Abstract
Nutmeg plant (Myristica fragrans Houtt) is known as one of traditional medicine. The nutmeg root has a strong potential in antioxidant and anticancer agents among other nutmeg plant parts. The n-hexane root extract has been carried out by thin-layer chromatography and obtained 8 fractions (labeled as Myristica fragrans Houtt Root: MFHR 1 − 8). Specifically, the MFHR 4 has been purified for several times to obtain a yellow-brown color. Furthermore, lignan compound 6′-methyl-(7‑hydroxy-8-methylbut-9-en)-3,2′-dimethoxybiphenyl-4,5-diol) was identified with chemical formula of C20H24O5 and analyzed using UV–vis spectroscopy, fourier-transform infrared spectroscopy (FTIR), 1D/2D nuclear magnetic resonance (NMR), and liquid chromatography–mass spectrometry (LC-MS). Based on MTT assay, MFHR demonstrated moderate anticancer activity against MCF-7 cell lines of 51.95 µM, meanwhile, DPPH activity confirmed the strong antioxidant activity with IC50 value of 12.67 ppm.
Keywords: Nutmeg root, N-hexane extract, Antioxidant, Anticancer, Lignan
Specifications Table
| Subject | Biochemistry, Organic Chemistry, Phytochemistry |
| Specific subject area | Antioxidant and anticancer |
| Type of data | Table, Figure, Graph |
| How data were acquired | UV–vis spectrophotometer, FTIR, 1D/2D NMR, LC-MS, and MTT assay |
| Data format | Analyzed |
| Parameters for data collection | Thin layer chromatography (TLC) were used to isolate n-hexane extract of nutmeg root (MFHR) where 8 fractions was obtained. |
| Description of data collection | The antioxidant activity of the extract was carried out by the free radical DPPH method and 3–4, 5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide (MTT) assay was used to evaluate the anticancer properties of MFHR against MCF-7 cell lines. |
| Data source location | City/Town/Region: Kampung Paya Village, North Kluet District, South Aceh Province Country: Indonesia |
| Data accessibility | The data are included in this article |
| Related research article | none |
Value of the Data
-
•
This data provides helpful leads for pharmacological field, n-hexane extracts of nutmeg root demonstrated great potential as antioxidant and anticancer activity.
-
•
The data reported here provides insight to identify the bioactive molecules such as lignan compound from the n-hexane extracts that possessed antioxidant and anticancer activity.
-
•
The dataset obtained from the UV–Vis, FTIR, LC-MS, NMR and MTT assay, will guide researchers with the utilization of lignan compound for further investigation in anticancer besides against the MCF-7 cell lines.
-
•
The LC-MS and NMR dataset from the characterization of the obtained extract from nutmeg root will provide researchers with the biochemical constituents and other possible applications of the nutmeg root.
1. Data description
The yield of n-hexane extract of nutmeg root of 18.21% was obtained. The thin layer chromatography (TLC) has been used to identify each compound in n-hexane root extracts and 8 fractions were obtained and labeled as Myristica fragrans Houtt Root (MFHR) 1 – 8. To observe the antioxidant activity, the DPPH assay was employed. The DPPH assay offers a low-cost method to evaluate potential antioxidants by spectrophotometry as indicate at λmax of 517 nm. The percentage of inhibition of n-hexane extract-based sample at various concentrations of 25, 50, and 100 ppm were 52.80%, 69.27%, and 75.00%, respectively. The MFHR 4 − 8 exhibit a strong antioxidant activity with relatively low percentage of half maximal inhibitory concentration (IC50) value implying higher percentage inhibition. Although, the MFHR 6 shows the strongest antioxidant activity with the lowest IC50 value of 6.00 ppm, however lower percentage inhibition with lower than 40%. Furthermore, the MFHR 1 – 3 had the weakest antioxidant activity as indicating of higher IC50 value of 380.02, 590.51, and 604.44 ppm, respectively. The results of antioxidant activity tests are summarized in Table 1.
Table 1.
