Abstract
Our purpose was to classify acute invasive fungal rhinosinusitis (AIFR) caused by Mucor versus Aspergillus species by evaluating computed tomography radiological findings. Two blinded readers retrospectively graded radiological abnormalities of the craniofacial region observed on craniofacial CT examinations obtained during initial evaluation of 38 patients with eventually pathology-proven AIFR (13:25, Mucor:Aspergillus). Binomial logistic regression was used to analyze correlation between variables and type of fungi. Score-based models were implemented for analyzing differences in laterality of findings, including the ‘unilateral presence’ and ‘bilateral mean’ models. Binary logistic regression was used, with Score as the only predictor and Group (Mucor vs Aspergillus) as the only outcome. Specificity, sensitivity, positive predictive value, negative predictive value and accuracy were determined for the evaluated models. Given the low predictive value of any single evaluated anatomical site, a ‘bilateral mean’ score-based model including the nasal cavity, maxillary sinuses, ethmoid air cells, sphenoid sinus and frontal sinuses yielded the highest prediction accuracy, with Mucor induced AIFR correlating with higher prevalence of bilateral findings. The odds ratio for the model while integrating the above anatomical sites was 12.3 (p < 0.001). PPV, NPV, sensitivity, specificity and accuracy were 0.85, 0.82, 0.92, 0.69 and 0.84 respectively. The abnormal radiological findings on craniofacial CT scans of Mucor and Aspergillus induced AIFR could be differentiated based on laterality, with Mucor induced AIFR associated with higher prevalence of bilateral findings.
Subject terms: Microbiology, Oncology, Risk factors
Introduction
Acute invasive fungal rhinosinusitis (AIFR) is a rapidly progressive and life-threatening infection involving the nasal cavity and paranasal sinuses1–3. Patients with early stage AIFR limited to the nasal cavity and paranasal sinuses, have relatively lower mortality rates2, while intracranial extension doubles the mortality4. While a variety of causative organisms have been identified, Aspergillus and Mucor fungal species are predominant3. The most commonly predisposing conditions involve immunodeficiency and include hematologic malignancies, poorly controlled diabetes mellitus, chemotherapy or immunosuppression due to hematopoietic stem cells or organ transplantation5,6. Even though AIFR is a rare disease, its high mortality rate of approximately 50%7 highlights the importance of an appropriate and early diagnosis followed by aggressive treatment utilizing a combination of surgical debridement, antifungal pharmacotherapy and restoration of the patient's immune system when possible8. In many cases, AIFR is a manifestation of an overall poor prognosis with mortality attributed to the underlying medical condition3.
Early diagnosis and treatment of AIFR is of paramount importance to reduce patient morbidity and mortality. Effective treatment consists of an early and aggressive debridement of the necrotic tissue to decrease the fungal load and reduce impediments (e.g. vascular thrombosis) to antifungal delivery to remaining viable tissue, along with antifungal therapy and reconstitution of the patient's immune system9–17. Due to the importance of rapid treatment initiation, empiric antifungal pharmacotherapy might be initiated when AIFR is suspected, and will be further modified, as needed, according to pathology results.
Craniofacial computed tomography (CT) is a valuable tool in the early evaluation and for surgical planning for patients with AIFR despite its low sensitivity and specificity18–20. In many cases, imaging is obtained prior to the consultation with an otolaryngologist and nasal endoscopy. The most common CT findings of early AIFR include sinonasal mucosal thickening, air/fluid levels, soft-tissue infiltration of the maxillary periantral fat planes, infiltration of the middle turbinate and sinus opacification1,2,18,21,22. Radiological findings of advanced disease include bony dehiscence, orbital invasion, and intracranial extension1,18,19,23,24.
To the best our knowledge, the differences between the radiological findings in CT scans of patients with AIFR induced by Mucor versus Aspergillus were not evaluated so far. In the present study, we aimed to detect the possible differences of the imaging abnormalities found on craniofacial CT obtained during initial evaluation of patients eventually diagnosed with AIFR caused by Mucor and Aspergillus species.
Materials and methods
Patient selection and study design
The study was approved by the institutional review board at the Pennsylvania State University, College of Medicine. Institutional review board that approved the study waived the need for informed consent as part of the study approval. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional guidelines on human experimentation, the human subjects protection office, and with the Helsinki Declaration of 1975, as revised in 2008.
