Abstract
Background
The 2017 KDIGO guidelines establish a 2B grade recommendation in favor of testing Bone Mineral Density (BMD) by DXA to assess osteoporotic fracture (OPF) risk in patients with CKD G3a-G5D. Still, controversy remains because large studies evaluating it for this particular population are lacking.
Aim
To establish the clinical performance of BMD measured by DXA in the evaluation of fracture risk in women with CKD.
Methods
We conducted a 43 year retrospective cohort study with 218 women ≥18 years-old with CKD and BMD measurement by DXA of total hip and lumbar spine. Clinical (age, year of CKD onset, comorbidities, BMI, transplant status, treatment), and biochemical (PTH, corrected calcium, phosphate, vitamin D [25 (OH) D3], creatinine, and albumin), parameters were collected from hospital records. All osteoporotic fractures (as defined by the WHO) found in the clinical and radiologic files were registered.
Results
218 women with a median age of 60 years (40–73 IQ range) and a CKD evolution time of 12 years (7–18 IQ range) were evaluated. Forty-eight (28.23%) presented an OPF. These women were older (57 vs 69 years, p =0.0072) and had a lower BMD. CKD stage did not influence fracture incidence. In the multivariate analysis we found that for each standard deviation decrease in hip and lumbar spine T-Score, the overall fracture risk was 2.7 and 2.04 times higher, respectively. More than 50% of fractures took place within the first ten years of follow-up, especially with GFR <30 mL/min/m2 and osteoporosis. Diabetes and hypothyroidism accelerated fracture onset, while renal transplant delayed it. In the ROC analysis, the AUC was largest with the total hip (0.7098, p =0.000) and lumbar spine (0.6916, p = 0.000).
Conclusions
BMD measured by DXA is a useful fracture prediction tool for women with CKD, having a sensibility and specificity similar to that in the general population. It seems to be appropriate for the diagnosis, treatment decisions, and follow-up of patients with renal failure.
Keywords: Osteoporosis, Chronic kidney disease, Osteoporotic fracture, Fragility fracture, Bone mineral density, Parathyroid hormone
1. Introduction
Patients with stages 3 to 5 of chronic kidney disease (CKD) have a twofold fracture risk and higher morbidity and mortality compared to the healthy population (Bucur et al., 2014; Goldenstein et al., 2015). The mechanisms thought to increase this fracture risk involve secondary hyperparathyroidism due to reduced phosphaturia and vitamin D deficiency secondary to poor calcitriol synthesis, both of which lead to detrimental bone effects (Chen et al., 2018).
The 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) establishes a grade 2B recommendation in favor of testing bone mineral density (BMD) by DXA to assess osteoporotic fracture (OPF) risk in patients with CKD G3a-G5 CKD with risk factors for osteoporosis as long as the results impact treatment decisions (KDIGO, 2017). Even so, the usefulness of DXA, which is the gold standard for evaluating BMD in healthy individuals, is controversial in CKD patients due to a lack of large studies evaluating it for this particular population (Bucur et al., 2014; Jamal, 2010). Furthermore, CKD patients have significant rates of cortical bone loss and underlying metabolic bone disease, which are undetectable by DXA (Nickolas et al., 2006; Babayev and Nickolas, 2015; Salam et al., 2014).
Our main objective was to establish the clinical performance of BMD as measured by DXA in the evaluation of fracture risk in women with CKD.
2. Methods
We conducted an observational, retrospective cohort study from 1977 to 2020 in a National Health Institute in Mexico City. The study group included 218 female patients ≥18 years who met the following criteria: glomerular filtration rate (GFR) ≤ 60 mL/min/m2 and total hip and lumbar spine BMD determination by DXA. The study population was divided into two categories by age: < 50 years and ≥ 50 years. We excluded patients with primary causes of osteoporosis including Cushing's syndrome, hyperthyroidism, primary hyperparathyroidism, primary hypogonadism, and central hypogonadism (i.e., Sheehan syndrome and tumors of the nervous system). Fractures and DXAs before CKD diagnosis (< 60 mL/min/m2) were also excluded.
2.1. Cohort
The clinical charts of 830 female patients diagnosed with CKD were reviewed. Among these, 218 patients met the inclusion criteria. All DXAs after CKD diagnosis were documented, resulting in a total of 314 BMD records. For statistical purposes, in non-fractured patients the last DXA was taken into consideration, whereas for fractured patients the DXA closest to the fracture was used.
2.2. Chronic kidney disease
The first patient with CKD was recorded in 1977, the year of CKD diagnosis was collected directly from the clinical records and verified using the KDIGO 2012 definition for kidney failure (GFR < 60 mL/min/m2 for >3 months) (Kellum et al., 2012). GFR was calculated using the CKD-EPI formula (Levey et al., 2009). Patients were classified using the KDIGO staging system: G3a (45–59 mL/min/m2), G3b (30–44 mL/min/m2), G4 (15–29 mL/min/m2), and G5 (< 15 mL/min/m2).
2.3. Dual-energy x-ray absorptiometry
The first DXA after CKD diagnosis was recorded in November 1998, and the last one in January 2020. Total hip and lumbar spine BMD determinations were measured using a Hologic QDR 1000 W DXA instrument (Hologic Inc., Waltham, MA, USA) with precisions (CV %) of 0.9% (L1–L4 spine) and 1.2% (hip). The BMD results were expressed in T-score and g/cm2 and classified according to WHO parameters as normal (−1.0 or above), osteopenia (between −1.0 and −2.5), and osteoporosis (−2.5 or below) (World Health Organization, 1998). A T-score < −1.0 indicated an abnormal bone diagnosis.
