Abstract
In this meta-analysis, we aimed to estimate and compare the efficacy of add-on treatment of antidepressants with esketamine nasal spray and second-generation antipsychotics in patients with nonpsychotic major depressive disorder and inadequate response to antidepressants. Searching for acute-phase, double-blind, placebo-controlled, randomized trials, we found 22 second-generation antipsychotic (n = 8363) and 3 intranasal esketamine (n = 641) studies. Mean change in the Montgomery Åsberg Depression Rating Scale total score served as outcome. We determined a higher mean difference (vs placebo) for the pooled esketamine nasal spray trials (mean difference = 4.09, 95% confidence interval: 2.01 to 6.17) than for the pooled second-generation antipsychotic augmentation trials (mean difference = 2.05, 95% confidence interval: 1.51 to 2.59). Thus, the effect size for intranasal esketamine was nearly twice as high as those for the second-generation antipsychotics. This indicates high efficacy of add-on esketamine nasal spray in treatment-resistant major depressive disorder compared with other well-established, evidence-based pharmacological options such as augmentation with second-generation antipsychotics.
Keywords: major depressive disorder, add-on treatment, esketamine nasal spray, second-generation antipsychotics, treatment resistance
Introduction
Add-on treatment with second-generation antipsychotics (SGAs) represents a well-established, evidence-based mainstay in the pharmacotherapy of treatment-resistant major depressive disorder (MDD) (Nelson and Papakostas, 2009; Blier, 2014; Zhou et al., 2015; Bauer et al., 2017). Moreover, some SGAs received the official approval for this indication by regulatory authorities (e.g., quetiapine XR [in the United States and Europe] and aripiprazole [in the United States] as adjunctive treatment of antidepressants, and olanzapine as fixed combination with fluoxetine in the United States). Accordingly, pharmacoepidemiological surveys consistently revealed a substantial increase of SGA prescriptions in MDD over the last decades (Konstantinidis et al., 2012; Dold et al., 2016; Halfdanarson et al., 2017). With esketamine, a new pharmacological compound characterized by a glutamatergic mechanism of action became most recently available to treat resistant MDD (Kraus et al., 2017). Meanwhile, the intranasal type of application of esketamine is officially licensed for this indication by the regulatory authorities in the United States and the European Union.
In our study, we aimed to categorize the efficacy of esketamine nasal spray. For this, it appears reasonable to compare the newly approved intranasal compound with SGAs, which represent up to now the first-line treatment option in resistant MDD. Moreover, both agents are (1) recommended to treat the same type of patients (MDD patients with inadequate response to antidepressants) and (2) both are administered as add-on medication to antidepressants. As no head-to-head randomized controlled trials (RCTs) are currently available, we sought to elucidate the evidence for both treatment strategies based on the findings of double-blind, placebo-controlled RCTs by comparing the effect sizes of esketamine nasal spray with those of the SGAs.
Methods
A detailed protocol of the applied methods is provided in the supplementary Material (Appendix 1).
Inclusion Criteria: Trial Design
We included all acute-phase, double-blind, placebo-controlled RCTs in which adult patients with nonpsychotic MDD received either add-on treatment of an antidepressant medication with SGAs or esketamine nasal spray in the intervention group (SGA/antidepressant or esketamine nasal spray/antidepressant) and add-on placebo in the control group (antidepressant/placebo). Further inclusion criteria comprised inadequate response to at least 1 antidepressant trial prior to randomization.
Search Strategy
We systematically screened without language restriction the electronic databases ClinicalTrials.gov, Clinicaltrialsregister.eu, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, PubMed/Medline, and PsycINFO for relevant publications (last search January 2020). Search terms were “major depressive disorder,” “depression,” and “antidepress*” together with 1 of the following phrases: “antipsychotic*,” “neurolept*,” “add-on,” “adjunctive,” “augmentation,” “amisulpride,” “aripiprazole,” “asenapine,” “brexpiprazole,” “cariprazine,” “clozapine,” “esketamine,” “iloperidone,” “olanzapine,” “lurasidone,” “paliperidone,” “pimozide,” “quetiapine,” “risperidone,” “sertindole,” “sulpiride,” and “ziprasidone.” In addition, we manually inspected the references of all included individual studies, related reviews, and treatment guidelines on this topic for further relevant publications.
