Abstract
Hematopoietic stem cells (HSCs) generated during embryonic development are able to maintain hematopoiesis for the lifetime, producing all mature blood lineages. HSC transplantation is a widely used cell therapy intervention in the treatment of hematologic, autoimmune and genetic disorders. Its use, however, is hampered by the inability to expand HSCs ex vivo, urging for a better understanding of the mechanisms regulating their physiological expansion. In the adult, HSCs reside in the bone marrow, in specific microenvironments that support stem cell maintenance and differentiation. Conversely, while developing, HSCs are transiently present in the fetal liver, the major hematopoietic site in the embryo, where they expand. Deeper insights on the dynamics of fetal liver composition along development, and on how these different cell types impact hematopoiesis, are needed. Both, the hematopoietic and hepatic fetal systems have been extensively studied, albeit independently. This review aims to explore their concurrent establishment and evaluate to what degree they may cross modulate their respective development. As insights on the molecular networks that govern physiological HSC expansion accumulate, it is foreseeable that strategies to enhance HSC proliferation will be improved.
Keywords: hematopoietic stem cells, fetal liver, fetal liver microenvironment, fetal hematopoiesis, hematopoietic stem cell expansion, hematopoietic stem cell niche, self-renewal, cytokine signaling
Introduction
In the adult organism, hematopoietic stem cells (HSCs) constitute a rare and largely quiescent cell population residing in the bone marrow (BM) (Cheshier et al., 1999). The current dogma states that HSCs self-renew to maintain their pool throughout life and reenter cell cycle in response to stress (Wilson et al., 2008). The balance between self-renewal and differentiation in adult BM has been extensively studied, with the identification of different cellular niches and molecular cues as important elements in HSC maintenance and differentiation – reviewed in Crane et al. (2017) and Pinho and Frenette (2019).
During ontogeny, HSCs undergo a high proliferative stage, expanding in the fetal liver (FL), one of the anatomical locations of embryonic hematopoiesis (Ema and Nakauchi, 2000). Therefore, it has long been assumed that the hepatic microenvironment may drive the proliferation of HSCs while sustaining their primary “stemness” hallmark (functional capacity to reconstitute the hematopoietic compartment of irradiated recipients). So far, however, limited information is available on an HSC supportive environment in the FL and the mechanisms conveying these functional properties remain elusive, hindering effective translation into clinical applications.
A thorough dissection of the architecture and cellular organization of the liver is critical to elucidate the nature of the hematopoietic FL niche and disclose the elements (soluble and/or cell-bound signals, cell-cell contact, cell-matrix interactions, physical properties, etc.) contributing for the regulation of HSCs. This review aims to discuss the role of the FL stroma (encompassing all non-hematopoietic FL cells) and explore the interplay of the two fetal systems – hepatic and hematopoietic – in mouse (or otherwise stated) and how they mutually influence their development.
The Emergence of the Hematopoietic System During Embryogenesis
The adult hematopoietic system relies on a robust process whereby HSCs divide and differentiate generating all mature blood lineages. In physiological conditions, this process takes place in the BM in both humans and mice. Even though adult hematopoiesis occurs in the BM, this is merely the end-site of an otherwise thrilling journey through different anatomic locations (Figure 1).
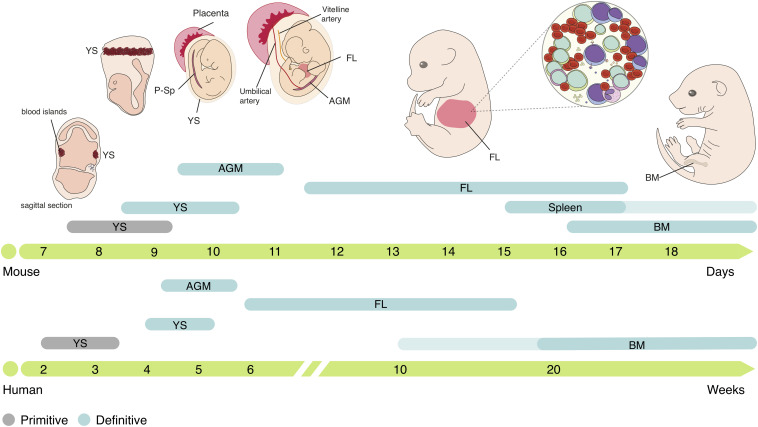
FIGURE 1.
Ontogeny of the hematopoietic system in mouse and human. Embryonic hematopoiesis is established in three distinct waves. The first hematopoietic cells emerge in YS blood islands, generating primitive erythrocytes, macrophages and megakaryocytes, constituting the primitive hematopoietic wave. The second hematopoietic wave initiates in the vascular plexus of the YS, generating EMPs that produce definitive erythrocytes, megakaryocytes, macrophages, neutrophils, granulocytes and mast cells, but lacks HSC activity. EMPs are the origin of tissue-resident macrophages that can persist throughout life. HSCs emerge in the AGM region and migrate to the FL, the major embryonic hematopoietic organ. In FL, HSCs expand and differentiate into all mature blood cell lineages. Small numbers of hematopoietic progenitors also colonize the fetal spleen and are still found few weeks after birth. Migration to the BM, where HSCs reside through adulthood, occurs as a continuous process and, in humans, can take several weeks. YS, yolk sac; P-Sp para-aortic splanchnopleura; FL, fetal liver; AGM, aorta-gonads-mesonephros; BM, bone marrow.
The first hematopoietic cells emerge in the yolk sac (YS) in extra-embryonic structures named blood islands at around embryonic day (E) 7.5 in mice and 2–3 weeks post-conception (wpc) in humans (Bloom and Bartelmez, 1940; Palis et al., 1999). Primitive erythroid progenitors (EryP) generate primitive erythrocytes, large nucleated cells that express embryonic globins (Kingsley et al., 2006), which are found in circulation after the onset of cardiac contractions at E8.25 (∼3 wpc in humans) (Ji et al., 2003; Tavian et al., 1999) and oxygenate the developing embryo. Myeloid progenitors such as macrophage colony-forming cells or megakaryocyte colony-forming cells are also represented during early stages, concomitantly with EryP (Palis et al., 1999), suggesting that primitive hematopoiesis is limited to these three lineages.
Around 24 h later, at E8.5 (4–5 wpc in humans), a second wave of hematopoiesis initiates, with erythro-myeloid progenitors (EMPs) (Migliaccio et al., 1986; Bertrand et al., 2005b) emerging in the vascular plexus of the YS, in a process denominated endothelial to hematopoietic transition (EHT) (Frame et al., 2016; Kasaai et al., 2017). EMPs proliferate and differentiate in the YS into erythroid and myeloid cells but can also be identified in circulation and in the developing liver at later stages (E10.5) via surface expression of c-Kit, CD16/32 and low levels of CD45 (McGrath et al., 2015). These progenitors generate the first definitive erythrocytes, megakaryocytes, macrophages and other myeloid lineages such as neutrophils, granulocytes and mast cells, but lack HSC activity (Palis et al., 1999; McGrath et al., 2015). Although EMPs are a transient population at early stages of embryonic development, they generate different tissue-resident macrophages that, depending on the organ, can persist throughout adulthood (Gomez-Perdiguero et al., 2015), mast cells that are maintained until birth (Gentek et al., 2018), and are the major producers of erythrocytes throughout embryonic life (Soares-da-Silva et al., 2020, Preprint).
A third wave of hematopoiesis occurs between E9.5-E11 in mice (∼4 wpc in humans), with HSCs emergence in the aorta-gonads-mesonephros (AGM) region (Cumano et al., 1996; Medvinsky et al., 1996; Tavian et al., 1996) through EHT (Bertrand et al., 2010; Kissa and Herbomel, 2010). After generation, immature HSCs (imHSCs) undergo a maturation process as they migrate to the FL (Taoudi et al., 2008; Kieusseian et al., 2012) where they proliferate [expanding in numbers by >30-fold (Ema and Nakauchi, 2000)] and differentiate into all blood lineages: erythroid, myeloid and lymphoid. HSCs can be defined by their ability to provide long-term multilineage hematopoietic reconstitution (LTR) when transplanted to lethally irradiated mice (Morrison et al., 1995) and further repopulate secondary recipients. These cells can be found within the Lin–CD45+Sca1+c-Kit+ (LSK) compartment and be further divided according to their reconstitution ability in long-term (LSK CD150+CD48– LT-HSC) or short-term (LSK CD150–CD48– ST-HSC) reconstituting cells (Kim et al., 2006). Downstream progenitors of HSCs such as multipotent progenitors (MPPs), lympho-myeloid-primed progenitors (LMPPs), common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs) can also be found in FL and are responsible for the seeding of other hematopoietic organs such as the thymus (Ramond et al., 2014). Although adult and embryonic HSCs have similar lineage potentials, some lymphoid lineages are only produced during embryonic development, namely dendritic epidermal T cells (Ikuta et al., 1990), lymphoid tissue–inducer cells (Eberl et al., 2004), and a subset of IL-17-producer γδ T cells (Haas et al., 2012). Embryonic hematopoiesis also takes place in the placenta, starting at E10.5-E11 (∼6 wpc in humans) and declining at around E15.5 (Gekas et al., 2005; Ottersbach and Dzierzak, 2005; Robin et al., 2009). HSCs and other progenitors are also found in the fetal spleen after E15.5 (Christensen et al., 2004), even though without evidence for significant expansion and mostly differentiating into the macrophage lineage (Bertrand et al., 2006). At around E16.5 (∼10 wpc in humans) HSCs migrate to the BM, where they are maintained through adulthood (Charbord et al., 1996; Christensen et al., 2004). In the adult, BM HSCs are largely quiescent (Cheshier et al., 1999) and only divide to maintain the stem cell pool, while the replenishment of blood lineages appears to be guaranteed by downstream MPPs (Sun et al., 2014; Busch et al., 2015; Pei et al., 2017; Rodriguez-Fraticelli et al., 2018).
Thus, embryonic hematopoiesis is characterized by an overlap in time and space of three waves with distinct anatomical origins and lineage potential. All waves converge to the FL, the major hematopoietic organ during embryogenesis.
The Colonization of the Fl by the Hematopoietic System
Which Cells Are Present When Hematopoietic Progenitors Arrive?
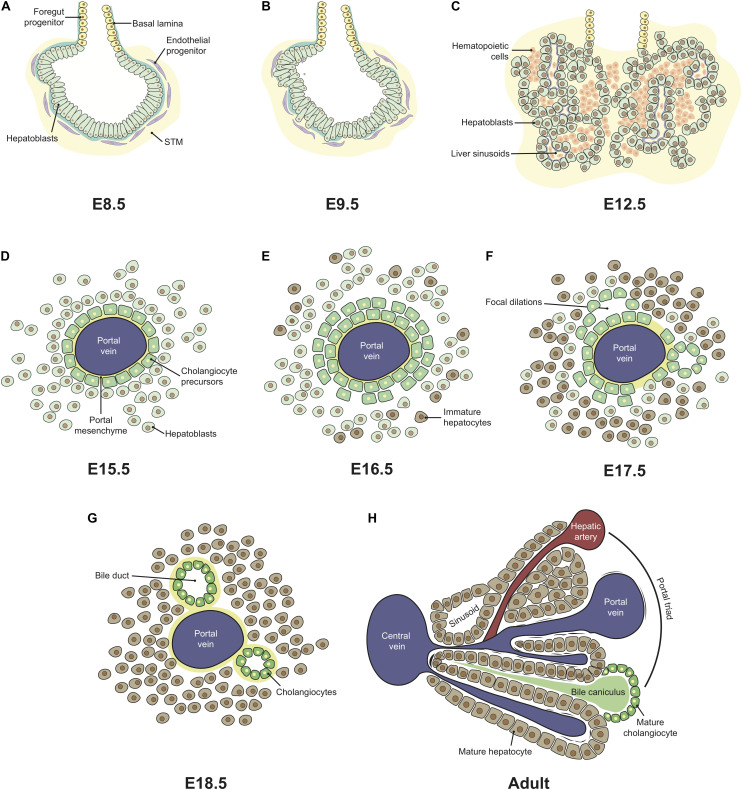
As the embryo gastrulates and folds, endoderm envelops the YS ultimately forming a hollow structure, the primitive gut tube, subsequently patterned into foregut, midgut and hindgut regions. The foregut, located in the anterior endoderm, adjacent to the developing heart, generates the liver, alongside with the lungs, thyroid, and pancreas (Tremblay and Zaret, 2005). Embryonic liver development starts at around E8.5-E9 (∼4 wpc in humans) with the formation of the hepatic diverticulum, an extension of the ventral foregut epithelium that invades the septum transverse mesenchyme (STM) and forms a liver bud, by E9.5 (Severn, 1971; Wilson et al., 2006). The liver bud originates from a single-sheet of columnar endodermal epithelium with a gut morphology (Figure 2A), which then transitions to pseudostratified epithelial hepatoblasts (Figure 2B; Bort et al., 2006). At this stage, these cells are separated from the STM by a basement membrane rich in laminin and composed of other extracellular matrix (ECM) molecules, such as nidogen, type IV collagen, fibronectin, and heparan sulfate proteoglycan (Shiojiri and Sugiyama, 2004). A process of extensive hepatoblast proliferation follows, during which the cells outgrow the liver bud, disrupting the basement membrane, into the STM (Figure 2C; Douarin, 1975). The other constituents of the organ, sinusoidal endothelial cells (SECs), mesothelial, sub-mesothelial and hepatic stellate cells have a mesoderm origin (reviewed by Yang et al., 2019), as described below. Angioblasts or endothelial progenitor cells are found delimiting the basement membrane (Figure 2A), resembling a loose “necklace” of cells, and were shown to promote liver organogenesis. In Flk-1–/– mutant embryos, which lack endothelial cells, hepatic specification occurs, but proliferation and migration into the STM are impaired (Matsumoto et al., 2001). At E10.5–E11.0 (∼5–6 wpc in humans), hematopoietic cells colonize the FL that rapidly becomes the major fetal hematopoietic organ (Johnson and Moore, 1975; Migliaccio et al., 1986; Palis et al., 2001).
FIGURE 2.
