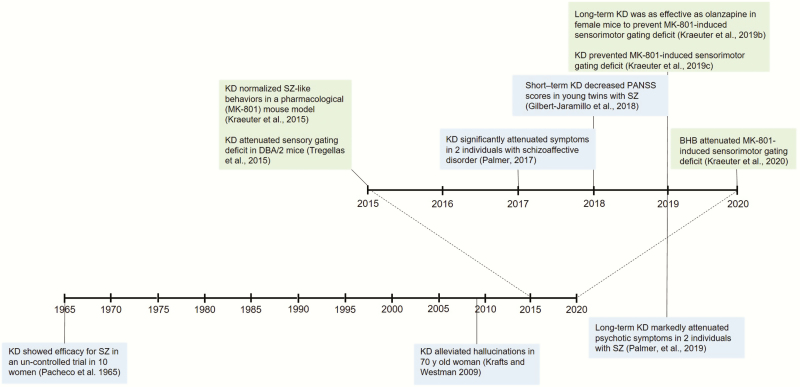
We have read the recent review article on the potential application of induced ketosis in psychiatry by Morris et al. (Morris et al., 2020) with great interest and shared enthusiasm. It is quite encouraging to see that others are recognizing the exciting potential of the ketogenic diet and other ketogenic therapies in the treatment of serious mental disorders. As with all research, however, it is important to be aware of what existing evidence is already accumulated. This is particularly pertinent as such published evidence further strengthens the case for induced ketosis in psychiatry proposed as “food for thought” (Morris et al., 2020). In this Commentary, we aim to fill this gap by providing a brief overview of the published preclinical and clinical evidence that clearly supports the advancement of ketogenic therapies in a variety of psychiatric disorders, especially in psychosis (Figure 1).
Figure 1.
Development of the use of ketogenic therapy in the treatment of schizophrenia: timeline.
The ketogenic diet is typically a high-fat, moderate-protein, and very low–carbohydrate diet. Ratios of fats to protein and carbohydrate are sometimes prescribed, typically in a 3:1 or 4:1 ratio. This results in 3 or 4 g of fat being consumed for every 1 g of carbohydrate or protein. Exogenous ketone supplements are also being studied to determine if these can mimic some of the effects of the ketogenic diet without requiring strict adherence to the diet.
Although preclinical animal models are unable to capture the full pathophysiology and symptomatology of psychotic disorders, converging evidence from pharmacological and genetic models have provided proof-of-concept level results supporting the potential therapeutic utility of ketogenic diet (Table 1). Taking advantage of the leading mechanistic hypothesis of schizophrenia, the NMDA receptor hypofunction (Jentsch and Roth, 1999; Coyle et al., 2012), pharmacological inhibition of the NMDA-type glutamate receptors by dizocilpine (MK-810) was used to induce a behavioral profile in rodents that captures a wide spectrum of the schizophrenia-like phenotype (Neill et al., 2014; Cadinu et al., 2018). A high-fat, very low–carbohydrate ketogenic diet was provided for 3 weeks, which resulted in metabolic features, such as elevated beta-hydroxybutyrate and decreased glucose levels in the plasma and decreased body weight, that correspond with nutritional ketosis (Kraeuter et al., 2015). The ketogenic diet prevented the schizophrenia-like abnormal behaviors induced by acute MK-801 administration, including hyperactivity, stereotyped behavior, decreased sociability, working memory deficit, and impaired pre-pulse inhibition of startle in male mice (Kraeuter et al., 2015, 2019c). Further evidence has been provided by the use of the DBA/2 mice, an inbred mouse strain, that have been proposed to exhibit a variety of behavioral and metabolic features that resemble those of schizophrenia (Olivier et al., 2001; Sarnyai et al., 2015). The effect of ketogenic diet was investigated on hippocampal P20/N40 gating in DBA/2 mice, a translational endophenotype that mirrors inhibitory deficits in P50 sensory gating in schizophrenia patients (Tregellas et al., 2015). Animals with the highest blood ketone levels showed the lowest P20/N40 gating ratios, indicating that a ketogenic diet normalizes sensory gating deficits (Tregellas et al., 2015), which are conceptualized as fundamental in the development of hallucinatory episodes in persons with schizophrenia (Javitt and Freedman, 2015). Furthermore, it was recently shown that the therapeutic effects of a ketogenic diet in the context of the NMDA-receptor hypofunction model can be generalized to female mice, and they are similar but nonadditive to the effect of olanzapine (Kraeuter et al., 2019b) and can be mimicked by chronic administration of the main circulating ketone body, beta-hydroxybutyrate (Kraeuter et al., 2020). The above strong, converging evidence for the efficacy of ketogenic therapies from pharmacological and genetic animal models paves the way for the introduction of this nutritional/metabolic approach to the clinical management of psychiatric disorders (Kraeuter et al., 2019a; Palmer, 2019a).
