Abstract
Background
Congenital hypothyroidism (CHT) affects approximately one in 3000 to 4000 infants. CHT is one of the most common preventable causes of learning difficulties. Optimal management of CHT requires early diagnosis and prompt treatment to avoid abnormal neurodevelopmental outcome. One of the main issues in the management of CHT relates to the initial dose of levothyroxine to be used in order to achieve optimal results in terms of intellectual development. Currently, it remains unclear whether high dose thyroid hormone replacement is more effective than low dose in the treatment of CHT. Further research is required to determine an appropriate dose that improves mental and psychomotor developmental outcomes.
Objectives
To determine the effects of high versus low dose of initial thyroid hormone replacement for congenital hypothyroidism.
Search methods
Randomised controlled trials were identified by searching The Cochrane Library, MEDLINE and EMBASE and reference lists of published papers.
Selection criteria
Randomised controlled clinical trials investigating the effects of high versus low dose of initial thyroid hormone replacement for congenital hypothyroidism were included.
Data collection and analysis
Both authors independently selected trials, assessed risk of bias and extracted data.
Main results
The initial search identified 1014 records which identified 13 publications for further examination. After screening the full text of the 13 selected papers, only one study evaluating 47 babies finally met the inclusion criteria. Using the same cohort at two different time periods, the study investigated the effects of high versus low dose thyroid hormone replacement in relation to (1) time taken to achieve euthyroid status and (2) neurodevelopmental outcome. The study reported that a high dose is more effective in rising serum thyroxine and free thyroxine concentrations to the target range and earlier normalisation of thyroid stimulating hormone compared to a lower dose. Similarly, full scale intelligence quotient was noted to be significantly higher in children who received the high dose compared to the lower dose. However, the verbal intelligence quotient and performance intelligence quotient were similar in both groups. Growth and adverse effects were not reported in the included trial.
Authors' conclusions
There is currently only one randomised controlled trial evaluating the effects of high versus low dose of initial thyroid hormone replacement for CHT. There is inadequate evidence to suggest that a high dose is more beneficial compared to a low dose initial thyroid hormone replacement in the treatment of CHT.
Plain language summary
High versus low dose of initial thyroid hormone replacement for congenital hypothyroidism
Congenital hypothyroidism is a condition that affects infants from birth and results from a missing or abnormally developed thyroid gland, abnormal production of thyroid hormones or a failure of the pituitary gland to stimulate thyroid hormone production. It affects approximately 1 in 3000 to 4000 newborn infants and early diagnosis and treatment is very important to achieve a good outcome.Treatment of congenital hypothyroidism consists of a daily dose of thyroid hormone (thyroxine). However, the initial dose of thyroxine required to improve outcomes for infants with this condition is unclear and has been the subject of several studies. Some studies have suggested that when infants with congenital hypothyroidism are treated with a higher dose of thyroxine compared with the standard dose, this results in earlier normalisation of the their thyroid hormones and leads to better developmental outcome and intelligence.
There is currently only one study reporting on 47 babies that fulfils our review criteria and compares different high dose versus low dose of initial replacement thyroxine for the treatment of congenital hypothyroidism. There is not enough evidence to suggest that a high dose is more beneficial than a low dose therapy. Growth and adverse effects were not reported in the included study. There should be more randomised controlled trials to assess the effects of high versus low dose of initial thyroid hormone replacement for congenital hypothyroidism.
Background
Description of the condition
Congenital hypothyroidism (CHT) is one of the most common preventable causes of learning difficulties with a prevalence of approximately 1 in 3000 to 4000 births (AAP 1993; LaFranchi 1999). In the majority of cases, the disorder is permanent and results from an abnormality in thyroid gland development (dysgenesis or agenesis) or a defect in thyroid hormonogenesis but rarely it can be due to pituitary or hypothalamic abnormality (central or secondary/tertiary hypothyroidism) (AAP 1993; Song 2001). Occasionally, the altered neonatal thyroid function is transient, due to the transplacental passage of maternal medication, maternal blocking antibodies, or iodine deficiency or excess (Brown 1996; Calaciura 2002; Haddow 1999).
CHT can result in poor cognitive development, school delay and behavioural problems (Gruters 2002; Oerbeck 2003; Rovet 2002). Routine screening programmes have resulted in early diagnosis and treatment with levothyroxine (L‐T4) which in turn have led to a marked improvement in overall prognosis (Hulse 1984; Murphy 1986). The age at which treatment is started, the initial dose and time take to achieve and maintain the target ranges for thyroxine (T4) and thyroid stimulating hormone (TSH) play an important role in achieving normal outcome (Grant 1992).
Description of the intervention
There are varying reports for follow‐up studies of intellectual development in children with CHT treated early (Heyerdahl 1991; Murphy 1986; Salerno 1999). It is recommended that early normalisation of TSH is one of the aims in the management of CHT (Heyerdahl 1991; Murphy 1986; Ng 2004). Studies have shown that delayed TSH normalisation was significantly related to plasma T4 levels and dosage of L‐T4, suggesting that this may be due to undertreatment in the initial stage (Abusrewil 1988; Brown 1996; Heyerdahl 1991). The initial starting dose of L‐T4 (conventional dose of 5 to 10 μg/kg/day versus high dose 10 to 15 μg/kg/day) has been the subject of previous studies (Campos 1995; Dubuis 1996; Salerno 2002). Initial doses of L‐T4 of 10 to 15 μg/kg/day have been reported to result in normalisation within three weeks whereas doses of 8 μg/kg/day result in normalisation within 6 to 8 weeks (Campos 1995; Fisher 1989; Germak 1990; Rovet 1995). In addition, early high dose L‐T4 has been reported to close the developmental gap and normalise intelligence quotient (IQ) (Dubuis 1996; Bongers‐Schokking 2000; Rovet 1995; Simoneau‐Roy 2004). A starting L‐T4 dose of 10 to 15 mcg/kg/day is currently recommended by the European Society for Paediatric Endocrinology (ESPE) (Working group 1999).
