Abstract
Microalgae with high growth rates have been considered as promising organisms to replace fossil resources with contemporary primary production as a renewable source. However, their microscopic size makes it hard to be harvested for industrial applications. In this regard, multicellular macroalgae are more suitable for harvesting. Here, we show that Ulva meridionalis has the highest growth rate ever reported for a multicellular autotrophic plant. Contrasted to the known bloom-forming species U. prolifera growing at an approximately two-fold growth rate per day in optimum conditions, U. meridionalis grows at a daily rate of over fourfold. The high growth ability of this multicellular alga would provide the most effective method for CO2 fixation and biomass production.
Subject terms: Biological techniques, Physiology, Plant sciences
Introduction
Microalgae and marine macroalgae (seaweeds) represent the most promising producers of renewable biological resources from carbon dioxide and inorganic nutrients by photosynthesis for the sustainable circular bioeconomy1,2. Initially in order to utilize their high growth rates and without using valuable arable land for farming, microalgae were explored to optimize the economics of the application process3. However, the harvesting process of microalgae is still a major problem, accounting for about 20–30% of the biomass production cost4. The main reasons for the high costs are the small size of microalgae and their culture in dilute media with densities close to that of water, making it difficult to separate the microalgae from the medium. There is currently no microalgal harvesting method that is both efficient and economically viable4. By contrast, seaweeds are much simpler to utilize because they can be harvested using a net or similar structure. Furthermore, the green seaweed Ulva having a growth rate nearly equal to microalgae can compete with microalgal production5. Ulva have a large biomass in coastal regions all over the world6. Particularly, U. prolifera commonly dominates in temperate brackish estuaries, having an ability to tolerate a wide range of salinities7. Some variants of this species cause spectacular blooms called green tides, covering several hundreds of kilometers of coastal waters only in a few months8,9. The rapid initial expansion of these blooms could be explained by the high growth rates of 10–37% increment per day in the field or under laboratory conditions10. From the viewpoint of industrial application, such a high growth rate of Ulva is the essential key for algal bioremediation and biomass production for sustainable feed, fuel and chemical generation5,11,12.
The Yoshino River estuary on Shikoku Island supports Japan’s largest production of U. prolifera by setting out culture nets in winter which yearly attains 60–70 dry-ton as edible green powder13. There is also another Ulva species occasionally blooming in summer14. This species is like U. prolifera having a thin and branched morphology. However, based on the microscopic cellular morphology and comparison of a DNA marker, it has been identified as a new species designated as U. meridionalis in 201115. Through our field observations of the excessive growth of these two species even in highly variable temperature and salinity conditions in the Yoshino River estuary (Supplementary Fig. 1 and Supplementary Table 1), they would be expected to have high growth potential. Here we examined which conditions are optimal for their growth and report that U. meridionalis has an extremely high growth rate.
Results
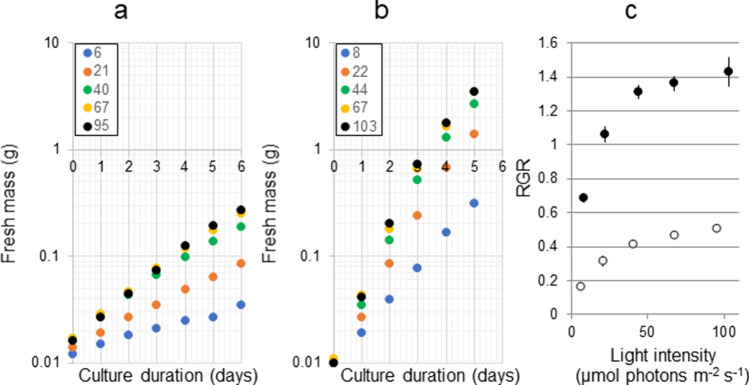
For determination of light intensity saturation for growth in U. prolifera and U. meridionalis, five light intensities were initially tested. Consecutive data of the fresh mass values from 0.01 to 1 g in our experimental setup (Supplementary Fig. 2) were logarithmically transformed and linearly arranged in each of the light conditions, indicating that the two species grow exponentially (Fig. 1a,b). However, the relative growth clearly declined when the fresh mass was over 1 g due to self-shading (Fig. 1b). Therefore, relative growth rates (RGRs) were calculated from the consecutive fresh mass values of < 1 g and plotted in relation to the light intensities (Fig. 1c). Light intensities of > 67 µmol photons m−2 s−1 gave saturated growth. Consequently, light intensity of 100–200 µmol photons m−2 s−1 was used as the standard light condition for all other experiments.