Table 1.
Preclinical Studies on the Effects of KD on Animal Models of Schizophrenia
| Reference (author and journal) | Animals | Model | Schizophrenia-like behaviors/endophenotype | Findings |
|---|---|---|---|---|
| Kraeuter et al., 2015 Schizophrenia Res. | C57Bl/6 mice (males) | Std and KDa (3 wk) MK-801–induced (0.1, 0.2, and 0.4 mg/kg) hypo-glutamatergic state | Locomotor hyperactivity Stereotyped behaviors Ataxia Decreased sociability Spatial working-memory impairment | KD prevented MK-801–induced behavioral abnormalities |
| Tregellas et al., 2015 Schizophrenia Res. | DBA/2 mice | KDb only Auditory gating deficit in DBA/2 mice | Hippocampal P20/N40 gating in DBA/2 mice | Animals with highest blood ketone levels showed lowest P20/N40 gating ratios |
| Kraeuter et al., 2019c Schizophrenia Res. | C57Bl/6 mice (males) | Std and KDa (7 wk) MK-801–induced (0.25 mg/kg) hypo-glutamatergic state | Sensorimotor gating deficit (prepulse inhibition of startle) | KD prevented MK-801–induced sensorimotor gating deficit (independent of decreased caloric intake related to KD) |
| Kraeuter et al., 2019b Schizophrenia Res. | C57Bl/6 mice (females) | SD and KDa (6 mo) Olanzapine (2 mg/kg, 8 wk) MK-801–induced (0.25 mg/kg) hypo-glutamatergic state | Sensorimotor gating deficit (prepulse inhibition of startle) | Long-term KD and chronic olanzapine prevented MK-801–induced sensorimotor gating deficit in female mice No interaction between KD and olanzapine |
| Kraeuter et al., 2020 Psychopharmacology | C57Bl/6 mice (males) | BHB (3 wk) MK-801–induced (0.25 mg/kg) hypo-glutamatergic state | Sensorimotor gating deficit (prepulse inhibition of startle) | BHB prevented MK-801–induced sensorimotor gating deficit |
Abbreviations: BHB, beta-hydroxybutyrate; KD, ketogenic diet; Std, standard diet.
aKetogenic diet composition: carbohydrates: 9.4%, protein: 9.5%, fat: 77.6%.
bKD composition: carbohydrates: 0.6%, protein: 22.4%, fat: 77%.
The first suggestive clinical evidence for the potential efficacy of the ketogenic diet in psychotic disorders emerged 55 years ago in a small, open-label, uncontrolled trial in 10 women hospitalized with schizophrenia. The ketogenic diet was added to their existing treatment for 4 weeks. The researchers reported a significant decrease in symptoms after 2 weeks on the diet (Pacheco et al., 1965).
In 2009, a 70-year-old woman with chronic schizophrenia since her teens was reported to have improved significantly after starting a ketogenic diet for weight loss (Kraft and Westman, 2009). Within 8 days of starting the diet, she reported no hallucinations and improved energy. After 1 year, she lost 5 kg and remained free of hallucinations.
More rigorous and longer term case studies have been conducted (Table 2) in different populations by one of us (C.M.P.) and have provided encouraging results both in terms of symptom control and safety/tolerability (Palmer, 2017, 2019b; Gilbert-Jaramillo et al., 2018), which we summarized recently (Sarnyai et al., 2019).
Table 2.
