Abstract
We describe 2 cases of pseudotumors induced by an unusual size of polyethylene wear particle after metal-on-polyethylene total hip arthroplasty (MoP THA). The supra-macroparticles of size >100 μm originated from a polyethylene liner with relatively small cup anteversion, potentially leading to excessive loading and increased wear of the anterior edge of the polyethylene liner. Histopathology showed a foreign-body reaction to the polyethylene particles without an adverse reaction to metal debris and with no severe signs of corrosion at the head-neck junction, which have been noted in past reports of pseudotumors in MoP THA. It has been suggested that the large polyethylene wear particles might be the cause of pseudotumor formation in MoP THA.
Keywords: Metal-on-polyethylene, Pseudotumor, Revision total hip arthroplasty, Polyethylene wear
Introduction
An abnormal periprosthetic soft-tissue reaction resulting in a granulomatous or destructive cystic lesion has been recognized as a serious postoperative complication in total hip arthroplasty (THA). It generally causes discomfort, pain, severe destruction of the periarticular soft tissue, instability, osteolysis, and implant loosening, ultimately leading to revision surgery [1,2]. There is now an extensive body of literature on metal-induced periprosthetic soft-tissue reaction, known as adverse reaction to metal debris (ARMD), after metal-on-metal (MoM) THA [[3], [4], [5]]. ARMD includes necrosis, lymphocytosis, vasculitis, and the development of pseudotumors.
Pseudotumors are nonmalignant soft-tissue growths arising because of particulate debris irritation, and their histopathological findings often include features consistent with foreign-body reactions and hypersensitivity induced by metal particles and/or ions, such as macrophages containing metal particles, necrosis, lymphocytic aggregates, and granulomas [1,5]. Recent reports [[6], [7], [8]] have described pseudotumor formation in metal-on-polyethylene (MoP) bearings although it occurs less frequently than with MoM bearings. The pseudotumor formation is thought to be due to mechanically assisted crevice fretting/corrosion at the head-neck tapers (so-called trunnionosis) and excessive articular wear causing metal particle/ion release [2,[6], [7], [8]]. Nevertheless, the detailed mechanism of pseudotumor formation remains to be fully elucidated, especially in MoP THA (with a possible coexistence of metal and polyethylene particles). The influence of polyethylene wear particles on pseudotumor formation is unclear. In this context, the present report presents 2 patients with symptomatic pseudotumors after cementless MoP THAs, which were associated with tissue reactions to extremely large polyethylene debris—called supra-macroparticles [9].
Case histories
This article presents 2 patients (cases 1 and 2) with symptomatic pseudotumor formation after primary cementless THAs using MoP bearings performed in our institution in 2003. They received RingLoc® press-fit cups with ArCom® RingLoc acetabular liners (Zimmer Biomet, Warsaw, IN). The femoral components were collarless, proximally porous-coated, Bi-Metric® stems with 28-mm CoCr heads (Zimmer Biomet, Warsaw, IN). Two-dimensional head penetration into the polyethylene liners was analyzed on anteroposterior (AP) pelvic radiographs using the Martell’s Hip Analysis Suite software (version 8.0.4.5; University of Chicago, IL), which is a computer-assisted semiautomated edge detection system. The patient demographics are summarized in Table 1. Prior studies have reported a desirable combined anteversion in a range of 25°-50° [10,11]; therefore, the implant alignment would be considered acceptable in both cases (29° and 30° in cases 1 and 2, respectively). However, the cup anteversion alone (12° and 7° in cases 1 and 2, respectively) was relatively smaller than the safe zones reported by recent studies (31° ± 8° [12] and 17° ± 4° [13]).
Table 1.
Demographic information of the patients.
| Age/ gender | Diagnosis | Time to revision (y) | Cup abduction angle | Cup anteversion angle | Stem anteversion angle | Total penetration (mm) | Penetration rate (mm/y) |
|---|---|---|---|---|---|---|---|
| 53/ female | OA | 10.4 | 42 | 12 | 17 | 1.45 | 0.14 |
| 44/ male | ION | 11.3 | 43 | 7 | 23 | 3.42 | 0.30 |
OA, osteoarthritis; ION, idiopathic osteonecrosis of the femoral head.
