Abstract
Rationale & Objective:
Safe analgesic choices are limited in chronic kidney disease (CKD). We conducted a comparative analysis of harm from opioids versus nonsteroidal anti-inflammatory drugs in CKD.
Study Design:
Prospective cohort study
Setting & Participants:
3939 patients with CKD in the Chronic Renal Insufficiency Cohort (CRIC) study.
Exposures:
30-day analgesic use reported at annual visits.
Outcomes:
a composite outcome of 50% glomerular filtration rate (GFR) reduction and kidney failure requiring kidney replacement therapy (KRT), as well as the outcomes of kidney failure requiring KRT, hospitalization, and pre-kidney failure death.
Analytical Approach:
Marginal structural models with time-updated exposures.
Results:
Participants were followed for a median of 6.84 years with 391 (9.9%) and 612 (15.5%) reporting baseline opioid and NSAID (non-steroidal anti-inflammatory drug) use, respectively. Time-updated opioid use was associated with the kidney disease composite outcome, kidney failure with KRT, death (HRs of 1.4 [95% CI, 1.2-1.7], 1.4 [95% CI, 1.1-1.7], and 1.5 [95% CI, 1.2-2.0], respectively), and hospitalization (rate ratio (RR), 1.7; 95% CI, 1.6-1.9) versus opioid non-users. Similar results were found in an analysis restricted to a sub-cohort of participants reporting ever using other (non-opioid, non-NSAID) analgesics or tramadol. Time-updated non-steroidal anti-inflammatory drug use was associated with an increased risk for the kidney disease composite (HR, 1.2; 95% CI, 1.0-1.5) and hospitalization (RR, 1.1; 95% CI, 1.0-1.3); however, these associations were not significant in the sub-cohort. The association of non-steroid anti-inflammatory drug use with the kidney disease composite outcome varied by race, with a significant risk in blacks (HR, 1.3; 95% CI, 1.0-1.7). NSAID use was associated with a lower risk of kidney failure with KRT in women and individuals with GFR < 45 mL/min/1.73 m2 (HRs of 0.63 [95% CI, 0.45-0.88], 0.77 [95% CI, 0.59-0.99], respectively).
Limitations:
Limited periods of recall of analgesic use and potential confounding by indication
Conclusion:
Opioid use had a stronger association with adverse events than nonsteroidal anti-inflammatory drugs, with the latter’s association with kidney disease outcomes limited to specific sub-groups, notably those of black race.
Keywords: chronic kidney disease (CKD), analgesics, opioids, non-steroidal anti-inflammatory drug (NSAID), end-stage renal disease (ESRD), pain management, COX-2 inhibitor, kidney function, kidney disease progression, drug safety, outcomes
Introduction:
Pain is common among patients with all stages of chronic kidney disease (CKD), but safe treatment options are not well-defined 1, 2. Much literature describes the ill-effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the kidney3, 4. Nevertheless, the National Kidney Foundation endorses limited NSAID use for management of occasional pain in CKD, but advises against long-term use 5. Other disease-specific guidelines exclude NSAIDs from chronic pain treatment algorithms 6. However, recent studies demonstrate the renal safety of NSAIDs in some chronic diseases, adding to the controversy regarding their use in CKD7, 8. As a result, recommendation for NSAID avoidance have been challenged, even in Stage 3 to 5 CKD 7 .
Coincident with the concern for NSAID use in CKD is acknowledgment of the opioid epidemic and recommendations to avoid long-term opioid use. In 2013, American healthcare providers wrote ~ 250 million opioid prescriptions9, and in 2014 almost 2 million persons either misused opioids, or suffered from an opioid use disorder (OUD)10. About 1 in 4 people prescribed long-term opioids for non-cancer pain struggle with an OUD11. More than 183,000 adults died from opioid overdose between 1999-201510. Government and health agencies have established pain management guidelines intent on directing prescribers towards more judicious opioid use. Notable examples include the World Health Organization (WHO) 3-step analgesic ladder for cancer-related pain12, and the Center for Disease Control (CDC) Guideline for Prescribing Opioids for Chronic Pain10. The CDC Guide for chronic pain management recommends non-pharmacologic and non-opioid therapies including acetaminophen, selected antidepressant, anticonvulsants, as well as NSAIDs before long-term opioid use10.
Prior work from the Chronic Renal Insufficiency Cohort (CRIC) study revealed that 24% of study participants reported NSAID use at study entry or at least one follow-up visit13. In addition, initiation or discontinuation of NSAIDs is often associated with supplementation or replacement, respectively, with opioids 13. Hence these analgesic classes are both likely to be used, and interchanged in CKD; however, comparative outcomes of using drugs from these analgesic classes is not known. In this analysis of the CRIC study, our objective is to evaluate the association of opioid and NSAID use with clinical outcomes in patients with CKD not requiring KRT.
Methods:
Study Design and Participants:
The CRIC study commenced in 2003 with Phase I&II enrollment completed in 2006, and continued follow-up to date with the design previously described 14 . This analysis examined 3,939 participants who gave informed consent and were enrolled at 21 to 74 years old with age-specific eGFR eligibility criteria of 20-70 ml/min/1.73 m2, from seven U.S. centers with 13 clinical sites, and Institutional Review Board approval at each site. Briefly, CRIC participants underwent annual in-center visits providing demographic information, medical history and status update, vital signs, blood and urine samples, and other survey-based information. GFR was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation, the prevailing clinical measure of kidney function at study commencement15.
