All Treatments ‐ Incomplete outcome data
The majority of studies in this review (57.6%) were categorized as having low risk for attrition bias. In over a quarter of studies (28.8%), insufficient information was given to determine the risk of attrition bias (Figure 2).
No significant difference was found between studies at high and low risk for attrition bias (P = 0.87).
Selective reporting
See: Analysis 7.8
7.8. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 8: All Treatments ‐ Selective reporting
Selective data reporting was not a major issue in the studies included in this review; 57.6% of studies were at low risk for reporting bias while only 5.9% were determined to be at high risk (Figure 2).
Despite the low percentage of high‐risk studies, analysis demonstrated a significant difference between studies at high and low risk for reporting bias (P = 0.01). Sensitivity analyses considering only studies at low risk of bias resulted in a slight increase towards 1 in the summary estimates of beta‐blockers (OR 0.39; 95% CI 0.28 to 0.54) and posterior pericardiotomy (OR 0.50; 95% CI 0.23 to 1.07).
Other potential sources of bias
Intention‐to‐treat analysis
See: Analysis 7.9
7.9. Analysis.

Comparison 7: Risk of Bias Assessment, Outcome 9: All Treatments ‐ Intention‐to‐treat analysis
Only 18 studies (15.3%) specifically stated that they undertook an intention‐to‐treat analysis. Information to determine whether an intention‐to‐treat analysis was undertaken was insufficient in 44.9% of the studies. This category of bias had the greatest amount of studies in the high‐risk category (39.8%) (Figure 2).
The results of analysis based on the use of an intention‐to‐treat analysis demonstrated a significant difference between all three subgroups (P = 0.01) but not between high and low risk groups (P = 0.17). Considering only studies that specifically stated that they undertook an intention‐to‐treat analysis, the summary odds ratios for post‐operative atrial fibrillation for amiodarone treatment increased towards 1 (OR 0.56; 95% CI 0.45 to 0.69).
Effects of interventions
The effect of interventions on post‐operative atrial fibrillation and supraventricular tachycardia
All included trials evaluated the effect of various pharmacological and non‐pharmacological interventions on post‐operative atrial fibrillation or supraventricular tachycardia. Definitions of an event varied between studies, from any occurrence of supraventricular tachycardia to atrial fibrillation lasting at least one hour or requiring therapy for hemodynamic compromise. Nearly all studies monitored patients for events using continuous electrocardiogram telemetry or Holter monitoring. The majority of studies followed patients until discharge from the intensive care unit or hospital while Forlani 2002 and Pfisterer 1997 followed up patients until 30 and 90 days post‐operative, respectively. Jacquet 1994; Khuri 1987; White 2002; White 2003 and Yagdi 2003 re‐evaluated patients at a follow‐up clinic visit approximately 30 days after surgery. The median length of follow‐up was five days (interquartile range: three to seven).
Considering all 118 studies, with 18,381 counted participants (including those control groups counted in multiple comparisons, as described in the Data extraction and management section), prophylactic intervention was associated with a significant reduction in atrial fibrillation in the treatment group (17.7%) compared to the control group (32.3%) (OR 0.41; 95% CI 0.37 to 0.47; I2 = 56%; Figure 4). Pharmacological interventions were evaluated in a total of 93 studies with 14,685 participants. Analysis of these studies demonstrated a reduction in atrial fibrillation in the treatment group (17.7%) compared to the control group (32.2%) (OR 0.40; 95% CI 0.35 to 0.46; I2 = 57%; Figure 4). Non‐pharmacological interventions were associated with a reduction in atrial fibrillation in the treatment group (17.7%) compared to the control group (32.9%) (OR 0.44; 95% CI 0.34 to 0.57; I2 = 54%; Figure 4) in 27 trials with 3696 participants.
4.

Forest plot: 1 Atrial fibrillation or Supraventricular tachycardia; 1.1 All Treatments.
Amiodarone
Thirty‐three of the studies included in this review, with a total of 5402 participants, evaluated the effect of amiodarone on post‐operative atrial fibrillation and supraventricular tachycardia. Dosing regimens, including loading doses and infusion rates, varied between studies and were delivered both orally and intravenously. Approximately half of the studies began amiodarone administration pre‐operatively and half post‐operatively. Dörge 2000 and White 2002 each contained two separate treatment groups. The former utilized a high‐ and a low‐dose group while the groups in the latter differed by the rate of the loading dose. Amiodarone was associated with a significant reduction in post‐operative atrial fibrillation in the treatment group (19.4%) compared with the control group (33.3%) (OR 0.43; 95% CI 0.34 to 0.54; I2 = 63%; Figure 5)
5.

Forest plot: 1 Atrial fibrillation or Supraventricular tachycardia; 1.2 Amiodarone.
Beta‐blockers
Thirty‐three of the studies included in this review evaluated the effects of beta‐blockers on post‐operative atrial fibrillation and supraventricular tachycardia. These trials included 4698 participants. Half of these studies investigated propranolol. Dosing regimens varied between studies and were delivered both orally and intravenously. The majority of studies (81.8%) began beta‐blocker administration post‐operatively. Treatment with beta‐blockers demonstrated a reduction in post‐operative atrial fibrillation in the treatment group (16.3%) compared to the control group (31.7%) (OR 0.33; 95% CI 0.26 to 0.43; I2 = 55%; Figure 6).
6.

Forest plot: 1 Atrial fibrillation or Supraventricular tachycardia; 1.3 Beta‐Blockers.
Sotalol
Eleven studies with 1609 participants evaluated the effects of sotalol on post‐operative atrial fibrillation and supraventricular tachycardia. Dosing regimens varied between studies. All studies delivered sotalol orally but two studies (Jacquet 1994; Janssen 1986) began with intravenous infusions until the patients were able to receive pills. Six of the studies used a dose of 80 mg twice a day. The majority of studies (54.5%) began sotalol administration post‐operatively. Sotalol was associated with a significant reduction in post‐operative atrial fibrillation in the treatment group (18.1%) compared to the control group (40.0%) (OR 0.34; 95% CI 0.26 to 0.43; I2 = 3%; Figure 7).
7.

Forest plot:: 1 Atrial fibrillation or Supraventricular tachycardia; 1.4 Sotalol.
Magnesium
Twenty‐one of the studies included in this review investigated the effects of magnesium on post‐operative atrial fibrillation and supraventricular tachycardia. These studies included 2988 participants. Dosing regimens varied between studies but all administration of magnesium was done intravenously. In 12 (57.1%) of these studies, magnesium was first administered intra‐operatively. This analysis demonstrated a significant reduction in post‐operative atrial fibrillation in the treatment group (16.5%) compared to the control group (26.2%) (OR 0.55; 95% CI 0.41 to 0.73; I2 = 51%; Figure 8).
8.

Forest plot: 1 Atrial fibrillation or Supraventricular tachycardia; 1.5 Magnesium.
Atrial Pacing
Twenty‐one of the papers included in this review studied the effects of atrial pacing on post‐operative atrial fibrillation and supraventricular tachycardia. These trial included 2933 participants. Nine studies (Chavan 2010; Eslami 2005; Fan 2000; Gerstenfeld 1999; Goette 2002; Greenberg 2000; Hakala 2005; Mirkhani 2005; Neto 2007) contained more than one treatment group. Each of these groups were based on an alternate pacing location except for those in Hakala 2005, which were both right atrial pacing but differed in the target heart rate algorithms. There were 32 treatment groups in total, including three Bachmann's bundle pacing, 13 biatrial pacing, four left atrial pacing, 10 right atrial pacing, one triple‐site atrial pacing and one not specified. The incidence of post‐operative atrial fibrillation across all studies was 18.7% in the treatment group and 32.8% in the control group, a difference that was statistically significant (OR 0.47; 95% CI 0.36 to 0.61; I2 = 50%; Figure 9).
9.

Forest plot: 1 Atrial fibrillation or Supraventricular tachycardia; 1.6 Atrial Pacing.
Posterior Pericardiotomy
There were six papers with 763 participants identified in this review that investigated posterior pericardiotomy for its effects on post‐operative atrial fibrillation and supraventricular tachycardia. Surgical technique was similar in each of the studies and involved a 4 cm longitudinal incision in the pericardium posterior and parallel to the phrenic nerve. Posterior pericardiotomy was associated with a significant reduction in post‐operative atrial fibrillation in the treatment group (14.0%) compared to the control group (33.1%) (OR 0.35; 95% CI 0.18 to 0.67; I2 = 66%; Figure 10).
10.

Forest plot: 1 Atrial fibrillation or Supraventricular tachycardia; 1.7 Posterior Pericardiotomy.
The effect of interventions on post‐operative stroke and cerebrovascular accident
To determine if prophylactic treatment to prevent post‐operative atrial fibrillation has a substantial effect on post‐operative stroke, we collected all available data regarding this outcome from the studies included in this review. Twenty‐eight studies with 34 treatment groups and 6361 participants provided data on the number of strokes. No patients in the Rubin 1987 study suffered a stroke and therefore this study did not contribute to the overall pooled summary estimate. Follow‐up for stroke was identical to follow‐up for atrial fibrillation in all studies. Only Auer 2004 specifically defined this outcome and required confirmation by brain computed tomography. Considering all interventions together, prophylactic treatment was associated with a borderline significant reduction in post‐operative atrial fibrillation in the treatment group (1.5%) compared to the control group (2.3%) (OR 0.69; 95% CI 0.47 to 1.01; I2 = 0%; Figure 11). There was insufficient data to judge the individual effects of beta‐blockers, sotalol, magnesium or posterior pericardiotomy on post‐operative stroke.
11.

Forest plot: 2 Stroke or Cerebrovascular Accident; 2.1 All Treatments.
Amiodarone
Fourteen studies, with 3087 participants, investigated amiodarone for its effect on post‐operative atrial fibrillation provided data on post‐operative stroke or cerebrovascular accident. The White 2002 study contained two separate treatment groups. Treatment with amiodarone demonstrated a borderline significant reduction in post‐operative stroke in the treatment group (1.6%) compared to the control group (2.8%) (OR 0.60; 95% CI 0.35 to 1.02; I2 = 0%; Figure 11).
Atrial Pacing
Six studies with 10 treatment groups and 832 participants provided data on the effect of atrial pacing on post‐operative stroke or cerebrovascular accident. Atrial pacing was associated with an insignificant reduction in post‐operative stroke in the treatment group (3.6%) compared to the control group (4.8%) (OR 0.72; 95% CI 0.36 to 1.46; I2 = 0%; Figure 11).
The effect of interventions on post‐operative mortality
Sixty‐one studies with 72 treatment groups and 10,986 participants provided post‐operative all‐cause mortality rates for each group. The majority of studies recorded patient death for the duration of the follow‐up for atrial fibrillation. Twenty studies (Auer 2004; Bert 2001; Butler 1993; Connolly 2003; Debrunner 2004; England 1992; Forlani 2002; Gerstenfeld 2001; Haddad 2009; Ivey 1983; Janssen 1986; Martinussen 1988; Matsuura 2001; Mulay 1995; Nyström 1993; Oka 1980; Paull 1997; Speziale 2000; Suttorp 1991; Zangrillo 2005) did not report an event in the control group and at least one of their treatment groups. Four studies (Beaulieu 2010; Crystal 2003; Giri 2001; Yeatman 2002) contacted study participants at 30 days post‐operatively to assess mortality but not occurrence of atrial fibrillation. Considering all interventions together, prophylactic treatment for atrial fibrillation was not associated with a difference in all‐cause post‐operative mortality between the treatment group (1.9%) and the control group (1.8%) (OR 1.03; 95% CI 0.77 to 1.39; I2 = 0%; Figure 12). No individual intervention was associated with a significant effect on post‐operative mortality.
12.

Forest plot: 3 Mortality; 3.1 All Treatments.
The effect of interventions on post‐operative cardiovascular mortality
Of the studies that reported on post‐operative mortality, 40 with 44 treatment groups and 6750 participants described the cause of death and allowed for categorization of certain events as cardiovascular mortality. The majority of these studies did not have any cardiovascular mortality events. Considering all interventions together, treatment was not associated with a difference in post‐operative cardiovascular mortality between the treatment group (0.6%) and the control group (0.7%) (OR 0.87; 95% CI 0.47 to 1.62; I2 = 0%; Figure 13). No individual intervention was associated with a significant effect on post‐operative cardiovascular mortality.
13.

Forest plot: 4 Cardiovascular Mortality; 4.1 All Treatments.
The effect of interventions on hospital length of stay
There were 51 studies with 63 treatment groups and 9661 participants that reported on hospital length of stay. Three trials (Farsak 2002; Kuralay 1999; Redle 1999) did not contribute to the final pooled analysis due to lack of standard deviation data. Interventions were associated with a significant reduction in length of stay in the treatment group, demonstrated by a mean difference of ‐0.69 days (95% CI ‐0.95 to ‐0.43). All individual interventions were associated with similar significant or borderline significant reductions except for magnesium. Nine studies evaluating the effects of magnesium demonstrated a mean difference of 0.05 days (95% CI ‐0.47 to 0.57; I2 = 69%; Figure 14).
14.

Forest plot: 5 Length of Stay; 5.1 All Treatments.
The effect of interventions on cost of treatment during hospital stay
Twelve studies with 14 treatment groups and 2790 participants reported data on the cost of treatment during the hospital stay. Four trials (Connolly 2003; Eslami 2005; Fan 2000; Guarnieri 1999) did not contribute to the final pooled analysis due to lack of standard deviation data. Interventions were associated with a small but significant reduction in cost in the treatment group, demonstrated by a mean difference of ‐1.25 [1000s of US dollars] (95% CI ‐1.97 to ‐0.52; I2 = 13%; Figure 15).
15.

