Abstract
Background/Objectives:
Exercise is a front-line countermeasure used to maintain astronaut health during long-duration spaceflight; however, reductions in metabolic health still occur. Accordingly, we evaluated serial changes in metabolic parameters in a spaceflight analog and evaluated the efficacy of exercise with or without the addition of low-dose testosterone treatment on mitigating adverse metabolic changes.
Subjects/Methods:
Healthy young (<50 years) men were randomly assigned to one of three groups during 70-days of a strict, diet controlled, 6° head-down bed rest: Control (CON, n=9), exercise plus testosterone countermeasure (TEX, n=8), or exercise countermeasure plus placebo (PEX, n=9). Basal metabolic rate (BMR), glucose tolerance, and insulin sensitivity were measured before, during, and after bed rest. Exercise energy expenditure and excess post-exercise oxygen consumption (EPOC) were measured in TEX and PEX subjects during bed rest.
Results:
Leptin decreased during HDBR (Pre to BR+0 changed from 6.9 ± 5.1, 5.8 ± 4.2, and 4.7 ± 4.1 to 7.9 ±3.6, 6.5 ± 4.6, and 4.1 ±3.0 ug· L−1 for CON, PEX, and TEX respectively). Bed rest induced a decrease in BMR (Pre to BR66 changed from 1655 ± 212, 1629 ± 108, and 1706 ± 146 to 1476 ± 166, 1668 ± 142, and 1603 ± 132 95 kcal · day−1 ± 95%CI for CON, PEX, and TEX respectively). Similarly, bed rest negatively affected glucose metabolism assessed by 2hr OGTT glucose (Pre to BR66 changed from 6.29 ± 0.72, 5.13 ± 0.72, and 5.87 ± 0.73 to 6.62 ± 0.72, 5.83 ± 0.72, and 7.08 ± 0.72 mmol · L−1 ± 95%CI). Reambulation following bed rest positively affected glucose tolerance in CON (2hr OGTT glucose at BR+12: 5.3 ± 0.72, 6.42 ± 0.73, and 6.04 ± 0.73 mmol · L−1 ± 95%CI). Testosterone protected against bed rest induced insulin resistance (HOMA-IR from Pre to BR+66 changed from 1.74 ± 0.54, 1.18 ± 0.55, and 1.45 ± 0.56 to 2.24 ± 0.56, 1.47 ± 0.54, and 1.07 ± 0.54).
Conclusion:
This study confirmed that inactivity during 70 days of head-down bed rest adversely affects metabolic health. The daily exercise countermeasures were beneficial but not completely protective of bed rest induced decrements in metabolic health. Supplementary countermeasures such as testosterone may provide additional benefits not provided by exercise alone.
Keywords: Energy Balance, Metabolic Rate, Glucose Tolerance, Insulin Sensitivity, Spaceflight
1. Introduction
Exercise is the most effective countermeasure currently available against spaceflight-induced alterations in muscle, bone, and cardiac metabolism. As skeletal muscle is a major driver of energy metabolism, disuse of skeletal muscle directly affects substrate utilization and results in metabolic dysregulation including insulin resistance and muscle loss [1]. Despite the benefits of exercise, skeletal muscle deconditioning during flight is not completely prevented and there is an interest in additional countermeasures that can be implemented to optimize astronaut health. Head-down bed rest (HDBR) provides a unique and relevant model to investigate physiologic and metabolic adaptations to musculoskeletal unloading in healthy subjects while also controlling for and monitoring dietary intake. Previous bed rest studies have demonstrated increases in plasma insulin and insulin resistance, a metabolic shift favoring dyslipidemia, impaired microvascular function, and impaired glucose metabolism [2–4]. As exercise is a standard countermeasure during long-duration spaceflight, bed rest investigations that include exercise protocols are better approximations of spaceflight conditions than bed rest alone. The addition of exercise during bed rest appears to have varying effects on the insulin response, and the dose of exercise (intensity, frequency, volume) necessary to offset the effects of unloading is unknown. We previously showed that a high intensity integrated aerobic and resistance training program effectively improved cardiorespiratory fitness (VO2peak) and maintained leg muscle size, muscle strength, and basal metabolic rate during 14 days of HDBR [5; 6]. However, most of these studies were limited in duration and diet was often not tightly controlled. Therefore, a need remained to investigate the effects of exercise on decrements in metabolic health during long-duration HDBR under strictly controlled conditions.
In addition to optimizing nutrition and exercise routines for astronauts during long-duration spaceflight, there is a growing interest in additional countermeasures that can be employed to augment the existing exercise countermeasures. Testosterone has long been known to increase muscle strength and size in athletes [7]. We previously demonstrated that even low doses of testosterone are anabolic to muscle mass and strength in untrained, but ambulatory older men with testosterone levels < 500 ng/dL [8–11]. However, the effectiveness of exercise with or without a low-dose testosterone countermeasure on metabolic health in healthy younger men during long-duration bed rest was unknown.
Recently, we evaluated the impact of 70 days of strict 6° HDBR alone, or bed rest paired with a daily high intensity resistance and aerobic exercise program with and without a testosterone supplement, on body composition, cardiac function, muscle strength, and the skeletal muscle proteome [12–15]. Here we examined the impact of these changes on energy balance, glucose tolerance, and insulin sensitivity.
2. Methods
2.1. Study Overview
This research was part of an integrated study registered with ClinicalTrials.gov (NCT00891449). Sample sizes were determined based on the primary outcomes from several independently funded investigations involved in the bed rest study campaign conducted between 2010 and 2014 [16]. Because of overlap in start-time between funded investigations, subject numbers are not identical between the various reports that emanated from this bed rest campaign [12–16]. For the parameters included in this report, data were available from twenty-six healthy individuals that volunteered to participate in the 70-day HDBR study. Determination of subject eligibility was based on a screening evaluation by the NASA Johnson Space Center’s Human Test Subject Facility and Exercise Physiology & Countermeasures Laboratory as previously described [16]. All participants were nonsmokers and were given a detailed explanation of the experimental protocol before obtaining informed consent and random assignment (in blocks of three) to one of three groups: Control (CON, n=9, 38.1 ± 7.8 yr; 178.4 ± 4.6 cm; 81.4 ± 7.7 kg), exercise and testosterone countermeasure (TEX, n=8, 42.3 ± 5.7 yr; 182.2 ± 8.0 cm; 77.0 ± 13.9 kg), or exercise countermeasure alone (PEX, n=9, 42.0 ± 6.5 yr; 179.4 ± 4.5 cm; 75.4 ± 6.4 kg). Assignment to Exercise versus Exercise and Testosterone treatment was double-blinded to the subjects, staff and investigators. The UTMB investigational drug services Pharmacy staff as well as designated physicians and staff remained unblinded to subject treatments throughout the study. This study was approved by both Institutional Review Boards at the Johnson Space Center and the University of Texas Medical Branch, and all participants provided written informed consent before the experiment.