Medication ascertainment:
Coordinators recorded participants’ prescription, over the counter (OTC) medications, supplements, and vitamins from 30 days preceding the study visit. To reduce recall bias, participants were asked to maintain a list or bring medications to visits. The drug name, frequency, total daily dosage (TDD), dosage units, and administration route were documented. Individual medications and constituents of combinations were identified using the First Databank® dictionary
Classification of analgesics:
The CRIC data file was closed in May 2014 to permit data cleaning and preparation for this analysis. The NSAID category included all oral NSAIDs and cyclooxygenase 2 (COX-2) inhibitors. Aspirin was classified as an NSAID if the TDD was greater than 325 mg, the dose frequency was more than once daily, or the drug was part of a combination analgesic (excluding aspirin & dipyridamole). Members of the opioid class included all orally administered narcotics as designated in First Databank® such as hydrocodone, codeine, oxycodone. Methadone represented less than 0.1% of opioid entries and no buprenorphine/naloxone use was reported. Opioids used as cold or cough remedy were excluded. Members of the other (non-opioid, non-NSAID) class included predominately acetaminophen. Since tramadol has overlapping but distinct pharmacological properties from narcotics 16, it was considered as a separate class unless taken in combination with an NSAID or an opioid, in which case the medication was classified in the latter’s category. We defined the time-updated opioid and NSAID use at each clinical visit as whether a patient had reported NSAID or opioid at that visit or any one of the previous CRIC visits. The class of other (non-opioid, non-NSAID) analgesics included all other oral analgesics heretofore not classified. Of note, more than 90% of those entries were acetaminophen alone or in combination with another agent (e.g, diphenhydramine). No intravenous or topical analgesics were included in the analysis.
Outcomes:
We examined four clinical outcomes including kidney failure requiring KRT, the composite of kidney failure with KRT and 50% reduction of eGFR from baseline, pre-kidney failure death, and number of pre-kidney failure hospitalizations between two consecutive annual visits. GFR was estimated annually15. Death was ascertained through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Mortality Master File17. Kidney failure with KRT was ascertained by CRIC study personnel and cross-reference to the US Renal Data System18 . For Kidney failure with KRT and the composite kidney disease outcome, participant follow-up was censored at the time of death, withdrawal, loss to follow-up, or the end of the follow-up period, whichever occurred first. For pre-kidney failure death and number of hospitalizations, participant follow-up was censored at the time of KRT initiation, withdrawal, loss to follow-up, or the end of the follow-up period, whichever occurred first.
Covariates:
We considered several clinically relevant covariates including baseline factors: gender, race, education level, and income reported at study entry. Time-dependent covariates included age, any alcohol drinking, comorbidities (diabetes, cardiovascular disease, hypertension, asthma, non-skin cancer, hyperkalemia, and arthritis), GFR, urinary protein-creatinine ratio (UPCR), response on the Beck’s depression, symptom severity, and Kidney Disease Quality of Life 36 questionnaire (KDQOL-36) burden and symptoms sub-scales, SF-12 physical composite (including a question asking how much pain impeded activities of daily living), SF-12 mental composite, nephrologist visits, and other analgesic use (non-opioid/non-NSAID analgesic, tramadol) collected at annual visits. Urinary protein and creatinine were also measured using standard assays. Urinary Protein-creatinine ratios from 24-hour and spot urine specimens were combined to a single urinary protein-creatinine ratio (UPCR) variable.
Statistical Methods:
For descriptive analyses, X2-tests and t-tests compared discrete characteristics and continuous variables, respectively, across groups. We examined the association between time-updated opioid and NSAID use and the study outcomes while controlling for time-dependent covariates. We applied joint marginal structural models (MSMs) as several time-dependent covariates including eGFR could both be a consequence and a predictor for analgesic use. The challenges of making causal inferences from observational data have been previously discussed and illustrated with steps of fitting joint MSMs described 19.
In brief, we used a pooled logistic regression model to predict the probability of time-updated NSAID use at each visit based on NSAID use and opioid use at the previous visit and covariates at the previous visit. Another pooled logistic regression model was applied to predict the probability of time-updated opioid use at each visit based on NSAID use at both the current and the previous visit, opioid use at the previous visit, and covariates at the previous visit. The inverse probability weights were computed and stabilized. To control for informative censoring, the inverse probability of censoring weight was also computed and stabilized. The final weight was the product of the NSAID and opioid exposure, and the censoring weight. The final weight was also truncated at 99th percentile. Finally, we fit a weighted discrete failure time model for each of the survival outcomes through generalized estimating equations (GEE) and using the final weight developed from the logistic regression models. Only baseline covariates were included in the discrete failure time models. For the hospitalizations, we fit a weighted Poisson regression model using GEE and the final weight.
We performed the analyses using the full cohort and a sub-cohort including participants who ever used another (non-opioid, non-NSAID) analgesic or tramadol at baseline or during follow-up as a surrogate for need of pain relief. We also performed stratified analyses using demographic variables, and key predictors of kidney outcomes: baseline age (<65, >=65), sex, race (Non-Black, Black), diabetes status, eGFR (<= vs > 45 ml/min/1.79 m2), and UPCR dichotomized at the sample median.
To demonstrate our results were robust and not due to unmeasured confounding, we examined the association of opioids and NSAIDs with risk of incident diabetes as a negative control outcome among CRIC participants without diabetes at enrollment and using the joint marginal structural models as described above.
