Abstract
Background
The identification of frailty is considered an effective means of enhancing healthy aging. The definition of frailty affects its prevalence and associated institutionalization and mortality. This study aimed to identify the prevalence of frailty among community-dwelling older Korean adults according to different frailty scales.
Methods
This cross-sectional study based on the Korean Frailty and Aging Cohort Study represents a population of 1,318 people aged 70 years and older. Discrepancies in the prevalence of frailty were evaluated among six validated assessment tools. Multivariate logistic regression analysis was used to evaluate the prevalence of frailty according to its predictors (age, sex, and socioeconomic status).
Results
The mean age of the participants was 76.1 (standard deviation, 3.9) years, and females comprised 51.0%. The prevalence of frailty varied from 2.5% to 12.4% using the Study of Osteoporotic Fracture frailty index and the Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of Weight scale, respectively. The prevalence of frailty was higher among women and frailty rates increased with age on all scales. The risks of prefrailty and frailty were increased among participants with a low education level after adjusting for age, sex, residence, and income level.
Conclusion
In this study, the prevalence of frailty was found to vary depending on the scale used. Efforts aimed at screening and providing intervention for frailty and frail adults at risk, respectively, are needed to improve health outcomes considering the characteristics of each frailty scale and the determined prevalence.
Keywords: Frailty, Prevalence, Frailty scale, Older, Korean Frailty and Aging Cohort Study
INTRODUCTION
The aging population is increasing in Korea at a more rapid rate compared to other countries due to the persistently low birth rate and extended life expectancy.1) The proportion of adults aged 65 years and older is about 14% and is predicted to increase to 20% with a super-aged society in 2026.2) These situations affect the incidence of health problems among older adults and could create an enormous socioeconomic burden on the Korean society. Health or functional status may vary diversely among individuals of the same chronological age, although frailty often comes with increasing age.3) Frailty may lead to functional decline and increased rates of hospitalization, institutionalization, and mortality. According to the Cardiovascular Health Study (CHS), among 5,317 subjects aged 65 years or older, the 3-year mortality among frail persons was twice as high as that among robust older adults.3) A study of 11,844 older Korean adults also showed a significantly higher mortality risk among frail participants (hazard ratio, 2.28; 95% confidence interval [CI], 1.61–3.22).1) A systematic review reported a 3%–5% decrease in mortality risk in older adults with the prevention of frailty, considering the decrease in its prevalence and relative risk.4) Therefore, comprehensive assessment and prevention of frailty are critical to enhancing the quality of life, preventing functional declines, and decreasing mortality and institutionalization.3–5)
The prevalence of frailty tends to increase with age and is higher among women and subjects with chronic diseases.3,6,7) However, the prevalence of frailty is extremely heterogeneous with proportions ranging from 10%–21.3% as frail and 43%–59% as prefrail, depending on the definition or components of frailty.8–10) A systematic review reported a wide range of prevalence rates for frailty, from 4.0%–59.1%, although different definitions of frailty were used in the 21 studies.11) Moreover, this discrepancy in the prevalence of phenotypic frailty has been found in different countries, populations, and individuals of different economic statuses.12,13)
Frailty is considered a clinical syndrome that results from increased vulnerability and results in disabilities. Considering this, it is essential to identify the frail population by establishing a specific and universal definition of frailty to prevent disabilities. The present study was designed to estimate the difference in prevalence of frailty using known validated frailty scales in the same populations and to evaluate the associations between frailty and age and sex and its relation to socioeconomic status.
