Abstract
Background
This is an updated version of the original Cochrane review published in Issue 4, 2006. Patients may control postoperative pain by self administration of intravenous opioids using devices designed for this purpose (patient controlled analgesia or PCA). A 1992 meta‐analysis by Ballantyne et al found a strong patient preference for PCA over non‐patient controlled analgesia, but disclosed no differences in analgesic consumption or length of postoperative hospital stay. Although Ballantyne's meta‐analysis found that PCA did have a small but statistically significant benefit upon pain intensity, a 2001 review by Walder et al did not find statistically significant differences in pain intensity or pain relief between PCA and groups treated with non‐patient controlled analgesia.
Objectives
To evaluate the efficacy and safety of patient controlled intravenous opioid analgesia (termed PCA in this review) versus non‐patient controlled opioid analgesia of as‐needed opioid analgesia for postoperative pain relief.
Search methods
We ran the search for the previous review in November 2004. For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 12), MEDLINE (1966 to 28 January 2015), and EMBASE (1980 to 28 January 2015) for randomized controlled trials (RCTs) in any language, and reference lists of reviews and retrieved articles.
Selection criteria
We selected RCTs that assessed pain intensity as a primary or secondary outcome. These studies compared PCA without a continuous background infusion with non‐patient controlled opioid analgesic regimens. We excluded studies that explicitly stated they involved patients with chronic pain.
Data collection and analysis
Two review authors independently extracted data, which included demographic variables, type of surgery, interventions, efficacy, and adverse events. We graded each included study for methodological quality by assessing risk of bias and employed the GRADE approach to assess the overall quality of the evidence. We performed meta‐analysis of outcomes that included pain intensity assessed by a 0 to 100 visual analog scale (VAS), opioid consumption, patient satisfaction, length of stay, and adverse events.
Main results
Forty‐nine studies with 1725 participants receiving PCA and 1687 participants assigned to a control group met the inclusion criteria. The original review included 55 studies with 2023 patients receiving PCA and 1838 patients assigned to a control group. There were fewer included studies in our updated review due to the revised exclusion criteria. For the primary outcome, participants receiving PCA had lower VAS pain intensity scores versus non‐patient controlled analgesia over most time intervals, e.g., scores over 0 to 24 hours were nine points lower (95% confidence interval (CI) ‐13 to ‐5, moderate quality evidence) and over 0 to 48 hours were 10 points lower (95% CI ‐12 to ‐7, low quality evidence). Among the secondary outcomes, participants were more satisfied with PCA (81% versus 61%, P value = 0.002) and consumed higher amounts of opioids than controls (0 to 24 hours, 7 mg more of intravenous morphine equivalents, 95% CI 1 mg to 13 mg). Those receiving PCA had a higher incidence of pruritus (15% versus 8%, P value = 0.01) but had a similar incidence of other adverse events. There was no difference in the length of hospital stay.
Authors' conclusions
Since the last version of this review, we have found new studies providing additional information. We reanalyzed the data but the results did not substantially alter any of our previously published conclusions. This review provides moderate to low quality evidence that PCA is an efficacious alternative to non‐patient controlled systemic analgesia for postoperative pain control.
Plain language summary
Patient controlled opioid analgesia versus non‐patient controlled opioid analgesia for controlling postoperative pain
Patients may control pain after surgery by self administration of analgesics (pain killers) using devices designed for this purpose (patient controlled analgesia or PCA). PCA involves self administration (by pushing a button) of small doses of opioids (such as morphine) intravenously by means of a programmable pump. Previous studies have shown that often patients prefer PCA to traditional methods of pain management, such as a nurse administering an analgesic upon a patient's request. This review demonstrated moderate to low quality evidence that PCA provided slightly better pain control and increased patient satisfaction when compared with non‐patient controlled methods. Patients tended to use slightly higher doses of medication with PCA and suffered a higher occurrence of itching, but otherwise side effects were similar between groups.
Summary of findings
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 4, 2006) on 'Patient controlled opioid analgesia versus conventional opioid analgesia for controlling postoperative pain'. The title has been amended slightly for this update to reflect changes in terminology for 'conventional analgesia' to 'non‐patient controlled analgesia'.
Description of the condition
Pain after surgery is common. Many postoperative analgesic regimens rely upon a patient to self administer analgesics. For example, a patient may be given a prescription for tablets and instructed to take one every few hours as needed. The development in the late 1960s of devices (Evans 1976; Keeri‐Szanto 1971; Harmer 1985; Schezer 1968; Schug 2000) for the precise intravenous (or, on occasion, subcutaneous) delivery of bolus (single) doses of opioids upon the demand of the patient, with provision of regulation by their healthcare provider, led to coinage of the term 'patient controlled analgesia' (PCA).
Description of the intervention
PCA is now routinely used in postoperative care throughout much of the developed world (Carr 1998; Warfield 1995). PCA devices are programmable by the healthcare provider to deliver a specific amount of medication upon each request by the patient. A continuous 'background' infusion may be co‐administered in addition to patient controlled bolus doses. Bolus doses are limited by a programmed 'lockout interval' within which subsequent requests are ignored or a cumulative limit to drug dose permitted in a fixed interval, such as one or more hours (Ferrante 1990). PCA may be applied via intravenous, subcutaneous, transdermal, epidural or intrathecal routes (Crews 2000; Viscusi 2004), and other routes (for example, pulmonary or nasal) have also been investigated (Christensen 2008).
How the intervention might work
Commonly, PCA devices are applied to deliver intravenous opioids after operations, although PCA has also been used following trauma or to treat cancer pain (Lehmann 1999), and to deliver non‐opioids such as non‐steroidal anti‐inflammatory drugs (Cepeda 1995) or local anesthetics (Cepeda 1996; DeKock 1994). Opioids provide analgesia by binding to opioid receptors of the mu and kappa class, blocking the release of neurotransmitters such as substance P and enhancing descending inhibitory pain pathways. Opioid receptors are expressed both centrally and peripherally during the inflammatory response in injured tissue.
Why it is important to do this review
PCA is a widely applied modality although its costs (particularly in comparison to those of non‐patient controlled intramuscular analgesics) are not fully determined (Jacox 1997). A previous systematic review, Ballantyne 1993, found pain control during PCA to be superior to non‐patient controlled postoperative analgesia. However, the magnitude of the difference (6 mm on a zero to 100 mm visual analog scale (VAS)) was small. A later systematic review, Walder 2001, did not find differences in pain intensity or pain relief between PCA and non‐patient controlled treatment, although patients expressed a preference for PCA. Those findings suggest that the patient preference for PCA over non‐patient controlled analgesia described in both reviews reflects factors other than analgesia per se, such as increased autonomy (Ferrante 1989; Kiecolt‐Glaser 1998). The present review examines randomized controlled trials (RCTs) of patient controlled intravenous analgesia versus non‐patient controlled postoperative opioid analgesia to treat postoperative pain. The updated review was undertaken in order to re‐examine the previous review by applying advances in Cochrane methodology and to incorporate evidence from newly published studies. The update was also prompted by evidence, published after our original review, raising safety concerns (particularly risk of respiratory depression) in patients using PCA (Overdyk 2007).
Objectives
To evaluate the efficacy and safety of patient controlled intravenous opioid analgesia (termed PCA in this review) versus non‐patient controlled regimens of as‐needed opioid analgesia for postoperative pain relief.
Methods
Criteria for considering studies for this review
Types of studies
For the updated review we excluded studies with fewer than 10 participants per arm (Moore 1998), and abstracts that were more than three years old.
We included RCTs in this review if they compared the efficacy of opioid PCA versus non‐patient controlled (conventional) opioid regimens. We included studies with pain intensity as the primary or secondary outcome. We excluded non‐randomized studies and case reports as well as retrieved trials that presented insufficient data to allow assessment of outcomes of interest or study quality.
Types of participants
For the updated review, we performed sensitivity analysis with studies of pediatric participants removed.
We set no age limits (but see above) for patient inclusion except to require that the participant (and not a surrogate such as a parent or nurse) operated the PCA and reported pain intensity. Thus, participants in the enrolled studies had to have the cognitive ability to understand the concept of PCA and to report pain intensity on a standardized scale. We excluded trials in which participants received an initial period of analgesia other than PCA postoperatively (for example, those sedated and ventilated for one to two days after surgery). However, we included studies in which nurses administered analgesia immediately after surgery in order to stabilize the patient. We also excluded trials that explicitly stated they enrolled patients with chronic pain or who were receiving chronic opioid therapy, if data from such participants were not separable from those of participants without preoperative chronic pain or opioid therapy.
Types of interventions
We compared intermittent doses of opioids self administered to participants via PCA pumps to non‐patient controlled administration of opioids. For the updated review, because of the evolution of postoperative analgesic regimens since our original review, we have more clearly defined our interpretation of 'conventional' analgesia. We included studies where an opioid was administered as needed, but not if opioid administration was scheduled, i.e., around the clock. We considered regimens where an opioid was administered by any of the following routes to be conventional (non‐patient controlled): intravenous (bolus or intermittent infusion), intramuscular, subcutaneous, oral, and rectal. We performed a sensitivity analysis where only parenteral routes were analyzed. As non‐opioids, i.e., non‐steroidal anti‐inflammatory drugs (NSAIDs) and acetaminophen, are now routinely a component of postoperative multimodal analgesic regimens, we revised our previous exclusion criteria for studies administering such drugs to exclude only those studies where an non‐opioid was physically added to the PCA solution, where only one group received a non‐opioid, or where both groups received non‐opioids, but the specific non‐opioid, dose or schedule differed between groups.
The opioids included in this review were limited to morphine and other full mu opioid agonists (a drug that binds to and activates an opioid receptor) such as hydromorphone, meperidine (synonymous with pethidine), codeine, fentanyl, piritramide, and ketobemidone. We excluded trials in which PCA was used to administer opioids whose actions are pharmacologically distinct from those of morphine or that display a plateau dose response (for example, partial mu opioid agonists such as buprenorphine, or mixed kappa opioid agonist and mu opioid antagonist compounds such as butorphanol). We excluded studies in which non‐opioids were co‐administered during opioid PCA (except as stated above) because the opioid‐sparing effect of non‐opioids might decrease the generalizability of study results by decreasing opioid requirements or pain intensity, or both, in participants in the trial (Souter 1995). We excluded studies in which continuous (background) intravenous opioid infusion was provided in the PCA group from this review. Trials frequently rely on nurses to administer non‐patient controlled analgesics, but the lack of information on this aspect of a trial was not an exclusion criterion.
Types of outcome measures
For the updated review, we added the following outcomes to reflect advances in Cochrane methodology: serious adverse events; withdrawals due to adverse events; and withdrawals due to lack of efficacy. We also added the safety outcome 'respiratory depression', as evidence published since our original review suggests that this may be more common and serious than previously thought (Overdyk 2007). The existing outcome, 'length of stay', we renamed 'length of stay: time to readiness for discharge' as time to actual discharge may be affected by non‐clinical factors; however all included studies reported the former outcome only.
Primary outcomes
The primary outcome was pain intensity assessed via a visual analog scale (VAS). Pain intensity data assessed by means other than a zero to 100 VAS were normalized to such a scale. To do so, we either multiplied the original scale employed by an appropriate factor (for example, by 10 if the original scale ranged from zero to 10) or by assigning values on a zero to 100 scale that corresponded to choices on the original assessment scale. For example, if a participant was offered a five‐point scale, selection of the second point was scored as 50 on a zero to 100 scale (0 = no pain, 1 = 25, 2 = 50, 3 = 75, 4 = 100).
Secondary outcomes
We extracted data on the following secondary outcomes:
Opioid consumption (type and amount of opioid used, converted to intravenous morphine equivalents)
Patient satisfaction
Length of hospital stay: time to readiness for discharge
Serious adverse events
Incidence and severity of individual adverse events: sedation; nausea and vomiting; pruritus; respiratory depression; and urinary retention
Withdrawals due to adverse events and due to lack of efficacy
Search methods for identification of studies
This search was run for the original review in November 2004 and subsequent searches were run on 28 January 2015. In addition, we included a search of the US National Institutes of Health website ClinicalTrials.gov in the updated review.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 12)
MEDLINE (OVID) (1966 to 28 January 2015)
EMBASE (OVID) (1980 to 28 January 2015)
We applied no language restrictions.
We combined search terms for RCTs with terms for patient controlled analgesia and terms for postoperative pain. Our updated search strategies can be found in Appendix 1, Appendix 2, and Appendix 3.
Searching other resources
We identified additional reports from the reference lists of retrieved papers. Lastly, we searched the US National Institutes of Health website ClinicalTrials.gov (Appendix 4).
Data collection and analysis
We made several changes to our methods based on updated Cochrane standards, such as assessing risk of bias and incorporating GRADE, dealing with unit of analysis issues and missing data, and assessing heterogeneity, as detailed below.
Selection of studies
We determined eligibility by reading the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy our inclusion criteria, and obtained full copies of the remaining studies. Two review authors read these studies independently and reached agreement by discussion. The studies were not anonymized in any way before assessment.
Data extraction and management
Two review authors duplicate extracted and agreed on data, using an adaptation of a standard Cochrane form, before entry into Review Manager (RevMan) 5.3 (RevMan 2014). In the event of a disagreement, a third review author was asked to adjudicate. Data extracted included the following.
Age and sex of participants.
Number of participants enrolled and completing the study.
Type of operation.
Pain intensity for all time points at which it was measured.
PCA settings (bolus dose, lockout, limit dose).
Non‐patient controlled (conventional) treatment (control) regimen (dose, route, frequency).
Total opioid consumption expressed as mg of intravenous morphine sulfate or equivalent, where equivalents were calculated using equianalgesic conversion tables for commonly used opioids (APS 2008). We converted less commonly employed opioids, not routinely included in equianalgesic conversion tables, as follows: for ketobemidone a 1:1 conversion was used (Micromedex 2014); papaveretum was considered 0.85 times as strong as morphine (an approximation based on inconsistency of proportion of constituents) (Micromedex 2014); and for piritramide, 15 mg was considered equivalent to 10 mg of morphine (Micromedex 2014).
Patient satisfaction (preference for PCA versus non‐patient controlled (conventional) analgesic regimen).
Length of hospital stay (readiness for discharge).
Severity or incidence of adverse events.
Assessment of risk of bias in included studies
In our original review, we graded included studies for methodological quality using the Oxford Quality Scale (Jadad 1996). In this updated review, we also used the 'Risk of bias' tool for both the original included studies and those included from the updated search. Two review authors independently assessed the risk of bias of all included studies. The review authors made critical assessments for each of the following domains: sequence generation (randomization), allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other risks of bias (e.g., small sample sizes). For sample size, we considered studies to be at low risk of bias if they had 200 participants or more, at unknown risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants (Derry 2013). We entered the review author judgment for each domain into a 'Risk of bias' table, with answers 'low risk', 'high risk' or 'unclear risk' (indicating either lack of information or uncertainty over the potential for bias).
We employed the GRADE approach to assess the overall quality of evidence (GRADE 2004; Langendam 2013). We used the GRADE profiler (GRADEpro) to import data from Review Manager 5.3 to create 'Summary of findings' tables (Table 1; Table 2; Table 3). For each chosen comparison, these tables provide information concerning the overall quality of evidence from studies included in each outcome, and pooled estimates of the magnitude of effect of each intervention and differences between these interventions (PCA and non‐patient controlled analgesia). We included the following outcomes in the 'Summary of findings' tables.
Summary of findings 1. VAS pain scores (0 to 100): PCA versus non‐patient controlled opioid analgesia for postoperative pain.
| VAS pain scores (0 to 100): PCA versus non‐patient controlled opioid analgesia for postoperative pain | ||||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: patient‐controlled analgesia (PCA) Comparison: conventional opioid analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐patient controlled opioid analgesia | Patient‐controlled analgesia (PCA) | |||||
| Pain scores 0 to 24 h Visual analog scale Scale from: 0 to 100 | The mean pain scores 0 to 24 h ranged across control groups from 16 to 47 | The mean pain scores 0 to 24 h in the intervention groups was 8.82 lower (13.09 to 4.54 lower) | 1516 (23 studies) | ⊕⊕⊕⊝ moderate1 | When sub‐analyzed by type of surgery, considerable heterogeneity exists between and within surgeries | |
| Pain scores 25 to 48 h Visual analog scale Scale from: 0 to 100 | The mean pain scores 25 to 48 h ranged across control groups from 16 to 37 | The mean pain scores 25 to 48 h in the intervention groups was 8.82 lower (14.15 to 3.49 lower) | 609 (13 studies) | ⊕⊕⊝⊝ low1,2 | When sub‐analyzed by type of surgery, considerable heterogeneity exists between and within surgeries | |
| Pain scores 49 to 72 h Visual analog scale Scale from: 0 to 100 | The mean pain scores 49 to 72 h ranged across control groups from 20 to 38 | The mean pain scores 49 to 72 h in the intervention groups was 12.11 lower (26.04 lower to 1.83 higher) | 231 (3 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | Insufficient data for sub‐analysis by type of surgery | |
| Pain scores 0 to 48 h Visual analog scale Scale from: 0 to 100 | The mean pain scores 0 to 48 h ranged across control groups from 21 to 46 | The mean pain scores 0 to 48 h in the intervention groups was 9.74 lower (12.49 to 6.99 lower) | 372 (7 studies) | ⊕⊕⊝⊝ low1,3 | Insufficient data for sub‐analysis by type of surgery | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; h: hour | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1All studies unblinded. 2Unexplained heterogeneity. 3Total population size < 400. 495% confidence interval includes no effect.
Summary of findings 2. Consumption of intravenous morphine equivalents (mg): PCA versus non‐patient controlled opioid analgesia for postoperative pain.
| Consumption of intravenous morphine equivalents (mg): PCA versus non‐patient controlled opioid analgesia for postoperative pain | ||||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: patient‐controlled analgesia (PCA) Comparison: conventional opioid analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐patient controlled opioid analgesia | Patient‐controlled analgesia (PCA) | |||||
| Consumption of morphine equivalents 0 to 24 h mg | The mean consumption of morphine equivalents 0 to 24 h ranged across control groups from 12 mg to 88 mg | The mean consumption of morphine equivalents 0 to 24 h in the intervention groups was 7.21 higher (1.44 to 12.98 higher) | 1586 (25 studies) | ⊕⊕⊝⊝ low1,2 | When sub‐analyzed by type of surgery, considerable heterogeneity exists between and within surgeries | |
| Consumption of morphine equivalents 25 to 48 h mg | The mean consumption of morphine equivalents 25 to 48 h ranged across control groups from 12 mg to 53 mg | The mean consumption of morphine equivalents 25 to 48 h in the intervention groups was 5.37 higher (2.82 to 7.92 higher) | 449 (9 studies) | ⊕⊕⊕⊝ moderate1 | Insufficient data for sub‐analysis by type of surgery | |
| Consumption of morphine equivalents 0 to 48 h mg | The mean consumption of morphine equivalents 0 to 48 h ranged across control groups from 16 mg to 185 mg | The mean consumption of morphine equivalents 0 to 48 h in the intervention groups was 17.5 higher (4.75 lower to 39.75 higher) | 334 (8 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | Insufficient data for sub‐analysis by type of surgery | |
| Consumption of morphine equivalents 0 to 72 h mg | The mean consumption of morphine equivalents 0 to 72 h ranged across control groups from 51 mg to 65 mg | The mean consumption of morphine equivalents 0 to 72 h in the intervention groups was 21.06 higher (5.18 to 36.94 higher) | 244 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | Insufficient data for sub‐analysis by type of surgery | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; h: hour | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1All studies unblinded. 2Unexplained heterogeneity. 3Total population size < 400. 495% confidence interval includes no effect.
Summary of findings 3. Patient satisfaction: PCA versus non‐patient controlled opioid analgesia for postoperative pain.
| Patient satisfaction: PCA versus non‐patient controlled opioid analgesia for postoperative pain | ||||||
| Patient or population: patients with postoperative pain Settings: hospital Intervention: patient‐controlled analgesia (PCA) Comparison: conventional opioid analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐patient controlled opioid analgesia | Patient‐controlled analgesia (PCA) | |||||
| Satisfaction on a continuous scale Measured with different scales in the different studies. Higher scores mean greater satisfaction | The mean satisfaction on a continuous scale in the intervention groups was 0.55 standard deviations higher (0.13 to 0.97 higher) | 427 (7 studies) | ⊕⊕⊝⊝ low1,2 | SMD 0.55 (95% CI 0.13 to 0.97). A standard effect size of 0.55 represents a moderate difference between groups | ||
| Number of patients in arm satisfied with therapy | 61 per 100 | 80 per 100 (68 to 93) | See comment | 547 (11 studies) | ⊕⊕⊝⊝ low1,2 | Risks were calculated from pooled risk differences. RR 1.32 (95% CI 1.12 to 1.53) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardized mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1All studies unblinded. 2Unexplained heterogeneity.
VAS pain scores
Opioid consumption (morphine equivalents)
Patient satisfaction
Where there was disagreement between review authors (EM, MF), we achieved consensus by discussion or by the involvement of the third review author (JH).
Measures of treatment effect
Dichotomous data
We used discrete events, such as preference for PCA versus non‐patient controlled analgesic regimens, or the number of participants with adverse events, to calculate the risk difference, risk ratio, or both (we calculated odds ratios in our original review, but these are more difficult to interpret (Sackett 1996)) using Review Manager 5.3 software. When a statistically significant risk difference existed between interventions, we derived the number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH) (Cook 1995). Additionally, dichotomous outcomes are presented in terms of both raw numbers and percentages of participants in each study arm benefiting from therapy or suffering adverse events.
Continuous data
We undertook meta‐analyses when comparable data were available from continuous outcomes, such as pain intensity, analgesic consumption in mg of morphine equivalents, or intensity of a specific adverse event, using mean differences (MD). Where we were unable to convert scales to a common unit of measurement we used standard mean differences (SMD).
Unit of analysis issues
In our original review, we split the control treatment arm between active treatment arms in a single study if the active treatment arms were not combined for analysis. In the updated review this was not necessary for any of the studies.
Dealing with missing data
We did not contact authors for original data unless data were missing or unclear. If, despite attempts to contact study authors, participant data were missing, we based analyses on participant populations in which outcomes were reported. Discrepancies between the number of participants enrolled and the number of participants in whom outcomes were reported are noted in the Characteristics of included studies tables. Where studies reported statistics based on intention‐to‐treat (ITT) or modified ITT populations, we performed available case analyses. The ITT population consisted of participants who were randomized, received the assigned intervention, and provided at least one post‐baseline assessment.
Assessment of heterogeneity
We evaluated heterogeneity between and within trials using both the Chi² test and the I² statistic. The Chi² test assesses whether observed differences in results are compatible with chance alone. A low P value (or a large Chi² statistic relative to its degrees of freedom) provides evidence of heterogeneity of treatment effects (variation in effect estimates beyond chance). The Chi² test has low power in estimating heterogeneity in the common situation where few trials are analyzed or where included trials have small sample sizes. Although a statistically significant result may indicate a problem with heterogeneity, a non‐significant result is not necessarily evidence of lack of heterogeneity. Methods developed for quantifying inconsistency across studies that move the focus away from testing whether heterogeneity is present to assessing its impact on the meta‐analysis include the I² statistic. I² = [(Q ‐ df)/Q] x 100%, where Q is the Chi² statistic and df is its degrees of freedom (Deeks 2011; Higgins 2003). The I² statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). A value greater than 50% may be considered substantial heterogeneity (Deeks 2011). We also assessed heterogeneity by visually studying forest plots.
Assessment of reporting biases
We made no attempt to assess reporting bias. We attempted to mitigate the potential for publication bias by searching the website ClinicalTrials.gov.
Data synthesis
We employed the random‐effects model by DerSimonian and Laird (Deeks 2011), using Review Manager 5.3 (RevMan 2014), to combine outcomes data related to pain intensity and opioid consumption across trials at comparable time points (for example, average pain score per 24‐hour interval).
Subgroup analysis and investigation of heterogeneity
Where possible we performed subgroup analysis based on type of surgery (upper or lower abdominal, cardiothoracic, orthopedic, neurosurgical, mixed/other).
Sensitivity analysis
In our original review we performed sensitivity analyses by eliminating:
cross‐over studies;
inadequately randomized studies.
For our updated review, we performed additional sensitivity analyses by eliminating:
studies with pediatric participants (Berde 1991);
studies where the control intervention was not administered parenterally (Precious 1997).
Results
Description of studies
Results of the search
The 2004 literature search yielded 3462 citations (2043 from MEDLINE; 845 from CENTRAL; and 574 from EMBASE), of which 52 papers, incorporating 56 studies, met the criteria for inclusion in the original review. Given changes to Cochrane methodology and our amendment of the inclusion and exclusion criteria, we excluded 13 studies originally included in our 2004 review in the 2015 update. We excluded three due to their enrolling fewer than 10 participants per arm (Hecker 1988a; Hecker 1988b; Walson 1992); we excluded seven because participants in the control group received a scheduled opioid regimen (Boulanger 1993; Ceriati 2003; Choiniere 1998; Ferrante 1988; Kleiman 1988; Martinez‐Ubieto 1992; Paoletti 1993 (gyn)); we excluded one because participants in the non‐patient controlled group also received acetaminophen (Sanansilp 1995); and we excluded two because they were published only as abstracts and were more than three years old (Coyle 1990; Taylor 1994). The literature search covering 2004 to 2015 yielded an additional 4450 citations (1262 from MEDLINE; 1191 from CENTRAL; and 1997 from EMBASE) of which we selected seven for inclusion (Figure 1) (Boulanger 2002; Crisp 2012; Egbert 1990; Hu 2006; Morad 2009; Morad 2012; Sudheer 2007). We found no completed or ongoing studies on ClinicalTrials.gov, other than those already included from our database search.
1.
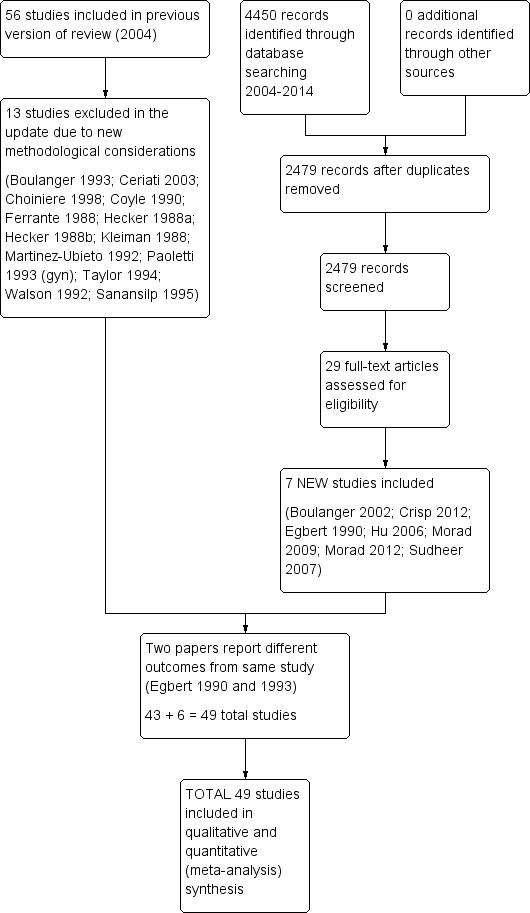
Study flow diagram.
Included studies
Forty‐eight papers met the inclusion criteria. Two papers reported demographics and outcomes for different operations separately (Chan 1995 (chole); Chan 1995 (laminectomy); Ellis 1982 (chole); Ellis 1982 (hysterectomy)). These two papers were analyzed as comprising two different studies in each paper. Conversely, two papers reported different outcomes from the same study (Egbert 1990; Egbert 1993). As a result, the 48 papers produced 49 studies eligible for analysis.
In the 49 included studies, 1725 patients were randomly allocated to PCA groups and 1687 patients to control groups. All analyzed studies were conducted in inpatient settings. In aggregate, the studies spanned all ages (children, adolescents, elderly) with the youngest participant being seven years old. One study, Berde 1991, exclusively evaluated children and adolescents aged seven to 19 years. For the updated review, we conducted a sensitivity analysis in which this study was excluded. Seven of the 49 studies enrolled more than 100 patients. The largest study involved 328 patients (PCA: n = 143; control: n = 185) (Jackson 1989). Twenty‐four studies included fewer than 50 patients. The smallest study consisted of 20 patients, i.e., the minimum number for inclusion in our review (Bhise 1997). Nine studies did not present data describing the numbers of males and females. Of the remaining studies, 24 studies enrolled males and females, one study only included males, and 15 studies included only females. In the studies that reported outcomes in both sexes, females outnumbered males, mostly because of the large number of studies evaluating lower abdominal gynecologic procedures (15 studies). Patients underwent various operations; the most common surgeries were abdominal procedures (29 studies) followed by cardiothoracic surgeries (nine studies).
In the control groups, analgesia was administered intramuscularly (34 studies), subcutaneously (three studies), as intravenous boluses (six studies), as intravenous infusions with and without intravenous boluses (four studies), as combined intravenous and intramuscular injections (one trial), and with combined oral and intramuscular administration (one trial). We performed a sensitivity analysis for the one trial that evaluated a non‐parenteral route of administration (Precious 1997). While not all papers described who administered opioid in the control groups, in those that did, a nurse was responsible for its delivery. Forty studies compared the same analgesic in both groups: morphine (29), meperidine (five), fentanyl (two), hydromorphone (one), piritramide (one), ketobemidone (one), and papaveretum (one). Nine studies compared two different opioids: meperidine PCA versus morphine control (two studies); morphine PCA versus codeine (two); morphine or meperidine PCA versus meperidine (two); morphine PCA versus morphine or codeine (one); morphine PCA versus meperidine (one); and meperidine or morphine PCA versus morphine (one).
The most frequently used opioid in the PCA arms was morphine (33 studies). In these 33 studies the most commonly administered dose of morphine was 1 mg (16 studies) (range: 0.5 mg to 2.5 mg). The most frequent lockout intervals were 10 minutes (11 studies) and five to six minutes (18 studies) (range: 5 minutes to 20 minutes). In the majority of studies there was no reported dose limit (33 studies).
We were not able to include data from every study in all of our meta‐analyses. Some studies did not assess or report all outcomes of interest (for example, Bedder 1991 assessed morphine consumption, VAS, and some adverse effects but did not examine patient satisfaction and length of stay). In some of the papers the data were incomplete (for example, missing standard deviations (SD)) and so could not be used for statistical analysis. Other data could not be used because they were not clearly defined or were presented in an idiosyncratic manner (for example, in Harrison 1988 analgesia was assessed according to the percentages of patients reporting mild, moderate, or severe pain).
Excluded studies
In total, we excluded 58 papers from the original and updated review because they did not meet the inclusion criteria. The numbers below add up to more than 58 due to some studies failing to meet multiple criteria; see the Characteristics of excluded studies table). We excluded four because they enrolled fewer than 10 participants per arm (Atwell 1984; Hecker 1988a; Hecker 1988b; Walson 1992); we excluded 10 because the control group received a scheduled opioid regimen (Boulanger 1993; Ceriati 2003; Choiniere 1998; Dieterich 2012; Ferrante 1988; Gursoy 2006; Kleiman 1988; Martinez‐Ubieto 1992; Rothwell 2011; Vengadesh 2005). We excluded four because they were published only as abstracts and were more than three years old (Coyle 1990; Halilotlu 2010; Jabri 2010; Taylor 1994). From the original review, we excluded Paoletti 1993 (ortho) (orthopedic study) based on our updated definition of a conventional regimen (the control arm utilized continuous infusion). A continuous background infusion was administered in the PCA group in 19 studies (Bayar 2008; Bell 2007; D'haese 1998; Davis 2006; Duggleby 1992; Eremenko 2011; Gao 2007; Khalili 2013; Kilbride 1992; Knudsen 1993; Nitschke 1996; Peters 1999; Rundshagen 1999; Sanansilp 1995; Searle 1994; Tsang 1999; Weldon 1993; Zacharias 1990). Opioids other than pure mu agonists were used in six studies. In three of these six studies buprenorphine (a partial agonist) was used in either control or both groups (Gaitini 1996; Lange 1988; Lee 2013); in another two studies nalbuphine (a mixed agonist‐antagonist) was evaluated (Shin 2001; Woods 1991); and in another study the PCA solution contained the tranquilizer droperidol (Liu 2005). NSAIDs (ketorolac or indomethacin) and acetaminophen were added to opioids or used as the sole analgesic in four studies (Gust 1999; Moreno 2000; Searle 1994; Shin 2001). Tramadol, which is not considered a conventional mu opioid, was used in two studies (Forst 1999; Jellinek 1990). Comparison of two different PCA regimens instead of PCA and non‐patient controlled analgesia was performed in five studies (Robinson 1991; Viscusi 2004; Weldon 1993; Woodhouse 1997; Xiao 2011). In two studies, the PCA and non‐patient controlled groups received different non‐opioid regimens (Cho 2011; Lee 2010). Two studies evaluated outcomes other than those considered in the present review: plasma catecholamines, blood cortisol and glucose levels (Moller 1988), and cost (Rittenhouse 1999). One study assessed patients with both acute and chronic pain but did not report results separately for each group (White 1998). In one paper the control group was from a retrospective chart review (Spetzler 1987), and lastly two studies were not randomized (Knapp‐Spooner 1995; Yost 2004).
We included Crisp 2012 and Dahl 1987 in the updated analysis despite their having semi‐scheduled regimens. In Crisp 2012, participants had the option to decline dosing at the specified interval. Dahl 1987 had scheduled intramuscular morphine plus an option for intravenous as needed.
Risk of bias in included studies
Our original review used the Oxford Quality Scale to assess the quality of each included study. Each report was scored independently for quality by two of the review authors using a three‐item scale (Jadad 1996). The review authors then met to agree a 'consensus' score for each report. The quality scores for individual studies are reported in the notes section of the Characteristics of included studies table. These scores were not used to weight the results in any way. The maximum possible score on the Oxford scale (indicating a trial of high methodological quality) is five. None of the studies comparing PCA with non‐patient controlled analgesia was double‐blinded, therefore we could not assign any points based upon blinding. Therefore, the highest possible score for included studies was three. The median quality score of the included studies was two.
In the updated review we supplemented the Jadad scale with the 'Risk of bias' tool, applying it both to new studies and to those from the original review. Summaries of the 'Risk of bias' assessments can be found in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Note: Empty cells denote study where risk of bias was judged for each subgroup (Chan 1995) or for original study only (Egbert 1990)
Allocation
Seventeen studies adequately described methods for randomization and we assigned them a low risk of bias for sequence generation. Adequate description of randomization included details in relation to use of computer‐generated randomization, use of a random numbers table, shuffled sealed envelopes, or coin toss. The majority of studies (41) presented an unclear risk of selection bias due to lack of reporting of methods of allocation concealment. Three studies were at high risk of bias due to a lack of randomized allocation based on PCA availability (Ellis 1982 (hysterectomy)), or because no attempt was made to conceal allocation (Myles 1994; Perez‐Woods 1991).
Blinding
Participants in all studies were unblinded to the mode of analgesia, creating a high risk of performance and detection bias. In many studies, although blinding was not explicitly mentioned, descriptions of interventions led us to conclude that they were unblinded. Given the different modalities being evaluated, PCA versus non‐patient controlled analgesia, it is not unexpected that the studies included in this review would be open‐label. Four studies included in the review mentioned some blinding of healthcare providers: one study stated part of the study was blinded but participants were aware of treatment (Boulanger 2002); one study blinded research assistants and ward staff (Chang 2004); one study stated that anesthesia teams were blinded (Morad 2012); and in another study pain was assessed by blinded nurses but patients and anesthesiologists were unblinded (Sudheer 2007).
Incomplete outcome data
We assessed 32 studies as having a low risk of attrition bias. Many studies accounted for all participants randomized in the study. We classified three studies as high risk in this category based on the following characteristics: high numbers of participants were unaccounted for (Bedder 1991); more than 10% did not complete the study (Boulanger 2002); and prescriber authority to change the medication and/or participation in the study (Snell 1997). We classified the remaining 14 studies as unclear risk for various reasons including: no mention of how missing data were handled (Bennett 1982; Chan 1995 (chole); Chan 1995 (laminectomy); Colwell 1995; Harrison 1988; Jackson 1989; Myles 1994), a lack of a statement that all participants completed the study or lack of clarity regarding the number of participants that withdrew (Bhise 1997; Chan 1995 (chole); Chan 1995 (laminectomy); Chang 2004; Colwell 1995; Harrison 1988; Jackson 1989; Kenady 1992; McGrath 1989; Munro 1998; Myles 1994), and no mention of how participants switching regimens after postoperative day one from PCA to codeine were imputed (Precious 1997).
Selective reporting
A total of 34 studies had a low risk of reporting bias due to consistency in outcomes described in the Methods and reported in the Results. Only five studies had a high risk of bias in this category. Reasons for high risk were a lack of reporting of data for all specified outcomes (Bennett 1982; Jackson 1989; Sudheer 2007), a lack of adverse event reporting (Bennett 1982), and/or not reporting mean data with measure of variation (Gillman 1995; Jackson 1989; Paoletti 1993 (gyn)). We designated the remaining 10 studies as an unclear risk of bias for various reasons including incomplete reporting of secondary outcomes (see 'Risk of bias' tables located with the Characteristics of included studies).
Other potential sources of bias
Sample size was an issue for most studies in the analysis. We classified only three studies out of 49 as having a low risk of sample size bias (Brewington 1989; Jackson 1989; Murphy 1994). We classified 24 studies as high risk due to very small sample size and we classified the remaining 22 studies as unclear risk. In addition, we categorized Snell 1997 as high risk of other bias because of the fact that there was bias towards who would benefit from PCA over non‐patient controlled treatment and this was left to the physician's discretion.
Effects of interventions
See: Table 1; Table 2; Table 3
'Summary of findings' tables are presented for the following outcomes: visual analog scale (VAS) pain scores, opioid consumption, and patient satisfaction. Quality of evidence is reported with these results based on GRADE criteria. We classified no studies in any 'Summary of findings' tables as high quality, based on the lack of blinding. For this reason, moderate quality evidence is the highest level presented in the 'Summary of findings' tables. Low quality evidence was established as meeting two criteria for low quality, including lack of blinding and one of the following: unexplained heterogeneity, total population size below 400, and if the 95% confidence interval (CI) included no effect. Very low quality evidence was established as meeting all of the previously listed criteria.
Pain intensity
See Table 1: VAS pain scores (0 to 100): patient controlled analgesia (PCA) versus control for postoperative pain.
Quality of analgesia was assessed by asking participants to report their pain intensity using a VAS. Different investigators recorded this outcome on different scales and at different intervals. We normalized all VAS to a zero to 100 range. The majority of authors reported average results over the following intervals: zero to 24 hours, 25 to 48 hours, 49 to 72 hours, and zero to 48 hours. One trial, Bedder 1991, reported the average VAS over 36 hours and we included it in the zero to 48 hours analysis. Data were generally reported as the average pain intensity of multiple observations over any given time period; however, in studies in which the only data available were single measurements at the end of a time period (for example, 24 hours) we used this measurement.
Pain intensity over the first 24 hours was reported in 23 studies, which involved 1516 participants with 780 in the PCA group and 736 in the control group (moderate quality of evidence according to the GRADE criteria). Participants in the PCA group reported a mean difference (MD) in pain intensity nine points lower than in the control group (95% CI ‐13 to ‐5) (Analysis 1.1, Figure 4). Average pain intensity in the postoperative 25 to 48 hours was described in 13 studies (609 participants, 321 with PCA and 288 controls; low quality of evidence according to the GRADE criteria). Meta‐analysis favored the PCA group: participants in the PCA group had lower pain scores than their counterparts (MD ‐9, 95% CI ‐14 to ‐3) (Analysis 1.2). Three studies (231 participants, 117 participants with PCA and 114 controls) analyzed pain intensity in the interval from 49 to 72 hours (very low quality of evidence according to the GRADE criteria). Our analysis again favored the PCA group, although the results were not statistically significant (P value = 0.09, Analysis 1.3). Seven studies examined pain scores over the zero to 48 hours interval (372 participants, 206 with PCA and 166 controls; low quality of evidence according to the GRADE criteria). Participants in the PCA group rated their pain 10 points less than those given conventional therapy (95% CI ‐12 to ‐7) (Analysis 1.4). Only one study including 83 participants, Egbert 1990, reported results of pain intensity in the zero to 72‐hour interval and demonstrated an eight‐point difference between PCA and control (95% CI ‐15 to ‐1, Analysis 1.5).
1.1. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 1: Pain scores 0 to 24 h
4.

