Summary
Objectives
To evaluate the biomechanical performance of the Femoral Neck System (FNS) versus the Hansson Pin System (Hansson Pins) with two parallel pins in a Pauwels II femoral neck fracture model with posterior comminution.
Methods
Forty-degree Pauwels II femoral neck fractures AO 31-B2.1 with 15° posterior wedge were simulated in fourteen paired fresh-frozen human femora, followed by instrumentation with either FNS or Hansson Pins in pair-matched fashion. Implant positioning was quantified by measuring shortest implant distances to inferior cortex (DI) and posterior cortex (DP) on anteroposterior and axial X-rays, respectively. Biomechanical testing was performed in 20° adduction and 10° flexion with simulated iliopsoas muscle tension. Progressively increasing cyclic loading was applied until construct failure. Interfragmentary femoral head-to-shaft movements were measured with optical motion tracking.
Results
Cycles to 10° varus deformation were significantly higher for FNS (23007 ± 5496) versus Hansson Pins (17289 ± 4686), P = 0.027. Cycles to 10° femoral head dorsal tilting (FNS: 12765 ± 3425; Hansson Pins: 13357 ± 6104) and cycles to 10° rotation around the femoral neck axis (FNS: 24453 ± 5073; Hansson Pins: 20185 ± 11065) were comparable between the implants, P ≥ 0.314. For Hansson Pins, the outcomes for varus deformation and dorsal tilting correlated significantly with DI and DP, respectively (P ≤ 0.047), whereas these correlations were not significant for FNS (P ≥ 0.310).
Conclusions
From a biomechanical perspective, by providing superior resistance against varus deformation and performing in a less sensitive way to variations in implant placement, the angular stable Femoral Neck System can be considered as a valid alternative to the Hansson Pin System for the treatment of Pauwels II femoral neck fractures.
Level of evidence
therapeutic, Level V.
The Translational potential of this article
The translational potential of this article is to compare the performance of the FNS with Hansson Pins in a AO 31-B2.1 fracture model featuring a 15 posterior wedge to show the implants behavior concerning the dorsal tilting tendency.
Keywords: Femoral neck fracture, Posterior comminution, Dorsal tilting, Femoral Neck System, Hansson Pin System, Biomechanics
Introduction
Femoral neck fractures account for half of all hip fractures and are recognized as a major public health problem associated with a high socioeconomic burden [1]. Whilst internal fixation is preferred over arthroplasty for physiologically younger patients, no consensus exists about the optimal fixation device yet. The complication rate related to healing of surgically treated femoral neck fractures ranges from 15% to 40% [[1], [2], [3]]. Clinical investigations show a clear relationship between unsatisfactory reduction in the region of the femoral neck and the likelihood of a surgical reintervention [4]. In the course of fracture management, following the establishment of anatomical reduction, its maintenance is the subsequent logical crucial demand on a fixation device. In this particular anatomical region of the femoral neck, such an implant should ideally provide best possible mechanical resistance against varus deformation, femoral head dorsal tilting, rotation of the femoral head around the neck axis, as well as neck shortening [5,6].
The recently introduced implant Femoral Neck System (FNS) (DePuy Synthes, Zuchwil, Switzerland) was developed for dynamic fixation of femoral neck fractures and is characterized by providing angular stability combined with a minimally invasive surgical technique and the opportunity of additional intraoperative compression at the fracture site if needed. In addition, the implant allows postoperative dynamization at the fracture site. Its biomechanical performance proved to be superior versus fixations with 3 parallel cannulated screws and comparable to both dynamic hip screw (DHS) with antirotation screw and DHS with blade in terms of sustainability of the restored neck length for unstable Pauwels III fractures [7]. In addition, a completely guided instrumentation procedure renders the surgical technique simple. An alternative approach for fixation of femoral neck fractures relies on internal buttressing of the implant being in contact with the cervical cortex to provide enhanced stability. One of the implants exploiting the advantages of internal buttressing is the Hansson Pin System (Hansson Pins, Swemac, Linköping, Sweden). Each pin of this implant has an integrated hook at the tip, deployed after insertion to provide rotational stability of the femoral head. Hansson Pins demonstrated a high user friendliness leading to good clinical outcomes provided that proper implant positioning is achieved [4,8]. Moreover, its well-known application for treatment of slipped capital femoral epiphyses [9,10] is related to reduced rates of avascular necrosis compared to the three-cannulated-screw fixation [4]. The system also allows postoperative dynamization at the fracture site. Although both implants FNS and Hansson Pins represent minimally invasive solutions and are constructed around a hollow, blunt-surfaced main component to provide rotational stability, their functioning concepts are fundamentally different. Considering the pinning principle of Hansson Pins, higher stability may be anticipated for FNS over the former. However, the application of Hansson Pins is preferred in more stable Pauwels II fractures with existing calcar support, for which no comparison to FNS has been undertaken yet. In addition, little knowledge about the biomechanical performance of both implants can be retrieved from the literature. By offering internal buttressing and rotational stability, Hansson Pins proved to be an interesting and conclusive minimal invasive alternative to the three-cannulated-screw fixation. Therefore, the aim of this study was to evaluate the biomechanical performance of FNS versus Hansson Pins in a Pauwels II femoral neck fracture model with simulated posterior comminution. We hypothesized that FNS would perform equally to Hansson Pins in terms of resistance to varus collapse, dorsal head tilting and head rotation around the femoral neck axis.