CON subjects reported to the Flight Analog Research Unit (FARU) 14 days before the start of HDBR and PEX and TEX subjects arrived 21 days before the start of HDBR. During the pre-HDBR familiarization period (denoted as BR-21 through −1), exercise subjects gained experience with the exercise equipment and trained 5 days per week. During bed rest (denoted as BR1 through 70), subjects were confined to strict 6° HDBR for 70 days and were continually monitored by medical and nursing staff. All subjects remained in the FARU for 12 days after HDBR (denoted as BR+0 through +12), to perform reconditioning and post-HDBR testing activities. All subjects participated in pre-, during-, and post-HDBR activities that included tests of aerobic fitness and muscle size, strength, and function [12]. Daily activities such as reading, computer use, and television viewing were conducted while subjects maintained a supine position. Hygiene activities, bathing, and urine collection also were performed in bed while maintaining a supine position. Full body Dual-energy X-ray absorptiometry (DXA) scans were performed before and during HDBR on a GE Lunar iDXA system as previously reported [13].
2.2. Exercise Training
During HDBR, subjects in exercise groups performed resistance exercise 3 days per week and aerobic exercise 6 days per week [12; 16]. Briefly, resistance exercise sessions were performed every other day and consisted of three sets of 6–12 repetitions on each of four exercises (supine squat, supine leg press, supine calf raise, and prone lying leg curl). The resistance exercise and 30 minutes continuous aerobic exercise on the supine cycle ergometer were completed on the same day and were separated by at least 4 hours. Continuous aerobic exercise sessions targeted intensity was set at a heart rate equivalent to 80% of VO2peak achieved during the pre-bed rest peak cycle test. Interval aerobic exercise sessions were performed supine on the stand-alone zero-gravity locomotion simulator treadmill (sZLS), three times per week on nonresistance/continuous exercise days and consisted of three different sessions: 6 × 2-minute stages at a heart rate equivalent to 70%, 80%, 90%, 100%, 90%, and 80% of VO2peak with 2 minutes of active rest between intervals; 8 × 30 seconds at maximal effort with 15 seconds of active rest between intervals; 4 × 4 minutes at a heart rate equivalent to 85% VO2peak with 3 minutes of active rest between intervals. Additional details on the exercise protocol have been previously provided [5; 12].
2.3. Testosterone Supplementation
Placebo (saline) or testosterone enanthate injections (100 mg·week−1, intramuscular, alternating left vs. right gluteus maximus) were administered in 2-week intervals (i.e., weekly testosterone enanthate for 2 weeks, followed by 2 weeks with no treatment) for the duration of the 70-day HDBR period [13].
2.4. Bed Rest Diet
The bed rest diet was tightly controlled, designed to maintain body weight, and consisted of a 55% carbohydrate (4.97 ± 0.30 g∙kg−1), 30% fat (1.21 ± 0.07 g∙kg−1), and 15% protein (1.36 ± 0.08 g∙kg−1) macronutrient profile. Energy requirements for exercise groups were calculated using the Harris-Benedict equation [17] with an activity factor of 1.6 and the thermal effect of food [18]. The CON subject energy requirements were calculated using the Harris-Benedict equation with an activity factor of 1.4 and the thermal effect of food. An 8-day menu cycle was used during the study; subjects consumed the research diet pre-, during-, and post-HDBR. Subjects ate three times per day (at approximately 0700, 1200, and 1700 hours) with no snacking permitted between meals. Subjects were asked to eat all of their food and any leftover food was weighed on a Mettler balance to determine actual intake. Nutrient data for all foods were obtained using Nutritionist IV (First DataBank, The Hearst Corporation, San Bruno, CA, USA) [19].
2.5. Daily Energy Expenditure
Basal metabolic rate (BMR) was measured pre-HDBR on days −14 and −2, during-HDBR on days 15, 28, 43, and 57, and post-HDBR on day +2 using indirect calorimetry [20]. Following a standard evening meal, subjects fasted at least 12 hours before BMR measurement with only water permitted ad libitum thereafter. No exercise was conducted 24 hours preceding BMR assessment. On the day of testing, subjects were awakened at 0600 hours and instructed to limit their physical activity to minimal grooming and slow movement. Body mass was measured after voiding and subjects were transported to a dimly lit room isolated from disturbing noise. Subjects rested quietly for 10 minutes in the supine position and were asked to breathe normally, minimize movement, and then to remain as quiet as possible for an additional 45-minute testing period. CO2 and O2 were measured using a ventilated hood (ParvoMedics Inc, Sandy, UT, USA). Heart rate (HR; Polar 810i; Polar USA Inc, Montvale, NJ) was continuously monitored throughout the test. Basal steady-state was defined as a minimum of 10 minutes during which the volume of oxygen consumed (VO2), minute ventilation (VE), and respiratory quotient (RQ) did not vary by more than 10%. Data from the first 10 minutes were excluded; thereafter, VO2 and CO2 production were averaged over 30-second intervals for the 45-minute test to calculate BMR [20].
Exercise energy expenditure and excess post-exercise oxygen consumption (EPOC) were collected one time per week during a continuous, 2-minute interval, or 4-minute interval aerobic exercise session as described previously [6]. Briefly, subjects were fitted with a heart rate monitor (Polar 810i; Polar USA Inc, Montvale, NJ) and a facemask connected to a metabolic cart (TrueOne 2400, ParvoMedics; Sandy, UT) before the start of each exercise session. Baseline metabolic rate was established during a 10-minute pre-exercise rest period, metabolic data were collected throughout the exercise session and for 30 minutes of post-exercise recovery. Exercise energy expenditure was calculated from the average exercise VO2 and respiratory exchange ratio (RER) [21]. EPOC was measured for 30 minutes following the completion of exercise. Total daily energy expenditure was calculated by summing the energy cost of daily activities (BMR × 1.2), exercise energy expenditure (including EPOC), and the thermal effect of food [18]. Note that the daily caloric intake was prescribed based on the Harris-Benedict equation, as noted above, not based on the calculated daily energy expenditure.
