Abstract
Background
Gene therapy has held promises for treating specific genetic diseases. However, the key to clinical application depends on effective gene delivery.
Methods
Using a large animal model, we developed two pharmaceutical formulations for gene delivery in the pigs’ vagina, which were made up of poly (β-amino ester) (PBAE)-plasmid polyplex nanoparticles (NPs) based two gel materials, modified montmorillonite (mMMT) and hectorite (HTT).
Findings
By conducting flow cytometry of the cervical cells, we found that PBAE-GFP-NPs-mMMT gel was more efficient than PBAE-GFP-NPs-HTT gel in delivering exogenous DNA intravaginally. Next, we designed specific CRISPR/SpCas9 sgRNAs targeting porcine endogenous retroviruses (PERVs) and evaluated the genome editing efficacy in vivo. We discovered that PERV copy number in vaginal epithelium could be significantly reduced by the local delivery of the PBAE-SpCas9/sgRNA NPs-mMMT gel. Comparable genome editing results were also obtained by high-fidelity version of SpCas9, SpCas9-HF1 and eSpCas9, in the mMMT gel. Further, we confirmed that the expression of topically delivered SpCas9 was limited to the vagina/cervix and did not diffuse to nearby organs, which was relatively safe with low toxicity.
Interpretation
Our data suggested that the PBAE-NPs mMMT vaginal gel is an effective preparation for local gene therapy, yielding insights into novel therapeutic approaches to sexually transmitted disease in the genital tract.
Funding
This work was supported by the National Science and Technology Major Project of the Ministry of science and technology of China (No. 2018ZX10301402); the National Natural Science Foundation of China (81761148025, 81871473 and 81402158); Guangzhou Science and Technology Programme (No. 201704020093); National Ten Thousand Plan-Young Top Talents of China, Fundamental Research Funds for the Central Universities (17ykzd15 and 19ykyjs07); Three Big Constructions—Supercomputing Application Cultivation Projects sponsored by National Supercomputer Center In Guangzhou; the National Research FFoundation (NRF) South Africa under BRICS Multilateral Joint Call for Proposals; grant 17–54–80078 from the Russian Foundation for Basic Research.
Keywords: CRISPR/Cas9, Gene therapy, Local delivery, Poly (β-amino ester), Vaginal gel
1. Introduction
Genome editing has shown promisingresults to treat genetic disorders [1,2]. Nevertheless, one of the keys to successful gene therapy is the delivery of the genome engineering tools [3,4]. Owing to the nanoscale sizes, low toxicity, long cycle time and excellent plasticity, polymer based nanoparticles (NPs) have achieved efficient delivery for gene therapy [5], [6], [7]. In recent years, we and other groups demonstrated PBAE NPs had advantages of low toxicity, structural diversity, easy to synthesis and high transfection efficiency [8], [9], [10]. PBAE contained multiple amine groups which are able to interact with the negatively-charged DNAs, thus resulting in effective DNA condensation for gene delivery [11,12]. In addition, when entrapped into (endo)lysosome, multiple amine groups of PBAE could facilitate (endo)lysosome escape, and avoid the degradation of DNAs by the acidic environment and enzymes [13,14].
Research in context.
Evidence before this study
The large contact surface of the vagina provides possibilities for local gene therapy application, but one major difficulty for developing vaginal gene therapy drugs is the low delivery efficiency for exogenous DNA going through the vaginal mucus.
Added value of this study
In this study, we developed a poly (β-amino ester) (PBAE)-plasmid polyplex nanoparticles (NPs) containing gels for efficient delivery of genome editing tools. Two PBAE-based vaginal gels, modified montmorillonite (mMMT) and hectorite (HTT) were separately tested for their efficacy and safety. We found that PBAE-GFP-NPs-mMMT gel was more efficient than PBAE-GFP-NPs-HTT gel in delivering exogenous DNA intravaginally. Next, we evaluated the genome editing efficacy in vivo by transfecting specific CRISPR/SpCas9 sgRNAs targeting porcine endogenous retroviruses (PERVs). PERV copy number was significantly reduced in the PBAE-CRISPR/Cas9-NPs-mMMT gel treated group. Further, we confirmed that the gel was relatively safe because the expression of topically delivered SpCas9 was limited in the vagina/cervix.
Implications of all the available evidence
We developed a new pharmaceutical formulation for treating specific genetic diseases in the vagina. And our data showed that the PBAE-NPs mMMT vaginal gel is a safe and effective preparation for local gene therapy. The PBAE-NPs mMMT vaginal gel may become a new delivery method for local gene therapy, yielding insights into the therapeutic approaches to sexually transmitted disease in the genital tract.
Alt-text: Unlabelled box
Based on specific therapeutics aims and pharmaceutical dynamics, gene therapy could be delivered systematically or locally [15,16]. Compared to systemic delivery, local vaginal delivery has its unique advantages in treatment of genital tract infection [17]. For instance, the topical delivery of genome editing tools to treat HPV infections could maximize the therapeutic effects and minimize the off-target side-effects locally [18]. However, before translating such technology into clinical medicine, major obstacles still remained to be overcome [19]. First, to avoid flowing away from the open genital tract, PBAE NPs need to be formulated into the form of suppository [20], gels [21], films [22], intravaginal rings [23], etc., to achieve genital tract retention [24]. Second, PBAE NPs may be affected by vaginal and cervical mucus, which posed as a natural barrier to penetrate [25]. Third, large animal models that infected with sextually transmitted virus including human immunodeficiency virus (HIV) [26], human papillomavirus (HPV) [27], and herpes simplex virus (HSV) [28] still lacks, making translational medicine studies of gene therapy difficult. Last but not the least, many more important steps including clinical trial, upscaled manufacturing, quality assurance, etc. are warranted to translate the genome editing technology into clinical application [29].
Porcine endogenous retroviruses (PERVs) are integrated in the genome of all pigs and are released as infectious particles [30]. Under certain conditions, they can infect human cells and share similarity with human sexually transmitted viruses [31,32]. Therefore, in this study, we tried to develop a new gel delivery system based on PBAE-CRISPR/Cas9 plasmid polyplex NPs in big mammalian pigs and chose PERVs as the therapeutic target. In order to substantially enhance muco-retention and obtain a sustained drug-release profile, PBAE-plasmid polyplex NPs were further incorporated into gels [33]. We demonstrated that PBAE NPs based mMMT gel could effectively deliver PERVs-targeted CRISPR/Cas9 system into the vaginal epithelium and significantly reduced the copy number of the virus, yielding more insights into the development of vaginal gene therapy for the prevention and treatment of sexually transmitted viruses.
2. Materials and method
2.1. Materials
1,4-butanediol diacrylate (BDD) and 5-amino-1-pentanol (AP) were purchased from TCI (Shanghai, China). 1-(3-aminopropyl)-4-methylpiperazine (AMP) was purchased from Alfa Aesar (L04876, USA). MMT was natural clay composed by Al2O3, MgO and SiO2 while HTT was synthesized lithium magnesium silicate. Polyethyleneimine 25 kD (PEI 25 kD) (Cat, 28,968) was purchased from Polyscience (400valley Road, Warrington). pMAX-GFP (CAS: 8,603,168) was purchased from Bio Vector NTCC (Beijing, China). The plasmid encoding Cas9 and sgRNA was purchased from Addgene (#58,778). Three guide RNAs (gRNAs) were designed against/targeting PERV-pol gene (Accession No. AJ279056.1) following the protocol of Mali et al. [34], and synthesized by GENEWIZ (Jiangsu, China).The specific sequences of double-strand oligo tag [35] are: forward, 5´ G*T*TTAATTGAGTTGTCATATGTTAATAACGGT*A*T 3´; reverse, 5´ A*T*ACCGTTATTAACATATGACAACTCAATTAA*A*C 3´(* means Phosphorylation) .The protocol to make “oligo-tag” is: dissolve two oligos with STE buffer (10 mM Tris pH8.0, 50 mM NaCl, 1 mM EDTA) at a concentration of 2OD/100μL, equimolar mixing them and incubate at 94° for 5 min, cool to room temperature. All sgRNAs used in this study were presented in Table 1.
