Abstract
Background
Pitavastatin is the newest statin on the market, and the dose‐related magnitude of effect of pitavastatin on blood lipids is not known.
Objectives
Primary objective To quantify the effects of various doses of pitavastatin on the surrogate markers: LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides in participants with and without cardiovascular disease.
To compare the effect of pitavastatin on surrogate markers with other statins.
Secondary objectives To quantify the effect of various doses of pitavastatin on withdrawals due to adverse effects.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for trials up to March 2019: the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 2, 2019), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. The searches had no language restrictions.
Selection criteria
RCT and controlled before‐and‐after studies evaluating the dose response of different fixed doses of pitavastatin on blood lipids over a duration of three to 12 weeks in participants of any age with and without cardiovascular disease.
Data collection and analysis
Two review authors independently assessed eligibility criteria for studies to be included, and extracted data. We entered data from RCT and controlled before‐and‐after studies into Review Manager 5 as continuous and generic inverse variance data, respectively. Withdrawals due to adverse effects (WDAE) information was collected from the RCTs. We assessed all included trials using the Cochrane 'Risk of bias' tool under the categories of allocation (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias.
Main results
Forty‐seven studies (five RCTs and 42 before‐and‐after studies) evaluated the dose‐related efficacy of pitavastatin in 5436 participants. The participants were of any age with and without cardiovascular disease, and pitavastatin effects were studied within a treatment period of three to 12 weeks. Log dose‐response data over doses of 1 mg to 16 mg revealed strong linear dose‐related effects on blood total cholesterol and LDL cholesterol and triglycerides. There was no dose‐related effect of pitavastatin on blood HDL cholesterol, which was increased by 4% on average by pitavastatin. Pitavastatin 1 mg/day to 16 mg/day reduced LDL cholesterol by 33.3% to 54.7%, total cholesterol by 23.3% to 39.0% and triglycerides by 13.0% to 28.1%. For every two‐fold dose increase, there was a 5.35% (95% CI 3.32 to 7.38) decrease in blood LDL cholesterol, a 3.93% (95% CI 2.35 to 5.50) decrease in blood total cholesterol and a 3.76% (95% CI 1.03 to 6.48) decrease in blood triglycerides. The certainty of evidence for these effects was judged to be high. When compared to other statins for its effect to reduce LDL cholesterol, pitavastatin is about 6‐fold more potent than atorvastatin, 1.7‐fold more potent than rosuvastatin, 77‐fold more potent than fluvastatin and 3.3‐fold less potent than cerivastatin. For the placebo group, there were no participants who withdrew due to an adverse effect per 109 subjects and for all doses of pitavastatin, there were three participants who withdrew due to an adverse effect per 262 subjects.
Authors' conclusions
Pitavastatin lowers blood total cholesterol, LDL cholesterol and triglyceride in a dose‐dependent linear fashion. Based on the effect on LDL cholesterol, pitavastatin is about 6‐fold more potent than atorvastatin, 1.7‐fold more potent than rosuvastatin, 77‐fold more potent than fluvastatin and 3.3‐fold less potent than cerivastatin. There were not enough data to determine risk of withdrawal due to adverse effects due to pitavastatin.
Plain language summary
Pitavastatin for lowering lipids
Review question
How do different doses of pitavastatin affect fats in our blood?
Background
Pitavastatin is the newest statin on the market. We don't know the effect of different sizes of dose on the amount of fats in our blood.
Search date
We looked at research up to March 2019.
Study characteristics
We looked for high‐quality randomised trials (RCTs) and before‐and‐after studies with pitavastatin in different dose sizes . The trials were between three and 12 weeks long.
Participants in the trials could be of any age and gender, with or without cardiovascular disease.
Key results
People taking 1 mg to 16 mg of pitavastatin per day lowered their LDL cholesterol by 33.3% to 54.7%. The higher the dose, the lower the levels of three measures of cholesterol. The average increase in HDL cholesterol for all doses was 4%.
For lowering LDL cholesterol, pitavastatin is 6‐times stronger than atorvastatin, 1.7‐times stronger than rosuvastatin, 77‐times stronger than fluvastatin and 3.3‐times weaker than cerivastatin.
In the RCTS, no person out of 109 in the placebo group and three out of 262 people in the pitavastatin group dropped out due to adverse effects.
Certainty of the evidence
There is a high level of trust around the effects of pitavastatin on total cholesterol, LDL cholesterol and triglycerides.
Summary of findings
Background
Description of the condition
Cardiovascular disease is a major cause of death and disability in the developed world, accounting for more than one‐third of total deaths (Kreatsoulas 2010). In the USA, cardiovascular disease causes one in three reported deaths each year (CDC 2011; Roger 2011). Existing evidence shows a weak association between adverse cardiovascular events and blood concentrations of low‐density lipoprotein (LDL) cholesterol in adults (Grundy 2004). The current recommended treatment for secondary prevention of adverse cardiovascular events consists of diet and lifestyle changes plus drug therapy with the drug class widely known as ’statins’(CTT 2005).
Description of the intervention
Pitavastatin is a new synthetic potent statin that received FDA approval in the USA in 2009. In a long‐term trial, pitavastatin has been reported to increase high‐density lipoprotein (HDL) cholesterol in people with low HDL cholesterol levels < 40 mg/dL (Teramoto 2009). Pitavastatin is rapidly absorbed, reaching peak plasma concentration within one hour and has a half‐life of 11 hours. Pitavastatin is metabolised to a small degree by cytochromes P‐450 2C8 and P‐450 2C9 to 8‐hydroxy‐pitavastatin (M13) (Mukhtar 2005). Statins as a class have been shown in individual randomised controlled trials (RCTs) and systematic reviews of RCTs to reduce mortality and major vascular events in people with occlusive vascular disease (CTT 2005).
How the intervention might work
Pitavastatin acts in the liver by inhibiting an enzyme early in the pathway for cholesterol synthesis, 3‐hydroxy‐3‐methyl‐glutarylcoenzyme A reductase (HMG‐CoA reductase). This enzyme irreversibly converts 3‐hydroxy‐3‐methylglutaryl CoA to mevalonate (Moghadasian 1999). This reaction is the third step in a sequence of reactions resulting in the production of many compounds including cholesterol and its circulating blood derivatives, LDL cholesterol and very low‐density (VLDL) cholesterol (Gaw 2000). The prevailing hypothesis is that statins reduce mortality and morbidity in patients with occlusive vascular disease by reducing liver production of cholesterol and thus causing a reduction in blood LDL cholesterol and a resulting decrease in atherogenesis. However, the HMG CoA reductase enzyme is also responsible for the production of ubiquinone (co‐enzyme Q10), heme a, vitamin D, steroid hormones and many other compounds. It remains possible that the beneficial effects of statins are due to actions other than the reduction of cholesterol. These other actions have been referred to as the pleiotropic effects of statins (Liao 2005).
Why it is important to do this review
Statins are the most widely prescribed class of drugs in the world. Prescribing of statins is increasing, as are average prescribed doses. At the present time, clinicians have only an approximate sense of the different potency of the different statins. Previous systematic reviews have assessed the effect of statins on serum lipids (Bandolier 2004; Edwards 2003; Law 2003; Ward 2007). They have demonstrated that different statins have different potencies in terms of lipid‐lowering and that higher doses of statins cause greater lowering of serum lipids than lower doses (Kellick 1997; Schaefer 2004; Schectman 1996).
However, a systematic assessment of the potency, dose‐response relationship, and variability of effect has only been published for atorvastatin (Adams 2015), rosuvastatin (Adams 2014), fluvastatin (Adams 2016; Adams 2018) and cerivastatin (Adams 2017; Adams 2020). It is possible that pitavastatin will have different potency, slope of the dose‐response or variability of response. Statin‐induced myopathy is common to all statins, and limits the use of statins in many patients. Knowledge of the effects of statins on blood lipids can help us to use them more effectively. We will use the percentage reduction from baseline on the following surrogate markers to describe the dose‐response relationship of the effect of pitavastatin: total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides (Boekholdt 2012). We will use the results of this review to compare pitavastatin with rosuvastatin, atorvastatin, cerivastatin and fluvastatin. Subsequent reviews of other drugs in the class (i.e. lovastatin, pravastatin, simvastatin) will also be done, in order to compare the results of all the statins.
Objectives
Primary objective
To quantify the effects of various doses of pitavastatin on the surrogate markers: blood LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides in participants with and without cardiovascular disease.
We recognise that the outcomes important to patients are mortality and cardiovascular morbidity, however, that is not the objective of this systematic review. The aim of this review is to examine the pharmacology of pitavastatin by characterising the dose‐related effect and variability of the effect of pitavastatin on surrogate markers and to compare these to the other statins.
Secondary objectives
To quantify the effect of various doses of pitavastatin on withdrawals due to adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised placebo‐controlled trials (RCTs) as well as controlled before‐and‐after studies. Before‐and‐after studies were included, because it has been shown that there is no placebo effect of statins on lipid parameters and that a placebo control is therefore not essential (Tsang 2002). We included data from cross‐over trials if the authors reported data for the initial treatment period versus parallel treatment groups followed by an adequate washout period before crossing over to the other active treatments and if data were reported in a similar manner during all treatment periods.
Types of participants
Participants may be of any age, with and without cardiovascular disease. They could have normal lipid parameters or any type of hyperlipidaemia or dyslipidaemia. We included participants with various comorbid conditions, including type 2 diabetes mellitus, hypertension, metabolic syndrome, chronic renal failure or cardiovascular disease.
Types of interventions
Pitavastatin had to be administered at a constant daily dose defined as a single dose per day compared to placebo or alone defined as a single pitavastatin dose per day for a period of three to 12 weeks. We chose this administration time window to allow at least three weeks for a steady‐state effect of pitavastatin to occur and to keep it short enough to minimise participants dropping out. We included studies where pitavastatin was administered at any time during the day. Trials required a washout baseline dietary stabilisation period of at least three weeks, where all previous lipid‐altering medication was withdrawn. This baseline phase ensured participants follow a standard lipid‐regulating diet and helped to stabilise baseline lipid values prior to treatment. In trials where participants were not receiving lipid‐altering medications or dietary supplements before receiving the test drug, we did not require washout baseline dietary stabilisation periods.
Types of outcome measures
Pitavastatin 1 mg/day, 2 mg/day and 4 mg/day were the primary doses as these are the recommended and predominantly prescribed doses.
Lipid parameters: for the RCTs, we presented the mean percentage change from baseline for different doses of pitavastatin minus the mean percentage change from baseline with placebo for LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides. For the before‐and‐after studies, we presented the mean percentage change from baseline of different doses of pitavastatin. RCT data and before‐and‐after study data were combined because it was shown previously (Adams 2014; Adams 2015) that the two study designs yielded similar results (Tsang 2002).
Primary outcomes
LDL cholesterol
Secondary outcomes
Total cholesterol
HDL cholesterol
Triglycerides
End of treatment variability (standard deviation (SD)) and coefficient of variation of all lipid measurements for each dose of pitavastatin. It is important to know whether pitavastatin has an effect on the variability of lipid measures and ultimately to compare this with the effect of other statins.
Withdrawals due to adverse effects (WDAEs) could only be assessed in the placebo‐controlled trials.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist searched the following databases without language, publication year or publication status restrictions:
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (searched 4 March 2019);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 4 March 2019);
Embase Ovid (from 1974 onwards) (searched 4 March 2019);
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 4 March 2019);
World Health Organization International Clinical Trials Registry Platform (www.who.it.trialsearch) (searched 4 March 2019).
Epistemonikos (https://www.epistemonikos.org) searched 4 March 2019).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. We present the search strategies for major databases in Appendix 1.
Systematic searches in the following databases were conducted for before‐and‐after studies in MEDLINE, Embase, CENTRAL, CRS‐Web, ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform, and Epistemonikos. No filters were used to retrieve RCTs and before‐and‐after studies because we wanted to retrieve all available documents on the subject.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches for controlled trials in the Allied and Complementary Medicine Database (AMED), CAB Abstracts & Global Health, CINAHL, and Web of Science.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
We included grey literature by searching other resources.
ProQuest Dissertations and Theses (search.proquest.com/pqdtft/).
OpenTrials (opentrials.net).
US Food and Drug Administration (www.fda.gov/).
European Patent Office (worldwide.espacenet.com).
These resources were searched using the following keywords: pitavastatin, alipza, itavastatin, nisvastatin, livalo, livazo, NK‐104.
Data collection and analysis
Selection of studies, data extraction and management and assessment of risk of bias in included studies were all done using Covidence systematic review software (Covidence 2019).
Selection of studies
Initial selection of RCTs and before‐and after‐studies involved retrieving and reading the titles and abstracts of each paper found from the electronic search databases or bibliographic citations (see Figure 1 for PRISMA flow diagram (Moher 2009)). Two review authors (SA and NA) analysed the full‐text papers independently, to decide on the studies to be included. We resolved disagreements by recourse to the third review author (JMW). Two review authors (SA and NA) independently extracted the appropriate data from each of the included studies.
1.
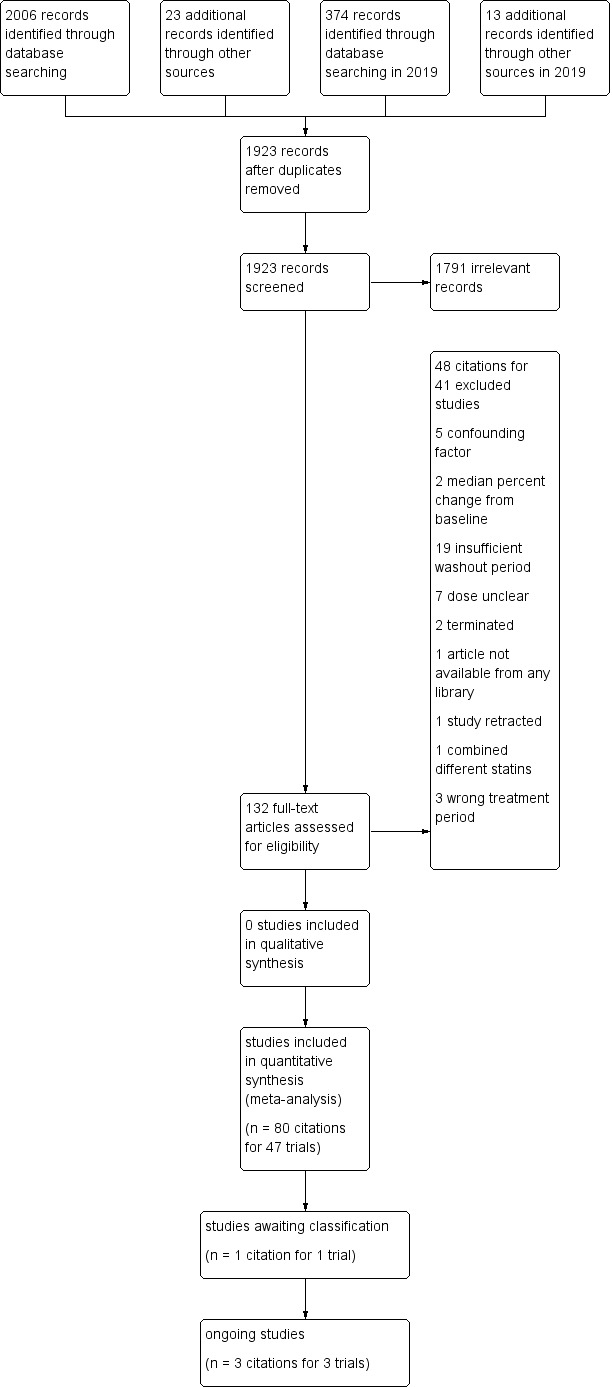
Study flow diagram for pitavastatin.
Data extraction and management
We extracted the mean percentage change directly from the data, or we calculated it from the baseline and endpoint values using the calculation found in Appendix 2. We added the calculated data to the Data and analyses section of the review. If the calculated data differed from the given data by more than 10%, the data were not included in the review. We extracted standard deviations (SDs) and standard errors (SEs) from the report or calculated them when possible using the following calculations (Appendix 3). We entered the data from RCT and controlled before‐and‐after studies into Review Manager 5 (RevMan 2020) as continuous and generic inverse variance data, respectively.
Assessment of risk of bias in included studies
We assessed all RCTs and before‐and‐after studies using the Cochrane 'Risk of bias' tool under the categories of allocation (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias. We produced 'Risk of bias' tables' as outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8 (Higgins 2011). Controlled before‐after‐studies were considered 'high risk' and 'low grading' compared to RCTs. With respect to before‐and‐after studies, having only one study group is considered 'high risk' for random sequence generation and allocation concealment but other features in this study design might be at lower risk, and there may be unidentified differences between the intervention and control groups that may affect changes in the outcome measure. We appreciate that blinding of participants and personnel and blinding of outcome assessment are inappropriate for before‐and‐after studies and that this is a limitation. However, because the lipid parameter measurements are unlikely to be influenced by lack of blinding and were measured in a remote laboratory, they were considered unlikely to be affected by the study design. We were able to use the Cochrane 'Risk of bias' tool for the controlled before‐and‐after studies because there was a lack of difference in the mean differences between the two type of studies (Tsang 2002).
Measures of treatment effect
We analysed the treatment effects as mean difference (MD) for each dose in the RCTs and generic inverse variance for each dose in the before‐and‐after controlled studies separately. In the event that the mean effects from the two study designs were not statistically different, we re‐analysed all efficacy study data using the generic inverse variance to determine the overall weighted treatment effects and their 95% confidence intervals (CIs) for blood total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides.
Unit of analysis issues
The unit of analysis is the mean value for the people completing the study. We expected follow‐up to be reasonably high for these short‐term studies. The data, however, represented treatment efficacy and not real world effectiveness of pitavastatin on these lipid parameters.
Dealing with missing data
We expected follow‐up to be reasonably high for these short‐term trials. The data, however, represented treatment efficacy and not real‐world effectiveness of pitavastatin on these lipid parameters. When data were missing, we requested them from the authors. The most common type of value that was not reported was the SD of the change.
In the case of a missing SD for the change in lipid parameters, we imputed the SD using the following hierarchy (listed from high to low preference).
SD calculated either from the t statistics corresponding to the exact P value reported or from the 95% CI of the mean difference between treatment groups.
Average weighted standard deviation of the change from other trials in the review (Furukawa 2006).
Because it is common for the SD to be miscalculated and in order not to overweight studies where it was inaccurately calculated and lower than expected, when SD values were less than 40% of the average weighted SDs, we used the imputed value by the method Furukawa 2006.
Assessment of heterogeneity
The Chi2 test to identify heterogeneity was not appropriate because it has low power when there are few studies but has excessive power to detect clinically unimportant heterogeneity when there are many studies (Higgins 2002). The I² is a better statistic. I² calculates between‐study variance/(between‐study variance + within‐study variance). This measures the proportion of total variation in the estimate of the treatment effect that is due to heterogeneity between studies. This statistic is also independent of the number of studies in the analysis (Higgins 2002).
The I2 was assessed as moderate heterogeneity when ranging from 30% to 50% and high heterogeneity when greater than 50%.
Assessment of reporting biases
We assessed publication bias using funnel plots, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 13 (Page 2019) when there were ten studies or more examining the same outcome (dose).
Data synthesis
We entered all RCTs into Review Manager 5 (RevMan 2020) as mean difference fixed‐effect model data to determine the weighted treatment effect and 95% CIs for blood total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides. We entered all controlled before‐and‐after studies as generic inverse variance fixed‐effect model data to determine the weighted treatment effect. If the effects in the RCTs were not statistically significantly different from the before‐and‐after studies, we entered all trials for each dose as generic inverse variance to determine the best overall weighted treatment effect for each dose.
We recorded data of each study and dose in GraphPad Prism 4, to yield a weighted least squares analysis based on the inverse of the square of the SE for each lipid parameter, to generate weighted log dose response curves. We entered the number of participants in placebo‐controlled trials, who prematurely withdrew due to at least one adverse effect in Review Manager 5 (RevMan 2020) as dichotomous data for each dose and all combined doses of pitavastatin and reported these as RR versus placebo.
The relative potency of pitavastatin with respect to fluvastatin, atorvastatin, rosuvastatin and cerivastatin, was determined as the ratio of the milligram (mg) amount of pitavastatin to the mg amount of fluvastatin or atorvastatin or rosuvastatin or cerivastatin needed to produce the same specified effect. These values were calculated from the log dose response curves of pitavastatin, fluvastatin, atorvastatin, rosuvastatin and cerivastatin for LDL cholesterol, total cholesterol and triglycerides. The relative potencies were estimated from these dose ratios. The relative potency results are mentioned in the Effects of interventions subsection of the Results section.
Subgroup analysis and investigation of heterogeneity
The main subgroup analyses were the different doses of pitavastatin. We assessed heterogeneity using the I² (Higgins 2002). If an I² value was ≥ 50%, we attempted to identify possible causes for this by carrying out a number of planned subgroup analyses, provided there were sufficient numbers of trials (see below).
We analysed subgroups based on the following factors.
Placebo‐controlled trials versus before‐and‐after trials (described above)
Men versus women
Morning administration time versus evening administration time as defined by the cut‐offs 6:00 am to noon and 6:00 pm to midnight
Kowa funded versus non‐Kowa funded trials
Twice‐daily versus once‐daily administration
Sensitivity analysis
We conducted sensitivity analyses to assess the effect of different comorbidities, such as familial hyperlipidaemia, on the treatment effect. We compared the treatment effects as generic inverse variance between trials whose subjects were reported to have type IIa or familial hypercholesterolaemia versus trials whose subjects were not reported to have genetic hypercholesterolaemia. Trials were not included in the comparison if the subjects had both familial and non‐familial hypercholesterolaemia. We conducted sensitivity analyses to assess the effect of different methods of dosing, such as twice daily versus single dose, on the treatment effect.
RCTs and before‐and‐after studies were analysed separately in the Data and analyses section.
Summary of findings and assessment of the certainty of the evidence
We used Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the certainty of the supporting evidence behind each estimate of treatment effect (Schünemann 2019a; Schünemann 2019b). We presented key findings of the review, including a summary of the amount of data, the magnitude of the effect size and the overall certainty of the evidence, in the Table 1, Table 2 and Table 3. We did not summarise the findings on HDL cholesterol in a 'Summary of findings' table because pitavastatin doses ranging from 1 mg/day to 8 mg/day had no dose‐related effect on HDL cholesterol. There were not enough data to determine risk of withdrawal due to adverse effects due to pitavastatin, therefore, there was no 'Summary of findings' table for WDAEs.
Summary of findings 1. Low‐density lipoprotein (LDL) cholesterol‐lowering efficacy of pitavastatin.
| Low‐density lipoprotein (LDL) cholesterol‐lowering efficacy of pitavastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory clinics Intervention: different fixed doses of pitavastatin Comparison: placebo or baseline | ||||||
| pitavastatin dose |
Anticipated absolute effects mmol/L (95%CI) |
Percentage change from baseline (95% CI) | No of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| LDL‐cholesterol before exposure to pitavastatina | LDL‐cholesterol after exposure to pitavastatin | |||||
| 1 mg/day | 5.06 (4.39 to 5.74) |
3.38 (3.32 to 3.44) |
‐33.2 (‐34.3 to ‐32.1) |
759 (10) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response curve, ‐33.3% |
| 2 mg/day | 4.51 (4.24 to 4.79) |
2.77 (2.75 to 2.79) |
‐38.65 (‐39.1 to ‐38.2) |
3847 (36) |
⊕⊕⊕⊕ high | Effect predicted from log dose‐response curve, ‐38.6% |
| 4 mg/day | 5.04 (4.24 to 5.85) |
2.82 (2.73 to 2.91) |
‐44.0 (‐45.8 to ‐42.3) |
469 (7) |
⊕⊕⊕⊕ high | Effect predicted from log dose‐response curve, ‐44.0% |
| CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aMean baseline values.
Summary of findings 2. Total cholesterol‐lowering efficacy of pitavastatin.
| Total cholesterol‐lowering efficacy ofpitavastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory clinics Intervention: different fixed doses of pitavastatin Comparison: placebo or baseline | ||||||
| Pitavastatin dose |
Anticipated absolute effects mmol/L (95%CI) |
Percentage change from baseline (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Total cholesterol before exposure to pitavastatina | Total cholesterol after exposure to pitavastatin | |||||
| 1 mg/day | 7.24 (6.67 to 7.82) |
5.55 (5.49 to 5.60) |
‐23.4 (‐24.2 to ‐22.7) |
777 (10) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is ‐23.3% |
| 2 mg/day | 6.65 (6.33 to 6.97) |
4.84 (4.81 to 4.87) |
‐27.25 (‐27.65 to ‐26.84) |
2789 (32) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is ‐27.3% |
| 4 mg/day | 7.21 (6.49 to 7.94) |
4.97 (4.87 to 5.07) |
‐31.1 (‐32.4 to ‐29.7) |
477 (7) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is ‐31.2% |
| CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aMean baseline values.
Summary of findings 3. Triglyceride‐lowering efficacy of pitavastatin.
| Triglyceride‐lowering efficacy of pitavastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory clinics Intervention: different fixed doses of pitavastatin Comparison: placebo or baseline | ||||||
| Pitavastatin dose |
Anticipated absolute effects mmol/L (95%CI) |
Percentage change from baseline (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Triglycerides before exposure to pitavastatina | Triglycerides after exposure to pitavastatin | |||||
| 1 mg/day | 1.71 (1.27 to 2.15) |
1.49 (1.45 to 1.52) |
‐13.1 (‐15.4 to ‐10.85) |
673 (8) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is ‐13.0% |
| 2 mg/day | 1.88 (1.75 to 2.02) |
1.56 (1.54 to 1.59) |
‐16.8 (‐18.2 to ‐15.5) |
2035 (26) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is ‐16.8% |
| 4 mg/day | 2.04 (1.29 to 2.79) |
1.67 (1.57 to 1.77) |
‐18.0 (‐23.0 to ‐13.0) |
424 (6) |
⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is ‐20.6% |
| CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aMean baseline values.
Results
Description of studies
This review included 47 studies involving 5659 intention‐to‐treat participants of whom 5436 (96.1%) participants had at least one lipid parameter measured and of whom 5127 (90.6%) had LDL cholesterol measured. There were 42 before‐and‐after studies, four double‐blind and one single‐blind RCT. The number of placebo and pitavastatin participants were 213 and 5223 respectively. The number of male and female participants reported in 44 of the 47 trials were 2,214 and 2,558, respectively. Participants could be of any age. There were five familial hypercholesterolaemia studies and 11 non‐familial hypercholesterolaemia studies.
Results of the search
Database searching identified 2380 citations and 36 other resource citations giving a total of 2416 records. After the duplicates were removed, 1923 records remained. The number of irrelevant records was 1791. From these remaining records, 132 were obtained as full‐text articles and assessed for eligibility. There were 48 citations for 41 excluded studies with reasons. The final number of included studies was 47 (Figure 1).
Included studies
Eighty citations to 47 studies met the inclusion criteria and had extractable data to evaluate the dose‐related blood lipid‐lowering effect of pitavastatin. Each included study is summarized in the Characteristics of included studies table. The publication languages of the 47 included studies were 40 (85.1%) English, three (6.4%) Japanese, four (8.5%) Chinese. The RCTs consisted of four double‐blind and one single‐blind randomised trial. Trials evaluating the lipid‐altering efficacy of pitavastatin were first published in 2000 and continued to be published until 2018 (Figure 2).
2.
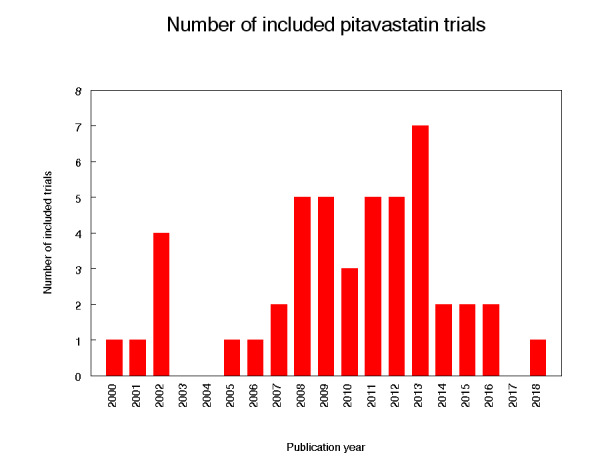
Number of included trials according to publication year
The baseline mean (range) lipid parameters were as follows: total cholesterol, 6.80 mmol/L (4.58 mmol/L to 8.91 mmol/L), 263 mg/dL (177 mg/dL to 344 mg/dL); LDL cholesterol, 4.56 mmol/L (2.97 mmol/L to 6.97 mmol/L), 176 mg/dL (115 mg/dL to 270 mg/dL); HDL cholesterol, 1.38 mmol/L (1.09 mmol/L to 1.71 mmol/L), 53 mg/dL (42 mg/dL to 66 mg/dL) and triglycerides 1.86 mmol/L (0.79 mmol/L to 3.10 mmol/L), 165 mg/dL (70 mg/dL to 274 mg/dL).
Excluded studies
Forty‐one studies were excluded because they did not meet the inclusion criteria. Reasons for exclusion included confounding in which participants were receiving drugs that affect blood lipid concentrations: immunosuppressants such as cyclosporine, protease inhibitors such as ritonavir and indinavir, food supplements such as fish oils, fibrates such as gemfibrozil, fenofibrate and clofibrate, bile acid sequestrants such as cholestyramine, colestipol, colesevelam, the cholesterol absorption inhibitor ezetimibe, the vitamin niacin and the anti‐oxidant drug probucol, inappropriate dosing, inappropriate outcomes such as median percent change from baseline, and inadequate dietary baseline stabilisation period. The reasons for excluding each trial are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed all included trials using the Cochrane 'Risk of bias' tool under the categories of allocation (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias.
Allocation
Random sequence generation bias was judged to be high in the 42 before‐and‐after studies. In the five RCTs, one was judged to have a low risk of bias for random sequence generation, and four were judged to be unclear. Allocation concealment was not applicable to the 42 before‐and‐after studies, but in the 'Risk of bias' tables they were judged to have high risk of bias. Of the five RCTs, two were judged to have a low risk of bias, one single‐blind RCT was judged to have a high risk of bias and two were judged unclear.
Blinding
We judged the risk of performance and detection bias for lipid parameters to be low for all the trials as lipid parameter measurements are unlikely to be influenced by lack of blinding.
We judged the risk of blinding of outcome assessment for lipids to be low for all the trials as lipid parameters were measured in a remote laboratory.
For withdrawals due to adverse effects (WDAEs), in the five RCTs, there was a high risk of detection bias in the single‐blind RCT and in two double‐blind RCTs. It was judged unclear in the other two.
Incomplete outcome data
Incomplete outcome reporting leading to attrition bias was not a problem in this review as few participants were lost to follow‐up and were balanced across the groups in the RCTs. Overall, 96.1% of the participants completed the treatment.
Selective reporting
Out of 47 trials, 45 (95.7%) reported the primary lipid outcome LDL‐C, thus selection bias was not a potential source of bias for this outcome.
Out of five RCTs, only three (60%) reported WDAEs. The trials that did not report these could have deliberately not done so because WDAEs were increased. Therefore, selective reporting bias was judged an important source of bias for this outcome. See 'Risk of bias' tables in Characteristics of included studies, and for the overall risk of bias.
Other potential sources of bias
The main other potential source of bias is industry funding. Out of the 47 trials, 15 (32%) reported funding by industry, 2 (4.4%) reported partial funding by industry and government, 7 (14.9%) reported no industry funding and in 23 (48.9%) trials the source of funding was not reported. Out of 15 industry funded trials, 10 (67%) were funded by Kowa, marketers of pitavastatin and 5 (33%) was funded by another pharmaceutical company. The Kowa‐funded trials might be biased in favour of pitavastatin and would be expected to overestimate the treatment effect while trials funded by rival pharmaceutical companies might be biased against pitavastatin and be expected to underestimate the treatment effect. In trials where the source of funding was not reported, bias could be for or against pitavastatin. Kowa‐funded versus non‐Kowa‐funded LDL cholesterol efficacy data were available for the doses of 2 mg/day and 4 mg/day. These data were analysed separately using the generic inverse variance fixed‐effect model in RevMan 5. The sensitivity analysis revealed that the lipid‐lowering efficacy of pitavastatin in Kowa‐funded versus non‐Kowa‐funded trials was not different for the doses analysed; 2 mg/day (‐38.8% vs ‐39.5%; P = 0.43) and 4 mg/day (‐39.5% vs ‐35.2%; P = 0.36). Assessment for publication bias was done by reviewing the funnel plots for all lipid outcomes with 10 or more trials. None of these funnel plots suggested publication bias.
The determination of lipids in the blood samples was done by laboratories not connected to the trial personnel or participants, therefore, we judged the overall risk of bias to be low for both the placebo‐controlled RCTs and for the before‐and‐after studies.
Effects of interventions
See: Table 1; Table 2; Table 3
See: Summary of findings table 1, Summary of findings table 2 and Summary of findings table 3, for the LDL cholesterol‐lowering, total cholesterol‐lowering, and triglyceride‐lowering efficacy of pitavastatin for all trials. Relative potencies of pitavastatin with respect to fluvastatin, atorvastatin, rosuvastatin and cerivastatin for LDL cholesterol, total cholesterol and triglycerides were determined. For LDL cholesterol, pitavastatin is 77‐fold more potent than fluvastatin, 6.2‐fold more potent than atorvastatin, 1.7‐fold more potent than rosuvastatin and 3.3‐fold less potent than cerivastatin. For total cholesterol, pitavastatin is 71‐fold more potent than fluvastatin, 5.4‐fold more potent than atorvastatin, 1.8‐fold more potent than rosuvastatin and 3.3‐fold less potent than cerivastatin. For triglycerides, pitavastatin is 31‐fold more potent than fluvastatin, 2.8‐fold more potent than atorvastatin, 3.2‐fold more potent than rosuvastatin and 5.6‐fold less potent than cerivastatin.
Overall efficacy of pitavastatin
Values from all data describing the efficacy of pitavastatin to lower the lipid parameters from RCT and before‐and‐after studies from the Data and analyses section were entered as generic inverse variance data separately into GraphPad Prism 4 to yield log dose‐response curves for placebo and before‐and after studies. To compare slope results of RCT versus before‐and‐after studies, t‐tests from the formula t = (Placebo Slope‐Before‐and‐After Slope)/SQRT(SE2placebo slope + SE2before-and-after slope) were performed from the slopes and standard errors of the curves for total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides. The results showed that there were no differences between RCTs and before‐and‐after studies for total cholesterol (P = 0.2851), LDL cholesterol (P = 0.2723), HDL cholesterol (P = 0.406) and triglycerides (P = 0.686). This demonstrates that the two trial designs provide similar estimates of the lipid‐lowering efficacy of pitavastatin.
In addition, two‐tailed one sample t‐tests were performed from the RCTs to test for the difference between placebo mean effects and zero. The results of these tests demonstrated that the placebo means were not different from zero: total cholesterol: ‐2.53 (95% CI ‐7.41 to 2.36) P = 0.13; LDL cholesterol: 8.0 (95% CI ‐4.23 to 4.51) P = 0.95; HDL cholesterol 1.7 (95% CI ‐3.9 to 7.3) P = 0.23; and triglycerides: 1.6 (95% CI ‐4.0 to 7.2) P = 0.75. The evidence of lack of a placebo effect provided further justification for combining all the trials to determine the overall efficacy.
Validation for combining the results from the two trial designs has been previously shown in the atorvastatin, rosuvastatin, fluvastatin and cerivastatin reviews (Adams 2014; Adams 2015; Adams 2018; Adams 2020).
Combining the results from the two trial designs was done by entering all data into RevMan 5 using the generic inverse variance model outside of this review (data and analysis are not shown). The mean parameters from this analysis are summarised in Table 4. The results from the two trial designs were combined because the mean treatment effects were not statistically different between RCTs and before‐and‐after studies.
1. Pitavastatin overall efficacy.
| Pitavastatin dose mg/day | 1 | 2 | 4 | 8 | 16 |
| Mean percentage change from control of LDL‐Ca (95% confidence interval) |
‐33.2 (‐34.3 to ‐32.1) |
‐38.65 (‐39.1 to ‐38.2) |
‐44.0 (‐45.8 to ‐42.3) |
‐48.7 (‐52.4 to ‐45.0) |
‐54.5 (‐59.4 to ‐49.6) |
| Mean percentage change from control of total cholesterol (95% confidence interval) |
‐23.4 (‐24.2 to ‐22.7) |
‐27.25 (‐27.65 to ‐26.84) |
‐31.1 (‐32.4 to ‐29.7) |
‐37.0 (‐41.4 to ‐32.6) |
|
| Mean percentage change from control of triglycerides (95% confidence interval) |
‐13.1 (‐15.4 to ‐10.85) |
‐16.8 (‐18.2 to ‐15.5) |
‐18.0 (‐23.0 to ‐13.0) |
‐32.9 (‐45.0 to ‐20.8) |
aLDL‐C: low‐density lipoprotein cholesterol
Primary Outcome
LDL cholesterol
In total, 44/47 (93.6%) trials and 5127/5659 (90.6%) participants contributed to the LDL cholesterol data analysis. The effect of different doses of pitavastatin on LDL cholesterol are shown in the Data and analyses section (Analysis 1.1; Analysis 1.5; Analysis 2.1; Analysis 2.5; Analysis 3.1; Analysis 3.5; Analysis 4.1; Analysis 5.1). The analysis for LDL cholesterol yielded the log dose‐response straight‐line equation, y = ‐17.78 log(x) ‐ 33.27. This equation provides the best estimate of the mean reductions in blood LDL cholesterol from baseline for pitavastatin doses ranging from 1 mg/day to 16 mg/day as it uses all the available data. Using this formula, the calculated reductions in blood LDL cholesterol for doses of 1 mg per day to 16 mg per day were from 33.3% to 54.7%. For every two‐fold dose increase, there was a 5.35% (95% CI 3.32 to 7.38) percentage decrease in blood LDL cholesterol (Figure 3).
1.1. Analysis.