2.6. Oral Glucose Tolerance Test and Breath CO2 Protocols
Oral glucose tolerance tests (OGTTs) were conducted on 24 of the 26 total subjects (n=8 for each of the three groups). These tests were conducted after an overnight fast pre- (day −1), during- (days 38 and 66), and post-HDBR (day +12). In the morning, an antecubital intravenous line was inserted for the collection of timed blood samples. A baseline blood sample and breath sample were collected before the start of the tests [22]. After a baseline sample collection, subjects consumed a drink containing 75-g glucose isotopically labeled with 150 mg [U-13C6] glucose within 1 minute. From this point (t = 0 min), blood and breath samples were collected every 30 minutes for 2 hours. Baseline (reference) breath was collected in a 1.3-L bag fitted with a one-way valve while single timed breath samples were collected in individual 300 mL bags. For the collection of breath samples, the subjects were instructed to breathe normally, hold their breath for 3 seconds, and then exhale completely into the collection bag provided. Multiple breaths were collected for the baseline reference sample. Water was allowed ad libitum throughout the 120-minute OGTT. After collection of the final samples, the intravenous line was removed, and the subjects were fed and told to continue their daily routine as scheduled.
2.7. Blood and Breath Sample Analysis
Fasting blood samples were collected twice before bed rest (BR-11 and BR-4), during bed rest (BR30), and twice after bed rest (BR+0 and BR+5). Fasting leptin was measured in duplicate using radioimmunosassay (Lenco-Millipore).
Plasma glucose and lactate concentrations were determined using a 2300 STAT Plus Glucose analyzer. Plasma insulin concentrations were determined using an Immulite 2000 chemiluminescence immunoassay system (Siemens, Los Angeles, CA). The homeostatic model assessment for insulin resistance (HOMA-IR) [23] and the whole-body insulin sensitivity index (WBISI or Matsuda index) [24] were calculated using plasma glucose (mg/Dl) and insulin (μIU/mL) measurements obtained during the OGTT.
Glucose-derived breath CO2 data were analyzed by measuring the ratios of 13CO2 to 12CO2 in single breath samples using an UBiT-IR300 infrared spectrophotometer (Otsuka Electronics, Hirakata, Osaka, Japan) [22]. The UBIT-IR300 calculates the difference in 13CO2 abundance from the baseline breath sample to each timed sample and expresses this as per mille delta over baseline (‰DOB) [22].
2.8. Statistical Analysis
Statistical analyses were conducted using Stata, IC software (v14.21) and setting 2-tailed alphato reject the null hypothesis at 0.05. Our experimental design is a mixed-factorial, with repeated observations collected before, during, and after 70 days of bed rest among subjects randomized to one of three different groups described above (CON, PEX, and TEX). Multiple pre-HDBR blood (HDBR −12, −2) time points were averaged for the pre-HDBR data point. We evaluated the effects of condition (CON, PEX, and TEX), HDBR (pre, during, post), and their interaction effects in separate mixed-effects models per dependent variable, with a priori simple interaction terms comparing the changes relative to pre-HDBR among all possible pairs of condition (CON versus PEX, CON versus TEX, and PEX versus TEX), and false discovery rate adjustments [25] within outcome for the inflated Type 1 error risk. Each of these models included a random y-intercept to accommodate the within-subjects experimental design. Rigorous model assumption and outlier evaluations were conducted before hypothesis testing, resulting in the elimination of a small number of overly influential observations for some outcomes that we identify in the results section below. Changes (pre/during, and pre/post) in BMR, lean mass, and fat mass were correlated using the Somer’s d statistic [26] with appropriate adjustments for the repeated measures experimental design (i.e., two delta scores per subject; pre versus during, and pre versus post). Tables and figures were prepared using Excel (Excel 2016, Microsoft), Prism (Version 5.02, GraphPad Software Inc.), and RStudio (Version 1.2.1335, RStudio Inc.).
3. Results
3.1. Study Adherence
All subjects adhered strictly to the HDBR, exercise and testosterone interventions, and nutrition procedures as described in the study outcome papers by Ploutz-Snyder et al. and Dillon et al. [12; 13]. All men were eugonadal with baseline testosterone concentrations averaging 531 ± 194, 553 ± 96, and 553 ± 171 ng∙dL−1 for CON (n=8), PEX (n=8), and TEX (n=8), respectively. Circulating testosterone levels showed no changes within or between the groups when measured one week after each administration. As addressed previously, this likely indicates the normal response of the hypothalamic-pituitary-gonadal (HPG) axis to return endogenous testosterone levels to a physiological baseline [13]. No dose adjustments were made and there were no testosterone related adverse events.
3.2. Leptin
Leptin, which is directly related to changes in fat mass [27], showed a significant time × group interaction effect. Pre vs. post HDBR levels were 6.9 ± 5.1 vs 7.9 ± 3.6 for CON, 5.8 ± 4.2 vs. 6.5 ± 4.6 for PEX, and 4.7 ± 4.1 vs. 4.1 ± 3.0 ug· L−1 for TEX, although there were no significant differences between the groups. Pre and post HDBR levels of leptin correlated to subject total fat mass (Pearson r =0.6542, p < 0.0001, Figure 1). As previously reported, CON and PEX subjects experienced increases in fat mass compared to the TEX subjects from pre to post-HDBR while total body mass did not change in any of the groups during HDBR [13]. While lean mass decreased in the CON subjects compared to both exercise groups, improvements in body composition (gains in lean mass and maintenance in fat mass) were observed in the TEX group [13].
Figure 1. Total Body Mass vs. Leptin.
