Abstract
Background/aim
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes mellitus (DM). The Michigan Neuropathy Screening Instrument (MNSI) is a simple, brief, and useful screening tool that was designed to assess DPN. The aim of this study was to develop a Turkish version of the MNSI and assess its reliability and validity.
Materials and methods
Eighty-three patients with DM who were divided into two groups according the results of nerve conduction studies (NCS) as having DPN or without DPN were enrolled in this cross-sectional study. The Toronto clinical scoring system, pain detect questionnaire, and NCS were assessed along with the MNSI.
Results
Each section of the MNSI was internally consistent (Cronbach’s alpha > 0.70), and the scores of both sections were positively correlated with total MNSI score (r = 0.938; r = 0.908, respectively, p < 0.001). The test–retest reliability of the Turkish version of the MNSI was determined as 0.99 for the total score (intraclass correlation coefficient = 0.996). Using the agreement between MNSI scores and DPN diagnosis by NCS as a gold standard, receiver-operating characteristic (ROC) curve values for section A and section B were estimated as 0.973 and 1.00, respectively. When a cut-off value ≥ 3.0 in section A and a cut-off value ≥ 2.0 in section B were used, we obtained a sensitivity of 97.6% and 100%; a specificity of 63.4% and 97.6%; a positive predictive value of 72.7% and 97.6%; and a negative predictive value of 96.3% and 100%, respectively.
Conclusion
The Turkish version of MNSI is a reliable and valid tool for screening DPN in Turkish patients.
Keywords: Michigan Neuropathy Screening Instrument, Validity, Reliability, Diabetic neuropathy
Introduction
The incidence of diabetes mellitus (DM) continues to rise and has become one of the most costly chronic diseases and a major public health problem throughout the world. The most common complication of DM is neuropathy as a consequence of chronic hyperglycemia. Furthermore diabetic peripheral neuropathy (DPN) is the most common type of diabetic neuropathy [1]. DPN has been recently defined as a symmetric, length-dependent sensorimotor polyneuropathy attributable to metabolic and microvascular alterations as a result of chronic hyperglycemia exposure and cardiovascular risk covariates [2]. DPN is a leading cause for disability due to foot ulceration and amputation, gait disturbance, and fall-related injury [3]. DPN significantly lowers quality of life and substantially increases health costs associated with diabetes [4]. Therefore, early screening of DPN in patients with DM is important to prevent disease complications, especially diabetic foot and ulceration [5].
In clinical practice, DPN is diagnosed in the presence of signs and symptoms of peripheral nervous system dysfunction after other causes of neuropathy are excluded in patients with DM according to American Diabetes Association recommendations [6]. Early diagnosis of DPN is crucial, but there is not a single and simple method that can be used to diagnose DPN. Almost a half of the patients with DPN have no symptoms consistent with neuropathy; therefore, neurological examination of the patients should be carefully performed. Even so confirmed neuropathy requires abnormality of nerve conduction study (NCS) or a validated measure of small-fiber function [7]. NCS is the most efficient component of the electrodiagnostic evaluation, so a simple, objective, and sensitive measurement which is intended as a gold standard test for corroborating the diagnosis of DPN. In addition, there are some disadvantages when NCS are considered in clinical settings; NCS have limits on the availability for routine diagnostic evaluation of DPN and NCS are insensitive for the identification of small-fiber neuropathy. Therefore, various screening tools and clinical scoring systems have been developed in different countries for detecting DPN in patients with DM based on verbal pain description, with or without physical examination [8]. Although only one instrument is available in Turkish, the Michigan Neuropathy Screening Instrument (MNSI) validation process in Turkish population has not been done [9].
The MNSI is an easy-to-use questionnaire aimed at screening and detecting DPN [10]. The MNSI with questions about location and severity of clinical signs and symptoms of neuropathy is used to assess DPN, based on patient’s self-report and physician’s clinical examination [11]. It promotes the standardization of the clinical evaluation and ensures the proper referral for hospital evaluation and follow-up. Its utility has been discussed in several papers as well as by the American Diabetic Association [8, 10, 12]. The aim of this study was to translate and validate the MNSI into Turkish and to check its psychometric properties in the Turkish population.
