Abstract
Background
Ketamine has rapid-acting antidepressant effects but is associated with psychotomimetic and other adverse effects. A 7-chlorokynurenic acid is a potent and specific glycine site N-methyl-d-aspartate receptor antagonist but crosses the blood-brain barrier inefficiently. Its prodrug, L-4-chlorokynurenine (4-Cl-KYN), exerts acute and sustained antidepressant-like effects in rodents and has no reported psychotomimetic effects in either rodents or healthy volunteers. This study examined whether 4-Cl-KYN has rapid antidepressant effects in individuals with treatment-resistant depression.
Methods
After a 2-week drug-free period, 19 participants with treatment-resistant depression were randomized to receive daily oral doses of 4-Cl-KYN monotherapy (1080 mg/d for 7 days, then 1440 mg/d for 7 days) or placebo for 14 days in a randomized, placebo-controlled, double-blind, crossover manner. The primary outcome measure was the Hamilton Depression Rating Scale score, assessed at several time points over a 2-week period; secondary outcome measures included additional rating scale scores. Pharmacokinetic measures of 7-chlorokynurenic acid and 4-Cl-KYN and pharmacodynamic assessments were obtained longitudinally and included 1H-magnetic resonance spectroscopy brain glutamate levels, resting-state functional magnetic resonance imaging, and plasma and cerebrospinal fluid measures of kynurenine metabolites and neurotrophic factors.
Results
Linear mixed models detected no treatment effects, as assessed by primary and secondary outcome measures. No difference was observed for any of the peripheral or central biological indices or for adverse effects at any time between groups. A 4-Cl-KYN was safe and well-tolerated, with generally minimal associated adverse events.
Conclusions
In this small crossover trial, 4-Cl-KYN monotherapy exerted no antidepressant effects at the doses and treatment duration studied.
ClinicalTrials.gov identifier: NCT02484456.
Keywords: 4-Chlorokynurenine, depression, glutamate, glycine site antagonist, NMDA receptor
Significance Statement.
N-Methyl-D-aspartate receptor (NMDAR) antagonism has been implicated as the mechanism underlying ketamine’s rapid-acting antidepressant effects. The prodrug 4-Cl-kynurenine (4-Cl-KYN) converts to the NMDAR glycine site antagonist 7-Cl-kynurenic acid (7-Cl-KYNA). In animal models, 4-Cl-KYN demonstrated antidepressant effects without the dissociative side effects associated with ketamine. This randomized, placebo-controlled, crossover trial examined the antidepressant effects of 4-Cl-KYN vs placebo in 19 individuals with treatment-resistant depression and found that a 2-week trial of 4-Cl-KYN did not improve overall depressive symptomatology compared with placebo. Similarly, 4-Cl-KYN had no effects on various biological measures from baseline, including pharmacokinetic and pharmacodynamic measures of 7-Cl-KYNA and 4-Cl-KYN in plasma and cerebrospinal fluid, 1H-magnetic resonance spectroscopy (MRS) brain glutamate levels, and resting-state functional magnetic resonance imaging (rsfMRI). 4-Cl-KYN did not demonstrate antidepressant efficacy in this treatment-resistant sample. However, it is possible that CNS concentrations of the active drug, 7-Cl-KYNA, were insufficient to produce the desired effect.
Introduction
Current standard monoaminergic-based pharmacological approaches for treating major depressive disorder (MDD) are only modestly effective during acute depressive episodes and have a considerable lag of onset of action (Murrough et al., 2017; Park and Zarate, 2019). Modulation of the N-methyl-d-aspartate receptor (NMDAR) complex or other mechanisms of glutamatergic signaling may improve depressive symptoms and related constructs/dimensions of observable behavior and antidepressant response (Abdallah et al., 2018; Kadriu et al., 2019). Subanesthetic doses of ketamine have demonstrated rapid antidepressant effects that manifest within a few hours in individuals with treatment-resistant unipolar MDD (Berman et al., 2000; Zarate et al., 2006; aan het Rot et al., 2010; Murrough et al., 2013) and bipolar depression (Diazgranados et al., 2010; Zarate et al., 2012), a process proposed to occur via NMDAR inhibition (Trullas and Skolnick, 1990). These clinical studies have led to renewed optimism for the pharmacological treatment of depression, raising expectations for developing therapies with rapid response rates and greater efficacy. However, ketamine is also associated with psychotomimetic adverse effects and abuse potential, which has led to the study of its enantiomers and metabolites as a potential source of safer and better tolerated versions of this agent (Zanos et al., 2016, 2018). Esketamine nasal spray (Spravato) was recently approved by the FDA as an adjunctive therapy for treatment-resistant depression (TRD), but this agent shares all of ketamine’s aforementioned liabilities (Molero et al., 2018). Recent preclinical investigations, including comprehensive mechanistic cellular and molecular studies, support efforts to develop novel treatment approaches that robustly improve depressive symptoms within hours instead of weeks without ketamine’s most concerning adverse effects (Abdallah et al., 2018; Gould et al., 2019).