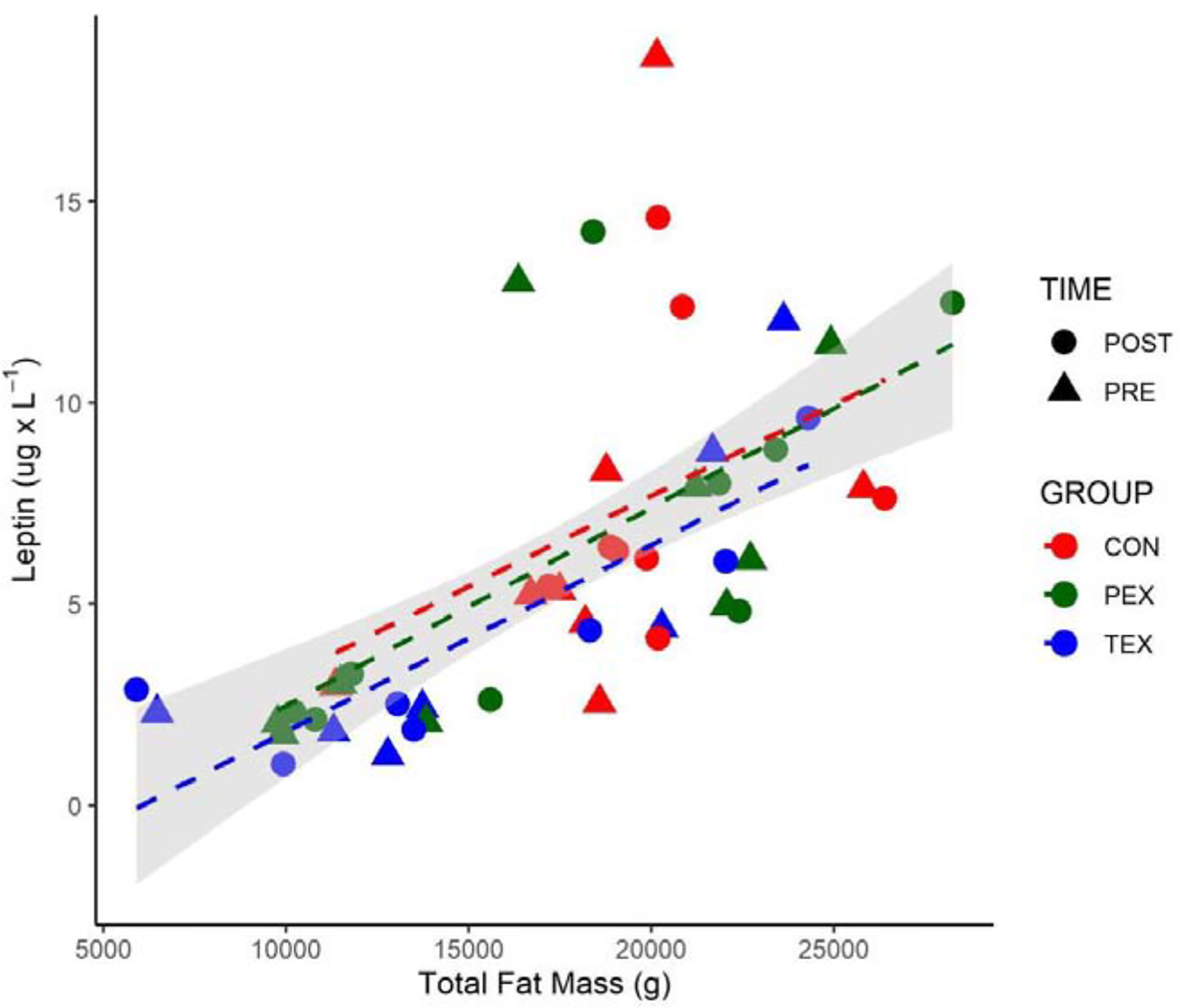
Total fat mass (g) vs. circulating leptin measurements (ug· L−1) before HDBR (triangle) and after HDBR (circle). Gray area represents 95% CI of the linear model regression for all points (Pearson r = 0.6542, p < 0001). For visualization, groups with regression lines are identified as follows: CON (red), PEX (green), and TEX (blue).
3.3. Basal Metabolic Rate (BMR)
A main effect was observed for a decrease in measured BMR from pre (1662 ± 201 kcal·d−1) to post-HDBR (1581 ± 199 kcal·d−1); however, no group interaction was detected (Figure 1). There was no significant main effect when BMR was normalized to lean mass. Changes in fat mass or lean mass did not correlate to changes in BMR observed during the 70 days of HDBR (data not shown). Fasting RERs were similar in all groups pre-HDBR (0.85 ± 0.05, 0.89 ± 0.04, and 0.86 ± 0.05, for CON, PEX, and TEX) and remained unaltered during HDBR (0.84 ± 0.05, 0.88 ± 0.05, and 0.85 ± 0.06, for CON, PEX, and TEX post-HDBR).
3.4. Metabolic Cost of Exercise and Daily Energy Balance
The average exercise intensity was not different between the PEX and TEX group for any of the aerobic exercise intervals. Average exercise energy expenditure + EPOC was 303±60 kcal for the 2-minute intervals, 314±59 kcal for the 4-minute intervals, and 390±59 kcal for the 30-minute continuous session. Although we did not measure energy expenditure during the resistance and 30-second interval sessions, we previously collected this data and showed resistance exercise averaged ~250 kcal per session and 30-second interval energy expenditure was similar to a subjects’ metabolic cost of the 2 and 4-minute intervals [6].
Calculated average daily energy expenditure was 2532 ± 421, 2851 ± 234, and 2866 ± 349 kcal in CON, PEX, and TEX, respectively. As prescribed, CON consumed fewer calories during HDBR compared to the exercise groups (CON = 2551 ± 328; PEX = 2776 ± 102; TEX = 2879 ± 338). Calculated daily energy expenditure was remarkably similar to actual energy intake in all groups.
3.5. Oral Glucose Tolerance Testing.
There were significant two-way interactions (group × time) for OGTT induced glucose, OGTT induced insulin, insulin resistance, insulin sensitivity, and glucose derived CO2 during the OGTT (Table 1). Exercise prevented HDBR induced increases in OGTT induced glucose and insulin responses when compared to CON. There were significant main effects between CON and PEX at BR+12 for OGTT induced glucose, insulin, and lactate. In general, post-HDBR reambulation (BR+12) induced more favorable changes in these OGTT responses for the CON subjects compared to the exercising groups. It is likely that the cessation of the exercise prescription in PEX and TEX subjects after completion of bed rest contributed to this contrast between the groups and suggests that continuation of exercise during post-bed rest recovery would have been desirable to maintain metabolic health post-bed rest. The HOMA insulin resistance index and Matsuda index for insulin sensitivity indicated favorable responses to TEX countermeasures during HDBR compared to CON. Glucose derived CO2 in breath increased during HDBR in CON compared to the exercise groups (significant difference between CON and PEX at BR66). There were no significant differences between the exercise groups (PEX vs. TEX). Combined-group and within-group responses of blood glucose, insulin, and lactate measured at each 30-minute time point during the 2-hour OGTTs across the study are shown in Supplementary Figures 1–3. No correlations were observed between changes in lean mass or fat mass and measures of glucose tolerance or insulin sensitivity (data not shown).
Table 1.
