Abstract
Background
The addition of a long acting beta agonist (LABA) to inhaled corticosteroid (ICS) therapy can improve asthma symptoms and reduce exacerbations. The addition of LABA may also have an ICS‐sparing effect and permit a reduction in ICS maintenance dose.
Objectives
To determine the efficacy of adding LABA to maintenance ICS therapy in reducing the requirement for ICS while maintaining control of chronic asthma.
Search methods
We searched the Cochrane Airways Group trials register and reference lists of articles. Date of last search: November 2004
Selection criteria
Parallel RCTs that compared reduced dose ICS in combination with LABA vs ICS alone in asthmatics requiring daily ICS.
Data collection and analysis
Trial quality was assessed and data were extracted independently by two reviewers. Study authors were contacted for confirmation. Trials were analysed according to the following ICS dose comparisons: a fixed moderate/high dose or a reduced/tapering dose of the same ICS.
Main results
19 publications describing 10 trials of adults were included in the review. Studies that compared reduced dose (mean 60% reduction) ICS/LABA combination to a fixed moderate/high dose ICS found no significant difference in severe exacerbations requiring oral corticosteroids (RR 1.0, 95%CI 0.76 to 1.32), withdrawal due to worsening asthma (RR 0.82, 95%CI 0.5 to 1.35) or airway inflammation. There were also significant improvements in FEV1 (change from baseline WMD 0.10, 95%CI 0.07 to 0.12), morning & evening PEF and percent rescue free days with LABA. Two studies provided outcomes for a reduced/tapering ICS dose comparison. More participants receiving the LABA/reduced ICS combination achieved a reduction in ICS dose reaching significance in one study. A significant reduction of 253 mcg BDP was achieved in one study.
Authors' conclusions
In adults with asthma, using moderate to high maintenance doses of ICS, the addition of LABA has an ICS‐sparing effect. The addition of LABA permits more participants on minimum maintenance ICS to reduce ICS. The precise magnitude of the ICS dose reduction requires further study.
Plain language summary
Long‐acting beta2‐agonists as an inhaled corticosteroid‐sparing agent for chronic asthma in adults and children
The combination of long acting beta agonist (LABA) with inhaled corticosteroid (ICS) is used frequently in asthma and a benefit from adding LABA to ICS has been described. This review compared reduced dose (mean 60% reduction in inhaled steroid) ICS/LABA combination to either a fixed moderate/high dose ICS or a reduced/tapering ICS dose. In adults with asthma, who use moderate to high maintenance doses of ICS, the addition of LABA has an ICS‐sparing effect. LABA permit a reduction of 37% (253 mcg BDP) in subjects on minimum maintenance ICS and up to 60% (300 mcg FP) in subjects on maintenance ICS without deterioration in asthma control.
Background
Asthma is characterised by airway inflammation and variable expiratory airflow obstruction. Inhaled corticosteroids (ICS) and long acting beta agonists (LABA) effectively target these pathophysiological processes in asthma. The combination of LABA with ICS is used frequently in asthma and a complementary effect from adding LABA to ICS has been described. This could result from a synergistic molecular mechanism where LABA increases the nuclear translocation of active glucocorticoid receptors (Barnes 2002). An additional mechanism relates to the heterogeneity of airway inflammation in asthma where ICS effectively target eosinophilic inflammation and LABA target the airway hyper responsiveness that occurs as a consequence of non eosinophilic asthma pathology (which is relatively resistant to ICS). These complementary effects of ICS and LABA in asthma translate into improved clinical efficacy in a variety of settings. In uncontrolled asthma, the addition of LABA to ICS gives better results than ICS alone for both initial asthma therapy (Ducharme 2004), and maintenance asthma therapy (Greenstone 2004). When asthma is controlled, ICS doses can often be reduced. In this setting, LABA may provide an ICS‐sparing effect permitting greater ICS dose reduction while maintaining asthma control. However, concern has been raised that reducing ICS while using LABA may mask worsening airway inflammation (McIvor 1998), and therefore the ICS‐sparing effect of LABA needs careful examination. The aim of this review is to assess the steroid‐sparing effect of the addition of LABA to maintenance moderate or high dose ICS in stable asthmatics.
Objectives
The objective of this review is to determine the efficacy of the addition of long acting beta agonists to maintenance ICS therapy in reducing the requirement for ICS while maintaining control of chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of parallel design only were considered. Double, single or unblinded studies were considered.
Types of participants
Adults and children > 2 years with stable asthma maintained on regular moderate to high dose ICS ( > 400 mcg/day BDP equivalent in adults, > 200 mcg/day BDP equivalent in children) for a minimum of one month prior to study entry, and not using LABA.
Types of interventions
The active intervention was long acting beta agonist combined with an inhaled corticosteroid at a reduced or tapering dose. The ideal design to determine the minimal effective ICS dose should include a gradual decrease in ICS dose to avoid artificial overinflation of the results and ensure similar asthma control at the end of tapering in both groups. Several comparison interventions were examined:
A fixed moderate or high dose of the same ICS,
A gradual dose reduction of the same ICS according to asthma control. This comparison controlled for the reduction in ICS maintenance dose that may be possible without additional therapy.
ICS alone in patients whose loss of control had been determined on reduced ICS prior to randomisation. This comparison permitted a more precise estimate of ICS dose reduction, since the minimum maintenance dose was defined before addition of LABA.
Several delivery systems were considered including metered dose inhalers (pMDI), with or without a valved holding chamber (spacer) or dry powder inhaler (DPI). Treatment duration needed to be one month or greater. Asthma control was assessed using symptom/lung function criteria at study completion in order to ascertain that control is maintained after tapering.
Types of outcome measures
1) Change in dose of ICS achieved at endpoint. 2) Any clinical outcome identifying asthma control such as: ‐ withdrawal due to worsening asthma (RR and 95% CI within 0.9 to 1.1), ‐ exacerbations requiring systemic steroids, ‐ exacerbations requiring hospital admission, ‐ lung function (change from baseline in FEV1 < 200 mL), ‐ asthma symptoms, ‐ airway hyporesponsives, ‐ rescue medication use, ‐ Emergency room (ER) visits, ‐ inflammatory mediators, and 3) Adverse events ‐ overall withdrawals
Search methods for identification of studies
The Cochrane Airways Group Specialised Register of asthma trials (derived from systematic searches of CENTRAL, MEDLINE, EMBASE, CINAHL; hand‐searched respiratory journals and meeting abstracts). A free‐text search of the Register was conducted in November 2004 using the following terms:
((inhale* AND (corticosteroid OR steroid) OR beclometh* OR triamcin* OR flutic* OR budes* OR betameth* OR flunis*) AND ((long AND (beta* OR *agonist* OR bronchodilator)) OR salmeterol OR eformoterol OR formoterol).
Full text versions of the relevant papers were obtained, and their bibliographic lists were hand searched for additional articles.
Data collection and analysis
Selection of studies
All identified abstracts were independently assessed by two reviewers. The full text version of each potential article was obtained for assessment by two independent reviewers to establish whether it met the inclusion criteria.
Data extraction and management
Authors were contacted to verify and provide further information about methodological approaches and outcomes data. 60% of authors responded to our request.
Assessment of risk of bias in included studies
Study quality was assessed with the Jadad system (Jadad 1996), allows for a score between 0 and 5 with higher scores indicating a better description of the study by two review authors independently. The Jadad system measures the clarity of the description of: randomisation (1 point, 2 points if well described and appropriate), blinding (1 point, 2 points if well described and appropriate) and treatment of withdrawals and dropouts (1 point). One point is deducted if the method of randomisation or blinding is inappropriate.
Studies were further assessed, using the Cochrane method, as low risk of bias, high risk of bias, or unclear to the actual methods used for randomisation, concealment of allocation and blinding. In this assessment, if studies are either not truly randomised (e.g. alternated) or if allocation to treatment or control groups is not truly blinded, studies are considered as being of at a high risk of bias. If the author does not fully state these methods, the study is characterised as "unclear" until the author is contacted and clarification can be made.
Data synthesis
The following comparisons were analysed: i) ICS & LABA vs ICS alone (fixed moderate or high dose) ii) ICS & LABA vs ICS alone (reduced/tapering dose) iii) ICS & LABA vs ICS alone in patients whose loss of control had been determined on reduced ICS prior to randomisation.
Outcomes were analysed as continuous and/or dichotomous variables, using standard statistical techniques: i) For continuous outcomes, the weighted mean difference (for outcomes reported in the same scale) or standardised mean difference (for outcomes reported in different scales) with 95% confidence intervals was calculated. ii) For dichotomous outcomes, the relative risk was calculated with 95% confidence interval. The number needed to treat (NNT) was calculated from the pooled odds ratio.
Heterogeneity was examined using both a Chi‐squared test and the I square percentage. A random effects model was used when the I‐Square was ≥ 25%. Funnel plots were used to examine for publication bias.
Subgroup analysis and investigation of heterogeneity
Outcomes were analysed according to: i) Age (children / adults) ii) Reported asthma control at randomisation (satisfactory versus unsatisfactory) iii) Delivery device (pMDI / DPI) for LABA iv) Delivery device (pMDI/DPI) for ICS v) Delivery device (single or separate inhalers for LABA / ICS) vi) Study quality vii) Treatment Duration (< 24 weeks vs > 24 weeks) viii) ICS type/LABA type Differences between subgroups were tested for using Chi‐square totals or the Peto OR and t‐test as appropriate. Where there were heterogenous results, these were explored using the subgroup analyses above. If imputed data were included, a sensitivity analysis was performed. ICS dose was reported as the dose mcg beclomethasone (BDP) equivalent ex‐valve and classified according to British Thoracic Society Guidelines as 1 mcg FP: 2 mcg BDP and 1 mcg:BUD:1 mcg BDP
Results
Description of studies
Results of the search
From electronic searches, 59 citations describing 42 RCTs were identified for possible inclusion. Ten of these met the eligibility criteria of the review. Details of individual eligible studies are given in Characteristics of included studies.
Included studies
The included trials were conducted in adults and adolescents. Three trials included participants > 12 years of age (Baranuik 1999; Busse 2003; Lemanske 2001). One trial did not state the age of the participants (Bloom 2003). Trials were analysed according to the design of the comparison intervention: a fixed moderate/high ICS dose or a reduced/tapering ICS dose.
1) Abrupt fixed reduced ICS + LABA versus fixed moderate or high dose of the same ICS
Seven trials (Baranuik 1999; Bloom 2003; Busse 2003; Dorinsky 2004; Kips 2000; Lalloo 2001; Pauwels 1997) compared treatment with a reduced ICS dose combined with a LABA to a fixed moderate to high dose of ICS alone to maintain asthma control. Asthma stability was assessed during the run in period in 6 trials ( Baranuik 1999; Bloom 2003; Busse 2003; Dorinsky 2004; Kips 2000; Pauwels 1997). Inadequate asthma control was an entry criteria for one trial (Lalloo 2001). Of these, two trials (Busse 2003; Dorinsky 2004) conducted 3 run‐in phases to establish that participants became unstable on reduced ICS doses and then regained asthma control on moderate to high dose ICS prior to randomisation. In one study (Baranuik 1999) participants were eligible for randomisation if they were symptomatic or had demonstrated a reduced FEV1 40‐65% predicted. The inclusion criterion for one study Bloom 2003 was that participants required moderate dose ICS to maintain control. In 2 trials (Kips 2000; Pauwels 1997) participants were eligible for randomisation if they demonstrated asthma stability and were compliant during a 4 week run in period on moderate to high dose ICS.
Fluticasone propionate (200 mcg/day) and salmeterol (100 mcg/day) were compared to fluticasone (500 mcg/day) alone in 4 trials (Busse 2003; Baranuik 1999; Bloom 2003; Dorinsky 2004). The remaining 3 trials (Kips 2000; Lalloo 2001; Pauwels 1997) compared budesonide (160‐200 mcg/day) and formoterol (9‐24 mcg/day) to budesonide (400‐800 mcg/day) alone. Two trials included other treatment arms that did not meet the criteria for this analysis and were not included: Baranuik 1999 included a third treatment arm of triamcinalone 1200 mcg/day and Pauwels 1997 had 4 treatment arms, two of which were not applicable for this analysis and were not included.
By design these 7 studies reduced ICS dosage with the addition of LABA by 60‐75% with a mean dose reduction of 64.3% (95%CI 57.5 to 71.1). The average ICS dose was 857 mcg/day BDP equivalent and this was compared to 309 mcg /day BDP equivalent with the addition of LABA. Treatment periods were 12 weeks in 2 trials (Baranuik 1999; Lalloo 2001), 24 weeks in two trials (Bloom 2003; Dorinsky 2004), and 12 months in 2 trials (Kips 2000; Pauwels 1997). One trial (Busse 2003) had an initial treatment period of 12 weeks with a proportion of participants randomised for 24 weeks. Outcome data collected at 12 weeks was used for this analysis. The delivery devices used were the same for both ICS and LABA delivery in 3 trials (DPI, n= 2 and pMDI, n = 1) and a single DPI device was used in 4 trials (Busse 2003; Bloom 2003; Dorinsky 2004; Lalloo 2001).
2) Reduced or tapering ICS + LABA versus reduced or tapering dose of the same ICS according to asthma control
ICS dose reduction was evaluated in 3 trials (Nielsen 1999; Lemanske 2001; Self 1998) that compared treatment with reducing doses of ICS with and without the addition of LABA.
In one trial participants received a three‐phase run‐in ( Nielsen 1999) where their minimum acceptable ICS dose (MAD) was determined. After receiving BDP via DPI at an equivalent dose to their maintenance ICS for 2 weeks their BDP was reduced by 200 mcg/week until they became unstable according to predefined criteria and MAD determined. Those who remained stable were withdrawn from the study. Participants were restabilised on 3 x MAD for 2 weeks then randomised to placebo or salmeterol 100 mcg/day and their ICS reduction recommenced until MAD determined .
Self 1998 conducted a 4 week run‐in where participants received their usual ICS (BDP, FP or TAA) at a minimum dose of 1000 mcg/day. Those who were stable were randomised to salmeterol 200 mcg/day or placebo plus their usual ICS. After 2 weeks ICS reduction was commenced in both treatment arms at a rate of 10% every 4 weeks if the participant was stable. ICS was not reduced > 25% or below 500 mcg/day for participants with moderate/severe asthma. Participants were monitored for 12 months. ICS was optimised with pMDI and spacer and education and crisis prednisolone provided at commencement of the study.
Lemanske 2001 randomised participants remaining uncontrolled after 6 weeks triamcinalone 800 mcg/day to two arms, namely the addition of salmeterol or placebo. After 8 weeks participants in the salmeterol treatment arm were further randomised to reduced TAA 400 mcg/day for 8 weeks then placebo TAA for 8 weeks or to remain on TAA 800 mcg/day for 16 weeks, while those in the placebo arm received reduced TAA (400 mcg/day) for 8 weeks then placebo TAA for 8 weeks.
3) Reduced ICS + LABA versus ICS alone in participants who demonstrate deteriorating asthma control when ICS are reduced
In six trials (Baranuik 1999; Busse 2003; Dorinsky 2004; Lalloo 2001; Lemanske 2001; Nielsen 1999) the inclusion criteria required either a demonstrated deterioration in asthma control if ICS were reduced or that participants had inadequate asthma control on their maintenance ICS prior to randomisation. These trials are included in comparisons 1 and 2 but are also included in this analysis since their design determines the ICS dose reduction possible that is attributable to the addition of LABA. A sensitivity analysis was conducted for the different control comparison:a fixed moderate or high ICS dose or reduced or tapering ICS dose.
Excluded studies
From 42 RCTs, 32 trials were excluded for the following reasons: subjects were not previously maintained on ICS (n=1); ICS dose was not reduced in the active intervention group (n=23); subjects were ICS naive (n=1); subjects were on LABA prior to study entry (n=1); both LABA and ICS were withdrawn (n=1); were a crossover design (n=4) or LABA not added in the active intervention group (n = 1). The remaining 19 citations (9 publications and 10 abstracts) describing 10 parallel designed RCTs met the inclusion criteria for the review. Reasons for the exclusion of these studies are given in Excluded studies.
Risk of bias in included studies
For four of the studies we were unable to determine the degree to which any aspect of study design protected against bias. Allocation generation and concealment was assessed as being of a low risk of bias in five studies. In six studies blinding procedures were adequate. Using the Jadad system, 80% of the studies scored three or higher, with 50% scoring 5.
Effects of interventions
1) Abrupt fixed reduced ICS + LABA versus fixed moderate or high dose of the same ICS
Seven trials randomised 2625 participants to two treatment arms applicable for this analysis. Where appropriate, subgroup analyses were conducted for asthma control at randomisation, delivery device and study quality. All trials were conducted in adults.
Worsening Asthma
Exacerbations requiring oral corticosteroids (OCS)
The number of participants who had a severe exacerbation that required OCS was reported in two trials (Kips 2000; Pauwels 1997), both comparing budesonide and formoterol to mod/high budesonide dose and were of 12 months duration. There was no significant difference between treatment groups when these results were pooled. No heterogeneity was present. In this analysis, LABA permitted a reduction in ICS dose without an increase in exacerbations. Exacerbations requiring OCS: RR 1.0 (95% CI 0.76 to 1.32), Analysis 1.1 .
1.1. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 1 Severe exacerbation requiring OCS.
Withdrawal due to worsening asthma
Four trials reported the number of subjects who withdrew from the study due to worsening asthma. Dorinsky 2004 reported no difference in the proportion of participants who withdrew due to worsening asthma in a post hoc analysis. Two studies comparing FP/salmeterol (Bloom 2003; Busse 2003) over 24 and 12 weeks respectively and one trial comparing BUD/formoterol (Pauwels 1997) over 12 months reported data that could be pooled in a meta analysis. The pooled results of these 3 trials show no significant group difference. There was no heterogeneity (withdrawal due to worsening asthma: RR 0.82 (95% CI 0.50 to 1.35), Analysis 1.2).
1.2. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 2 Withdrawal due to worsening asthma.
Hospitalisations
Two trials reported hospitalisations for worsening asthma. Pauwels 1997 reported a similar number of hospitalisations across all treatment groups and there were no hospitalisations for either treatment group in the trial conducted by Dorinsky 2004.
Lung Function
FEV1
FEV1 was reported in six studies as litres change from baseline, percent predicted change from baseline and percent predicted at end of study. Four studies reporting litres change from baseline provided sufficient data to be pooled using a WMD. There was a significant improvement in FEV1 in favour of the reduced ICS /LABA combination with no heterogeneity. FEV1 (L) change from baseline: WMD 0.10 L(95%CI 0.07 to 0.12), Analysis 1.3.
1.3. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 3 FEV1 (L) change from baseline.
The pooled result for the two studies reporting FEV1 percent predicted at the end of 12 months was also significant in favour of the reduced ICS/LABA group with no heterogeneity present. FEV1 percent predicted: WMD 3.62% (95%CI 1.65 to 5.59), Analysis 1.4.
1.4. Analysis.
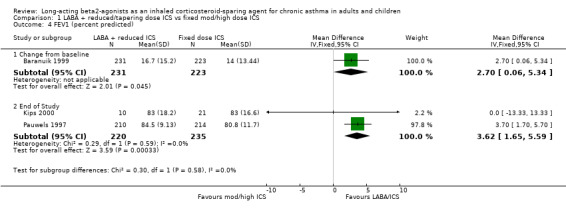
Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 4 FEV1 (percent predicted).
Morning Peak Expiratory Flow (PEF)
Morning PEF was reported in six studies. There was a significant improvement in morning PEF from baseline when the results of four studies all comparing FP/salmeterol combinations to FP alone were pooled. No heterogeneity was present. Morning PEF (L/min) change from baseline: WMD 16.68 (95%CI 9.13 to 24.24), Analysis 1.5.
1.5. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 5 Morning PEF (L/min) change from baseline.
Evening Peak Expiratory Flow (PEF)
Five studies reported evening PEF. Three studies comparing FP/salmeterol combination reported the change in evening PEF from baseline and were pooled using a WMD. There was a small but significant improvement in evening PEF with no heterogeneity present. Evening PEF (L/min) change from baseline: WMD 12.56 (95%CI 4.9 to 20.22), Analysis 1.6.
1.6. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 6 Evening PEF (L/min) change from baseline.
The remaining two studies reported that evening PEF improved significantly in the BUD/formoterol group (Lalloo 2001; Kips 2000).
Rescue Medications
Six studies reported the use of rescue medications. This was reported as the change in mean puffs per day from baseline in four studies comparing FP/salmeterol to FP alone. There was a non significant reduction in rescue medication use for the reduced ICS/LABA combination and no heterogeneity.
Rescue medication use (puffs/day) change from baseline: WMD ‐0.11 (95%CI ‐0.25 to 0.03), Analysis 1.7.
1.7. Analysis.
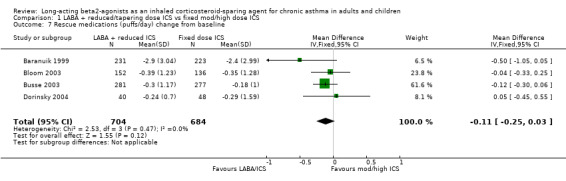
Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 7 Rescue medications (puffs/day) change from baseline.
Three of these studies also reported the percent rescue free days. There was a significant improvement in the percentage of days without rescue medication use for the reduced ICS/LABA group. Statistical heterogeneity was present.
Percent rescue free days change from baseline: WMD 9.21 (95%CI 1.36 to 17.05). Chi2 = 5.11, P = 0.08, I2 = 60.9%, Analysis 1.8.
1.8. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 8 Percent Rescue Free Days change from baseline.
When analysed by asthma control at randomisation there was no heterogeneity and significance maintained (comparison 4.08).
One study reported no difference in rescue medication use between the treatment groups (Kips 2000) and a significant reduction in day and night rescue medication use was reported for the formoterol group in another study (Pauwels 1997).
Symptoms
Percent Symptom Free Days
Symptom free days were reported in seven studies. There was a non significant improvement in the change in percentage of symptom free days from baseline for the FP/salmeterol treatment group in the results of four studies evaluating FP/salmeterol to FP alone. Low heterogeneity was present. In one study evaluating BUD/formoterol combination there was a non significant improvement in the percentage of symptom free days for the reduced ICS/LABA combination at the end of the study. Percent symptom free days change from baseline: WMD 3.71(95%CI ‐1.14 to 8.57). Chi2 = 4.35, P = 0.23, I2 = 31.1%, Analysis 1.9.
1.9. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 9 Percent Symptom Free Days.
When analysed by control at randomisation there was no heterogeneity and statistical significance for participants who were controlled at randomisation. Percent symptom free days: WMD 5.76 (95%CI 0.81 to 10.7), Analysis 4.2
4.2. Analysis.

