Abstract
In recent years, successive reports have been made on large-scale cardiovascular outcome trials using novel hypoglycemic drugs. Their results have shown that sodium–glucose cotransporter 2 (SGLT2) inhibitors are hypoglycemic drugs that could potentially greatly improve the heart failure-related outcomes in type 2 diabetes patients with a high cardiovascular risk. Further analyses have subsequently been performed from various perspectives, and SGLT2 inhibitors with their class effect have been indicated to be potentially useful for heart failure in type 2 diabetes patients with extensive clinical background. As a result, a clear concept has globally emerged with SGLT2 inhibitors as drugs of choice in clinical practice to prevent heart failure in type 2 diabetes patients. Further studies are needed to examine the next research topics on heart failure prevention using SGLT2 inhibitors, including their detailed pharmacological mechanism of action and their effectiveness and safety against heart failure in patients regardless of diabetes status. This paper outlines (1) the current evidence of heart failure prevention by SGLT2 inhibitors based on the results of recent large-scale cardiovascular outcome trials and (2) future research topics on their further applications in clinical practice.
Keywords: SGLT2 inhibitor, Heart failure, Cardiovascular outcome trial, Type 2 diabetes
Introduction
In recent years, major attention has been focused on heart failure (HF) as a cardiovascular complication in diabetes patients [1–4]. One reason behind this attention is that HF-related outcomes are becoming evaluated in large-scale clinical trials of antidiabetic drugs in addition to macroangiopathy from conventional arteriosclerosis. Thus, the effects on HF-related outcomes are gradually becoming evident for each antidiabetic drug, and a body of evidence has accumulated on the selection of antidiabetic drug with consideration for HF. The clinical features of comorbid HF in diabetes include the following: (1) although the comorbidity rate of HF increases with decreasing glycemic control [5], improvement in glycemic control has not been fully shown to prevent HF; (2) since diabetes patients tend to have HF with preserved ejection fraction (HFpEF) more than HF with reduced ejection fraction (HFrEF), there could be an unexpectedly high number of patients who have yet to be diagnosed definitively [6]; and (3) since preventive or therapeutic effects of conventional antidiabetic drugs have not been demonstrated against HF [7], novel prevention and treatment strategies are urgently needed. Meanwhile, large-scale clinical trials using SGLT2 inhibitors have indicated the potential in their strong preventive effect against HF, thereby building an expectation of a major breakthrough in the HF prevention strategy for diabetic patients. This paper outlines (1) the current state of HF prevention in diabetic patients based on evidence of such prevention, focusing on SGLT2 inhibitors used in large-scale clinical trials, and (2) future topics of research.
Clinical relevance of diabetes and HF
Diabetes, with obesity and metabolic syndrome at its foundation, is well accepted as a strong risk factor for macroangiopathies, such as myocardial infarction and stroke. Diabetes is also a risk factor for HF independently of hypertension and coronary artery disease. However, there is insufficient awareness of this reality reflected in the existing routine medical care, including among cardiologists. The Framingham study has found that the incidence of HF was two times higher in diabetic men and five times higher in diabetic women than in their non-diabetic counterparts [8], and approximately 20% of diabetic patients aged 65 or older were said to have HF. Another study examined type 2 diabetes patients without a past diagnosis of HF and found that 28% of these patients were diagnosed with HF after detailed examination, the majority of whom had HFpEF [6]. This result suggested that there is a certain frequency of subclinical HFpEF in type 2 diabetes patients. Still, another study has reported that the mortality rate was ten times higher in diabetic patients with comorbid HF than those without, and the former had a 5-year survival rate of only 12.5% [9]. In Japan, the survival rates of myocardial infarction and angina pectoris have greatly improved due to advancements in therapeutic techniques and devices, and HF in diabetic patients is a very clinically important target for prevention and treatment.
HF itself is closely associated with the onset of diabetes, and approximately 20–40% of HF patients have been reported to have comorbid diabetes based on Japanese studies, including the Chronic Heart Failure Analysis and Registry in the Tohoku District (CHART)-2 Study and the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). When the background was examined for registered patients in past multiple large-scale clinical trials, the diabetes comorbidity rate increased with the severity of HF (New York Heart Association functional classification). Thus, diabetes and HF are associated bidirectionally. The underlying pathology is considered to involve very diverse mechanisms, such as microcirculatory disturbance and vascular endothelial dysfunction, and metabolic abnormalities, such as lipotoxicity and glucotoxicity—mainly insulin resistance. Diabetic cardiopathy has been proposed as the disease concept [10]. However, the detailed molecular mechanism has not yet been sufficiently elucidated, and specific treatment for this pathology has not yet been fully established.
