Abstract
Rotavirus is the most common cause of acute gastroenteritis (AGE) in young children. Bacillus clausii (B. clausii) is a spore-forming probiotic that is able to colonize the gut. A mixture of four B. clausii strains (O/C, T, SIN and N/R) is commonly used for the treatment of AGE, and it has been demonstrated that it can reduce the duration and severity of diarrhea in children with AGE. Few studies have sought to characterize the mechanisms responsible for such beneficial effects. Intestinal effects of probiotics are likely to be strain-specific. We conducted a series of in vitro experiments investigating the activities of this mixture of B. clausii strains on biomarkers of mucosal barrier integrity and immune function in a cellular model of Rotavirus infection. B. clausii protected enterocytes against Rotavirus-induced decrease in trans-epithelial electrical resistance, and up-regulated expression of mucin 5AC and tight junction proteins (occludin and zonula occludens-1), all of which are important for effective mucosal barrier function. B. clausii also inhibited reactive oxygen species production and release of pro-inflammatory cytokines (interleukin-8 and interferon-β) in Rotavirus-infected cells, and down-regulated pro-inflammatory Toll-like receptor 3 pathway gene expression. Such mechanisms likely contributed to the observed protective effects of B. clausii against reduced cell proliferation and increased apoptosis in Rotavirus-infected enterocytes.
Subject terms: Gastroenteritis, Viral infection
Introduction
Acute gastroenteritis (AGE), defined as sudden-onset diarrhea that is unrelated to chronic disease, with or without nausea, vomiting, fever or abdominal pain, is disproportionately common among young children1,2. Rotavirus (RV) is the most common cause of AGE and the leading cause of AGE-associated mortality in children younger than 5 years of age2–6. In 2016, more than 258 million episodes of diarrhea and approximately 1.5 million hospitalizations and 128,500 deaths in children younger than 5 years were attributable to RV infection globally2,7. The highest rates of RV-associated mortality have been reported in sub-Saharan Africa, Southeast Asia, and South Asia7. The high cost of RV vaccination precludes its widespread use in such low-income settings8. However, even in developed countries, AGE remains a considerable burden, despite the implementation of RV vaccination programs7. For example, routine RV vaccination was introduced in 2006 in the US, but there were 70,553 AGE-associated hospital admissions, about 20,000 due to RV infection, among US children younger than 5 years in 2013, which were associated with direct costs of more than US $226 million9.
Probiotics are living microorganisms that, when administered in adequate amounts, confer a health benefit on the host after colonizing the gut, and can help to prevent and treat AGE by supporting a healthy gut and immune system10,11. Short- and long-term beneficial effects of probiotics on the gut are the result of a range of mechanisms, including competitive exclusion and direct antagonism of gut pathogens, stimulation of host mucosal immune mechanisms, and reconstitution and enhancement of intestinal barrier function3,11,12. However, not all such beneficial effects can be ascribed to probiotics as a general class, as effects occurring at the intestinal or extra-intestinal level are likely to be strain-specific11.
Bacillus clausii (B. clausii) is a rod-shaped, spore-forming, aerobic, Gram-positive probiotic bacterium that is acid resistant and able to colonize the gut13–16. Data suggest that a mixture of four B. clausii probiotic strains (O/C, T, SIN and N/R) is effective in the treatment of pediatric AGE13. General antimicrobial and immunomodulatory properties of these B. clausii strains have been previously described17, but specific mechanisms of action against AGE are still largely undefined.
The current study aimed to investigate the protective activities of a mixture of four B. clausii strains (O/C, T, SIN and N/R) and their metabolites, on human enterocytes in basal conditions and in a model of RV infection. The effects of B. clausii on indicators of mucosal barrier integrity and innate immune function were also examined.
Results
Human beta defensin 2 and cathelicidin synthesis
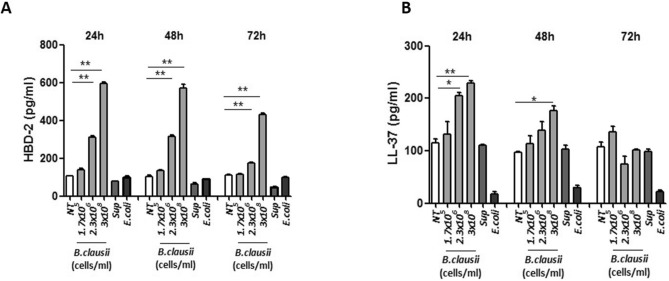
Relative to untreated enterocytes, B. clausii strains, but not its supernatant, elicited a dose-dependent increase of HBD-2 and LL-37 synthesis (Fig. 1A,B). Maximal effects were obtained after 48 h of treatment with B. clausii strains 3 × 108 cells/mL (P < 0.001 vs untreated cells).
Figure 1.
