Abstract
Background
Tobacco use is prevalent in individuals who are routinely exposed to stress. However, little is known about how nicotine affects responses to trauma. We examined in rats how nicotine exposure affects fear conditioning, a procedure often used to study stress-related psychiatric illness.
Methods
We examined 2 methods of nicotine exposure: self-administration, modeling voluntary use, and experimenter-programmed subcutaneous administration, modeling medicinal administration (nicotine patch). For self-administered nicotine, rats trained to self-administer nicotine i.v. were fear conditioned (via light cue preceding foot-shock) either immediately after a 12-hour self-administration session or 12 hours later during a period with somatic signs of nicotine withdrawal. For experimenter-delivered nicotine, rats were conditioned after 1–21 days of nicotine delivered by programmable (12 hours on) subcutaneous mini-pumps. Tests to evaluate acoustic startle responses to the conditioning environment (context-potentiated startle) and in the presence or absence of the light cue (fear-potentiated startle) occurred after a 10-day period.
Results
Rats fear conditioned immediately after nicotine self-administration showed reduced responses to the shock-associated context, whereas those trained during nicotine withdrawal showed exaggerated responses. Experimenter-programmed nicotine produced effects qualitatively similar to those seen with self-administered nicotine.
Conclusions
Self-administration or experimenter-programmed delivery of nicotine immediately before exposure to aversive events can reduce conditioned fear responses. In contrast, exposure to aversive events during nicotine withdrawal exacerbates fear responses. These studies raise the possibility of developing safe and effective methods to deliver nicotine or related drugs to mitigate the effects of stress while also highlighting the importance of preventing withdrawal in nicotine-dependent individuals.
Keywords: Fear conditioning, nicotine, PTSD, withdrawal
Significance Statement.
In human tobacco users, use of nicotine-containing products such as cigarettes and chewing tobacco is prevalent during times of stress. However, little is known about how nicotine affects responses to stress or traumatic experiences. Nicotine has anti-anxiety effects that might reduce the impact of stress, but it also enhances alertness and attention that might strengthen stress-related memories. We examined in rats how nicotine exposure affects fear conditioning, a procedure used to study stress-related psychiatric illness. We found that rats fear conditioned immediately after nicotine self-administration showed reduced responses to environments previously associated with aversive events, whereas those trained during nicotine withdrawal showed exaggerated responses. Nicotine delivered via a programmed subcutaneous minipump produced effects similar to those seen with self-administered nicotine. These studies raise the possibility of developing safe and effective methods to deliver nicotine or related drugs to mitigate the effects of stress while demonstrating the importance of preventing withdrawal.
Introduction
Stress disorders involve genetic and environmental factors (Knoll and Carlezon, 2010; Ressler et al., 2011; Gelernter et al., 2019). Use of tobacco products is particularly high during times of stress, consistent with reports that they have calming effects (Spielberger, 1986; Benowitz, 2010) and strengthening the theory that the anxiolytic effects of tobacco products and their primary psychoactive constituent, nicotine, may contribute to the development and maintenance of tobacco use (Weinberger et al., 2010). Nicotine also has psychomotor stimulant effects that enhance alertness and cognitive performance in humans (Spielberger, 1986, Newhouse et al., 2004; Semenova et al., 2007; Heishman et al., 2010) and laboratory animals (Raybuck and Gould, 2010). These combined effects—reduced anxiety that provides motivation to use tobacco and increased cognitive function that accompanies tobacco use—may have long-lasting and potentially unintended consequences. Tobacco use before, during, or after a stressful event may affect the perception of stress or the encoding, consolidation, and retrieval of stress-related memories. If the anxiolytic effects of nicotine prevail, tobacco use might have beneficial effects by reducing the impact of stress. If the cognitive-enhancing effects of nicotine prevail, tobacco use might increase the impact of stress, promoting persistent conditions such as post-traumatic stress disorder (PTSD). The effects of nicotine may also involve factors such as whether users are dependent and are under the influence of the drug or in withdrawal during stress.
Little is known about relationships between tobacco use and vulnerability to develop stress disorders. Tobacco use is prevalent in people who have already developed PTSD (Thorndike et al., 2006; Fu et al., 2007), and smokers with PTSD are more likely to smoke when experiencing stress (Beckham et al., 2008). Nicotine has complex effects in people with PTSD, a hallmark sign of which is an exaggerated startle reflex (Bremner et al., 1996). Although smokers with PTSD report that smoking relieves their symptoms, objective measurements indicate that smoking potentiates their already-exaggerated startle responses (Calhoun et al., 2011). These findings suggest that despite a tobacco user’s perceptions that nicotine has calming effects, continued use can exacerbate and perpetuate the symptoms of PTSD.
Nicotine is difficult to study in laboratory animals. Experimenter-delivered nicotine often produces aversion responses in rodents (Henningfield and Goldberg, 1983; Fudala and Iwamoto, 1987; Jackson et al., 2009; Risinger and Oakes, 2009). Indeed, passive delivery of even highly rewarding drugs (e.g., cocaine) can cause vastly different physiological responses than those seen with voluntary self-administration (Galici et al., 2000; Chen et al., 2008). Aversive effects can make it difficult to examine how a drug affects the perception of traumatic or fear-inducing stimuli. Furthermore, to model tobacco use as it occurs in humans, it is crucial to use self-administration procedures that are sufficient to produce nicotine dependence, a state where discontinuation of the drug produces physical signs of withdrawal.