Existing HF prevention strategies in diabetes
In diabetic patients, HF prevention strategies (including primary and secondary prevention) can be thought to have two aspects, diabetes treatment and HF treatment. In general, there is presently no clear difference in HF treatment between diabetic patients and non-diabetic patients. Renin–angiotensin system inhibitors, β-blockers, and diuretics are used particularly for patients with HFrEF. However, sufficient evidence has not yet been established globally for the treatment of HFpEF which is strongly associated with diabetes.
Management of diabetes holds major significance in HF prevention. Each 1% increase in HbA1c has been reported to result in an 8% increase in the risk of hospitalization for HF (HHF) or death from HF [5], and the prognosis of HF is considered to be proportionally worse with increasing severity of diabetes. However, the reduction of HbA1c by strict glycemic control and antidiabetic drugs has not yet been shown to be effective in HF prevention [7, 11]. Thiazolidine derivatives enhance sodium reabsorption, causing fluid retention and edema at a certain frequency. Thus, they are contraindicated in HF patients. Since biguanides were thought to increase the risk of lactic acidosis, they are contraindicated in patients with severe cardiovascular disorder or pulmonary dysfunction. In recent years, however, European and US studies have examined diabetic patients with HF and have shown that biguanides improved their HHF rate and mortality rate [12, 13]. Therefore, biguanides are no longer contraindicated drugs in these countries. Even in Europe and the US, they are still contraindicated in patients with HF with unstable hemodynamics such as acute HF. In Japan, they continue to be relatively contraindicated, because clear evidence on their effectiveness is limited in patients with chronic HF. Based on such a background, the Japanese Circulation Society has stated in its Guidelines for Treatment of Chronic Heart Failure (2010 revised version) that there is no firm evidence to recommend a particular diabetes treatment in patients with chronic HF with comorbid diabetes.
Incretin-based drugs and HF outcomes in large-scale clinical trials
In recent years, large-scale cardiovascular outcome trials (CVOTs) have been required in Europe and the US to obtain the approval for new antidiabetic drugs. Subsequently, the effects of individual antidiabetic drugs have been shown on the cardiovascular outcomes. There have been large-scale trials conducted on dipeptidyl peptidase-4 (DPP-4) inhibitors, which are frequently used in Japan. In the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)—thrombolysis in myocardial infarction (TIMI) 53 trial, HHF increased 27% in patients taking saxagliptin [14]. In the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome (EXAMINE) trial, HHF tended to increase, although not significantly, in diabetic patients with recent acute coronary syndrome and taking alogliptin [15]. However, an increase in HF risk was not seen in large-scale outcome trials using other DPP-4 inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, and these drugs showed neutral results compared with the placebo. Although there were some differences among trials in the effect of DPP-4 inhibitors on HF risk, their effects were considered largely neutral if concerns regarding some drugs were excluded. More recently, in the Cardiovascular and Renal Microvascular Outcome Study With Linaglipin (CARMELINA) trail, linagliptin at least did not increase the risk of HHF in patients with type 2 diabetes and established cardiovascular disease with albuminuria and/or kidney disease (HR 0.90; 95% CI 0.74–1.08) [16], suggesting its safety for HF. Furthermore, a substudy from the CARMELINA trial demonstrated that linagliptin was associated with a nominal decrease in the risk of HHF in Asian population (HR 0.47; 95% CI 0.24–0.95) [17]. The latest European Society of Cardiology (ESC) and European Association for the Study of Diabetes (EASD) Guidelines on diabetes established DPP-4 drugs as antidiabetic drugs for HF patients with class IIb recommendation, but excluded DPP-4 inhibitors suspected to increase the HF risk [18].