Forest plot of comparison: 1 VAS pain scores (0 to 100): PCA versus control, outcome: 1.1 Pain scores 0 to 24 h.
1.2. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 2: Pain scores 25 to 48 h
1.3. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 3: Pain scores 49 to 72 h
1.4. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 4: Pain scores 0 to 48 h
1.5. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 5: Pain scores 0 to 72 h
We subanalyzed pain intensity according to the type of surgery. We were able to create five subgroups for analysis of the zero to 24‐hour postoperative interval: lower abdominal surgery (seven studies), upper abdominal (six studies), cardiothoracic (four studies), neurosurgery (three studies), and mixed/other (three studies). Results of pain intensity statistically favored PCA over controls for lower abdominal and neurosurgery, but were not statistically significant for upper abdominal, cardiothoracic, and mixed/other surgery types (Analysis 1.1; Figure 4). There was evidence of substantial heterogeneity within many of the subanalyses, based on inspection of forest plots and I2 values greater than 50%.
For the 25 to 48‐hour postoperative interval, we evaluated five subgroups according to type of surgery: lower abdominal (three studies), upper abdominal (four studies), cardiothoracic (three studies), neurosurgery (one trial), and mixed/other (two studies). In the subcategories of lower and upper abdominal surgery and neurosurgery there were no statistical differences between groups. In the subcategories of cardiothoracic and mixed/other surgeries, meta‐analysis statistically favored PCA (MD ‐19, 95% CI –26 to –13; and MD ‐12, 95% CI –18 to –6, respectively).
We performed no subanalyses for the postoperative intervals 49 to 72 hours, zero to 48 hours or zero to 72 hours due to the small number of studies according to each type of surgery. Similarly, there were not enough studies to generate a meaningful subanalysis according to orthopedic surgery for any postoperative interval.
We performed sensitivity analysis based on removing studies that we considered to be inadequately randomized (see 'Risk of bias' tables located with the Characteristics of included studies). For this reason, we removed four studies from any meta‐analysis of pain intensity in which they had been included (Bollish 1985; Ellis 1982 (hysterectomy); Perez‐Woods 1991; Thomas 1995. We removed two studies, Ellis 1982 (hysterectomy) and Thomas 1995, from the lower abdominal subgroup of the zero to 24‐hour postoperative pain score analysis (Analysis 1.6); we removed one trial, Ellis 1982 (hysterectomy), from the lower abdominal subgroup of the 25 to 48‐hour postoperative pain score analysis (Analysis 1.7); and we removed two studies, Bollish 1985 and Perez‐Woods 1991, from the zero to 48‐hour postoperative analysis (Analysis 1.8). In all of these analyses, best point estimates were similar and statistical significance was unchanged when compared to our original analyses.
1.6. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 6: Pain scores 0 to 24 h minus inadequately randomized trials
1.7. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 7: Pain scores 25 to 48 h minus inadequately randomized trials
1.8. Analysis.