Fear-potentiated startle (FPS) is an acoustic startle–based procedure (Shi and Davis, 2001; Meloni et al., 2006; Ressler et al., 2011) frequently used in preclinical studies of PTSD. It has numerous advantages for studies of factors that contribute to stress-related behavioral adaptations, including the presence of an “index trauma” as a triggering mechanism, development of persistent fearful memories, and expression of hypervigilance (exaggerated startle responsiveness). Startle is also a translationally relevant endpoint that can be used in humans (Pitman et al., 1999), enabling the design of complementary preclinical and clinical studies. Exaggerated startle responses and deficits in extinction of fearful memories are hallmark features of PTSD (Morgan et al., 1995; Grillon et al., 2003; Parsons and Ressler, 2013) that are readily studied in rats. The FPS procedure has excellent predictive validity in identifying anxiolytic drugs that reduce fear and anxiety in people and is ideal to study processes involved in recovery from stress, such as extinction learning (Davis, 1986, 1993; Milad et al., 2006; Quirk et al., 2010). In addition to exaggerated startle responses, another sign of PTSD is inability to forget stressful memories or the cues that symbolize them (American Psychiatric Association, 2013). People with PTSD have deficits in extinction learning, a process that involves new learning that weakens the expression of old memories (Myers et al., 2011). In the case of FPS, extinction training involves repeatedly exposing the rats to a cue (e.g., a light) that once predicted shock without delivering shock, which gradually decreases startle reactivity.
The present studies were designed to determine how nicotine exposure affects fear conditioning in male rats. We first examined i.v. self-administration (IVSA) of nicotine, modeling voluntary use. On finding evidence that nicotine may have medically useful (protective) effects, we examined experimenter-programmed subcutaneous (s.c.) administration, modeling medicinal administration via methods such as a nicotine patch.
METHODS
Rats
Male Long-Evans rats (300–350 g; Charles River Laboratories, Charlotte, NC) were singly housed following either catheterization or minipump implantation and maintained on a 12-hour-light/-dark cycle (lights on 7:00 am) with ad libitum access to food and water except during testing. Procedures were approved by the McLean Hospital Institutional Care and Use Committee and consistent with the 1996 National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
(-)-Nicotine hydrogen tartrate was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in sterile 0.9 % saline solution. The pH was adjusted to 7.0 with 0.1 N sodium hydroxide. Doses are expressed as free base.
Experiment 1
Experiment 1 examined nicotine IVSA effects on fear conditioning endpoints. Rats stably self-administering nicotine in 12-hour sessions were fear conditioned immediately after the self-administration session during which they qualified for inclusion (below) and were tested after a 10-day period without additional nicotine access. This design is intended to model conditions in humans where trauma is experienced while under the influence of nicotine and followed by a period where tobacco products are not available or used.
IVSA
IVSA procedures were conducted as described (Thomsen and Caine, 2005). Rats were allowed to recover for at least 7 days, during which a prophylactic dose of cefazolin (17 mg/kg, i.v.) dissolved in saline containing heparin (3 USP U/0.1 mL) was delivered once daily to prevent infection and promote catheter patency. Thereafter, catheters were flushed daily with sterile physiological saline containing heparin (3 USP U/0.1 mL). Catheter patency was confirmed daily; rats with nonfunctional catheters were either implanted with a second catheter in the opposite jugular or terminated from the study.
Rats were allowed to self-administer nicotine (0.03 mg/kg/injection) in 12-hour (overnight) sessions in 2-lever standard operant conditioning chambers for a minimum of 14 sessions until IVSA had stabilized. Nicotine availability was signaled by illumination of a yellow LED light above the active lever, and each injection was accompanied by lever retraction and a 20-second timeout period. The criterion for inclusion was an IVSA >0.7 mg/kg/session for 4 of 5 consecutive sessions. Rats in the nicotine IVSA condition had counterparts that self-administered saline in an equivalent number of sessions; in cases where the counterpart was lost, rats were retained regardless as long as they had been through 14 self-administration sessions. This experiment yielded 15 rats that stably self-administered nicotine and 18 rats assigned to the saline self-administration condition.
Nicotine Withdrawal Signs
Nicotine dependence was operationally defined as a significant increase in the number of any of the well-validated somatic signs of nicotine withdrawal, including body shakes/tremor, eye blinks, gasps, writhes/stretches, teeth chattering/chewing, and ptosis (Kenny and Markou, 2001). Twelve hours after the final IVSA session, these signs were measured during a 10-minute observation period by an individual blinded to the treatment conditions.
Fear Conditioning
Fear conditioning training coincided with the end of a 12-hour IVSA session to minimize nicotine withdrawal. Training comprised 2 elements: collection of baseline data and fear conditioning itself. The baseline element of the session comprises exposure to ten 100-dB white noise bursts over a 5-minute period. Baseline data were used to (1) assign (match) rats to groups with equivalent startle baselines, (2) evaluate treatment-associated changes in startle responsivity that might be caused by sedative or activating drug effects, and (3) serve as the basis (denominator) for context-potentiated startle (CPS) calculations (Shi and Davis, 2001). This element was identical for all rats (n = 33). The fear conditioning element followed immediately and comprised 10 parings of a light cue (4 seconds) that co-terminated with a 0.5-second, 0.6-mA foot-shock over a 25-minute period, as described (Meloni et al., 2006). During this time, shock reactivity data were collected. To enable determination of whether self-administered nicotine affected sensitivity to acoustic startle, rats were further subdivided into nicotine-shock (n = 7), nicotine-no shock (n = 8), saline-shock (n = 9), and saline-no shock (n = 9) groups. Testing began 10 days later following a period without additional nicotine exposure. Test sessions included 2 elements: evaluation of CPS and FPS. For CPS, rats again received ten 100-dB white noise bursts over a 5-minute period. FPS was then quantified by comparing startle responses with a 100-dB white noise burst in the presence (10 trials) or absence of the light cue (10 trials). To evaluate extinction of these conditioned responses, rats receive a total of 3 test sessions conducted at 48-hour intervals. CPS (Shi and Davis, 2001) is expressed as a percent change from a pre-fear conditioning baseline and is calculated by dividing the difference between startle responses to the white noise bursts pre- and post-fear conditioning by the pre-fear conditioning baseline (%CPS = [(startle at test–startle before fear conditioning)/startle before fear conditioning] × 100); FPS is expressed as a percent change from baseline and is calculated by comparing with the difference in startle amplitude elicited in the presence or absence of the light (%FPS = [(startle in presence of light–startle in dark)/startle in dark] × 100).