Ontogeny of the fetal liver. Liver development is initiated with the formation of the hepatic diverticulum at E8.5 (A). The single layer of columnar endodermal epithelial cells (A) thickens and transitions to a pseudostratified epithelium, followed by the delamination and migration of hepatoblasts into the STM, forming the liver bud by E9.5 (B). Extensive hepatoblast proliferation follows, originating hepatic chords intermingled with the hepatic mesenchyme and surrounding the sinusoids, formed from pre-existing vitelline vessels. From E10.5, hematopoietic cells colonize the FL that rapidly becomes the major fetal hematopoietic organ up until E15.5 (C). From E12.5-E15.5, the FL continues to enlarge, expanding both hematopoietic and hepatic compartments. Structural changes are only evident after hepatoblast-to-cholangiocyte specification occurs around the portal vessels, forming a monolayered ductal plate (D) that evolves into a bi-layer at E16.5 (E). Focal dilations (F) evolve into bile duct structures in late gestation (G). Portal triads characterize adult liver architecture where a hepatic artery, a portal vein and a bile duct form a complex structure, and a central vein, to which plates of hepatocytes lined by sinusoids converge (H). This architecture is only recognizable few weeks after birth. STM, septum transversum mesenchyme.
Hepatoblasts
Hepatoblasts are bipotent immature FL cells that differentiate into hepatocytes – the hepatic parenchyma main constituent – or cholangiocytes – the biliary epithelial cells. At the onset of liver development, bipotent hepatoblasts express the liver-specific transcription factors (TFs) hematopoietically-expressed homeobox protein (HHEX) (Bogue et al., 2000), prospero homeobox protein 1 (PROX1) (Dudas et al., 2004), and co-express the hepatocytes’ markers alpha-fetoprotein (AFP), albumin (ALB) (Cascio and Zaret, 1991), cytokeratin 18 (CK18) (Tanimizu et al., 2003), hepatocyte nuclear factor 4α (HNF4α) (Li et al., 2000) and cholangiocytes’ markers such as cytokeratin 19 (CK19) (Tanimizu et al., 2004). Other typical hepatoblast markers are listed in Table 1.
TABLE 1.
Fetal liver non-hematopoietic compartment: cell types and associated markers.
| Cell type | Gene expression | Markers used for isolation |
| Hepatoblasts | Hhex, Prox1, Alb, Afp, Ck8, Ck18, Ck19, Met, Hnf6, Oc2, Hnf4a, Ttr, Foxm1, Foxa2, Tbx3, Dlk1, Lgr5, Epcam, Cdh1, Trgb1, Itga6, Liv2, Prom1, Anpep | DLK1+ (Tanimizu et al., 2003; Tan et al., 2017) E-Cadherin+ (Nitou et al., 2002; Nierhoff et al., 2005) EpCAM+DLK1+ (Tanaka et al., 2009) Liv2+CD31–CD45–Lgr5-eGFP+ (Prior et al., 2018) Ter119–CD45–c-Kit–CD49f+CD29+ (Suzuki et al., 2000) VCAM1+ALCAM+DLK1+ (Tsuneto et al., 2013) Ter119–CD45–CD51+VCAM1+PDGFRα– (Brouard et al., 2017) |
| Hepatocytes | Alb, Afp, Ttr, G6p, Apoa1, Apoh Por, Cps1 | – |
| Cholangiocytes | Sox9, Hnf6, Oc2, Spp1, Ck19, Epcam, Krt7 | EpCAM+ (Yang et al., 2017) |
| Endothelial cells | Flk1, Flt1, Ve-cadh, Pecam1, Mcam, Tek, Tie, Lyve1, Kdr | CD45–Ter119–CD31+ (Khan et al., 2016) DLK1–CD45–Ter119–CD31+LYVE-1+ and DLK1–CD45–Ter119–CD31+LYVE-1– (Tan et al., 2017) |
| Mesothelial cells | Cytokeratin, Cd200, Gpm6a, Alcam, Gp38, Wt1, Podxl, Msln | Flk1–PODXLhigh (Onitsuka et al., 2010) |
| Sub-mesothelial cells | Alcam, Desmin, Nestin, p75tnr, Pdgfra, Wt1 | – |
| Hepatic stellate cells | Vimentin, Acta2, Desmin, p75NTR, Foxf1, Lhx2, Hlx | DLK1–Ter119–CD45–CD31–LYVE-1–p75NTR+ (Tan et al., 2017) |
| Pericytes | NG2, Nestin, Vimentin, Acta2, Pdgfra, Pdgfrb, Dlk1, Itgav, Endoglin, Vcam, Mcam, Nr2f2 | Ter119–CD45–CD31–Nestin+NG2+ (Khan et al., 2016) CD45–CD56–CD34–CD146+1 (Gerlach et al., 2012) |
1Study performed in humans.
Different markers have been used to isolate hepatoblasts, namely delta like non-canonical Notch ligand [DLK1 or preadipocyte factor 1 (Pref-1)] (Tanimizu et al., 2003), epithelial cadherin (E-cadherin) or CD324 (Nitou et al., 2002), epithelial cell adhesion molecule (EpCAM) or CD326 (Tanaka et al., 2009), and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) (Prior et al., 2018). DLK1 is strongly expressed by hepatoblasts in the E10.5 liver bud and continues to be highly expressed until around E16.5, being significantly downregulated thereafter and absent in mature hepatocytes and cholangiocytes (Tanimizu et al., 2003; Tanaka et al., 2009). E-Cadherin, present at the onset of liver outgrowth, is downregulated by the time hepatoblasts migrate to the STM, disrupting the epithelial sheet, although it can be used as a hepatoblast-specific marker after E12.5 (Nitou et al., 2000; Margagliotti et al., 2007). Transient EpCAM expression labels newly formed hepatoblasts but is significantly reduced after E12, while expression after E16 specifically labels bile duct cells (Tanaka et al., 2009). Recently, combining multicolor clonal genetic lineage tracing, organoid cultures and analysis of single-cell RNA sequencing, LGR5 was shown to mark a subpopulation of bonafide bipotential hepatoblasts at E9.5–E10 as the origin of the hepatoblast pool (Prior et al., 2018).
Endothelial Cells
The main blood vessels in the adult liver are the portal and central veins and the hepatic artery. Up until birth, the hepatic artery is absent and embryonic circulation is sustained by a transient afferent vascular system, the extraembryonic umbilical and vitelline veins (Collardeau-Frachon and Scoazec, 2008). The portal vein arises early in the liver development, between E10.5–E12.5 in mouse (4–6 wpc in human) (Collardeau-Frachon and Scoazec, 2008; Swartley et al., 2016). The hepatic sinusoids are the first vessels to appear, by E10–E10.5, originating from the pre-existing vitelline vessels. The latter sprouts throughout the STM, by angiogenesis, receiving signals from the surrounding mesenchyme (Figure 2C; Swartley et al., 2016). Hepatoblasts were also identified as a positive stimulator of sinusoid morphogenesis and maturation (Takabe et al., 2012). Stabilin 2 (STAB-2) and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) (commonly used as a marker of lymphatics) – hyaluronan receptors – start to be expressed in SECs at E9.5 and E10.5, respectively, and continue to be expressed thereafter (Nonaka et al., 2007; Takabe et al., 2012). Of note, lymphatic vessels were only reported after birth (Swartley et al., 2016). At E9.5, endothelial cells located around the liver diverticulum (Figure 2A) express both CD31/PECAM-1 and Flk-1 (Sugiyama et al., 2010b). CD31 and Flk1 expression in SECs is strong in the early stages of liver development, but is downregulated with time. In adult livers, endothelial cells of portal and hepatic veins strongly express CD31, while it is absent or weakly detected in SECs (Sugiyama et al., 2010b; Takabe et al., 2012). Primitive SECs also strongly express Flk-1, contrarily to endothelial cells of portal and hepatic veins (Sugiyama et al., 2010b). During embryonic liver development, portal vessels express the arterial markers Ephrin-B2 and Neuropilin-1, but not the venous marker EphB4. This expression profile is inverted at the end of gestation, with the transition into a venular phenotype (Wang et al., 1998; Khan et al., 2016). Liver endothelial cells constitute a heterogeneous cellular compartment and different markers should be used for their identification according to vascular location and developmental stage.
Mesothelial and Sub-Mesothelial Cells
Mesothelial cells (MCs) compose a single epithelial layer (mesothelium) lining the liver parenchyma on the surface of lobes. From E12.5, MCs are characterized by the expression of cytokeratin, CD200, glycoprotein M6A (GPM6A), podoplanin (PDPN/Gp38), podocalyxin-like protein 1 (PODXL), and mesothelin (MSLN) (Lua and Asahina, 2016). PODXL is highly expressed in immature MCs, being downregulated during development, while MSLN is upregulated. MCs proliferate during liver development and remain quiescent after birth. Wilm’s tumor-1 (WT1) is mainly expressed by MCs (Onitsuka et al., 2010). WT1–/– embryos show incomplete lobulation compared to control littermates at E13.5, reduced numbers of Flk1–PODXLhigh MCs, DLK1+ hepatoblasts, and total FL cells, suggesting that hepatic development was impaired due to defective MCs (Ijpenberg et al., 2007; Onitsuka et al., 2010). This is supported by the observation that fetal MCs express growth factors (PTN, MDK, and HGF) involved in hepatic development (Onitsuka et al., 2010).
Underneath the MC sheet lays a population of cells expressing Desmin, Nerve growth factor receptor (NGFR/p75NTR) and platelet-derived growth factor receptor α (PDGFRα/CD140a), associated with type IV collagen of the basal lamina, commonly referred as “sub-mesothelial cells” (sub-MC) or capsular fibroblasts. The expression of activated leukocyte cell adhesion molecule (ALCAM/CD166) and WT1 was also observed in MC and sub-MC around E11–E14 and, before that, in the STM by E9–E10 (Asahina et al., 2011; Lua and Asahina, 2016).
Hepatic Stellate Cells and/or Pericytes
Although the terms hepatic stellate cells and pericytes have been used by many authors as synonyms, it is not consensual they represent the same population. In adult liver, there is a population of perisinusoidal cells residing in the space of Disse between hepatocytes and SECs, that stores vitamin D lipids (Wake, 1971), and is a major player in liver fibrogenesis (Guyot et al., 2006). MesP1-expressing mesoderm has been considered its earliest ancestry, as it gives rise to the STM – the origin of the liver mesothelium and mesenchymal cells. Migration inward of MC and sub-MC from the liver surface is assumed to give rise to hepatic stellate cells and perivascular mesenchymal cells (Asahina, 2012). Hepatic stellate cells express Desmin, p75NTR, but not the MC markers ALCAM, WT1, and Gp38 (Asahina et al., 2010).
Gerlach et al. (2012) isolated CD146+CD45–CD56–CD34– cells from fetal and adult human livers and identified them as pericytes, a distinct population from hepatic stellate cells. They showed that these cells express NG2 and vimentin, but not GFAP in situ, and are found around periportal but not pericentral blood vessels neither within the space of Disse. These cells exhibit high osteogenic and myogenic, but low adipogenic or chondrogenic differentiation potential, in in vitro differentiation assays. In mice, a population characterized by the expression of Nestin and NG2 was identified as periportal pericytes, which expresses mesenchymal markers and shows trilineage mesenchymal capacity in vitro (Khan et al., 2016).
Law of Attraction: What Brings Hematopoietic Progenitors to the Developing Liver?
Hematopoietic stem cells emerge from the dorsal aorta directly into circulation and can, therefore, be found in different locations (Cumano et al., 1996; Medvinsky et al., 1996). These cells can travel through the umbilical arteries to the placenta and return to the embryo through the umbilical veins. The umbilical veins drain directly into the liver by fusing with the intrahepatic vascular plexus of the vitelline veins, forming the hepatic sinus. Cells traveling in the right umbilical vein can bypass the liver directly to the vena cava through the ductus venosus, a structure only present during fetal development, and can be directed into other embryonic regions through the heart. The liver is also irrigated by the vitelline veins, which transport blood from the YS, and eventually mature to become the portal vein. Another route for newly formed HSCs is to travel from the dorsal aorta through the subcardinal vein to the liver or inferior cardinal vein to the heart. From the heart, circulating cells can reach the developing lungs or upper half of the body (Kiserud, 2005). However, it is in the developing FL that HSCs establish and remain until they migrate to the BM. Transplantation studies show that HSCs can also be found in the placenta (Gekas et al., 2005; Ottersbach and Dzierzak, 2005). The placenta of a mouse model lacking a functional circulatory system was shown to still harbor hematopoietic activity, suggesting that the placenta could generate de novo hematopoietic cells with multilineage potential (Rhodes et al., 2008), however, direct evidence for HSC emergence from the placenta is yet to attain.
Hematopoietic stem or progenitor cells (HSPCs) but also EMPs, colonize the FL after liver bud formation at E10.5 (Johnson and Moore, 1975; Palis et al., 2001). Distinct mechanisms of FL colonization have been proposed, mostly relying on cell adhesion-mediated processes and/or chemoattraction (cytokines, chemokine signaling, and growth factors) (Figure 3; Hayashi et al., 2019).
FIGURE 3.
Molecular mechanisms of fetal liver colonization. HSPC express a variety of cell adhesion molecules in their surface that can favor attachment to a specific location. β1 integrin is essential for colonization of FL by HSPC and can bind to the ECM components fibronectin and type IV collagen through dimerization with α4 and α5 or α2 integrins, respectively. VLA-4 and VLA-5 binding to fibronectin has been proposed to be modulated by CD41. VLA-4 (α4β1 integrin) can also mediate cell-cell interactions by binding with VCAM-1, expressed on the surface of FL stromal cells. Expression of the cadherin CD144 in HSCs as they emerge is assumed to play a role in the organ colonization, although no binding partner has been described in FL. CXCL12, a potent chemoattractant is expressed by FL stromal cells and binds to CXCR4, expressed on the surface of HSPC. CXCL12 gradients can be generated as this chemokine binds to proteoglycans in the ECM, namely to heparan sulfate (HS). HSPC, hematopoietic stem and progenitor cell; FL, fetal liver; ECM, extracellular matrix; VLA, very late antigen; Fn, fibronectin; HS, heparan sulfate.