Materials & methods
Specimens preparation
Fourteen paired fresh-frozen (−20 °C) human cadaveric femora from 5 male and 2 female donors aged 68.5 ± 4.2 years (mean ± standard deviation (SD), range 60–75 years) with caput-collum-diaphyseal (CCD) angle of 130 ± 3° (range 125–135°) were used in this study. All donors gave their informed consent inherent within the donation of the anatomical gift statement during their lifetime. Specimens with any signs of severe osteoporosis, grade 3 or 4 osteoarthritis, Paget disease, avascular necrosis or previous hip fractures were excluded. All included femora were with a maximum distance of 95 mm, as measured between the lateral cortex and the medial femoral head apex along the femoral neck axis. Bone mineral density (BMD) was evaluated in the femoral head using high-resolution peripheral quantitative computed tomography (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland). The femora were assigned in a paired fashion for fixation with either FNS or Hansson Pins, whereby the anatomical sites of each pair were randomized to either treatment group. They were thawed at room temperature for 24 h prior to preparation and biomechanical testing. The former was performed in three steps of partial osteotomy setting, instrumentation, and osteotomy completion (Fig. 1). Within the first step a dorsally located 15° wedge fragment was removed from the femoral neck by means of two cuts using an oscillating saw. The first cut was performed with 40° angulation according to the Pauwels II fracture type classification to a depth reaching 75% of the neck diameter. A custom-made saw guide was used for this purpose to obtain standardized cutting angles (Fig. 1A) [11]. Subsequently, a custom 15° wedge cutting template with extended plate was used to perform the second cut and separate the wedge fragment. The plate was inserted into the first cutting zone until stop, while the second cut was performed along the 15° guiding surface, which was aligned to the end of the extended plate and inclined from posterior to superior (Fig. 1B). This temporary incomplete fracture was set to preserve the anterior cortical buttress during implant placement. In the second step, the instrumentation of FNS and Hansson Pins was performed by an experienced surgeon according to the manufacturer’s guidelines using a conventional radiographic resource (Fig. 2). After insertion of a 3.2-mm guiding Kirschner wire, the dynamic FNS component was placed in a center–center position at a tip-to-apex distance of 10–20 mm between the implant tip and subchondral bone [12]. The antirotating FNS screw was inserted and tightened at 4 Nm, and the 130-degree one-hole side plate was fixed to the shaft using a 5.0-mm locking screw.
Figure 1.
Osteotomy setting. Creation of a 40° femoral neck fracture line with a customized sawing guide (A); setting of a 15° dorsally located wedge defect with a sawing block guide (B).
Figure 2.
Anteroposterior radiographs (A, B) and photographic images (C, D) of a femoral pair instrumented with FNS (A, C) and Hansson Pins (B, D).
The implantation of the two Hansson Pins was performed following the idea of internal buttressing by touching two cortices from inside, a validated and formerly described technique [[13], [14], [15], [16], [17], [18]]. Entering from the lateral cortex side, two Hansson Pins were implanted parallel to each other after insertion of guide wires. The posterior pin touched the posterior cortex and the inferior pin – the medial cortex of the femoral neck. The pins were spread as far as possible from each other in lateral view. Whereas the FNS was produced of titanium alloy Ti–6Al–7Nb (TAN), the Hansson Pins were made of Ti–6Al–4V (TAV). After completion of the instrumentation, the fracture model was completed in the third step by a single cut separating the cortical bridge at the anterior femoral neck. Following, in preparation for biomechanical testing, the lesser trochanter was opened twice with a 2.5-mm drill in horizontal direction. The drill holes were slung with a 1-mm wound steel cable simulating the iliopsoas tendon, and a cylindric steel bar was placed within the sling to reduce force concentration in the area of the lesser trochanter and to avoid cutting out of the cable. After shaft shortening of all femora at a total length of 175 mm, each specimen was embedded in a 65-mm high polymethylmetacrylate (PMMA, SCS-Beracryl D28, Suter Kunststoffe AG, Fraubrunnen, Switzerland) base.