Experiment 2
Experiment 2 examined the effects of nicotine withdrawal followed by sustained nicotine unavailability on fear conditioning endpoints. Rats stably self-administering nicotine in 12-hour sessions were fear conditioned 12 hours after the self-administration session during which they qualified for inclusion, during nicotine withdrawal, and tested after a 10-day period without additional access to nicotine. This design is intended to model conditions in humans where trauma is experienced during nicotine withdrawal and followed by a period when tobacco products are not available or not used. Previous data in rats demonstrate that the somatic and motivational signs of nicotine withdrawal are negligible after 10 days (Kenny and Markou, 2001).
IVSA
IVSA procedures were conducted as in Experiment 1. This experiment yielded 14 rats that stably self-administered nicotine and 15 rats assigned to the saline self-administration condition.
Nicotine Withdrawal Signs
Nicotine withdrawal signs were measured 12 hours after the final IVSA session immediately before fear conditioning.
Fear Conditioning
Fear conditioning procedures were conducted as in Experiment 1, except they occurred 12 hours after the end of a 12-hour IVSA session (coinciding with nicotine withdrawal). Rats were subdivided into nicotine-shock (n = 7), nicotine-no shock (n = 7), saline-shock (n = 8), and saline-no shock (n = 7) groups; comparisons between nicotine-no shock and saline-no shock were intended to provide insight on whether rats that had self-administered nicotine were still in withdrawal during CPS and FPS testing. Testing began 10 days later following a period without additional nicotine exposure.
Experiment 3
Experiment 3 examined the effects of acute nicotine withdrawal followed by sustained nicotine availability on fear conditioning endpoints. Unlike Experiment 2, rats were allowed to continue to self-administer nicotine during the 10-day period following fear conditioning and between the test sessions. This design is intended to model conditions in humans where trauma is experienced during nicotine withdrawal and followed by a period when tobacco products are used.
IVSA
IVSA procedures were conducted as described in Experiment 1, and nicotine withdrawal was quantified as in Experiment 2. This experiment yielded 17 rats that stably self-administered nicotine and 17 rats assigned to the saline self-administration condition.
Nicotine Withdrawal Signs
As in Experiment 2, nicotine withdrawal signs were measured 12 hours after the final IVSA session immediately before fear conditioning.
Fear Conditioning
Fear conditioning procedures were conducted as in Experiment 2. Rats were further subdivided into nicotine-shock (n = 8), nicotine-no shock (n = 9), saline-shock (n = 9), and saline-no shock (n = 8) groups. Testing began 10 days later following a period of continued (normal) nicotine availability.
Experiment 4
Experiment 4 examined the effects of experimenter-delivered nicotine on fear conditioning endpoints. The design was complementary to Experiment 1, but involving passive delivery of nicotine in 12-hours-on/-off periods, using programmable infusion mini-pumps. This design is intended to model conditions in humans where nicotine could be used prophylactically, such as in cases where trauma can be anticipated, by a safer delivery method (e.g., nicotine patch). The doses of nicotine and duration of the regimens were intended to approximate the parameters observed in the IVSA studies.
Programmable Mini-Pumps
Each rat was implanted with a s.c. programmable iPRECIO minipump (Model SMP-200; Alzet, CA) for administration of nicotine or saline. Mini-pumps were programmed to deliver nicotine in 12-hours-on/-off periods at 0.083 mg/h (total dose = 1.0 mg/d; “high dose”) or 0.025 mg/h (total dose = 0.3 mg/d; “low dose”) or an equivalent volume of saline (control rats). Prior to implantation, all pumps were pre-programmed and filled with nicotine solution or saline under sterile conditions. A single dose of the analgesic ketoprofen (5.0 mg/kg, s.c.) as well as the antibiotic amikacin (10 mg/kg, s.c.) were administered immediately prior to the surgery and for 2 days after surgery. On induction of isoflurane anesthesia, a small incision was made between the scapulae, and the minipump was placed in a subcutaneous pocket created behind the incision. The mini-pump was connected to an approximately 2-inch length of catheter tubing anchored and secured by sutures into a space below the incision. The wound was closed with silk sutures. Rats were allowed to recover for 7 days before any subsequent procedures began. Thereafter, rats were assigned to treatment groups (see below) and received scheduled replenishment of their mini-pumps. The mini-pumps were programmed such that the fear conditioning occurred 21 days after the final day of the surgical recovery period; that is, rats in the 21-day groups received treatment for the entire 21 days leading up to fear conditioning, whereas rats in the 10-day and 1-day groups received treatment only on the final 10 days and 1 day, respectively, and received saline on all other days. To ensure that the rats experienced similar light isoflurane (2%) anesthesia and minipump refill experience, mini-pumps were refilled with either nicotine or saline 3 times (days 1, 11, and 20 of the 21-day treatment regimen). Fear conditioning training coincided with the end of a 12 hour-on period to avoid nicotine withdrawal.
Fear Conditioning
Rats were divided into 9 conditions that differed with respect to treatment (nicotine and/or dose) and treatment duration (1, 10, or 21 days), yielding the following group assignments: 1-day/saline (n = 19), 1-day/low (n = 12), 1-day/high (n = 13), 10-day/saline (n = 18), 10-day/low (n = 14), 10-day/high (n = 13), 21-day/saline (n = 17), 21-day/low (n = 13), or 21-day/high (n = 15). No shock (control) groups were not used because no effects were seen in Experiments 1–3. Testing was conducted as described in Experiment 1.