Table 1.
sgRNA sequence and corresponding PAM sequences.
| Name | gRNA sequence (5′−3′) | PAM sequence (5′−3′) |
|---|---|---|
| sgRNA-1 | TTCGAATGGAGAGATCCAGG | CGG |
| sgRNA-2 | ACTCGACTGCCCCAAGGGTT | TGG |
| sgRNA-3 | GGTGACCCTCCTCCAGTACG | TGG |
2.2. PBAE polymer synthesis
BDD, AP, and AMP were used to form PBAE polymer by following method: first, BDD (stabilized with MEHQ) and AP were mixed with 1:1 molar ratio and stirred on a magnetic stir-plate at 90 °C for 36 h under N2 atmosphere. After above reaction, the mixture was diluted with dimethylformamide (DMF), and the precipitated and washed by cold ethyl ether. After remove of ethyl ether by vacuum drying, the intermediate product (i-PBAE) was dried at room temperature. Second, i-PBAE was dissolved in anhydrous DMF, and reacted with 5 times mole amount of AMP under stirring at room temperature for 24 h. Next, the reaction product was precipitated in cold anhydrous diethyl ether. Finally, the end polymer (PBAE) was washed three times with diethyl ether, dried under vacuum for 48 h and stored at 4 °C. For further use, the polymer was dissolved in Dimethyl sulfoxide (DMSO) at a concentration of 100 mg/mL and stored at 20 °C.
The structures of the products were characterized by 1H NMR spectra (Bruker AVANCE III 400 MHz NMR spectrometer, solvents: CDCl3) and Fourier transform infrared spectroscopy (FTIR, PerkinElmer Spectrum Two, 32 replicate scan setting). The molecular weight of PBAE was measured by gel permeation chromatography (GPC, Waters-2410 system) with a Waters 2414 refractive index detector (mobile phase: DMF, standard: narrow-disperse polystyrene). The sample was dissolved in dimethyl formamide (DMF) with the concentration of 0.3 wt%.
2.3. Preparation and characterization of PBAE-plasmid polyplex NPs
To prepare PBAE-plasmid polyplex NPs, PBAE was dissolved in citrate buffer at pH 5.0 and the polymer solution was obtained by ultrasonic dissolution. The polymer solution and plasmid were mixed evenly according to 75:1 wt ratio, and then oscillated by the vortex and incubated at room temperature for 30 min. PBAE/pDNA NPs were characterized dynamic light scattering (DLS, ZetaPALs, Brookhaven, USA) and transmission electron microscopy (Hitachi HT7700, Japan). When measured the particles size, the laser wavelength is 660 nm and scattering angles is 90° To prepared transmission electron microscopy (TEM) sample, fresh prepared NPs solution (PBAE: 15 mg/mL, plasmid: 0.2 mg/mL) was dropped onto a carbon-coated copper grid and then dried at room temperature. For PEI-plasmid polyplex NPs, plasmid DNA was mixed with 25-kDa liner PEI instead of PBAE at mass ratio of 10:1 [20].
2.4. Preparation of PBAE-plasmid polyplex NPs based vaginal gel
Preparation of NPs-HTT gel: 66.65 mg HTT mixed with 1 mL water at 1000–1500 rpm in magnetic stirrer and dispersed for 20–30 min. Then 0.11 mL 10 × citrate buffer (citric acid: 1 mol/L, sodium citrate: 1 mol/L, pH: 4.7) was added followed by addition of 1 mL PBAE-plasmid polyplex nanoparticle composite (containing plasmid DNA 250 μg and oligo-tag100 μl) until it is evenly mixed;
Preparation of NPs-mMMT gel: 60 mg modified MMT (mMMT) mixed with 1 mL water at 1000–1500 rpm in magnetic stirrer and dispersed for 20–30 min. Then 0.11 mL 10 × citrate buffer was added followed by addition of 1 mL PBAE-plasmid polyplex nanoparticle composite (containing plasmid DNA 250 μg and oligo-tag100 μL) until it is evenly mixed.
The NPs-HTT gel and NPs-mMMT gel, as well as HTT/mMMT matrix were observed by scanning electron microscopy (SEM, Hitachi SU8010, Japan) at 2 kV. Before the test, the samples were fixed on silicon chip as a thin film and then coated with gold. To see the stability of the formulations, the gels were stored at room temperature for 3 days and then observed by SEM. The release of the NPs from the gels was also tested by diluting the gels with 4 times volume of citrate buffer and filtering by a 0.45 μm filter. The solution was tested by DLS to measure the diameter of NPs released from the gels. The size of NPs was also measured in the same way after different storage time (1 day, 2 day and 3 day) at room temperature.
PEI-plasmid polyplex NPs based vaginal gels were prepared in the same way as PBAE -plasmid polyplex NPs based vaginal gels by simply replacing PBAE by PEI.
2.5. Animals experiment
Female Kunming mice (5 weeks) were purchased from Laboratory Animal Resources of Huazhong University of Science & Technology. To evaluate the vagina retention ability of NPs-mMMT gel and NPs-HTT gel in Kunming mice model, Ce-6 marked NPs (prepared as our previous publication [10])-mMMT/HTT gel and free Ce-6 was injected into vagina of Kunming mice and then observed by living imaging system (Pearl Imager, LICOR, USA) immediately and after 1, 2, 3 days. One-month-old pigs were purchased from Huazhong Agricultural University. For drug administration, each pig was given 2 mL of PBAE-plasmid polyplex NPs based vaginal gel for GFP and CRISPR/Cas9 plasmid (250 μg), and the vaginal gel was evenly spread on the vaginal surface, cervical surface and posterior iliac crest of a one-month-old pig. We administered the drugs on the 1st day and the 4th day, then the cells were collected on the 7th day. For dealing with PBAE-GFP polyplex NPs based vaginal gel, we rotate the brush 3 times around the sow's cervix to obtain cells (which is quite similar to the method of Pap smear collection in human patient) [36]. The sampled cervical cells were stored in cervical cell preservation solution and collected by centrifugation at 715 g for 5 min.Then, we resuspended the cells with the same volume of PBS, and used the cell counter to count the number of cells in a certain volume, thus calculated the total number of collected cells. After counting the collected cells, we diluted the cells to the same concentration and took about 2.5 × 104 cells for fluorescence microscope and flow cytometry, in which cell viability can be estimated from a simple plot of forward vs. side scatter (Fig. S1). Then the transfection conditions of each group were detected by fluorescence microscope (Leica DMI8) and flow cytometry (Accuri®C6, BD, USA). Meanwhile, the cervix along with organs (ovary, urethra, vagina and uterus) were collected and rinsed with PBS to remove any adhered fats or any other extra tissues, then fixed in 4% paraformaldehyde and sliced into sections of 3–5 μm thickness for Hematoxylin-Eosin (H&E) staining and immunohistochemistry (IHC).
The animal handling procedure was performed in accordance to the approved protocol for the use of experimental animals set by the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology (HUST, Wuhan, China).
All the animals (including pigs and mice) were managed in accordance with the regulations of Chinese law, and the research was approved by the Experimental Animal Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology.
2.6. DNA extraction and PCR
On the seventh day after the first admistration, the cervical cells at the cervix of the pigs were taken by medical DNA extraction flocking swab, and then the DNA was extracted with a Kit (69,506, Qiagen, Germany). The PCR reaction was conducted using Q5® Hot Start High-Fidelity 2X Master Mix (M0494S, NEW ENGLAND BioLabs, USA). The primers used were listed (Table 2).
Table 2.
Sequences of PCR and real-time PCR primers.
| Name | Forward | Reverse |
|---|---|---|
| PERV-PCR | aatactcccctgctaccggt | aatctgggccttcttagcgg |
| PERV-qPCR | gagactacatcccactagccaac | tagggcttcgtcaaagatggtc |
| GAPDH-pig | ccgcgatctaatgttctctttc | ttcactccgaccttcaccat |
2.7. Real-time pcr (qPCR)
The DNA extracted from the cervix of the pigs before the admistration and on the seventh day after admistration was used to conduct the Real-time PCR, and the qPCR was conducted using the Bio-Rad CFX96 system with PowerUp™ SYBR™ Green Master Mix (A25742, Applied Biosystems, USA). The experiments were performed in triplicates, and the relative expression change of PERV copy number was calculated using the comparative Ct method (which compared the Ct value before and after admistration) with GAPDH of pig as the reference gene. The primers used were listed (Table 2).