All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results:
The 3939 participants had a median follow-up of 6.84 years. There were a total of 24838 visits for time to kidney failure with KRT or pre-kidney failure death, and 24552 visits for the composite outcome of kidney failure with KRT and 50% reduction in eGFR. Tables 1 & 2 show the overall baseline characteristics of CRIC participants grouped by reported baseline opioid and NSAID use. Comparing the 391 (9.9%) participants who reported baseline opioid use to the 3548 (90.1%) who did not, the former group was more likely to be female, black, have an annual income of less than or equal to $50,000, have a history of rheumatoid arthritis, cardio-vascular disease, asthma, and non-skin cancer, and were less likely to report alcohol drinking. Compared to the 3327 (84.5%) participants who did not report baseline NSAID use, the 612 (15.5%) who did were more likely to be 45-64 years old, female, non-black, a college graduate or with higher education, with an income greater than $50,000, and to report drinking alcohol. Those reporting baseline NSAID use also were more likely to have a history of asthma, higher eGFR and were less likely to have diabetes, cardiovascular disease, hypertension, previous hyperkalemia and/or prior visit with a nephrologist. Opioid users were more likely to have depressive symptoms than non-users and lower KDQOL-36 and its components domains (Table 2). However, NSAID users had higher KDQOL-36 scores compared with non-users.
Table 1:
Baseline Characteristics of CRIC participants overall and by opioid and NSAID use
| Opioid use at baseline | NSAID use at baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n=3548) | Yes (n=391) | P | No (n=3327) | Yes (n=612) | P | |||||
| Age (years) | 0.3 | 0.003 | ||||||||
| 21-<45 | 494 (13.9%) | 44 (11.3%) | 462 (13.9%) | 76 (12.4%) | ||||||
| 45-<65 | 2025 | (57.1) | 236 | (60.4) | 1872 | (56.3) | 389 | (63.6) | ||
| 65+ | 1029 | (29.0) | 111 | (28.4) | 993 | (29.8) | 147 | (24.0) | ||
| Male sex | 2004 | (56.5) | 157 | (40.2) | <0.001 | 1908 | (57.3) | 253 | (41.3) | <0.001 |
| Black race | 1472 | (41.5) | 186 | (47.6) | 0.02 | 1432 | (43.0) | 226 | (36.9) | 0.005 |
| Diabetes | 1720 | (48.5) | 188 | (48.1) | 0.9 | 1668 | (50.1) | 240 | (39.2) | <0.001 |
| Hypertension | 3062 | (86.3) | 329 | (84.1) | 0.2 | 2892 | (86.9) | 499 | (81.5) | <0.001 |
| Any cardiovascular disease | 1159 | (32.7) | 157 | (40.2) | 0.003 | 1156 | (34.7) | 160 | (26.1) | <0.001 |
| Rheumatoid Arthritisa | 411 | (12.2) | 81 | (22.3) | <0.001 | 404 | (12.8) | 88 | (15.1) | 0.1 |
| Asthmab | 419 | (12.0) | 79 | (20.5) | <0.001 | 401 | (12.3) | 97 | (16.1) | 0.01 |
| Cancer (excluding non-melanoma skin cancer | 168 | (4.7) | 28 | (7.2) | 0.04 | 167 | (5.0) | 29 | (4.7) | 0.8 |
| Drinking alcohol | 743 | (20.9) | 50 | (12.8) | <0.001 | 649 | (19.5) | 144 | (23.5) | 0.02 |
| Prior visit with nephrologist | 2328 | (65.6) | 273 | (69.8) | 0.1 | 2295 | (69.0) | 306 | (50.0) | <0.001 |
| Educationc | 0.06 | <0.001 | ||||||||
| Less than high school | 752 | (21.2) | 76 | (19.4) | 739 | (22.2) | 89 | (14.6) | ||
| High school graduate | 660 | (18.6) | 81 | (20.7) | 631 | (19.0) | 110 | (18.0) | ||
| Some college | 1015 | (28.6) | 131 | (33.5) | 955 | (28.7) | 191 | (31.3) | ||
| College graduate or higher | 1120 | (31.6) | 103 | (26.3) | 1002 | (30.1) | 221 | (36.2) | ||
| Income | 0.006 | 0.001 | ||||||||
| $20,000 or under | 1102 | (31.1) | 138 | (35.3) | 1082 | (32.5) | 158 | (25.8) | ||
| $20,001 - $50,000 | 847 | (23.9) | 111 | (28.4) | 809 | (24.3) | 149 | (24.3) | ||
| $50,001 - $100,000 | 673 | (19.0) | 61 | (15.6) | 603 | (18.1) | 131 | (21.4) | ||
| More than $100,000 | 369 | (10.4) | 23 | (5.9) | 311 | (9.3) | 81 | (13.2) | ||
| Don’t wish to answer | 557 | (15.7) | 58 | (14.8) | 522 | (15.7) | 93 | (15.2) | ||
| Non-opioid/non-NSAID analgesic use | 560 | (15.8) | 114 | (29.2) | <0.001 | 532 | (16.0) | 142 | (23.2) | <0.001 |
| Tramadol use | 72 | (2.0) | 18 | (4.6) | 0.001 | 73 | (2.2) | 17 | (2.8) | 0.4 |
| Anxiolytic used | 107 | (3.0) | 38 | (9.7) | <0.001 | 109 | (3.3) | 36 | (5.9) | 0.002 |
| Anti-epileptic usee | 289 | (8.2) | 95 | (24.3) | <0.001 | 315 | (9.5) | 69 | (11.3) | 0.2 |
N=3939. Values given to count (percent).