Materials and methods
Patients
This cross-sectional study was carried out at the Rheumatology, and Endocrinology and Metabolism Departments of Pamukkale University between August and September 2019. The study protocol was approved by the Institutional Review Board of the University and each patient provided written informed consent to participate this study. Patients with each sex, aged 18 years or over, able to speak, and understand Turkish language, of whom diagnosis of DM was confirmed by endocrinologists according to the American Diabetes Association criteria [13] were included in this cross-sectional study. Exclusion criteria were: being illiterate, having altered mental status, requiring dialysis, alcoholism, drug abuse, peripheral vasculitis or autoimmune diseases, cerebrovascular disease, cancer, undergoing chemotherapy or radiotherapy treatment, clinical radicular neuropathy, and organs’ failure.
After demographic information was obtained, detailed medical history and physical examination were performed. The following information was gathered; sociodemographic characteristics, type of DM and disease duration, comorbidities, concomitant medications, and laboratory findings such as glycated hemoglobin (HbA1C), glucose plasma levels, and liver and renal function tests.
NCS was performed for all patients. The physician who performed the NCS was blinded to the MNSI score. Then, the patients were evaluated by another investigator who was blinded to the patients to apply Turkish version of the MNSI along with Toronto clinical scoring system (TCSS) and pain detect questionnaire (PD-Q).
Michigan Neuropathy Screening Instrument
The MNSI is an instrument including two parts; section A is self-administered by the patient and assesses the clinical symptoms through 15 “yes” or “no” questions and section B is based on a clinical evaluation [10]. The questionnaire inquires about positive (pain, temperature sensation, and tingling) and negative (numbness) sensory symptoms, cramps and muscle weakness, foots ulcers or cracks, and amputation. Items 4 which assess a circulation measurement and item 10 which is a general status measurement were excluded in the original scale. Neuropathy can be defined as seven or more positive responses on the MNSI questionnaire [10]. Studies have shown that a cut-off of ≥ 4 harmonizes the sensitivity and specificity of both sections of the MNSI [12, 14]. After the questionnaires, patients were evaluated neurologically. (i) In physical examination, feet were evaluated for deformity, dry skin, callus, infection, and ulceration. Foot deformities included prominent metatarsal heads, hallux valgus, joint subluxation, and Charcot joint. One point was given if any of these signs were present and an additional one point was given if ulceration was present. (ii) Vibration sense was evaluated using 128 Hz vibration fork. Vibrating fork was located on the interphalangeal joint of the right great toe. If the patient could not perceive vibration, two points were given. If the patient perceived vibration on the great toe, diaposone was located over ankle (inner malleolus), while it was still vibrating and the patient was asked to compare vibrations from two locations. If vibration was perceived better in ankle, “1” point was given. If no difference could be found, no point was given. Zero point was accepted as normal, “1” point showed mild moderate deficit, and “2” points showed a severe deficit. (iii) Achilles reflex was observed and reported as absent, decreased, or normal. Patients with normal Achilles reflex were given “0” point, while patients with decreased Achilles reflex got “0.5” point and patients with no reflex got “1” point. Positive responses and abnormal physical examination findings were recorded in the questionnaire form. In the questionnaire form, risk of neuropathy was accepted to increase with higher number of positive responses. DPN was diagnosed in patients with a physical examination score ≥ 2.5. The test was completed by expert physician. All subjects were re-evaluated 48 h later. In the second examination, the MNSI was administered again to assess time stability of measurements.
Toronto clinical scoring system
TCSS consists of three parts [15]. The first part involves scoring the symptoms at lower and upper extremity complaints (numbness, burning, weakness, pain, and ataxia). “0” indicates the absence of symptoms, and “1” indicates the presence of symptoms. The second part involves scoring the examination of the ankle and patellar reflexes. “0” indicates normal reflexes, “1” indicates reduced reflexes, and “2” indicates the absence of reflexes. The third part involves scoring the sensation of the big toe. Vibration, position, touch, pinprick, and thermal sensation are evaluated. “0” indicates normal sensory examination, and “1” indicates abnormal sensory examination. Total score ranges from 0 (no neuropathy) to 19 points. Six points are obtained from the symptoms, 8 points are obtained from the reflexes, and 5 points are obtained from the sensory examination of the big toe. The validity and reliability of the scale was proved in Turkish patients [9].