One proposed mechanism for such agents is inhibiting NMDAR function by targeting the NMDAR glycine site. NMDAR activity requires binding of glycine or D-serine to an obligatory co-agonist (“glycine”) site, which is located on the GluN1 subunit (Leeson and Iversen, 1994; Danysz and Parsons, 1998). The efficacy of NMDAR glycine receptor blockade by a full antagonist has been demonstrated in mouse tests sensitive to selective serotonin reuptake inhibition (Przegalinski et al., 1998; Poleszak et al., 2007). In addition, compared with classic NMDAR channel blocking antagonists such as ketamine, glycine site antagonists displayed a much safer profile in animal testing. Specifically, these agents do not induce behavioral arousal or neuropathological or hemodynamic changes, thus potentially reducing the clinical risks associated with conventional NMDAR antagonism, including motor deficits (Leeson and Iversen, 1994; Parsons et al., 1997; Wu et al., 1997; Zanos et al., 2015). Consistent with the possible antidepressant actions of NMDAR glycine site antagonists in humans, 1 clinical study found that adjunctive treatment with the broad spectrum antibiotic D-cycloserine (DCS; 1000 mg) had antidepressant effects in individuals with TRD (Heresco-Levy et al., 2013); at such high doses, DCS may function as a net NMDAR antagonist via the glycine site (Emmett et al., 1991).
L-4-chlorokynurenine (4-Cl-KYN) was developed as a prodrug that is rapidly converted in vivo to its active metabolite 7-Cl-kynurenic acid (7-Cl-KYNA), a well-characterized NMDAR antagonist at the glycine site (Wallace et al., 2017). 7-Cl-KYNA is potent (Kemp et al., 1988) and selective (Zanos et al., 2015) but does not readily cross the blood-brain barrier in rodents, although its bioprecursor 4-Cl-KYN does so rapidly (Zanos et al., 2015; Yaksh et al., 2017). In the brain, 4-Cl-KYN is accumulated by astrocytes and converted enzymatically to 7-Cl-KYNA (Hokari et al., 1996). This conversion is catalyzed predominantly by kynurenine aminotransferase II, the major enzyme responsible for kynurenic acid metabolism in the human brain (Guidetti et al., 2007). 7-Cl-KYNA is thought to be released extracellularly and to inhibit NMDAR activity by targeting the NMDAR glycine site (Kemp et al., 1988).
In mice, a single 4-Cl-KYN administration exerted rapid, dose-dependent, and persistent antidepressant-like effects similar to those of ketamine; these were abolished by pretreatment with the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid antagonist NBQX (Zanos et al., 2015). In rodent models, 4-Cl-KYN administration was not associated with ketamine’s abuse potential or psychotomimetic-like effects and did not induce locomotor sensitization or stereotypic behaviors (Zanos et al., 2015).
In humans, 4-Cl-KYN is readily absorbed when administered orally (Wallace et al., 2017). Studies found that mean half-life values were consistent across doses tested, ranging from 1.64 to 1.82 hours. Mean corresponding 4-Cl-KYN peak time values increased with increasing dose level, reaching nearly 2 hours for the highest dose group (1440 mg). The 4-Cl-KYN mean maximum concentration (Cmax) reached 64.4 μg/mL (Wallace et al., 2017). In addition, 4-Cl-KYN did not inhibit or induce human cytochrome P450 isoforms (Fleischer, 2008, unpublished data) and had no significant binding to off-site targets as identified by receptor screening (Zanos et al., 2015). Phase 1 safety and tolerability studies in humans found that 2 weeks of 4-Cl-KYN up to a dose of 1440 mg/d had a side effect profile similar to placebo, with no evidence of psychotomimetic effects (Wallace et al., 2017).
The present study assessed whether oral administration of 4-Cl-KYN would convert to 7-Cl-KYNA and result in rapid antidepressant effects in TRD participants. Given the favorable preclinical efficacy data associated with 7-Cl-KYNA, the primary hypothesis was that TRD participants treated with 4-Cl-KYN (1080 mg/d for 7 days followed by 1440 mg/d for 7 days) would experience a greater and more rapid decrease in depressive symptoms than those receiving placebo for 14 consecutive days. Pharmacokinetic measures of 7-Cl-KYNA, 4-Cl-KYN, and 4-chloro-3-hydroxyanthranilic acid were collected. Pharmacodynamic assessments were obtained longitudinally and included 1H-magnetic resonance spectroscopy (MRS) to measure brain glutamate levels, resting-state functional magnetic resonance imaging (rsfMRI) to investigate changes in connectivity between brain regions, and plasma and cerebrospinal fluid (CSF) measures of kynurenine metabolites and neurotrophic factors as putative biomarkers of antidepressant response and drug effects. Finally, to compare exposure levels in humans with those previously found to be effective in mice, 4-Cl-KYN was administered to mice with subsequent assessment of brain and plasma concentrations.
Methods
Participants
Participants were 19 men and women, 18 to 65 years old (demographic characteristics appear in Table 1). All were inpatients at the National Institute of Mental Health with a diagnosis of MDD and currently depressed without psychotic features. Participants were required to be experiencing a current major depressive episode lasting at least 4 weeks and have a current or past history of lack of antidepressant response to at least 1 adequate antidepressant trial. A minimum score of ≥18 on the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) at screening and before starting study medication was required for study participation. The study was approved by the Combined Neuroscience IRB at the National Institutes of Health. All participants provided written informed consent before entry into the study. A full description of inclusion/exclusion criteria and other characteristics of the participant sample can be found in the Supplemental Materials.
Table 1.