2.4. Clinical and biochemical parameters
The clinical parameters included age, year of CKD onset, anthropometry (weight, height, and body mass index [BMI]), BMD by DXA (BMD and T-scores for hip and lumbar spine), renal transplant status, current treatment regimens for osteoporosis (i.e., bisphosphonates, denosumab, teriparatide), immunosuppressive treatment (i.e., corticosteroids, post-transplant immunosuppression), and calcium and vitamin D supplementation. The biochemical parameters included serum intact parathyroid hormone (PTH), total serum calcium, phosphate, vitamin D (25 [OH] D3), creatinine, and albumin. PTH and 25 (OH) D3 were registered within ±12 months, whereas other biochemical parameters were assessed within ±3 months of the analyzed DXA.
Calcium correction was performed using the following formula: serum total calcium (mg/dL) + 0.8 × [4.0 − serum albumin (g/dL)] (Obi et al., 2017). Patients were classified into three groups according to their 25 (OH) D3 levels: deficiency (< 20 ng/mL), insufficiency (20–29 ng/mL), and normal (≥ 30 ng/mL) (Okazaki et al., 2017).
2.5. Parathyroid hormone levels
PTH levels were evaluated using the standard values established by the institutional laboratory. PTH levels were considered normal if they were < 88 ng/L and high if they were ≥ 88 ng/L. PTH levels were also divided into quartiles (Q) of the following values for operational purposes: Q1: 4.7 ng/L–59 ng/L; Q2: 59.3 ng/L–123.9 ng/L; Q3: 129.2 ng/L–294.8 ng/L; Q4: 309.7 ng/L–2535 ng/L.
2.6. Fractures
The WHO defines OPFs, including asymptomatic vertebral fractures, as those that occur in adults after minimal trauma, such as a fall from standing height or less (World Health Organization, 1994). All OPFs were registered from the clinical records and radiographic files throughout the study period. We collected data from radiography, computed tomography scan, and magnetic resonance imaging reports. All fractures were diagnosed by expert radiologists. The patients were classified according to fracture type: proximal femur (hip), vertebrae (lumbar spine), distal radius (wrist), or other fragility fractures (FF) (i.e., ankle, tibia, humerus). We excluded fractures caused by severe trauma (i.e., high-speed motor vehicle collision, injury from a projectile) and those related to malignancy.
2.7. Body mass index
We used BMI as a metric parameter for establishing anthropometry characteristics regarding the percentage of total body fat. Each patient was classified into one of three BMI categories: underweight (< 18.5 kg/m2), normal weight (≥ 18.5 and < 25 kg/m2), and overweight or obesity (≥ 25 kg/m2) (World Health Organization, 1995).
3. Statistical analysis
Results were expressed in mean and standard deviation. Variables without a normal distribution were expressed as proportion, median and interquartile range. Variables used to compare the fractured group and the non-fractured group were evaluated using the following: Pearson's chi squared test for qualitative variables, Student's t-test or Mann-Whitney U test for quantitative variables, Kruskal-Wallis for comparing groups, and Pearson's rho for correlation analysis between two quantitative variables. According to the fracture event we made a binary logistic regression. Univariate and multivariate models were adjusted for significant variables for lumbar spine and total hip T-score. P values < 0.05 were considered statistically significant. Kaplan-Meier survival analyses were conducted for fracture events for total hip and lumbar spine values associated with other variables. Receiver operating characteristic (ROC) analysis was made to estimate the area under curve (AUC), sensitivity, specificity, and cutoff values for total hip and lumbar spine T-score, and biochemical parameters; the reported cutoff values were the highest in terms of average sensitivity and specificity. Data was processed using Microsoft Office Excel 365 (Redmond, Washington, USA) and STATA/SE 14.0.
4. Results
During the study period, 218 women with CKD were evaluated (non-fractured = 170, fractured = 48). The median age was 60 years (40–73 IQ range). The CKD evolution median time was 12 years (7–18 IQ range), and CKD evolution time at the moment of the first DXA on record was 5 years (2–10 IQ range). The mean GFR was 29.63 mL/min/m2 (±17.44). No CKD stage (G3a–G5) predominated between fractured and non-fractured groups (p = 0.131). In the total group the average of DXAs performed was 1.3 per patient. Forty-eight women (28.23%) presented OPF, of which 12.5% had more than one fracture, most in the lumbar spine (36.3%, n = 20), whereas the others were in the hip (32.7%, n = 18), FF (23.6%, n = 13), and wrist (7.27%, n = 4).
Women in the fractured group were older than those in the non-fractured group (57 vs. 69 years, p = 0.0072) and had a lower BMD with osteoporosis more frequently found in the lumbar spine (52 vs. 25%, p = 0.000) and in the hip (46 vs. 13%, p = 0.000) (Table 1).
Table 1.
Baseline characteristics of the 218 patients§.