The recommended goal of therapy is to normalise blood T4 concentrations within 2 to 3 weeks and blood TSH concentrations within one month (AAP 1993; Song 2001). Outcome studies by Song et al showed that children with CTH who took the longest time to normalise their thyroid hormones had greater difficulty in tasks of attention and memory compared with children who normalised their thyroid function earlier (Song 2001). Although a lower initial dose (less than 10 µg/kg/day) of L‐T4 may achieve this, a higher dose (greater than 10 µg/kg/day) appears to normalise the serum T4 concentrations in three days and maintain the TSH in the target range within two weeks of therapy (Bakker 1995). However, the impact on long‐term outcomes is controversial. Consequently, one of the major outstanding issues in the management of CHT concerns the amount of the initial levothyroxine dose to be used in the initial treatment of CHT, which may give optimal result in terms of intellectual development. It is thus important to establish the evidence regarding potential benefits of initial dosage requirements in the treatment of CH.
Adverse effects of the intervention
The use of a high dose in the initial treatment is controversial as it is reported to be associated with behavioural problems, poor visual motor skills and numerical reasoning (Schwartz 1994; Rovet 1995) . There are studies that showed normal outcome in relation to IQ even with lower doses of L‐T4 (Campos 1995; Rovet 1995).
Why it is important to do this review
Although several reports have been published describing normal IQ values among patients with CHT treated initially with low dosages, more recent studies have demonstrated better outcomes with high L‐T4 dosages compared to low dosages.This review tries to establish evidence that high dose treatment of thyroxine in CHT improves neurodevelopmental outcome, growth or behaviour in infants with CHT.
Objectives
To assess the effects of high versus low dose of initial thyroid hormone replacement for congenital hypothyroidism.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials.
Types of participants
All children diagnosed with congenital hypothyroidism based on a neonatal congenital hypothyroidism screening programmes according to the appropriate diagnostic criteria of the time.
Types of interventions
Low dose of levothyroxine (LT‐4) of 5 to 9.9 μg/kg/day (standard dose regime) versus high dose of 10 to 15 μg/kg/day (high dose regime).
Types of outcome measures
Primary outcomes
cognitive development (for example intelligence quotient (IQ), language, visio‐spatial processing, selective memory, auditory discrimination, academic achievement, attention and behavioural problems;
growth and increased growth rate;
adverse effects.
Secondary outcomes
morbidity;
all‐cause mortality;
development of hyperthyroidism;
suppression of thyroid stimulating hormone (TSH),
health‐related quality of life;
costs.
Covariates, effect modifiers and confounders
compliance with the treatment;
age treatment is initiated;
age at which target range for TSH and thyroxine (T4) are achieved;
length of time TSH is maintained in the target range during first year of life.
Timing of outcome measurement
Outcome measurements will be assessed according to the following intervals:
short term: less than 12 months;
medium term: 1 to 5 years;
long‐term: more than 5 years.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library
MEDLINE
EMBASE
We also searched databases of ongoing trials: Current Controlled Trials (www.controlled‐trials.com ‐ with links to other databases of ongoing trials). The described search strategy (see for a detailed search strategy under Appendix 1) was used for MEDLINE. For use with EMBASE, The Cochrane Library and the other databases this strategy was slightly adapted.
If additional key words of relevance were detected during any of the electronic or other searches we planned to modify electronic search strategies incorporating these terms. Studies published in any language were included.
Searching other resources
We tried to identify additional studies by searching the reference lists of included trials and (systematic) reviews, meta‐analyses and health technology assessment reports noticed.
Data collection and analysis
No analysis was possible as only one RCT was included
Selection of studies
To determine the studies to be assessed further, two reviewers (SN, DA) independently scanned the abstract, title or both sections of every record retrieved. All potentially relevant articles were reviewed as full text. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Differences were marked and if these studies were later on included, the influence of the primary choice was subjected to a sensitivity analysis. Where differences in opinion existed, they were resolved by the third reviewer (AW). The third reviewer also proof read the review at different stages. If resolving disagreement was not possible, the article was added to those 'awaiting assessment' and authors were contacted for clarification. An adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection is attached (Moher 1999).
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) obtained priority.
Data extraction and management
For studies that fulfilled inclusion criteria, two authors (SN, DA) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies, Table 1, Appendix 2 and Appendix 3) with any disagreements being resolved by discussion, or if required by a third party. Any relevant missing information on the trial was sought from the original author(s) of the article, if required.