Data Analyses
Prism 8 (GraphPad Software, San Diego, CA) and SPSS 24 (IBM, Armonk, NY) were used to perform Student’s t tests or ANOVAs with repeated measures. Significant effects were further analyzed using Bonferroni tests, which adjust P values to control for multiple comparisons.
RESULTS
IVSA: Experiments 1–3
For each rat, we analyzed the self-administration session during which they fulfilled criteria for stability and inclusion in the study. Rats self-administering nicotine (n = 46) obtained more infusions than rats self-administering saline (n = 50): a 2-way ANOVA with time as the repeated factor revealed a significant drug × time interaction (F[11,1034] = 10.77, P < .0001). Post hoc (Bonferroni’s multiple comparisons) tests revealed that nicotine rats received significantly more infusions than saline rats during hours 1–3 (P < .0001), 4 (P = .0006), 5 (P = .0002), 6 (P = .0002), 10 (P = .0123), and 12 (P = .0345) (Fig. 1A). Averaged across all times, rats self-administering nicotine obtained more infusions than rats self-administering saline (t[94] = 7.508, P < .0001) (Fig. 1B). Rats required 21.5 ± 1.8 days (mean ± SEM) to meet criteria for stable nicotine IVSA and received a total of 1.28 ± 0.05 mg/kg/session, which corresponds well with the range of doses known to have rewarding effects in rats (Bauco and Wise, 1994; Kenny et al., 2009); these data served as the basis for Experiment 4. When assessed for withdrawal signs during a 10-minute session at the time corresponding to their next 12-hour IVSA session (i.e., approximately 12 hours after their last session), post hoc tests indicated that rats self-administering nicotine had statistically significant higher numbers of shakes/tremors (t[94] = 3.41, P = .00134) (Fig. 1C), a sign of nicotine dependence. Although there were nominal increases in other indices, none reached significance. Rats were then distributed into matched groups among Experiments 1–3; there were no substantive differences in nicotine IVSA or withdrawal scores among the experimental groups.
Figure 1.
(A) Intravenous self-administration (IVSA) of nicotine in 12-hour sessions. Data reflect the mean ± SEMs for the 4 days preceding fear conditioning. (B) Average infusions per session collapsed across the entire session during the final 4 IVSA sessions. (C) Nicotine withdrawal signs 12 hours after the last 12-hour self-administration session. *P < .05, **P < .01, ***P < .001, Bonferroni’s tests.
Fear Conditioning: Baselines for Experiments 1–3
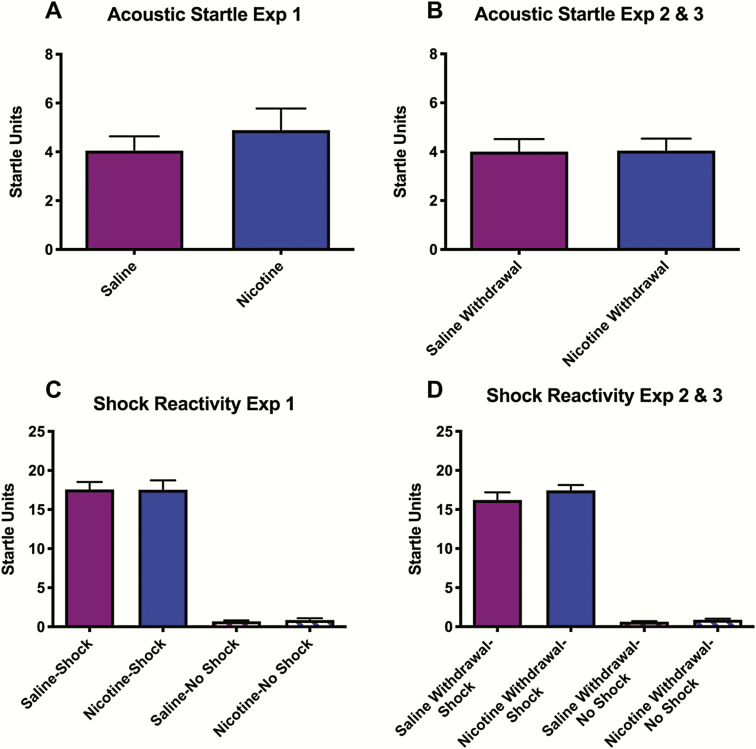
Fear conditioning was conducted either immediately after IVSA sessions (Experiment 1) or at what would be the time of the next IVSA session, during withdrawal (Experiments 2–3). Initial analyses examined treatment-related differences in sensitivity to the fear conditioning training stimuli. For rats in Experiment 1—before subdivision into shock and no shock groups—there were no differences in cage displacement in response to the acoustic startle stimuli (white noise bursts) between rats that had self-administered nicotine or saline (Fig. 2A). Similarly, for Experiments 2–3, there were no differences between rats that had self-administered nicotine or saline 12 hours earlier (Fig. 2B). These data suggest that neither self-administered nicotine nor withdrawal from self-administered nicotine affect acoustic startle. In addition, neither nicotine nor nicotine withdrawal affected shock reactivity. For Experiment 1, a 2-way ANOVA (drug × shock) on data collected during fear conditioning revealed a significant main effect of shock (F[1,29] = 514.1, P < .0001), but there were no effects of nicotine on shock-induced cage displacement nor a drug × shock interaction, suggesting that self-administered nicotine did not alter sensitivity to the foot-shock (Fig. 2C). Similarly, for Experiments 2–3, a 2-way ANOVA (drug × shock) on data collected during fear conditioning revealed a significant main effect of shock (F[1,59] = 652.8, P < .0001), but there were no effects of nicotine withdrawal on shock-induced cage displacement nor a drug × shock interaction, suggesting that withdrawal did not alter sensitivity to the foot-shock (Fig. 2D). These data rule out the possibility that effects of nicotine or nicotine withdrawal on fear conditioning are related to altered motor capacities or responsiveness to the acoustic startle stimulus or foot-shock. Rats in the nicotine withdrawal condition were then distributed into matched groups among the various conditions for Experiments 2–3; there were no substantive differences in these measures between the experimental groups.