197 missing
312 missing for opioid use and 69 missing for NSAID use
76 missing for opioid use and 1 missing for NSAID use
353 missing for opioid use and 28 missing for NSAID use
296 missing for opioid use and 28 missing for NSAID use
Table 2:
Baseline characteristics by opioid and NSAID use
| All participants | Opioid Use | NSAID Use | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |
| eGFR (ml/min/1.73 m2) | 3939 | 43 ± 13 | 3548 | 43 ± 14 | 391 | 43 ± 14 | 3327 | 42 ± 13 | 612 | 48 ± 13 |
| UPCR mcg/mg | 3772 | 1 ± 2 | 3397 | 1 ± 2.4 | 375 | 0.7 ± 2 | 3185 | 1 ± 2 | 587 | 0.6 ± 2 |
| Beck’s Score | 3889 | 8 ± 8 | 3506 | 8 ± 8 | 383 | 11 ± 9 | 3288 | 8 ± 8 | 601 | 8 ± 8 |
| Symptom Severity Score | 3919 | 151 ±177 | 3531 | 144 ±173 | 388 | 220 ±199 | 3307 | 150 ±176 | 612 | 159 ±184 |
| KDQOL Burden of Kidney Disease | 3913 | 82 ± 24 | 3527 | 83 ± 23 | 386 | 78 ± 26 | 3306 | 81 ± 24 | 607 | 87 ± 22 |
| KDQOL Symptoms | 3921 | 83 ± 15 | 3534 | 84 ± 14 | 387 | 77 ± 17 | 3312 | 84 ± 15 | 609 | 83 ± 14 |
| SF-12 Physical Composite | 3847 | 41 ± 12 | 3464 | 42 ± 11 | 383 | 33 ± 10 | 3245 | 41 ± 12 | 602 | 41 ± 12 |
| SF-12 Mental Composite | 3847 | 50 ± 11 | 3464 | 51 ± 10 | 383 | 47 ± 11 | 3245 | 50 ± 11 | 602 | 50 ± 11 |
N=3939
Table 3 displays the crude rates of outcomes in the year following observed visits classified by opioid and NSAID use alone, in combination, or use of neither class of analgesic. The crude incidence of death was highest in the opioid only group and opioid and NSAID group, with crude rates (per 100 person-years) of 3.5 (95% CI, 2.84.3) and 3.5 (95% CI, 2.2-5.5), respectively. The crude incidence of kidney failure with KRT in the opioid and other (non-opioid, non-NSAID group) were 4.2 (95% CI, 3.4-5.2) and 4.3 (95% CI, 4.0-4.6) per 100 person-years, respectively, appearing to be higher than the crude rates in NSAID-only group (1.9 (95% CI, 1.4-2.5) per 100 person-years) and opioid and NSAID group (2.5 [95% CI, 0.1-1.5] per 100 person-years). A similar pattern was observed for the composite kidney disease outcome, with crude rates in the opioid and other analgesic groups of 5.9 (95% CI, 5.3-6.3) and 5.9 (95% CI, 5.5-6.3) per 100 person-years, respectively. For hospitalizations, the opioid-only group had the highest crude rate of hospitalizations while the NSAID-only group had the lowest crude rate (108.6 [95% CI, 99.1-118.9] vs58.0 [95% CI, 51.5-65.3] per 100 person-years).
Table 3.
Crude rate of events (per 100 person-years) following annual visits classified by reported Opioid and NSAID use*
| Pre-kidney failure Death | Kidney Failure with KRT | Composite kidney disease outcome | Pre-kidney failure Hospitalization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analgesic use | events | total visits | Rate per 100 visits (95 % CI) | events | total visits | Rate per 100 visits (95 % CI) | events | total visits | Rate per 100 visits (95 % CI) | events | total visits | Rate per 100 visits (95 % CI) |
| Opioid (no NSAID) | 85 | 2420 | 3.5 (2.8, 4.3) | 84 | 1998 | 4.2 (3.4, 5.2) | 114 | 1942 | 5.9 (5.3, 6.3) | 2169 | 1998 | 108.6 (99.1, 118.9) |
| NSAID (no opioid) | 38 | 2780 | 1.4 (1.0, 1.9) | 46 | 2465 | 1.9 (1.4, 2.5) | 60 | 2454 | 2.4 (1.9, 3.2) | 1429 | 2465 | 58.0 (51.5, 65.3) |
| Other (non-opioid, non-NSAID) | 402 | 19097 | 2.1 (1.9, 2.3) | 683 | 15960 | 4.3 (4.0, 4.6) | 930 | 15744 | 5.9 (5.5, 6.3) | 9786 | 15960 | 61.3 (58.6, 64.2) |
| Opioid & NSAID | 19 | 541 | 3.5 (2.2, 5.5) | 12 | 476 | 2.5 (1.4, 4.4) | 11 | 473 | 2.3 (1.2, 4.2) | 426 | 476 | 89.5 (75.2, 106.6) |
Total number of visits used for pre-kidney failure death is 24838. Total number of visits for kidney failure with KRT, and pre-kidney failure hospitalization is 20899. Total number of visits for the composite kidney disease outcome is 20613.
Table 4 shows the association of time-updated opioid and NSAID exposure with outcomes in the full cohort with the risk estimates expressed as a hazard ratio (HR) for the kidney disease outcomes and as rate ratios (RR) for hospitalization. Time-updated opioid use was associated with increased adjusted risk of all four outcomes relative to never using opioids during CRIC participation, with HRs of 1.4 (95% CI, 1.2-1.7), 1.4 (95% CI, 1.1-1.7), 1.5 (95% CI, 1.2-2.0), and RR of 1.7 (95% CI, 1.6-1.9), for the kidney disease composite, kidney failure with KRT, death, and hospitalization, respectively. Time-updated NSAID use was associated with a modestly increased hazard of the kidney disease composite and risk of hospitalizations relative to never using NSAID with an HR of 1.2 (95% CI, 1.0-1.5) and a RR of 1.1 (95% CI, 1.0-1.3). However, there was no significant association between time-updated NSAID use and kidney failure with KRT or death.