Comparison 4 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (asthma control), Outcome 2 Percent Symptom Free Days change from baseline.
Two further studies, not included in the meta analysis, reported a significantly higher percentage of symptom free days for the reduced ICS/LABA treatment groups (Lalloo 2001; Pauwels 1997).
Symptom Score
There was a non significant reduction in symptom score from baseline for the reduced ICS/LABA combination when the results of three studies, all comparing FP/Salmeterol to FP alone and using either a 0‐4 or 0‐5 scale were pooled. Significant heterogeneity was present between the studies. Symptom score change from baseline: SMD ‐0.16 (95%CI ‐0.40 to 0.09). Chi2 = 6.85, P = 0.03, I2 = 70.8%, comparison 1.10. Heterogeneity could not be explained by asthma control at randomisation (comparison 4.10) or delivery device (comparison 5.10). When examined by study quality (comparison 6.10), there was no heterogeneity present for studies with a Jadad score > 3. Two other studies reported improvement in day and night symptom scores with the addition of formoterol to reduced dose BUD which was significant in one study (Pauwels 1997) and non significant in the other (Kips 2000).
Night Awakening
Night waking was reported in four studies. There was no difference in the change in mean number of night awakenings from baseline in the two FP/salmeterol studies that reported data that could be pooled. No heterogeneity was present. Night Waking change from baseline: WMD 0.02 (95%CI ‐0.09 to 0.12), Analysis 1.11.
1.11. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 11 Night Waking change from baseline.
One study reported the mean number of awakenings per night but significance between the reduced BUD/formoterol and BUD alone group was not stated (Pauwels 1997). The remaining study reported no difference between the treatment groups (Kips 2000).
Airway Inflammation
Airway inflammation was evaluated in two studies (Kips 2000; Dorinsky 2004). Neither study reported data in a way that could be pooled for analysis. Dorinsky 2004 reported no change from baseline between treatment groups in mean collagen band thickness or inflammatory cells within the submucosa obtained from conducting bronchoalveolar lavage (BAL) and biopsy. There was no difference between treatment groups in sputum inflammatory cells including eosinophils, EG2 cells or ECP reported in the study conducted by Kips 2000.
Adverse Events
There was no significant difference in the occurrence of adverse events between the treatment groups in the pooled result of three studies (FP/salmeterol: n= 2, BUD/formoterol: n=1) reporting this outcome. There was no heterogeneity present. Adverse Events: RR 0.92 (95%CI 0.79 to 1.07), Analysis 1.12.
1.12. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 12 Adverse Events.
Overall Withdrawals
There was no significant difference in the overall withdrawals between the treatment groups in the pooled result of three studies reporting this outcome. There was no heterogeneity present. Overall Withdrawals: RR 0.97 (95%CI 0.74 to 1.28), Analysis 1.13.
1.13. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 13 Overall Withdrawals.
2) Reduced or tapering ICS + LABA versus reduced or tapering dose of the same ICS according to asthma control
Due to the methods of reporting of the three studies (Nielsen 1999; Self 1998; Lemanske 2001) included in this comparison only data for lung function could be pooled for meta‐analysis.
Lung Function
FEV1
FEV1 was reported as Litres change from baseline in two studies. There was a non significant improvement in FEV1 for the reduced LABA/ICS group. No heterogeneity was present. FEV1(L) change from baseline: WMD 0.21 (95%CI 0.0 to 0.41), Analysis 2.1.
2.1. Analysis.