Biomechanical testing
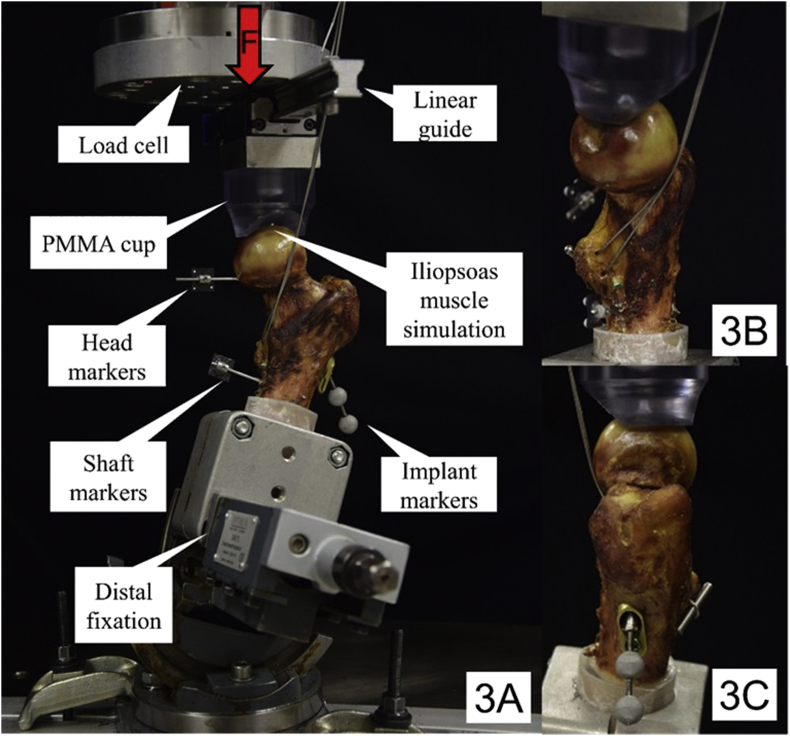
Biomechanical testing was performed on a servo-hydraulic material testing system (Bionix 858, MTS Systems, Eden Prairie, MN) equipped with a 4 kN/100 Nm load cell. All specimens were constrained in 20° adduction and 10° flexion during testing by means of a 3-axis vice (Fig. 3A) and loaded along the machine axis to mimic the action of the main force components in the frontal and sagittal planes during the human gait as measured by Bergmann et al. [19]. A PMMA shell cup was shaped as load mediator to enhance force transmission at the dorsal aspect of the femoral head. The cup was interconnected to the machine actuator via a frictionless linear guide able to move freely in anteroposterior direction for encouraged dorsal tilting of the femoral head fragment. In addition, static simulation of the iliopsoas muscle was achieved by the steel cable winding through the trochanter minor and passing below the medial cortex along to the anterior aspect of the femoral neck, oriented collinear with the shaft axis and fixed to the machine frame (Fig. 3B and C). This simulation was established to neutralize torsional forces at the femoral shaft resulting from the specimen position. The slightly lateral orientation of the cable respected the higher contribution of the Iliacus part over the Psoas part of the compound muscle [20]. To ensure prevention of cutting out of the steel cable from the lesser trochanter, a steel bolt was applied for a smoother force distribution over the area of the attachment (Fig. 3C).
Figure 3.
Setup with a specimen mounted for biomechanical testing. Each specimen was attached to the machine base via a 3-axis vice to achieve 20° adduction and 10° flextion position. Anterior view with vertical arrow denoting loading direction (3A); Medial (3B) and lateral (3C) view depicting simulation of the iliopsoas muscle attached to the lesser trochanter to counter torsional moments during loading.