Comparison 1: 1 mg vs control, Outcome 1: LDL cholesterol RCTs
1.5. Analysis.

Comparison 1: 1 mg vs control, Outcome 5: LDL‐cholesterol non‐RCTs
2.1. Analysis.

Comparison 2: 2 mg vs control, Outcome 1: LDL cholesterol RCTs
2.5. Analysis.

Comparison 2: 2 mg vs control, Outcome 5: LDL‐cholesterol non‐RCTs
3.1. Analysis.

Comparison 3: 4 mg vs control, Outcome 1: LDL‐cholesterol RCTs
3.5. Analysis.

Comparison 3: 4 mg vs control, Outcome 5: LDL‐cholesterol non‐RCTs
4.1. Analysis.

Comparison 4: 8 mg vs control, Outcome 1: LDL cholesterol RCTs
5.1. Analysis.

Comparison 5: 16 mg vs control, Outcome 1: LDL cholesterol RCTs
3.
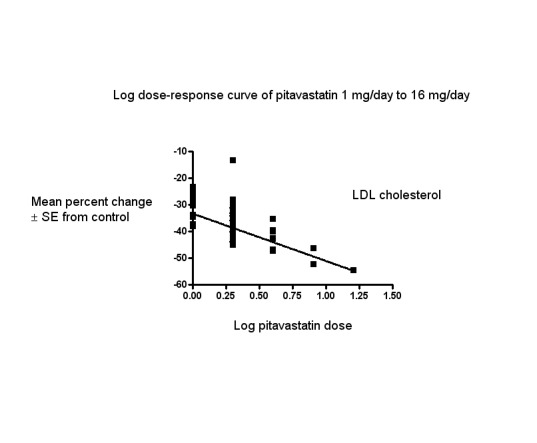
Log dose pitavastatin response curve for LDL cholesterol
Values represent the results of each trial for each dose comparison. The standard error bars cannot be seen because they all lie within the points
Secondary Outcomes
Total cholesterol
In total, 40/47 (85.1%) trials and 3836/5659 (67.8%) participants contributed to the total cholesterol data analysis. The effect of different doses of pitavastatin on total cholesterol are shown in the Data and analyses section (Analysis 1.2; Analysis 1.6; Analysis 2.2; Analysis 2.6; Analysis 3.2; Analysis 3.6; Analysis 4.2). The analysis for total cholesterol yielded the log dose‐response straight‐line equation, y = ‐13.04 log(x) ‐23.34. This equation provides the best estimate of the mean reductions in blood total cholesterol from baseline for pitavastatin doses ranging from 1 mg/day to 8 mg/day as it uses all the available data. Using this formula, the calculated reductions in blood total cholesterol for doses of 1 mg per day to 8 mg per day were from 23.3% to 35.1%. For every two‐fold dose increase, there was a 3.93% (95% CI 2.35 to 5.50) percentage decrease in blood total cholesterol (Figure 4).
1.2. Analysis.

Comparison 1: 1 mg vs control, Outcome 2: Total cholesterol RCTs
1.6. Analysis.

Comparison 1: 1 mg vs control, Outcome 6: Total cholesterol non‐RCTs
2.2. Analysis.

Comparison 2: 2 mg vs control, Outcome 2: Total cholesterol RCTs
2.6. Analysis.

Comparison 2: 2 mg vs control, Outcome 6: Total cholesterol non‐RCTs
3.2. Analysis.

Comparison 3: 4 mg vs control, Outcome 2: Total cholesterol RCTs
3.6. Analysis.

Comparison 3: 4 mg vs control, Outcome 6: Total cholesterol non‐RCTs
4.2. Analysis.

Comparison 4: 8 mg vs control, Outcome 2: Total cholesterol RCTs
4.
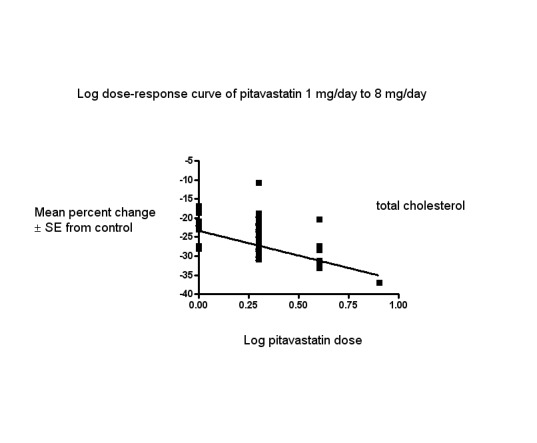
Log dose pitavastatin response curve for total cholesterol
Values represent the results of each trial for each dose comparison. The standard error bars cannot be seen because they all lie within the points
HDL cholesterol
In total, 40/47 (85.1%) trials and 3230/5659 (57.1%) participants contributed to the HDL cholesterol data analysis. The effect of different doses of pitavastatin on HDL cholesterol are shown in the Data and analyses section (Analysis 1.3; Analysis 1.7; Analysis 2.3; Analysis 2.7; Analysis 3.3; Analysis 3.7; Analysis 4.3). The GraphPad Prism 4 analysis showed that pitavastatin doses ranging from 0.1 mg/day to 0.8 mg/day had no dose‐related effect on blood HDL cholesterol. All doses of pitavastatin caused a small increase in HDL cholesterol. When all trials and doses were pooled using generic inverse variance, the magnitude of the increase was 4.11% (95% CI 3.61 to 4.61).
1.3. Analysis.

Comparison 1: 1 mg vs control, Outcome 3: HDL cholesterol RCTs
1.7. Analysis.

Comparison 1: 1 mg vs control, Outcome 7: HDL‐cholesterol non‐RCTs
2.3. Analysis.

Comparison 2: 2 mg vs control, Outcome 3: HDL cholesterol RCTs
2.7. Analysis.

Comparison 2: 2 mg vs control, Outcome 7: HDL‐cholesterol non‐RCTs
3.3. Analysis.

Comparison 3: 4 mg vs control, Outcome 3: HDL cholesterol RCTs
3.7. Analysis.

Comparison 3: 4 mg vs control, Outcome 7: HDL‐cholesterol non‐RCTs
4.3. Analysis.

Comparison 4: 8 mg vs control, Outcome 3: HDL cholesterol RCTs
Triglycerides
In total, 32/47 (68.1%) trials and 2979/5659 (52.6%) participants contributed to the triglyceride data analysis. The effect of different doses of pitavastatin on triglycerides are shown in the Data and analyses section (Analysis 1.4; Analysis 1.8; Analysis 2.4; Analysis 2.8; Analysis 3.4; Analysis 3.8; Analysis 4.4). The analysis for triglycerides yielded the log dose‐response straight‐line equation, y = ‐12.48 log(x) ‐ 13.04. This equation provides the best estimate of the mean reductions in blood triglycerides from baseline for pitavastatin doses ranging from 1 mg/day to 8 mg/day as it uses all the RCT data. Using this formula, the calculated reductions in total blood triglycerides for doses of 1 mg /day to 8 mg per day were from 13.0% to 24.3%. For every two‐fold dose increase, there was a 3.76% (95% CI 1.03 to 6.48) percentage decrease in blood triglycerides (Figure 5).
1.4. Analysis.

Comparison 1: 1 mg vs control, Outcome 4: Triglycerides RCTs
1.8. Analysis.

Comparison 1: 1 mg vs control, Outcome 8: Triglycerides non‐RCTs
2.4. Analysis.

Comparison 2: 2 mg vs control, Outcome 4: Triglycerides RCTs
2.8. Analysis.

Comparison 2: 2 mg vs control, Outcome 8: Triglycerides non‐RCTs
3.4. Analysis.

Comparison 3: 4 mg vs control, Outcome 4: Triglycerides RCTs
3.8. Analysis.

Comparison 3: 4 mg vs control, Outcome 8: Triglycerides non‐RCTs
4.4. Analysis.

Comparison 4: 8 mg vs control, Outcome 4: Triglycerides RCTs
5.
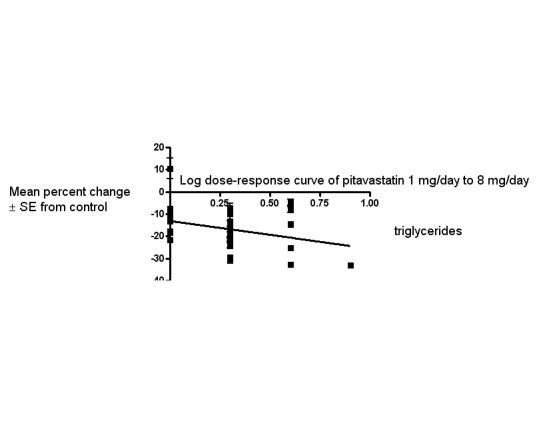
Log dose pitavastatin response curve for triglycerides
Values represent the results of each trial for each dose comparison. The standard error bars cannot be seen because they all lie within the points
End of treatment variability
Pitavastatin did not significantly affect the end‐of‐treatment variabilities of total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides.
Withdrawal data
Three (60%) of the five RCTs reported WDAEs during the three to 12‐week treatment period. In one trial, no participant discontinued treatment due to adverse effects or died during the study, therefore, risk reduction was not estimable. A pooled estimate for all doses compared to placebo showed a risk ratio (RR) of 1.35 (95% CI 0.15 to 12.04) for WDAEs in these short‐term trials (Analysis 6.1). There were not enough data to determine risk of withdrawal due to adverse effects due to pitavastatin. For the placebo group, there were 0 out of 109 participants who withdrew due to an adverse effect and, for all doses of pitavastatin, there were 3 out of 262 participants who withdrew due to an adverse effect.
6.1. Analysis.