Pain detect questionnaire
The PD-Q is simple, self-administered, useful screening questionnaires that allow to detect neuropathic pain components in patients with chronic pain. It consists off four sections in total. The final score is obtained summing up the scores of the last three sections with a total score of 1–38. Two cut-off values are used by developer of PD-Q for the presence of neuropathic pain. Scores ≤ 12 state that a neuropathic pain component is unlikely, and scores ≥ 19 indicate that neuropathic component is very likely to be present [16]. The Turkish version of the PD-Q was shown to be reliable and valid [17].
Nerve conduction study
DPN was diagnosed according to NCS results. The values that Ovayolu et al. referred to were taken into account when diagnosing DPN [18]. Study of NCS was performed by the Keypoint DANTEC device (Skovlunde, Denmark). Stimulation duration was 0.2 ms for motor, and 0.1 ms for sensory stimuli. All stimulations were performed supramaximally. Bipolar stimulus electrodes were used for all stimuli. NCS under limit for the lower extremity was 42 m/s for motor conduction velocity and sensory conduction velocity. Underlimit the amplitude of motor unit potential was taken as 3 mV for peroneal nerve and 4 mV for tibial nerve. The amplitude of the sensory nerve action potential was accepted 6 mV for sural nerve. Decrease of motor and sensorial amplitude more than 40% of normal value was evaluated as polyneuropathy.
Translation and face validity
Permission to use the instrument and to conduct reliability–validity study for Turkish version was obtained from Dr. Ewa Feldman. For the translation procedure, guidelines for cross-cultural modifying with five phases were applied [19]. The original text of the English version of the MNSI was translated to Turkish by two independent translators who were a native Turkish speaker fluent in English and were blinded to the instrument, one of the authors and a professional translator. These translations were done independently, and afterward, the translations were compared. The differences from the independent translations were discussed, and a final translation was agreed upon. This final Turkish version was translated back into English by two independent English native speaker who were blinded to the original scale. This version was compared to the original, and the discrepancies were then identified and reviewed. A comparison between the back translation and the original scale was made to point out the discrepancies between the original and the translated version. The differences between translated versions were evaluated, and a satisfactory compliance with the original scale was achieved by consensus of the translators. The translation and back-translation phase of the MNSI produced Turkish version of the questionnaire (Appendix). The final version of the MNSI was obtained and applied to a pilot sample of patients to find out whether the patients had any doubts about the meaning of the items. The instrument was applied by a researcher who was blinded to the presence of DPN in patients with DM.
Statistical analysis
Sample size was calculated assuming that at least 80 patients were needed to validate a scale, 40 in each group with an 85% power or above. A sample size of 40 produces two-sided 95% confidence interval with a distance from the mean to the limits that is equal to 6.7, with an estimated standard deviation of 2.7 for MNSI score.
All statistical analyses were performed using SPSS version 22.0 for Windows (Statistical Package for Social Sciences Inc., Chicago, IL, USA). Descriptive statistics were used to describe demographic characteristics. The Kolmogorov–Smirnov test was used to analyse normal distribution assumption of the data. As the distributions were not normal, non parametric tests were used in statistical evaluation. For continuous variables, the significance of the differences were analysed using the Mann–Whitney U test, while categorical variables were analysed with Chi-squared test. For reliability analysis, Cronbach’s α was used to assess the scale internal consistency, and intraclass correlation coefficient (ICC) between test and retest scores was used to assess stability over time. Cronbach’s α and ICC of > 0.7 show a good internal consistency and an excellent agreement between 2 moments, respectively. Factor analysis for dichotomous data was used to identify the dimensions of the MNSI. Convergent validity was assessed by examining correlation between MNSI with TCSS and PD-Q scores. The area under the receiver-operating characteristic (ROC) curves was estimated to measure the discriminatory power of the test. For each total MNSI section scores, sensitivity and specificity were computed and graphed in an ROC curve according to DPN diagnosis. The ROC curve was used to select optimal cut-off MNSI section scores for screening patients who had a DPN diagnosis according to NCS. Discriminant statistics, sensitivity, specificity, positive predictive value, and negative predictive value were also assessed. In all analyses, p values < 0.05 were considered as statistically significant.