Oral Glucose Tolerance Test
| CON | PEX | TEX | |||||
|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | ||
| Fasting Glucose (mmol - L−1) | |||||||
| Pre-BR | 4.97 | (4.75 – 5.19) | 4.74 | (4.51 – 4.96) | 4.95 | (4.73 – 5.17) | |
| BR38 | 5.02 | (4.80 – 5.24) | 4.96 | (4.74 – 5.18) | 5.05 | (4.83 – 5.27) | |
| BR66 | 5.15 | (4.93 – 5.37) | 4.90 | (4.68 – 5.12) | 4.94 | (4.72 – 5.16) | |
| BR+12 | 4.92 | (4.70 – 5.14) | 4.99 | (4.77 – 5.21)M | 4.96 | (4.74 – 5.19) | |
| Fasting Insulin (μIU - L−1) | |||||||
| Pre-BR | 7.93 | (5.45 – 10.4) | 5.71 | (3.16 – 8.26) | 6.57 | (4.02 – 9.12) | |
| BR38 | 8.60 | (6.05 – 11.2) | 6.12 | (3.64 – 8.60) | 6.21 | (3.73 – 8.69) | |
| BR66 | 9.81 | (7.25 – 12.4) | 6.81 | (4.33 – 9.29) | 4.75 | (2.27 – 7.23) | |
| BR+12 | 6.34 | (3.86 – 8.82) | 5.14 | (2.66 – 7.62) | 5.44 | (2.96 – 7.92) | |
| Fasting Lactate (mmol - L−1) | |||||||
| Pre-BR | 1.51 | (1.32 – 1.70) | 1.22 | (1.03 – 1.41) | 1.27 | (1.08 – 1.46) | |
| BR38 | 1.45 | (1.26 – 1.63) | 1.30 | (1.11 – 1.49) | 1.37 | (1.18 – 1.56) | |
| BR66 | 1.39 | (1.20 – 1.58) | 1.24 | (1.06 – 1.43) | 1.22 | (1.03 – 1.41) | |
| BR+12 | 1.17 | (0.99 – 1.36) | 1.18 | (0.99 – 1.37)s | 1.07 | (0.88 – 1.26) | |
| 2Hr OGTT Glucose (mmol - L−1)GxT | |||||||
| Pre-BR | 6.29 | (5.57 – 7.01) | 5.13 | (4.41 – 5.86) | 5.87 | (5.14 – 6.59) | |
| BR38 | 6.79 | (6.07 – 7.52) | 6.21 | (5.49 – 6.93) | 6.79 | (6.06 – 7.51) | |
| BR66 | 6.62 | (5.90 – 7.34) | 5.83 | (5.11 – 6.55) | 7.08 | (6.36 – 7.80) | |
| BR+12 | 5.30 | (4.58 – 6.03) | 6.42 | (5.69 – 7.14)M,s | 6.04 | (5.31 – 6.76) | |
| 2Hr OGTT Insulin (μIU - L−1)GxT | |||||||
| Pre-BR | 55.1 | (39.5 – 70.6) | 33.1 | (18.1 – 48.1) | 31.4 | (16.4 – 46.4) | |
| BR38 | 56.5 | (41.5 – 71.5) | 45.4 | (30.4 – 60.4) | 41.9 | (26.9 – 56.9) | |
| BR66 | 69.1 | (53.6 – 84.7) | 35.0 | (20.0 – 50.0) | 42.7 | (27.2 – 58.3) | |
| BR+12 | 35.2 | (20.2 – 50.2) | 38.9 | (23.9 – 53.8)M | 33.8 | (18.8 – 48.8)M | |
| 2Hr OGTT Lactate (mmol - L−1) | |||||||
| Pre-BP | 1.46 | (1.32 – 1.59) | 1.23 | (1.10 – 1.37) | 1.29 | (1.15 – 1.44) | |
| BR38 | 1.32 | (1.19 – 1.46) | 1.28 | (1.15 – 1.42) | 1.32 | (1.19 – 1.46) | |
| BR66 | 1.40 | (1.27 – 1.54) | 1.20 | (1.06 – 1.34) | 1.29 | (1.15 – 1.43) | |
| BR+12 | 1.39 | (1.26 – 1.53) | 1.39 | (1.25 – 1.52)M | 1.33 | (1.19 – 1.46) | |
| HOMA-IRGxT | |||||||
| Pre-BR | 1.74 | (1.20 – 2.29) | 1.18 | (0.63 – 1.74) | 1.45 | (0.89 – 2.01) | |
| BR38 | 1.91 | (1.35 – 2.47) | 1.35 | (0.81 – 1.90) | 1.39 | (0.85 – 1.93) | |
| BR66 | 2.24 | (1.68 – 2.80) | 1.47 | (0.93 – 2.02) | 1.07 | (0.53 – 1.61)M,s | |
| BR+12 | 1.38 | (0.84 – 1.93) | 1.15 | (0.61 – 1.69) | 1.22 | (0.68 – 1.76) | |
| MatsudaGxT | |||||||
| Pre-BR | 4.78 | (2.45 – 7.12) | 7.63 | (5.24 – 10.0) | 8.34 | (5.95 – 10.7) | |
| BR38 | 4.72 | (2.38 – 7.05) | 7.27 | (4.94 – 9.61) | 7.41 | (5.07 – 9.75) | |
| BR66 | 3.86 | (1.52 – 6.20) | 7.61 | (5.27 – 9.94) | 8.70 | (6.32 – 11.1) | |
| BR+12 | 6.50 | (4.16 – 8.84) | 7.33 | (4.99 – 9.67) | 7.65 | (5.27 – 10.0)M | |
| Glucose derived CO2 (% Baseline)GxT | |||||||
| Pre-BR | 37.2 | (33.3 – 41.1) | 37.0 | (33.1 – 40.9) | 32.6 | (28.5 – 36.7) | |
| BR38 | 36.0 | (32.0 – 40.1) | 36.2 | (32.3 – 40.1) | 34.9 | (31.0 – 38.8) | |
| BR66 | 46.0 | (41.9 – 50.0) | 37.8 | (33.9 – 41.7)M,s | 36.5 | (32.4 – 40.6) | |
| BR+12 | 37.9 | (33 8 – 419) | 34.9 | (310 – 388) | 34.2 | (30.3 – 38.1) | |
Values are presented as marginal means and 95% confidence intervals.
Two-way interaction (omnibus).
Main effect (Pre-HDBR and CON as references).
Simple interaction (Pre-HDBR and CON as references). No significant interaction effects between PEX and TEX.
4. Discussion
This is the first study to provide a thorough evaluation of the effects of strict long-duration bed rest alone, bed rest paired with a high intensity exercise countermeasure, and bed rest paired with a combined exercise and testosterone countermeasure on energy balance and glucose metabolism in healthy subjects. The primary results of this study 1) confirm that confinement to HDBR, even when paired with a World Health Organization (WHO) weight-maintaining diet and exercise, result in unfavorable changes in metabolic health, and modest reductions in BMR of healthy subjects; and 2) demonstrate that exercise and testosterone countermeasures help protect metabolic health of adult males during 70 days of HDBR. These changes are consistent with the changes in body composition and muscle strength previously reported [12; 13]
In this study, HDBR induced unfavorable changes in BMR and acute oral-glucose utilization (as assessed by an OGTT breath test). These changes are in agreement with Bergouignan et al. [3; 28] and further demonstrates that physical inactivity is detrimental to health in otherwise healthy individuals following a WHO weight maintaining diet. Exercise prevented changes in OGTT induced glucose oxidation observed in CON during HDBR (i.e. significant difference in glucose-derived CO2 in breath between CON and PEX at BR66 in Table 1). Increased glucose oxidation during an OGTT could be indicative of either improved glucose uptake from circulation or, more likely in the case of CON, be related to an overall decrease in the capacity for nonoxidative glucose disposal (glycogenesis) in response to long-term inactivity [29; 30]. While the development of metabolic inflexibility (the decreased ability for metabolism to switch between fatty acid oxidation and glucose oxidation in response to the availability of energy sources) remains a possibility, fasting RERs were similar in all groups indicating there were no overt HDBR-induced changes in basal fuel utilization in any of the groups [29; 31; 32]. Conversely, the post-HDBR recovery improvements in 2-hour OGTT glucose and insulin responses of non-exercising (CON) subjects further illustrate the importance of habitual physical activity because such changes during recovery from bed rest were absent in the subjects that had exercised during HDBR (PEX and TEX).