Comparison 2 LABA + reduced/tapering dose ICS vs reduced/tapering dose ICS, Outcome 1 FEV1 (L) change from baseline.
Morning PEF
There was no significant difference in morning PEF L/min in the pooled results of two studies and no heterogeneity. Morning PEF (L/min): WMD: 7.80 (95%CI ‐14.24 to 29.85), Analysis 2.2.
2.2. Analysis.

Comparison 2 LABA + reduced/tapering dose ICS vs reduced/tapering dose ICS, Outcome 2 Morning PEF (L/min) change from baseline.
Other Outcomes
Nielsen 1999 reported a significantly lower minimal acceptable ICS dose during the treatment period and a significantly greater reduction in mean (se) ICS mcg minimal acceptable dose for the LABA/ICS group (‐253 (196)mcg/day, 37% ) compared to a small increase for the group receiving ICS alone (42 (118) mcg/day) (P<0.01). A significantly greater proportion of participants in the LABA/ ICS group attained a > 50% reduction in ICS dose with no significant difference in FEV1(L), morning or evening PEF between treatment groups when compared to their baseline sensitivity period. In addition, there was a significantly greater reduction in rescue medication use, fewer daytime symptoms and more symptom free days between baseline sensitivity and treatment periods compared to reducing ICS alone and no difference in adverse events
There was no significant difference between treatment groups in the mean percent reduction of ICS dose (LABA/ICS combination, 18.7%; reduced ICS alone, 6.9%) reported by Self 1998. However more patients in the LABA/ICS group compared to ICS alone achieved a reduction in ICS. A significantly higher FEV1 percent predicted at end of the 12 month study and an improvement in asthma quality of life was reported for the group receiving LABA and a reduction in rescue medication use for both treatment groups. There was no difference in clinic PEF or adverse events for either treatment group.
Lemanske 2001concluded that reducing ICS to zero whilst taking additional LABA was not possible due to significant deterioration in asthma control but a 50% reduction of ICS was possible without loss of asthma control.
3) Reduced ICS + LABA versus ICS alone in participants who demonstrate deteriorating asthma control when ICS are reduced
Six trials randomised 1776 participants after determining their deterioration in asthma control on reduced dose ICS alone. By design a 60% (mean 510 mcg BDP equivalent) ICS dose reduction was achieved in the four studies comparing reduced ICS/LABA to a fixed moderate/high ICS dose and a 50% reduction was achieved for the two studies using a reduced/tapering ICS dose comparison whilst maintaining asthma control. The following are the results of the pooled analyses.
Withdrawals due to worsening asthma
There was no significant difference between treatment groups in the rate of withdrawal due to worsening asthma reported in one study (Busse 2003).
Lung Function
FEV1
There was a significant improvement in the change in FEV1 from baseline in favour of the reduced ICS /LABA combination in the four studies that provided sufficient data to be pooled using a WMD. There was no heterogeneity and the result remained unchanged with the sensitivity analysis. FEV1 (L) change from baseline: WMD 0.10 (95%CI 0.07 to 0.12), Analysis 3.2.
3.2. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 2 FEV1 (L) change from baseline.
Morning Peak Expiratory Flow (PEF)
There was a significant improvement in morning PEF from baseline when the results of four studies were pooled. No heterogeneity was present and significance maintained with the sensitivity analysis. Morning PEF (L/min) change from baseline: WMD 15.59 (95%CI 7.89 to 23.29), Analysis 3.3.
3.3. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 3 Morning PEF (L/min) change from baseline.
Evening Peak Expiratory Flow
Four studies reported the change in evening PEF from baseline and were pooled using a WMD. There was a small but significant improvement in evening PEF with no heterogeneity present. Significance was maintained with the sensitivity analysis. Evening PEF (L/min) change from baseline: WMD 12.45 (95%CI 5.05 to 19.84), Analysis 3.4.
3.4. Analysis.
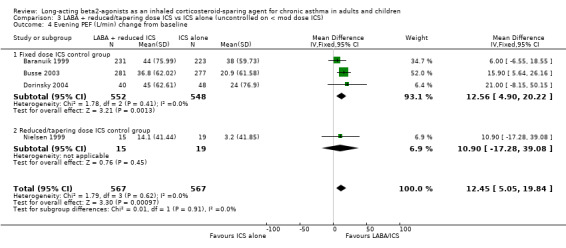
Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 4 Evening PEF (L/min) change from baseline.
Rescue Medications
Rescue medication use was reported as the change in mean puffs per day from baseline in three studies comparing FP/salmeterol to FP alone and the change in salbutamol mcg/day in one study comparing BDP/salmeterol to BDP alone. There was a non significant reduction in rescue medication use for the reduced ICS/LABA combination. Low heterogeneity was present. Rescue medication use (puffs/day) change from baseline: SMD ‐0.15 (95%CI ‐0.30 to 0.01). Chi 2=4.19, P= 0.24, I2 = 28.3%, Analysis 3.5.
3.5. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 5 Rescue medications change from baseline.
Three of these studies, all with a fixed ICS dose control group, also reported the percent rescue free days. There was a significant improvement in the percentage of days without rescue medication use for the reduced ICS/LABA group. However statistical heterogeneity was present. Percent rescue free days: WMD 9.21 (95%CI 1.36 to 17.05). Chi2 = 5.11, P= 0.08, I2 = 60.9%, Analysis 3.7. When analysed by control at randomisation there was no inconsistency and significance maintained (Analysis 4.1).
3.7. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 7 Percent symptom free days change from baseline.
4.1. Analysis.