Patients with nasal and paranasal sinus biopsies between January 1, 2007 and December 31, 2017 that were positive for invasive mucormycosis and invasive asperguillosis were included in this study. Inclusion criteria were: (1) Histopathologically proven invasive fungal rhinosinusitis with positive culture growth of either Mucor or Aspergillus species, and (2) CT imaging of the craniofacial region within 5 days preceding tissue biopsy. Subjects were excluded if the CT imaging was inadequate due to: (1) partial coverage of the nasal cavity, paranasal sinuses, orbits or intracranial space, or (2) the presence of severe dental artifacts impeding analysis of the imaging.
Clinical data was collected from the medical records including demographic data, surgical and histopathology reports, culture results, underlying medical conditions, antifungal treatment, absolute neutrophil count (ANC) and outcome. Outcome was classified as either deceased from AIFR, deceased not from AIFR, not deceased, and uncertain.
Imaging protocol
All studies were performed in our institution, using the institutional craniofacial CT protocol. According to our protocol, images were acquired as 1–3-mm-thick sections and an in-plane FOV from 170 to 190 mm. Soft tissue and bone algorithm reconstructions were performed.
Scans were obtained in an axial plane and included the paranasal sinuses and hard palate. Multiplanar reformations were obtained in the coronal and sagittal plane. None of the patients in the Aspergillus group received contrast while three subjects from the Mucor group had contrast enhanced CT scans.
Image analysis
Studies were reviewed independently by two readers, a neuroradiologist and an otolaryngologist, both blinded to the patients' clinical information and histopathology results. There was consensus between the readers. The readers evaluated and graded as 'present' or 'absent' mucosal thickening or infiltration in the following anatomical sites: anterior periantral fat (soft tissue anterior to the anterior wall of the maxillary sinus), posterior periantral fat, sphenopalatine foramen, pterygopalatine fossa, nasolacrimal duct, medial orbital fat,inferior orbital fat and facial soft tissue including other location not specified, including but not limiting to, the masticator space. Mucosal thickening of the nasal cavity was defined as more than 3 mm and was recorded as present or absent. Bony dehiscence of the sinonasal area or hard palate were recorded as present or absent.
Sinus opacification was graded according to the amount of mucosal thickening in the paranasal sinuses (maxillary sinuses, frontal sinuses, sphenoid sinuses and ethmoid air cells) on a scale of 0–3 (0; no mucosal thickening, 1; < 50% opacified, 2; > 50% opacified, 3; 100% opacified).
Statistical analysis
The R statistical programing language (version 3.4.4, 2018–03-15) was used to clean, transform, and analyze the results. Pearson’s correlation coefficient (ρ), Kendall’s correlation coefficient (τ), and Cramer’s V coefficient were used to analyze pairwise relationships between variables, both for identifying potential confounders and in guiding inclusion in models. Several modeling techniques were attempted including binomial logistic regression and Canonical correlation analysis.
A score-based model was implemented that included multiple factors, based on methodology by Middlebrooks et al.20. Two techniques for analyzing differences in laterality of findings were used to construct scores: the “unilateral presence” model assigns a value of one for a unilateral presence of a factor, and uses the lateral mean for each factor as its value, and the “bilateral mean” model scales each factor’s possible values between zero and one and uses the lateral mean for each factor as its value.
Values for each technique were summed to construct a score. Binary logistic regression was used, with Score as the only predictor and Group (Mucor vs Aspergillus) as the only outcome. Results were considered significant with α ≤ 0.05.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinski Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived from all individual participants included in the study.
Results
Thirty-eight patients met the inclusion criteria, thirteen patients (34.2%) diagnosed with invasive mucormycosis and twenty-five patients (65.8%) with invasive aspergillosis. Table 1 describes the demographic and clinical variables collected. There was no difference in the demographic characteristics between the two groups. The most prevalent predisposing condition was AML for both Mucor (76.9%) and Aspergillus (56%) patients without any statistically significant differences between the distributions of conditions. There was no significant difference in the ANC for both groups. One patient had an ANC > 500. This patient had poorly controlled diabetes without an underlying hematological malignancy.
Table 1.