Summary of Case Studies With KD in Schizophrenia
| Case | Gender and age | Primary diagnosis | Other psychiatric diagnoses | Medication | KD | Psychiatric symptoms (PANSS) | Metabolic features | Comments |
|---|---|---|---|---|---|---|---|---|
| Case #1 (63) | Male, 33 y | Schizoaffective disorder | ADHD, MDD | History: methylphenidate, amphetamine salts, dextroamphetamine, bupropion, sertraline, paroxetine, buspirone, lamotrigine, lorazepam, clonazepam, gabapentin, haloperidol, perphenazine, aripiprazole, olanzapine, quetiapine, and clozapine; lack of symptom control | Coffee with MCT oil and butter (“bulletproof coffee”), eggs, meat, fish, poultry, spinach, kale, and olive oil for 3 wk; ketosis measured by urine strips | Before KD: Total = 98 Positive = 27 Negative = 25 General = 46 After KD: Total = 49 Positive = 13 Negative = 8 General = 28 Symptoms worsened within 1–2 d after breaking KD | Before KD: 146 kg After KD: 98.9 kg [Weight loss of 47.2 kg after 1 y] | Completed certification course, successfully participates in online college program, has friends, began dating, lives independently |
| Case #2 (63) | Female, 31 y | Schizoaffective disorder | MDD, anorexia nervosa | History: sertraline, bupropion, amphetamine salts, lorazepam, lamotrigine, divalproex, topiramate, risperidone, aripiprazole, quetiapine, olanzapine, and clozapine; 23 ECT; lack of symptom control | Coffee, eggs, poultry, and lettuce for 4 mo | Before KD: Total = 107 Positive = 24 Negative = 29 General = 54 After KD: Total = 70 Positive = 15 Negative = 18 General = 38 Severe paranoia and delusion after breaking KD but improvement after a 3-d fast | Weight loss of 13.6 kg after 4 mo | Symptom control on previously insufficient dose of aripiprazole |
| Case #3 (64) | Female, 22 y (twin of case #4) | Schizophrenia (diagnosed at age 14) | None | For 5 mo before study: clozapine (300 mg), risperidone (6 mg), clonazepam (3 mg), and biperiden (6 mg) | 3:1 ratio KD plan set to standard 2000 kcal/d and mainly consisted of avocado, olive oil, butter, eggs, cheese, meat, spinach, and broccoli; for 15 d Ketosis determined by daily urine analysis | Before KD: Total = 101 Positive = 28 Negative = 16 General = 57 After KD: Total = 91 Positive = 26 Negative = 15 General = 50 | Before KD: BMI = 21.3 kg/m2 Body fat = 24.5% After KD: BMI = 19.8 kg/m2 Body fat = 19.8% | Blood clinical analysis remained unremarkable after KD |
| Case #4 (64) | Male, 22 y (twin of case #4) | Schizophrenia (diagnosed at age 18) | None | For 23 mo before study: levomepromazine (150 mg), quetiapine (100 mg), valproic acid (1000 mg), biperiden (6 mg), and risperidone (4 mg) | Same as case #3 | Before KD: Total = 82 Positive = 19 Negative = 18 General = 45 After KD: Total = 75 Positive = 16 Negative = 17 General = 42 | Before KD: BMI = 25.1 kg/m2 Body fat = 21.7% After KD: BMI = 22.9 kg/m2 Body fat = 16.8% | Blood clinical analysis remained unremarkable after KD |
| Case #5 (65) | Female, 82 y (same as in Ref. 62) | Schizophrenia (diagnosed at age 17) | None | Before start of KD in 2008: haldol-decanoate, risperidone, atenolol, furosemide, trazodone, and sertraline [Before 2008: lithium, olanzapine, ziprasidone, aripiprazole, lamotrigine, quetiapine, haloperidol, perphenazine, and risperidone] | Patient-directed KD initiated in 2008, maintains KD until today | Before KD: Chronic paranoia, disorganized speech, and both visual and auditory hallucinations, suicide attempts After KD: Marked reduction in psychotic symptoms; stopped taking all medication, hallucinations and paranoia remitted completely, improved mood, no suicidal ideations | Before KD: 150 kg After KD: 82 kg [Weight loss of 68 kg] | Regained her independence, no longer need a guardian |
| Case #6 (65) | Female, 39 y | Schizophrenia (hallucinations and paranoia started at age 14; diagnosed at age 24) | MDD, anxiety, anorexia nervosa | Haloperidol, clozapine, ziprasidone, risperidone, quetiapine, aripiprazole, olanzapine, sertraline, paroxetine, citalopram, fluoxetine, duloxetine, and venlafaxine | KD started in 2003 by functional medicine practitioner for symptoms of chronic gastrointestinal distress | Remained free of psychotic symptoms for past 5 y off antipsychotic medications | Weight loss of 32 kg, anorexia exacerbated, regained 13.6 kg, currently at normal body weight | Finished graduate school and works full time |
Abbreviations: ADHD, attention deficit hyperactivity disorder; ECT, electroconvulsive therapy; KD, ketogenic diet; MCT, medium chain triglyceride; MDD, major depressive disorder; PANSS, Positive and Negative Symptoms Scale.