Figure. 1.
Determination of light intensity saturation for growth in Ulva prolifera and U. meridionalis. (a) U. prolifera at 20 °C. (b), U. meridionalis at 25 °C. Numerical values in key show light intensities (µmol photons m−2 s−1). (c) Changes in RGRs to light intensity gradient. The RGRs (n = 3) were calculated from 4 consecutive samples linearly arranged between 0.01 and 1 g in (a) and (b). Open circle, U. prolifera. Filled circle, U. meridionalis. Bar is standard error.
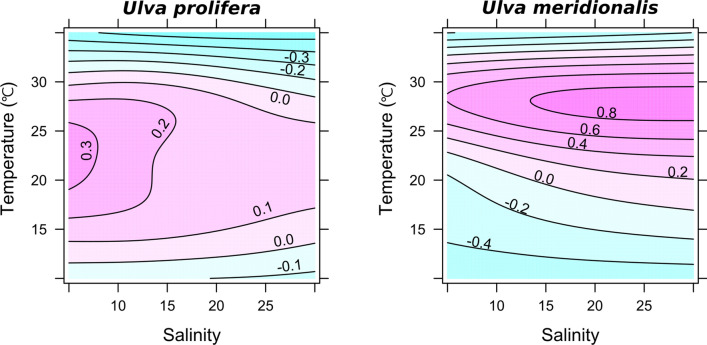
The RGRs of U. prolifera and U. meridionalis were obtained at practical ranges of temperature and salinity in their brackish habitats (Figs. 2, 3). Ulva prolifera showed consistently high RGRs (0.37–0.89 day−1) over broad ranges of salinities 5–30 and temperatures of 10–30 °C. In contrast, U. meridionalis showed extremely high RGRs of > 1.4 day−1 at salinities 10–30 at 30 °C, although the RGRs clearly declined as temperatures decreased. In the optimum condition of salinity 30 and 30 °C, U. meridionalis showed RGR 1.44 day−1, increasing 18-fold in fresh mass after 48 h of culture (Fig. 4). To check the intrinsic variability of the highest RGR, additional RGRs were repeatedly measured for samples taken from different generations. The result confirmed that the extremely high RGRs of around 1.4 day−1 are stable over generations (Table 1).
Figure. 2.
Growth characteristics in various combinations of temperature (10–35 °C) and salinity (5–30) of Ulva prolifera and U. meridionalis. RGRs in each combination (n = 4 in U. prolifera, n = 3 in U. meridionalis). The average RGRs (column) were calculated from consecutive fresh mass samples between 0.01 g and 1 g as in Fig. 1. Bar is standard error.
Figure. 3.
Estimation of optimal growth conditions by the generalized additive model using the data in Fig. 2. The areas (°C × psu) of the optimal growth conditions for temperature and salinity (≥ 0 = average RGR) were detected using the thin plate smoothing splines and 456.23 for U. prolifera and 348.80 for U. meridionalis.
Figure 4.

The maximum growth of thallus clusters of U. meridionalis. (a) The early growth stage. (b) The same thallus clusters after 48 h of culture at 30 °C and salinity 30. Scale bars, 1 cm (a,b).
Table 1.