MATERIALS AND METHODS
Study Design and Data Collection
The Korean Frailty and Aging Cohort Study (KFACS) was designed as a multicenter, longitudinal study; the baseline survey was conducted from January 2016 to December 2016. Data were obtained from participants aged 70–84 years, stratified by age and sex and recruited from urban and rural regions nationwide. Ten medical centers were selected, and 1,318 community-dwelling older adults without dependency were included in this population-based, cross-sectional study. The interviewers completed training on how to study subjects using specific interviewing skills and physical performance measurements. The components of frailty were determined via in-person interview and health examination. Frailty phenotypes and socioeconomic and demographic indicators were obtained by trained investigators. We compared the prevalences of frailty according to six validated frailty scales commonly used in Korea: the CHS frailty index,3) the Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of Weight scale (K-FRAIL scale),14) the Korean Frailty Index (KFI),10) the Frailty Instrument (FI),15) the Korean Longitudinal Study on Health and Aging Frailty Index (KLoSHA),8) and the Study of Osteoporotic Fracture (SOF) frailty index.16)
Definitions of Frailty Scales
CHS frailty index3)
To assess frailty, a modified version of the well-validated CHS frailty index was used. Five components were included as follows: unintentional weight loss, exhaustion, low physical activity, weakness, and slowness. Weight loss was defined as an unintentional loss of more than 4.5 kg or 5% of the body weight recorded in the preceding year. Exhaustion was evaluated by self-reporting either the feeling that every activity required effort or being unable to “get going” in the preceding week. The level of activity was calculated as the energy expenditure for the preceding 1 week using the International Physical Activity Questionnaire-Short Form and was said to be low if the energy expenditure was less than 20% among participants of the KFACS (<494.65 kcal for men and <283.50 kcal for women). Weakness was assessed by grip strength (<26 kg for men and <18 kg for women). Slowness was measured using a 4-m walking speed, where 1.0 m/sec was considered as the cutoff point. The CHS frailty scale score, with the sum of each component scored as one point, was classified as follows: frail (3–5), prefrail (1–2), and robust (0) health status.
K-FRAIL
The K-FRAIL scale14) has 5 components: fatigue, resistance, ambulation, illness, and loss of weight. Fatigue was assessed by asking participants how much time during the preceding 4 weeks they felt tired, with responses of “all of the time” or “most of the time” scored as one point. Resistance was defined as difficulty in walking up ten stair steps alone without resting and without aids, and ambulation was assessed by asking whether they had any difficulty in walking 300 m alone and without aids. Illness was defined as having five or more conditions out of 11: hypertension, diabetes mellitus, chronic obstructive pulmonary disease, angina, myocardial infarction, heart failure, asthma, arthritis, stroke, renal disease, and cancer. Loss of weight was recorded as the loss of at least 5% of the body weight within the preceding year. These scale scores were classified as frail (3–5), prefrail (1–2), and robust (0) health status.
Korean frailty index
The KFI scale10) was developed by experts from the Korean Geriatrics Society. This scale includes an 8-item questionnaire. Hospital admission was assessed by asking how many times participants had been admitted to the hospital in the preceding year and a response of “more than once” was scored one point. Self-assessment of health status was evaluated by asking what they thought of their health, and response of “poor” was scored one point. Polypharmacy was defined if participants took more than four drugs, including herbal medications, regularly. Weight loss was identified by asking if they had lost weight to such an extent that they had observed loosening of clothing recently. Depressed mood, incontinence, and auditory and visual disturbance were evaluated by asking “have you been depressed or sad in recent months?,” “have you had any involuntary leakage of urine or defecation in the last month?,” or “have you ever had problems hearing or visual disturbance in your daily life?,” respectively. The timed up and go test was performed, and one point was scored if the test took more than 10 seconds to perform. The scores, one point for each component, ranged from 0 to 8 cutoff values for prefrail (≥2.5) and frail (≥4.5) and were defined by distributions of frailty.
Frailty instrument
The FI scale consists of weakness of grip strength, exhaustion, and social isolation.15) Weakness was measured by grip strength (<24 kg for men and <15 kg for women). Exhaustion was evaluated by self-reporting either of the feeling that every task required effort or they could not “get going” in the preceding week. Isolation was assessed by asking if they were participating in meetings or group activities. This scale score ranged from 0–3 and was categorized as follows: frail (≥2), prefrail (≥1), and robust (0) health status.
KLoSHA frailty index
The KLoSHA frailty index scale8) has five components: functional status, physical performance, cognitive function, mood, and nutritional status using serum albumin level.
Functional status is calculated using the Korean Activities of Daily Living scale and the Korean Instrumental Activities of Daily Living scale.17) Physical performance was measured using the short physical performance battery.18) Cognition was evaluated using the Korean Mini-Mental State Examination19) and mood using the Korean version of the Geriatric Depression Scale (GDS-K).20) Weighted values were applied for each domain, rather than a simple summation, by clinical importance. With a possible total score of 1, cutoff values for prefrail (0.2) and frail (0.35) are defined by distributions of frailty.