Table 4:
Associations of time-updated cumulative NSAID and opioid exposure with outcomes, adjusting for time-dependent covariates in the full cohort and a sub-cohort comprised of participants who ever used other analgesics or tramadol during CRIC study
| Outcome | Full cohort | Sub-cohort | ||
|---|---|---|---|---|
| HR* (95% CI) | P value | HR* (95% CI) | P value | |
| Composite kidney disease outcome** | ||||
| Opioid use | 1.4 (1.2, 1.7) | <0.001 | 1.6 (1.3, 2.0) | <0.001 |
| NSAID use | 1.2 (1.0, 1.5) | 0.04 | 1.1 (0.9, 1.4) | 0.2 |
| Kidney failure with KRT | ||||
| Opioid use | 1.4 (1.1, 1.7) | 0.005 | 1.5 (1.2, 2.0) | <0.001 |
| NSAID use | 1.1 (0.8, 1.3) | 0.6 | 1.0 (0.7, 1.3) | 0.9 |
| Pre-kidney failure death | ||||
| Opioid use | 1.5 (1.2, 2.0) | 0.002 | 1.6 (1.1, 2.2) | 0.009 |
| NSAID use | 0.9 (0.7, 1.1) | 0.3 | 1.2 (0.8, 1.7) | 0.5 |
| Hospitalization | ||||
| Opioid use | 1.7 (1.6, 1.9) | <0.001 | 1.7 (1.5, 1.9) | <0.001 |
| NSAID use | 1.1 (1.0, 1.3) | 0.01 | 1.1 (0.9, 1.3) | 0.08 |
Other analgesics are non-opioid, non-NSAID.
Table 4 demonstrates the associations of time-updated opioid and NSAID exposure with outcomes in the sub-cohort comprising those participants ever exposed to other analgesics (non-opioid, non-NSAID) or tramadol during the study. The strength of association between opioid use and the outcomes were comparable to the full cohort. The association between NSAID use and hospitalization, kidney failure with KRT, the kidney disease composite, and death was no longer significant.
The forest plots (Figure 1, Table S1) display the varying HRs for the association of each analgesic group and the outcomes within sub-groups designated by age, gender, race, diabetes, GFR, and UPCR at baseline. The association between time-updated NSAID use and the composite kidney disease outcome was stronger in blacks versus non-blacks (HRs of 1.31 [95% CI, 1.01-1.69] and 0.83 [95% CI, 0.64-1.09], respectively, p=0.02 for effect modification). The association of time-updated NSAID use and kidney failure with KRT also varied across gender and baseline eGFR with a higher HR for males versus females (HRs of 1.21 [95% CI, 0.91-1.61] and 0.63 [95% CI, 0.45-0.88], respectively, p=0.004 for effect modification) and a significantly lower risk of kidney failure with KRT in the lower versus higher eGFR sub-groups (HRs of 0.77 [95% CI, 0.56-1.0] and 1.38 [95% CI, 0.89-2.14], respectively, for eGFR <= and >45 mL/min/1.73 m2; p=0.02 for effect modification). The association of pre-kidney failure hospitalization with opioid use (Figure S1a, Table S1) was higher in the lower versus higher baseline UPCR sub-groups (RRs of 1.90 [95% CI, 1.66-2.17] and 1.54 [95% CI, 1.36-1.74], respectively, for UPCRs below and above the median; p=0.02 for effect modification). Varying association between NSAID use and pre-kidney failure hospitalization had no significant effect modification (Figure S1b, Table S1).
Figure 1.
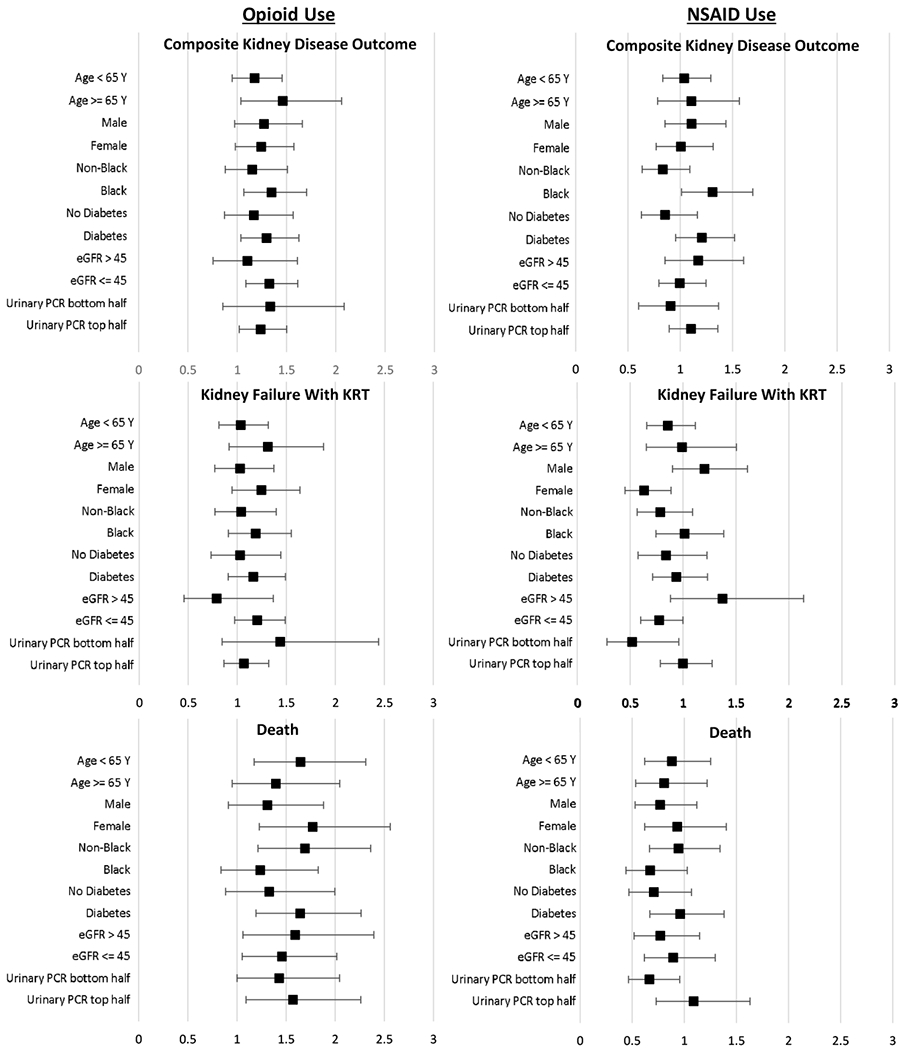
Forest plots of adjusted hazard ratios of the composite kidney disease outcome, kidney failure with KRT, death, comparing time-updated opioid use in the past versus none, and time-updated NSAID use versus none, stratified by key demographic and case-mix subgroups and adjusted for all other covariates in primary analysis.