Comparison 6: All doses of pitavastatin vs placebo, Outcome 1: WDAE
Subgroup Analyses
Male versus female participant data were available for the 1 mg/day, 2 mg/day and 4 mg/day doses. These data were analysed separately for LDL cholesterol‐lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5 outside of this review. The subgroup analysis revealed that the efficacy of pitavastatin was greater in females than in males. The efficacy for the 1 mg/day dose (male versus female) was: (‐30.61 vs ‐31.70; P = 0.49), for the 2 mg/day dose (male versus female) was: (‐36.05 vs ‐40.91; P < 0.0001) and for the 4 mg/day dose (male versus female) was: (‐43.64 vs ‐45.30; P = 0.17).
Morning versus evening dose administration data were available for the 1 mg/day, 2 mg/day, and the 4 mg/day doses. These data were analysed separately for LDL cholesterol‐lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5 outside of this review. The subgroup analysis revealed that the efficacy of pitavastatin was greater when pitavastatin was administered in the evening versus when administered in the morning. The efficacy for the 1 mg/day dose (morning versus evening) was: (‐25.91 vs ‐33.78; P = 0.0002) for the 2 mg/day dose (morning versus evening) was: (‐29.20 vs ‐39.07; P < 0.0007) and for the 4 mg/day dose (morning versus evening) was: (‐39.50 vs ‐45.08; P = 0.08). Comparison of twice‐daily administration versus single‐dose comparison was not possible because no trial provided the appropriate data.
Sensitivity Analyses
Familial versus non‐familial hypercholesterolaemia participant data were available for the doses 1 mg/day, 2 mg/day, and 4 mg/day. These data were analysed separately for LDL cholesterol‐lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5. There was no difference in the efficacy of pitavastatin between familial versus non‐familial hypercholesterolaemia participants. The efficacy for the 1 mg/day dose (familial versus non‐familial) was: (‐25.91 vs ‐28.02; P = 0.41), for the 2 mg/day dose (familial versus non‐familial) was: (‐37.00 vs ‐37.31; P = 0.85) and for the 4 mg/day dose (familial versus non‐familial) was: (‐39.50 vs ‐41.77; P = 0.49).
Discussion
Summary of main results
Daily pitavastatin intake is effective in lowering LDL cholesterol concentrations and does so in a predictable, dose‐related manner. The Summary of findings table 1 documents the effect of pitavastatin on LDL cholesterol over the dose range of 1 to 4 mg/day, the range for which this systematic review has the most data. Over this range, LDL cholesterol is decreased by 33.2% to 44.0% (Summary of findings table 1). These large reductions reflect a reduction in synthesis of cholesterol by the liver and indicate that liver HMG CoA reductase is being inhibited by approximately two‐fifths over this dose range. This has significant implications beyond circulating LDL cholesterol, as LDL cholesterol is only one of many important biochemical products that are produced by the HMG CoA reductase pathway. Those other products, including co‐enzyme Q10, heme A, vitamin D, steroid hormones and many other compounds are also likely to be reduced by about 40% over this dose range. It is important to recognise that the long‐term consequences of reduction of these products is presently unknown.
In the Data and analyses section, it can be seen that there are more trials and data with the before‐and‐after design than from placebo‐controlled trials. Slope results from placebo‐controlled versus before‐and‐after studies showed no differences for all outcomes, therefore, the effect of pitavastatin on the lipid parameters is similar with the two different trial designs. This, plus the demonstration that the placebo effect was not different from zero, justified using generic inverse variance and displaying the combined estimates in Table 1. In addition, all trial data were entered into GraphPad Prism 4 to calculate the regression lines shown in Figure 3, Figure 4 and Figure 5. The overall efficacy results from GraphPad Prism 4 provide the best estimate of the treatment effect, because they are based on a regression line calculated from all the data for all the doses. The estimates of the average treatment effect from the regression lines are similar to the mean value for all the data for each dose (see Table 1). It was important that we used before‐and‐after studies for this review as we would have only had five RCTs if we limited it to placebo‐controlled trials.
In this review, it was established, using regression analysis, that there was a correlation between the baseline value and pitavastatin effect on LDL cholesterol when the effect was expressed as absolute change from baseline (P < 0.0001). There was no correlation between the baseline value and the pitavastatin effect when the effect was expressed as percent reduction from baseline (P = 0.3140). This finding provides support for the fact that systematic reviews reporting the effect of statins on absolute changes in lipid parameters are problematic and potentially misleading.
What is the effect of pitavastatin on end‐of‐treatment variability?
End‐of‐treatment variabilities of pitavastatin and placebo were compared to determine the effect of pitavastatin on variability of blood lipids when expressed as a coefficient of variation. Compared with placebo, pitavastatin at all doses did not affect the coefficient of variation of blood total cholesterol, LDL cholesterol, HDL cholesterol or triglycerides. However, the fact that we only had five trials to test this makes this a weak finding.
Does pitavastatin increase withdrawals due to adverse effects?
There were not enough data in the review to determine risk of withdrawal due to adverse effects due to pitavastatin.
Pitavastatin 8 mg/day, 16 mg/day, 32 mg/day and 64 mg/day from phase 2 and phase 3 clinical trials had rates of discontinuation of 4.6%, 19.6%, 11.8% and 36.4% respectively due to treatment‐emergent adverse effects. In these phase 2 trials, nine subjects developed rhabdomyolysis: two cases for pitavastatin 8 mg/day, one case for 16 mg/day, three for 32 mg/day and three for 64 mg/day (Chowdhury 2009).
Symptomatic myopathy occurred in 8.6% of participants and asymptomatic myopathy in 27.5% of participants. Pitavastatin doses of 8 mg and above were not well tolerated. These were associated with an increased rate of SAEs, of treatment discontinuations, of CK, AST and ALT elevations and of haematuria (Australian Government 2013) and, therefore, it is important to not exceed 4 mg/day.
Overall completeness and applicability of evidence
This review included 47 trials with 5436 out of 5659 intention‐to‐treat participants of which 5127 participants had their LDL cholesterol reported. Even though the number of participants in the review was small, the amount of data retrieved provided us with robust evidence of the dose‐related lipid‐lowering effects of pitavastatin. Practitioners can use this evidence to calculate the expected effect of doses of pitavastatin commonly utilised in society. It is likely that further research will change these estimates appreciably. There was a fair amount of heterogeneity in many of the estimates and it is possible that this was due to differences in the populations being studied (e.g. gender or genetic differences) (Thompson 2005). To explore this, where it was possible, we compared the effect of pitavastatin in males and females. A subgroup analysis comparing male versus female subject data was available for the doses 1, 2 and 4 mg/day and suggested that efficacy of pitavastatin was greater in females than in males. A greater effect in females than males could be because females on average weigh less than males. This subgroup analysis in the atorvastatin, rosuvastatin and cerivastatin reviews also showed a larger effect in females than males (Adams 2014; Adams 2015; Adams 2020). In the fluvastatin review, there was no statistically significant difference of the effect in males and females (Adams 2018).
When we did the review, it was unknown whether the time of pitavastatin administration was important with respect to lipid‐lowering. A subgroup analysis comparing morning versus evening dose administration was available for the doses 1 mg/day, 2 mg/day and 4 mg/day. This comparison showed that the LDL‐lowering efficacy of pitavastatin was greater when pitavastatin was administered in the evening than when administered in the morning. This suggests that evening administration is preferred for this statin and it is similar to simvastatin where evening dose administration is recommended. This finding is clearly worth exploring further as, at the present time, the pitavastatin product monograph does not recommend a time of administration.
Familial versus non‐familial hypercholesterolaemia participant data were available for a sensitivity analysis of the pitavastatin doses of 1 mg/day, 2 mg/day and 4 mg/day. These data were analysed separately for LDL cholesterol‐lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5. There was no difference in the efficacy of pitavastatin between familial versus non‐familial hypercholesterolaemia participants. This finding is consistent with what was found in the rosuvastatin and cerivastatin reviews (Adams 2014; Adams 2020). However, it is in contrast to the findings in the atorvastatin and fluvastatin reviews where the LDL‐lowering effect was less in participants with familial hypercholesterolaemia (Adams 2015; Adams 2018).
The profound and relatively consistent effect of pitavastatin on lipid parameters shown in this review is probably appreciated by clinicians who treat patients with these drugs. Investigators involved in placebo‐controlled RCTs are likely to know whether participants are taking statins or not. Knowledge of the lipid parameters almost certainly leads to loss of blinding in statin RCTs. The present review calls attention to that problem and efforts to prevent this loss of blinding are needed in future statin RCTs (Higgins 2011).
Quality of the evidence
The summary of all ’Risk of bias’ tools for the lipid effects suggests a high risk of bias (Figure 6). However, the lipid parameter outcomes are probably relatively resistant to bias. If anything, a high risk of bias would lead to an overestimate of the lipid‐lowering effects rather than an underestimate. However, because of the objectivity of the measurement of the lipid parameters, we think that the lipid measures effects are reasonably accurate. This view is strengthened by the fact that we could not show evidence of funding bias. Comparing Kowa‐funded trials with non‐Kowa‐funded trials showed no differences. Furthermore, review of funnel plots did not suggest any publication bias.
6.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
There were not enough data to determine risk of withdrawal due to adverse effects due to pitavastatin.
Potential biases in the review process
Combining the placebo‐controlled trials with the before‐and‐after studies is a limitation of the review. We have explained why the increased risk of bias associated with the before‐and‐after design is less in this instance because the lipid parameters were measured in remote laboratories. Another limitation of this review is that many trials did not report standard deviations for the lipid‐lowering effects. In those trials, the standard deviation of the per cent change from baseline of the blood lipid parameters were imputed as the average of this parameter from trials that reported it. These values were determined by the method of (Furukawa 2006), from t‐statistics corresponding to the exact P values reported or from the 95% CI of the mean difference between treatment groups. Such imputation might weight some studies more or less; however, this has been shown in other reviews to not have much effect on the estimate of the effect size (Heran 2008; Musini 2014). Another limitation is that, in this review, few studies were available to demonstrate the lipid‐lowering effect of pitavastatin at doses of < 1 mg/day and > 16 mg/day. We did not downgrade the certainty of evidence due to heterogeneity of LDL cholesterol because the confidence intervals for the pooled result estimates were narrow.
Agreements and disagreements with other studies or reviews
The best estimate of the mean per cent reduction in blood LDL cholesterol for any dose of pitavastatin can be calculated from our log dose‐response equation. Using this equation y = ‐17.78 log(x) ‐ 33.27, a pitavastatin dose of 2 mg/day reduces LDL cholesterol by an average of 38.6%. This is similar to the estimate of 36% reduction in LDL cholesterol in 527 participants in Edwards 2003.
Comparison of the efficacy of pitavastatin with other statins
The greatest value in doing this type of review is the ability to compare pitavastatin to other statins. At present, we can compare it to atorvastatin, rosuvastatin, fluvastatin and cerivastatin, which have been reviewed using the same protocol. The most important finding in this review is that the slope of the dose‐response effect for pitavastatin on LDL cholesterol, total cholesterol and triglycerides is not different from the slope of the dose‐response curves for atorvastatin (Adams 2015), rosuvastatin (Adams 2014), fluvastatin (Adams 2018) and cerivastatin (Adams 2020). This provides some confirmation that the five statins are all causing lipid‐lowering by a similar mechanism. However, it also demonstrates that pitavastatin is more potent than fluvastatin, atorvastatin, rosuvastatin and less potent than cerivastatin at lowering LDL cholesterol: pitavastatin is 77‐fold more potent than fluvastatin, 6.2‐fold more potent than atorvastatin and 1.7‐fold more potent than rosuvastatin. Pitavastatin is 3.3‐fold less potent than cerivastatin but, since cerivastatin is no longer on the market, pitavastatin is the most potent statin currently on the market.
When we compare pitavastatin 1 mg/day which reduces LDL cholesterol by 33.3% on average with the other statins, the dose of fluvastatin, atorvastatin, rosuvastatin and cerivastatin to achieve the same reduction in LDL cholesterol is 106 mg/day, 7.7 mg/day, 1.9 mg/day and 0.3 mg/day, respectively.
When the statins effect in the recommended dose range are compared for their effect to lower LDL cholesterol, pitavastatin has a greater effect than fluvastatin and cerivastatin and a lesser effect than atorvastatin and rosuvastatin.
Pitavastatin 1 mg to 4 mg (33% to 44%) decrease in LDL cholesterol
Fluvastatin 20 mg to 80 mg (21 to 33%) decrease in LDL cholesterol
Cerivastatin 0.1 mg to 0.8 mg (23% to 41%) decrease in LDL cholesterol
Atorvastatin 10 mg to 80 mg (37% to 52%) decrease in LDL cholesterol
Rosuvastatin 5 mg to 40 mg (41% to 55%) decrease in LDL cholesterol
It has been suggested that pitavastatin may be better at increasing HDL than the other statins (Teramoto 2009). This review demonstrates that that is not the case. The average increase in HDL for all doses of pitavastatin was 4.1%. This is not greater than the other statins which, on average, increase HDL by 4.0% for atorvastatin, 5.0% for cerivastatin, 3.7% for fluvastatin and 7.3% for rosuvastatin.
Authors' conclusions
Implications for practice.
1. Pitavastatin causes a linear dose‐response reduction in the per cent change from control of blood LDL cholesterol, total cholesterol and blood triglycerides. There is no dose‐response relationship for HDL cholesterol which is increased by 4% on average for all doses. This effect on HDL is not different from the other studied statins. Pitavastatin doses of 1 mg/day to 16 mg/day resulted in a range of 33.3% to 54.7% decrease of LDL cholesterol. From the slope of the lines for every 2‐fold dose increase, there was a 3.93%, 5.35%, and 3.76% decrease in blood total cholesterol, LDL cholesterol, and triglycerides, respectively. The slope of the dose response is similar to the other studied statins: atorvastatin, rosuvastatin, fluvastatin and cerivastatin.
2. For reducing LDL cholesterol, pitavastatin is about 77‐fold more potent than fluvastatin, 6.2‐fold more potent than atorvastatin, 1.7‐fold more potent than rosuvastatin and 3.3‐fold less potent than cerivastatin.
3. There was not enough data to determine risk of withdrawal due to adverse effects due to pitavastatin.
Implication of these findings:
In the recommended dose range, pitavastatin lowers LDL cholesterol more than fluvastatin and cerivastatin and less than atorvastatin and rosuvastatin.
Implications for research.
More data from randomised placebo‐controlled trials are needed to know the harms of pitavastatin including withdrawals due to adverse effects. This review shows that pitavastatin lowers LDL cholesterol more in females than in males and more when it is given in the evening than when it is given in the morning. These findings need to be further studied.
What's new
| Date | Event | Description |
|---|---|---|
| 1 July 2020 | Amended | corrected minor errors in the list of references |
History
Protocol first published: Issue 7, 2017 Review first published: Issue 6, 2020
Acknowledgements
The review authors would like to acknowledge assistance provided by staff of the Cochrane Hypertension group.
Appendices
Appendix 1. Search strategies
Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search Date: 4 March 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 pitavastatin AND CENTRAL:TARGET #2 alipza AND CENTRAL:TARGET #3 itavastatin AND CENTRAL:TARGET #4 lippiza AND CENTRAL:TARGET #5 livalo AND CENTRAL:TARGET #6 livazo AND CENTRAL:TARGET #7 nikita AND CENTRAL:TARGET #8 nisvastatin AND CENTRAL:TARGET #9 pitava AND CENTRAL:TARGET #10 trolise AND CENTRAL:TARGET #11 vezepra AND CENTRAL:TARGET #12 zypitamag AND CENTRAL:TARGET #13 ("nk 104" OR nk104 OR "nks 104" OR nks104) AND CENTRAL:TARGET #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 AND CENTRAL:TARGET ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to March 01, 2019> Search Date: 4 March 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 pitavastatin.mp. 2 alipza.mp. 3 itavastatin.mp. 4 lippiza.mp. 5 livalo.mp. 6 livazo.mp. 7 nikita.mp. 8 nisvastatin.mp. 9 pitava.mp. 10 trolise.mp. 11 vezepra.mp. 12 zypitamag.mp. 13 147526‐32‐7.mp. 14 ("nk 104" or nk104 or "nks 104" or nks104).mp. 15 or/1‐14 16 animals/ not (humans/ and animals/) 17 15 not 16 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2019 March 01> Search Date: 4 March 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 pitavastatin.mp. (2891) 2 alipza.mp. 3 itavastatin.mp. 4 lippiza.mp. 5 livalo.mp. 6 livazo.mp. 7 nikita.mp. 8 nisvastatin.mp. 9 pitava.mp. 10 trolise.mp. 11 vezepra.mp. 12 zypitamag.mp. 13 147526‐32‐7.mp. 14 ("nk 104" or nk104 or "nks 104" or nks104).mp. 15 or/1‐14 16 cholesterol$.mp. 17 (HDL or LDL).mp. 18 lipoprotein?.mp. 19 lipid$.mp. 20 triglyceride$.mp. 21 triacylglycerol.mp. 22 or/16‐21 23 15 and 22 24 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 25 23 not 24 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 4 March 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: Pitavastatin Study type: Interventional Studies ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 4 March 2019 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Intervention: pitavastatin Recruitment status: ALL
Appendix 2. Mean Percentage Change
[(Endpoint‐Baseline)/Baseline]*100
Appendix 3. Extracted SDs and SEs
SE = |MD/t|
SD = (√n)*SE
SD = √n( upper confidence limit ‐ lower confidence limit)/2t
Data and analyses
Comparison 1. 1 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 LDL cholesterol RCTs | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐26.85 [‐29.89, ‐23.81] |
| 1.2 Total cholesterol RCTs | 3 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐19.43 [‐21.90, ‐16.97] |
| 1.3 HDL cholesterol RCTs | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | 6.28 [3.36, 9.20] |
| 1.4 Triglycerides RCTs | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | ‐19.22 [‐28.52, ‐9.91] |
| 1.5 LDL‐cholesterol non‐RCTs | 7 | 504 | Mean Difference (IV, Random, 95% CI) | ‐33.37 [‐35.87, ‐30.86] |
| 1.6 Total cholesterol non‐RCTs | 7 | 522 | Mean Difference (IV, Random, 95% CI) | ‐23.51 [‐25.98, ‐21.04] |
| 1.7 HDL‐cholesterol non‐RCTs | 5 | 402 | Mean Difference (IV, Random, 95% CI) | 3.71 [‐1.29, 8.70] |
| 1.8 Triglycerides non‐RCTs | 6 | 471 | Mean Difference (IV, Fixed, 95% CI) | ‐12.72 [‐15.05, ‐10.38] |
| 1.9 WDAE | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
1.9. Analysis.

Comparison 1: 1 mg vs control, Outcome 9: WDAE
Comparison 2. 2 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 LDL cholesterol RCTs | 3 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐31.00 [‐34.09, ‐27.90] |
| 2.2 Total cholesterol RCTs | 3 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐22.77 [‐25.32, ‐20.22] |
| 2.3 HDL cholesterol RCTs | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 6.25 [3.32, 9.19] |
| 2.4 Triglycerides RCTs | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐24.63 [‐33.45, ‐15.80] |
| 2.5 LDL‐cholesterol non‐RCTs | 33 | 3594 | Mean Difference (IV, Random, 95% CI) | ‐37.97 [‐39.53, ‐36.41] |
| 2.6 Total cholesterol non‐RCTs | 29 | 2536 | Mean Difference (IV, Fixed, 95% CI) | ‐27.36 [‐27.77, ‐26.96] |
| 2.7 HDL‐cholesterol non‐RCTs | 28 | 1996 | Mean Difference (IV, Random, 95% CI) | 3.98 [2.40, 5.55] |
| 2.8 Triglycerides non‐RCTs | 24 | 1835 | Mean Difference (IV, Fixed, 95% CI) | ‐16.66 [‐18.00, ‐15.31] |
| 2.9 WDAE | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
2.9. Analysis.

Comparison 2: 2 mg vs control, Outcome 9: WDAE
Comparison 3. 4 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 LDL‐cholesterol RCTs | 4 | 315 | Mean Difference (IV, Fixed, 95% CI) | ‐39.97 [‐42.86, ‐37.08] |
| 3.2 Total cholesterol RCTs | 4 | 315 | Mean Difference (IV, Random, 95% CI) | ‐28.09 [‐32.73, ‐23.46] |
| 3.3 HDL cholesterol RCTs | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | 6.65 [3.57, 9.73] |
| 3.4 Triglycerides RCTs | 3 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐24.81 [‐32.20, ‐17.41] |
| 3.5 LDL‐cholesterol non‐RCTs | 3 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐46.39 [‐48.54, ‐44.24] |
| 3.6 Total cholesterol non‐RCTs | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐32.28 [‐33.95, ‐30.60] |
| 3.7 HDL‐cholesterol non‐RCTs | 4 | 319 | Mean Difference (IV, Random, 95% CI) | 6.69 [‐1.04, 14.43] |
| 3.8 Triglycerides non‐RCTs | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐12.00 [‐18.87, ‐5.14] |
| 3.9 WDAE | 3 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
3.9. Analysis.