2.8. IHC staining
After the pigs were euthanized, the cervix, ovary, urethra, vagina and uterus were isolated and fixed (4% paraformaldehyde). Paraffin-embedded sections (5 mm) were subjected to IHC staining according to the Proteintech protocol (http://www.ptgcn.com/support/protocols). The slides were incubated overnight at 4 °C with mouse anti-Flag M2 (F1804, Sigma-Aldrich), IFN-γ Rabbit pAb (384,740, ZEN BIO), TNF-α Rabbit pAb (251,341, ZEN BIO). Photographs were taken of three randomly chosen fields using cellSens Dimension (version 1.8.1, Olympus).
2.9. Statistical analysis
All quantitative data is represented as mean ± SD from at least triple parallel measurements. Statistical analysis was calculated using one-way ANOVA, Tukey's post hoc test and two-side Student's t-test by GraphPad Prism software (GraphPad Prism 8) with P<0.05 as the significant difference.
3. Results
3.1. Synthesis and characterization of PBAE-plasmid NPs
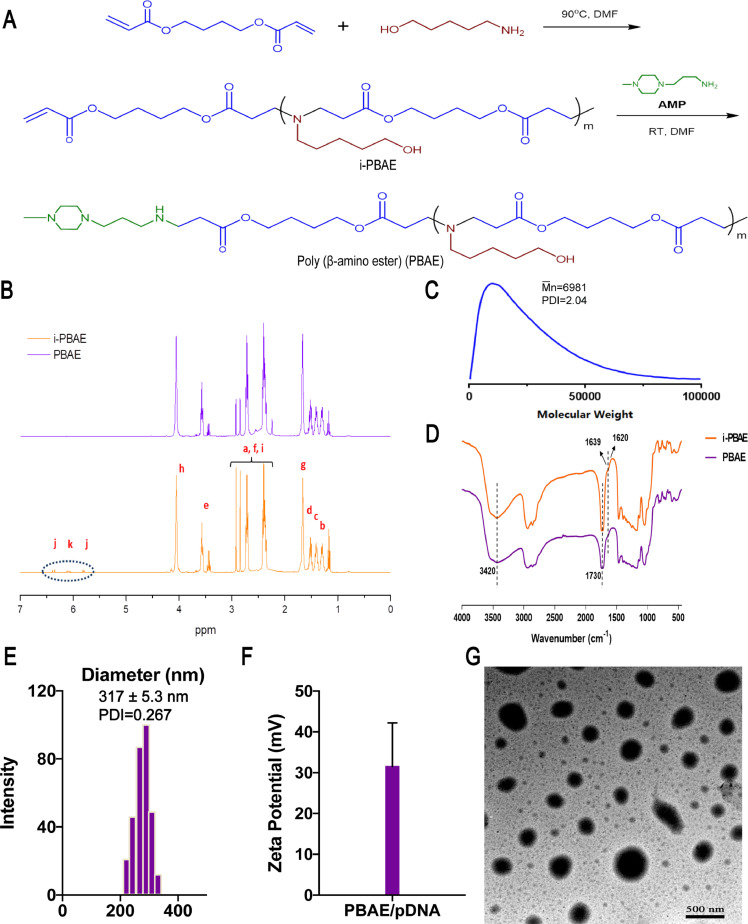
PBAE was synthesized via Michael addition polymerization method for plasmid delivery (Fig. 1A), and the 1H-NMR spectra of the i-PBAE and PBAE are shown in Fig. 1B. The peak with chemical shift value of 4.062 ppm belonged to the hydrogen signal peak of -COOCH2- on the BDD unit of PABE and i-PABE, which was in accordance with our previous results [10]. Besides, 1.32–1.53 ppm region was the hydrogen signal peak of methylene -CH2- on the AP, while the 2.42, 2.65, and 2.85 ppm peaks were the hydrogen signal peaks of -N (CH2)2-, -NCH2CH2OCO and -NCH2CH2OCO-. Of note, the 5.80–6.40 ppm region of i-PABE's spectra was the hydrogen signal peak of allyl end group, which was not detected from the spectra of synthesized PABE, indicating the successful reaction between the C=C double bond and AMP. The number-average molecular weight (Mn) of PBAE was 6981 Da, as measured by GPC (Fig. 1C).
Fig. 1.
Synthesis and Characteristic of PBAE and PBAE-pDNA polyplex NPs.
A) Synthesis of PBAE. B)1H NMR spectra of i-PABE and PABE. C) Gel Permeation Chromatography result of PBAE; D) FTIR spectra of i-PBAE and PBAE. E) Particle sizes of PBAE-pDNA polyplex NPs with 75:1 PBAE/pDNA weight ratio and F) Zeta potentials of PBAE-pDNA polyplex NPs. The data represented the mean ± SD (n = 3 per group). G) TEM image of PBAE/pDNA NPs (weight ratio 75:1, without staining). Scale bar, 500 nm.
Furthermore, we examined the Fourier transform infrared spectrum (FTIR) of PBAE and i-PBAE (Fig. 1D). The peak at 1730 cm−1 was specially derived from the fatty ester -CH2-COO-CH2- on the BDD chain of i-PABE and PBAE. In addition, the broad peak between 3200 and 3500 cm−1 proved the presence of -OH on the main chain, while unique 1639 and 1620 cm−1 peaks of i-PBAE were the hydrocarbon stretching vibration peaks of C=C double bond, which also disappeared in PBAE spectrum. Above results were consistent with that of 1H NMR, indicating that the PBAE was successfully synthesized.
In our previous studies [10], we came to the conclusion that the optimal PBAE/pDNA ratio was 75:1, while the corresponding smallest particle size was 317.1 ± 5.3 nm, the PDI was 0.267 (Fig. 1E) and surface charge was 34.9 ± 6.5 mV (Fig. 1F). We measured the particle size of NPs in different batches, and the diameter was close to each other, illustrating our NPs had good reproducibility (Fig. S2). The carrier material PBAE and pDNA were compressed by electrostatic interaction, therefore the NPs were compacted and densely charged. By TEM, we observed the morphology of the composite NPs (Fig. 1G). Electron micrographs showed that the composite NPs formed by PBAE all had spherical-like particles, and the size distribution was around 240 nm with excellent dispersion performance, which was smaller than the result of DLS test. This phenomenon was caused by the dehydration and shrink of the polyplex NPs.