Demographic and Clinical Characteristics of the Participant Sample (n = 19)
| Mean | SD | |
|---|---|---|
| Age (y) | 41.28 | 11.8 |
| Age of onset (y) | 14.5 | 7.2 |
| Length of current episode (y) | 8.6 | 10.97 |
| Antidepressant trials (lifetime) | 7.8 | 5.67 |
| Antidepressant trials (current episode) | 2.6 | 5.06 |
| Body mass index | 25.67 | 2.86 |
| n | % | |
| Sex (male) | 10 | 52.6 |
| Number of lifetime episodes | ||
| • 1 | 4 | 21 |
| • 2 | 1 | 5 |
| • 3 or more | 9 | 47 |
| • More than 1 but count unclear | 5 | 26 |
| Race | ||
| • Black or African American | 1 | 5 |
| • Multiple races | 1 | 5 |
| • White | 17 | 89 |
| Marital status | ||
| • Married | 5 | 26 |
| • Separated | 1 | 5 |
| • Single | 12 | 63 |
| • Single/divorced | 1 | 5 |
| Education | ||
| • Graduate school | 6 | 32 |
| • College graduate | 7 | 37 |
| • Some college | 5 | 26 |
| • GED | 1 | 5 |
| • Disability | 1 | 6* |
| • Unemployed | 11 | 58 |
| Family history | ||
| • Mood disorder | 15 | 79 |
| • Alcohol abuse or dependence | 1 | 5 |
| Lifetime diagnosis | ||
| • Alcohol abuse or dependence | 5 | 26 |
| • Substance abuse or dependence | 4 | 21 |
| • Anxiety disorder | 17 | 89 |
| • Suicide attempt | 4 | 21 |
| Depression subtype | ||
| • Atypical | 5 | 26 |
| • Melancholic | 10 | 53 |
| • Both | 2 | 11 |
| • Neither | 5 | 26 |
A priori power calculations indicated that a sample size of 20 participants was required to detect a treatment effect size of Cohen’s d = 0.67 with 81% power (alpha = .05, 2-tailed).
Study Design
This single-center, double-blind, randomized, crossover, placebo-controlled study was conducted to assess the efficacy and safety of daily doses of 4-Cl-KYN monotherapy in individuals with TRD. During the entirety of the study, participants were not allowed to receive any other psychotropic medications (including benzodiazepines) or structured psychotherapy. Electrocardiogram, complete blood counts, electrolyte panels, and liver function tests were obtained at baseline and at the end of the study.
After consenting to the study, participants were tapered off their psychotropic medications while remaining on the research unit, then underwent a 2-week drug-free period (5 weeks for fluoxetine and aripiprazole). In phase 1 of the study, participants were blindly randomized to receive an oral daily dose of 4-Cl-KYN (1080 mg/d for 7 days followed by 1440 mg/d for 7 days) or placebo for 14 days. Given that this was a first-in-patient administration, a conservative approach to dosing was taken (i.e., starting below the maximal demonstrated pharmacokinetic dose). After a 2-week wash-out period (3 weeks if treatment response, defined as 50% improvement from baseline on the HAM-D, was met), participants crossed over to the other treatment condition in phase 2; participants maintaining antidepressant response after the 3-week washout were withdrawn from the study and received standard treatment. In phase 1 of the study, 10 participants received 4-Cl-KYN and 9 received placebo. One participant dropped out during phase 1 (due to a death in the family), and 1 participant completed phase 1 (on placebo) but did not enter phase 2 (on study drug) due to continued clinical response. During phase 2, there were 2 participants receiving 4-Cl-KYN who dropped out (1 because of bilateral pulmonary emboli and 1 due to worsening depressive symptoms).
A flexible dose design was used. That is, all participants were administered a starting dose of placebo or 4-Cl-KYN (1080 mg/d given as a single morning dose for 7 days); the treatment target for all participants was 1440 mg/d for an additional 7 days unless tolerability issues were a concern. All participants who received 4-Cl-KYN tolerated the higher 1440-mg/d dose well, and there was no need for dose reduction throughout the course of the study. All staff was blind to whether drug or placebo was being administered.
Outcome Measures
Participants were rated at 60 minutes before their first 1080-mg dose of study medication or placebo, and on days 1, 2, 3, 7, and 13 (before the final 1440-mg dose or final placebo dose) of each phase of the study. The primary outcome measure was the 17-item HAM-D (Hamilton, 1960); secondary outcome measures included the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), the Beck Depression Inventory (Beck and Beamesderfer, 1974), the Hamilton Anxiety Rating Scale (Hamilton, 1959), the Positive and Negative Affect Schedule (Watson et al., 1988), the Snaith-Hamilton Pleasure Scale (Snaith et al., 1995), the Temporal Experience of Pleasure Scale (Gard et al., 2006), the Scale for Suicide Ideation (Beck et al., 1979), the Brief Psychiatric Rating Scale (Overall and Gorham, 1962), the Clinician Administered Dissociative States Scale (Bremner et al., 1998), and the Young Mania Rating Scale (Young et al., 1978). High inter-rater reliability was obtained for the HAM-D, MADRS, and Young Mania Rating Scale (all Intraclass Correlation Coefficient > 0.81). Throughout the study, the same rater conducted most ratings for an individual participant.
Pharmacokinetic, Pharmacodynamic, and Safety Evaluation
Human Study
Before and after starting study drug/placebo, blood samples for all 19 participants were collected at baseline (−60 minutes) and at various time points over the next 13 days. CSF samples were obtained for 5 participants who consented to the procedure. Plasma and CSF were assayed for potential biomarkers of antidepressant response and drug effects. Additional details are provided in the supplemental Materials.
Mouse Study
To compare human exposure levels with those previously found to be effective in mice, 4-Cl-KYN (25 or 125 mg/kg, doses previously found to induce antidepressant-like effects in preclinical behavioral tests [Zanos et al., 2015]) was administered i.p. (injection volume: 7.5 mL/kg) to 56 male CD-1 mice (10 weeks old; Charles River Laboratories, Raleigh, NC) at 0.0083, 0.25, 0.5, 0.75, 1, 2, and 4 hours (4 mice per dose per time point) prior to collecting brain and plasma samples. Additional details are provided in the supplemental Materials.
