Abstract
Recently, it is widely recognized that microinflammation plays important roles in the pathophysiology of metabolic diseases, especially obesity-related disorders, diabetes and their complications. Lipopolysaccharide-binding protein (LBP) is a liver-derived acute-phase protein responsive to lipopolysaccharides (LPS) produced by gram-negative bacteria, thus reflects the systemic inflammation caused by the infection of those bacteria including gut dysbiosis. In this study, we evaluated the plasma LBP levels and investigated its clinical significance in 67 Japanese patients with type 1 diabetes. Univariable analysis showed that LBP levels were significantly associated with body mass index (BMI; r = 0.43, p < 0.01) and serum high-sensitivity C-reactive protein (hs-CRP; r = 0.64, p < 0.001) levels. However, there was no significant association between plasma LBP levels and diabetic complications. Mediation analysis revealed that LBP had significant mediation effects on the association between hs-CRP and BMI (0.27 [95% confidence interval 0.10–0.48]). These results suggest that the systemic condition where the LBP level increases, such as gut dysbiosis, at least partly, impacts on chronic microinflammation in patients with type 1 diabetes.
Keywords: Type 1 diabetes, Lipopolysaccharide-binding protein, Body mass index, Microinflammation
Introduction
Type 1 diabetes is a chronic metabolic disease characterized by absolute insulin deficiency and hyperglycemia, and is associated with various chronic complications [1]. In addition to the ‘classical’ concept of hyperglycemia-induced complications, the involvement of microinflammation in the development and progression of various complications in numbers of metabolic diseases has been revealed [2]. For example, microinflammation in obese adipose tissue is known to contribute to the development of adipocyte dysfunction, systemic insulin resistance and vascular damages through the production of proinflammatory cytokines by invaded immune cells [3, 4]. On the other hand, recently, the contribution of gut microbiota in various diseases has also been highlighted [5–7]. Gut dysbiosis represents an imbalanced alteration of microbiota, especially associated with diseases, and is induced by energy-rich foods or a high-fat diet [8]. Indeed, close relationship between gut dysbiosis and metabolic diseases including obesity and type 2 diabetes together with their complications suggests the pathophysiological significance of gut microbiota in these diseases. In gut dysbiosis, gram-negative bacteria-producing toxin lipopolysaccharide (LPS) is translocated to the bloodstream from a leaky gut, and induces systemic inflammation [9, 10].
Lipopolysaccharide-binding protein (LBP) is a liver-derived acute-phase protein responsive to LPS, thus reflects the systemic inflammation including infection of those bacteria and gut dysbiosis [8–11]. Recent studies have found that plasma LBP levels were higher [12], and associated with complications (arterial stiffness) [13] in patients with type 2 diabetes, suggesting its clinical significance in the development of complications. In contrast, the clinical significance of LBP in patients with type 1 diabetes remains unclear while the involvement of gut dysbiosis in the development of type 1 diabetes is reported especially through the increased risk of disease onset and/or autoimmune reactions [14, 15]. Hence, in this study, we evaluated the plasma LBP levels and explored its pathophysiological significance in patients with type 1 diabetes.
Materials and methods
Study population
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Osaka University Clinical Research Review Committee (Number 12,372, date of approval: 7/04/2017). Seventy-seven Japanese type 1 diabetes patients receiving the annual health checkups were recruited and enrolled in our study after having provided written informed consent. All participants were diagnosed as type 1 diabetes patients by diabetes specialists at the time of presenting with symptoms of hyperglycemia and/or ketosis in their clinical history, and since that time all had been under treatment with insulin therapy. Most of the participants are considered to be having the autoimmune, acute-onset type 1 diabetes, but the inclusion of the fulminant type patients cannot be completely excluded when they are diagnosed before the establishment of the concept and diagnostic criteria of the fulminant type 1 diabetes. The patients with neoplasm, autoimmune diseases, and administration of oral anti-diabetic agents were excluded. We also excluded patients with current smoking and those with missing data of the analyses.