Sensitivity analyses examined 1442 CRIC participants without diabetes at enrollment. In this subgroup, neither opioid nor NSAID use were associated with incident diabetes (HRs of 1.27 [95% CI, 0.87-1.84] and 1.03 [95% CI, 0.73-1.45], respectively).
To further explore the impact of potential unmeasured confounders on the associations between opioids and NSAIDs and outcomes, we computed E-values for potential unmeasured confounders for each risk estimate and the corresponding confidence interval (see Table S2) 20, 21. E-values ranged from 2.2 to 2.8 for the risk estimates determined for the associations between opioids and all outcomes, and between 1.4 and 2.6 for the corresponding lower confidence intervals. The E-values were in the higher portion of that range for the sub-cohort risk estimates, but closer to the null for the weaker associations reported for NSAIDs and outcomes.
We also explored potential confounding of other drug groups that may be used as analgesics including anxiolytics and antiepileptics (Table 1); however only antiepileptics were associated with the outcome of death. Hence, we repeated the analysis including antiepileptics with the time-dependent covariates input to the marginal structural models for death. The results were essentially unchanged from those reported in Table 4.
Discussion:
In this cohort of adults with CKD, we demonstrated that reported opioid use within 30 days of ascertainment and treated as a time-updated exposure was associated with a substantial risk of adverse kidney disease outcomes, death, and hospitalization. This was in contrast with the unexpected and modest relationship of NSAID use with adverse outcomes. The association between NSAID use and adverse kidney disease events was most prominent in blacks, with a potentially beneficial association with outcomes observed in sub-groups including women and those with a lower eGFR.
Physicians have long reported associations between various analgesics and kidney disease. “Analgesic nephropathy” is characterized by papillary necrosis, chronic interstitial nephritis, and progressive decreases in GFR22. Phenacetin was implicated as the principal causative agent, followed by aspirin and NSAIDs. Addition of caffeine to analgesic formulations may also exacerbate kidney injury22, 23. Reports of analgesic nephropathy led to the worldwide ban of phenacetin and several combination analgesics 24, 25. However, epidemiologic studies examining analgesics and kidney failure have revealed mixed findings23, 26. The Physician Health Study showed no risk of decreased GFR among moderate users of aspirin, acetaminophen, or NSAIDs 27,28. Several case-control studies found substantial consumption of acetaminophen, aspirin, and NSAIDs were associated with an increased risk of kidney failure 29, 30. A study of adults with CKD and comparable controls found acetaminophen, but not aspirin, to be associated with incident disease31. Another study of advanced CKD not requiring KRT revealed cumulative acetaminophen and aspirin exposure was associated with a risk for CKD 32. However, another study of patients with rheumatoid arthritis using COX-2 inhibitors showed no harmful effects from these analgesics except in advanced CKD 8. NSAID use in a large cohort of active, healthy US soldiers demonstrated a dose-dependent increased but modest risk of both AKI and CKD.33
Studies, including this group’s, reveal a higher frequency (9-36%) of NSAID use in CKD than one might expect given recommendations against their use 34–37. Approximately 20% of dialysis patients are found to have used NSAIDs consistently during years preceding KRT initiation38. In our report of NSAID use in the CRIC study, a quarter of study participants reported NSAID use at baseline or at least one annual visit, with a substantial proportion of users reporting treatment over the study 13. Relatively few reports describe opioid use in non-KRT-requiring CKD, with most data coming from KRT patients 39. One study revealed almost a third of non-KRT-requiring CKD patients were prescribed an opioid with the likelihood increasing with declining GFR40. This is in contradistinction to NSAID prescriptions, which diminished with declining GFR40. Besides the well-documented hazards of opioid use in the general population including mortality 41, 42, and lack of a beneficial treatment effect versus non-opioid analgesics 43, their risks are compounded in CKD where dosing of many opioids is affected by decreased clearance. Accumulation of both parent drugs’ metabolites44, 45, and enhanced adverse effects with use of these drugs in CKD make the adverse outcomes described here highly plausible46, 47.