SOF frailty index
A modified SOF frailty index16) was used with the following components: (1) unintentional weight loss of 5% or more over 1 year; (2) time for standing up 5 times over 60 seconds; and (3) reduced level of energy indicated by the question “Do you feel full of energy?” on the GDS-K.20) The scores, one point for each component, ranging from 0 to 3, were classified as follows: frail (2–3), prefrail (1), and robust (0) health status.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). We used an independent t-test for continuous variables and a chi-square test for discrete variables to evaluate the sociodemographic characteristics by sex. Frailty prevalence was measured using the CHS,3,21) K-FRAIL scale,14) KFI,10) FI,15) KLoSHA,8) and SOF.16) We calculated the age-adjusted prevalence rates (age-adjusted to the 2016 standard population) for frailty to make fairer comparisons among these scales with different age distribution. Odds ratios (ORs) of prefrailty and frailty were evaluated by age distribution after adjusting for age, sex, residence, education, and income level using multivariate logistic regression analysis. We conducted further analyses to investigate the effect of socioeconomic status on the risk of frailty.
This study was approved by the Institutional Review Board of the Clinical Trial Review Committee of the Kyung Hee University Medical Center and complied with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each participant before or at registration (KMC IRB 2015-12-103).
RESULTS
Table 1 shows the sociodemographic characteristics of the study participants. The mean age of participants was 76.1 (standard deviation, 3.9) years. Among the participants, 24.1% comprised the oldest old age group (≥80 years), and women comprised 51%. Participants with urban residence and low education and income levels were more commonly women than men.
Table 1.
Sociodemographic characteristics of the study sample
| Characteristic | Total (n=1,318) | Men (n=647) | Women (n=671) | p-value |
|---|---|---|---|---|
| Age (yr) | 76.1±3.9 | 76.4±3.9 | 75.8±3.9 | 0.014 |
| 70–74 | 521 (39.5) | 239 (36.9) | 282 (42.0) | 0.140 |
| 75–79 | 479 (36.3) | 241 (37.2) | 238 (35.5) | |
| ≥80 | 318 (24.1) | 167 (25.8) | 151 (22.5) | |
| Residence | 0.012 | |||
| Urban | 745 (56.5) | 343 (53.0) | 402 (59.9) | |
| Rural | 573 (43.5) | 304 (47.0) | 269 (40.1) | |
| Education level | <0.001 | |||
| <High school | 851 (64.6) | 322 (49.8) | 529 (78.8) | |
| High school | 244 (18.4) | 155 (24.0) | 87 (13.0) | |
| ≥College or greater | 225 (17.1) | 170 (26.3) | 55 (8.2) | |
| Income level | <0.001 | |||
| 1st quartile, Low | 280/1,116 (25.1) | 101/561 (18.0) | 179/555 (32.3) | |
| 2nd quartile | 292/1,116 (26.2) | 131/561 (23.4) | 161/555 (29.0) | |
| 3rd quartile | 269/1,116 (24.1) | 151/561 (26.9) | 118/555 (21.3) | |
| 4th quartile, High | 275/1,116 (24.6) | 178/561 (31.7) | 97/555 (17.5) |
Values are presented as mean ± standard deviation or number (%).
Table 2 shows the prevalence of frailty according to the six frailty scales. The prevalence of frailty varied according to frailty scale (SOF, 2.5%; K-FRAIL, 12.4%). The K-FRAIL yielded the highest prevalence of frailty among the scales. Even when the same frailty scale was considered, the prevalence of frailty among women was higher than that among men (CHS: 14.6% of women, 7.6% of men). These trends were confirmed by almost all frailty scales except the SOF frailty index. Particularly, the gap was increased in the K-FRAIL (18.2% of women and 6.5% of men). On the other hand, the lowest prevalence of frailty (4.3% of women, 3.2% of men) was determined using the KLoSHA. However, the KLoSHA yielded the highest prevalence of frailty among women. The SOF did not yield this sex difference in frailty prevalence.
Table 2.