Comparison 1: VAS pain scores (0 to 100): PCA versus control, Outcome 8: Pain scores 0 to 48 h minus inadequately randomized trials
One of the studies included in the analyses employed a cross‐over design (Bollish 1985). The Cochrane Handbook for Systematic Reviews of Interventions suggests three approaches towards incorporating cross‐over studies into a meta‐analysis (Deeks 2011). One approach involves calculating a correlation coefficient to describe how similar the measurements on interventions A and B were within a participant. The study by Bollish did not provide sufficient information to calculate this coefficient. A second approach involves including data from only the first period; however, these data were not reported separately. A third approach is to simply treat results as if they were from a parallel trial. We used this approach for calculating differences between the two groups in pain scores at zero to 48 hours and opioid consumption at zero to 24 hours. All three approaches carry the potential for bias. For this reason, and again as suggested by the Cochrane Handbook for Systematic Reviews of Interventions, we performed a sensitivity analysis with this study removed from relevant comparisons, but given the low sample size of the study, it had no effect on the overall best point estimate or statistical significance of the differences found.
Lastly, a sensitivity analysis with removal of the one study which utilized a non‐parenteral conventional regimen, Precious 1997, resulted in no difference in effect size or statistical significance of the quality of analgesia.
Opioid consumption
See Table 2: Consumption of intravenous morphine equivalents: PCA versus control for postoperative pain.
Opioid consumption was analyzed in 33 studies. The total number of participants in those studies was 1586, with 803 participants in a PCA group and 783 participants in a control group (low quality of evidence according to the GRADE criteria). Different authors reported opioid consumption across different intervals. The most frequently reported results were over the first 24 hours (25 studies); nine studies continued to report results over the next 24 hours (25 to 48 hours post‐operation); and eight studies reported opioid consumption from zero to 48 hours. Four studies described opioid consumption over the first 72 hours. Some investigators reported opioid consumption during more than one interval.
The first analysis, for opioid consumption in the zero to 24‐hour postoperative interval, showed a statistically significantly higher consumption of morphine equivalents in the PCA group (MD 7 mg, 95% CI 1 to 13) (Analysis 2.1; Figure 5). In the interval from 25 to 48 hours, opioid consumption was also statistically higher with PCA (MD 5 mg, 95% CI 3 to 8); moderate quality of evidence according to the GRADE criteria (Analysis 2.2).
2.1. Analysis.

Comparison 2: Opioid consumption: PCA versus control, Outcome 1: Consumption of morphine equivalents 0 to 24 h
5.

Forest plot of comparison: 2 Opioid consumption: PCA versus control, outcome: 2.1 Consumption of morphine equivalents 0 to 24 h.
2.2. Analysis.

Comparison 2: Opioid consumption: PCA versus control, Outcome 2: Consumption of morphine equivalents 25 to 48 h
At the time interval zero to 48 hours there were no statistically significant differences and there was wide variation in cumulative opioid consumption between the PCA and control groups (MD 18 mg, 95% CI ‐ 5 to 40) (Analysis 2.3). Evaluation of cumulative opioid consumption over 72 hours (zero to 72 hours) showed a statistically significant difference in consumption of opioids between groups (MD 21 mg, 95% CI 5 to 37; very low quality of evidence according to the GRADE criteria (Analysis 2.4)).
2.3. Analysis.

Comparison 2: Opioid consumption: PCA versus control, Outcome 3: Consumption of morphine equivalents 0 to 48 h
2.4. Analysis.

Comparison 2: Opioid consumption: PCA versus control, Outcome 4: Consumption of morphine equivalents 0 to 72 h
In a similar manner to our analysis of the quality of analgesia results, we explored subcategories based on type of surgery and performed a sensitivity analysis based upon eliminating inadequately randomized studies.
We performed subgroup meta‐analysis for the following types of surgery to analyse opioid consumption over the first 24 hours: lower abdominal (504 participants, 259 with PCA and 245 controls), upper abdominal (252 participants, 125 with PCA and 127 controls), cardiothoracic (334, 171 with PCA and 163 controls), neurosurgery (213 participants, 105 with PCA and 108 controls), and mixed/other surgery types (283 participants, 143 with PCA and 140 controls). In all subcategories except neurosurgery, opioid consumption was not statistically different between the PCA and the control group. In the neurosurgery analysis, opioid consumption was statistically significantly higher with PCA versus control (MD 20 mg, 95% CI 4 to 35) (Analysis 2.1; Figure 5). There was evidence of substantial heterogeneity in many of the subanalyses, based on inspection of forest plots and I2 values greater than 50%.
Exclusion of three inadequately randomized studies, Bollish 1985, Ellis 1982 (hysterectomy) and Thomas 1995, from opioid consumption meta‐analyses at the postoperative time intervals zero to 24 hours did not alter the significance, and only slightly altered the magnitude of the lower opioid consumption in the control group (Analysis 2.5).
2.5. Analysis.

Comparison 2: Opioid consumption: PCA versus control, Outcome 5: Consumption of morphine equivalents 0 to 24 h minus inadequately randomized trials
As with analyses of pain scores, we performed a sensitivity analysis with removal of the Bollish 1985 cross‐over study from relevant comparisons, but it had no effect on the size or statistical significance of the differences found. The sensitivity analysis that excluded Precious 1997 due to use of a non‐parenteral route of administration led to a statistically significant change in opioid consumption from zero to 48 hours (MD 23 mg, 95% CI 2 to 45).
Patient satisfaction
See Table 3: Patient satisfaction: PCA versus control for postoperative pain.
Patient satisfaction results were presented as either continuous or dichotomous data, i.e., on a scale (usually zero to 10, where 10 is the most satisfied) or as the number of participants in a study arm satisfied with therapy.
Seven studies were available for analysis of satisfaction on a scale (427 participants, 233 with PCA and 194 controls; low quality of evidence according to the GRADE criteria). We reported standardized mean difference as we were unable to normalize the data to a 0 to 100 range. The mean satisfaction in the PCA groups was 0.55 standard deviations higher versus control (95% CI 0.13 to 0.97) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Patient satisfaction: PCA versus control, Outcome 1: Satisfaction on a continuous scale
The incidence of patient satisfaction was determined in 11 studies with a total of 547 participants (272 with PCA and 275 in control groups; low quality of evidence according to the GRADE criteria). More participants in the PCA groups were satisfied with their mode of analgesia (81% versus 61%; risk difference (RD) 0.20, 95% CI 0.07 to 0.32) (Analysis 3.2; Figure 6). We calculated the corresponding number needed to treat for an additional beneficial outcome (NNTB) as 5 (95% CI 4 to 15).
3.2. Analysis.

Comparison 3: Patient satisfaction: PCA versus control, Outcome 2: Number of participants in arm satisfied with therapy
6.

Forest plot of comparison: 3 Patient satisfaction: PCA versus control, outcome: 3.2 Number of participants in arm satisfied with therapy.
We were not able to perform subanalyses according to type of surgery or sensitivity analyses by removal of inadequately randomized studies, due to an insufficient number of studies reporting data. A sensitivity analysis with removal of the one study which utilized a non‐parenteral conventional regimen, Precious 1997, resulted in no difference in effect size or statistical significance related to patient satisfaction.
Length of hospital stay: time to readiness for discharge
In the updated review, we amended this outcome to 'readiness for discharge' but since no studies reported results in this manner, we simply analyzed length of stay, as with the original review.
In the updated analysis, 14 studies reported differences in length of stay between participants using PCA and those in the control groups; however, not all reported data in a manner compatible with meta‐analyses (e.g., stating that length of stay was similar between groups without reporting actual data). The 10 remaining studies that were suitable for meta‐analysis (550 participants, 302 with PCA and 248 controls) demonstrated a slight but non‐statistically significant reduction in length of stay in those participants using PCA (MD ‐0.18, 95% CI ‐0.63 to 0.26) (Analysis 4.1). Again, there were an insufficient number of studies to perform subanalyses based on type of surgery.
4.1. Analysis.

Comparison 4: Length of stay: time to readiness for discharge, Outcome 1: Number of days: PCA versus control
We performed sensitivity analysis with removal of inadequately randomized studies (Thomas 1995), which changed neither the direction of effect estimate nor the statistical significance of the original analysis (Analysis 4.2).
4.2. Analysis.

Comparison 4: Length of stay: time to readiness for discharge, Outcome 2: Number of days: PCA versus control minus inadequately randomized trials
Adverse events
For the purposes of the updated analysis, an adverse event was defined as any undesirable experience associated with the use of a medical product in a patient. The most frequently reported adverse events were sedation, nausea and vomiting, pruritus, and urinary retention. Most studies did not specify the timing of adverse events. In the updated review, we added serious adverse events, withdrawals (due to lack of efficacy or adverse events), and respiratory depression to the safety analyses. For respiratory depression, we also performed subanalyses based on type of surgery.
Serious adverse events
Nineteen studies reported data related to serious adverse events (1284 participants, 632 with PCA and 652 controls). No statistically significant difference was noted in serious adverse events between PCA and control groups. A total of 10 (1.6%) serious adverse events were reported in the PCA group from four studies (one death from Boulanger 2002; four wound infections from Kyzer 1995; one report of atelectasis from Rogers 1990; four severe adhesions from Rosen 1998). Seven (1.1%) serious adverse events were reported in the control group from three studies (one wound infection from Kyzer 1995; three from Myles 1994 (two deaths and one cerebrovascular event); and three severe adhesions from Rosen 1998) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Serious adverse events, Outcome 1: Number of participants with serious adverse event
Incidence and severity of individual adverse events
Sedation
Twenty‐seven studies evaluated sedation, but not all studies provided data suitable for meta‐analysis. Results were presented either on a continuous scale (usually zero to 10, where 10 is the most sedated) or as the number of participants in a study arm that experienced sedation.
Data suitable for analysis were reported in 20 studies (1323 participants). Ten studies (514 participants, 270 with PCA and 244 in controls) evaluated sedation by means of a scale. Where described, severity was predominately nurse evaluated. In one study, Berde 1991, severity was reported by both the participant and a nurse. Different scales were used (zero to 100, zero to 10, one to five, and a four‐point scale). We reported standardized mean difference (SMD) as we were unable to normalize the data to a 0 to 100 range. Meta‐analysis demonstrated that participants in the PCA group reported a non‐statistically significant reduction in the degree of sedation (SMD ‐0.4, 95% CI ‐1.1 to 0.2) (Analysis 8.1).
8.1. Analysis.

Comparison 8: Sedation, Outcome 1: Sedation on a continuous scale
Ten studies (809 participants, 403 with PCA and 406 in control groups) expressed sedation as the number of participants reporting sedation. Incidence was similar between groups, with 15% of participants in the PCA group versus 16% of those in the control group reporting sedation (RD ‐0.01, 95% CI ‐0.03 to 0.02) (Analysis 8.2).
8.2. Analysis.

Comparison 8: Sedation, Outcome 2: Number of participants in arm reporting sedation
Nausea or vomiting, or both
Nausea and vomiting were evaluated in 25 studies (1652 participants).
Three studies (127 participants, 67 with PCA and 60 in control groups) assessed severity of nausea and vomiting using a scale (all utilized a zero to 10‐point scale). Meta‐analysis yielded no clinical or statistical evidence of a difference in severity of nausea between PCA and control groups (Analysis 9.1). As noted above, because of the inclusion of pediatric participants in one study (Berde 1991), we performed sensitivity analysis where the study was removed from analysis. Exclusion of this study resulted in a statistically significant reduction in severity of nausea and vomiting in the PCA group (RD ‐1.3, 95% CI ‐2.3 to ‐0.3), but this was based on only two studies (Ellis 1982 (chole); Ellis 1982 (hysterectomy)).
9.1. Analysis.

Comparison 9: Nausea and vomiting, Outcome 1: Nausea and vomiting on a 0 to 10 scale (10 = most severe)
Twenty‐two studies (1525 participants, 766 with PCA and 759 in control groups) expressed numbers of participants in each group reporting nausea or vomiting, or both. Dichotomous data again demonstrated no statistically significant difference between groups (30% versus 32%; RD 0, 95% CI ‐0.06 to 0.06) (Analysis 9.2).
9.2. Analysis.

Comparison 9: Nausea and vomiting, Outcome 2: Number of participants reporting nausea or vomiting, or both
Pruritus
The incidence of pruritus was evaluated in 10 studies (544 participants, 272 with PCA and 272 in control groups). All studies used the same opioid in each arm. Meta‐analysis yielded a non‐statistically significant RD of 0.05 (95% CI ‐0.02 to 0.12), but a statistically significant risk ratio (RR) of 1.8 (95% CI 1.1 to 2.8), where more participants complained of pruritus in the PCA groups (15%) than in the control groups (8%) (Analysis 10.1).
10.1. Analysis.

Comparison 10: Pruritus, Outcome 1: Number of participants reporting pruritus
Respiratory depression
Respiratory depression was a safety outcome added to the updated analysis. In studies that did not specifically define respiratory depression we evaluated the outcome as oxygen desaturations to 90% and below, respiratory rate less than 10/min, and/or if naloxone was required.
Twenty‐nine studies reported data for respiratory depression (1914 participants, 947 with PCA and 967 in control groups). Meta‐analysis noted no meaningful evidence of an effect between PCA and controls in regards to occurrence of respiratory depression. In the PCA group, 22 participants (2.3%) experienced respiratory depression versus 19 in the control groups (2%) (RD 0, 95% CI ‐0.01 to 0.01). Almost half of all reports of respiratory depression were reported from cardiothoracic surgeries (12 events with PCA versus eight events in control groups) (Analysis 11.1).
11.1. Analysis.

Comparison 11: Respiratory depression, Outcome 1: Number of participants affected
Meta‐analyses revealed no statistically significant evidence of an effect in respiratory depression in any subgroup by type of surgery (Analysis 11.1). Orthopedic surgeries reported no events of respiratory depression in either the PCA or control groups.
Urinary retention
The incidence of urinary retention was reported in six studies (467 participants, 239 with PCA and 228 in control groups). There was no clinical or statistical difference in the incidence of urinary retention between groups (23% versus 25%; RD ‐0.04, 95% CI ‐0.11 to 0.03) (Analysis 12.1).
12.1. Analysis.

Comparison 12: Urinary retention, Outcome 1: Number of participants reporting urinary retention
Withdrawals due to adverse events or lack of efficacy
Eighteen studies reported on withdrawals due to adverse events (1281 participants, 650 with PCA and 631 controls). No statistically significant difference was noted in withdrawals due to adverse events between PCA and control groups. A total of 16 withdrawals (2.5%) due to adverse events were reported from seven studies in the PCA group (Boulanger 2002; Brewington 1989; Gillman 1995; Morad 2009; Morad 2012; Paoletti 1993 (gyn); Smythe 1994). Twelve withdrawals (1.9%) due to adverse events were reported in the control group from three studies (Boulanger 2002; Brewington 1989; Smythe 1994) (Analysis 6.1).
6.1. Analysis.