3.2. PBAE-plasmid NPs based vaginal gel is effective for DNA delivery into cervical cells in vivo
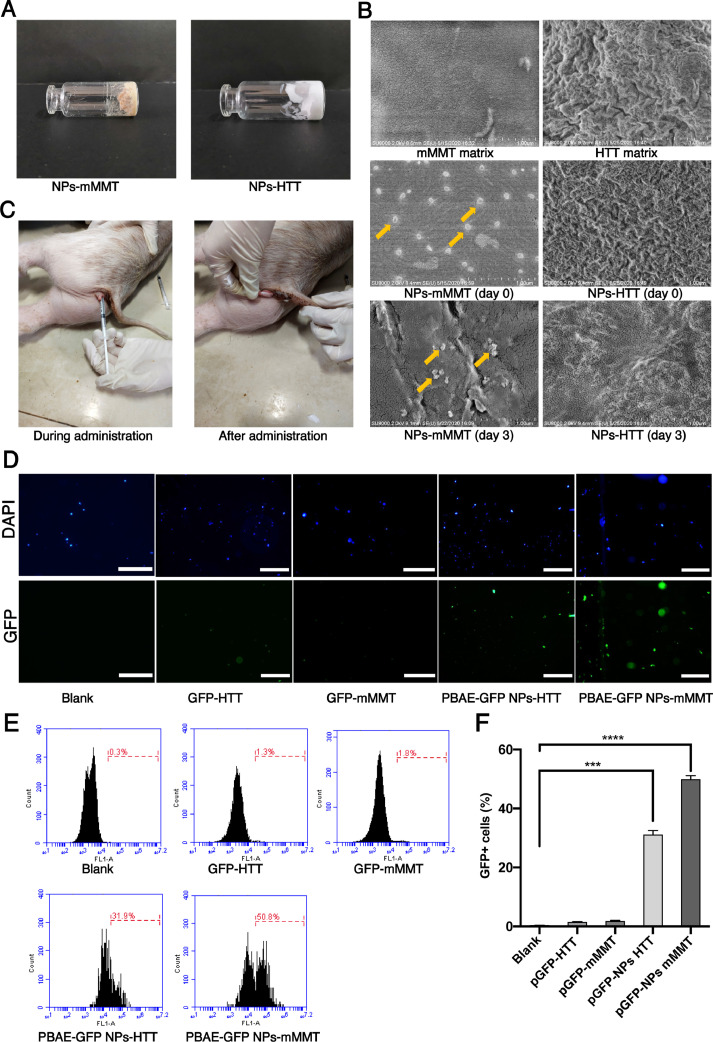
Generally, our in vivo DNA delivery system was made up of PBAE-plasmid polyplex NPs mixed with vaginal gels. Two materials for vaginal gels, mMMT and HTT, were tested in this investigation (Fig. 2A). We evaluated the NPs in the mMMT and HTT gels by SEM. As shown in the SEM image, the NPs remained intact in mMMT even after 3 day's storage. The mMMT matrix did not disrupt the PBAE-plasmid polyplex NPs (Fig. 2B and Fig. S3). In contrast, we cannot observe intact NPs in HTT gels, indicating HTT gels could affect the NPs. We further evaluated the vagina retention ability of NPs-mMMT gel and NPs-HTT gel in Kunming mice model by living image. As shown in Fig. S4, the signal of free fluorescent dye (Ce-6) disappeared within one day. For mMMT gel, strong fluorescence can be found at day 3. For HTT gel, the fluorescence became weak at day 2 and disappeared at day 3. To evaluate the transfection efficiency of our system, we first developed these two kinds of vaginal gels which carrying NPs consisted of PBAE polyplex and green fluorescence protein (GFP) plasmid. For experimental animal, we chose large mammalian pig as the experimental subjects because the genome of the pig is very close to that of the human and they are infected with PERVs. Routine drugs were administered on the 1st day and the 4th day (Fig. 2C). On the 7th day, cervical cells were collected by brushes (similar to Pap smear), washed with PBS and stained with DAPI, and were immediately analyzed without culturing them. For the controls of PBAE-pGFP-NPs alone without gel and plasmid alone without gel, the two groups were difficult to achieve vaginal retention because they flowed away when applied into the vaginal of pigs without gels. Under fluorescence microscopy, GFP postive cervical cells could be observed in PBAE-GFP-NPs-HTT group and PBAE-GFP-NPs-mMMT group (Fig. 2C). The cells were then subjected to flow cytometry to determine the specific transfection efficiency in each group (Fig. 2D). The proportion of GFP+ cells in the vaginal smear were 0.30%±0.05% in blank group; 1.41%±0.28% in GFP-HTT group; 1.80%±0.24% in GFP-mMMT group; 25.79%±1.56% in PBAE-GFP NPs-HTT group; 51.12%±1.23% in PBAE-GFP NPs-mMMT group, respectively (Fig. 2E). Obviously, much higher proportion of GFP-positive cell was obtained when using mMMT material to make the vaginal gel (p value<0.0001). Meanwhile, the delivery without PBAE showed low transfection efficiency, which indicated the cationic polymer, PBAE was essential as a gene delivery vehicle. Together, the data suggested that our PBAE-GFP NPs-mMMT gel was more efficient than PBAE-GFP NPs-HTT gel for in vivo intravaginal DNA delivery.
Fig. 2.
The DNA delivery efficiency of PBAE-GFP plasmid NPs based vaginal gel.
A) Photos of the PBAE-based vaginal gel manufactured by two materials: mMMT and HTT. B)SEM images of mMMT/HTT matrix, NPs-mMMT/HTT and NPs-mMMT/HTT storing at room temperature for 3 days, Scale bar, 1μm. C) One-month-old pig being administered and after administration. D) Fluorescence images of pig cervical cells on the 7th day after treated with different component of the delivery system. Blank means without treatment (n = 6 per group). Scale bars, 50 μm; E) and F) GFP+ cells by flow cytometry on the 7th day after treated with different component of the delivery system (n = 6 per group, ***P < 0.001, ****P < 0.0001, one-way ANOVA).
3.3. The comparison of PBAE NPs and PEI NPs for the delivery of GFP plasmid in mMMT gels
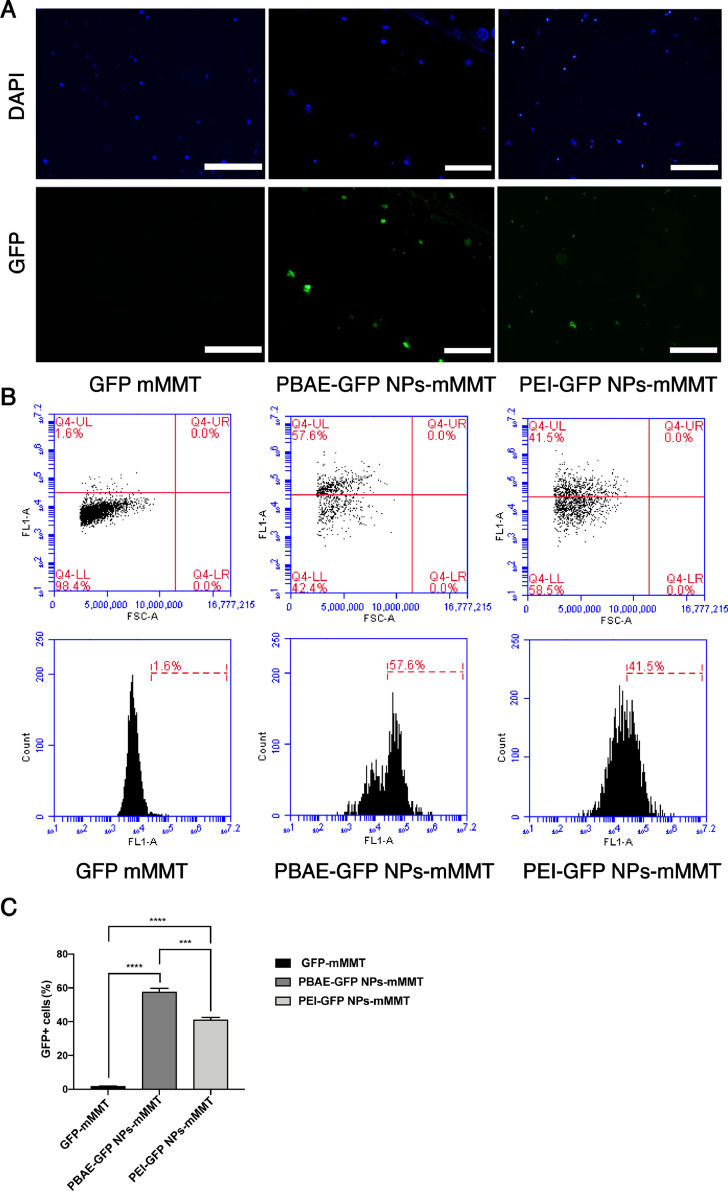
We next explored to compare the effects of PBAE NPs and PEI NPs in our mMMT gel system due to PEI is one of the most commonly used transfection reagents with high transfection efficiency [37,38]. Because PEI with the molecular weight in the range of 20–30 kDa exhibits high transfection efficiency, the plasmid DNA was polymerized with 25-kDa linear PEI at mass ratio of 10:1 in our experiment. Similarly, GFP positive cells were observed in both PBAE-GFP NPs-mMMT group and PEI-GFP NPs-mMMT group under fluorescence microscopy (Fig. 3A). By flow cytometry, we observed that the transfection efficiency of PBAE NPs (57.1%±0.82%) was higher than that of PEI NPs (41.4%±0.73%) in our mMMT gel system (p value<0.001) (Fig. 3B and 3C).
Fig. 3.
The comparison of PBAE NPs and PEI NPs in mMMT gels.
A) Fluorescence images of pig cervical cells treated PBAE/PEI NPs-mMMT gel (PBAE/GFP, weight ratio 75:1, PEI/GFP, weight ratio 10:1), Scale bars, 50 μm; B) Flow cytometry analysis of cervical cells treated with PBAE/PEI NPs-mMMT gel, blank means only mMMT gel was administered. C) GFP+ cells by flow cytometry on the 7th day after treated with different component of the delivery system (n = 6 per group, ***P < 0.001, ****P < 0.0001, one-way ANOVA).