Comparison 4 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (asthma control), Outcome 1 Percent Rescue Free Days change from baseline.
Symptoms
Percent Symptom Free Days
There was a significant improvement in the percentage of symptom free days from baseline for the FP/salmeterol treatment group in the results of three studies evaluating FP/salmeterol to fixed dose FP alone. Percent symptom free days change from baseline: WMD 6.0 (95%CI 1.86 to 10.15), Analysis 3.6.
3.6. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 6 Percent Rescue Free Days change from baseline.
Symptom Score
There was a non significant reduction in symptom score from baseline for the reduced ICS/LABA combination when the results of three studies, all comparing FP/Salmeterol to FP alone and using either a 0‐4 or 0‐5 scale were pooled. Significant heterogeneity was present between the studies. Symptom score change from baseline: SMD ‐0.16 (95%CI ‐0.40 to 0.09). Chi2 = 7.41, P= 0.03, I2 = 70.8%, Analysis 3.8. Heterogeneity could not be explained by asthma control at randomisation or delivery device. When examined by study quality low heterogeneity remained for studies with a Jadad score > 3.
3.8. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 8 Symptom Score change from baseline.
Night Waking
There was no significant difference in the change in mean number of night awakenings from baseline in the two FP/salmeterol studies with a fixed dose control group that reported data that could be pooled. No heterogeneity was present. Night Waking change from baseline: WMD 0.02 (95%CI ‐0.09 to 0.12), comparison 3.11.
Adverse Events
There was no significant difference in the occurrence of adverse events between the treatment groups in the pooled result of three studies (FP/salmeterol: n= 2, BUD/formoterol: n=1) reporting this outcome. There was no heterogeneity present and no difference with the sensitivity analysis. Adverse Events: RR 0.92 (95%CI 0.80 to 1.07), Analysis 3.10.
3.10. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 10 Adverse Events.
Overall Withdrawals
There was no significant difference in the overall withdrawals between the treatment groups in the pooled result of two studies reporting this outcome. There was no heterogeneity present. Overall Withdrawals: RR 01.03 (95%CI 0.65 to 1.61), Analysis 3.11.
3.11. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 11 Overall Withdrawals.
Discussion
This systematic review of 10 trials suggests that in adults with asthma maintained on regular ICS it is possible to reduce maintenance ICS dose by the addition of a LABA without compromising asthma control. The results also showed that the addition of a LABA led to improved indices of airway calibre and reduced short acting beta agonist use. This is consistent with the known actions of LABA as a bronchodilator. In addition, maintenance ICS dose could be reduced without loss of asthma control or excess exacerbations. With the increasing preference for prescribed maintenance ICS/LABA combinations and the subsequent opportunities to reduce ICS doses the need to establish the efficacy of reducing ICS dose in combination with LABA whilst still maintaining asthma control is important. As there were no studies in children that met the inclusion criteria an evaluation for this group could not be conducted.
We sought to answer whether an ICS dose reduction was possible with the addition of LABA and, if so, by how much the maintenance ICS dose could be reduced. When evaluating the seven studies included in comparison 1 that compared reduced ICS doses in combination with a LABA to a fixed moderate/high ICS dose there was no significant difference between treatment groups for those experiencing exacerbations requiring OCS or withdrawing due to worsening asthma despite a reduction in their maintenance ICS dose. We defined maintenance of asthma control a priori as a change in baseline FEV1 < 200 mls. This was achieved with the reduced ICS/LABA combination treatment group also experiencing clinically small but consistent benefits in comparison to the fixed moderate/high ICS dose treatment groups. This included significant improvements in FEV1, morning and evening PEF with no heterogeneity. For FEV1 this equated to a 100 ml (95% CI 70 to 120 mls) greater improvement than those receiving ICS alone, despite receiving a lower ICS dose, which is similar to the 180 ml (95%CI 110 to 240 mls) improvement reported by Ni Chroinin 2005 for participants who received LABA in addition to their maintenance ICS. There were also significant improvements in the percent of days without rescue medications but there was moderate inconsistency among these studies. When this was explored further with a subgroup analysis based on whether their asthma was controlled at randomisation there was no inconsistency and the significance was maintained for those who were controlled at randomisation. These effects are consistent with the known bronchodilator effects of LABA.
There have been concerns that the introduction of LABA may mask airway inflammation. There were only two studies that reported this outcome. These two studies reported no differences between treatment groups in inflammatory cells when ICS was reduced in combination with LABA. These two studies, one of 12 months duration, suggest that masking of airway inflammation with the addition of LABA may not occur when ICS are continued at 200‐400 mcg day BDP equivalent. The current evidence supports that ICS dose reduction is possible when in combination with a LABA. Reducing ICS to zero (i.e.. complete cessation) whilst continuing LABA is contraindicated (Lemanske 2001) because of potential adverse effects. The studies were varied in design. Three different study designs were examined: the effect of reducing ICS in combination with a LABA was compared to a fixed moderate high dose ICS, a reduced/tapering dose ICS and in participants whose loss of control on reduced ICS alone had been determined. In six studies the inclusion criteria included either a demonstrated deterioration in asthma control if ICS were reduced or participants had inadequate asthma control on their maintenance ICS prior to randomisation. This design provides a more accurate assessment of the degree of ICS reduction possible that is attributable to the addition of LABA. In the remaining four studies, where deterioration in asthma control was not assessed with ICS dose reduction prior to randomisation, a proportion of the ICS dose reduction may have been possible without the addition of LABA and the precise estimate of ICS reduction that could be attributed to the addition of a LABA cannot be determined.
This systematic review indicates the extent that ICS could be reduced by the addition of LABA. One study attempted to reduce ICS to zero with the addition of LABA. Lemanske 2001 reported a substantial deterioration in asthma control when ICS was reduced to zero in combination with LABA and concluded that complete ICS elimination was not achievable but that a 50% reduction in ICS dose was possible without loss of asthma control. The 6 studies in our third comparison have also demonstrated that ICS can be reduced by a mean 57% with the addition of a LABA in people whose asthma deteriorates when ICS is reduced alone. In two studies (Busse 2003; Dorinsky 2004) this was achieved for subjects requiring high dose FP (500 mcg day). We have previously reported that the dose response curve for FP reaches a plateau at 500 mcg day with minimal benefits at higher doses and a sharp increase in side effects between a moderate dose (200 mcg day) and high dose FP (500 mcg day) (Powell 2003). The addition of LABA to reduced dose ICS therefore has the potential to substantially reduce the side effects of ICS whilst maintaining asthma control.
To establish a more precise estimate of the ICS dose reduction possible we were able to evaluate the results of two studies that compared reducing ICS in combination with a LABA to reducing ICS alone. The primary outcome for these studies was the dose reduction in ICS achieved. For both studies more patients in the reduced ICS/LABA group achieved a greater reduction in ICS dose and a greater proportion attained an ICS dose reduction. However these results reached significance in only one study where Nielsen 1999 was able to reduce ICS by a mean 253 mcg day BDP. The sample size for these two studies was small leading to the possibility of a type II error in the study that did not reach significance. Due to the methods of reporting these results could not be pooled in a meta analysis. In conjunction with the attained ICS dose reduction there was no concurrent deterioration in lung function, symptoms, rescue medication use or adverse events. Overall, the results suggest that the ICS‐sparing effect of LABA is equivalent to approximately 60% of the maintenance ICS dose. In patients on high dose FP (500 mcg day) this equated to an ICS reduction of 300 mcg day FP as determined by study design. While the improvement shown in lung function and symptoms for participants who received the reduced ICS in combination with a LABA is consistent with a bronchodilator effect of LABA. The mechanism of the ICS‐sparing effect is not defined. Possible explanations of the ICS‐sparing effect are that LABAs have anti‐inflammatory effects, that the mechanism that induces bronchodilation is the same mechanism that provides the corticosteroid‐sparing effect, or that the combination of LABA with ICS may have complementary effects on asthma.
Potential anti‐inflammatory effects of LABA are an inhibition of mast cell mediator release, and potentiation of ICS effects by increasing nuclear translocation of corticosteroid receptors. However LABA are only partially effective in asthma, since complete ICS cessation while continuing LABA is deleterious (Lemanske 2001). Potential complementary effects of LABA and ICS in asthma relate to the heterogeneity of airway inflammation in asthma.
In studying the ICS‐sparing effect of LABA there is an assumption that the asthma population is homogenous and responds to treatment in the same way. However we know that there is heterogeneity of the asthma population and that some people with asthma are not responsive to ICS, in particular people with non eosinophilic asthma. It could be argued that the ICS‐sparing qualities seen occur in this portion of the asthma population. In comparison 3 of this review however, we have demonstrated that ICS can be reduced with the addition of LABA in participants who have demonstrated deterioration in asthma control when ICS is reduced alone. This would indicate that it is not just that portion of people with asthma who do not respond to ICS that are able to reduce their ICS with the addition of LABA but also those who are ICS dependent.
This review supports the ICS‐sparing qualities of ICS/LABA combination therapy. ICS dose reduction in adults may be attained with the use of ICS/LABA combination therapy without deterioration in asthma control. However no studies have been carried out in children so the results may not be generalisable under the age of 12 years.
Authors' conclusions
Implications for practice.
Maintenance ICS dose reduction in adults may be attained with the use of ICS/LABA combination therapy without deterioration in asthma control.
Implications for research.
RCTs to assess the ICS‐sparing qualities of ICS/LABA combination therapy in children. Further RCTs to evaluate the effects of reduced ICS dose in combination with LABA on airway inflammation. Further RCTs using the ideal design (a gradual decrease in ICS dose to avoid artificial overinflation of the results and ensure similar asthma control at the end of tapering in both groups) to quantify the achievable ICS dose reduction. Further research to identify the mechanisms of the ICS‐sparing effect with the addition of LABA.
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 26 July 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Airways Review Group for their assistance with this review with data base searches, obtaining studies, translations and statistical assistance: Toby Lasserson, Liz Arnold and Chris Cates.
We would also like to thank the following for providing information regarding their studies: Shailesh Patel for Baranuik 1999; Dorinsky 2004; Nielsen 1999, Lemanske 2001, Nils Gundstrom for Pauwels 1997, and Klas Svensson for Kips 2000.
We would like to thank the Cooperative Research Centre for Asthma for financial support.
Data and analyses
Comparison 1. LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe exacerbation requiring OCS | 2 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
| 2 Withdrawal due to worsening asthma | 3 | 1270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.35] |
| 3 FEV1 (L) change from baseline | 4 | 1388 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.07, 0.12] |
| 4 FEV1 (percent predicted) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Change from baseline | 1 | 454 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [0.06, 5.34] |
| 4.2 End of Study | 2 | 455 | Mean Difference (IV, Fixed, 95% CI) | 3.62 [1.65, 5.59] |
| 5 Morning PEF (L/min) change from baseline | 4 | 1388 | Mean Difference (IV, Fixed, 95% CI) | 16.68 [9.13, 24.24] |
| 6 Evening PEF (L/min) change from baseline | 3 | 1100 | Mean Difference (IV, Fixed, 95% CI) | 12.56 [4.90, 20.22] |
| 7 Rescue medications (puffs/day) change from baseline | 4 | 1388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.25, 0.03] |
| 8 Percent Rescue Free Days change from baseline | 3 | 1100 | Mean Difference (IV, Random, 95% CI) | 9.21 [1.36, 17.05] |
| 9 Percent Symptom Free Days | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Change from baseline | 4 | 1388 | Mean Difference (IV, Random, 95% CI) | 3.71 [‐1.14, 8.57] |
| 9.2 End of Study | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 10.90 [‐7.40, 29.20] |
| 10 Symptom Score change from baseline | 3 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.40, 0.09] |
| 11 Night Waking change from baseline | 2 | 1012 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.09, 0.12] |
| 12 Adverse Events | 3 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.07] |
| 13 Overall Withdrawals | 3 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.28] |
1.10. Analysis.

Comparison 1 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS, Outcome 10 Symptom Score change from baseline.
Comparison 2. LABA + reduced/tapering dose ICS vs reduced/tapering dose ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) change from baseline | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.00, 0.41] |
| 2 Morning PEF (L/min) change from baseline | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | 7.80 [‐14.24, 29.85] |
Comparison 3. LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to worsening asthma | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.34] |
| 1.1 Fixed dose ICS control group | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.34] |
| 1.2 Reduced/tapering dose ICS control group | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (L) change from baseline | 4 | 1134 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.07, 0.12] |
| 2.1 Fixed dose ICS control group | 3 | 1100 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.07, 0.12] |
| 2.2 Reduced/tapering dose ICS control group | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.2 [‐0.33, 0.73] |
| 3 Morning PEF (L/min) change from baseline | 4 | 1134 | Mean Difference (IV, Fixed, 95% CI) | 15.59 [7.89, 23.29] |
| 3.1 Fixed dose ICS control group | 3 | 1100 | Mean Difference (IV, Fixed, 95% CI) | 15.79 [7.70, 23.87] |
| 3.2 Reduced/tapering dose ICS control group | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 13.7 [‐11.43, 38.83] |
| 4 Evening PEF (L/min) change from baseline | 4 | 1134 | Mean Difference (IV, Fixed, 95% CI) | 12.45 [5.05, 19.84] |
| 4.1 Fixed dose ICS control group | 3 | 1100 | Mean Difference (IV, Fixed, 95% CI) | 12.56 [4.90, 20.22] |
| 4.2 Reduced/tapering dose ICS control group | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [‐17.28, 39.08] |
| 5 Rescue medications change from baseline | 4 | 1134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, 0.01] |
| 5.1 Fixed dose ICS control group | 3 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.24, ‐0.00] |
| 5.2 Reduced/tapering dose ICS control group | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.50, ‐0.09] |
| 6 Percent Rescue Free Days change from baseline | 3 | 1100 | Mean Difference (IV, Random, 95% CI) | 9.21 [1.36, 17.05] |
| 6.1 Fixed dose ICS control group | 3 | 1100 | Mean Difference (IV, Random, 95% CI) | 9.21 [1.36, 17.05] |
| 6.2 Reduced/tapering dose ICS control group | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Percent symptom free days change from baseline | 3 | 1100 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [1.86, 10.15] |
| 7.1 Fixed dose ICS control group | 3 | 1100 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [1.86, 10.15] |
| 7.2 Reduced/tapering dose ICS control group | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Symptom Score change from baseline | 3 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.40, 0.09] |
| 8.1 Fixed dose ICS control group | 3 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.40, 0.09] |
| 8.2 Reduced/tapering dose ICS control group | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Night Waking change from baseline | 2 | 1012 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.09, 0.12] |
| 9.1 Fixed dose ICS control group | 2 | 1012 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.09, 0.12] |
| 9.2 Reduced/tapering dose ICS control group | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse Events | 3 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.80, 1.07] |
| 10.1 Fixed dose ICS control group | 2 | 1012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.08] |
| 10.2 Reduced/tapering dose ICS control group | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.36] |
| 11 Overall Withdrawals | 2 | 1012 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.61] |
| 11.1 Fixed dose ICS control group | 2 | 1012 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.61] |
| 11.2 Reduced/tapering dose ICS control group | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 1 Withdrawal due to worsening asthma.
3.9. Analysis.
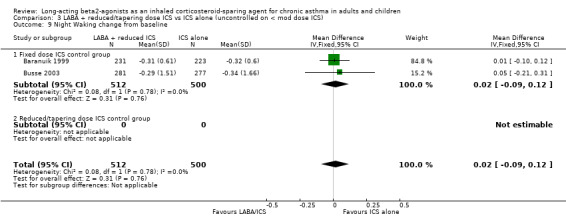
Comparison 3 LABA + reduced/tapering dose ICS vs ICS alone (uncontrolled on < mod dose ICS), Outcome 9 Night Waking change from baseline.
Comparison 4. LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (asthma control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent Rescue Free Days change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Controlled | 2 | 646 | Mean Difference (IV, Random, 95% CI) | 5.34 [0.16, 10.52] |
| 1.2 Uncontrolled | 1 | 454 | Mean Difference (IV, Random, 95% CI) | 16.1 [8.33, 23.87] |
| 1.3 Unknown | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Percent Symptom Free Days change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Controlled | 2 | 646 | Mean Difference (IV, Random, 95% CI) | 5.76 [0.81, 10.70] |
| 2.2 Uncontrolled | 1 | 454 | Mean Difference (IV, Random, 95% CI) | 6.60 [‐1.03, 14.23] |
| 2.3 Unknown | 1 | 288 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐13.17, 4.57] |
| 3 Symptom Score change from baseline | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Controlled | 2 | 646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.76, 0.14] |
| 3.2 Uncontrolled | 1 | 454 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.21] |
| 3.3 Unknown | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.3. Analysis.