Demographics and clinical characteristics of both patient groups.
| Characteristics | Mucor | Aspergillus | p value |
|---|---|---|---|
| Mean age, years (SD) | 52.5 (12.5) | 51.6 (17.2) | 0.979 |
| M:F | 7:6 | 14:11 | 0.468 |
| ANCa < 500 | 10/13 | 23/25 | |
| ANC > 500 | 0/13 | 1/25 | |
| ANC unknown | 3/13 | 1/25 | |
| Outcome | 0.223 | ||
| AIFR-related mortality | 7 | 9 | |
| Non-AIFRb related mortality | 1 | 8 | |
| Not deceased | 3 | 7 | |
| Condition | 0.292 | ||
| AMLc | 10 | 14 | |
| ALLd | 2 | 3 | |
| MDSe | 0 | 3 | |
| MMf | 1 | 1 | |
| Others | 0 | 4 |
aANC, absolute neutrophil count; bAIFR, acute invasive fungal rhinosinusitis; cAML, acute myeloid leukemia; dALL, acute lymphocytic leukemia; eMDS, myelodysplastic syndrome; fMM, multiple myeloma.
Severe soft tissue thickening, including the turbinates, nasal walls, septum, and anterior and posterior periantral fat was present in 5 of the 13 Mucor patients in this study and in 9 of the 25 Aspergillus patients in this study. Severe orbital soft tissue thickening and fatty standing was present in 3 of the 13 Mucor patients in this study and in 5 of the 9 Aspergillus patients in this study. Sinus mucosal thickening was present in 13 out of 13 Mucor patients and in 24 out of 25 Aspergillus patients in this study.
The average interval between CT examination and surgical intervention was 0.92 days (range 0–3 days) for Mucor patients and 1.1 days (range 0–5 days) for Aspergillus patients. By reviewing radiological abnormalities distribution for each of the aforementioned anatomical regions, no individual region or radiological pattern significantly correlated with either Mucor or Aspergillus AIFR. Hence, score-based models evaluating the laterality of findings from each of the aforementioned anatomical regions were applied. Examples for abnormal radiological findings in patients with Mucor and Aspergillus are presented in Figs. 1 and 2.
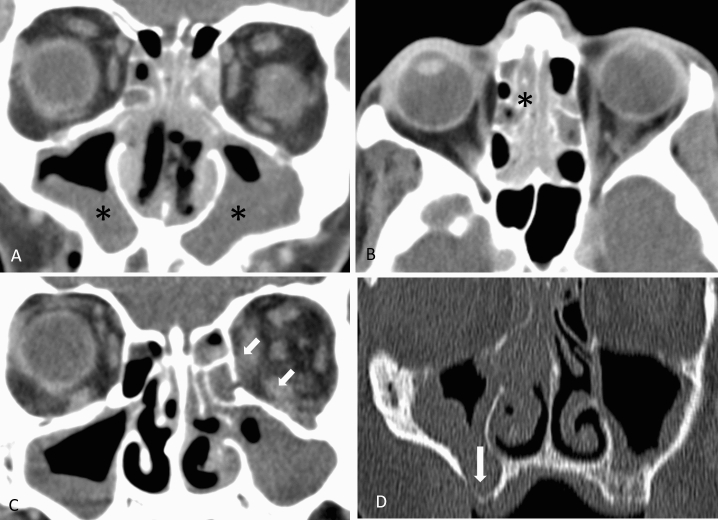
Figure 1.
Examples of findings in patients with Mucor AIFR. (A) Coronal CT image shows bilateral mucosal thickening involving the maxillary sinuses (asterisks) and the nasal cavity. (B) Axial image in the same patient demonstrates bilateral mucosal thickening of ethmoid air cells (asterisk). (C) Coronal image in a different patient shows bilateral maxillary sinuses involvement and left orbital involvement with fatty infiltration of the left medial and inferior extraconal orbital fat (arrows). (D) Coronal CT of a different patient shows bilateral mucosal thickening of the maxillary sinuses with bony dehiscence along the inferior aspect of the right maxillary sinus.
Figure 2.
Examples of findings in patients with Aspergillus AIFR. (A) Coronal CT image shows unilateral mucosal thickening of the right maxillary sinus (asterisk) and the ethmoid air cells. (B, C) Axial images from the same patient shows fatty infiltration of the anterior periantral fat and the posterior periantral fat, without bony dehiscence. (D) Another patient with Aspergillus AIFR with unilateral mucosal thickening of the maxillary sinus and the nasal cavity (asterisk).