Metal artifact reduction sequence–magnetic resonance imaging (MARS-MRI) was used to visualize the periprosthetic soft tissues. The pseudotumors were graded using a previously validated classification system by Hauptfleisch et al. [14] based on their compositions and wall thickness on MARS-MRI (type I: cystic lesions with wall thickness <3 mm, type II: cystic lesions with wall thickness >3 mm, and type III: solid lesions). Goldberg’s 4-point visual scoring system [15] was used to determine the severity of corrosion based on the extent and severity of surface damage seen at the head-neck taper. The scores represent the following: 1 = no corrosion; 2 = mild corrosion; 3 = moderate corrosion; and 4 = severe corrosion. The metal-ion levels in the serum of the patients were measured using the inductively coupled plasma mass spectrometry method for Co-ion levels (Mayo Medical Laboratories, Rochester, MN) and the atomic absorption spectrometry method for Cr-ion levels (LSI Medience Corporation; Tokyo). Histopathological analyses of abnormal periprosthetic soft tissues (pseudotumor) were performed. Both cases were also given an aseptic lymphocyte-dominated vasculitis–associated lesion (ALVAL) score on a scale of 1 to 10 (low: 0-4, moderate: 5-8, and high: 9-10) diagnosed by a pathologist. The higher ALVAL scores occurred in patients suspected to have metal hypersensitivity.
Both patients have given their written informed consent to having their data submitted for publication.
Case 1
A 53-year-old woman with a preoperative diagnosis of secondary osteoarthritis underwent THA of the right hip in 2003. At the 7-year follow-up, she complained of right hip discomfort when riding a bicycle. No radiolucent lines or osteolysis was evident on the AP radiograph (Fig. 1a). The setting angles of the cup and stem are listed in Table 1. MARS-MRI showed a pseudotumor (classified as type I) measuring 48 × 17 × 14 mm in front of the cup (Fig. 1b and c). The serum Co- and Cr-ion levels were both 0.3 μg/L.
Figure 1.
(a) Anteroposterior (AP) radiograph of case 1 showing no evidence of osteolysis, radiolucency, and head penetration of the polyethylene liner (total penetration: 1.73 mm) 10 y after primary THA. Coronal T2 (b) and axial T2 (c) MARS-MRI showing pseudotumor (type I) (white arrow).
The patient underwent revision surgery in 2014. For the revision procedure, the stem and cup were retained, and the CoCr head was replaced with a 32-mm head and the polyethylene liner with a vitamin E–infused highly cross-linked liner (E1®; Zimmer Biomet, Warsaw, IN). The visual corrosion scores at the head and neck were both evaluated as grade 2. An impingement scar was found on the anterior peripheral rim of the polyethylene liner. The pseudotumor was resected completely, and histopathological examination showed a foreign-body reaction containing numerous multinucleated giant cells with large polyethylene wear with 20- to 120-μm particles (supra-macroparticles) (Fig. 2a). However, no metal particles or related tissue reactions were discernible in the histopathological images. Immunohistochemical staining revealed CD68+ macrophage infiltration around the polyethylene particles (Fig. 2b), whereas only a few lymphocytes (CD20+ and CD3+) were observed. The ALVAL score was moderate at 5 points. The patient had an uneventful postoperative course and remained well at her 2-year follow-up visit.
Figure 2.
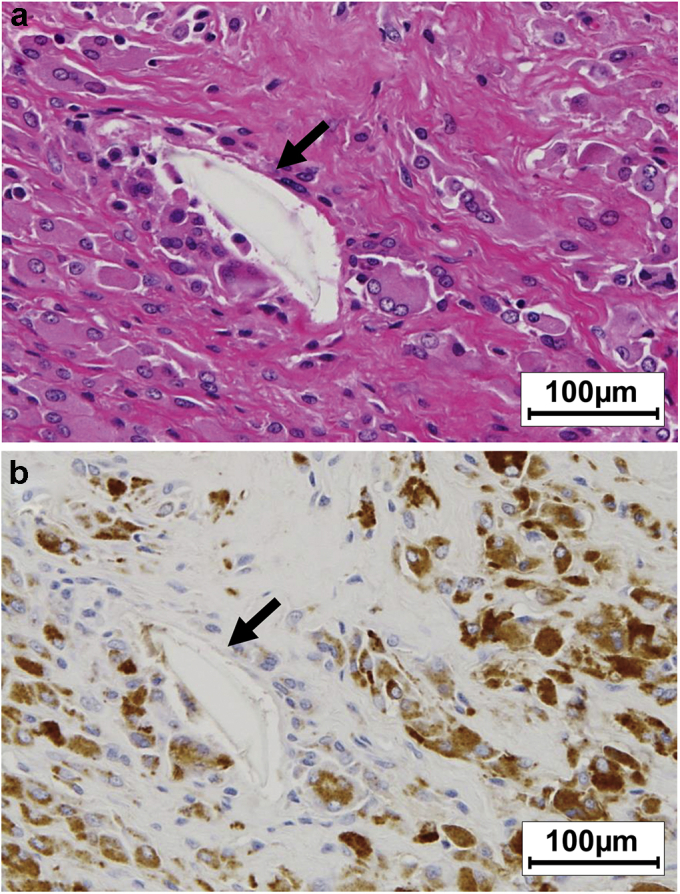
Histopathological (hematoxylin and eosin [H&E] staining; 100× magnification) (a) and immunopathological staining images (100×) (b) of the pseudotumor in case 1. The arrows indicate the polyethylene supra-macroparticles.