Outcome Criteria
Mean change (from study baseline to endpoint) in the Montgomery Åsberg Depression Rating Scale (MADRS) total score (Montgomery and Asberg, 1979) served as outcome. If change data were not available, we considered mean values at study endpoint.
Study Selection and Data Extraction
Study selection and data extraction were independently performed by at least 2 reviewers and discrepancies were resolved through discussion. Intention-to-treat data were used whenever available. The workflow was elaborated following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement to ensure a standardized data collection procedure (Moher et al., 2009).
Statistical Analyses
As effect size, we calculated mean differences (MDs) with the 95% confidence intervals (CIs). This appears appropriate as we only used the MADRS total score as outcome. Therefore, no standardization of the effect size (i.e., calculation of the standardized mean difference) was necessary. Statistical significance was assumed if the 95% CI did not include the numerical value of 0.
To consider variability between the different individual RCTs, the random-effects model was applied to estimate the pooled continuous effect sizes (i.e., the mean difference). The degree of heterogeneity between the studies was explored statistically with I2 statistic and chi2 test of homogeneity (significance level: I2 > 50%). Significant heterogeneity was reported, and outlier studies were removed in post-hoc sensitivity analyses. The likelihood for the presence of a publication bias was investigated by funnel-plot visualization. All meta-analytic analyses were carried out with the software STATA Version 16.0 and Review Manager (RevMan) Version 5.3.
In addition to the meta-analytic calculations, we determined the MADRS total score reduction in the intervention and control groups separately employing descriptive statistics.
Risk of Bias Assessment
Every included RCT was rated independently by 2 reviewers with respect to its methodological quality by the “risk of bias” tool implemented by the Cochrane Collaboration (Higgins et al., 2019). This set of criteria contains a rating of sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and risk for other possible biases.
Results
Search Results and Characteristics of Included Studies
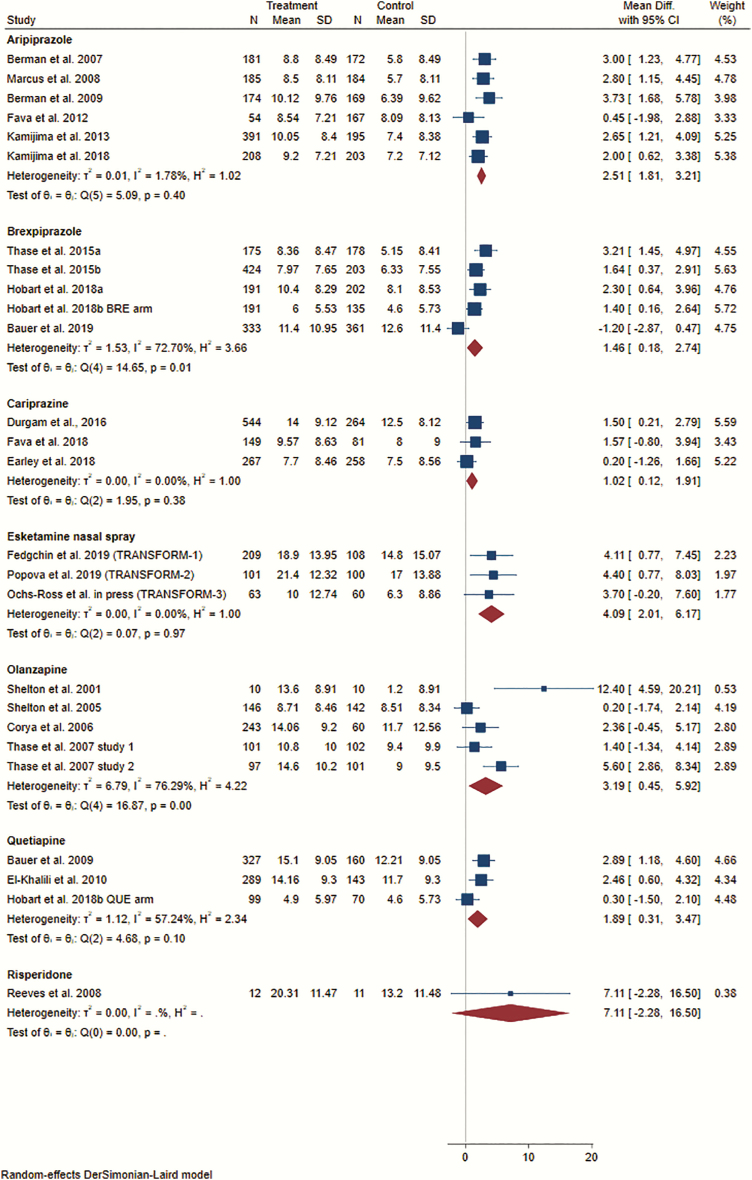