Seven CVOTs have been conducted on other incretin-based drugs, GLP-1 receptor agonists. A meta-analysis study on these seven trials was published in 2019 and showed that GLP-1 receptor agonists significantly decreased HHF by 9% [19]. Existing GLP-1 receptor agonists have been implicated to improve cardiovascular outcomes, mainly by antiarteriosclerotic effects [20]. Since the aforementioned decrease in HHF occurred in parallel with the inhibitory effect on myocardial infarction, secondary HF was suggested to be likely inhibited as a result of inhibition of myocardial infarction (Fig. 1). Because a GLP-1 receptor agonist was implicated to potentially increase mortality and HHF in a clinical trial with acute HF patients [21], careful follow-up examinations are necessary for HF patients, particularly when they are administered at the acute stage.
Fig. 1.
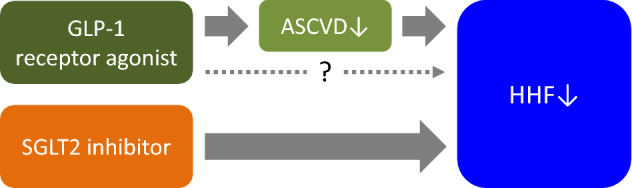
Speculated differences in reduction of hospitalization for heart failure: comparison between GLP-1 receptor agonists and SGLT2 inhibitors. The results of large-scale trials have indicated the possibility that GLP-1 receptor agonists contribute to the inhibition of secondary heart failure onset by inhibiting atherosclerotic cardiovascular disease, mainly myocardial infarction. Primary preventive effect of GLP-1 receptor agonists on heart failure remains to be clinically determined. When GLP-1 receptor agonists are compared to SGLT2 inhibitors, the latter can be expected to have a more direct effect on the reduction of heart failure based on the multidisciplinary effects of SGLT2 inhibitors and their consistent results against heart failure in large-scale trials. GLP-1 glucagon-like peptide-1, SGLT2 sodium–glucose cotransporter 2, ASCVD atherosclerotic cardiovascular disease, HHF hospitalization for heart failure
Basic pharmacological effects of SGLT2 inhibitors
SGLT2 inhibitors selectively inhibit SGLT2, which is the major mechanism of glucose reabsorption in the renal proximal tubules. Thus, these inhibitors are antidiabetic drugs with the plain and simple effect of increasing urinary glucose excretion, thereby decreasing glucose reabsorption into the blood. When SGLT2 inhibitors are compared with conventional antidiabetic drugs, the most characteristic feature of SGLT2 inhibitors is their unique and efficient pharmacological effect which enables glucose (+ sodium) excretion in the urine in an insulin-independent manner. Although SGLT2 inhibitors have this seemingly simple effect, they are also considered to have a complex effect on the pathology of diabetes. In pathologies such as hyperglycemia and diabetes, the renal threshold for glucose increases in the tubule simultaneously with the increase in SGLT2 expression at the same site. Then, glucose and sodium reabsorption increase, and subsequently glucose metabolism is worsened and blood pressure increases, resulting in a vicious cycle of pathology [22]. In other words, SGLT2 inhibitors can effectively eliminate glucotoxicity and demonstrate their effect in such pathological situations. Since their effects are also insulin-independent, they can be expected to safely lower glucose. In addition, the results on various metabolic pathways have shown that SGLT2 inhibitors can be expected to have diverse effects on other important clinical indices such as body weight, blood pressure, and uric acid [23]. Many clinicians have likely seen such effects in clinical practice, and SGLT2 inhibitors are recognized to exert comprehensive benefits of reducing multiple cardiovascular risks. This point indicates the characteristic pharmacological effect of SGLT2 inhibitors, that is not seen in other hypoglycemic drugs. In the undermentioned large-scale cardiovascular outcome trials, the consistent improvement of cardiovascular outcomes, including the HF-related outcomes, was speculated to be largely attributable to SGLT2 inhibitors.
Summaries of large-scale cardiovascular outcome trials
This section will summarize the results, mainly of cardiovascular outcomes, in large-scale cardiovascular outcome trials using SGLT2 inhibitors. These results were reported in publications, such as main research papers and subgroup analysis papers, as of November 2019.