| No fractures (n = 170) | Fractures (n = 48) | PValue | |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 57 (37–70) | 69 (51–75) | 0.0072† |
| CKD evolution (years) | 12 (7–18) | 11 (7.5–16) | 0.56† |
| CKD to first DXA (years) | 4 (1–9) | 5 (2–8.5) | 0.29† |
| BMI (Kg/m2) | 26.4 (±6.37) | 26.1 (±4.57) | 0.27 |
| Lumbar spine BMD (g/cm2) | 0.872 (±0.151) | 0.767 (±0.151) | <0.000⁎ |
| Lumbar spine T-score | −1.57 (±1.37) | −2.51 (±1.39) | <0.000⁎ |
| Total hip BMD (g/cm2) | 0.784 (±0.151) | 0.674 (±0.160) | <0.000⁎ |
| Total hip T-score | −1.27 (±1.11) | −2.13 (±1.18) | <0.000⁎ |
| aLumbar spine diagnosis | |||
| Normal | 53 (31.1) | 5 (10.4) | |
| Osteopenia | 75 (44.1) | 18 (37.5) | |
| Osteoporosis | 42 (24.7) | 25 (52.1) | <0.000⁎ |
| aTotal hip diagnosis | |||
| Normal | 72 (42.3) | 10 (20.8) | |
| Osteopenia | 76 (44.7) | 16 (33.3) | |
| Osteoporosis | 22 (12.9) | 22 (45.8) | <0.000⁎ |
| Fracture events | NS‡ | ||
| Lumbar spine | – | 20 (36.3) | |
| Femur | – | 18 (32.7) | |
| Radius | – | 4 (7.27) | |
| FFb | – | 13 (23.6) | |
| CKD stage | |||
| 3a | 51 (30) | 7 (14.5) | |
| 3b | 42 (24.7) | 11 (22.9) | |
| 4 | 37 (21.7) | 15 (31.2) | |
| 5 | 40 (23.5) | 15 (31.2) | 0.12 |
| Laboratory values | |||
| GFR mL/min/m2 | 30.6 (±17.6) | 25.92 (±16.4) | 0.09 |
| PTH pg/mL, n = 126 | 140.2 (62.7–341.4) | 139.2 (71.9–322.7) | 0.52† |
| Serum calcium mg/dL, n = 189 | 9.32 (±0.79) | 9.33 (±0.75) | 0.99 |
| Corrected calcium mg/dL, n = 177 | 9.48 (±0.67) | 9.45 (±0.67) | 0.83 |
| Serum phosphate mg/dL, n = 184 | 4.04 (±1.11) | 4.05 (±1.15) | 0.95 |
| 25 (OH) D3 ng/mL, n = 95 | 20.4 (±10.3) | 17.2 (±9.02) | 0.17 |
| Albumin g/dL, n = 191 | 3.86 (±0.65) | 3.85 (±0.56) | 0.96 |
| Comorbidities | |||
| Type 2 Diabetes | 70 (41.1) | 26 (54.1) | 0.10 |
| Systemic lupus erythematosus | 28 (16.4) | 7 (14.5) | 0.75 |
| Hypothyroidism | 46 (27) | 17 (35.4) | 0.25 |
| Renal transplant | 51 (30) | 8 (16.6) | 0.06 |
| Calcium treatment | 70 (41.1) | 26 (54.1) | 0.14 |
| Vitamin D treatment | 72 (42.3) | 25 (52) | 0.11 |
| Osteoporosis treatment | 12 (7) | 8 (16.6) | 0.61 |
| Immunosuppressive treatment | 64 (37.6) | 10 (20.8) | 0.03⁎ |
Data are given as the mean ± SD or percentage.
Data are given as median and range.
Any statistical difference between fracture site.
T-score diagnosis based on the WHO standards.
Fragility fractures as defined by WHO guidelines.
Statistical significance (p < 0.05).
No differences were observed between the groups in terms of weight, CKD evolution time, systemic lupus erythematosus, type 2 diabetes (T2D), primary hypothyroidism, kidney transplant history, GFR, PTH levels, corrected calcium, P, 25 (OH) D3, and albumin. There was no significant difference in fracture incidence among patients who received calcium or 25 (OH) D3 supplementation or those under osteoporosis. However, we found an association between immunosuppressive treatment and a lower fracture incidence (p = 0.03) (Table 1).
Regarding CKD evolution time and fracture onset, lumbar spine fractures developed earlier when compared to hip fractures (5.25 years vs. 9.42 years, p = 0.0174). It is worth mentioning that no association was found between BMD, BMI or biochemical parameters with fracture incidence in any specific site.
BMI had a significant positive correlation with total hip and lumbar spine BMD (p = 0.0000, p = 0.0000), whereas age (p = 0.013) had a negative correlation with hip BMD, and PTH (p = 0.0046, p = 0.008) had a negative correlation with hip and lumbar spine BMD (Table 2). However, there were no significant differences between fracture incidences when compared with BMI classification, age (< 50 and ≥ 50 years), and PTH.
Table 2.
Correlations between lumbar spine and total hip BMD with other variables.
| Lumbar spine BMD (r) | PValue | Total hip BMD (r) | PValue | |
|---|---|---|---|---|
| Age | −0.088 | 0.192 | −0.166 | 0.013 |
| Weight | 0.381 | <0.000 | 0.429 | <0.000 |
| Height | 0.147 | 0.029 | 0.111 | 0.10 |
| BMI | 0.337 | <0.000 | 0.402 | <0.000 |
| Years with CKD | 0.034 | 0.61 | −0.013 | 0.84 |
| Lumbar spine BMD | – | – | 0.686 | <0.000 |
| Total hip BMD | 0.686 | <0.000 | – | – |
| GFR | −0.014 | 0.83 | 0.078 | 0.24 |
| PTH | −0.167 | 0.046 | −0.236 | 0.008 |
| Corrected calcium | 0.103 | 0.17 | 0.107 | 0.15 |
| Serum phosphate | 0.093 | 0.20 | 0.022 | 0.75 |
| 25 (OH) D3 | 0.091 | 0.37 | 0.152 | 0.14 |
Bold numbers show statistical significance (p < 0.05).