Comparison 3: 4 mg vs control, Outcome 9: WDAE
Comparison 4. 8 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 LDL cholesterol RCTs | 2 | 256 | Mean Difference (IV, Random, 95% CI) | ‐48.96 [‐54.93, ‐43.00] |
| 4.2 Total cholesterol RCTs | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐37.00 [‐41.46, ‐32.54] |
| 4.3 HDL cholesterol RCTs | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [0.44, 11.56] |
| 4.4 Triglycerides RCTs | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐32.90 [‐45.17, ‐20.63] |
Comparison 5. 16 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 LDL cholesterol RCTs | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐54.50 [‐59.47, ‐49.53] |
Comparison 6. All doses of pitavastatin vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 WDAE | 3 | 371 | Risk Ratio (IV, Fixed, 95% CI) | 1.35 [0.15, 12.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braamskamp 2015.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Methods: 5‐week dietary run‐in period; 12‐week randomised, double‐blind, placebo‐controlled trial |
|
| Participants |
Baseline Characteristics 1 mg
2 mg
4 mg
Placebo
Overall
Included criteria: Children, aged 6‐17 years were eligible if they had diet‐controlled fasting LDL‐C ≥ 160 (4.1 mmol/L) mg/dL,or LDL‐C ≥ 130 mg/dL (3.4 mmol/L) with one of the following risk factors: male; family history of premature cardiovascular disease; presence of low high‐density lipoprotein cholesterol (HDL‐C) 45 mg/dL or high triglycerides > 150 mg/dL; increased lipoprotein(a) > 75 nmol/L; type 2 diabetes mellitus diagnosed by treating physician according to current guidance; or systolic and diastolic blood pressures above the 95th percentile for age and height. Originally, children with an LDL‐C > 190 mg/dL without a risk factor and > 160 mg/dL with a risk factor could be enroled. These levels were changed to > 160 mg/dL without a risk factor and > 130 mg/dL with a risk factor Excluded criteria: None Baseline Group Characteristics: In general, the treatment groups were comparable with respect to all demographic and clinical characteristics |
|
| Interventions |
Intervention Characteristics 1 mg 2 mg 4 mg Placebo |
|
| Outcomes |
Total cholesterol
LDL‐cholesterol
WDAE
|
|
| Notes | Stephen P on 01/02/2018 10:33 Outcomes Given versus calculated percentage changes from baseline for HDL cholesterol for each dose had a greater than 10% difference (placebo 1.1 vs ‐0.8), (1 mg/day 6.1 vs 4.9), (2 mg/day 2.4 vs ‐3.5) and (4 mg/day 3.1 vs ‐4.2) and triglycerides were expressed as medians; therefore, HDL cholesterol and triglyceride outcomes could not be reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement Comment: method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: double‐blind placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐C outcome was reported. |
| Other bias | High risk | Judgement Comment: Kowa Research Europe Ltd. supported the trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All placebo and pitavastatin 1 mg/day participants were included in the efficacy analysis, [(27 ‐ 26)/27]*100 = 3.7%, pitavastatin 2 mg/day participants were not included in the efficacy analysis and [(26 ‐ 24)/26]*100 = 7.7% pitavastatin 4 mg/day participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All placebo and pitavastatin 1 mg/day participants were included in the efficacy analysis, [(27 ‐ 26)/27]*100 = 3.7%, pitavastatin 2 mg/day participants were not included in the efficacy analysis and [(26 ‐ 24)/26]*100 = 7.7% pitavastatin 4 mg/day participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | Given versus calculated percentage changes from baseline for HDL cholesterol for each dose had a greater than 10% difference; HDL cholesterol was not included in the analysis |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | Triglycerides were expressed as medians, therefore triglycerides were not included in the analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | Unclear risk | Blinding method was not described. |
| Selective reporting (reporting bias) for WDAEs | Low risk | WDAE outcome reported |
Budinski 2009.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Parallel group Methods: 6 to 8‐week dietary lead‐in period; 12‐week before‐and‐after trial |
|
| Participants |
Baseline Characteristics 2 mg
2 mg then uptitrated to 4 mg at week 4
2 mg combined data
Overall
Included criteria: men and non‐pregnant, nonlactating women, aged 18 to 75 years, diagnosed with primary hypercholesterolaemia or combined dyslipidaemia. mean fasting LDL‐C levels of 160 mg/dL or more (4.1 mmol/L) and less than or equal to 220 mg/dL (5.7mmol/L), and TG levels of less than or equal to 400 mg/dL (4.5 mmol/L) at the end of the lead‐in period Excluded criteria: previous contraindications or intolerance to statin therapy, homozygous familial hypercholesterolaemia, familial hypoalpha‐lipoproteinaemia, conditions that might have caused secondary dyslipidaemia, uncontrolled diabetes mellitus, pregnancy, conditions affecting absorption, distribution, metabolism or excretion of drugs, symptomatic heart failure (New York Heart Association classification III or IV), significant cardiovascular disease, impaired pancreatic function, liver enzyme levels greater than 1.5‐times the upper limit of normal, impaired renal function, impaired urinary tract function, uncontrolled hypothyroidism, symptomatic cerebrovascular disease, left ventricular ejection fraction less than 0.25, uncontrolled hypertension, muscular or neuromuscular disease, neoplastic disease, treatment with other lipid‐lowering drugs and treatment that would interact with the pharmacokinetics of statins. Women of childbearing potential were only allowed to participate if they were using a reliable contraceptive method. Baseline Group Characteristics: The treatment groups were well matched in terms of age, vital statistics (height, weight and BMI) and disease diagnosis and duration. Approximately 79% of patients in each treatment group had primary hypercholesterolaemia (78.4 to 79.1%) and most of the remainder had combined dyslipidaemia. The groups were also well matched in diagnosis of hypertension and in baseline lipid values. Study subjects included slightly more women than men, and this balance was reflected across the treatment groups |
|
| Interventions |
Intervention Characteristics 2 mg 2 mg then uptitrated to 4 mg at week 4 2 mg combined data |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Kowa Research Europe Ltd. sponsored the trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(316 ‐ 315)/316]*100 = 0.3% were not included in the efficacy analysis for the pitavastatin 2 mg/day; [(300 ‐ 298)/300]*100 = 0.7% were not included in the efficacy analysis for the pitavastatin 2 mg/day then titrated to 4 mg/day at week 4. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(316 ‐ 315)/316]*100 = 0.3% were not included in the efficacy analysis for the pitavastatin 2 mg/day; [(300 ‐ 298)/300]*100 = 0.7% were not included in the efficacy analysis for the pitavastatin 2 mg/day then titrated to 4 mg/day at week 4. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(316 ‐ 315)/316]*100 = 0.3% were not included in the efficacy analysis for the pitavastatin 2 mg/day; [(300 ‐ 298)/300]*100 = 0.7% were not included in the efficacy analysis for the pitavastatin 2 mg/day then titrated to 4 mg/day at week 4. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(316 ‐ 315)/316]*100 = 0.3% were not included in the efficacy analysis for the pitavastatin 2 mg/day; [(300 ‐ 298)/300]*100 = 0.7% were not included in the efficacy analysis for the pitavastatin 2 mg/day then titrated to 4 mg/day at week 4. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Chen 2012.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Methods: Participants were not on lipid medications within 2 months of the trial; therefore, no washout required. 3 months before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: participants have primary hypertension, men and women aged 18 to 85 years. Excluded criteria: people who were taking lipid‐lowering drugs, nonsteroidal anti‐inflammatory drugs, anticoagulants, angiotensin receptor antagonists; also with liver and kidney and other organ dysfunction; other cardiovascular diseases; having infectious diseases; acute and chronic inflammatory disease, connective tissue disease, cancer, diabetes, thyroid disease; pregnant and lactating women Baseline Group Characteristics: There was no significant difference between the BMI and smoking status between groups |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: source of funding not reported |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Chen 2015.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Methods: Washout period of 1 month; 3 months before‐and‐after trial |
|
| Participants |
Baseline Characteristics 2 mg
|
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Source of funding not reported |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Eriksson 2011.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Methods: 6‐8 week washout period, 4 week before‐and‐after trial with evening dosing |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Patients of either gender were eligible for inclusion in the study if they were aged 18‐75 years and had primary hypercholesterolaemia or combined dyslipidaemia that was uncontrolled (LDL‐C ≥ 3.4 mmol/L [130 mg/dL] and ≤ 5.7mmol/L [220 mg/dL]; triglycerides ≤ 4.6 mmol/L [400 mg/dL]) despite dietary measures. In addition, patients were required to have at least two of the following cardiovascular risk factors: cigarette smoking; blood pressure of 140/90 mmHg or above or receiving antihypertensive therapy; a high‐density lipoprotein cholesterol (HDL‐C) concentration of 1 mmol/L (40 mg/dL) or below; a family history of CHD in a male or female first‐degree relative below 55 or below 65 years of age, respectively; age above 45 years in men or above 55 years in women. An HDL‐C concentration above 1.55 mmol/L (60 mg/dL) was considered to offset one risk factor. Patients who were receiving lipid‐modifying therapies were eligible for inclusion if such treatment was withdrawn at least 8 weeks before randomisation. Excluded criteria: The principal exclusion criteria were homozygous familial hypercholesterolaemia, unstable medical conditions, or conditions associated with secondary dyslipidaemia, conditions that might affect drug pharmacokinetics, significant cardiovascular disease, or symptomatic heart failure (left ventricular ejection fraction 0.25) or cerebrovascular disease, uncontrolled or poorly controlled hypertension, uncontrolled diabetes (> 8% glycated haemoglobin), impaired liver or kidney function, or other serious medical conditions. Women of childbearing potential were required to have a negative pregnancy test at the start of the dietary run‐in period and before starting treatment, and to use adequate contraception throughout the study. Baseline Group Characteristics: The two groups were well matched in terms of their baseline characteristics. The mean age of the patients was approximately 60 years, about two‐thirds were male, and all except one were white. The majority of patients (>80%) had primary hypercholesterolaemia, and approximately three‐quarters were at moderate or high cardiovascular risk according to the NCEP criteria. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
LDL‐cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: The study was supported by Kowa Research Europe Ltd. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(236 ‐ 233)/236]*100 =1.3% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(236 ‐ 233)/236]*100 =1.3% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(236 ‐ 233)/236]*100 =1.3% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(236 ‐ 233)/236]*100 =1.3% participants were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Gumprecht 2011.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 6‐8 week washout period, 12 week before‐and‐after study with evening dosing |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Eligible patients were aged 18 – 75 years with type 2 diabetes [haemoglobin A1c (HbA1c) ≤ 7.5%] and combined dyslipidaemia [plasma LDL‐C ≥ 100 and ≤ 220 mg/dL (≥ 2.6 and ≤ 5.7 mmol/L) and triglycerides (TG) ≥ 150 mg/dL ( ≥1.7 mmol/L)], despite dietary therapy, and were receiving an oral antidiabetic treatment (not including glitazones) or insulin. Eligible patients had a body mass index (BMI) of not more than 35 kg/m2, and women of childbearing potential had to use reliable contraception. Excluded criteria: homozygous familial hypercholesterolaemia; other conditions with potential to cause secondary dyslipidaemia, including human immunodeficiency virus infection; HbA1c more than 7.5%; significant cardiovascular disease; history of cerebrovascular disease, neoplastic disease within 10 years, unexplained increased serum creatine kinase (CK) of more than five times the upper limit of the reference range (ULRR); systolic blood pressure above 160 mmHg and diastolic blood pressure above 90 mmHg; history of muscular or neuromuscular disease; and history of resistance to lipid‐lowering therapy or use of supplements that affect lipid metabolism Baseline Group Characteristics: Baseline characteristics were generally well matched across randomised treatment groups in the core study. Most patients were Caucasian (88%) and male (57%), with a mean age of 59 years. All patients were in the NCEP high‐risk category; most were hypertensive (77%) and 49% had previously received a lipid‐modifying therapy. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
LDL cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Kowa Research Europe Ltd. supported this trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(279 ‐ 274)/279]*100 = 1.8% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(279 ‐ 274)/279]*100 = 1.8% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(279 ‐ 274)/279]*100 = 1.8% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(279 ‐ 274)/279]*100 = 1.8% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
HaeKim 2018.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No washout required because no subject was receiving lipid medications within 3 months of trial, 3‐month before‐and‐after trial |
|
| Participants |
Baseline Characteristics 4 mg
Included criteria: age from 40 to 80 years, statin‐naïve subjects, and low‐density lipoprotein cholesterol (LDL‐C) ≥ 130 mg/dL with more than or equal to two major risk factors for coronary heart disease (CHD) or LDL‐C ≥ 160 mg/dL with less than two risk factors. Major risk factors for CHD include ≥ 45 years of age for men or ≥ 55 years of age for women, family history of premature CHD (CHD in male first‐degree relative < 55 years or in female first‐degree relative < 65 years), current cigarette smoking, blood pressure (BP) ≥ 140/90 mmHg or on antihypertensive medication, and high‐density lipoprotein cholesterol (HDL‐C) < 40 mg/dL Excluded criteria: hospitalisation for acute coronary syndrome or cerebrovascular disease within the previous 2 months; the use of statin or other lipid‐lowering medication within the previous 3 months; impaired hepatic function or a history of liver disease; chronic renal failure; a history of malignancy; any known contraindication to statin therapy, such as statin allergy, ciclosporin use, pregnancy, breastfeeding, myopathy, or lactose intolerance; and failure to obtain informed consent from participants Baseline Group Characteristics: The mean age was 58 ±8 years, and female predominance was observed (70.8%, [34/48]). Thirty‐two patients (66.7%) had hypertension, and 8 patients (16.7%) had diabetes. None of the patients had coronary artery disease. Of the patients, 25 (52.1%) were taking angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), 10 (20.8%) were taking beta blockers, and 15 (31.3%) were taking calcium‐channel blockers (CCB). |
|
| Interventions |
Intervention Characteristics 4 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Study was supported by grants from Il‐dong Pharmaceutical Co. Ltd. (Republic of Korea) |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | Judgement Comment: (60 ‐ 48)*100/60 = 20% were not included n the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | Judgement Comment: (60 ‐ 48)*100/60 = 20% were not included n the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | Judgement Comment: (60 ‐ 48)*100/60 = 20% were not included n the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | Judgement Comment: (60 ‐ 48)*100/60 = 20% were not included n the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | Judgement Comment: No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Han 2012.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No washout required because no participant received lipid medications, 4 weeks before‐and‐after trial |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Adult subjects, aged 25 to 75 years, with elevated ALT concentrations (≥ 1.25 times and ≤ 2.5 times the upper limit of the normal range [ULN]; 40 IU/L) were recruited from the lipid clinics of 10 major hospitals in Korea. All subjects were non‐alcoholics and serologically negative for viral hepatitis markers. None had been treated with statins for more than 3 months before screening, and all had basal fasting LDL‐C concentration levels ≥ 3.36 mmol/L (≥ 130 mg/dL).After 2 weeks of an intervention‐free screening period, subjects were re‐evaluated, including by measurements of serum ALT levels. Subjects who did not meet any of the exclusion criteria were grouped into those with persistently elevated (> 1.25 times and ≤ 2.5 times ULN; 50–100 IU/L) and reduced (50 IU/L) concentrations. Excluded criteria: Overt and irreversible liver cirrhosis, serologically positive for viral markers or active viral hepatitis, cholestatic features (serum bilirubin > 2 X ULN), fasting triglyceride levels ≥ 4.52 mmol/L (400mg/dL), acute or unstable conditions, including a recent history (3 months) of myocardial infarction, advanced heart failure (New York Heart Association class III‐IV), renal dysfunction (serum creatinine ≥ 2.0 mg/dL), uncontrolled hypertension (DBP ≥ 100 mmHg), and thyroid dysfunction (TSH ≥ 1.5 X ULN). Medications prohibited from 1 month before the screening period until the completion of study included drugs that could potentially improve liver function test results (betaine, thiazolidinediones), those that could induce significant hepatic damage (synthetic estrogens, androgen, oral contraceptives, amiodarone, tamoxifen, erythromycin, tetracycline, sulfa antibiotics, methotrexate, perhexiline maleate, valproic acid, cocaine, zidovudine, didanosine, fialuridine, isoniazid, rifampicin, other anti‐tuberculosis medications, trazodone, nefazodone, venlafaxine, chlorpromazine, quinidine, gemfibrozil, herb medication), those that could affect lipid profiles (other HMG‐CoA reductase inhibitors, fibrates, niacin, ezetimibe, steroids, anti‐obesity drugs), and those known to have serious drug interactions with statins (ketoconazole, itraconazole, erythromycin, clarithromycin, cyclosporin, norethindrone, ethyl estradiol). Baseline Group Characteristics: Of these subjects, 129 were male and 60 were female, their mean age was 55.1 years, and 28% had been diagnosed with diabetes, 68% with hypertension, and 72% with metabolic syndrome. All demographic features; habits such as exercise, smoking, and drinking; and medication profiles were similar between the two groups. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: The study was supported by JW Pharmaceutical Co, Korea. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(103 ‐ 88)/103)]*100 = 14.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(103 ‐ 88)/103)]*100 = 14.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | [(103 ‐ 88)/103)]*100 = 14.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(103 ‐ 88)/103)]*100 = 14.6% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Harada Shiba 2016.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Parallel group Method: 3 month dietary run‐in period, 12‐week before‐and‐after study with morning dosing |
|
| Participants |
Baseline Characteristics 1 mg
1 mg then titrated to 2 mg at week 4
Overall
Included criteria: (1) patients with an LDL‐C level of ≥ 190 mg/dL or LDL‐C level of ≥ 160 mg/dL with one or more of the following risk factors, a family history of coronary artery disease (relation in the second degree), obesity (obesity index ≥ 20%), type 2 diabetes, hypertension (systolic blood pressure ≥ 125 mmHg or diastolic blood pressure ≥ 70 mmHg) and a low HDL cholesterol level ( < 40 mg/dL); (2) Japanese male children 10 to 15 years of age inclusive at the time of informed consent; (3) patients who had received diet therapy for at least three months before screening based on a physician’s instructions or those whose diet for at least three months before screening was judged by the investigator not to require more intensive dietary restrictions (in either case, the patients were required to have received a fixed regimen of diet or diet/exercise therapy for at least four weeks before screening); (4) outpatients; and (5) patients for whom written informed consent was obtained from the patients themselves and their parents or guardians Excluded criteria: (1) patients with homozygous familial hypercholesterolaemia; (2) patients with secondary hyperlipidaemia; (3) patients on apheresis therapy; (4) patients who had recently experienced a cerebrovascular disorder, anginal attack or myocardial infarction; (5) patients with severe hepatic or renal disorders, poor glycaemic control, severe hypertension, a history of allergies to drugs or serious adverse drug reactions (ADRs); (6) patients who had participated in other clinical trial(s) and received investigational drug(s) within 12 weeks before screening; and (7) patients judged inappropriate for the study by the investigator Baseline Group Characteristics: The mean age was 11.8 years, the mean height was 147.6 cm, and the mean weight was 41.1 kg. The patient characteristics were generally similar between 1 mg and 2 mg treatment groups, except for the obesity index. |
|
| Interventions |
Intervention Characteristics 1 mg 1 mg then titrated to 2 mg at week 4 Both Groups |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Kowa Co. Ltd funded this trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Huang 2012.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: Participants were not on any lipid medications, therefore no washout required, 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Age > 60 years of age, conformity to the 1999 World Health Organization (WHO) criteria for the diagnosis of type 2 diabetes, blood glucose resulting in a HbA1c of 7%, stable blood sugar, fasting blood glucose (FBS) 70 mmol/L blood glucose, 2 h after three meals (PBC) 11.1 mmoI/L, not to have taken statins and other lipid‐regulating drugs within one month, expresses the intention of both patients and their family members, and signed informed consent Excluded criteria: type 1 diabetes, uncooperative, poor compliance, hypersensitivity to pitavastatin and atorvastatin, homozygous familial hypercholesterolaemia, patients with active liver disease or unexplained persistent transaminase elevation (alanine aminotransferase and/or aspartate aminotransferase are 3 times greater than normal), patients taking immunosuppressants such as cyclosporine, severe cases of kidney disease, severe cardiovascular and cerebrovascular diseases (such as myocardial infarction, acute cerebral infarction, acute heart failure, etc.), severe infection, trauma, stress ulcer hyperthyroidism, malignancy, diabetic ketosis, diabetic patients with non‐ketotic hyperosmolar coma or patients with psychiatric symptoms and patients receiving glucocorticoids and postoperative patients Baseline Group Characterisitcs: Baseline characteristics were generally well matched across age, gender, smoking, BMI, drinking history, drug use, and disease diagnosis |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Unclear risk | [(40 ‐ 36)/40)]*100 = 10% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Unclear risk | [(40 ‐ 36)/40)]*100 = 10% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Unclear risk | [(40 ‐ 36)/40)]*100 = 10% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Unclear risk | [(40 ‐ 36)/40)]*100 = 10% participants were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Ikegami 2012.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No participants were receiving lipid medications, therefore, washout period not required; 3‐month before‐and‐after trial |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: 15 men and women with hypercholesterolaemia Excluded criteria: alcohol consumption of more than 20 g per week; evidence of pregnancy, treatment with corticosteroid, and hormone replacement therapy. Subjects using lipid‐lowering medication or food enriched with functional plant stanols or sterols were excluded from the study. Subjects with positive test results for the following disorders were also excluded: secondary causes of steatohepatitis and drug‐induced liver injury, viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, a‐1‐antitrypsin deficiency, haemochromatosis, Wilson’s disease, and biliary obstruction. Baseline Group Characteristics: The mean ages and ratios of male/female subjects were not significantly different between the control and NAFLD groups. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
|
|
| Notes | Stephen P on 27/01/2018 11:00 Included Post hoc of Hyogo 2011 trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | High risk | Judgement Comment: LDL‐C outcome was not reported. |
| Other bias | Low risk | Judgement Comment: This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | LDL cholesterol was not reported. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | HDL cholesterol was not reported. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | Triglycerides were not reported. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Kajinami 2000.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐8 week placebo washout period; 8‐week before‐and‐after study with evening dosing |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: 30 patients (15 men and 15 women, aged 51 ± 13 [mean ± SD] years) with heterozygous FH. All patients fulfilled the diagnostic criteria: primary hypercholesterolaemia (> 230 mg/dL) with tendon xanthoma or first‐degree relatives of previously diagnosed heterozygous FH patients showing primary hypercholesterolaemia (> 230 mg/dL). The mean ± SD of body mass index was 23.9 ± 2.9 kg/m2. Coronary artery disease had already been documented in 9 patients (30%), and no patient had cerebral atherosclerotic vascular disease. Excluded criteria: not reported. Baseline Group Characteristics: The study population consisted of 30 patients (15 men and 15 women, aged 51±13 [mean ± SD] years) with heterozygous FH. All patients fulfilled our diagnostic criteria: primary hypercholesterolaemia ( > 230 mg/dl) with tendon xanthoma or first‐degree relatives of previously diagnosed heterozygous FH patients showing primary hypercholesterolaemia (> 230 mg/dl). The mean ± SD of body mass index was 23.9 ± 2.9 kg/m2 . Coronary artery disease had already been documented in 9 patients (30%), and no patient had cerebral atherosclerotic vascular disease. |
|
| Interventions |
Intervention Characteristics 2 mg
|
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Kakuda 2013.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: Participants were not on any medication; no washout required; 4‐week before‐and‐after trial |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Japanese men, who agreed to undergo pitavastatin treatment and mixed meal test, were involved in this study (n = 10;age: 33.9 ± 10.1 years; body height 172.0 ± 4.3 cm; body weight 80.2 ± 25.3 kg; body mass index (BMI) 27.0 ± 8.3kg/m2; waist circumference 88.5 ± 18.9 cm) Excluded criteria: not reported. Baseline Group Characteristics: Japanese men, (n = 10; age 33.9 ± 10.1 years; body height 172.0 ± 4.3 cm; body weight 80.2 ± 25.3 kg; body mass index (BMI) 27.0 ± 8.3 kg/m2 ; waist circumference 88.5 ± 18.9 cm). None of them had received medication. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
|
|