-
Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial [24]
The subjects were 7020 high-risk type 2 diabetes patients with a history of cardiovascular disease (all patients were secondary prevention cases). As a result, empagliflozin showed a significant 35% risk reduction in the risk of HHF. It is still fresh in our memory that SGLT2 inhibitors drew major attention which was triggered by the results of this trial. Subsequently, a stratified analysis was performed on patients with a history of HF and those without. Only about 10% of the total patients had a history of HF, but empagliflozin was statistically shown to improve HF-related outcomes independently of history of HF [25]. A stratified analysis was performed on HF risk in patients without a history of HF, who accounted for the majority of the patients in this trial. The HF risk was stratified into three groups using the 9-variable Health ABC HF Risk score: low-to-average (< 10%), high (10–20%), and very high (≥ 20%). All three groups showed a consistent improvement of HF-related outcomes [26]. When stratification was performed by cardiovascular risk based on the ten-point TIMI Risk Score and by the absence/presence of macrovascular diseases (defined by the absence/presence of myocardial infarction, coronary artery bypass graft, or stroke), empagliflozin was also found to have a consistent inhibitory effect on cardiovascular events, including HF [27, 28]. In an interesting subgroup analysis, short-term effects were examined on re-hospitalization for HF (+ death) in these patients. The empagliflozin groups had HF readmission rates that were approximately half of that of the placebo group during 45–90 days after first admission. When the composite outcome of HF readmission and death was examined, the inhibitory effect on this outcome manifested 30 days after the first hospitalization, suggesting a major effect of empagliflozin even on short-term prognosis after the event of HHF [29]. Furthermore, in a subgroup analysis of only Asian patients [30], empagliflozin demonstrated its effectiveness in the inhibition of cardiovascular events, including HF, at a level similar to that of the overall trial population. These results led to further attention on SGLT2 inhibitors also in Japan.
-
Canagliflozin Cardiovascular Assessment Study (CANVAS) Program [31]
CANVAS and CANVAS-Renal (CANVAS-R) are known collectively as the CANVAS Program, which integrated analyses of these two trials. Of a total of 10,142 participants of the CANVAS Program, 70% were secondary prevention patients with a history of cardiovascular disease similar to patients in the EMPA-REG OUTCOME trial. The results were similar to those of the EMPA-REG OUTCOME trial; canagliflozin showed a significant 33% reduction in the risk of HHF. When a stratified analysis was performed on the presence/absence of history of cardiovascular disease [32], no statistical evidence of heterogeneity was observed for the effects of canagliflozin on either outcome of MACE or HHF in the primary and secondary prevention groups. When a stratified analysis was performed on the presence/absence of HF history (14% of the total patients had an HF history), more remarkable reductions of HHF risk and cardiovascular mortality risk were seen in patients with HF history than patients without [33]. In an interesting subgroup analysis on HF events, the phenotypes of HF (HFpEF and HFrEF) at onset were analyzed in participants of this trial. Canagliflozin was found to reduce the risk for both phenotypes compared with placebo. The result of this analysis also suggested a potential for greater inhibition of HFrEF onset than of HFpEF onset [34].
-
Dapagliflozin effect on cardiovascular events-thrombolysis in myocardial infarction 58 (DECLARE-TIMI 58) trial [35]
This trial was performed on a much larger scale with a total of 17,160 patients compared with the aforementioned trials. Since approximately 40% of the total patients had a history of cardiovascular disease, the most notable characteristic of this trial is that many primary prevention patients were enrolled. Dapagliflozin showed a significant 17% risk reduction compared with the placebo in the primary outcome measure—a composite of cardiovascular death or HHF. It also showed a significant 27% risk reduction in HHF. When a stratified analysis was performed by the presence/absence of history of myocardial infarction [36], dapagliflozin tended to show a strong risk-reduction effect for cardiovascular death or HHF in patients with a history of myocardial infarction. It also showed a certain level of risk reduction in patients without a history of myocardial infarction, suggesting the possibility that dapagliflozin improves HF-related outcomes independent of myocardial infarction history. When a stratified analysis was performed based on the left-ventricular ejection fraction at registration (HFrEF patients with EF < 45% accounted for 3.9% of the total patients; HF patients with unknown EF accounted for 7.7%) [37], dapagliflozin reduced the risk for HHF independent of EF. However, it markedly reduced the risk of cardiovascular death and all-cause mortality in HFrEF patients, suggesting that an SGLT2 inhibitor might have a differing effect on some cardiovascular prognoses depending on EF.