B. clausii increases HBD-2 and LL-37 expression in human enterocytes. Cells were exposed to B. clausii probiotic strains mix at different concentrations; B. clausii supernatant (Sup, dilution 1:100) or E. coli K12 (1 × 106 cells/mL) as control. Only the exposure to B. clausii strains was able to elicit a significant increase in HBD-2 (A) and LL-37 (B) production by human enterocytes. HBD 2, human beta defensin 2; LL-37, cathelicidin; NT, untreated. *p < 0.05 vs NT; **p < 0.001 vs NT.
Proliferation, cell cycle and apoptosis analysis by flow cytometry
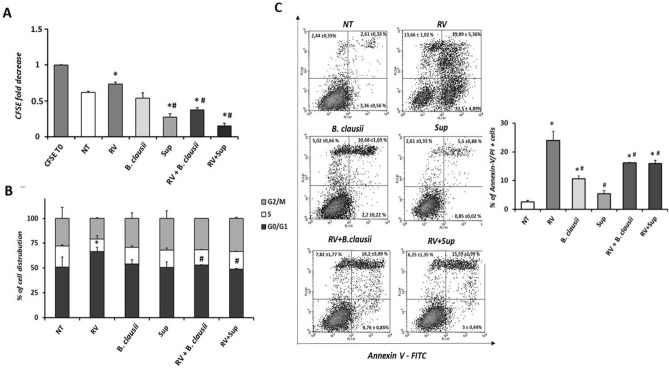
After 24 h of treatment with B. clausii probiotic strains or B. clausii supernatant, cell proliferation was comparable to that of the untreated cells, whereas RV significantly reduced cell growth (Fig. 2A). The combination of RV with B. clausii probiotic strains or B. clausii supernatant partially restored the proliferation rate (P < 0.05 vs RV alone). Rotavirus exposure blocked proliferation, with almost 70% of cells arrested in G0/G1 phase (Fig. 2B). We observed a G1/S transition block with RV compared with untreated cells and compared with infected cells stimulated with B. clausii probiotic strains or B. clausii supernatant for 24 h (P < 0.05). Compared with RV alone, greater proportions of cells exposed to a combination of RV and B. clausii probiotic strains or B. clausii supernatant were in the G2/M phase. Double staining with Annexin V and PI to evaluate apoptosis induction showed a toxic effect of RV stimulation (Fig. 2C), as demonstrated by an increase in necrotic cells (positive only for PI) and late apoptotic cells (positive for both PI and Annexin V) relative to untreated cells and uninfected cells treated with B. clausii probiotic strains or B. clausii supernatant. Treatment of RV-infected cells with B. clausii strains or B. clausii supernatant reduced the proportion of necrotic and apoptotic cells.
Figure 2.
B. clausii counteracts the Rotavirus effects on human enterocytes proliferation and viability. (A) Rotavirus (RV) (10 pfu/cell) reduced human enterocytes proliferation rate. B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (Sup, dilution 1:100) were able to inhibit the RV effect. (B) Cell cycle analysis confirmed the reduction in proliferation and a block in G0/G1 phases induced by RV. Again, the effect was inhibited by the incubation with B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (dilution 1:100). (C) Apoptosis analysis showed that the exposure to RV resulted in pro-apototic effect on human enterocytes. Again, both B. clausii and its supernatant were able to inhibit this effect. *p < 0.05 vs NT, #p < 0.05 vs RV.
Transepithelial electrical resistance
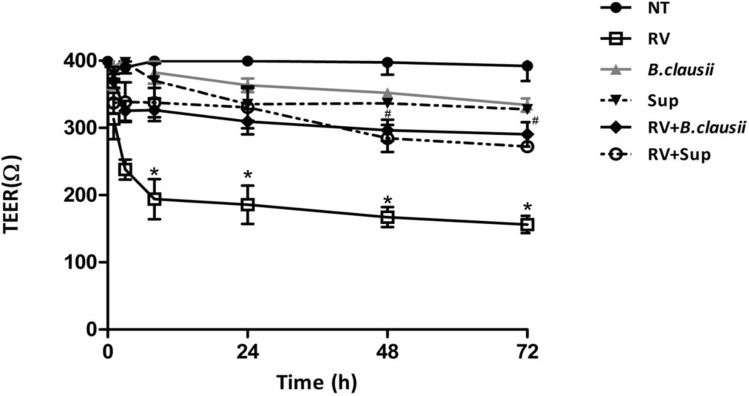
Treatment of uninfected cells with B. clausii probiotic strains or with B. clausii supernatant did not affect TEER, but RV-infected cells had decreased TEER (P < 0.05 vs untreated cells from 8 to 72 h; Fig. 3). Stimulation with B. clausii probiotic strains or B. clausii supernatant protected against a RV-induced decrease in TEER (P < 0.05 vs RV alone from 8 to 72 h).
Figure 3.