The RGRs of Ulva prolifera and U. meridionalis at each temperature and salinity condition for the optimum growth. In addition to the RGR data in Fig. 2, further RGRs were measured of 2 and 5 seedling stocks of different generations and expressed as mean ± s.d. and range.
| Species (optimum growth condition) | RGR |
|---|---|
| Ulva prolifera (20 °C, salinity 5) | 0.81 ± 0.098, 0.70–0.89 (n = 3) |
| Ulva meridionalis (30 °C, salinity 30) | 1.41 ± 0.081, 1.28–1.46 (n = 6) |
Table 2 shows that C content in dry mass was not significantly different between U. meridionalis and U. prolifera. However, major nutrient contents of N and P in U. meridionalis were significantly lower than those in U. prolifera.
Table 2.
Chemical composition of cultured Ulva prolifera and U. meridionalis. Data are mean (± s.e., n = 4). Except for ash and C contents, average values of moisture and N and P contents were significantly different between the two species (P < 0.05).
| Moisture (%) | Ash (dry mass %) | C (dry mass %) | N (dry mass %) | P (dry mass %) | |
|---|---|---|---|---|---|
| Ulva prolifera | 85.2 ± 0.19 | 14.75 ± 0.74 | 35.01 ± 0.37 | 4.62 ± 0.07 | 0.59 ± 0.03 |
| Ulva meridionalis | 81.0 ± 0.58 | 12.75 ± 0.42 | 34.86 ± 0.43 | 3.74 ± 0.08 | 0.15 ± 0.01 |
Discussion
The results imply that U. prolifera is a generalist species having a stable growth ability over a wide range of environmental conditions, while U. meridionalis is a specialist species showing rapid growth in a narrow range of high temperatures (Fig. 3). The different growth characteristics reasonably correspond with their spatiotemporal growth patterns observed in the habitat. That is, Ulva prolifera occurs in a wide range of brackish estuaries with an extensive seasonal period of luxuriant growth, while U. meridionalis has extremely rapid growth in a limited area during a short summer period (Supplementary Fig. 1). Because various strains of U. meridionalis have been collected in tropical Okinawan islands15, the extremely high growth ability would be selected while being distributed in the high temperature environment.
The somatic cells of Ulva divide synchronously under standardized conditions once a day6. Accordingly, the RGRs of 0.37–0.89 day−1 in U. prolifera (Fig. 1a) are equal to a 1.4–2.4-fold increase per day, indicating that almost all the cells divide once a day. The highest RGRs ever reported in autotrophic multicellular algae are 1.03 day−1 and 1.00 day−1 (reported as 179.2 and 172.7% increment per day) in U. prolifera and U. linza, respectively16, around 0.67 day−1 in U. tepida17 and 0.68 day−1 in a filamentous Chaetomorpha species causing green tides in tropical waters18, which are similar RGRs as in U. prolifera in the present study. However, the maximum RGR of 1.41 day−1 in U. meridionalis (Table 1) means a 4.1-fold daily increase, suggesting that all the cells would divide at least twice a day. Two consecutive cell divisions per day have been reported in microscopic Ulva germlings for the early developmental stage during a single dark phase under ordinary light: dark cycle conditions19. However, Ulva meridionalis cells seem to be able to divide twice a day even in well-developed thalli as in Fig. 4.
Carbon content in dry mass was almost the same in U. meridionalis and U. prolifera (Table 2), demonstrating that U. meridionalis quickly builds up its plant body without reducing the carbon mass percentage and essentially has a carbon fixation ability twice as high as U. prolifera. Nevertheless, N and P contents in U. meridionalis were clearly lower than those in U. prolifera, especially the P content being only a quarter. The typical C to N to P stoichiometry by moles for algal biomass is C106: N16: P1, generally referred to as the Redfield ratio20. This average stoichiometry allows quantitative predictions to be made about the quantities of C, N and P required for algal production21. Our calculated value of C154: N17: P1 in U. prolifera is comparatively close to the typical stoichiometry. However, U. meridionalis has a much higher ratio of C to nutrients (= C595: N55: P1), implying that a larger biomass production would be effectively gained even with a lower nutrient supply, particularly for P. In our preliminary culture experiment using an outdoor tank with an upper one square meter of open area continuously supplied with natural seawater adjusted to a concentration of 20–30 µM nitrate and 2–3 µM phosphate, this species showed a high productivity of approximately 60 g-dry m−2 day−1 (ref. 14). Commercially prosperous Ulva production rates have been estimated to be 20–26 g-dry m−2 day−1 over a full year from pond raceway systems in South Africa5. If the same systems are operated in tropical regions in which seawater with the optimal high temperature for U. meridionalis can be constantly supplied, the production rates would be 2–3 times by using U. meridionalis. Biomass productivities of common industrial microalgae such as Chlorella and Spirulina have been reviewed to be 11–69 g-dry m−2 day−1 for open pond production systems or closed photobioreactors22. We have acquired a unique multicellular algal strain with easy handling for harvest and, in addition, having a high productivity nearly equal to the maximum values of microalgae. Ulva meridionalis cultivation would be one of the most effective options for CO2 fixation and biomass production in the future.