Score based models
Unilateral presence and bilateral mean score-based models were applied for the data collected from each of the aforementioned anatomical regions.
Unilateral presence, though close, did not reach statistical significance and yielded lower PPV, NPV, sensitivity, specificity and accuracy and thus was not selected. The bilateral mean model was tested with various combinations of the evaluated anatomical regions, yielded statistically significant results and was found to be the most predictive model to classify between Mucor and Aspergillus AIFR.
Furthermore, the scores for the bilateral mean model yielded the highest accuracy when data from the following anatomical regions, as well as the presence of bony dehiscence, were included:
Nasal cavity
Maxillary sinuses
Ethmoid air cells
Sphenoid sinus
Frontal sinuses
Bony dehiscence
The odds ratio for the model while integrating the above anatomical sites was 12.3 (p < 0.001). PPV, NPV, sensitivity, specificity and accuracy were 0.85, 0.82, 0.92, 0.69 and 0.84 respectively. The addition of orbital involvement to the data resulted in a statistically significant model although with slightly reduced PPV, NPV, sensitivity and accuracy, and therefore was not included (Table 2).
Table 2.
AIFR classification of Aspergillus versus Mucor in various combinations of the bilateral mean model.
| Item | Bilateral meana | Bilateral mean with bony dehiscenceb | Bilateral mean with orbitsc | Bilateral mean with orbits and bony dehiscenced |
|---|---|---|---|---|
| Effect from score on odds for Mucor | 12.43 | 12.30 | 5.27 | 6.45 |
| p value for effect from score | 0.01 | 0.00 | 0.01 | 0.00 |
| PPV: Mucor | 0.81 | 0.85 | 0.76 | 0.83 |
| NPV: Mucor | 0.80 | 0.82 | 0.58 | 0.69 |
| Sensitivity: Mucor | 0.92 | 0.92 | 0.79 | 0.83 |
| Specificity: Mucor | 0.62 | 0.69 | 0.54 | 0.69 |
| Accuracy: Mucor | 0.81 | 0.84 | 0.70 | 0.78 |
aModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus and frontal sinus.
bModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus, frontal sinus and bony dehiscence.
cModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus, frontal sinus and orbital involvement.
dModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus, frontal sinus, bony dehiscence and orbital involvement.
Cutoff score
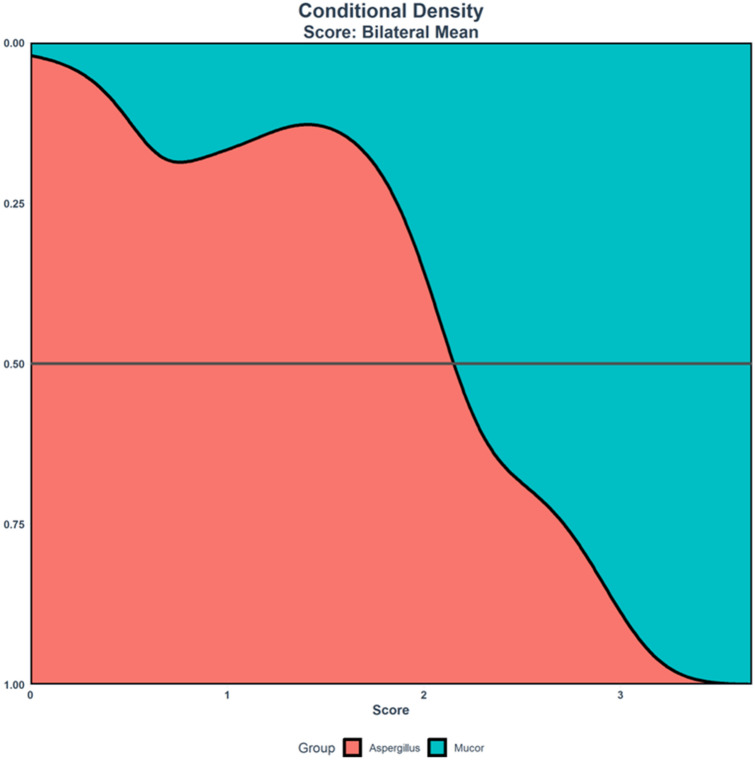
The cutoff (transition) score to predict Mucor versus Aspergillus AIFR was calculated for the bilateral mean model. Scores above the cuttoff are more likely to be Mucor than Aspergillus (Table 3). The cut off score at 50% for the bilateral mean model was 2.15. This can also be appreciated by the conditional density diagram (Fig. 3).