Comparison 4 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (asthma control), Outcome 3 Symptom Score change from baseline.
Comparison 5. LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (device).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent Rescue Free Days change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 pMDI | 1 | 454 | Mean Difference (IV, Random, 95% CI) | 16.1 [8.33, 23.87] |
| 1.2 DPI | 2 | 646 | Mean Difference (IV, Random, 95% CI) | 5.34 [0.16, 10.52] |
| 2 Percent Symptom Free Days change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 pMDI | 1 | 454 | Mean Difference (IV, Random, 95% CI) | 6.60 [‐1.03, 14.23] |
| 2.2 DPI | 3 | 934 | Mean Difference (IV, Random, 95% CI) | 2.29 [‐4.68, 9.25] |
| 3 Symptom Score change from baseline | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 pMDI | 1 | 454 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.21] |
| 3.2 DPI | 2 | 646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.76, 0.14] |
5.1. Analysis.

Comparison 5 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (device), Outcome 1 Percent Rescue Free Days change from baseline.
5.2. Analysis.

Comparison 5 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (device), Outcome 2 Percent Symptom Free Days change from baseline.
5.3. Analysis.

Comparison 5 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (device), Outcome 3 Symptom Score change from baseline.
Comparison 6. LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (quality).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent Rescue Free Days change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Jadad >=3 | 2 | 1012 | Mean Difference (IV, Random, 95% CI) | 10.39 [‐0.07, 20.85] |
| 1.2 Jadad < 3 | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 4.90 [‐9.61, 19.41] |
| 2 Percent Symptom Free Days change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Jadad >=3 | 3 | 1300 | Mean Difference (IV, Random, 95% CI) | 3.44 [‐2.63, 9.52] |
| 2.2 Jadad < 3 | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐10.14, 18.14] |
| 3 Symptom Score change from baseline | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Jadad >=3 | 2 | 1012 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| 3.2 Jadad < 3 | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.02, ‐0.16] |
6.1. Analysis.

Comparison 6 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (quality), Outcome 1 Percent Rescue Free Days change from baseline.
6.2. Analysis.

Comparison 6 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (quality), Outcome 2 Percent Symptom Free Days change from baseline.
6.3. Analysis.

Comparison 6 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (quality), Outcome 3 Symptom Score change from baseline.
Comparison 7. LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (ICS/LABA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent Rescue Free Days change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 FP/Salm | 3 | 1100 | Mean Difference (IV, Random, 95% CI) | 9.21 [1.36, 17.05] |
| 1.2 BUD/Form | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Percent Symptom Free Days change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 FP/Salm | 4 | 1388 | Mean Difference (IV, Random, 95% CI) | 3.71 [‐1.14, 8.57] |
| 2.2 BUD/Form | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Symptom Score change from baseline | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 FP/Salm | 3 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.40, 0.09] |
| 3.2 BUD/Form | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.

Comparison 7 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (ICS/LABA), Outcome 1 Percent Rescue Free Days change from baseline.
7.2. Analysis.

Comparison 7 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (ICS/LABA), Outcome 2 Percent Symptom Free Days change from baseline.
7.3. Analysis.

Comparison 7 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (ICS/LABA), Outcome 3 Symptom Score change from baseline.
Comparison 8. LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (duration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percent Rescue Free Days change from baseline | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 <= 24 weeks | 3 | 1100 | Mean Difference (IV, Random, 95% CI) | 9.21 [1.36, 17.05] |
| 1.2 > 24 weeks | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Percent Symptom Free Days change from baseline | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 <= 24 weeks | 4 | 1388 | Mean Difference (IV, Random, 95% CI) | 3.71 [‐1.14, 8.57] |
| 2.2 > 24 weeks | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Symptom Score change from baseline | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 <= 24 weeks | 3 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.40, 0.09] |
| 3.2 > 24 weeks | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.

Comparison 8 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (duration), Outcome 1 Percent Rescue Free Days change from baseline.
8.2. Analysis.

Comparison 8 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (duration), Outcome 2 Percent Symptom Free Days change from baseline.
8.3. Analysis.