Forest plot: 6 Cost; 6.1 All Treatments.
Heterogeneity
Considerable heterogeneity was found in the primary outcome analyses for each of the reviewed interventions, except for sotalol (I2 = 3%). However, much of this heterogeneity may be explained by primary trial characteristics.
In the amiodarone analysis, difference in treatment regimens between the studies, including dosages and timing of initial administration, likely contributed substantially to the heterogeneity. When considering only studies that began administration of amiodarone post‐operatively, heterogeneity was numerically decreased (I2 = 37%).
A source of heterogeneity in the beta‐blocker analysis may have been the type of beta‐blocker used. When considering only studies evaluating the effects of propranolol, only 18% of the variation between studies was found to be due to heterogeneity.
The pooled analysis of trials studying the efficacy of magnesium demonstrate borderline significant heterogeneity (I2 = 51%). Treatment regimens varied from small doses added to the cardioplegia solution to continuous infusions over several days and possibly accounted for a large part of this variance.
Heterogeneity in the atrial pacing analysis may have been due to the pooling of studies utilizing different pacing methods, including Bachmann's bundle pacing, left atrial pacing, right atrial pacing and biatrial pacing.
Due to the small number of trials contained in the analysis of posterior pericardiotomy for prevention of post‐operative atrial fibrillation, much of the heterogeneity found in this analysis (I2 = 66%) was due to one study (Asimakopoulos 1997). This study differed from the others investigating this intervention in its definition of the primary outcome. Asimakopoulos 1997 considered all instances of supraventricular tachycardia while the other studies in this analysis specified the primary outcome as incidence of atrial fibrillation or set a minimum duration required for the event to be considered.
Discussion
Summary of main results
This meta‐analysis demonstrated that each of the studied interventions significantly reduced the rate of post‐operative atrial fibrillation after cardiac surgery compared with a placebo control. Beta‐blockers and sotalol appeared to have similar efficacy. Amiodarone, atrial pacing and posterior pericardiotomy were found to be effective. However, the summary estimate for the latter was based on only six trials. The ability of magnesium to prevent atrial fibrillation may be slightly less than that of the other pharmacological agents.
Prophylactic intervention of any kind in this setting appeared to reduce the odds of post‐operative stroke, though this reduction did not reach statistical significance. Intervention also decreased the hospital length of stay by approximately two‐thirds of a day and decreased the cost of hospital treatment by roughly $1250 US. There was insufficient evidence to appropriately compare the efficacy of the individual interventions for these secondary outcomes. However, it is important to note that magnesium and posterior pericardiotomy were not associated with a decreased length of hospital stay. With regards to the latter, this can possibly be explained by other positive effects of alternate interventions, including influencing hemodynamic stability, that are not benefits of posterior pericardiotomy. Atrial pacing was not associated with a decrease in the cost of hospital treatment, possibly due to the equipment costs associated with this intervention.
None of the interventions demonstrated a significant protective effect against post‐operative all‐cause or cardiovascular mortality.
Limitations
This meta‐analysis was primarily limited by the lack of availability of relevant secondary outcome data. The incidence of stroke, all‐cause mortality and cardiovascular mortality, as well as length of hospital stay and cost of hospital treatment were not collected in many of the included studies. Further, this review is limited by the quality of the available studies. Improper allocation concealment, lack of blinding within pharmacological trials, selective reporting and failure to utilize an intention‐to‐treat analysis were all associated with variation in the pooled summary estimates for prevention of post‐operative atrial fibrillation. Although the results of sensitivity analyses based on the level of risk demonstrated some numerical differences, these adjusted results would not influence the overall implications of this review. Though many of the analyses in this review demonstrated significant heterogeneity, much of this variance may be explained by individual study characteristics that can be taken into account when applying the results of this review in the clinical setting. This review was not designed or powered to evaluate drug dosage or timing of interventions, which varied considerably between studies. Consensus on these factors through further research or more detailed analysis of data specific to these questions is warranted.
Agreements and disagreements with other studies or reviews
This review sought to update the previous version (Crystal 2004) with the available evidence from the seven years since its publication. In addition, the interventions of magnesium and posterior pericardiotomy were included due to their significant presence within the literature. Overall, the results of this meta‐analysis are largely similar to those of the previous version. Increased evidence has lead to an improved estimated efficacy for both amiodarone and atrial pacing in preventing atrial fibrillation. Though the summary estimate for all interventions in relation to stroke still did not reach statistical significance, it is clearer from this updated review that there is a trend towards the protective effects of prophylaxis. The estimated reduction in hospital length of stay due to preventative intervention was nearly identical in the two versions of this review. Finally, additional evidence allowed this review to confirm the hypothesis of the previous version's authors that intervention in this setting leads to a decreased cost of hospital stay.
Since the publication of the previous version of this meta‐analysis, a number of studies have reviewed the state of the literature surrounding this topic. However, few have done so in a systematic fashion. The most recent complete systematic review of this literature evaluated each of the pharmacological interventions we considered in this paper, as well as atrial pacing, digoxin and calcium‐channel blockers (Burgess 2006). Our updated results are in agreement with those presented in this previous review.
A more recent analysis (Shepherd 2008) summarized the efficacy of magnesium for preventing post‐operative atrial fibrillation after CABG. The authors reported a pooled odds ratio in a random‐effects model of 14 studies of 0.61 (95% CI 0.41 to 0.90). Our summary estimate was similar.
A meta‐analysis of posterior pericardiotomy in this setting retrieved the same six studies identified in our search and presented a nearly identical pooled summary estimate (Biancari 2010).
Clinical research has also focused on the use of steroids to decrease post‐operative mortality and morbidity following cardiac surgery, including for prevention of atrial fibrillation. This class of drugs was not studied within this meta‐analysis because several recent extensive reviews are readily available. Cappabianca 2011 reported in a review of 31 randomized trials that steroids were associated with a pooled odds ratio of 0.56 (95% CI 0.44 to 0.72) for atrial fibrillation, a result comparable to those of the interventions studied in this review.
Authors' conclusions
Implications for practice.
Recent guidelines from both Canadian (Mitchell 2011) and American (Bradley 2005) expert groups have suggested that beta‐blockers be adopted for routine prophylactic use following cardiac surgery to prevent post‐operative atrial fibrillation in both patients regularly taking this type of medication at home and those not. In patients where the use of beta‐blockers is contraindicated, amiodarone is considered to be the second choice. Due largely to possible adverse effects, sotalol is only recommended by these guidelines in patients at high risk of atrial fibrillation (e.g. the elderly, valvular surgery patients or those with congestive heart disease) (Shirzad 2010). The more recent Canadian guidelines (Mitchell 2011) state that magnesium and atrial pacing may be beneficial, but are only recommended if the patient has contraindications for both beta‐blockers and amiodarone. This review largely supports these guidelines. Each of these interventions demonstrated strong efficacy in preventing post‐operative atrial fibrillation. The selection of prophylaxis should reflect the individual patient's condition and take into account the risks and adverse events associated with each intervention. While the studies analyzed in this review were generally not powered to evaluate the adverse effects of each of the interventions and there was no standardized method of reporting these events, the known risks associated with each of the interventions are outlined in Table 1. These interventions should be administered by health professionals familiar with their use and with the assessment for and treatment of potential complications. The evidence presented in this review on posterior pericardiotomy is based on only a few randomized trials. Therefore, although it demonstrated promising results, we cannot recommend use of this surgical prophylactic intervention at this time.
Implications for research.
This review presents evidence that post‐operative atrial fibrillation can be reduced by several interventions with different mechanism of actions, for example, beta‐blockers via catecholamine pathways and posterior pericardiotomy through diminution of cardiac irritation by blood. Further research should consider examining synergism between interventions for greater efficacy.
This review also demonstrates that intervention for preventing post‐operative atrial fibrillation leads to a decreased hospital length of stay, lower costs of hospital treatment and a trend towards decreased risk of post‐operative stroke. However, these data were unavailable from a number of the trials included in this review. Future studies should make note to properly collect and present these data as conclusive evidence of the beneficial effects of prophylactic intervention beyond the direct prevention of atrial fibrillation would be beneficial.
Finally, continued investigation into the physiological mechanisms of atrial fibrillation and especially the circumstances that lead to thrombus formation during this arrhythmia would benefit future research and lead to greater understanding of how to prevent this outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2021 | Review declared as stable | This review question is considered low priority for the current scope of Cochrane Heart. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 30 October 2012 | New citation required but conclusions have not changed | Increased evidence has lead to an improved estimated efficacy for both amiodarone and atrial pacing in preventing atrial fibrillation. Though the summary estimate for all interventions in relation to stroke still did not reach statistical significance, it is clearer from this updated review that there is a trend towards the protective effects of prophylaxis. Finally, additional evidence allowed this review to confirm the hypothesis of the previous version's authors that intervention in this setting leads to a decreased cost of hospital stay. No major changes to the conclusions were made. |
| 30 July 2011 | New search has been performed | We reran the search strategy up to July 2011. Two additional interventions, magnesium and posterior pericardiotomy, were included due to their significant presence within the literature. Three studies that were awaiting assessment (Gerstenfeld 2001; Matsuura 2001; White 2002) and two that were excluded (Kurz 1999; Tokmakoglu 2002) in the original review were included. Twenty‐one additional studies from the time period covered in the original literature search were identified, The background section was updated. The methodology section was updated to include the 'Risk of bias' assessment suggested in the Cochrane Handbook and sensitivity analyses on this assessment. |
Appendices
Appendix 1. CENTRAL Search Strategy
1. MeSH descriptor Atrial Fibrillation explode all trees
2. MeSH descriptor Atrial Flutter explode all trees
3. MeSH descriptor Tachycardia, Supraventricular explode all trees
4. atrial NEAR/ fibrillat*
5. atrial NEAR/ flutter*
6. auricular* NEAR/ fibrillat*
7. auricular* NEAR/ flutter*
8. atrium NEAR/ fibrillat*
9. atrium NEAR/ flutter*
10. tachycardia NEAR/ supraventricular
11. (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
12. MeSH descriptor Cardiac Surgical Procedures explode all trees
13. heart NEAR/ surg*
14. cardiac NEAR/ surg*
15. coronary NEAR/ surg*
16. coronary NEAR/ bypass
17. CABG
18. valv* NEAR/ surg*
19. valv* NEAR/ replace*
20. (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19)
21. (#11 AND #20)
Appendix 2. CINAHL Search Strategy
1. (MH "Atrial Fibrillation")
2. (MH "Atrial Flutter")
3. atrial N5 fibrillat*
4. atrial N5 flutter*
5. auricular* N5 fibrillat*
6. auricular* N5 flutter*
7. atrium N5 fibrillat*
8. atrium N5 flutter*
9. (MH "Tachycardia, Supraventricular")
10. tachycardia N5 supraventricular
11. S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10
12. (MH "Surgery, Cardiovascular")
13. heart N5 surg*
14. cardiac N5 surg*
15. coronary N5 surg*
16. coronary N5 bypass
17. CABG
18. valv* N5 surg*
19. valv* N5 replace*
20. S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19
21. S11 and S20
Appendix 3. EMBASE Search Strategy
RCT Search Filter:
1. random*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
2. factorial*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
3. (crossover* or cross‐over*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
4. placebo*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
5. (doubl* adj blind*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
6. (singl* adj blind*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
7. assign*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
8. allocat*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
9. volunteer*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
10. crossover procedure.sh.
11. double‐blind procedure.sh.
12. randomized controlled trial.sh.
13. single‐blind procedure.sh.
14. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
Search:
15. heart atrium fibrillation/
16. heart atrium flutter/
17. (atrial adj6 fibrillat*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
18. (atrial adj6 flutter*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
19. (auricular* adj6 fibrillat*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
20. (auricular* adj6 flutter*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
21. (atrium adj6 fibrillat*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
22. (atrium adj6 flutter*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
23. supraventricular tachycardia/
24. (tachycardia adj6 supraventricular).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
25. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26. exp heart surgery/
27. (heart adj6 surg*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
28. (cardiac adj6 surg*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
29. (coronary adj6 surg*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
30. (coronary adj6 bypass).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
31. CABG.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
32. (valv* adj6 surg*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
33. (valv* adj6 replace*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
34. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33
35. 14 and 25 and 34
Appendix 4. MEDLINE Search Strategy
RCT Search Filter:
1. randomized‐controlled‐trial.pt.
2. Randomized Controlled Trial/
3. Random Allocation/
4. Double‐Blind Method/
5. Single‐Blind Method/
6. 1 or 2 or 3 or 4 or 5
7. limit 6 to humans
8. clinical trial.pt.
9. exp Clinical Trial/
10. (clin* adj5 trial*).ti,ab.
11. ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
12. ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab.
13. Placebos/
14. placebo*.ti,ab.
15. random*.ti,ab.
16. Research Design/
17. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
18. limit 17 to humans
19. exp Evaluation Studies/
20. Follow‐Up Studies/
21. Prospective Studies/
22. (control* or prospectiv* or volunteer*).mp,ti,ab.
23. 18 or 19 or 20 or 21 or 22
24. limit 23 to humans
25. 7 or 18 or 24
Search:
26. Atrial Fibrillation/
27. Atrial Flutter/
28. (atrial adj5 fibrillat*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
29. (atrial adj5 flutter*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
30. (auricular* adj5 fibrillat*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
31. (auricular* adj5 flutter*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
32. Tachycardia, Supraventricular/
33. (tachycardia adj5 supraventricular).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
34. 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33
35. Cardiac Surgical Procedures/
36. (heart adj5 surg*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
37. (cardiac adj5 surg*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
38. (coronary adj5 surg*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
39. (coronary adj5 bypass).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
40. CABG.mp.
41. (valv* adj5 surg*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
42. (valv* adj5 replace*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
43. 35 or 36 or 37 or 38 or 39 or 41 or 42
44. 34 and 43
45. 25 and 44
Data and analyses
Comparison 1. Atrial fibrillation or Supraventricular tachycardia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All Treatments | 118 | 18381 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.37, 0.47] |
| 1.1.1 Pharmacological Interventions | 93 | 14685 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.46] |
| 1.1.2 Non‐pharmacological Interventions | 27 | 3696 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.34, 0.57] |
| 1.2 Amiodarone | 33 | 5402 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.34, 0.54] |
| 1.3 Beta‐Blockers | 33 | 4698 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.26, 0.43] |
| 1.4 Sotalol | 11 | 1609 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.26, 0.43] |
| 1.5 Magnesium | 21 | 2988 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 1.6 Atrial Pacing | 21 | 2933 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.36, 0.61] |
| 1.7 Posterior Pericardiotomy | 6 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.67] |
1.1. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 1: All Treatments
1.2. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 2: Amiodarone
1.3. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 3: Beta‐Blockers
1.4. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 4: Sotalol
1.5. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 5: Magnesium
1.6. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 6: Atrial Pacing
1.7. Analysis.