Case 2
A 44-year-old man with a preoperative diagnosis of idiopathic osteonecrosis of the femoral head underwent THA of the right hip in 2003. At the 10-year follow-up, he complained of feeling a mass and discomfort around his right hip. There was no radiographic evidence of osteolysis or implant loosening, but polyethylene wear (penetration by the head into the liner) was noted on the AP radiograph (Fig. 3a). The setting angles of the cup and stem are listed in Table 1. MARS-MRI showed a pseudotumor measuring 109 × 56 × 27 mm (classified as type II) in front of the cup (Fig. 3b and c). The serum Co- and Cr-ion levels were 0.3 and 0.5 μg/L, respectively.
Figure 3.
(a) AP radiograph of case 2 showing no clear osteolysis and radiolucency and head penetration of the polyethylene liner (total penetration: 3.42 mm) 11 y after primary THA. Coronal T2 (b) and axial T2 (c) MARS-MRI showing pseudotumor (type II) (white arrow).
The patient underwent revision THA in 2014. For the revision procedure, the stem was retained and the cup was replaced to increase the anteversion angle. The CoCr head was replaced with a 32-mm head and the polyethylene liner with E1®. There was no visual evidence of fretting corrosion on the stem trunnion (Fig. 4a), and the corrosion scores at the head and neck were both evaluated as grade 1. An impingement scar was found on the anterior peripheral rim of the polyethylene liner (Fig. 4b). All visible pseudotumors were resected. Histopathological analysis of the resected pseudotumor showed intense macrophage reaction to extremely large polyethylene wear debris with 200- to 1000-μm particles (supra-macroparticles) (Fig. 5a). No metal particles or related tissue reactions were discernible in the histopathological images. According to the immunohistochemical staining image (Fig. 5b), CD68+ macrophage was confirmed around the polyethylene particles, whereas only a few lymphocytes (CD20+ and CD3+) were observed. The ALVAL score was moderate at 6 points. The patient had an uneventful postoperative course and continued to do well at his 1-year follow-up visit.
Figure 4.

An intraoperative photograph of the stem taper in case 2 (a) clearly showing no corrosion at the head-neck junction after removing the head. Photograph of the removed polyethylene liner (b) showing impingement scar of the anterior edge (black arrow).
Figure 5.

Histopathological (H&E staining; 200× magnification) (a) and immunopathological staining images (200×) (b) of the pseudotumor in case 2. The arrows indicate polyethylene supra-macroparticles.
Discussion
Pseudotumor formation after THA is a rare complication, and most recent reports [[3], [4], [5]] focused on the problem of ARMD in MoM bearings. Nevertheless, there are some reports [2,[6], [7], [8]] on revision THA of periprosthetic pseudotumor formation associated with MoP bearings. Whitehouse et al. [6] reported 17 pseudotumor cases after MoP THAs due to metal corrosion products released from the head-neck junction. Nodzo et al. [2] also reported severe trunnionosis findings in 8 of 11 patients who underwent revision surgery. Recent studies of the causes of pseudotumors have shown the potential for taper corrosion and fretting damage at the head-neck junction, which leads to the release of metal debris and adverse local-tissue reactions [16,17]. Recent retrospective reports [7,18] document that the risk of ARMD or ALVAL revision surgeries is increasing in MoP THAs.
The histopathological findings in our cases, however, showed a foreign-body reaction to large polyethylene wear particles without ALVAL or ARMD. Furthermore, no visual signs of severe corrosion were noted at the head-neck junction at surgery. In addition, serum metal-ion levels measured before revision surgery were within the normal range. Therefore, our findings suggest that the development of pseudotumor was more likely to be associated with the wear particles from the polyethylene liner than debris and ion release from the metal implants.
