Abstract
To determine the pathophysiology of gestational diabetes (GDM) in lean Japanese pregnant women in relation to insulin secretion or insulin resistance. The 75-g oral glucose tolerance test (OGTT) was performed in case of positive results of universal screening of a 50-g glucose challenge test at 24–28 weeks’ gestation in Japanese pregnant women. These women were treated in our hospital between 2012 and 2016. Among these women, 30 with a body mass index of < 18.5 kg/m2 were selected as lean subjects. Nine women were diagnosed with GDM (GDM group) and the remaining 21 had normal glucose tolerance (control group). For evaluating insulin secretion or resistance, the following parameters were compared between the two groups together with a family history of diabetes mellitus (DM) among first-degree relatives: (1) plasma glucose and immnunoreactive insulin (IRI) levels after glucose loading, (2) insulinogenic index (I.I), (3) homeostasis model assessment of β-cell function (HOMA-β), (4) homeostasis model assessment of insulin resistance (HOMA-IR), and (5) insulin sensitivity index (ISI) composite. The percentage of having a family history of DM was significantly higher in the GDM group (3/9, 33.3%) than in the control group (0/21, 0.0%, P < 0.001). Serum glucose levels at 30, 60, and 120 min after glucose loading were significantly higher in the GDM group than in the control group (all P < 0.05). IRI levels at 60 and 120 min were significantly higher in the GDM group than in the control group (both P < 0.05), and they showed persistent insulin secretion patterns. Values of the I.I. and ISI composite were significantly lower in the GDM group than in the control group (both P < 0.05), with no differences in HOMA-β, HOMA-IR and HbA1c levels between the groups. Lean Japanese pregnant women with GDM have impaired β-cell function, which is in part associated with hereditary traits.
Keywords: Gestational diabetes mellitus, Lean pregnant women, Insulin sensitivity, Insulin secretion
Introduction
Gestational diabetes mellitus (GDM) is a major complication during pregnancy in developed countries, including Japan, and approximately 10% of Japanese pregnant women are diagnosed with GDM [1, 2]. Because of current screening protocols during pregnancy, most patients with GDM are cared for under proper management, and the number of various complications, such as macrosomia, has decreased [3, 4]. Even if glucose intolerance is normalized after pregnancy, patients with GDM have a high probability of developing type 2 diabetes mellitus (DM) in the future [5]. Therefore, follow-up strategies for patients who have a history of GDM are necessary to detect and intervene in glucose intolerance in the early stage.
Glucose intolerance is caused by an imbalance between insulin secretion capacity of pancreatic β-cells and insulin resistance in peripheral target organs, including the liver, skeletal muscles, and adipose tissues. The relationship between insulin resistance and insulin response is different between races. In most cases of GDM, elevated insulin resistance during pregnancy is the major cause of glucose intolerance [6]. However, an increase in β-cell function in response to elevated insulin resistance is lower for Asians compared with Caucasians [7]. This suggests a different pathophysiology of GDM during pregnancy and the clinical course after parturition depending on the race.
Obesity is an important factor for deterioration of insulin resistance [8]. Therefore, lean subjects are a good model to evaluate intrinsic abnormalities by minimizing additional contributing factors to insulin resistance. Because most Caucasians with GDM are obese, there have been few reports regarding the pathophysiology of GDM in lean women [9–11], and no reports in Japanese lean women with GDM.
In the present study, we aimed to determine the pathophysiology of GDM in lean Japanese pregnant women by examining insulin secretion and insulin resistance using data that were obtained from the 75-g oral glucose tolerance test (OGTT).
Materials and methods
For screening of glucose intolerance during pregnancy, a 50-g glucose challenge test (GCT) was performed at 24–28 weeks of gestation in all patients who were treated in Kurume University Hospital. Among 1820 Japanese singleton pregnant women between January 2012 and June 2016, 172 were positive for the 50-g GCT (i.e., ≥ 1.4 g/L at 1 h after glucose loading), and the 75-g OGTT was performed at 24–34 weeks of gestation after more than 12 h of fasting.