-
Canagliflozin and renal events in diabetes with established nephropathy clinical evaluation (CREDENCE) [38]
Unlike the aforementioned three trials, this trial examined the effects of an SGLT2 inhibitor (canagliflozin in this trial) on composite renal outcomes in type 2 diabetes patients with chronic kidney disease (CKD). The secondary outcome measures of this trial were MACE and HHF. The trial included a total of 4401 patients, and 50% of the total patients had a history of cardiovascular disease. In the trial, canagliflozin not only had markedly inhibited the composite renal events but also had significantly reduced MACE by 20% and HHF by 39%. These findings were the first results to show the effectiveness and safety of an SGLT2 inhibitor in CKD patients.
Dapagliflozin and prevention of adverse outcomes in heart failure trial (DAPA-HF) [39]
This trial largely differed from the aforementioned four trials in that its subjects were HFrEF patients only, regardless of whether or not they had diabetes. This trial had a total of 4744 patients, of whom 45% had a history of diabetes. Dapagliflozin showed a significant 26% reduction in the incidence of primary outcome measure (composite of cardiovascular death or aggravated HF) and a significant 30% reduction in the aggravation of the initial HF. An interesting point is that dapagliflozin showed similar effectiveness regardless of whether or not the patients had diabetes, suggesting a possible therapeutic effect of SGLT2 inhibitor on HF itself.
Inhibition of cardiovascular event and HF prevention using SGLT2 inhibitors
Zelniker et al. [40] performed a meta-analysis on the effects of SGLT2 inhibitors on the risk of cardiovascular events in the earlier three trials described above (EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58). The following summary is based on their results.
The SGLT2 inhibitors have a higher potential to be effective in the secondary prevention patients than the primary prevention patients against the primary outcome measure of MACE (cardiovascular death, myocardial infarction, and stroke).
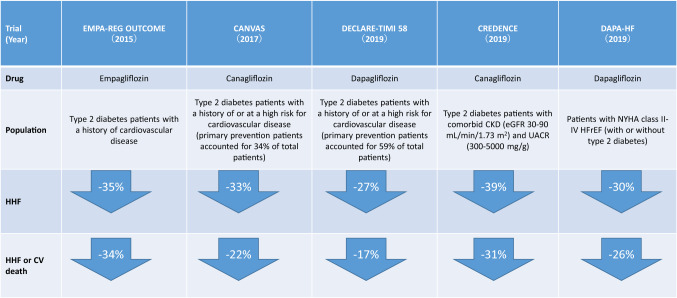
In all trials, there was a consistent risk reduction of approximately 30% for HHF (+ cardiovascular death) (Fig. 2), and the SGLT2 inhibitors have a high potential to be effective in a wide variety of type 2 diabetes patients independent of their history of HF or atherosclerotic cardiovascular disease.
The SGLT2 inhibitors also showed a consistent risk reduction of composite renal events similar to the risk reduction of HHF. These inhibitors not only are effective in patients at high risk for cardiovascular events, but also have a high potential to be similarly effective in type 2 diabetes patients with a history of CKD (details are not presented in our paper).
Fig. 2.
Summary of impact on heart failure-related outcomes observed in the cardiovascular outcome trials with SGLT2 inhibitors
As discussed above, the results of recent large-scale trials using SGLT2 inhibitors advanced the substance of diabetes treatment onto a new stage. The substance of diabetes treatment included the prevention of cardiovascular disease and the improvement in prognosis for survival in type 2 diabetes patients. In particular, the dramatic effects of SGLT2 inhibitors have clearly been demonstrated for the prevention of HF, which is the theme of our paper, and for renal events. Such dramatic effects were thought to be very characteristic results of SGLT2 inhibitors which had not seen ever in other antidiabetic drugs. Thus, SGLT2 inhibitors are the promising class of hypoglycemic drug in which the preventive effect on HF has been demonstrated [41].
The above results have led to a high level of global attention on SGLT2 inhibitors’ positioning in diabetes treatment strategy, which aims to improve outcomes, such as renal prognosis, and to prevent HF in diabetic patients. The descriptions that follow explain the treatment policy, particularly in Europe and the U.S., for patients who fail to achieve target glycemic control using metformin—the first-line hypoglycemic drug. An SGLT2 inhibitor or GLP-1 receptor agonist is indicated for type 2 diabetes patients with a history of atherosclerotic cardiovascular disease, and an SGLT2 inhibitor is indicated in patients where HF or CKD predominates [18, 42]. In Japan, the Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure (revised 2017) indicate SGLT2 inhibitors, with a high level of evidence and class of recommendation, as a treatment for diabetic patients with HF and HF prevention in obese and diabetic patients [43].