| Notes | Stephen P on 02/02/2018 08:13 Outcomes Triglyceride outcome was not included in the efficacy analysis because the values were expressed as medians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | Triglyceride data were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Lee 2007.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week dietary washout period; 4‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Korean men and women aged 20 to 79 years who had untreated hypercholesterolaemia Excluded criteria: Pregnant and breastfeeding women were excluded. Other exclusion criteria were current use of lipid‐lowering therapy, uncontrolled diabetes mellitus (fasting plasma glucose concentration > 180 mg/dL), uncontrolled hypertension (diastolic blood pressure > 115 mm Hg), a history of cerebrovascular disease or myocardial infarction within 3 months of enrolment, congestive heart failure, a serum creatinine concentration > 2.0 mg/dL, hepatic dysfunction (transaminase levels > 2.5 times the upper limit of normal [ULN]), or an unexplained serum creatine kinase (CK) elevation > 2.5 times the ULN Baseline Characteristics: The characteristics of the 2 groups were similar at baseline. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | Nima Alaeiilkhchi on 27/01/2018 11:48 Included We calculated the incomplete outcome data based on best estimates from the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Study was funded by Choongwae Pharma Corp. Seoul, Republic of Korea. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(136 ‐ 110)/136] X 100 = 19.1% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(136 ‐ 110)/136] X 100 = 19.1% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | [(136 ‐ 110)/136] X 100 = 19.1% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(136 ‐ 110)/136] X 100 = 19.1% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Liu 2013.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week dietary lead‐in period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Eligible patients were men and women aged 20 or older with fasting LDL‐C higher than 100 mg/dL. In addition, to be considered “high‐risk”, a patient had to meet at least one of the following criteria (NCEP ATP III guideline): documented CHD; type 2 DM; the patient had fewer than 2 risk factors (other than LDL) present in the following items without CHD or a CHD risk equivalent, a 10‐year (short‐term) CHD risk had to be assessed with a Framingham score > 20%: female: ≥ 55 years old, or male: ≥ 45 years old; fasting high‐density lipoprotein cholesterol (HDL‐C) 40 mg/dL; a family history of premature CHD (CHD in first‐degree male relative 55 years; CHD in first‐degree female relative 65 years); hypertension (BP ≥ 140/90 mm Hg or treated with anti‐hypertensive agents); HDL‐C ≥ 60 mg/dL counted as a“negative” risk factor; its presence removed one risk factor from the total count. Excluded criteria: a history of hypersensitivity to statins, hepatic dysfunction [aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 100 IU/L], suspected hepatic metabolism disorders or biliary obstruction (acute hepatitis, acute exacerbation of chronic hepatitis, liver cirrhosis, liver cancer and jaundice), or renal dysfunction (serum creatinine > 1.5 mg/dL), pregnancy, possible pregnancy, or breastfeeding and poorly controlled diabetes (HbA1C > 9.0%) Baseline Group Characteristics: The characteristics of the 2 groups were similar at baseline. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Low risk | Judgement Comment: The associated study (Lin 2014) was partly supported by research grants V101B‐023, V102B‐048 and V103B‐019 toL.Y. Lin. from the Taipei Veterans General Hospital, Taipei, Taiwan. Kowa company supplied the medicine for the clinical trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(113 ‐ 94)/113]*100 = 16.8% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(113 ‐ 94)/113]*100 = 16.8% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | [(113 ‐ 94)/113]*100 = 16.8% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(113 ‐ 94)/113]*100 = 16.8% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Majima 2007.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No participant received lipid medications within 3 months of the trial, therefore, no washout required; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 1 mg
Included criteria: 101 Japanese patients (57 men and 44 women, mean age 58.58 ± 12.0 years) with untreated hypercholesterolaemia, who attended the clinic of Rakuwakai Otowa Hospital between March 2006 and December 2006, were selected for this study. The diagnosis of hypercholesterolaemia was established on the basis of laboratory findings, including an elevated serum total cholesterol (TC) level (> 220 mg/dL) and an elevated serum low density lipoprotein cholesterol (LDL‐C) level (> 140 mg/dL). Excluded criteria: current smokers and those who had a history of fractures and/or of other diseases (type 1 diabetes mellitus, liver disease, renal dysfunction, malignancy, hyperthyroidism, hyperparathyroidism, hypercortisolism, or hypogonadism) and those taking medications (active vitamin D3, bisphosphonates, calcitonin, selective oestrogen receptor modulators, estrogens, testosterones, steroids, thyroid hormones, diuretics, heparin or anticonvulsants) that could influence bone metabolism Baseline Group Characteristics: At baseline, all differences between group A and group B were non significant. |
|
| Interventions |
Intervention Characteristics 1 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐C outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(66 ‐ 63)/66]*100 = 4.5% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(66 ‐ 63)/66]*100 = 4.5% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(66 ‐ 63)/66]*100 = 4.5% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(66 ‐ 63)/66]*100 = 4.5% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Mao 2012.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Age 18‐75 years of age, gender is not limited; clinic or hospitalised patients diagnosed as hypercholesterolaemia or willing to accept for the elder brother of patients, the fasting LDL‐C ≥ 3.37 mmol/L; has not received pitavastatin in the past; signed informed consent Excluded criteria: Patients with pitavastatin calcium allergy; had a history of active arterial disease within 3 months such as acute coronary syndromes (i.e. unstable angina and myocardial infarction), angioplasty, angioplasty, coronary artery bypass grafting, transient ischaemic attacks, and stroke; familial cardiogenic sudden death; patients with acute heart failure; poorly controlled refractory hypertension (systolic > 160 mm Hg, diastolic > 100 mm Hg, 1 mm Hg 0.133 kPa); patients with active liver disease, severe liver and kidney dysfunction with ALT, AST and Cr values exceeding 2 times the upper limit of normal; malignancy, bed history of thyroid disease and uncontrolled acute inflammation; patients given hormones or immunosuppressants within the last 1 month; patients in the field or those who are unable to follow up because of mobility; pregnant, breastfeeding, or within 6 months of delivery Baseline Group Characteristics: NR |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐C outcome was reported. |
| Other bias | High risk | Judgement Comment: Kowa (Shanghai) Pharma Consulting Co. Ltd. sponsored the trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(397 ‐ 295)/397]*100 = 25.7% were not included in the efficacy analysis because they were receiving lipid medications at baseline. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(397 ‐ 295)/397]*100 = 25.7% were not included in the efficacy analysis because they were receiving lipid medications at baseline. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | HDL‐cholesterol was not included in the analysis because the values were expressed as medians. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | Triglycerides were not included in the analysis because they were expressed as medians. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Motomura 2009.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout period; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: more than 20 years old, no chronic or acute inflammatory diseases diagnosed by clinical symptoms and/or laboratory data, no corticosteroid administration, serum creatinine concentrations < 2.0 mg/dL, serum transaminase concentrations less than twice the upper limit of control ranges, and no viral hepatitis Excluded criteria: none reported Baseline Group Characteristics: NR |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: This work was supported in part by a grant from the Ministry of Education, Science and Sports of Japan and "an unrestricted grant" from Kowa Pharmaceutical. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(91 ‐ 65)/91]*100 = 28.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(91 ‐ 65)/91]*100 = 28.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | [(91 ‐ 65)/91]*100 = 28.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(91 ‐ 65)/91]*100 = 28.6% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Nakamura 2008.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Method: No participant received lipid medications within 6 months of randomisation therefore no washout required 1 month single‐blind randomised placebo‐controlled study only the patients were blinded to the content of the tablets |
|
| Participants |
Baseline Characteristics 4 mg
Placebo
Overall
Included criteria: The study enroled 65 consecutive patients with ACS, the presence of carotid plaque [intima‐media thickness (IMT) ≥ 1.1 mm], and hypercholesterolaemia (260 > total cholesterol levels ≥ 220 mg/dL). ACS was diagnosed by the presence of acute ischaemic symptoms lasting ≥ 20 minutes within 48 hours before admission to our hospital, and electrocardiographic (ECG) changes consistent with ACS. The presence of ACS was confirmed by coronary angiography in all patients. Acute myocardial infarction (AMI) was diagnosed when creatine kinase‐MB levels increased by at least 2 times the upper level of normal (3.5 ng/mL). Patients without AMI were considered to have unstable angina pectoris (u‐AP). According to these inclusion criteria, 47 patients were diagnosed as u‐AP, and 18 patients were diagnosed as AMI. Excluded criteria: (1) use of statin or other lipid‐lowering agents during the preceding 6 months, (2) history of hepatic disease, (3) untreated endocrine disorder, (4) history of systemic inflammatory diseases, (5) infectious diseases, (6) history of hypersensitivity to statins, (7) cardiogenic shock or pulmonary oedema at admission, or (8) stroke at admission Baseline Group Characteristics: 65 consecutive patients with ACS were randomised. Profiles of traditional risk factors, calibrated IBS values, and levels of CRP, VEGF, and TNFa were similar between the 2 treatment groups. All ACS patients took aspirin and ticlopidine orally during the follow‐up period, and other medications and invasive therapy for culprit lesions were similar in both treatment groups |
|
| Interventions |
Intervention Characteristics 4 mg Placebo |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
WDAE
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement Comment: The ACS patients were randomly assigned using a random number table generated by a computer. |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Single‐blind allocation concealment was not applied to the investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Single‐blind (only participants); however, lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported and WDAEs were reported; all of the study participants completed the trial with no WDAEs. |
| Other bias | Low risk | Judgement Comment: This study was supported by Grants‐in‐Aid for (B)(2)‐15390244 and (B)‐19390209, Priority Areas (C) ‘‘Medical Genome Science 15012222’’ from the Ministry of Education, Culture, Sports, Science, and Technology, and by Health and Labor Sciences Research Grants for Comprehensive Research on Aging and Health (H15‐Choju‐012), Tokyo, Japan, government grants |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | Single‐blinded trial |
| Selective reporting (reporting bias) for WDAEs | Low risk | WDAE outcome reported |
NK‐104.202 2013.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Method: 4‐week single‐blind placebo run‐in period; 12‐week randomised double‐blind placebo‐controlled study with evening dosing |
|
| Participants |
Baseline Characteristics 1 mg
2 mg
4 mg
8 mg
Placebo
Overall
Included criteria: 18‐75 years; females of childbearing potential to be on oral contraception of at least 3 months; and willingness to adhere to the National Cholesterol Education Program (NCEP) Step‐1 or equivalent diet. Subjects had primary hypercholesterolaemia with LDL‐cholesterol ≥ 160 mg/dL (4.13 mmol/L) but ≤ 250 mg/dL (6.46 mmol/L), and triglyceride (TG) level ≤ 300 mg/dL (3.43 mmol/L) at Visit 3. Excluded criteria: pregnancy, or not taking oral contraception; • BMI > 30 kg/m2; • alcohol abuse; • hypersensitivity to HMG‐CoA reductase inhibitors; • use of prohibited concomitant medications; • compliance 80% during run‐in period; • diabetes or fasting serum glucose ≥ 7 mmol/L at Visit 2; • renal impairment or serum creatinine > 1.8 mg/dL or nephrotic syndrome; • uncontrolled hypertension or diastolic blood pressure (DBP) ≥ 110 mmHg or systolic blood pressure (SBP) ≥ 180 mmHg; • history of myocardial infarction, unstable angina, stroke, transient ischaemic attack (TIA), percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG); • NYHA class 3 or 4 congestive cardiac failure; • active liver disease or AST or alanine aminotransferase (ALT) > 2 times ULN; • muscular or neuromuscular disease or creatinine kinase (CK) > 3 times ULN without explanation; • malignancy within past 10 years; • known cataracts; • human immunodeficiency virus (HIV) infection; • severe depression or suicidal tendencies; • Type I, IIb, III, IV or V hyperlipidaemia; • familial hypercholesterolaemia or LDL‐C > 250 mg/dL at Visit 3; and • hypercholesterolaemia secondary to hypothyroidism Baseline Group Characteristics:NR |
|
| Interventions |
Intervention Characteristics 1 mg 2 mg 4 mg 8 mg Placebo |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | Stephen P on 22/11/2018 13:02 Included 8 mg dose: we used the adjusted mean and imputed SDs of the percentage change but adjusted means were not calculated for total cholesterol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement Comment: Method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Judgement Comment: A centralised randomising system was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Double‐blind. Study medication was blinded and investigators did not receive lipid values during treatment period until after study completion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Trial sponsored by laboratories Negma, a pharmaceutical company in France. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(252 ‐ 249)/252]*100 = 1.2 % participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(252 ‐ 249)/252]*100 = 1.2 % participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(252 ‐ 249)/252]*100 = 1.2 % participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(252 ‐ 249)/252]*100 = 1.2 % participants were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No WDAEs were reported. |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
NK‐104.203 2013.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Methods: 4‐week placebo washout run‐in period; 12‐week randomised double‐blind placebo‐controlled study with evening dosing |
|
| Participants |
Baseline Characteristics 1 mg
2 mg
4 mg
Placebo
Overall
Included criteria: 18‐75 years; females of childbearing potential to be on oral contraception of at least 3 months; and willingness to adhere to the National Cholesterol Education Program (NCEP) Step‐1 or equivalent diet. Subjects had primary mixed or combined hyperlipidaemia with LDL‐C level ≥ 135 and ≤ 300 mg/dL (≥ 3.5 and ≤ 7.8 mmol/L) and TG ≥ 175 and ≤ 500 mg/dL (≥ 2.0 and ≤ 5.7 mmol/L). Excluded criteria: pregnancy, or not taking oral contraception; • BMI > 33 kg/m2; • alcohol abuse; • hypersensitivity to HMG‐CoA reductase inhibitors; • use of prohibited concomitant medications; • compliance 80% during run in period; • diabetes or fasting serum glucose ≥ 7 mmol/L at Visit 2; • renal impairment or serum creatinine > 1.8 mg/dL or nephrotic syndrome; • uncontrolled hypertension or diastolic blood pressure (DBP) ≥110 mmHg or systolic blood pressure (SBP) ≥ 180 mmHg; • history of myocardial infarction, unstable angina, stroke, transient ischaemic attack (TIA), percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG); • NYHA class 3 or 4 congestive cardiac failure; • active liver disease or AST or alanine aminotransferase (ALT) > 2 times ULN; • muscular or neuromuscular disease or creatinine kinase (CK) > 3 times ULN without explanation; • malignancy within past 10 years; • known cataracts; • human immunodeficiency virus (HIV) infection; • severe depression or suicidal tendencies; • Type I, IIb, III, IV or V hyperlipidaemia; • familial hypercholesterolaemia or LDL‐C > 250 mg/dL at Visit 3; and • hypercholesterolaemia secondary to hypothyroidism Baseline Group Characteristics:NR |
|
| Interventions |
Intervention Characteristics 1 mg 2 mg 4 mg Placebo |
|
| Outcomes |
LDL cholesterol
Total cholesterol
HDL cholesterol
Triglycerides
WDAE
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement Comment: Method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Judgement Comment: A centralised randomising system was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: double‐blind. Study medication was blinded and investigators did not receive lipid values during treatment period until after study completion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: The study was sponsored by Laboratories Negma, a pharmaceutical company in France. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(261 ‐ 251)/261)]*100 = 3.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(261 ‐ 251)/261)]*100 = 3.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(261 ‐ 251)/261)]*100 = 3.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(261 ‐ 251)/261)]*100 = 3.8% participants were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | Unclear risk | Blinding method was not described. |
| Selective reporting (reporting bias) for WDAEs | Low risk | WDAE outcome reported |
NK‐104.209 2013.
| Study characteristics | ||
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Methods: 6 to 8‐week dietary washout period; 8‐week randomised double‐blind placebo‐controlled trial |
|
| Participants |
Baseline Characteristics none reported Included criteria: participants 18 to 80 years with plasma mean LDL‐C levels at two consecutive qualifying visits ≥ 130 mg/dL and ≤ 220 mg/dL, and triglycerides ≤ 400 mg/dL Excluded criteria: pregnancy, or not taking oral contraception; • BMI > 30 kg/m2; • alcohol abuse; • hypersensitivity to HMG‐CoA reductase inhibitors; • use of prohibited concomitant medications; • compliance < 80% during run‐in period; • diabetes or fasting serum glucose ≥ 7 mmol/L at Visit 2; • renal impairment or serum creatinine > 1.8 mg/dL or nephrotic syndrome; • uncontrolled hypertension or diastolic blood pressure (DBP) ≥ 110 mmHg or systolic blood pressure (SBP) ≥ 180 mmHg; • history of myocardial infarction, unstable angina, stroke, transient ischaemic attack (TIA), percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG); • NYHA class 3 or 4 congestive cardiac failure; • active liver disease or AST or alanine aminotransferase (ALT) > 2 times ULN; • muscular or neuromuscular disease or creatinine kinase (CK) > 3 times ULN without explanation; • malignancy within past 10 years; • known cataracts; • human immunodeficiency virus (HIV) infection; • severe depression or suicidal tendencies; • Type I, IIb, III, IV or V hyperlipidaemia; • familial hypercholesterolaemia or LDL‐C > 250 mg/dL at Visit 3; and • hypercholesterolaemia secondary to hypothyroidism Baseline Group Characteristics:NR |
|
| Interventions |
Intervention Characteristics 8 mg 16 mg Placebo |
|
| Outcomes |
LDL cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement Comment: Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Judgement Comment: Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Double‐blind treatment lipid parameter measurements unlikely to be influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: Sankyo Pharma sponsored the trial. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | No total cholesterol data reported |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(54 ‐ 53)/54]*100 = 1.9% participants for placebo group [(107 ‐ 103)/107]*100 = 3.7% participants for the pitavastatin 8 mg/day group [(107 ‐ 103)/107]*100 = 3.7% participants for the pitavastatin 16 mg/day group |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | No HDL cholesterol data reported |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | No triglyceride data reported |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No WDAE data reported |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Noji 2002.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐8 week placebo washout period; 8‐week before‐and‐after study with evening dosing |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: 25 patients with heterozygous familial hypercholesterolaemia with primary hypercholesterolaemia (TC > 230 mg/dL) with tendon xanthoma or first‐degree relatives of previously diagnosed heterozygous FH patients. Achilles’ tendon xanthoma was observed in 21 patients and xanthelasma in four. Excluded criteria: not reported. Baseline Group Characteristics:NR |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
|
|
| Notes | Stephen P on 02/02/2018 08:37 Outcomes Given versus calculated percentage changes from baseline for triglycerides had a greater than 10% difference (‐14.0 vs 0.0), therefore, the triglyceride outcome was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(36 ‐ 25)/36]*100 = 30.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(36 ‐ 25)/36]*100 = 30.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | [(36 ‐ 25)/36]*100 = 30.6% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(36 ‐ 25)/36]*100 = 30.6% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Nozue 2008.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 8‐week washout period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Eight patients with heterozygous familial hypercholesterolaemia (male/female = 1/7, mean age = 62 ± 6 years) were studied. FH was diagnosed according to the following two criteria: primary hypercholesterolaemic patients (TC level above 230 mg/dL in any age group) with tendon xanthomas, or primary hypercholesterolaemic patients with and without tendon xanthomas in a first‐degree relative of familial hypercholesterolaemic patients. Excluded criteria: Not reported Baseline Group Characteristics: There was no significant difference in age, gender, body mass index, TC, LDL‐C, HDL‐C, TG, sd‐LDL‐C and RLP‐C levels between the two groups |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Ohbayashi 2009.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No washout required because participants were not treated for hypercholesterolaemia; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: None of the patients had any current or past history of ischaemic heart disease. Excluded criteria: Previous usage of stains; taking any medication that might potentially affect the PPAR and insulin resistance, such as pioglitazone and fibrates; taking medication for anticoagulation, or antiplatelet drugs; severe hepatic, respiratory or renal disease failure, haematologic diseases or other grave complications; oral steroid use; and a history of poor drug compliance Baseline Group Characteristics: None of the patients had any current or past history of ischaemic heart disease |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Ose 2009.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 6‐8 week washout dietary lead‐in period; 12‐week before‐and‐after trial with evening dosing |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: men and non‐pregnant, non‐lactating women aged 18–75 years, Women of childbearing potential were only permitted to participate if they were using a reliable method of contraception throughout; mean LDL‐C levels ≥ 160 mg/dL (4.1 mmol/L) and ≤ 220 mg/dL (5.7 mmol/L) and mean TG levels of ≤ 400 mg/dL (4.6 mmol/L at the end of the run‐in qualified for randomisation) Excluded criteria: previous contraindications or intolerance to statin therapy, homozygous familial hypercholesterolaemia, familial hypoalphalipoproteinaemia, medical conditions that might cause secondary dyslipidaemia, uncontrolled diabetes mellitus, pregnancy, conditions affecting absorption, distribution, metabolism, or excretion of drugs, symptomatic heart failure (New York Heart Association classification III or IV), significant cardiovascular disease, such as myocardial infarction, coronary or peripheral artery angioplasty, bypass graft surgery, or severe or unstable angina pectoris, impaired pancreatic function, liver enzyme levels greater than 1.5 times the upper limit of normal, impaired renal function, impaired urinary tract function, uncontrolled hypothyroidism, symptomatic cerebrovascular disease, left ventricular ejection fraction lower than 0.25, uncontrolled hypertension, muscular or neuromuscular disease, neoplastic disease, treatment with other lipid‐lowering drugs and medications potentially altering the pharmacokinetics of statins. Patients with serum creatine kinase (CK) activity greater than the upper limit of the reference range (ULRR) without clinical explanation Baseline Group Characteristics: The four treatment groups were similar with respect to age, sex, and race. Overall, there were more females than males 61 vs. 39% – in the safety population. Average age was 58 years and mean values for height, weight and BMI were very similar across the four treatment groups. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐C outcome was reported. |
| Other bias | High risk | Judgement Comment: The trial was supported by Kowa Research Europe Ltd. L.O. is a consultant/advisor to Kowa. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(315 ‐ 307)/315)]*100 = 2.5% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(315 ‐ 307)/315)]*100 = 2.5% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(315 ‐ 307)/315)]*100 = 2.5% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(315 ‐ 307)/315)]*100 = 2.5% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Park 2005.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout dietary lead‐in period; 8‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Korean men and women who were aged between 20 and 75 years with fasting triglyceride levels 600 mg/dL and LDL cholesterol levels > 130 mg/dL. The dietary guidelines recommended by the Guideline Committee for Hyperlipidemia Management in Korea were used for the lead‐in period, and the patients continued the diet after randomisation. These recommendations were defined as a diet comprising ≤ 20% of total calories from fat, ≤ 6% of total calories from saturated fat, and a daily cholesterol intake of 200 mg/dL. Excluded criteria: participation in other studies 3 months before enrolment; currently taking any kind of antihyperlipidaemic drug; suffering from uncontrolled diabetes (fasting plasma glucose, ≥ 180 mg/dL), thyroid dysfunction (abnormal thyroid‐stimulating hormone values 0.035 lalU/mL or > 3.1 plU/mL), or uncontrolled hypertension (diastolic blood pressure, > 115 mm Hg); symptomatic cerebrovascular disease or myocardial infarction within 3 months of enrolment; creatine kinase (CK) levels > 2 times the upper limit of normal (ULN); aspartate or alanine aminotransferase levels > 2.5 times ULN; and serum creatinine levels > 2.5 times ULN. Pregnant or breastfeeding women. Baseline Group Characteristics: No significant between‐group differences were found for baseline total cholesterol (P = 0.316), triglyceride (P = 0.278), LDL cholesterol (P = 0.403), or HDL cholesterol (P = 0.615) levels between groups. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | Stephen P on 30/06/2018 10:40 Included Only subjects with triglycerides greater than or equal to 150 mg/dL were included in the efficacy analysis (bias reporting). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: This study was sponsored by Choongwae Pharma Corporation, Seoul, South Korea. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(52 ‐ 49)/52)]*100 = 5.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(52 ‐ 49)/52)]*100 = 5.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(52 ‐ 49)/52)]*100 = 5.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(52 ‐ 28)/52]*100 = 46.2% participants were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
PREVAIL‐US 2016.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 6‐week washout/dietary stabilisation; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 4 mg
Included criteria: Participants were aged 18 to 80 years with either primary hyperlipidaemia or mixed dyslipidaemia. After a 6‐week washout/dietary stabilisation, subjects had LDL‐C levels of 130 to 220 mg/dL and TG levels ≤ 400 mg/dL. Excluded criteria: Familial hypercholesterolaemia, secondary causes of dyslipidaemia such as nephrotic syndrome, previous intolerance or allergy to statins, uncontrolled diabetes mellitus (defined as a glycosylated haemoglobin level > 8%), poorly controlled hypertension (blood pressure ≥ 160/100 mm Hg), or the presence of any unstable medical conditions Baseline Group Characteristics: The majority of participants in both groups were white (89.0% and 86.0%, respectively). Approximately 70% of participants in both groups were diagnosed with primary hyperlipidaemia and the other 30% with mixed dyslipidaemia. |
|
| Interventions |
Intervention Characteristics 4 mg |
|
| Outcomes |
HDL cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | High risk | Judgement Comment: LDL‐cholesterol outcome was not reported. |
| Other bias | High risk | Judgement Comment: Dr. Sponseller is an employee of Kowa Pharmaceuticals America, Inc. Kowa Pharmaceuticals sell pitavastatin |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | No outcome reported |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | No outcome reported |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(164 ‐ 157)/164]*100 = 4.3% participants were not included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | No outcome reported |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Saito 2002a.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Parallel group Method: 4‐week or longer run‐in period; 12‐week before‐and‐after study with evening dosing |
|
| Participants |
Baseline Characteristics 1 mg
2 mg
4 mg
Included criteria: 273 patients with hyperlipidaemia with serum total cholesterol levels ≥ 220 mg/dL, aged 25‐75 years Excluded criteria: None reported Baseline Characteristics: There was no imbalance between the 3 dose groups with regard to age, sex ratio, WHO classification for hyperlipidaemia, weight or height, although imbalances were noted with regard to the familial hypercholesterolaemia (FH) and baseline serum lipid levels (TC, TG, HDL‐C and LDL‐C) |
|
| Interventions |