Comparison 1: Atrial fibrillation or Supraventricular tachycardia, Outcome 7: Posterior Pericardiotomy
Comparison 2. Stroke or Cerebrovascular Accident.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All Treatments | 28 | 6361 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.01] |
| 2.1.1 Amiodarone | 14 | 3087 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.02] |
| 2.1.2 Beta‐Blockers | 5 | 1554 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.46, 3.93] |
| 2.1.3 Sotalol | 1 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.47] |
| 2.1.4 Magnesium | 3 | 760 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.20] |
| 2.1.5 Atrial Pacing | 6 | 832 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.36, 1.46] |
2.1. Analysis.

Comparison 2: Stroke or Cerebrovascular Accident, Outcome 1: All Treatments
Comparison 3. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All Treatments | 61 | 10986 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.39] |
| 3.1.1 Amiodarone | 23 | 4177 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.56] |
| 3.1.2 Beta‐Blockers | 16 | 2671 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.34, 2.22] |
| 3.1.3 Sotalol | 8 | 1092 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.08, 5.37] |
| 3.1.4 Magnesium | 12 | 1764 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.31, 2.24] |
| 3.1.5 Atrial Pacing | 7 | 1082 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.32, 2.47] |
| 3.1.6 Posterior Pericardiotomy | 2 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 16.44] |
3.1. Analysis.

Comparison 3: Mortality, Outcome 1: All Treatments
Comparison 4. Cardiovascular Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All Treatments | 40 | 6750 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.47, 1.62] |
| 4.1.1 Amiodarone | 14 | 2515 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.46, 1.86] |
| 4.1.2 Beta‐Blockers | 11 | 2011 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.10, 9.66] |
| 4.1.3 Sotalol | 7 | 964 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.1.4 Magnesium | 9 | 962 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.09, 3.13] |
| 4.1.5 Atrial Pacing | 2 | 198 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.1.6 Posterior Pericardiotomy | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
4.1. Analysis.

Comparison 4: Cardiovascular Mortality, Outcome 1: All Treatments
Comparison 5. Length of Stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All Treatments | 51 | 9661 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.95, ‐0.43] |
| 5.1.1 Amiodarone | 18 | 3497 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.37, ‐0.52] |
| 5.1.2 Beta‐Blockers | 6 | 1676 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.48, ‐0.00] |
| 5.1.3 Sotalol | 7 | 911 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.77, ‐0.02] |
| 5.1.4 Magnesium | 9 | 1589 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.47, 0.57] |
| 5.1.5 Atrial Pacing | 12 | 1525 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.72, ‐0.55] |
| 5.1.6 Posterior Pericardiotomy | 3 | 463 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐1.99, 3.12] |
5.1. Analysis.

Comparison 5: Length of Stay, Outcome 1: All Treatments
Comparison 6. Cost.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 All Treatments | 12 | 2790 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.97, ‐0.52] |
| 6.1.1 Amiodarone | 8 | 1344 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.65, ‐0.53] |
| 6.1.2 Beta‐Blockers | 2 | 1140 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐7.77, ‐0.83] |
| 6.1.3 Atrial Pacing | 3 | 306 | Mean Difference (IV, Random, 95% CI) | 8.22 [‐17.89, 34.33] |
6.1. Analysis.