B. clausii and its supernatant significantly inhibit Rotavirus-induced TEER reduction in human enterocytes. The incubation with Rotavirus (RV) (10 pfu/cell), but not with B. clausii probiotic strains (3 × 108 cells/mL) or with B. clausii supernatant (Sup, dilution 1:100), elicited a significant reduction of TEER. B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (Sup, dilution 1:100) significantly inhibited the RV-induced TEER decrease. *p < 0.05 vs NT; #p < 0.05 vs RV.
ROS production
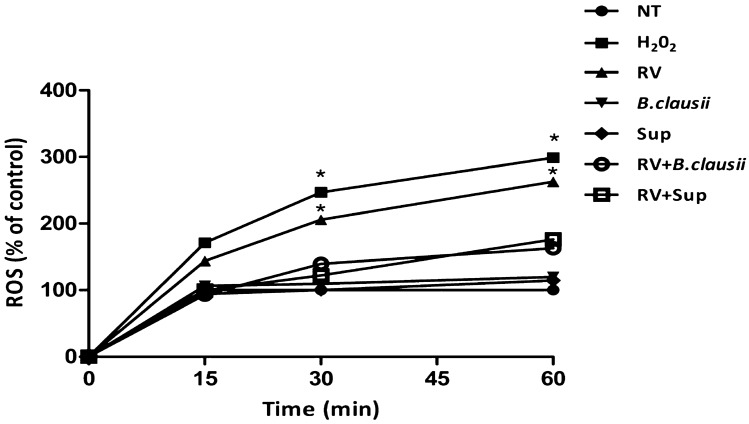
Rotavirus significantly increased ROS production in a time-dependent manner (Fig. 4). B. clausii probiotic strains and B. clausii supernatant inhibited the RV-induced increase in ROS.
Figure 4.
B. clausii and its supernatant significantly inhibit Rotavirus-induced ROS production in human enterocytes. Rotavirus (RV) (10 pfu/cell), but not with B. clausii probiotic strains (3 × 108 cells/mL) or with B. clausii supernatant (Sup, dilution 1:100), induced a significant increase in ROS production in a time-dependent manner. B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (Sup, dilution 1:100) significantly inhibited the RV-induced increase in ROS. H2O2 as a positive control. *p < 0.05 vs NT.
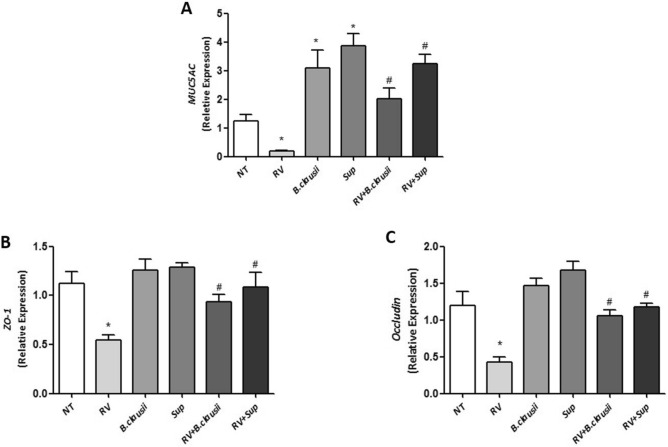
Expression of MUC5AC and tight junction proteins
Compared with untreated cells, B. clausii probiotic strains and B. clausii supernatant significantly increased MUC5AC expression under basal conditions, while RV infection alone significantly reduced MUC5AC expression (Fig. 5A). B. clausii probiotic strains and B. clausii supernatant upregulated MUC5AC expression in RV-infected cells (P < 0.05 vs RV alone).
Figure 5.
B. clausii and its supernatant significantly counteract the Rotavirus-induced alteration in MUC-5, ZO-1 and Occludin expression in human enterocytes. Rotavirus (RV) (10 pfu/cell) significantly reduced the expression of MUC5AC (A), ZO-1 (B) and Occludin (C) in human enterocytes, whereas both B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (Sup, dilution 1:100) significantly increased the expression of these proteins in basal condition and blunted the RV effect. *p < 0.05 vs NT; #p < 0.05 vs RV.
Rotavirus infection significantly reduced ZO-1 and occludin expression (Fig. 5B,C). The combination of RV with B. clausii probiotic strains or B. clausii supernatant significantly increased expression of both tight junction proteins expression levels. These results were confirmed when analyzed the protein for occludin and ZO-1, as showed in Fig. S1A,B.