1. Study populations.
| study ID | intervention | [n] screened | [n] randomised | [n] safety | [n] ITT | [n] finishing study | comments |
| Selva 2002 | Intervention (I): L‐thyroxine 50 mcg/day Control1 (C1): L‐thyroxine 37.5mcg/day Control 2 (C2): loading dose 50 mcg/day for 3 days, followed by 37.5mcg/day |
I: nr C1: nr C2: nr |
I: 15 C1: 15 C2: 17 Total: 47 |
I: nr C1: nr C2: nr |
I: nr C1: nr C1: nr |
I: 15 C1: 15 C2: 17 Total: 47 |
nr = not reported
Assessment of risk of bias in included studies
Two authors (SN, DA) assessed each trial independently (for details see Characteristics of included studies). Possible disagreement would be resolved by consensus, or with consultation of a third party in case of disagreement. We planned to explore the influence of individual quality criteria in a sensitivity analysis (see under 'sensitivity analyses'). Interrater agreement for key quality indicators would be calculated using the kappa statistic (Cohen 1960). In cases of disagreement, the rest of the group would consult and a judgement would be made based on consensus.
Measures of treatment effect
The authors planned to carry out statistical analysis using the Review Manager software. Fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar would be used. If heterogeneity was found this would have been explored by sensitivity analysis followed by random‐effects meta‐analysis if required.
Dealing with missing data
Relevant missing data were planned to be obtained from authors, if feasible. Evaluation of important numerical data such as screened, eligible and randomised patients as well as intention‐to‐treat (ITT) and per‐protocol (PP) population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data, ITT and PP were critically appraised and compared to specification of primary outcome parameters and power calculation.
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, study results would not be combined by means of meta‐analysis. Heterogeneity would be identified by visual inspection of the forest plots, by using a standard χ2‐test and a significance level of α = 0.1, in view of the low power of such tests. Heterogeneity would be specifically examined with I2 (Higgins 2002), where I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). If heterogeneity was found, we would attempt to determine potential reasons for it by examining individual study characteristics and those of subgroups of the main body of evidence.
Assessment of reporting biases
Funnel plots would be used in an exploratory data analysis to assess for the potential existence of small study bias. There are a number of reasons for the asymmetry of a funnel plot, including true heterogeneity, design of studies and publication bias (Sterne 2001). Therefore, we did not place undue emphasis on this tool (Lau 2006).
Data synthesis
Data would be summarised statistically if they were available, sufficiently similar and of sufficient quality. Statistical analysis were performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of study quality, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results would be tested by repeating the analysis using different measures of effects size (relative risk, odds ratio etc.) and different statistical models (fixed‐ and random‐effects models).
Results
Description of studies
Results of the search
The initial search identified 1014 records, from these,13 full publications were identified for further examination. The other studies were excluded on the basis of their abstracts because they were not relevant to the question under study (see Figure 1 for details of the amended QUOROM (quality of reporting of meta‐analyses) statement) flow‐chart of study selection). After screening the full text of the selected publications, only one study finally met the inclusion criteria.
1.
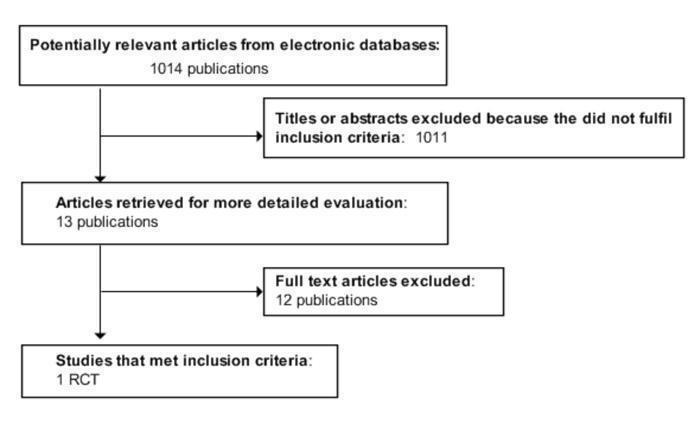
Amended QUOROM (quality of reporting of meta‐analyses) statement) flow‐chart of study selection
Included studies
One trial was included in this review . This reported a sample size of 47 participants and the trial was published in two phases described below.
A single centre, randomised controlled trial of 47 infants investigating the effectiveness of three different initial doses in achieving early euthyroid status. The trial had three arms that were randomly assigned to three different starting dosages for the initiation of treatment for congenital hypothyroidism (CHT) detected through a screening programme and confirmed by laboratory investigations. The infants in the first arm received a dose of 37.5 mcg/day (group 1), the second arm 62.5 mcg/day for three days followed by 37.5 mcg/day (group 2) and the third arm 50 mcg/day (group 3). The serum T4, free T4, free T3 and TSH concentrations were measured at baseline before onset of treatment and after 1, 2, 4, 8 and 12 weeks. No changes were made to the initial treatment dose during the first two weeks of treatment but subsequent dosages were altered to maintain the T4 concentrations in the target range using a standard protocol.
In the second follow‐up phase of the study , children from the above study were followed up between 21 months and 8 years of age to assess their neurodevelopment. Sixteen children were lost to follow‐up and the remaining 31 children were assessed. In addition to comparing the neurodevelopmental outcomes of children who received varying initial doses of treatment, a comparison was also made with unaffected sibling controls aged between 43 months and 14 years. All cognitive tests were administered by the same person blinded to the study cohort, disease severity and control participant status. Three different age appropriate cognitive tests were used. Children below four years of age underwent the 'Mullen Scales for Early Learning' test, those between 4 and 6 years were assessed with the 'Wechsler Preschool and Primary Scale of Intelligence‐ Revised (WPPSI‐R)' test and children more than six years were evaluated with the 'Wechsler Intelligence Scale for Children, Third edition (WISC III)' test.