Prevalence of frailty according to different frailty scales
| Frailty scale | Total (n=1,318) | Men (n=647) | Women (n=671) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Robust | Prefrail | Frail | Robust | Prefrail | Frail | Robust | Prefrail | Frail | |
| CHS | 516 (39.2) | 655 (49.7) | 147 (11.2) | 311 (48.1) | 287 (44.4) | 49 (7.6) | 205 (30.6) | 368 (54.8) | 98 (14.6) |
|
| |||||||||
| K-FRAIL | 555 (42.1) | 599 (45.4) | 164 (12.4) | 348 (53.8) | 257 (39.7) | 42 (6.5) | 207 (30.8) | 342 (51.0) | 122 (18.2) |
|
| |||||||||
| KFI | 833 (63.2) | 365 (27.7) | 120 (9.1) | 451 (69.7) | 148 (22.9) | 48 (7.4) | 382 (56.9) | 217 (32.3) | 72 (10.7) |
|
| |||||||||
| FI | 820 (62.2) | 422 (32.0) | 76 (5.8) | 451 (69.7) | 164 (25.3) | 32 (4.9) | 369 (55.0) | 258 (38.5) | 44 (6.6) |
|
| |||||||||
| KLoSHA | 833 (63.2) | 435 (33.0) | 50 (3.8) | 394 (60.9) | 232 (35.9) | 21 (3.2) | 439 (65.4) | 203 (30.3) | 29 (4.3) |
|
| |||||||||
| SOF | 862 (65.4) | 423 (32.1) | 33 (2.5) | 465 (71.9) | 165 (25.5) | 17 (2.6) | 397 (59.2) | 258 (38.5) | 16 (2.4) |
Values are presented as number (%).
CHS, Cardiovascular Health Study; K-FRAIL, Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight scale; KFI, Korean Frailty Index; FI, Frailty Instrument; KLoSHA, Korean Longitudinal Study on Health and Aging; SOF, Study of Osteoporotic Fracture.
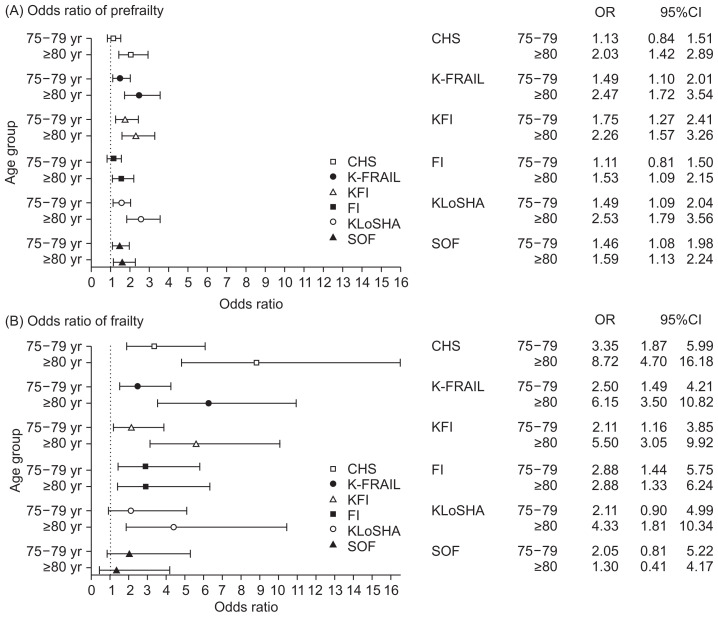
The proportion of robustness tended to decrease as those of frailty and prefrailty increased with age regardless of frailty scale (Table 3). Frailty prevalence appeared to vary from 22.0% on the CHS to 3.1% on the SOF, particularly in the oldest old-age group. We estimated an OR for pre-frailty and frailty to ascertain the difference according to age distribution using a logistic regression model (Fig. 1). Compared to ages 70–74 years, the risk of prefrailty significantly increased with age, except for participants aged 75 to 79 years evaluated using the CHS (OR, 1.13; 95% CI, 0.84–1.51) and FI (OR, 1.11; 95% CI, 0.81–1.50). The statistical significance of frailty is also shown in Fig. 1, B. This significance disappeared on the SOF (75–79 years: OR, 2.05; 95% CI, 0.81–5.22; ≥80 years: OR, 1.30; 95% CI, 0.41–4.17). Age-adjusted prevalence yielded similar results to unadjusted prevalence, making objective comparisons possible by adjusting different age structures between frailty scales (Table 4).
Table 3.