3.4. PBAE-plasmid NPs based vaginal gel is effective for DNA editing in vivo
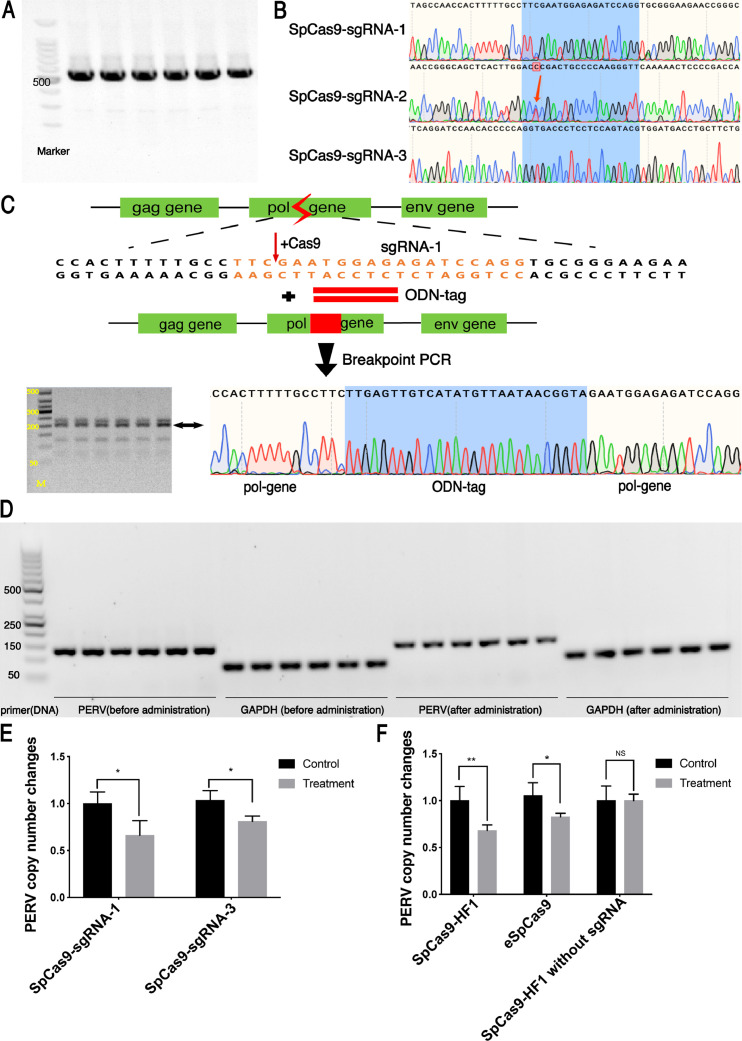
PERV is a retrovirus that can integrate its DNA into the pig genome to form proviruses. Its genome mainly consists of three genes, PERV-gag, pol, and env, which can replicate along with host genome [30,32]. Previous research had confirmed pol gene had the more copy number in tissues compared with gag and env gene [32,39], therefore we designed sgRNAs targeting PERV-pol gene. According to Zhang lab's website [40], we selected three highest-scoring sgRNAs and constructed SpCas9-sgRNA plasmid (SpCas9-sgRNA-1, SpCas9-sgRNA-2 and SpCas9-sgRNA-3). Then we produced vaginal mMMT gels containing the corresponding PBAE-plasmid NPs. Because our previous experiment showed that if the target had mutations, the editing efficiency of CRISPR/Cas9 system would be compromised. Therefore, we routinely performed Sanger sequencings of the PERV DNA to confirm PERV sequence did not have mutations before animal experiments (Fig. 4A). As the result showed, the target DNA of sgRNA-2 had one base mutation, which may affect the editing efficiency of CRISPR/Cas9 system (Fig. 4B). So, we selected sgRNA-1 and sgRNA-3 for further experiment.
Fig. 4.
The gene editing efficiency of PBAE-plasmid NPs based vaginal gel.
A) Agarose gel electrophoresis and B) Sanger sequencing of PCR products to verify whether the cleavage sites of PERV at the cervical epithelium of pigs have mutations; C) Description of PERV-tag breakpoint PCR after treated with PBAE-CRISPR-tag NPs based vaginal gel; D) DNA gel electrophoresis of qPCR products. E) The copy number changes of PERV of SpCas9-sgRNA-1,3. Control is the PERV copy number before treatment. The data represent the mean ± SD (n = 6 per group). F) The copy number changes of PERV of eSpCas9, SpCas9-HF1 and SpCas9-HF1 without sgRNA. SpCas9-HF1: PBAE-SpCas9-HF1NPs mMMT gel; eSpCas9: PBAE-eSpCas9 NPs mMMT gel; SpCas9-HF1 without sgRNA: PBAE-SpCas9-HF1 without sgRNA NPs mMMT gel. Control is the PERV copy number before treatment. The data represent the mean ± SD (n = 6 per group, *P < 0.05, **P < 0.01, Student's t-test).
If the SpCas9 cleaved the target PERV DNA in the vagina in vivo, then oligo would incorporate into the cutting site as a DNA scar [35]. And PCR of inserted oligo DNA (with one primer on the oligo and the other on the genomic site near the target) would produce the correct size of around 200 bp (Fig. 4C). As expected, Sanger-Sequencings proved that the SpCas9-PERV sgRNA plasmid effectively cut the targeted PERV sequence of the cervical cells in vivo, with oligo correctly inserted (Fig. 4C). Further qPCR also demonstrated a decrease in PERV copy number in PBAE-SpCas9 NPs mMMT gel treated groups. Compared to the PERV copy number before treatment, PERV copy number of sgRNA-1 group was reduced by 34% and that of sgRNA-3 group was reduced by 17%, indicating the efficacy of sgRNA-1 was superior than that of sgRNA-3 (Fig. 4D).
To reduce the off-targets without compromising the genome editing efficiency, the specificity of the SpCas9 nuclease was improved by its two variants: eSpCas9 [41] and SpCas9-HF1 [42]. Therefore, in this study, we further tested the efficacies of eSpCas9 and SpCas9-HF1 along with sgRNA-1 in mMMT gel. Compared to controls, the copy number of PERV was decreased by approximately 42% in SpCas9-HF1 group while that of the eSpCas9 group was declined by 34%, indicating the SpCas9-HF1 based vaginal gel was more effective (P<0.05, Fig. 4E). Because that plasmid delivery could trigger an inflammatory response that can affect the copy numbers of the PBAE, we added experimental group, PBAE-SpCas9-HF1 (without the effecting sgRNA) NPs-mMMT gel, as another control to observe the phenomenon. We found that the copy number of PERV in SpCas9-HF1 without sgRNA treatment group remained unchanged compared to that before treatment (Fig. 4E), indicating that plasmid delivery did not cause an immune response that can affect the copy numbers of PERV.
Together, the data suggested our in vivo DNA delivery based on PBAE-Cas9 NPs-mMMT vaginal gel has achieved effective local genome editing.