Methods
Strains and preparation of seedling stocks
The U. prolifera strain E1823 and U. meridionalis strain E1614,15 respectively maintained as unialgal isolates in Usa Marine Biological Institute, Kochi University were used. Their seedling stocks for the growth experiments were prepared according to the ‘germling cluster’ method for unattached macroalgal culture in the free-floating form24. Synchronous zoid formation in each strain was induced by cutting a well-developed thallus into small fragments of 1–2 mm length. Several tens of the fragments were cultured in a Petri dish containing 40 mL of enriched natural seawater (ES) medium25 at 20 °C for U. prolifera or 25 °C for U. meridionalis with a 12 h:12 h L:D cycle at 100–200 µmol photons m−2 s−1. Under these conditions, thallus fragments released zoids within 3 days. Aliquots of the zoid suspension densely concentrated using their phototactic response were placed in Petri dishes, adjusted to a density of > 104 zoids per 1 mL medium, and incubated under the same condition as mentioned above. After 2–3 weeks, germlings grew at a high density on the bottom of the dish and attached to one another to form aggregations that appear like a green mat. The aggregations were scraped off the dish, torn into numerous small clusters of germlings and cultured with aeration, drifting freely with the current in a vessel. When they attained a length of 1 mm or more, they were statically stocked under weak light (12 h:12 h L:D cycle at < 50 µmol photons m−2 s−1) at 20 °C until being used for the growth experiments.
Relative growth rate measurement
To reduce the lag phase caused by the inactive condition of the stocked materials, hundreds of the germling clusters were pre-cultured in a round 1L-flask with continuous aeration for several days. The flask was filled with 1/2 ES medium for which half the amount of the enrichment solution for the standard ES medium was added to artificial seawater adjusted to salinity 15 (Supplementary Table 2). Temperature and light conditions were set as above for the germling growth condition. The medium was exchanged every day. When the thalli of the clusters grew to 5–10 mm in length in this pre-culture, they were subsequently cultured in 500 mL-flasks at various experimental conditions set in the incubator (Supplementary Fig. 2).
Five light intensities from 6 to 103 µmol photons m−2 s−1 with a 12 h:12 h L:D cycle were provided by placing optical neutral filters (ND filter, Fuji Film, Tokyo, Japan) between the light source and the water bath in the setup (Supplementary Fig. 2). Light intensity was measured at the bottom of the flask with a LI-190SA quantum sensor (Li-Cor Biosciences, Lincoln, NE, USA). Around 0.01 g fresh mass of the thallus clusters was initially set in the flask filled with 500 mL of the 1/2 ES medium (Salinity 32) which was exchanged every other day. In order to determine the fresh mass of the living materials without causing damage by drying, the thallus clusters were held between sterilized paper towels more than five times to carefully remove water on the surface, immediately put in a Petri dish (6 cm in diameter) filled with each medium on the balance, quantified and returned to the same culture condition. This mass measurement was made within a few minutes at the end of the light period every day, equally spaced at 24 h-intervals. Relative growth rate expresses the continuously accelerating growth of algae during the exponential phase, represented by RGR = (ln W1—ln W0) day−1 in which W0 is the initial fresh mass in the culture at zero time, W1 being the mass after 24 h. The RGRs of U. prolifera and U. meridionalis were measured in a total of 36 conditions of various salinities (5, 10, 15, 20, 25, 30) and temperatures (10, 15, 20, 25, 30, 35 °C) under the standard light condition (100–200 µmol photons m−2 s−1). To confirm the stability of the highest growth performance, seedlings from different generations were produced by repeating subculture through zoids and their RGRs were measured at the optimum growth condition for each species.