Summary of the antioxidant activity of n-hexane nutmeg root extracts at various concentrations.
| Sample | Inhibition of concentration (%) | IC50 (ppm) | ||
|---|---|---|---|---|
| 25 ppm | 50 ppm | 100 ppm | ||
| n-hexane extract | 52.80 | 69.27 | 75.00 | 0.216 |
| MFHR-1 | 14.55 | 21.33 | 22.55 | 380.02 |
| MFHR-2 | 12.24 | 12.85 | 17.09 | 590.51 |
| MFHR-3 | 19.64 | 20.61 | 23.52 | 604.44 |
| MFHR-4 | 29.33 | 46.18 | 60.85 | 69.64 |
| MFHR-5 | 44.61 | 71.27 | 85.21 | 24.54 |
| MFHR-6 | 27.15 | 34.55 | 36.55 | 6.00 |
| MFHR-7 | 57.94 | 72.24 | 84.12 | 39.51 |
| MFHR-8 | 41.45 | 58.18 | 73.58 | 55.32 |
The antioxidant activity is expressed by the IC50 value of each fraction. The IC50 of MFHR 1 − 8 are higher than the result of the antioxidant activity of n-hexane extract which has an IC50 value of 0.216 ppm. This shows that the n-hexane extract-based sample has better inhibitory activity due to the presence of several active compounds, which is in good accordance with previous report that nutmeg root extracts consists of seven main compounds namely 5-octadecanoic acid, methyl ester, linalool, cyclopropanepentanoic acid-2-undercyl-methyl ester-trans-, methoxyeugenol, eugenol, hexadecenoic acid, methyl ester, and myristicin [1]. The complex fat compounds can synergize in inhibiting free radicals, thus will help to make the antioxidant activity of pristine n-hexane extract MFHR stronger than its fractions.
Although the fraction exhibit lower antioxidant activity than the pristine n-hexane extracts, the specific compound that plays a role as an antioxidant agent is still not clear. To identify it, the fractionated extract needs further analysis. Among 8 fractions, the MFHR-4 exhibited better separation, therefore the purification of MFHA-4 crystals was carried out using a column chromatography method with eluent n-hexane: ethyl acetate (9:1) gradient. The results of TLC monitor had a staining pattern and purification by washing the MFHR-4 with acetone and n-hexane for several times to obtain yellow brownish color powder of 4 mg and melting point of 132 - 136 °C. After several times of purifications, the MFHR subfraction shows better antioxidant activity with lower IC50 of 12.67 ppm than the first extraction process of MFHR-4.
The nutmeg root extracts are expected to have a high potential activity for the anticancer treatment. The anticancer activity of nutmeg plant methanol extract has been tested on osteosarcoma cells (MG-63) using the MTT assay and exhibited an IC50 value of 40.39 μg/mL (Chakraborty et al., 2015). Furthermore, the lower IC50 value of 24.83 μg / mL has been also reported on Vero Cell Line using the MTT assay (Piaru et al., 2012). To determine the anticancer activity level, the toxicity level was measured by analyzing the inhibitor concentration 50 (IC50). The standard guideline has been established by NCI to analyze the IC50 value. The smaller the IC50 value means the stronger the anticancer activity. It has been reported that if IC50 below 20 ppm is classified to have strong anticancer activity for extract compounds, while below 4 ppm was for pure compound [2].
The breast cancer against MCF-7 cell lines has been examined in order to evaluate the anticancer activity of n-hexane extract at various concentrations of 25, 50, 100, and 200 ppm. Doxorubicin is also tested as a control sample. The absorbance of each sample was analyzed at a wavelength of 595 nm and relatively high IC50 of 51.95 µM of n-hexane extract of nutmeg root was obtained. Based on the IC50 value, the MFHR-4 demonstrated a moderate anticancer activity due to the higher IC50 value above 20 µM [3]. Although it has a weak anticancer activity, the n-hexane extract of nutmeg root has a high potential to further develop as a natural antioxidant and anticancer agent.