7T MRS and rsfMRI Scans
To assess brain glutamate levels and resting state activity, MRS and rsfMRI scans were obtained as a baseline/Cmax (after first dose) pair during each phase of the trial for a total of 4 time points in a subset of 12 participants who consented to the procedure. Additional details are provided in the supplemental Materials.
Statistics
Clinical Measures
Briefly, linear mixed models were used to estimate and test the efficacy of 4-Cl-KYN monotherapy relative to placebo on depression rating scale scores as measured over time; effect sizes were calculated using means and standard error estimates from the mixed models. Additional details regarding the primary and secondary outcome measure analyses can be found in the supplemental Materials. As established in the protocol, the primary outcome (alpha) was set at .05, 2-tailed; in the event of a significant treatment-by-time interaction, we planned to use a Bonferroni correction for post-hoc tests of the treatment effect at each time.
Biomarkers
A linear mixed effects model was used to estimate mean concentrations for each endogenous kynurenine pathway analyte (kynurenine, kynurenic acid, 3-hydroxyanthranilic acid [3-HAA], quinolinic acid) measured in plasma at baseline, 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12, and 24 hours, and on days 3, 7, and 13 post-4-Cl-KYN or placebo. Separate models were run for each time point due to confounding factors surrounding day and batch (specifically, days 3, 7, and 13 were assayed together). In addition to the main effect of treatment, each model included period-specific baseline concentration and crossover period as covariates and a random intercept per person. A mixed model was used to estimate mean MRS glutamate values for 4-Cl-KYN, placebo, and their difference. For vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) levels, the trapezoidal rule was used to calculate 2 areas under the curve (AUC) measures for each person (1 for 4-Cl-KYN and 1 for placebo); means and SDs were then calculated for each treatment condition, as were the mean and SD of the within-person difference. For all biomarkers, estimated means, SDs, or standard errors, 95% confidence intervals (CIs), rather than P values, are reported. Additional details are provided in the supplemental Methods.
Results
Demographic and treatment characteristics of the participants appear in Table 1 and supplemental Table 1, respectively. Notably, the average number of lifetime antidepressant trials was 7.8 (SD = 5.67), and the average number of antidepressant trials associated with the current episode was 2.6 (SD = 5.06), underscoring the substantial treatment-resistant status of this sample. Nineteen participants were randomized to receive either 4-Cl-KYN (1080 mg/d for 7 days followed by 1440 mg/d for 7 days) or placebo for 14 days before crossing over into the opposite treatment arm 2 weeks later (supplemental Fig. 1). Over the entire course of the study, 17 of 19 participants were exposed to both placebo and active drug, and 15 of 19 completed both phases.
Efficacy
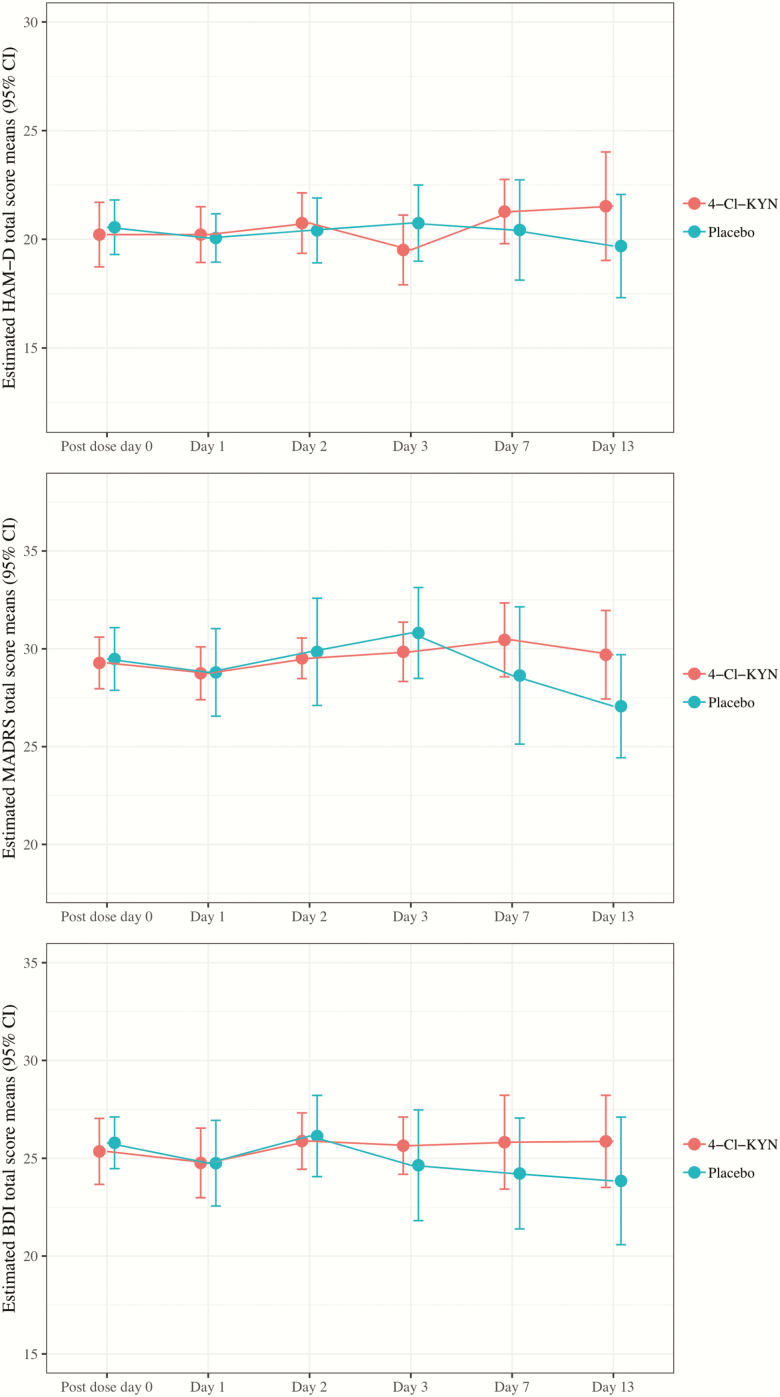