Comparison 8 LABA + reduced/tapering dose ICS vs fixed mod/high dose ICS (duration), Outcome 3 Symptom Score change from baseline.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baranuik 1999.
| Methods | Study Design: Parallel RCT Randomisation: computer generated random numbers Concealment of Allocation: coded identical inhalers Double Blinding: Yes Withdrawals / dropouts: Described Adverse events: Described Statistical analysis: Intention to treat Jadad Score: 5 | |
| Participants | Study site: Multi‐centred pulmonary/allergy clinics No eligible: No randomised: Interventions 1&2: 454 (231;223); Intervention 3: 226 (N/A) No completed: Interventions 1&2: 425 (215;210); Intervention 3: 205 (N/A) Sex: Males ‐ Intervention 1: 95 (45%); Intervention 2: 86 (39%); Intervention 3: N/A Age (mean, range): 1: 41 (12‐79); 2: 40 (12‐74); 3: N/A Diagnostic criteria for asthma: ATS criteria Inclusion criteria: >= 12 yrs, FEV1 40‐85% predicted, >= 15% bronchodilator reversibility, nonsmokers, requiring maintenance ICS for 3 months prior to study Exclusion criteria: pregnancy, lactation, use of methotrexate, gold, cyclosporine, or azathioprine, inhaled cromolyn, nedocromil, oral or injectable steroids in past month. Significant comorbidity, other prescription medication. Baseline severity of asthma: FEV1 (mean, SE): 1: 2.14 (0.04); 2: 2.12 (0.04), FEV1 % (mean, SE): 1: 63.1(0.78); 2: 63.1 (0.82), PEF % (mean, SE): 1: 68 (1); 2: 65 (1) | |
| Interventions | Run in phase: 2 weeks on usual ICS, If FEV1 40‐65% predicted or 65.1%‐85% and symptomatic at end of run‐in they were eligible for randomisation. 1: FP 100mcg BID (200mcg/day, ex‐valve) & salmeterol 50mcg BID (100mcg/day, ex‐valve) 2: FP 250mcg BID (FP 500mcg/day, ex‐valve) 3: Triamcinalone 600mcg BID (1200mcg/day) ‐ Not applicable for this review Delivery device: MDI plus spacer Duration of treatment: 12 weeks Other: Albuterol allowed prn and theophylline at fixed dose if part of usual treatment regimen. Albuterol and theophylline were withheld >= 6 hours prior to study visits. | |
| Outcomes | Adverse events, withdrawal due to adverse events,overall withdrawals, physician global assessment. Measured as change from baseline: Morning & evening PEF (L/min), PEF % predicted, morning & evening FEV1 (L), FEV1 % predicted, rescue medications (puffs/day), % rescue free days, night awakenings, asthma symptom score, % symptom free days. SDs were calculated based on an ITT analysis | |
| Notes | Comparison 1: Constant dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random numbers |
| Allocation concealment? | Low risk | Investigators unaware as to order of randomisation |
| Blinding? All outcomes | Low risk | Identical inhaler devices used |
Bloom 2003.
| Methods | Study Design: Parallel RCT Randomisation: Random stated, method not described. Concealment of Allocation: not stated. Double Blinding: Yes. Withdrawals / dropouts: Described. Adverse events: not stated. Statistical analysis: not stated. Jadad Score: 3 | |
| Participants | Study site: No eligible: No randomised: 574 No completed: 288 Sex: Males ‐not stated; Females ‐not stated Age: not stated. Diagnostic criteria for asthma: not stated. Inclusion criteria: Requiring moderate dose maintenance ICS (FP 220bid equivalent) to maintain asthma control. Exclusion criteria: not stated. Baseline severity of asthma: FEV1 mean 78% predicted | |
| Interventions | Run in phase: not stated
1: Fluticasone/Salmeterol 100/50mcg combination bid (200/100mcg/day)
2: Fluticasone 250mcg bid (500mcg/day)
Delivery device: DPI, single device
Duration of treatment: 24 weeks Other: |
|
| Outcomes | All measured as change from baseline: Withdrawal due to worsening asthma, FEV1(L), morning PEF (L/min), rescue medication use (puffs/day), % symptom free days | |
| Notes | Abstract only. Comparison 1: Constant dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Busse 2003.
| Methods | Study Design: Parallel RCT Randomisation: random stated, method not described. Concealment of Allocation: not stated. Double Blinding: Yes. Withdrawals / dropouts: described. Adverse events: described. Statistical analysis: Intention to Treat Jadad Score: 3 | |
| Participants | Study site: multicentre No eligible: 760 No randomised: 558 (281/277) No completed: 235/223 at 12 weeks Sex: Males 42%; Females 58% Age (mean,range): Intervention1: 38 (12‐77); Intervention 2: 39 (12‐72). Diagnostic criteria for asthma: objective lung function. Inclusion criteria: asthma > 6 months and treated with medium dose ICS for at least 30 days prior to study entry, FEV1 65‐95% predicted, >12% reversibility. Exclusion criteria: No OCS or parenteral corticosteroids past 30 days, pregnancy or lactation, life‐threatening asthma, asthma hospitalisation in past 3 months, change in asthma maintenance in past 30 days, significant concurrent disease including upper or lower respiratory tract infection. Baseline severity of asthma (mean, SD): 80.5 (9.7)/80.9 (9.4)% predicted | |
| Interventions | Run in 1: FP 220mcg bid for 10‐14 days. If stable continue to Run in 2: FP 100 bid for 5‐28 days then if unstable continue to Run in 3: FP 250mccg bid for 4 weeks to regain asthma control, once controlled ‐randomised to; Intervention 1: Fluticasone/salmeterol 100/50 mcg bid (200/100mcg/day) Intervention 2: Fluticasone 250 mcg bid (500mcg/day) Delivery device: DPI, single device Duration of treatment: 12 weeks with a proportion randomised to 24 weeks. Other: No theophylline, other bronchodilators, anticholinergics, leukotriene modifiers, cromolyn, nedocromil, salmeterol or formoterol throughout study. | |
| Outcomes | Withdrawal due to worsening asthma, adverse events, overall withdrawals. Measured as change from baseline:FEV1(L), morning and evening PEF (L/min), rescue medication use (puffs/day), % rescue medication free days, % symptom free days, daily asthma symptom score, night‐time waking. SDs were calculated based on an ITT analysis | |
| Notes | Comparison 1: Constant dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Dorinsky 2004.
| Methods | Study Design: Parallel RCT Randomisation: Random stated, method not described Concealment of Allocation: Not stated. Double Blinding: Yes Withdrawals / dropouts: Not described Adverse events: Not described Statistical analysis: Described Jadad Score: 2 | |
| Participants | Study site: No eligible: No randomised: 88 (40;48) No completed: Not stated Sex: Intervention 1: 43% males; Intervention 2: 46% males Age (mean, SD): 1: 34.3 (11.3); 2: 35.3 (14.6) Diagnostic criteria for asthma: Not stated Inclusion criteria: FEV1 65‐95% predicted and at least 30 days of treatment with medium dose ICS, patients had to become clinically unstable when ICS was decreased to FP 100mcg bid. Exclusion criteria: Not stated Baseline severity of asthma: FEV1 (mean, SE) Intervention 1: 84.5% (1.5); Intervention 2: 81.2% (1.4) | |
| Interventions | Run in phase 1: 2 weeks. Patients stable on FP 250 mcg bid or equivalent dose. Run in phase 2: 5‐28 days. Stable patients decreased ICS to FP 100mcg bid ‐ those who became unstable were eligible to continue in trial. Run in phase 3: 4 weeks. FP dose increased to 250mcg bid. If stable on FP 250mcg bid eligible for trial Intervention 1: FSC 100/50mcg bid (200/100mcg daily) Intervention 2: FP 250mcg bid (FP 500mcg daily) Delivery device: DPI, single device Duration of treatment: 24 weeks Other: | |
| Outcomes | BAL/biopsy ‐ airway inflammation Measured as change from baseline: Morning & evening PEF (l/min), FEV1 (L), rescue medications (puffs/day), % rescue free days), daily symptom score, % symptom free days, | |
| Notes | Poster only. Comparison 1: Constant dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Kips 2000.
| Methods | Study Design: Parallel RCT Randomisation: computer generated random numbers Concealment of Allocation: opaque consecutive numbered envelopes, identical placebo Double Blinding: Yes Withdrawals / dropouts: Described Adverse events: exacerbations only Statistical analysis: 'per protocol ' analysis Jadad Score: 5 | |
| Participants | Study site: multicentre No eligible: 70 No randomised: 60 (29/31) No completed: not stated Sex: Males‐24 (40%);Females‐36 (60%) Age (mean, range): Intervention1:34.7 (19‐59); Intervention 2: 37.6 (19‐69) Diagnostic criteria for asthma: doctor diagnosis and objective lung function Inclusion criteria: asthma diagnosis > 6 months, maintenance ICS > 3 months and constant ICS dose in past month, FEV1 > 50% predicted, > 15% reversibility. Exclusion criteria: > 2000mcg BDP, >1600mcg BUD, or > 800mcg FP per day, 3 courses OCS or hospitalisation in past 6 months Baseline severity of asthma: FEV1 (mean, se): 2.87(0.18)/2.52(0.14)L; FEV1 % predicted: 82.6(3.6)/76.1(3.0) | |
| Interventions | Run in phase: BUD 800mcg/day for 1 month then randomised if compliance 75‐125% recommended dose and asthma stable for last 10 days of run in. 1: BUD 100mcg + Formoterol 12mcg twice daily 2: BUD 400mcg + placebo twice daily Delivery device: DPI Duration of treatment: 12 months Other: | |
| Outcomes | Exacerbations, % episode free days, rescue medications, nocturnal awakenings, airway inflammation, FEV1 % predicted, evening PEF, morning and evening symptom scores. | |
| Notes | Comparison 1: Constant dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random numbers |
| Allocation concealment? | Low risk | Opaque consecutive numbered envelopes |
| Blinding? All outcomes | Low risk | Identical placebo |
Lalloo 2001.
| Methods | Study Design: Parallel RCT Randomisation: Random stated, method not described. Concealment of Allocation: Not stated. Double Blinding: Yes Withdrawals / dropouts: Not described. Adverse events: Not described. Statistical analysis: Not described. Jadad Score: 2 | |
| Participants | Study site: No eligible: No randomised: 467 No completed: Not stated Sex: Not stated Age: mean 41 yrs Diagnostic criteria for asthma: Objective lung function Inclusion criteria: mild to moderate asthma not fully controlled on ICS alone, > 12% bronchodilator reversibility. Exclusion criteria: Baseline severity of asthma: Mean FEV1 82% predicted, mean ICS/day= 390mcg | |
| Interventions | Run in phase: Not stated 1: Budesonide/formoterol 80/4.5mcg x 2 (160/9 mcg/day) 2: Budesonide 200mcgx2 (400mcg/day) Delivery device: DPI, single device Duration of treatment: 12 weeks Other: | |
| Outcomes | Morning & evening PEF, % symptom free days, exacerbations, asthma control days | |
| Notes | Abstract only Comparison 1: Constant dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Lemanske 2001.
| Methods | Study Design: Parallel RCT Randomisation: Described Concealment of Allocation: Described. Double Blinding: triple blind Withdrawals / dropouts: Described Adverse events: described Statistical analysis: Intention to treat Jadad Score: 5 | |
| Participants | Study site: multicentre No eligible: 339 (164 controlled asthmatics entered SOCS trial) No randomised: 175 No completed: 160 ( Intervention 1:18; Intervention 2A: 71; Intervention 2B:71) at end of TAA reduction phase Sex: Males ‐intervention 1: 8(42%); Intervention 2B: 35(47.3%) Age(mean, SD): 1: 35.6 14.4); 2B: 34.2 (10.8) yrs Diagnostic criteria for asthma: ATS criteria Inclusion criteria: 12‐65 yrs, persistent asthma (FEV1<=80% and >=12% bronchodilator reversibility, if on ICS & FEV1 > 80% then needed to demonstrate a 20% reduction in FEV1 to provocative methacholine challenge. Exclusion criteria: Smoking history, >=10 pack years, use of other medicines except oral contraceptive and nasal beclomethasone, respiratory infection or asthma exacerbation in past 6 weeks, comorbid serious illness. Baseline severity of asthma: mean(SD) FEV1 pre bronchodilator: Intervention 1: 72.5% (12.5); Intervention 2B: 73.8% (11.2) | |
| Interventions | Run in phase: 6 weeks Triamcinolone 800mcg/day. If controlled entered in SOCS trial. If uncontrolled (FEV1 <=80% or PEF variability > 2‐%) randomised to:
Salmeterol introduction phase
1: TAA 400mcg bid(800mcg/day) + placebo
2: TAA 400mcg bid (800mcg/day) + salmeterol 42mcg bid(84mcg/day)
After 8 weeks enter:
TAA reduction phase
1: TAA 200mcg bid(400mcg/day) + placebo
2: Subjects randomised to
2A: TAA 400mcg bid (800mcg/day) + salmeterol 42mcg bid(84mcg/day)
or
2B: TAA 200mcg bid(400mcg/day) + salmeterol 42mcg bid (84mcg/day)
After 8 weeks enter:
TAA elimination phase
1: placebo + placebo
2A: TAA 400mcg bid (800mcg/day) + salmeterol 42mcg bid(84mcg/day)
2B: placebo + salmeterol 42mcg bid(84mcg/day)
Delivery device: pMDI
Duration of treatment: 24 weeks total, Outcomes used after TAA reduction phase (16 weeks treatment) Other: |
|
| Outcomes | Time to treatment failure, PEF, FEV1, AHR (methacholine), Asthma symptoms, QOL | |
| Notes | Comparison 2: Reduced dose ICS Interventions 1 vs intervention 2B outcomes at end TAA reduction phase eligible for review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Patient randomization was performed online via an Internet connection to the computer system at the data coordinating centre (...) The first randomization at the end of the triamcinolone run‐in period was stratified according to clinical centre, and the second randomization before the triamcinolone reduction phase was stratified according to ethnic group, sex, and age.' |
| Allocation concealment? | Low risk | 'Staff members entered and verified the pertinent data and received a drug packet number to give each eligible patient at the 2 randomizations.' |