Prevalence of frailty according to age distribution
| Frailty scale | 70–74 Years (n=521) | 75–79 Years (n=479) | ≥80 Years (n=318) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Robust | Prefrail | Frail | Robust | Prefrail | Frail | Robust | Prefrail | Frail | |
| CHS | 237 (45.5) | 261 (50.1) | 23 (4.4) | 199 (41.5) | 226 (47.2) | 54 (11.3) | 80 (25.2) | 168 (52.8) | 70 (22.0) |
|
| |||||||||
| K-FRAIL | 268 (51.4) | 218 (41.8) | 35 (6.7) | 195 (40.7) | 224 (46.8) | 60 (12.5) | 92 (28.9) | 157 (49.4) | 69 (21.7) |
|
| |||||||||
| KFI | 382 (73.3) | 113 (21.7) | 26 (5.0) | 297 (62.0) | 145 (30.3) | 37 (7.7) | 154 (48.4) | 107 (33.6) | 57 (17.9) |
|
| |||||||||
| FI | 349 (67.0) | 155 (29.8) | 17 (3.3) | 304 (63.5) | 143 (29.9) | 32 (6.7) | 167 (52.5) | 124 (39.0) | 27 (8.5) |
|
| |||||||||
| KLoSHA | 384 (73.7) | 126 (24.2) | 11 (2.1) | 302 (63.0) | 160 (33.4) | 17 (3.5) | 147 (46.2) | 149 (46.9) | 22 (6.9) |
|
| |||||||||
| SOF | 367 (70.4) | 145 (27.8) | 9 (1.7) | 310 (64.7) | 155 (32.4) | 14 (2.9) | 185 (58.2) | 123 (38.7) | 10 (3.1) |
Values are presented as number (%).
CHS, Cardiovascular Health Study; K-FRAIL, Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight scale; KFI, Korean Frailty Index; FI, Frailty Instrument; KLoSHA, Korean Longitudinal Study on Health and Aging; SOF, Study of Osteoporotic Fracture.
Fig. 1.
Odds ratio of prefrailty and frailty according to age distribution. Multiple logistic regression analysis was performed after adjusting for sex, income, residence, and education level. (A) Odds ratio of prefrailty among older adults, compared with that of robustness. (B) Odds ratio of frailty among older adults, compared with that of robustness. CHS, Cardiovascular Health Study; K-FRAIL, Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight scale; KFI, Korean Frailty Index; FI, Frailty Instrument; KLoSHA, Korean Longitudinal Study on Health and Aging; SOF, Study of Osteoporotic Fracture.
Table 4.
Age-adjusted prevalence of frailty according to different frailty scales
| Frailty scale | Total (n=1,318) | Men (n=647) | Women (n=671) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Robust | Pre-frail | Frail | Robust | Pre-frail | Frail | Robust | Pre-frail | Frail | |
| CHS | 39.7 | 49.6 | 10.7 | 49.9 | 43.7 | 6.4 | 30.2 | 54.8 | 15.0 |
|
| |||||||||
| K-FRAIL | 42.7 | 45.3 | 12.0 | 56.0 | 38.3 | 5.7 | 30.4 | 51.0 | 18.6 |
|
| |||||||||
| KFI | 63.9 | 27.3 | 8.8 | 71.5 | 21.8 | 6.7 | 56.4 | 32.6 | 11.0 |
|
| |||||||||
| FI | 62.6 | 31.8 | 5.6 | 70.5 | 25.0 | 4.5 | 54.6 | 38.7 | 6.7 |
|
| |||||||||
| KLoSHA | 64.0 | 32.4 | 3.6 | 62.5 | 34.7 | 2.8 | 64.8 | 30.8 | 4.4 |
|
| |||||||||
| SOF | 65.8 | 31.8 | 2.4 | 74.0 | 24.6 | 2.4 | 58.9 | 38.7 | 2.4 |
Values are presented as percentage.
CHS, Cardiovascular Health Study; K-FRAIL, Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight scale; KFI, Korean Frailty Index; FI, Frailty Instrument; KLoSHA, Korean Longitudinal Study on Health and Aging; SOF, Study of Osteoporotic Fracture.
The association between sociodemographic factors and frailty is shown in Table 5. Participants residing in rural areas had statistically higher risks of frailty (CHS: OR, 2.65; 95% CI, 1.67–4.19; K-FRAIL: OR, 3.62; 95% CI, 2.30–5.70; KFI: OR, 1.65; 95% CI, 1.03–2.62; FI: OR, 3.37; 95% CI, 1.82–6.24; SOF: OR, 3.68; 95% CI, 1.41–9.57). A level of education of less than high school significantly increased the risk of both prefrailty and frailty. A low income level was also associated with an increased risk of frailty (CHS; OR, 1.83; 95% CI, 1.14–2.93; K-FRAIL: OR, 1.97; 95% CI, 1.25–3.12; KFI: OR, 1.64; 95% CI, 1.01–2.67; FI: OR, 1.83; 95% CI, 1.02–3.26; KLoSHA: OR, 2.62; 95% CI, 1.32–5.21) except when obtained using the SOF (OR, 1.66; 95% CI, 0.71–3.90).
Table 5.