None of the 17 participants who received 4-Cl-KYN monotherapy responded to the drug, and 1 of 19 (5.3%) responded to placebo. The participant who achieved remission in phase 1 on placebo experienced sustained response and did not proceed to the 4-Cl-KYN arm. No treatment effect was detected as assessed by our primary outcome measure (HAM-D total score) (treatment-by-time F = 1.755,24.95, P = .16; main effect of treatment F = 0.141,11.62, P = .71), and Cohen’s d values at each time point all had corresponding CIs that crossed zero (Figure 1). In addition, no significant treatment or treatment-by-time interactions were detected for any of the secondary global measures of depression, including MADRS score (treatment F = 0.221,23.9, P = .64; treatment-by-time F = 1.315,26.8, P = .29), Beck Depression Inventory score (treatment F = 0.331,29.2, P = .571; treatment-by-time F = 0.555,27.3, P = .77) (Figure 1; supplemental Table 2), or any other secondary clinical measure (for additional details, see supplemental Table 3).
Figure 1.
Depression rating scale scores for 4-Cl-KYN and placebo over time. BDI, Beck Depression Inventory; CI, Confidence Interval HAM-D, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale.
Adverse Events
No differences were noted between treatment groups in the emergence of side effects (Table 2) or laboratory tests. One participant experienced a serious adverse event (bilateral pulmonary emboli); subsequent work-up revealed that this individual had a previously undiagnosed clotting abnormality (antiphospholipid syndrome), though relatedness to study drug could not be absolutely ruled out.
Table 2.
Occurrence of Side Effects [Participants reporting (percentage)]a
| Side Effect | 4-Cl-KYN | Placebo |
|---|---|---|
| Headache | 6 (0.35) | 3 (0.16) |
| Chest pain | 1 (0.06) | 1 (0.05) |
| Stomach or abdominal discomfort | 1 (0.06) | 1 (0.05) |
| Diarrhea | 1 (0.06) | 1 (0.05) |
| Appetite decrease | 2 (0.12) | 1 (0.05) |
| Cramps | 1 (0.06) | 1 (0.05) |
| Eye irritation | 1 (0.06) | 1 (0.05) |
| Muscle/bone/joint pain | 5 (0.29) | 4 (0.21) |
| Tiredness/fatigue | 2 (0.12) | 3 (0.16) |
| Decreased motor activity | 2 (0.12) | 2 (0.11) |
| Difficulty falling asleep | 4 (0.24) | 2 (0.11) |
| Early morning awakening | 3 (0.18) | 6 (0.32) |
| Interrupted sleep | 4 (0.24) | 5 (0.26) |
| Drowsiness/sedation | 7 (0.41) | 2 (0.11) |
| Concentration difficulty | 2 (0.12) | 2 (0.11) |
| Memory problems | 3 (0.18) | 2 (0.11) |
| Anxiety | 2 (0.12) | 2 (0.11) |
| Irritability | 4 (0.24) | 4 (0.21) |
| Suicidal ideas | 1 (0.06) | 1 (0.05) |
| Not otherwise listed or unknown | 3 (0.18) | 1 (0.05) |
Abbreviation: 4-Cl-KYN, L-4-chlorokynurenine.
a Counts include all participants who reported moderate or severe symptoms after having mild or absent symptoms at baseline
Plasma and CSF 4-Cl-KYN and 7-Cl-KYNA Levels
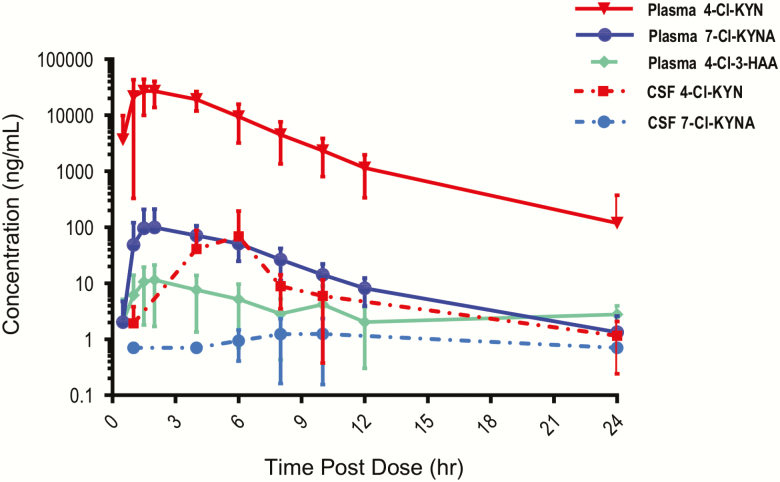
Concentrations of 4-Cl-KYN and 7-Cl-KYNA appeared to follow bi-exponential decay; that is, because the distribution phase preceded the terminal elimination phase, multi-compartmental kinetics were likely (supplemental Table 4; Figure 2). To accurately estimate the apparent elimination rate constant (λ Z), at least 3 observed terminal data points were used for the terminal slope. Graphical demonstration of estimated λ Z for each participant and matrix is available on request. For participants with fewer than 3 terminal data points, λ Z was not estimated (CSF 4-Cl-KYN for participant 11; CSF 7-Cl-KYNA for participants 2, 8, 9, 11, and 13). Following the first single dose of oral 4-Cl-KYN (1080 mg/d), plasma and CSF 4-Cl-KYN concentrations peaked at 2.2 and 4.7 hours, respectively, and then declined with an estimated half-life of 2.5 and 4 hours, respectively.