Ultraviolet-visible (UV–vis) absorption spectra and Fourier-transform infrared spectrum (FTIR) were performed to identify the specific compound in the MFHR. There are two peaks at 269 and 275 nm which indicate the presence of two substituted benzene rings of the compound as shown in Fig. 1a. Moreover, according to the UV absorption existence at a wavelength of 250−280 nm is a typical type of spectrum of phenolic or lignan compound, which is in good agreement with previous report (Filleur et al., 2002; Zhang et al., 2014). Fig. 1b shows the FTIR analysis results. It shows several peaks at wavenumbers of 3446−3408 cm−1, 3143−3014 cm−1, and 2937−2858 cm−1 which indicate the presence of OH stretch, C—H stretch, and the C—H stretch for methyl compound. Moreover, the C = C stretch ring and C—O-C stretch are found at peaks at 1604−1452 cm−1 and 1271−1138 cm−1 which is correspond to lignan compound.
Fig. 1.
(a) UV–vis absorption spectrum and (b) FT-IR analysis results of MFHR 4 according to Tables 1 and 2 data.
Table 1. UV–Vis spectrum data of MFHR 4 compound based on Fig. 1a.
| Wavelength (nm) | Absorption (a.u.) |
|---|---|
| 226.8 | 0.92041 |
| 228.3 | 0.77877 |
| 228.7 | 0.61789 |
| 230.9 | 0.50251 |
| 231.8 | 0.40989 |
| 233.5 | 0.35761 |
| 235.0 | 0.34595 |
| 241.0 | 0.31506 |
| 242.3 | 0.31049 |
| 246.9 | 0.32021 |
| 247.2 | 0.32186 |
| 252.5 | 0.34786 |
| 252.8 | 0.38198 |
| 252.8 | 0.37711 |
| 258.7 | 0.47465 |
| 260.9 | 0.56589 |
| 262.8 | 0.53826 |
| 264.7 | 0.56731 |
| 265.2 | 0.57239 |
| 265.6 | 0.62114 |
| 266.7 | 0.65851 |
| 268.0 | 0.64714 |
| 269.1 | 0.66989 |
| 270.1 | 0.64226 |
| 270.6 | 0.60849 |
| 271.0 | 0.57889 |
| 271.9 | 0.53826 |
| 272.9 | 0.47001 |
| 274.2 | 0.53826 |
| 274.5 | 0.59676 |
| 275.5 | 0.68614 |
| 276.5 | 0.61364 |
| 276.8 | 0.60814 |
| 277.2 | 0.52201 |
| 277.4 | 0.45214 |
| 279.0 | 0.35111 |
| 281.7 | 0.23249 |
| 282.4 | 0.22754 |
| 288.4 | 0.18636 |
| 294.3 | 0.16062 |
| 295.9 | 0.15449 |
| 300.2 | 0.14517 |
| 306.1 | 0.14003 |
| 312.1 | 0.12973 |
| 315.8 | 0.12361 |
| 318.0 | 0.12458 |
| 323.9 | 0.11429 |
| 329.8 | 0.10914 |
| 330.9 | 0.11061 |
| 335.8 | 0.10399 |
| 341.7 | 0.10399 |
| 347.6 | 0.10399 |
Table 2. FTIR spectrum data of MFHR 4 compound based on Fig. 1b.