What is the mechanism of HF prevention?
As discussed above, SGLT2 inhibitors have a very characteristic preventive effect on HF. Was this effect within the initially predicted range or an unexpected result [44]? What is its mechanism? When SGLT2 inhibitors are re-examined from the viewpoint of pharmacological effect, the most significant effect is the inhibition of glucose and sodium reabsorption in the proximal tubule. From the perspective of HF prevention, it is not difficult to imagine that this inhibition of sodium reabsorption (≈ promotion of natriuresis) is the leading trigger in the HF prevention mechanism [45]. In clinical practice, the diuretic effect of SGLT2 inhibitors is temporary, occurring only in the initial stage of their administration. Thus, any clinicians who prescribed SGLT2 inhibitors would have personally experienced that their diuretic effect is not sustained long term. That is, HF prevention occurs not only due to the diuretic effect from natriuresis, but also with natriuresis as the starting point. The mechanism is speculated to involve the following effects which are downstream of this starting point and occur in a continuous fashion: preload/afterload reduction, cardiorenal-related improvement, inhibition of sympathetic nervous system activity, and hemodynamic effects such as due to improvement in vascular compliance and increase in hematocrit level. Furthermore, it is speculated that the mechanism is in continuous action which results in beneficial effects on various metabolic aspects in tissues of the heart, kidneys, and vessels. In fact, recent basic research has also inferred that although SGLT2 receptors are not expressed in the heart, SGLT2 inhibitors have protective cardiac effects via wide-ranging (broadly defined) metabolic pathways [46, 47]): a direct anti-inflammatory effect on cardiac tissue; an inhibitory effect on a Na+ /H+ exchanger (NHE3)—which increases its activity due to HF and then imposes impairments of ionic homeostasis in the heart (Na+ and Ca2+ overload in the myocardium and decrease in mitochondrial Ca2+)—and thereby improving the cardiac calcium handling and mitochondrial function [48]; and improvement in myocardial energy metabolism, resulting from improved mitochondrial function and increased ketone body production [49]. From these results, SGLT2 inhibitors are speculated to have direct inhibitory effects on HF, compared to the GLP-1 receptor agonists (Fig. 1). We clinicians may think that the main mechanism of SGLT2 inhibitors in HF prevention involves their effect on macro-hemodynamic parameters, such as those causing body weight and blood pressure reduction due to their diuretic effect. The greatest potential of SGLT2 inhibitors is not limited to such macro-effects, but also extends to the improvement of glucose and lipid metabolism and triggering of various micro-effects on metabolic and biological remodeling in many organs and tissues. The resulting effects are very diverse and demonstrated synergistically and comprehensively with time and without interruption. We think that such multidisciplinary effects constitute the mechanism of their protective effects against renal and cardiovascular systems, including against HF [50]. Furthermore, in the DAPA-HF trial, an SGLT2 inhibitor also showed effectiveness against HF in non-diabetic patients, and the mechanism is predicted to be at the least independent of glucose metabolism improvement and elimination of glucotoxicity. However, HF is often complicated with not only overt ‘diabetes’, but also several degrees of glucose intolerance, namely ‘pre-diabetes’. To date, whether SGLT2 inhibitors can exert beneficial impacts on HF in patients with pre-diabetes remains to be elucidated. Thus, their detailed mechanism of action against HF has not yet been fully elucidated and is a topic of future research [51].
Future research topic and outlook
The understanding of SGLT2 inhibitors greatly affected by the results of trials, such as the CVOTs in type 2 diabetes patients at a high cardiovascular risk and the DAPA-HF trial in HFrEF patients with or without diabetes. SGLT2 inhibitors, a class of antidiabetic drug, have garnered large attention because of their potential of beneficial effects against HF itself. We, as cardiologists, await with great anticipation their drug repositioning for HF treatment. However, the effects of SGLT2 inhibitors on HF itself have not yet been fully elucidated. In fact, enrolled subjects with a history of HF accounted for only 10–15% of the total subjects in the earlier CVOTs. Furthermore, specific effects have not yet been fully examined on clinical indicators related to HF, such as biomarkers and cardiac function, and there are only limited reports on patients irrespective of HF at baseline [52–54]. Therefore, multiple clinical studies are being performed specifically on indices related to HF using surrogate markers for HF (such as echocardiographic index, natriuretic peptide, and exercise tolerance) in diabetic patients with HF. There should be future progress in the elucidation of specific clinical mechanisms and effects.