In the univariate analysis of fractures, we found that age (p = 0.043), hip and lumbar spine T-scores (p = 0.000, p = 0.000), G4 (p = 0.032) and G5 stages (p = 0.046) were statistically significant, whereas only hip and lumbar spine T-scores (p =0.000, p =0.002) remained significant in the multivariate analysis. For each standard deviation decrease in T-score, the risk of hip and lumbar spine fracture increased by 2.7 (95% CI 1.56 to 4.76, p = 0.000) and 2.04 times (95% CI 1.29 to 3.12, p = 0.002) (Table 3).
Table 3.
Odds ratios according to binary logistic regression by fracture for univariate and multivariate analysis
| Univariate | 95% IC | PValue |
Spine |
Total Hip |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariatea | 95% CI | PValue | Multivariateb | 95% CI | PValue | ||||
| Age | 1.01 | 1.00–1.03 | 0.043 | 1.02 | 0.98–1.05 | 0.21 | 1.01 | 0.98–1.04 | 0.38 |
| Weight | 0.99 | 0.97–1.01 | 0.66 | 1.02 | 0.98–1.00 | 0.21 | 1.04 | 0.99–1.00 | 0.06 |
| Height | 0.15 | 0.00–9.59 | 0.37 | 0.37 | 0.00–425 | 0.78 | 0.30 | 0.00–367 | 0.74 |
| Years with CKD | 0.98 | 0.94–1.03 | 0.56 | 1.04 | 0.96–1.13 | 0.25 | 1.02 | 0.93–1.11 | 0.62 |
| Lumbar spine T-Score | 0.59 | 0.45–0.77 | 0.000 | 0.49 | 0.32–0.77 | 0.002 | – | – | – |
| Total hip T-Score |
0.51 | 0.38–0.70 | 0.000 | – | – | – | 0.37 | 0.21–0.64 | 0.000 |
| G3b | 1.90 | 0.67–5.35 | 0.22 | 5.09 | 0.34–75.6 | 0.23 | 3.85 | 0.25–58.4 | 0.33 |
| G4 | 2.95 | 1.09–7.96 | 0.032 | 9.51 | 0.76–118 | 0.08 | 11.3 | 0.88–146 | 0.06 |
| G5 | 2.73 | 1.01–7.33 | 0.046 | 10.5 | 0.81–136 | 0.07 | 10.4 | 0.78–139 | 0.07 |
| PTH | 1.00 | 0.99–1.00 | 0.52 | 1.00 | 0.99–1.00 | 0.96 | 0.99 | 0.99–1.00 | 0.90 |
| Corrected calcium | 0.94 | 0.56–1.59 | 0.83 | 1.07 | 0.76–3.95 | 0.18 | 2.42 | 0.94–6.24 | 0.06 |
| Serum phosphate | 1.00 | 0.73–1.38 | 0.95 | 0.85 | 0.52–1.39 | 0.52 | 0.79 | 0.48–1.31 | 0.38 |
| Albumin | 0.98 | 0.58–1.66 | 0.96 | 1.03 | 0.54–3.11 | 0.55 | 1.29 | 0.50–3.35 | 0.59 |
Bold numbers show statistical significance (p < 0.05).
Adjusted by age, weight, height, years with CKD, lumbar spine T-Score, GFR, PTH, corrected calcium, phosphorus and albumin.
Adjusted by age, weight, height, years with CKD, total hip T-Score, GFR, PTH, corrected calcium, phosphorus and albumin.
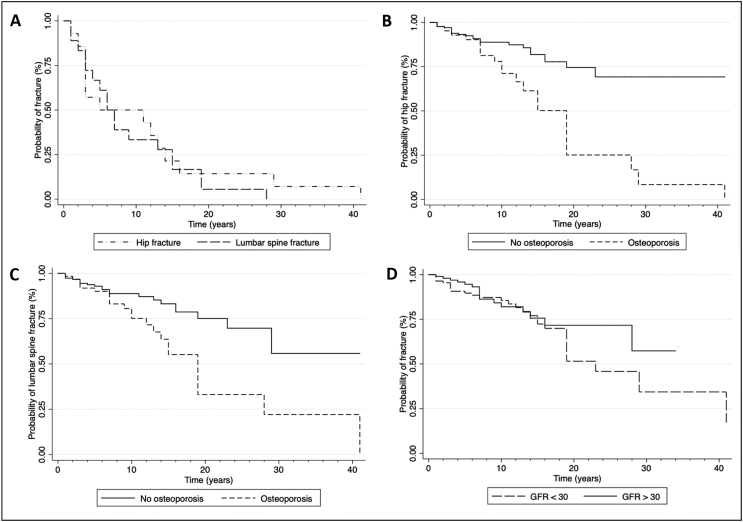
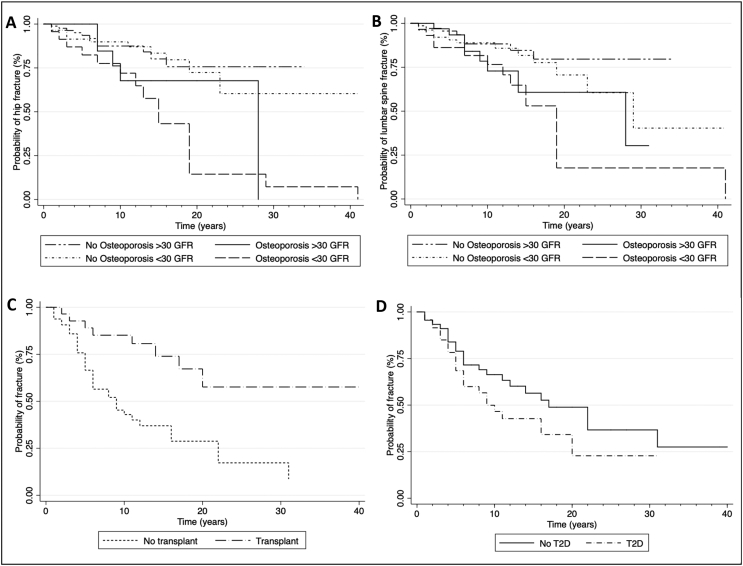
More than 50% of fractures took place during the first ten years of follow-up. With lower GFR (< 30 mL/min/m2), fracture events occurred earlier when compared to higher GFR (≥ 30 mL/min/m2) (Fig. 1). Comorbidities, such as T2D and hypothyroidism, accelerated fracture onset, whereas renal transplant delayed it (Fig. 2).