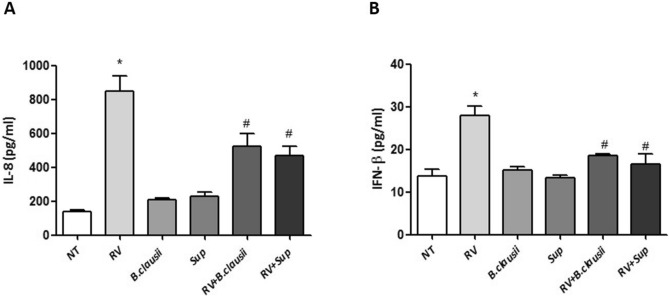
Analysis of interleukin-8 and interferon-β production
There was a significant increase in IL-8 and IFN-β production in RV-infected enterocytes (Fig. 6A,B). These effects were blunted by B. clausii probiotic strains and B. clausii supernatant (P < 0.05 vs RV alone).
Figure 6.
B. clausii and its supernatant significantly reduce Rotavirus-induced expression of pro-inflammatory cytokines in human enterocytes. Rotavirus (RV) (10 pfu/cell) elicited a significant increase in IL-8 (A) and IFN-β (B) production. B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (Sup, dilution 1:100) significantly inhibited the RV-induced increase in IL-8 and IFN-β production. *p < 0.05 vs NT; #p < 0.05 vs RV.
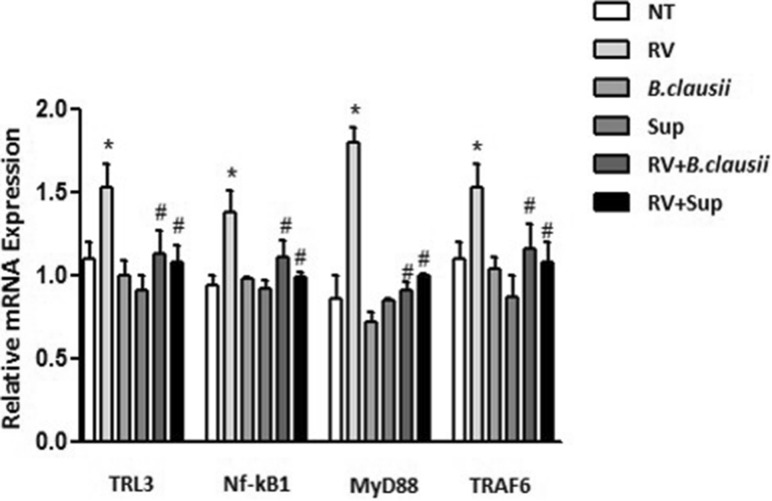
Toll-like receptor 3 pathway analysis
TLR3, NF-κB1, MyD88 and TRAF6 expression were all significantly up-regulated in cells infected with RV compared with untreated cells (Fig. 7). Down-regulation of pro-inflammatory TLR3 pathway gene expression was observed in RV-infected cells treated with B. clausii probiotic strains and B. clausii supernatant (P < 0.05 vs RV alone). The respective proteins of these genes showed the same trend of mRNA expression (Fig. S2A,B).
Figure 7.
B. clausii and its supernatant contrast Rotavirus-mediated activation of Toll-like receptor-3 pathway in human enterocytes. Rotavirus (RV) (10 pfu/cell) significantly up-regulated TLR3, NF-κB1, MyD88 and TRAF6 expression. B. clausii probiotic strains (3 × 108 cells/mL) and B. clausii supernatant (Sup, dilution 1:100) significantly inhibited such effects. *p < 0.05 vs NT; #p < 0.05 vs RV.
Discussion
Children are constantly being exposed to infectious agents in the gastrointestinal tract and are able to resist these infections due to the action of two main defense mechanisms: the non-immune mucosal barrier and immune response18,19. The results of our study provide evidence that a mixture of four B. clausii probiotic strains, namely O/C, T, SIN, and N/R, and their metabolites modulate a number of non-immune and immune defense mechanisms against RV-induced AGE.
To determine whether treatment with B. clausii probiotic strains and their supernatant induce a protective action against RV infection, we evaluated enterocyte proliferation and survival. Rotavirus reduced cell growth in association with a cytotoxic effect. We demonstrated that B. clausii probiotic strains and their metabolites (from the supernatant) were able to restore cell proliferation, inducing a restart in cell cycle progression and protecting against apoptosis, in RV-infected human enterocytes.
The non-immune mucosal barrier acts through a well-modulated network involving epithelial cell layers that express tight cell–cell contacts (tight-junctions regulated by several proteins including occludin and ZO-1), and the secreted mucus layer that overlays the epithelium in the intestinal tract20. The integrity of the non-immune mucosal barrier, which is of pivotal importance in protecting children against pathogens, is disrupted by RV21. We found that B. clausii probiotic strains and their metabolites had beneficial effects on markers of epithelial barrier damage and enterocyte monolayer permeability in cells infected by RV. Whereas B. clausii probiotic strains or supernatant did not affect epithelial integrity as assessed by TEER in uninfected cells, they protected infected cells against a RV-induced increase in TEER. B. clausii probiotic strains and supernatant also up-regulated the expression of mucin protein MUC5AC and occluding and ZO-1 tight junction proteins, all of which are important for effective mucosal barrier function20.