Figure 2.
Human plasma and CSF concentrations (ng/mL) of 4-Cl-KYN and 7-Cl-KYNA vs time (hour) post-4-Cl-KYN dose (1080 mg).
Following the first 1080-mg oral dose of 4-Cl-KYN, plasma 7-Cl-KYNA concentrations peaked at an estimated 3 hours, but CSF concentrations of 7-Cl-KYNA in 4 of the 5 participants were too low to accurately calculate peak time. The plasma Cmax geometric means were 32.11 μg/mL (coefficient of variation [CV%] = 21) for 4-Cl-KYNA and 0.073 μg/mL (CV% = 33) for 7-Cl-KYNA. Plasma concentrations of 7-Cl-KYNA declined with an estimated half-life of 4.6 hours. When pharmacokinetic values from all participants were compared with pharmacokinetic values from the 5 participants with both CSF and plasma levels (supplemental Table 5), pharmacokinetic differences were not significant. Total daily exposure (AUC0-24hr) closely matched AUC0-∞ as the percent extrapolated AUC was small (i.e., much less than 25%), indicating a more accurate estimation of AUC0-∞. The volume of distribution (V/F) of 4-Cl-KYN was large (greater than the typical blood volume in humans, approximately 7 L), indicating widespread distribution in bodily tissues, and more so in the brain as supported by the large V/F in CSF (53 157 L). V/F and clearance values assume oral bioavailability of 0.85 (Food and Drug Administration, 1997).
4-Cl-KYN and 7-Cl-KYNA Levels in Mice
To compare these clinical results with those previously observed in mice, two 4-Cl-KYN doses were administered (25 or 125 mg/kg, i.p., both previously found to be antidepressant-like doses in mice [Zanos et al., 2015]). Brain and plasma samples were collected at 7 different time points after drug administration. Of the analyte data provided, only 4-Cl-KYN had sufficient data points to perform the noncompartmental analysis. Tissue concentrations of 7-Cl-KYNA were summarized at each time point where at least 1 value below quantifiable levels was available. Following doses of 25 mg/kg and 125 mg/kg of 4-Cl-KYN, the CV% for 4-Cl-KYNA was 24.6 μg/mL (16%) and 90.0 μg/mL (7.1%), respectively, and the CV% for 7-Cl-KYNA was 3.9 μg/mL (39.6%) and 18.0 μg/mL (25%), respectively (supplemental Fig. 2). Mouse brain Cmax for 7-Cl-KYNA did not have sufficient data points to perform an noncompartmental analysis.
Peripheral and Central Levels of Surrogate Biomarkers
Model adjusted means, standard errors, and 95% CIs are presented in supplemental Figure 3 for kynurenine pathway analyte plasma concentrations measured 1, 3, 7, and 13 days following initial doses of 4-Cl-KYN or placebo. Descriptive statistics for CSF kynurenine concentrations for placebo and 4-Cl-KYN (1080 mg) conditions are reported in supplemental Table 6 for 7 time points spanning baseline to day 1. No significant associations were observed. The remaining CSF analytes (3-HAA, kynurenic acid, quinolinic acid, and picolinic acid) all had 2 or fewer values above detectable levels for each time point.
Concentrations for the neurotrophic factors VEGF and BDNF did not differ significantly between conditions; details are available in the supplemental Materials.
MRS
MRS glutamate levels were measured in 12 participants, 11 of whom had data of acceptable quality (measured water line width not exceeding 16). The adjusted (i.e., controlling for baseline and period) treatment difference in glutamate measured 2 to 4 hours post–4-Cl-KYN (1080 mg) or placebo administration was 0.03 (95% CI = −1.79–1.85) (adjusted means: 4-Cl-KYN = 1.21 [SE = 0.04], placebo = 1.25 [SE = 0.14]).
rsfMRI
rsfMRI data from 12 participants were available for analysis following the first single 1080-mg oral dose of 4-Cl-KYN. Two participants had excess motion, leaving 10 datasets for analysis. No significant changes in connectivity were found between the placebo and 4-Cl-KYN scans (supplemental Fig. 4).
Discussion
This double-blind, placebo-controlled, proof-of-concept monotherapy study found that administration of the 4-Cl-KYN prodrug of the NMDAR glycine site antagonist 7-Cl-KYNA did not improve overall depressive symptomatology in individuals with TRD compared with placebo. Commensurately, oral administration of 4-Cl-KYN (1080 mg/d) resulted in no significant changes in glutamate brain levels (as measured by 7T 1H-MRS at Cmax), rsfMRI connectivity, or peripheral and central measures (including kynurenine metabolites). These findings contrast with previous results that found that 2 weeks of adjunctive treatment with high-dose DCS (1000 mg/d) had significant antidepressant effects in individuals with TRD (Heresco-Levy et al., 2013). It should be noted that no DCS replication studies have been reported since that initial finding, nor have biomarker/target engagement studies demonstrated the therapeutic relevance of glycine-site antagonism with regard to the effectiveness of DCS in treating depressive symptoms. However, in a recent 6-week study of TRD participants, Chen and colleagues found that although DCS demonstrated no initial effectiveness as an augmentation agent compared with placebo, it maintained the antidepressant and antisuicidal effects of low-dose ketamine (Chen et al., 2019).