Excluded studies
Twelve studies had to be excluded after careful evaluation of the full publication. Main reasons for exclusion were non‐randomisation (for details see Characteristics of excluded studies).
Risk of bias in included studies
In order to assess the risk of bias in the included study, the authors assessed the methodological quality of the included trial using criteria suggested by Jüni for the following dimensions: concealment of allocation; generation of the randomisation sequence; intention‐to‐treat; and level of blinding reported Juni 2001. The dimensions, concealment of allocation, generation of the randomisation sequence and intention‐to‐treat were categorised as adequate, unclear or inadequate which related to a low, unclear or high risk of bias respectively. RCTs were planned to be categorised according to whether double‐blinding had been reported or not. The more people blinded to an intervention relates to a decreasing risk of bias to the results.
Allocation
The included trial stated that allocation was randomised, but did not describe the method of randomisation used. We therefore judged the risk of bias due to the generation of the randomisation sequence as unclear in that trial. Allocation concealment was not described.
Blinding
The trial did not report blinding conditions.
Incomplete outcome data
The trial did not explicitly state that an intention‐to‐treat analysis was performed. Neither did it state that any participants deviated from the randomised group to which they were assigned to. Therefore we judged the risk of bias to be unclear in this trial. There were 16 participants that were lost to follow‐up and these were described. We judged this risk of this bias to be low.
Selective reporting
We were unable to identify any selective reporting in the included trial, but did not have any access to the original trial protocols to definitely confirm this; we therefore conclude that there is an unclear risk of bias due to selective reporting.
Other potential sources of bias
Participants were selected from a restricted geographical area in proximity to the hospital on the basis of the screening program for identifying CHT babies. This may present a potential risk of bias due to pre‐selection of participants.
Effects of interventions
Primary outcomes
Cognitive development: Participants who were on higher initial dose of 50 mcg/day L‐thyroxine had higher full scale IQ scores compared to participants who were on lower initial dose of 37.5mcg/day. Verbal IQ, performance IQ and achievement scores did not differ among the three groups.
Growth and increased growth rate: These outcomes were not reported.
Adverse effects: No adverse effects were described.
Secondary outcomes
The outcomes morbidity, all‐cause mortality, development of hyperthyroidism, suppression of thyroid stimulating hormone (TSH), health‐related quality of life and costs were not reported.
Covariates, effect modifiers and confounders
Selva 2002 reported full compliance with treatment during the 12 week study follow‐up. The age at which treatment was initiated was not reported. Participants on an initial dose of 50 mcg/day (12.5 to 16 mcg/kg/day) (group 3) normalised TSH within two weeks of treatment. Participants on an initial dose of 37.5 mcg/day (9.3 to 12 mcg/kg/day) (group 1) normalised TSH within 12 weeks of treatment. Participants on an initial dose of 62.5 mcg/day for three days followed by 37.5 mcg/day never achieved normal TSH concentrations. Length of time TSH was maintained in the target range during the first year of life was not reported.
Discussion
This review found only one randomised controlled trial which examined the effects of high versus low dose of initial thyroid hormone replacement for congenital hypothyroidism (CHT).
This study had limitations in methodological quality. The trial (Selva 2002) was conducted in a single centre comprising of 47 infants with a birth weight between 3 to 4 kg, of which 37% (16 children) were lost to follow‐up for the second phase of the study (Selva 2005). The trial had three arms that were randomly assigned to three different starting dosages for the initiation of treatment for CHT. Consequently, it did not fully match our initial protocol description of groups as 'low dose' (L‐thyroxine (LT‐4): 5 to 9.9 μg/kg/day standard dose regime) and 'high dose' (10 to 15 μg/kg/day). For the purpose of this review, we assigned group 1 of the trial as low initial dose based on the calculated dose of 9.3 to 12 μg/kg/day and group 3 of the trial as high initial dose based on the calculated dose of 12.5 to 16 μg/kg/day.
The study found that the serum T4 concentrations reached the recommended target range by three days of treatment in the high dose group but took one week in the low dose group. However, the infants in the high dose group exceeded the upper limits of target range during the first two weeks albeit without any adverse side effects. Similarly, infants in the high dose group had their TSH levels in the normal range by two weeks whereas in the low dose group a normal range was achieved at about 12 weeks.
In the second phase of the study (Selva 2005), 16 children were lost to follow‐up and the remaining 31 children were assessed between 21 months and 8 years of age for their neurodevelopment. Consequently, different tests were applied to different age groups. The study noted that the full scale IQ scores in the high dose group was significantly higher than in the low dose group. However, verbal IQ, performance IQ and achievement scores did not differ among the three groups
These findings must be interpreted with caution because of several limitations. The number of participants in the trial is very small and authors do not explain how the sample size was calculated. Similarly, they do not describe the randomisation process and allocation concealment and how successful it was except in relation to screening and diagnostic criteria. In the first phase of the study, authors report P‐values to illustrate significant differences between high and low dose groups in achieving early euthyroid status but do not provide confidence intervals to estimate the effect size. Similar limitations apply to the second follow‐up phase of the study. In addition, different assessment methods were used to assess results at different ages but the results of these assessments were combined in calculating mean scores for the two groups.