Results
A total of 97 patients were screened for eligibility for this study, 14 of whom had to be excluded from the study; six of them due to having altered mental status, two of them requiring dialysis, four of them due to peripheral vasculitis or autoimmune diseases, and two had organs failure. Therefore, 83 patients with DM who were divided into two groups according the results of NCS as having DPN or without DPN were included in this study. The median age of the patients was 62 years, and the majority of the patients were female (63 women and 20 men), with a median disease duration of 10 years. There were no statistically significant difference between groups regarding the sociodemographic and clinical characteristics except DPN screening tools and neuropathic pain scores, as shown in Table 1 (p > 0.05). The median overall score of MNSI, TCSS and PD-Q scores were significantly higher in diabetic patients with DPN than diabetic patients without DPN (p < 0.001).
Table 1.
Demographic and clinical characteristics of patients with diabetes mellitus
| Group 1 DM with PNP (n = 42) |
Group 2 DM without PNP (n = 41) |
p | |
|---|---|---|---|
| Age (years), median (IQR) | 64 (12.25) | 58 (12.00) | 0.516 |
| Gender, n (%) | 0.951 | ||
| Male | 10 (23.8%) | 10 (24.4%) | |
| Female | 32 (76.2%) | 31 (75.6%) | |
| Medical treatment, n (%) | 0.994 | ||
| OAD | 16 (38.1%) | 16 (39%) | |
| Insulin | 21 (50%) | 20 (48.8%) | |
| OAD + insulin | 5 (11.9%) | 5 (12.2%) | |
| Education levels (years), median (IQR) | 7.25 (2.13) | 8 (3) | 0.516 |
| Type of DM, n (%) | 0.667 | ||
| Type 1 | 5 (11.9%) | 5 (12.2%) | |
| Type 2 | 37 (81.1%) | 36 (87.8%) | |
| Disease duration (years), median (IQR) | 10 (8.25) | 10 (11) | 0.717 |
| HbA1C, median (IQR) | 8.6 (2.7) | 8.3 (2.95) | 0.410 |
| PD-Q, median (IQR) | 24 (9) | 15 (11) | < 0.001* |
| TCSS, median (IQR) | 17 (6) | 9 (5) | < 0.001* |
| Total MNSI, median (IQR) | 19 (3.25) | 4 (2) | < 0.001* |
| MNSI section A, median (IQR) | 12 (3) | 3 (2) | < 0.001* |
| MNSI section B, median (IQR) | 7.5 (1.5) | 1 (1) | < 0.001* |
DM diabetes mellitus, PNP polyneuropathy, IQR interquartile range, OAD oral antidiabetic, PD-Q pain detect, TCSS Toronto clinical scoring system, MNSI Michigan Neuropathy Screening Instrument
Each sections of the MNSI showed good internal consistency (Cronbach’s alpha > 0.70) as shown in Table 2. In section A, all items showed a moderate-strong factor loading ranging from 0.433 to 0.713. In section A, when item 7 was excluded, Cronbach’s α value increased to 0.896. It did not show a strong factor loading when compared to the other items. Accordingly, this item might be excluded from the Turkish version of the scale. Also in section B, all items showed a strong factor loading ranging from 0.464 to 0.832 as stated in Table 2.
Table 2.