Among these women, 30 with a body mass index (BMI) of < 18.5 kg/m2 before pregnancy were selected as lean subjects and evaluated in this study. The subjects comprised nine women with a GDM pattern and 21 with a normal pattern. GDM was diagnosed on the basis of criteria of the international association of diabetes and pregnancy study groups [12]. These criteria were fasting plasma glucose levels or plasma gluose levels at 1 or 2 h after glucose loading of ≥ 92, 180, or 153 mg/dL, respectively. In the nine women with GDM, none of them were positive for anti-glutamic acid decarboxylase antibody. There was no difference in gestational age at the 75-g OGTT between the groups (GDM [median, range between the 25th and 75th percentiles]: 273/7, 261/7–321/7 weeks vs. control: 276/7, 252/7–294/7 weeks) (P = 0.21).
Informed consent was obtained from all participants in this study. The study was approved by the Ethical Committee of Kurume University (#12021, approved on April 19, 2012). During the 75-g OGTT, plasma glucose and immunoreactive insulin (IRI) levels were simultaneously measured at before and 30, 60, and 120 min after glucose loading. IRI levels were measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Basel, Switzerland). The insulinogenic index (I.I.) [13], homeostasis model assessment of β-cell function (HOMA-β) [14], homeostasis model assessment of insulin resistance (HOMA-IR) [14], and insulin sensitivity index (ISI) composite [15] were calculated according to the formulae shown in the appendix.
The following parameters were compared between the groups: maternal age, parity, BMI before pregnancy, presence of a family history of DM among first-degree relatives, gestational age at delivery, birth weight, plasma glucose and IRI levels before and after glucose loading, I.I., HOMA-β, HOMA-IR, ISI composite, and HbA1c levels. Statistical analysis was performed using the Mann–Whitney U test or Chi-square test with the statistical software JMP®14 (SAS institute, Cary, NC, USA). P values < 0.05 were considered as statistically significant.
Results
With regard to the clinical background, there were no significant differences in maternal age, parity, BMI before pregnancy, gestational age at the 75-g OGTT and delivery, and neonatal birth weight between the groups (Table 1). However, the percentage of having a history of DM in first-degree relatives was significantly higher in the GDM group than in the control group (P < 0.001).
Table 1.
Comparisons of maternal and perinatal background between the gestational diabetes and control groups
| Gestational diabetes (n = 9) | Control (n = 21) | P value | |
|---|---|---|---|
| Maternal age (years) | 34.0 (29.5–35.5) | 31.0 (30.0–33.0) | 0.31 |
| Nulliparous | 7 (77.8) | 9 (42.8) | 0.11 |
| Body mass index before pregnancy (kg/m2) | 17.8 (15.1–18.2) | 18.0 (17.4–18.1) | 0.41 |
| Presence of diabetes mellitus in the 1st degree relatives | 3 (33.3) | 0 (0.0) | < 0.001* |
| Gestational age at delivery (weeks) | 386/7 (375/7–394/7) | 381/7 (374/7–394/7) | 0.60 |
| Neonatal birth weight (g) | 2804 (2155–2968) | 2802 (2458–3106) | 0.57 |
Data are presented as medians (ranges between the 25th and 75th percentiles) or numbers of subjects (%)
The Mann–Whitney U test or chi-squire test was used for statistical analysis
* Significantly different
Figure 1 shows serum glucose levels during the 75-g OGTT in the GDM and control groups. There was no difference in fasting glucose levels between the groups. However, plasma glucose levels at 30, 60, and 120 min after glucose loading in the GDM group were significantly higher than those in the control group (P = 0.0032, P = 0.0001, and P = 0.0029, respectively).
Fig. 1.