Maintaining daily energy balance is important as it relates to metabolic health and body composition. The calculations in our study indicate that subjects were in a state of energy balance that allowed for weight maintenance. Importantly, diet alone and even the addition of exercise were not completely effective in preventing gains in fat mass and decreases in lean mass during HDBR without the addition of testosterone [13]. Although there was no statistically significant difference between the groups, the mean decline in BMR from pre- to post-HDBR was visual only in the CON group (Figure 1) and are consistent with losses of lean mass during this study. Conversely, the relative increase in leptin during HDBR in the PEX and CON groups compared to the TEX group is likely the result of gains in fat mass [27] observed in the PEX and CON groups .
Despite the robust exercise routine followed by the PEX and TEX subjects in the present study, the added energy expenditure was still insufficient to fully offset the effects of physical inactivity due to confinement to HDBR. In attempt to understand specific exercise and energy expenditure requirements to maintain metabolic health during bed rest, Dolkas and Greenleaf investigated the effectiveness of resistance versus aerobic exercise on the insulin and glucose response during OGTT over 2 weeks of bed rest in young healthy men [33]. Their results showed that 1 hour of aerobic exercise was more effective than resistance exercise on insulin response to a glucose load. These authors also predicted (by calculating the relationships between daily energy expenditure and insulin area under the curve) daily exercise-induced energy expenditure during bed rest needs to reach ~1000 kcal·d−1 [33] to mitigate changes in insulin sensitivity and glucose utilization. In our study, subjects expended significantly less than 1000 kcal/d of exercise-induced energy expenditure (~350 kcal·d−1). Additionally, other factors that are associated with bed rest such as high stress environments, inflammation, and oxidative stress are known to negatively impact body composition and metabolic health. Thus, there remains a need to further understand exercise intensity and modality, as well as the potential for nutrition, hormones, or other mitigations to optimize the benefits of countermeasures against metabolic dysregulation during prolonged inactivity and/or unloading.
While the proposition of testosterone treatment to promote lean mass is not new, the use of a cycled testosterone treatment approach is novel and allows for a substantially lowered administration of this hormone than traditional regimens. As previously reported, LBM increased while the accretion of fat mass was prevented during HDBR in the TEX group in response to testosterone treatment [13]. The acute dose of testosterone enanthate (100mg) as well as the method of administration (im) used in this investigation was identical to that used in previous studies and is commonly used during clinical treatment of hypogonadal men [8; 34; 35]. As both benefits and risks are dose-dependent, clinical testosterone regimens typically range 75–100 mg every week (or 150–200 mg every 2 weeks, etc.) [36]. Among the concerns with long-term and continuous testosterone treatment is the possibility of chronically disrupting the HPG axis. These results further demonstrate that the intermittent treatment approach was sufficient to deliver benefits on body composition (i.e. increased LBM and reduced fat mass) and allowed for repeated drug-naive periods that minimized disruptions of the HPG axis [22] and indicators of metabolic health (present study) in eugonadal men. Although the precise mechanism of testosterone signaling remains obscure, we previously identified several proteomic responses consistent with an upregulation of hypertrophy in the TEX group [14]. The testosterone data from this investigation are consistent with the intent of the demonstrated treatment design; are promising for its safe use in healthy males and support the existing data on benefits for patients with metabolic syndrome [37].
4.1. Limitations
Despite, or perhaps due to, the high level of control under which this study was conducted to best model the conditions encountered during spaceflight, the data are limited in their interpretation and application. First, the general population is not likely to follow a tightly controlled WHO diet. The moderate decline in metabolic parameters observed in this study may therefore be a testament to the importance of quality nutrition for maintenance of overall health during long-term bed rest. A sedentary lifestyle paired with a poor diet would likely result in greater decrements in metabolic health than we observed in our CON group. Second, the CON subjects in this study participated in four supine VO2peak cycle tests and nine power cycle tests (5-second supine cycle test) during HDBR. Although this was very little activity, it is possible that even this limited stimulus prevented some of the decrements in metabolic health previously observed with strict bed rest alone. Third, these data were only collected in healthy males. It is not clear whether the magnitude of response would be different in older men or women following an exercise program during bed rest, or how women would respond to a testosterone countermeasure during bed rest or spaceflight. Fourth, glucose derived CO2 during standard OGTTs were measured using a novel non-invasive breath test shown to correlate with conventional OGTT measurements [22]. While promising as a noninvasive tool for distinguishing healthy adults from those with impaired glucose tolerance, this method has not been previously validated during bed rest. Fifth, this investigation did not examine the pharmacokinetic responses of testosterone immediately following administration. Although standard practices were followed, it is unknown whether individual differences in systemic drug delivery or distribution contributed to the reported findings.
4.2. Application of Results
Maintenance of energy balance is critical to prevent metabolic inefficiencies and prevent losses in muscle mass and strength during spaceflight. In this study we demonstrated that when WHO dietary recommendations with an activity factor of 1.6 are maintained, an exercise prescription and testosterone supplementation during unloaded conditions protects against declines in metabolic health during 70 days of bed rest. These results also indicate that a combined exercise and testosterone countermeasure may be an effective approach to optimize exercise time and frequency when the desired outcome is the maintenance of metabolic health and body composition. Higher exercise induced energy expenditure may be required to fully maintain muscle mass and metabolic health during long-duration missions (i.e., longer than 70 days) without the added testosterone supplement. From a clinical perspective, physical inactivity is a growing epidemic among young and otherwise healthy individuals as only 5% of the American population is considered active as measured by accelerometry [38]. These bed rest data confirm the negative effects of physical inactivity, even with a well-controlled diet.