Figure 2.
Effect of i.v. self-administration (IVSA) treatment on responsiveness to the acoustic startle stimulus and foot-shock. Data reflect mean (±SEM) platform displacement in arbitrary startle units. Neither nicotine (A) nor nicotine withdrawal (B) affected responsiveness to the acoustic startle stimulus (100-dB white noise burst) in rats before group assignment into shock or no shock conditions. Likewise, neither nicotine (C) nor nicotine withdrawal (D) affected responsiveness to foot-shock (0.5 seconds at 0.6 mA).
Experiment 1: CPS and FPS
Following a 10-day period without nicotine, rats received 3 test sessions separated by 48 hours to examine CPS and FPS. To determine if nicotine IVSA alters responsiveness to the trauma (shock)-associated context, we first examined CPS, a metric that compares startle reactivity to the startle stimuli before fear conditioning with that seen after fear conditioning (prior to the presentation of the first light + startle stimulus) during the test sessions. A 3-way (drug × shock × test) ANOVA with repeated measures on test session revealed a main effect of drug (F[1,29] = 4.39, P = .045) and a significant test × shock interaction (F[2,58] = 11.59, P < .0001) (Fig. 3A). Post hoc tests revealed that rats that had self-administered nicotine immediately before fear conditioning exhibited significantly less CPS than rats that had self-administered saline before fear conditioning during Test 1 (P = .018), although they did not differ on Tests 2 or 3. For FPS, a 3-way (drug × shock × test) ANOVA with repeated measures on test session revealed a significant main effect of shock (F[1,29] = 13.73, P = .009), indicating that rats that received fear conditioning with foot-shock had higher %FPS than those that did not receive foot-shock, as expected, but no other main effects or interactions (Fig. 3B). These data suggest that nicotine self-administration immediately preceding fear conditioning reduces reactivity to the shock-associated context, reflected by CPS, without affecting learning and memory of the fear conditioning, reflected by FPS.
Figure 3.
Startle responsiveness during testing in rats that received fear conditioning immediately after i.v. self-administration (IVSA) sessions when access to nicotine was unavailable during the 10 days between training and testing. (A) Context-potentiated startle (CPS) was reduced in rats fear conditioned immediately following the final nicotine IVSA session compared with saline controls during Test 1, but there were no differences Test 2 or Test 3. As expected, no-shock controls did not exhibit contextual fear at any time point. (B) Fear-potentiated startle (FPS) did not differ between rats fear conditioned immediately following the final nicotine IVSA session and saline controls. *P < .05, Bonferroni’s tests.
Experiment 2: CPS and FPS
The design of Experiment 2 was similar to that of Experiment 1 except that rats were in nicotine withdrawal during fear conditioning. Following the 10-day period of no additional access to nicotine, we first examined CPS. A 3-way (drug × shock × test) ANOVA with repeated measures on test session revealed significant main effects of shock (F[1,25] = 10.57, P = .0033) and test (F[2,50] = 4.967, P = .0108) on context-potentiated startle (Fig. 4A). No other main effects or interactions were observed. Post hoc tests revealed no differences in CPS between nicotine withdrawal and saline rats at Test 1. However, saline rats that received fear conditioning showed reductions in CPS from Test 1 to Test 2 (P = .0032) and from Test 1 to Test 3 (P = .0306), indicating between-session extinction. This reduction was not observed in the nicotine withdrawal rats. For FPS, a 3-way (drug × shock × test) ANOVA with repeated measures on test session revealed a significant main effect of shock (F[1,25] = 8.60, P = .0071), indicating that rats that received fear conditioning with foot-shock had higher %FPS than those that did not receive foot-shock but no other main effects or interactions (Fig. 4B). These data suggest that nicotine withdrawal immediately preceding fear conditioning disrupts extinction to the shock-associated context, reflected by CPS, without affecting learning and memory of the fear conditioning, reflected by FPS.
Figure 4.
Startle responsiveness during testing in rats that received fear conditioning 12 hours after i.v. self-administration (IVSA) sessions, during nicotine withdrawal, when access to nicotine was unavailable during the 10 days between training and testing. (A) There were no group differences in CPS during Test 1, but rats trained during nicotine withdrawal had persistently higher CPS during Test 2 and Test 3. As expected, no-shock controls did not exhibit contextual fear at any time point. (B) Fear-potentiated startle (FPS) did not differ between nicotine withdrawal rats and saline controls. *P < .05, **P < .01, Bonferroni’s tests.
Experiment 3: CPS and FPS
Experiment 3 was similar to Experiment 2 except that rats had normal access to nicotine during the 10-day period between fear conditioning and testing. For CPS, a 3-way (drug × shock × test) ANOVA with repeated measures on test session revealed significant main effects of shock (F[1,30] = 14.63, P = .0006) and test (F[2,50] = 4.69, P = .0127) (Fig. 5A). No other main effects or interactions were observed. As in Experiment 2, post hoc tests revealed no differences in CPS between nicotine withdrawal and saline rats at Test 1. However, saline rats that received fear conditioning showed significant reductions in CPS from Test 1 to Test 3 (P = .0138), indicating between-session extinction. For FPS, a 3-way (drug × shock × test) ANOVA with repeated measures on test session revealed significant main effects of shock (F[1,30] = 5.83, P = .022), again indicating that rats that received fear conditioning with foot-shock had higher FPS than those that did not receive foot-shock, as expected, but no other main effects or interactions were observed (Fig. 5B). These data are consistent with the findings in Experiment 2 showing that nicotine withdrawal immediately preceding fear conditioning disrupts extinction to the shock-associated context without affecting learning and memory of the fear conditioning and further indicate that this is not affected by continued access to nicotine during the period between fear conditioning and testing.