Sociodemographic factors associated with frailty
| Frailty scale | Residence (rural)* | Education (<high school)† | Income (low income)‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Prefrail | Frail | Prefrail | Frail | Prefrail | Frail | |||||||
|
|
|
|
|
|
|
|||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| CHS | 1.26 | 0.96–1.66 | 2.65 | 1.67–4.19 | 1.68 | 1.27–2.22 | 2.98 | 1.68–5.29 | 0.96 | 0.69–1.33 | 1.83 | 1.14–2.93 |
|
| ||||||||||||
| K-FRAIL | 1.43 | 1.08–1.89 | 3.62 | 2.3–5.7 | 2.00 | 1.51–2.66 | 4.89 | 2.63–9.08 | 1.14 | 0.82–1.6 | 1.97 | 1.25–3.12 |
|
| ||||||||||||
| KFI | 1.21 | 0.91–1.61 | 1.65 | 1.03–2.62 | 1.91 | 1.39–2.62 | 2.51 | 1.41–4.45 | 1.44 | 1.05–1.99 | 1.64 | 1.01–2.67 |
|
| ||||||||||||
| FI | 1.16 | 0.88–1.53 | 3.37 | 1.82–6.24 | 2.13 | 1.57–2.88 | 2.84 | 1.35–5.98 | 1.25 | 0.91–1.71 | 1.83 | 1.02–3.26 |
|
| ||||||||||||
| KLoSHA | 1.25 | 0.95–1.64 | 1.64 | 0.83–3.24 | 2.36 | 1.74–3.21 | 8.87 | 2.59–30.39 | 1.57 | 1.15–2.14 | 2.62 | 1.32–5.21 |
|
| ||||||||||||
| SOF | 1.65 | 1.26–2.17 | 3.68 | 1.41–9.57 | 1.89 | 1.40–2.56 | 7.88 | 1.78–34.92 | 1.15 | 0.85–1.57 | 1.66 | 0.71–3.90 |
OR, odds ratio; CI, confidence interval; CHS, Cardiovascular Health Study; K-FRAIL, Korean version of the Fatigue, Resistance, Ambulation, Illnesses, and Loss of weight scale; KFI, Korean Frailty Index; FI, Frailty Instrument; KLoSHA, Korean Longitudinal Study on Health and Aging; SOF, Study of Osteoporotic Fracture.
Odds of rural participants compared to that of urban adjusted for age, sex, education, and income level.
Odds of participants with lower education compared to high school graduates adjusted for age, sex, residence, and income level.
Odds of participants with low-income level adjusted for age, sex, residence, and education.
DISCUSSION
The prevalence of frailty varied from 2.5% to 12.4% among 6 commonly used frailty scales among community-dwelling Korean older adults. Despite evaluating the same population, the wide range of prevalence rate appears to have been attributable to the diversity in the components of each frailty scale found in the literature. These differences led to the creation of a gap between selected frail populations from each frailty scale, due to the nature of each scale and development background. The CHS, mainly represented as physical phenotype, is estimated depending on relatively objective measures and therefore consists of physical components only.3) However, the KFI, FI, and SOF have a broader definition of frailty, including social and psychological aspects. The K-FRAIL scale was validated only for the urban population in Korea; the mean age and proportion of women in the study population was slightly higher than that of our study.14) Furthermore, as this scale depends on self-reported estimation, personal or cultural differences in perceptions of health may lead to misestimation of respondents’ health statuses.22) For this reason, the prevalence rate would differ from that obtained in the original validation study.14) The KFI, FI, and KLoSHA were developed and validated only in Korea. The proportion of women included in a validation study of the KFI was 72.1%, which supports the high prevalence of frailty (21.3%) in an original KFI validation study.10) Since the FI scale was developed to evaluate frailty from the perspective of prevention for long-term care, the average age of the study participants was lower (70.6 years) and the proportion of women was higher (56.6%) than that of our study.15) Furthermore, components such as grip strength and walking speed, associated with survival and life expectancy23,24) and that reflect on health and functional status25) among older adults, were missing in the FI because of usability and quickness of the assessment process.15) These support the lower prevalence of frailty in our study. As a result, we can summarize the difference in prevalence of frailty between our study and other studies as follows. The prevalence rate determined via the CHS (11.2%) in this study was higher than that in the original CHS study (6.9%),3) but was similar to that obtained in another study conducted in the Korean population (13.2%).8) The prevalence rate of frailty determined via the K-FRAIL in this study was lower (12.4%) than that obtained in an original validation study (17.5%).14) Similarly, the frailty prevalence rate was lower in this study (9.1%) than that obtained in a validation study of the KFI (23.1%).10) With regard to FI, the frailty prevalence rate was 5.8% in this study, and 21.3% in a validation study.15) The rates of frailty in this study were lower in both the KLoSHA and SOF frailty scales.8) These differences in prevalence could be attributable to the characteristics of the study population such as age, sex, socioeconomic status, and regional difference.26,27) Another noticeable point was the agreement to consider frailty as a predisability stage.28) Considering frailty as a continuum from robustness to disability, we should understand disability as a consequence of frailty and various diseases as a leading cause of frailty that trigger physiological vulnerability. Thus, disability might not be included in the definitions of frailty. Having this factor as one of the components of a frailty scale could increase the association with frailty.