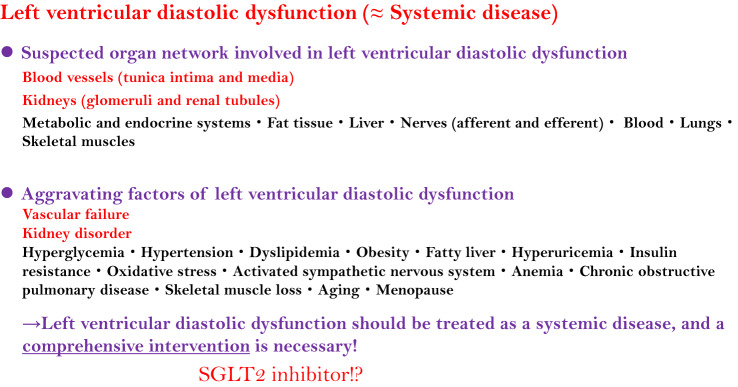
Importantly, effective treatment strategy and drugs have not yet been established for HFpEF, resulting in unmet clinical needs in HF treatment. It differs greatly from HFrEF in which the effectiveness of drugs, such as renin–angiotensin–aldosterone system inhibitors and β blockers, and treatment strategies have been sufficiently established. Since many diabetes patients are likely to suffer from HFpEF, a treatment for HFpEF is also thought to be of great future interest in diabetes treatment. Left-ventricular diastolic dysfunction—a factor of these clinical unmet needs and the main pathology of HFpEF—is a cardiac phenotype in the failure of the multi-organ network, in other words systemic disease [55]. That is, comprehensive care is thought necessary for these multiple organs in the HFpEF treatment. Under these circumstances, the aforementioned multidisciplinary effects of SGLT2 inhibitors are considered the very effects that are needed in an effective comprehensive intervention, mainly targeting kidney disorders and vascular failure (Fig. 3). A multinational clinical trial is also presently being conducted to examine the effects of SGLT2 inhibitors on HFpEF [56], resulting in great anticipation among cardiologists for the potential of these inhibitors in HFpEF treatment.
Fig. 3.
Roles of SGLT2 inhibitors in left-ventricular diastolic dysfunction. There is much anticipation in the multidisciplinary effects of SGLT2 inhibitors in a comprehensive intervention for left-ventricular diastolic dysfunction (≈ HFpEF), a systemic disease
Thus, we anticipate further accumulation of evidence for the scope of indications, examination of effectiveness in clinical practice, and the elucidation of mechanism of action against a specific type of HF.
Conclusions
The recent results of large-scale clinical trials have attracted major attention in the treatment of HF as a cardiovascular complication in diabetic patients. Under such a circumstance, there has been great anticipation for SGLT2 inhibitors in a strategy for HF prevention in diabetic patients. The concept of such SGLT2-inhibitor use has just begun to be advanced in the light of major evidence being accumulated. In the future, it will be necessary to elucidate the effectiveness and mechanism of action against more specific types of HF such as HFpEF, to examine clinical long-term safety, and to carefully examine the scope of clinical indications.
Acknowledgements
The authors would like to thank Ms. Aya Yamada (Saga University) for her excellent support. This work was partly supported by Taiju Life Social Welfare Foundation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Atsushi Tanaka, Email: tanakaa2@cc.saga-u.ac.jp.
Koichi Node, Email: node@cc.saga-u.ac.jp.