4.3. Conclusion
This study design allowed for the unique opportunity to describe a cause-and-effect relationship between physical inactivity and the effectiveness of adding exercise during an otherwise inactive condition on metabolic health in an extremely well-controlled environment. This study supports the notion that confinement to bed rest, even with adequate nutritional support, results in moderate metabolic impairments in healthy individuals that is difficult to fully prevent by exercise alone. Exercise countermeasures will likely prevent metabolic dysregulation during mission durations up to 70 days and these data provide critical information to support the need for daily exercise during periods of unloading to not only protect muscle, bone, and cardiac health, but also metabolic health. The addition of an intermittent and low-dose testosterone supplement mitigates HDBR-induced increases in insulin resistance. These results indicate that this may be a beneficial supplemental countermeasure to counteract metabolic dysregulation, especially during long-duration spaceflight beyond the time-frame studied here. Future work should aim at understanding the specific exercise and nutritional requirements needed to maintain metabolic health, body composition, muscle strength, and aerobic fitness in men and women during well-controlled conditions of prolonged unloading or disuse.
Supplementary Material
Figure 2. Basal Metabolic Rate (BMR).
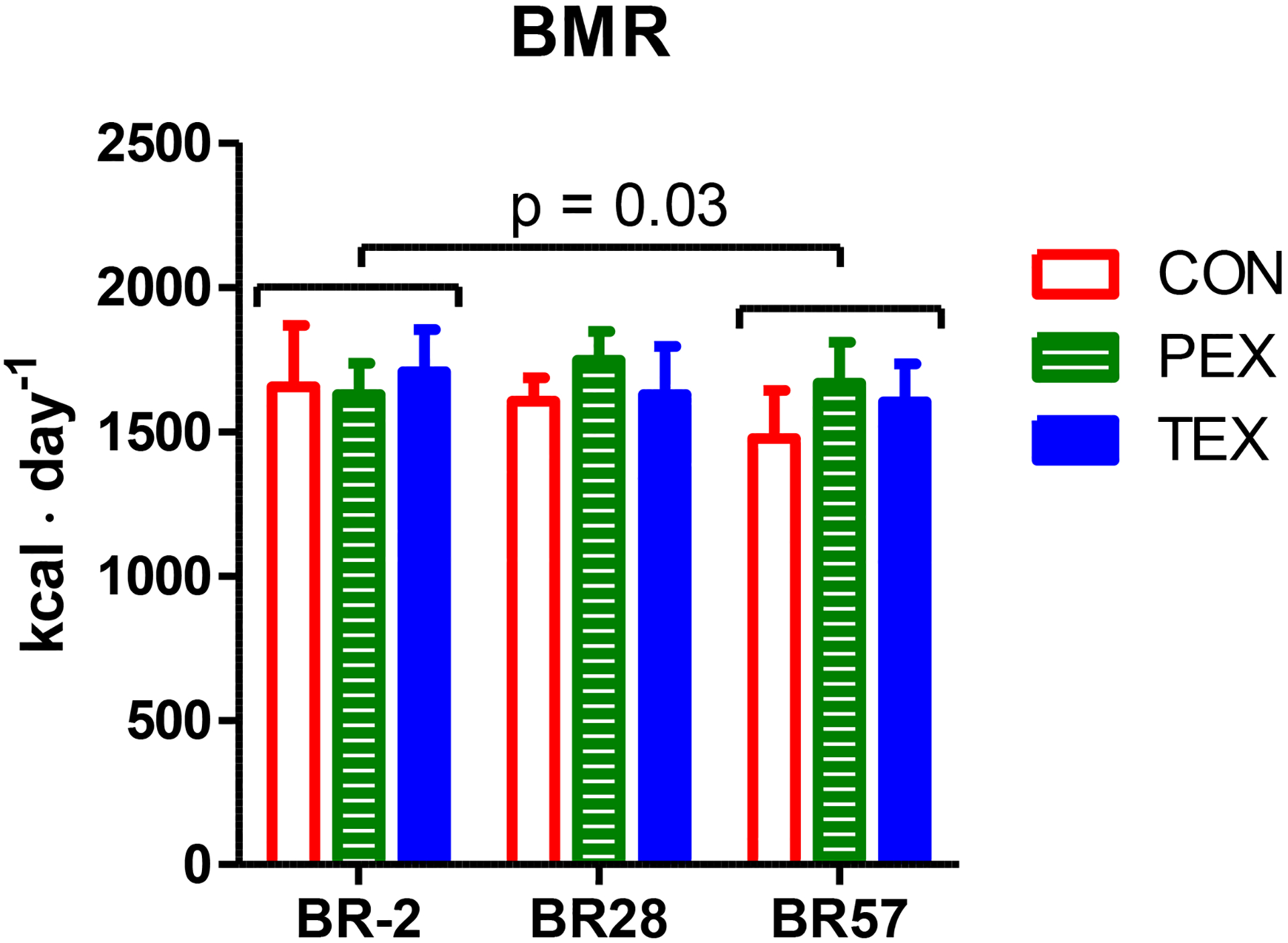
Measured basal metabolic rates (BMR) pre-, during, and post-HDBR. CON (red, open bars), PEX (green, striped bars), and TEX (blue, closed bars). Measures are presented as means and 95% CI. *Significant main effect (time) from pre to post-HDBR (P<0.05); no between-group differences.
Acknowledgments
The authors would like to thank all the subject volunteers, the UTMB nurses and staff at the NASA FARU, and all colleagues that assisted with this project. This type of project takes a considerable amount of effort and we thank everyone who took part in the process.