| Blinding? All outcomes | Low risk | Triple blind. 'Medication for each patient was packaged together, labelled with a unique number, and distributed to the clinical centres. The contents of the drug packages were known only to administrative personnel at the data coordinating centre.' |
Nielsen 1999.
| Methods | Study Design: Parallel RCT Randomisation: computer generated Concealment of Allocation: opaque consecutive numbered envelopes Double Blinding: Yes Withdrawals / dropouts: described Adverse events: described Statistical analysis: described Jadad Score: 5 | |
| Participants | Study site: Multi‐centred No eligible: 77 No randomised: 34 (15/19) No completed: 34 Sex: Males: 15 (44%); Females:19 (56%) Age: mean 43.9 yrs; range: 19‐65yrs Diagnostic criteria for asthma: Clinically diagnosed asthma defined by ATS criteria Inclusion criteria: stable asthma (according to predefined criteria), controlled on ICS (BDP or BUD) of 800‐1600mcg/day; symptoms of episodic cough , wheezing and dyspnoea for > 1 year prior to Visit 1; reversible airway obstruction with >=15% reversibility with bronchodilator;positive PC20 documented up to 6 months prior to study;20% PEF diurnal variation; FEV1 > 60% predicted; adults 18‐65 yrs. Exclusion criteria: excluded if remained stable when ICS was withdrawn during the sensitivity period. Baseline severity of asthma: mean FEV1: 2.9L; mean FEV1 % predicted: 86.4%; Mean BDP dose at study entry: 1224mcg/day; smokers: 14(41%); atopy:15 (44%). | |
| Interventions | Run in phase: 2 weeks with BDP dose equivalent to maintenance ICS to assess stability. Run in phase 2: 'sensitivity period': BDP reduced by 200mcg/week until asthma became unstable according to predefined criteria. Minimal acceptable dose (MAD) BDP determined as the dose one step above the dose precipitating unstable asthma. Those remaining stable after ICS withdrawal excluded from study. Run in phase 3: given 3 times MAD for 2 weeks then if stable randomised to: 1: Salmeterol 50mcg bd , OR 2: Placebo AND ICS dose reduction repeated and MAD determined. Run out phase: 2 weeks follow‐up on appropriate ICS at conclusion of study. Delivery device: DPI Duration of treatment: Max 14 weeks Other: salbutamol prn | |
| Outcomes | ICS dose, morning & evening PEF, FEV1, day a& nighttime symptom scores, rescue medication, adverse events | |
| Notes | Comparison 2: Reduced dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation schedule |
| Allocation concealment? | Low risk | Opaque consecutive numbered envelopes |
| Blinding? All outcomes | Low risk | Identical inhaler devices used |
Pauwels 1997.
| Methods | Study Design: Parallel RCT Randomisation: randomised in balanced blocks of 4 at each treatment centre. Concealment of Allocation: opaque consecutive numbered envelopes, identical placebo Double Blinding: Yes Withdrawals / dropouts: described Adverse events: described Statistical analysis: intention to treat Jadad Score: 5 | |
| Participants | Study site: multicentre No eligible: 1114 No randomised: 852 (213/210/214/215) No completed: 694 Sex: Males 416 (49%); Females 436 (51%) Age (mean, range): Intervention 1: 42 (18‐70); Intervention 2: 41 (18‐68); Intervention 3: 44 (18‐70); Intervention 4: 42 (17‐70) Diagnostic criteria for asthma: doctor diagnosis and objective lung function Inclusion criteria: asthma > 6 months, ICS maintenance >3 months, FEV1 > 50% predicted, > 15% reversibility. Exclusion criteria: 3 or more courses OCS or hospitalisation in past 6 months, ICS doses > 2000mcg BDP, > 1600mcg BUD(pMDI), > 800mcg FP, or > 800mcg BUD(DPI). Baseline severity of asthma: FEV1% (mean): 75.8/75.7/75.4/76.3; Morning PEF: 397/399/381/394; Evening PEF: 402/402/387/402 | |
| Interventions | Run in phase: BUD 800mcg twice daily for 4 weeks then randomised if compliant (75‐125% ) and stable asthma. 1: BUD 100mcg + placebo twice daily (N/A) 2: BUD 100mcg + Formoterol 12mcg twice daily 3: BUD 400mcg + placebo twice daily 4: BUD 400mcg + Formoterol 12mcg twice daily (N/A) Delivery device: DPI Duration of treatment: 12 months Other: | |
| Outcomes | Withdrawal due to severe exacerbations, overall withdrawals, exacerbations, FEV1 % predicted, morning PEF, episode free days, symptoms, rescue medications (no of puffs), hospitalisations, QOL. SDs were calculated based on an ITT analysis | |
| Notes | Comparison 1: Constant dose ICS (interventions 2&3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation scheme |
| Allocation concealment? | Low risk | Opaque consecutive numbered envelopes |
| Blinding? All outcomes | Low risk | 'All medications were inhaled by means of a multidose Turbuhaler.' |
Self 1998.
| Methods | Study Design: Parallel RCT Randomisation: random stated, method not described Concealment of Allocation: Double Blinding: Yes. Plain MDI used without spacer for both placebo and salmeterol Withdrawals / dropouts: described Adverse events: described Statistical analysis: described Jadad Score: 3 | |
| Participants | Study site: multicentre No eligible: 46 No randomised: 42 No completed: 24 (12/12) Sex: Males:25%/0%; Females: 75%/100% Age (mean, range): Intervention1:39.6yrs (30‐57); Intervention 2: 46.6yrs (22‐68) Diagnostic criteria for asthma: According to criteria of the National Institutes of Health (NIH) International Consensus report Inclusion criteria: > 18 yrs, on maintenance ICS >= 1000mcg/day Exclusion criteria: Chronic bronchitis, emphysema, pregnancy, inability to use peak flow meter or pMDI/spacer, clinically significant cardiac disease, psychosis, or substance abuse. Baseline severity of asthma: PEF (mean,SD): 386 (76)/388 (107); childhood onset asthma: 83%/67%; Rhinitis: 100%/75%; Sinusitis history: 58%/42%; History GERD: 42%/25% | |
| Interventions | Run in phase: 4 weeks usual ICS (BDP, triamcinolone, flunisolide) to assess stability then randomised if stable to either: 1: Salmeterol 100mcg bd , OR 2: Placebo PLUS usual ICS for two weeks then ICS reduction commenced. ICS reduction: ICS reduced by at least 10% (but not more than 25%) every 4 weeks if stable (PEF >=80%; FEV1>=80%; and rescue mediation use < 4 x day). ICS was not reduced below 500mcg/day for patients with mod/severe asthma. Delivery device: pMDI Duration of treatment: 12 months Other: ICS optimised (with use of spacer), patient education and crisis prednisolone provided with written instruction on its use (PEF < 50% personal best) at commencement of study. | |
| Outcomes | % ICS dose reduction, FEV1 % predicted, PEF, rescue medications, ER visits, hospitalisations, days off work, adverse event (tremor), QOL. | |
| Notes | Comparison 2: Reduced dose ICS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Plain MDI used without spacer for both placebo and salmeterol |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aziz 2000 | Crossover design |
| Bouchard 2000 | ICS not reduced |
| Buhl 2001 | ICS not reduced |
| Buhl 2003 | ICS not reduced |
| Creemers 2002 | ICS not reduced |
| Creticos 1999 | ICS not reduced |
| Dorinsky 2004 (b) | ICS not reduced |
| Dorinsky 2004 (c) | No previous ICS use |
| Feschenko 2003 | ICS not reduced |
| Hawkins 2003 | No LABA in active intervention group |
| Heuck 2000 | Crossover design |
| Hyland 1995 | ICS not reduced |
| Ind 2003 | ICS not reduced |
| Kavuru 2000 | ICS not reduced |
| Lazarus 2001 | No ICS in active intervention group |
| Lundback 2001 | ICS not reduced |
| Matz 2001 | ICS not reduced |
| McCarthy 2001 | ICS not reduced |
| McIvor 1998 | Crossover design |
| Miraglia del Giudice | ICS not reduced |
| Ortega‐Cisneros 1998 | ICS not reduced |
| Ozkaya 1999 | ICS not reduced |
| Pljaskic Kamenov | ICS not reduced |
| Price 2002 | On LABA prior to study entry |
| Rooklin 2001 | ICS not reduced |
| Shapiro 2000 | ICS not reduced |
| Stojkovic‐Andjelkovi | Initial ICS/LABA dose comparison |
| Tal 2002 | ICS not reduced |
| Wallin 2003 | ICS not reduced |
| Weinstein 2001 | ICS not reduced |
| Wilding 1997 | Crossover design |
| Zetterstrom 2001 | ICS not reduced |
Contributions of authors
Gibson PG ‐ conception, protocol design, inclusion/exclusion, quality assessment, data extraction, analysis and interpretation, writing and editing. Powell H ‐ protocol design, inclusion/exclusion, quality assessment, data extraction, analysis, interpretation and writing. Ducharme F ‐ conception, protocol design, study identification and editing.
Sources of support
Internal sources
No sources of support supplied
External sources
Cooperative Research Centre for Asthma, Australia.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Baranuik 1999 {published data only}
- Baraniuk J, Murray JJ, Nathan RA, Berger WE, Johnson M, Edwards LD, et al. Fluticasone alone or in combination with salmeterol vs triamcinolone in asthma. Chest 1999;116:625‐32. [DOI] [PubMed] [Google Scholar]
Bloom 2003 {published data only}
- Bloom J, Calhoun W, Koenig S, Yancey S, Reilly D, Edwards L, et al. Fluticasone propionate/salmeterol 100/50mcg is inhaled steroid sparing in patients who require fluticasone propionate 250mcg for asthma stability [abstract]. ATS 99th International Conference, SEATTLE. 2003:D034 Poster C33.
Busse 2003 {published data only}
- Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, Edwards LD. Steroid‐sparing effects of fluticasone propionate 100 microg and salmeterol 50 microg administered twice daily in a single product in patients previously controlled with fluticasone propionate 250 microg administered twice daily. Journal of Allergy & Clinical Immunology 2003;111:57‐65. [DOI] [PubMed] [Google Scholar]
- Sahn S, Yancey S, Reilly D, Edwards L, Rickard K, Dorinsky P. The fluticasone propionate/salmeterol (FSC) combination product 100/50 mcg bid is steroid‐sparing in patients who require FP 250 mcg bid for asthma stability [Abstract]. Chest: Conference, San Diego, California. 2002:P417.
Dorinsky 2004 {published data only}
- Dorinsky P, Yancey S, Reilly D, Stauffer J, Edwards L, Sutton L. Control of airway inflammation is maintained in asthma patients following a 60% reduction in ICS dose with fluticasone propionate/salmeterol (FSC) compared with higher dose fluticasone propionate (FP) alone [Abstract]. European Respiratory Journal 2004;24(Suppl 48):308s. [Google Scholar]
- Dorinsky PM, Yancey SW, Reilly D, Edwards L. The effectiveness of fluticasone propionate/salmeterol 100/50mcg diskus (R) (FSC) as an inhaled corticosteroid‐sparing agent: effect of baseline asthma severity [Abstract]. Chest 2003;124(4):87S‐b. [Google Scholar]
- Dorinsky PM, Yancey SW, Waitkus‐Edwards K, Edwards L. Clinical markers of worsening asthma with the fluticasone propionate/salmeterol 100/50 mcg diskus (R) (FSC) vs fluticasone propionate (FP) 250mcg alone in patients requiring FP 250mcg BID for asthma stability [Abstract]. Chest 2003;124(4):88S. [Google Scholar]
- Wilson SJ, Rigden HM, Jarjour N, Laviolette M, Moore WC, Dorinsky PM, et al. Effect of switching from high dose fluticasone propionate (FP) to FP/salmeterol (FSC) on airway inflammation in asthma [Abstract]. European Respiratory Society Annual Congress 2004;24(Suppl 48):44s. [Google Scholar]
Kips 2000 {published data only}
- Kips JC, O'Connor BJ, Inman MD, Svensson K, Pauwels RA, O'Byrne PM. A long‐term study of the antiinflammatory effect of low‐dose budesonide plus formoterol versus high‐dose budesonide in asthma. American Journal of Respiratory & Critical Care Medicine 2000;161(3 I):996‐1001. [DOI] [PubMed] [Google Scholar]
- Kips JC, O'Connor BJ, Svensson K, Pauwels RA, O'Byrne PM. Low dose budesonide plus formoterol versus high dose budesonide in asthma therapy: effects on markers of airway inflammation [Abstract]. European Respiratory Journal 1997;10(Suppl 25):1S. [DOI] [PubMed] [Google Scholar]
Lalloo 2001 {published data only}
- Lalloo UG, Malolepsky J, Kozma D, Krofta K, Ankerst J, Johansen B, Thompson NC. Budesonide and formoterol in a single inhaler controls exacerbations more effectively than a higher dose of inhaled corticosteroids alone, in mild‐moderate persistent asthma [Abstract]. European Respiratory Journal 2001;18(Suppl 33):43s. [Google Scholar]
- Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, Johansen B, Thompson NC. Budesonide and formoterol in a single inhaler is more effective than a higher dose of inhaled corticosteroid in mild‐moderate persistent asthma [Abstract]. European Respiratory Journal 2001;18(Suppl 33):159s. [Google Scholar]
Lemanske 2001 {published data only}
- Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285(20):2594‐603. [DOI] [PubMed] [Google Scholar]
Nielsen 1999 {published data only}
- Nielsen LP, Pedersen B, Faurschou P, Madsen F, Wilcke JT, Dahl R. Salmeterol reduces the need for inhaled corticosteroid in steroid‐dependent asthmatics. Respiratory Medicine 1999;93:863‐8. [DOI] [PubMed] [Google Scholar]
- Pedersen B, Faurschou P, Madsen F, Viskum K, Wilcke T, Dahl R. Salmeterol treatment reduces the need for inhaled steroids in steroid dependent asthmatics‐a randomised, double‐blind, placebo‐controlled parallel group study. European Respiratory Journal 1996;9(Suppl 23):50s‐1s. [Google Scholar]
Pauwels 1997 {published data only}