The systematic literature search yielded a total of 12 181 citations (without duplications), and finally, we were able to include 22 SGA augmentation trials (21 publications) with 23 relevant comparisons (n = 8363) and 3 intranasal esketamine RCTs (n = 641) (supplementary Appendix 3). Five RCTs (n = 691) were ultimately excluded because no MADRS data were available (details are provided in the supplementary Material). Supplementary Figure 1 illustrates the flow diagram of the literature search with a detailed description of the individual steps based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement requirements. The examined SGAs were aripiprazole (n = 6, n = 2284), brexpiprazole (n = 5, n = 2393), cariprazine (n = 3, n = 1563), olanzapine (n = 5, n = 1012), quetiapine (n = 3, n = 1088), and risperidone (n = 1, n = 23).
Risk of Bias Assessment
Supplementary Figures 2 and 3 illustrate graphically the single ratings for each item of the “risk of bias” tool. Briefly, all studies were clearly stated to be randomized, and 14 of the 25 included RCTs described an appropriate randomization procedure and 10 adequate concealment of allocation. The mechanism of blinding was sufficiently indicated in 13 trials for participants and personnel (performance bias) and in 10 studies for outcome assessment (detection bias). Overall attrition (incomplete outcome data) was low (<10%) in 7 studies, moderate (10–25%) in 16, and high (>25%) in 2 trials. With one exception, all studies appeared to be free of selective reporting.
Outcome: Mean MADRS Total Score Change
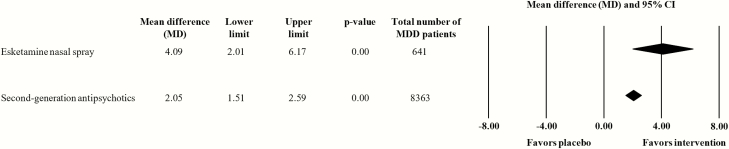
We determined a higher mean difference (vs antidepressant/placebo) for the pooled add-on esketamine nasal spray trials (n = 3, n = 641; MD = 4.09, 95% CI: 2.01 to 6.17) than for the pooled SGA augmentation trials (n = 23, n = 8363; MD = 2.05, 95% CI: 1.51 to 2.59) (Figure 1). This comparison was not accompanied by significant heterogeneity (I2 = 43.9%). Itemization according to the individual SGA/antidepressant medications revealed significant superiority over antidepressant/placebo for aripiprazole (n = 6, n = 2284; MD = 2.51, 95% CI 1.81 to 3.21), brexpiprazole (n = 5, n = 2393; MD = 1.46, 95% CI 0.18 to 2.74), cariprazine (n = 3, n = 1563; MD = 1.02, 95% CI 0.12 to 1.91), olanzapine (n = 5, n = 1012; MD = 3.19, 95% CI 0.45 to 5.92), and quetiapine (n = 3, n = 1088; MD = 1.89, 95% CI 0.31 to 3.47). Risperidone (n = 1, n = 23) failed to differentiate significantly from antidepressant/placebo (Figure 2). Visual inspection of the funnel plot did not provide any evidence for a potential publication bias (supplementary Figure 4).