Early high dose L‐T4 has been reported to close the developmental gap and normalise IQ (Dubuis 1996 ; Salerno 2002, Simoneau‐Roy 2004), but may be associated with internalising behavioral and concentration problems later in life (Rovet 1995). However, a cohort study of 49 adults with early treated CHT and sibling controls showed that no adverse effects of high dose thyroxine were found on measures of memory, attention and behavioural problems (Oerbeck 2003). A recent retrospective study of high versus low initial doses of L‐T4 has found no evidence of sustained overgrowth up to three years of age while a higher dose of L‐T4 achieved normalisation of thyroid hormones several months earlier than the lower dose regime (Jones 2008).
Although results from one randomised controlled trial suggest that a high initial dose in the treatment of CHT is likely to achieve early euthyroid status and improve full‐scale IQ scores, the evidence is insufficient to suggest a high dose is more effective than a low dose in the initial management of CHT. Important quality criteria of the trial included were not reported adequately. Therefore, we were unable to make firm conclusions about the quality of the trial based on the evidence that was reported in the trial.
From our review, no conclusive benefit of improved neurodevelopmental outcome can be deduced from the effects of high dose of initial thyroid hormone replacement for congenital hypothyroidism. Although results from one randomised controlled trial found significant improvements in full‐scale IQ scores but no significant differences in verbal IQ, performance IQ and achievement scores, this raises the question if there is any real benefit from this intervention while current guidelines nevertheless advocate the use of higher thyroxine treatment doses in CHT.
The importance of establishing clear beneficial outcomes for high dose initial replacement of thyroxine in CHT is therefore of major clinical relevance.
Authors' conclusions
Implications for practice.
At present, there is insufficient evidence from randomised controlled trials to evaluate the effectiveness of high versus low dose of initial thyroid hormone replacement for congenital hypothyroidism (CHT). Trials with definite clinical outcomes are essential in evaluating the potential of high dose treatment of CHT. There is still a need for RCTs to evaluate safety and efficacy in the treatment of CHT.
Implications for research.
This systematic review has identified the need for a well‐designed, adequately powered, multi‐centred randomised controlled trial on evaluating the effects of high versus low dose of initial thyroid hormone replacement for the treatment of congenital hypothyroidism (CHT). Outcome measures such cognitive development (for example intelligence quotient (IQ), language, visio‐spatial processing, selective memory, auditory discrimination, academic achievement, attention and behavioural problems), growth and increased growth rate should be stated and trials be adequately powered to obtain reliable results. At present, with only one RCT, it cannot be stated that high doses of initial thyroid hormone replacement are efficacious and safe in infants with CHT. Therefore, more RCTs are needed to evaluate the effects of high dose versus low dose in the treatment of CHT. We would urge trialists to recognise that the results of individual randomised controlled trials are likely to be included in systematic reviews such as this. They should therefore consider standardising the presentation of outcomes to enable the data to be aggregated.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank Bernd Richter for the useful suggestions and assistance with the review, and the Cochrane group for assisting us with running the search strategies.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical Subject Heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = substitute for one or no characters; ab = abstract; ti = titel; ot = original titel; pt = publication type; sh = MeSH: Medical subject heading (MEDLINE medical index term); adj = adjacency. 1.exp Congenital Hypothyroidism/ 2.(congenital adj3 (myx?edema$ or hypothyroidism$)).tw,ot. 3.1 or 2 4.Randomized Controlled Trial.pt. 5.Controlled Clinical Trial.pt. 6.Randomized Controlled Trials.sh. 7.Random Allocation.sh. 8.Double‐Blind Method.sh. 9.Single‐Blind Method.sh. 10.or/4‐9 11.clinical trial.pt. 12.Clinical Trials.sh. 13.(clinic$ adj25 trial$).tw,kf,ot. 14.((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).tw,kf,ot. 15.Placebos.sh. 16.placebo$.tw,kf,ot. 17.random$.tw,kf,ot. 18.Research Design.sh. 19.(latin adj square).tw,kf,ot. 20.or/11‐19 21.comparative study.pt. 22.Evaluation Studies.sh. 23.Follow‐up Studies.sh. 24.Prospective Studies.sh. 25.(control$ or prospectiv$ or volunteer$).tw,kf,ot. 26.Cross‐Over Studies.sh. 27.or/21‐26 28.10 or 20 or 27 29.limit 28 to animals 30.limit 28 to humans 31.29 not 30 32.28 not 31 33.exp "Review Literature"/ 34.exp Technology Assessment, Biomedical/ 35.exp Meta‐Analysis/ 36.meta‐analysis.pt. 37.((review$ or search$) adj10 (literature or medical database$ or medline or pubmed or embase or cochrane or cinhal or psychinfo or psychlit or healthstar or biosis or current content$ or systemat$)).tw,kf,ot. 38.hta.tw,kf,ot. 39.(health technology adj6 assessment$).tw,kf,ot. 40.(meta analy$ or metaanly$ or meta?analy$).tw,kf,ot. 41.or/33‐40 42.32 or 41 43.3 and 42 |
Appendix 2. Baseline characteristics
| Characteristic | Selva 2002 |
| Intervention: L‐thyroxine 50 mcg/day, n=15 Control1: L‐thyroxine 37.5mcg/day, n=15 Control 2: loading dose 50 mcg/day for 3 days, followed by 37.5mcg/day, n=17 |
|
| Sex [female% / male%] | I1: nr C1: nr C2: nr Total: 47 |
| Age [mean days (SD)] | I1: nr, < 1 year C1: nr, < 1 year C2: nr, < 1 year Total: mean age at which newborn screenign test was performed: 1.6 ( SD 1.4) days |
| Ethnic groups [%] | I1: nr C1: nr C2: nr |
| Notes | all infants recruited were enrolled in the study after detection by the Northwest Regional Newborn Screening Program |
|
Footnotes nr = not reported | |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Selva 2002.