Comparison 6: Cost, Outcome 1: All Treatments
Comparison 7. Risk of Bias Assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 All Treatments ‐ Randomization sequence generation | 118 | 18393 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.37, 0.47] |
| 7.1.1 High Risk | 7 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.49] |
| 7.1.2 Unclear Risk | 71 | 10437 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.34, 0.47] |
| 7.1.3 Low Risk | 40 | 7171 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.38, 0.55] |
| 7.2 All Treatments ‐ Allocation concealment | 118 | 18393 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.37, 0.47] |
| 7.2.1 High Risk | 6 | 630 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.40] |
| 7.2.2 Unclear Risk | 83 | 12185 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.35, 0.48] |
| 7.2.3 Low Risk | 29 | 5578 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.37, 0.54] |
| 7.3 Pharmacological Treatments ‐ Blinding of participants and personnel | 93 | 14617 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.46] |
| 7.3.1 High Risk | 19 | 1873 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.26, 0.48] |
| 7.3.2 Unclear Risk | 18 | 2388 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.21, 0.39] |
| 7.3.3 Low Risk | 56 | 10356 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.39, 0.55] |
| 7.4 Non‐Pharmacological Treatments ‐ Blinding of participants and personnel | 27 | 3696 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.34, 0.57] |
| 7.4.1 High Risk | 13 | 1691 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.30, 0.65] |
| 7.4.2 Unclear Risk | 7 | 1047 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.25, 0.81] |
| 7.4.3 Low Risk | 7 | 958 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.29, 0.59] |
| 7.5 Pharmacological Treatments ‐ Blinding of outcome assessment | 93 | 14697 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.46] |
| 7.5.1 High Risk | 19 | 2066 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.22, 0.47] |
| 7.5.2 Unclear Risk | 21 | 2518 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.26, 0.45] |
| 7.5.3 Low Risk | 53 | 10113 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.38, 0.53] |
| 7.6 Non‐Pharmacological Treatments ‐ Blinding of outcome assessment | 27 | 3696 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.34, 0.57] |
| 7.6.1 High Risk | 10 | 1317 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.25, 0.70] |
| 7.6.2 Unclear Risk | 7 | 1017 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.99] |
| 7.6.3 Low Risk | 10 | 1362 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.31, 0.51] |
| 7.7 All Treatments ‐ Incomplete outcome data | 118 | 18393 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.37, 0.47] |
| 7.7.1 High Risk | 14 | 1905 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.30, 0.59] |
| 7.7.2 Unclear Risk | 34 | 4249 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.34, 0.55] |
| 7.7.3 Low Risk | 70 | 12239 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.47] |
| 7.8 All Treatments ‐ Selective reporting | 118 | 18233 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.37, 0.47] |
| 7.8.1 High Risk | 5 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.05] |
| 7.8.2 Unclear Risk | 43 | 5712 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.47] |
| 7.8.3 Low Risk | 70 | 11970 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.37, 0.49] |
| 7.9 All Treatments ‐ Intention‐to‐treat analysis | 118 | 18393 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.37, 0.47] |
| 7.9.1 High Risk | 49 | 8017 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.37, 0.52] |
| 7.9.2 Unclear Risk | 53 | 6676 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.27, 0.43] |
| 7.9.3 Low Risk | 16 | 3700 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.44, 0.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abel 1983.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG using a saphenous vein graft only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | AF or atrial flutter; Mortality | |
| Follow‐Up | 6 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization based on last digit of hospital clinical record |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information of attempts to blind control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawn patients from treatment arm with high rate of clinical events |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Akbarzadeh 2009.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. Biatrial Pacing vs. Control | |
| Outcomes | AF lasting at least 1 hour or associated with hemodynamic compromise; Mortality | |
| Follow‐Up | ICU discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Externally validated sequence generator |
| Allocation concealment (selection bias) | Low risk | Allocation based on computer‐generated randomizer |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Insufficient information; Pacing, therefore unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blinded, therefore physicians know treatment regimen |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Alcalde 2006.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting at least 10 minutes or associated with hemodynamic instability; Stroke; LOS | |
| Follow‐Up | 8.9‐11.5 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers (75% of patients) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, identical looking pills |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Ali 1997.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Beta‐blocker (metoprolol, atenolol, sotalol or inderal) vs. Control | |
| Outcomes | AF; Stroke; Mortality; CV Mortality | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Alves 2007.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump cardiac surgery (79.7% CABG only, 2% mitral commissurotomy, 2% mitral valveplasty; 2% CABG + mitral valveplasty, 2% CABG + mitral valve replacement, 4.1% aortic valve replacement, 8.2% mitral valve replacement) | |
| Interventions | Amiodarone vs. Control | |
| Outcomes | AF | |
| Follow‐Up | 7 days or hospital discharge | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Arbatli 2003.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective, on‐pump CABG only | |
| Interventions | Posterior pericardiotomy vs. Control | |
| Outcomes | AF lasting at least 15 minutes; LOS | |
| Follow‐Up | 2 days | |
| Concurrent Antiarrhythmic Medications | Diltiazem; Magnesium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Asimakopoulos 1997.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Posterior pericardiotomy vs. Control | |
| Outcomes | SVT; Mortality | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgical Procedure |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgical Procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Assefi 2010.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | CABG only | |
| Interventions | Amiodarone vs. Control | |
| Outcomes | AF | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Alternate Randomization" used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Auer 2004.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Cardiac surgery (Valve surgery in 44.7% [Aortic 36%, Mitral 8.7%, Mitral and aortic 0.4%, Tricuspid 0.4%]; Combined CABG + valve in 9.9%) | |
| Interventions | Metoprolol vs. sotalol vs. placebo | |
| Outcomes | AF lasting at least 5 minutes or requiring intervention for angina or hemodynamic compromise; Stroke; Mortality; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generation of a randomization table |
| Allocation concealment (selection bias) | Low risk | Assignment and treatment was blinded and isolated from patients and treaters |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, identical looking pills |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Low risk | Yes |
Avila Neto 2007.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Right atrial pacing vs. Control | |
| Outcomes | AF | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Amiodarone and cardioversion in cases of clinical AF | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment distribution |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgical Procedure |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgical Procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Babin‐Ebell 1996.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | SVT | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Due to discontinuations in treatment group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Beaulieu 2010.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Nonurgent on‐pump valve surgery (isolated or CABG + valve) | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting more than 30 minutes or requiring treatment for hemodynamic compromise or discomfort; Stroke; Mortality; CV Mortality; LOS | |
| Follow‐Up | Hospital Discharge; Telephone follow‐up at 1 month | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization program |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Bert 2001.
| Study characteristics | ||
| Methods | Single‐blind, randomized, controlled | |
| Participants | On‐pump CABG, CABG + mitral valve | |
| Interventions | Magnesium vs. propranolol vs. control | |
| Outcomes | AF, atrial flutter or SVT lasting more than 5 minutes and requiring treatment; Mortality; CV Mortality; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers, digoxin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of placebo use |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | AF assessed by blinded physician |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Blommaert 2000.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Right atrial pacing vs. Control | |
| Outcomes | AF lasting at least 15 minutes | |
| Follow‐Up | 1 day | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgical Procedure |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgical Procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Budeus 2006.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | Af lasting at least 10 minutes; Mortality; CV Mortality; LOS; Cost | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization program |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Butler 1993.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | SVT lasting more than 5 minutes and requiring treatment; Stroke; Mortality; CV Mortality | |
| Follow‐Up | 6 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Cagli 2006.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting more than 30 minutes or requiring treatment for hemodynamic compromise or symptoms | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Casthely 1994.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. Control | |
| Outcomes | SVA | |
| Follow‐Up | 2 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of placebo use |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Chavan 2010.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | CABG only | |
| Interventions | Bachmann's bundle pacing vs. Right atrial pacing vs. Control | |
| Outcomes | AF | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Chung 2000a.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Atrial pacing vs. Control | |
| Outcomes | AF or atrial flutter requiring treatment; LOS | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers, digoxin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization program |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surgical Procedure |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Surgical Procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Connolly 2003.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump cardiac surgery | |
| Interventions | Metoprolol vs. placebo | |
| Outcomes | AF or atrial flutter; Stroke; Mortality; CV Mortality; LOS; Cost | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers, digoxin, diltiazem/verapamil | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Crystal 2003.
| Study characteristics | ||
| Methods | Multicentre double‐blind, randomized, placebo‐controlled | |
| Participants | CABG (n = 69); Aortic valve (n = 1); Mitral valve (n = 2); Aneurysm resection (n = 6); Other (n = 2) | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting at least 30 minutes | |
| Follow‐Up | Not reported (30‐day mortality) | |
| Concurrent Antiarrhythmic Medications | Verapamil, diltiazem, Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Dagdelen 2002.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | On‐pump valvular surgery with or without CABG | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF lasting at least 2 minutes | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Metoprolol, Nifedipine | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Daoud 1997.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting at least 5 minutes; Stroke; Mortality; CV Mortality; LOS; Cost | |
| Follow‐Up | Discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers, CCBs, Digitalis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Da Silva 2008.
| Study characteristics | ||
| Methods | Single‐blind, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial pacing vs. Control | |
| Outcomes | AF and atrial flutter | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Digoxin, beta‐blockers, calcium channel blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numerical table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pacing, therefore unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Daudon 1986.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Acebutolol vs. Control | |
| Outcomes | AF lasting at least 5 minutes | |
| Follow‐Up | 9 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of placebo use |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Debrunner 2004.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Biatrial pacing | |
| Outcomes | SVA (AF lasting at least 20 minutes atrial flutter or SVT); Mortality; CV Mortality | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pacing procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluated by 2 separate physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Dörge 2000.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone (150mg) vs. Amiodarone (300mg) vs. placebo | |
| Outcomes | AF; Mortality; LOS | |
| Follow‐Up | 10 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Ekim 2006.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump cardiac surgery (CABG n = 84; Valve n = 10; CABG + Valve n = 6) | |
| Interventions | Posterior pericardiotomy vs. Control | |
| Outcomes | SVT | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | During surgical procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent ECG monitoring |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
England 1992.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium | |
| Outcomes | AF; Mortality; CV Mortality; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Calcium channel blockers, Beta‐blockers, Digoxin, Other antiarrythmic agents | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized through pharmacy list system |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, identical looking placebo therapy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Eslami 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial pacing vs. Left atrial pacing vs. Control | |
| Outcomes | AF; Stroke; LOS; Cost | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers, Digitalis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Evrard 2000.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Sotalol vs. Control | |
| Outcomes | AF lasting at least 10 minutes; Mortality; CV Mortality | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of placebo use |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Fan 2000.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial pacing vs. Left atrial pacing vs. Right atrial pacing vs. Control | |
| Outcomes | AF lasting at least 10 minutes | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent ECG monitoring |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some drop‐out, but might not impact |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Low risk | Yes |
Fanning 1991.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | SVT lasting at least 30 minutes; Mortality; CV Mortality | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers until surgery; None postoperatively | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Farsak 2002.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Posterior pericardiotomy vs. Control | |
| Outcomes | AF requiring treatment for symptoms or hemodynamic deterioration; LOS | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random digits, where first 75 are treatment, next 75 control |
| Allocation concealment (selection bias) | High risk | Randmization method visible |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded patients, and outcome assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Forlani 2002.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. sotalol vs. Control | |
| Outcomes | AF requiring treatment for symptoms or hemodynamic deterioration; Mortality; CV Mortality; LOS | |
| Follow‐Up | 30 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization program |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Gerstenfeld 1999.
| Study characteristics | ||
| Methods | Single‐blind, randomized, controlled | |
| Participants | Elective on‐pump cardiac surgery (CABG n = 172; Valve n = 19; CABG + Valve n = 29) | |
| Interventions | Biatrial Pacing vs. Right atrial pacing vs. Control | |
| Outcomes | AF lasting at least 5 minutes or symptomatic or associated with hemodynamic compromise requiring treatment; LOS | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blinded, participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | AF determined through ECG |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Gerstenfeld 2001.
| Study characteristics | ||
| Methods | Multicentre, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial Pacing vs. Control | |
| Outcomes | AF; Stroke; Mortality; CV Mortality; LOS | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | AF determined through ECG |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Giri 2001.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG (86%) or CABG + valve (14%) | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting at least 30 minutes or requiring treatment due to symptoms or hemodynamic compromise; Stroke; Mortality; LOS; Cost | |
| Follow‐Up | Hospital discharge, Follow‐up at 30 days for mortality | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization software |
| Allocation concealment (selection bias) | Low risk | Pharmacy controlled groupings |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Goette 2002.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG or AVR | |
| Interventions | Bachmann's bundle pacing vs. Right atrial pacing vs. Control | |
| Outcomes | AF lasting at least 1 hour or requiring therapy for hemodynamic compromise; Mortality; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Intention‐to‐treat analysis | High risk | No |