Comparison 6: Withdrawals due to adverse events, Outcome 1: Number of participants withdrawing
Withdrawals due to lack of efficacy were reported for 18 studies (1347 participants, 681 with PCA and 666 controls). No statistically significant difference was noted in withdrawals due to lack of efficacy between PCA and control groups. Four participants receiving PCA withdrew (0.6%) from two studies (Brewington 1989; Morad 2012) versus seven participants (1%) in the control groups from three studies (Morad 2009; Morad 2012; Sudheer 2007) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Withdrawals due to lack of efficacy, Outcome 1: Number of participants withdrawing
Discussion
Summary of main results
Data from six additional studies were available for this updated review, four of which contributed data to the primary outcome. Conversely, 13 studies that met the inclusion criteria in our original review were excluded from our updated review, eight of which had contributed data to our primary outcome.
Primary outcome: pain intensity scores
The results of our meta‐analyses demonstrate that patient controlled analgesia (PCA) provided better pain control than non‐patient controlled analgesia. Pain intensity on a visual analog scale (VAS) was statistically significantly lower in participants using PCA versus those receiving non‐patient controlled analgesia at all time intervals, with the exception of the small meta‐analysis of results reported over 49 to 72 hours, which showed a trend towards lower scores. Addition of data from the updated search and elimination of data from previously included studies (due to the updated exclusion criteria) had little effect on results at any time point. For example, for the 0 to 24‐hour time period, 23 studies contributed data to the updated review and demonstrated an approximately nine‐point reduction in pain on a 0 to 100 scale, whereas our original review demonstrated an approximately eight‐point overall reduction from 27 studies.
PCA may have varying effectiveness depending on the extent of invasiveness of the surgery after which it is administered. For the updated review we created six subgroups according to type of surgery (versus only two in our original review). There were sufficient data for subgroup analysis by type of surgery for the time periods 0 to 24 hours and 25 to 48 hours. Reductions in pain differed by surgery type, but were also inconsistent across time periods, making conclusions about variations in effectiveness challenging.
Secondary outcomes
Opioid consumption
Opioid consumption was higher in participants using PCA than those administered non‐patient controlled analgesia, although results between studies displayed substantial heterogeneity over the intervals 0 to 24 hours and 0 to 48 hours. This difference was statistically significant over the postoperative intervals of 0 to 24 hours, 25 to 48 hours, and 0 to 72 hours: 7 mg, 5 mg and 21 mg of morphine equivalents, respectively. Again, the updated meta‐analyses demonstrated similar results to those in our original review. The clinical significance of this small difference is questionable. A single intravenous or intramuscular dose of morphine for moderate‐to‐severe pain in a healthy adult may be 5 mg to 10 mg (often given incrementally). Taking into consideration its elimination half‐life (1.7 hours to 3.3 hours (Stoelting 1999)) and duration of effect (three to four hours after either intravenous or intramuscular dosing (Fee 1996)), the daily dose could conceivably reach 80 mg to 120 mg. Thus, an increased consumption of morphine by less than 8 mg/24 hours does not seem important clinically. On the other hand, in the included PCA trials the average morphine equivalent consumption during the first 24 hours was about 45 mg in the PCA group, so 8 mg/24 hours would represent about 20% of this dose.
The conversion of doses of opioids other than morphine to morphine equivalents may have affected our results, especially where different opioids were used in comparator arms within a study (Ellis 1982 (chole); Ellis 1982 (hysterectomy); Kyzer 1995; Precious 1997; Stoneham 1996). Some studies reported amounts of both the particular opioid used and the conversion to morphine equivalents. Most stated the conversion factor used (Eisenach 1988; Kyzer 1995; Precious 1997; Stoneham 1996). In those trials that did not convert to morphine equivalents (Boldt 1998; Crisp 2012; Ellis 1982 (chole); Ellis 1982 (hysterectomy); Morad 2012; Murphy 1994; Pettersson 2000; Rayburn 1988; Thomas 1995; Wang 1991), we used standard conversion factors (APS 2008; Micromedex 2014).
There were sufficient data for subanalysis by type of surgery for only the 0 to 24‐hour interval. Only analysis of participants undergoing neurosurgery demonstrated a statistically significant increase in opioid consumption, perhaps as a reflection of the unusually high doses consumed in both groups. Conventionally, low doses of opioids are employed in many neurosurgeries because of beliefs that requirements are low and concerns over clouding diagnosis or worsening the patient's condition (Stoneham 1995). Abdominal (upper and lower) and cardiothoracic surgeries showed a non‐statistically significant trend towards higher opioid consumption in the PCA groups, but the magnitude of this difference was small, ranging from 4 mg to 10 mg of morphine equivalents. In cardiothoracic surgical patients this negative finding might be explained by the residual effect of large intraoperative opioid doses into the postoperative period.
Our results could also have been affected by the fact that the opioid administration regimens in the respective arms varied considerably between studies. However, in studies in which flexibility in dosing regimens was permitted, this was generally equally distributed among groups and we do not think that it contributed to bias in our results. Lastly, the observed disparity in opioid consumption may be related to factors like nurse availability or nurse assessment of the need for pain medication rather than true difference in analgesic requirements.
Patient satisfaction
Although many studies investigated patient satisfaction, several did not supply extractable data. We analyzed 17 trials (939 participants). In common with our original review, none of the studies in our updated review demonstrated that participants were more satisfied with conventional treatment. Meta‐analysis of both the degree of satisfaction and the number of participants satisfied with therapy statistically significantly favored participants in the PCA group, again in agreement with our 2006 analysis. Most of the studies did not indicate why participants reported satisfaction with a given therapy. It is not surprising to find greater satisfaction with PCA. Patients are given a greater degree of autonomy which, in turn, may reduce fears of insufficient analgesia. Instant availability of the medication may also contribute to greater satisfaction with the mode of treatment. We had insufficient data to perform subanalyses based upon type of surgery. It would be interesting to investigate whether patients undergoing more invasive surgeries would be less inclined to be in charge of their own pain management.
The measurement of satisfaction in trials where participants are not blinded to study arm assignment creates a potential for bias. All studies in our analysis were unblinded. In the older studies, participants who received a 'new breakthrough' treatment may have expressed a preference compared to those who 'missed out'. Alternatively, elderly and/or patients with higher acuity conditions may prefer conservative and established treatments or may not wish to be responsible for their analgesic regimen. However, advanced age does not appear to be the explanation in our meta‐analysis, since the mean age of participants was only around 50 years after excluding pediatric participants and women who underwent cesarean section. Additionally, Egbert 1990, which enrolled frail elderly men, demonstrated a statistically significant preference for PCA.
Length of stay
Two additional studies from our updated search contributed data for analysis of length of stay, which included a total of 10 studies. Two trials reported that length of stay was statistically significantly shorter in the PCA group, one trial favored non‐patient controlled analgesia, and seven did not find a statistically significant difference between groups. Similar to our original analysis, this updated meta‐analysis showed that length of stay was 0.18 days shorter in the PCA group but, again, the difference was not statistically significant. The most appropriate approach would be to control for factors affecting the length of stay, such as comorbidities, or to assess readiness for discharge, rather than time to actual discharge. However, none of the included studies reported this outcome. These factors, in combination with the relatively small number of trials available for analysis, may contribute to absence of difference between the analyzed modes of analgesia.
Adverse events
For our updated review we added the outcomes 'serious adverse events', 'withdrawals due to adverse events', 'withdrawals due to lack of efficacy', and 'respiratory depression', in addition to our original analyses of sedation, nausea and vomiting, pruritus, and urinary retention. We analyzed respiratory depression, potentially the most serious adverse event, separately since its inclusion in a larger analysis of adverse or serious adverse events may have caused obscuration of any difference between groups. We chose this approach in order to address recent evidence suggesting increased risk of respiratory depression in patients receiving PCA (Overdyk 2007).
Our original analyses failed to show any differences, either clinical or statistical, for all but one of the most commonly reported adverse events ‐ participants using PCA had a higher incidence of pruritus. The same was true for our updated review. In addition there were no statistically or clinically significant differences between groups for any of the new outcomes. While the possibility that there is, in fact, no difference in adverse event occurrence between interventions cannot be discounted, the lack of difference may be explained by the rarity with which many of the new adverse outcomes occur, the manner in which adverse event data are assessed in randomized controlled trials (RCTs), or both. Consistent and widely accepted definition of adverse events and prospective, scheduled timing of their collection facilitates accurate comparisons. Some of the adverse events, such as sedation, may be subjective unless strictly defined. Others are easier to quantify, however definitions differ across the studies. Respiratory depression, for example, was defined and assessed in numerous ways in the studies that reported it, including respiratory rates of less than 10 breaths per minute or oxygen saturation of less than 90%. It is acknowledged that reporting of adverse events in RCTs is often inadequate (Edwards 1999), and that small studies are unable to detect differences in rare but serious adverse events (Liu 2007). Only sedation, nausea and vomiting, pruritus, and urinary retention occurred in more than 10% of participants. Our meta‐analysis demonstrated that only 22 of 947, or 2.3% of participants receiving PCA, were assessed as suffering from respiratory depression versus 19 of 967 participants (2.0%) in the conventional groups. Conversely, an observational study of patients using PCA, where respiratory depression was the primary outcome, reports much higher occurrences, i.e., 12% and 41% for desaturation (less than 90%) and bradypnea (respiratory rate less than 10 for three minutes or more), respectively (Overdyk 2007). Liu 2007 points out that rare incidences of morbidity and mortality in modern surgery require subject samples of 500 to 50,000 to detect 50% reductions in incidence. Given these numbers, it is not surprising that our analyses of adverse events did not show a difference between groups. Lastly, adverse events occurring due to device malfunction or programming errors must be considered, but there were very few reports of these in the included studies. United States Food and Drug Administration (FDA) data have identified both as being major causes of adverse event reporting in hospitals using PCA (Hankin 2007).
Overall completeness and applicability of evidence
The included studies reported relevant data for both the primary and secondary outcomes. For the primary outcome, VAS pain scores, for the interval 0 to 24 hours 1516 participants contributed data. The studies enrolled participants undergoing a wide variety of surgeries for which PCA is commonly employed, although almost one‐third of these studies were in females undergoing gynecologic surgery. Participants ranged from children to elderly, although there were few participants at the extremes of this range. Pediatric or geriatric patients may be more susceptible to both the positive and adverse effects of opioids. As mentioned in Types of interventions, we restricted our definition of 'conventional analgesia' in this updated review. We therefore did not include studies where participants received scheduled opioid regimens as opposed to those on an 'as needed' basis.
Quality of the evidence
We assessed all studies as having a high risk of performance bias in that none of them were blinded. While theoretically possible in studies of PCA, blinding of participants appears to be impractical. It has been suggested that trials of low methodological quality may overestimate differences between therapies (Moher 1998). From the information provided in the included studies we were able to ascertain that five studies were inadequately randomized. Exclusion of these trials made little difference either statistically or clinically to any of our analyses. Lastly, we assessed only three studies, Brewington 1989, Jackson 1989 and Murphy 1994, as having low risk of bias due to adequate sample sizes (Moore 1998).
When assessing the quality of findings using GRADE, we ranked them from moderate to very low across the different efficacy outcomes, primarily due to risk of performance bias from lack of blinding. In some outcomes, unexplained heterogeneity, low overall population sizes, and imprecision of results also contributed to our downgrading of the evidence. We attempted to explain heterogeneity by performing subgroup and sensitivity analyses but, for the most part, heterogeneity remained. We can, therefore, only speculate as to possible sources. We noted above the potential for between‐study variability in conventional groups. In participants assigned to PCA groups, evidence of substantial variability in opioid demands between individuals has been documented (Moore 2011).
Potential biases in the review process
We carried out a comprehensive search for relevant studies. While we did not assess publication bias, we attempted to mitigate its potential effect by searching for non‐published data via the website clinicaltrials.gov.
We analyzed data from cross‐over studies in the same manner as that from parallel studies. This approach may give rise to a unit of analysis error (Higgins 2011). However, only one study employed a cross‐over method (Bollish 1985), and removal of this study from each meta‐analysis made negligible differences in estimates of effect for either efficacy or safety.
We excluded studies that explicitly mentioned that they enrolled participants with chronic pain. A potential weakness of our analysis is that, while not specified, some participants, particularly orthopedic patients, might have experienced some degree of chronic pain preoperatively.
Advances in postoperative pain management suggest that the groups in newer studies might receive analgesic regimens superior to those used in older studies. We attempted to mitigate this by excluding studies employing scheduled regimens in the control group and studies where non‐opioid regimens differed between groups. Similarly, recent improvements in surgical technique, such as using minimally invasive methods, may reduce pain and other complications from surgery, potentially lessening the superiority of PCA (Liu 2007). However, for our primary outcome, VAS pain scores, included studies from 2006 and later actually demonstrated greater mean improvements versus earlier studies during the 0 to 24‐hour interval and values remained similar over all time periods for each meta‐analysis.
Agreements and disagreements with other studies or reviews
Two previous reviews compared PCA with non‐patient controlled analgesia (Ballantyne 1993; Walder 2001). Walder and colleagues' meta‐analysis did not reach the same conclusions as ours for our primary outcome (Walder 2001). In that analysis, neither continuous data of pain intensity nor dichotomous data of combined pain intensity and pain relief produced statistically significant differences. The different results between the present analysis and that of Walder's may result from the different inclusion criteria employed. Walder's meta‐analysis included studies in which both partial mu agonists and background infusions were employed in the PCA groups. Alternatively, the discordance between the two reviews may simply be due to our having a greater number of studies available for analysis and, therefore, a greater possibility of achieving statistical significance. Conversely, findings for our primary outcome are consistent with Ballantyne and colleagues' 1993 meta‐analysis (Ballantyne 1993). Ballantyne's review concluded that patients treated with PCA were more comfortable than patients given non‐patient controlled analgesia, even though the authors questioned the clinical significance of these findings (six points lower pain score in PCA patients on a zero to 100 VAS). Although the difference is greater in the present review, it is still questionable whether a nine‐point lowering of pain intensity is clinically significant.
In contrast to both earlier reviews, we found that opioid consumption was higher in participants using PCA than those administered conventional analgesia. Exclusion criteria employed in our meta‐analysis may have played a role in the observed finding. Walder's review included studies in which participants using PCA also had 'background infusions' of opioids. The continuous infusion of opioid in these studies may have contributed to more constant plasma levels and decreased demand for bolus doses. However, the use of a background infusion is generally discouraged in opioid‐naive patients (APS 2008), as it may lead to opioid overdosage.
Our analysis of patient satisfaction is consistent with the results of Ballantyne 1993, even though the meta‐analysis involved only 160 participants in that review. Walder and colleagues' analysis did not find a difference in patient satisfaction between groups, although they did report that more participants expressed a preference for PCA over conventional therapy. Our analysis of length of stay is consistent with Ballantyne's and Walder's results. Lastly, with regards to adverse events, both previous meta‐analyses were unable to find significant differences in the incidence or severity of adverse events between groups.
Authors' conclusions
Implications for practice.
A limited amount of additional data were identified for this update, reinforcing the conclusions of our previous review. The fact that patient controlled analgesia (PCA) is now standard practice may account for the scarcity of new randomized controlled trials (RCTs) assessing its efficacy.
For people with postoperative pain
PCA has gained acceptance among patients despite the lack of evidence supporting clinical advantages from this review or previous reviews. Low quality evidence demonstrates that patients report greater satisfaction with, and in general prefer, PCA. While the reasons for this preference are unclear, they may be a function of increased patient autonomy and absence of delay in opioid administration. Patients should expect pain relief to be marginally superior to that achieved with nurse‐administered analgesia and a similar degree of side effects.
For clinicians
Our meta‐analysis provides moderate to very low quality evidence that PCA provides superior analgesia in comparison to non‐patient controlled regimens. Length of stay was similar in both groups. Despite slightly higher opioid consumption in participants using PCA, we found no increase in the occurrence of opioid‐induced adverse effects. Where available and appropriate, PCA should be offered to patients.
For policy makers
PCA for postoperative pain control continues to be commonly used in many hospitals in the western world, and in the absence of new evidence or advances in technology, is likely to remain so in the near future.
For funders
Very limited evidence suggests that PCA may be more costly than nurse‐administered analgesia. Our review does not demonstrate the potential for savings through reduced time to discharge. However, given that hospital reimbursement is, in part, contingent on patient satisfaction data in countries such as the United States, increases in direct costs may be offset by such policies.
Implications for research.
General
While intravenous administration remains the most commonly used mode of PCA, several alternative modes have been applied in the clinical setting or in controlled clinical trials. Alternative routes of administration include oral, transdermal, inhaled, intranasal, and epidural, each with their own potential benefits and disadvantages. Oral, transdermal, inhaled, and intranasal administration modalities offer the potential advantage of reductions in cost, labor, and required expertise of staff, and increased patient mobility when compared to intravenous PCA. There are currently insufficient RCTs available to determine whether any of the above modes of PCA will prove more safe or effective than intravenous PCA.
Design
While further trials investigating different surgeries may be helpful, the number of trials currently available for assessing overall efficacy of intravenous PCA is already extensive. More studies enrolling geriatric or pediatric populations, and those patients with risk factors such as chronic pain and substance abuse disorders, should be conducted. If possible, the quality of the future trials could be improved by introducing double‐blinding and by clearly defining criteria for inclusion.
The safety profile of PCA has not been fully established in this review. Further research from large epidemiological studies that include high‐risk patients and that assess programming error and device malfunction data are needed to provide a more complete picture of the risks associated with PCA.
Measurement (endpoints)
Most studies used standard validated pain intensity scales and widely accepted opioid conversion values. However, mean differences in pain scores or opioid consumption may not accurately reflect differences between PCA and nurse‐administered analgesia. The use of dichotomous outcomes, such as the number of participants administering less than a predetermined cumulative amount of opioid may have greater validity.
Comparison between active treatments
There is a lack of either indirect or head‐to‐head comparisons of different opioids administered via PCA. While head‐to‐head studies were not considered for this analysis, evidence supporting the superiority of one opioid versus others or, conversely, a class effect would be helpful.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 11 January 2019 | Amended | Contact details updated. |
| 20 May 2015 | Review declared as stable | This review will be assessed for further updating in 2020. See Published notes. |
| 15 October 2014 | New citation required and conclusions have changed | Results from original outcomes unchanged. New comparisons added related to safety and withdrawals. |
| 15 October 2014 | New search has been performed | Six new studies added; 13 from previous review excluded. Conclusions from original outcomes unchanged. Changes in methodology, including incorporation of 'Risk of bias' assessments and GRADE. New outcomes: withdrawals due to lack of efficacy, withdrawals due to adverse events, serious adverse events, respiratory depression. |
| 1 May 2012 | Amended | The review has been amended to denote assessment of the potential impact of exclusion of one study from meta‐analysis (Boldt 1998). See Published notes. |
| 8 February 2011 | Amended | Contact details updated. |
| 6 November 2008 | Amended | Further changes as a result of the RevMan 5 conversion. |
| 22 April 2008 | Amended | Converted to new review format. |
Notes
2012
We assessed the impact of the exclusion of one included study (Boldt 1998) on 1 May 2012. In 2011, 89 published studies in each of which Dr. Boldt was an author were retracted due to lack of evidence that they had received approval from an institutional review board (http://www.reuters.com/article/2011/03/04/us-journals-retractions-idUSTRE7235J820110304). The study included in this review, Boldt 1998, is not one of those retracted (http://www.aaeditor.org/EIC.Joint.Statement.on.Retractions.pdf). However, as a precautionary measure, we re‐analyzed all meta‐analyses that contained data from the study with the data excluded. In total, 13 meta‐analyses contained data, including comparisons of pain scores, opioid consumption, patient satisfaction, and various adverse events. Exclusion of these data did not affect either the statistical or clinical significance of any of our findings.
2015
At 2015, the authors and editors agreed to re‐assess this review for updating in 2020, as it is unlikely that further research will change conclusions.
Assessed for updating in 2020
At June 2020 we are not aware of any potentially relevant studies likely to change the conclusions. This is not an active area of research and so this review has now been stabilised following discussion with the authors and editors. If appropriate we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
We are sad to confirm the death of co‐author Jana Hudcova in June.
Acknowledgements