| Methods | Randomised controlled trial | |
| Participants | 47 babies with congenital hypothyroidism detected by newborn screening | |
| Interventions | Participants were randomly assigned to 3 dosage arms of 37.5 mcg/day (group 1), 62.5 mcg/day for 3 days followed by 37.5 mcg/day (group 2) and 50 mcg/day (group 3) | |
| Outcomes | 1) Time to normalisation of TSH during the first 12 weeks of CHT treatment | |
| Study details | Initial dose of 50mcg/day (12‐17mcg/kg/day) (group 3) normalised TSH by 2 weeks of treatment. Initial dose of 37.5mcg/day (9‐3‐12 mcg/kg/day) (group 1) normalised TSH by 12 weeks of treatment. Initial dose of 62.5mcg/day for 3 days followed by 37.5 mcg/day never achieved normal TSH concentrations | |
| Publication details | J Pediatr 2002; 141:786‐92 | |
| Stated aim of study | Quote "The first objective of our study was to determine the ideal replacement dose of L‐thyroxine in infants with CH as judged by the time course of rise in T4 and free T4 and fall in TSH, by using 3 treatment arms. A second objective was to compare the rise in T4 and free T4 and fall in TSH between mild and severe cases of CH within each treatment group. A third objective was to reexamine the “target” T4 and free T4 range in the first 2 weeks of therapy, with the use of the above regimens, as judged by the corresponding TSH concentrations". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, but no details of method given |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Did not report blinding of investigators |
| Incomplete outcome data addressed? All outcomes | Low risk | Described as no losses to follow‐up |
| Free of selective reporting? | Unclear risk | Unable to identify any selective reporting in the included trial as did not have any access to the original trial protocols |
| Free of other bias? | Unclear risk | Participants were selected from a restricted geographical area in proximity to the hospital on the basis of the screening program for identifying congenital hypothyroidism babies. This may present a potential risk of bias due to pre‐selection of participants. |
Selva 2005.
| Methods | Randomised controlled trial. follow‐up study | |
| Participants | 47 babies with congenital hypothyroidism detected by newborn screening | |
| Interventions | Participants were randomly assigned to 3 dosage arms of 37.5 mcg/day (group 1), 62.5 mcg/day for 3 days followed by 37.5 mcg/day (group 2) and 50 mcg/day (group 3) | |
| Outcomes | Neurodevelopment assessment using Mullen Scales of Early learning, Weschler Preschool and Primary Scale of Intelligence‐revised, Weschler Intelligence Scale for Children, Wide Range of Acheivement Test and Child Behavioural Checklist | |
| Study details | Participants who were on higher initial dose of 50mcg/day L‐thyroxine had higher full scale IQ scores compared to participants who were on lower initial dose of 37.5mcg/day. verbal IQ, performance IQ and achievement scores did not differ among the three groups. | |
| Publication details | J Pediatr 2005; 147:775‐80 | |
| Stated aim of study | Quote "Our goal was to compare neurodevelopmental outcomes, particularly cognition, academic achievement, and attention/behavior, among the 3 treatment groups. In addition, we investigated the effect of CH severity, differences (if any) between CH subjects and their unaffected siblings, and the effect of the timing of thyroid function normalization on neurodevelopmental outcomes in all treatment cohorts". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, but no details of method given. |
| Allocation concealment? | Unclear risk | Not described |
| Blinding? All outcomes | Unclear risk | Did not report blinding of investigators |
| Incomplete outcome data addressed? All outcomes | Low risk | 16 participants were lost to follow up and reasons were described. |
| Free of selective reporting? | Unclear risk | Unable to identify any selective reporting in the included trial, but did not have any access to the original trial protocols |
| Free of other bias? | Unclear risk | Participants were selected from a restricted geographical area in proximity to the hospital on the basis of the screening program for identifying congenital hypothyroidism babies. This may present a potential risk of bias due to pre‐selection of participants. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bongers‐Schokking 2000 | This is a non‐randomised clinical controlled trial studying the timing and dose of thyroid hormone replacement on development in infants with CHT. |
| Bongers‐Schokking 2005 | This is a non‐randomised clinical controlled trial studying the timing and dose of thyroid hormone replacement on development in infants with CHT. |
| Campos 1995 | This is a non‐randomised clinical controlled trial studying the outcome of low dose initial thyroxine therapy for CHT. |
| Cassio 2003 | This is a randomised controlled clinical trial of thyroxine (T4) plus triiodothyronine (T3) versus T4 alone in CH patients. |
| Chiesa 1994 | This is a non‐randomised clinical controlled trial studying the growth of children with CHT before and during treatment. |
| Dubuis 1996 | This is a non‐randomised clinical controlled trial studying the outcome of severe CHT using early high dose T4 therapy. |
| Gunn 1996 | This is a non‐randomised clinical controlled trial. |
| Salerno 2002 | This is a non‐randomised clinical controlled trial studying the effect of different starting doses of T4 on growth and outcome at four years of age in CHT. |
| Schwartz 1994 | This is a non‐randomised clinical controlled trial studying the outcome in children with CHT treated with varying amounts of T4 during first 2 years of life. This does not fulfill the objective of the review. |
| Simoneau‐ Roy 2005 | This is a non‐randomised clinical controlled trial. |
| Touati 1997 | This is a non‐randomised clinical controlled trial. |
| Vanhole 1997 | This is a randomised clinical controlled trial looking at replacement thyroxine therapy in hypothyroxinaemia of prematurity and not in congenital hypothyroidism. |
CHT = congenital hypothyroidism
Contributions of authors
SZE MAY NG and DHULLIPALA ANAND independently selected trials, assessed methodological quality and extracted data. ALAN WEINDLING proof read the review.