- Juniper EF, Svensson K, O'Byrne PM, Barnes PJ, Bauer CA, Lofdahl CG, et al. Asthma quality of life during 1 year of treatment with budesonide with or without formoterol. European Respiratory Journal 1999;14:1038‐43. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Lorfdahl C‐G, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. New England Journal of Medicine 1997;337:1405‐11. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations.. American Journal of Respiratory & Critical Care Medicine 1999;160:594‐9. [DOI] [PubMed] [Google Scholar]
Self 1998 {published data only}
- Self T, Rumbak MJ, Kelso T, Eberle L, Abou‐Shala N, Learned CC, et al. Does salmeterol facilitate 'step‐down' therapy in patients with asthma receiving moderate to high doses of inhaled corticosteroids?. Current Therapeutic Research 1998;59(11):803‐811. [Google Scholar]
References to studies excluded from this review
Aziz 2000 {published data only}
- Aziz I, Wilson AM, Lipworth BJ. Effects of once‐daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest 2000;118:891‐3. [DOI] [PubMed] [Google Scholar]
Bouchard 2000 {published data only}
- Bouchard J, Arkinstall W, Tesarowski D. Efficacy of salmeterol and fluticasone propionate (FP) combination therapy vs FP alone in mild/moderate asthma [Abstract]. American Journal of Respiratory & Critical Care Medicine 2000;161:A197. [Google Scholar]
Buhl 2001 {published data only}
- Buhl R, Zetterstrom O, Mellem H, Perpina M, Hedman J, O'Neill S, Peterson S. Improved asthma control with budesonide/formoterol via a single inhaler compared with budesonide alone, in moderate persistent asthma [Abstract]. European Respiratory Journal 2001;18:48s. [DOI] [PubMed] [Google Scholar]
Buhl 2003 {published data only}
- Buhl R, Creemers JPHM, Vondra V, Martelli NA, Naya IP, Ekstrom T. Once‐daily budesonide/formoterol in a single inhaler in adults with moderate persistent asthma. Respiratory Medicine 2003;97(4):323‐30. [DOI] [PubMed] [Google Scholar]
Creemers 2002 {published data only}
- Creemers JPHM, Bantje T, Eliraz A, Ekstrom T, Buhl R. Budesonide/formoterol in a single inhaler once or twice daily provides better asthma control than inhaled fluticasone or budesonide alone in patients with moderate persistent asthma [Abstract]. European Respiratory Journal 2002;20(Suppl 38):387s. [Google Scholar]
Creticos 1999 {published data only}
- Creticos PS, Freidhoff LR, Bernstein DI, Chu T, Khattignavong AP, Pasatiempo AM, Lim JC. Comparison of an inhaled corticosteroid (triamcinolone acetonide) to a long‐acting bronchodilator (salmeterol), the combination, and placebo in mild‐moderate adult asthmatic patients. International Archives of Allergy & Immunology 1999;1181(2‐4):345‐6. [DOI] [PubMed] [Google Scholar]
Dorinsky 2004 (b) {published data only}
- Dorinsky P, Stauffer J, Waitkus‐Edwards K, Yancey S, Prillaman BA, Sutton L. "Stepping down" from fluticasone propionate/salmeterol 100/50mcg diskus results in loss of asthma control [Abstract]. European Respiratory Journal 2004;24(Suppl 48):309s. [Google Scholar]
Dorinsky 2004 (c) {published data only}
- Dorinsky P, Kerwin E, Schoaf L, Ellsworth A, House K. Effectiveness and safety of fluticasone propionate/salmeterol 250/50 mcg administered once daily to patients with persistent asthma. European Respiratory Journal 2004;Suppl 48(309s):Glasgow, Scotland. [Google Scholar]
Feschenko 2003 {published data only}
- Feschenko YI, Yashyna LA, Polyanska MA, Gymenuk GL, Sidun GC, Klochko LT. Improvement of asthma control with the use of combined medicine salmeterol + fluticasone (two‐in‐one) [Abstract]. Allergy & Clinical Immunolgy International 2003;1(Suppl):P‐2‐16. [Google Scholar]
Hawkins 2003 {published data only}
- Hawkins G, McMahon A, Twaddle, S, Wood S, Ford I, Thompson N. Stepping down inhaled corticosteroids in asthma: randomised controlled trial. BMJ 2003;326:1115‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See S, Rubin S. Tapering inhaled steroids effective for chronic asthma. Journal of Family Practice 2003;52(10):748‐51. [PubMed] [Google Scholar]
Heuck 2000 {published data only}
- Heuck C, Heickendorff L, Wolthers OD. A randomised controlled trial of short term growth and collagen turnover in asthmatics treated with inhaled formoterol and budesonide. Archives of Diseases in Childhood 2000;83:334‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyland 1995 {published data only}
- Hyland ME, Crocker GR. Validation of an asthma quality of life diary in a clinical trial. Thorax 1995;50:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ind 2003 {published data only}
- Ind PW, Dal Negro R, Colman NC, Fletcher CP, Browning D, James MH. Addition of salmeterol to fluticasone propionate treatment in moderate‐to‐severe asthma. Respiratory Medicine 2003;97:555‐62. [DOI] [PubMed] [Google Scholar]
- Ind PW, Dal Negro R, Fletcher CP, Browning DC, James MH. Title Inhaled salmeterol and fluticasone propionate therapy in moderate adult asthma [Abstract]. European Respiratory Journal 1997;10:1S. [Google Scholar]
Kavuru 2000 {published data only}
- Braunstein G. Optimization of asthma control with fluticasone/salmeterol (seretide) combination: new clinical data. Revue de Pneumologie Clinique 2002;58(3 pt 2):1S12‐5. [PubMed] [Google Scholar]
- Kavuru M, Melamed J, Gross G, Laforce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6 Pt 1):1108‐16. [DOI] [PubMed] [Google Scholar]
- Nathan RA, Dorinsky P, Carranza Rosenweig JR, Shah T, Edin H, Prillaman B. Improved ability to perform strenuous activities after treatment with fluticasone propionate/salmeterol combination in patients with asthma. Journal of Asthma 2003;40(7):815‐22. [DOI] [PubMed] [Google Scholar]
Lazarus 2001 {published data only}
- Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long‐acting beta2‐agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;20:2583‐93. [DOI] [PubMed] [Google Scholar]
Lundback 2001 {published data only}
- Lundback B, Ronmark E, Jonsson AC, Larsson LG, Petavy F, James MH. Airway hyperresponsiveness and treatment effectiveness during a one year study of the combination of salmeterol and fluticasone propionate (FP) compared with FP and salmeterol alone in mild to moderate asthma [Abstract]. European Respiratory Journal. 2002; Vol. 20, issue Suppl 38:386s.
- Lundbäck B, Rönmark E, Jonsson AC, Lindberg A, Larsson LG, James MH. Treatment effectiveness and exacerbations during one year with seretide compared to fluticasone propionate and salmeterol in mild to moderate asthma [Abstract]. European Respiratory Journal 2001;18(Suppl 33):176s. [Google Scholar]
Matz 2001 {published data only}
- Matz J, Emmett A, Rickard K, Kalberg C. Addition of salmeterol to low‐dose fluticasone versus higher‐dose fluticasone: An analysis of asthma exacerbations. Journal of Allergy & Clinical Immunology 2001;107:783‐9. [DOI] [PubMed] [Google Scholar]
McCarthy 2001 {published data only}
- McCarthy TP, Edin HM, House K, Vandermeer AK. Quality of life and asthma control assessment in patients previously on inhaled corticosteroids (ICS) treated with salmeterol/fluticasone combination (SFC) metered dose inhaler (MDI) [Abstract]. Thorax 2001;56(Suppl 3):iii64. [Google Scholar]
- McCarthy TP, Edin HM, House K, Yan SK, Vandermeer AK. The effects of salmeterol/fluticasone combination (SFC) dry powder inhaler (DPI) on asthma control and quality of life in patients previously treated with inhaled corticosteroids (ICS).. Thorax 2001;56(Suppl 3):iii63. [Google Scholar]
McIvor 1998 {published data only}
- Mcivor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158:924‐30. [DOI] [PubMed] [Google Scholar]
- Pizzichini E, McIvor RA, Turner MO, Hussack P, Hargreave FE, Sears MR. Masking effect of salmeterol in exacerbations of asthma induced by steroid reduction. A randomized controlled trial [Abstract]. European Respiratory Journal 1997;10:1S. [Google Scholar]
Miraglia del Giudice {published data only}
- Miraglia del Giudice M, Capristo M, Amelio R, Rocco A, Fusco N, Brunese FP. Combined fluticasone propionate/salmeterol (diskus) vs fluticasone propionate (Diskus) in the control of persistent asthma in children [Abstract]. ATS 99th International Conference, SEATTLE 2003:A117 Poster D78. [Google Scholar]
Ortega‐Cisneros 1998 {published data only}
- Ortega‐Cisneros M, Maldonado‐Alaniz ML, RosasVargas MA, Sienra‐Monge JJL. Salmeterol and inhaled beclomethasone versus high dose inhaled beclomethasone in the control of pediatric patients with moderate asthma [Abstract]. Annals of Allergy Asthma & Immunology 1998;80:131. [Google Scholar]
Ozkaya 1999 {published data only}
- Ozkaya O, Turktas I, Cengizlier R. Low dose budesonide plus formoterol versus high dose budesonide in children with bronchial asthma [Abstract]. European Respiratory Society; 1999 Annual Congress; Oct 9‐13; Madrid, Spain P365. [Google Scholar]
Pljaskic Kamenov {published data only}
- Pljaskic Kamenov SS, Filipovic MD, Kamenov BA. Comparison of addition of salmeterol xinafoate to budesonide with budesonide alone on symptoms and quality of life in asthmatic children [Abstract]. European Respiratory Journal 2000;16(Suppl 31):518s. [Google Scholar]
Price 2002 {published data only}
- Price D, Dutchman D, Mawson A, Bodalia B, Duggan S, Todd P. Early asthma control and maintenance with eformoterol following reduction of inhaled corticosteroid dose. Thorax 2002;57(9):791‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rooklin 2001 {published data only}
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The Fluticasone Propionate/Salmeterol HFA MDI is significantly more efficacious in treating asthma that placebo HFA MDI, Fluticasone Propionate CFC MDI or Salmeterol CFC MDI [Abstract]. Journal of Allergy & Clinical Immunology 2001;107(2):S100. [Google Scholar]
Shapiro 2000 {published data only}
- Shapiro G, Lumry W, Wolfe J, Given J, White MV, Woodring A, et al. Combined salmeterol 50mcg and fluticasone propionate 250 mcg in the diskus device for the treatment of asthma. American Journal of Respiratory & Critical Care Medicine 2000;161(2 Pt 1):527‐34. [DOI] [PubMed] [Google Scholar]
Stojkovic‐Andjelkovi {published data only}
- Stojkovic‐Andjelkovic AK, Pajovic DM, Protrka OJ, Ugrinovic BS, Obradovic SM, Pavicevic MD. Effect of combination fluticasone propionate and salmeterol diskhaler in treatment of moderate to severe asthma: comparison of initial high dose, constant medium dose and placebo [Abstract]. European Respiratory Journal 2001;18(Suppl 33):123s. [Google Scholar]
Tal 2002 {published data only}
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, Boeck K. Budesonide/Formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatric Pulmonology 2002;34(5):342‐50. [DOI] [PubMed] [Google Scholar]
Wallin 2003 {published data only}
- Wallin A, Sue‐Chu M, Bjermer L, Ward J, Sandstrom T, Lindberg A, et al. Effect of inhaled fluticasone with and without salmeterol on airway inflammation in asthma. Journal of Allergy & Clinical Immunology 2003;112:72‐8. [DOI] [PubMed] [Google Scholar]
- Wison SJ, Ward JA, Djukanovic R, Wallin A, Sue‐Chu M, Sandstrom T, Bjermer L. Effects of high and low dose inhaled fluticasone propionate (FP) compared to low dose FP combined with salmeterol (SAL) on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1997;161(3 Suppl):A196. [Google Scholar]
Weinstein 2001 {published data only}
- Weinstein SF, Pearlman DS, Condemi JJ, Herrle MR, Scott CA, Payne JE, et al. Superior efficacy of the Fluticasone Propionate/Salmeterol (88/42MCG) HFA‐MDI combination product versus the individual components in asthmatics previously treated with either short or long‐acting Beta2‐agonist or inhaled corticosteroids [Abstract]. Journal of Allergy & Clinical Immunology 2001;107(2):S102. [Google Scholar]
Wilding 1997 {published data only}
- Wilding P, Clark M, Thompson Coon J, Lewis S, Rushton L, Bennett J, et al. Effect of long‐term treatment with salmeterol on asthma control: a double blind, randomised crossover study. BMJ 1997;314:1441‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zetterstrom 2001 {published data only}
- Zetterstrom O, Buhl R, Mellem H, Perpina M, Hedman J, O'Neill S, Ekstrom T. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. European Respiratory Journal 2001;18:262‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Barnes 2002
- Barnes PJ. Scientific rationale for inhaled combination therapy with long‐acting beta2‐agonists and corticosteroids. European Respiratory Journal 2002;19:182‐91. [DOI] [PubMed] [Google Scholar]
Ducharme 2004
- Ducharme F. Personal communication 2004.
Greenstone 2004
- Greenstone IR, Ducharme FM. Addition of inhaled long‐acting beta2‐agonists to inhaled corticosteroids in chronic asthma in children and adults (Protocol). The Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI] [PubMed] [Google Scholar]
Hawkins 2003
- Hawkins G, McMahon AD, Twaddle S, Wood SF, Ford I, Thomson NC. Stepping down inhaled corticosteroids in asthma: randomised controlled trial. BMJ 2003;326:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
McIvor 1998
- McIvor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158:924‐30. [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, Ducharme FM. Combination of inhaled corticosteroids and long‐acting beta‐agonists versus a similar dose of inhaled corticosteroids alone in children and adults with chronic asthma. The Cochrane Database of Systematic Review [pending publication] 2005. [DOI] [PubMed]
Powell 2003
- Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence‐based approach. Medical Journal of Australia 2003;178:223‐5. [DOI] [PubMed] [Google Scholar]