Several cell adhesion molecules have been identified in embryonic HSPCs, including integrins, selectins, cadherins, and others. Hematopoietic progenitors express vascular-endothelial cadherin (VE-Cadherin/CD144) as they emerge from the YS (in the case of EMPs) or AGM (in the case of HSCs) that is downregulated thereafter and undetectable in BM HSCs (Fraser et al., 2002; Taoudi et al., 2005). FL CD34+ progenitors express higher levels of the integrins β1 (CD29), α2 (CD49b), and α5 (CD49e), similar levels of integrins α4 (CD49d) and α6 (CD49f), E- and P-selectins (CD62E and CD62P, respectively) and CD11b and CD11c molecules, but lower levels of integrin β2 (CD18), CD11a and CD44 than their adult BM equivalent (Roy and Verfaillie, 1999). Seminal studies showed that HSPCs lacking β1 integrin were unable to colonize the FL but were still present in the circulation and capable of generating all blood lineages, suggesting a role for integrin-mediated cell adhesion in FL colonization (Hirsch et al., 1996). Integrin receptors result from the dimerization of α and β subunits and analysis of the expression of the α chain partner of β1 integrin in LSK progenitors revealed integrins α4 and α6 as the most predominant (Sugiyama et al., 2013). FL CD34+ progenitors bind the ECM component fibronectin through the integrin receptors α4β1 and α5β1 (also known as VLA-4 and VLA-5) and this binding has been proposed to be modulated by the integrin α2b (GPIIb or CD41), also expressed in these cells (Figure 3; Roy and Verfaillie, 1999; Emambokus and Frampton, 2003). Other ECM components have been tested for adhesion of FL CD34+ progenitors such as type I and type IV collagen and laminin, however, only type IV collagen promotes adherence at levels equivalent to that of fibronectin, through α2β1 integrin (VLA-2) (Roy and Verfaillie, 1999). In the FL, hepatoblasts (defined as DLK1+ cells) are the major producers of ECM components, namely vitronectin and fibronectin (Sugiyama et al., 2013). Embryos lacking hepatoblasts can still form the liver bud but die between E10.5–E12.5 (Nishina et al., 1999). These FL show decreased expression of vitronectin and fibronectin that may play an important role in FL colonization by HSCs and YS EMPs, although this role has not been specifically assessed (Sugiyama et al., 2013). Integrins can also mediate cell-cell interactions. Cellular bound counterparts of VLA-4 include the vascular cell adhesion molecule-1 (VCAM-1/CD106), expressed by FL hepatoblasts (Sugiyama et al., 2010a).
Cytokine and chemokine signaling can also stand at the basis of FL colonization. The stromal cell-derived factor-1 (SDF-1), commonly known as CXC chemokine ligand 12 (CXCL12), acts through binding to its receptor CXCR4 present in HSPCs and has been extensively studied in the adult BM – reviewed in Yu and Scadden (2016); and Wei and Frenette (2018). CXCL12 expression is stabilized at the cell surface or in the surrounding ECM through proteoglycans binding, allowing the creation of chemokine gradients essential for cell migration (Schumann et al., 2010). In the FL, CXCL12 is expressed by DLK1+ hepatoblasts (Chou and Lodish, 2010) and Nestin+NG2+ pericytes (Khan et al., 2016). The role of CXCL12 in FL colonization was analyzed using CXCL12–/– embryos. At early stages (E12.5–E14.5), the number of HSCs was similar in the FL of mutant animals and controls. By E16.5 FL HSCs were reduced by more than twofold and an abnormally high number was found in circulation. These observations indicate that CXCL12 is an important factor for retaining HSCs in FL, but not for its initial colonization (Ara et al., 2003).
Owing to the particular architecture of fetal circulation, FL is in an anatomically privileged location. Even if HSCs are traveling directly within the embryo through the subcardinal vein, or the umbilical veins after passing in the placenta, the FL is the first intraembryonic organ they encounter. Whether a passive retainment of circulating cells (e.g., through β1-integrin) occurs, or specific signals directly promote chemoattraction of HSPCs to FL is still unclear.
How Do the Hepatic and Hematopoietic Cell Types Organize During Development?
The structure of the FL changes dramatically during embryonic development. Crawford et al. (2010) extensively characterized the mouse developing hepatobiliary system from E9.5 to E18.5 creating a histology atlas. At initial stages (E10.5–E12.5), the liver is mainly constituted by a vascular plexus and migrating hepatoblasts that later form hepatic chords. At E11.5, the hepatic sinusoids are wide, which may favor the access and establishment of the newly generated hematopoietic progenitors. From E12.5 onward, the organization of the cells in the FL changes as the frequency of hematopoietic cells increases. At E13.5, the most frequent FL population are nucleated erythrocytes that, at early stages, are located throughout the liver parenchyma, in between the hepatic chords, but after E14.5 more mature enucleated erythroid cells are found within the vessels (Ayres-Silva et al., 2011). At this stage, megakaryocytes and erythroblastic islands, which consist of a central macrophage surrounded by erythroid cells, are also distinguishable in the liver parenchyma. These macrophages are responsible for the phagocytosis of the expelled nuclei during erythroid maturation (Bessis et al., 1978). Megakaryocytes are essential to thrombosis and hemostasis and may be determinant in an organ that is mostly constituted by erythroid cells. Moreover, the developing liver seems to provide a unique microenvironment for the expansion of megakaryocyte progenitors (Brouard et al., 2017). From E13.5 to late gestation, no dramatic changes occur in the histology of the FL. Other hematopoietic cells such as B cell progenitors identified by Pax5 expression, can be found interspersed in the tissue by E12.5 and also forming perivascular aggregates by E18.5. Granulocytes are scattered throughout the tissue from E16.5 onward, concentrating/converging around central veins and in the periphery by E17.5, correlating with the presence of mesenchymal cells and suggesting a crosstalk between these distinct cell types (Ayres-Silva et al., 2011). By this time-point, as hematopoietic cells exit the organ and migrate to the BM, hepatoblasts and hepatocytes regain contact (Crawford et al., 2010).
Disclosure of HSCs distribution within the FL has been hindered by the multi-marker assessment required, i.e., Lin–c-Kit+Sca1+CD150+CD48–, to phenotypically identify these cells. Hematopoietic progenitors (defined by c-Kit expression) are found in close association with DLK1+ hepatoblasts at E14.5 (Sugiyama et al., 2013). Nevertheless, c-Kit+ cells could mostly represent erythroid progenitors as they are the most frequent population at this stage (Soares-da-Silva et al., 2020, Preprint). Other approaches include the use of transgenic Ly6a-GFP (labeling Sca1+ cells) mice, that together with Runx1 localized HSPCs at E11.5 in close contact with endothelial cells (Tamplin et al., 2015). HSCs, profiled as CD150+CD48–Lin–, have been found in close association with Nestin+NG2+ pericytes surrounding the portal vessels (Khan et al., 2016). Although this characterization more closely identifies a potential HSC, FL studies using mouse models that directly label HSCs are still missing. Generation of a mouse with a single-color reporter driven by endogenous Hoxb5 (Hoxb5–tri-mCherry), which expression in the BM is limited to LT-HSCs and in situ imaging evidenced the close proximity of LT-HSCs with VE-Cadherin+ cells (Chen et al., 2016). Recently, another HSC-specific reporter line has been described, yet also only analyzed in the adult bone (Christodoulou et al., 2020).
The Interplay Between the Developing Hepatic-Hematopoietic Tissues
How Does the FL Environment Modulate Hematopoiesis? A Role in Maturation, Expansion and Differentiation of HSCs?
Emerging imHSCs lack long-term reconstitution activity in conventional or Rag2–/– immunocompromised mice but can reconstitute NK-deficient Rag2γc–/– animals (Cumano et al., 2001; Bertrand et al., 2005a). After co-culture with the OP9 BM stromal cell line in the presence of thrombopoietin (TPO) or with E10.5 FL rudiments, CD31+c-Kit+CD45– imHSCs acquire an adult HSC phenotype (LSK CD150+CD48–) and develop LTR activity in Rag2–/– or conventional mice as they upregulate MHC class I molecules (Kieusseian et al., 2012). These experiments suggest that the FL provides the signals necessary for the maturation of newly formed HSCs. FL stroma also supports the differentiation of committed hematopoietic progenitors towards distinct lineages. Interleukine 7 (IL-7) promotes the survival and proliferation of lymphoid progenitors and controls the determination of the B cell lineage (Sudo et al., 1989; Peschon et al., 1994). In FL, IL-7 is produced by VCAM1+ALCAM+DLK1+ hepatoblasts (Tsuneto et al., 2013) and controls the number of lymphoid progenitors that develop into the B-cell lineage by stabilizing the B-cell transcriptional signature (Berthault et al., 2017). For instance, erythropoietin (EPO) is produced by DLK1+ hepatoblasts and is required for proliferation and terminal differentiation of erythroid progenitors (Sugiyama et al., 2011). Also, TPO expressing Ter119–CD45–CD51+VCAM1+PDGFRα– FL hepatoblasts support the production of megakaryocytes from adult BM HSCs in a contact-dependent manner (Brouard et al., 2017). TPO is the main regulator of megakaryocyte differentiation and platelet production (Kaushansky, 1995; Eaton and de Sauvage, 1997) but has also been shown to promote survival and proliferation of BM HSPCs in vitro (Borge et al., 1996; Ku et al., 1996), the proliferation of fetal hematopoietic progenitors in vivo (Alexander et al., 1996) or expansion of BM HSCs following transplantation (Fox et al., 2002). Lack of TPO signaling causes decreased HSC function and numbers (Kimura et al., 1998; Solar et al., 1998), a consequence from the exit of a quiescent state (Nakamura et al., 2007; Qian et al., 2007), possibly leading to a premature exhaustion of the stem cell pool. The survival and proliferation effects of TPO are enhanced when used in combination with other early cytokines, namely FMS-like tyrosine kinase 3 ligand (FLT3L) and c-Kit ligand [KITL, also known as stem cell factor (SCF) or steel factor (SF)] both in murine and human adult BM cells (Ramsfjell et al., 1996; Borge et al., 1997). The highest levels of TPO in the adult are found in the liver (Lok et al., 1994). Systemic TPO produced by hepatocytes, but not by hematopoietic, osteoblast or BM mesenchymal stromal cells is required for BM HSC maintenance (Decker et al., 2018). It can be detected in FL as early as E10.5, having a strong impact on HSC expansion and survival in this organ (Petit-Cocault et al., 2007). Indeed, several cytokines/chemokines/growth factors are important for HSC proliferation, maintenance and survival, namely KITL, FLT3L, insulin growth factor (IGF), angiopoietin-3, angiopoietin-like 2, Wnt family growth factors, Ephrin2a, CSF1, EPO, CXCL12, and IL-6 – reviewed in Sauvageau et al. (2004). In FL, some of these cytokines are expressed by hepatoblasts or other stromal cells, such as stellate cells or pericytes (see Table 2 and Figure 4; Charbord and Moore, 2005; Chou and Lodish, 2010; Khan et al., 2016; Tan et al., 2017).
TABLE 2.
Cytokine signaling in fetal liver.
|
FL Supportive Stroma |
Hematopoietic progenitors |
||
| Pathway involved | Expressing cell | Receptor | Effect |
| KITL | DLK1+ hepatoblasts (Chou and Lodish, 2010; Sugiyama et al., 2011) Nestin+ cells (Khan et al., 2016) Stellate cells (Tan et al., 2017) | c-Kit (CD117) (Yarden et al., 1987) | BM HSC survival and self-renewal (Barker, 1994; Miller et al., 1997) |
| ANGPTL2 | Nestin+ cells (Khan et al., 2016) | PirB (Zheng et al., 2012) | BM HSC proliferation (Zhang et al., 2006) |
| ANGPTL3 | DLK1+ hepatoblasts (Chou and Lodish, 2010) | PirB (Zheng et al., 2012) | BM HSC maintenance (Zheng et al., 2011) BM HSC proliferation (Zhang et al., 2006) |
| FLT3L | Stellate cells (Tan et al., 2017) | Flt3 (CD135) (Matthews et al., 1991) | FL HSPC proliferation (Lyman et al., 1993) |
| TPO | DLK1+ hepatoblasts (Chou and Lodish, 2010; Sugiyama et al., 2011; Brouard et al., 2017) Stellate cells (Tan et al., 2017) | MPL (TPO-R, CD110) (Vigon et al., 1992) | FL HSC survival and proliferation (Petit-Cocault et al., 2007) BM HSC quiescence (Nakamura et al., 2007; Qian et al., 2007) |
| CSF1 | Stellate cells (Tan et al., 2017) | Csf-1 Receptor (Guilbert and Stanley, 1980) | Commitment to macrophage lineage (Rieger et al., 2009) |
| EPO | DLK1+ hepatoblasts (Sugiyama et al., 2011) Stellate cells (Tan et al., 2017) | EPO receptor (Sawyer et al., 1987) | Proliferation and differentiation of FL erythroid progenitors (Lin et al., 1996) |
| CXCL12 | Dlk1+ hepatoblasts (Chou and Lodish, 2010) Nestin+ cells (Khan et al., 2016) Stellate cells (Kubota et al., 2007) | CXCR4 (Bleul et al., 1996) | FL and FBM B-cell lymphopoiesis and FBM myelopoiesis (Nagasawa et al., 1996) BM HSC engraftment post-transplantation (Lai et al., 2014; McDermott et al., 2015) FL HSC retainment (Ara et al., 2003) BM HSC retainment (Sugiyama et al., 2006) |
| IL-7 | VCAM1+ALCAM+DLK1+ hepatoblasts (Tsuneto et al., 2013) | IL-7Rα (Park et al., 1990) | Lymphocyte expansion (Peschon et al., 1994) |
| IGF2 | Dlk1+ hepatoblasts (Chou and Lodish, 2010) Nestin+ cells (Khan et al., 2016) Stellate cells (Tan et al., 2017) | IGF1-R (Rubin et al., 1983) IGF2-R (Morgan et al., 1987) Insulin receptor (House and Weidemann, 1970) | F L and BM HSC proliferation (Zhang and Lodish, 2004) |
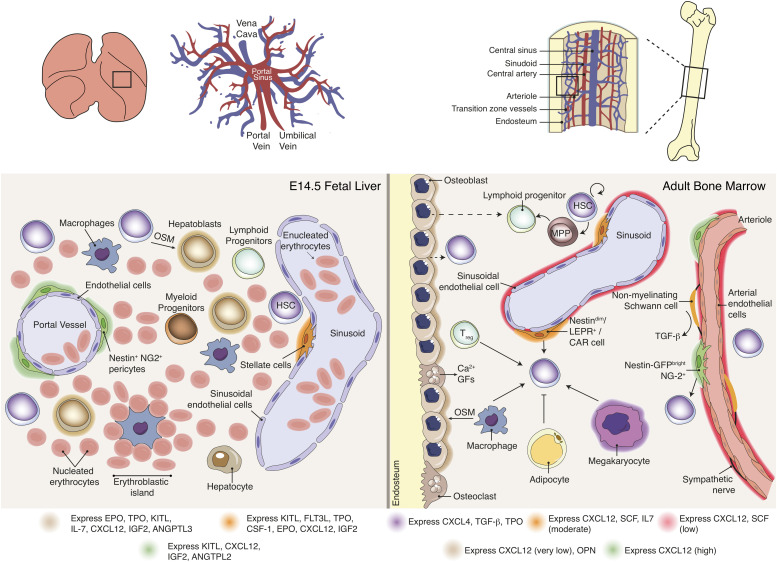
FIGURE 4.