The lead author completed the write‐up of the review with contributions from the co‐authors and acts as guarantor of the review.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Selva 2002 {published data only}
- Selva KA, Mandel SH, Rein L, Sesser D, Miyahira R, Skeels M, Nelson JC, Franchi SH. Initial treatment dose of L‐thyroxine in congenital hypothyroidism. J Peadiatr 2002;141:786‐92. [DOI] [PubMed] [Google Scholar]
Selva 2005 {published data only}
- Selva KA, Harper A, Downs A, Blasco PA, Franchi SH. Neurodevelopmental outcomes in congenital hypothyroidism: comparison of initial T4 dose and time to reach target T4 and TSH . J Pediatr 2005;147:775‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bongers‐Schokking 2000 {published data only}
- Bongers‐Schokking JJ, Koot HM, Wiersma D, Verkerk PH, Muinck Keizer‐Schrama SMPF. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. Journal of Pediatrics 2000;136:292‐7. [DOI] [PubMed] [Google Scholar]
Bongers‐Schokking 2005 {published data only}
- Bongers‐Schokking JJ, Muinck Keizer‐Schrama SM. Influence of timing and dose of thyroid hormone replacement on mental, psychomotor, and behavioral development in children with congenital hypothyroidism. J Pediatr 2005;147:768‐74. [DOI] [PubMed] [Google Scholar]
Campos 1995 {published data only}
- Campos SP, Sadberg DE, Barric C, Vorhess ML, Mcgillivary MH. Outcome of low dose initial thyroxine therapy for congenital hypothyroidism. Clinical Pediatrics 1995;34:514‐20. [DOI] [PubMed] [Google Scholar]
Cassio 2003 {published data only}
- Cassio A, Cacciari E, Cicognani A, Damiani G, Missiroli G, Corbelli E. Treatment for congenital hypothyroidism: thyroxine alone or thyroxine plus triiodothyronine?. Pediatrics 2003;111:1055‐60. [DOI] [PubMed] [Google Scholar]
Chiesa 1994 {published data only}
- Chiesa A. Growth follow‐up in 100 children with congenital hypothyroidism before and during treatment. The Journal of Pediatric Endocrinology and Metabolism 1994;7(3):211‐7. [DOI] [PubMed] [Google Scholar]
Dubuis 1996 {published data only}
- Dubuis J, Glorieux J, Richer F, Dussault JH, Vliet GV. Outcome of severe congenital hypothyroidism: closing the developmental gap with early high dose levothyroxine therapy. Journal of Clinical Endocrinology and Metabolism 1996;81:222‐7. [DOI] [PubMed] [Google Scholar]
Gunn 1996 {published data only}
- Gunn AJ, Wake M, Cutfield WS. High and low dose initial thyroxine therapy for congenital hypothyroidism. J Paediatr Child Health 1996;32:242‐5. [DOI] [PubMed] [Google Scholar]
Salerno 2002 {published data only}
- Salerno M, Militerni R, Bravaccio C, Micillo M, Capalbo D, Di MS, Tenore A. Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid 2002;12(1):45‐52. [1050‐7256 (Print)] [DOI] [PubMed] [Google Scholar]
Schwartz 1994 {published data only}
- Schwartz ID, Turner K, Kruger T, Bennet D, Howard CP, Grunt JA. Neuropsychological outcome in children with congenital hypothyroidism treated with varying amounts of levothyroxine during first 2 years of life. International Pediatrics 1994;9:254‐9. [Google Scholar]
Simoneau‐ Roy 2005 {published data only}
- Simoneau‐Roy J. Cognition and behavior at school entry in children with congenital hypothyroidism treated early with high‐dose levothyroxine. J Pediatr 2004;144:747‐52. [DOI] [PubMed] [Google Scholar]
Touati 1997 {published data only}
- Touati G, Leger J, Toublanc JC. A thyroxine dose of 8 mg/kg per day is appropriate for initial treatment of the majority of infants with congenital hypothyroidism. Eur J Pediatr 1997;156:94‐8. [DOI] [PubMed] [Google Scholar]
Vanhole 1997 {published data only}
- Vanhole C, Aerssens P, Naulaers G, Casneuf A, Devlieger H, BG. L thyroxine treatment of preterm newborns: Clinical and endocrine effects. Pediatr Res 1997;8:87‐92. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 1993
- American Academy of Paediatrics. Newborn screening for congenital hypothyrodism: Recommended guidelines. Pediatrics 1993;91:1203‐9. [PubMed] [Google Scholar]
Abusrewil 1988
- 8.Abusrewil SSA, Tyfield L, Savage DCL. Serum thyroxine and thyroid stimulating hormone concentrations after treatment of congenital hypothyroidism. Archives of Disease in Childhood 1988;63:1368‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakker 1995
- Bakker B, Kempers MJ, Vijdler JJ. Dynamics of plasma concentrations of TSH, FT4 and T3 following thyroxine supplementation in congenital hypothyroidism. Clinical Endocrinology 2002;57:529‐37. [DOI] [PubMed] [Google Scholar]
Brown 1996