Gomes 1999.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG or AVR | |
| Interventions | Sotalol vs. placebo | |
| Outcomes | AF lasting at least 1 hour or requiring therapy for hemodynamic compromise; Mortality; CV Mortality; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | External randomization through pharmacy registry |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled groupings |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Greenberg 2000.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | Off‐pump CABG only | |
| Interventions | Biatrial Pacing vs. Left atrial pacing vs. Right atrial pacing vs. Control | |
| Outcomes | AF lasting at least 10 minutes; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers (20mg qid) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded physician assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Low risk | Yes |
Gu 2009.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Valve surgery (with or without CABG) | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF; Mortality; LOS; Cost | |
| Follow‐Up | 14 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers, Calcium channel blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization software |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Guarnieri 1999.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Amiodarone vs. Placebo | |
| Outcomes | AF; Stroke; Mortality; CV Mortality; LOS; Cost | |
| Follow‐Up | 30 days | |
| Concurrent Antiarrhythmic Medications | Continuation if already using beta‐blockers, calcium channel blockers or digitalis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | Low risk | Yes |
Gun 1998.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. placebo | |
| Outcomes | AF | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Haddad 2009.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF; Mortality; CV Mortality; LOS | |
| Follow‐Up | 42 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Full details of outcomes not provided, although significance/non‐sig stated |
| Selective reporting (reporting bias) | Unclear risk | Annotated version, not all outcome data reported |
| Intention‐to‐treat analysis | High risk | No |
Hakala 2005.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Right atrial pacing (atrial overdrive) vs. Right atrial pacing (bradycardia prevention) vs. Control | |
| Outcomes | AF | |
| Follow‐Up | From 24 to 72 hours postoperative | |
| Concurrent Antiarrhythmic Medications | Metoprolol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Allocation based on sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pacing procedure, therefore unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Patients excluded if ICU stay longer than 24hr; excluded if AF event within first 24 hrs postop |
| Intention‐to‐treat analysis | Low risk | Yes |
Hamid 2008.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Off‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF lasting at least 15 minutes or requiring therapy for instability | |
| Follow‐Up | 1 day | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacy‐controlled, computer‐generated randomization software |
| Allocation concealment (selection bias) | Low risk | Allocation from pharmacy, administration of drug concealed through identical syringes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Harahsheh 2001.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | On‐pump CABG only (with saphenous vein graft) | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | SVT; Mortality | |
| Follow‐Up | 42 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate from treatment arm that could be related to therapy |
| Selective reporting (reporting bias) | High risk | No defined protocol, limited outcomes reported, an outcome seems to be measured at an illogical time point |
| Intention‐to‐treat analysis | High risk | No |
Hazelrigg 2004.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF, atrial flutter or SVT lasting more than one minute with hemodynamic deterioration; Mortality; LOS | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacy‐controlled, statistician developed randomization sequence |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐controlled randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded, placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Hohnloser 1991.
| Study characteristics | ||
| Methods | Single‐blind, randomized, placebo‐controlled | |
| Participants | CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | SVT | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Continuation if already on inotropic drugs, digitalis, nitrates, B‐blockers, CCBs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of patients, no mention of physicians |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some missing data, but from both study arms that are likely to balance out |
| Selective reporting (reporting bias) | Unclear risk | Events only recorded within 2 day postop time frame |
| Intention‐to‐treat analysis | High risk | No |
Imren 2007.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, randomized, controlled | |
| Participants | Off‐pump CABG only | |
| Interventions | Metoprolol vs. Control | |
| Outcomes | AF lasting more than 15 minutes; Stroke; Mortality; LOS | |
| Follow‐Up | 6 days | |
| Concurrent Antiarrhythmic Medications | Esmolol during surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Isolated physician regulated randomization schemed based on random number list |
| Allocation concealment (selection bias) | Low risk | Allocation from a non‐study related physician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Ivey 1983.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. placebo | |
| Outcomes | SVT; Mortality; CV Mortality | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacy‐controlled randomization and drug administration |
| Allocation concealment (selection bias) | Low risk | Double‐blind, and pharmacy‐controlled groupings |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Intention‐to‐treat analysis | High risk | No |
Jacquet 1994.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Sotalol vs. Control | |
| Outcomes | SVA lasting at least 1 minute with a heart rate of greater than 120 bpm; LOS | |
| Follow‐Up | Hospital discharge; 1‐3 month postoperative follow‐up | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomization method mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Event rates based on post‐randomization drop‐outs, but baseline characteristics not adjusted for |
| Selective reporting (reporting bias) | High risk | Adverse outcomes such as bradycardia not reported as risk factor, although high dropout rate due to complications |
| Intention‐to‐treat analysis | High risk | No |
Janssen 1986.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Metoprolol vs. Sotalol vs. Control | |
| Outcomes | SVT; Mortality; CV Mortality | |
| Follow‐Up | 2 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using sealed envelope |
| Allocation concealment (selection bias) | Low risk | Randomization using sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment allocation was marked on patient chart. No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Treatment allocation was marked on patient chart. No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Jensen 1997.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF lasting more than 1 minute | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers; Calcium antagonists; Nitroglycerin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Kanchi 2004.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Off‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF | |
| Follow‐Up | 1 day | |
| Concurrent Antiarrhythmic Medications | 50 mg atenolol; 10 mg diazepam | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Kaplan 2003.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Elective CABG only, both on and off‐pump | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF; LOS | |
| Follow‐Up | Hospital stay (6 days) | |
| Concurrent Antiarrhythmic Medications | Calcium‐channel blockers; ACE inhibitors; Digoxin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Karmy‐Jones 1995.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective CABG, valve or combination | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | SVT; Mortality; CV Mortality; LOS | |
| Follow‐Up | Hospital discharge (7 days) | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Physicians directing therapy were also blinded to all but the initial postoperative serum magnesium results" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Khuri 1987.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Nadolol vs. placebo | |
| Outcomes | SVT | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, done centrally |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "7 patients were excluded from the evaluation of efficacy because of insufficient postoperative data" ‐ Allocation not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Kuralay 1999.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Posterior pericardiotomy vs. Control | |
| Outcomes | SVT lasting at least 30 minutes | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Kurz 1999.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump cardiac surgery | |
| Interventions | Biatrial pacing vs. Control | |
| Outcomes | Af lasting at least 2 minutes | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | Trial Prematurely Aborted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization stratified by CABG vs valve |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial prematurely aborted ‐ Loss of atrial sensing in Tx group led to AF development |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Low risk | |
Lamb 1988.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Atenolol vs. Control | |
| Outcomes | AF | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Lee 2000.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting at least 10 minutes; Stroke; Mortality; CV Mortality; LOS, Cost | |
| Follow‐Up | 14 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers; Calcium channel blockers; Digitalis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Levy 2000.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial pacing vs. Control | |
| Outcomes | AF lasting at least 1 hour; Stroke; Mortality; LOS | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
Lúcio 2004.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Metoprolol vs. Control | |
| Outcomes | Sustained AF and atrial flutter; Mortality | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
Maras 2001.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting at least 1 hour or associated with hemodynamic compromise; Stroke; Mortality; CV Mortality; LOS | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | Not reported (control group did remain on usual medications) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
Markovic 2010.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Martinussen 1988.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. placebo | |
| Outcomes | SVT; Mortality; CV Mortality | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 patients removed from analysis because treatment/placebo not given |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Matangi 1985.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | SVA (AF or atrial flutter or atrial tachycardia); Mortality | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Low risk | |
Matangi 1989.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Atenolol vs. placebo | |
| Outcomes | SVA | |
| Follow‐Up | 8 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Materne 1985.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Acebutolol vs. Control | |
| Outcomes | SVT | |
| Follow‐Up | Hospital discharge (mean 10 days) | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Matsuura 2001.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Sotalol vs. Control | |
| Outcomes | AF lasting at least 5 minutes; Mortality; CV Mortality; LOS | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | Digitalis; ACE inhibitors; Dihydropyridine calcium antagonists | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was administered to controls |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Mirkhani 2005.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Biatrial pacing vs. Left atrial pacing vs. Control | |
| Outcomes | AF; Stroke; LOS | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Mitchell 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective CABG, valve or combo | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | SVT lasting at least 5 minutes and requiring treatment; Stroke; Mortality; CV Mortality; LOS | |
| Follow‐Up | 6 days | |
| Concurrent Antiarrhythmic Medications | Digoxin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated"; Stratified by age, type of surgery and use of preoperative beta‐blockers |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated" and "implemented by a hospital pharmacist who was not otherwise involved in the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study personnel were not aware of the allocation sequences or of patient allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Study personnel were not aware of the allocation sequences or of patient allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
Mohr 1981.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | SVT | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization by odd or even last numbers on medical records |
| Allocation concealment (selection bias) | High risk | Randomization by odd or even last numbers on medical records |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Mulay 1995.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Posterior pericardiotomy vs. Control | |
| Outcomes | SVT (AF or atrial flutter) that persisted despite correction of hypoxia or electrolyte imbalance; Mortality; CV Mortality | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | High risk | No |
Myhre 1984.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | "Clnically important SVA"; | |
| Follow‐Up | 8 days | |
| Concurrent Antiarrhythmic Medications | Nitroglycerin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "One patient in group B died peri‐operatively and thus one more patient was included" |
| Allocation concealment (selection bias) | High risk | "One patient in group B died peri‐operatively and thus one more patient was included" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Four patients were excluded from group B. Two patients did not receive propranolol at all, one because of arterial hypotension and one because propranolol was not administered as ordered" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | High risk | No |
Najafi 2007.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. Control | |
| Outcomes | AF | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The assignment of 345 patients to study group and control group was made possible with random number table at the time of admission to the operating room" |
| Allocation concealment (selection bias) | Low risk | "The assignment of 345 patients to study group and control group was made possible with random number table at the time of admission to the operating room" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients... were blind to the random allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Clinical confirmation of occurrence of AF was made by a cardiologist who was blind to the random allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Eleven patients were dropped from the study due to... Mistake in Mg infusion dosage calculation (n = 2)" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | High risk | No |
Neto 2007.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial pacing vs. Right atrial pacing vs. Control | |
| Outcomes | AF; Mortality | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers (preoperative) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Nurözler 1996.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. Control | |
| Outcomes | AF that did not convert to sinus rhythm in 1 minute | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Nygård 2004.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. Control | |
| Outcomes | AF lasting at least 5 minutes; Mortality; CV Mortality | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated from a computerized table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "The randomization list was generated from a computerized table of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was conducted in an open manner" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The study was conducted in an open manner" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Nyström 1993.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Sotalol | |
| Outcomes | AF; Mortality; CV Mortality | |
| Follow‐Up | 6 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Oka 1980.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | Paroxysmal atrial tachyarrhythmia | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Fifty‐four consecutive patients... receiving long‐term propranolol therapy, were entered in a randomized trial. They were compared with 17 patients... who were receiving no propranolol therapy prior to surgery" |
| Allocation concealment (selection bias) | High risk | "Fifty‐four consecutive patients... receiving long‐term propranolol therapy, were entered in a randomized trial. They were compared with 17 patients... who were receiving no propranolol therapy prior to surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Orboric 2010.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective CABG only | |
| Interventions | Amiodarone vs. Control | |
| Outcomes | AF | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Ormerod 1984.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | AF | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Ozin 2005.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Triple‐site Atrial Pacing | |
| Outcomes | AF; Stroke; LOS | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Low risk | "randomized blindly" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Parikka 1993.
| Study characteristics | ||
| Methods | Single‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF | |
| Follow‐Up | 10 days | |
| Concurrent Antiarrhythmic Medications | Metoprolol (25‐50 mg tid), Digoxin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Paull 1997.
| Study characteristics | ||
| Methods | Single‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Metoprolol vs. placebo | |
| Outcomes | AF; Mortality; CV Mortality | |
| Follow‐Up | Hospital discharge; First post‐op visit (not specified) | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Pfisterer 1997.
| Study characteristics | ||
| Methods | ||
| Participants | Elective on‐pump CABG or aortic valve or both | |
| Interventions | Sotalol vs. placebo | |
| Outcomes | AF or atrial flutter; LOS | |
| Follow‐Up | 90 days | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | Yes |
Redle 1999.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF; Mortality; CV Mortality; LOS; Cost | |