The intestinal epithelium is an integral component of innate immunity, which provides the initial host response to invading pathogens22. Soluble proteins and bioactive small molecules are involved in the innate immune response. These are either constitutively present at a systemic level in many biological fluids (e.g., complement proteins and defensins) or are released from activated cells resulting in inflammation (e.g., cytokines, reactive free radical species and bioactive amines)22. In experiments conducted in Caco-2 enterocytes under basal conditions, B. clausii probiotic strains and supernatant increased the synthesis of the innate immunity antimicrobial peptides HBD-2 and LL-37, which are responsible for effective defense mechanisms against several pathogens in the gastrointestinal tract23. B. clausii probiotic strains and supernatant also inhibited ROS production, and the release of pro-inflammatory cytokines (IL-8 and IFN-β) in RV-infected cells.
The innate immune system also includes membrane-bound receptors and cytoplasmic proteins that bind invading microbes22. For example, RV double stranded RNA binds TLR3, expressed in intestinal epithelial cells, and this interaction elicits an upregulation of the expression of type I IFN (IFN-α, IFN-β)24–26 that are crucial to limit RV infection27 to block cell replication, and a secretion of various cytokines and chemokines, including IL-8 and IFN-β22. We showed that B. clausii probiotic strains were able to reduced secretion of IL-8 and IFN-β, and down-regulate the expression of pro-inflammatory TLR3 pathway genes (TLR3, NF-κB1, MyD88, TRAF6) activate by RV infection. Our results are consistent with clinical trials13, and help to explain the beneficial effects of the same mixture of four B. clausii probiotic strains (O/C, T, SIN, N/R) in children with AGE. In a meta-analysis of randomized controlled trials, the same B. clausii strains mixture reduced the duration of diarrhea by 9.12 h relative to control (oral rehydration solutions or placebo), and duration of hospitalization was reduced by 0.85 days13. There was also a trend towards a reduction in stool frequency. In line with the findings of the meta-analysis, data from a large observational study indicated a reduction in diarrhea duration (median duration 3 days) and stool frequency in children with viral or antibiotic-associated AGE who were treated with the O/C, T, SIN, N/R mix of B. clausii strains28. There was no significant difference in the mean duration of diarrhea between patients with viral diarrhea and those with antibiotic-associated diarrhea. The B. clausii O/C strain has previously been reported to produce bacteriocin and protease antimicrobials with activity against Gram-positive bacteria, which may be partially responsible for the protective effects of this probiotic in antibiotic-associated diarrhea17,29 .
Although a specific recommendation for B. clausii in the treatment of childhood AGE has hitherto been missing because of limited data, there is now a growing body of clinical evidence in support of the O/C, T, SIN, N/R mix of B. clausii probiotic strains as an effective therapeutic option in this clinical setting13,28,30. Modulation of the immune response with this B. clausii strains mix may also have health benefits beyond the treatment of AGE, ranging from the prevention of recurrent respiratory infections in allergy-prone children to influencing outcomes in cancer patients31,32. In addition to its documented efficacy and safety in childhood AGE13,28, B. clausii has the practical advantage of being a spore-forming probiotic, making it heat stable and able to be transported and stored at room temperature without loss of viability33.
Given that certain effects of probiotics, including immunomodulatory effects, are likely to be strain-specific11, a key strength of our study and the aforementioned clinical studies is that a clearly defined mix of four B. clausii strains was used. Just as the clinical effects of a particular strain of probiotic should not be extrapolated to other strains, intestinal mechanisms of action should not be ascribed to all strains8,11. Another strength of our study is the use of a validated in vitro model of enterocyte infection with RV34, which is the most relevant AGE-causing pathogen in the pediatric age worldwide2,7. Additionally, we assessed a wide range of variables that are indicative of intestinal mucosal barrier integrity and innate immune function. As well as testing a mixture of four B. clausii strains, we tested the effects of its supernatant on all variables. This is important to assess as it is often mainly the metabolites produced by probiotics could modulate intestinal epithelial cell functions35.
The main limitation of our study is related to the fact that we did not explore the potential protective effects of B. clausii against other viral and non-viral agents responsible for AGE in the pediatric age group. Evaluation of the efficacy and mechanisms of action of B. clausii probiotic strains on more complex systems, such as human biopsies and/or organoids exposed to different gastrointestinal pathogens, is advocated to further explore the potential of such therapeutic approach.
Conclusion
The mixture of four B. clausii probiotic strains investigated in this study has protective effects and stimulates various non-immune mucosal barrier and innate immune system defense mechanisms in a human enterocyte model of RV infection. These observations provide insights into the mechanisms that are potentially responsible for the beneficial effects of this B. clausii mixture in pediatric patients with AGE, and should encourage further study into the effects of this probiotic on AGE caused by other viral and non-viral pathogens.