The specimens were tested under axial loading applying a protocol adapted from previous studies [7,11,21,22]. It comprised an initial quasi-static ramp between 50 N and 200 N compression at a rate of 15 N/s, followed by cyclic loading at 2 Hz with a physiological profile of each cycle [21]. While the valley load was kept constant at 200 N, the peak load, starting at 500 N, was progressively increased at a rate of 0.1 N/cycle. Test stop criterion was defined by reaching 30 mm axial displacement of the machine actuator relative to its position at the test begin.
Data acquisition and evaluation
Implant positioning was quantified based on anteroposterior and axial radiological images post instrumentation. The anteroposterior images were used to measure the shortest distance between the inferior contour of the FNS bolt component or the inferior Hansson Pin and the medial femoral neck cortex in the calcar region, which was defined as Distance Inferior (DI) parameter of interest (Fig. 4A). Moreover, the shortest distance between the posterior contour of the FNS bolt component or the posterior Hansson Pin and the posterior femoral neck cortex – defined as Distance Posterior (DP) parameter of interest – was measured on the axial radiological images (Fig. 4B).
Figure 4.
Parameters indicating pin positioning in relation to the femoral neck cortex. Distance Posterior (DP) (4A); Distance Inferior (DI) (4B).
Machine data in terms of axial load and displacement were recorded from the machine controllers at 128 Hz. Based on that, axial construct stiffness was calculated from the slope of the load–displacement curve within initial quasi-static ramped loading between 150 and 200 N.
Relative movements between the femoral head and shaft were captured in all six degrees of freedom by means of three-dimensional optical motion tracking using 5 infrared cameras (ProReflex MCU, Qualisys AB, Gothenburg, Sweden). Retro-reflective marker sets were attached to the femoral head (4 markers), shaft (4 markers) and implant (2 markers). The head and shaft markers were glued on carbon fiber plates, each one of them building a set of four coplanar markers arranged in a square, allowing to virtually represent the corresponding rigid bodies. A local coordinate system was defined by orienting the shaft markers in the coronal plane so that the first principal axis (x) was oriented perpendicular to the femoral shaft axis in this plane, the second axis (y) passed parallel to the femoral axis, and the third axis (z) represented the anteroposterior anatomical axis. Based on this, varus deformation was computed from the rotational movement of the femoral head in the coronal plane, and dorsal tilting was calculated from the movement of the femoral head around the axis defined by crossing of the coronal and the first osteotomy planes. In addition, the rotation of the femoral head around the neck axis was evaluated. The neck axis was reconstructed based on virtual rotation of the shaft coordinate system around its anteroposterior axis by the amount given from the measured CCD angles. Furthermore, the two implant markers attached to the FNS antirotation screw and the inferior Hansson Pin were used to capture the implant dynamics within the femoral head in terms of telescoping (for FNS) or backing-out (for Hansson Pins).
The parameters derived from motion tracking were analyzed after 5000, 10000 and 15000 test cycles to evaluate the degradation of the construct stability within the course of cycles.
Furthermore, the numbers of cycles until 10° angular displacement and the corresponding peak load were calculated based on the progressions of these three parameters over time. Each of the outcomes was derived in peak loading condition determined by the absolved cyclic number, and with respect to the value at the beginning of the cyclic test after 3 cycles, the latter considered as baseline for exclusion of any initial settling effects. A C-arm was used to take axial radiological images at the beginning and end of the quasi-static ramp, and then in triggered mode at timed intervals every 250 cycles during the cyclic test at 200 N valley load. For that purpose, the cyclic test was interrupted for 2 s.
Statistical analysis upon the parameters of interest was performed with SPSS software package (IBM SPSS Statistics, V23, IBM, Armonk, NY). Shapiro–Wilk test was conducted to screen the data for normality of distribution. Three-step General Linear Model Repeated Measures test was applied to detect significant differences between the groups with regard to the parameters evaluated over the three time points after 5000, 10000 and 15000 cycles. Furthermore, significant differences between the two groups with regard to the cycles to 10° varus deformation, dorsal tilting and rotation around the neck axis were identified with Paired-Samples t-tests. In addition, Pearson correlation tests were performed to investigate the influence of BMD, DP and DI on the outcomes. Level of significance was set to 0.05 for all statistical tests.
Results
BMD was equally distributed between FNS (261.3 ± 34.4 mgHA/cm3) and Hansson Pins (265.1 ± 33.6 mgHA/cm3) with no significant differences between the 2 implant systems (P = 0.425).