- Brown RS, Bellisaro RL, Botero D. Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor‐blocking antibodies in over one million babies. Journal of Clinical Endocrnology and Metabolism 1996;81:1147‐51. [DOI] [PubMed] [Google Scholar]
Calaciura 2002
- Calaciura F, Motto RM, Miscio G. Subclinical hypothyroidism in early childhood: a frequent outcome of transient neonatal hyperthyrotropinemia. Journal of Clinical Endocrinology and Metabolism 2002;87:3209‐14. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Fisher 1989
- Fisher DA, Foley TP. Early treatment of congenital hypothyroidism. Journal of Pediatrics 1989;117:211‐9. [PubMed] [Google Scholar]
Germak 1990
- Germak JA, Foley TP. Longitudinal assessment of L‐thyroxine therapy for congenital hypothyroidism. Journal of Pediatrics 1990;117:211‐9. [DOI] [PubMed] [Google Scholar]
Grant 1992
- Grant DB, Smith I, Fuggle PW, Tokar S, Chapple J. Congenital hypothyroidism detected by neonatal screening: relationship between biochemical severity and early clinical features. Archives of Disease in Childhood 1992;67:87‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruters 2002
- Gruters A, Jenner A, Krude H. Long term consequences of congenital hypothyroidism in the era of screening programmes. Best Practice Research Clinical Endocrinology and Metabolism 2002;16:369‐82. [DOI] [PubMed] [Google Scholar]
Haddow 1999
- Haddow JE, Palomaki GE, Allan WC. Maternal thyroid function during pregnancy and subsequent neuropsychological development of the child. New England Journal of Medicine 1999;341:549‐55. [DOI] [PubMed] [Google Scholar]
Heyerdahl 1991
- Heyerdahl S, Kase BF, Lie SO. Intellectual development in children with congenital hypothyroidism in relation to recommended thyroxine treatment. Journal of Pediatrics 1991;118:850‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. In: The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org..
Hulse 1984
- Hulse JA. Outcome for congenital hypothyroidism. Archives of Disease in Childhood 1984;59:23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2008
- Jones JH, Gellen G, Paterson WF, Beaton S, Donaldson MD. Effect of high versus low initial doses of L‐thyroxine for congenital hypothyroidism on thyroid function and somatic growth. Arch Dis Child 2008;93(11):940‐4. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care:Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
LaFranchi 1999
- LaFranchi S. Thyroid function in the preterm infant. Thyroid 1999;9(1):71‐8. [1050‐7256 (Print)] [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Murphy 1986
- Murphy G, Hulse JA, Jackson D. Early treated hypothyroidism:development at 3 years. Archives of Disease in Childhood 1986;61:761‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2004
- Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, Wong E. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Archives of Disease in Childhood Fetal Neonatal Ed 2004;89(2):F119‐26. [1359‐2998 (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oerbeck 2003
- Oerbeck B, Sundet K, Kase BF, Heyerdahl S. Congenital hypothyroidism: influence of disease severity and L‐thyroxine treatment on intellectual, motor and school‐associated outcomes in young adults. Pediatrics 2003;112:923‐30. [DOI] [PubMed] [Google Scholar]
Rovet 1995
- Rovet JF, Ehrlich RM. Long term effects of L‐thyroxine therapy for congenital hypothyrodism. Journal of Pediatrics 1995;126:380‐6. [DOI] [PubMed] [Google Scholar]
Rovet 2002
- Rovet JF. Congenital hypothyroidism: an analysis of persisting deficits and associated factors. Child Neuropsychology 2002;8:150‐62. [DOI] [PubMed] [Google Scholar]
Salerno 1999
- Salerno M, Militerni R, Maio S, Bravaccio C, Gasparini N, Tenore A. Intellectual outcome at 12 years of age in congenital hypothyroidism. European Journal of Endocrinology 1999;141(2):105‐10. [0804‐4643 (Print)] [DOI] [PubMed] [Google Scholar]
Simoneau‐Roy 2004
- Simoneau‐Roy J, Marti S, Deal C, huot C, Robaey P, Vliet G. Cognition and behaviour at school entry with children with congenital hypothyroidism treated early with high dose levothyroxine. J Pediatr 2004;144:747‐52. [DOI] [PubMed] [Google Scholar]
Song 2001
- Song SI, Daneman D, Rovet J. The influence of etiology and treatment factors on intellectual outcome in congenital hypothyroidism. Journal of Developmental Behavioural Pediatrics 2001;22(6):376‐84. [0196‐206X (Print)] [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Working group 1999
- Working group on neonatal screening of the European Society of Paediatric Endocrinology. Revised guidelines for neonatal screening programmes for primary congenital hypothyroidism. Horm Res 1999;52:49‐52. [DOI] [PubMed] [Google Scholar]