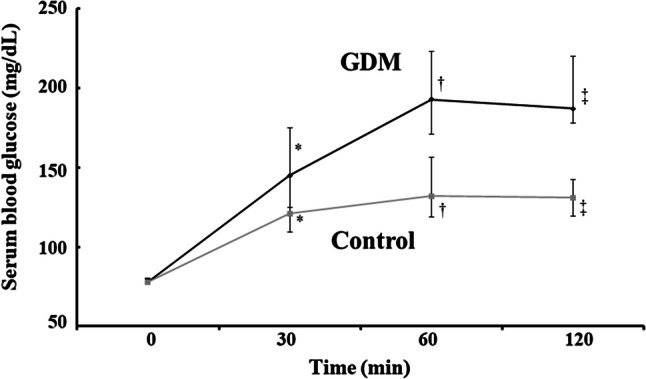
Changes in plasma glucose levels during the 75-g oral glucose tolerance test in the gestational diabetes and control groups. *P = 0.0032, †P = 0.0001, ‡P = 0.0029
Figure 2 shows plasma IRI levels during the 75-g OGTT in the GDM and control groups. There was no difference in IRI levels at fasting and 30 min after glucose loading (P = 0.0032, P= 0.0001 and P = 0.0029 respectively). However, IRI levels at 60 and 120 min after glucose loading in the GDM group were significantly higher than those in the control group (P = 0.0019 and P = 0.011, respectively). The changes in IRI levels in the GDM group showed a persistent insulin secretion pattern.
Fig. 2.
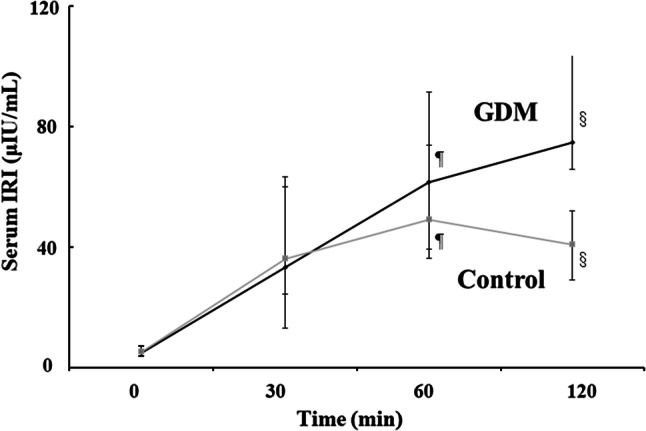
Changes in plasma immunoreactive insulin levels during the 75-g oral glucose tolerance test in the gestational diabetes and control groups. ¶P = 0.019, §P = 0.011
Values of the I.I. and ISI composite in the GDM group were significantly lower than those in the control group (P = 0.0017 and P = 0.037, respectively). However, there were no differences in HOMA-β and HOMA-IR between the groups (Table 2).
Table 2.
Comparisons of the indices for insulin secretion and resistance between the gestational diabetes and control groups
| Gestational diabetes (n = 9) | Control (n = 21) | P value | |
|---|---|---|---|
| Insulinogenic index | 0.49 (0.39–0.57) | 0.76 (0.66–0.99) | 0.0017* |
| HOMA-β | 99.4 (81.5–146.7) | 124.8 (75.1–153.5) | 0.95 |
| HOMA-IR | 0.92 (0.72–1.43) | 1.00 (0.54–1.36) | 0.50 |
| Insulin sensitivity index composite | 4.73 (2.73–5.52) | 6.90 (5.54–12.7) | 0.037* |
| HbA1c (%) | 5.2 (4.95–5.60) | 5.0 (4.90–5.20) | 0.1354 |
Data are presented as medians (ranges between the 25th and 75th percentiles). The Mann–Whitney U test was used for statistical analysis
HOMA-β homeostasis model assessment of β-cell function, HOMA-IR homeostasis model assessment of insulin resistance
* Significantly different
There were no differences in HbA1c values between the groups.