Exploratory factor analysis and internal consistency of the Turkish version of the Michigan Neuropathy Screening Instrument Section A and B
| Questionnaire item | Factor loading | Item total correlation | Cronbach’s alpha when item was excluded |
|---|---|---|---|
| Section A | |||
| Question 1 | 0.707 | 0.611 | 0.873 |
| Question 2 | 0.781 | 0.705 | 0.868 |
| Question 3 | 0.810 | 0.746 | 0.866 |
| Question 5 | 0.724 | 0.643 | 0.872 |
| Question 6 | 0.725 | 0.641 | 0.872 |
| Question 7 | – | 0.159* | 0.896* |
| Question 8 | 0.639 | 0.569 | 0.876 |
| Question 9 | 0.725 | 0.573 | 0.875 |
| Question 11 | 0.710 | 0.611 | 0.873 |
| Question 12 | 0.637 | 0.567 | 0.876 |
| Question 13 | 0.640 | 0.572 | 0.875 |
| Question 14 | 0.623 | 0.549 | 0.877 |
| Question 15 | 0.551 | 0.476 | 0.880 |
| All | 0.884 | ||
| Section B | |||
| Foot appearance | 0.740 | 0.818 | 0.866 |
| Ulceration | 0.464 | 0.704 | 0.938 |
| Ankle reflexes | 0.771 | 0.827 | 0.863 |
| Vibration | 0.812 | 0.866 | 0.854 |
| Monofilament | 0.832 | 0.885 | 0.849 |
| All | 0.901 | ||
* When question 7 was excluded, Cronbach’s alpha value increased to 0.896
**Scores are the sum of the left and right lower limbs for section B
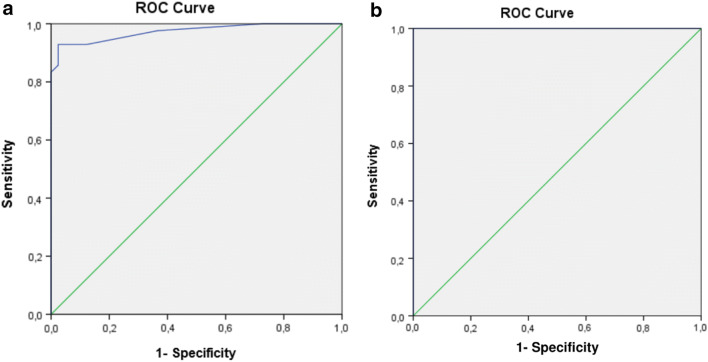
The test–retest reliability of the Turkish version of the MNSI was determined as 0.99 for the total score (ICC = 0.996) as stated in Table 3. Using the agreement between MNSI scores and DPN diagnosis, ROC curve values for section A and section B were estimated as 0.973 and 1.00, respectively, as shown in Fig. 1. When a cut-off value ≥ 3.0 in section A and a cut-off value ≥ 2.0 in section B were used, we obtained a sensitivity of 97.6% and 100%; a specificity of 63.4% and 97.6%; a positive predictive value of 72.7% and 97.6%; and a negative predictive value of 96.3% and 100%, respectively.
Table 3.
Stability of the Turkish version of the Michigan Neuropathy Screening Instrument
| Initial score Median (IQR) (n = 83) |
Retest score Median (IQR) (n = 83) |
ICC (95% CI) | |
|---|---|---|---|
| MNSI score | 8.5 (11.25) | 9 (10.25) | 0.996 (0.989–0.998) |
IQR interquartile range, MNSI Michigan Neuropathy Screening Instrument, ICC intraclass correlation coefficient
Fig. 1.
a Receiver-operating characteristic curve for Michigan Neuropathy Screening Instrument Section A. Area under the curve: 0.973. b Receiver-operating characteristic curve for Michigan Neuropathy Screening Instrument Section B. Area under the curve: 1.00
Total MNSI score was positively correlated with TCSS (r = 0.665; p < 0.001) and PD-Q (r = 0.657; p < 0.001) scores. Also both section A and B scores of MNSI were positively correlated with total MNSI score (r = 0.938; r = 0.908, respectively, p < 0.001) as stated in Table 4. Also scores of section A and section B were significantly and positively correlated (r = 0.604; p < 0.001).
Table 4.