Interpretation of the findings warrant consideration of the limitations inherent to its design. With observational analyses, one cannot overlook confounding by indication whereby use, or non-use, of one or the other analgesic is driven by factors which may be associated with the outcome of interest rather than the primary exposure, in this case, analgesic choice. To minimize confounding by indication we employed causal inference models with inverse probability weighting by expected analgesic use in the examination of the association of analgesics with outcomes. More extensive characterization of the time-updated exposure including variations in dosage and drug discontinuation was limited by the modest sample size. Additionally, the CRIC study was limited by its lack of a detailed pain assessment including measures of severity and type of pain. However, we used a wide array available measures of severity of illness and function embedded in the KDQOL −36 and SF-12, which include a gauge of pain’s impedance of work and ability to perform activities of daily living.
Notably, analgesic use exposure ascertainment in CRIC was restricted to selfreport, limited to the 30 days preceding an annual visit, and did not necessarily reflect actual use over more distant time intervals. Previous studies have examined the fidelity of self-report of NSAID and acetaminophen use when compared to urine drug screening 48. While the study did evaluate the use of NSAID and opioids versus non-users of these drugs, and the sub-group who were ever treated with any analgesics including the broader range of pain modulators such as acetaminophen, it did not assess the independent effect of the latter since this group served as the analysis reference group. Also, one cannot rule out the possibility analgesic choice may have been different during the years of this cohort before the opioid epidemic was more broadly recognized; however, we expect the reported associations would only be minimally influenced by secular trends in usage.
Of note, this is the first study we are aware of examining the comparative harm of NSAID vs opioid use in CKD. Both classes of agents have recognized risk profiles that are likely amplified in CKD, justifying close consideration of their risk versus benefit. Perhaps most importantly, the equipoise may be avoided with consideration of non-pharmacologic analgesic interventions that often show promising effectiveness in pain syndromes 49, 50. In conclusion, our study findings suggest opioid use is associated with greater harm in CKD than NSAIDs, with a substantial increase in risk for death and poor kidney outcomes. The adverse effects of NSAIDs appears to be less consistent across sub-groups with evidence for patient strata where NSAID use is at least neutral and possibly beneficial. Further studies needed to confirm such variable findings. While a prospective trial comparing analgesics in CKD patients with comparable degrees of pain and indications for analgesics is desirable, such a study is unlikely. Future guidance for strategies for patients with non-KRT-requiring CKD, therefore, will be based on comparative harm studies such as this and further studies are needed to verify the reported findings.
Supplementary Material
Figure S1: Forest plots of adjusted RR of hospitalization comparing participants with and without time-updated (A) opioid and (B) NSAID use.
Table S1: Point estimates and confidence intervals for all values depicted in forest plots for all outcomes.
Table S2: Hazard and rate ratios and corresponding E-values for each analgesic and associated outcomes.
Acknowledgments
Support: JCF, RMD, and MZ were supported by NIH/NIDDK R01 DK090008. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) V 2014.07.28 UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References:
- 1.Pham PC, Dewar K, Hashmi S, et al. Pain prevalence in patients with chronic kidney disease. Clin Nephrol. 2010;73(4):294–9. [PubMed] [Google Scholar]
- 2.Wu J, Ginsberg JS, Zhan M, et al. Chronic pain and analgesic use in CKD: implications for patient safety. Clin J Am Soc Nephrol. 2015;10(3):435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984;310(9):563–72. [DOI] [PubMed] [Google Scholar]
- 4.Murray MD, Brater DC. Adverse effects of nonsteroidal anti-inflammatory drugs on renal function. Ann Intern Med. 1990;112(8):559–60. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation. Pain Medicine and Your Kidneys. https://www.kidney.org/sites/default/files/01-10-7201.pdf.Accessed February 5, 2019.
- 6.Koncicki HM, Unruh M, Schell JO. Pain Management in CKD: A Guide for Nephrology Providers. Am J Kidney Dis. 2017;69(3):451–460. [DOI] [PubMed] [Google Scholar]
- 7.Hiremath S, Goldfarb DS, Juurlink DN. Opioid Overuse or NSAID Underuse? A Response to the Pain Guide. Am J Kidney Dis. 2017;69(6):865. [DOI] [PubMed] [Google Scholar]
- 8.Moller B, Pruijm M, Adler S, Scherer A, Villiger PM, Finckh A. Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann Rheum Dis. 2015;74(4):718–23. [DOI] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration. 2014 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf.Accessed February 5, 2019.
- 10.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among outpatients on opioid therapy in a large US health-care system. Addiction (Abingdon, England). 2010;105(10):1776–82. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO’s cancer pain ladder for adults. https://www.who.int/cancer/palliative/painladder/en/.Accessed October 9, 2018.