Fig. 1.
Fracture survival rate among women with CKD
(A) Stratified by hip and lumbar spine fracture (B) Hip fracture stratified by osteoporosis and no osteoporosis (normal and osteopenia) BMD by DXA (C) Lumbar spine stratified by osteoporosis and no osteoporosis (normal and osteopenia) BMD by DXA (D) Stratified by the KDIGO G3a and G3b (GFR > 30 mL/min); G4 and G5 (GFR <30 mL/min).
Fig. 2.
Fracture survival rate among women with CKD and comorbidities.
(A) Lumbar spine fracture stratified by the KDIGO G3a and G3b (GFR > 30 mL/min); G4 and G5 (GFR > 30 mL/min), diagnosis of osteoporosis and no osteoporosis (normal and osteopenia) by DXA (B) Hip fracture stratified by the KDIGO G3a and G3b (GFR > 30 mL/min); G4 and G5 (GFR > 30 mL/min), diagnosis of osteoporosis and no osteoporosis (normal and osteopenia) BMD by DXA (C) Stratified by transplant and no transplant (D) Stratified by T2D and no T2D.
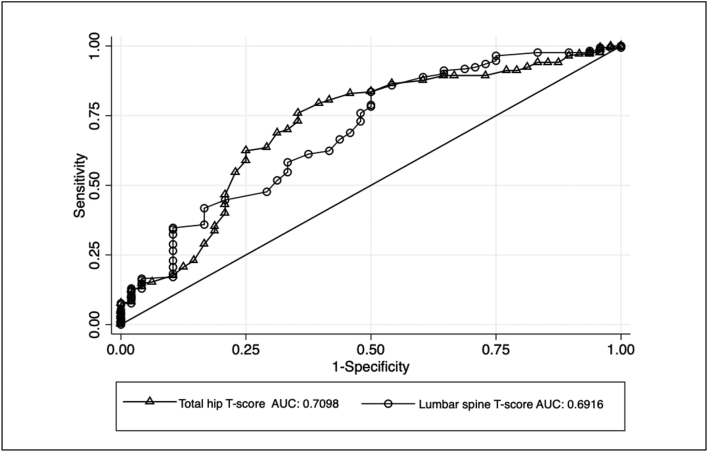
The ROC analysis for fracture incidence demonstrated that the AUC was larger with the total hip (0.7098, p = 0.000) and lumbar spine T-score (0.6916, p = 0.000). Biochemical parameters did not show any predictive value for the fracture event. (Fig. 3 and Table 4).
Fig. 3.
ROC analysis on the prediction for any type of fracture for total hip and lumbar spine T-score.
Table 4.
ROC analysis on bone mineral densities for fracture.
| AUC | Cutoff value | PValue | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| T-score⁎ | |||||
| Lumbar spine | ≤ − 1 | 32.35 | 89.58 | ||
| ≤ −1.7 | 54.71 | 66.67 | |||
| 0.6916 | ≤ −2.5 | <0.000 | 78.24 | 50 | |
| ≤ −2.7 | 83.53 | 50 | |||
| ≤ −2.9 | 88.82 | 39.58 | |||
| Total hip | 0.7098 | ≤ − 1 | <0.000 | 42.94 | 79.17 |
| ≤ −1.7 | 70 | 66.67 | |||
| ≤ −2.5 | 87.65 | 39.58 | |||
| ≤ −2.7 | 89.41 | 27.08 | |||
| ≤ −2.9 | 91.18 | 20.83 | |||
Bold numbers show statistical significance (p < 0.05).
BMD cutoff values, sensitivity and specificity were equivalent
5. Discussion
We found that total hip and lumbar spine BMD as measured by DXA is a useful tool for predicting fractures in female patients with CKD independently of age or CKD stage. Our data show that there is a statistically significant correlation between total hip and lumbar spine BMD (r = 0.68, p = 0.000), which suggests that both measurements are equally useful in predicting fracture risk. Supporting our findings, a meta-analysis by Bucur et al. found that BMD predicts fracture risk in CKD patients regardless of the measuring site (Bucur et al., 2014). This suggests that the pathophysiology of CKD bone disorders is complex and does not depend solely on PTH levels (Salam et al., 2014; Waziri et al., 2019). Despite this, hip BMD is recognized as the best fracture predictor among CKD patients (Jamal et al., 2012; Iimori et al., 2012; Yenchek et al., 2012) due to the PTH predominantly exercising its effect on cortical bone, which is the main form in the hip whereas the lumbar spine is formed mostly by trabecular bone (Yenchek et al., 2012; Duan et al., 1999; Denburg et al., 2013; Clarke, 2008). We did not find a significant correlation between total hip and lumbar spine BMD with PTH levels (r = −0.23, p = 0.008) (Table 2).
Our cohort contained 48 fractures, of which 38 (79%) occurred in women age 50 or older. Of these, 92% were found in women with an abnormal bone diagnosis (osteopenia or osteoporosis). However, we found that even though only 20% of fractures occurred in women younger than 50 years, 90% of these were also found in women with an abnormal bone diagnosis, meaning that BMD is a useful fracture risk prediction tool independently of age for this particular population. This finding is supported by the fact that age was not significant in the multivariate model analysis (Table 3).