Initial stiffness remained without significant differences between FNS (311.2 ± 121.3 N/mm) and Hansson Pins (335.4 ± 126.7 N/mm, P = 0.770).
Descriptive outcome measures of the parameters evaluated over the three time points after 5000, 10000 and 15000 cycles are summarized in Table 1, showing significant increase of each parameter between those cycle numbers (P < 0.001). However, the differences between the groups remained non-significant for each parameter (P ≥ 0.135).
Table 1.
Dorsal tilting, varus deformation, rotation around neck axis and implant telescoping/backing-out after 5000, 10000 and 15000 cycles, presented in terms of mean value and standard deviation for each study group separately, together with P-values from the statistical comparisons between the study groups (right column) and P-values indicating the temporal change over cycles (bottom row for each parameter separately).
| Group | Cycles |
P-value FNS vs Hansson Pins |
||
|---|---|---|---|---|
| 5000 | 10000 | 15000 | ||
| Dorsal tilting (°) | ||||
| FNS | 2.62 ± 0.82 | 4.80 ± 0.96 | 5.96 ± 0.96 | 0.135 |
| Hansson Pins | 1.63 ± 1.38 | 3.59 ± 1.54 | 5.64 ± 1.76 | |
| P-value over cycles | <0.001 | |||
| Varus deformation (°) | ||||
| FNS | 0.87 ± 0.70 | 2.09 ± 1.11 | 2.88 ± 1.15 | 0.465 |
| Hansson Pins | 1.36 ± 0.83 | 2.86 ± 1.48 | 3.83 ± 1.74 | |
| P-value over cycles | <0.001 | |||
| Rotation around neck (°) | ||||
| FNS | 2.15 ± 0.75 | 4.26 ± 1.17 | 5.41 ± 1.38 | 0.869 |
| Hansson Pins | 2.10 ± 3.61 | 3.61 ± 4.59 | 5.14 ± 5.63 | |
| P-value over cycles | <0.001 | |||
| Implant telescoping/backing-out (mm) | ||||
| FNS | 1.11 ± 1.05 | 1.96 ± 1.30 | 2.62 ± 1.41 | 0.292 |
| Hansson Pins | 1.40 ± 0.74 | 2.47 ± 0.75 | 3.91 ± 0.96 | |
| P-value over cycles | <0.001 | |||
Descriptive data for cycles to 10° varus deformation, dorsal tilting and rotation around the neck axis is summarized in Table 2 for each group separately. FNS revealed significantly higher number of cycles to 10° varus deformation and load at 10° varus deformation compared to Hansson Pins (P = 0.027). However, number of cycles to 10° dorsal tilting and rotation around neck axis, as well as the corresponding load at 10° dorsal tilting and rotation around neck axis were not significantly different between FNS and Hansson Pins (P ≥ 0.314), Fig. 5. In addition, no significant correlation was indicated between BMD and cycles to 10° varus deformation, dorsal tilting and rotation around neck axis (P ≥ 0.071).
Table 2.
Cycles to 10° dorsal tilting, varus deformation and rotation around the neck axis, presented with the corresponding loads in terms of mean value and standard deviation for each study group separately, together with P-values from the statistical comparisons between the study groups.
| Parameter | FNS | Hansson Pins | P-value |
|---|---|---|---|
| Cycles to 10° dorsal tilting | 12765 ± 3425 | 13357 ± 6104 | 0.768 |
| Load at 10° dorsal tilting | 1776.5 ± 342.5 | 1835.7 ± 610.4 | |
| Cycles to 10° varus deformation | 23007 ± 5496 | 17289 ± 4686 | 0.027 |
| Load at 10° varus deformation | 2800.7 ± 549.6 | 2228.9 ± 468.6 | |
| Cycles to 10° rotation around neck axis | 24453 ± 5073 | 20185 ± 11065 | 0.314 |
| Load at 10° rotation around neck axis | 2945.3 ± 507.3 | 2518.5 ± 1106.5 |
Figure 5.
Cycles to 10° dorsal tilting, varus deformation and rotation around neck axis, presented in terms of mean value and standard deviation for each group separately. Star indicates significant difference.
Implant positioning-related outcomes DI and DP amounted to 10.9 ± 3.1 mm and 8.1 ± 2.6 mm for FNS, and 4.6 ± 1.8 mm and 2.9 ± 1.7 mm for Hansson Pins, respectively. For Hansson Pins, significant negative correlations were indicated between DI and cycles to 10° varus deformation, as well as between DP and cycles to 10° dorsal tilting (P ≤ 0.047), while for FNS these correlations remained non-significant (P ≥ 0.310), Fig. 6. Finally, for Hansson Pins, DP tended to negatively correlate with cycles to 10° rotation around the neck axis (P = 0.070), whereas for FNS this correlation was not significant (P = 0.223).