Despite these differences, some properties of frailty have been manifested repeatedly across studies using different frailty scales. The finding of this study that frailty increases with age is consistent with those of previous studies.11,22,29) In the present study, the oldest adults (age≥80 years) were estimated to comprise about 24% of all participants, and the prevalence of frailty for the oldest responders ranged from 3.1%–22.0%, depending on the frailty scale used in this study. The frailty prevalence could vary by measurement setting and may be underestimated in current community-based studies.
The finding that women generally have higher frailty prevalence than men on all scales is in keeping with the well-accepted knowledge,11,30) given that women have a longer life expectancy and lower average scores of lean body mass and muscle strength, which are related to frailty.7,31) These physiological characteristics have been posited to explain the differences between men and women.
This study showed that residency in rural areas and having lower education and income levels were associated with overall frailty, which is consistent with previous studies. A study from the Canadian Study of Health and Aging showed that the rural-urban difference of frailty in the oldest and rural residents tended to reflect a higher frailty risk for rural residents.32) The increase in the prevalence of frailty with low educational level was explained in a 13-year longitudinal study. Low income was the strongest contributing factor to the educational difference. Lifestyle, such as smoking, obesity, unhealthy eating habits, and poor healthcare seeking behavior due to low income, can explain these associations.33) All 3 explained the characteristics of frailty; low education level additionally explained the characteristics of prefrailty much better than other so-ciodemographic factors in our study.
This study has some limitations. First, almost all participants registered in this study were healthier and better able to undergo several examinations. Thus, frailty prevalence might be underestimated in this study conducted among community-dwelling older adults compared with the general population. Second, modified frailty scales were used in the identification of frailty in this study. For example, slowness in the original CHS was defined by the lowest 20% of the subjects on a walk of 15 feet after adjustment for sex and height. However, in the modified CHS, a speed of 1.0 m/sec on a 4-m walk was the cut off point for frailty. On the modified SOF, we replaced “inability to stand from a chair 5 times” in the original version with “time to stand up 5 times over 60 seconds” in the short physical performance battery. These alterations in some components of the original version may result in differences in the prevalence of frailty.
However, the present study may be meaningful in that it compared the prevalence of frailty according to different validated frailty scales in the same Korean populations using a prospective cohort study designed to develop a frailty scale. We reconfirmed that the frailty prevalence increases with age and is higher among women. Sociodemographic factors, including residence in a rural area, low education, and low-income level, were associated with an increased frailty prevalence. This cohort study was extended to most areas of Korea and may be generalized.
One of the most important reasons for assessing frailty is to classify a population for preventing frailty. The use of different scales leads to the selection of different sets of frail adults. For this reason, efforts aimed at screening and providing intervention for frailty and frail adults at risk, respectively, considering the characteristics of each frailty scale and prevalence of frailty should be the first step before organizing appropriate treatment and preventive measures for the underlying causes of frailty.
In conclusion, the prevalence of frailty may differ significantly according to frailty scale. Therefore, we may choose the frailty scale with consideration of the difference in prevalence rates and characteristics of each frailty scale when classifying frail populations.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C3153).
Footnotes
The researchers claim no conflicts of interest.