Discussion
There is heterogeneity of metabolic abnormalities in GDM and several factors are related to development of gestational diabetes, including maternal age at delivery, pre-pregnancy BMI, and a history of abnormal glucose tolerance in a previous pregnancy [16, 17]. Because obesity is one of the most influential factors of insulin resistance, we only included extremely lean women with a preconceptional BMI of < 18.5 kg/m2 to avoid possible secondary effects of obesity. The median maternal age of subjects in the GDM group was 34.0 years old, which is slightly higher than the average age of Japanese pregnant women, but it was not different from that in the control group. In the GDM group, there were no women who were multipara with abnormal glucose tolerance in a previous pregnancy or with the presence of islet cell antibodies.
There have been a few reports regarding the characteristics of insulin secretion and resistance during the third trimester of pregnancy in non-obese women. Catalano et al. and Kautzky-Willer et al. showed that subjects with GDM had more pronounced insulin resistance and inadequate insulin secretion compared with those with normal glucose tolerance, especially first-phase responsiveness to glucose of β-cells [10, 11]. Buchanan et al. showed that women with GDM had a decreased first-phase insulin response, but insulin sensitivity was similar to that in women in the control group [9]. To date, there have been no conclusive results obtained regarding the etiology or pathogenesis of the lean type of GDM.
HOMA-β and HOMA-IR represent insulin secretion capacity and hepatic insulin sensitivity during the fasting state, respectively. In contrast, the I.I. and ISI composite indicate insulin secretion capacity and whole body insulin sensitivity during both fasting and post-prandial states, respectively [13–15]. In our study, there were no differences in HOMA parameters between the groups. However, the I.I. and ISI composite were significantly lower in the GDM group than in the control group. Taking into account delayed enhancement of glucose and IRI responses during post-glucose loading, the lower ISI composite in the GDM group could be attributable to a delayed response in these two parameters. Therefore, a deficit in early-phase insulin secretion and a delayed pattern of insulin secretion might be characteristics of the lean type of GDM, and they are probably due to impaired function of pancreatic β-cells.
In the present study, 33.3% of lean subjects with GDM had a family history of DM among their first-degree relatives. The number of women in this study was limited. However, this rate is higher than that of a previous report (23.4%) concerning the prevalence of GDM diagnosed using the same criteria as our subjects in Japanese pregnant women in which BMI before pregnancy was not considered [2]. Most genetic variants associated with type 2 diabetes are related to insulin secretion rather than insulin resistance, except for variants that change fat mass and predispose to diabetes via the effect of obesity [18]. Therefore, a decreased insulin secretion capacity is likely to be associated with hereditary traits, which could explain the decreased insulin secretion capacity in the GDM group in the present study.
Our study shows that impaired β-cell function is the main pathogenesis for development of GDM in lean Japanese pregnant women and this is in part related to a hereditary trait. Insulin profiles in women with GDM (i.e., a decrease in early-phase insulin secretion capacity and following persistent insulin secretion) are also observed in cases with borderline glucose intolerance and a mild form of type 2 DM [19, 20]. Therefore, the pathogenesis of lean pregnant women with GDM is similar to that of the early phase of type 2 DM. This finding indicates that lean pregnant women with GDM after pregnancy are at great risk for developing type 2 DM in the future, similar to obese women with GDM. Consequently, careful monitoring of the glucose profile is required after pregnancy. Further studies are necessary to determine the natural clinical course of lean pregnant women with GDM who might develop type 2 DM.
The major limitation of this study is that the number of subjects was small. This is partly because the number of lean women with a BMI of < 18.5 kg/m2 was limited, even though Japanese pregnant women tend to be leaner compared with those in other developed countries. In fact, there have been few reports on the characteristics of insulin secretion and insulin resistance in lean pregnant women in North America and Europe, and the number of subjects in these reports was small [9–11]. Additionally, the characteristics of insulin secretion and insulin resistance in pregnant women are different depending on body mass. Insulin resistance tends to be increased in pregnant women with obesity (data not shown). Therefore, subjects in this study were limited to lean pregnant women with a BMI of < 18.5 kg/m2. This is only a preliminary study and the results obtained in this study need to be confirmed in a large-scale population.