Some interesting contrasts can also be made between these results and previous pharmacokinetic findings from phase 1 studies with 4-Cl-KYN (Wallace et al., 2017). The exposure of both 4-Cl-KYN and 7-Cl-KYNA, as measured by AUC0-24hr and AUC0-∞, was lower (12% and 26% lower, respectively) in this cohort of TRD participants than in the healthy reference population in the phase 1 studies (see discussion in supplemental Materials and supplemental Table 7). The lack of biochemical changes (peripheral or central measures) or signal of behavioral effects (across the multiple symptom domains of depression, anhedonia, anxiety, and suicide) suggests that there could have been insufficient CNS exposure to 7-Cl-KYNA. In this context, it should be noted that all participants who received 4-Cl-KYN demonstrated excellent tolerability on the higher dose of 1440 mg/d. Although 7-Cl-KYNA achieved study serum target levels of approximately 150 ng/mL (comparable with previous results [Wallace et al., 2017]), average CSF levels of 4-Cl-KYN were approximately 22 ng/mL (n = 5) and 7-Cl-KYNA levels were mostly below detection (<1.4 ng/mL).
Thus, in this cohort of TRD participants, measurable concentrations of 7-Cl-KYNA in CSF were not achieved with either dose of 4-Cl-KYN, indicating that the doses of 4-Cl-KYN used in this study were not sufficient to induce conversion to the active metabolite. This may have resulted from CNS concentrations of the prodrug that were too low to support adequate conversion to 7-Cl-KYNA. Given the short half-life of 4-Cl-KYN, this suggests that either more frequent dosing and/or an overall higher dose may be required to observe an antidepressant effect. Another explanation that cannot be excluded at this time is that conversion of 4-Cl-KYN to 7-Cl-KYNA did not proceed as expected in the CNS of this human sample with TRD. It is possible that kynurenine aminotransferase II activity in the brain and/or genes involved in the transport of 4-Cl-KYN or 7-Cl-KYNA may function with different efficiencies or kinetics in humans (compared with mice) and/or TRD (compared with healthy) participants.
Coincidentally, the kynurenine pathway may play a role in the pathophysiology of depression (Reus et al., 2015), interfacing with inflammatory/immune response, neurotrophic factors such as VEGF and BDNF, and glutamatergic neurotransmission (Miller, 2013). Kynurenine is converted to a series of metabolites, including several neuroprotectants (picolinic acid, kynurenic acid) and neurotoxins (3-HAA, quinolinic acid). A prior study found that response to ketamine may depend on ketamine’s ability to elicit a pronounced effect on kynurenine pathway metabolites (not related to glycine activity) (Moaddel et al., 2018). In this study, no significant effects on kynurenine pathway metabolites were observed. This corresponds with the lack of clinical response seen in this trial and is distinct from changes seen in response to ketamine administration.
This study had several strengths. First, participants were hospitalized for an average of 6 weeks prior to their first 1080-mg dose of 4-Cl-KYN, which allowed sufficient time to characterize them and document the stability of depressive symptoms during their current episode. Second, the study was randomized and placebo controlled; placebo treatment effects were unusually low, increasing the probability of identifying a treatment effect if there was one. Third, adherence to study drug intake was monitored by nursing staff and documented by plasma and CSF levels. Fourth, to examine the biological effects of the drug, both peripheral and central measures were obtained from participants as possible measures of drug activity; however, given the lack of efficacy as assessed by a variety of measures, the relationship between biomarkers and treatment response was not examined.
This study was also associated with several limitations and qualifications. First, the group size was small (n = 19). In this context, high variability was observed for the pharmacokinetic parameters, particularly for the CSF matrix of the 5 participants assessed; a larger sample size might have reduced this variability. Second, the participants had a significant history of treatment resistance, and it is possible that treatment effects might have been observed in a less treatment-refractory group. Third, almost 90% of the participants had a lifetime history of anxiety disorder (see Table 1). Individuals with TRD and comorbid anxiety disorder are known to have a lower response rate to medication and incomplete remission of symptoms (Kornstein and Schneider, 2001). Fourth, given the preclinical data and our focus on rapid antidepressant interventions, the duration of the treatment phase was only 1 week on the lower dose of study drug and 1 week on the higher dose. Thus, it is possible that antidepressant effects might have emerged with longer treatment periods if the higher dose (1440 mg/d) had been given for the entire 2 weeks or if 4-Cl-KYN had been administered as a single bolus administration (as is effective with ketamine). Fifth, 4-Cl-KYN was administered as monotherapy, and it is possible that antidepressant effects might have emerged if it had been administered adjunctively with standard antidepressant treatment.
The present results do not support the hypothesis that monotherapeutic administration of 7-Cl-KYNA (derived from the prodrug 4-Cl-KYN) at the doses and duration used in this study had antidepressant effects in this sample of MDD participants with a significant history of treatment resistance. Given the low CSF concentrations of 7-Cl-KYNA seen in this study, further investigations should determine whether this is a dose-dependent phenomenon or if substantive differences exist in the conversion of 4-Cl-KYN to 7-Cl-KYNA in the CNS of TRD participants compared with mouse brain models. Future studies should also examine additional therapeutic options, including administration of higher doses of 4-Cl-KYN, varying the route and schedule of administration, use as an adjunctive treatment, excluding co-morbid anxiety disorders, or studying a less treatment-refractory population.