We found no relationship between fracture risk and CKD stage, as seen in previous studies. However, when we followed each CKD stage separately, G4 and G5 presented fracture events earlier (Fig. 1). Even though advanced CKD (G4, G5) increased the fracture risk by 2.95 and 2.73 times in the univariate analysis, respectively. In the multivariate analysis, only BMD remained significant. This finding reflects that BMD measurement is the most important parameter for predicting fracture risk in these patients (Table 3).
According to S. L. West and the National Kidney Foundation, in patients with CKD stages 1 to 4, the primary cause of fractures is osteoporosis (Chen et al., 2018; West et al., 2015). However, stage 5 patients have many mineral metabolism disturbances, resulting in a more challenging diagnosis. Therefore, the KDIGO guidelines suggest that it is reasonable to perform a bone biopsy on these patients to guide treatment course (KDIGO, 2017). In our Kaplan-Meier analysis, we found that fracture events occurred earlier in patients with osteoporosis. Half of them reached fracture events at approximately fifteen years, whereas only 25% of non-osteoporotic patients experienced a fracture during follow-up (Fig. 1). Even when following patients with advanced kidney failure (GFR < 30 mL/min/m2) and osteoporosis, the latter was the main factor fracture determinant over time (Fig. 2).
In the general population with osteoporosis, the most common fractures occur in the lumbar spine (500,000 symptomatic fractures and more than one million subclinical fractures yearly in the United States), followed by hip fractures (300,000 yearly) (Lindsay and Cosman, 2020). There is a significantly increased risk of hip fracture in patients with CKD (Kazama, 2017). A GFR ≤ 60 mL/min/m2 confers a twofold risk for this event (OR 2.12) (Nickolas et al., 2006). However, the frequency of vertebral fractures is similar to that in the general population (Fusaro et al., 2013). This phenomenon is explained by the PTH mechanism. Both statements agree with our findings, in which we observed a major incidence of lumbar spine fractures (36.5%) but a similar incidence of total hip fractures (32.7%). Nevertheless, most of the vertebral fractures that we reported were incidental findings in the radiologic files, which could be explained because of their subclinical presentation. On the other hand, 250,000 fractures at other sites occur yearly in the general population with osteoporosis (Lindsay and Cosman, 2020). In our study, there was an important proportion of FF (23.6%) in which age, GFR, and biomarkers were similar, reinforcing BMD as the main predictive risk factor for overall fractures.
Interestingly, in kidney transplant patients, fractures occurred later than the average fracture onset, which suggests that the recovery of renal function delays OPF events. The latter is supported by the fact that we also observed that the use of immunosuppressors was associated with a lower fracture incidence. According to Ball et al., transplanted patients initially have a 34% higher fracture risk than dialyzed patients; however, after the first 630 postoperative days, this relationship reverts (Ball et al., 2002; Naylor et al., 2016). Iyer et al. demonstrated that during the first year after transplant, central BMD remains the same or even increases, whereas peripheral BMD decreases (Iyer et al., 2014). Based on these results, we found that transplanted patients had a higher incidence of FF and wrist fractures than did non-transplanted patients.
According to Guowei Li et al., T2D patients have a propensity for OPF with higher BMD values (Iyer et al., 2014; Li et al., 2019). In fact, menopausal women with T2D, experienced more fractures compared to individuals without diabetes, especially in the hip (De Liefde et al., 2005; Janghorbani et al., 2007; Bonds et al., 2006; Khalil et al., 2011). Hyperinsulinism has a trophic effect on bone initially; however, this bone matrix deteriorates due to advanced glycation end products, resulting in an overall defective bone (Ivaska et al., 2015; Compston, 2018; Asadipooya and Uy, 2019). In support of this statement, we found that 54% of patients in the fractured group had T2D, and these patients presented fractures earlier than non-diabetic patients (Fig. 2D).
The WHO's osteoporosis guidelines estimate that the risk of fracture increases for each standard deviation decrease in BMD, peaking at −2.5 SD (World Health Organization, 1994). We consider evaluating BMD using DXA in women with CKD appropriate because the AUC obtained was 0.70 for total hip and 0.69 for lumbar spine, with good sensitivity (88% and 78%, respectively) and specificity (40% and 50%, respectively) for a cutoff T-score value of ≤ −2.5 SD. Iimori et al. reported similar performance in the evaluation of total hip and lumbar spine T-scores for fracture prediction in women with CKD with an AUC of 0.74 and 0.67, respectively, and a cutoff value of < −2.7 SD in total hip with 80% sensitivity and 63.8% specificity (Jamal et al., 2012).
It is worth mentioning that only 43.73% of the patients who experienced a fracture received treatment for osteoporosis; as evidenced by Andrade et al. who found that only 24% of postmenopausal women received therapy during the year following a fracture (Andrade et al., 2003). According to Weycker et al. and the International Osteoporosis Foundation, about 50% of women who started osteoporosis therapy abandoned it within 12 months due to side effects and lack of information (Weycker et al., 2006; International Osteoporosis Foundation, 2005). Osteoporosis treatment reduces risk of vertebral fracture by 77% and risk of non-vertebral fracture by 49%; it also decreases mortality by up to 10% in older and fragile patients (Bolland et al., 2010). This is especially true in patients with CKD, for whom therapeutic adherence becomes even more complicated because primary pharmacological treatments for osteoporosis, such as bisphosphonates, are contraindicated in advanced renal failure (Levey et al., 2009; Lindsay and Cosman, 2020). However, patients with CKD have many comorbidities that represent excessive costs and make osteoporosis treatment less important from the perspective of patients and sometimes that of medical staff (KDIGO, 2017; Lindsay et al., 2016).