| Wavenumber (cm−1) | Transmittance (%) |
|---|---|
| 3982.58 | 87.91161 |
| 3937.82 | 86.57552 |
| 3932.85 | 85.93652 |
| 3893.07 | 86.0527 |
| 3873.17 | 85.41371 |
| 3858.25 | 86.28507 |
| 3818.47 | 84.13571 |
| 3828.42 | 85.29752 |
| 3788.63 | 84.60043 |
| 3748.84 | 84.65852 |
| 3728.95 | 83.72907 |
| 3728.95 | 83.03198 |
| 3694.14 | 82.79962 |
| 3664.30 | 80.81485 |
| 3634.46 | 79.47876 |
| 3594.68 | 77.96839 |
| 3549.92 | 79.42066 |
| 3525.05 | 80.81485 |
| 3485.27 | 80.58248 |
| 3425.59 | 81.39575 |
| 3360.94 | 81.1053 |
| 3326.13 | 79.65303 |
| 3301.26 | 78.43312 |
| 3291.32 | 76.51612 |
| 3266.45 | 74.94767 |
| 3226.67 | 75.76094 |
| 3196.83 | 74.94767 |
| 3191.85 | 76.74849 |
| 3176.93 | 77.96839 |
| 3162.01 | 79.07212 |
| 3082.44 | 79.07212 |
| 3027.74 | 78.02648 |
| 2947.34 | 78.02648 |
| 2872.74 | 77.38748 |
| 2827.99 | 76.6323 |
| 2758.36 | 76.39994 |
| 2693.71 | 75.93521 |
| 2624.09 | 75.4124 |
| 2509.70 | 75.29621 |
| 2415.21 | 74.54103 |
| 2335.64 | 73.84394 |
| 2280.94 | 73.08876 |
| 2231.21 | 72.15931 |
| 2191.42 | 72.50785 |
| 2111.85 | 71.52031 |
| 2101.91 | 70.00995 |
| 2057.15 | 67.68631 |
| 2037.26 | 69.89376 |
| 2007.42 | 71.46222 |
| 1957.69 | 69.54522 |
| 1952.71 | 67.51204 |
| 1922.87 | 65.17872 |
| 1882.26 | 66.5729 |
| 1847.45 | 65.87581 |
| 1807.66 | 67.62822 |
| 1787.77 | 69.25476 |
| 1733.07 | 67.57013 |
| 1683.33 | 66.22436 |
| 1648.52 | 67.68631 |
| 1603.76 | 66.75686 |
| 1608.74 | 65.64345 |
| 1559.01 | 66.34054 |
| 1544.09 | 65.41109 |
| 1514.25 | 64.59781 |
| 1474.46 | 66.81495 |
| 1444.62 | 65.52727 |
| 1419.76 | 64.6559 |
| 1384.95 | 66.68908 |
| 1350.13 | 68.90622 |
| 1295.43 | 67.39586 |
| 1255.64 | 66.28245 |
| 1225.81 | 68.15104 |
| 1195.97 | 66.34054 |
| 1181.05 | 65.23681 |
| 1166.13 | 63.95881 |
| 1116.40 | 65.58536 |
| 1091.53 | 65.12063 |
| 1026.88 | 64.24927 |
| 987.10 | 64.94636 |
| 957.26 | 64.19118 |
| 912.50 | 65.46918 |
| 852.82 | 64.6559 |
| 787.34 | 63.37791 |
| 717.72 | 62.56463 |
| 638.15 | 61.34473 |
| 613.28 | 62.50654 |
| 583.44 | 63.26172 |
| 538.68 | 61.92563 |
| 518.79 | 60.70573 |
| 488.95 | 59.25345 |
| 454.14 | 61.22854 |
| 424.30 | 59.02109 |
| 404.41 | 61.69327 |
Table 2.
The chemical shifts of 1H and 13C NMR of MFHR 4.
