Abstract
Background
An objective evaluation of coronavirus disease 2019 in the first days of infection is almost impossible, as affected individuals are generally in home quarantine, and there is limited accessibility for the operator who should perform the test. To overcome this limitation, a recently validated psychophysical self-administered test was used, which can be performed remotely in the assessment of early-stage coronavirus disease 2019 patients.
Methods
Olfactory and gustatory functions were objectively assessed in 300 patients in the first 7 days from coronavirus disease 2019 symptom onset.
Results
Seventy per cent of the patients presented olfactory and/or gustatory disorders. The dysfunctions detected were mainly complete anosmia (47 per cent) or ageusia (38 per cent). A significant correlation was found between taste dysfunction and female gender (odds ratio = 1.936, p = 0.014) and fever (odds ratio = 2.132, p = 0.003).
Conclusion
The psychophysical evaluation protocol proposed is an effective tool for the fast and objective evaluation of patients in the early stages of coronavirus disease 2019. Chemosensitive disorders have been confirmed to be frequent and early symptoms of the coronavirus infection, and, in a significant number of cases, they are the first or only manifestation of coronavirus disease 2019.
Key words: COVID-19, Taste, Smell, Anosmia, Ageusia, SARS-CoV-2 Infection, Coronavirus
Introduction
The high frequency of chemosensitive disorders in patients with coronavirus disease 2019 (Covid-19) is a clinical finding reported by several authors in Europe and America.1–10 These symptoms are typical of the early stages of the disease,11,12 and, considering their specificity, they may be useful as a screening marker.13,14
Psychophysical tests are crucial to determine the exact frequency, extent and clinical characteristics of these chemosensitive disorders, and to monitor their recovery over time. However, most of the studies currently present in the literature are based on anamnestic or observational evidence. As of 25 May 2020, only four objective studies had been published.15–18 These few studies mainly include patients with advanced stages of Covid-19 at the time of worst impairment, preventing the evaluation of chemosensitive functions. An objective evaluation in the first days of the disease is almost impossible, as these patients are generally in home quarantine, and there is limited accessibility for the operator who should perform the test.
A few weeks ago, Vaira et al.19 proposed and validated a new self-administered psychophysical test that can be performed remotely for the evaluation of olfactory and gustatory functions in patients in home quarantine. Using this test, we have objectively evaluated the chemosensitive functions of 300 Covid-19 patients, belonging to the healthcare staff of the Bellaria-Maggiore Hospital in Bologna, within the first 7 days of Covid-19 clinical onset.
Materials and methods
This cohort study was conducted on severe acute respiratory syndrome 2 (SARS-CoV-2) positive patients monitored by the Surveillance and Prevention Department of Bellaria-Maggiore Hospital in Bologna, between 16 April and 2 May 2020. All the subjects were healthcare staff at the hospital.
In order to be eligible for enrolment in this study, patients had to meet the following inclusion criteria: adult over 18 years of age; SARS-CoV-2 infection confirmed by nasopharyngeal swab; and Covid-19 clinical onset (or positive swab in asymptomatic patients) less than 7 days previously.
Patients were excluded from the study if they did not answer the phone call twice, refused to participate, or presented a history of: previous gustatory or olfactory disorders, chronic rhinosinusitis, surgery, or radiotherapy or trauma to the oral and nasal cavities. The study protocol was approved by the Azienda Unità Sanitaria Locale di Bologna Ethical Committee (number: 378-2020-OSS-AUSLBO).
The lists of SARS-CoV-2 positive subjects were obtained from the Surveillance and Prevention Department of Bellaria-Maggiore Hospital. Two operators called each individual from the lists within 7 days of Covid-19 clinical onset.
Each telephone interview started with the request for the patient's explicit consent to participate in the study. Next, the patient's demographics (i.e. age, gender and professional role), co-morbidities or conditions that could be a cause for exclusion from the study were recorded. The presence of other Covid-19 symptoms and the date of onset were also recorded for each patient.
Finally, the chemosensitive psychophysical test procedure was explained to the subjects. Specifically, the patients were asked to collect seven common household odorants and prepare four basic flavoured solutions, before calling the operator again to perform the test. The test methodology and the scoring system have already been described by Vaira et al.19 Both the olfactory and gustatory functions were objectively evaluated.