Table 3.
Cutoffs for scores for predicting Aspergillus versus Mucor in various combinations of the bilateral mean model.
| Model | Threshold score | Probability of Mucor at threshold |
|---|---|---|
| Bilateral meana | 2.06 | 0.51 |
| Bilateral mean with bony dehiscenceb | 2.15 | 0.5 |
| Bilateral mean with orbitsc | 2.34 | 0.5 |
| Bilateral mean with orbits and bony dehiscenced | 2.43 | 0.5 |
aModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus and frontal sinus.
bModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus, frontal sinus and bony dehiscence.
cModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus, frontal sinus and orbital involvement.
dModel included nasal cavity, maxillary sinus, ethmoid air cells, sphenoid sinus, frontal sinus, bony dehiscence and orbital involvement.
Figure 3.
Conditional density diagram of the bilateral mean model. Patients with AIFR caused by Mucor species had higher mean score values compared with patients with AIFR caused by Aspergillus species.
Discussion
We retrospectively evaluate the presence and distribution of radiological abnormalities in patients with histopathologically proven Mucor or Aspergillus induced AIFR. Since Mucor and Aspergillus species constitute the vast majority of the offending organisms in AIFR, other rare types of fungi were not included in our study.
Our cohort included a total of 38 patients. Thirteen patients with invasive mucormycosis and twenty-five patients with invasive aspergillosis. By incorporating various abnormal radiological craniofacial CT findings from specific anatomical regions into the bilateral mean model, we found that AIFR caused by Mucor species demonstrated a higher degree of bilateral sinonasal involvement. Furthermore, as the calculated score rose above the cutoff score (more bilateral findings), the probability for Mucor (vs Aspergillus) increased. The application of this model demonstrated the radiological differences in the laterality of findings between Aspergillus and Mucor AIFR.
Identifying radiological differences for AIFR induced by Mucor versus Aspergillus species on craniofacial CT scans, has the potential to predict the inciting pathogen and potentially facilitate guidance of a more targeted pharmacological treatment prior to definite tissue diagnosis in patients with a high index of suspicion for AIFR.
Interestingly, previous studies found unilateral mucosal thickening and soft tissue infiltration to be associated with AIFR1. Middlebrooks et al.20 found that 78.6% of their patients had unilateral predominant findings. Those studies evaluated cohorts of patients with AIFR induced by various fungi species but did not contrast these findings in Mucor versus Aspergillus species. Therefore, as Aspergillus is a considerably more prevalent pathogen in AIFR compared to Mucor, it could possibly shift the pendulum towards unilaterality of the abnormal CT findings, which is in line with our results that demonstrated a higher likelihood of unilateral findings with Aspergillus.
Tissue biopsy is the gold standard for diagnosing and classifying the pathogen in AIFR, with histology demonstrating fungal invasion. It mandates intranasal endoscopy performed either at bedside or in the operating room. In most cases, AIFR is the hallmark of an overall severely deteriorated medical state which may include severe thrombocytopenia and even hemodynamic instability which may delay obtaining tissue biopsy.
Craniofacial CT scan will typically be the imaging modality of choice for the initial patient's evaluation, while MRI is usually reserved for cases of clinically suspected intracranial, base of skull or orbital involvement. Bony dehiscence is a rare and insensitive radiological finding that is usually detected in cases of very advanced disease1,20,24,26. Generally, craniofacial CT will demonstrate nonspecific findings similar to acute or chronic rhinosinusitis1,18,21,26. Finkelstein et al.26 compared craniofacial CT abnormalities of 14 patients with AIFR (8 patients with Mucor and 6 with Aspergillus species), with those of 20 patients with suspected, but finally excluded, AIFR. Thirteen imaging parameters were evaluated from which bony dehiscence, facial soft tissue thickening, extra sinus extension and unilaterality were statistically associated with AIFR. Nevertheless, they found that craniofacial CT findings on the early stages of AIFR are nonspecific and must be complemented with additional diagnostic tools. In a retrospective case/control study, DelGuadio et al.18 compared CT findings of 23 patients with AIFR (9 patients with Mucor and 14 with Aspergillus species) with those of 10 control patients with acute myelocytic leukemia (AML) and nonfungal rhinosinusitis. They found severe unilateral nasal mucosal thickening to be the most consistent finding in AIFR. Middlebrooks et al.20 analyzed 23 variables of CT findings from 42 patients with AIFR (10 patients with Mucor, 18 with Aspergillus, and the rest with various fungal species) versus 42 control patients proved negative for AIFR, in order to design a diagnostic imaging model. By multivariate analysis, the group finally developed a model consisting of 7 variables including bony dehiscence, orbital invasion, and septal ulceration, involvement of periantral fat, pterygopalatine fossa, nasolacrimal duct and lacrimal sac. The presence of an abnormality of any additional variable increased the positive/negative predictive value, sensitivity and specificity regarding the diagnosis of AIFR. Bony dehiscence was reported to have 100% specificity and 35% sensitivity for AIFR. None of the aforementioned studies discriminated Mucor versus Aspergillus species in their data analysis.