| Number of Carbon | 500 MHz; CDCl3 ppm J in Hz | |
|---|---|---|
| 1HNMR δ | 13CNMR δ | |
| 1 | – | 132.35 (s) |
| 2 | 6.97 | 109.00 (d) |
| 3 | 6.89 | 114.21 (d) |
| 4 | 6.78 | 113.45 (d) |
| 5 | −3.88 | 146.70 (s) 56.14 (d) |
| 6 | – | 132.22 (s) |
| 7 | 5.63 | 93.98 (d) |
| 8 | 3.45 | 45.78 (d) |
| 8 | 1.38 | 17.70 (q) |
| 9 | 6.35 | 131.07 (d) |
| 10 | 6.13 | 123.69 (d) |
| 11 | 1.86 | 18.58 (q) |
| 1′ | – | 133.42 (s) |
| 2′ | 6.97 | 109.16 (d) |
| 3′ | - 3.88 |
146.81 (s) 56.14 (d) |
| 4′ | – | 144.31 (s) |
| 5′ | – | 145.92 (s) |
| 6′ | 6.89 | 120.16 (d) |
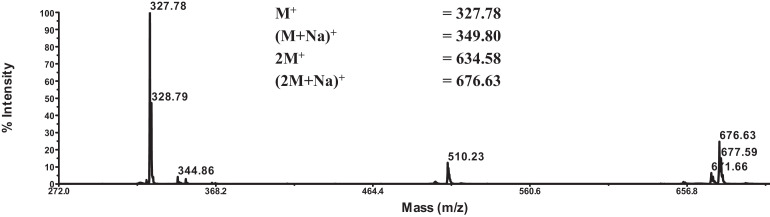
The structure of pure isolate n-hexane MFHR-4 is expected to be a group of phenolic compounds or lignans. The similar chemical structure of lignan “argenteane” from nutmeg has been reported with only different on the aliphatic chain [4, 5]. Mass spectra analysis was performed with the liquid chromatography-mass (LC-MS). As a result, it was found the present of molecular ions at m/z 344.86 (M + H)+ as shown in Fig. 2. It confirms that the MFHR 4 compound has a molecular weight of 344.86 with the chemical formula of C20H24O5 which is consistent with UV–Vis and FTIR result.
Fig. 2.
LC-MS spectrum analysis of MFHR 4.
To further elucidate the lignan compound in the MFHR 4, NMR analysis was carried out. NMR spectroscopy provides comprehensive and more accurate information on both quantitative and qualitative analysis. The 1H NMR and 13C NMR spectra of the MFHR 4 compound were obtained by dissolving the sample in the chloroform-d (CDCl3) as a solvent. The spectra recorded on the JEOL spectrophotometer (1H NMR 500 MHz and 13C NMR 125 MHz) are summarized in Table 2 and the raw data can be referred to Supplementary Materials.
The 1H NMR spectra in Fig. 3a shows the presence of two methyl protons (CH3) in the δH 1.37 (d) and δH 1.86 regions (d). Two methoxyl (OCH3) groups are in the δH 3.88 (s) area and nine methine (CH) groups in the 97H 6.97 (d) area, δH 6.89 (d) two protons, δH 6.78 (d) two protons, δH 6.35 (d), δH 6.13 (d), δH 3.45 (d), δH 5.10 (d) as shown in Fig. 3b.
Fig. 3.
1H—NMR spectra for MFHR-4.
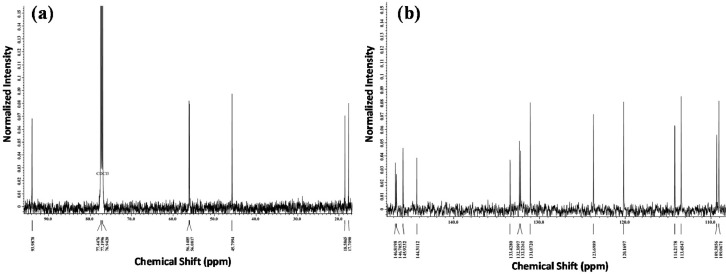
Fig. 4 shows the 13C NMR spectra of MFHR 4. It shows the presence of 20 carbon atoms consisting of seven quaternary carbon that found in the region of δC 132.2 (s), δC 132.3 (s), δC 133.4 (s), δC 144, 3 (s), δC 145.9 (s), δC 146.7 (s) and δC 146.8 (s). Two methyl carbon (CH3) are shown in the δC 17.7 (q) region, and δC 18.5 (q). Furthermore, nine carbon methins (CH) are also shown in the δC 45.7 (d), δC 93.9 (d), δC 109.0 (d), δC 109.3 (d), δC 113, 4 (d), δC 114.2 (d), δC 120.1 (d), δC 123.6 (d) and 131.0 (d). Besides, the existence of two methoxyl groups (OCH3) in the δC 56.14 (d) and δC 56.14 (d) regions were also obtained.