Elemental composition of algal biomass
For analyses of elemental composition of the cultured Ulva, initial fresh mass of 0.01 g of U. prolifera and U. meridionalis were cultured in 1L-flasks filled with 1/2 ES medium at 20 °C and 25 °C, respectively. The medium was exchanged every other day. The samples were harvested after 6 days of culture, when they were in exponential growth. The samples were repeatedly rinsed with distilled water to remove residual salts from the culture medium and surface moisture was carefully removed with paper towels. Average fresh mass of the samples harvested from four replicates (n = 4) were 0.195 (± 0.009 s.e.) g for U. prolifera and 0.320 (± 0.006 s.e.) g for U. meridionalis. The water content of the fresh mass was determined by the weight difference before and after freeze drying of the thalli. The carbon (C) and nitrogen (N) content in the dried thalli was measured using CHNS analyzer (Flash EA, Thermo Fischer Scientific Inc. MA, USA). Phosphorus (P) content was determined by ICP-AES (Optima 4,300 DV CYCRON, PerkinElmer Inc., MA, USA).
Statistical analysis
Optimal growth conditions for U. prolifera and U. meridionalis were estimated from the RGR data in the tested combinations of temperature and salinity using thin plate smoothing splines of the generalized additive model. Then, we defined conditions indicating average and higher RGRs as the optimal growth conditions. Differences between the two species in chemical components were assessed with unpaired t-test.
Supplementary information
Acknowledgments
This work was supported by the Kochi University research project of the Biomass Refinery of Marine Algae and the Wood and Cabinet Office grant in aid, the Advanced Next-Generation Greenhouse Horticulture by IoP (Internet of Plants), Japan.
Author contributions
M.H. designed the experiments. M.H., Y.K. and A.P.M. performed the algal growth experiments. Mo.H. analysed the growth data. S.T. and A.O. analysed the chemical composition of the algal samples. M.H. and A.D. made the field samplings. M.H. wrote the manuscript with the help of all other authors. A.O. supervised the project.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-69536-4.
References
- 1.Georgianna DR, Mayfield SP. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature. 2012;488:329–335. doi: 10.1038/nature11479. [DOI] [PubMed] [Google Scholar]
- 2.Mohan SV, et al. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016;215:2–12. doi: 10.1016/j.biortech.2016.03.130. [DOI] [PubMed] [Google Scholar]
- 3.Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science. 2010;329:796–799. doi: 10.1126/science.1189003. [DOI] [PubMed] [Google Scholar]
- 4.Barros AI, Gonçalves AL, Simões M, Pires JCM. Harvesting techniques applied to microalgae: a review. Renew. Sustain. Energy Rev. 2015;41:1489–1500. doi: 10.1016/j.rser.2014.09.037. [DOI] [Google Scholar]
- 5.Bolton JJ, Cyrus MD, Brand MJ, Joubert M, Macey BM. Why grow Ulva? Its potential role in the future of aquaculture. Perspect. Phycol. 2016;3:113–120. doi: 10.1127/pip/2016/0058. [DOI] [Google Scholar]
- 6.Wichard T, et al. The green seaweed Ulva: a model system to study morphogenesis. Front. Plant Sci. 2015;6:72. doi: 10.3389/fpls.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiraoka M, Higa M. Novel distribution pattern between coexisting sexual and obligate asexual variants of the true estuarine macroalga Ulva prolifera. Ecol. Evol. 2016;6:3658–3671. doi: 10.1002/ece3.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smetacek V, Zingone A. Green and golden seaweed tides on the rise. Nature. 2013;504:84–88. doi: 10.1038/nature12860. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, et al. Taxonomic reassessment of Ulva prolifera (Ulvophyceae, Chlorophyta) based on specimens from the type locality and Yellow Sea green tides. Phycologia. 2018;57:692–704. doi: 10.2216/17-139.1. [DOI] [Google Scholar]