Figure 1.
Effect sizes (mean differences) for the outcome mean change in Montgomery Åsberg Depression Rating Scale (MADRS) total score from baseline to study endpoint. Comparison: pooled esketamine nasal spray group vs placebo nasal spray group and pooled second-generation antipsychotic (SGA) group vs placebo group. Data synthesis: random-effects model. The forest plot illustrates the mean differences with the associated 95% confidence intervals (CIs). Numerical values greater than 0 indicate a larger MADRS total score reduction in the esketamine/SGA group than in the placebo group. Statistical significance can be assumed if the 95% CI does not comprise the numerical value of 0, and/or if the P-value of the comparison is <.05.
Figure 2.
Effect sizes (mean differences) for the outcome mean change in Montgomery Åsberg Depression Rating Scale (MADRS) total score from baseline to study endpoint. Comparison: esketamine nasal spray vs placebo nasal spray and second-generation antipsychotic (SGA) vs placebo. Data synthesis: random-effects model. The forest plot illustrates the mean differences with the associated 95% confidence intervals (CIs). Numerical values greater than 0 indicate a larger MADRS total score reduction in the esketamine/SGA group than in the placebo group. Statistical significance can be assumed if the 95% CI does not comprise the numerical value of 0.
When additionally analyzing the intervention and control groups separately using descriptive statistics, we estimated concerning the intervention groups a higher pooled mean reduction in the MADRS total score for esketamine nasal spray/antidepressant pharmacotherapy (−18.08 points; n = 3, n = 373) than for SGA/antidepressant medication (−10.72 points; n = 23, n = 4792). With respect to the control conditions, we found higher mean MADRS reduction in the antidepressant/placebo groups of the esketamine trials (−13.72 points; n = 3, n = 268) compared with the SGA studies (−8.45 points; n = 23, n = 3571).
Discussion
This meta-analysis comprising a total of 25 RCTs (26 relevant study arms) with altogether 9004 MDD patients revealed a higher mean difference for add-on pharmacotherapy with intranasal esketamine (4.09 MADRS total score points) compared with SGAs (2.05 points). The effect size for esketamine nasal spray/antidepressant treatment was nearly twice as high as those for SGA/antidepressant medication. Moreover, the mean MADRS difference of intranasal esketamine is much higher than the 2-point difference suggested by Montgomery and Moller (2009) to consider an antidepressant-placebo separation to be clinically meaningful.
Comparing the different effect sizes for add-on intranasal esketamine and SGA treatment, it should be considered that the large majority of SGA augmentation trials methodologically differ from those investigating esketamine. In the latter, MDD patients with insufficient response to antidepressant treatment were randomized to either esketamine nasal spray in the intervention group or placebo nasal spray in the control group. In addition, their current antidepressant medication was discontinued, and, instead, pharmacotherapy with a new antidepressant drug (duloxetine, escitalopram, sertraline, or venlafaxine XR) was initiated. Thus, a switch of the concurrently administered antidepressant drug was performed at the timepoint of the randomization. This was, however, not the case in most of the SGA augmentation trials. In these RCTs, patients were randomized to add-on SGAs or adjunctive placebo, but the augmented antidepressant medication remained unchanged during the double-blind study phase. Consequently, the mean reductions in the MADRS assessed for intranasal esketamine cannot be directly compared with those for add-on SGA treatment without reservation.