Clinical examinations
The clinical parameters were evaluated by a medical history interview, physical examination, blood sampling and indices of atherosclerosis. The details of the study methods are previously reported [16]. Blood sampling was performed at the time of the visit irrespective of the time of meal and insulin injection. Plasma LBP levels were measured using the specific ELISA (HK315-02, HyCult Biotech Inc., Uden, the Netherlands), and other blood samples were measured at SRL Inc. (Tokyo, Japan). All data and blood samples were collected on July 29th at Osaka Police Hospital or August 26th at Osaka University Medical Hospital in 2017.
Statistical analyses
Data on baseline characteristics are presented as means ± standard deviations for continuous variables or percentages for discrete variables. All statistical analysis was performed using JMP version 14 (SAS Institute, Cary, NC, USA). A p value < 0.05 was considered statistically significant. A mediated effect of LBP level on the relationship between variables of interest was assessed via a mediation analysis that can evaluate the causal pathway between the factors and the outcome [17]. We calculated the 95% confidence intervals (CIs) of the mediation effect using bootstrap sampling with 2000 repeated samples.
Mediation analysis
During the mediation analysis, the total, direct, and indirect effects of an exposure on an outcome were derived as follows; the total effect (Effect c) was evaluated to be the standardized partial regression coefficient in the univariate linear regression model. The direct effect (Effect c′), i.e., the effect not via a mediator of interest, was estimated as the standardized partial regression coefficient adjusted for the mediator in the multivariate linear regression model. The indirect effect, i.e., the effect that acted through the mediator, was calculated as [Effect a] × [Effect b], where Effect a and Effect b denoted the total effect of the exposure on the mediator (equal to the standardized partial regression coefficient in the univariate linear regression model), and the direct effect of the mediator on the outcome (equal to the standardized partial regression coefficient adjusted for the exposure in the multivariate linear regression model), respectively. This indirect effect of the exposure on the outcome via the mediator could be also calculated as [Effect c] − [Effect c′].
Results
The clinical characteristics of the 67 patients included in the study are presented in Table 1. The median serum hs-CRP and mean plasma LBP levels were within the normal ranges (0.03 mg/dL and 8.2 µg/mL, respectively) [18, 19]. The mean body mass index (BMI) was 23.8 kg/m2, and 12 patients with overweight (25 ≤ BMI < 30) and five patients with obesity (BMI > 30) were included among the subjects. The levels of hs-CRP and LBP of 17 overweight or obese patients were higher than those of non-obese patients (log[hs-CRP]; 6.6 ± 1.0 vs 5.7 ± 1.1, p < 0.01, LBP; 9.9 ± 3.0 vs 7.6 ± 2.2, p < 0.01), but both remained within the normal ranges. In univariate analyses, log[hs-CRP] was significantly correlated with log[triglyceride (TG)] (r = 0.39, p < 0.05), systolic blood pressure (r = 0.27, p = 0.03), BMI (r = 0.43, p < 0.05), and LBP (r = 0.64, p < 0.05), whereas LBP was only significantly correlated with BMI (r = 0.47, p < 0.05) and log[hs-CRP] (Table 2). Parameters of diabetic complications, including diabetic retinopathy, were not statistically correlated with log[hs-CRP] and LBP. We subsequently analyzed a possible effect of LBP as a mediator, on the relationship between hs-CRP and BMI via a mediation analysis (Fig. 1). The indirect effect of LBP on the relationship between BMI and hs-CRP was significant (0.27; 95% CI 0.10–0.48), whereas the direct effect of BMI on hs-CRP was 0.16; 95% CI 0.04–0.37.
Table 1.