Methods
B. clausii probiotic strains and supernatant
The commercially available mixture of B. clausii strains investigated in this study (Enterogermina) had the following composition: O/C (3 × 108), T (1.7 × 105), SIN (2.3 × 106), N/R (1.7 × 107). B. clausii supernatant was prepared as previously described32. Briefly, B. clausii supernatant was obtained by centrifugation and filtration of a suspension of the four B. clausii probiotic strains (3.2 × 108 cells/mL), which was cultured aerobically in a specific fermentation medium at 37 °C in a rotary shaker for 3 days. B. clausii supernatant was harvested by centrifugation at 4 °C (10,000g for 5 min) and filtered onto 0.2 μm cellulose membrane.
Cell line
All experiments were conducted using the Caco-2 cell line of human enterocytes (American Type Culture Collection, Middlesex, UK; accession number: HTB-37). Cells were grown to confluence in Dulbecco’s modified Eagle’s medium (Gibco, Berlin, Germany) supplemented with 20% fetal bovine serum (FBS; Lonza, Visp, Switzerland), 1% l-glutamine (Lonza), 1% non-essential amino acids, and 1% penicillin/streptomycin (Lonza). Cells were cultured at 37 °C in a water-saturated atmosphere consisting of 95% air and 5% CO2. The medium was changed every 2 days and Caco-2 cells were grown for 14 days after confluence and cultured in 6-well plates.
Rotavirus strain and infection protocol
The simian RV strain SA11 was used as previously described30. Briefly, the virus (10 pfu/cell) was activated with 20 µg/mL porcine trypsin for 1 h at 37 °C. The viral suspension was added to the apical side of Caco-2 cell monolayers. After 1 h, the cells were washed and incubated in FBS-free medium for the indicated time periods after infection.
B. clausii cell stimulation protocol
For dose–response and time-course experiments, uninfected Caco-2 cells were stimulated for 24, 48 and 72 h with the mixture of four B. clausii probiotic strains (O/C, T, SIN and N/R) at three different doses (1.7 × 105, 2.3 × 106, 3 × 108 cells/mL), serial dilutions of B. clausii supernatants or medium only (untreated cells). Serial dilutions of B. clausii supernatants were prepared in phosphate buffered saline (PBS) as previously described36. E. coli K12 strain (1 × 106 cells/mL) served as control.
For RV infection experiments, Caco-2 cells were pre-treated for 12 h with B. clausii probiotic strains (3 × 108 cells/mL) or B. clausii supernatant (dilution 1:100). The cells were then washed in PBS and activated RV was added in FBS-free medium for 1 h at 37 °C. Infected cells were then re-suspended in medium with B. clausii probiotic strains (3 × 108 cells/mL) or its supernatant (dilution 1:100) for 24 or 48 h. Infected and uninfected cells that were not pretreated with B. clausii probiotic strains or B. clausii supernatant were incubated in medium alone.
Human beta defensin 2 and cathelicidin synthesis
Concentrations of the antimicrobial peptides human beta defensin 2 (HBD-2) and cathelicidin LL-37 in the cell supernatant were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits specific for human HBD-2 (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) or LL-37 (Hycult biotechnology, Uden, The Netherlands), respectively. ELISA was performed according to the manufacturers’ recommendations.
Proliferation, cell cycle and apoptosis analysis by flow cytometry
To perform proliferation analysis, undifferentiated Caco-2 cells were stained with carboxyfluorescein succinimidyl ester (CFSE) at a final concentration of 2.5 μM (Cell-Trace CFSE Proliferation Kit, Molecular Probes, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Briefly, cells were incubated with CFSE 2.5 μM for 15 min in darkness, stirring occasionally. The reaction was stopped by adding PBS with 20% FBS followed by centrifugation and repeated twice. Cells were then counted and 0.5 × 106 cells/well were plated in 6-well plates, stimulated and analyzed after 24 h.
To perform cell cycle analysis, 0.5 × 106 un-differentiated Caco-2 cells were plated in 6-well plates. After stimulation for 24 h, cells were collected and stained with propidium iodide (PI) 50 μg/mL (Sigma-Aldrich, St. Louis, MO, USA) in the presence of RNase A 100 μg/mL (Serva, Heidelberg, Germany).
To analyze cell apoptosis rates, Annexin V Apoptosis Detection Kit APC was used (eBioscience; San Diego, CA, USA) according to the manufacturer’s protocol. After 48 h of treatment, the cells were washed with PBS and incubated with 1 × Annexin V binding buffer, then 5 × 105 cells were stained with Annexin V-fluorescein isothiocyanate (FITC) for 10 min at room temperature in the dark. Before reading with a BD FACS Calibur flow cytometer flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), PI 5 μg/mL was added.