Intervention Characteristics 1 mg 2 mg 4 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(90 ‐ 84)/90)]*100 = 6.7% participants were not included in the efficacy analysis for pitavastatin 1 mg/day; [(90 ‐ 82)/90]*100 = 8.9% participants were not included in the efficacy analysis for pitavastatin 2 mg/day; [(86 ‐ 85)/86]*100 = 1.2% participants were not included in the efficacy analysis for pitavastatin 4 mg/day. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(90 ‐ 81)/90]*100 = 10% participants were not included in the efficacy analysis for pitavastatin 1 mg/day; [(90 ‐ 73)/90]*100 = 18.9% participants were not included in the efficacy analysis for pitavastatin 2 mg/day; [(86 ‐ 77)/86]*100 = 10.5% participants were not included in the efficacy analysis for pitavastatin 4 mg/day; [(266 ‐ 231)/266]*100 = 13.2% participants were not included in the efficacy analysis for all doses. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(90 ‐ 84)/90]*100 = 6.7% participants were not included in the efficacy analysis for pitavastatin 1 mg/day; [(90 ‐ 82)/90]*100 = 8.9% participants were not included in the efficacy analysis for pitavastatin 2 mg/day; [(86 ‐ 85)/86]*100 = 1.2% participants were not included in the efficacy analysis for pitavastatin 4 mg/day. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(90 ‐ 83)/90]*100 = 7.8% participants were not included in the efficacy analysis for pitavastatin 1 mg/day; [(90 ‐ 80)/90]*100 = 11.1% participants were not included in the efficacy analysis for pitavastatin 2 mg/day; [(86 ‐ 83)/86]*100 = 3.5% participants were not included in the efficacy analysis for pitavastatin 4 mg/day; [(266 ‐ 246)/266]*100 = 7.5% participants were not included in the efficacy analysis for all doses. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Saito 2002b.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: more than 4 weeks run‐in period; 12‐week before‐and‐after study with evening dosing |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Women and men between the ages of 20 and 75 years with primary hyperlipidaemia, with a TC value ≥ 220 mg/dL and TG values ≤ 400 mg/dL Excluded criteria: Pregnant women and those who were breastfeeding, subjects who had taken pitavastatin, had participated in other studies 4 months prior to the study, suffered from uncontrolled diabetes mellitus or severe hypertension or had a cerebrovascular disorder or myocardial infarction diagnosed 3 months prior to the study, heart failure, hepatic or renal dysfunction or drug allergy. Baseline Group Characteristics: Based on baseline characteristics, all demographic and prognostic factors and lipid parameters were well balanced between the two treatment groups. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(125 ‐ 120)]*100 = 4% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(125 ‐ 120)]*100 = 4% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(125 ‐ 120)]*100 = 4% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(125 ‐ 75)]*100 = 40% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Sakabe 2008.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: Participants were not receiving any concomitant drug, therefore, no washout required; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Patients with primary hypercholesterolaemia with a low‐density lipoprotein (LDL) cholesterol concentration > 160 mg/dL and a triglyceride concentration ≤ 400 mg/dL Excluded criteria: Patients who smoked or had diabetes, hypertension, vascular events, revascularisation procedures, coronary artery disease, or active liver disease, or took any concomitant drug Baseline Characteristics: NR |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Sansanayudh 2010.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No washout required because participants were not on lipid medications; 8‐week before‐and‐after study with evening dosing |
|
| Participants |
Baseline Characteristics 1 mg
Included criteria: patients older than 18 years of age with hypercholesterolaemia who had an indication for statin therapy according to the NCEP‐ATPIII guidelines (i.e. CHD or CHD risk equivalents and LDL‐C ≥ 100 mg/dL; ≥ 2 risk factors [10‐y risk 10‐20%] and LDL‐C ≥ 130 mg/dL; ≥ 2 risk factors [10‐y risk 10%] and LDL‐C ≥ 160 mg/dL; 0‐1 risk factor and LDL‐C ≥ 190 mg/dL) Excluded criteria: currently taking drugs known to affect lipid metabolism or interact with pitavastatin or atorvastatin (e.g. estrogen, corticosteroids, azole antifungals, fibrates, ticlopidine, thiazolidinedione, phenobarbital, and valproic acid), had previously been treated with statins, had active liver disease or elevated liver enzyme levels (AST or ALT > 3 times the upper limit of normal[ULN]), had CK levels more than 10 times the ULN, or had severe renal impairment (creatinine clearance 30 mL/min), pregnancy or lactation and triglyceride (TG) level > 400 mg/dL. Baseline Group Characteristics: Baseline characteristics were similar between treatment groups with the exception of mean AST. |
|
| Interventions |
Intervention Characteristics 1 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
|
|
| Notes | Stephen P on 01/02/2018 09:23 Outcomes Given versus calculated percentage changes from baseline for HDL cholesterol and triglycerides had a greater than 10% difference (2.76 vs ‐0.4) and (‐10.4 vs ‐18.4) therefore HDL cholesterol and triglyceride outcomes were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(50 ‐ 48)/50]*100 = 4% missing data were replaced by series mean, see Table 3. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(50 ‐ 48)/50]*100 = 4% missing data were replaced by series mean, see Table 3. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(50 ‐ 48)/50]*100 = 4% missing data were replaced by series mean, see Table 3. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(50 ‐ 48)/50]*100 = 4% missing data were replaced by series mean, see Table 3. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Sasaki 2008.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout period for participants who had been taking lipid‐lowering drugs before enrolment; 2 to 4‐week run‐in period for all participants; 8‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Eligible patients were men or postmenopausal women aged ≥ 20 years; had LDL‐C levels ≥ 140 mg/dL, HDL‐C levels 80 mg/dL, and TG levels 500 ≤ mg/dL; and had glucose intolerance. Glucose intolerance was defined as receipt of pharmacologic treatment for diabetes (excluding insulin therapy) or a glucose measurement in the past 3 months indicative of glucose intolerance (i.e. fasting blood glucose ≥ 110 mg/dL, 1‐hour blood glucose ≥ 180 mg/dL, or 2‐hour blood glucose ≥ 140 mg/dL after a 75‐g oral glucose challenge, or a casual blood glucose level ≥ 140 mg/dL). This definition was based on the criteria for borderline diabetes used in Japan and on World Health Organization criteria for impaired fasting glucose and impaired glucose tolerance. Excluded criteria: contraindications to statin use (i.e. hepatic impairment or biliary tract obstruction, cyclosporine use, and use of fibrates with an abnormal renal function test result); severe renal impairment or dysfunction (serum creatinine ≥ 2 mg/dL); secondary hyperlipidaemia associated with conditions such as hypothyroidism or Cushing’s syndrome; use of steroid hormones, including topical and nasal forms; severe hypertension; cerebrovascular disease in the past 3 months; myocardial infarction or coronary artery reconstruction in the past 3 months; heart failure (New York Heart Association class 3 or higher); history of allergy or serious adverse reactions to the study drugs; poorly controlled diabetes, based on the study physician’s judgement; and type 1 diabetes. Patients could also be excluded if their participation was considered inappropriate by the study physician Baseline Group Characteristics: There were no significant differences between the 2 groups in terms of any parameter |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
HDL cholesterol
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | High risk | Judgement Comment: No 3‐12 week data for LDL cholesterol |
| Other bias | Low risk | Judgement Comment: Clinical research grant from the International University of Health and Welfare, Tochigi, Japan |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | No 3‐12 week data |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | No 3‐12 week data |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis at 8 weeks. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | No 3‐12 week data |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Shimabukuro 2011.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week dietary lead‐in period; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: men or women aged 30‐79 with type 2 diabetes with hypercholesterolaemia [total cholesterol ≥ 5.70 mmol/L (220 mg/dL)] and/or hypertriglyceridaemia [triglycerides 1.70–3.96 mmol/L (150–350 mg/dL)] Excluded criteria: a past history of hypersensitivity to statins; hepatic dysfunction [serum levels of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥ 100 IU], suspected disorder of hepatic metabolism or biliary obstruction (acute hepatitis, acute exacerbation of chronic hepatitis, liver cirrhosis, liver cancer and jaundice); renal dysfunction [serum creatinine ≥ 133 umol/L(1.5 mg/dL)], pregnant, possibly pregnant or breastfeeding women; patients with poorly controlled diabetes mellitus [HbA1c > 9.4% (National Glycohemoglobin Standardization Program), 79 mmol/L (International Federation of Clinical Chemistry and Laboratory Medicine)], recent history of cerebrovascular disease, coronary heart disease or congestive heart failure; familial hypercholesterolaemia; secondary hyperlipidaemia other than that associated with diabetes mellitus Baseline Group Characteristics: There were no differences in characteristics between two groups |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Sone 2002.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week run‐off period; 8‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: 13 men and 20 women with type 2 diabetes mellitus whose serum cholesterol levels were more than 5.7 mmol/L (220 mg/dL) and changes in HbA1c levels were less than 10% in the previous two months Excluded criteria: The use of other drugs to treat hyperlipidaemia was not permitted during the study Baseline Group Characteristics: The study subjects consisted of 13 men and 20 women with type 2 diabetes mellitus whose serum cholesterol levels were more than 5.7 mmol/L ( = 220 mg/dL) and changes in HbA1c levels were less than 10% in the previous two months |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
|
|
| Notes | Stephen P on 17/02/2018 09:46 Included Source of funding was from industry and government. Stephen P on 14/07/2018 10:18 Included HDL‐cholesterol and triglyceride data differed by more than 10% between given and calculated values in percentage change from baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Kowa, Co. supported this study. This work was also supported by the Promotion of Fundamental Studies in Health Science in the Organization for Pharmaceutical Safety and Research (OPSR), Health Sciences Research Grants (Research on Human Genome and Gene Therapy) from the Ministry of Health and Welfare, and a Research Project Grant from the University of Tsukuba. H.S. is a recipient of Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | No data included in the efficacy analysis because the data differed by more than 10% between given and calculated values |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | No data included in the efficacy analysis because the data differed by more than 10% between given and calculated values |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Stender 2013.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Parallel group Method: 6 to 8‐week washout/dietary period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 1 mg
2 mg
2 mg then titrated to 4 mg at 4 weeks
Included criteria: Enroled patients were at least 65 years of age with a diagnosis of primary hypercholesterolaemia or combined (mixed) dyslipidaemia [plasma LDL‐C between 3.4 mmol/L (130 mg/dL) and 5.7 mmol/L (220 mg/dL) despite dietary therapy, and moderately elevated triglyceride levels ≤ 4.6 mmol/L (400 mg/dL)] at two consecutive assessments during a washout and dietary lead‐in period. Excluded criteria: Exclusion criteria included homozygous familial hypercholesterolaemia or condition(s) that could cause secondary dyslipidaemia; uncontrolled medical conditions, including diabetes mellitus (glycated haemoglobin > 8%), uncontrolled hypothyroidism, defined as concentrations of thyroid‐stimulating hormone above the upper limit of the reference range (ULRR); and poorly controlled or uncontrolled hypertension (systolic blood pressure > 160 mmHg and diastolic blood pressure > 90 mmHg) with or without antihypertensive therapy; gastrointestinal conditions that may have interfered with drug absorption; impaired liver or renal function; serum creatine kinase (CK) more than five times the ULRR; or significant CVD, such as MI, coronary or peripheral artery angioplasty, bypass graft surgery, or severe or unstable angina pectoris. Baseline Group Characteristics: Groups were well matched in terms of their demographic and other baseline characteristics. |
|
| Interventions |
Intervention Characteristics 1 mg 2 mg 2 mg then titrated to 4 mg at 4 weeks |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | High risk | Judgement Comment: This study was supported by Kowa Research Europe. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(209 ‐ 207)/209]*100 = 1.0% participants were not included in the efficacy analysis for 1 mg/day dose; [(226 ‐ 224)/226]*100 = 0.9% participants were not included in the efficacy analysis for 2 mg/day dose. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | Pitavastatin 1 mg/day: 4 weeks [(209 ‐ 197)/209]*100 = 5.7%, 8 weeks [(209 ‐ 192)/209]*100 = 8.1%, 12 weeks [(209 ‐ 188)/209]*100 = 10.0% participants were not included in the efficacy analysis. Pitavastatin 2 mg/day: 4 weeks [(226 ‐ 217)/226]*100 = 4.0%, 8 weeks [(226 ‐ 214)/226]*100 = 5.3%, 12 weeks [(226 ‐ 208)/226]*100 = 8.0% participants were not included in the efficacy analysis. Pitavastatin 2 mg/day at 4 weeks then uptitrated to 4 mg/day from 8‐12 weeks: [(216 ‐ 203)/216]*100 = 6.0% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(209 ‐ 207)/209]*100 = 1.0% participants were not included in the efficacy analysis for 1 mg/day dose; [(226 ‐ 224)/226]*100 = 0.9% participants were not included in the efficacy analysis for 2 mg/day dose. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(209 ‐ 207)/209]*100 = 1.0% participants were not included in the efficacy analysis for 1 mg/day dose; [(226 ‐ 224)/226]*100 = 0.9% participants were not included in the efficacy analysis for 2 mg/day dose. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Suzuki 2009.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Patients with high cholesterol having serum TC ≥ 220 mg/dL; fasting LDL‐C ≥ 140 mg/dL and serum TG ≤ 400 mg/dL Excluded criteria: Patients previously taking dextran sulfate sodium sulfur 18 (DS), patients who are contraindicated for statins or DS, patients who can not be discontinued from previous medication, patients with HbA1c > 8% or poor glycaemic control, patients with fasting serum TG ≥ 600 mg/dL, patients not suitable for the study according to the investigators. Baseline Group Characteristics: NR |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | [(26 ‐ 18)/26]*100 = 30.8% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | High risk | [(26 ‐ 11)/26]*100 = 57.7% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | [(26 ‐ 13)/26]*100 = 50.0% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(26 ‐ 18)/26]*100 = 30.8% participants were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Tateishi 2011.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No participant received lipid medications, therefore no washout required; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: participants with hypercholesterolaemia and women over 20 years old who had not been receiving statins beforehand Excluded criteria: not reported Baseline Group Characteristics: NR |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Teramoto 2001.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4 to 6‐week washout period; 8‐week before‐and‐after study with evening dosing; HDL cholesterol data weres not included in the efficacy analysis because the calculated percentage change versus given percentage change was greater than 10%. |
|
| Participants |
Baseline Characteristics Pitavastatin 2 mg/day
Included criteria: Serum total cholesterol value of 220 mg/dL (5.69 mmol/L) or more. Ages: 20‐75 years old. Excluded criteria: Patients with poor control of diabetes and patients with severe hypertension, patients with severe hepatopathy, renal impairment, myocardial infarction and cerebrovascular disorder attack (within 3 months after the attack) and patients with heart failure, women who are breastfeeding, pregnant women or women who desire pregnancy, patients with a history of drug hypersensitivity or a history of serious side effects, other patients who were judged inappropriate as subjects of this trial by the investigators, no consent of trial participation Baseline Group Characteristics: NR |
|
| Interventions |
Intervention Characteristics Pitavastatin 2 mg/day
|
|
| Outcomes |
Total cholesterol
LDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(313 ‐ 295)/313]*100 = 5.8% were not included in the efficacy analysis for total cholesterol at 4 weeks; [(313 ‐ 277)/313]*100 = 11.5% were not included in the efficacy analysis for total cholesterol at 8 weeks. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Unclear risk | [(313 ‐ 289)/313]*100 = 7.7% were not included in the efficacy analysis for LDL cholesterol at 4 weeks; [(313 ‐ 268)/313]*100 = 14.4% were not included in the efficacy analysis for LDL cholesterol at 8 weeks. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | No data included in the efficacy analysis because the data differed by more than 10% between given and calculated values |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | [(313 ‐ 138)/313]*100 = 55.9% were not included in the efficacy analysis for triglycerides at 4 weeks; [(313 ‐ 133)/313]*100 = 57.5% were not included in the efficacy analysis for triglycerides at 8 weeks. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Uzui 2014.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No washout required because participants were not receiving anti‐dyslipidaemic agents; 2‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: patients who had not received anti‐dyslipidaemic agents and had LDL‐C levels greater than 140 mg/dL Excluded criteria: not reported Baseline Group Characteristics: There were no differences in the baseline characteristics of the patients in each group. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: This work was partially supported by a Research Grant from the University of Fukui. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yamasaki 2014.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Parallel group Method: Participants were not receiving lipid medication for at least 3 months; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 1 mg
4 mg
Included criteria: 63 essential hypertensive patients with dyslipidaemia with LDL‐cholesterol level higher than the National Cholesterol Education Program Adult Treatment Panel III recommendations (100 mg/dL for moderately high/high‐risk subjects without atherosclerotic vascular disease, 70 mg/dL for high‐risk subjects with atherosclerotic vascular disease) Excluded criteria: Aged 20 years, treatment for dyslipidaemia within the preceding 3 months, current treatment with progesterone or other hormone therapy within the previous 3 months, familial hypercholesterolaemia, acute coronary syndrome, congestive heart failure (New York Heart Association class II or greater), liver dysfunction, chronic kidney disease requiring regular haemodialysis, endocrine disease, secondary hypertension, and administration of agents affecting lipid metabolism Baseline Group Characteristics: Baseline total cholesterol, LDL‐cholesterol and apolipoprotein B were significantly higher in the pitavastatin 4 mg/day group than in the pitavastatin 1 mg/day group. |
|
| Interventions |
Intervention Characteristics 1 mg 4 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | Stephen P on 21/02/2018 09:30 Included Authors had no support or funding to report; they may not have wanted to report source of funding but I did not know for sure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Low risk | Judgement Comment: Authors had no support or funding to report. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yanagi 2011.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Parallel group Method: No lipid‐lowering medication had been administered, therefore no washout period required; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg PIT‐ROS
2 mg PIT‐PIT
2 mg PIT‐ROS and PIT‐PIT
Included criteria: outpatients with type 2 diabetes with fasting serum LDL‐C ≥ 140 mg/dL and triglyceride 300 mg/dL; no lipid‐lowering medication had been administered; glycated haemoglobin A1c (HbA1c) 8.5%; serum creatinine 2.0 mg/dL; urinary albumin excretion 300 mg/Cr; no concomitant use of insulin, fibrates, thyroid hormone, or corticosteroid hormone; and no changes in medications during the previous 3 months Excluded criteria: history of stroke or other cardiovascular events Baseline Group Characteristics: No significant differences in any parameters at baseline were seen among the four groups. |
|
| Interventions |
Intervention Characteristics 2 mg PIT‐ROS 2 mg PIT‐PIT 2 mg PIT‐ROS and PIT‐PIT |
|
| Outcomes |
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | High risk | Total cholesterol data were not reported. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(45 ‐ 43)/45]*100 = 4.4% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | [(45 ‐ 43)/45]*100 = 4.4% were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | [(45 ‐ 43)/45]*100 = 4.4% were not included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yokote 2008.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Men and women aged 20 or older with hypercholesterolaemia (TC ≥ 220 mg/dL) and TG ≤ 400 mg/dL, including familial hypercholesterolaemia Excluded criteria: During the study period, administration of fibrates, other statins, probucol and cyclosporine (because of drug–drug interaction) was prohibited. Patients with a past history of hypersensitivity to statins; patients with hepatic dysfunction [aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥ 100 IU/L], suspected hepatic metabolism disorders or biliary obstruction (acute hepatitis, acute exacerbation of chronic hepatitis, liver cirrhosis, liver cancer and jaundice), or renal dysfunction (serum creatinine ≥ 1.5 mg/dL); pregnant women, women who may be pregnant, and breastfeeding women; patients with poorly controlled diabetes (HbA1c > 8.0%) Baseline Group Characteristics: Baseline incidence of hypertension was significantly higher in the pitavastatin group, but no significant differences were observed in the other parameters. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
|
|
| Notes | Stephen P on 02/02/2018 10:36 Outcomes Given versus calculated percentage changes from baseline for HDL cholesterol and triglycerides had a greater than 10% difference (3.2 vs 1.9) and (‐17.3 vs ‐23.6), therefore HDL cholesterol and triglyceride outcomes were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Low risk | Quote: "This study was supported by the grant from Non‐Profit Organization for Medical Frontier, Chiba, Japan. It was also supported in part by a Grant‐in‐Aids for Scientific Research from the Ministry of Health, Labour and Welfare to K.Y." Judgement Comment: government grants |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | [(101 ‐ 93)/101]*100 = 7.9% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | [(101 ‐ 93)/101]*100 = 7.9% participants were not included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | High risk | No data included in the efficacy analysis because the data differed by more than 10% between given and calculated values |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | No data included in the efficacy analysis because the data differed by more than 10% between given and calculated values |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yoshida 2010.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: Participants were not receiving any medications, therefore washout not required; 4‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: male chronic smokers, who were newly diagnosed with mild hypercholesterolaemia Excluded criteria: history of malignancy, cardiovascular events or active inflammatory diseases, other cardiovascular risk factors or taking other medications Baseline Characteristics: There were no significant differences between the 2 groups in any clinical parameters. |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Low risk | Judgement Comment: Sources of Funding: A grant from the Smoking Research Foundation to T.M |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yoshida 2013.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: No participant was on lipid medications, therefore no washout required; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: Participants with hyperlipidaemia, aged 45 to 75 years Excluded criteria: Participants aged 20 years, premenopausal females, diabetes, CVD, liver dysfunction, renal dysfunction, endocrine disease, or administration of agents affecting lipid metabolism and lipid oxidation Baseline Group Characteristics: There were no significant baseline characteristic differences between groups |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
HDL cholesterol
|
|
| Notes | Stephen P on 31/01/2018 05:52 Outcomes Given versus calculated percentage change from baseline for triglycerides was greater than 10%, therefore the triglyceride outcome could not be reported in RevMan 5 for this trial (‐12.5 vs ‐17.1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Low risk | Judgement Comment: government grant |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | High risk | No data included in the efficacy analysis because the data differed by more than 10% between given and calculated values |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yoshika 2010.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: Participants were not on any drug therapy for at least 6 months, therefore no washout required; 3‐month before‐and‐after study |
|
| Participants |
Baseline Characteristics 2 mg
Included criteria: participants had hypercholesterolaemia (LDL‐C > 140 mg/dL). Excluded criteria: diabetes and renal dysfunction and CRP levels > 5.0 mg/L Baseline Group Characteristics: There were no significant differences in age, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) |
|
| Interventions |
Intervention Characteristics 2 mg |
|
| Outcomes |
LDL cholesterol
HDL cholesterol
Triglycerides
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
Yoshitomi 2006.
| Study characteristics | ||
| Methods |
Study design: Historically controlled trial Study grouping: Method: 4‐week washout period; 12‐week before‐and‐after study |
|
| Participants |
Baseline Characteristics 1 mg
Included criteria: men and women who were at least 18 years old treated with or without lipid‐lowering agents, plasma LDL cholesterol level above 140 mg/dL and a plasma TG level below 400 mg/dL Excluded criteria: pregnant or had familial hyperlipoproteinaemia, acute phase coronary artery disease, active liver disease, hepatic or renal dysfunction, or uncontrolled hypertension, concurrently taking drugs known to affect lipid levels or known to interact with the study medication Baseline Group Characteristics: There were no significant differences in age, sex, and body mass index between groups. Risk factors and complications did not differ between the two groups. |
|
| Interventions |
Intervention Characteristics 1 mg |
|
| Outcomes |
Total cholesterol
LDL cholesterol
Triglycerides
|
|
| Notes | Stephen P on 31/01/2018 08:13 Outcomes Given versus calculated percentage changes from baseline for HDL cholesterol had a greater than 10% difference (‐3 vs 1.7), therefore, HDL cholesterol outcome wasl not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Allocation concealment (selection bias) | High risk | Judgement Comment: Controlled before‐and‐after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Judgement Comment: Lipid parameter measurements unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement Comment: Lipid parameters were measured in a remote laboratory. |
| Selective reporting (reporting bias) | Low risk | Judgement Comment: LDL‐cholesterol outcome was reported. |
| Other bias | Unclear risk | Judgement Comment: Source of funding was not reported. |
| Incomplete outcome data (attrition bias) Total cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) LDL cholesterol) | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) HDL cholesterol | Low risk | All participants were included in the efficacy analysis. |
| Incomplete outcome data (attrition bias) Triglycerides | Low risk | All participants were included in the efficacy analysis. |
| Blinding of outcome assessment (detection bias)WDAEs | High risk | No comparison possible |
| Selective reporting (reporting bias) for WDAEs | High risk | No WDAE outcome reported |
ACS: Acute coronary syndromes ADRs: Adverse drug reactions ALT: Alanine aminotransferase AMI: Acute myocardial infarction AST: Aspartate aminotransferase ATP: Adult treatment panel BMI: Body mass index BP: Blood pressure BUN: Blood urea nitrogen CABG: Coronary artery bypass grafting CHD: Coronary heart disease CK: Creatine kinase Cr: Creatinine DBP: Diastolic blood pressure DM: Diabetes mellitus DS: Dextran sulfate ECG: Electrocardiogram FBS: Fetal bovine serum FH: Familial hypercholesterolaemia HbA1C: Hemoglobin A1c HDL‐C: High density lipoprotein cholesterol HIV: Human immunodeficiency virus HMG‐CoA: 3‐hydroxy‐3‐methylglutaryl coenzyme A IMT: Intima media thickness KPa: Kilo Pascals LDL‐C: Low density lipoprotein cholesterol MB: Myocardial band NCEP: National Cholesterol Education Program NYHA: New York Heart Association PBC: Primary biliary cholangitis PIT: Pitavastatin PPAR: Peroxisome proliferator‐activated receptor PTCA: Percutaneous transluminal coronary angioplasty
RLP‐C: Reminant‐like particle cholesterol ROS: Rosuvastatin SBP: Systolic blood pressure