Baseline characteristics of patients enrolled in the study (n = 67)
| Male n (%) | 18 (27) |
| Age (years) | 34 ± 7 |
| Duration of diabetes (years) | 25 ± 7 |
| Body mass index (kg/m2) | 23.8 ± 3.5 |
| HbA1c (%) | 7.4 ± 1.0 |
| Systolic blood pressure (mmHg) | 120 ± 17 |
| Diastolic blood pressure (mmHg) | 71 ± 11 |
| Hypertension n (%) | 10 (15) |
| Dyslipidemia n (%) | 6 (9) |
| Aspartate transaminase (U/L) | 19 ± 5 |
| Alanine transaminase (U/L) | 15 ± 9 |
| U-ACR (mg/gCr) | 4.1 (3.0–6.0) |
| Uric acid (mg/dL) | 4.0 ± 1.2 |
| Creatinine (mg/dL) | 0.64 ± 0.10 |
| eGFR (mL/min/1.73 m2) | 95 ± 13 |
| TG (mg/dL) | 63 (48–96) |
| HDL-cholesterol (mg/dL) | 72 ± 17 |
| LDL-cholesterol (mg/dL) | 107 ± 22 |
| Max carotid IMT (mm) | 0.95 (0.85–1.10) |
| ABI | 1.0 ± 0.1 |
| baPWV (cm/s) | 1318 ± 198 |
| hs-CRP (mg/dL) | 0.03 (0.02–0.07) |
| LBP (µg/mL) | 8.2 ± 2.6 |
Data are shown as n (%), mean ± standard deviation, or median (interquartile range)
U-ACR urinary albumin creatinine ratio, eGFR estimated glomerular filtration rate, TG triglycerides, HDL high-density lipoprotein, LDL low-density lipoprotein, IMT intima media thickness, ABI ankle brachial pressure index, baPWV brachial-ankle pulse wave velocity, hs-CRP high-sensitivity C-reactive protein, LBP lipopolysaccharide-binding protein
Table 2.
Correlation between serum LBP and hs-CRP levels
| Parameters | log[hs-CRP] | LBP | ||
|---|---|---|---|---|
| r | p value | r | p value | |
| Sex | – | 0.29 | – | 0.52 |
| Age | 0.04 | 0.76 | − 0.10 | 0.42 |
| Duration of diabetes | − 0.07 | 0.56 | − 0.14 | 0.27 |
| Body mass index | 0.43 | < 0.01 | 0.47 | < 0.01 |
| HbA1c | 0.22 | 0.07 | 0.20 | 0.11 |
| Systolic blood pressure | 0.20 | 0.10 | 0.27 | 0.03 |
| Diastolic blood pressure | 0.21 | 0.08 | 0.22 | 0.08 |
| Aspartate transaminase | − 0.004 | 0.97 | 0.04 | 0.74 |
| Alanine transaminase | 0.19 | 0.12 | 0.20 | 0.11 |
| log[U-ACR] | − 0.03 | 0.79 | 0.12 | 0.33 |
| Uric acid | 0.14 | 0.26 | 0.02 | 0.86 |
| Creatinine | − 0.002 | 0.99 | 0.03 | 0.78 |
| eGFR | 0.12 | 0.32 | 0.10 | 0.43 |
| log[TG] | 0.31 | < 0.01 | 0.39 | < 0.01 |
| HDL-cholesterol | − 0.04 | 0.74 | 0.07 | 0.60 |
| LDL-cholesterol | − 0.11 | 0.37 | 0.14 | 0.25 |
| Max carotid IMT | 0.03 | 0.82 | 0.07 | 0.60 |
| ABI | − 0.11 | 0.37 | − 0.11 | 0.39 |
| baPWV | 0.02 | 0.88 | 0.11 | 0.37 |
| LBP | 0.64 | < 0.01 | – | – |
p values were determined using single linear regression analysis except sex, whose p value was assessed using unpaired t-test
U-ACR urinary albumin creatinine ratio, eGFR estimated glomerular filtration rate, TG triglycerides, HDL high-density lipoprotein, LDL low-density lipoprotein, IMT intima media thickness, ABI ankle brachial pressure index, baPWV brachial-ankle pulse wave velocity, hs-CRP high-sensitivity C-reactive protein, LBP lipopolysaccharide-binding protein
Fig. 1.