Relationship between Michigan Neuropathy Screening Instrument Scores and other Neuropathy Scales in patients with diabetes mellitus
| MNSI total score Spearman (Rho) |
p | |
|---|---|---|
| MNSI Section A score | 0.938 | < 0.001 |
| MNSI Section B Score | 0.908 | < 0.001 |
| TCSS | 0.665 | < 0.001 |
| PD-Q | 0.657 | < 0.001 |
MNSI Michigan Neuropathy Screening Instrument, TCSS Toronto clinical scoring system, PD-Q pain detect questionnaire, PD-Q pain detect questionnaire
Discussion
In this cross-sectional study, we aimed to develop a Turkish version of the MNSI and assess its reliability and validity. The main finding of this study was that the Turkish version of the MNSI shows good psychometric and discriminant properties for detecting the presence of a DPN in patients with DM. The test–retest reliability of the Turkish version of the MNSI seemed to be good. It was also shown that self-questionnaire section had a similar discriminatory power as compared to physical examination section.
The original instrument was developed by the Neurology Department of Michigan University, using a sample of 56 patients with type 1 or 2 DM, and the NCS was the gold standard to confirm the diagnosis and to grade neuropathy. Of 29 patients with a clinical MNSI score > 2, 28 had neuropathy [10]. Contrary to MNSI examination tool, MNSI questionnaire has a lower sensitivity. A large study conducted on type 1 diabetic patients suggested that when the threshold to define an abnormal MNSI examination was set at 2.5, the MNSI examination was 61% sensitive and 79% specific in defining confirmed clinical neuropathy and had a positive predictive value of 55% and a negative predictive value of 83% [14]. In another cross-sectional study, MNSI scores of 1.5, 2.0, 2.5, and 3.0 were assessed as cut-off values. They stated that sensitivities were 79%, 65%, 50%, and 35% and specificities were 65%, 83%, 91%, and 94%, respectively, for each scores. As a result of the study, it was found that the sensitivity and specificity were higher when the cut-off value of MNSI was taken as 2 according to the Youden’s index [20]. In our study, when the cut-off value of MNSI was taken as 1, 1.5, and 2, respectively, the sensitivity was 100% for all three scores, and the specificity was 82.9%, 87.8%, and 97.6%, respectively. Therefore, we found the best cut-off value ≥ 2 for section B with higher Youden’s index.
To our knowledge, MNSI was only validated for the Iranian and Portuguese languages and populations [20, 21]. Our results revealed an ICC of 0.996 in the test/retest score, which supports a high stability of the MNSI, as obtained in other validation studies [20, 21]. In a recent validation study conducted on 76 patients, it was shown that the Portuguese version of the MNSI had good reliability/stability and validity and that it defined the best cut-off points to detect neuropathy. The best cut-off for section A was ≥ 3, and section B was ≥ 2 with higher Youden’s index. It was also shown that section A had a similar discriminatory power as total MNSI [21]. In accordance with these finding, we also reported good psychometric and discriminant properties for detecting the presence of a DPN in patients with DM for Turkish version of the MNSI. It was also shown that self-questionnaire section had a similar discriminatory power as compared to physical examination section in the present study. We also reported the same cut-off values for section A and B as reported in Portuguese validation study.
In a recent study, it was concluded that MNSI is a simple and validated diagnostic tool for DNP with a strong correlation to electrophysiological parameters [22]. Moreover, in cross-sectional studies, moderate correlation was stated between MNSI and the other neuropathy scales such as TCSS, United Kingdom screening test [7, 23]. Similarly, we also demonstrated a moderate correlation between MNSI and TCSS in the present study. The MNSI has a section to assess history of neuropathic symptoms which can explain the correlation between MNSI and PD-Q shown in our study.
Feasibility was assessed with difficulties found by patients when answering items, and the number of items not answered by patients. In linguistic validation phase, the most difficult items in ensuring the compliance were questions 7 and 15 during the forward translation process. There were also minor differences among the translators during the forward translation process in question 7 of self-questionnaire section of the MNSI that were amended by translators. All MNSI items were answered by all patients, and multiple answers were not noted for any item, showing that they were well understood. However, question 7 may be misunderstood by the individuals who respond to the questionnaire as it did not show good internal consistency. A possible explanation may be related to the fact that the question did not mention specifically the words “legs or feet” and, therefore, might be misunderstood by the individuals who respond to the questionnaire (section A), even in those who had unilateral amputation. Also, when the outcome of all scores was taken into consideration, it is clear that the translation procedure was completed successfully. In this study, it was shown that the Turkish version of MNSI can be used to discriminate between diabetic patients with DPN or without DPN.