sd: small dense SD: Standard deviation TC: Total cholesterol TG: Triglyceride TIA: Transient ischaemic attack TSH: Thyroid stimulating hormone u‐AP: Unstable angina pectoris ULN: Upper limit of normal ULRR: Upper limit of the reference range WDAE: Withdrawal due to adverse effects WHO: World health organisation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aberg 2017 | Confounding factor: protease inhibitors of treating HIV |
| CTRI201305003686 2013 | Dose is 2‐4 mg/day pitavastatin one/two Pitavastatin 2 mg tablets per day |
| CTRI201307003842 2013 | Washout period unclear |
| CTRI201309004003 2013 | Washout period unclear |
| Horiuchi 2010 | Combined different statins |
| Huang 2016 | Trial excluded because dose was 1‐2 mg/day and lipids had median percentage change from baseline |
| Hyogo 2011 | Run‐in washout period not reported |
| Inami 2007 | Some participants may have had at least 3‐week washout period but others only 2 weeks. |
| Jing 2008 | Dose unclear |
| Joshi 2017 | Confounding factor: protease inhibitors of treating HIV |
| JPRN JMA IIA00056 2011 | Dose is 1‐4 mg/day dose range |
| JPRN UMIN000000685 2007 | Participants may be receiving lipid altering agents at baseline no washout period reported |
| JPRN UMIN000001600 2009 | Dose is 1 or 2 mg/day dose not specific |
| JPRN UMIN000002507 2009 | Participants were receiving lipid altering agents at baseline |
| JPRN UMIN000002680 2009 | Patients received cholesterol lowering drugs at baseline |
| JPRN UMIN000003554 2010 | no 3‐12 week lipid data 12‐16 weeks |
| JPRN UMIN000003628 2010 | Dose is 1‐4 mg/day dose range |
| JPRN UMIN000005489 2011 | Participants may be receiving lipid altering agents at baseline no washout period reported |
| JPRN UMIN000005501 2011 | Participants may be receiving lipid altering agents at baseline no washout period reported |
| JPRN UMIN000007130 2012 | Participants may be receiving non statin lipid altering agents at baseline no washout period reported |
| JPRN UMIN000007695 2012 | Participants may be receiving non statin lipid altering agents at baseline no washout period reported |
| JPRN UMIN000009241 2012 | Participants may be receiving lipid altering agents at baseline no washout period reported |
| JPRN UMIN000013384 2014 | Participants are receiving a statin excluding pitavastatin at baseline |
| JPRN UMIN000019020 2015 | Participants may be receiving lipid altering agents at baseline no washout period reported |
| Kawashiri 2008 | Confounding factor in 5 of the 19 subjects |
| Matsubara 2012 | Confounding factor |
| Minai 2008 | Trial period reported as 8 weeks or more |
| Muto 2013 | Some participants received lipid‐lowering agents at baseline |
| Nakamura 2006 | Subjects may be receiving lipid altering agents at baseline |
| Nakaya 2001 | Baseline washout period too short |
| NCT02670434 2016 | Terminated The study was aborted and the investigators provided no data |
| NCT02799758 2016 | Terminated The study was aborted and the investigators provided no data |
| Nishiguchi 2018 | Median percent change from baseline |
| Nomura 2008 | Some participants may have had at least a 3‐week washout period but others only 2 weeks. |
| Nozue 2015 | Median percent change from baseline |
| Rui 2014 | Article not available from any library |
| Watanabe 2015 | Dose unclear |
| Wongprikorn 2016 | Confounding factor |
| Yagi 2011 | Some participants were treated for 12 weeks while some were treated for more than 12 weeks. |
| Yao 2016 | Article retracted |
| Zhu 2010 | Washout period of all previous lipid‐lowering drugs not clear |
HIV: Human Immunodeficiency Virus
Characteristics of studies awaiting classification [ordered by study ID]
JPRN UMIN000003055 2010.
| Methods | Historically controlled trial Participants were not receiving lipid altering medications within 4 weeks of the study no washout required |
| Participants | Male and Female 20‐80 years old with NASH/NAFLD Included criteria: 1)The patients with fatty liver 2)The patients with the level of ALT (42‐120IU/L) 3)The patients with high cholesterol levels Exclusion criteria: 1)The patients with the use of drugs for dyslipidaemia within 4 weeks 2)The patients with chronic kidney disease (serum Cre>2.0) or severe liver dysfunction 3)The patients who are pregnant or giving the breast to a baby or want to be pregnant within the period of trial 4)The patients with cyclosporin 5)The patients with liver dysfunction by drug, alcohol, virus, or autoimmune factors |
| Interventions | pitavastatin calcium |
| Outcomes | The level of alanine aminotransferase |
| Notes | treatment period not reported |
ALT: Alanine aminotransferase
CK: Creatine kinase
Cre: Creatinine
ECG: electrocardiogram
GPT: Glutamic‐pyruvic transaminase
HDL‐C: High density lipoprotein cholesterol
HMG‐CoA: 3‐hydroxy‐3‐methylglutaryl coenzyme A
IU: International unit
LDL‐C: Low density lipoprotein cholesterol
MB: Myocardial band
NAFLD: Nonalcohol fatty liver disease
NASH: Nonalcohol steatohepatitis
NYHA: New York Heart Association
PCI: Percutaneous coronary intervention
ST: End of S and start of T wave between ventricular depolarisation and repolarization electrocardiogram
TC: Total cholesterol
TG: Triglycerides
Characteristics of ongoing studies [ordered by study ID]
KCT0001730 2015.
| Study name | STA study |
| Methods | Historically controlled trial Parallel Participants were not receiving lipid altering medications no washout required 8 week trial |
| Participants | Male and Female 18 years or greater Included criteria: 1) Unstable angina, non‐ST‐segment elevation acute coronary syndrome receiving optimal reperfusion therapy 2) Evidence of coronary artery disease 3) low density lipoprotein cholesterol >70mg/dl, triglyceride <500mg/dl 4) agree to informed consent Exclusion criteria: 1) contraindication to statin therapy 2) pregnant women 3) life expectancy less than 2 years 4) abnormal renal, liver function 5) prescribed other lipid lowering medication (fibrate, omega 3, niacin) |
| Interventions | pitavastatin 2 mg/day pitavastatin 4 mg/day |
| Outcomes | Lipid profile (total cholesterol, triglyceride, LDL‐C, HDL‐C) in each group |
| Starting date | 2015‐06‐23 |
| Contact information | Kyeong Ho Yun MD Wonkwang University Hospital 895 Muwang‐ro, Iksan, Korea, 54538 |
| Notes |
NCT01402843 2011.
| Study name | COCTAIL |
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Methods: 8 week randomised, double‐blind, placebo‐controlled trial |
| Participants | Males and females aged 20 years or older Included criteria:
Exclusion criteria:
|
| Interventions | Placebo Pitavastatin 4 mg/day |
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, Triglycerides |
| Starting date | June 2011 |
| Contact information | Gyu Rok Han, MD Dept. of Cardiology, Hallym University Medical Center |
| Notes |
NCT01710007 2011.
| Study name | Efficacy and Safety Study of Pitavastatin for Hypercholesterolemia |
| Methods | Historically controlled trial 4 week washout period of all previous lipid lowering drugs 12 week study |
| Participants | Females or males aged between 20 and 80 years Included criteria:
Exclusion criteria:
|
| Interventions | 1PC002 (Pitavastatin) 2 mg/day |
| Outcomes | Total cholesterol, LDL cholesterol, HDL cholesterol, Triglycerides |
| Starting date | October 18, 2012 |
| Contact information | Orient Pharma Co., Ltd. |
| Notes |
ALT: Alanine aminotransferase
AST: Aspartate aminotransferase
CK: Creatine kinase
CVD: Cardiovascular disease
HbA1c: Haemoglobin A1c
HDL‐C: High density lipoprotein cholesterol
HIV: Human immunodeficiency virus
ICF: Informed consent form
LDL‐C: Low density lipoprotein cholesterol
PCI: Percutaneous coronary intervention
ST: End of S and start of T wave between ventricular depolarisation and repolarization electrocardiogram
TC: Total cholesterol
TG: Triglycerides
ULN: Upper limit of normal
Differences between protocol and review
Attempting to compare the efficacy of pitavastatin between twice‐daily versus single dose was not mentioned in the protocol but was attempted in the review under the heading 'subgroup analysis'. The secondary objective to quantify the relative potency of pitavastatin with respect to fluvastatin, atorvastatin, rosuvastatin and cerivastatin for total cholesterol, LDL cholesterol and triglycerides was not mentioned in the protocol.
Contributions of authors
Stephen P Adams: contributed to the design of the protocol, screened the citations, assessed all trials for inclusion or exclusion, extracted the data, analysed the data and made contributions to the discussion.
Nima Alaeiilkhchi: screened the citations, assessed all trials for inclusion or exclusion and extracted the data.
James M Wright: interpreted the data, made contributions to the discussion and conclusions.
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology & Therapeutics, University of BC, Canada
Office space
External sources
-
BC Ministry of Health grant to the Therapeutics Initiative, Canada
Salary support
Declarations of interest
Stephen P Adams: None known.
Nima Alaei: None known.
James M Wright: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Braamskamp 2015 {published data only}
- Braamskamp MJ, Stefanutti C, Langslet G, Drogari E, Wiegman A, Hounslow N, et al. Efficacy and safety of pitavastatin in children and adolescents at high future cardiovascular risk. Journal of Pediatrics 2015;167(2):338-43.e5. [DOI: 10.1016/j.jpeds.2015.05.006] [DOI] [PubMed] [Google Scholar]
- Daniels SR. Pitavastatin in children. Journal of Pediatrics 2015;167(2):219-21. [DOI: ] [Google Scholar]
- World Health Organization. Investigating pitavastatin for high cholesterol in children [A double-blind, randomised, placebo-controlled, parallel-group, 12-week study of pitavastatin in high-risk hyperlipidaemia in childhood P/266/2011, P267/2011, P268/2011 - PASCAL 401]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011-004964-32-NL first received 9 February 2012. [WHO 2020]
Budinski 2009 {published data only}
- Budinski D, Arneson V, Hounslow N, Gratsiansky N. Pitavastatin compared with atorvastatin in primary hypercholesterolemia or combined dyslipidemia. Clinical Lipidology 2009;4(3):291-302. [EMBASE: 354734823] [Google Scholar]
- Budinski D. Study comparing pitavastatin and atorvastatin in patients with type II diabetes mellitus and combined dyslipidemia. ClinicalTrials.gov . [CLINICALTRIALS.GOV ID: NCT00309751]
- Study to compare the efficacy of pitavastatin with that of atorvastatin in lowering cholesterol levels. ClinicalTrials.gov . [CLINICALTRIALS.GOV ID: NCT00249249]
- Study to compare the efficacy of pitavastatin with that of atorvastatin in lowering cholesterol levels. ICH Good Clinical Practice Clinical Trials Registry . [ICH CTR ID: NCT00249249]
Chen 2012 {published data only}
- Chen L, Qu P, Lou D, Liu Q, Wang J, Zhang C. Effect of pitavastatin on high-sensitive c-reactive protein levels in patients with hypertension [Pǐ fá tātīng duì gāo xiěyā huànzhě gāomǐn c fǎnyìng dànbái shuǐpíng de yǐngxiǎng]. Zhongguo xin yao yu lin chuang za zhi [Chinese Journal of New Drugs and Clinical Remedies] 2012;31(6):328-31. [Google Scholar]
Chen 2015 {published data only}
- Chen H, Zhang Y, Wei X, Han Z, Liu Z, Liu S. Clinical observation of pitavastatin in the treatment of hyperlipidemia [pi fa ta ting zhi liao gao zhi xue zheng lin chuang liao xiao guan cha]. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease 2015;13(06):822-4. [DOI: 10.3969/j.issn.1672-1349.2015.06.044] [DOI] [Google Scholar]
Eriksson 2011 {published data only}
- EUCTR2005 001037 15. Study of pitavastatin 4 mg vs simvastatin 40 mg (following up-titration) in patients with primary hypercholesterolema or combined dyslipidemia and 2 or more risk factors for coronary heart disease. EU Clinical Trials Register/show/EUCTR2005 001037 15 (first received 29 December 2005). [EUCTR2005 001037 15]
- Eriksson M, Budinski D, Hounslow N. Comparative efficacy of pitavastatin and simvastatin in high-risk patients: a randomized controlled trial. Advances in Therapy 2011;28(9):811-23. [DOI: 10.1007/s12325-011-0056-7] [DOI] [PubMed] [Google Scholar]
- Good Clinical Practice Network. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin 4 mg vs simvastatin 40 mg (following up-titration) in patients with primary hypercholesterolema or combined dyslipidemia and 2 or more risk factors for coronary heart disease]. Good clinical practice network/show/NCT00309738 (first received September 2005). [URL: ichgcp.net/clinical-trials-registry/NCT00309738]
- NCT00309738. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin vs. simvastatin (following up-titration) in patients with primary hypercholesterolemia or combined dyslipidemia and 2 or more risk factors for coronary heart disease]. clinicaltrials.gov/show/NCT00309738 (first received September 2005). [NCT00309738]
Gumprecht 2011 {published data only}
- Center for Drug Evaluation and Research. Active-controlled study with atorvastatin in patients with type II diabetes mellitus (NK-104-305) [Study of pitavastatin 4 mg vs atorvastatin 20 mg (following up-titration) in subjects with type II diabetes mellitus and combined dyslipidemia [NK-104-305]]. FDA Open Trials/show/NK-104-305 first received 28 February 2012:21-2. [www.documentcloud.org/documents/3199241.html]
- Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20-40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes, Obesity & Metabolism 2011;13(11):1047-55. [DOI: 10.1111/j.1463-1326.2011.01477.x] [DOI] [PubMed] [Google Scholar]
HaeKim 2018 {published data only}
- Hae Kim C, Wang S, Park JB, Jung KH, E Yoon Y, Lee SP, et al. Assessing impact of high-dose pitavastatin on carotid artery elasticity with speckle-tracking strain imaging. Journal of Atherosclerosis & Thrombosis 2018;25(11):1137-48. [DOI: 10.5551/jat.42861] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2012 {published data only}
- Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: pitch study (pitavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). Journal of Clinical Lipidology 2012;6(4):340-51. [DOI: 10.1016/j.jacl.2012.01.009] [DOI] [PubMed] [Google Scholar]
- NCT01166633. Efficacy and safety study of pitavastatin versus atorvastatin to treat hypercholesterolemia (pitch) [A randomized, open label, dose titration study to evaluate the effect of pitavastatin versus atorvastatin in patients with hypercholesterolemia and mild to moderate hepatic damage]. clinicaltrials.gov/show/NCT01166633 (first received June 2009). [NCT01166633]
Harada Shiba 2016 {published data only}
- Harada-Shiba M, Arisaka O, Ohtake A, Okada T, Suganami, H. Efficacy and safety of pitavastatin in Japanese male children with familial hypercholesterolemia. Journal of Atherosclerosis & Thrombosis 2016;23(1):48-55. [DOI: 10.5551/jat.28753] [DOI] [PubMed] [Google Scholar]
- Harada-Shiba M, Kastelein JJP, Hovingh GK, Ray KK, Ohtake A, Arisaka O, et al. Efficacy and safety of pitavastatin in children and adolescents with familial hypercholesterolemia in Japan and Europe. Journal of Atherosclerosis and Thrombosis 2018;25(5):422-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang ZQ, Wu YT, Wang R, Huang W, Chen L. Effects of pitavastatin and atorvastatin on blood lipids and blood glucose in elderly type 2 diabetic patients [Pǐ fá tātīng jí ā tuō fá tātīng duì lǎonián 2 xíng tángniàobìng huànzhě xuèzhī, xiětáng de yǐngxiǎng]. Zhongguo xin yao yu lin chuang za zhi [Chinese Journal of New Drugs and Clinical Remedies] 2012;31(10):614-7. [CENTRAL: CN-00972987] [Google Scholar]
Ikegami 2012 {published data only}
- Ikegami T, Hyogo H, Honda A, Miyazaki T, Tokushige K, Hashimoto E, et al. Increased serum liver x receptor ligand oxysterols in patients with non-alcoholic fatty liver disease. Journal of Gastroenterology 2012;47(11):1257-66. [DOI: 10.1007/s00535-012-0585-0] [DOI] [PubMed] [Google Scholar]
Kajinami 2000 {published data only}
- Kajinami K, Koizumi J, Ueda K, Miyamoto S, Takegoshi T, Mabuchi H, Hokuriku nk-104 study Group. Effects of nk-104, a new hydroxymethylglutaryl-coenzyme reductase inhibitor, on low-density lipoprotein cholesterol in heterozygous familial hypercholesterolemia. American Journal of Cardiology 2000;85(2):178-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kakuda 2013 {published data only}
- Hae Kim C, Wang S, Park JB, Jung KH, E Yoon Y, Lee SP, et al. Assessing impact of high-dose pitavastatin on carotid artery elasticity with speckle-tracking strain imaging. Journal of Atherosclerosis & Thrombosis 2018;25(11):1137-48. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda H, Kobayashi J, Nakato M, Takekoshi N. Short-term effect of pitavastatin treatment on glucose and lipid metabolism and oxidative stress in fasting and postprandial state using a test meal in Japanese men. Cholesterol 2013;2013(Article ID 314170):1-7. [DOI: 10.1155/2013/314170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee SH, Chung N, Kwan J, Kim DI, Kim WH, Kim CJ, et al. Comparison of the efficacy and tolerability of pitavastatin and atorvastatin: an 8-week, multicenter, randomized, open-label, dose-titration study in Korean patients with hypercholesterolemia. Clinical Therapeutics 2007;29(11):2365-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Lin LY, Huang CC, Chen JS, Wu TC, Leu HB, Huang PH, et al. Effects of pitavastatin versus atorvastatin on the peripheral endothelial progenitor cells and vascular endothelial growth factor in high-risk patients: a pilot prospective, double-blind, randomized study. Cardiovascular Diabetology 2014;13:111. [DOI: 10.1186/s12933-014-0111-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Lin LY, Lin HJ, Hsia CH, Hung YR, Yeh HI, et al. Pitavastatin and atorvastatin double-blind randomized comparative study among high-risk patients, including those with type 2 diabetes mellitus, in Taiwan (papago-t study). PLOS One 2013;8(10):e76298 [erratum appears in PLOS One. 2014;9(11):e114175]. [DOI: 10.1371/journal.pone.0076298] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01386853. Efficacy and safety of pitavastatin and atorvastatin in high risk hypercholesterolemic patients [A 12-week, randomized, multicenter, double-blind, active-controlled, non-inferiority study to compare the efficacy and safety of pitavastatin and atorvastatin in high risk hypercholesterolemic patients]. clinicaltrials.gov/show/NCT01386853 (first received July 2011). [NCT01386853]
Majima 2007 {published data only}
- Majima T, Shimatsu A, Komatsu Y, Satoh N, Fukao A, Ninomiya K. Short-term effects of pitavastatin on biochemical markers of bone turnover in patients with hypercholesterolemia. Internal Medicine 2007;46(24):1967-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mao 2012 {published data only}
- Mao Y, Yu JM, Zhan YQ, Hu DY, Ding RJ, Zhang F, et al. Safety and efficacy of pitavastatin in patients with hypercholesterolemia: a multicenter study [Pǐ fá tātīng zhìliáo gāo dǎngùchún xiě zhèng ānquán xìng hé yǒuxiào xìng de duō zhōngxīn guānchá]. Chung Hua I Hsueh Tsa Chih [Chinese Medical Journal (Taipei)] 2012;92(14):968-73. [MEDLINE: ] [PubMed] [Google Scholar]
- Mao Y, Yu JM, Zhang F, Hu DY, Ding RJ, Zhan YQ, et al. The effect of pitavastatin on blood glucose and its efficacy in diabetic patients with hypercholesterolemia [Pǐ fá tātīng duì tángniàobìng gāo dǎngùchún xiě zhèng huànzhě xiětáng de yǐngxiǎng jí qí liáoxiào]. Chung Hua Nei Ko Tsa Chih [Chinese Journal of Internal Medicine] 2012;51(7):508-12. [MEDLINE: ] [PubMed] [Google Scholar]
- Wang C. Clinical study on coronary heart disease. Chinese Medical Journal 2014;127(Suppl 2):49-54. [EMBASE: 2015362190] [Google Scholar]
Motomura 2009 {published data only}
- Motomura T, Okamoto M, Kitamura T, Yamamoto H, Otsuki M, Asanuma N, et al. Effects of pitavastatin on serum lipids and high sensitivity c-reactive protein in type 2 diabetic patients. Journal of Atherosclerosis & Thrombosis 2009;16(5):546-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2008 {published data only}
- Nakamura T, Obata JE, Kitta Y, Takano H, Kobayashi T, Fujioka D, et al. Rapid stabilization of vulnerable carotid plaque within 1 month of pitavastatin treatment in patients with acute coronary syndrome. Journal of Cardiovascular Pharmacology 2008;51(4):365-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NK‐104.202 2013 {published data only}
- Australian Government. Australian public assessment report for pitavastatin. Australian Government Department of Health Therapeutics Goods Administration 2013:49-52. [URL: www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf]
- Chowdhury IN, Gortler D. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary hypercholesterolaema [HEC/NK98402N/NK-104.202]. Center for Drug Evaluation and Research Application Number 22-363 Medical Reviews 2009:39-45. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
- Mahayni H, Marroum P. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary hypercholesterolaema [HEC/NK98402N/NK-104.202]. Center for Drug Evaluation and Research Application Number 22-363 Clinical Pharmacology and Biopharmaceutics Review(s) 2009;Part 2:102. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_ClinPharmR_P2.pdf]
- Min M, Lin K, Elmore C, Johnson K. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary hypercholesterolaema;[HEC/NK98402N/NK-104.202]. Center for Drug Evaluation and Research Application Number 22-363 Statistical Review(s) 2009:28. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_StatR.pdf]
NK‐104.203 2013 {published data only}
- Australian Government. Australian public assessment report for pitavastatin. Australian Government Department of Health Therapeutics Goods Administration 2013:49-52. [URL: www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf]
- Chowdhury IN, Gortler D. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary mixed or combined hyperlipidaemia [HEC/NK98402N/NK-104.203]. Center for Drug Evaluation and Research Application Number: 22-363 Medical Review(s) 2009:39-45. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
- Mahayni H, Marroum P. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4mg, and 8 mg compared to placebo in patients with primary mixed or combined hyperlipidaemia; [HEC/NK98402N/NK-104.203]. Center for Drug Evaluation and Research Application Number: 22-363 Clinical Pharmacology and Biopharmaceutics Review(s) 2009;Part 2:102. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_ClinPharmR_P2.pdf]
- Min M, Lin K, Elmore C, Johnson K. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4mg, and 8 mg compared to placebo in patients with primary mixed or combined hyperlipidaemia;[HEC/NK98402N/NK-104.203]. Center for Drug Evaluation and Research Application Number: 22-363 Statistical Review(s) 2009:28. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_StatR.pdf]
NK‐104.209 2013 {published data only}
- Australian Government. Australian public assessment report for pitavastatin. Australian Government Department of Health Therapeutics Goods Administration 2013:52-53. [URL: www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf]
- Mahayni H, Marroum P. A dose ranging study of NK-104 in patients with primary hypercholesterolema; [NK-104-209]. Center for Drug Evaluation and Research Application Number: 22-363 Clinical Pharmacology and Biopharmaceutics Review(s) 2009;Part 2:102. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_ClinPharmR_P2.pdf]
- Min M, Lin K, Elmore C, Johnson K. A dose ranging study of NK-104 in patients with primary hypercholesterolema; [NK-104-209]. Center for Drug Evaluation and Research Application Number: 22-363 Statistical Review(s) 2009:28. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_StatR.pdf]
Noji 2002 {published data only}
- Mabuchi H, Koizumi J, Kajinami K, Miyamoto S, Takegoshi T. Long-term effects of NK-104, a new HMG-CoA reductase inhibitor, in patients with heterozygous familial hypercholesterolemia. Rinsho Iyaku 2001;17(6):915-43. [Google Scholar]
- Noji Y, Higashikata T, Inazu A, Nohara A, Ueda K, Miyamoto S, et al. Long-term treatment with pitavastatin (nk-104), a new HMG-CoA reductase inhibitor, of patients with heterozygous familial hypercholesterolemia. Atherosclerosis 2002;163(1):157-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nozue 2008 {published data only}
- Nozue T, Michishita I, Ito Y, Hirano T. Effects of statin on small dense low-density lipoprotein cholesterol and remnant-like particle cholesterol in heterozygous familial hypercholesterolemia. Journal of Atherosclerosis & Thrombosis 2008;15(3):146-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ohbayashi 2009 {published data only}
- Ohbayashi H, Miyazawa C, Miyamoto K, Sagara M, Yamashita T, Onda R. Pitavastatin improves plasma pentraxin 3 and arterial stiffness in atherosclerotic patients with hypercholesterolemia. Journal of Atherosclerosis & Thrombosis 2009;16(4):490-500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ose 2009 {published data only}
- Center for Drug Evaluation and Research. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin 2 mg vs. simvastatin 20 mg and pitavastatin 4 mg (following up-titration) in subjects with primary hypercholesterolemia or combined dyslipidemia [NK-104-302]]. FDA Open Trials/show/NK-104-302 (first received 1 October 2008). [www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
- EUCTR2005-001033-15. Study of pitavastatin 2 mg vs. simvastatin 20 mg and pitavastatin 4 mg vs. simvastatin 40 mg (following up-titration) in patients with primary hypercholesterolemia or combined dyslipidemia. EU Clinical Trials Register/show/EUCTR2005-001033-15 (first received 1 August 2005). [EUCTR2005-001033-15]
- NCT00309777. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin vs. simvastatin (following up-titration) in patients with primary hypercholesterolemia or combined dyslipidemia]. clinicaltrials.gov/show/NCT00309777 (first received 1 July 2011). [NCT00309777]
- Ose L, Budinski D, Hounslow N, Arneson V. Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Current Medical Research and Opinion 2009;25(11):2755-64 [erratum appears in Current Medical Research and Opinion. 2010;26(5):1046 Note: dosage error in article text]. [DOI: 10.1185/03007990903290886] [DOI] [PubMed] [Google Scholar]
Park 2005 {published data only}
- Park S, Kang HJ, Rim SJ, Ha JW, Oh BH, Chung N, et al. A randomized, open-label study to evaluate the efficacy and safety of pitavastatin compared with simvastatin in Korean patients with hypercholesterolemia. Clinical Therapeutics 2005;27(7):1074-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PREVAIL‐US 2016 {published data only}
- Kryzhanovski V, Morgan R, Sponseller C, Davidson M. Pitavastatin 4 mg provides significantly greater reduction in LDL-C compared to pravastatin 40 mg with neutral effects on glucose metabolism: prespecified safety analysis from the short-term phase 4 prevail US trial in patients with primary hyperlipidemia or mixed dyslipidemia. Journal of the American College of Cardiology 2012;59(13 Suppl 1):E1692. [EMBASE: 70715132] [Google Scholar]
- Miller PE, Martin SS, Joshi PH, Jones SR, Massaro JM, D'Agostino RB, et al. Pitavastatin 4 mg provides significantly greater reduction in remnant lipoprotein cholesterol compared with pravastatin 40 mg: results from the short-term phase IV Prevail-US trial in patients with primary hyperlipidemia or mixed dyslipidemia. Clinical Therapeutics 2016;38(3):603-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morgan R, Campbell SE, Kryzhanovski VA, Yu CY, Sponseller CA, Davidson MH. Pitavastatin 4 mg is superior to pravastatin 40 mg in LDL-C reduction: results from Prevail US trial in primary hyperlipidemia or mixed dyslipidemia. Journal of Clinical Lipidology 2012;6(3):280-2. [EMBASE: 70819817] [Google Scholar]
- NCT01256476. Prevail-US: a study of pitavastatin 4 mg vs. pravastatin 40 mg in patients with primary hyperlipidemia or mixed dyslipidemia (Prevail-US) [A randomized, double-blind, active controlled, parallel group study of pitavastatin 4 mg vs. pravastatin 40 mg in patients with primary hyperlipidemia or mixed dyslipidemia]. clinicaltrials.gov/show/NCT01256476 (first received 8 December 2010). [NCT01256476]
- Sponseller CA, Morgan RE, Campbell SE, Yu CY, Davidson MH. Pitavastatin 4 mg significantly reduces LDL-P and increases HDL size compared with pravastatin 40 mg: results from Prevail US. Journal of Clinical Lipidology 2012;6(3):288-9. [EMBASE: 70819827] [Google Scholar]
- Sponseller CA, Morgan RE, Kryzhanovski VA, Campbell SE, Davidson MH. Comparison of the lipid-lowering effects of pitavastatin 4 mg versus pravastatin 40 mg in adults with primary hyperlipidemia or mixed (combined) dyslipidemia: a phase IV, prospective, US, multicenter, randomized, double-blind, superiority trial. Clinical Therapeutics 2014;36(8):1211-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Toth P, Martin SS, Joshi P, Jones S, Massaro J, D'Agostino R, et al. Pitavastatin 4 mg provides significantly greater reduction in remnant lipoprotein cholesterol compared to pravastatin 40 mg: results from the Prevail trial. Atherosclerosis 2014;235(2):e261-2. [EMBASE: 71752224] [DOI] [PubMed] [Google Scholar]
Saito 2002a {published data only}
- Saito Y, Teramoto T, Yamada N, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy of nk-104 (pitavastatin), a new synthetic HMG-CoA reductase inhibitor, in the dose finding, double blind, three-group comparative study [Yōryō hakken, nijūmōken, 3-gun hikaku shiken ni okeru atarashī gōsei HMG - CoA kangen kōso sogai-zaidearu nk - 104 (pitabasutachin) no rinshō kōka]. Rynshouiyaku 2001;17(6):829-55. [Google Scholar]
- Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy of pitavastatin, a new 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, in patients with hyperlipidemia. Dose-finding study using the double-blind, three-group parallel comparison. Arzneimittelforschung 2002;52(4):251-5. [DOI: 10.1055/s-0031-1299888] [DOI] [PubMed] [Google Scholar]
Saito 2002b {published data only}
- Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, et al. A randomized, double-blind trial comparing the efficacy and safety of pitavastatin versus pravastatin in patients with primary hypercholesterolemia. Atherosclerosis 2002;162(2):373-9 [erratum appears in Atherosclerosis. 2003 Jun;168(2):401]. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sakabe 2008 {published data only}