3.5. The biosafety profile of PBAE-plasmid NPS-mMMT vaginal gel in pigs
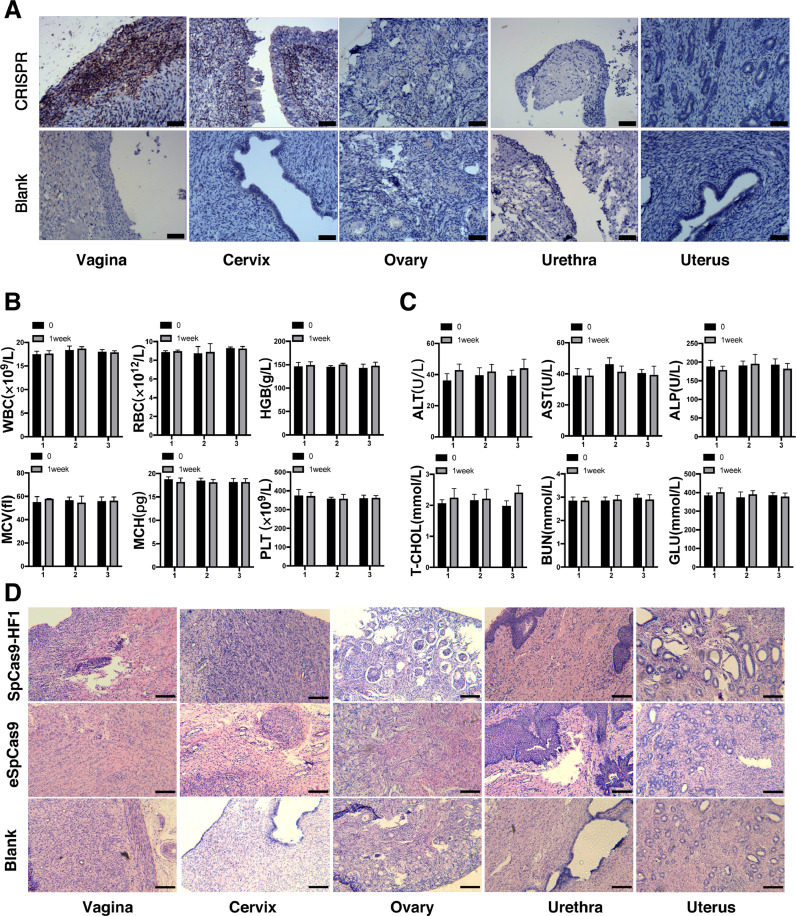
The biosafety profile of our vaginal delivery method is always the primary concern for their potential application in human subjects [43]. During administration, pigs in the experimental and control groups did not have weight loss or changes in eating habits, and the hair of the pigs was shiny and healthy. We first assessed the biodistribution of the delivery system. Because the CRISPR/Cas9 plasmid in our system contains the Flag tag, thus Flag [36] will be expressed when the gels reach a certain place in the organ of the treated pigs. We performed IHC staining on the expression of the Flag tag in different organs including vagina, urethra, cervix, ovary and uterus. It could be seen that only vaginal and cervical tissues expressed Flag, between which the vagina was the strongest. Flag tag was not expressed in the tissues of other organs, indicating that our system worked locally in the vagina and cervix and was less likely to spread to nearby organs (Fig. 5A). Next, routine blood tests and blood biochemical tests were conducted in pigs treated with the NPs-mMMT gel to assess their toxicity. The blood tests reflected the normal metabolism of multiple systems throughout the body, including hematopoietic system and immune system. Among blood biochemical tests, ALT, AST and ALP are related to the function of liver and BUN is an important indicator of kidney. Routine mMMT gels were administered on the 1st day and the 4th day, and we collected the blood and serum to run the blood tests and blood biochemical tests on the day before treatment and on the 7th day. The values of WBC, RBC and other indicators in routine blood tests were not significantly different from those before administration (P<0.05, Fig. 5B). Similar results were obtained in blood biochemistry tests (Fig. 5C). H&E stainings of cervix, ovary, urethra, vagina and uterus were further performed to evaluate the toxicity profile of the drug, and showed no morphological disorders, no pathological edema, no inflammatory necrosis and no apoptotic bodies (Fig. 5D). We also performed immunochemistry of the inflammatory cytokines IFN-γ and TNF-α in the vagina, cervix and urethral cavity in both PBAE-SpCas9/sgRNA NPs-mMMT treated and control group to confirm the results. No obvious inflammatory cytokine stainings were observed (Fig. S5). The data indicated our drug delivery formulation was relatively safe with local vaginal biodistribution and low toxicity, which was necessary for future applications in clinical trials.
Fig. 5.
Analysis of PBAE-plasmid NPs based vaginal gel toxicity in pigs.
A) Representative images of IHC staining of the Flag protein expression at major organs cervix, ovary, urethra, vagina and uterus. CRISPR: PBAE-CRISPR/sgRNA NPs mMMT vaginal gel; Blank: PBAE NPs mMMT gel. Scale bars, 500 μm; B) Routine blood test of chronic toxicity experiment at 0 day and 1 weeks for every group. Quantitative analysis of white blood cell (WBC), red blood cell (RBC), Hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), platelet (PLT). The results are expressed as the mean ± SEM. n = 6 pigs per group. *P < 0.05 (Student's t-test); C) Blood biochemical test of toxicity experiment at 0 day and 1 week for each group. Quantitative analysis of alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total cholesterol (T-CHOL), blood urea nitrogen (BUN), Glucose (GLU). The results are expressed as the mean ± SEM. n = 6 pigs per group. *P < 0.05 (Student's t-test); D) H&E staining of cervix, ovary, urethra, vagina and uterus of pigs. SpCas9-HF1: PBAE-SpCas9-HF1NPs mMMT gel; eSpCas9: PBAE-eSpCas9 NPs mMMT gel; Blank: PBAE NPs mMMT gel, all the system contained 250ug plasmid. Scale bars, 200 μm.
4. Discussion
The vagina presents several features that favor the delivery of therapeutic molecules, including DNAs, RNAs and proteins [44]. Vaginal route possesses sufficient blood supply, high contact surface area and suitable permeability to several substances, allowing both systemic and local drug delivery [33]. In addition, it avoids the hepatic first-pass effects and gastrointestinal fluids encountered by oral administration [45]. Nevertheless, one major difficulty for developing vaginal gene therapy drugs is the low delivery efficiency of exogenous DNA when going through the vaginal mucus [46]. Accordingly, mucoadhesive and mucus-penetrating systems were developed and exhibit possibilities to realize enhanced vaginal treatment [46]. Still, further development of an optimal formulation for the vaginal gene therapy is in urgent need [47].
In this study, we developed a PBAE-based vaginal gel that consisted of two important components, one was the PBAE-DNA complex NPs and the other was the mMMT gel formulation. When we changed mMMT to HTT, the transfection efficiency decreased significantly (Fig. 2C and 2E). To investigate the differences of the two gels, we evaluated the NPs in the mMMT and HTT gels by SEM and DLS. As shown in SEM and DLS results, the NPs in mMMT gel kept unchanged (Fig. 2B) and could be release directly in the form of nanoparticle from gel matrix after dilution (Fig. S6). While in HTT gel, the NPs were greatly affected by the matrix and no NPs could be observed (Fig. 2B). Although NPs re-formed after dilution, their diameter was larger than the origin formulation which may lower the transfection efficacy of the whole system (Fig. S6). These results suggested mMMT gel had better stability to maintain the NP structure than HTT gel, which may be the reason why the transfection efficiency of mMTT gel was much better than HTT gel. When the PBAE-SpCas9/sgRNA-1 NPs-mMMT vaginal gels were applied to the vagina of pigs, local copy number of PERV infection was successfully reduced (Fig. 3E; 4C). Our pre-clinical study in large animal suggested that PBAE-plasmid NPs based vaginal mMMTs gel is a safe and efficient approach for vaginal gene editing, providing new insights into the clinical translational medicine.
Compared with traditional vaginal gels, our PBAE-plasmid based mMMT gels have following advantages. First, the preparation process is relatively simple. And the materials are common compared to other nanocarrier system [48]. Second, the reagent formulation is flexible that the mMMT gel can be adjusted to suit different dosage requirements. Third, it was also approved by FDA as a biocompatible clay material and have been applied as safe vaginal hydrogel material in researchs [49,50]. These guaranteed the safety of our vaginal gels. Furthermore, the storage condition only requires room temperature around 18–25 °C. And the vaginal gel can be self-applicated, which provides conveniences for both the patients and the doctors [18].
However, there also are several limitations in this study that needed to be addressed. First, there are possible unwanted genetic changes induced by genome editing, namely off-target effects, which may bring harm to the patient health/life [51]. Second, double stranded plasmid used to express CRISPR/Cas9 may accidently integrate into human genome, causing insertional mutations [52]. Regarding the above two issues, genome editing tools administered topically in genital tract may limit the off-target effects/insertional mutations locally. Besides, high-fidelity version of SpCas9, eSpCas9 and SpCas9-HF1, were also tested in our system and achieved comparable genome editing efficiencies (Fig. 4D and 4E). Nevertheless, it may still have the risk to cause local unwanted genetics aberrations that could further lead to cancer [53]. Further genetic evaluations including genome-wide off-target profiles are warranted to monitor these issues to ensure the safety of gene therapy [54]. Lastly, the pH value of prepubertal and sexually mature pig vaginal was neutral [55] (approximately ranging from 6.4 to 7.6) [56], which was different from that of the human (pH 3.5–4.5) [57]. Therefore, when future testing the delivery system in human, confounding factors including vaginal pH value should be further taken into considerations.