Entrapment neuropathy, amyotrophy, distal symmetric polyneuropathy, focal neuropathy, cranial neuropathy, and small-fiber neuropathy are the neuropathic forms seen in diabetic patients. NCS is considered as the gold standard in the diagnosis of neuropathy. However, NCS is detected to be normal in small-fiber neuropathy presenting with burning, pain, and hyperesthesia. Because NCS evaluates large and myelinated nerves, while small-fiber neuropathy involves myelinated and unmyelinated small nerve fibers. The diagnosis of this form is made by neurophysiological tests such as quantitative sensory testing, quantitative sudomotor axon reflex test, and skin biopsy that evaluate intraepidermal nerve fibers [24]. In our study, skin biopsy was not performed, since it was painful and invasive. However, not performing biopsy was an important limitation of our study, since it is the gold standard in small-fiber neuropathy.
Age, duration of diagnosis, and gender are among the risk factors for neuropathy in diabetic patients [25]. Although there was no significant difference between the groups in terms of age and gender in our study, the female-to-male ratio was higher in both groups. A study found female gender as a risk factor for the severity of neuropathic pain [26]. Another study found that neuropathic pain was almost 50% higher in female patients than in male patients [27]. Therefore, the high female-to-male ratio in our study is one of the points that should be taken into consideration.
However, this study has some limitations such as difficulty to determine if the outcome of MNSI influenced by sociodemographic characteristics of participant due to small-sample size. Moreover, this validation study was conducted in a single university hospital by the contributions of two separate departments, but the strength of representation of the whole Turkish community could be better if it was a multicenter study. Although in the linguistic validation section, two native speakers translate the original version to Turkish and back translation which were done by two independent English native speakers, there might be a doubt whether the Turkish version was appropriate for people who could not understand the Turkish.
In conclusion, the Turkish version of the MNSI shows good psychometric and discriminant properties for detecting the presence of DPN in patients with DM. Therefore, the Turkish version of MNSI is a reliable and valid tool for screening DPN in Turkish patients.
Acknowledgements
The authors would like to thank Füsun Ardıç and Necmettin Yıldız.
Appendix
Hasta Versiyonu
Compliance with ethical standards
Conflict of interest
Authors do not have anything to disclose and declare no conflict of interest.
Human rights statement
The study “Turkish version of the Michigan Neuropathy Screening Instrument in the assessment of diabetic peripheral neuropathy: A validity and reliability study” was conducted in agreement with the Institutional Ethical Review Board of Pamukkale University Institute of Higher Education and Research, Denizli, Turkey (Approval Number: 60116787-020/54845, Date: 09/08/2019).
Informed consent
Informed consent was obtained from the participants before they were included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Serdar Kaymaz, Email: dr.serdarkymaz@gmail.com.
Hakan Alkan, Email: alkangsc@yahoo.com.
Ugur Karasu, Email: u_karasu@yahoo.com.
Veli Çobankara, Email: vcobankara@hotmail.com.