This study was supported by the NASA Human Research Program, the National Space Biomedical Research Institute (NSBRI) and NASA grant #NNX10AP86G (RJU/MSM) The study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch through funding by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health. Data were submitted to the NASA Life Science Data Archive (https://lsda.jsc.nasa.gov/).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☐The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- [1].Goodpaster BH, Sparks LM, 2017. Metabolic Flexibility in Health and Disease. Cell Metab 25(5):1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bergouignan A, Blanc S, 2006. [The energetics of obesity]. J Soc Biol 200(1):29–35. [DOI] [PubMed] [Google Scholar]
- [3].Bergouignan A, Schoeller DA, Normand S, Gauquelin-Koch G, Laville M, Shriver T, et al. , 2006. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary Fatty acids: results of a randomized trial. PLoS Clin Trials 1(5):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brooks N, Cloutier GJ, Cadena SM, Layne JE, Nelsen CA, Freed AM, et al. , 2008. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J Appl Physiol (1985) 105(1):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ploutz-Snyder LL, Downs M, Ryder J, Hackney K, Scott J, Buxton R, et al. , 2014. Integrated resistance and aerobic exercise protects fitness during bed rest. Med Sci Sports Exerc 46(2):358–368. [DOI] [PubMed] [Google Scholar]
- [6].Scott JM, Hackney K, Downs M, Guined J, Ploutz-Snyder R, Fiedler J, et al. , 2014. The metabolic cost of an integrated exercise program performed during 14 days of bed rest. Aviat Space Environ Med 85(6):612–617. [DOI] [PubMed] [Google Scholar]
- [7].Harridge SD, 2007. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol 92(5):783–797. [DOI] [PubMed] [Google Scholar]
- [8].Sheffield-Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon-Jones D, Durham WJ, et al. , 2011. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab 96(11):E1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fitts RH, Peters JR, Dillon EL, Durham WJ, Sheffield-Moore M, Urban RJ, 2015. Weekly versus monthly testosterone administration on fast and slow skeletal muscle fibers in older adult males. J Clin Endocrinol Metab 100(2):E223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. , 2002. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282(3):E601–607. [DOI] [PubMed] [Google Scholar]
- [11].Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ, 2003. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88(1):358–362. [DOI] [PubMed] [Google Scholar]
- [12].Ploutz-Snyder LL, Downs M, Goetchius E, Crowell B, English KL, Ploutz-Snyder R, et al. , 2018. Exercise Training Mitigates Multisystem Deconditioning during Bed Rest. Med Sci Sports Exerc 50(9):1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dillon EL, Sheffield-Moore M, Durham WJ, Ploutz-Snyder LL, Ryder JW, Danesi CP, et al. , 2018. Efficacy of Testosterone plus NASA Exercise Countermeasures during Head-Down Bed Rest. Med Sci Sports Exerc 50(9):1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dillon EL, Soman KV, Wiktorowicz JE, Sur R, Jupiter D, Danesi CP, et al. , 2019. Proteomic investigation of human skeletal muscle before and after 70 days of head down bed rest with or without exercise and testosterone countermeasures. PLoS One 14(6):e0217690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scott JM, Martin D, Ploutz-Snyder R, Downs M, Dillon EL, Sheffield-Moore M, et al. , 2018. Efficacy of Exercise and Testosterone to Mitigate Atrophic Cardiovascular Remodeling. Med Sci Sports Exerc 50(9):1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cromwell RL, Scott JM, Downs M, Yarbough PO, Zanello SB, Ploutz-Snyder L, 2018. Overview of the NASA 70-day Bed Rest Study. Med Sci Sports Exerc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harris JA, Benedict FG, 1919. A biometric study of basal metabolism in man, Publ 279. Washington, DC: Carnegie Institute of Washington. [Google Scholar]
- [18].Aksnes AK, Brundin T, Hjeltnes N, Maehlum S, Wahren J, 1993. Meal-induced rise in resting energy expenditure in patients with complete cervical spinal cord lesions. Paraplegia 31(7):462–472. [DOI] [PubMed] [Google Scholar]
- [19].Inniss AM, Rice BL, Smith SM, 2009. Dietary support of long-duration head-down bed rest. Aviat Space Environ Med 80(5 Suppl):A9–14. [DOI] [PubMed] [Google Scholar]
- [20].Compher C, Frankenfield D, Keim N, Roth-Yousey L, Evidence Analysis Working G, 2006. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106(6):881–903. [DOI] [PubMed] [Google Scholar]
- [21].Jequier E, Acheson K, Schutz Y, 1987. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr 7:187–208. [DOI] [PubMed] [Google Scholar]
- [22].Dillon EL, Janghorbani M, Angel JA, Casperson SL, Grady JJ, Urban RJ, et al. , 2009. Novel noninvasive breath test method for screening individuals at risk for diabetes. Diabetes Care 32(3):430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blanc S, Normand S, Pachiaudi C, Fortrat JO, Laville M, Gharib C, 2000. Fuel homeostasis during physical inactivity induced by bed rest. J Clin Endocrinol Metab 85(6):2223–2233. [DOI] [PubMed] [Google Scholar]
- [24].Brooks NE, Cadena SM, Cloutier G, Vega-Lopez S, Roubenoff R, Castaneda-Sceppa C, 2014. Influence of exercise on the metabolic profile caused by 28 days of bed rest with energy deficit and amino acid supplementation in healthy men. Int J Med Sci 11(12):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics 29:1165–1188. [Google Scholar]
- [26].Somers RH, 1962. A new asymmetric measure of association for ordinal variables. American Sociological Review 27:799–811. [Google Scholar]
- [27].Oswal A, Yeo G, 2010. Leptin and the Control of Body Weight: A Review of Its Diverse Central Targets, Signaling Mechanisms, and Role in the Pathogenesis of Obesity. Obesity 18:221–229. [DOI] [PubMed] [Google Scholar]
- [28].Bergouignan A, Trudel G, Simon C, Chopard A, Schoeller DA, Momken I, et al. , 2009. Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes 58(2):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bienso RS, Ringholm S, Kiilerich K, Aachmann-Andersen NJ, Krogh-Madsen R, Guerra B, et al. , 2012. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes 61(5):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yokoyama H, Mori K, Emoto M, Araki T, Teramura M, Mochizuki K, et al. , 2008. Nonoxidative glucose disposal is reduced in type 2 diabetes, but can be restored by aerobic exercise. Diabetes Obes Metab 10(5):400–407. [DOI] [PubMed] [Google Scholar]
- [31].Novikova NV, 1989. [Sulfide ooze mud and sodium chloride baths in treating osteoarthrosis patients]. Vopr Kurortol Fizioter Lech Fiz Kult(2):35–37. [PubMed] [Google Scholar]
- [32].Gunn J, Gristwood J, 1975. Use of the Buss-Durkee hostility inventory among British prisoners. J Consult Clin Psychol 43(4):590. [DOI] [PubMed] [Google Scholar]
- [33].Dolkas CB, Greenleaf JE, 1977. Insulin and glucose responses during bed rest with isotonic and isometric exercise. J Appl Physiol Respir Environ Exerc Physiol 43(6):1033–1038. [DOI] [PubMed] [Google Scholar]
- [34].Dillon EL, Durham WJ, Urban RJ, Sheffield-Moore M, 2010. Hormone treatment and muscle anabolism during aging: androgens. Clin Nutr 29(6):697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Urban RJ, Dillon EL, Choudhary S, Zhao Y, Horstman AM, Tilton RG, et al. , 2014. Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc 125:27–42; discussion 42–24. [PMC free article] [PubMed] [Google Scholar]
- [36].Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. , 2010. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95(6):2536–2559. [DOI] [PubMed] [Google Scholar]
- [37].Kovac JR, Pastuszak AW, Lamb DJ, Lipshultz LI, 2014. Testosterone supplementation therapy in the treatment of patients with metabolic syndrome. Postgrad Med 126(7):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M, 2008. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40(1):181–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