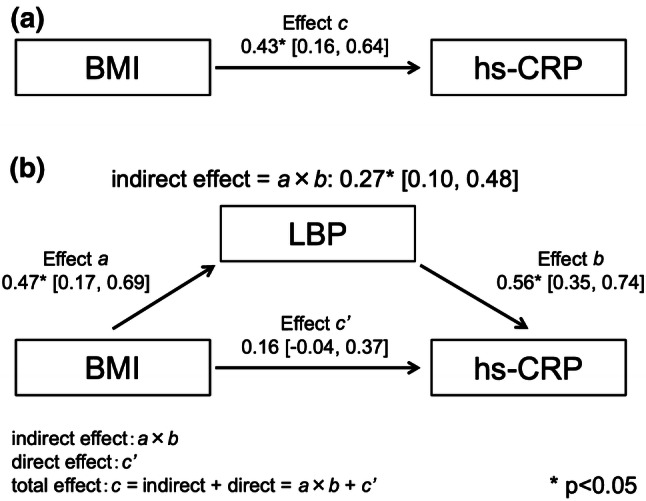
Mediation effects of LBP on the association between BMI and hs-CRP using bootstrap sampling with 2000 repeated samples. a The total effect of BMI on hs-CRP, b the mediation (indirect) effect of LBP estimated by Effect a × Effect b, and the direct effect of BMI on hs-CRP (Effect c′) after adjusting for a mediator
Discussion
In this study, plasma levels of LBP were significantly associated with BMI and serum levels of hs-CRP, and LBP was found to be a statistically significant mediator of the relationship between BMI and hs-CRP. These results suggest that the condition of inflammation represented by LBP levels including infection and gut dysbiosis, at least in part, impacts on the chronic microinflammation linked to body weight gain in Japanese patients with type 1 diabetes.
In this study, we found that LBP had a significant impact on the pathophysiology of type 1 diabetes since LBP significantly mediated the relationship between BMI and hs-CRP. Both obesity and microinflammation are well known to play important roles in the development of various diabetic complications. It should be noted that LBP levels and BMI in the study participants were both within the normal ranges [18], which is in contrast to previous reports targeting gut dysbiosis and obesity in type 2 diabetes. It is reported that gut microbial properties differ between obese and non-obese Japanese subjects [20], and the difference would impact on the increased microinflammation associated with body weight gain [21]. This is similar to previous reports from other country groups, suggesting that it is not specific to the Japanese population. Indeed, the overweight or obese subjects in our study exhibited significantly higher hs-CRP and LBP levels than other subjects, even within normal ranges. This suggests that microinflammation is also increased as patients with type 1 diabetes become obese, and the significant association between LBP and BMI or hs-CRP, and the mediation of these parameters in our study suggest a pathophysiological significance of microinflammation represented by plasma LBP levels even in a small range.
Previous studies reporting elevated levels of LBP in patients with obesity and/or metabolic syndrome [22, 23] indicated a possible contribution of gut dysbiosis in these metabolic disorders. Indeed, the existence of gut dysbiosis has been shown by rRNA-targeted reverse transcription-quantitative polymerase chain reaction (RT-PCR), and the plasma LBP levels have been found to be higher in patients with type 2 diabetes [12]. In addition, serum LBP levels were reported to be significantly associated with arterial stiffness evaluated by aortic pulse wave velocity (PWV), in patients with type 2 diabetes [13]. These results suggest that the existence of gut dysbiosis in type 2 diabetes has an impact on macrovascular complication. In contrast, there have been very few reports evaluating LBP in type 1 diabetes. The serum LBP level in Indian patients with type 1 diabetes was lower than that in healthy control subjects, and no significant association was found between serum LBP levels and vascular complications [24]. The reduced LBP levels in this report are different from our current data, but the associations between LBP levels and other clinical parameters were not assessed. To our knowledge, there is no other report describing the analysis of LBP in Japanese type 1 diabetes population. It is possible that there are some differences in the levels or tendencies of LBP between ethnicities due to environmental or cultural influences.
The current study has some limitations. First, it is a single-center study with small sample size, lacking age matched suitable healthy controls mainly due to clinical limitations. Second, the participants were relatively well-controlled with less vascular complications so may not be representative of patients with type 1 diabetes. Third, the current study was observational in nature and was unable to distinguish the origin of LPS and the followed LBP response due to gut dysbiosis or concomitant infections of LPS-producing bacteria in other sites, and/or the LBP is directly linked to an increase in body weight and chronic inflammation. We did not examine the existence of gut dysbiosis through direct analyses such as rRNA-targeted quantitative PCR. Thus, future studies collecting more detailed information will be needed to validate the current findings.
In conclusion, the study revealed that LBP significantly mediated the association between BMI and hs-CRP in patients with type 1 diabetes. This suggests that microinflammation represented by LBP is one of the etiologies of complications in type 1 diabetes and could be a potential diagnostic and therapeutic target in the future.