Summary of case studies with ketogenic diet in schizophrenia. Reprinted with permission by Wolters Kluwer from Sarnyai et al., 2019.
In the first case studies (Palmer, 2017), 2 patients with longstanding, treatment-resistant schizoaffective disorder started the ketogenic diet for weight loss. Within 2 months of starting the diet, both patients experienced significant reductions in psychotic symptoms as measured by the Positive and Negative Symptom Scale. Both patients either purposely or inadvertently stopped the ketogenic diet, and their psychotic symptoms returned quickly. When they resumed the diet, the symptoms abated again, suggesting an “on/off” effect of this dietary intervention similar to a medication. In addition to improvements in psychotic symptoms, both patients lost significant amounts of weight, with one losing 30 pounds in 4 months and the other losing 104 pounds over a 1-year period. Given the high rates of obesity, diabetes, cardiovascular disease, and premature death in people with schizophrenia and schizoaffective disorder (Olfson et al., 2015), these weight loss effects add to the therapeutic value of the ketogenic diet.
Two additional case studies (Palmer et al, 2019b) highlight the long-term potential of the ketogenic diet as a sustainable treatment that can induce full remission of psychotic symptoms, at least in some people. The first case was a 12-year follow-up of the 70-year-old woman first described by Kraft and Westman (Kraft and Westman, 2009). At age 82, this woman who suffered from treatment-resistant schizophrenia for 53 years remained alive and well on the ketogenic diet. She was off all psychotropic medications for 11 years, including antipsychotic medications, and remained free of psychotic symptoms. She lost 150 pounds over this 12-year period. Additionally, she no longer needed a court-appointed guardian or Program of Assertive Community Treatment (PACT) team and was thriving independently. The second case was a 39-year-old woman who had suffered from treatment-resistant psychotic symptoms for 20 years. She started the ketogenic diet at the instruction of a medical practitioner and, after several months on the diet, experienced complete remission of her psychotic symptoms. She continued the diet and lost 70 pounds. She was also able to stop antipsychotic medications and remain free of psychotic symptoms for 5 years on the ketogenic diet. Her ability to function in society has been greatly improved. She completed graduate school and now works full time while continuing the ketogenic diet.
These encouraging preclinical data and the emerging evidence from recent case studies certainly support our enthusiasm about the application of ketogenic therapies in serious mental illness, including psychosis (Sarnyai et al., 2019). It is intriguing to speculate about the variety of mechanisms, including the ones reviewed by Morris et al (Morris et al., 2020), as well as others such as mitochondrial Complex 1 and oxidative phosphorylation deficits (Ben-Shachar, 2017; Ni et al., 2019) and the role of the gut microbiome (Olson et al., 2018; Zhu et al., 2020) through which ketogenic therapies may exert their effect. However, randomized controlled clinical trials are needed to demonstrate the efficacy as well as the safety of this proposed novel treatment approach.
Acknowledgments
None.
Statement of Interest
None.