| Follow‐Up | 4 days | |
| Concurrent Antiarrhythmic Medications | Digoxin; Calcium channel blockers; Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomized list" |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomized list that remained confidential until formal unblinding at the end of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | Yes |
Roshanali 2009.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | ||
| Follow‐Up | Hospital discharge (approximately 7 days) | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers; Calcium channel blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Rubin 1987.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | AF lasting at least 30 seconds ("chosen because all episodes in this series lasting longer than 30 seconds persisted until pharmacologic intervention"); Stroke | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized by lot" |
| Allocation concealment (selection bias) | Low risk | "randomized by lot" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Twenty‐seven patients were excluded... 19 patients, protocol deviations" |
| Selective reporting (reporting bias) | High risk | Not all follow‐up data collected were reported on |
| Intention‐to‐treat analysis | High risk | No |
Salazar 1979.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | Paroxysmal atrial tachycardia, Flutter‐fibrillation, multiple atrial/nodal premature contractions | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Not reported (No beta‐blockers) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Schweikert 1998.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Biatrial Pacing vs. Control | |
| Outcomes | AF of at least 10 minutes requiring intervention | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All patients were connected via temporary epicardial wires..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | High risk | Abstract only |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Sezai 2011.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Landiolol vs. placebo | |
| Outcomes | AF lasting at least 5 minutes or requiring intervention for hemodynamic compromise; Stroke; Mortality; CV Mortality; LOS; Cost | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by lottery method |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | High risk | No |
Silverman 1982.
| Study characteristics | ||
| Methods | Multicentre, randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | ||
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was by birthdate" |
| Allocation concealment (selection bias) | High risk | "Randomization was by birthdate" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Speziale 2000.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF; Mortality; CV Mortality | |
| Follow‐Up | 2 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Stephenson 1980.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | AF or atrial flutter | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was by birthdate" |
| Allocation concealment (selection bias) | High risk | "Randomization was by birthdate" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Two patients were excluded from this group because the resident responsible for writing the transfer orders had prescribed a higher dose of propranolol than 10mg every 6 hours"; "Other patients were excluded from the propranolol group, even though they had been randomly assigned to it by one of us, because the resident writing the transfer orders had neglected to prescribe the drug" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | High risk | No |
Suttorp 1991.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | CABG, valve, combined, aortic or arrhythmia surgery | |
| Interventions | Sotalol vs. placebo | |
| Outcomes | SVA; Mortality; CV Mortality | |
| Follow‐Up | Not reported | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The data from 3 patients were excluded from analysis because of protocol violations (2 patients had taken the trial medication improperly)" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | High risk | No |
Tokmakoglu 2002.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | Elective CABG only | |
| Interventions | Amiodarone vs. Control | |
| Outcomes | AF | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Toraman 2001.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF lasting 10 minutes or requiring therapy | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Metoprolol only (19 in Treatment group, 23 in Control group) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All electrocardiograms were analyzed by a cardiologist who was blinded to the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Treggiari‐Venzi 2000.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. Magnesium vs. placebo | |
| Outcomes | AF; Mortality; CV Mortality | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Metoprolol only | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Turk 2007.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Elective off‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting 10 minutes or longer; Stroke; Mortality; CV Mortality | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Vecht 1986.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Timolol vs. placebo | |
| Outcomes | SVT lasting at least 30 minutes | |
| Follow‐Up | 1 day | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers until day of surgery; Postop digoxin not allowed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Weber 1998.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Sotalol vs. placebo | |
| Outcomes | AF, atrial flutter, SVT or AVNRT lasting at least 30 seconds; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Calcium channel blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | Yes |
Wenke 1999.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | CABG only | |
| Interventions | Metoprolol vs. Control | |
| Outcomes | SVA, AF or atrial flutter; LOS | |
| Follow‐Up | 10 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number series |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
White 1984.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Timolol vs. placebo | |
| Outcomes | AF or aflutter (divided into mild [< 30 sec, < 150 bpm], moderate [30 sec ‐ 5 min, 150 ‐ 200 bpm] or severe [> 5min, > 200 bpm]); Mortality; CV Mortality | |
| Follow‐Up | 7 days | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
White 2002.
| Study characteristics | ||
| Methods | Double‐blind, randomized, palcebo‐controlled | |
| Participants | Any open heart surgery (randomization was stratified by CABG vs valve) | |
| Interventions | Fast‐load amiodarone vs. Slow‐load amiodarone vs. placebo | |
| Outcomes | AF lasting more than 5 minutes or symptomatic; Mortality; Stroke; LOS | |
| Follow‐Up | Hospital discharge, 1 month postop | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The four randomization tables used in the study... were generated using commercially available statistical software" |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacy dispensed all study medication..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Low risk | Yes |
White 2003.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | Any cardiothoracic surgery (randomization stratified by CABG vs valve) | |
| Interventions | Amiodarone vs. Bachmann's Bundle Pacing vs. placebo | |
| Outcomes | AF; LOS; Cost | |
| Follow‐Up | Hospital discharge, 1 week postop, 1 month postop | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This study was designed as a management trial in that the recommended treatment regimens were established by study investigators and available to clinicians but the patient's physician determined whether to adjust regimen intensity or to discontinue therapy without investigator consultation" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This study was designed as a management trial in that the recommended treatment regimens were established by study investigators and available to clinicians but the patient's physician determined whether to adjust regimen intensity or to discontinue therapy without investigator consultation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Wilkes 2002.
| Study characteristics | ||
| Methods | Double‐blind, randomized, controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Magnesium vs. Control | |
| Outcomes | AF; Mortality; CV Mortality | |
| Follow‐Up | 3 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to one of two groups by random numbers generated from random‐number tables" |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated to one of two groups by random numbers generated from random‐number tables" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To administer magnesium sulfate on the basis of ionized magnesium plasma levels and repeat this intervention as required, one nominated investigator remained unblinded to treatment group. All other clinicians involved in the care of patients and technicians concerned with the analysis of Holter tapes were rigorously blinded throughout the study period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To administer magnesium sulfate on the basis of ionized magnesium plasma levels and repeat this intervention as required, one nominated investigator remained unblinded to treatment group. All other clinicians involved in the care of patients and technicians concerned with the analysis of Holter tapes were rigorously blinded throughout the study period." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Another two patients underwent mediastinal reexploration for bleeding complications (one in each group). All of these patients were excluded from further analysis of postoperative cardiac rhythm" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Williams 1982.
| Study characteristics | ||
| Methods | Randomized, controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Propranolol vs. Control | |
| Outcomes | AF | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Digoxin (9 patients), Quinidine or procainamide (5 patients) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Yagdi 2003.
| Study characteristics | ||
| Methods | ||
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting more than 5 minutes or requiring therapy; Stroke; Mortality; LOS | |
| Follow‐Up | Hospital discharge, 30 days postop | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Yazicioglu 2002.
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled | |
| Participants | On‐pump CABG only | |
| Interventions | Atenolol vs. placebo | |
| Outcomes | AF; Mortality; CV Mortality | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Yazigi 2002.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective on‐pump CABG only | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF lasting for > 5 minutes; Stroke; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | None | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacy dispensed all study medication..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | Yes |
Yeatman 2002.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only (elective and urgent but not emergency) | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF; Stroke; Mortality; LOS | |
| Follow‐Up | Hospital discharge (mortality = 30 days) | |
| Concurrent Antiarrhythmic Medications | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was performed by card allocation immediately before surgery" |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was performed by card allocation immediately before surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Unclear risk | Not reported |
Zangrillo 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | Elective off‐pump CABG only | |
| Interventions | Magnesium vs. placebo | |
| Outcomes | AF lasting for > 10 minutes or requiring treatment; Stroke; Mortality; CV Mortality; LOS | |
| Follow‐Up | Hospital discharge | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated list"; "Independent nurses dispensed either Mg or placebo in the operating room" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Intention‐to‐treat analysis | Low risk | Yes |
Zebis 2007.
| Study characteristics | ||
| Methods | Double‐blind, randomized, placebo‐controlled | |
| Participants | On‐pump CABG only; Randomization stratified by age (65 yrs) and pre‐op use of Beta‐blockers | |
| Interventions | Amiodarone vs. placebo | |
| Outcomes | AF; Stroke; Mortality | |
| Follow‐Up | 5 days | |
| Concurrent Antiarrhythmic Medications | Beta‐blockers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized prospective randomization schedule" |
| Allocation concealment (selection bias) | Low risk | "Each patient received a randomization number, which was recorded and sent by fax to the pharmacy... The pharmacy decoded the randomization number, prepared the appropriate infusion and pills, and forwarded them, together with a sealed opaque envelope containing the randomization assignment..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis stated, but not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Intention‐to‐treat analysis | High risk | No |
ACE: angiotensin‐converting enzyme AF: atrial fibrillation AVNRT: atrioventricular nodal reentry tachycardia AVR: aortic valve replacement bpm: beats per minute CABG: coronary artery bypass graft CCBs: calcium channel blockers CV: cardiovascular ICU: intensive care unit LOS: length of stay SVT: supraventricular tachycardia tid: three times per day
Differences between protocol and review
Two additional interventions, magnesium and posterior pericardiotomy, were included due to their significant presence within the literature.
Contributions of authors
Kyle Arsenault
‐ Protocol preparation
‐ Search strategy development and initiation
‐ Study selection
‐ Obtaining copies of studies
‐ Data extraction
‐ Data analysis and interpretation
‐ Manuscript preparation
‐ Final editing
Arif Yusuf
‐ Study selection
‐ Obtaining copies of studies
‐ Data extraction
‐ Final editing
Eugene Crystal
‐ Original review
‐ Final editing
Carlos Morillo
‐ Protocol preparation
‐ Data interpretation
‐ Final editing
Jeff Healey
‐ Data interpretation
‐ Final editing
Girish Nair
‐ Data interpretation
‐ Final editing
Richard Whitlock
‐ Lead research effort
‐ Protocol preparation
‐ Data interpretation
‐ Manuscript preparation
‐ Final editing
Sources of support
Internal sources
No sources of support supplied
External sources
-
Beamish Family Foundation Chair in Vascular Surgery, Canada
Kyle Arsenault was supported by the Beamish Family Foundation Chair in Vascular Surgery, held by Dr. Jacques G. Tittley
Declarations of interest
The authors have no declarations of interest.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abel 1983 {published data only}
- Abel RM, Van Gelder HM, Pores IH, Ligouri J, Gelchinsky I, Parsonnet V. Continued propanolol administration following coronary bypass surgery: antiarrhythmic effects. Archives of Surgery 1983;118:727-31. [DOI] [PubMed] [Google Scholar]
Akbarzadeh 2009 {published data only}
- Akbarzadeh F, Kazemi-Arbat B, Golmohammadi A, Pourafkari L. Biatrial pacing vs. intravenous amiodarone in prevention of atrial fibrillation after coronary artery bypass surgery. Pakistan Journal of Biological Sciences 2009;12(19):1325-9. [DOI] [PubMed] [Google Scholar]
Alcalde 2006 {published data only}
- Alcalde RV, Guaragna JC, Bodanese LC, Castro I, Sussenbach E, Noer R, et al. High dose of amiodarone in a short-term period reduces the incidence of postoperative atrial fibrillation and atrial flutter. Arquivos Brasileiros de Cardiologia 2006;87(3):202-5. [DOI] [PubMed] [Google Scholar]
Ali 1997 {published data only}
- Ali IM, Sanalla AA, Clark V. Beta-blocker effects on postoperative atrial fibrillation. European Journal of Cardio-Thoracic Surgery 1997;11(6):1154-7. [DOI] [PubMed] [Google Scholar]
Alves 2007 {published data only}
- Alves RJ, Geovanini GR, De Brito G, Miguel GA, Glauser VA, Nakiri K. Prevention of atrial fibrillation with moderate doses of amiodarone in the postoperative period of cardiac surgery is safe and effective in patients with high risk for developing this arrhythmia. Arquivos Brasileiros de Cardiologia 2007;89(1):20-4. [PubMed] [Google Scholar]
Arbatli 2003 {published data only}
- Arbatli H, Demirsoy E, Aytekin S, Rizaoglu E, Unal M, Yagan N, et al. The role of posterior pericardiotomy on the incidence of atrial fibrillation after coronary revascularization. Journal of Cardiovascular Surgery 2003;44:713-7. [PubMed] [Google Scholar]
Asimakopoulos 1997 {published data only}
- Asimakopoulos G, Della Santa R, Taggart DP. Effects of posterior pericardiotomy on the incidence of atrial fibrillation and chest drainage after coronary revascularization: a prospective randomized trial. Journal of Thoracic and Cardiovascular Surgery 1997;113(4):797-9. [DOI] [PubMed] [Google Scholar]
Assefi 2010 {published data only}
- Assefi M, Najafi F, Salehi K. Effect of amiodarone in preventing atrial fibrillation in high risk patients after CABG surgery. International Journal of Medicine 2010;40(Suppl. 1):70. [Google Scholar]
Auer 2004 {published data only}
- Auer J, Weber T, Berent R, Puschmann R, Hartl P, Ng CK, et al. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: The Pilot Study of Prevention of Postoperative Atrial Fibrillation (SPPAF), a randomized, placebo-controlled trial. American Heart Journal 2004;147(4):636-43. [DOI] [PubMed] [Google Scholar]
Avila Neto 2007 {published data only}
- Avila Neto V, Costa R, Silva KR, Martins AL, Moreira LF, Santos LB, et al. Effect of temporary right atrial pacing in prevention of atrial fibrillation after coronary artery bypass graft surgery [Efeitos da estimulação temporária atrial direita na prevenção da fibrilação atrial no pós-operatóriode revascularização do miocárdio com circulação extracorpórea]. Revista Brasileira de Cirurgia Cardiovascular 2007;22(3):332-40. [DOI] [PubMed] [Google Scholar]
Babin‐Ebell 1996 {published data only}
- Babin-Ebell J, Keith PR, Elert O. Efficacy and safety of low-dose propranolol versus diltiazem in the prophylaxis of supraventricular tachyarrhythmia after coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery 1996;10(6):412-6. [DOI] [PubMed] [Google Scholar]
Beaulieu 2010 {published data only}
- Beaulieu Y, Denault AY, Couture P, Roy D, Talajic M, O'Meara E, et al. Perioperative intravenous amiodarone does not reduce the burden of atrial fibrillation in patients undergoing cardiac valvular surgery. Anesthesiology 2010;112(1):128-37. [DOI] [PubMed] [Google Scholar]
Bert 2001 {published data only}
- Bert AA, Reinert SE, Singh AK. A beta-blocker, not magnesium, is effective prophylaxis for atrial tachyarrhythmias after coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2001;15(2):204-9. [DOI] [PubMed] [Google Scholar]
Blommaert 2000 {published data only}
- Blommaert D, Gonzalez M, Mucumbitsi J, Gurné O, Evrard P, Buche M, et al. Effective prevention of atrial fibrillation by continuous atrial overdrive pacing after coronary artery bypass surgery. Journal of the American College of Cardiology 2000;35(6):1411-5. [DOI] [PubMed] [Google Scholar]
Budeus 2006 {published data only}