Krenn et al. [9] defined the morphological characteristics (the size, shape, and color) of wear particulate components of implant materials in a particle algorithm that classifies polyethylene particles into 3 sizes: microparticles (<5 μm), macroparticles (>5-100 μm), and supra-macroparticles (>100 μm). Polyethylene wear usually produces relatively small particles up to 5 μm in size, which are thought to be phagocytosed predominantly by macrophages and causes the release of inflammatory cytokines and chemokines [19]. Particles of sizes 1 μm or less are the most prominent and reactive ones [20]. The cytokines stimulate the release of mediators that initiate an inflammatory cascade, which results in periprosthetic osteolysis and aseptic loosening.
The proinflammatory effects of the polyethylene supra-macroparticles are unclear. Macrophages can phagocytose microparticles (<5 μm), whereas particles of larger sizes (>10 μm) induce the formation of foreign-body giant cells [19]. Therefore, the function of foreign-body giant cells may be to phagocytose foreign bodies too large for macrophages. In our cases, it was suggested that large polyethylene particles (20-1000 μm) were phagocytosed by foreign body–type giant cells, resulting in reactive pseudotumor formation. Although the mechanism of pseudotumor formation is unknown, it occurred in these patients after MoP THA, suggesting that large polyethylene wear particles played a role. To our knowledge, this is the first study analyzing pseudotumors associated with large polyethylene particles after MoP THA.
The polyethylene supra-macroparticles (>100 μm) were described by Krenn et al. [9] who observed a previously unreported and unusual-sized particle in their series of 13 cases that showed loosening of the implants. The survival rate of the prostheses with polyethylene supra-macroparticles was lower than expected [21]. The polyethylene supra-macroparticles could be a consequence of excessive mechanical loading such as dislocation, subluxation, and impingement [9,21]. In our cases, a conventional polyethylene liner, ArCom®, was used; however, the amount of polyethylene wear was significant (0.14-0.30 mm/year; Table 1). We believe that the large number and size of polyethylene particles is related to the formation of pseudotumors. Furthermore, the cup anteversion in both cases was smaller than the safe zones suggested by Murphy et al. (31° ± 8°) [12] and Danoff et al. (17° ± 4°) [13], potentially leading to the generation of large wear particles due to anterior edge loading or rim impingement of the polyethylene liner [9,21].
With the improvement of polyethylene liners in recent years, low wear resistance can be expected, and vitamin E addition has improved the tolerance to edge loading [22]. Besides, the recent in vitro studies of vitamin E–diffused polyethylene have suggested that there is a reduction in the biological activity of polyethylene debris such as reduced osteolysis and pseudotumor potential [23]. However, there is still much to learn about the biological reaction to polyethylene particles in vivo, and it is necessary to investigate the number, size, and types of polyethylene particles that promote pseudotumor development in MoP THA. Furthermore, apart from the periprosthetic osteolysis, we must remember that a pseudotumor adjacent to the hip can present as polyethylene failure after THA.
Summary
Pseudotumor formation after MoP THA may be due to an adverse reaction to metal particles from trunnion corrosion and foreign-body reaction to polyethylene wear particles. In our cases, we found that polyethylene supra-macroparticles were produced by increased anterior edge loading of the polyethylene liner and by neck-liner impingement. The pseudotumor developed as a foreign-body reaction to large polyethylene wear particles. Although the reaction to large polyethylene particles in vivo is still unclear, the possibility of their involvement in pseudotumor formation is suggested.
Conflict of interest
The authors declare there are no conflicts of interest.