Transepithelial electrical resistance measurement
The Caco-2 cell monolayer transepithelial electrical resistance (TEER) was measured using a Millicel-ERS resistance monitoring apparatus (Merck Millipore, Billerica, MA, USA). TEER was measured at 1, 3, 8, 24, 48, and 72 h after Rotavirus infection.
Reactive oxygen species production
Reactive oxygen species (ROS) production was measured using 7′-dichlorofluorescein diacetate (DCFH-DA) spectrofluorometry. After stimulation, cells were exposed to DCFH-DA 20 µL (D6665; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37 °C in the dark, with 10 mM of H2O2 (Sigma-Aldrich) used as a positive control. Intracellular ROS production was measured using a fluorometer (SFM 25; Kontron Instruments; Japan).
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT‐PCR) was used to analyze the effect of intestinal exposure to B. clausii probiotic strains on gene expression of mucin 5AC (MUC5AC) and the tight junction proteins occludin and zonula occludens-1 (ZO-1), as well as toll-like receptor 3 (TLR3), nuclear factor κ B subunit 1 (NF-κB1), myeloid differentiation primary response 88 (MyD88), and tumor necrosis factor receptor-associated factor 6 (TRAF6), as previously described37. Briefly, qRT‐PCR was performed with the TaqMan gene expression assay kit, (Applied Biosystems; Grand Island, NY, USA) according to the manufacturer's instructions. Samples were run in duplicate at 95 °C for 15 s and 60 °C for 1 min using an ABI Prism 7900 Sequence Detection System (Applied Biosystems). Data were analyzed using the comparative threshold cycle method. We used the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene to normalize the level of mRNA expression.
Western blot analysis
Total proteins were extracted from Caco-2 cells using Trizol reagent (Invitrogen, Life Technologies, CA, USA) following the manufacturer’s instructions. The concentrations were determined by using a protein assay kit adopting bovine serum albumin standards, according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA). A total of 30 μg protein was separated by SDS-polyacrylamide gel electrophoresis and blots were prepared on a nitrocellulose membrane Amersham Hybond-ECL (Amersham, GEhealthcare, USA). The membranes was then incubated with anti-occludin (#31721, AbCam, Cambridge, UK), anti-ZO-1 (#96587, AbCam), anti-TRL3 (PA520183, Thermofisher, Waltham, MA, USA) anti-TRAF6 (#40675, AbCam), anti-MyD88 (#133739, AbCam), anti-NF-κB1 (#13586, Cell signaling Technology, Danvers, MA, USA) and anti-β actin (ACTBD11B7, Santa Cruz, CA, USA) primary antibodies at different dilutions. A goat anti-rabbit or anti-mouse horseradish peroxidase-linked antibody (Amersham, Orsay, France) was used at a 1:2,000 and 1:500 dilution, respectively, as a secondary antibody. Bound immunoglobulins was revealed by the ECL-detection system and the quantification was performed by Chemidoc (Biorad, Hercules, CA, USA).
Analysis of interleukin-8 and interferon-β production
Interleukin 8 (IL-8) production was measured in the cell supernatant using a commercially available ELISA kit specific for IL-8 (Abcam; Cambridge, UK). Interferon-β (IFN-β) concentration was measured using the specific human ELISA assay kit (BioVendor; Brno, Czech Republic). All ELISA assays were performed according to the manufacturer’s recommendations, and results expressed as pg/mL.
Statistical analysis
All experiments were performed in triplicate and were repeated twice. The Kolmogorov–Smirnov test was used to determine whether variables were normally distributed. We used the t test to evaluate differences among continuous variables. The level of significance for all statistical tests was 2-sided, P < 0.05. All analyses were conducted in SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5 (La Jolla, CA, USA)37.
Supplementary information
Acknowledgements
The authors thank Andrea Bothwell and Joanne Dalton who provided medical writing assistance for writing the outline and the first draft of the manuscript, respectively, on behalf of Springer Healthcare Communications. This medical writing assistance was funded by Sanofi Aventis.
Author contributions
L.P. and R.B.C. designed the study and coordinated the research team; L.P. and L.T. contributed to the study design, and performed the experiments; C.B., L.P. and C.D. performed the experiments and statistical analyses. All authors read and approved the drafts.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The study was supported by a Sanofi Aventis research grant devoted to the CEINGE Biotecnologie Avanzate at the University of Naples Federico II, Naples Italy. However, Sanofi Aventis had no influence on the study design, the collection, analysis, and interpretation of data, or the decision to submit the manuscript for publication.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lorella Paparo and Lorella Tripodi.
Supplementary information
is available for this paper at 10.1038/s41598-020-69533-7.