6. Conclusions
BMD measured by DXA is a useful fracture prediction tool for women with CKD independently of age, as it has a sensitivity and specificity similar to that of the general population. It appears to be appropriate for the diagnosis, follow-up, and treatment of patients with renal failure. In addition, it might eliminate the need for a bone biopsy in advanced cases. Unfortunately it is poorly used, as we found in our study the average of DXAs was 1.3 despite the long CKD evolution.
CKD stage alone correlates poorly with an increased fracture risk; however, the combination of advanced renal failure and osteoporosis results in earlier fracture events. Although PTH clearly plays a key role in the physiopathology of bone disease, it does not seem to be a strong fracture predictor and therefore should not be used alone. Given that patients with T2D present fractures earlier, they should be followed closely. Finally, kidney transplant may delay fractures events, but prospective studies are needed (Sidibé et al., 2019).
7. Limitations
Given the fact that this was a retrospective study, some data regarding patient records are lacking. For instance, total and bone-specific alkaline phosphatase were inconsistently measured and thus not taken into account for statistical analyses. Furthermore, tobacco and alcohol use were seldom reported and therefore we were unable to calculate the FRAX score.
Ethical standards
The present study has been approved by the ethics committee and has been performed in accordance with the ethical standards. All patients gave their informed consent prior to their inclusion in the study.
CRediT authorship contribution statement
Valeria E. Gómez-Islas: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization. Kevin R. García-Fong: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization. Rosa E. Aguilar-Fuentes: Data curation, Methodology, Investigation, Writing - review & editing. Salvador Hernández-Castellanos: Data curation, Investigation, Methodology, Writing - review & editing. Alfredo Pherez-Farah: Data curation, Investigation, Writing - original draft, Writing - review & editing. Sofía A. Méndez-Bribiesca: Investigation, Writing - original draft, Writing - review & editing. Juan M. López-Navarro: Conceptualization, Data curation. Hillary K. Osorio-Landa: Conceptualization, Data curation. Sergio L. Carbajal-Morelos: Conceptualization, Data curation. Areli M. Zúñiga-Guzmán: Data curation. Iván Pérez-Díaz: Conceptualization, Supervision, Validation, Project administration, Methodology, Formal analysis, Writing - review & editing.
Declaration of competing interest
The authors state that we have read and agree to abide by the Bone Reports conflict of interest policy. Hereby, we acknowledge that we do not have any conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Furthermore, we understand that we are obliged to promptly reveal any changes in our interests, affiliations or activities which may give rise to a conflict of interest and in such case the conflict of interest statement will be updated.
References
- Andrade S.E., Majumdar S.R., Arnold Chan K., Buist D.S.M., Go A.S., Goodman M. Low frequency of treatment of osteoporosis, among postmenopausal women following a fracture. Arch. Intern. Med. 2003;163:2052–2057. doi: 10.1001/archinte.163.17.2052. [DOI] [PubMed] [Google Scholar]
- Asadipooya K., Uy E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J Endocr Soc. 2019;3:1799–1818. doi: 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayev R., Nickolas T.L. Bone disorders in chronic kidney disease: an update in diagnosis and management. Semin. Dial. 2015;28:645–653. doi: 10.1111/sdi.12423. [DOI] [PubMed] [Google Scholar]
- Ball A.M., Gillen D.L., Sherrard D., Weiss N.S., Emerson S.S., Seliger S.L. Risk of hip fracture among dialysis and renal transplant recipients. J. Am. Med. Assoc. 2002;288:3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- Bolland M.J., Grey A.B., Gamble G.D., Reid I.R. Effect of osteoporosis treatment on mortality: a meta-analysis. J. Clin. Endocrinol. Metab. 2010;95:1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- Bonds D.E., Larson J.C., Schwartz A.V., Strotmeyer E.S., Robbins J., Rodriguez B.L. Risk of fracture in women with type 2 diabetes: the women's health initiative observational study. J. Clin. Endocrinol. Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- Bucur R.C., Panjwani D.D., Turner L., Rader T., West S.L., Jamal S.A. Low bone mineral density and fractures in stages 3–5 CKD: an updated systematic review and meta-analysis. Osteoporos. Int. 2014;26:449–458. doi: 10.1007/s00198-014-2813-3. [DOI] [PubMed] [Google Scholar]
- Chen H., Lips P., Vervloet M.G., van Schoor N.M., de Jongh R.T. Association of renal function with bone mineral density and fracture risk in the longitudinal aging study Amsterdam. Osteoporos. Int. 2018;29:2129–2138. doi: 10.1007/s00198-018-4592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3(Suppl. 3):131–139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J. Type 2 diabetes mellitus and bone. J. Intern. Med. 2018;283:140–153. doi: 10.1111/joim.12725. [DOI] [PubMed] [Google Scholar]
- De Liefde I.I., Van Der Klift M., De Laet C.E.D.H., Van Daele P.L.A., Hofman A., Pols H.A.P. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam study. Osteoporos. Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- Denburg M.R., Tsampalieros A.K., De Boer I.H., Shults J., Kalkwarf H.J., Zemel B.S. Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J. Clin. Endocrinol. Metab. 2013;98:1930–1938. doi: 10.1210/jc.2012-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., De Luca V., Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J. Clin. Endocrinol. Metab. 1999;84:718–722. doi: 10.1210/jcem.84.2.5498. [DOI] [PubMed] [Google Scholar]
- Fusaro M., Tripepi G., Noale M., Vajente N., Plebani M., Zaninotto M. High prevalence of vertebral fractures assessed by quantitative morphometry in hemodialysis patients, strongly associated with vascular calcifications. Calcif. Tissue Int. 2013;93:39–47. doi: 10.1007/s00223-013-9722-x. [DOI] [PubMed] [Google Scholar]
- Goldenstein P.T., Jamal S.A., Moysés R.M.A. Fractures in chronic kidney disease: pursuing the best screening and management. Curr. Opin. Nephrol. Hypertens. 2015;24:317–323. doi: 10.1097/MNH.0000000000000131. [DOI] [PubMed] [Google Scholar]
- Iimori S., Mori Y., Akita W., Kuyama T., Takada S., Asai T. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients-a single-center cohort study. Nephrol. Dial. Transplant. 2012;27:345–351. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- International Osteoporosis Foundation. The adherence gap: why osteoporosis patients don't continue with treatment. 2005.