It can be assumed that due to the switch of the antidepressant drug in the esketamine studies, the probability for treatment response provoked by this measure meaningfully increased. Even if a switch to another, new antidepressant drug after insufficient response to the currently administered antidepressant cannot generally be regarded as evidence-based strategy in resistant MDD (Souery et al., 2011; Bauer et al., 2017; Dold and Kasper, 2017; Bartova et al., 2019), there is some evidence that at least some patients appear to benefit even from a within-class switch of the antidepressant compound (Papakostas et al., 2008). Hence, the antidepressant switch conducted in the intranasal esketamine trials represented active comparator treatment every participant received. In this context, it should be considered that investigations focusing on the mechanisms of placebo response in clinical trials revealed an association between high likelihood for receiving active treatment and increased response, mainly due to enhanced hope induction (Rutherford and Roose, 2013; Kasper and Dold, 2015). This increased response preferentially affects placebo groups rather than active treatment groups, resulting in a lower verum-placebo separation at study endpoint (Papakostas and Fava, 2009; Sinyor et al., 2010). Even if we assume that hope induction is preferentially relevant for receiving esketamine as it represents treatment with a completely new, glutamatergic mode of action, the antidepressant switch in the esketamine studies could, nevertheless, have provoked an additional expectation bias in study participants. On the contrary, in the SGA augmentation studies, the trial design minimized the likelihood for increased placebo response by maintaining the stable antidepressant medication during the double-blind study phase. Considering these methodological aspects, we anticipated a higher difficulty for esketamine nasal spray to achieve significant verum-placebo separation compared with the SGAs. In our descriptive analyses of the MADRS change in the intervention and control groups, we determined for the intervention groups a higher pooled reduction for intranasal esketamine than for the SGAs. In addition, we revealed higher MADRS reduction in the control groups of the esketamine trials compared with the SGA studies. The latter finding was in accordance with our assumption that the switch of the antidepressant at the beginning of the double-blind phase in the esketamine trials provoked higher MADRS improvement in the control group. Contrary to our assumption, however, increased treatment response was not limited to the antidepressant/placebo group.
Our finding of a higher effect size for intranasal esketamine than for the SGAs is especially remarkable against the background that achieving significant verum-placebo separation was more difficult for esketamine than for the SGAs due to the different study design evoking higher placebo response in the esketamine trials. Hence, the effect sizes in the intranasal esketamine studies indicate high efficacy of add-on esketamine in treatment-resistant MDD conditions compared with other well-established, evidence-based pharmacological options such as augmentation with SGAs.
Another methodological difference between the esketamine nasal spray and SGA trials probably influencing treatment response affects the higher frequency and intensity of the study visits in the intranasal esketamine trials (twice a week with sessions lasting 2–3 hours) compared with the large majority of SGA trials.
Several clinical and methodological limitations confining the conclusions of this meta-analysis should be considered. First, our meta-analysis exclusively evaluated the efficacy of the compounds. However, safety aspects should also be considered in individual drug choice, and an improvement in pure efficacy-related outcomes such as the MADRS total score is not necessarily accompanied by increased quality of life. A further limitation arises from the exclusion of 5 RCTs without MADRS assessments. In this meta-analysis, we have focused on the MADRS scale as it can be regarded as an appropriate measurement instrument to display the improvement in overall symptoms of depression. Moreover, change in this rating scale is intuitive to understand and served as primary outcome in most pharmacological trials within the field of MDD. This meta-analysis included RCTs with nonpsychotic MDD. In this context, it should be considered that SGAs are especially recommended if MDD is accompanied by psychotic symptoms (Bauer et al., 2017; Dold et al., 2018). Even if the symmetrical funnel plot did not provide any evidence for the existence of a publication bias, it should be taken into account that we cannot definitely rule out that some (particularly negative) study findings were not published and subsequently not covered by our systematic literature search.
Supplementary Material
Acknowledgments
The present study received no funding.