- Budeus M, Hennersdorf M, Perings SR, Röhlen S, Schnitzler S, Felix O, et al. Amiodarone prophylaxis for atrial fibrillation of high-risk patients after coronary bypass grafting: a prospective, double-blinded, placebo-controlled, randomized study. European Heart Journal 2006;27(13):1584-91. [DOI] [PubMed] [Google Scholar]
Butler 1993 {published data only}
- Butler J, Harriss DR, Sinclair M, Westaby S. Amiodarone prophylaxis for tachycardias after coronary artery surgery: a randomised, double blind, placebo controlled trial. British Heart Journal 1993;70(1):56-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cagli 2006 {published data only}
- Cagli K, Ozeke O, Ergun K, Budak B, Demirtas E, Birincioglu CL, et al. Effect of low-dose amiodarone and magnesium combination on atrial fibrillation after coronary artery surgery. Journal of Cardiac Surgery 2006;21(5):458-64. [DOI] [PubMed] [Google Scholar]
Casthely 1994 {published data only}
- Casthely PA, Yoganathan T, Komer C, Kelly M. Magnesium and arrhythmias after coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 1994;8(2):188-91. [DOI] [PubMed] [Google Scholar]
Chavan 2010 {published data only}
- Chavan C, Karmalkar M, Badani R, Sharada K, Rani U, Rao P, et al. Evaluation of Bachmann Bundle pacing versus right atrial pacing in prevention of atrial fibrillation after coronary artery bypass surgery. Indian Pacing and Electrophysiology Journal 2010;10(12):529-35. [PMC free article] [PubMed] [Google Scholar]
Chung 2000a {published data only}
- Chung MK, Augostini RS, Asher CR, Pool DP, Grady TA, Zikri M, et al. Ineffectiveness and potential proarrhythmia of atrial pacing for atrial fibrillation prevention after coronary artery bypass grafting. Annals of Thoracic Surgery 2000;69(4):1057-63. [DOI] [PubMed] [Google Scholar]
Connolly 2003 {published data only}
- Connolly SJ, Cybulsky I, Lamy A, Roberts RS, O'Brien B, Carroll S, et al. Double-blind, placebo-controlled, randomized trial of prophylactic metoprolol for reduction of hospital length of stay after heart surgery: The beta-Blocker Length Of Stay (BLOS) study. American Heart Journal 2003;145(2):226-32. [DOI] [PubMed] [Google Scholar]
Crystal 2003 {published data only}
- Crystal E, Kahn S, Roberts R, Thorpe K, Gent M, Cairns JA, et al. Long-term amiodarone therapy and the risk of complications after cardiac surgery: Results from the Canadian Amiodarone Myocardial Infarction Arrhythmia Trial (CAMIAT). Journal of Thoracic and Cardiovascular Surgery 2003;125(3):633-7. [DOI] [PubMed] [Google Scholar]
Dagdelen 2002 {published data only}
- Dagdelen S, Toraman F, Karabulut H, Alhan C. The value of P dispersion on predicting atrial fibrillation after coronary artery bypass surgery: effect of magnesium on P dispersion. Annals of Noninvasive Electrocardiology 2002;7(3):211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdelen S, Yuce M, Toraman F, Karabulut H, Alhan C. The value of P dispersion on predicting atrial fibrillation after coronary artery bypass surgery; effect of magnesium on P dispersion. Cardiac Electrophysiology Review 2003;7(2):162-4. [DOI] [PubMed] [Google Scholar]
Daoud 1997 {published data only}
- Daoud EG, Strickberger SA, Man KC, Goyal R, Deeb GM, Bolling SF, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. New England Journal of Medicine 1997;337(25):1785-91. [DOI] [PubMed] [Google Scholar]
Da Silva 2008 {published data only}
- Da Silva FM, Milani R, Precoma D, Guimaraes M, Moutinho JA, Barboza L, et al. Influence of external temporary biatrial pacing on the prevention of atrial fibrillation after coronary artery bypass without extracorporeal circulation. Arquivos Brasileiros de Cardiologia 2008;90(2):80-5. [PubMed] [Google Scholar]
Daudon 1986 {published data only}
- Daudon P, Corcos T, Gandjbakhch I. Prevention of atrial fibrillation or flutter by acebutolol after coronary bypass grafting. American Journal of Cardiology 1986;58(10):933-6. [DOI] [PubMed] [Google Scholar]
Debrunner 2004 {published data only}
- Debrunner M, Naegeli B, Genoni M, Turina M, Bertel O. Prevention of atrial fibrillation after cardiac valvular surgery by epicardial, biatrial synchronous pacing. European Journal of Cardio-Thoracic Surgery 2004;25(1):16-20. [DOI] [PubMed] [Google Scholar]
Dörge 2000 {published data only}
- Dörge H, Schoendube FA, Schoberer M, Stellbrink C, Voss M, Messmer BJ. Intraoperative amiodarone as prophylaxis against atrial fibrillation after coronary operations. Annals of Thoracic Surgery 2000;69(5):1358-62. [DOI] [PubMed] [Google Scholar]
Ekim 2006 {published data only}
- Ekim H, Kutay V, Hazar A, Akbayrak H, Başel H, Tuncer M. Effects of posterior pericardiotomy on the incidence of pericardial effusion and atrial fibrillation after coronary revascularization. Medical Science Monitor 2006;12(10):CR431-4. [PubMed] [Google Scholar]
England 1992 {published data only}
- England MR, Gordon G, Salem M, Chernow B. Magnesium administration and dysrhythmias after cardiac surgery. A placebo-controlled, double-blind, randomized trial. JAMA 1992;268(17):2395-402. [PubMed] [Google Scholar]
Eslami 2005 {published data only}
- Eslami M, Mirkhani HS, Sanatkar M, Bayat H, Sattarzadeh R, Mirhoseini M. Role of biatrial pacing in prevention of atrial fibrillation after coronary artery bypass surgery. Indian Pacing & Electrophysiology Journal 2005;5(1):5-11. [PMC free article] [PubMed] [Google Scholar]
Evrard 2000 {published data only}
- Evrard P, Gonzalez M, Jamart J, Malhomme B, Blommaert D, Eucher P, et al. Prophylaxis of supraventricular and ventricular arrhythmias after coronary artery bypass grafting with low-dose sotalol. Annals of Thoracic Surgery 2000;70(1):151-6. [DOI] [PubMed] [Google Scholar]
Fan 2000 {published data only}
- Fan K, Lee KL, Chiu CS, Lee JW, He GW, Cheung D, et al. Effects of biatrial pacing in prevention of postoperative atrial fibrillation after coronary artery bypass surgery. Circulation 2000;102(7):755-60. [DOI] [PubMed] [Google Scholar]
Fanning 1991 {published data only}
- Fanning WJ, Thomas CS, Roach A, Tomichek R, Alford WC, Stoney WS. Prophylaxis of atrial fibrillation with magnesium sulfate after coronary artery bypass grafting. Annals of Thoracic Surgery 1991;52(3):529-33. [DOI] [PubMed] [Google Scholar]
Farsak 2002 {published data only}
- Farsak B, Günaydin S, Tokmakoglu H, Kandemir Ö, Yorgancioglu C, Zorlutuna, Y. Posterior pericardiotomy reduces the incidence of supra-ventricular arrhythmias and pericardial effusion after coronary artery bypass grafting. European Journal of Cardio-Thoracic Surgery 2002;22(2):278-81. [DOI] [PubMed] [Google Scholar]
Forlani 2002 {published data only}
- Forlani S, De Paulis R, Notaris S, Nardi P, Tomai F, Proietti I, et al. Combination of sotalol and magnesium prevents atrial fibrillation after coronary artery bypass grafting. Annals of Thoracic Surgery 2002;74(3):720-6. [DOI] [PubMed] [Google Scholar]
Gerstenfeld 1999 {published data only}
- Gerstenfeld EP, Hill MR, French SN, Mehra R, Rofino K, Vander Salm TJ, et al. Evaluation of right atrial and biatrial temporary pacing for the prevention of atrial fibrillation after coronary artery bypass surgery. Journal of the American College of Cardiology 1999;33(7):1981-8. [DOI] [PubMed] [Google Scholar]
Gerstenfeld 2001 {published data only}
- Gerstenfeld EP, Khoo M, Martin RC, Cook JR, Lancey R, Rofino K, Vander Salm TJ, Mittleman RS. Effectiveness of bi-atrial pacing for reducing atrial fibrillation after coronary artery bypass graft surgery. Journal of Interventional Cardiac Electrophysiology 2001;5(3):275-83. [DOI] [PubMed] [Google Scholar]
Giri 2001 {published data only}
- Giri S, White CM, Dunn AB, Felton K, Freeman-Bosco L, Reddy P, et al. Oral amiodarone for prevention of atrial fibrillation after open heart surgery, the Atrial Fibrillation Suppression Trial (AFIST): a randomised placebo-controlled trial. Lancet 2001;357:830-6. [DOI] [PubMed] [Google Scholar]
- Giri S. Erratum: Oral amiodarone for prevention of atrial fibrillation after open heart surgery, the Atrial Fibrillation Suppression Trial (AFIST): A randomised placebo-controlled trial. Lancet 2001;358:246. [DOI] [PubMed] [Google Scholar]
- Kluger J, White CM. Amiodarone prevents symptomatic atrial fibrillation and reduces the risk of cerebrovascular accidents and ventricular tachycardia after open heart surgery: Results of the atrial fibrillation suppression trial (AFIST). Cardiac Electrophysiology Review 2003;7(2):165-7. [DOI] [PubMed] [Google Scholar]
Goette 2002 {published data only}
- Goette A, Mitag J, Friedl A. Effectiveness of atrial pacing inpreventing atrial fibrillation after cardiovascular surger [abstract]. Pacing and Clinical Electrophysiology 1999;23:700. [Google Scholar]
- Goette A, Mittag J, Friedl A, Busk H, Jepsen MS, Hartung WM, et al. Pacing of Bachmann's bundle after coronary artery bypass grafting. Pacing and Clinical Electrophysiology 2002;25(7):1072-8. [DOI] [PubMed] [Google Scholar]
- Goette A. Pacing to prevent atrial fibrillation after coronary artery bypass grafting. What works, what doesn't: insights from Bachmann's Bundle pacing. Cardiac Electrophysiology Review 2003;7(2):154-7. [DOI] [PubMed] [Google Scholar]
Gomes 1999 {published data only}
- Gomes JA, Ip J, Santoni-Rugiu F, Mehta D, Ergin A, Lansman S, et al. Oral d,l sotalol reduces the incidence of postoperative atrial fibrillation in coronary artery bypass surgery patients: a randomized, double-blind, placebo-controlled study. Journal of the American College of Cardiology 1999;34(2):334-9. [DOI] [PubMed] [Google Scholar]
Greenberg 2000 {published data only}
- Greenberg MD, Katz NM, Iuliano S, Tempesta BJ, Solomon AJ. Atrial pacing for the prevention of atrial fibrillation after cardiovascular surgery. Journal of the American College of Cardiology 2000;35(6):1416-22. [DOI] [PubMed] [Google Scholar]
Gu 2009 {published data only}
- Gu S, Su PX, Liu Y, Yan J, Zhang XT, Wang TY. Low-dose amiodarone for the prevention of atrial fibrillation after coronary artery bypass grafting in patients older than 70 years. Chinese Medical Journal 2009;122(24):2928-32. [PubMed] [Google Scholar]
Guarnieri 1999 {published data only}
- Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR. Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial. Journal of the American College of Cardiology 1999;34(2):343-7. [DOI] [PubMed] [Google Scholar]
Gun 1998 {published data only}
- Gun C, Bianco AC, Freire RB, Ramos RF, Timernan A, Piegas LS. Beta-blocker effects on postoperative atrial fibrillation after coronary artery bypass surgery. In: Journal of American College of Cardiology/CD-ROM of Abstracts from World Cardiology Congress. 1998:3801.
Haddad 2009 {published data only}
- Haddad M, Nair R, Hendry P, Coyle D, Mesana T. Peri-operative amiodarone for post-operative atrial fibrillation prophylaxis in valve surgery patients. Critical Care Medicine 2009;37(12 (Suppl.)):A400. [Google Scholar]
Hakala 2005 {published data only}
- Hakala T, Valtola AJ, Turpeinen AK, Hedman AE, Vuorenniemi RE, Karjalainen JM, et al. Right atrial overdrive pacing does not prevent atrial fibrillation after coronary artery bypass surgery. Europace 2005;7(2):170-4. [DOI] [PubMed] [Google Scholar]
Hamid 2008 {published data only}
- Hamid M, Kamal RS, Sami SA, Atiq F, Shafquat A, Naqvi HI, et al. Effect of single dose magnesium on arrhythmias in patients undergoing coronary artery bypass surgery. Journal of the Pakistan Medical Association 2008;58(1):22-7. [PubMed] [Google Scholar]
Harahsheh 2001 {published data only}
- Harahsheh BS, Sawalha WA. Effect of amiodarone on atrial fibrillation after coronary artery bypass surgery. Saudi Medical Journal 2001;22(9):797-9. [PubMed] [Google Scholar]
Hazelrigg 2004 {published data only}
- Hazelrigg SR, Boley TM, Cetindag IB, Moulton KP, Trammell GL, Polancic JE, et al. The efficacy of supplemental magnesium in reducing atrial fibrillation after coronary artery bypass grafting. Annals of Thoracic Surgery 2004;77(3):824-30. [DOI] [PubMed] [Google Scholar]
- Hazelrigg SR, Boley TM, Moulton KP, Trammel GL, Palancic JE, Shawgo TS. The efficacy of supplemental magnesium in reducing atrial fibrillation after coronary artery bypass grafting abstract. American Journal of Respiratory and Critical Care Medicine 2002;165(8 (Suppl.)):A790. [DOI] [PubMed] [Google Scholar]
Hohnloser 1991 {published data only}
- Hohnloser SH, Meinertz T, Dammbacher T, Steiert K, Jähnchen E, Zehender M, et al. Electrocardiographic and antiarrhythmic effects of intravenous amiodarone: results of a prospective, placebo-controlled study. American Heart Journal 1991;121:89-95. [DOI] [PubMed] [Google Scholar]
Imren 2007 {published data only}
- Imren Y, Benson AA, Zor H, Tasoglu I, Ereren E, Sinci V, et al. Preoperative beta-blocker use reduces atrial fibrillation in off-pump coronary bypass surgery. Australian and New Zealand Journal of Surgery 2007;77(6):429-32. [DOI] [PubMed] [Google Scholar]
Ivey 1983 {published data only}
- Ivey MF, Ivey TD, Bailey WW, Williams DB, Hessel EA, Miller DW. Influence of propranolol on supraventricular tachycardia early after coronary artery revascularization. A randomized trial. Journal of Thoracic and Cardiovascular Surgery 1983;85(2):214-8. [PubMed] [Google Scholar]
Jacquet 1994 {published data only}
- Jacquet L, Evenepoel M, Marenne F, Evrard P, Verhelst R, Dion R, et al. Hemodynamic effects and safety of sotalol in the prevention of supraventricular arrhythmias after coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 1994;8(4):431-6. [DOI] [PubMed] [Google Scholar]
Janssen 1986 {published data only}
- Janssen J, Loomans L, Harink J, Taams M, Brunninkhuis L, Starre P, et al. Prevention and treatment of supraventricular tachycardia shortly after coronary artery bypass grafting: a randomized open trial. Angiology 1986;37(8):601-9. [DOI] [PubMed] [Google Scholar]
Jensen 1997 {published data only}
- Jensen BM, Alstrup P, Klitgård NA. Magnesium substitution and postoperative arrhythmias in patients undergoing coronary artery bypass grafting. Scandinavian Cardiovascular Journal 1997;31(5):265-9. [DOI] [PubMed] [Google Scholar]
Kanchi 2004 {published data only}
- Kanchi M, Prasad N, Garg D, Banakal SK. Prophylactic magnesium sulphate vs. lidocaine during off-pump coronary artery bypass grafting. European Journal of Anaesthesiology 2004;21(11):914-5. [DOI] [PubMed] [Google Scholar]
Kaplan 2003 {published data only}
- Kaplan M, Kut MS, Icer UA, Demirtas MM. Intravenous magnesium sulfate prophylaxis for atrial fibrillation after coronary artery bypass surgery. Journal of Thoracic and Cardiovascular Surgery 2003;125(2):344-52. [DOI] [PubMed] [Google Scholar]
Karmy‐Jones 1995 {published data only}
- Karmy-Jones R, Hamilton A, Dzavik V, Allegreto M, Finegan BA, Koshal A. Magnesium sulfate prophylaxis after cardiac operations. Annals of Thoracic Surgery 1995;59(2):502-7. [DOI] [PubMed] [Google Scholar]
Khuri 1987 {published data only}
- Khuri SF, Okike ON, Josa M, Vander Salm TJ, Assoussa S, Leone L, et al. Efficacy of nadolol in preventing supraventricular tachycardia after coronary artery bypass grafting. American Journal of Cardiology 1987;60(6):51D-58D. [DOI] [PubMed] [Google Scholar]
Kuralay 1999 {published data only}
- Kuralay E, Ozal E, Demirkili U, Tatar H. Effect of posterior pericardiotomy on postoperative supraventricular arrhythmias and late pericardial effusion. Journal of Thoracic and Cardiovascular Surgery 1999;118(3):492-5. [DOI] [PubMed] [Google Scholar]
Kurz 1999 {published data only}
- Kurz DJ, Naegeli B, Kunz M, Genoni M, Niederhauser U, Bertel O. Epicardial, biatrial synchronous pacing for prevention of atrial fibrillation after cardiac surgery. Pacing and Clinical Electrophysiology 1999;22(5):721-6. [DOI] [PubMed] [Google Scholar]
Lamb 1988 {published data only}
- Lamb RK, Prabhakar G, Thorpe JA, Smith S, Norton R, Dyde JA. The use of atenolol in the prevention of supraventricular arrhythmias following coronary artery surgery. European Heart Journal 1988;9(1):32-6. [PubMed] [Google Scholar]
Lee 2000 {published data only}
- Lee SH, Chang CM, Lu MJ, Lee RJ, Cheng JJ, Hung CR, et al. Intravenous amiodarone for prevention of atrial fibrillation after coronary artery bypass grafting. Annals of Thoracic Surgery 2000;70(1):157-61. [DOI] [PubMed] [Google Scholar]
Levy 2000 {published data only}
- Levy T, Fotopoulos G, Walker S, Rex S, Octave M, Paul V, et al. Randomized controlled study investigating the effect of biatrial pacing in prevention of atrial fibrillation after coronary artery bypass grafting. Circulation 2000;102(12):1382-7. [DOI] [PubMed] [Google Scholar]
Lúcio 2004 {published data only}
- Lúcio E de A, Flores A, Blacher C, Leães PE, Lucchese FA, Ribeiro JP. Effectiveness of metoprolol in preventing atrial fibrillation and flutter in the postoperative period of coronary artery bypass graft surgery. Arquivos Brasileiros de Cardiologia 2004;82(1):42-6. [PubMed] [Google Scholar]
Maras 2001 {published data only}
- Maraš D, Bošković SD, Popović Z, Nešković AN, Kovačević S, Otašević P, et al. Single-day loading dose of oral amiodarone for the prevention of new-onset atrial fibrillation after coronary artery bypass surgery. American Heart Journal 2001;141(5):E8. [DOI] [PubMed] [Google Scholar]
Markovic 2010 {published data only}
- Markovic D, Radovic M, Terzic M, Soskic L, Kovacevic Kostic N, Minic M. Low dose of intravenous amiodarone for the prevention postoperative atrial fibrillation in CABG patients. Intensive Care Medicine 2010;36:S320. [Google Scholar]
Martinussen 1988 {published data only}
- Martinussen HJ, Lolk A, Szczepanski C, Alstrup P. Supraventricular tachyarrhythmias after coronary bypass surgery--a double blind randomized trial of prophylactic low dose propranolol. Thoracic and Cardiovascular Surgeon 1988;36(4):206-7. [DOI] [PubMed] [Google Scholar]
Matangi 1985 {published data only}
- Matangi MF, Neutze JM, Graham KJ, Hill DG, Kerr AR, Barratt-Boyes BG. Arrhythmia prophylaxis after aorta-coronary bypass: the effect of minidose propanolol. Journal of Thoracic and Cardiovascular Surgery 1985;89:439-43. [PubMed] [Google Scholar]
Matangi 1989 {published data only}
- Matangi MF, Strickland J, Garbe GJ, Habib N, Basu AK, Burgess JJ, et al. Atenolol for the prevention of arrhythmias following coronary artery bypass grafting. Canadian Journal of Cardiology 1989;5(4):229-34. [PubMed] [Google Scholar]
Materne 1985 {published data only}
- Materne P, Larbuisson R, Collignon P, Limet R, Kulbertus H. Prevention by acebutolol of rhythm disorders following coronary bypass surgery. International Journal of Cardiology 1985;8(3):275-86. [DOI] [PubMed] [Google Scholar]
Matsuura 2001 {published data only}
- Matsuura K, Takahara Y, Sudo Y, Ishida K. Effect of sotalol in the prevention of atrial fibrillation following coronary artery bypass grafting. Japanese Journal of Thoracic and Cardiovascular Surgery 2001;49(10):614-7. [DOI] [PubMed] [Google Scholar]
Mirkhani 2005 {published data only}
- Mirkhani S, Eslami M, Bayat H, Sanatkar M, Sattarzadeh R, Naseri M. Biatrial pacing as cost-effective strategy for prevention of atrial fibrillation after coronary artery bypass surgery. Archives of Iranian Medicine 2005;8(1):21-6. [PMC free article] [PubMed] [Google Scholar]
Mitchell 2005 {published data only}
- Mitchell LB, Exner DV, Wyse DG, Connolly CJ, Prystai GD, Bayes AJ, et al. Prophylactic oral amiodarone for the prevention of arrhythmias that begin early after revascularization, valve replacement, or repair. PAPABEAR: A randomized controlled trial. JAMA 2005;294(24):3093-100. [DOI] [PubMed] [Google Scholar]