Hematopoietic stem cell niche in fetal liver versus bone marrow. Distinct BM stromal cell populations have been described as modulators of HSC functions via expression of soluble and membrane-bound factors. Data available for FL stromal populations is scarce and consequently evidence for a direct role of stromal populations in HSC expansion or differentiation is not reported. The FL microenvironment includes cytokine-producing hepatoblasts, pericytes and stellate cells, together with high number of hematopoietic progenitors and distinct developmental stage erythroid cells. BM Nestin-GFPhigh, NG2+ perisinusoidal cells and Nestin-GFPdim, LepR+, CAR sinusoidal cells are key regulators of HSC maintenance, contributing differentially for cytokine production. Macrophages, Treg and megakaryocytes were also reported to contribute to HSC maintenance and mobilization. Sympathetic nerves regulate HSC mobilization through a tightly control of CXCL12 expression by stromal cells. Non-myelinating Schwann cells activate latent TGF-β into its active form, which may contribute for HSC maintenance. The role of osteoblasts in HSPC is still unclear. Adipocytes may negatively affect HSC maintenance.
The hypothesis that stem cells are regulated by their environment has been proposed by Schofield (1978) and postulates that stem cell properties are maintained by the surrounding cells designated “niche.” Stromal regulation of hematopoiesis has been proposed by many and early studies of co-culture of hematopoietic progenitors with either FL fibroblast or epithelial-stromal cell lines showed erythroid and myeloid support (Tsai et al., 1986; Hata et al., 1993). More than 200 FL stromal cell lines have been developed and tested for maintenance or expansion of HSCs, however, only a few were able to maintain their repopulating activity over a 3-week co-culture period (Wineman et al., 1996). The functional heterogeneity observed was not due to distinct cytokine production as all cell lines expressed similar cytokine profiles, including Flt3l, Kitl, Tpo, Igf1, Il6, Il11, leukemia inhibitory factor (Lif), granulocyte-colony stimulating factor (G-CSF/Csf3), granulocyte/macrophage-colony stimulating factor (GM-CSF/Csf2), and transforming growth factor, beta 1 (Tgfb1). Importantly, the successful stromal cell lines have in common the expression of Dlk1, and overexpression of this factor is sufficient to enable hematopoietic support (Moore et al., 1997). Although these cell lines only maintain HSC potential and do not promote HSC expansion, some FL cells have been described to do so. Recent studies have addressed the role of specific FL populations in vivo or in vitro. It is the case of DLK1+ hepatoblasts that are able to expand LT-HSC around 20-fold after a 3-week co-culture period (Chou et al., 2013). This expansion seems to be contact-dependent as DLK1+ conditioned medium only allows expansion of ST-HSC and cells cultured in transwell inserts did not show the same expansion levels. Although promising, it is worth mentioning that these cultures were supplemented with KITL, TPO, and FLT3L and, therefore, it remains unclear which mechanisms underlie HSCs expansion in DLK1+ cell co-cultures. Moreover, DLK1 knockdown in human hepatoblasts results in decreased hematopoietic support in vitro (Gerlach et al., 2019). The role of hepatoblasts in vivo has been difficult to assess as transgenic mice with hepatoblast deficiencies die between E10.5 and E12.5 and studies of liver development do not usually investigate the hematopoietic compartment (Nishina et al., 1999). DLK1 has been used to identify hepatoblasts but it is also expressed by the majority of Nestin+ cells surrounding the portal vessels, a cell type that has been implicated as part of the FL niche (Tanimizu et al., 2003; Chou and Lodish, 2010; Chou et al., 2013; Khan et al., 2016). HSCs numbers are modestly reduced when Nestin+NG2+ pericytes are selectively eliminated and the remaining HSCs are less proliferative (Khan et al., 2016). So far, this is the only study that addresses the role of a specific cell type in HSC expansion and maintenance in vivo. Nestin+ cells are the major producers of Cxcl12 at E14.5 when compared to Nestin– cells, therefore, the reduction in FL HSCs could result from a defect in HSC expansion together with a displacement of cells into circulation. Moreover, Nestin+ cells seem to be a transient population, important for HSC localization around the portal vessels during FL hematopoiesis but are no longer present postnatally, a stage at which HSCs migrate and reside in the BM. These cells have also been described in adult BM and ablation of Nestin+NG2+ pericytes alters HSCs localization away from arterioles (Kunisaki et al., 2013). In the rat, fetal hepatic stellate cells were shown to express VCAM-1 and to secrete Cxcl12 and hepatocyte growth factor (Hgf), revealing a potential role for the hematopoietic and hepatic development (Kubota et al., 2007). Accordingly, mouse FL hepatic stellate cells (defined as p75NTR+) express a range of hematopoietic cytokines, Csf1, Igf2, Tpo, Kitl, Epo, Igf1, Il11, Flt3l and Oncostatin M (Osm, involved in hepatic maturation) and were therefore proposed as potential niche components (Tan et al., 2017).
To date, researchers have undertaken a cell type-directed approach, however, it is conceivable that different FL populations play distinct roles in the maintenance and expansion of HSCs and act through cellular networks. Only an unsupervised analysis of the FL constituents as a whole will shed light on the part each cell type takes in the support of hematopoiesis.
The vascular labyrinthine of the placenta (where embryonic circulation meets maternal circulation) has also been proposed as a niche for HSC expansion (Gekas et al., 2005; Ottersbach and Dzierzak, 2005; Robin et al., 2009). Human placenta-derived stromal cell lines with pericyte characteristics were shown to support in vitro maintenance of cord blood (CB) hematopoietic progenitors and hematopoietic cells were found in close contact with pericytes/perivascular cells in the placenta by immunostaining (Robin et al., 2009). Taken together with what has already been described for the FL (Khan et al., 2016), pericyte-like cells are likely to play a role on supporting hematopoiesis.
Can the Adult Liver Be a Hematopoietic Site?
Extramedullary hematopoiesis (EMH) is a process in which HSPCs leave their microenvironment in the BM and establish in distinct anatomical locations wherein they continue to produce mature blood cells. Although it is a physiologic process during embryonic development (YS, AGM, FL, and fetal spleen), in the adult it only occurs in pathological settings of BM failure, myelostimulation, tissue inflammation, or abnormal cytokine production (Johns and Christopher, 2012). EMH can occur sporadically in lymph nodes, spinal cord, kidneys, gastrointestinal tract, and lung (Chiu et al., 2015). It is interesting, however, that the predominant sites of EMH are shared between the embryo and the adult: the spleen and the liver. In fact, splenic or liver hematopoiesis can still be observed postnatally in many mammals but disappears before adulthood. Hematopoietic foci in the adult liver can be found within sinusoids and in close association with macrophages (Barberá-Guillem et al., 1989). BM HSPCs co-cultured with liver sinusoid endothelial cells (LSECs) were maintained for more than 6 weeks in cytokine supplemented media, demonstrating a putative niche role of endothelial cells in the adult liver (Cardier and Barberá-Guillem, 1997). An adherent layer of liver cells has been suggested to support megakaryopoiesis by the production of TPO and B lymphopoiesis by the production of Il7 and Flt3l (Cardier and Dempsey, 1998; Wittig et al., 2010). Although endothelial cells have been reported to express TPO, this cytokine is mostly produced by the liver parenchyma (hepatocytes) (Nomura et al., 1997). In the adult, EPO is produced by the kidney, although hepatocytes can also support hepatic erythropoiesis in physiological or pathological conditions (Ploemacher and van Soest, 1977; Semenza et al., 1991; Eckardt et al., 1994; Weidemann and Johnson, 2009). In conclusion, under physiologic conditions, the liver harbors low numbers of HSPCs and supports extra-medullary hematopoiesis (Taniguchi et al., 1996; Watanabe et al., 1996). Thus, it is conceivable that adult liver may keep some of its embryonic niche properties.
Is the Development of the FL and Hematopoietic Cells Symbiotic?
In mid-gestation, embryonic liver functions as a “bag” accommodating the expanding hematopoietic system before BM development. During the temporal window in which the organ is essentially a hematopoietic tissue – from E12.5 to E16.5 (∼6–18 wpc in humans) – and the ratio of non-hematopoietic cells/total liver cells is very low, the organ’s architecture is far less complex than that of its adult counterpart. In the adult, hepatocytes are the main parenchymal cell type, organized in cords interspaced by an intricate vascular and biliary system (Figure 2H). Alongside with the massive migration of hematopoietic cells to the BM at E16.5 (Christensen et al., 2004), the liver tissue starts to mature – hepatocytes and cholangiocytes differentiate from hepatoblasts and cellular adhesion increases, creating tight hepatic parenchyma with dense hepatocyte cords (Crawford et al., 2010). Transcriptomic and proteomic analysis throughout FL development identified E15.5 as the time of onset of metabolic, detoxification and immune programs (Guo et al., 2009).
Hepatoblasts differentiate into hepatocytes starting at ∼E13.5 in mice and around 14 wpc in humans (Haruna et al., 1996; Yang et al., 2017). Single-cell transcriptomic studies along development (E10.5–E17.5) of hepatoblasts/hepatocytes/cholangiocytes (sorted based on the expression of DLK-1 and EpCAM) suggest that hepatoblast-to-hepatocyte lineage specification is the default process. Cholangiocyte specification occurs as early as E11.5 and is completed by E14.5 (Yang et al., 2017). Hepatoblasts fate decision is modulated by a gradient of Activin/TGF-β signaling, controlled by Onecut (OC) TFs (OC-1/HNF-6 and OC-2) (Clotman, 2005). Cholangiocyte-primed hepatoblasts appear in low numbers at E13.5 around the portal vein and portal sinus, forming a single-layered ductal plate at E15.5, that evolves to a double-layer by E16.5 (Figures 2D,E), characterized by CK19 and CK8 cytokeratins and β2 integrin (CD18) (Van Eyken et al., 1988; Tanimizu et al., 2009). At this stage, CK19 expression becomes specific to ductal plate cells (Van Eyken et al., 1988). Focal dilations between the two ductal plate cell layers give rise to the bile ducts (Figures 2F,G), whereas the remaining tissue progressively regresses (Clotman et al., 2002). This ductal plate remodeling involves tubulogenesis (Antoniou et al., 2009) and apoptosis (Terada and Nakanuma, 1995). Around birth, the portal mesenchyme encircles the cells of the ductal plate (Swartley et al., 2016). Hepatoblasts located away from the portal vein will gradually differentiate into hepatocytes and by E17 start to exhibit a characteristic polarized epithelial morphology disposed in hepatic cords alongside the bile canaliculi (Zorn, 2008). Because cholangiocyte differentiation occurs along the hilum-to-periphery axis, different maturation states can be observed at a given developmental time (Yang et al., 2017). Three-dimensional reconstructions of serial cross-sections/whole-mount immunostained FL and carbon ink injection have been used to disclose the morphogenesis of intrahepatic bile ducts (Vestentoft et al., 2011; Takashima et al., 2015; Tanimizu et al., 2016).
Kinoshita et al. (1999) showed that hematopoietic cells expand when cultured in a monolayer of primary fetal hepatic cells (in presence of hematopoietic cytokines) and that the addition of OSM suppresses in vitro hematopoiesis, by inducing the maturation of the hepatic cells. Since hematopoietic cells produce OSM, its expansion increases the local OSM concentration, consequently promoting hepatic development (Kamiya et al., 1999). It was hypothesized that a metabolically active liver no longer supports hematopoiesis (Miyajima et al., 2000). However, it is still not clear whether the displacement of the hematopoietic cells out of the FL facilitates liver maturation or if the changes in the microenvironment no longer support hematopoiesis.
Impaired hematopoiesis in c-Myb mutant (Mucenski et al., 1991) or Ubc–/– mice (Ryu et al., 2012) and abnormal erythropoiesis in Rb-deficient mice (Lee et al., 1992) also results in impaired liver growth. However, the early embryonic lethality (at around E15) has hindered the analysis of the impact of the hematopoietic compartment in liver development.
The vasculature remodeling at the end of gestation was also correlated with the rapid loss of HSCs in the postnatal liver (Khan et al., 2016). After birth, the portal vein no longer receives blood from the vitelline vein, collecting the blood from the gut, draining it to the central vein and the hepatic artery arises. With the ligation of the umbilical inlet, the portal vessels acquire a vein phenotype and lose the periportal pericytes (Khan et al., 2016).
Hematopoietic Exit From Fl and Establishment in the BM
What Makes the Hematopoietic System Move? How Is the BM Niche Established?
The BM is the ultimate destination of the hematopoietic system journey in the embryo, being the production hub for blood cells throughout life. LSK cell homing and colonization of mouse BM was reported at E15.5–E16.5, coinciding with the fetal bone marrow (FBM) vascularization (Gekas et al., 2005; Coşkun et al., 2014; Cao et al., 2019), although LT-HSC activity cannot be detected before E17.5 (Christensen et al., 2004). In mice, HSCs migrating out of the FL also seed the spleen, starting at E15.5, with HSC activity still being detected a few weeks after birth (Wolber et al., 2002; Christensen et al., 2004; Bertrand et al., 2006). In humans, although early colonization of fetal long bones was reported at ∼10 wpc (Charbord et al., 1996), the hematopoietic shift from the FL to the FBM occurs later, around 20 wpc (Figure 1).
Coşkun et al. (2014) showed that hematopoietic progenitor cells reside in the vascularized regions of fetal long bones, stage at which they are still proliferative. Previous work showed that in the BM, HSCs are cycling during the first 3 weeks after birth and become quiescent thereafter (Bowie et al., 2006). The shift to a quiescence state seems to be dependent on the cellular composition of the microenvironment (Coşkun et al., 2014). LSK cells isolated from Osx–/– FBM [that lack osteolineage cells (Nakashima et al., 2002) and some stromal populations (Mizoguchi et al., 2014)] form multi-lineage colonies in vitro, but fail to repopulate transplanted recipients (Mizoguchi et al., 2014). LSK cells exhibited dysregulated cell cycle progression and defective homing ability, suggesting that osteolineage and/or mesenchymal cells are necessary to establish and sustain BM LT-HSCs (Coşkun et al., 2014). Additionally, E15.5 CD105+Thy1– mesenchymal progenitors transplanted under the kidney capsule give rise to donor-derived chondrocytes and can create an ectopic niche with the recruitment of host-derived marrow and HSCs, evidencing the importance of endochondral ossification for HSC niche formation (Chan et al., 2009).
Osteopontin (OPN), also known as secreted phosphoprotein 1 (SPP1) has been suggested to be an important niche factor in BM. Its concentration in stromal cells is inversely correlated with HSC proliferation in the adult BM (Stier et al., 2005). The OPN dominant form thrombin-cleaved osteopontin (trOPN) is highly expressed in FBM (at the trabecular bone surface), but neglectable-to-none in the early-mid gestation FL (Cao et al., 2019). trOPN receptor α4β1 integrin is upregulated in fetal compared to adult HSCs. The differential concentration of the divalent metal cations, Ca2+, Mg2+, and Mn2+ between FL and FBM, being highly prevalent in the latter, was assumed to activate α4β1 in HSCs, possibly hindering their expansion in the BM (Cao et al., 2019).