- Sakabe K, Fukuda N, Fukuda Y, Wakayama K, Nada T, Morishita S, et al. Comparisons of short- and intermediate-term effects of pitavastatin versus atorvastatin on lipid profiles, fibrinolytic parameter, and endothelial function. International Journal of Cardiology 2008;125(1):136-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sansanayudh 2010 {published data only}
- Sansanayudh N, Wongwiwatthananukit S, Putwai P, Dhumma-Upakorn R. Comparative efficacy and safety of low-dose pitavastatin versus atorvastatin in patients with hypercholesterolemia. Annals of Pharmacotherapy 2010;44(3):415-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasaki 2008 {published data only}
- Sasaki J, Ikeda Y, Kuribayashi T, Kajiwara K, Biro S, Yamamoto K, et al. A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance. Clinical Therapeutics 2008;30(6):1089-101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shimabukuro 2011 {published data only}
- Shimabukuro M, Higa M, Tanaka H, Shimabukuro T, Yamakawa K, Masuzaki H. Distinct effects of pitavastatin and atorvastatin on lipoprotein subclasses in patients with type 2 diabetes mellitus. Diabetic Medicine 2011;28(7):856-64. [DOI: 10.1111/j.1464-5491.2011.03240.x] [DOI] [PubMed] [Google Scholar]
Sone 2002 {published data only}
- Sone H, Takahashi A, Shimano H, Ishibashi S, Yoshino G, Morisaki N, et al. HMG-CoA reductase inhibitor decreases small dense low-density lipoprotein and remnant-like particle cholesterol in patients with type-2 diabetes. Life Sciences 2002;71(20):2403-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stender 2013 {published data only}
- Center for Drug Evaluation and Research. Study to compare the efficacy and safety of pitavastatin and pravastatin in elderly patients [Study of pitavastatin 1 mg vs. pravastatin 10 mg, pitavastatin 2 mg vs. pravastatin 20 mg and pitavastatin 4 mg vs pravastatin 40 mg ( following up-titration) in elderly subjects with primary hypercholesterolemia or combined dyslipidemia [NK-104-306]]. FDA Open Trials/show/NK-104-306 (first received 1 October 2008). [www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
- EUCTR2005 001039 31. Study to compare the efficacy and safety of pitavastatin and pravastatin in elderly patients [Study of pitavastatin 1 mg vs pravastatin 10 mg, pitavastatin 2 mg vs. pravastatin 20 mg and pitavastatin 4 mg vs. pravastatin (following up-titration) in elderly patients with primary hypercholesterolemia or combined dyslipidemia]. EU Clinical Trials Register/show/EUCTR2005 001039 31 (first received 8 November 2005). [regroup-production.s3.amazonaws.com/documents/ReviewReference/45536664/EUCTR2005-001039-31%20%20%20NK-104-306.pdf?AWSAccessKeyId=AKIAJBZQODCMKJA4H7DA&Expires=1546977580&Signature=SUINaIvFS8nPa9srlEYWrB25ytc%3D]
- NCT00257686. Study to compare the efficacy and safety of pitavastatin and pravastatin in elderly patients [Study Of pitavastatin 1 mg vs. pravastatin 10 mg, pitavastatin 2 mg vs. pravastatin 20 mg and pitavastatin 4 mg vs. pravastatin 40 mg ( following up-titration) in elderly patients with primary hypercholesterolemia or combined dyslipidemia]. clinicaltrials.gov/show/NCT00257686 (first received 23 November 2005). [NCT00257686]
- Stender S, Budinski D, Gosho M, Hounslow N. Pitavastatin shows greater lipid-lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolaemia or combined (mixed) dyslipidaemia. European Journal of Preventive Cardiology 2013;20(1):40-53. [DOI: 10.1177/2047487312451251] [DOI] [PubMed] [Google Scholar]
Suzuki 2009 {published data only}
- Suzuki S, Hattori H, Kato K, Kanemaki K, Kimura A, Haga M, et al. Clinical results of combination therapy with pitavastatin calcium and dextran sulfate sodium in patients with mixed hyperlipidemia [Kongō kōshikesshō kanja ni okeru pitabasutachinkarushiumu to ryūsan dekisutoran'natoriumu no heiyō ryōhō no rinshō kekka]. Japanese Pharmacology and Therapeutics 2009;37(7):587-96. [EMBASE: 2009464427] [Google Scholar]
Tateishi 2011 {published data only}
- Tateishi J. Efficacy of three potent statins in patients with hypercholesterolemia who were newly prescribed statins [Atarashiku shohō sa reta sutachindeatta kō koresuterōru kesshō kanja ni okeru 3 ttsu no kyōryokuna sutachin no yūkōsei]. Therapeutic Research 2011;32(12):1653-61. [EMBASE: 2012051834] [Google Scholar]
Teramoto 2001 {published data only}
- Teramoto T, Saito Y, Yamada N, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy and safety of NK-104 (pitavastatin), a new synthetic HMG-CoA reductase inhibitor, in the long-term treatment of hyperlipidemia [Kōshikesshō no chōki chiryō ni okeru atarashī gōsei HMG - CoA redakutāze sogai-zaidearu nk - 104 (pitabasutachin) no rinshō kōka to anzen-sei]. Rinsho Iyaku 2001;17(6):885-913. [Google Scholar]
Uzui 2014 {published data only}
- Uzui H, Hayashi H, Nakae I, Matsumoto T, Uenishi H, Hayasaki H, et al. Pitavastatin decreases serum lox-1 ligand levels and mt1-mmp expression in cd14-positive mononuclear cells in hypercholesterolemic patients. International Journal of Cardiology 2014;176(3):1230-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamasaki 2014 {published data only}
- Yamasaki T, Iwashima Y, Jesmin S, Ohta Y, Kusunoki H, Hayashi S, et al. Comparison of efficacy of intensive versus mild pitavastatin therapy on lipid and inflammation biomarkers in hypertensive patients with dyslipidemia. PLOS One 2014;9(2):e89057. [DOI: 10.1371/journal.pone.0089057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yanagi 2011 {published data only}
- Yanagi K, Monden T, Ikeda S, Matsumura M, Kasai K. A crossover study of rosuvastatin and pitavastatin in patients with type 2 diabetes. Advances in Therapy 2011;28(2):160-71. [DOI: 10.1007/s12325-010-0098-2] [DOI] [PubMed] [Google Scholar]
Yokote 2008 {published data only}
- Yokote K, Bujo H, Hanaoka H, Shinomiya M, Mikami K, Miyashita Y, et al. Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients: collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (Chiba study). Atherosclerosis 2008;201(2):345-52. [DOI: 10.1016/j.atherosclerosis.2008.02.008] [DOI] [PubMed] [Google Scholar]
- Yokote K, Saito Y. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (Chiba study). Journal of Atherosclerosis & Thrombosis 2009;16(3):297-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshida 2010 {published data only}
- Yoshida O, Kondo T, Kureishi-Bando Y, Sugiura T, Maeda K, Okumura K, et al. Pitavastatin, an HMG-CoA reductase inhibitor, ameliorates endothelial function in chronic smokers. Circulation Journal 2010;74(1):195-202 [erratum appears in Circ J. 2010 Feb;74(2):386]. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshida 2013 {published data only}
- JPRN UMIN000001783. Value of oxidant lipid lowering effect by statin intervention in hypercholesterolemia [Value of oxidant lipid lowering effect by statin intervention in hypercholesterolemia]. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN UMIN000001783 (first received 25 March 2009). [JPRN UMIN000001783]
- Yoshida H, Shoda T, Yanai H, Ikewaki K, Kurata H, Ito K, et al. Effects of pitavastatin and atorvastatin on lipoprotein oxidation biomarkers in patients with dyslipidemia. Atherosclerosis 2013;226(1):161-4. [DOI: 10.1016/j.atherosclerosis.2012.10.069] [DOI] [PubMed] [Google Scholar]
Yoshika 2010 {published data only}
- Yoshika M, Komiyama Y, Masuda M, Yokoi T, Masaki H, Ohkura H, et al. Pitavastatin further decreases serum high-sensitive c-reactive protein levels in hypertensive patients with hypercholesterolemia treated with angiotensin II, type-1 receptor antagonists. Clinical & Experimental Hypertension (New York) 2010;32(6):341-6. [DOI: 10.3109/10641961003628460] [DOI] [PubMed] [Google Scholar]
Yoshitomi 2006 {published data only}
- Yoshitomi Y, Ishii T, Kaneki M, Tsujibayashi T, Sakurai S, Nagakura C, et al. Efficacy of a low dose of pitavastatin compared with atorvastatin in primary hyperlipidemia: results of a 12-week, open label study. Journal of Atherosclerosis & Thrombosis 2006;13(2):108-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aberg 2017 {published data only}
- Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (intrepid): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017;4(7):e284-94 Correction: (S2352301817300759), (10.1016/S2352-3018(17)30075-9)). [DOI: 10.1016/S2352-3018(17)30075-9] [DOI] [PubMed] [Google Scholar]
- NCT01301066. A 12-week study comparing pitavastatin 4 mg vs. pravastatin 40 mg in HIV-infected subjects [A 12-week, randomized, double-blind, active-controlled, parallel-group study comparing pitavastatin 4 mg vs. pravastatin 40 mg in HIV-infected subjects with dyslipidemia, followed by a 40-week safety extension study]. clinicaltrials.gov/show/NCT01301066 (first received December 2010). [NCT01301066]
- Sponseller CA, Morgan R, Campbell S, Kryzhanovski BV, Kartman C, Aberg J, et al. Pitavastatin 4 mg provides superior LDL-C reduction vs. pravastatin 40 mg over 12 weeks in HIV-infected adults with dyslipidemia, the Intrepid trial. Journal of Clinical Lipidology 2013;7(3):260. [EMBASE: 71086780] [Google Scholar]
- Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. Aids 2017;31(6):797-806. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI201305003686 2013 {published data only}
- CTRI/2013/05/003686. Post marketing surveillance study of pitavastatin for evaluation of its safety and efficacy in patients with diabetes and high cholesterol [A post marketing surveillance study to assess the efficacy and safety of pivasta (pitavastatin) tablets in patients of type 2 diabetes mellitus and dyslipidemia]. Clinical Trials Registry - India/show/CTRI/2013/05/003686 (first received 27 May 2013). [CTRI/2013/05/003686]
CTRI201307003842 2013 {published data only}
- CTRI/2013/07/003842. To compare the effectiveness of two cholesterol lowering drugs in patients with high cholesterol [Efficacy of pitavastatin vs rosuvastatin in hypercholesterolemic patients - an open labeled study]. Clinical Trials Registry - India/show/CTRI/2013/07/003842 (first received 26 July 2013). [CTRI/2013/07/003842]
CTRI201309004003 2013 {published data only}
- CTRI/2013/09/004003. Evaluation of pitavastatin for efficacy and safety in dyslipidemic patients: a comparative randomized controlled trial [A prospective, controlled, randomized, double blind, comparative, parallel, 2-arm study to evaluate the efficacy and safety of pitavastatin (4 mg) vs. atorvastatin (20 mg) in dyslipidemic patients associated with hypertension, diabetes and coronary artery disease]. Clinical Trials Registry - India/show/CTRI/2013/09/004003 (first received 19 September 2013). [CTRI/2013/09/004003]
Horiuchi 2010 {published data only}
- Horiuchi Y, Hirayama S, Soda S, Seino U, Kon M, Ueno T, et al. Statin therapy reduces inflammatory markers in hypercholesterolemic patients with high baseline levels. Journal of Atherosclerosis and Thrombosis 2010;17(7):722-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2016 {published data only}
- Huang CH, Huang YY, Hsu BR. Pitavastatin improves glycated hemoglobin in patients with poorly controlled type 2 diabetes. Journal of Diabetes Investigation 2016;7(5):769-76. [DOI: 10.1111/jdi.12483] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyogo 2011 {published data only}
- Hyogo H, Ikegami T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, et al. Efficacy of pitavastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatology Research 2011;41(11):1057-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Inami 2007 {published data only}
- Inami N, Nomura S, Shouzu A, Omoto S, Kimura Y, Takahashi N, et al. Effects of pitavastatin on adiponectin in patients with hyperlipidemia. Pathophysiology of Haemostasis & Thrombosis 2007;36(1):1-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jing 2008 {published data only}
- Jing S, Sun NL, Wang HY, Lu XN. Efficacy and safety of pitavastatin calcium tablets in the treatment of primary hypercholesterolemia [Pǐ fá tātīng gài piàn zhìliáo yuán fā xìng gāo dǎngùchún xiě zhèng liáoxiào hé ānquán xìng 匹伐他汀钙片治疗原发性高胆固醇血症疗效和安全性]. Chinese Journal of New Drugs 2008;17(9):775-7+86. [EMBASE: 2010307143] [Google Scholar]
Joshi 2017 {published data only}
- Joshi PH, Miller PE, Martin SS, Jones SR, Massaro JM, D'Agostino RB, et al. Greater remnant lipoprotein cholesterol reduction with pitavastatin compared with pravastatin in HIV-infected patients. AIDS 2017;31(7):965-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
JPRN JMA IIA00056 2011 {published data only}
- JPRN-JMA-IIA00056. The effect of pitavastatin on the lipoprotein subfraction profile and lipid metabolism. JMACCT Clinical Trials Registry/show/PRN-JMA-IIA00056 (first received 27 January 2011). [JPRN-JMA-IIA00056]
JPRN UMIN000000685 2007 {published data only}
- JPRN-UMIN000000685. Stanford type B aortic dissection patients with normally hypotensive medical therapy and pitavastatin treatment effect. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000000685 (first received 1 June 2007). [JPRN-UMIN000000685]
JPRN UMIN000001600 2009 {published data only}
- JPRN-UMIN000001600. Effect of pitavastatin on amelioration for renal function in patients with chronic kidney disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000000685 (first received 1 June 2007). [URL: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000001922]
JPRN UMIN000002507 2009 {published data only}
- JPRN-UMIN000002507. Effects of aggressive lipid-lowering therapy on atherosclerosis and multi-lipoprotein profiling in patients with coronary artery disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002507 (first received 1 October 2009). [JPRN-UMIN000002507]
JPRN UMIN000002680 2009 {published data only}
- JPRN-UMIN000002680. Randomized evaluation of aggressive or moderate lipid lowering therapy with pitavastatin in coronary artery disease (Real-Cad). UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002680 (first received 27 October 2009). [JPRN-UMIN000002680]
JPRN UMIN000003554 2010 {published data only}
- JPRN-UMIN000003554. Comparison of effects of pitavastatin and atorvastatin on glucose and lipid metabolism in diabetic patients with hyperlipidemia. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000003554 (first received 1 May 2010). [JPRN-UMIN000003554]
JPRN UMIN000003628 2010 {published data only}
- JPRN-UMIN000003628. Toyama anti-arteriosclerosis and plaque assessment trial by aggressive lipid lowering therapy with pitavastatin in peripheral artery disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000003628 (first received 1 June 2010). [JPRN-UMIN000003628]
JPRN UMIN000005489 2011 {published data only}
- JPRN-UMIN000005489. Effect of pitavastatin on the coronary endothelial dysfunction after PCI with DES (drug-eluting stent). UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000005489 (first received 22 April 2011). [JPRN-UMIN000005489]
JPRN UMIN000005501 2011 {published data only}
- JPRN-UMIN000005501. Effect of pitavastatin on restenosis with carotid artery stenting. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000005501 (first received 25 April 2011). [JPRN-UMIN000005501]
JPRN UMIN000007130 2012 {published data only}
- JPRN-UMIN000007130. A multicenter randomized study evaluating the efficacy and safety of HMG-CoA reductase inhibitors (statins) in dyslipidemic patients with nephrosclerosis. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000007130 (first received 24 January 2012). [JPRN-UMIN000007130]
JPRN UMIN000007695 2012 {published data only}
- JPRN-UMIN000007695. Comparison of effects of pitavastatin and rosuvastatin on patients with chronic heart failure. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000007695 (first received 10 April 2012). [URL: JPRN-UMIN000007695]
JPRN UMIN000009241 2012 {published data only}
- JPRN-UMIN000009241. The comparison of the effects of rosuvastatin 5 mg and pitavastatin 2 mg on hypertensive patients. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000009241 (first received 1 November 2012). [JPRN-UMIN000009241]
JPRN UMIN000013384 2014 {published data only}
- JPRN-UMIN000013384. Effect of pitavastatin on high density lipoprotein cholesterol levels in hyperlipidemia. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000013384 (first received 10 March 2014). [JPRN-UMIN000013384]
JPRN UMIN000019020 2015 {published data only}
- JPRN-UMIN000019020. Investigation of lipid-improving and pleiotrophic effects of pitavastatin. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000019020 (first received 15 September 2015). [URL: JPRN-UMIN000019020]
Kawashiri 2008 {published data only}
- Kawashiri MA, Nohara A, Tada H, Mori M, Tsuchida M, Katsuda S, et al. Comparison of effects of pitavastatin and atorvastatin on plasma coenzyme q10 in heterozygous familial hypercholesterolemia: results from a crossover study. Clinical Pharmacology & Therapeutics 2008;83(5):731-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsubara 2012 {published data only}
- Matsubara T, Naruse K, Arakawa T, Nakao M, Yokoi K, Oguri M, et al. Impact of pitavastatin on high-sensitivity c-reactive protein and adiponectin in hypercholesterolemic patients with the metabolic syndrome: the Premium study. Journal of Cardiology 2012;60(5):389-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- NCT00444717. Impact of pitavastatin in hypercholesterolemic patients with metabolic syndrome [Multicenter study for anti-oxidative and anti-inflammatory effects of pitavastatin in hypercholesterolemic patients with metabolic syndrome]. clinicaltrials.gov/show/NCT00444717 (first received 8 March 2007). [NCT00444717]
Minai 2008 {published data only}
- Minai K, Inoue Y, Miyata S, Nakae S, Azuma Y, Uehara Y, et al. Efficacy and safety of pitavastatin, HMG-CoA reductase inhibitor, in patients of coronary heart disease with hyperlipidemia. Therapeutic Research 2008;29(9):1611-9. [EMBASE: 2008509864] [Google Scholar]
Muto 2013 {published data only}
- Muto E. Pitavastatin ameliorates endothelial function in diabetics. Japanese Pharmacology and Therapeutics 2013;41(7):685-8. [EMBASE: 2013528826] [Google Scholar]
Nakamura 2006 {published data only}
- Nakamura T, Sugaya T, Kawagoe Y, Suzuki T, Inoue T, Node K. Effect of pitavastatin on urinary liver-type fatty-acid-binding protein in patients with nondiabetic mild chronic kidney disease. American Journal of Nephrology 2006;26(1):82-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakaya 2001 {published data only}
- Nakaya N, Saito Y, Morisaki N, Tamura K, Shirai K, Shinomiya M, et al. Phase II clinical study of NK-104 (pitavastatin) in patients with hyperlipidemia. Rinsho Iyaku 2001;17(6):789-806. [Google Scholar]
NCT02670434 2016 {published data only}
- NCT02670434. Safety and efficacy comparison study of NK-104-CR (controlled release) in patients with primary hyperlipidemia or mixed dyslipidemia. clinicaltrials.gov/show/NCT02670434 (first received 1 February 2016). [NCT02670434]
NCT02799758 2016 {published data only}
- NCT02799758. Efficacy & long-term safety comparison study of NK-104-CR & livalo® IR with primary hyperlipidemia or mixed dyslipidemia. clinicaltrials.gov/show/NCT02799758 (first received 15 June 2016). [NCT02799758]
Nishiguchi 2018 {published data only}
- JPRN-UMIN000002526. Effects of statin on renal function in patients with chronic kidney disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002526 (first received 18 September 2009). [JPRN-UMIN000002526]
- JPRN-UMIN000002678. Effect of pitavastatin on coronary fibrous-cap thickness - assessment by fourier-domain optical coherence tomography (Escort). UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002678 (first received 1 November 2009). [JPRN-UMIN000002678]
- Nishiguchi T, Kubo T, Tanimoto T, Ino Y, Matsuo Y, Yamano T, et al. Effect of early pitavastatin therapy on coronary fibrous-cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome: the Escort study. Journal of the American College of Cardiology: Cardiovascular Imaging 2018;11(6):829-38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nomura 2008 {published data only}
- Nomura S, Shouzu A, Omoto S, Inami N, Tanaka A, Nanba M, et al. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thrombosis Research 2008;122(1):39-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nozue 2015 {published data only}
- Nozue T, Michishita I. Statin treatment alters serum n-3 to n-6 polyunsaturated fatty acids ratio in patients with dyslipidemia. Lipids in Health & Disease 2015;14:67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rui 2014 {published data only}
- Rui Y, Youyou D, Yanzhou Z, Qinghua C, Luosha Z, Ling L. Comparison of clinical efficacy of different statins on cardiovascular events following percutaneous coronary intervention. Experimental and Clinical Cardiology 2014;20(8):2598-614. [EMBASE: 2014538748] [Google Scholar]
Watanabe 2015 {published data only}
- Watanabe E, Yamaguchi J, Arashi H, Ogawa H, Hagiwara N. Effects of statin versus the combination of ezetimibe plus statin on serum lipid absorption markers in patients with acute coronary syndrome. Journal of Lipids 2015;2015(Article ID 109158):1-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wongprikorn 2016 {published data only}
- NCT02442700. Effects of pitavastatin on lipid profiles in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir [Effects of pitavastatin on lipid profiles in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir: a randomized, double-blind, crossover study]. clinicaltrials.gov/show/NCT02442700 (first received 13 May 2015). [NCT02442700]
- Wongprikorn A, Sukasem C, Puangpetch A, Numthavej P, Thakkinstian A, Kiertiburanakul S. Effects of pitavastatin on lipid profiles in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir: a randomized, double-blind, crossover study. PLOS One [Electronic Resource] 2016;11(6):e0157531. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yagi 2011 {published data only}
- Yagi S, Akaike M, Aihara K, Iwase T, Ishikawa K, Yoshida S, et al. Effect of low-dose (1 mg/day) pitavastatin on left ventricular diastolic function and albuminuria in patients with hyperlipidemia. American Journal of Cardiology 2011;107(11):1644-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yao 2016 {published data only}
- Yao R, Du Y, Zhang Y, Chen Q, Zhao L, Li L. Comparison of clinical efficacy of different statins on cardiovascular events following percutaneous coronary intervention. International Journal of Clinical and Experimental Medicine 2016;9(2):4356-63. [EMBASE: 20160256110] [Google Scholar]
Zhu 2010 {published data only}
- Zhu H, Li D, Xia Y, Zhang Y, Xu T, Pan D, et al. Therapeutic effects of domestic pitavastatin calcium tablets and atorvastatin calcium tablets on hypercholesterolemia. Xuzhou Yixueyuan Xuebao 2010;30(11):719-21. [Google Scholar]
References to studies awaiting assessment
JPRN UMIN000003055 2010 {published data only}
- JPRN-UMIN000003055. Effectiveness of ezetimibe or pitavastatin calcium calcium in Nash/Nafld patients with high cholesterol levels: a randomized controlled study. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000003055 (first received 18 January 2010). [JPRN-UMIN000003055]
References to ongoing studies
KCT0001730 2015 {published data only}
- Clinical Research Information Service. Effects of lipid lowering on endothelial function [Effects of lipid lowering on endothelial function: statin treatment for atherosclerotic vascular disease (STA study)]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=5808 (first received 8 December 2015). [KCT0001730 ]
NCT01402843 2011 {published data only}
- NCT01402843. Efficacy, safety of coadministered pitavastatin and valsartan in patients with hypertension and dyslipidemia (Coctail) [A randomized, double blind, double dummy, placebo controlled phase III trial to evaluate the efficacy, safety of coadministered pitavastatin and valsartan in patients with hypertension and dyslipidemia (Coctail study)]. clinicaltrials.gov/show/NCT01402843 (first received 26 July 2011). [NCT01402843]
NCT01710007 2011 {published data only}
- NCT01710007. Efficacy and safety study of pitavastatin for hypercholesterolemia [A prospective, double-blind, randomized, parallel, multiple-center study to compare the efficacy and safety of 1PC002 and atorvastatin in Taiwanese patients with hypercholesterolemia]. clinicaltrials.gov/show/NCT01710007 (first received 18 October 2012). [NCT01710007]
Additional references
Adams 2014
- Adams SP, Sekhon SS, Wright JM. Rosuvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010254.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2015
- Adams SP, Tsang M, Wright JM. Atorvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD008226.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2016
- Adams SP, Sekhon SS, Wright JM, Tsang M. Fluvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2017
- Adams SP, Tiellet N, Wright JM. Cerivastatin for lowering lipids. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012501] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2018
- Adams SP, Sekhon SS, Wright JM, Tsang M. Fluvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012282.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2020
- Adams SP, Tiellet N, Alaeiilkhchi N, Wright JM. Cerivastatin for lowering lipids. Cochrane Database of Systematic Reviews 2020, Issue 1. [DOI: 10.1002/14651858.CD012501.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Government 2013
- Department of Health Therapeutic Goods Administration. Australian public assessment report for pitavastatin. www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf (accessed prior to 30 May 2020).
Bandolier 2004
- Bandolier. Cholesterol lowering with statins. Bandolier 2004:121-2.
Boekholdt 2012
- Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosaJC, Nestel PJ, et al. Association of ldl cholesterol, non-hdl cholesterol, and apolipoprotein b levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA 2012;307(12):1302–9. [DOI] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention (CDC). Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors - United States, 2011. MMWR - Morbidity & Mortality Weekly Report 2011;60(36):1248-51. [MEDLINE: ] [PubMed] [Google Scholar]
Chowdhury 2009
- Chowdhury IN, Gortler D. Center for Drug Evaluation and Research application number: 22-363 Medical Review(s). assets.documentcloud.org/documents/3199266/NDA022363-0-Approval-Medical-Review.pdf (accessed prior to 30 May 2020).
Covidence 2019 [Computer program]
- Veritas Health Innovation Covidence. Version accessed 1 August 2017. Melbourne, Australia: Veritas Health Innovation, 2019.Available at covidence.org.
CTT 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al, Cholesterol Treatment Trialists (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267-78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edwards 2003
- Edwards JE, Moore RA. Statins in hypercholesterolaemia: a dose-specific meta-analysis of lipid changes in randomised, double blind trials. BMC Family Practice 2003;4:18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaw 2000
- Gaw A, Packard CJ, Shepherd J. Statins: the HMG CoA Reductase Inhibitors in Perspective. London, England: Martin Dunitz Ltd, 2000. [1853174688] [Google Scholar]
GraphPad Prism 4 [Computer program]
- GraphPad Software Inc. GraphPad Prism 4. Version 4.0. La Jolia: GraphPad Software Inc., 2003.
Grundy 2004
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Journal of the American College of Cardiology 2004;44(3):720–32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kellick 1997
- Kellick KA, Burns K, McAndrew E, Haberl E, Hook N, Ellis AK. Focus on atorvastatin: an HMG-CoA reductase inhibitor for lowering both elevated ldl cholesterol and triglycerides in hypercholesterolemic patients. Formulary 1997;32(4):352-63. [EMBASE: 1997129035] [Google Scholar]
Kreatsoulas 2010
- Kreatsoulas C, Anand SS. The impact of social determinants on cardiovascular disease. Canadian Journal of Cardiology 2010;26(Suppl C):8C–13C. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2003
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326(7404):1423. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2005
- Liao JK, Laufs U. Pleiotropic effects of statins. Annual Review of Pharmacology and Toxicology 2005;45:89-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moghadasian 1999
- Moghadasian MH. Clinical pharmacology of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Life Sciences 1999;65(13):1329–37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukhtar 2005
- Mukhtar RYA, Reid J, Reckless JPD. Pitavastatin. International Journal of Clinical Practice 2005;59(2):239-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
RevMan 2020 [Computer program]
- Nordic Cochrane Centre, the Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, the Cochrane Collaboration, 2020.
Roger 2011
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics - 2011 update: a report from the American Heart Association. Circulation 2011;123(4):e18–e209. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaefer 2004
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schectman 1996
- Schectman G, Hiatt J. Dose-response characteristics of cholesterol-lowering drug therapies: implications for treatment. Annals of Internal Medicine 1996;125(12):990-1000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2019a
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Schünemann 2019b
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Teramoto 2009
- Teramoto T, Shimano H, Yokote K, Urashima M. Effects of pitavastatin (livalo tablet) on high density lipoprotein cholesterol (hdl-c) in hypercholesterolemia. Journal of Atherosclerosis and Thrombosis 2009;16(5):654-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thompson 2005
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, et al. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics Journal 2005;5(6):352-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsang 2002
- Tsang MB, Adams SP, Jauca C, Wright JM. In some systematic reviews placebos may not be necessary: an example from a statin dose-response study. In: 10th Cochrane Colloquium; 2002 31 July - August 2002; Stavanger, Norway. Vol. Abstracts. Stavanger, 2002:Poster 29.
Ward 2007
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment (Winchester, England) 2007;11(14):1-160, iii-iv. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