- 10.Liu D, et al. The world's largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuar. Coast. Shelf Sci. 2013;129:2–10. doi: 10.1016/j.ecss.2013.05.021. [DOI] [Google Scholar]
- 11.Lawton RJ, Mata L, de Nys R, Paul NA. Algal bioremediation of waste waters from land-based aquaculture using Ulva: selecting target species and strains. PLoS ONE. 2013;8:e77344. doi: 10.1371/journal.pone.0077344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang R, et al. Thermochemical hydrolysis of macroalgae Ulva for biorefinery: Taguchi robust design method. Sci. Rep. 2016;6:27761. doi: 10.1038/srep27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dan A. Physiological and ecological studies on Enteromorpha prolifera (Müller) J. Agardh and its applications for cultivation. Bull. Tokushima Pref. Fish. Res. Ins. 2008;6:1–78. [Google Scholar]
- 14.Tsubaki S, Zhu W, Hiraoka M. Production and conversion of green macroalgae (Ulva spp.) In: Kerton FM, Yan N, editors. Fuels, Chemicals and Materials from the Oceans and Aquatic Sources. Hoboken: Wiley; 2017. pp. 19–41. [Google Scholar]
- 15.Horimoto R, Masakiyo Y, Ichihara K, Shimada S. Enteromorpha-like Ulva (Ulvophyceae, Chlorophyta) growing in the Todoroki River, Ishigaki Island, Japan, with special reference to Ulva meridionalis Horimoto et Shimada, sp. nov. Bull. Natl. Mus. Nat. Sci., Ser. B. Natl. 2011;37:155–167. [Google Scholar]
- 16.Xie E, et al. Growth characteristics of hybrids produced by closely related Ulva species. Aquaculture. 2020;519:734902. doi: 10.1016/j.aquaculture.2019.734902. [DOI] [Google Scholar]
- 17.Carl C, de Nys R, Paul NA. The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS ONE. 2014;9:e98700. doi: 10.1371/journal.pone.0098700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutsui I, et al. High tolerance of Chaetomorpha sp. to salinity and water temperature enables survival and growth in stagnant waters of central Thailand. Int. Aquatic. Res. 2015;7:47–62. doi: 10.1007/s40071-014-0092-4. [DOI] [Google Scholar]
- 19.Kuwano K, Sakurai R, Motozu Y, Kitade Y, Saga N. Diurnal cell division regulated by gating the G1/S transition in Enteromorpha compressa (Chlorophyta) J. Phycol. 2014;44:364–373. doi: 10.1111/j.1529-8817.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 20.Redfield AC. The biological control of chemical factors in the environment. Am. Sci. 1958;46:205–221. [PubMed] [Google Scholar]
- 21.Shurin JB, et al. Industrial-strength ecology: trade-offs and opportunities in algal biofuel production. Ecol. Lett. 2014;16:1393–1404. doi: 10.1111/ele.12176. [DOI] [PubMed] [Google Scholar]
- 22.Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;14:557–577. doi: 10.1016/j.rser.2009.10.009. [DOI] [Google Scholar]
- 23.Hiraoka M, et al. Different life histories of Enteromorpha prolifera (Ulvales, Chlorophyta) from four rivers on Shikoku Island Japan. Phycologia. 2003;42:275–284. doi: 10.2216/i0031-8884-42-3-275.1. [DOI] [Google Scholar]
- 24.Hiraoka M, Oka N. Tank cultivation of Ulva prolifera in deep seawater using a new “germling cluster” method. J. Appl. Phycol. 2008;20:97–102. doi: 10.1007/s10811-007-9186-3. [DOI] [Google Scholar]
- 25.Andersen RA, Berges JA, Harrison PJ, Watanabe MM. Appendix A—recipes for freshwater and seawater media. In: Andersen RA, editor. Algal Culturing Techniques. Cambridge: Elsevier Academic Press; 2005. pp. 429–538. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.