Acknowledgments
The authors thank Ms. Satomi Takebe, Drs. Naoki Shimo, Hiroyo Ninomiya, Kazuo Omori, Ihoko Sato, Yasumitsu Takahi, Yasuki Nagai, Azusa Shiraki, and Naohiro Taya (Department of Metabolic Medicine, Graduate School of Medicine, Osaka University, Osaka, Japan), Prof. Munehide Matsuhisa and Dr. Akio Kuroda (Diabetes Therapeutics and Research Center, Tokushima University, Tokushima, Japan), Dr. Tetsuyuki Yasuda (Osaka Police Hospital, Osaka, Japan), Drs. Ken Kato, Toko Oida, Takafumi Masuda, and Saki Kawamoto (National Hospital Organization Osaka National Hospital, Osaka, Japan), Dr. Ayaha Kawashima (Kanda Naika Clinic, Osaka, Japan), and Dr. Fumiyo Kubo (Yao Municipal Hospital, Osaka, Japan) for their assistance in clinical examinations. This study was partly supported by a research grant from the Japan Diabetes Foundation (to DK).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Investig. 2017;127:83–93. doi: 10.1172/JCI88884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 4.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Investig. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab. 2014;99:656–663. doi: 10.1210/jc.2013-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepper PM, Schumann C, Triantafilou K, et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol. 2007;50:25–31. doi: 10.1016/j.jacc.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz AG, Casafont F, Crespo J, et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374–1380. doi: 10.1007/s11695-007-9243-7. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.Amar J, Chabo C, Waget A, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 12.Sato J, Kanazawa A, Ikeda F, et al. Gut dysbiosis and detection of ‟live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37:2343–2350. doi: 10.2337/dc13-2817. [DOI] [PubMed] [Google Scholar]
- 13.Sakura T, Morioka T, Shioi A, et al. Lipopolysaccharide-binding protein is associated with arterial stiffness in patients with type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2017;16:62. doi: 10.1186/s12933-017-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis-Richardson AG, Triplett EW. A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia. 2015;58:1386–1393. doi: 10.1007/s00125-015-3614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavin PG, Mullaney JA, Loo D, et al. Intestinal metaproteomics reveals host-microbiota interactions in subjects at risk for type 1 diabetes. Diabetes Care. 2018;41:2178–2186. doi: 10.2337/dc18-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osawa S, Kawamori D, Katakami N, et al. Significant elevation of serum dipeptidyl peptidase-4 activity in young-adult type 1 diabetes. Diabetes Res Clin Pract. 2016;113:135–142. doi: 10.1016/j.diabres.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Mascha EJ, Dalton JE, Kurz A, et al. Statistical grand rounds: understanding the mechanism: mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117:980–994. doi: 10.1213/ANE.0b013e3182a44cb9. [DOI] [PubMed] [Google Scholar]
- 18.Asada M, Oishi E, Sakata S, et al. Serum lipopolysaccharide-binding protein levels and the incidence of cardiovascular disease in a general Japanese population: the hisayama study. J Am Heart Assoc. 2019;8:e013628. doi: 10.1161/JAHA.119.013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamaki S, Kanazawa A, Sato J, et al. Clinical factors associated with bacterial translocation in Japanese patients with type 2 diabetes: a retrospective study. PLoS One. 2019;14:e0222598. doi: 10.1371/journal.pone.0222598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasai C, Sugimoto K, Moritani I, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. doi: 10.1186/s12876-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Lu L, Yao P, et al. Lipopolysaccharide binding protein, obesity status and incidence of metabolic syndrome: a prospective study among middle-aged and older Chinese. Diabetologia. 2014;57:1834–1841. doi: 10.1007/s00125-014-3288-7. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Navarrete JM, Ortega F, Serino M, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2012;36:1442–1449. doi: 10.1038/ijo.2011.256. [DOI] [PubMed] [Google Scholar]
- 24.Alexender V, Nayar PG, Murugesan R, et al. CardioGenBase: a literature based multi-omics database for major cardiovascular diseases. PLoS One. 2015;10:e0143188. doi: 10.1371/journal.pone.0143188. [DOI] [PMC free article] [PubMed] [Google Scholar]