We appreciated the contributions of the authors of the original review, Dr. Daniel Carr, Dr. Joseph Lau, and Dr. Cheng Quah. We are grateful to Zehui He, PhD from the Department of Big Medical Data, Guangzhou University of Chinese Medicine, Guangzhou, China, for translation and extraction of all Chinese language articles. Lastly, we would like to thank Joanne Abbott, Trials Search Co‐ordinator, from the Cochrane Pain, Palliative and Supportive Care Group for running and compiling all of the literature searches for our review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Pain, Postoperative] this term only
#2 ((postoperative near/4 pain*) or (post‐operative near/4 pain*) or post‐operative‐pain* or (post* near/4 pain*) or (postoperative near/4 analgesi*) or (post‐operative near/4 analgesi*) or "post‐operative analgesi*"):ti,ab,kw (Word variations have been searched)
#3 ((post‐surgical near/4 pain*) or ("post surgical" near/4 pain*) or (post‐surgery near/4 pain*)):ti,ab,kw (Word variations have been searched)
#4 ("pain‐relief after surg*" or "pain following surg*" or "pain control after"):ti,ab,kw (Word variations have been searched)
#5 (("post surg*" or post‐surg*) and (pain* or discomfort)):ti,ab,kw (Word variations have been searched)
#6 ((pain* near/4 "after surg*") or (pain* near/4 "after operat*") or (pain* near/4 "follow* operat*") or (pain* near/4 "follow* surg*")):ti,ab,kw (Word variations have been searched)
#7 ((analgesi* near/4 "after surg*") or (analgesi* near/4 "after operat*") or (analgesi* near/4 "follow* operat*") or (analgesi* near/4 "follow* surg*")):ti,ab,kw (Word variations have been searched)
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 MeSH descriptor: [Analgesia, Patient‐Controlled] this term only
#10 (patient‐controlled near/2 analgesi*):ti,ab,kw (Word variations have been searched)
#11 (PCA or PCEA or PCIA):ti,ab,kw (Word variations have been searched)
#12 #9 or #10 or #11
#13 #8 and #12 from 2004 to 2013
Appendix 2. MEDLINE (OVID) search strategy
1. Pain, Postoperative/
2. ((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or "post‐operative analgesi*").mp.
3. ((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp.
4. ("pain‐relief after surg*" or "pain following surg*" or "pain control after").mp.
5. (("post surg*" or post‐surg*) and (pain* or discomfort)).mp.
6. ((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp.
7. ((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp.
8. or/1‐7
9. Analgesia, Patient‐Controlled/
10. (patient‐controlled adj2 analgesi*).tw.
11. (PCA or PCEA or PCIA).tw.
12. 9 or 10 or 11
13. 8 and 12
14 (2004* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013*).ed.
15 randomized controlled trial.pt.
16 controlled clinical trial.pt.
17 randomized.ab.
18 placebo.ab.
19 clinical trials as topic.sh.
20 randomly.ab.
21 trial.ti.
22 15 or 16 or 17 or 18 or 19 or 20 or 21
23 exp animals/ not humans.sh.
24 22 not 23
25 13 and 14 and 24
Appendix 3. EMBASE (OVID) search strategy
1. Pain, Postoperative/
2. ((postoperative adj4 pain*) or (post‐operative adj4 pain*) or post‐operative‐pain* or (post* adj4 pain*) or (postoperative adj4 analgesi*) or (post‐operative adj4 analgesi*) or "post‐operative analgesi*").mp.
3. ((post‐surgical adj4 pain*) or ("post surgical" adj4 pain*) or (post‐surgery adj4 pain*)).mp.
4. ("pain‐relief after surg*" or "pain following surg*" or "pain control after").mp.
5. (("post surg*" or post‐surg*) and (pain* or discomfort)).mp.
6. ((pain* adj4 "after surg*") or (pain* adj4 "after operat*") or (pain* adj4 "follow* operat*") or (pain* adj4 "follow* surg*")).mp.
7. ((analgesi* adj4 "after surg*") or (analgesi* adj4 "after operat*") or (analgesi* adj4 "follow* operat*") or (analgesi* adj4 "follow* surg*")).mp.
8. or/1‐7
9. Analgesia, Patient‐Controlled/
10. (patient‐controlled adj2 analgesi*).tw.
11. (PCA or PCEA or PCIA).tw.
12. 9 or 10 or 11
13. 8 and 12
14. random$.tw.
15. factorial$.tw.
16. crossover$.tw.
17. cross over$.tw.
18. cross‐over$.tw.
19. placebo$.tw.
20. (doubl$ adj blind$).tw.
21. (singl$ adj blind$).tw.
22. assign$.tw.
23. allocat$.tw.
24. volunteer$.tw.
25. Crossover Procedure/
26. double‐blind procedure.tw.
27. Randomized Controlled Trial/
28. Single Blind Procedure/
29. or/14‐28
30. (animal/ or nonhuman/) not human/
31. 29 not 30
32. 13 and 31
33. (2004* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013*).dd.
34. 32 and 33
Appendix 4. Clinicaltrials.gov search strategy
Search terms: patient controlled analgesia
Recruitment: all studies
Study Results: all studies
Study Type: interventional
Conditions: pain, postoperative
Outcome measures: pain
Data and analyses
Comparison 1. VAS pain scores (0 to 100): PCA versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain scores 0 to 24 h | 23 | 1516 | Mean Difference (IV, Random, 95% CI) | ‐8.82 [‐13.09, ‐4.54] |
| 1.1.1 Abdominal ‐ lower | 7 | 576 | Mean Difference (IV, Random, 95% CI) | ‐14.54 [‐20.53, ‐8.54] |
| 1.1.2 Abdominal ‐ upper | 6 | 280 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐6.93, 4.92] |
| 1.1.3 Cardiothoracic | 4 | 154 | Mean Difference (IV, Random, 95% CI) | ‐6.81 [‐17.72, 4.10] |
| 1.1.4 Neurosurgical | 3 | 173 | Mean Difference (IV, Random, 95% CI) | ‐17.60 [‐26.06, ‐9.14] |
| 1.1.5 Mixed/other | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐12.06, 6.54] |
| 1.2 Pain scores 25 to 48 h | 13 | 609 | Mean Difference (IV, Random, 95% CI) | ‐8.82 [‐14.15, ‐3.49] |
| 1.2.1 Abdominal ‐ lower | 3 | 88 | Mean Difference (IV, Random, 95% CI) | ‐3.44 [‐22.91, 16.03] |
| 1.2.2 Abdominal ‐ upper | 4 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐10.82, 6.73] |
| 1.2.3 Cardiothoracic | 3 | 134 | Mean Difference (IV, Random, 95% CI) | ‐19.12 [‐25.51, ‐12.74] |
| 1.2.4 Neurosurgical | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐8.47, 2.47] |
| 1.2.5 Mixed/other | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐12.09 [‐18.39, ‐5.80] |
| 1.3 Pain scores 49 to 72 h | 3 | 231 | Mean Difference (IV, Random, 95% CI) | ‐12.11 [‐26.04, 1.83] |
| 1.4 Pain scores 0 to 48 h | 7 | 372 | Mean Difference (IV, Random, 95% CI) | ‐9.74 [‐12.49, ‐6.99] |
| 1.5 Pain scores 0 to 72 h | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐8.00 [‐15.40, ‐0.60] |
| 1.6 Pain scores 0 to 24 h minus inadequately randomized trials | 21 | 1366 | Mean Difference (IV, Random, 95% CI) | ‐7.71 [‐11.88, ‐3.54] |
| 1.6.1 Abdominal ‐ lower | 5 | 426 | Mean Difference (IV, Random, 95% CI) | ‐12.02 [‐17.12, ‐6.92] |
| 1.6.2 Abdominal ‐ upper | 6 | 280 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐6.93, 4.92] |
| 1.6.3 Cardiothoracic | 4 | 154 | Mean Difference (IV, Random, 95% CI) | ‐6.81 [‐17.72, 4.10] |
| 1.6.4 Neurosurgical | 3 | 173 | Mean Difference (IV, Random, 95% CI) | ‐17.60 [‐26.06, ‐9.14] |
| 1.6.5 Mixed/other | 3 | 333 | Mean Difference (IV, Random, 95% CI) | ‐2.76 [‐12.06, 6.54] |
| 1.7 Pain scores 25 to 48 h minus inadequately randomized trials | 12 | 569 | Mean Difference (IV, Random, 95% CI) | ‐9.79 [‐15.15, ‐4.43] |
| 1.7.1 Abdominal ‐ lower | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐8.92 [‐31.84, 14.00] |
| 1.7.2 Abdominal ‐ upper | 4 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐10.82, 6.73] |
| 1.7.3 Cardiothoracic | 3 | 134 | Mean Difference (IV, Random, 95% CI) | ‐19.12 [‐25.51, ‐12.74] |
| 1.7.4 Neurosurgical | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐8.47, 2.47] |
| 1.7.5 Mixed/other | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐12.09 [‐18.39, ‐5.80] |
| 1.8 Pain scores 0 to 48 h minus inadequately randomized trials | 5 | 290 | Mean Difference (IV, Random, 95% CI) | ‐9.67 [‐12.58, ‐6.76] |
Comparison 2. Opioid consumption: PCA versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Consumption of morphine equivalents 0 to 24 h | 25 | 1586 | Mean Difference (IV, Random, 95% CI) | 7.21 [1.44, 12.98] |
| 2.1.1 Abdominal ‐ lower | 7 | 504 | Mean Difference (IV, Random, 95% CI) | 9.62 [‐4.97, 24.21] |
| 2.1.2 Abdominal ‐ upper | 5 | 252 | Mean Difference (IV, Random, 95% CI) | 4.45 [‐7.50, 16.40] |
| 2.1.3 Cardiothoracic | 7 | 334 | Mean Difference (IV, Random, 95% CI) | 4.43 [‐1.83, 10.68] |
| 2.1.4 Neurosurgical | 4 | 213 | Mean Difference (IV, Random, 95% CI) | 19.65 [4.23, 35.07] |
| 2.1.5 Mixed/other | 2 | 283 | Mean Difference (IV, Random, 95% CI) | ‐3.85 [‐10.34, 2.64] |
| 2.2 Consumption of morphine equivalents 25 to 48 h | 9 | 449 | Mean Difference (IV, Random, 95% CI) | 5.37 [2.82, 7.92] |
| 2.3 Consumption of morphine equivalents 0 to 48 h | 8 | 334 | Mean Difference (IV, Random, 95% CI) | 17.50 [‐4.75, 39.75] |
| 2.4 Consumption of morphine equivalents 0 to 72 h | 4 | 244 | Mean Difference (IV, Random, 95% CI) | 21.06 [5.18, 36.94] |
| 2.5 Consumption of morphine equivalents 0 to 24 h minus inadequately randomized trials | 22 | 1396 | Mean Difference (IV, Random, 95% CI) | 8.42 [2.63, 14.22] |
| 2.5.1 Abdominal ‐ lower | 6 | 408 | Mean Difference (IV, Random, 95% CI) | 13.15 [5.54, 20.75] |
| 2.5.2 Abdominal ‐ upper | 4 | 212 | Mean Difference (IV, Random, 95% CI) | 6.14 [‐8.04, 20.32] |
| 2.5.3 Cardiothoracic | 7 | 334 | Mean Difference (IV, Random, 95% CI) | 4.43 [‐1.83, 10.68] |
| 2.5.4 Neurosurgical | 3 | 159 | Mean Difference (IV, Random, 95% CI) | 26.34 [6.75, 45.92] |
| 2.5.5 Mixed/other | 2 | 283 | Mean Difference (IV, Random, 95% CI) | ‐3.85 [‐10.34, 2.64] |
Comparison 3. Patient satisfaction: PCA versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Satisfaction on a continuous scale | 7 | 427 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.13, 0.97] |
| 3.2 Number of participants in arm satisfied with therapy | 11 | 547 | Risk Difference (M‐H, Random, 95% CI) | 0.20 [0.07, 0.32] |
Comparison 4. Length of stay: time to readiness for discharge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Number of days: PCA versus control | 10 | 550 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.63, 0.26] |
| 4.2 Number of days: PCA versus control minus inadequately randomized trials | 9 | 440 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.51, 0.36] |
Comparison 5. Serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Number of participants with serious adverse event | 19 | 1284 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
Comparison 6. Withdrawals due to adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Number of participants withdrawing | 18 | 1281 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
Comparison 7. Withdrawals due to lack of efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Number of participants withdrawing | 18 | 1347 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
Comparison 8. Sedation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Sedation on a continuous scale | 10 | 514 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.11, 0.23] |
| 8.2 Number of participants in arm reporting sedation | 10 | 809 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
Comparison 9. Nausea and vomiting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Nausea and vomiting on a 0 to 10 scale (10 = most severe) | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.75, 0.40] |
| 9.2 Number of participants reporting nausea or vomiting, or both | 22 | 1525 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
Comparison 10. Pruritus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Number of participants reporting pruritus | 10 | 544 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
Comparison 11. Respiratory depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Number of participants affected | 29 | 1914 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 11.1.1 Abdominal ‐ lower | 8 | 435 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 11.1.2 Abdominal ‐ upper | 5 | 194 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 11.1.3 Cardiothoracic | 7 | 356 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 11.1.4 Orthopedic | 2 | 95 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 11.1.5 Neurosurgical | 3 | 173 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 11.1.6 Mixed/other | 4 | 661 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
Comparison 12. Urinary retention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Number of participants reporting urinary retention | 6 | 467 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albert 1988.
| Study characteristics | ||
| Methods | Parallel, 72 h administration | |
| Participants | PCA 32, control 30 Partial or total colon resection | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg (increases by 0.5 mg on physician order)/10 min/NR Control: IM morphine 5 mg to 12 mg every 3 to 4 h |
|
| Outcomes | Pain intensity, opioid consumption, level of sedation/activity, day of resolution of ileus, duration and total cost of hospitalization, individual cost of either PCA or conventional IM analgesic | |
| Source of funding | Not reported | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Method of analysis and number of participants completing study not described, but appears from results that all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Bedder 1991.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 20, control 18 Non‐thoracic elective or emergency surgery ‐ ICU environment | |
| Interventions | PCA: morphine. Bolus/lockout/ 4 h limit: 2 mg/10 min/NR Control: IV morphine 2 mg every 10 min prn |
|
| Outcomes | Pain intensity, opioid consumption, sedation scores, oxygen saturation | |
| Source of funding | "Supported in part by BARD and Nellcor" (no description of nature of funding groups) | |
| Notes | Oxygen saturation < 90%: 2/20 PCA vs 1/18
No respiratory rate < 10 QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Method of analysis and number of participants completing study not described. Figures suggest that several participants in both groups did not complete study |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Bennett 1982.
| Study characteristics | ||
| Methods | Parallel, 60 h | |
| Participants | PCA 12, control 12 Gastric bypass in morbidly obese patients | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 0.6 mg/m2 BSA (increases by 0.2 mg/m2 BSA)/6 min/NR Control: IM morphine 8 mg to 12 mg every 4 to 6 h prn |
|
| Outcomes | Nurse‐recorded: opioid use, level of analgesia, sedation Patient‐reported questionnaire: pain, sedation, activity levels, preference |
|
| Source of funding | Not reported | |
| Notes | QS = 3 (R = 2, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that all participants completed the study, but no mention of how missing data were imputed |
| Selective reporting (reporting bias) | High risk | Opioid consumption and patient preference were assessed, but not reported. No adverse event reporting (other than sedation) |
| Other bias | High risk | Very small sample size |
Berde 1991.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 32, control 23 Children and adolescents, major orthopedic surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 0.025 mg/kg/10 min/0.24 mg/kg Control: IM morphine 0.1 to 0.18 mg/kg every 3 h prn |
|
| Outcomes | Patients: pain intensity, sedation, nausea, anxiety, and satisfaction every 2 h Nurses: pain intensity, sedation, nausea, anxiety, asleep or awake, RR, vital signs, vomiting, urinary retention, and global assessment |
|
| Source of funding | Supported in part by a grant from Abbott Laboratories, and by contributions from the Christopher Coakley Memorial Fund, from the Karen Grunebaum Cancer Research Fund, and from a Student Research Award, Federal Republic of Germany | |
| Notes | No respiratory depression in either group QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17 participants did not complete the study, but reasons for withdrawal unrelated to interventions |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported, some secondary outcomes (e.g., anxiety) reported only as "non‐significant" |
| Other bias | Unclear risk | Small sample size |
Bhise 1997.
| Study characteristics | ||
| Methods | Parallel, time frame unclear | |
| Participants | PCA 10, control 10 Coronary artery bypass graft | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 2 mg/15 min/15 mg Control: IV morphine with same parameters as PCA group |
|
| Outcomes | Pain intensity, opioid consumption, sedation, HR, BP, pulmonary artery pressure, oxygen saturation, arterial blood gases, patient acceptability, and side effects | |
| Source of funding | Not reported | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that all participants completed the study, but not explicit |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Boldt 1998.
| Study characteristics | ||
| Methods | Parallel, 72 h | |
| Participants | PCA 30, control 30 First time elective cardiac surgery | |
| Interventions | PCA: piritramide. Bolus/lockout/1 h limit: 2 mg/10 min/6 doses Control: IV piritramide 5 mg, on demand or as determined by nurse |
|
| Outcomes | Pain intensity, sedation, patient satisfaction, pulmonary function, cortisol and troponin levels, O2 saturation, PaO2 and PaCO2, adverse events | |
| Source of funding | Not reported | |
| Notes | See Published notes for details regarding study retraction QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants completing study not reported, imputation methods not described |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Bollish 1985.
| Study characteristics | ||
| Methods | Cross‐over, 48 h | |
| Participants | PCA 20, control 20 (20 participants in total, each received both PCA and conventional) Abdominal surgery (colostomy, cholecystectomy, appendectomy, etc.) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/10 min/NR Control: IM morphine, typically 6 mg to 10 mg every 4 to 6 h prn |
|
| Outcomes | Pain intensity and relief, sedation, activity, opioid use, respiratory rate, ability to sleep, ability to carry out "pulmonary toilet", patient preference | |
| Source of funding | Not reported | |
| Notes | QS = 1 (R = 0, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were assigned alternately into groups |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study (one was replaced) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Boulanger 2002.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 22, control 20 Cardiac surgery with cardiopulmonary bypass (coronary artery bypass, valve replacement, atrial septal defect closure) |
|
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 0.015 mg/kg/6 min/NR Control: SC morphine 0.15 mg/kg every 4 h prn Upward and downward titration permitted in both groups |
|
| Outcomes | Primary: time to extubation Secondary: pain, opioid requirements, patient satisfaction, treatment acceptability, adverse events, recovery parameters |
|
| Source of funding | Development Fund of the Department of Anesthesiology of University of Montreal, the Quebec Anesthesiology Research Foundation, the Cardiac Surgery Foundation of Hôtel‐Dieu du Centre Hospitalier de l’Université de Montréal, and a posthumous donation from the Aon Reed Stenhouse Company | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Part of the study was blinded, but participants were aware of group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% of patients in both groups did not complete the study. Type of analysis not specified (LOCF, BOCF, or 'completer'), but graphs, figures suggest completer analysis only |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered in clinicaltrials.gov. All pre‐specified outcomes in the publication were addressed. Some outcomes (e.g., pain assessments) were reported incompletely – these data were presented only graphically vs raw values |
| Other bias | High risk | Very small sample size |
Brewington 1989.
| Study characteristics | ||
| Methods | Parallel, 72 h | |
| Participants | PCA 112, control 112 Gynecologic oncology surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/12 minutes/NR Control: IM morphine 8 mg to 12 mg every 3 to 4 h prn |
|
| Outcomes | Pain intensity, opioid usage, sedation, nausea/vomiting, patient preference | |
| Source of funding | Not mentioned | |
| Notes | QS = 1 (R = 0, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation made on alternating basis |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of dropouts described and similar between groups, remaining participants completed study |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Low risk | Adequate sample size |
Chan 1995 (chole).
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 12, control 11 (cholecystectomy via laparotomy) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1.5 to 2 mg/5 to 10 min/NR Control: IM morphine 0.15 to 0.2 mg/kg every 4 h prn |
|
| Outcomes | Pain relief, satisfaction with analgesia, nursing time spent on each participant, time to ambulation, resumption of activities of daily living, return of bowel function, return of oral feeding, tolerance of oral analgesia, LOS | |
| Source of funding | Abbott Laboratories | |
| Notes | QS = 3 (R = 2, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that all participants completed study, but not stated. Imputation methods not described |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Chan 1995 (combined).
| Study characteristics | ||
| Methods | — | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Source of funding | — | |
| Notes | — | |
Chan 1995 (laminectomy).
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 24, control 20 (laminectomy) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1.5 to 2 mg/5 to 10 min/NR Control: IM morphine 0.15 to 0.2 mg/kg every 4 h prn |
|
| Outcomes | Pain relief, satisfaction with analgesia, nursing time spent on each participant, time to ambulation, resumption of activities of daily living, return of bowel function, return of oral feeding, tolerance of oral analgesia, LOS | |
| Source of funding | Abbott Laboratories | |
| Notes | QS = 3 (R = 2, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that all participants completed study, but not stated. Imputation methods not described |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Chang 2004.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 62, control 63 (142 total agreed to participate, but initial group assignment numbers before withdrawal not described) abdominal gynecologic surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: NR/8 to 10 min/NR Control: IM morphine 0.1 to 0.2 mg/kg (max 10 mg every 3 h) prn |
|
| Outcomes | Pain intensity, patient satisfaction, cost‐effectiveness, opioid use, side effects | |
| Source of funding | Grant from the Hong Kong Health Services Research Committee | |
| Notes | Method of measurement of respiratory depression not defined QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Double blinding was not adopted, as there were obvious differences in each method of pain management. However, the research assistant and ward staff were blind to the research hypotheses." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants withdrew in each group (17 total). Results reported for completers only |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Colwell 1995.
| Study characteristics | ||
| Methods | Parallel, 72 h | |
| Participants | PCA 91, control 93 (195 enrolled, reasons for 11 withdrawals reported, but initial group assignment numbers not specified) Elective joint replacement or spinal procedure | |
| Interventions | PCA: morphine or meperidine (converted to morphine equivalents). Bolus/lockout/4 h limit: 0.25 to 0.5 mg/6 min/10 to 20 mg Control: IM morphine or meperidine (doses converted to morphine equivalents) 8 mg to 15 mg every 3 h prn |
|
| Outcomes | Pain intensity and relief, opioid consumption, ability to ambulate, pulse oximetry, nursing time and cost of materials, patient and nurse satisfaction, side effects | |
| Source of funding | Baxter Healthcare funded study, but authors did not receive personal compensation | |
| Notes | Withdrawals: n = 11. Unplanned admission to ICU, lack of preoperative instruction, allergy to a medication used, operation canceled (numbers not specified) QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 participants excluded after randomization, but before intervention. Appears that all remaining participants completed study. Methods of imputation of missing data not mentioned |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section, although patient satisfaction only reported as non‐significant between groups |
| Other bias | Unclear risk | Small sample size |
Crisp 2012.
| Study characteristics | ||
| Methods | Parallel, participants received IV opioids on postoperative day 0 only, with follow‐up to 14 days | |
| Participants | PCA 32, control 27 Vaginal reconstructive surgery | |
| Interventions | PCA: hydromorphone. Bolus/lockout/4 h limit: 0.2 mg/8 min/5 mg Control: IV hydromorphone 0.5 mg every 2 h with option to decline |
|
| Outcomes | Primary: pain intensity and satisfaction with pain control Secondary: opioid use, side effects |
|
| Source of funding | Not mentioned | |
| Notes | QS = 3 (R = 2, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (nQuery Advisor) randomization table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned – assume no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low numbers of dropouts, not related to interventions |
| Selective reporting (reporting bias) | Low risk | Trial registered on clinicaltrials.gov NCT01442818. No results posted, but amount of opioid used not listed as secondary outcome. Primary outcomes same as in manuscript |
| Other bias | Unclear risk | Small sample size |
Dahl 1987.
| Study characteristics | ||
| Methods | Parallel, 16 h | |
| Participants | PCA 18, control 18 Lower abdominal surgery (hysterectomy, hysterosalpingo‐oophorectomy, oophorectomy, second look) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 2.5 mg/10 min/NR Control: scheduled IM morphine (7.5 to 12.5 mg depending on body weight), with IV morphine 2.5 mg as required |
|
| Outcomes | Pain intensity and relief, opioid consumption, vital signs, nausea/vomiting, consciousness | |
| Source of funding | Not mentioned | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section, although consciousness only reported at 4 h |
| Other bias | High risk | Very small sample size |
Egbert 1990.
| Study characteristics | ||
| Methods | Parallel, 72 h | |
| Participants | PCA 43, control 40 Mixed major elective surgery in frail elderly men | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 0.01 mg/kg (titration allowed)/10 min/NR Control: IM morphine 0.1 mg/kg every 3 h prn (titration allowed) |