CXCL12 expression in the vicinity of vascular endothelial cells in FBM supports the hematopoietic colonization of the organ (Nagasawa et al., 1996; Ara et al., 2003). HSCs isolated from CXCL12–/– embryos failed to colonize the BM in long-term repopulation assays whereas their migration ability could be rescued by enforced expression of CXCL12 under the control of vascular-specific Tie-2 regulatory sequences (Ara et al., 2003). Moreover, the CXCL12-mediated migration of HSPCs in vitro seems to be enhanced by the presence of KITL, indicating an additive effect, only found in fetal HSCs (Christensen et al., 2004). This suggests an important role of KITL for HSC seeding and homing during embryonic development.
CXCL12-GFP knock-in mice allowed the identification of CXCL12-abundant reticular (CAR) cells, a population with high expression of CXCL12, distributed near SECs and at a lower extent near the endosteum (Sugiyama et al., 2006). These cells show in vitro potential to differentiate into adipocytic and osteoblastic lineages (Omatsu et al., 2010). Specific markers have been identified for mesenchymal cells in BM. Leptin receptor (Lepr) is highly enriched in Scf-GFP-expressing perivascular stromal cells (Ding et al., 2012) and Lepr+ cells were shown to largely overlap with CAR cells (Zhou et al., 2014). A rare population of Nestin+ cells that contains all of the fibroblastic CFU (CFU-F) activity within the mouse BM, capable of generating mesenspheres (mesenchymal spheres) in vitro, multipotent and with self-renewal potential was identified as a mesenchymal stem cell (MSC) population (Méndez-Ferrer et al., 2010). Later, two different Nestin-GFP populations were discriminated based on the fluorescence intensity by microscopy. Rare quiescent Nestin-GFPbright cells, positive for the pericyte marker NG2 and α-smooth muscle actin, are enriched for CFU-F activity and express Cxcl12 and Kitl. These cells are located alongside arterioles, close to dormant HSCs (Kunisaki et al., 2013), and associated with sympathetic nerves, that regulate their CXCL12 expression through the β3-adrenergic receptor (Méndez-Ferrer et al., 2010). Nestin-GFPdim cells have a reticular shape, are mitotically active and line sinusoids, largely overlapping with Lepr+ cells (∼80%) (Kunisaki et al., 2013). Selective ablation of mouse Nestin+ cells (Méndez-Ferrer et al., 2010) or CAR cells (Omatsu et al., 2010) significantly impacts the maintenance of HSCs. The structural differences of the blood vessels and perivascular populations seem to be associated with heterogeneity in HSC function. Besides, the selective deletion of Cxcl12 from arteriolar NG2+ cells, but not sinusoidal Lepr+ cells, significantly reduced the HSCs compartment in the BM and a similar effect was observed by deletion of Kitl in LepR+; but not NG2+ cells, evidencing the differential contribution of perivascular populations in the cytokine production (Asada et al., 2017). BM microenvironment is illustrated in Figure 4.
Nestin+ cells can be prospectively isolated using PDGFRα and CD51 markers in the mouse and human fetal and adult BM (Pinho et al., 2013) and show MSC’s properties and enrichment of Cxcl12, Vcam1, Angpt1, Opn, and Scf genes. Of note, in humans, these cells represent a small subset of CD146+ cells (Pinho et al., 2013), the latter harboring all the CFU-F activity in BM (Sacchetti et al., 2007). Co-culture of human PDGFRα+CD51+ mesenspheres with human FBM CD34+ cells in a serum-free, but cytokine supplemented (TPO, SCF, FLT3L) culture media can expand MPPs that engraft immunodeficient mice (Pinho et al., 2013).
In the FL, Nestin-GFP+NG2+ cells, associated with portal vessels, form a niche promoting HSC expansion during the FL development that is no longer found after birth. Concomitantly, the phenotype of the portal vessel transits from Neuropilin-1+Ephrin-B2+ arterial to EphB4+ venular vessels (Khan et al., 2016). This role of Nestin+NG2+ cells in FL is opposite to that in BM, where Nestin+NG2+arteriolar pericytes were proposed to maintain HSC quiescent (Kunisaki et al., 2013).
Unresolved Questions – Ex vivo Expansion of HSCs
Hematopoietic stem cells are the only cells of the hematopoietic compartment with the potential to replenish all mature blood cells and to divide without triggering differentiation programs, a process known as self-renewal. The mechanisms conveying these properties have been under investigation over the last 60 years, however, to date, they remain poorly understood. The concept that specific BM niche/microenvironment components regulate the fate of HSCs has been proposed by many authors. Cordeiro Gomes et al. (2016) analyzed not only HSCs but also different hematopoietic progenitors and found both HSCs and MPPs locate near or in contact with the same mesenchymal progenitor CAR-cells, expressing CXCL12 and SCF, fundamental to maintain the HSC pool and IL-7 that acts as a short-range signal for lymphoid differentiation. These observations suggest that both maintenance and multilineage differentiation are locally regulated by the same niche (Cordeiro Gomes et al., 2016). In the FL, HSCs expand considerably and differentiate, producing different mature lineages. If a given stromal population is also involved in the regulation of both processes, by the expression of various cytokines, or different cell populations contribute with distinct cytokines is still unresolved.
Hematopoietic stem cell transplantation is a widely used cell therapy intervention in the treatment of hematologic, autoimmune and genetic disorders. However, this therapy is still associated with high mortality rates, mainly due to infection, graft-versus-host disease (GvHD) and organ dysfunction, urging the need for improvement (Tanaka et al., 2016). The most common source of HSCs for transplantation is the BM or mobilized circulating HSPCs. However, matching of major histocompatibility complex antigens is needed to avoid GvHD (Schuster et al., 2012; Walasek et al., 2012). CB can be successfully used as a source of partially mismatched HSCs, as it is readily available through CB banks and elicits low levels of GvHD. Low numbers of HSCs in CB and consequent absent or delayed reconstitution leads to post-transplantation infections, limiting the use of CB in adult patients (Ruggeri et al., 2014).
In vitro generation of HSCs would overcome some of the current clinical difficulties that transplantation faces, however, despite countless efforts to derive HSCs from pluripotent stem cell sources, generation of HSC in vitro has not yet been achieved – reviewed in Freire and Butler (2020). Another possibility to obtain higher numbers of HSCs would be to expand them ex vivo prior to transplantation. Distinct cytokine/growth factor cocktails have shown promising for expansion of HSCs, yet, limited success was reported in clinical studies due to a lack of LT-HSC expansion and rather proliferation of downstream progenitors together with undesirable stem cell differentiation – reviewed in Kumar and Geiger (2017) and Tajer et al. (2019). Cytokines currently used for ex vivo HSC expansion include KITL, TPO, IL-3, and FLT3L. Kitl and Tpo knockout mice show normal fetal development but reduced HSC numbers in the adult, suggesting that these cytokines are important for stem cell survival and proliferation in adulthood but might not be the drivers of HSC expansion during the embryonic period (Fujita et al., 1989; de Sauvage et al., 1996). There is growing evidence that the physical and mechanical properties of the microenvironment could impact on HSC decisions – reviewed in Kumar and Geiger (2017). A combination of cytokines with stroma derived ECM components – fibronectin and collagen – has shown encouraging results (Wohrer et al., 2014; Wilkinson et al., 2019). Moreover, culture of BM HSPCs in tropoelastin, the most elastic biomaterial known, induces a sixfold increase of LSK cells without supplementation with cytokines, suggesting that tropoelastin mediates a similar effect in survival and proliferation of LSK cells and its use could replace exogenous cytokines (Holst et al., 2010). Most cytokines studied have a role in HSC function in the BM, a site where HSC expansion does not occur in physiological conditions. The same cytokines have been found in FL stroma, but whether these are responsible for fetal HSC expansion is not known. Most likely, the FL expansion of newly generated HSCs results from intrinsic cellular properties together with a suitable microenvironment, physical cues included. Efforts have been made to replicate the embryonic microenvironment where HSCs expand, particularly by co-culture with FL hepatic or mesenchymal cells (Wineman et al., 1996; Chou and Lodish, 2010; Chou et al., 2013; Khan et al., 2016). Most strategies used so far focused on specific cell populations, either hepatoblasts or mesenchymal cells, but overlooked the possibility that distinct populations might need to interact. Only the co-culture of HSCs in a system where all the cytokine-expressing populations of the FL are present would reproduce the FL microenvironment. Such a system where hepatoblasts, endothelial and mesenchymal cells are cultured together in a 3D aggregate has already been devised, but no co-culture with hematopoietic cells has been attempted (Takebe et al., 2015). Liver cell culture models have been extensively developed for pharmacological and toxicological research or as a source for transplantation, to obtain an in vitro system that resembles a mature liver – reviewed in Godoy et al. (2013), and for that reason are not suitable for recreating the FL niche environment.
Whereas the FL interactions between different cell types have been correlated with their contribution to the massive transient expansion of the hematopoietic system, other niche factors need to be addressed. Sigurdsson et al. (2016) reported the role of FL bile acids (BAs) as chemical chaperones, critical to sustain high protein production by expanding LT-HSCs without triggering endoplasmic reticulum (ER) stress. Inhibiting the biosynthesis of BAs in vivo resulted in reduced numbers of Lin–, LSKs, and LSK CD48–CD150+ in the FL, with no apparent effect in the number of HSCs in the mother’s BM (Sigurdsson et al., 2016). Comparison of FL and BM HSCs transcriptomes demonstrated that FL HSCs metabolism relies on oxygen-dependent pathways, which may be a requirement for extensive energy production during expansion. Contrary to BM HSCs, FL HSCs use oxidative phosphorylation (aside from glycolysis), have higher number of mitochondria and up regulate genes associated with antioxidant and DNA repair pathways, that are speculated to confer protection from reactive oxygen species-mediated (geno)toxicity (Manesia et al., 2015).
Exploring the complexity of HSC niches (cellular composition, cytokine and growth factors milieu, physical properties, oxygen availability, etc.) will improve into our understanding of HSC self-renewal capacity (recently reviewed by Wilkinson et al., 2020), that is currently insufficient to devise efficient strategies for HSC expansion ex vivo.
Concluding Remarks
Distinct studies have identified and characterized FL stromal populations that may contribute with specific cues, enabling the HSC expansion in this organ. Despite the efforts, the role of the FL microenvironment has not been directly shown. The possibility that HSCs proliferate exclusively due to intrinsic properties is however remote. Therefore, a model in which distinct populations cooperate is conceivable. An analysis that contemplates such complex multicellular networks has not been attempted. This review aimed to compile information on the cellular populations that could signal to HSCs during development, highlighting the advances and the unresolved questions in the field.
Author Contributions
FS-d-S and MP: conceptualization and writing. AC: discussion and revision. PP-d-Ó: conceptualization and revision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the members of PP-d-Ó and AC laboratories for critical discussions.
Footnotes
Funding. FS-d-S funded by FCT grants PD/BD/114128/2015 and POCI-0100145-FEDER-01638. MP funded by FCT grant SFRH/BD/143605/2019. AC financed by the Institut Pasteur, INSERM, ANR (grant Twothyme and EpiDev) and REVIVE Future Investment Program and Pasteur-Weizmann Foundation. PP-d-Ó financed by Portuguese funds through FCT/MCTES in the framework of the projects PTDC/MED-OUT/32656/2017, POCI-01-0145-FEDER-032656, and by – FCT – UIDB/04293/2020.