Supplementary Material
Acknowledgments
The authors thank the 7SE research unit and staff for their support.
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (ZIAMH002857; NCT02484456), by a NARSAD Independent Investigator Award to Dr Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr Zarate.
Statement of Interest
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the US government but will share a percentage of any royalties that may be received by the government. Dr Gould has received research funding from Allergan and Roche Pharmaceuticals during the preceding 3 years and is a consultant for FSV7 LLC. The mouse pharmacokinetic studies were supported by a gift donation from VistaGen Therapeutics to Dr Gould. Drs Smith and Snodgrass are employed by and have an equity interest in VistaGen Therapeutics, which has commercial rights to the study drug. Dr Machado-Vieira has received consulting fees from Eurofarma Pharmaceuticals and BioStrategies group and has research contracts with Janssen Pharmaceuticals. Dr Machado-Vieira has also received speaker fees from Otsuka and Cristalia and is a member of the scientific board of Symbinas Pharmaceuticals. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH (2018) The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther 190:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A (1974) Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry 7:151–169. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A (1979) Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol 47:343–352. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Chen MH, Cheng CM, Gueorguieva R, Lin WC, Li CT, Hong CJ, Tu PC, Bai YM, Tsai SJ, Krystal JH, Su TP (2019) Maintenance of antidepressant and antisuicidal effects by D-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: a double-blind randomized placebo-control study. Neuropsychopharmacology 44:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG (1998) Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50:597–664. [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett MR, Mick SJ, Cler JA, Rao TS, Iyengar S, Wood PL (1991) Actions of D-cycloserine at the N-methyl-D-aspartate-associated glycine receptor site in vivo. Neuropharmacology 30:1167–1171. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (1997) Guidance document: dissolution testing of immediate release solid oral dosage forms (No. 97D-0331). Rockville, MD. [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP (2006) Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Personal 40:1086–1102. [Google Scholar]
- Gould TD, Zarate CA Jr, Thompson SM (2019) Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol 59:213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R (2007) Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem 102:103–111. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, Kremer I (2013) A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol 16:501–506. [DOI] [PubMed] [Google Scholar]
- Hokari M, Wu HQ, Schwarcz R, Smith QR (1996) Facilitated brain uptake of 4-chlorokynurenine and conversion to 7-chlorokynurenic acid. Neuroreport 8:15–18. [DOI] [PubMed] [Google Scholar]
- Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr (2019) Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol 22:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN (1988) 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A 85:6547–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Schneider RK (2001) Clinical features of treatment-resistant depression. J Clin Psychiatry 62(Suppl 16):18–25. [PubMed] [Google Scholar]
- Leeson PD, Iversen LL (1994) The glycine site on the NMDA receptor: structure-activity relationships and therapeutic potential. J Med Chem 37:4053–4067. [DOI] [PubMed] [Google Scholar]
- Miller AH. (2013) Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology 38:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, Morris PJ, Yuan P, Thomas CJ, Gould TD, Ferrucci L, Zarate CA (2018) Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl) 235:3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ (2018) Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs 32:411–420. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Mathew SJ (2017) Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 16:472–486. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Park LT, Zarate CA Jr (2019) Depression in the primary care setting. N Engl J Med 380:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G, Hartmann S, Lorenz B, Wollenburg C, Baran L, Przegalinski E, Kostowski W, Krzascik P, Chizh B, Headley PM (1997) Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J Pharmacol Exp Ther 283:1264–1275. [PubMed] [Google Scholar]
- Poleszak E, Wlaź P, Wróbel A, Dybała M, Sowa M, Fidecka S, Pilc A, Nowak G (2007) Activation of the NMDA/glutamate receptor complex antagonizes the NMDA antagonist-induced antidepressant-like effects in the forced swim test. Pharmacol Rep 59:595–600. [PubMed] [Google Scholar]
- Przegaliński E, Tatarczyńska E, Chojnacka-Wójcik E (1998) Anxiolytic- and antidepressant-like effects of an antagonist at glycineB receptors. Pol J Pharmacol 50:349–354. [PubMed] [Google Scholar]
- Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J (2015) Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res 68:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P (1995) A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 167:99–103. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P (1990) Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol 185:1–10. [DOI] [PubMed] [Google Scholar]
- Wallace M, White A, Grako KA, Lane R, Cato AJ, Snodgrass HR (2017) Randomized, double-blind, placebo-controlled, dose-escalation study: Investigation of the safety, pharmacokinetics, and antihyperalgesic activity of l-4-chlorokynurenine in healthy volunteers. Scand J Pain 17:243–251. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Salituro FG, Schwarcz R (1997) Enzyme-catalyzed production of the neuroprotective NMDA receptor antagonist 7-chlorokynurenic acid in the rat brain in vivo. Eur J Pharmacol 319:13–20. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Schwarcz R, Snodgrass HR (2017) Characterization of the effects of L-4-chlorokynurenine on nociception in rodents. J Pain 18:1184–1196. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
- Zanos P, Piantadosi SC, Wu HQ, Pribut HJ, Dell MJ, Can A, Snodgrass HR, Zarate CA Jr, Schwarcz R, Gould TD (2015) The Prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/GlycineB-site inhibition. J Pharmacol Exp Ther 355:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD (2018) Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 70:621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.