Acknowledgements
We thank Ellen Knapp, PhD, from Edanz Group (https://www.edanzediting.com/ac) for editing a draft of this manuscript. This study was supported in part by a Grant-in-Aid for Scientific Research (C) (JP18K09306).
Appendix
BG0, BG30, BG60, and BG120 represent plasma glucose concentrations in units of mg/dL at 0, 30, 60, and 120 min, respectively. IRI0, IRI30, IRI60, and IRI120 represent plasma insulin concentrations in units of µIU/mL at 0, 30, 6, and 120 min, respectively.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iwama N, Sugiyama T, Metoki H, Kusaka H, Yaegashi N, Sagawa N, et al. Difference in the prevalence of gestational diabetes mellitus according to gestational age at 75-g oral glucose tolerance test in Japan: the Japan assessment of gestational diabetes mellitus screening trial. J Diabetes Investig. 2019 doi: 10.1111/jdi.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikenoue S, Miyakoshi K, Saisho Y, Sakai K, Kasuga Y, Fukutake M, et al. Clinical impact of women with gestational diabetes mellitus by the new consensus criteria: two year experience in a single institution in Japan. Endocrine J. 2014;61:353–358. doi: 10.1507/endocrj.EJ13-0496. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama T, Metoki H, Hamada H, Nishigori H, Saito M, Yaegashi N, et al. A retrospective multi-institutional study of treatment for mild gestational diabetes in Japan. Diabetes Res Clin Pract. 2014;103:412–418. doi: 10.1016/j.diabres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama T, Nagao K, Metoki H, Nishigori H, Saito M, Tokunaga H, et al. Pregnancy outcomes of gestational diabetes mellitus according to pre-gestational BMI in a retrospective multi-institutional study in Japan. Endocr J. 2014;61:373–380. doi: 10.1507/endocrj.EJ13-0541. [DOI] [PubMed] [Google Scholar]
- 5.O’Sallivan JB. Body weight and subsequent diabetes mellitus. JAMA. 1982;248:949–952. doi: 10.1001/jama.1982.03330080031024. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan TA, Kjos SL, Xiang A, Watanabe R. What is gestational diabetes? Diabet Care. 2007;30:S105–111. doi: 10.2337/dc07-s201. [DOI] [PubMed] [Google Scholar]
- 7.Mørkrid K, Jenum AK, Sletner L, Vårdal MH, Waage CW, Nakstad B, et al. Failure to increase insulin secretion capacity during pregnancy-induced insulin resistance is associated with ethnicity and gestational diabetes. Eur J Endocrinol. 2012;167:579–588. doi: 10.1530/EJE-12-0452. [DOI] [PubMed] [Google Scholar]
- 8.Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, et al. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162:1008–1014. doi: 10.1016/0002-9378(90)91306-W. [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 11.Kautzky-Willer A, Prager R, Waldhausl W, Pacini G, Thomaseth K, et al. Pronounced insulin resistance and inadequate β-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care. 1997;20:1717–1723. doi: 10.2337/diacare.20.11.1717. [DOI] [PubMed] [Google Scholar]
- 12.International association of diabetes and pregnancy study groups consensus panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. [DOI] [PMC free article] [PubMed]
- 13.Kosaka K, Hagura R, Kuzuya T, Kuzuya N. Insulin secretory response of diabetes during the period of improvement of glucose tolerance to normal range. Diabetologia. 1974;10:775–782. doi: 10.1007/BF01219540. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI200524531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan BM, Breheny PJ, Robinson JG, Baer RJ, Saftlas AF, Bao W, et al. Development and validation of a clinical model for preconception and early pregnancy risk prediction of gestational diabetes mellitus in nulliparous women. PLoS ONE. 2019;14(4):e0215173. doi: 10.1371/journal.pone.0215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–418. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabet Care. 2006;29:1130–1139. doi: 10.2337/dc05-2179. [DOI] [PubMed] [Google Scholar]
- 20.Faerch K, Vaag A, Holst JJ, Hansen T, Jørgensen T, Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care. 2009;32:439–444. doi: 10.2337/dc08-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]