Figure 5.
Startle responsiveness during testing in rats that received fear conditioning 12 hours after i.v. self-administration (IVSA) sessions, during nicotine withdrawal, when access to nicotine was available during the 10 days between training and testing. (A) There were no group differences in CPS during Test 1, but responsiveness in saline-treated rats diminished between Test 1 and Test 3, whereas it remained persistently high in nicotine withdrawal rats. As expected, no-shock controls did not exhibit contextual fear at any time point. (B) Fear-potentiated startle (FPS) did not differ between nicotine withdrawal rats and saline controls. *P < .05, Bonferroni’s tests.
Experiment 4
Experiment 4 was designed to determine if the CPS-reducing effects of self-administered nicotine (Experiment 1) could be recapitulated by experimenter-programmed administration. Rats were fear conditioned immediately following the termination of a 12 hours-on/12 hours-off exposure regimen to a low (0.3 mg/kg/d) dose of nicotine, a high (1.0 mg/kg/d) dose of nicotine, or saline for 1, 10, or 21 days. Initial analyses examined treatment-related differences in sensitivity to the training stimuli used for fear conditioning. A 2-way (dose × duration) ANOVA revealed a significant interaction (F[4,125] = 2.46, P = .049). Post hoc tests revealed no group differences within each of the exposure durations (i.e., within the 1-, 10-, and 21-day exposures) and that the only differences were between groups in different treatment durations: specifically, low-dose nicotine in the 10-day condition was different from saline and the high-dose nicotine in the 21-day condition (P = .0428 and P = .0238, respectively) (Fig. 6A-C). A parallel 2-way ANOVA revealed no effects on cage displacement in response to the acoustic startle, nor was there an interaction between variables (Fig. 6E,F). These data suggest that experimenter-programmed nicotine did not cause systematic alterations in sensitivity to the acoustic startle or foot-shock.
Figure 6.
Effect of programmed s.c. treatment with nicotine at various doses and durations (1, 10, and 21 days) on responsiveness to the acoustic startle stimulus and foot-shock. There were no group differences in responsiveness to the acoustic startle stimulus (A–C) or foot-shock (D–F).
Following a 10-day period without additional nicotine exposure (as in Experiment 1), rats received 3 test sessions separated by 48 hours to examine CPS and FPS. For CPS, a 3-way (dose × duration × test) ANOVA with repeated measures on test session revealed a significant dose × duration interaction (F[2,375] = 8.25, P < .0001) but no other interactions. Because there was no significant effect of test, data were collapsed across sessions for subsequent analyses. A 2-way (dose × duration) ANOVA revealed a significant interaction (F[4,125] = 4.21, P = .0038), and post hoc tests revealed significant differences in CPS between saline and both low (P = .047) and high (P = .0038) doses of nicotine in the 10-day exposure condition (Fig. 7A–C). While there was a nominal increase in CPS in the high-dose nicotine group after 1 day of exposure—possibly reflecting acute stimulant effects of the drug—this effect did not reach statistical significance. No other between-group differences were observed. For FPS, a 3-way (dose × duration × test) ANOVA with repeated measures on test session revealed a significant main effect of dose (F[2,375] = 3.00, P = .05) but no effect of duration or test; nor were there significant interactions. Because there was no significant effect of test, we used a similar approach as with the CPS data and collapsed across sessions for subsequent analyses. A 2-way (dose × duration) ANOVA did not reveal main effects of dose or duration on FPS, nor was there a significant interaction (Fig. 7D–F). This pattern of data—nicotine-induced reductions in responsivity to the shock-related context without changes in learning and memory—broadly resembles that seen in Experiment 1, suggesting that experimenter-programmed administration of nicotine for a moderate duration produces the same types of effects as nicotine IVSA.
Figure 7.
Startle responsiveness during testing in rats that received fear conditioning immediately following programmed s.c. treatment with nicotine when access to nicotine was unavailable during the 10 days between training and testing. (A–C) CPS was reduced in rats that received 10 days of programmed nicotine exposure but not 1 day or 21 days of exposure. (D–F) FPS was not affected by programmed nicotine exposure at any dose or duration. *P < .05, Bonferroni’s tests.
Discussion
Nicotine exposure preceding aversive events has complex behavioral effects in male rats. Some effects can be conceptualized as being beneficial (stress-reducing), whereas others can be conceptualized as detrimental (stress-exacerbating). Factors including the nicotine dose, duration of treatment, and time since last nicotine exposure have major influences on outcomes, whereas whether the drug is self-administered or experimenter delivered and the route of administration (i.v. or s.c.) seem less critical.
We first examined the effects of i.v. nicotine self-administration in amounts sufficient to produce dependence, as indicated by stable drug intake and expression of classic nicotine withdrawal signs (shakes and tremors) 12 hours after the last exposure to nicotine. As expected, we found that control (saline-treated) rats showed elevated startle responses on reexposure to the shock-associated context, defined as CPS, reflecting a characteristic sign of fear conditioning (Shi and Davis, 2001). Increases in startle are thought to reflect hypervigilance, a diagnostic criteria for PTSD (American Psychiatric Association, 2013). In people with fear and anxiety disorders, hypervigilance is persistent and disruptive to normal behavior rather than reflecting heightened cognitive function or enhanced performance. In contrast, we discovered that rats fear conditioned immediately after nicotine self-administration showed reduced CPS, suggesting anxiolytic effects. Rats that were instead fear conditioned during nicotine withdrawal showed exaggerated CPS, suggesting anxiogenic effects. The anxiogenic effects of nicotine withdrawal at the time of fear conditioning persisted when nicotine self-administration was continued following fear conditioning, suggesting that nicotine withdrawal status at the time trauma is experienced is critical. In experiments designed to extend the finding that nicotine IVSA produces outcomes in fear conditioning tests that could be conceptualized as beneficial, we examined the effects of experimenter-programmed nicotine, discovering that nicotine delivered via subcutaneous minipump produced anxiolytic-like effects on CPS that were qualitatively similar to self-administered nicotine. None of the nicotine exposure regimens affected FPS, suggesting effects specific to the context in which the aversive learning event was experienced rather than broad effects on learning and memory processes. Likewise, none of the effects can be attributed to nicotine or nicotine withdrawal-induced alterations in motor capacities or sensitivity to shock, which would have been identified in the acoustic startle and shock reactivity tests. These results suggest that, under the conditions examined, nicotine’s anxiolytic effects prevail over its cognitive-enhancing effects. These studies raise the possibility of developing safe and effective methods to deliver nicotine or related drugs to mitigate stress effects, while also highlighting the importance of preventing withdrawal in nicotine-dependent individuals.