Fig. 4.
13C—NMR spectra for MFHR 4.
The 1H–1H COSY (Correlation Spectroscopy) spectrum is shown in Fig. 5a. It shows that the proton from methyl (CH3) δH 1.38 ppm is associated with protons 3,H 3.45 ppm (H-8) and δH 6.35 ppm (H-9). The proton from methyl at 1,H 1.86 ppm (H-11) corresponds to the proton dH 6.13 ppm (H-10) and dH 6.35 ppm (H-9). Protons from metin δH 3.45 ppm (H-8) correspond to protons δH 5.10 ppm (H-7) as well as protons from methine (CH) 5,H 5.10 ppm (H-7) associated with proton dH 3.45 ppm (H-8). The methane proton (CH) δH 6.35 ppm (H-9) corresponds to the proton δH 6.13 ppm (H-10) and δH 1.86 ppm (H-11). In the A ring, the proton of methine (CH) δH 6.78 ppm (H-3) corresponds to protons δH 6.89 (H-4) and δH 6.97 ppm (H-5). The proton from methine (CH) δH 6.89 (H-4) corresponds to the proton δH 6.78 ppm (H-3) and δH 6.97 ppm (H-5). Protons from methine (CH) δH 6.97 ppm (H-5) are associated with protons δH 6.89 ppm and δH 6.89 ppm (H-4).
Fig. 5.
Key 1H- 1H COSY and HMBC correlations of compound.
Furthermore, hetero multiple bond connectivity (HMBC) spectra show the existence of a proton relationship with carbon more than one bond apart as shown in Fig. 5b. The ordinate axis in the HMBC spectrum was plotted with the chemical nucleus shift (13C) and the x-axis was plotted with the proton-nucleus chemical shift (1H). In the HMBC spectrum of the MFHR 4 exhibit 3 bonds of the proton signal δH 6.97 ppm (H-2 ′) associated with δC 133.42 ppm (C-1 ′), δC 109.0 ppm (C-2), C 114.21 ppm (C-3). Moreover, the protons at δH 6.89 ppm (H-6 ′) give 2 bonds associated with δC 133.42 (C-1 ′) and 132.22 ppm (C-6). The 78H signal 6.78 ppm (H-4) corresponds to δC 114.21 ppm (C-3) and δC 146.70 ppm (C-5). The proton δH 6.13 ppm (H-10) corresponds to δC 131.07 ppm (C-9). Based on the result of 2D NMR from COSY and HMBC, the suggested chemical structure of the lignin compound is successfully demonstrated in Fig. 5. This result is in good agreement with LC-MS, FT-IR and UV–vis results.
2. Experimental design, materials and methods
2.1. Material and sample preparation
All the solvent used in this work such as methanol, petroleum ether, n-hexane, and ethyl acetate were purchased from Merck Chemicals without further purification. Furthermore, the same solvent that functions as an eluent is used as a pro analysis solvent, 60 G silica, and 2,2-diphenyl-1-picrylhydrazyl (DPPH).
The nutmeg roots were collected from Kampung Paya Village, North Kluet District, South Aceh Province. The sample was carried out with several stages of work to separate the secondary metabolites of nutmeg root. Nutmeg root extraction was obtained by flowing distilled water to remove the dirt and followed by oven dry for overnight. Subsequently, the dried nutmeg root was blend and the powder of 483 g was macerated with n-hexane for 24 h. After that, the solution was filtered and evaporated with a rotary evaporator. As a result, the extract of n-hexane nutmeg root was obtained.
2.2. Isolation of n-hexane extract of nutmeg root
Nutmeg root n-hexane extract 4.35 g was further isolated using column chromatography, using 60 G silica gel as the stationary phase and then the eluent was determined by thin layer chromatography (TLC). The n-hexane extract was separated by its components and each fraction was collected in a test tube.