References
- Alexander W. S., Roberts A. W., Nicola N. A., Li R., Metcalf D. (1996). Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 87 2162–2170. [PubMed] [Google Scholar]
- Antoniou A., Raynaud P., Cordi S., Zong Y., Tronche F., Stanger B. Z., et al. (2009). Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 136 2325–2333. 10.1053/j.gastro.2009.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T., Tokoyoda K., Sugiyama T., Egawa T., Kawabata K., Nagasawa T. (2003). Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19 257–267. 10.1016/S1074-7613(03)00201-2 [DOI] [PubMed] [Google Scholar]
- Asada N., Kunisaki Y., Pierce H., Wang Z., Fernandez N. F., Birbrair A., et al. (2017). Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19 214–223. 10.1038/ncb3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K. (2012). Hepatic stellate cell progenitor cells. J. Gastroenterol. Hepatol. 27 (Suppl. 2), 80–84. 10.1111/j.1440-1746.2011.07001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K., Tsai S. Y., Li P., Ishii M., Jr., Henry M. (2010). Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 49 998–1011. 10.1002/hep.22721.Mesenchymal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K., Zhou B., Pu W. T., Tsukamoto H. (2011). Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53 983–995. 10.1002/hep.24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres-Silva J. D. P., Manso P. P. D. A., Madeira M. R. D. C., Pelajo-Machado M., Lenzi H. L. (2011). Sequential morphological characteristics of murine fetal liver hematopoietic microenvironment in Swiss Webster mice. Cell Tissue Res. 344 455–469. 10.1007/s00441-011-1170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberá-Guillem E., Ayala R., Vidal-Vanaclocha F. (1989). Differential location of hemopoietic colonies within liver acini of postnatal and phenylhydrazine-treated adult mice. Hepatology 9 29–36. 10.1002/hep.1840090106 [DOI] [PubMed] [Google Scholar]
- Barker J. E. (1994). Sl/Sld hematopoietic progenitors are deficient in situ. Exp. Hematol. 22 174–177. [PubMed] [Google Scholar]
- Berthault C., Ramond C., Burlen-Defranoux O., Soubigou G., Chea S., Golub R., et al. (2017). Asynchronous lineage priming determines commitment to T cell and B cell lineages in fetal liver. Nat. Immunol. 18 1139–1149. 10.1038/ni.3820 [DOI] [PubMed] [Google Scholar]
- Bertrand J., Chi N. C., Santoso B., Teng S., Stainier D. Y. R., Traver D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464 108–111. 10.1038/nature08738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J., Desanti G. E., Lo-Man R., Leclerc C., Cumano A., Golub R. (2006). Fetal spleen stroma drives macrophage commitment. Development 133 3619–3628. 10.1242/dev.02510 [DOI] [PubMed] [Google Scholar]
- Bertrand J., Giroux S., Golub R., Klaine M., Jalil A., Boucontet L., et al. (2005a). Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc. Natl. Acad. Sci. U.S.A. 102 134–139. 10.1073/pnas.0402270102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J., Jalil A., Klaine M., Jung S., Cumano A., Godin I. (2005b). Three pathways to mature macrophages in the early mouse yolk sac. Blood 106 3004–3011. 10.1182/blood-2005-02-0461 [DOI] [PubMed] [Google Scholar]
- Bessis M., Mize C., Prenant M. (1978). Erythropoiesis: comparison of in vivo and in vitro amplification. Blood Cells 4 155–174. [PubMed] [Google Scholar]
- Bleul C. C., Farzan M., Choe H., Parolin C., Clark-Lewis I., Sodroski J., et al. (1996). The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382 829–833. 10.1038/382829a0 [DOI] [PubMed] [Google Scholar]
- Bloom W., Bartelmez G. W. (1940). Hematopoiesis in young human embryos. Am. J. Anat. 67 21–53. 10.1002/aja.1000670103 [DOI] [Google Scholar]
- Bogue C. W., Ganea G. R., Sturm E., Ianucci R., Jacobs H. C. (2000). Hex expression suggests a role in the development and function of organs derived from foregut endoderm. Dev. Dyn. 219 84–89. [DOI] [PubMed] [Google Scholar]
- Borge O., Ramsfjell V., Cui L., Jacobsen S. E. (1997). Ability of early acting cytokines to directly promote survival and suppress apoptosis of human primitive CD34+CD38- bone marrow cells with multilineage potential at the single-cell level: key role of thrombopoietin. Blood 90 2282–2292. [PubMed] [Google Scholar]
- Borge O., Ramsfjell V., Veiby O., Murphy M., Lok S., Jacobsen S. (1996). Thrombopoietin, but not erythropoietin promotes viability and inhibits apoptosis of multipotent murine hematopoietic progenitor cells in vitro. Blood 88 2859–2870. 10.1182/blood.V88.8.2859.bloodjournal8882859 [DOI] [PubMed] [Google Scholar]
- Bort R., Signore M., Tremblay K., Barbera J. P. M., Zaret K. S. (2006). Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev. Biol. 290 44–56. 10.1016/j.ydbio.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Bowie M. B., McKnight K. D., Kent D. G., McCaffrey L., Hoodless P. A., Eaves C. J. (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest. 116 2808–2816. 10.1172/JCI28310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard N., Jost C., Matthias N., Albrecht C., Egard S., Gandhi P., et al. (2017). A unique microenvironment in the developing liver supports the expansion of megakaryocyte progenitors. Blood Adv. 1 1854–1866. 10.1182/bloodadvances.2016003541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K., Klapproth K., Barile M., Flossdorf M., Holland-Letz T., Schlenner S. M., et al. (2015). Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518 542–546. 10.1038/nature14242 [DOI] [PubMed] [Google Scholar]
- Cao H., Cao B., Heazlewood C. K., Domingues M., Sun X., Debele E., et al. (2019). Osteopontin is an important regulative component of the fetal bone marrow hematopoietic stem cell niche. Cells 8:985 10.3390/cells8090985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardier J., Barberá-Guillem E. (1997). Extramedullary hematopoiesis in the adult mouse liver is associated with specific hepatic sinusoidal endothelial cells. Hepatology 26 165–175. 10.1002/hep.510260122 [DOI] [PubMed] [Google Scholar]
- Cardier J., Dempsey J. (1998). Thrombopoietin and its receptor, c-mpl, are constitutively expressed by mouse liver endothelial cells: evidence of thrombopoietin as a growth factor for liver endothelial cells. Blood 91 923–929. 10.1182/blood.V91.3.923 [DOI] [PubMed] [Google Scholar]
- Cascio S., Zaret K. S. (1991). Hepatocyte differentiation initiates during endodermal-mesenchymal interactions prior to liver formation. Development 113 217–225. [DOI] [PubMed] [Google Scholar]
- Chan C. K. F., Chen C.-C., Luppen C. A., Kim J.-B., DeBoer A. T., Wei K., et al. (2009). Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457 490–494. 10.1038/nature07547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbord P., Moore K. A. (2005). Gene expression in stem cell-supporting stromal cell lines. Ann. N. Y. Acad. Sci. 1044 159–167. 10.1196/annals.1349.020 [DOI] [PubMed] [Google Scholar]
- Charbord P., Tavian M., Humeau L., Peault B. (1996). Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood 87 4109–4119. 10.1182/blood.V87.10.4109.bloodjournal87104109 [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Miyanishi M., Wang S. K., Yamazaki S., Sinha R., Kao K. S., et al. (2016). Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530 223–227. 10.1038/nature16943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier S. H., Morrison S. J., Liao X., Weissman I. L. (1999). In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 96 3120–3125. 10.1073/pnas.96.6.3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.-C., Liu H.-H., Chen C.-L., Chen P.-R., Liu M.-C., Lin S.-Z., et al. (2015). Extramedullary hematopoiesis (EMH) in laboratory animals: offering an insight into stem cell research. Cell Transplant. 24 349–366. 10.3727/096368915X686850 [DOI] [PubMed] [Google Scholar]
- Chou S., Flygare J., Lodish H. F. (2013). Fetal hepatic progenitors support long-term expansion of hematopoietic stem cells. Exp. Hematol. 41 479–490. 10.1016/j.exphem.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Lodish H. F. (2010). Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 107 7799–7804. 10.1073/pnas.1003586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. L., Wright D. E., Wagers A. J., Weissman I. L. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2:e020075 10.1371/journal.pbio.0020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou C., Spencer J. A., Yeh S.-C. A., Turcotte R., Kokkaliaris K. D., Panero R., et al. (2020). Live-animal imaging of native haematopoietic stem and progenitor cells. Nature 578 278–283. 10.1038/s41586-020-1971-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotman F. (2005). Control of liver cell fate decision by a gradient of TGF signaling modulated by Onecut transcription factors. Genes Dev. 19 1849–1854. 10.1101/gad.340305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotman F., Lannoy V. J., Reber M., Cereghini S., Cassiman D., Jacquemin P., et al. (2002). The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development 129 1819–1828. [DOI] [PubMed] [Google Scholar]
- Collardeau-Frachon S., Scoazec J. Y. (2008). Vascular development and differentiation during human liver organogenesis. Anat. Rec. 291 614–627. 10.1002/ar.20679 [DOI] [PubMed] [Google Scholar]
- Cordeiro Gomes A., Hara T., Lim V. Y., Herndler-Brandstetter D., Nevius E., Sugiyama T., et al. (2016). Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity 45 1219–1231. 10.1016/j.immuni.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coşkun S., Chao H., Vasavada H., Heydari K., Gonzales N., Zhou X., et al. (2014). Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 9 581–590. 10.1016/j.celrep.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane G. M., Jeffery E., Morrison S. J. (2017). Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17 573–590. 10.1038/nri.2017.53 [DOI] [PubMed] [Google Scholar]
- Crawford L. W., Foley J. F., Elmore S. A. (2010). Histology atlas of the developing mouse hepatobiliary system with emphasis on embryonic days 9.5-18.5. Toxicol. Pathol. 38 872–906. 10.1177/0192623310374329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Dieterlen-Lievre F., Godin I. (1996). Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86 907–916. 10.1016/S0092-8674(00)80166-X [DOI] [PubMed] [Google Scholar]
- Cumano A., Ferraz J. C., Klaine M., Di Santo J. P., Godin I. (2001). Intraembryonic, but Not Yolk Sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15 477–485. 10.1016/S1074-7613(01)00190-X [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Carver-Moore K., Luoh S. M., Ryan A., Dowd M., Eaton D. L., et al. (1996). Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J. Exp. Med. 183 651–656. 10.1084/jem.183.2.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M., Leslie J., Liu Q., Ding L. (2018). Hepatic thrombopoietin is required for bone marrow hematopoietic stem cell maintenance. Science 360 106–110. 10.1126/science.aap8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Saunders T. L., Enikolopov G., Morrison S. J. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481 457–462. 10.1038/nature10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douarin N. M. (1975). An experimental analysis of liver development. Med. Biol. 53 427–455. [PubMed] [Google Scholar]
- Dudas J., Papoutsi M., Hecht M., Elmaouhoub A., Saile B., Christ B., et al. (2004). The homeobox transcription factor Prox1 is highly conserved in embryonic hepatoblasts and in adult and transformed hepatocytes, but is absent from bile duct epithelium. Anat. Embryol. 208 359–366. 10.1007/s00429-004-0403-4 [DOI] [PubMed] [Google Scholar]
- Eaton D. L., de Sauvage F. J. (1997). Thrombopoietin: the primary regulator of megakaryocytopoiesis and thrombopoiesis. Exp. Hematol. 25 1–7. [PubMed] [Google Scholar]
- Eberl G., Marmon S., Sunshine M. J., Rennert P. D., Choi Y., Littmann D. R. (2004). An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5 64–73. 10.1038/ni1022 [DOI] [PubMed] [Google Scholar]
- Eckardt K. U., Pugh C. W., Meier M., Tan C. C., Ratcliffe P. J., Kurtz A. (1994). Production of erythropoietin by liver cells in vivo and in vitro. Ann. N. Y. Acad. Sci. 718 50–60. 10.1111/j.1749-6632.1994.tb55703.x [DOI] [PubMed] [Google Scholar]
- Ema H., Nakauchi H. (2000). Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95 2284–2289. [PubMed] [Google Scholar]
- Emambokus N. R., Frampton J. (2003). The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity 19 33–45. 10.1016/S1074-7613(03)00173-0 [DOI] [PubMed] [Google Scholar]
- Fox N., Priestley G., Papayannopoulou T., Kaushansky K. (2002). Thrombopoietin expands hematopoietic stem cells after transplantation. J. Clin. Invest. 110 389–394. 10.1172/JCI15430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame J. M., Fegan K. H., Conway S. J., McGrath K. E., Palis J. (2016). Definitive hematopoiesis in the yolk sac emerges from wnt-responsive hemogenic endothelium independently of circulation and arterial identity. Stem Cells 34 431–444. 10.1002/stem.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S. T., Ogawa M., Yu R. T., Nishikawa S., Yoder M. C., Nishikawa S. I. (2002). Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin+ population. Exp. Hematol. 30 1070–1078. 10.1016/S0301-472X(02)00887-1 [DOI] [PubMed] [Google Scholar]
- Freire A. G., Butler J. M. (2020). Blood making: learning what to put into the dish. F1000Research 9:38 10.12688/f1000research.21245.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J., Onoue H., Ebi Y., Nakayama H., Kanakura Y. (1989). In vitro duplication and in vivo cure of mast-cell deficiency of Sl/Sld mutant mice by cloned 3T3 fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 86 2888–2891. 10.1073/pnas.86.8.2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C., Dieterlen-Lièvre F., Orkin S. H., Mikkola H. K. A. (2005). The placenta is a niche for hematopoietic stem cells. Dev. Cell 8 365–375. 10.1016/j.devcel.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Gentek R., Ghigo C., Hoeffel G., Bulle M. J., Msallam R., Gautier G., et al. (2018). Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity 48 1160–1171. 10.1016/j.immuni.2018.04.025 [DOI] [PubMed] [Google Scholar]
- Gerlach J. C., Over P., Turner M. E., Thompson R. L., Foka H. G., Chen W. C. W., et al. (2012). Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev. 21 3258–3269. 10.1089/scd.2012.0296 [DOI] [PubMed] [Google Scholar]
- Gerlach J. C., Thompson R. L., Gridelli B., Schmelzer E. (2019). Effects of delta-like noncanonical Notch ligand 1 expression of human fetal liver hepatoblasts on hematopoietic progenitors. Stem Cells Intern. 2019:7916275 10.1155/2019/7916275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy P., Hewitt N. J., Albrecht U., Andersen M. E., Ansari N., Bhattacharya S., et al. (2013). Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87:1315 10.1007/s00204-013-1078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., et al. (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518 547–551. 10.1038/nature13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. (1980). Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J. Cell Biol. 85 153–159. 10.1083/jcb.85.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Zhang X., Huang J., Zeng Y., Liu W., Geng C., et al. (2009). Relationships between hematopoiesis and hepatogenesis in the midtrimester fetal liver characterized by dynamic transcriptomic and proteomic profiles. PLoS One 4:e7641 10.1371/journal.pone.0007641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot C., Lepreux S., Combe C., Doudnikoff E., Bioulac-Sage P., Balabaud C., et al. (2006). Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Intern. J. Biochem. Cell Biol. 38 135–151. 10.1016/j.biocel.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Haas J. D., Ravens S., Düber S., Sandrock I., Oberdörfer L., Kashani E., et al. (2012). Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37 48–59. 10.1016/j.immuni.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Haruna Y., Saito K., Spaulding S., Nalesnik M. A., Gerber M. A. (1996). Identification of bipotential progenitor cells in human liver development. Hepatology 23 476–481. 10.1002/hep.510230312 [DOI] [PubMed] [Google Scholar]