Our results demonstrate that while AIFR caused by Mucor and Aspergillus share overall similar abnormal findings on craniofacial CT, the two pathogens may be differentiated by laterality of radiological findings as demonstrated by the bilateral mean model.
The limitations of our study include a retrospective study, a limited cohort of patients and the exclusion of fungi species other than Mucor and Aspergillus. These limitations are derived from the fact that AIFR is rare, and although this is one of the largest cohorts of AIFR patients reported, the power of statistical analysis is limited.
In this study we demonstrated that although the abnormal radiological finding on craniofacial CT scans of Mucor and Aspergillus induced AIFR are similar, they could be differentiated based on laterality. By using the bilateral mean model, we demonstrated that Mucor induced AIFR is associated with higher prevalence of bilateral findings. Similar to prior studies evaluating patients with AIFR, facial soft tissue thickening and orbital involvement were not common features4,25. Mucosal thickening in the sinuses was a common feature both in Mucor and Aspergillus AIFR as has been previously reported18,20,26.
It is important to highlight that our model does not intend to make a diagnosis of Mucor versus Aspergillus AIFR based on imaging findings solely. The workflow should be imaging findings that raise concern of AIFR specifically in at risk population presenting with relevant clinical symptoms and with suspicious finding on physical examination/nasal endoscopy concerning for AIFR, and then apply the model to further predict Mucor versus Aspergillus (as most likely pathogen) while waiting for final pathological results. Besides a potential clinical significance, we believe that this model provides a better understanding of the radiological manifestation of AIFR specifically understanding the subtle differences between Mucor and Aspergillus inflicted disease.
To the best of our knowledge, this is the first study to directly compare radiological finding of patients with Mucor versus Aspergillus induced AIFR. We believe that our findings could potentially contribute to future studies incorporating the rapidly evolving machine learning algorithms for better classification of diseases by imaging.
Acknowledgements
No funding was received for this study.
Author contributions
G.S.: substantial contributions to the conception, design of the work, interpretation of data and have drafted and revised the work. G.S. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. J.D.M.: substantial contributions to the conception of the work and have drafted the work and substantively revised it. J.D.M. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. N.G.: substantial contributions to the conception of the work and have drafted the work and substantively revised it. N.G. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. H.C.: substantial contributions to the conception of the work and the acquisition of data. H.C. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. M.H.: substantial contributions to the conception of the work and the acquisition of data. M.H. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. E.G.: substantial contributions to the conception of the work and the acquisition of data. E.G. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. E.S.: substantial contributions to the conception of the work, the acquisition, analysis, and interpretation of data; and have drafted the work or substantively revised it. E.S. approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Data availability
The authors declare to make materials, data and associated protocols promptly available to readers without undue qualifications.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aribandi M, McCoy VA, Bazan C. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2007;27:1283–1296. doi: 10.1148/rg.275065189. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie MB, O'Malley BW, Jr, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch. Otolaryngol. Head Neck Surg. 1998;124:520–526. doi: 10.1001/archotol.124.5.520. [DOI] [PubMed] [Google Scholar]
- 3.Parikh SL, Venkatraman G, DelGaudio JM. Invasive fungal sinusitis: a 15-year review from a single institution. Am. J. Rhinol. 2004;18:75–81. doi: 10.1177/194589240401800202. [DOI] [PubMed] [Google Scholar]