In summary, as a state-of-art DNA delivery material, the PBAE-based mMMT vaginal gel can be safely and effectively used for the CRISPR/Cas9 system delivery towards cervical cells, providing new delivery strategy for clinical application of vaginal gene therapy.
Author contributions
Z.H. took full responsibility for the study, especially in conceiving, designing and supervising the research together with S.T. and S.C.. G.N., Z.J. and C.Z. designed the study and drafted the manuscript. X.G., CM.Z., W.Z., J.D. and J.W. took part in the pharmaceutical design and material synthesis. B.D., K.S., I.H. and P.D. critically reviewed and revised the manuscript. D.H. and X.M. performed data analysis and interpretation. X.T., Q.G., Z.Y., R.T., Z.C. and W.F. performed the animal experiment. W.X., ZY.H., C.C., W.X. and HX.X. performed the DLS and SEM experiment. HY.X., XZ.T., Y.W., Z.Y. and H.H. performed the molecular biology experiments. All authors contributed to writing the manuscript, discussing the results and implications and editing the manuscript at all stages.
Declaration of Competing Interests
The authors declare that there is no conflict of interest to disclose.
Acknowledgments
This work was supported by the National Science and Technology Major Project of the Ministry of science and technology of China (No. 2018ZX10301402); the National Natural Science Foundation of China (81761148025, 81871473 and 81402158); Guangzhou Science and Technology Programme (No. 201704020093); National Ten Thousand Plan-Young Top Talents of China, Fundamental Research Funds for the Central Universities (17ykzd15 and 19ykyjs07); Three Big Constructions—Supercomputing Application Cultivation Projects sponsored by National Supercomputer Center In Guangzhou; the National Research foundation (NRF) South Africa under BRICS Multilateral Joint Call for Proposals; grant 17–54–80078 from the Russian Foundation for Basic Research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102897.
Contributor Information
Songwei Tan, Email: tansongwei@gmail.com.
Shuqin Chen, Email: chenshuqin1021@163.com.
Zheng Hu, Email: huzheng1998@163.com.
Appendix. Supplementary materials
Fig. S1: Animal experiment information
A) the cells number for fluorescence microscope and flow cytometry (n\elsamp #x00A0;\elsamp #x003D;\elsamp #x00A0;6 per group); B) live cells by flow cytometry for each group (n\elsamp #x00A0;\elsamp #x003D;\elsamp #x00A0;6 per group). C) Representative scatter profile of sampled cervical cells for each group (FSC vs SSC).
Fig. S2: DLS results of PBAE-GFP polyplex NP in different batches
Fig. S3: SEM images of PBAE-GFP polyplex NPs
SEM images of PBAE-GFP polyplex NPs, Scale bar, 1\elsamp #x00A0;\elsamp #x03BC;m.
Fig. S4: Living images of different formulations after different time interval
A) in vivo image, and B)ex vivo image at day 3. (day 0: just after gel prepared and injected; day 1: 1 day later; day 2: 2 days later; day 3: 3 days later)
Fig. S5: Analysis of PBAE-plasmids NPs based vaginal gel inflammatory effects in pigs
Representative images of IHC staining of the IFN-\elsamp #x03B3; and TNF-\elsamp #x03B1; expression at major organs cervix, ovary and uterus. CRISPR: PBAE-CRISPR/sgRNA NPs mMMT vaginal gel, Blank: PBAE NPs mMMT gel, Vagina. Scale bars, 500 \elsamp #x03BC;m
Fig. S6: DLS results of nanoparticle released from gels.
A) Particle sizes change of NPs, NPs-mMMT gel and NPs-HTT gel after different time interval (day 0: just after gel prepared; day 1: 1 day later; day 2: 2 days later; day 3: 3 days later;) and B) detailed DLS results.
References
- 1.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359(6372) doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 2.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526(7573):351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 3.Yin H., Kauffman K.J., Anderson D.G. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017;16(6):387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 4.Yin H., Song C.Q., Dorkin J.R., Zhu L.J., Li Y., Wu Q. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao Y., Hou X., Zuo F., Li X., Pang Y., Jiang G. Application of nanoparticle-based siRNA and CRISPR/Cas9 delivery systems in gene-targeted therapy. Nanomedicine (Lond) 2019;14(5):511–514. doi: 10.2217/nnm-2018-0522. [DOI] [PubMed] [Google Scholar]
- 6.Wong J.K.L., Mohseni R., Hamidieh A.A., MacLaren R.E., Habib N., Seifalian A.M. Will nanotechnology bring new hope for gene delivery? Trends Biotechnol. 2017;35(5):434–451. doi: 10.1016/j.tibtech.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordeiro R.A., Serra A., Coelho J.F.J., Faneca H. Poly(beta-amino ester)-based gene delivery systems: from discovery to therapeutic applications. J Control Release. 2019;310:155–187. doi: 10.1016/j.jconrel.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Li Y., Keskin D., Shi L. Poly(beta-Amino Esters): synthesis, formulations, and their biomedical applications. Adv Healthc Mater. 2019;8(2) doi: 10.1002/adhm.201801359. [DOI] [PubMed] [Google Scholar]
- 10.Gao X., Jin Z., Tan X., Zhang C., Zou C., Zhang W. Hyperbranched poly(β-amino ester) based polyplex nanopaticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J Controlled Release. 2020;321:654–668. doi: 10.1016/j.jconrel.2020.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Miao L., Guo S., Lin C.M., Liu Q., Huang L. Nanoformulations for combination or cascade anticancer therapy. Adv Drug Deliv Rev. 2017;115:3–22. doi: 10.1016/j.addr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen D.N., Green J.J., Chan J.M., Longer R., Anderson D.G. Polymeric materials for gene delivery and DNA vaccination. Adv Mater. 2009;21(8):847–867. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J.J., Langer R., Anderson D.G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41(6):749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai W.F., Wong W.T. Design of polymeric gene carriers for effective intracellular delivery. Trends Biotechnol. 2018;36(7):713–728. doi: 10.1016/j.tibtech.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Zuris J.A., Thompson D.B., Shu Y., Guilinger J.P., Bessen J.L., Hu J.H. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015;33(1):73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landegger L.D., Pan B., Askew C., Wassmer S.J., Gluck S.D., Galvin A. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35(3):280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Gascon A., Del Pozo-Rodriguez A., Isla A., Solinis M.A. Vaginal gene therapy. Adv Drug Deliv Rev. 2015;92:71–83. doi: 10.1016/j.addr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Hu Z., Ding W., Zhu D., Yu L., Jiang X., Wang X. TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J Clin Invest. 2015;125(1):425–436. doi: 10.1172/JCI78206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensign L.M., Cone R., Hanes J. Nanoparticle-based drug delivery to the vagina: a review. J Control Release. 2014;190:500–514. doi: 10.1016/j.jconrel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren C., Li X., Mao L., Xiong J., Gao C., Shen H. An effective and biocompatible polyethylenimine based vaginal suppository for gene delivery. Nanomedicine. 2019 doi: 10.1016/j.nano.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Furst T., Dakwar G.R., Zagato E., Lechanteur A., Remaut K., Evrard B. Freeze-dried mucoadhesive polymeric system containing pegylated lipoplexes: towards a vaginal sustained released system for siRNA. J Control Release. 2016;236:68–78. doi: 10.1016/j.jconrel.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Basnet P., Hussain H., Tho I., Skalko-Basnet N. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharm Sci. 2012;101(2):598–609. doi: 10.1002/jps.22785. [DOI] [PubMed] [Google Scholar]