- Hata M., Nanno M., Doi H., Satomi S., Sakata T., Suzuki R., et al. (1993). Establishment of a hepatocytic epithelial cell line from the murine fetal liver capable of promoting hemopoietic cell proliferation. J. Cell. Physiol. 154 381–392. 10.1002/jcp.1041540222 [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Sezaki M., Takizawa H. (2019). Development of the hematopoietic system: Role of inflammatory factors. Wil. Interdiscipl. Rev. Dev. Biol. 8 1–17. 10.1002/wdev.341 [DOI] [PubMed] [Google Scholar]
- Hirsch E., Iglesias A., Potocnik A. J., Hartmann U., Fässler R. (1996). Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature 380 171–175. 10.1038/380171a0 [DOI] [PubMed] [Google Scholar]
- Holst J., Watson S., Lord M. S., Eamegdool S. S., Bax D. V., Nivison-Smith L. B., et al. (2010). Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 28 1123–1128. 10.1038/nbt.1687 [DOI] [PubMed] [Google Scholar]
- House P. D. R., Weidemann M. J. (1970). Characterization of an [125I]-Insulin binding plasma membrane fraction from rat liver. Biochem. Biophys. Res. Commun. 41 541–548. 10.1016/0006-291X(70)90046-X [DOI] [PubMed] [Google Scholar]
- Ijpenberg A., Pérez-Pomares J. M., Guadix J. A., Carmona R., Portillo-Sánchez V., Macías D., et al. (2007). Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev. Biol. 312 157–170. 10.1016/j.ydbio.2007.09.014 [DOI] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y., et al. (1990). A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 62 863–874. 10.1016/0092-8674(90)90262-D [DOI] [PubMed] [Google Scholar]
- Ji R. P., Phoon C. K. L., Aristizábal O., McGrath K. E., Palis J., Turnbull D. H. (2003). Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 92 133–135. 10.1161/01.RES.0000056532.18710.C0 [DOI] [PubMed] [Google Scholar]
- Johns J. L., Christopher M. M. (2012). Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet. Pathol. 49 508–523. 10.1177/0300985811432344 [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Moore M. A. S. (1975). Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature 258 726–728. 10.1038/258726a0 [DOI] [PubMed] [Google Scholar]
- Kamiya A., Kinoshita T., Ito Y., Matsui T., Morikawa Y., Senba E., et al. (1999). Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 18 2127–2136. 10.1093/emboj/18.8.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaai B., Caolo V., Peacock H. M., Lehoux S., Gomez-Perdiguero E., Luttun A., et al. (2017). Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling. Sci. Rep. 7 25–28. 10.1038/srep43817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K. (1995). Thrombopoietin: the primary regulator of platelet production. Blood 86 419–431. 10.1182/blood.V86.2.419.bloodjournal862419 [DOI] [PubMed] [Google Scholar]
- Khan J., Mendelson A., Kunisaki Y., Birbrair A., Kou Y., Arnal-Estapé A., et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351 176–180. 10.1126/science.aad0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieusseian A., de la Grange P. B., Burlen-Defranoux O., Godin I., Cumano A. (2012). Immature hematopoietic stem cells undergo maturation in the fetal liver. Development 139 3521–3530. 10.1242/dev.079210 [DOI] [PubMed] [Google Scholar]
- Kim I., He S., Yilmaz O. H., Kiel M. J., Morrison S. J., Yilmaz H. (2006). Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood 108 737–744. 10.1182/blood-2005-10-4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Roberts A. W., Metcalf D., Alexander W. S. (1998). Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc. Natl. Acad. Sci. U.S.A. 95 1195–1200. 10.1073/pnas.95.3.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley P. D., Malik J., Emerson R. L., Bushnell T. P., McGrath K. E., Bloedorn L. A., et al. (2006). “Maturational” globin switching in primary primitive erythroid cells. Blood 107 1665–1672. 10.1182/blood-2005-08-3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Sekiguchi T., Xu M.-J., Ito Y., Kamiya A., Tsuji K.-I., et al. (1999). Hepatic differentiation induced by oncostatin M attenuates fetal liver hematopoiesis. Proc. Natl. Acad. Sci. U.S.A. 96 7265–7270. 10.2177/jsci.21.supplement_215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiserud T. (2005). Physiology of the fetal circulation. Semin. Fet. Neonat. Med. 10 493–503. 10.1016/j.siny.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Kissa K., Herbomel P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464 112–115. 10.1038/nature08761 [DOI] [PubMed] [Google Scholar]
- Ku H., Yonemura Y., Kaushansky K., Ogawa M. (1996). Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood 87 4544–4551. [PubMed] [Google Scholar]
- Kubota H., Yao H., Reid L. M. (2007). Identification and characterization of vitamin a-storing cells in fetal liver: implications for functional importance of hepatic stellate cells in liver development and hematopoiesis. Stem Cells 25 2339–2349. 10.1634/stemcells.2006-0316 [DOI] [PubMed] [Google Scholar]
- Kumar S., Geiger H. (2017). HSC niche biology and hsc expansion Ex Vivo. Trends Mol. Med. 23 799–819. 10.1016/j.molmed.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y., Bruns I., Scheiermann C., Ahmed J., Pinho S., Zhang D., et al. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502 637–643. 10.1038/nature12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-Y., Yamazaki S., Okabe M., Suzuki S., Maeyama Y., Iimura Y., et al. (2014). Stage-specific roles for Cxcr4 signaling in murine hematopoietic stem/progenitor cells in the process of bone marrow repopulation. Stem Cells 32 1929–1942. 10.1002/stem.1670 [DOI] [PubMed] [Google Scholar]
- Lee E. Y.-H. P., Chang C.-Y., Hu N., Wang Y.-C. J., Lai C.-C., Herrup K., et al. (1992). Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359 288–294. 10.1038/359288a0 [DOI] [PubMed] [Google Scholar]
- Li J., Ning G., Duncan S. A. (2000). Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 14 464–474. 10.1128/MMBR.66.1.39-63.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Lim S. K., D’Agati V., Costantini F. (1996). Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 10 154–164. 10.1101/gad.10.2.154 [DOI] [PubMed] [Google Scholar]
- Lok S., Kaushansky K., Holly R. D., Kuijper J. L., Lofton-Day C. E., Oort P. J., et al. (1994). Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature 369 565–568. 10.1038/369565a0 [DOI] [PubMed] [Google Scholar]
- Lua I., Asahina K. (2016). The role of mesothelial cells in liver development. Injury Regener. Gut Liver 10:166 10.5009/gnl15226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman S. D., James L., Bos T., Vanden de Vries P., Brasel K., Gliniak B., et al. (1993). Molecular cloning of a ligand for the flt3flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75 1157–1167. 10.1016/0092-8674(93)90325-K [DOI] [PubMed] [Google Scholar]
- Manesia J. K., Xu Z., Broekaert D., Boon R., van Vliet A., Eelen G., et al. (2015). Highly proliferative primitive fetal liver hematopoietic stem cells are fueled by oxidative metabolic pathways. Stem Cell Res. 15 715–721. 10.1016/j.scr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Margagliotti S., Clotman F., Pierreux C. E., Beaudry J. B., Jacquemin P., Rousseau G. G., et al. (2007). The onecut transcription factors HNF-6/OC-1 and OC-2 regulate early liver expansion by controlling hepatoblast migration. Dev. Biol. 311 579–589. 10.1016/j.ydbio.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yoshitomi H., Rossant J., Zaret K. S. (2001). Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294 559–563. 10.1126/science.1063889 [DOI] [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Wiegand G. W., Pardoll D., Lemischka I. R. (1991). A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 65 1143–1152. 10.1016/0092-8674(91)90010-V [DOI] [PubMed] [Google Scholar]
- McDermott D. H., Gao J.-L., Liu Q., Siwicki M., Martens C., Jacobs P., et al. (2015). Chromothriptic cure of WHIM syndrome. Cell 160 686–699. 10.1016/j.cell.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K. E., Frame J. M., Fegan K. H., Bowen J. R., Conway S. J., Catherman S. C., et al. (2015). Distinct sources of hematopoietic progenitors emerge before HSCS and provide functional blood cells in the mammalian Embryo. Cell Rep. 11 1892–1904. 10.1016/j.celrep.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A., Dzierzak E., Ridgeway T., Hill M. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86 897–906. 10.1016/S0092-8674(00)80165-8 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Michurina T. V., Ferraro F., Mazloom A. R., MacArthur B. D., Lira S. A., et al. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466 829–834. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G., Migliaccio A. R., Petti S., Mavilio F., Russo G., Lazzaro D., et al. (1986). Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac-liver transition. J. Clin. Invest. 78 51–60. 10.1172/JCI112572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Rebel V., I, Helgason C. D., Lansdorp D., Eaves C. J. (1997). Impaired steel factor responsiveness differentially affects the detection and long-term maintenance of fetal liver hematopoietic stem cells in vivo. Blood 89 1214–1223. 10.1182/blood.v89.4.1214 [DOI] [PubMed] [Google Scholar]
- Miyajima A., Kinoshita T., Tanaka M., Kamiya A., Mukouyama Y., Hara T. (2000). Role of oncostatin M in hematopoiesis and liver development. Cytokine Growth. Factor. Rev. 11 177–183. 10.1016/S1359-6101(00)00003-4 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Pinho S., Ahmed J., Kunisaki Y., Hanoun M., Mendelson A., et al. (2014). Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29 340–349. 10.1016/j.devcel.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A., Pytowski B., Witte L., Hicklin D., Lemischka I. R. (1997). Hematopoietic activity of a stromal cell transmembrane protein containing epidermal growth factor-like repeat motifs. Proc. Natl. Acad. Sci. U.S.A. 94 4011–4016. 10.1073/pnas.94.8.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., et al. (1987). Insulin-like growth factor II receptor as a multifunctional binding protein. Nature 329 301–307. 10.1038/329301a0 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Hemmati H. D., Wandycz A. M., Weissman I. L. (1995). The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 92 10302–10306. 10.1073/pnas.92.22.10302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Mclain K., Kier A., Swerdlow S. H., Schreiner C. M., Miller T. A., et al. (1991). A functional c-myb Gene is required for normal murine fetal hepatic hematopoiesis. Cell 65 677–689. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S. I., Kitamura Y., et al. (1996). Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382 635–638. 10.1038/382635a0 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Hagiwara T., Hosokawa K., Iwasaki H., Matsuoka S., Takahashi T., et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1 685–697. 10.1016/j.stem.2007.10.020 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J. M., Behringer R. R., et al. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108 17–29. 10.1016/S0092-8674(01)00622-5 [DOI] [PubMed] [Google Scholar]
- Nierhoff D., Ogawa A., Oertel M., Chen Y.-Q., Shafritz D. A. (2005). Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology 42 130–139. 10.1002/hep.20735 [DOI] [PubMed] [Google Scholar]
- Nishina H., Vaz C., Billia P., Nghiem M., Sasaki T., De la Pompa J. L., et al. (1999). Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development 126 505–516. [DOI] [PubMed] [Google Scholar]
- Nitou M., Ishikawa K., Shiojiri N. (2000). Immunohistochemical analysis of development of desmin-positive hepatic stellate cells in mouse liver. J. Anat. 197 635–646. 10.1046/j.1469-7580.2000.19740635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitou M., Sugiyama Y., Ishikawa K., Shiojiri N. (2002). Purification of fetal mouse hepatoblasts by magnetic beads coated with monoclonal anti-E-cadherin antibodies and their in vitro culture. Exper. Cell Res. 279 330–343. 10.1006/excr.2002.5615 [DOI] [PubMed] [Google Scholar]
- Nomura S., Ogami K., Kawamura K., Tsukamoto I., Kudo Y., Kanakura Y., et al. (1997). Cellular localization of thrombopoietin mRNA in the liver by in situ hybridization. Exp. Hematol. 25 565–572. [PubMed] [Google Scholar]
- Nonaka H., Tanaka M., Suzuki K., Miyajima A. (2007). Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev. Dyn. 236 2258–2267. 10.1002/dvdy.21227 [DOI] [PubMed] [Google Scholar]
- Omatsu Y., Sugiyama T., Kohara H., Kondoh G., Fujii N., Kohno K., et al. (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33 387–399. 10.1016/j.immuni.2010.08.017 [DOI] [PubMed] [Google Scholar]
- Onitsuka I., Tanaka M., Miyajima A. (2010). Characterization and functional analyses of hepatic mesothelial cells in mouse liver development. Gastroenterology 138 1525–1535. 10.1053/j.gastro.2009.12.059 [DOI] [PubMed] [Google Scholar]
- Ottersbach K., Dzierzak E. (2005). The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell 8 377–387. 10.1016/j.devcel.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Palis J., Chan R. J., Koniski A., Patel R., Starr M., Yoder M. C. (2001). Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 98 4528–4533. 10.1073/pnas.071002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J., Robertson S., Kennedy M., Wall C., Keller G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126 5073–5084. [DOI] [PubMed] [Google Scholar]
- Park L. S., Friend D. J., Schmierer A. E., Dower S. K., Namen A. E. (1990). Murine interleukin 7 (IL-7) receptor: characterization on an IL-7-dependent Cell Line. J. Exper. Med. 171 1073–1089. 10.1084/jem.171.4.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W., Feyerabend T. B., Rössler J., Wang X., Postrach D., Busch K., et al. (2017). Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 548 456–460. 10.1038/nature23653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon J. J., Morrissey P. J., Grabstein K. H., Ramsdell F. J., Maraskovsky E., Gliniak B. C., et al. (1994). Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180 1955–1960. 10.1084/jem.180.5.1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Cocault L., Volle-Challier C., Fleury M., Peault B., Souyri M. (2007). Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development 134 3031–3040. 10.1242/dev.001818 [DOI] [PubMed] [Google Scholar]
- Pinho S., Frenette P. S. (2019). Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20 303–320. 10.1038/s41580-019-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y., et al. (2013). PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exper. Med. 210 1351–1367. 10.1084/jem.20122252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploemacher R. E., van Soest P. L. (1977). Morphological investigation on phenylhydrazine-induced erythropoiesis in the adult mouse liver. Cell Tissue Res. 178 435–461. 10.1007/bf00219567 [DOI] [PubMed] [Google Scholar]
- Prior N., Hindley C. J., Rost F., Esteban E. M., Lau W. W. Y., Göttgens B., et al. (2018). Lgr5+ stem/progenitor cells reside at the apex of the embryonic hepatoblast pool. bioRxiv [Preprint]. 10.1101/485870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Buza-Vidas N., Hyland C. D., Jensen C. T., Antonchuk J., Månsson R., et al. (2007). Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1 671–684. 10.1016/j.stem.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Ramond C., Berthault C., Burlen-Defranoux O., De Sousa A. P., Guy-Grand D., Vieira P., et al. (2014). Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat. Immunol. 15 27–35. 10.1038/ni.2782 [DOI] [PubMed] [Google Scholar]
- Ramsfjell V., Borge O. J., Veiby O. P., Cardier J., Murphy M. J., Lyman S. D., et al. (1996). Thrombopoietin, but not erythropoietin, directly stimulates multilineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokines: distinct interactions with the ligands for c-kit and FLT3. Blood 88 4481–4492. [PubMed] [Google Scholar]
- Rhodes K. E., Gekas C., Wang Y., Lux C. T., Francis C. S., Chan D. N., et al. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2 252–263. 10.1016/j.stem.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M. A., Hoppe P. S., Smejkal B. M., Eitelhuber A. C., Schroeder T. (2009). Hematopoietic cytokines can instruct lineage choice. Science 325 217–218. 10.1126/science.1171461 [DOI] [PubMed] [Google Scholar]