References
- 1.Hartman S, Brown E, Loomis E, Russell HA. Gastroenteritis in children. Am. Fam. Physician. 2019;99:159–165. [PubMed] [Google Scholar]
- 2.G. B. D. Diarrhoeal Disease Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron D, et al. Probiotics for gastrointestinal disorders: Proposed recommendations for children of the Asia-Pacific region. World J. Gastroenterol. 2017;23:7952–7964. doi: 10.3748/wjg.v23.i45.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiejina, M. & Samant, H. Viral Diarrhea. In StatPearls (2020). [PubMed]
- 5.Lanata CF, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nirwati H, et al. Norovirus and rotavirus infections in children less than five years of age hospitalized with acute gastroenteritis in Indonesia. Arch. Virol. 2019;164:1515–1525. doi: 10.1007/s00705-019-04215-y. [DOI] [PubMed] [Google Scholar]
- 7.Troeger C, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172:958–965. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szajewska H, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2014;58:531–539. doi: 10.1097/MPG.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 9.Leshem E, et al. National estimates of reductions in acute gastroenteritis-related hospitalizations and associated costs in us children after implementation of rotavirus vaccines. J. Pediatr. Infect. Dis. Soc. 2018;7:257–260. doi: 10.1093/jpids/pix057. [DOI] [PubMed] [Google Scholar]
- 10.Berni Canani R, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335:340. doi: 10.1136/bmj.39272.581736.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill C, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 12.Guarnier F, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J. Clin. Gastroenterol. 2012;46:468–481. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- 13.Ianiro G, et al. Bacillusclausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018 doi: 10.3390/nu10081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghelardi E, et al. Survival and persistence of Bacillusclausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 2015;119:552–559. doi: 10.1111/jam.12848. [DOI] [PubMed] [Google Scholar]
- 17.Urdaci MC, Bressollier P, Pinchuk I. Bacillusclausii probiotic strains: antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004;38:S86–S90. doi: 10.1097/01.mcg.0000128925.06662.69. [DOI] [PubMed] [Google Scholar]
- 18.Allaire JM, et al. The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Takiishi T, Fenero CIM, Camara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capaldo CT, Powell DN, Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J. Mol. Med. (Berl). 2017;95:927–934. doi: 10.1007/s00109-017-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta. 2009;1788:832–841. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Chaplin DD. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobo ER, Chadee K. Antimicrobial human beta-defensins in the colon and their role in infectious and non-infectious diseases. Pathogens. 2013;2:177–192. doi: 10.3390/pathogens2010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen A, et al. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-zoster virus promote replication and skin pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 2012;109:600–605. doi: 10.1073/pnas.1114232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 27.Lin JD, et al. Distinct roles of type I and type III interferons in intestinal immunity to homologous and heterologous rotavirus infections. PLoS Pathog. 2016;12:e1005726. doi: 10.1371/journal.ppat.1005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Castro JA, Guno MJV, Perez MO. Bacillusclausii as adjunctive treatment for acute community-acquired diarrhea among Filipino children: a large-scale, multicenter, open-label study (CODDLE) Trop. Dis. Travel Med. Vaccines. 2019;5:14. doi: 10.1186/s40794-019-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripert G, et al. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by clostridium difficile and Bacilluscereus toxins. Antimicrob. Agents Chemother. 2016;60:3445–3454. doi: 10.1128/AAC.02815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guarino A, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J. Pediatr. Gastroenterol. Nutr. 2014;59:132–152. doi: 10.1097/MPG.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 31.Marseglia GL, et al. Efficacy of Bacillus clausii spores in the prevention of recurrent respiratory infections in children: a pilot study. Ther. Clin. Risk Manag. 2007;3:13–17. doi: 10.2147/tcrm.2007.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riquelme E, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795–806 e712. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayanthi N, Ratna Sudha M. Bacillus clausii—the probiotic of choice in the treatment of diarrhoea. J. Yoga Phys. Ther. 2015;5:4. doi: 10.4172/2157-7595.1000211. [DOI] [Google Scholar]
- 34.De Marco G, et al. Rotavirus induces a biphasic enterotoxic and cytotoxic response in human-derived intestinal enterocytes, which is inhibited by human immunoglobulins. J. Infect. Dis. 2009;200:813–819. doi: 10.1086/605125. [DOI] [PubMed] [Google Scholar]
- 35.De Marco S, et al. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with lPS. Evid. Based Complement Alternat. Med. 2018;2018:1756308. doi: 10.1155/2018/1756308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medrano M, Perez PF, Abraham AG. Kefiran antagonizes cytopathic effects of Bacilluscereus extracellular factors. Int. J. Food Microbiol. 2008;122:1–7. doi: 10.1016/j.ijfoodmicro.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 37.Paparo L, et al. Direct effects of fermented cow's milk product with Lactobacillusparacasei CBA L74 on human enterocytes. Benef. Microbes. 2018;9:165–172. doi: 10.3920/BM2017.0038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.