|
| Outcomes | Pain intensity, opioid consumption, vital signs, ear oximetry, pulmonary function tests, sedation, mental status, morphine levels, patient satisfaction, complications | |
| Source of funding | Veterans Administration Merit Review Grant 452‐0001 | |
| Notes | Asymptomatic desaturation: 6/43 vs 6/40 QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all randomized participants completed the study (although not explicitly stated) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section. Time to return of bowel sounds, urinary catheter placement and LOS reported despite not being mentioned in Methods section |
| Other bias | Unclear risk | Small sample size |
Egbert 1993.
| Study characteristics | ||
| Methods | see Egbert 1990 | |
| Participants | see Egbert 1990 | |
| Interventions | see Egbert 1990 | |
| Outcomes | see Egbert 1990, plus anxiety | |
| Source of funding | see Egbert 1990 | |
| Notes | see Egbert 1990 | |
Eisenach 1988.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 20, control 20 (third group receiving epidural morphine not reported here) Repeat Cesarean section | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 2 mg/15 min/NR Control: IM morphine 10 mg to 15 mg or meperidine 25 mg to 75 mg every 2 to 4 h prn (doses converted to morphine equivalents) |
|
| Outcomes | Pain intensity, morphine consumption, sedation, nausea, pruritus, respiratory rate, patient perception of postoperative analgesia | |
| Source of funding | Not mentioned | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned, assumed to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Ellis 1982 (chole).
| Study characteristics | ||
| Methods | Parallel, 5 days | |
| Participants | PCA 15, control 17 (third group receiving sublingual buprenorphine not reported here) Cholecystectomy | |
| Interventions | PCA: meperidine. Bolus/lockout/4 h limit: NR Control: IM morphine 10 mg prn according to usual practice of nursing staff |
|
| Outcomes | Pain intensity, opioid consumption, pulmonary function tests, sedation, nausea, patient satisfaction and preference | |
| Source of funding | Not reported | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all participants completed the study (although not explicitly stated) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section. |
| Other bias | High risk | Very small sample size |
Ellis 1982 (hysterectomy).
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 20, control 20 (matched with participants who received conventional analgesia but were not directly enrolled in study) hysterectomy |
|
| Interventions | PCA: meperidine. Bolus/lockout/4 h limit: NR Control: IM morphine 10 mg prn according to usual practice of nursing staff |
|
| Outcomes | Pain intensity, opioid consumption, pulmonary function tests, sedation, nausea, patient satisfaction and preference | |
| Source of funding | Not reported | |
| Notes | QS = 1 (R = 0, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization based on availability of PCA apparatus |
| Allocation concealment (selection bias) | High risk | Allocation based on availability of PCA apparatus |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all participants completed the study (although not explicitly stated) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Gillman 1995.
| Study characteristics | ||
| Methods | Parallel, 42 h | |
| Participants | PCA 11, control 11 Total abdominal hysterectomy | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/6 min/NR Control: IM morphine 0.04 to 0.14 mg/kg (2.5 to 10 mg) every 4 h prn (titration permitted) |
|
| Outcomes | Pain intensity, opioid consumption, number of patients satisfied with therapy, vital signs, urinary retention, nausea, vomiting, pruritus, sedation, cost of therapy (including comparison with regimen not used in the study) | |
| Source of funding | Not mentioned | |
| Notes | NSAIDs not used for 42 h postoperatively QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants had PCA discontinued due to infusion site problems, but data included in analysis |
| Selective reporting (reporting bias) | High risk | All outcomes described in Methods section are reported in Results section; however mean data not presented with accompanying spread for some outcomes |
| Other bias | High risk | Very small sample size |
Harrison 1988.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 18, control 20 (third group receiving epidural morphine not reported here) Cesarean section | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 2 mg/6 min/NR Control: IM morphine 10 mg to 15 mg every 4 h prn |
|
| Outcomes | Pain intensity, opioid consumption, pruritus, nausea/vomiting, respiratory rates, patient satisfaction, LOS | |
| Source of funding | Not reported | |
| Notes | No respiratory rate < 10/min in either group QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear if all participants completed study, methods of imputation not mentioned |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section. Opioid consumption not mentioned in Methods, but reported in Results |
| Other bias | High risk | Very small sample size |
Hu 2006.
| Study characteristics | ||
| Methods | Parallel, 72 h | |
| Participants | PCA 40, control 40 (third group receiving epidural analgesia not reported here) Lower abdominal surgery |
|
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/6 to 8 min/25 mg Control: IM meperidine ‐ regimen not reported |
|
| Outcomes | Pain intensity at rest and with coughing, opioid use, interleukin‐1 and interleukin‐6 levels | |
| Source of funding | Not reported | |
| Notes | Chinese language journal QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section. |
| Other bias | Unclear risk | Small sample size |
Jackson 1989.
| Study characteristics | ||
| Methods | Parallel, data recorded until participant discharged | |
| Participants | Cholecystectomy group: PCA 71, control 34 Hysterectomy group: PCA 72, control 151 | |
| Interventions | PCA: meperidine. Bolus/lockout/4 h limit: 10 mg/8 min/NR (titration permitted) Control: IM meperidine (with hydroxyzine or promethazine) at physician's discretion ‐ usually 75 mg to 100 mg every 3 to 4 h prn |
|
| Outcomes | Pain intensity, opioid consumption, pulmonary complications, vital signs, level of consciousness/sedation, levels of activity, safety, patient preference, LOS, cost‐effectiveness | |
| Source of funding | Not reported | |
| Notes | Study not split by type of surgery for this review as any data presented separately were not usable in analysis 96% of patients in PCA groups preferred PCA to IM therapy, 100% of nurses thought patients pain controlled better with PCA QS = 1 (R = 1, DB = 0, W = 0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear if all participants completed study, methods of imputation not mentioned |
| Selective reporting (reporting bias) | High risk | Several outcomes described in Methods section (pain intensity, vital signs, sedation, level of consciousness) are not reported in Results section. For some reported outcomes data reported incompletely (e.g., no standard deviations) |
| Other bias | Low risk | Adequate sample size |
Keita 2003.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 20, control 20 Total hip replacement in elderly patients | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/8 min/NR Control: SC morphine 0.1 mg/kg every 4 h for pain ≥ 30/100 |
|
| Outcomes | Pain intensity at rest and with mobilization, opioid consumption, side effects (hypotension, respiratory depression, sedation, urinary retention, nausea/vomiting, pruritus), LOS, cognitive function | |
| Source of funding | Supported in part by a research grant from Fondation de l'Avenir pour la Recherche Medicale Appliquee | |
| Notes | Respiratory depression defined as ventilatory frequency less than 8 breaths per minute QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not explicitly stated, but appears that all participants completed study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section. Continuous data reported as medians/percentiles, suggesting non‐normal distribution ‐ not used in meta‐analyses |
| Other bias | High risk | Very small sample size |
Kenady 1992.
| Study characteristics | ||
| Methods | Parallel, 72 h | |
| Participants | PCA 35, control 18 Cholecystectomy | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: NR Control: IM morphine ‐ no details |
|
| Outcomes | Pain relief and intensity, opioid consumption, level of sedation, interference of pain with postoperative recovery, dimensions of pain experience (tiring, frightening, punishing), ability to move around/breath/rest, LOS | |
| Source of funding | Not reported | |
| Notes | Withdrawals: admitted to ICU (n = 1), PCA malfunction (n = 1) QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 withdrawals, as detailed in Notes above. Not explicitly stated, but appears that all remaining participants completed study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section. P values only reported for some secondary outcomes |
| Other bias | Unclear risk | Small sample size |
Kyzer 1995.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 12, control 11 Gastroplasty (1 participant in each group also had cholecystectomy) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 2 mg/15 min/NR Control: IM meperidine (converted to morphine equivalents) 50 mg to 100 mg every 3 to 4 h prn |
|
| Outcomes | Pain intensity, opioid consumption, sedation, respiratory rate, blood gas levels, LOS, duration of ileus, incidence of pruritus/nausea/vomiting/complications | |
| Source of funding | Not reported | |
| Notes | SAE: wound infections requiring increased length of hospitalization (n), PCA 4 vs control 1 QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not explicitly stated, but appears that all participants completed study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section. Adverse event outcomes mostly presented only as not significantly different between groups |
| Other bias | High risk | Very small sample size |
McGrath 1989.
| Study characteristics | ||
| Methods | Parallel, approximately 48 h of PCA administration, outcomes measured to 72 h | |
| Participants | PCA 44, control 44 Cholecystectomy | |
| Interventions | PCA: meperidine. Bolus/lockout/4 h limit: 0.25 mg/kg (titration allowed)/20 min/NR Control: IM meperidine "in the conventional manner" up to 1.25 mg/kg every 3 h prn |
|
| Outcomes | Pain intensity, opioid consumption, Health Locus of Control, patient satisfaction, method participant would recommend | |
| Source of funding | Funded by the Alberta Foundation for Nursing Research | |
| Notes | Adverse events NR Lockout considerably longer than most included studies ‐ authors hypothesize that this may account for lack of superiority of PCA QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 106 patients initially approached. Unclear if 18 withdrawals occurred before or after randomization/enrollment. One participant (group not stated) withdrew due to inadequate pain relief, otherwise reasons for withdrawal appear unrelated to interventions |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section. No adverse events mentioned or reported |
| Other bias | Unclear risk | Small sample size |
Morad 2009.
| Study characteristics | ||
| Methods | Parallel, outcomes assessed until participants discharged from neuroscience critical care unit (10 h or longer) | |
| Participants | PCA 39, control 40 Elective supratentorial craniotomy, mostly due to tumor |
|
| Interventions | PCA: fentanyl. Bolus/lockout/1 h limit: 0.5 µg/kg/15 min/4 demand dose per h Control: IV fentanyl 25 to 50 µg every 30 min prn |
|
| Outcomes | Pain intensity, incidence of uncontrolled pain (defined as a pain score ≥ 5/10 for > 2 hours), incidence of respiratory depression requiring an opioid antagonist or institution of ventilatory support, neurological changes including the number of emergency postoperative imaging studies obtained for evaluation of neurological changes, incidence of pruritus, incidence, duration, and intensity of nausea and vomiting, vital signs | |
| Source of funding | Supported in part by grants from the Jacob and Hilda Blaustein Foundation, National Institutes of Health Grant No. NS041865 | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most dropouts occurred before PCA or PRN were started, i.e., during the operative phase, and were mostly due to protocol violations or unanticipated neurological outcomes. In remaining participants, no withdrawals occurred before 10 h of data collection and were balanced between groups (3 PCA vs 3 PRN) |
| Selective reporting (reporting bias) | Low risk | Protocol not available. All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Morad 2012.
| Study characteristics | ||
| Methods | Parallel, outcomes assessed until participants discharged from neuroscience critical care unit or the collection of 16 h of data | |
| Participants | PCA 40, control 40 Elective posterior fossa surgery: suboccipital craniectomy for Chiari‐type malformations (34% of patients), craniectomy for acoustic neuroma (35% of patients), and suboccipital craniotomy for other pathologies (31% of patients) |
|
| Interventions | PCA: fentanyl. Bolus/lockout/1 h limit: 0.5 µg/kg (max dose 50 µg)/15 min/4 demand dose per h Control: IV fentanyl 25 µg to 50 µg every 30 min prn |
|
| Outcomes | Pain intensity at rest, opioid consumption, changes in neurologic status with Glasgow Coma Scale, Ramsay Sedation Scale, the number of emergency postoperative imaging studies obtained for evaluation of neurologic changes, incidence of pruritus/nausea and vomiting, vital signs | |
| Source of funding | Financial support from the Jacob and Hilda Blaustein Foundation and the Richard J. Traystman Endowed Chair | |
| Notes | QS = 3 (R = 2, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was determined using a randomization scheme with random block lengths generated by the medical centre’s research pharmacy service." |
| Allocation concealment (selection bias) | Low risk | "Group assignments were communicated electronically by the research pharmacy service to the study coordinator after informed consent was obtained" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The surgical and anesthesia teams were masked to group assignments, but participants and nurses were not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number of dropouts in each group: PCA 6 dropouts, 5 prior to intervention, control 9 dropouts, 7 prior to intervention Final number of patients analyzed: PCA 34, control 31. Majority of dropouts occurred during operative phase, before participants received intervention Per protocol analysis, but majority of protocol violations also occurred before interventions administered |
| Selective reporting (reporting bias) | Unclear risk | Protocol (but not results) posted on clinicaltrials.gov – mentions outcomes of patient satisfaction and time to discharge. Neither reported in paper. Otherwise, all outcomes mentioned in Methods reported in Results |
| Other bias | Unclear risk | Small sample size |
Munro 1998.
| Study characteristics | ||
| Methods | Parallel, interventions administered for 2 days, outcomes assessed for 4 days | |
| Participants | PCA 39, control 41 Elective cardiac surgery | |
| Interventions | PCA: morphine. Bolus/lockout/1 h limit: 1 mg/6 min/10 mg Control: SC morphine 0 to 7.5 mg every 1 to 2 h based on pain scores and nursing assessment of vital signs |
|
| Outcomes | Pain intensity and relief scores, at rest and on movement, opioid consumption; nausea and pruritus, patient satisfaction, success of physiotherapy and ease of obtaining preemptive analgesia before physiotherapy | |
| Source of funding | Not mentioned | |
| Notes | QS = 3 (R = 2, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 withdrawals occurred after randomization, but before interventions administered because of delays in extubation (numbers in each group not specified). Appears that remaining participants completed the study, but unclear how many participants contributed data |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Murphy 1994.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 100, control 100 Laparotomy, thoracotomy | |
| Interventions | PCA: meperidine. Bolus/lockout/4 h limit: 20 mg/5 min/NR (titration permitted) Control: IV meperidine nurse controlled infusion, 0 to 40 mg/h with bolus doses of 20 to 40 mg and titration permitted |
|
| Outcomes | Pain intensity, opioid consumption, levels of nausea and sedation, incidence of adverse events | |
| Source of funding | Supported by the Dr. John Boyd Craig Bursary of the Australian and New Zealand College of Anaesthetists | |
| Notes | Respiratory depression requiring treatment with naloxone: 1/100 vs 1/100
Withdrawals: previous neurological deficit preventing use of PCA (n = 1) QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 withdrawal (PCA group), unrelated to intervention |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section. |
| Other bias | Low risk | Adequate sample size |
Myles 1994.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 36, control 36 Elective cardiac surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/5 min/no limit Control: IV morphine nurse controlled infusion (dose range not specified) |
|
| Outcomes | Pain intensity, opioid usage, intensity of nausea, time to extubation, cortisol levels | |
| Source of funding | PCA devices funded by Alfred Hospital Whole‐time Medical Specialists' Private Practice Fund | |
| Notes | Withdrawals: control, n = 3: 2 deaths, 1 cerebrovascular accident QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not adequately described |
| Allocation concealment (selection bias) | High risk | Blocked randomization with stratification ‐ study is not blinded, therefore allocation can be established |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data of 69 participants analyzed: PCA 36 and control 33 (3 withdrawals all in the control group). Data for 16 participants incomplete, due to reasons apparently unrelated to interventions ‐ not clear whether included in analyses. Group assignment and methods of imputation (or completer analysis only) not specified |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
O'Halloran 1997.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 44, control 46 (participants were randomized before consent) Elective cardiac surgery (mostly coronary artery bypass grafting) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/5 min/no limit Control: IV morphine nurse controlled infusion, 1 to 3 mg/h, with 1 mg boluses every 1 h prn |
|
| Outcomes | Pain at rest and with movement, opioid usage, respiratory rate, nausea/vomiting requiring anti‐emetic, sedation score | |
| Source of funding | Abbott Laboratories loaned additional PCA pumps | |
| Notes | Withdrawals: PCA vs control (n): consent withdrawn: 2 vs 4; protocol violation: 2 vs 5; late extubation: 1 vs 3; postoperative complications: 4 vs 2; postoperative confusion: 0 vs 1 QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | More withdrawals in control group, but appear to be or were stated as being unrelated to interventions (see notes) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Paoletti 1993 (gyn).
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 22, control 22 Gynecologic surgery (second study within paper not reported here as control group received continuous infusion not titrated to patient requirements) | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/6 to 15 min/NR Control: IM morphine 10 mg every 6 h prn |
|
| Outcomes | Pain, opioid usage, sedation, respiratory rate, nausea/vomiting, itch, sweating, quality of sleep, patient satisfaction | |
| Source of funding | Not mentioned | |
| Notes | Withdrawal: PCA, n = 1: hypotension/apnea QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported on all but 1 participant (PCA, withdrew due to AE) |
| Selective reporting (reporting bias) | High risk | All outcomes described in Methods section are reported in Results section; however spread for continuous outcomes not reported |
| Other bias | High risk | Very small sample size |
Passchier 1993.
| Study characteristics | ||
| Methods | Parallel, outcomes assessed through 96 h (unclear how long PCA or control administered) | |
| Participants | PCA 21, control 19 Elective upper abdominal surgery (cholecystectomy, intestinal resection) | |
| Interventions | PCA: morphine. Bolus/lockout/1 h limit: 1 mg/5 min/10 mg Control: IM morphine 10 mg prn (no schedule specified) |
|
| Outcomes | Pain intensity and relief, opioid consumption, state anxiety, patient satisfaction, distress, Profile of Mood State questionnaire, locus of control | |
| Source of funding | Support from Bard, the Netherlands | |
| Notes | Withdrawals: PCA vs control (n): refused to continue postoperatively: 4 vs 5 (not included in analyses) QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals balanced between groups and apparently unrelated to interventions |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section. No adverse events assessed or reported |
| Other bias | High risk | Very small sample size |
Perez‐Woods 1991.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 25, control 17 Cesarean section | |
| Interventions | PCA: morphine. Bolus/lockout/1 h limit: 0.5‐1.5 mg/6 min/10 doses Control: IM morphine every 3 to 4 h prn. Dose per "dosage chart", not shown |
|
| Outcomes | Pain intensity (patient and nurse reported), opioid consumption, LOS, time and frequency of ambulation, patient satisfaction, lung function, degree of sedation and other adverse events (frequency) | |
| Source of funding | Baxter Corporation and the Research Fund of the Marcella Niehoff School of Nursing Loyola University of Chicago | |
| Notes | QS = 1 (R = 0, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Based on week of admission |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but apears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods section are reported in Results section; however LOS only described as being not different between groups and adverse events not described other than being "few" |
| Other bias | High risk | Very small sample size |
Pettersson 2000.
| Study characteristics | ||
| Methods | Parallel, study terminated on morning of third postoperative day | |
| Participants | PCA 25, IV 25 Coronary artery bypass graft | |
| Interventions | PCA: ketobemidone. Bolus/lockout/4 h limit: 1 mg/6 min/30 mg (adjustment permitted, same settings in both ICU and ward) Control: IV ketobemidone. ICU: nurse controlled infusion, adjusted according to nurse assessment of need (dose range not specified); ward: 2 mg to 5 mg prn patient request or VAS > 3 Both groups also received acetaminophen 1 g every 6 h |
|
| Outcomes | Pain intensity on coughing/deep breathing, opioid consumption, patient and nurse satisfaction, LOS, side effects (nausea/vomiting, respiratory rate < 10 or > 20 breaths per minute, degree of sedation) | |
| Source of funding | Supported, in part, by a grant from The Karolinska Institute, Stockholm, Sweden | |
| Notes | No somnolence or arterial desaturation
Withdrawals (1 from each group): incomplete protocol (n = 1), minor neurological deficit (n = 1) (group for each not specified, not included in analyses) QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was achieved by placing instructions for one of the two analgesia alternatives in each of 50 envelopes. The envelopes were sealed, thoroughly mixed, and assigned a number from 1 to 50. Each participating patient was consecutively assigned an envelope number and ketobemidone was administered according to the instructions in the envelope." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated, but appears to be unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of dropouts, equally distributed between groups and apparently unrelated to interventions |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section. LOS reported despite not being listed in Methods |
| Other bias | Unclear risk | Small mple size |
Precious 1997.
| Study characteristics | ||
| Methods | Parallel, up to 56 h postoperatively | |
| Participants | PCA 25, control 25 (third group receiving po/pr naproxen not reported here) Orthognathic surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/10 min/NR Control: IM/PO codeine 30 to 60 mg every 4 h prn or IM morphine 0.1 to 0.2 mg/kg every 4 h prn severe pain |
|
| Outcomes | Pain intensity, opioid consumption, overall rating of analgesia, nausea/vomiting/other adverse events, vital signs | |
| Source of funding | Not mentioned | |
| Notes | No respiratory depression in either group QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts, but participants in PCA group were permitted to discontinue PCA any time after the end of day 1 postoperatively and switch to codeine regimen. 7 participants discontinued PCA at the end of day 1, 15 terminated PCA during day 2 postsurgery, and 3 maintained PCA until the end of the study. No mention of how data from participants changing regimen were imputed |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Small sample size |
Rayburn 1988.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 68, control 62 Cesarean section | |
| Interventions | PCA: meperidine. Bolus/lockout/1 h limit: 10 mg/10 min/60 mg Control: IM meperidine 75 mg to 100 mg (based on body weight) every 3 h prn |
|
| Outcomes | Pain intensity and relief, sedation, other adverse effects, cost, nurse satisfaction | |
| Source of funding | Not reported | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was accidentally overdosed on PCA and was excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Sall sample size |
Rogers 1990.
| Study characteristics | ||
| Methods | Parallel, over 24 h | |
| Participants | PCA 34, control 35 (72 enrolled, 3 excluded due to undergoing bile duct exploration, group not specified) Cholecystectomy | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 0.6 mg/m2/6 min/NR Control: IM/IV morphine (surgeon determined route/dose used) |
|
| Outcomes | Opioid requirements, LOS, complications (urinary retention, atelectasis, return of bowel function) | |
| Source of funding | Not reported | |