These studies were designed to approximate conditions relevant to humans. Humans who smoke or chew tobacco tend to do so on a daily basis, with periods where nicotine use is likely (during wakefulness) and unlikely (during sleep). Accordingly, we utilized long (12 hours) access to nicotine in the IVSA studies and programmed the mini-pumps to deliver the nicotine on a 12 hours-on/12-hours off schedule. Nicotine abstinence in humans who are dependent causes signs of withdrawal, so we designed the studies to examine whether there would be behavioral differences if the trauma had been encountered shortly after nicotine exposure or during withdrawal. Some types of trauma result in reduced nicotine availability for extended periods—for example, during hospitalization—so we examined parallel conditions where nicotine access was either available or discontinued during the period between the fear conditioning and the testing. We used a 10-day period because previous work indicates that the physical and motivational aspects of nicotine withdrawal are resolved within this period (Kenny and Markou, 2001), making it unlikely that nicotine withdrawal at the time of testing affected CPS or FPS. Indeed, while withdrawal was not explicitly measured following the 10-day period between fear conditioning and testing, no physical signs were observed before the tests. In humans, nicotine intake via tobacco products is linked to decreased fitness and increased risk of diseases such as cancer. As such, we used SC mini-pumps to model a safer route of delivery (transdermal patch). The doses of nicotine and durations of exposure used for the programmed nicotine delivery studies were derived from the IVSA studies (which showed how long it took rats to fulfill stability criteria and how much nicotine they self-administered each day on meeting that criteria) according to the principle that minimizing nicotine doses and exposure durations would be desirable when exploring its potential benefits.
An important premise for this work is the fact that nicotine can enhance alertness and cognitive performance across species (Spielberger, 1986; Newhouse et al., 2004; Semenova et al., 2007; Heishman et al., 2010; Raybuck and Gould, 2010). However, the effects of nicotine on learning and memory are complex and can vary due to a number of factors including dose, administration route, treatment duration, age at exposure, biological sex, and the specific task examined (Kenney and Gould, 2008; Mateos et al., 2011). For our studies, we selected fear conditioning because it is a translationally relevant endpoint (Pitman et al., 1999) often used in PTSD research. Indeed, we showed that different doses and durations of nicotine exposure can produce fundamentally different outcomes. Rats that received 1 day of nicotine by exposure via minipump had, if anything, elevated responses to the fear conditioned environment, raising the possibility that a period of nicotine exposure is needed before beneficial (anxiolytic-like) effects emerge. Similarly, the therapeutic-like effects seen after 10 days of minipump exposure were not seen after 21 days, suggesting that there is an optimal duration of nicotine treatment and effects may be lost after longer periods. Possible explanations for differences between 21 days of IVSA and 21 days of programmed nicotine delivery include differences in the moment-to-moment patterns in nicotine intake and blood levels between these routes. There are dynamic interactions between various types of neuroplasticity, including tolerance and sensitization, which differ according to drug dose, patterns of exposure (intermittent vs continuous), and exposure duration (Stewart and Badiani, 1993).
Although we intended to model the human condition, there are obvious caveats. While IVSA in rats models voluntary drug self-administration seen in humans, there are major differences between this route and smoking or chewing tobacco. Likewise, programmed delivery via s.c. minipump differs from delivery via transdermal patch. While there are cases where transdermal delivery of a drug creates adherence issues, the nicotine patch is widely and safely used for smoking cessation. There is a large number of additional permutations that could be examined in the future. Our studies were performed in nicotine-naïve rats; considering that nicotine effects can change with repeated exposure (Bauco and Wise, 1994; Tapper et al., 2004), the results may be considerably different in individuals previously exposed to nicotine. Delivery of nicotine by transdermal patch is likely safer than by smoking or chewing tobacco, but more work is needed to determine if outcomes could be further improved by altering exposure patterns or durations. It may be possible to utilize other drugs that share nicotine’s pharmacological actions but have fewer side effects or superior pharmacokinetic/pharmacodynamic profiles. The present studies set the stage for additional work to optimize drug delivery parameters and identify mechanisms.
Many factors that influenced our experimental design derive from the fact that the research was originally conceptualized to address issues relevant to the military, a population in which tobacco use is prevalent—and historically condoned—and for which stress exposure can often be predicted in advance (Grier et al., 2010; Hoge, 2004). While the demographics of soldiers serving in combat were predominantly male when we started this work, the prevalence of women in this role is increasing (Patten and Parker, 2011). It will be important to determine in future work if the findings with nicotine discovered here are broadly applicable to females, which show differences in sensitivity to other self-administered substances (Becker and Chartoff, 2019), in light of gender differences in the prevalence of stress-related illness (Ressler et al., 2011; Kimerling et al., 2018).