Statement of Interest
Dr Dold has received grants/honoraria from Medizin Medien Austria and Janssen. Dr Bartova has received travel grants and consultant/speaker honoraria from AOP Orphan, Medizin Medien Austria, Vertretungsnetz, Austroplant, and Janssen. Within the last 3 years, Dr Kasper has received grant/research support, consulting fees, and/or honoraria from Angelini, AOP Orphan Pharmaceuticals, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier.
References
- Bartova L, Dold M, Kautzky A, Fabbri C, Spies M, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, Schosser A, Kasper S (2019) Results of the European Group for the Study of Resistant Depression (GSRD) - basis for further research and clinical practice. World J Biol Psychiatry 20:427–448. [DOI] [PubMed] [Google Scholar]
- Bauer M, Severus E, Möller HJ, Young AH; WFSBP Task Force on Unipolar Depressive Disorders (2017) Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract 21:166–176. [DOI] [PubMed] [Google Scholar]
- Blier P. (2014) Rational site-directed pharmacotherapy for major depressive disorder. Int J Neuropsychopharmacol 17:997–1008. [DOI] [PubMed] [Google Scholar]
- Dold M, Bartova L, Kautzky A, Serretti A, Porcelli S, Souery D, Mendlewicz J, Montgomery S, Zohar J, Kasper S (2018) Clinical factors associated with augmentation treatment with second-generation antipsychotics and lithium in major depression - results from a European multicenter study. Eur Neuropsychopharmacol 28:1305–1313. [DOI] [PubMed] [Google Scholar]
- Dold M, Kasper S (2017) Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J Psychiatry Clin Pract 21:13–23. [DOI] [PubMed] [Google Scholar]
- Dold M, Kautzky A, Bartova L, Rabl U, Souery D, Mendlewicz J, Porcelli S, Serretti A, Zohar J, Montgomery S, Kasper S (2016) Pharmacological treatment strategies in unipolar depression in European tertiary psychiatric treatment centers - a pharmacoepidemiological cross-sectional multicenter study. Eur Neuropsychopharmacol 26:1960–1971. [DOI] [PubMed] [Google Scholar]
- Halfdanarson O, et al. (2017) International trends in antipsychotic use: a study in 16 countries, 2005-2014. Eur Neuropsychopharmacol 27:1064–1076. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). (2019) Cochrane handbook for systematic reviews of interventions version 6.0 [updated July 2019]. Cochrane Available from www.training.cochrane.org/handbook (accessed 3 February 2020). [DOI] [PMC free article] [PubMed]
- Kasper S, Dold M (2015) Factors contributing to the increasing placebo response in antidepressant trials. World Psychiatry 14:304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis A, Papageorgiou K, Grohmann R, Horvath A, Engel R, Kasper S (2012) Increase of antipsychotic medication in depressive inpatients from 2000 to 2007: results from the AMSP International Pharmacovigilance Program. Int J Neuropsychopharmacol 15:449–457. [DOI] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S (2017) Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Moller HJ (2009) Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol 24:111–118. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166:980–991. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fava M (2009) Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 19:34–40. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fava M, Thase ME (2008) Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry 63:699–704. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Roose SP (2013) A model of placebo response in antidepressant clinical trials. Am J Psychiatry 170:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y, Lanctôt KL (2010) Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry 71:270–279. [DOI] [PubMed] [Google Scholar]
- Souery D, Serretti A, Calati R, Oswald P, Massat I, Konstantinidis A, Linotte S, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Zohar J, Mendlewicz J (2011) Switching antidepressant class does not improve response or remission in treatment-resistant depression. J Clin Psychopharmacol 31:512–516. [DOI] [PubMed] [Google Scholar]
- Zhou XP, Keitner GIP, Qin BM, Ravindran AVP, Bauer MP, Del Giovane CP, Zhao JP, Liu YM, Fang YP, Zhang YP, Xie P (2015) Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol 25:pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.