- 23.Woolfson A.D., Malcolm R.K., Morrow R.J., Toner C.F., McCullagh S.D. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325(1–2):82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Ndesendo V.M., Pillay V., Choonara Y.E., Buchmann E., Bayever D.N., Meyer L.C. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS PharmSciTech. 2008;9(2):505–520. doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanic Z., Skalko-Basnet N. Nanopharmaceuticals for improved topical vaginal therapy: can they deliver? Eur J Pharm Sci. 2013;50(1):29–41. doi: 10.1016/j.ejps.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Yu L., Tian X., Gao C., Wu P., Wang L., Feng B. Genome editing for the treatment of tumorigenic viral infections and virus-related carcinomas. Front Med. 2018;12(5):497–508. doi: 10.1007/s11684-017-0572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Z., Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7(10):5217–5236. doi: 10.1002/cam4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh J., Michel D., Getson H.M., Chitanda J.M., Verrall R.E., Badea I. Development of amino acid substituted gemini surfactant-based mucoadhesive gene delivery systems for potential use as noninvasive vaginal genetic vaccination. Nanomedicine (Lond) 2015;10(3):405–417. doi: 10.2217/nnm.14.123. [DOI] [PubMed] [Google Scholar]
- 29.Kempf L., Goldsmith J.C., Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176(4):773–783. doi: 10.1002/ajmg.a.38413. [DOI] [PubMed] [Google Scholar]
- 30.Niu D., Wei H.J., Lin L., George H., Wang T., Lee I.H. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357(6357):1303–1307. doi: 10.1126/science.aan4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denner J. Can Antiretroviral Drugs Be Used to Treat Porcine Endogenous Retrovirus (PERV) Infection after Xenotransplantation? Viruses. 2017;9(8) doi: 10.3390/v9080213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L., Guell M., Niu D., George H., Lesha E., Grishin D. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015;350(6264):1101–1104. doi: 10.1126/science.aad1191. [DOI] [PubMed] [Google Scholar]
- 33.Mei L., Chen J., Yu S., Huang Y., Xie Y., Wang H. Expansible thermal gelling foam aerosol for vaginal drug delivery. Drug Deliv. 2017;24(1):1325–1337. doi: 10.1080/10717544.2017.1375575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai S.Q., Zheng Z., Nguyen N.T., Liebers M., Topkar V.V., Thapar V. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol. 2015;33(2):187–197. doi: 10.1038/nbt.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenzen E., Follmann F., Jungersen G., Agerholm J.S. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet Res. 2015;46:116. doi: 10.1186/s13567-015-0241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wegmann F., Gartlan K.H., Harandi A.M., Brinckmann S.A., Coccia M., Hillson W.R. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol. 2012;30(9):883–888. doi: 10.1038/nbt.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Chen Z., Du M., Li Y., Chen Y. Enhanced gene transfection efficiency by low-dose 25 kDa polyethylenimine by the assistance of 1.8 kDa polyethylenimine. Drug Deliv. 2018;25(1):1740–1745. doi: 10.1080/10717544.2018.1510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang P., Yu P., Wang W., Zhang L., Li S., Bu H. An effective method for the quantitative detection of porcine endogenous retrovirus in pig tissues. In Vitro Cell Dev Biol Anim. 2010;46(5):408–410. doi: 10.1007/s11626-009-9264-8. [DOI] [PubMed] [Google Scholar]
- 40.Doench J.G., Fusi N., Sullender M., Hegde M., Vaimberg E.W., Donovan K.F. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34(2):184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351(6268):84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J.S., Dagdas Y.S., Kleinstiver B.P., Welch M.M., Sousa A.A., Harrington L.B. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017 doi: 10.1038/nature24268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pineda M., Lear A., Collins J.P., Kiani S. Safe CRISPR: challenges and Possible Solutions. Trends Biotechnol. 2019;37(4):389–401. doi: 10.1016/j.tibtech.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 44.El-Hammadi M.M., Arias J.L. Nano-sized platforms for vaginal drug delivery. Curr Pharm Des. 2015;21(12):1633–1644. doi: 10.2174/1381612820666141029150427. [DOI] [PubMed] [Google Scholar]
- 45.Cook M.T., Brown M.B. Polymeric gels for intravaginal drug delivery. J Control Release. 2018;270:145–157. doi: 10.1016/j.jconrel.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Mirza M.A., Panda A.K., Asif S., Verma D., Talegaonkar S., Manzoor N. A vaginal drug delivery model. Drug Deliv. 2016;23(8):3123–3134. doi: 10.3109/10717544.2016.1153749. [DOI] [PubMed] [Google Scholar]
- 47.Remaut K., De Clercq E., Andries O., Rombouts K., Van Gils M., Cicchelero L. Aerosolized non-viral nucleic acid delivery in the vaginal tract of pigs. Pharm Res. 2016;33(2):384–394. doi: 10.1007/s11095-015-1796-x. [DOI] [PubMed] [Google Scholar]
- 48.das Neves J., Nunes R., Machado A., Sarmento B. Polymer-based nanocarriers for vaginal drug delivery. Adv Drug Deliv Rev. 2015;92:53–70. doi: 10.1016/j.addr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Güler M.A., Gök M.K., Figen A.K., Özgümüş S. Swelling, mechanical and mucoadhesion properties of Mt/starch-g-PMAA nanocomposite hydrogels. Appl Clay Sci. 2015;112-113:44–52. [Google Scholar]
- 50.Sharifzadeh G., Hezaveh H., Muhamad I.I., Hashim S., Khairuddin N. Montmorillonite-based polyacrylamide hydrogel rings for controlled vaginal drug delivery. Mater Sci Eng C Mater Biol Appl. 2020;110 doi: 10.1016/j.msec.2019.110609. [DOI] [PubMed] [Google Scholar]
- 51.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki K., Tsunekawa Y., Hernandez-Benitez R., Wu J., Zhu J., Kim E.J. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540(7631):144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haapaniemi E., Botla S., Persson J., Schmierer B., Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018;24(7):927–930. doi: 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 54.Cameron P., Fuller C.K., Donohoue P.D., Jones B.N., Thompson M.S., Carter M.M. Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat Methods. 2017;14(6):600–606. doi: 10.1038/nmeth.4284. [DOI] [PubMed] [Google Scholar]
- 55.Lorenzen E., Kudirkiene E., Gutman N., Grossi A.B., Agerholm J.S., Erneholm K. The vaginal microbiome is stable in prepubertal and sexually mature Ellegaard Gottingen Minipigs throughout an estrous cycle. Vet Res. 2015;46:125. doi: 10.1186/s13567-015-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenzen E., Agerholm J.S., Grossi A.B., Bojesen A.M., Skytte C., Erneholm K. Characterization of cytological changes, IgA, IgG and IL-8 levels and pH value in the vagina of prepubertal and sexually mature Ellegaard Göttingen minipigs during an estrous cycle. Develop Comparative Immunol. 2016;59:57–62. doi: 10.1016/j.dci.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Nunn K.L., Forney L.J. Unraveling the Dynamics of the Human Vaginal Microbiome. Yale J Biol Med. 2016;89(3):331–337. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Animal experiment information
A) the cells number for fluorescence microscope and flow cytometry (n\elsamp #x00A0;\elsamp #x003D;\elsamp #x00A0;6 per group); B) live cells by flow cytometry for each group (n\elsamp #x00A0;\elsamp #x003D;\elsamp #x00A0;6 per group). C) Representative scatter profile of sampled cervical cells for each group (FSC vs SSC).
Fig. S2: DLS results of PBAE-GFP polyplex NP in different batches
Fig. S3: SEM images of PBAE-GFP polyplex NPs
SEM images of PBAE-GFP polyplex NPs, Scale bar, 1\elsamp #x00A0;\elsamp #x03BC;m.
Fig. S4: Living images of different formulations after different time interval
A) in vivo image, and B)ex vivo image at day 3. (day 0: just after gel prepared and injected; day 1: 1 day later; day 2: 2 days later; day 3: 3 days later)
Fig. S5: Analysis of PBAE-plasmids NPs based vaginal gel inflammatory effects in pigs
Representative images of IHC staining of the IFN-\elsamp #x03B3; and TNF-\elsamp #x03B1; expression at major organs cervix, ovary and uterus. CRISPR: PBAE-CRISPR/sgRNA NPs mMMT vaginal gel, Blank: PBAE NPs mMMT gel, Vagina. Scale bars, 500 \elsamp #x03BC;m
Fig. S6: DLS results of nanoparticle released from gels.
A) Particle sizes change of NPs, NPs-mMMT gel and NPs-HTT gel after different time interval (day 0: just after gel prepared; day 1: 1 day later; day 2: 2 days later; day 3: 3 days later;) and B) detailed DLS results.