References
- Ben-Shachar D. (2017) Mitochondrial multifaceted dysfunction in schizophrenia; complex I as a possible pathological target. Schizophr Res 187:3–10. [DOI] [PubMed] [Google Scholar]
- Cadinu D, Grayson B, Podda G, Harte MK, Doostdar N, Neill JC (2018) NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology 142:41–62. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G (2012) Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol 213:267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Jaramillo J, Vargas-Pico D, Espinosa-Mendoza T, Falk S, Llanos-Fernandez K, Guerrero-Haro J, Orellana-Roman C, Poveda-Loor C, Valdevila-Figueira J, Palmer C (2018) The effects of the ketogenic diet on psychiatric symptomatology, weight and metabolic dysfunction in schizophrenia patients. Clin Nutr Metab 1: doi: 10.15761/CNM.1000105. [DOI] [Google Scholar]
- Javitt DC, Freedman R (2015) Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH (1999) The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Loxton H, Lima BC, Rudd D, Sarnyai Z (2015) Ketogenic diet reverses behavioral abnormalities in an acute NMDA receptor hypofunction model of schizophrenia. Schizophr Res 169:491–493. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Guest PC, Sarnyai Z (2019a) The therapeutic potential of ketogenic diet throughout life: focus on metabolic, neurodevelopmental and neurodegenerative disorders. Adv Exp Med Biol 1178:77–101. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Archambault N, Van Den Buuse M, Sarnyai Z (2019b) Ketogenic diet and olanzapine treatment alone and in combination reduce a pharmacologically-induced prepulse inhibition deficit in female mice. Schizophr Res 212:221–224. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Van Den Buuse M, Sarnyai Z (2019c) Ketogenic diet prevents impaired prepulse inhibition of startle in an acute NMDA receptor hypofunction model of schizophrenia. Schizophr Res 206:244–250. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Mashavave T, Suvarna A, Van Den Buuse M, Sarnyai Z (2020) Effects of beta-hydroxybutyrate administration on MK-801-induced schizophrenia-like behaviour in mice. Psychopharmacology. doi: 10.1007/s00213-020-05467-2. [DOI] [PubMed] [Google Scholar]
- Kraft BD, Westman EC (2009) Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab (Lond) 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Puri BK, Carvalho A, Maes M, Berk M, Ruusunen A, Olive L (2020) Induced ketosis as a treatment for neuroprogressive disorders: food for thought? Int J Neuropsychopharmacol pyaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM (2014) Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol 24:822–835. [DOI] [PubMed] [Google Scholar]
- Ni P, Noh H, Park GH, Shao Z, Guan Y, Park JM, Yu S, Park JS, Coyle JT, Weinberger DR, Straub RE, Cohen BM, Mcphie DL, YIN C, Huang W, Kim HY, Chung S (2019) iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Mol Psychiatry. doi: 10.1038/s41380-019-0423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS (2015) Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 72:1172–1181. [DOI] [PubMed] [Google Scholar]
- Olivier B, Leahy C, Mullen T, Paylor R, Groppi V, Sarnyai Z, Brunner D (2001) The DBA/2J strain and prepulse inhibition of startle: a model system to test antipsychotics? Psychopharmacology 156:284–290. [DOI] [PubMed] [Google Scholar]
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173:1728–1741.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A, Easterling WS, Pryer MW (1965) A pilot study of the ketogenic diet in schizophrenia. Am J Psychiatry 121:1110–1111. [DOI] [PubMed] [Google Scholar]
- Palmer CM. (2017) Ketogenic diet in the treatment of schizoaffective disorder: two case studies. Schizophr Res 189:208–209. [DOI] [PubMed] [Google Scholar]
- Palmer CM. (2019a) Diets and disorders: can foods or fasting be considered psychopharmacologic therapies? J Clin Psychiatry 81:19ac12727. [DOI] [PubMed] [Google Scholar]
- Palmer CM, Gilbert-Jaramillo J, Westman EC (2019b) The ketogenic diet and remission of psychotic symptoms in schizophrenia: two case studies. Schizophr Res 208:439–440. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Jashar C, Olivier B (2015) Modeling combined schizophrenia-related behavioral and metabolic phenotypes in rodents. Behav Brain Res 276:130–142. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kraeuter AK, Palmer CM (2019) Ketogenic diet for schizophrenia: clinical implication. Curr Opin Psychiatry 32:394–401. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Legget KT, Stevens KE (2015) Effects of a ketogenic diet on auditory gating in DBA/2 mice: a proof-of-concept study. Schizophr Res 169:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Ju Y, Wang W, Wang Q, Guo R, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, Jie Z, Zhao B, Xiao L, Yang L, Zhang T, Feng J, Guo L, He X, Chen Y, Chen C, Gao C, Xu X, Yang H, Wang J, Dang Y, Madsen L, Brix S, Kristiansen K, Jia H, Ma X(2020) Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun 11:1612. [DOI] [PMC free article] [PubMed] [Google Scholar]