- 13.Zhan M, St Peter WL, Doerfler RM, et al. Patterns of NSAIDs Use and Their Association with Other Analgesic Use in CKD. Clin J Am Soc Nephrol. 2017;12(11):1178–86. [Google Scholar]
- 14.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–53. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;111:155A. [Google Scholar]
- 16.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. [DOI] [PubMed] [Google Scholar]
- 17.Deo R, Shou H, Soliman EZ, et al. Electrocardiographic Measures and Prediction of Cardiovascular and Noncardiovascular Death in CKD. J Am Soc Nephrol. 2016;27(2):559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Xie D, Anderson AH, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63(2):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe CJ, Cole SR, Mehta SH, Kirk GD. Estimating the effects of multiple time-varying exposures using joint marginal structural models: alcohol consumption, injection drug use, and HIV acquisition. Epidemiology. 2012;23(4):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA. 2019;321(6):602–603. [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 22.Buckalew VM Jr, Schey HM. Analgesic nephropathy: a significant cause of morbidity in the United States. Am J Kidney Dis. 1986;7(2):164–8. [DOI] [PubMed] [Google Scholar]
- 23.Dubach UC, Rosner B, Sturmer T. An epidemiologic study of abuse of analgesic drugs. Effects of phenacetin and salicylate on mortality and cardiovascular morbidity (1968 to 1987). N Engl J Med. 1991;324(3):155–60. [DOI] [PubMed] [Google Scholar]
- 24.Noels LM, Elseviers MM, de Broe ME. Impact of legislative measures on the sales of analgesics and the subsequent prevalence of analgesic nephropathy: a comparative study in France, Sweden and Belgium. Nephrol Dial Transplant. 1995;10(2):167–74. [PubMed] [Google Scholar]
- 25.Henrich WL, Agodoa LE, Barrett B, et al. Analgesics and the kidney: summary and recommendations to the Scientific Advisory Board of the National Kidney Foundation from an Ad Hoc Committee of the National Kidney Foundation. Am J Kidney Dis. 1996;27(1):162–5. [DOI] [PubMed] [Google Scholar]
- 26.Elseviers MM and De Broe ME. A long-term prospective controlled study of analgesic abuse in Belgium. Kidney Int. 1995;48(6):1912–9. [DOI] [PubMed] [Google Scholar]
- 27.Rexrode KM, Buring JE, Glynn RJ, Stampfer MJ, Youngman LD, Gaziano JM. Analgesic use and renal function in men. JAMA. 2001;286(3):315–21. [DOI] [PubMed] [Google Scholar]
- 28.Kurth T, Glynn RJ, Walker AM, et al. Analgesic use and change in kidney function in apparently healthy men. Am J Kidney Dis. 2003;42(2):234–44. [DOI] [PubMed] [Google Scholar]
- 29.Pommer W, Bronder E, Greiser E, et al. Regular analgesic intake and the risk of end-stage renal failure. Am J Nephrol. 1989;9(5):403–12. [DOI] [PubMed] [Google Scholar]
- 30.Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N Engl J Med. 1994;331 (25):1675–9. [DOI] [PubMed] [Google Scholar]
- 31.Sandler DP, Smith JC, Weinberg CR, et al. Analgesic use and chronic renal disease. N Engl J Med. 1989;320(19):1238–43. [DOI] [PubMed] [Google Scholar]
- 32.Fored CM, Ejerblad E, Lindblad P, et al. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001;345(25):1801–8. [DOI] [PubMed] [Google Scholar]
- 33.Nelson DA, Marks ES, Deuster PA, O’Connor FG, Kurina LM. Association of Nonsteroidal Anti-inflammatory Drug Prescriptions With Kidney Disease Among Active Young and Middle-aged Adults. JAMA Netw Open. 2019;2(2):e187896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gooch K, Culleton BF, Manns BJ, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120(3):280, e1–7. [DOI] [PubMed] [Google Scholar]
- 35.Hull S, Mathur R, Dreyer G, Yaqoob MM. Evaluating ethnic differences in the prescription of NSAIDs for chronic kidney disease: a cross-sectional survey of patients in general practice. Br J Gen Pract. 2014;64(624):e448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plantinga L, Grubbs V, Sarkar U, et al. Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011. ;9(5):423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel K, Diamantidis C, Zhan M, et al. Influence of creatinine versus glomerular filtration rate on non-steroidal anti-inflammatory drug prescriptions in chronic kidney disease. American journal of nephrology. 2012;36(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristensen SL, Fosbol EL, Kamper AL, et al. Use of nonsteroidal anti-inflammatory drugs prior to chronic renal replacement therapy initiation: a nationwide study. Pharmacoepidemiol Drug Saf. 2012;21(4):428–34. [DOI] [PubMed] [Google Scholar]
- 39.Wyne A, Rai R, Cuerden M, Clark WF, Suri RS. Opioid and benzodiazepine use in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2011. ;6(2):326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick TK, Surapaneni A, Shin JI, et al. Prevalence of Opioid, Gabapentinoid, and NSAID Use in Patients with CKD. Clin J Am Soc Nephrol. 2018;13(12):1886–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–20. [DOI] [PubMed] [Google Scholar]
- 42.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The Burden of Opioid-Related Mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krebs EE, Gravely A, Nugent S, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA. 2018;319(9):872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osborne RJ, Joel SP, Slevin ML. Morphine intoxication in renal failure: the role of morphine-6-glucuronide. Br Med J (Clin Res Ed). 1986;292(6535):1548–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conway BR, Fogarty DG, Nelson WE, Doherty CC. Opiate toxicity in patients with renal failure. BMJ. 2006;332(7537):345–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: a review of available evidence. Am J Kidney Dis. 2003;42(2):217–28. [DOI] [PubMed] [Google Scholar]
- 47.Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497–504. [DOI] [PubMed] [Google Scholar]
- 48.Loo RL, Chan Q, Brown IJ, et al. A comparison of self-reported analgesic use and detection of urinary ibuprofen and acetaminophen metabolites by means of metabonomics: the INTERMAP Study. Am J Epidemiol. 2012;175(4):348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou R, Deyo R, Friedly J, et al. Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(7):493–505. [DOI] [PubMed] [Google Scholar]
- 50.Bonakdar RA. Integrative Pain Management. Med Clin North Am. 2017;101(5):987–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Forest plots of adjusted RR of hospitalization comparing participants with and without time-updated (A) opioid and (B) NSAID use.
Table S1: Point estimates and confidence intervals for all values depicted in forest plots for all outcomes.
Table S2: Hazard and rate ratios and corresponding E-values for each analgesic and associated outcomes.