The olfactory threshold was established by means of solutions with increasing dilutions of denatured ethyl-alcohol. Each patient was asked to prepare a 40 per cent solution of ethyl-alcohol in 100 ml water (bottle 0) using a syringe or a graduated container. In the subsequent bottles (bottles 1–8), the patients diluted a part of the previous solution with two parts of water, thus obtaining solutions with consequent 1:3 dilutions. A bottle filled with water was used as control. The test was performed by asking the patients to smell the alcohol-containing bottles, starting with the least concentrated (bottle 8), reporting if they found any differences compared to the control bottle. In the case of a negative answer, the examination continued with the subsequent bottle.19
The discriminative test was performed by asking each patient to collect some common household odorants: group A – orange juice, lemon juice or other citrus fruit juice; group B – pepper, rosemary, bay leaf or sage; group C – ‘Marseille’ or neutral soap; group D – wine or vinegar; group E – chocolate, Nutella® or coffee; and group F – Vicks VapoRub®, mint toothpaste or mouthwash. The subjects were then asked to smell one odorant from each group, giving a score reflecting their discriminative ability from 0 (no discrimination) to 10 (normal discrimination). The discriminative score was obtained from the average of the ratings for the six odorants.
The olfactory threshold and identification test scores were summed to provide an overall composite score; this allows a clinical classification of olfactory function (Table 1).19
Table 1.
Self-administered chemosensory test scoring systems: olfactory test
| Olfactory threshold score | Olfactory threshold composite score | Odour discrimination score | Odour discrimination composite score | Overall composite score* | Clinical diagnosis |
|---|---|---|---|---|---|
| 7–8 | 50 | 8–10 | 50 | 90–100 | Normal |
| 6 | 40 | 6–7.9 | 40 | 70–80 | Mild hyposmia |
| 5 | 30 | 4–5.9 | 30 | 50–60 | Moderate hyposmia |
| 4 | 20 | 3–3.9 | 20 | 20–40 | Severe hyposmia |
| 2–3 | 10 | 1–2.9 | 10 | 0–10 | Anosmia |
| 0–1 | 0 | 0–0.9 | 0 | 90–100 | Normal |
Olfactory threshold plus odour discrimination scores
The gustatory function was assessed by means of four solutions, one for each primary taste. These were prepared by each patient as follows: sweet solution – 60 g of refined sugar in 1 litre of water; sour solution – 90 ml of 100 per cent lemon juice in 1 litre of water; salted solution – 30 g of table salt in 1 litre of water; and bitter solution – unsweetened decaffeinated coffee.19 The patients were asked to taste a teaspoon of each solution, scoring the quality of their taste perception from 0 (ageusia) to 10 (normal perception). The bitter solution was always administered last. The gustatory score was obtained by averaging the values reported for each of the primary tastes (Table 2).
Table 2.
Self-administered chemosensory test scoring systems: gustatory test
| Patient administered scoring | Taste scoring system | Clinical diagnosis |
|---|---|---|
| 10–7 | 4 | Normal |
| 5–6.9 | 3 | Mild hypogeusia |
| 3–4.9 | 2 | Moderate hypogeusia |
| 1–2.9 | 1 | Severe hypogeusia |
| 0–0.9 | 0 | Ageusia |
Statistical analysis was performed using SPSS software, version 26.0 (IBM, Armonk, New York, USA). Categorical variables are reported in terms of numerals and percentages of the total. Descriptive statistics for quantitative variables are given as the mean ± standard deviation. Logistic regression analysis and the Fisher's exact test were used to evaluate the significance of the correlations between the chemosensitive disorders and the general and clinical variables. The level of statistical significance was set at p ≤ 0.05, with a 95 per cent confidence interval (CI).
Results
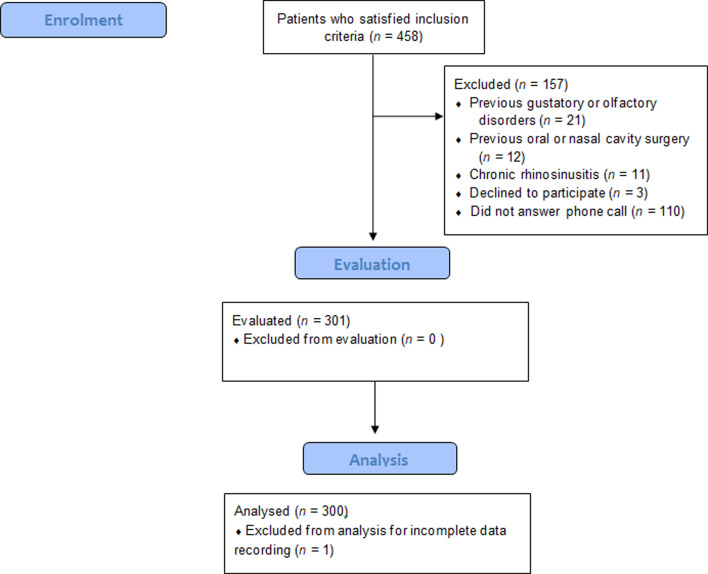
Of the 458 eligible patients, 157 were excluded in accordance with the exclusion criteria (Figure 1). The 301 remaining patients undertook the chemosensitive psychophysical test. One patient was excluded from the analysis because of an error in the recorded data. The general and clinical features of the patients are summarised in Table 3.