2.3. Antioxidant and anticancer activity test
The antioxidant activity of the extract was carried out by the free radical DPPH method where vitamin C (0.01–0.1 mg) was used as a reference according to previous report [6]. To investigate the anticancer activity of n-hexane extract of nutmeg root, the breast cancer (MCF-7) cells was obtained from Animal Study Center in Institut Pertanian Bogor, Indonesia according to ATCC protocols [7] has been carried out at various concentrations of 25, 50, 100, and 200 ppm. As a positive control, the doxorubicin was employed, where the cells were grown at a concentration of 5000 cells in 100 µL growth medium. The extract was added after the cells reached 50% confluence (24 h). The 3–4, 5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide (MTT) assay test was carried out on day 3, by adding MTT concentration of 5 mg / mL in 10 µl per tube and incubated for 4 h at 37 °C. Then, the formazan crystals are dissolved in ethanol. The absorbance reading was carried using VersaMax Elisa Microplate Reader at a wavelength of 595 nm.
2.4. Characterizations
The structure of the isolated compound was determined by NMR (1H and 13C-on JEOL 500 spectrometer; 500 MHz for 1H NMR; 125 MHz for 13C NMR). UV–vis spectra were obtained using a Shimadzu Variant Cary 100. Fourier transform infrared spectroscopy (FTIR) analysis was perform using Perkin Elmer FTS FT-IR spectrometer. EI-MS mass spectra by LC-MS / MS (HPLC Alliance 2695, Waters Detector Photodiode Array 2996).
Ethics statement
None
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Acknowledgments
The authors thank the Lembaga Penelitian dan Pengabdian Masyarakat (LPPM) of Universitas Syiah Kuala, Banda Aceh for facilitating this research and the Ministry of Health of the Republic of Indonesia, Head of the Center for Research and Development of Medicinal Plants and Traditional Medicines who have provided funding for this research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.105997.
Appendix. Supplementary materials
References
- 1.Ginting B., Marpaung L., Barus T., Simanjuntak P. Isolation and identification of flavonoid compound from nutmeg leaves (Myristica fragrans Houtt. Asian J. Chem. 2016;28:199. doi: 10.14233/ajchem.2016.19342. [DOI] [Google Scholar]
- 2.Piaru S.P., Mahmud R., Majid A.M.S.A., Nassar Z.D.M. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Trop. Med. 2012;5:294–298. doi: 10.1016/S1995-7645(12)60042-X. [DOI] [PubMed] [Google Scholar]
- 3.Duong T.-.H., Nguyen H.-.H., Le T.-.T., Tran T.-.N., Sichaem J., Nguyen T.-.T., Nguyen T.-.P., Mai D.-.T., Nguyen H.-.H., Le H.-.D. Subnudatones A and B, new trans-decalin polyketides from the cultured lichen mycobionts of Pseudopyrenula subnudata. Fitoterapia. 2020;142 doi: 10.1016/j.fitote.2020.104512. [DOI] [PubMed] [Google Scholar]
- 4.Abourashed E.A., El-Alfy A.T. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt. Phytochem. Rev. 2016;15:1035–1056. doi: 10.1007/s11101-016-9469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A.D., Bansal V.K., Babu V., Maithil N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt. J. Genet. Eng. Biotechnol. 2013;11:25–31. doi: 10.1016/j.jgeb.2012.12.001. [DOI] [Google Scholar]
- 6.Ramasubburayan R., Sumathi S., Bercy D.M., Immanuel G., Palavesam A. Antimicrobial, antioxidant and anticancer activities of mangrove associated bacterium Bacillus subtilis subsp. subtilis RG. Biocatal. Agric. Biotechnol. 2015;4:158–165. [Google Scholar]
- 7.Abas A.-S.M., Naguib D.M. Effect of germination on anticancer activity of Trigonella foenum seeds extract. Biocatal. Agric. Biotechnol. 2019;18 doi: 10.1016/j.bcab.2019.101067. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.