| Notes | SAE: atelectasis occurred in one patient in PCA group QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded because they underwent bile duct exploration (group not specified) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results section |
Rosen 1998.
| Study characteristics | ||
| Methods | Parallel, 48 h for all outcomes other than LOS | |
| Participants | PCA 36, control 36 Major gynecologic laparoscopy | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1.5 mg/5 min/NR Control: IM morphine 7.5 mg to 10 mg (frequency not specified) |
|
| Outcomes | Pain intensity, opioid consumption, patient satisfaction, LOS, sedation, nausea, vomiting | |
| Source of funding | Not mentioned | |
| Notes | QS = 2 (R = 1, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Method of analysis and number of participants completing study not described, but appears from results that all participants were included in analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Smallsample size |
Smythe 1994.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 19, control 17 Hysterectomy | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/6 min/NR or meperidine. Bolus/lockout/4 h limit: 10 mg/6 min/NR Control: IM meperidine 75 mg to 100 mg every 3 to 4 h prn |
|
| Outcomes | Pain intensity, satisfaction, cost, adverse effects, nursing and pharmacy time | |
| Source of funding | American Association of Colleges of Pharmacy New Investigator grant | |
| Notes | Withdrawals: PCA ‐ severe nausea: 1 patient discontinued after 2 h QS = 1 (R = 1, DB = 0, W = 0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 orthopedic surgery patients were excluded as a result of few orthopedic surgeons willing to allow their patients the chance of PCA and conventional options; ITT analysis based on the way Results are presented |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Snell 1997.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 53, control 38 (44 and 23 included in analysis) Major abdominal surgery | |
| Interventions | PCA: morphine or meperidine. Bolus/lockout/4 h limit: NR Control: IM meperidine |
|
| Outcomes | Pain intensity, opioid consumption, LOS, time to ambulation, adverse effects; satisfaction with pain control and satisfaction with transition from parenteral to oral pain medication | |
| Source of funding | Ottawa General Hospital Medical Research Committee | |
| Notes | Patients in IM group received almost 3x as much antiemetic (mg) as PCA group
Withdrawals: PCA vs control (n): 6 vs 12: physician changing analgesic route (n = 8), change in operation (n = 7), other (not specified, n = 3) QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants were no longer included in the study if the surgeon/anesthetist did not wish the patient to receive the assigned analgesia or if no pumps were available. Out of 91 patients that agreed to participate in the study 18 were excluded mostly because of physician changed analgesic route (n = 8) or medication (n = 7) and 3 unknown. Out of 73 remaining in the study only those who stayed for at least 48 hours (n = 67) were included in analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | choice and amount of analgesia was left to physician discretion Small sample size |
Stoneham 1996.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 15, control 15 Craniotomy | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/10 min/NR Control: IM codeine 30 mg to 60 mg every 4 h prn |
|
| Outcomes | Pain intensity (patient and nurse assessed), opioid consumption, nausea/vomiting, respiratory rate, Glasgow Coma score | |
| Source of funding | Not reported | |
| Notes | Median nausea score = 0 (0 = none) and Glasgow coma score = 15 in both groups at 24 h QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated to groups by a sealed envelope random number schedule |
| Allocation concealment (selection bias) | Low risk | A sealed envelope technique was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Method of analysis and number of participants completing the study not described, but appears from Results that all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Sudheer 2007.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 20, control 20 Craniotomy |
|
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/5 min/50 mg Control: IM codeine 60 mg up to 2 times in the first h; then 60 mg every 4 h prn |
|
| Outcomes | Pain intensity, patient satisfaction, adverse effects, PaCO2; arterial blood gases; vital signs: heart rate, blood pressure, oxygen saturation, respiratory rate; cumulative total dose of analgesia; Glasgow Coma score; pupil size; sedation; nausea/vomiting, patient satisfaction | |
| Source of funding | Not reported | |
| Notes | Data reported as median likely because not normally distributed; primary outcome was PaCO2 4 hours after eye opening QS = 3 (R = 2, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated to 1 of 3 groups by a computer‐generated code, using a closed envelope technique |
| Allocation concealment (selection bias) | Low risk | A closed envelope technique was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pain was assessed by blinded nurses. Patients and anesthesiologists were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomized were analyzed in the data despite 3 dropouts in the control group. Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Respiratory rate, cumulative total dose of analgesia were specified in publication but not reported in the results |
| Other bias | High risk | Very small sample size |
Thomas 1995.
| Study characteristics | ||
| Methods | Parallel, 24 h other than LOS | |
| Participants | PCA 61, control 49 Total abdominal hysterectomy | |
| Interventions | PCA: papaveretum. Bolus/lockout/4 h limit: 2 mg to 4 mg/10 to 15 min/NR; anesthetists were free to choose the demand dose and lockout interval Control: IM papaveretum 15 mg to 20 mg every 4 h prn |
|
| Outcomes | Pain, opioid consumption, LOS; relationship between psychological outcomes and pain intensity/opoid consumption | |
| Source of funding | Lewisham and North Southwark Health Authority and Hambland Foundation grants | |
| Notes | Adverse events NR QS = 1 (R = 0, DB = 0, W = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation on an alternating basis to ensure PCA availability |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Method of analysis and number of participants completing the study not described, but appears from Results that all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | Unclear risk | Smal sample size |
Wang 1991.
| Study characteristics | ||
| Methods | Parallel, 48 h | |
| Participants | PCA 13, IM 13 Thoracotomy | |
| Interventions | PCA: meperidine. Bolus/lockout/4 h limit: 8 mg to 15 mg/6 to 12 min/100 mg to 150 mg Control: IM meperidine 50 mg (40 mg if participant < 50 kg) every 4 hours prn |
|
| Outcomes | Pain intensity, opioid consumption, forced vital capacity, nocturnal sleep disturbance due to pain | |
| Source of funding | Not reported | |
| Notes | IM group had more disturbance of nocturnal sleep, slower recovery of lung function QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts in either group |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Wasylak 1990.
| Study characteristics | ||
| Methods | Parallel, interventions administered over approximately 48 h, outcomes assessed until discharge, questionnaire administered 2 weeks post‐discharge | |
| Participants | PCA 20, control 18 Gynecologic surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 2 mg/5 min in recovery room then 1 mg/10 min/NR in the ward Control: recovery room 2 mg IV as necessary then IM morphine 5 mg to 20 mg (with promethazine) every 4 to 6 h prn |
|
| Outcomes | Pain intensity, opioid consumption, ambulation, LOS, respiratory function, functional status 2 weeks post‐discharge | |
| Source of funding | Medical Research Council of Canada grant #MA8914 | |
| Notes | Respiratory rate reduced to greater extent in PCA group, but never < 10/min. Reduction in vital capacity and recovery rate similar QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Method of analysis not described but appears from Results that all participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods section are reported in Results section |
| Other bias | High risk | Very small sample size |
Wheatley 1992.
| Study characteristics | ||
| Methods | Parallel, 24 h | |
| Participants | PCA 20, control 20 (19 and 20 included in analysis) Upper abdominal surgery | |
| Interventions | PCA: morphine. Bolus/lockout/4 h limit: 1 mg/5 min/NR Control: IM morphine 0.15 mg/kg every 4 h prn |
|
| Outcomes | Pain intensity and relief, opioid consumption, hypoxemia | |
| Source of funding | Not reported | |
| Notes | Respiratory depression: oxygen saturation < 85% for > 6 min (severe) or < 90% for > 12 min (moderate)
Withdrawals: PCA, n = 1: insufficient data collected for technical reasons QS = 2 (R = 1, DB = 0, W = 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient was not included in the analysis due to insufficient data collection for technical reasons; method of analysis not described |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods were reported in Results |
| Other bias | High risk | Very small sample size |
AE = adverse event; BOCF = baseline observation carried forward; BP = blood pressure; BSA = body surface area; DB = double‐blind; h = hour; HR = heart rate; ICU = intensive care unit; IM = intramuscular; ITT = intention‐to‐treat; IV = intravenous; LOCF = last observation carried forward; LOS = length of stay; NR = not reported; NSAID = non‐steroidal anti‐inflammatory drug; PCA = patient controlled analgesia; po = by mouth; pr = by rectum; prn = as needed; QS = quality score; R = randomization; RR = respiratory rate; SAE = serious adverse event, SC = subcutaneous; VAS = visual analog score; VRS = verbal rating scores; W = withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atwell 1984 | < 10 participants in each arm |
| Bayar 2008 | PCA regimen had background infusion |
| Bell 2007 | PCA regimen had background infusion |
| Boulanger 1993 | Control regimen scheduled |
| Ceriati 2003 | Control regimen scheduled |
| Cho 2011 | Groups received different non‐opioid regimens |
| Choiniere 1998 | Control regimen scheduled |
| Coyle 1990 | Abstract > 3 years old |
| D'haese 1998 | PCA regimen had background infusion |
| Davis 2006 | PCA regimen had background infusion |
| Dieterich 2012 | Control regimen scheduled |
| Duggleby 1992 | PCA regimen had background infusion |
| Eremenko 2011 | PCA regimen had background infusion |
| Ferrante 1988 | Control regimen scheduled |
| Forst 1999 | Control group administered tramadol (non‐conventional opioid) |
| Gaitini 1996 | Control group administered buprenorphine (partial agonist) |
| Gao 2007 | PCA regimen had background infusion |
| Gursoy 2006 | Control regimen scheduled |
| Gust 1999 | Control group also received NSAID |
| Halilotlu 2010 | Abstract > 3 years old |
| Hecker 1988a | < 10 participants in each arm |
| Hecker 1988b | < 10 participants in each arm |
| Jabri 2010 | Abstract > 3 years old |
| Jellinek 1990 | Both groups administered tramadol (non‐conventional opioid) |
| Khalili 2013 | PCA regimen had background infusion |
| Kilbride 1992 | PCA regimen had background infusion |
| Kleiman 1988 | Control regimen scheduled |
| Knapp‐Spooner 1995 | Non‐RCT |
| Knudsen 1993 | PCA regimen had background infusion |
| Lange 1988 | Administered buprenorphine, a partial agonist |
| Lee 2010 | Groups received different non‐opioid regimens |
| Lee 2013 | PCA solution contained butorphanol, a partial agonist, along with an NSAID |
| Liu 2005 | PCA solution also contained droperidol |
| Martinez‐Ubieto 1992 | Control regimen scheduled |
| Moller 1988 | Outcomes presented (plasma catecholamines, cortisol and glucose levels) were not those listed in inclusion criteria |
| Moreno 2000 | PCA solution contained non‐opioid only |
| Nitschke 1996 | PCA regimen had background infusion |
| Paoletti 1993 (ortho) | Control regimen scheduled |
| Peters 1999 | PCA regimen had background infusion |
| Rittenhouse 1999 | Outcomes presented (costs) were not those listed in inclusion criteria |
| Robinson 1991 | Both groups received PCA |
| Rothwell 2011 | Control regimen scheduled |
| Rundshagen 1999 | PCA regimen had background infusion |
| Sanansilp 1995 | Control group also received acetaminophen prn |
| Searle 1994 | PCA regimen had background infusion Control group could receive opioid/acetaminophen combination |
| Shin 2001 | PCA group administered nalbuphine (agonist/antagonist) combined with NSAID |
| Spetzler 1987 | Control group from retrospective chart review, one time questionnaire assessment, no VAS, poor quality paper |
| Taylor 1994 | Abstract > 3 years old |
| Tsang 1999 | PCA regimen had background infusion |
| Vengadesh 2005 | Control regimen scheduled |
| Viscusi 2004 | Both groups received PCA |
| Walson 1992 | < 10 participants in each group |
| Weldon 1993 | Both groups received PCA Used continuous background infusion with PCA in one group |
| White 1998 | Proportion of patients had chronic pain Results not provided separately |
| Woodhouse 1997 | Both groups received PCA ‐ study compared outcomes based on age of patients |
| Woods 1991 | Administered nalbuphine (agonist/antagonist) |
| Xiao 2011 | All groups received PCA |
| Yost 2004 | Not randomized |
| Zacharias 1990 | PCA regimen had background infusion |
NSAID = non‐steroidal anti‐inflammatory drug PCA = patient controlled analgesia prn = as needed RCT = randomized controlled trial VAS = visual analog scale
Characteristics of studies awaiting classification [ordered by study ID]
Legeby 2002.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | This study was discovered in 2015 while conducting a separate review of persistent postoperative pain. It was not captured by either the 2005 or 2015 PCA search strategies. The journal in which it was published is referenced in MEDLINE and EMBASE, but only from 2003 onwards. |
Differences between protocol and review
For the 2015 update, we made several changes based on the evolution of Cochrane methodology and advances in postoperative pain management since our original review. For the former, we added searching for unpublished data, 'Risk of bias' assessments, GRADE assessments, 'Summary of findings' tables and analyses of additional adverse event outcomes, while we excluded studies with fewer than 10 participants in each arm and abstracts that were more than three years old. For the latter, we excluded studies with scheduled regimens in the non‐patient controlled analgesia arm, but we included studies that also administered non‐opioids as long as the non‐opioid regimens were the same between arms. We amended the title from 'Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain' to 'Patient controlled opioid analgesia versus non‐patient controlled opioid analgesia for postoperative pain' to reflect changes in clinical practice and terminology, and to add clarity to the review.
Contributions of authors
2015 review
Ewan McNicol: co‐ordinating the review, organizing retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, entering data into RevMan, analysis of data, updating of 'Characteristics of studies' tables, incorporating GRADE and 'Summary of findings' tables, writing and editing the review.
McKenzie Ferguson: screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, entering data into RevMan, analysis of data, writing and editing the review.
Jana Hudcova: screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, providing clinical perspective, editing the review.
2006 review
Jana Hudcova: organizing retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, writing the review.
Ewan McNicol: appraising quality of papers, extracting data from papers, entering data into RevMan, analysis of data, compiling of 'Characteristics of included studies' and 'Characteristics of excluded studies' tables, writing the review.
Cheng Quah: design, co‐ordination, data collection, screening search results, organizing retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers.
Daniel Carr: conceiving the review, design, co‐ordination, developing search strategy. Providing a methodological, clinical, policy, and consumer perspective. Providing general advice on the review. Securing funding for the review.
Joseph Lau: analysis of data. Providing a methodological and clinical perspective. Providing general advice on the review.
Sources of support
Internal sources
Richard Saltonstall Charitable Foundation, USA
Evenor Armington Fund, USA
External sources
No sources of support supplied
Declarations of interest
EM has no relevant conflicts of interest to declare
MF has no relevant conflicts of interest to declare
JH has no relevant conflicts of interest to declare
deceased
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Albert 1988 {published data only}
- Albert JM, Talbott TM. Patient-controlled analgesia vs conventional intramuscular analgesia following colon surgery. Diseases of the Colon and Rectum 1988;31:83-6. [DOI] [PubMed] [Google Scholar]
Bedder 1991 {published data only}
- Bedder MD, Soifer BE, Mulhall JJ. A comparison of patient-controlled analgesia and bolus PRN intravenous morphine in the intensive care environment. Clinical Journal of Pain 1991;7(3):205-8. [DOI] [PubMed] [Google Scholar]
Bennett 1982 {published data only}
- Bennett RL, Batenhorst RL, Bivins BA, Bell RM, Graves DA, Foster TS, et al. Patient-controlled analgesia: a new concept of postoperative pain relief. Annals of Surgery 1982;195(6):700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berde 1991 {published data only}
- Berde CB, Lehn BM, Yee JD, Sethna NF, Russo D. Patient-controlled analgesia in children and adolescents: a randomized prospective comparison with intramuscular administration of morphine for postoperative analgesia. Journal of Pediatrics 1991;118(3):460-6. [DOI] [PubMed] [Google Scholar]
Bhise 1997 {published data only}
- Bhise M, Mehta V, Dhole S, Trehan N. Patient controlled analgesia (PCA) following coronary artery bypass graft surgery. Journal of Anaesthesiology Clinical Pharmacology 1997;13:113-5. [Google Scholar]
Boldt 1998 {published data only}
- Boldt J, Thaler E, Lehmann A, Papsdorf M, Isgro F. Pain management in cardiac surgery patients: comparison between standard therapy and patient-controlled analgesia regimen. Journal of Cardiothoracic & Vascular Anesthesia 1998;12(6):654-8. [DOI] [PubMed] [Google Scholar]
Bollish 1985 {published data only}
- Bollish SJ, Collins CL, Kirking DM, Bartlett RH. Efficacy of patient-controlled versus conventional analgesia for postoperative pain. Clinical Pharmacy 1985;4(1):48-52. [PubMed] [Google Scholar]
Boulanger 2002 {published data only}
- Boulanger A, Perreault S, Choiniere M, Prieto I, Lavoie C, Laflamme C. Intrathecal morphine after cardiac surgery. Annals of Pharmacotherapy 2002;36:1337-43. [DOI] [PubMed] [Google Scholar]
Brewington 1989 {published data only}
- Brewington KC. Patient-controlled analgesia in gynecologic oncology surgery. Alabama Medicine 1989;59(5):15-7. [PubMed] [Google Scholar]
Chan 1995 (chole) {published data only}
- Chan VWS, Chung F, McQuestion M, Gomez M. Impact of patient-controlled analgesia on required nursing time and duration of postoperative recovery. Regional Anesthesia 1995;20:506-14. [PubMed] [Google Scholar]
Chan 1995 (combined) {published data only}
- Chan VWS, Chung F, McQuestion M, Gomez M. Impact of patient-controlled analgesia on required nursing time and duration of postoperative recovery. Regional Anesthesia 1995;20:506-14. [PubMed] [Google Scholar]
Chan 1995 (laminectomy) {published data only}
- Chan VWS, Chung F, McQuestion M, Gomez M. Impact of patient-controlled analgesia on required nursing time and duration of postoperative recovery. Regional Anesthesia 1995;20:506-14. [PubMed] [Google Scholar]
Chang 2004 {published data only}
- Chang AM, Ip WY, Cheung TH. Patient-controlled analgesia versus conventional intramuscular injection: a cost effectiveness analysis. Journal of Advanced Nursing 2004;46(5):531-41. [DOI] [PubMed] [Google Scholar]
Colwell 1995 {published data only}
- Colwell CW, Morris BA. Patient-controlled analgesia compared with intramuscular injection of analgesics for the management of pain after an orthopaedic procedure. Journal of Bone and Joint Surgery 1995;77-A(5):726-33. [DOI] [PubMed] [Google Scholar]
Crisp 2012 {published data only}
- Crisp CC, Bandi S, Kleeman SD, Oakley SH, Vaccaro CM, Estanol MV, et al. Patient-controlled versus scheduled, nurse-administered analgesia following vaginal reconstructive surgery: a randomized trial. American Journal of Obstetrics and Gynecology 2012;207:433.e1-6. [DOI] [PubMed] [Google Scholar]
Dahl 1987 {published data only}
- Dahl JB, Daugaard JJ, Larsen HV, Mouridsen P, Nielsen TH, Kristoffersen E. Patient controlled analgesia: a controlled trial. Acta Anaesthesiologica Scandinavica 1987;31:744-7. [DOI] [PubMed] [Google Scholar]
Egbert 1990 {published data only}
- Egbert AM, Parks HL, Short LM, Burnett ML. Randomized trial of postoperative patient controlled analgesia vs. intramuscular narcotics in frail elderly men. Archives of Internal Medicine 1990;150:1897-903. [PubMed] [Google Scholar]
Egbert 1993 {published data only}
- Egbert AM, Lampros LL, Parks LL. Effects of patient-controlled analgesia on postoperative anxiety in elderly men. American Journal of Critical Care 1993;2(2):118-24. [PubMed] [Google Scholar]
Eisenach 1988 {published data only}
- Eisenach JC, Grice SC, Dewan DM. Patient-controlled analgesia following cesarean section: a comparison with epidural and intramuscular narcotics. Anesthesiology 1988;68:444-8. [DOI] [PubMed] [Google Scholar]
Ellis 1982 (chole) {published data only}
- Ellis R, Haines D, Shah R, Cotton BR, Smith G. Pain relief after abdominal surgery - a comparison of im morphine, sublingual buprenorphine and self-administered iv pethidine. British Journal of Anaesthesia 1982;54:421-8. [DOI] [PubMed] [Google Scholar]
Ellis 1982 (hysterectomy) {published data only}
- Ellis R, Haines D, Shah R, Cotton BR, Smith G. Pain relief after abdominal surgery - a comparison of im morphine, sublingual buprenorphine and self-administered iv pethidine. British Journal of Anaesthesia 1982;54:421-8. [DOI] [PubMed] [Google Scholar]
Gillman 1995 {published data only}
- Gillman RD, Robertson MS. A cost-effectiveness study of intramuscular when required, pain relief against intravenous patient-controlled pain relief after total abdominal hysterectomy. British Journal of Medical Economics 1995;9(1):73-80. [W1 BR573] [Google Scholar]
Harrison 1988 {published data only}
- Harrison DM, Sinatra R, Morghese L, Chung JH. Epidural narcotic and patient controlled analgesia for post-cesarean pain relief. Anesthesiology 1988;68:454-7. [DOI] [PubMed] [Google Scholar]
Hu 2006 {published data only}
- Hu Y, Wang Y, Li YT. Effects of different analgesic methods on immune function after lower abdominal surgery. Chinese Journal of Clinical Rehabilitation 2006;10:62-4. [Google Scholar]
Jackson 1989 {published data only}
- Jackson D. A study of pain management: patient controlled analgesia versus intramuscular analgesia. Journal of Intravenous Nursing 1989;12(1):42-51. [PubMed] [Google Scholar]
Keita 2003 {published data only}
- Keita H, Geachan N, Dahmani S, Couderc E, Armand C, Quazza M, et al. Comparison between patient-controlled analgesia and subcutaneous morphine in elderly patients after total hip replacement. British Journal of Anaesthesia 2003;90(1):53-7. [DOI] [PubMed] [Google Scholar]
Kenady 1992 {published data only}
- Kenady DE, Wilson JF, Schwartz RW, Bannon CL, Wermeling D. A randomized comparison of patient-controlled versus standard analgesia requirements in patients undergoing cholecystectomy. Surgery, Gynecology & Obstetrics 1992;174:216-20. [PubMed] [Google Scholar]
Kyzer 1995 {published data only}
- Kyzer S, Ramadan E, Gersch M Chaimoff C. Patient-controlled analgesia following vertical gastroplasty: a comparison with intramuscular narcotics. Obesity Surgery 1995;5:18-21. [DOI] [PubMed] [Google Scholar]
McGrath 1989 {published data only}
- McGrath D, Thurston N, Wright D, Preshaw R, Fermin P. Comparison of one technique of patient-controlled postoperative analgesia with intramuscular meperidine. Pain 1989;37:265-70. [DOI] [PubMed] [Google Scholar]
Morad 2009 {published data only}
- Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, et al. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: a prospective randomized controlled trial. Journal of Neurosurgery 2009;111:343-50. [DOI] [PubMed] [Google Scholar]
Morad 2012 {published data only}
- Morad A, Winters B, Stevens R, White E, Weingart J, Yaster M, et al. The efficacy of intravenous patient-controlled analgesia after intracranial surgery of the posterior fossa: a prospective, randomized controlled trial. Anesthesia and Analgesia 2012;114:416-23. [DOI] [PubMed] [Google Scholar]
Munro 1998 {published data only}
- Munro AJ, Long GT, Sleigh JW. Nurse administered subcutaneous morphine is a satisfactory alternative to intravenous patient-controlled analgesia morphine after cardiac surgery. Anesthesia and Analgesia 1998;87(1):11-5. [DOI] [PubMed] [Google Scholar]
Murphy 1994 {published data only}
- Murphy DF, Graziotti P, Chalkiadis G, McKenna M. Patient-controlled analgesia: a comparison with nurse-controlled intravenous opioid infusions. Anaesthesia and Intensive Care 1994;22(5):589-92. [DOI] [PubMed] [Google Scholar]
Myles 1994 {published data only}
- Myles PS, Buckland MR, Cannon GB, Bujor MA, Langley M, Breaden A, et al. Comparison of patient-controlled analgesia and nurse-controlled infusion analgesia after cardiac surgery. Anaesthesia and Intensive Care 1994;22(6):672-8. [DOI] [PubMed] [Google Scholar]
O'Halloran 1997 {published data only}
- O'Halloran P, Brown R. Patient-controlled analgesia compared with nurse-controlled infusion analgesia after heart surgery. Intensive & Critical Care Nursing 1997;13(3):126-9. [DOI] [PubMed] [Google Scholar]
Paoletti 1993 (gyn) {published data only}
- Paoletti F, Ciammitti B, Tosti F, Boanelli A, Pasqualucci V. Postoperative analgesia i.v. [Analgesia postoperatoria endovenosa]. Minerva Anestesiologica 1993;59:523-30. [PubMed] [Google Scholar]