Figure 6.
Cycles to 10° dorsal tilting versus Distance Posterior (DP) (circles) and cycles to 10° varus deformation versus Distance Inferior (DI) (squares) plotted separately for each specimen instrumented with Hansson Pins. Blue and red lines denote linear regressions with the respective linear functions and coefficients of determination (R2).
Discussion
This study evaluated the biomechanical performance of fixations with FNS versus Hansson Pins in Pauwels II (AO/OTA 31-B2.1) fractures with posterior comminution and revealed significant superiority of the former in terms of resistance to varus collapse, despite the use of a more stable fracture model in comparison to previous work [7]. On the other hand, both implant systems resulted in comparable stability with regard to initial stiffness and the relative head-to-shaft fragment movements evaluated over the three time points, namely varus deformation, dorsal tilting of the femoral head fragment and its rotation around the neck axis. These outcomes demonstrate that the stability of FNS comes into complete effect under ultimate loads during biomechanical testing. Being a free-handed fixation solution, the implantation of the two Hansson Pins needs a careful execution to achieve internal buttressing on the inferior and posterior cortices of the cervical neck [4,15,16]. To account for this, two parameters – DP and DI – were introduced as measures of the implant positioning, and they correlated significantly with the dorsal tilting and varus deformation of the Hansson Pins, in contrast to FNS, the latter showing less sensitivity towards precise implant positioning in terms of stability. Hence, in comparison to the Hansson Pins, FNS seems to offer biomechanical behavior which is less dependent on the quality of implantation. It is known from previous studies that polyaxial anchorage is advantageous versus monoaxial fixation in terms of coronal and sagittal femoral head displacement [10,23,24]. In contrast to Hansson Pins, by offering a diverging lag screw, FNS offers polyaxiality [13] and has already proven its superiority in a more unstable fracture model in terms of less neck and leg shortening and higher resistance to varus collapse over similar implants, such as three cannulated screws [7]. The latter advantage was confirmed in the current study. Moreover, the outcomes for Hansson Pins were afflicted with higher variability, which could be attributed to the more sensitive implant positioning and the free-hand implantation. In contrast, FNS allows more consistent implant positioning, which may lead to higher reproducibility in its clinical use.
Regardless of the functional difference between the smoothly surfaced Hansson Pins and partially threaded cannulated screws, both implants rely on buttressing in the region of the cervical neck, postulating on that basis a similar demand for their positioning. Some authors recommend to place the screws in a range within 3 mm distance to the cortex to achieve the desired effect of internal buttressing [16], whereas others advise a position at 16 mm distance to the calcar for the anterior placed screw in a 2-screw fixation [14]. In this study the average distances from the anterior and the posterior Hansson Pin to the calcar (DI) and the posterior cortex (DP) were 4 mm and 3 mm, respectively, being close to the former recommendations for positioning [16].
A special focus of the present study was investigation of the dorsal femoral head tilting in the Pauwels II fractures as frequently observed failure in the clinical practice. To address this, the test setup implemented axial loading with reduced contact area and increased stress concentration at the dorsal aspects of the femoral head with specimen position in 20° adduction and 10° flexion. In addition, static mechanical simulation of the iliopsoas muscle with stabilization effect independent from the direction of action of the applied forces was used. With the presented novel setup, the reached failure loads are in agreement previously reported hip loads during everyday patient activities [21] and other biomechanical findings [7], although no one-to-one comparison is possible due to the diversity in the used parameters. In the current study, it is evident that 10° dorsal tilting – as one of the three failure criteria – was fulfilled first, given its lowest cyclic numbers and loads, which confirms the inherent characteristic of the novel setup to promote femoral head dorsal tilting.
The findings of this study elucidated a further worth-mentioning advantage of FNS over the Hansson Pins, namely the telescoping capability of its bolt, enabling controlled collapse of the femoral head without lateral hardware protrusion.