- Robin C., Bollerot K., Mendes S., Haak E., Crisan M., Cerisoli F., et al. (2009). Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell 5 385–395. 10.1016/j.stem.2009.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli A. E., Wolock S. L., Weinreb C. S., Panero R., Patel S. H., Jankovic M., et al. (2018). Clonal analysis of lineage fate in native haematopoiesis. Nature 553 212–216. 10.1038/nature25168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V., Verfaillie C. M. (1999). Expression and function of cell adhesion molecules on fetal liver, cord blood and bone marrow hematopoietic progenitors: implications for anatomical localization and developmental stage specific regulation of hematopoiesis. Exp. Hematol. 27 302–312. 10.1016/S0301-472X(98)00031-9 [DOI] [PubMed] [Google Scholar]
- Rubin J. B., Shia M. A., Pilch P. F. (1983). Stimulation of tyrosine-specific phosphorylation in vitro by insulin-Like growth factor I. Nature 305 438–440. 10.1038/305438a0 [DOI] [PubMed] [Google Scholar]
- Ruggeri A., Labopin M., Sormani M. P., Sanz G., Sanz J., Volt F., et al. (2014). Engraftment kinetics and graft failure after single umbilical cord blood transplantation using a myeloablative conditioning regimen. Haematologica 99 1509–1515. 10.3324/haematol.2014.109280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu K. Y., Park H., Rossi D. J., Weissman I. L., Kopito R. R. (2012). Perturbation of the hematopoietic system during embryonic liver development due to disruption of polyubiquitin gene Ubc in mice. PLoS One 7:32956 10.1371/journal.pone.0032956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131 324–336. 10.1016/j.cell.2007.08.025 [DOI] [PubMed] [Google Scholar]
- Sauvageau G., Iscove N. N., Humphries R. K. (2004). In vitro and in vivo expansion of hematopoietic stem cells. Oncogene 23 7223–7232. 10.1038/sj.onc.1207942 [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Luna J. (1987). Identification of the receptor for erythropoietin by cross-linking to Friend virus-infected erythroid cells. Proc. Natl. Acad. Sci. U.S.A. 84 3690–3694. 10.1073/pnas.84.11.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4 7–25. [PubMed] [Google Scholar]
- Schumann K., Lämmermann T., Bruckner M., Legler D. F., Polleux J., Spatz J. P., et al. (2010). Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32 703–713. 10.1016/j.immuni.2010.04.017 [DOI] [PubMed] [Google Scholar]
- Schuster J. A., Stupnikov M. R., Ma G., Liao W., Lai R., Ma Y., et al. (2012). Expansion of hematopoietic stem cells for transplantation: current perspectives. Exper. Hematol. Oncol. 1:12 10.1186/2162-3619-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L., Koury S. T., Nejfelt M. K., Gearhart J. D., Antonarakis S. E. (1991). Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 88 8725–8729. 10.1073/pnas.88.19.8725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severn C. B. (1971). A morphological study of the development of the human liver. I. Development of the hepatic diverticulum. Am. J. Anat. 131 133–158. 10.1002/aja.1001310202 [DOI] [PubMed] [Google Scholar]
- Shiojiri N., Sugiyama Y. (2004). Immunolocalization of extracellular matrix components and integrins during mouse liver development. Hepatology 40 346–355. 10.1002/hep.20303 [DOI] [PubMed] [Google Scholar]
- Sigurdsson V., Takei H., Soboleva S., Radulovic V., Galeev R., Siva K. (2016). Bile acids protect expanding hematopoietic stem cells from unfolded protein stress in fetal liver short article bile acids protect expanding hematopoietic stem cells from unfolded protein stress in fetal liver. Stem Cell 18 522–532. 10.1016/j.stem.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Soares-da-Silva F., Burlen-Defranoux O., Elsaid R., Iturri L., Freyer L., Sismeiro O., et al. (2020). Yolk sac erythromyeloid progenitors sustain erythropoiesis throughout embryonic life. bioRxiv [Preprint]. 10.1101/2020.02.27.968230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar G. P., Kerr W. G., Zeigler F. C., Hess D., Donahue C., Sauvage F. J., et al. (1998). Role of c-mpl in early hematopoiesis. Blood 92 4–10. [PubMed] [Google Scholar]
- Stier S., Ko Y., Forkert R., Lutz C., Neuhaus T., Grunewald E., et al. (2005). Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exper. Med. 201 1781–1791. 10.1084/jem.20041992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., et al. (1989). Interleukin 7 production and function in stromal cell-dependent B cell development. J. Exp. Med. 170 333–338. 10.1084/jem.170.1.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D., Kulkeaw K., Mizuochi C. (2013). TGF-beta-1 up-regulates extra-cellular matrix production in mouse hepatoblasts. Mech. Dev. 130 195–206. 10.1016/j.mod.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Sugiyama D., Kulkeaw K., Mizuochi C., Horio Y., Okayama S. (2011). Hepatoblasts comprise a niche for fetal liver erythropoiesis through cytokine production. Biochem. Biophys. Res. Commun. 410 301–306. 10.1016/j.bbrc.2011.05.137 [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. (2006). Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25 977–988. 10.1016/j.immuni.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Koike T., Shiojiri N. (2010a). Developmental changes of cell adhesion molecule expression in the fetal mouse liver. Anat. Rec. 293 1698–1710. 10.1002/ar.21204 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Takabe Y., Nakakura T., Tanaka S., Koike T., Shiojiri N. (2010b). Sinusoid development and morphogenesis may be stimulated by VEGF-Flk-1 signaling during fetal mouse liver development. Dev. Dyn. 239 386–397. 10.1002/dvdy.22162 [DOI] [PubMed] [Google Scholar]
- Sun J., Ramos A., Chapman B., Johnnidis J. B., Le L., Ho Y.-J., et al. (2014). Clonal dynamics of native haematopoiesis. Nature 514 322–327. 10.1038/nature13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Zheng Y. W., Kondo R., Kusakabe M., Takada Y., Fukao K., et al. (2000). Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 32 1230–1239. 10.1053/jhep.2000.20349 [DOI] [PubMed] [Google Scholar]
- Swartley O. M., Foley J. F., Livingston D. P., Cullen J. M., Elmore S. A. (2016). Histology atlas of the developing mouse hepatobiliary hemolymphatic vascular system with emphasis on embryonic days 11.5-18.5 and early postnatal development. Toxicol. Pathol. 44 705–725. 10.1177/0192623316630836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajer P., Pike-Overzet K., Arias S., Havenga M., Staal F. (2019). Ex vivo expansion of hematopoietic stem cells for therapeutic purposes: lessons from development and the niche. Cells 8:169 10.3390/cells8020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe Y., Yagi S., Koike T., Shiojiri N. (2012). Immunomagnetic exclusion of E-cadherin-positive hepatoblasts in fetal mouse liver cell cultures impairs morphogenesis and gene expression of sinusoidal endothelial cells. J. Anat. 221 229–239. 10.1111/j.1469-7580.2012.01532.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y., Terada M., Kawabata M., Suzuki A. (2015). Dynamic three-dimensional morphogenesis of intrahepatic bile ducts in mouse liver development. Hepatology 61 1003–1011. 10.1002/hep.27436 [DOI] [PubMed] [Google Scholar]
- Takebe T., Enomura M., Yoshizawa E., Kimura M., Koike H., Ueno Y., et al. (2015). Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16 556–565. 10.1016/j.stem.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Tamplin O. J., Durand E. M., Carr L. A., Childs S. J., Hagedorn E. J., Li P., et al. (2015). Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160 241–252. 10.1016/j.cell.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. S., Kulkeaw K., Nakanishi Y., Sugiyama D. (2017). Expression of cytokine and extracellular matrix mRNAs in fetal hepatic stellate cells. Genes Cells 22 836–844. 10.1111/gtc.12517 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Okabe M., Suzuki K., Kamiya Y., Tsukahara Y., Saito S., et al. (2009). Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: drastic change of EpCAM expression during liver development. Mech. Dev. 126 665–676. 10.1016/j.mod.2009.06.939 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kurosawa S., Tajima K., Tanaka T., Ito R., Inoue Y., et al. (2016). Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 51 553–559. 10.1038/bmt.2015.330 [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Toyoshima T., Fukao K., Nakauchi H. (1996). Presence of hematopoietic stem cells in the adult liver. Nat. Med. 2 198–203. 10.1038/nm0296-198 [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Kaneko K., Itoh T., Ichinohe N., Ishii M., Mizuguchi T., et al. (2016). Intrahepatic bile ducts are developed through formation of homogeneous continuous luminal network and its dynamic rearrangement in mice. Hepatology 64 175–188. 10.1002/hep.28521 [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Miyajima A., Mostov K. E. (2009). Liver progenitor cells fold up a cell monolayer into a double-layered structure during tubular morphogenesis. Mol. Biol. Cell 20 2486–2494. 10.1091/mbc.e08-02-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu N., Nishikawa M., Saito H., Tsujimura T., Miyajima A. (2003). Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 116 1775–1786. 10.1242/jcs.00388 [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Saito H., Mostov K., Miyajima A. (2004). Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J. Cell Sci. 117 6425–6434. 10.1242/jcs.01572 [DOI] [PubMed] [Google Scholar]
- Taoudi S., Gonneau C., Moore K., Sheridan J. M., Blackburn C. C., Taylor E., et al. (2008). Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-Cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell 3 99–108. 10.1016/j.stem.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Taoudi S., Morrison A. M., Inoue H., Gribi R., Ure J., Medvinsky A. (2005). Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development 132 4179–4191. 10.1242/dev.01974 [DOI] [PubMed] [Google Scholar]
- Tavian M., Coulombel L., Luton D., Clemente H. S., Dieterlen-Lièvre F., Péault B. (1996). Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood 87 67–72. [PubMed] [Google Scholar]
- Tavian M., Hallais M. F., Péault B. (1999). Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development 126 793–803. [DOI] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y. (1995). Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am. J. Pathol. 146 67–74. [PMC free article] [PubMed] [Google Scholar]
- Tremblay K. D., Zaret K. S. (2005). Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 280 87–99. 10.1016/j.ydbio.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Tsai S., Emerson S. G., Sieff C. A., Nathan D. G. (1986). Isolation of a human stromal cell strain secreting hemopoietic growth factors. J. Cell. Physiol. 127 137–145. 10.1002/jcp.1041270117 [DOI] [PubMed] [Google Scholar]
- Tsuneto M., Tokoyoda K., Kajikhina E., Hauser A. E., Hara T., Tani-ichi S., et al. (2013). B-cell progenitors and precursors change their microenvironment in fetal liver during early development. Stem Cells 31 2800–2812. 10.1002/stem.1421 [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Callea F., Steen K., Van Der Moerman P., Desmet V. J. (1988). The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology 8 1586–1595. 10.1002/hep.1840080619 [DOI] [PubMed] [Google Scholar]
- Vestentoft P. S., Jelnes P., Hopkinson B. M., Vainer B., Møllgård K., Quistorff B., et al. (2011). Three-dimensional reconstructions of intrahepatic bile duct tubulogenesis in human liver. BMC Dev. Biol. 11:56 10.1186/1471-213X-11-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigon I., Mornon J. P., Cocault L., Mitjavila M. T., Tambourin P., Gisselbrecht S., et al. (1992). Molecular cloning and characterization of MPL, the human homolog of the v- mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc. Natl. Acad. Sci. U.S.A. 89 5640–5644. 10.1073/pnas.89.12.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake K. (1971). “Sternzellen” in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am. J. Anat. 132 429–461. 10.1002/aja.1001320404 [DOI] [PubMed] [Google Scholar]
- Walasek M. A., van Os R., de Haan G. (2012). Hematopoietic stem cell expansion: challenges and opportunities. Ann. N. Y. Acad. Sci. 1266 138–150. 10.1111/j.1749-6632.2012.06549.x [DOI] [PubMed] [Google Scholar]
- Wang H. U., Chen Z. F., Anderson D. J. (1998). Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93 741–753. 10.1016/S0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Seki S., Abo T. (1996). c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J. Exp. Med. 184 687–693. 10.1084/jem.184.2.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Frenette P. S. (2018). Niches for hematopoietic stem cells and their progeny. Immunity 48 632–648. 10.1016/j.immuni.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A., Johnson R. S. (2009). Nonrenal regulation of EPO synthesis. Kidney Int. 75 682–688. 10.1038/ki.2008.687 [DOI] [PubMed] [Google Scholar]
- Wilkinson A. C., Igarashi K. J., Nakauchi H. (2020). Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 27 273–278. 10.1038/s41576-020-0241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson A. C., Ishida R., Kikuchi M., Sudo K., Morita M., Crisostomo R. V., et al. (2019). Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571 117–121. 10.1038/s41586-019-1244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., et al. (2008). Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135 1118–1129. 10.1016/j.cell.2008.10.048 [DOI] [PubMed] [Google Scholar]
- Wilson W., Groat C. S., Leduc E. H. (2006). Histogenesis of the liver. Ann. N. Y. Acad. Sci. 111 8–24. 10.1111/j.1749-6632.1963.tb36945.x [DOI] [PubMed] [Google Scholar]
- Wineman J., Moore K., Lemischka I., Müller-Sieburg C. (1996). Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood 87 4082–4090. [PubMed] [Google Scholar]
- Wittig O., Paez-Cortez J., Cardier J. E. (2010). Liver sinusoidal endothelial cells promote b lymphopoiesis from primitive hematopoietic cells. Stem Cells Dev. 19 341–350. 10.1089/scd.2009.0300 [DOI] [PubMed] [Google Scholar]
- Wohrer S., Knapp D. J. H. F., Copley M. R., Benz C., Kent D. G., Rowe K., et al. (2014). Distinct stromal cell factor combinations can separately control hematopoietic stem cell survival, proliferation, and self-renewal. Cell Rep. 7 1956–1967. 10.1016/j.celrep.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber F. M., Leonard E., Michael S., Orschell-Traycoff C. M., Yoder M. C., Srour E. F. (2002). Roles of spleen and liver in development of the murine hematopoietic system. Exp. Hematol. 30 1010–1019. 10.1016/S0301-472X(02)00881-0 [DOI] [PubMed] [Google Scholar]
- Yang L., Li L. C., Lamaoqiezhong L., Wang X., Wang W. H., Wang Y. C., et al. (2019). The contributions of mesoderm-derived cells in liver development. Semin. Cell Dev. Biol. 92 63–76. 10.1016/j.semcdb.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Yang L., Wang W. H., Qiu W. L., Guo Z., Bi E., Xu C. R. (2017). A single-cell transcriptomic analysis reveals precise pathways and regulatory mechanisms underlying hepatoblast differentiation. Hepatology 66 1387–1401. 10.1002/hep.29353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., et al. (1987). Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 6 3341–3351. 10.1002/j.1460-2075.1987.tb02655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. W. C., Scadden D. T. (2016). Hematopoietic stem cell and its bone marrow niche. Curr. Top. Dev. Biol. 118 21–44. 10.1016/bs.ctdb.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. C., Kaba M., Ge G., Xie K., Tong W., Hug C., et al. (2006). Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12 240–245. 10.1038/nm1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. C., Lodish H. F. (2004). Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 103 2513–2521. 10.1182/blood-2003-08-2955 [DOI] [PubMed] [Google Scholar]
- Zheng J., Huynh H., Umikawa M., Silvany R., Zhang C. C. (2011). Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche. Blood 117 470–479. 10.1182/blood-2010-06-291716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Umikawa M., Cui C., Li J., Chen X., Zhang C. C. C., et al. (2012). Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature 485 656–660. 10.1038/nature11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. O., Yue R., Murphy M. M., Peyer J. G., Morrison S. J. (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15 154–168. 10.1016/j.stem.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn A. M. (2008). Liver development. Stembook 2 1–26. 10.3824/stembook.1.25.1 [DOI] [Google Scholar]