Passchier 1993 {published data only}
- Passchier JP, Koenders MEF, Plree M, Luitwieler RL, Bonke B. Patient-controlled analgesia (PCA) leads to more postoperative pain relief, but also to more fatigue and less vigour. Acta Anesthesiologica Scandinavica 1993;37:659-63. [DOI] [PubMed] [Google Scholar]
Perez‐Woods 1991 {published data only}
- Perez-Woods R, Grohar JC, Skaredoff M, Rock SG, Tse AM, Tomich P, et al. Pain control after cesarean birth. Efficacy of patient-controlled analgesia vs traditional therapy (IM morphine). Journal of Perinatology 1991;11(2):174-81. [PubMed] [Google Scholar]
Pettersson 2000 {published data only}
- Pettersson PH, Lindskog EA, Owall A. Patient-controlled versus nurse-controlled pain treatment after coronary artery bypass surgery. Acta Anaesthesiologica Scandinavica 2000;44(1):43-7. [DOI] [PubMed] [Google Scholar]
Precious 1997 {published data only}
- Precious DS, Multari J, Finley GA, Mcgrath P. A comparison of patient-controlled and fixed schedule analgesia after orthognathic surgery. Journal of Oral and Maxillofacial Surgery 1997;55:33-9. [DOI] [PubMed] [Google Scholar]
Rayburn 1988 {published data only}
- Rayburn WF, Geranis BJ, Ramadei CA, Woods RE, Patil KD. Patient-controlled analgesia for post-caesarean section pain. Obstetrics and Gynecology 1988;72:136-9. [PubMed] [Google Scholar]
Rogers 1990 {published data only}
- Rogers DA, Dingus D, Stanfield J, Dipiro JT, May JR, Bowden TA Jr. A prospective study of patient controlled analgesia. Impact on overall hospital course. The American Surgeon 1990;56:86-9. [PubMed] [Google Scholar]
Rosen 1998 {published data only}
- Rosen DM, Lam AM, Carlton MA, Carlo GM, McBride L. Analgesia following major gynecological laparoscopic surgery - PCA versus intermittent intramuscular injection. Journal of the Society of Laparoendoscopic Surgeons 1998;2(1):25-9. [PMC free article] [PubMed] [Google Scholar]
Smythe 1994 {published data only}
- Smythe M, Loughlin K, Schad RF, Lucarroti RL. Patient-controlled analgesia versus intramuscular analgesic therapy. American Journal of Hospital Pharmacy 1994;51:1433-40. [PubMed] [Google Scholar]
Snell 1997 {published data only}
- Snell CC, Fothergill-Bourbonnais F, Durocher-Hendriks S. Patient-controlled analgesia and intramuscular injections: a comparison of patient pain experiences and postoperative outcomes. Journal of Advanced Nursing 1997;25:681-90. [DOI] [PubMed] [Google Scholar]
Stoneham 1996 {published data only}
- Stoneham MD, Cooper R, Quiney NF, Walters FJM. Pain following craniotomy: a preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia 1996;51:1176-8. [DOI] [PubMed] [Google Scholar]
Sudheer 2007 {published data only}
- Sudheer PS, Logan SW, Terblanche C, Ateleanu B, Hall JE. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia 2007;62:555-60. [DOI] [PubMed] [Google Scholar]
Thomas 1995 {published data only}
- Thomas V, Heath M, Rose D, Flory P. Psychological characteristics and the effectiveness of patient-controlled analgesia. British Journal of Anaesthesia 1995;74:271-6. [DOI] [PubMed] [Google Scholar]
Wang 1991 {published data only}
- Wang CS, Chou YP, Hou CC, Li CY, Lin BH, Yen FW. Efficiency of patient-controlled analgesia versus conventional analgesia in patients after thoracotomy (Chinese). Ma Tsui Hsueh Tsa Chi Anaesthesiologica Sinica 1991;29(2):604-9. [PubMed] [Google Scholar]
Wasylak 1990 {published data only}
- Wasylak TJ, Abbott FV, English MJ, Jeans ME. Reduction of postoperative morbidity following patient-controlled morphine. Canadian Journal of Anesthesia 1990;37:726-31. [DOI] [PubMed] [Google Scholar]
Wheatley 1992 {published data only}
- Wheatley RG, Sheperd D, Jackson IJB, Madej TH, Hunter D. Hypoxaemia and pain relief after upper abdominal surgery: comparison of i.m. and patient-controlled analgesia. British Journal of Anaesthesia 1992;69:558-61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atwell 1984 {published data only}
- Atwell JR, Flanigan RC, Bennet RL, Allen DC, Lucas BA, McRoberts JW. The efficacy of patient-controlled analgesia in patients recovering from flank incisions. Journal of Urology 1984;132:701-3. [DOI] [PubMed] [Google Scholar]
Bayar 2008 {published data only}
- Bayar U, Basaran M, Atasoy N, Ayoglu H, Sade H, Altunkaya H. Comparison of satisfaction and pain relief between patients-controlled analgesia and interval analgesia after laparoscopic ovarian cystectomy. Journal of Psychosomatic Obstetrics and Gynaecology 2008;29:139-45. [DOI] [PubMed] [Google Scholar]
Bell 2007 {published data only}
- Bell JG, Shaffer LET, Schrickel-Feller T. Randomized trial comparing 3 methods of postoperative analgesia in gynecology patients: patient-controlled intravenous, scheduled intravenous, and scheduled subcutaneous. American Journal of Obstetrics and Gynecology 2007;197:472.e1-e7. [DOI] [PubMed] [Google Scholar]
Boulanger 1993 {published data only}
- Boulanger A, Choinere M, Roy D, Boure B, Chartrand D, Choquette R, et al. Comparison between patient-controlled analgesia and intramuscular meperidine after thoracotomy. Canadian Journal of Anaesthesia 1993;40:409-15. [DOI] [PubMed] [Google Scholar]
Ceriati 2003 {published data only}
- Ceriati F, Tebala GD, De Cosmo G, Saraceni C, Coco C, Bosco F, et al. A prospective randomized clinical trial on pain control after major abdominal surgery. Chirurgia Italiana 2003;55:481-9. [PubMed] [Google Scholar]
Cho 2011 {published data only}
- Cho CH, Song KS, Min BW, Lee KJ, Ha E, Lee YC, et al. Multimodal approach to postoperative pain control in patients undergoing rotator cuff repair. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19:1744-8. [DOI] [PubMed] [Google Scholar]
Choiniere 1998 {published data only}
- Choiniere M, Rittenhouse BE, Perreault S, Chartrand D, Smith B, Pepler C. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Anesthesiology 1998;89:1377-88. [DOI] [PubMed] [Google Scholar]
Coyle 1990 {published data only}
- Coyle JP, Steele J, Cutrone F, Higgins TL, Taylor PC. Patient controlled analgesia after cardiac surgery. Anesthesia and Analgesia 1990;70(2):S71. [Google Scholar]
D'haese 1998 {published data only}
- D'haese C, Vanlersberghe V, Umbrain V, Camu F. Pharmaco-economic evaluation of a disposable patient-controlled analgesia device and intramuscular analgesia in surgical patients. European Journal of Anesthesiology 1998;15:297-303. [DOI] [PubMed] [Google Scholar]
Davis 2006 {published data only}
- Davis KM, Esposito MA, Meyer BA. Oral analgesia compared with intravenous patient-controlled analgesia for pain after cesarean delivery: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:967-71. [DOI] [PubMed] [Google Scholar]
Dieterich 2012 {published data only}
- Dieterich M, Muller-Jordan K, Stubert J, Kundt G, Wagner K, Gerber B. Pain management after cesarean: a randomized controlled trial of oxycodone versus intravenous piritramide. Archives of Gynecology and Obstetrics 2012;286:859-65. [DOI] [PubMed] [Google Scholar]
Duggleby 1992 {published data only}
- Duggleby W, Lander J. Patient-controlled analgesia for older adults. Clinical Nursing Research 1992;1:107-13. [DOI] [PubMed] [Google Scholar]
Eremenko 2011 {published data only}
- Eremenko AA, Sorokina LS. Analysis of patients control analgesia with trimeperidine and ketoprofen for postoperative pain management in patients who underwent cardiac surgery. Anesteziologiia i Reanimatologiia 2011:43-7. [PubMed] [Google Scholar]
Ferrante 1988 {published data only}
- Ferrante FM, Orav EJ, Rocco AG, Gallo J. A statistical model for pain in patient-controlled analgesia and conventional intramuscular opioid regimens. Anesthesia and Analgesia 1988;67:457-61. [PubMed] [Google Scholar]
Forst 1999 {published data only}
- Forst J, Wolff S, Thamm P, Forst R. Pain therapy following joint replacement. Archives of Orthopaedic and Trauma Surgery 1999;119:267-70. [DOI] [PubMed] [Google Scholar]
Gaitini 1996 {published data only}
- Gaitini L, Moskovitz B, Katz E, Vaisberg A, Vaida S, Nativ O. Sublingual buprenorphine compared to morphine delivered by a patient-controlled analgesia system as postoperative analgesia after prostatectomy. Urologia Internationalis 1996;57:227-9. [DOI] [PubMed] [Google Scholar]
Gao 2007 {published data only}
- Gao YL, Dai ZG, Guo SX, Chen L. Evaluation on analgesia methods in patients undergoing thoracic surgery [Chinese]. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11:3248-50. [Google Scholar]
Gursoy 2006 {published data only}
- Gursoy S, Kaygusuz K, Demirel Y, Duran B, Kafali H, Mimaroglu C. Comparison of postoperative analgesia methods in lower abdominal surgery [Turkish] [Alt batin cerrahilerinde postoperatif analjezi yontemlerinin karsilastirilmasi]. Turkiye Klinikeri Journal of Medical Science 2006;26:146-52. [Google Scholar]
Gust 1999 {published data only}
- Gust R, Pecher S, Gust A, Hoffmann V, Bohrer H, Martin E. Effect of patient-controlled analgesia on pulmonary complications after coronary artery bypass grafting. Critical Care Medicine 1999;27:2218-23. [DOI] [PubMed] [Google Scholar]
Halilotlu 2010 {published data only}
- Halilotlu M, Eti Z, Gogus FY. The effects of epidural PCA, intravenous PCA and conventional opioid administration on postoperative pulmonary functions and analgesia after laparotomy. Regional Anesthesia & Pain Medicine 2010;35:E93. [Google Scholar]
Hecker 1988a {published data only}
- Hecker BR, Albert L. Patient-controlled analgesia: a randomized prospective comparison between two commercially available PCA pumps and conventional analgesic therapy for postoperative pain. Pain 1988;35:115-20. [DOI] [PubMed] [Google Scholar]
Hecker 1988b {published data only}
- Hecker BR, Albert L. Patient-controlled analgesia: a randomized prospective comparison between two commercially available PCA pumps and conventional analgesic therapy for postoperative pain. Pain 1988;35:115-20. [DOI] [PubMed] [Google Scholar]
Jabri 2010 {published data only}
- Jabri H, Rais K, Chahed S, Bouali Y, Ben Hassen K, Kaabachi O. Management of post operative pain in TKR: Oral opioid versus IV PCA morphine. A preliminary study. Regional Anesthesia and Pain Medicine 2010;35:E116. [Google Scholar]
Jellinek 1990 {published data only}
- Jellinek H, Haumer H, Grubhofer G, Klappacher G, Jenny T, Weindlmayr-Goettel M, et al. Tramadol in postoperative pain therapy. Patient-controlled analgesia versus continuous infusion. Anaesthetist 1990;39:513-20. [PubMed] [Google Scholar]
Khalili 2013 {published data only}
- Khalili GR, Heidari SM, Sorani Heidari M. Comparison of intravenous morphine with morphine sulfate suppository in control of pain after knee and hip arthroplasty. Journal of Babol University Medical Sciences 2013;15:19-24. [Google Scholar]
Kilbride 1992 {published data only}
- Kilbride MJ, Senagore AJ, Mazier WP, Ferguson C, Ufkes T. Epidural analgesia. Surgery, Gynecology & Obstetrics 1992;174:137-40. [PubMed] [Google Scholar]
Kleiman 1988 {published data only}
- Kleiman RL, Lipman AG, Hare BD, MacDonald SD. A comparison of morphine administered in patient-controlled analgesia and regularly scheduled intramuscular injection in severe, postoperative pain. Journal of Pain Symptom Management 1988;3:15-22. [DOI] [PubMed] [Google Scholar]
Knapp‐Spooner 1995 {published data only}
- Knapp-Spooner C, Karlik BA, Pontieri-lewis V, Yarcheski A. Efficacy of patient-controlled analgesia in women cholecystectomy patients. International Journal of Nursing Studies 1995;32(5):434-42. [DOI] [PubMed] [Google Scholar]
Knudsen 1993 {published data only}
- Knudsen WP, Boettcher R, Vollmer WM, Griggs DK. A comparison of patient-controlled and intramuscular morphine in patients after abdominal surgery. Hospital Pharmacy 1993;28(2):117-22. [PubMed] [Google Scholar]
Lange 1988 {published data only}
- Lange MP, Dahn MS, Jacobs LA. Patient-controlled analgesia versus intermittent analgesia dosing. Heart and Lung 1988;17:495-8. [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee A, Chan SKC, Chen PP, Gin T, Lau ASC, Chiu CH. The costs and benefits of extending the role of the acute pain service on clinical outcomes after major elective surgery. Anesthesia and Analgesia 2010;111:1042-50. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee SK, Lee JW, Choy WS. Is multimodal analgesia as effective as postoperative patient-controlled analgesia following upper extremity surgery? Orthopaedics & Traumatology: Surgery & Research 2013;99:895-901. [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu XR, Wang YL, Wang CY. Effects of different patient-controlled analgesic methods on circulative function in hypertensive patients after upper abdominal surgery [Chinese]. Chinese Journal of Clinical Rehabilitation 2005;9:29-31. [Google Scholar]
Martinez‐Ubieto 1992 {published data only}
- Martinez-Ubieto J, Ortega-Lahuerta JP, Laglera S, Sanchez-Tirado JA, Carrion JC, Temino M. Usefulness of intravenous patient-controlled analgesia in the treatment of postoperative pain [Utilidad de la analgesia controlada por el paciente por via intravenosa en el tratamiento del dolor postoperatorio]. Revista Espanola de Anestesiologia y Reanimacion 1992;39:388-9. [PubMed] [Google Scholar]
Moller 1988 {published data only}
- Moller IW, Dinesen K, Sondergard S, Knigge U, Kehlet H. Effect of patient-controlled analgesia on plasma catecholamine, cortisol and glucose concentrations after cholecystectomy. British Journal of Anaesthesia 1988;61:160-4. [DOI] [PubMed] [Google Scholar]
Moreno 2000 {published data only}
- Moreno M, Castejon FJ, Palacio MA. Patient-controlled analgesia with ketorolac in pediatric surgery. Journal of Physiology & Biochemistry 2000;56:209-16. [DOI] [PubMed] [Google Scholar]
Nitschke 1996 {published data only}
- Nitschke LF, Schlosser CT, Berg RL, Sethafner JV, Wegner TJ, Avecilla CS. Does patient-controlled analgesia achieve better control of pain and fewer adverse effects than intramuscular analgesia? Archives of Surgery 1996;131:417-23. [DOI] [PubMed] [Google Scholar]
Paoletti 1993 (ortho) {published data only}
- Paoletti F, Ciammitti B, Tosti F, Boanelli A, Pasqualucci V. Postoperative analgesia i.v. [Analgesia postoperatoria endovenosa]. Minerva Anestesiologica 1993;59:523-30. [PubMed] [Google Scholar]
Peters 1999 {published data only}
- Peters JWB, Hoekstra B, Abu-saad HH, Bouwmester J, Meursing AEE, Tibboel D. Patient-controlled analgesia in children and adolescents: a randomized controlled trial. Paediatric Anesthesia 1999;9:235-41. [DOI] [PubMed] [Google Scholar]
Rittenhouse 1999 {published data only}
- Rittenhouse BE, Choiniere M. An economic evaluation of pain therapy after hysterectomy. Patient-controlled analgesia versus regular intramuscular opioid therapy. International Journal of Technology Assessment in Health Care 1999;15:548-62. [PubMed] [Google Scholar]
Robinson 1991 {published data only}
- Robinson SL, Rowbotham DJ, Smith G. Morphine compared with diamorphine. A comparison of dose requirements and side-effects after hip surgery. Anaesthesia 1991;46:538-40. [DOI] [PubMed] [Google Scholar]
Rothwell 2011 {published data only}
- Rothwell MP, Pearson D, Hunter JD, Mitchell PA, Graham-Woollard T, Goodwin L, et al. Oral oxycodone offers equivalent analgesia to intravenous patient-controlled analgesia after total hip replacement: a randomized, single-centre, non-blinded, non-inferiority study. British Journal of Anaesthesia 2011;106:865-72. [DOI] [PubMed] [Google Scholar]
Rundshagen 1999 {published data only}
- Rundshagen I, Schnabel K, Standl T, Schulte J. Patients' vs nurses' assessments of postoperative pain and anxiety during patient- or nurse-controlled analgesia. British Journal of Anaesthesia 1999;82:374-8. [DOI] [PubMed] [Google Scholar]
Sanansilp 1995 {published data only}
- Sanansilp V, Lertakyamanee J, Udompunturak S. Cost-effectiveness analysis of patient-controlled analgesia, intramuscular q.i.d. injection and p.r.n. injection for postoperative pain relief. Journal of the Medical Association of Thailand 1995;78:600-4. [PubMed] [Google Scholar]
Searle 1994 {published data only}
- Searle NR, Roy M, Bergeron G, Perrault J, Roof J, Heermans C, et al. Hydromorphone patient-controlled analgesia (PCA) after coronary artery bypass surgery. Canadian Journal of Anaesthesia 1994;41:198-205. [DOI] [PubMed] [Google Scholar]
Shin 2001 {published data only}
- Shin D, Kim S, Kim CS, Kim HS. Postoperative pain management using intravenous patient-controlled analgesia for pediatric patients. Journal of Craniofacial Surgery 2001;12:129-33. [DOI] [PubMed] [Google Scholar]
Spetzler 1987 {published data only}
- Spetzler B, Anderson L. Patient-controlled analgesia in the total joint arthroplasty patient. Clinical Orthopaedics and Related Research 1987;215:122-5. [PubMed] [Google Scholar]
Taylor 1994 {published data only}
- Taylor AM, Arthurs GA, Rosen M, Power I. A randomized controlled study of patient controlled analgesia versus intramuscular analgesia in 542 patients. European Journal of Anaesthesiology 1994;11:149-50. [Google Scholar]
Tsang 1999 {published data only}
- Tsang J, Brush B. Patient-controlled analgesia in postoperative cardiac surgery. Anaesthesia and Intensive Care 1999;27:464-70. [DOI] [PubMed] [Google Scholar]
Vengadesh 2005 {published data only}
- Vengadesh GS, Chandra SS, Robinson SS. Postoperative pain relief following abdominal operations: A prospective randomised study of comparison of patient controlled analgesia with conventional parenteral opioids. Indian Journal of Surgery 2005;67:34-7. [Google Scholar]
Viscusi 2004 {published data only}
- Viscusi ER, Reynolds L, Chung F, Atkinson LE, Khanna S. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA 2004;291:1333-41. [DOI] [PubMed] [Google Scholar]
Walson 1992 {published data only}
- Walson PD, Graves PS, Mortensen ME, Kern RA, Torch MA. Patient-controlled versus conventional analgesia for postsurgical pain relief in adolescents. Developmental Pharmacology and Therapeutics 1992;19:32-9. [DOI] [PubMed] [Google Scholar]
Weldon 1993 {published data only}
- Weldon BC, Connor M, White PF. Pediatric PCA: The role of concurrent opioid infusions and nurse-controlled analgesia. Clinical Journal of Pain 1993;9:26-33. [PubMed] [Google Scholar]
White 1998 {published data only}
- White CL, Pokrupa RP, Chan MH. An evaluation of the effectiveness of patient-controlled analgesia after spinal surgery. Journal of Neuroscience Nursing 1998;30:225-32. [DOI] [PubMed] [Google Scholar]
Woodhouse 1997 {published data only}
- Woodhouse A, Mather LE. The influence of age upon opioid analgesic use in the patient-controlled analgesia (PCA) environment. Anaesthesia 1997;52:949-55. [DOI] [PubMed] [Google Scholar]
Woods 1991 {published data only}
- Woods MP, Rayburn WF, McIntosh DG, Scott JC Jr, Smith ML, Anderson JR. Nalbuphine after major gynecologic surgery. Comparison of patient-controlled analgesia and intramuscular injections. Journal of Reproductive Medicine 1991;36:647-50. [PubMed] [Google Scholar]
Xiao 2011 {published data only}
- Xiao JF, Liu GW, Liu XJ, Hou XM, Gu MN. Effects of parecoxib on morphine dosage in postoperative patient-controlled analgesia following thoracoscope-assisted thoracotomy [Chinese]. Journal of Southern Medical University 2011;31:338-40. [PubMed] [Google Scholar]
Yost 2004 {published data only}
- Yost NP, Bloom SL, Sibley MK, Lo JY, McIntire DD, Leveno KJ. A hospital-sponsored quality improvement study of pain management after cesarean delivery. American Journal of Obstetrics and Gynecology 2004;190:1341-6. [DOI] [PubMed] [Google Scholar]
Zacharias 1990 {published data only}
- Zacharias M, Pfeifer MV, Herbison P. Comparison of two methods of intravenous administration of morphine for postoperative pain relief. Anaesthesia and Intensive Care 1990;18:205-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Legeby 2002 {published data only}
- Legeby M, Segerdahl M, Sandelin K, Wickman M, Ostman K, Olofsson C. Immediate reconstruction in breast cancer surgery requires intensive post-operative pain treatment but the effects of axillary dissection maybe more predictive of chronic pain. The Breast 2002;11:156-62. [DOI] [PubMed] [Google Scholar]
Additional references
APS 2008
- American Pain Society (APS). Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 6th edition. Glenview, IL: American Pain Society, 2008. [Google Scholar]
Ballantyne 1993
- Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo LF, Mosteller F. Postoperative patient-controlled analgesia: meta-analyses of initial randomized control trials. Journal of Clinical Anesthesia 1993;5:182-93. [DOI] [PubMed] [Google Scholar]
Carr 1998
- Carr DB, Miaskowski C, Dedrick SC, Williams GR. Management of perioperative pain in hospitalized patients: a national survey. Journal of Clinical Anesthesia 1998;10(1):77-85. [DOI] [PubMed] [Google Scholar]
Cepeda 1995
- Cepeda MA, Vargas L, Ortegon G, Sanchez MA, Carr DB. Comparative analgesic efficacy of patient-controlled analgesia with ketorolac versus morphine after elective intraabdominal operations. Anesthesia and Analgesia 1995;80:1150-3. [DOI] [PubMed] [Google Scholar]
Cepeda 1996
- Cepeda MS, Delgado M, Ponce M, Cruz C, Carr DB. Equivalent outcomes during postoperative patient-controlled intravenous analgesia with lidocaine plus morphine versus morphine alone. Anesthesia and Analgesia 1996;83:102-6. [DOI] [PubMed] [Google Scholar]
Christensen 2008
- Christensen KS, Adamson DN, Cohen AE, Mermelstein FH, Hamilton DA, McNicol E, et al. Analgesic efficacy and safety of a novel intranasal morphine formulation (morphine plus chitosan), immediate release oral morphine, intravenous morphine, and placebo in a postsurgical dental pain model. Anesthesia and Analgesia 2008;107:2018-24. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crews 2000
- Crews JC. New developments in epidural anesthesia and analgesia. Anesthesiology Clinics of North America 2000;18(2):251-6. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
DeKock 1994
- DeKock M, Levandhomme P, Scholtes JL. Intraoperative and postoperative analgesia using intravenous opioid, clonidine and lidocaine. Anaesthesia and Intensive Care 1994;22:15-22. [DOI] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD004233.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 1999
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18:427-37. [DOI] [PubMed] [Google Scholar]
Evans 1976
- Evans JM, Rosen M, MacCarthy J, Hogg MI. Apparatus for patient-controlled administration of intravenous narcotics during labour. Lancet 1976;1(7949):17-8. [DOI] [PubMed] [Google Scholar]
Fee 1996
- Fee H. Drugs used to supplement anaesthesia. In: Aitkenhead AR, Smith G, editors(s). Textbook of Anaesthesia. Third edition. United Kingdom: Churchill and Livingstone, 1996:159-77. [Google Scholar]
Ferrante 1989
- Ferrante FM, Orav E, Rocco AG, Gallo J. A statistical model for pain in patient-controlled analgesia and conventional intramuscular opioid regimen. Anesthesia and Analgesia 1989;67:457-61. [PubMed] [Google Scholar]
Ferrante 1990
- Ferrante FM, Ostheimer GW, Covino BG (editors). Patient Controlled Analgesia. Oxford: Blackwell Scientific, 1990. [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hankin 2007
- Hankin CS, Schein J, Clark JA, Panchal S. Adverse events involving intravenous patient-controlled analgesia. American Journal of Health-System Pharmacy 2004;64:1492-9. [DOI] [PubMed] [Google Scholar]
Harmer 1985
- Harmer M, Rosen M, Vickers MD (editors). Patient-Controlled Analgesia. Oxford: Blackwell Scientific, 1985. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jacox 1997
- Jacox A, Carr DB, Mahrenholz DM, Ferrell BM. Cost considerations in patient-controlled analgesia. Pharmacoeconomics 1997;12:109-20. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
Keeri‐Szanto 1971
- Keeri-Szanto M. Apparatus for demand analgesia. Canadian Anaesthetists' Society Journal 1971;18:581-2. [DOI] [PubMed] [Google Scholar]
Kiecolt‐Glaser 1998
- Kiecolt-Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery. Perspectives from psychoneuroimmunology. The American Psychologist 1998;53(11):1209-18. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehmann 1999
- Lehmann KA. Patient-controlled analgesia: an efficient therapeutic tool in the postoperative setting. European Surgical Research 1999;31(2):112-21. [DOI] [PubMed] [Google Scholar]
Liu 2007
- Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesthesia and Analgesia 2007;104:689-702. [DOI] [PubMed] [Google Scholar]
Micromedex 2014
- DRUGDEX System (2.0). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Updated 25 September 2014 (accessed 2 October 2014).
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;7:209-16. [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post-operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28:427-32. [DOI] [PubMed] [Google Scholar]
Overdyk 2007
- Overdyk FJ, Carter R, Maddox RR, Callura J, Herrin AE, Henriquez C. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesthesia and Analgesia 2007;105:412-8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sackett 1996
- Sackett DL, Deeks JJ, Altman DG. Down with odds ratios! Evidence Based Medicine 1996;1:164-6. [Google Scholar]
Schezer 1968
- Schezer PH. Objective measurement of pain. Anesthesiology 1968;29:209-10. [Google Scholar]
Schug 2000
- Schug AS. Patient controlled analgesia - the good, the bad and the ugly! Acute Pain 2000;3(2):60-1. [Google Scholar]
Souter 1995
- Souter AJ, Fredman B, White PF. Controversies in the perioperative use of non-steroidal anti-inflammatory drugs. Anesthesia and Analgesia 1995;79(6):1178-90. [DOI] [PubMed] [Google Scholar]
Stoelting 1999
- Stoelting R. Opioid agonists and antagonists. In: Stoelting R, editors(s). Pharmacology and Physiology in Anesthetic Practice. Third edition. Philadelphia: Lippincott-Raven, 1999:77-112. [Google Scholar]
Stoneham 1995
- Stoneham MD, Walters FJ. Post-operative analgesia for craniotomy patients: current attitudes among neuroanaesthetists. European Journal of Anaesthesiology 1995;12:571-5. [PubMed] [Google Scholar]
Walder 2001
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiologica Scandinavica 2001;45:795-84. [DOI] [PubMed] [Google Scholar]
Warfield 1995
- Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology 1995;83(5):1090-4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hudcova 2006
- Hudcova J, McNicol ED, Quah CS, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003348.pub2] [DOI] [PubMed] [Google Scholar]