The limitations of the current study are similar to those inherent in all cadaveric biomechanical investigations. A limited number of specimens was used for testing over a certain number of cycles. Postoperative bone healing was not simulated, but the worst case scenario was reflected in cadaveric setting instead. Cyclic loading was applied until catastrophic failure with frequently observed head split fractures, not necessarily reproducing the clinically observed modes of failure represented by screw cut-out, varus collapse and neck/leg shortening [7]. Since the completion of the fracture was performed after implant insertion, the compression feature offered by the FNS instrumentation set could not be applied. Finally, the relatively stable fracture model impeded the expression of the failure criteria in terms of translational interfragmentary displacements, therefore, angular displacement of the femoral head under consideration of all three degrees of freedom was chosen instead. Although the amount of 10° was selected on an arbitrary basis, it can be considered as clinically relevant [25].
Conclusion
In conclusion, this cadaveric study demonstrated that under cyclic loading the angular stable Femoral Neck System provides superior resistance against varus deformation and performs less sensitive to variations in implant placement as compared to the Hansson Pin System with regard to such clinically relevant parameters as varus deformation and dorsal tilting of the femoral head. Therefore, from a biomechanical perspective the Femoral Neck System can be considered as a valid alternative to the Hansson Pin System for treatment of Pauwels II femoral neck fractures.
Funding
This investigation was performed with the assistance of the AO Foundation via the AOTC System. DePuy Synthes is acknowledged for the delivery of all implants.
Informed consent was obtained for all human subjects used in this experimental study.
Authorship
Clemens Schopper, MD: Idea, instrumentation, writing, correction; Ivan Zderic, MSc: Mechanical testing, statistical evaluation, writing, correction; Johanna Menze, MSc: Assistance instrumentation, Expert opinion, correction; David Müller, Dipl Ing FH: Expert opinion, correction; Mirko Rocci, MSc: Expert opinion, correction; Matthias Knobe, MD, PhD: Expert opinion, correction; Etsuo Shoda, MD: Expert opinion, correction; Geoff Richards, PhD: Expert opinion, correction; Boyko Gueorguiev, PhD: Expert opinion, correction; Karl Stoffel, MD, PhD: Idea, Expert opinion, guidance, correction
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
References
- 1.Parker M.J. The management of intracapsular fractures of the proximal femur. J Bone Jt Surg Br. 2000;82(7):937–941. doi: 10.1302/0301-620x.82b7.11595. [DOI] [PubMed] [Google Scholar]
- 2.Stromqvist B., Nilsson L.T., Thorngren K.G. Femoral neck fracture fixation with hook-pins. 2-year results and learning curve in 626 prospective cases. Acta Orthop Scand. 1992;63(3):282–287. doi: 10.3109/17453679209154783. [DOI] [PubMed] [Google Scholar]
- 3.Rehnberg L., Olerud C. Uppsala screw fixation versus the von Bahr technique in displaced cervical hip fractures: preliminary report. J Orthop Trauma. 1989;3(1):48–52. doi: 10.1097/00005131-198903010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lykke N., Lerud P.J., Stromsoe K., Thorngren K.G. Fixation of fractures of the femoral neck. A prospective, randomised trial of three Ullevaal hip screws versus two Hansson hook-pins. J Bone Jt Surg Br. 2003;85(3):426–430. doi: 10.1302/0301-620x.85b3.13788. [DOI] [PubMed] [Google Scholar]
- 5.Knobe M., Altgassen S., Maier K.J., Gradl-Dietsch D., Kaczmarek C., Nebelung S. Screw-blade fixation systems in Pauwels three femoral neck fractures: a biomechanical evaluation. Int Orthop. 2018;42(2):409–418. doi: 10.1007/s00264-017-3587-y. [DOI] [PubMed] [Google Scholar]
- 6.Knobe M., Bettag S., Kammerlander C., Altgassen S., Maier K.J., Nebelung S. Is bone-cement augmentation of screw-anchor fixation systems superior in unstable femoral neck fractures? A biomechanical cadaveric study. Injury. 2019;50(2):292–300. doi: 10.1016/j.injury.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Stoffel K., Zderic I., Gras F., Sommer C., Eberli U., Mueller D. Biomechanical evaluation of the femoral neck system in unstable Pauwels III femoral neck fractures: a comparison with the dynamic hip screw and cannulated screws. J Orthop Trauma. 2017;31(3):131–137. doi: 10.1097/BOT.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 8.Mjorud J., Skaro O., Solhaug J.H., Thorngren K.G. A randomised study in all cervical hip fractures osteosynthesis with Hansson hook-pins versus AO-screws in 199 consecutive patients followed for two years. Injury. 2006;37(8):768–777. doi: 10.1016/j.injury.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Hagglund G., Hannson L.I., Sandstrom S. Slipped capital femoral epiphysis in southern Sweden. Long-term results after nailing/pinning. Clin Orthop Relat Res. 1987;(217):190–200. [PubMed] [Google Scholar]