Appendix A. Supplementary data
References
- 1.Daniel J., Holland J., Quigley L., Sprague S., Bhandari M. Pseudotumors associated with total hip arthroplasty. J Bone Joint Surg Am. 2012;94:86. doi: 10.2106/JBJS.J.01612. [DOI] [PubMed] [Google Scholar]
- 2.Nodzo S.R., Esposito C.I., Potter H.G., Ranawat C.S., Wright T.M., Padgett D.E. MRI, retrieval analysis, and histologic evaluation of adverse local tissue reaction in metal-on-polyethylene total hip arthroplasty. J Arthroplasty. 2017;32:1647. doi: 10.1016/j.arth.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Matthies A.K., Skinner J.A., Osmani H., Henckel J., Hart A.J. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin Orthop Relat Res. 2012;470:1895. doi: 10.1007/s11999-011-2201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutphen S.A., MacLaughlin L.H., Madsen A.A., Russell J.H., McShane M.A. Prevalence of pseudotumor in patients after metal-on-metal hip arthroplasty evaluated with metal ion analysis and MARS-MRI. J Arthroplasty. 2016;31:260. doi: 10.1016/j.arth.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Wiley K.F., Ding K., Stoner J.A., Teague D.C. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: a meta-analysis. J Arthroplasty. 2013;28:1238. doi: 10.1016/j.arth.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Whitehouse M.R., Endo M., Zachara S. Adverse local tissue reactions in metal-on-polyethylene total hip arthroplasty due to trunnion corrosion: the risk of misdiagnosis. Bone Joint J. 2015;97:1024. doi: 10.1302/0301-620X.97B8.34682. [DOI] [PubMed] [Google Scholar]
- 7.Manthe M., Blasser K., Beauchamp C., O'Connor M.I. Trunnion corrosion causing failure in metal-on-polyethylene total hip arthroplasty with monolithic femoral components. Reconstr Rev. 2016;6 [Google Scholar]
- 8.Siljander M.P., Baker E.A., Baker K.C., Salisbury M.R., Thor C.C., Verner J.J. Fretting and corrosion damage in retrieved metal-on-polyethylene modular total hip arthroplasty systems: what is the importance of femoral head size? J Arthroplasty. 2018;33:931. doi: 10.1016/j.arth.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Krenn V., Morawietz L., Perino G. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract. 2014;210:779. doi: 10.1016/j.prp.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Dorr L.D., Malik A., Dastane M., Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009;467:119. doi: 10.1007/s11999-008-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohmori T., Kabata T., Kajino Y. Differences in range of motion with the same combined anteversion after total hip arthroplasty. Int Orthop. 2018;42:1021. doi: 10.1007/s00264-017-3653-5. [DOI] [PubMed] [Google Scholar]
- 12.Murphy W.S., Yun H.H., Hayden B., Kowal J.H., Murphy S.B. The safe zone range for cup anteversion is narrower than for inclination in THA. Clin Orthop Relat Res. 2018;476:325. doi: 10.1007/s11999.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danoff J.R., Bobman J.T., Cunn G. Redefining the acetabular component safe zone for posterior approach total hip arthroplasty. J Arthroplasty. 2016;31:506. doi: 10.1016/j.arth.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Hauptfleisch J., Pandit H., Grammatopoulos G., Gill H.S., Murray D.W., Ostlere S. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty. Skeletal Radiol. 2012;41:149. doi: 10.1007/s00256-011-1329-6. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg J.R., Gilbert J.L., Jacobs J.J., Bauer T.W., Paprosky W., Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002;401:149. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Cooper H.J., Della Valle C.J., Berger R.A. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindgren J.U., Brismar B.H., Wikstrom A.C. Adverse reaction to metal release from a modular metal-on-polyethylene hip prosthesis. J Bone Joint Surg Br. 2011;93:1427. doi: 10.1302/0301-620X.93B10.27645. [DOI] [PubMed] [Google Scholar]
- 18.Persson A., Eisler T., Bodén H., Krupic F., Sköldenberg O., Muren O. Revision for symptomatic pseudotumor after primary metal-on-polyethylene total hip arthroplasty with a standard femoral stem. J Bone Joint Surg Am. 2018;100:942. doi: 10.2106/JBJS.17.00616. [DOI] [PubMed] [Google Scholar]
- 19.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell P., Ma S., Yeom B., McKellop H., Schmalzried T.P., Amstutz H.C. Isolation of predominantly submicron sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res. 1995;29:127. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 21.Krenn S., Thomsen M., Usbeck S. Supramacroparticulate PE in 6 different joint endoprostheses localisations: an indicator for PE damage? Pathol Res Pract. 2017;213:987. doi: 10.1016/j.prp.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi Y., Tateiwa T., Pezzotti G., Shishido T., Masaoka T., Yamamoto K. Improved resistance to neck-liner impingement in second-generation highly cross-linked polyethylene—the role of vitamin E and crosslinks. J Arthroplasty. 2016;31:2926. doi: 10.1016/j.arth.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Bichara D.A., Malchau E., Sillesen N.H., Cakmak S., Nielsen G.P., Muratoglu O.K. Vitamin E-diffused highly cross-linked UHMWPE particles induce less osteolysis compared to highly cross-linked virgin UHMWPE particles in vivo. J Arthroplasty. 2014;29:232. doi: 10.1016/j.arth.2014.03.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