- 4.Ingley AP, Parikh SL, DelGaudio JM. Orbital and cranial nerve presentations and sequelae are hallmarks of invasive fungal sinusitis caused by Mucor in contrast to Aspergillus. Am. J. Rhinol. 2008;22:155–158. doi: 10.2500/ajr.2008.22.3141. [DOI] [PubMed] [Google Scholar]
- 5.Kasapoglu F, Coskun H, Ozmen OA, Akalin H, Ener B. Acute invasive fungal rhinosinusitis: evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol. Head Neck Surg. 2010;143:614–620. doi: 10.1016/j.otohns.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Saghrouni F, et al. Twenty-nine cases of invasive aspergillosis in neutropenic patients. Med. Mal. Infect. 2011;41:657–662. doi: 10.1016/j.medmal.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. The Laryngoscope. 2013;123:1112–1118. doi: 10.1002/lary.23912. [DOI] [PubMed] [Google Scholar]
- 8.Suslu AE, Ogretmenoglu O, Suslu N, Yucel OT, Onerci TM. Acute invasive fungal rhinosinusitis: our experience with 19 patients. Eur. Arch. Otorhinolaryngol. Off. J. Eur. Fed. Otorhinolaryngol. Soc. 2009;266:77–82. doi: 10.1007/s00405-008-0694-9. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J. Infect. 2009;59:134–138. doi: 10.1016/j.jinf.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008;47:503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 11.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis RE, et al. How does antifungal pharmacology differ for mucormycosis versus aspergillosis? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;54(Suppl 1):S67–72. doi: 10.1093/cid/cir884. [DOI] [PubMed] [Google Scholar]
- 13.Neofytos D, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009;48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 14.Roden MM, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 15.Shibuya K, et al. Pathophysiology of pulmonary aspergillosis. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2004;10:138–145. doi: 10.1007/s10156-004-0315-5. [DOI] [PubMed] [Google Scholar]
- 16.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 18.DelGaudio JM, Swain RE, Jr, Kingdom TT, Muller S, Hudgins PA. Computed tomographic findings in patients with invasive fungal sinusitis. Arch. Otolaryngol. Head Neck Surg. 2003;129:236–240. doi: 10.1001/archotol.129.2.236. [DOI] [PubMed] [Google Scholar]
- 19.Groppo ER, El-Sayed IH, Aiken AH, Glastonbury CM. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch. Otolaryngol. Head Neck Surg. 2011;137:1005–1010. doi: 10.1001/archoto.2011.170. [DOI] [PubMed] [Google Scholar]
- 20.Middlebrooks EH, et al. Acute invasive fungal rhinosinusitis: a comprehensive update of CT findings and design of an effective diagnostic imaging model. AJNR Am. J. Neuroradiol. 2015;36:1529–1535. doi: 10.3174/ajnr.A4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamba JL, Woodruff WW, Djang WT, Yeates AE. Craniofacial mucormycosis: assessment with CT. Radiology. 1986;160:207–212. doi: 10.1148/radiology.160.1.3715034. [DOI] [PubMed] [Google Scholar]
- 22.Silverman CS, Mancuso AA. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: CT and MR findings. AJNR Am. J. Neuroradiol. 1998;19:321–325. [PMC free article] [PubMed] [Google Scholar]
- 23.Mossa-Basha M, et al. The many faces of fungal disease of the paranasal sinuses: CT and MRI findings. Diagn. Interv. Radiol. 2013;19:195–200. doi: 10.5152/dir.2012.003. [DOI] [PubMed] [Google Scholar]
- 24.Slonimsky G, et al. The significance of Computed Tomography in invasive paranasal mucormycosis. Rhinology. 2018;56:54–58. doi: 10.4193/Rhin17.153. [DOI] [PubMed] [Google Scholar]
- 25.Payne SJ, Mitzner R, Kunchala S, Roland L, McGinn JD. Acute invasive fungal rhinosinusitis: a 15-year experience with 41 patients. Otolaryngol. Head Neck Surg. 2016;154:759–764. doi: 10.1177/0194599815627786. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein A, et al. Paranasal sinuses computed tomography in the initial evaluation of patients with suspected invasive fungal rhinosinusitis. Eur. Arch. Otorhinolaryngol. Off. J. Eur. Fed. Otorhinolaryngol. Soc. 2011;268:1157–1162. doi: 10.1007/s00405-011-1561-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare to make materials, data and associated protocols promptly available to readers without undue qualifications.