- 10.Hansson L.I., Hagglund G., Ordeberg G. Slipped capital femoral epiphysis in southern Sweden 1910-1982. Acta Orthop Scand Suppl. 1987;226:1–67. doi: 10.3109/17453678709154165. [DOI] [PubMed] [Google Scholar]
- 11.Windolf M., Braunstein V., Dutoit C., Schwieger K. Is a helical shaped implant a superior alternative to the Dynamic Hip Screw for unstable femoral neck fractures? A biomechanical investigation. Clin Biomech (Bristol, Avon) 2009;24(1):59–64. doi: 10.1016/j.clinbiomech.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Baumgaertner M.R., Curtin S.L., Lindskog D.M., Keggi J.M. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Jt Surg Am. 1995;77(7):1058–1064. doi: 10.2106/00004623-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y.Q., Chang S.M., Huang Y.G., Wang X. The femoral neck safe zone: a radiographic simulation study to prevent cortical perforation with multiple screw insertion. J Orthop Trauma. 2015;29(5):e178–e182. doi: 10.1097/BOT.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi Y., Hiranaka T., Shirahama M., Uesugi M., Okimura K., Tsubosaka M. Ideal screw positions for multiple screw fixation in femoral neck fractures - study of proximal femur morphology in a Japanese population. J Orthop Sci. 2018;23(3):521–524. doi: 10.1016/j.jos.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Lindequist S. Cortical screw support in femoral neck fractures. A radiographic analysis of 87 fractures with a new mensuration technique. Acta Orthop Scand. 1993;64(3):289–293. doi: 10.3109/17453679308993627. [DOI] [PubMed] [Google Scholar]
- 16.Lindequist S., Tornkvist H. Quality of reduction and cortical screw support in femoral neck fractures. An analysis of 72 fractures with a new computerized measuring method. J Orthop Trauma. 1995;9(3):215–221. doi: 10.1097/00005131-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Probe R., Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565–571. doi: 10.5435/00124635-200609000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Pelet S., Leyvraz P.F., Garofalo R., Borens O., Mouhsine E. Sub- or intertrochanteric fracture following screw fixation of an intracapsular proximal femoral fracture: true complication or technical error? Swiss Surg. 2003;9(2):82–86. doi: 10.1024/1023-9332.9.2.82. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann G., Bender A., Dymke J., Duda G., Damm P. Standardized loads acting in hip implants. PloS One. 2016;11(5):e0155612. doi: 10.1371/journal.pone.0155612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blemker S.S., Delp S.L. Three-dimensional representation of complex muscle architectures and geometries. Ann Biomed Eng. 2005;33(5):661–673. doi: 10.1007/s10439-005-1433-7. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann G., Deuretzbacher G., Heller M., Graichen F., Rohlmann A., Strauss J. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34(7):859–871. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 22.Bonnaire F., Weber A., Bosl O., Eckhardt C., Schwieger K., Linke B. “Cutting out” in pertrochanteric fractures--problem of osteoporosis? Unfallchirurg. 2007;110(5):425–432. doi: 10.1007/s00113-007-1248-0. [DOI] [PubMed] [Google Scholar]
- 23.Santoni B.G., Nayak A.N., Copper S.A., Simthson I.R., Cox J.L., Marberry S.T. Comparison of femoral head rotation and varus collapse between a single lag screw and integrated dual screw intertrochanteric hip fracture fixation device using a cadaveric Hemi-Pelvis biomechanical model. J Orthop Trauma. 2016;30(4):164–169. doi: 10.1097/BOT.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 24.Kouvidis G.K., Sommers M.B., Giannoudis P.V., Katonis P.G., Bottlang M. Comparison of migration behavior between single and dual lag screw implants for intertrochanteric fracture fixation. J Orthop Surg Res. 2009;4:16. doi: 10.1186/1749-799X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zlowodzki M., Ayieni O., Petrisor B.A., Bhandari M. Femoral neck shortening after fracture fixation with multiple cancellous screws: incidence and effect on function. J Trauma. 2008;64(1):163–169. doi: 10.1097/01.ta.0000241143.71274.63. [DOI] [PubMed] [Google Scholar]