In addition to military relevance, these findings might also be applicable to civilian populations. While everyday stress can be unpredictable, there is often some lead-time preceding exposure to some of the most severe, debilitating, and costly forms of stress. Prophylactic treatments for stress could be useful for civilian first-responders or individuals assigned to assist in recovery or investigation efforts after accidents, natural disasters, or terrorist acts (Van’t Veer and Carlezon, 2013). Our findings suggest that passive nicotine delivery might reduce pathological responses that occur in contexts that have broad similarities with those in which a trauma was experienced. Although there are always risks associated with using addictive substances for medicinal benefit, it is well established that the nicotine patch and other nicotine replacement therapies have very low abuse liability (Stead et al., 2012). In fact, transdermal nicotine causes only mild subjective effects that are typically described as aversive, consistent with low abuse liability (Barr et al., 2008; Ashare et al., 2010). Additionally, the potential benefits of using nicotine to mitigate the persistent and debilitating effects of traumatic stress should be balanced with long-term risks of exposure, including putative transgenerational effects (Goldberg et al., 2019). Regardless of the medical possibilities for enhancing resilience and/or reducing new cases of stress-related illness, a critical finding is that nicotine withdrawal can exacerbate behavioral signs of stress susceptibility. Thus, if making nicotine available either for voluntary or medicinal use, it is essential that it always be available in sufficient quantities to avoid withdrawal.
Acknowledgments
This work was supported by research contracts from the United States Army (W81XWH-12-1-0454 and W81XWH-17-1-0001 to W.C.).
Interest Statement
Within the last 2 years, W.C. has served as a consultant for Psy Therapeutics. None of the other authors report any disclosures.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Ashare RL, Baschnagel JS, Hawk LW Jr (2010) Subjective effects of transdermal nicotine among nonsmokers. Exp Clin Psychopharmacol 18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Evins AE(2008) The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology 33:480–490. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA (1994) Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther 271:294–301. [PubMed] [Google Scholar]
- Becker JB, Chartoff E (2019) Behavioral pharmacology of novel kappa opioid receptor antagonists in rats. Neuropsychopharmacology 44:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Wiley MT, Miller SC, Dennis MF, Wilson SM, McClernon FJ, Calhoun PS (2008) Ad lib smoking in post-traumatic stress disorder: an electronic diary study. Nicotine Tob Res 10:1149–1157. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. (2010) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. New Engl J Med 62:2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS (1996) Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 153:369–375. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Wagner HR, McClernon FJ, Lee S, Dennis MF, Vrana SR, Clancy CP, Collie CF, Johnson YC, Beckham JC (2011) The effect of nicotine and trauma context on acostic startle in smokers with and without posttraumatic stress disorder. Psychopharmacol 215:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A (2008) Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. (1986) Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci 100:814–824. [DOI] [PubMed] [Google Scholar]
- Davis M. (1993) Pharmacological analysis of fear-potentiated startle. Braz J Med Biol Res 26:235–260. [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET (1987) Conditioned aversion after delay place conditioning with nicotine. Psychopharmacology (Berl) 92:376–381. [DOI] [PubMed] [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM (2007) Post-traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res 9:1071–1084. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP (2000) Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol 387:59–62. [DOI] [PubMed] [Google Scholar]
- Gelernter J, et al. ; Department of Veterans Affairs Cooperative Studies Program (#575B) and Million Veteran Program (2019) Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci 22:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR, Zeid D, Kutlu MG, Cole RD, Lallai V, Sebastian A, Albert I, Fowler CD, Parikh V, Gould TJ (2019) Paternal nicotine enhances fear memory, reduces nicotine administration, and alters hippocampal genetic and neural function in offspring. Addict Biol:e12859. doi: 10.1111/adb.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier T, Knapik JJ, Canada S, Canham-Chervak M, Jones BH (2010) Tobacco use prevalence and factors associated with tobacco use in new U.S. Army personnel. J Addict Dis 29:284–293. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J (2003) A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 114:1557–1579. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 210:453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR (1983) Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav 19:989–992. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL (2004) Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 351:13–22. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI (2009) The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology 56:970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ (2008) Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol 38:101–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA Jr, Markou A (2009) NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology 34:266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A (2001) Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70:531–549. [DOI] [PubMed] [Google Scholar]
- Kimerling R, Allen MC, Duncan LE (2018) Chromosomes to social contexts: sex and gender differences in PTSD. Curr Psychiatry Rep 20:114. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA Jr (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Viveros MP (2011) Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. J Psychopharmacol 25:1676–1690. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA Jr (2006) Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Ann N Y Acad Sci 1071:538–541. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ (2006) Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol 73:61–71. [DOI] [PubMed] [Google Scholar]
- Morgan CA 3rd, Grillon C, Southwick SM, Davis M, Charney DS (1995) Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry 38:378–385. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA Jr, Davis M (2011) Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A (2004) Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol 4:36–46. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ (2013) Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 16:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten E, Parker K (2011) Women in the U.S. Military: growing share, distinctive profile. Retrieved from www.pewsocialtrends.org/files/2011/12/22/women-in-the-u-s-military-growing-share-distinctive-profile/.
- Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA (1999) Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry 4:234–241. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A (2010) Erasing fear memories with extinction training. J Neurosci 30:14993–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ (2010) The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem 94:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V (2011) Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Oakes RA (2009) Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav 51:457–461. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A (2007) Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol Biochem Behav 87:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Davis M (2001) Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. J Neurosci 21:9844–9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. (1986) Physiological determinants of smoking behavior. In: Smoking and society: toward a more balanced assessment (Tollinson RD, ed), pp 89–134. Lexington, MA: Lexington Books. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T (2012) Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 11:CD000146. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4:289–312. [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB (2005) Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci 32:9–20. [DOI] [PubMed] [Google Scholar]
- Thorndike FP, Wernicke R, Pearlman MY, Haaga DA (2006) Nicotine dependence, PTSD symptoms, and depression proneness among male and female smokers. Addict Behav 31:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA Jr (2013) Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 229:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Desai RA, McKee SA (2010) Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend 108:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]