- Ivaska K.K., Heliövaara M.K., Ebeling P., Bucci M., Huovinen V., Kalervo Väänänen H. The effects of acute hyperinsulinemia on bone metabolism. Endocr Connect. 2015;4:155–162. doi: 10.1530/EC-15-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S.P., Nikkel L.E., Nishiyama K.K., Dworakowski E., Cremers S., Zhang C. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J. Am. Soc. Nephrol. 2014;25:1331–1341. doi: 10.1681/ASN.2013080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal S.A. Bone mass measurements in men and women with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2010;19:343–348. doi: 10.1097/MNH.0b013e328338f520. [DOI] [PubMed] [Google Scholar]
- Jamal S.A., Cheung A.M., West S.L., Lok C.E. Bone mineral density by DXA and hr pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos. Int. 2012;23:2805–2813. doi: 10.1007/s00198-012-1908-y. [DOI] [PubMed] [Google Scholar]
- Janghorbani M., Van Dam R.M., Willett W.C., Hu F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- Kazama J.J. Chronic kidney disease and fragility fracture. Clin. Exp. Nephrol. 2017;21:46–52. doi: 10.1007/s10157-016-1368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDIGO. Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). (Ki. Kidney Int. Suppl. 2017;7:e1. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed]
- Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- Khalil N., Sutton-Tyrrell K., Strotmeyer E.S., Greendale G.A., Vuga M., Selzer F. Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos. Int. 2011;22:1367–1376. doi: 10.1007/s00198-010-1357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A.S., Stevens L.A., Schmid C.H. Zhang Y (Lucy), Castro AF, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Prior J.C., Leslie W.D., Thabane L., Papaioannou A., Josse R.G. Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019;42:507–513. doi: 10.2337/dc18-1965. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Cosman F. 20th ed. NY: McGraw-Hill; New York: 2020. Chapter 404: Osteoporosis. [Google Scholar]
- Lindsay B.R., Olufade T., Bauer J., Babrowicz J., Hahn R. Patient-reported barriers to osteoporosis therapy. Arch. Osteoporos. 2016;11:3–10. doi: 10.1007/s11657-016-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K.L., Jamal S.A., Zou G., McArthur E., Lam N.N., Leslie W.D. Fracture incidence in adult kidney transplant recipients. Transplantation. 2016;100:167–175. doi: 10.1097/TP.0000000000000808. [DOI] [PubMed] [Google Scholar]
- Nickolas T.L., McMahon D.J., Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J. Am. Soc. Nephrol. 2006;17:3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- Obi Y., Nguyen D.V., Streja E., Rivara M.B., Rhee C.M., Lau W.L. Development and validation of a novel laboratory-specific correction equation for total serum calcium and its association with mortality among hemodialysis patients. J. Bone Miner. Res. 2017;32:549–559. doi: 10.1002/jbmr.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Ozono K., Fukumoto S., Inoue D., Yamauchi M., Minagawa M. Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research a. J. Bone Miner. Metab. 2017;35 doi: 10.1007/s00774-016-0805-4. [DOI] [PubMed] [Google Scholar]
- Salam S.N., Eastell R., Khwaja A. Fragility fractures and osteoporosis in CKD: pathophysiology and diagnostic methods. Am. J. Kidney Dis. 2014;63:1049–1059. doi: 10.1053/j.ajkd.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Sidibé A., Auguste D., Desbiens L.-C., Fortier C., Wang Y.P., Jean S. Fracture risk in dialysis and kidney transplanted patients: a systematic review. JBMR Plus. 2019;3:45–55. doi: 10.1002/jbm4.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waziri B., Duarte R., Naicker S. Chronic kidney disease–mineral and bone disorder (CKD-MBD): current perspectives. Int J Nephrol Renovasc Dis. 2019;12:263–276. doi: 10.2147/IJNRD.S191156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.L., Patel P., Jamal S.A. How to predict and treat increased fracture risk in chronic kidney disease. J. Intern. Med. 2015;278:19–28. doi: 10.1111/joim.12361. [DOI] [PubMed] [Google Scholar]
- Weycker D., Macarios D., Edelsberg J., Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos. Int. 2006;17:1645–1652. doi: 10.1007/s00198-006-0179-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis : report of a WHO study group. World Heal Organ 1994:129. [PubMed]
- World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee 1995:1–463. [PubMed]
- World Health Organization Guidelines for preclinical evaluation and clinical trials in osteoporosis. 1998:68. [Google Scholar]
- Yenchek R.H., Ix J.H., Shlipak M.G., Bauer D.C., Rianon N.J., Kritchevsky S.B. Bone mineral density and fracture risk in older individuals with CKD. Clin. J. Am. Soc. Nephrol. 2012;7:1130–1136. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]