Mohr 1981 {published data only}
- Mohr R, Smolinsky A, Goor DA. Prevention of supraventricular tachyarrhythmia with low-dose propranolol after coronary bypass. Journal of Thoracic and Cardiovascular Surgery 1981;81(6):840-5. [PubMed] [Google Scholar]
Mulay 1995 {published data only}
- Mulay A, Kirk AJ, Angelini GD, Wisheart JD, Hutter JA. Posterior pericardiotomy reduces the incidence of supra-ventricular arrhythmias following coronary artery bypass surgery. European Journal of Cardio-Thoracic Surgery 1995;9(3):150-2. [DOI] [PubMed] [Google Scholar]
Myhre 1984 {published data only}
- Myhre ES, Sørlie D, Aarbakke J, Hals PA, Straume B. Effects of low dose propranolol after coronary bypass surgery. Journal of Cardiovascular Surgery 1984;25(4):348-52. [PubMed] [Google Scholar]
Najafi 2007 {published data only}
- Najafi M, Hamidian R, Haghighat B, Fallah N, Tafti HA, Karimi A, et al. Magnesium infusion and postoperative atrial fibrillation: a randomized clinical trial. Acta Anaesthesiologica Taiwanica 2007;45(2):89-94. [PubMed] [Google Scholar]
Neto 2007 {published data only}
- Neto VA, Costa R, Da Silva KR, Martins AL, Escobar LF, Moreira LF, et al. Temporary atrial pacing in the prevention of postoperative atrial fibrillation. Pacing and Clinical Electrophysiology 2007;30(Suppl. 1):S79-81. [DOI] [PubMed] [Google Scholar]
Nurözler 1996 {published data only}
- Nurözler F, Tokgözoglu L, Pasaoglu I, Böke E, Ersoy U, Bozer AY. Atrial fibrillation after coronary artery bypass surgery: predictors and the role of MgSO4 replacement. Journal of Cardiac Surgery 1996;11(6):421-7. [DOI] [PubMed] [Google Scholar]
Nygård 2004 {published data only}
- Nygård E, Sørensen LH, Hviid LB, Pedersen FM, Ravn J, Thomassen L, et al. Effects of amiodarone and thoracic epidural analgesia on atrial fibrillation after coronary artery bypass grafting. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(6):709-14. [DOI] [PubMed] [Google Scholar]
Nyström 1993 {published data only}
- Nyström U, Edvardsson N, Berggren H, Pizzarelli G-P, Rädegran K. Oral sotalol reduces the incidence of atrial fibrillation after coronary artery bypass surgery. Thoracic and Cardiovascular Surgeon 1993;41(1):34-7. [DOI] [PubMed] [Google Scholar]
Oka 1980 {published data only}
- Oka Y, FrishmanW, Becker RM, Kadish A, Strom J, Matsumoto M, et al. Clinical pharmacology of the new beta-andrenegic blocking drugs, Part 10 Beta-adenoceptor blockade and coronary artery surgery. American Heart Journal 1980;99:255-69. [DOI] [PubMed] [Google Scholar]
Orboric 2010 {published data only}
- Orbovic B, Obrenovic-Kircanski B, Ristic S, Soskic LJ. Amiodarone prophylaxis of postoperative atrial fibrillation after coronary-artery bypass graft surgery reference to left atrium dimension. European Journal of Echocardiography 2010;11:ii89-90. [Google Scholar]
Ormerod 1984 {published data only}
- Ormerod OJM, McGregor CGA, Stone DL, Wisbey C, Petch MC. Arrhythmias after coronary bypass surgery. British Heart Journal 1984;51(6):618-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozin 2005 {published data only}
- Özin B, Sezgin A, Atar I, Gülmez Ö, Saritaş B, Gültekin B, et al. Effectiveness of triple-site triggered atrial pacing for prevention of atrial fibrillation after coronary artery bypass graft surgery. Clinical Cardiology 2005;28(10):479-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parikka 1993 {published data only}
- Parikka H, Toivonen L, Pellinen T, Verkalla K, Järvinen A, Nieminen MS. The influence of intravenous magnesium sulphate on the occurrence of atrial fibrillation after coronary artery by-pass operation. European Heart Journal 1993;14(2):251-8. [DOI] [PubMed] [Google Scholar]
Paull 1997 {published data only}
- Paull DL, Tidwell SL, Guyton SW, Harvey E, Woolf RA, Holmes JR, et al. Beta blockade to prevent atrial dysrhythmias following coronary bypass surgery. American Journal of Surgery 1997;173(5):419-21. [DOI] [PubMed] [Google Scholar]
Pfisterer 1997 {published data only}
- Pfisterer ME, Klöter-Weber UC, Huber M, Osswald S, Buser PT, Skarvan K, et al. Prevention of supraventricular tachyarrhythmias after open heart operation by low-dose sotalol: a prospective, double-blind, randomized, placebo-controlled study. Annals of Thoracic Surgery 1997;64(4):1113-9. [DOI] [PubMed] [Google Scholar]
Redle 1999 {published data only}
- Redle JD, Khurana S, Marzan R, McCullough PA, Stewart JR, Westveer DC, et al. Prophylactic oral amiodarone compared with placebo for prevention of atrial fibrillation after coronary artery bypass surgery. American Heart Journal 1999;138:144-50. [DOI] [PubMed] [Google Scholar]
Roshanali 2009 {published data only}
- Roshanali F, Mandegar MH, Yousefnia MA, Alaeddini F, Saidi B. Prevention of atrial fibrillation after coronary artery bypass grafting via atrial electromechanical interval and use of amiodarone prophylaxis. Interactive Cardiovascular and Thoracic Surgery 2009;8(4):421-5. [DOI] [PubMed] [Google Scholar]
Rubin 1987 {published data only}
- Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. Journal of Thoracic and Cardiovascular Surgery 1987;94(3):331-5. [PubMed] [Google Scholar]
Salazar 1979 {published data only}
- Salazar C, Frishman W, Friedman S, Patel J, Lin YT, Oka Y, et al. Beta-blockade therapy for supraventricular tachyarrhythmias after coronary surgery: a propranolol withdrawal syndrome? Angiology 1979;30(12):816-9. [DOI] [PubMed] [Google Scholar]
Schweikert 1998 {published data only}
- Schweikert RA, Grady TA, Gupta N, Augostini RS, Horiatis VI, French SN. Atrial pacing in the prevention of atrial fibrillation after cardiac surgery; results of the second PostOperative Pacing Study (POPS-2). Journal of the American College of Cardiology 1998;31:117A. [Google Scholar]
Sezai 2011 {published data only}
- Sezai A, Minami K, Nakai T, Hata M, Yoshitake I, Wakui S, et al. Landiolol hydrochloride for prevention of atrial fibrillation after coronary artery bypass grafting: New evidence from the PASCAL trial. Journal of Thoracic and Cardiovascular Surgery 2011;141(6):1478-87. [DOI] [PubMed] [Google Scholar]
Silverman 1982 {published data only}
- Silverman NA, Wright R, Levitsky S. Efficacy of low-dose propranolol in preventing postoperative supraventricular tachyarrhythmias: A prospective, randomized study. Annals of Surgery 1982;196(2):194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speziale 2000 {published data only}
- Speziale G, Ruvolo G, Fattouch K, Macrina F, Tonelli E, Donnetti M, et al. Arrhythmia prophylaxis after coronary artery bypass grafting: regimens of magnesium sulfate administration. Thoracic and Cardiovascular Surgeon 2000;48(1):22-6. [DOI] [PubMed] [Google Scholar]
Stephenson 1980 {published data only}
- Stephenson LW, MacVaugh HI, Tomasello DN, Josephson ME. Propranolol for prevention of postoperative cardiac arrhythmias: A randomized study. Annals of Thoracic Surgery 1980;29(2):113-6. [DOI] [PubMed] [Google Scholar]
Suttorp 1991 {published data only}
- Suttorp MJ, Kingma JH, Peels HO, Koomen EM, Tijssen JG, Hemel NM, et al. Effectiveness of sotalol in preventing supraventricular tachyarrhythmias shortly after coronary artery bypass grafting. American Journal of Cardiology 1991;68(11):1163-9. [DOI] [PubMed] [Google Scholar]
Tokmakoglu 2002 {published data only}
- Tokmakoglu H, Kandemir O, Gunaydin S, Catav Z, Yorgancioglu C, Zorlutuna Y. Amiodarone versus digoxin and metoprolol combination for the prevention of postcoronary bypass atrial fibrillation. European Journal of Cardio-Thoracic Surgery 2002;21(3):401-5. [DOI] [PubMed] [Google Scholar]
Toraman 2001 {published data only}
- Toraman F, Karabulut EH, Alhan HC, Dagdelen S, Tarcan S. Magnesium infusion dramatically decreases the incidence of atrial fibrillation after coronary artery bypass grafting. Annals of Thoracic Surgery 2001;72(4):1256-62. [DOI] [PubMed] [Google Scholar]
Treggiari‐Venzi 2000 {published data only}
- Treggiari-Venzi MM, Waeber JL, Perneger TV, Suter PM, Adamec R, Romand JA. Intravenous amiodarone or magnesium sulphate is not cost-beneficial prophylaxis for atrial fibrillation after coronary artery bypass surgery. British Journal of Anaesthesia 2000;85(5):690-5. [DOI] [PubMed] [Google Scholar]
Turk 2007 {published data only}
- Turk T, Ata Y, Vural H, Ozkan H, Yavuz S, Ozyazicioglu A. Intravenous and oral amiodarone for the prevention of postoperative atrial fibrillation in patients undergoing off-pump coronary artery bypass surgery. Heart Surgery Forum 2007;10(4):E299-303. [DOI] [PubMed] [Google Scholar]
Vecht 1986 {published data only}
- Vecht RJ, Nicolaides EP, Ikweuyke JK, Liassides C, Cleary J, Cooper WB. Incidence and prevention of supraventricular tachyarrhythmias after coronary bypass surgery. International Journal of Cardiology 1986;13(2):125-34. [DOI] [PubMed] [Google Scholar]
Weber 1998 {published data only}
- Weber UK, Osswald S, Buser P, Huber M, Skarvan K, Stulz P, et al. Significance of supraventricular tachyarrhythmias after coronary artery bypass graft surgery and their prevention by low-dose sotalol: A prospective double-blind randomized placebo-controlled study. Journal of Cardiovascular Pharmacology and Therapeutics 1998;3(3):209-16. [DOI] [PubMed] [Google Scholar]
Wenke 1999 {published data only}
- Wenke K, Parsa MH, Imhof M, Kemkes BM. Efficacy of metoprolol in prevention of supraventricular arrhythmias after coronary artery bypass grafting. Zeitschrift fur Kardiologie 1999;88(9):647-52. [DOI] [PubMed] [Google Scholar]
White 1984 {published data only}
- White HD, Antman EM, Glynn MA, Collins JJ, Cohn LH, Shemin RJ, et al. Efficacy and safety of timolol for prevention of supraventricular tachyarrhythmias after coronary artery bypass surgery. Circulation 1984;70(3):479-84. [DOI] [PubMed] [Google Scholar]
White 2002 {published data only}
- White CM, Giri S, Tsikouris JP, Dunn A, Felton K, Reddy P, et al. A comparison of two individual amiodarone regimens to placebo in open heart surgery patients. Annals of Thoracic Surgery 2002;74(1):69-74. [DOI] [PubMed] [Google Scholar]
White 2003 {published data only}
- White CM, Caron MF, Kalus JS, Rose H, Song J, Reddy P, et al. Intravenous plus oral amiodarone, atrial septal pacing, or both strategies to prevent post-cardiothoracic surgery atrial fibrillation: the Atrial Fibrillation Suppression Trial II (AFIST II). Circulation 2003;108(Suppl. 1):II200-6. [DOI] [PubMed] [Google Scholar]
Wilkes 2002 {published data only}
- Wilkes NJ, Mallett SV, Peachey T, Di Salvo C, Walesby R. Correction of ionized plasma magnesium during cardiopulmonary bypass reduces the risk of postoperative cardiac arrhythmia. Anesthesia and Analgesia 2002;95(4):828-34. [DOI] [PubMed] [Google Scholar]
Williams 1982 {published data only}
- Williams JB, Stephensen LW, Holford FD, Langer T, Dunkman WB, Josephson ME. Arrhythmia prophylaxis using propranolol after coronary artery surgery. Annals of Thoracic Surgery 1982;34(4):435-8. [DOI] [PubMed] [Google Scholar]
Yagdi 2003 {published data only}
- Yagdi T, Nalbantgil S, Ayik F, Apaydin A, Islamoglu F, Posacioglu H, et al. Amiodarone reduces the incidence of atrial fibrillation after coronary artery bypass grafting. Journal of Thoracic and Cardiovascular Surgery 2003;125(6):1420-5. [DOI] [PubMed] [Google Scholar]
Yazicioglu 2002 {published data only}
- Yazicioglu L, Eryilmaz S, Sirlak M, Inan MB, Aral A, Tasoz R, et al. The effect of preoperative digitalis and atenolol combination on postoperative atrial fibrillation incidence. European Journal of Cardio-Thoracic Surgery 2002;22(3):397-401. [DOI] [PubMed] [Google Scholar]
Yazigi 2002 {published data only}
- Yazigi A, Rahbani P, Zeid HA, Madi-Jebara S, Haddad F, Hayek G. Postoperative oral amiodarone as prophylaxis against atrial fibrillation after coronary artery surgery. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(5):603-6. [DOI] [PubMed] [Google Scholar]
Yeatman 2002 {published data only}
- Yeatman M, Caputo M, Narayan P, Lotto AA, Ascione R, Bryan AJ, et al. Magnesium-supplemented warm blood cardioplegia in patients undergoing coronary artery revascularization. Annals of Thoracic Surgery 2002;73(1):112-8. [DOI] [PubMed] [Google Scholar]
Zangrillo 2005 {published data only}
- Zangrillo A, Landoni G, Sparicio D, Pappalardo F, Bove T, Cerchierini E, et al. Perioperative magnesium supplementation to prevent atrial fibrillation after off-pump coronary artery surgery: a randomized controlled study. Journal of Cardiothoracic and Vascular Anesthesia 2005;19(6):723-8. [DOI] [PubMed] [Google Scholar]
Zebis 2007 {published data only}
- Zebis LR, Christensen TD, Thomsen HF, Mikkelsen MM, Folkersen L, Sørensen HT, et al. Practical regimen for amiodarone use in preventing postoperative atrial fibrillation. Annals of Thoracic Surgery 2007;83(4):1326-31. [DOI] [PubMed] [Google Scholar]
Additional references
Almassi 1997
- Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: A major morbid event? Annals of Surgery 1997;226(4):501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angelini 1987
- Angelini GD, Penny WJ, el-Ghamary F, West RR, Butchart EG, Armistead SH, et al. The incidence and significance of early pericardial effusion after open heart surgery. European Journal of Cardio-Thoracic Surgery 1987;1:165-8. [DOI] [PubMed] [Google Scholar]
Aranki 1996
- Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996;94(3):390-7. [DOI] [PubMed] [Google Scholar]
Biancari 2010
- Biancari F, Mahar MAA. Meta-analysis of randomized trials on the efficacy of posterior pericardiotomy in preventing atrial fibrillation after coronary artery bypass surgery. Journal of Thoracic and Cardiovascular Surgery 2010;139(5):1158-61. [DOI] [PubMed] [Google Scholar]
Bradley 2005
- Bradley D, Creswell LL, Hogue CW Jr, Epstein AE, Prystowsky EN, Daoud EG. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005;128:39S-47S. [DOI] [PubMed] [Google Scholar]
Bryan 1990
- Bryan AJ. Pericardial effusion after open heart surgery. Thorax 1990;45:655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgess 2006
- Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. European Heart Journal 2006;27:2846-57. [DOI] [PubMed] [Google Scholar]
Cappabianca 2011
- Cappabianca G, Rotunno C, Luca L, Schinosa T, Ranieri VM, Paparella D. Protective effects of steroids in cardiac surgery: A meta-analysis of randomized double-blind trials. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(1):156-65. [DOI] [PubMed] [Google Scholar]
Chung 2000b
- Chung MK. Cardiac surgery: Postoperative arrhythmias. Critical Care Medicine 2000;28(10):N136-44. [DOI] [PubMed] [Google Scholar]
Creswell 1993
- Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Annals of Thoracic Surgery 1993;56(3):539-49. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(12):1286-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frost 1992
- Frost L, Mølgaard H, Christiansen EH, Hjortholm K, Paulsen PK, Thomsen PEB. Atrial fibrillation and flutter after coronary arteryby-pass surgery: epidemiology, risk factors and preventive trials. International Journal of Cardiology 1992;36:253–61. [DOI] [PubMed] [Google Scholar]
Guazzi 2009
- Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart 2009;95(2):102-6. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. http://www.cochrane-handbook.org/.
Kowey 1992
- Kowey PR, Taylor JE, Rials SJ, Marinchak RA. Meta-Analysis of the effectiveness of prophylactic Drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. American Journal of Cardiology 1992;69:963-5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials publishedworld-wide – the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The CochraneLibrary. Emerging Themes in Epidemiology 2008;5:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leitch 1990
- Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. Journal of Thoracic and Cardiovascular Surgery 1990;100(3):338-42. [PubMed] [Google Scholar]
Loubani 2000
- Loubani M, Hickey MS, Spyt TJ, Galiñanes M. Residual atrial fibrillation and clinical consequences following postoperative supraventricular arrhythmias. International Journal of Cardiology 2000;74(2-3):125-32. [DOI] [PubMed] [Google Scholar]
Marik 2009
- Marik PE, Fromm R. The efficacy and dosage effect of corticosteroids for the prevention of atrial fibrillation after cardiac surgery: A systematic review. Journal of Critical Care 2009;24(3):458-63. [DOI] [PubMed] [Google Scholar]
Mathew 1996
- Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, et al. Atrial fibrillation following coronary artery bypass graft surgery. Predictors, outcomes, and resource utilization. JAMA 1992;276:300-6. [PubMed] [Google Scholar]
Mitchell 2011
- Mitchell LB, CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: Prevention and treatment of atrial fibrillation following cardiac surgery. Canadian Journal of Cardiology 2011;27:91-7. [DOI] [PubMed] [Google Scholar]
Müller 2002
- Müller I, Massberg S, Zierhut W, Binz C, Schuster A, Rüdiger-von Hoch S, et al. Effects of aspirin and clopidogrel versus oral anticoagulation on platelet function and on coagulation in patients with nonvalvular atrial fibrillation (CLAFIB). Pathophysiology of Haemostasis and Thrombosis 2002;32(1):16-24. [DOI] [PubMed] [Google Scholar]
Reed 1998
- Reed GL 3rd, Singer DE, Picard EH, DeSanctis RW. Stroke following coronary-artery bypass surgery. A case-control estimate of the risk from carotid bruits. New England Journal of Medicine 1988;319(19):1246-50. [DOI] [PubMed] [Google Scholar]
Shepherd 2008
- Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A. Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation. Health Technology Assessment 2008;12(28):iii-iv, ix-95. [DOI] [PubMed] [Google Scholar]
Shirzad 2010
- Shirzad M, Karimi A, Tazik M, Aramin H, Hossein Ahmadi S, Davoodi S, et al. Determinants of postoperative atrial fibrillation and associated resource utilization in cardiac surgery. Revista Espanola de Cardiologia 2010;63(9):1054-60. [DOI] [PubMed] [Google Scholar]
SPAF Investigators Committee 1998
- SPAF Investigators Committee. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Annals of Internal Medicine 1998;128(8):639-47. [DOI] [PubMed] [Google Scholar]
Taylor 1990
- Taylor GJ, Mikell FL, Moses HW, Dove JT, Katholi RE, Malik SA, et al. Determinants of hospital charges for coronary artery bypass surgery: The economic consequences of postoperative complications. American Journal of Cardiology 1990;65:309-13. [DOI] [PubMed] [Google Scholar]
Watson 2009
- Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373(9658):155-66. [DOI] [PubMed] [Google Scholar]
Whitlock 2008
- Whitock RP, Chan S, Devereaux PJ, Sun J, Rubens FD, Thorlund K, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: A meta-analysis of randomized trials. European Heart Journal 2008;29(21):2592-600. [DOI] [PubMed] [Google Scholar]
Whitlock 2009
- Whitlock RP, Healey JS, Connolly SJ. Left atrial appendage occlusion does not eliminate the need for warfarin. Circulation 2009;120:1927-32. [DOI] [PubMed] [Google Scholar]
Wolf 1991
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991;22(8):983-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crystal 2004
- Crystal E, Garfinkle MS, Connolly S, Ginger T, Sleik K, Yusuf S. Interventions for preventing post-operative atrial fibrillationin patients undergoing heart surgery. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD003611. [DOI: 10.1002/14651858.CD003611.pub2] [DOI] [PubMed] [Google Scholar]