REFERENCES
- 1.Lee Y, Kim J, Han ES, Ryu M, Cho Y, Chae S. Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology. 2014;60:475–82. doi: 10.1159/000362330. [DOI] [PubMed] [Google Scholar]
- 2.Korean Statistical Information Service . Population projections for Korea: 2010–2060 (Based on the 2010 Census) Daejoen (Korea): Korean Statistical Information Service; 2012. [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 4.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–36. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Jung HW, Kim KI. Multimorbidity in older adults. J Korean Geriatr Soc. 2014;18:65–71. doi: 10.4235/jkgs.2014.18.2.65. [DOI] [Google Scholar]
- 6.Rockwood K, Howlett SE, MacKnight C, Beattie BL, Bergman H, Hébert R, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–7. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 7.Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc. 2014;15:853–9. doi: 10.1016/j.jamda.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Jung HW, Kim SW, Ahn S, Lim JY, Han JW, Kim TH, et al. Prevalence and outcomes of frailty in Korean elderly population: comparisons of a multidimensional frailty index with two phenotype models. PLoS One. 2014;9:e87958. doi: 10.1371/journal.pone.0087958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung HW, Jang IY, Lee YS, Lee CK, Cho EI, Kang WY, et al. Prevalence of frailty and aging-related health conditions in older Koreans in rural communities: a cross-sectional analysis of the aging study of Pyeongchang rural area. J Korean Med Sci. 2016;31:345–52. doi: 10.3346/jkms.2016.31.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang HS, Kwon IS, Park BJ, Cho B, Yoon JL, Won CW. The validity and reliability of Korean frailty index. J Korean Geriatr Soc. 2010;14:191–202. doi: 10.4235/jkgs.2010.14.4.191. [DOI] [Google Scholar]
- 11.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 12.Payne CF, Wade A, Kabudula CW, Davies JI, Chang AY, Gomez-Olive FX, et al. Prevalence and correlates of frailty in an older rural African population: findings from the HAALSI cohort study. BMC Geriatr. 2017;17:293. doi: 10.1186/s12877-017-0694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8:e018195. doi: 10.1136/bmjopen-2017-018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung HW, Yoo HJ, Park SY, Kim SW, Choi JY, Yoon SJ, et al. The Korean version of the FRAIL scale: clinical feasibility and validity of assessing the frailty status of Korean elderly. Korean J Intern Med. 2016;31:594–600. doi: 10.3904/kjim.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim C, Sunwoo D. A frailty instrument to predict disability, institutionalization, and mortality: findings from the living profiles of older people survey. J Korean Gerontol Soc. 2015;35:451–74. [Google Scholar]
- 16.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 17.Won CW, Yang KY, Rho YG, Kim SY, Lee EJ, Yoon JL, et al. The development of Korean activities of daily living (K-ADL) and Korean Instrumental activities of daily living (K-IADL) scale. J Korean Geriatr Soc. 2002;6:107–20. [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–8. [Google Scholar]
- 20.Jung IK, Kwak DI, Joe SH, Lee HS. A study of standardization of Korean Form of Geriatric Depression Scale (KGDS) J Korean Geriatr Psychiatry. 1997;1:61–72. [Google Scholar]
- 21.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 22.Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Aihie Sayer A. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39:197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 24.Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, Grandjean H. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol. 2006;21:113–22. doi: 10.1007/s10654-005-5458-x. [DOI] [PubMed] [Google Scholar]
- 25.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 26.Song X, MacKnight C, Latta R, Mitnitski AB, Rockwood K. Frailty and survival of rural and urban seniors: results from the Canadian Study of Health and Aging. Aging Clin Exp Res. 2007;19:145–53. doi: 10.1007/BF03324681. [DOI] [PubMed] [Google Scholar]
- 27.Yu P, Song X, Shi J, Mitnitski A, Tang Z, Fang X, et al. Frailty and survival of older Chinese adults in urban and rural areas: results from the Beijing Longitudinal Study of Aging. Arch Gerontol Geriatr. 2012;54:3–8. doi: 10.1016/j.archger.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 29.Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age Ageing. 2015;44:162–5. doi: 10.1093/ageing/afu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard RE, Rockwood K. Frailty in older women. Maturitas. 2011;69:203–7. doi: 10.1016/j.maturitas.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Song WC, Koh KS, Kim SH, Hu KS, Kim HJ, Park JC, et al. Horizontal angular asymmetry of the face in korean young adults with reference to the eye and mouth. J Oral Maxillofac Surg. 2007;65:2164–8. doi: 10.1016/j.joms.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Hoogendijk EO, van Hout HP, Heymans MW, van der Horst HE, Frijters DH, Broese van Groenou MI, et al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol. 2014;24:538–44.e2. doi: 10.1016/j.annepidem.2014.05.002. Erratum in: Ann Epidemiol. 2014 Aug;24:628. [DOI] [PubMed] [Google Scholar]