References
- 1.Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853–872. doi: 10.1002/ejhf.1170. [DOI] [PubMed] [Google Scholar]
- 2.Marx N. Heart failure: an underestimated therapeutic target in diabetes. Cardiovasc Endocrinol Metab. 2018;7(1):10–12. doi: 10.1097/XCE.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–351. doi: 10.1016/j.jacc.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–e324. doi: 10.1161/CIR.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 5.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103(22):2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 6.Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, Rutten GE, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012;55(8):2154–2162. doi: 10.1007/s00125-012-2579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3(5):356–366. doi: 10.1016/S2213-8587(15)00044-3. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 9.Khan SS, Butler J, Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311(23):2379–2380. doi: 10.1001/jama.2014.4115. [DOI] [PubMed] [Google Scholar]
- 10.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57(4):660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 12.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6(3):395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 13.Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166(3):191–200. doi: 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 15.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 16.McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139(3):351–361. doi: 10.1161/CIRCULATIONAHA.118.038352. [DOI] [PubMed] [Google Scholar]
- 17.Inagaki N, Yang W, Watada H, Ji L, Schnaidt S, Pfarr E, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA® trial. Diabetol Int. 2019 doi: 10.1007/s13340-019-00412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 19.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka A, Node K. Clinical application of glucagon-like peptide-1 receptor agonists in cardiovascular disease: lessons from recent clinical cardiovascular outcomes trials. Cardiovasc Diabetol. 2018;17(1):85. doi: 10.1186/s12933-018-0731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316(5):500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54(12):3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 23.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes Vasc Dis Res. 2015;12(2):90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 25.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37(19):1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2018;39(5):363–370. doi: 10.1093/eurheartj/ehx511. [DOI] [PubMed] [Google Scholar]
- 27.Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139(11):1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma S, Mazer CD, Fitchett D, Inzucchi SE, Pfarr E, George JT, et al. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME(R) randomised trial. Diabetologia. 2018;61(8):1712–1723. doi: 10.1007/s00125-018-4644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savarese G, Sattar N, Januzzi J, Verma S, Lund LH, Fitchett D, et al. Empagliflozin is associated with a lower risk of post-acute heart failure rehospitalization and mortality. Circulation. 2019;139(11):1458–1460. doi: 10.1161/CIRCULATIONAHA.118.038339. [DOI] [PubMed] [Google Scholar]
- 30.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. Empagliflozin and cardiovascular outcomes in asian patients with type 2 diabetes and established cardiovascular disease- results from EMPA-REG OUTCOME((R)) Circ J. 2017;81(2):227–234. doi: 10.1253/circj.CJ-16-1148. [DOI] [PubMed] [Google Scholar]
- 31.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 32.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation. 2018;138(5):458–468. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figtree GA, Radholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation. 2019;139(22):2591–2593. doi: 10.1161/CIRCULATIONAHA.119.040057. [DOI] [PubMed] [Google Scholar]
- 35.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 36.Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516–2527. doi: 10.1161/CIRCULATIONAHA.119.039996. [DOI] [PubMed] [Google Scholar]
- 37.Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139(22):2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 38.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 39.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 40.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 41.Fitchett DH, Udell JA, Inzucchi SE. Heart failure outcomes in clinical trials of glucose-lowering agents in patients with diabetes. Eur J Heart Fail. 2017;19(1):43–53. doi: 10.1002/ejhf.633. [DOI] [PubMed] [Google Scholar]
- 42.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Management of Hyperglycemia in Type 2 Diabetes et al. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure-digest version. Circ J. 2019;83(10):2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 44.Verma S, McMurray JJV. The serendipitous story of SGLT2 inhibitors in heart failure. Circulation. 2019;139(22):2537–2541. doi: 10.1161/CIRCULATIONAHA.119.040514. [DOI] [PubMed] [Google Scholar]
- 45.Farkouh ME, Verma S. Prevention of heart failure with SGLT-2 inhibition: insights from CVD-REAL. J Am Coll Cardiol. 2018;71(22):2507–2510. doi: 10.1016/j.jacc.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 46.Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. 2018;17(1):101. doi: 10.1186/s12933-018-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–1029. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 49.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3(5):575–587. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka A, Node K. Emerging roles of sodium-glucose cotransporter 2 inhibitors in cardiology. J Cardiol. 2017;69(3):501–507. doi: 10.1016/j.jjcc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Packer M. Lessons learned from the DAPA-HF trial concerning the mechanisms of benefit of SGLT2 inhibitors on heart failure events in the context of other large-scale trials nearing completion. Cardiovasc Diabetol. 2019;18(1):129. doi: 10.1186/s12933-019-0938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Januzzi JL, Jr, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. 2017;70(6):704–712. doi: 10.1016/j.jacc.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME Trial? Diabetes Care. 2016;39(12):e212–e213. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]
- 54.Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140(18):1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929. [DOI] [PubMed] [Google Scholar]
- 55.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 56.Butler J, Hamo CE, Filippatos G, Pocock SJ, Bernstein RA, Brueckmann M, et al. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19(11):1390–1400. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]