References
- 1.Tesfaye S, Boulton JMA, Dyck JP, Freeman R, Horowitz M, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan BC, Cheng HT, Stables CL, Smith AL, Felmen EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen J, et al. Health care utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29(2):212–217. doi: 10.1016/j.jdiacomp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Q, Lu B, Ye HW, Wu X, Zhang T, et al. The diagnostic value of neuropathy symptom and change score, neuropathy impairment score and Michigan Neuropathy Screening Instrument for diabetic peripheral neuropathy. Eur Neurol. 2015;74:323–327. doi: 10.1159/000441449. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJM, Vinik AI, Arezzo JC, Bril V, Felmen EL, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliability and validity of the modified Toronto clinical neuropathy score in diabetic sensorimotor polyneuropathy. Diabet Med. 2009;26:240–246. doi: 10.1111/j.1464-5491.2009.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Chen R, Zhang Y, Huang Y, Hong T, et al. Scoring systems to screen for diabetic peripheral neuropathy. Cochrane Database of Syst Rev. 2018 doi: 10.1002/14651858.cd010974. [DOI] [Google Scholar]
- 9.Kaymaz S, Koylu S, Kaymaz TI, Aykan AA. The Validity and reliability of the Turkish version of the Toronto clinical scoring system (TCSS) J PMR Sci. 2019;22(2):41–47. doi: 10.31609/jpmrs.2019-65511. [DOI] [Google Scholar]
- 10.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, et al. A practical two step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 11.Mete T, Aydin Y, Saka M, Cınar YH, Bılen S, et al. Comparison of efficiencies of Michigan Neuropathy Screening Instrument, neurothesiometer, and electromyography for diagnosis of diabetic neuropathy. Int J Endocrinol. 2013 doi: 10.1155/2013/821745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, et al. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the diabetic neuropathy symptom score. Diabet Med. 2002;19:962–965. doi: 10.1046/j.1464-5491.2002.00819.x. [DOI] [PubMed] [Google Scholar]
- 13.Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zummerman RS, et al. American Association of Clinical Endocrinologists and American College of Endocrinology: clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr Pract. 2015;21:1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman WH, Pop Busui R, Braffett BH, Martin CL, Cleary PA, et al. Use the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 2012;29:937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care. 2002;25:2048–2052. doi: 10.2337/diacare.25.11.2048. [DOI] [PubMed] [Google Scholar]
- 16.Freynhagen R, Baron R, Gockel U, Tölle TR. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488&#xB7. [DOI] [PubMed] [Google Scholar]
- 17.Alkan H, Ardic F, Erdogan C, Sarsan A, Sahin F, et al. Turkish version of the pain DETECT questionnaire in the assessment of neuropathic pain: a validity and reliability study. Pain Med. 2013;14:1933–1943. doi: 10.1111/pme.12222. [DOI] [PubMed] [Google Scholar]
- 18.Ovayolu N, Akarsu E, Madenci E, Torun S, Ucan O, et al. Clinical characteristics of patients with diabetic polyneuropathy: the role of clinical and electromyographic evaluation and the effect of the various types on the quality of life. Int J Clin Pract. 2008;62:1019–1025. doi: 10.1111/j.1742-1241.2008.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-reportmeasures. Spine. 2000;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108:477–481. doi: 10.1016/j.clineuro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa M, Saavedra A, Severo M, Majer C, Carvelho D. Validation and reliability of the Portuguese version of the Michigan Neuropathy Screening Instrument. Pain Pract. 2017;17(4):514–521. doi: 10.1111/papr.12479. [DOI] [PubMed] [Google Scholar]
- 22.Muntean C, Catalin B, Tudorica V, Mota M. Efficiency of Michigan Neuropathy Screening Instrument and Nerve Conduction Studies for diagnosis of diabetic Distal symmetric polyneuropathy. Rom J Diabetes Nutr Metab Dis. 2016;23(1):55–65. doi: 10.1515/rjdnmd-2016-0007. [DOI] [Google Scholar]
- 23.Fateh HR, Madani SP, Heshmat R, Larijani B. Correlation of Michigan neuropathy screening instrument, United Kingdom screening test and electrodiagnosis for early detection of diabetic peripheral neuropathy. J Diabetes Metab Disord. 2016;15:8. doi: 10.1186/s40200-016-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hovaguimian A, Gibbons CH. Diagnosis and Treatment of Pain in Small Fiber Neuropathy. Curr Pain Headache Rep. 2011;15(3):193–200. doi: 10.1007/s11916-011-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res. 2016 doi: 10.1155/2016/3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, et al. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of healthy subjects. Neurology. 1995;45(6):1115–1121. doi: 10.1212/WNL.45.6.1115. [DOI] [PubMed] [Google Scholar]
- 27.Abbott CA, Malik RA, Van Ross ERE, Kulkarni J, Boulton AJM. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]