Fig. 1.
Consolidated Standards of Reporting Trials (‘CONSORT’) flowchart.
Table 3.
General and clinical features of study population
| Characteristic | Value |
|---|---|
| Gender (n (%)) | |
| – Male | 75 (25) |
| – Female | 225 (75) |
| Age (mean ± SD (IQR); years) | 43.6 ± 12.2 (33–53) |
| Reported symptoms (n (%)) | |
| – Asymptomatic | 28 (9.3) |
| – Fever | 204 (68) |
| – Headache | 133 (44.3) |
| – Myalgia | 128 (42.7) |
| – Asthenia | 109 (36.3) |
| – Cough | 95 (31.7) |
| – Pneumonia | 74 (24.7) |
| – Diarrhoea | 70 (23.3) |
| – Nausea | 35 (11.7) |
| – Dyspnoea | 31 (10.3) |
| – Nasal obstruction | 27 (9) |
| – Sore throat | 24 (8) |
| – Conjunctivitis | 11 (3.7) |
SD = standard deviation; IQR = interquartile
The objective evaluation revealed that 210 patients (70 per cent) had a chemosensitive disorder. In 54.7 per cent of cases, both taste and smell were affected, 8.7 per cent of patients had isolated olfactory disorders, while in 6.7 per cent of cases only taste was affected (Table 4). Both the olfactory and gustatory disturbances detected were, in the majority of cases, severe. In fact, 47 per cent and 38 per cent of patients presented olfactory and gustatory scores indicative of complete anosmia and ageusia, respectively (Table 4).
Table 4.
Chemosensory function assessment results
| Parameter | Patients (n (%)) |
|---|---|
| Chemosensory dysfunctions | |
| – Olfactory & taste disorders | 164 (54.7) |
| – Only olfactory disorders | 26 (8.7) |
| – Only taste disorders | 20 (6.7) |
| – Total | 210 (70) |
| – No taste or olfactory disorders | 90 (30) |
| Olfactory dysfunctions | |
| – Anosmia | 141 (47) |
| – Severe hyposmia | 22 (7.3) |
| – Moderate hyposmia | 19 (6.3) |
| – Mild hyposmia | 8 (2.7) |
| – Normal | 110 (36.7) |
| Gustatory dysfunctions | |
| – Ageusia | 114 (38) |
| – Severe hypogeusia | 22 (7.3) |
| – Moderate hypogeusia | 30 (10) |
| – Mild hypogeusia | 18 (6) |
| – Normal | 116 (38.7) |
The statistical significance of the associations between olfactory and gustatory disorders and some general and clinical characteristics of the patients was assessed. No significant correlations were found between the olfactory disturbances and the independent variables tested (Table 5). On the contrary, statistically significant correlations were found between taste dysfunctions and female gender (odds ratio = 1.936, 95 per cent CI = 1.139–3.386, p = 0.014) and fever (odds ratio = 2.132, 95 per cent CI = 1.299–3.499, p = 0.003) (Table 6).
Table 5.
Olfactory logistic regression and cross-tabulation analysis results
| Parameter | Olfactory dysfunction patients (n (%)) | No olfactory dysfunction (n (%)) | OR | 95% CI for OR | Fisher's exact test value | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Gender*:olfactory dysfunction† | ||||||
| – Female | 149 (66.2) | 76 (33.8) | 1.626 | 0.955 | 2.768 | 0.073 |
| – Male | 41 (54.7) | 34 (45.3) | ||||
| Age*:olfactory dysfunction† | ||||||
| – <50 years old | 128 (65) | 69 (35) | 1.227 | 0.751 | 2.005 | 0.415 |
| – ≥50 years old | 62 (60.2) | 41 (39.8) | ||||
| Fever*:olfactory dysfunction† | ||||||
| – Fever | 136 (71.6) | 54 (28.4) | 1.556 | 0.946 | 2.558 | 0.082 |
| – No fever | 68 (61.8) | 42 (38.2) | ||||
| Headache*:olfactory dysfunction† | ||||||
| – Headache | 89 (66.9) | 44 (33.1) | 1.322 | 0.821 | 2.128 | 0.251 |
| – No headache | 101 (60.5) | 66 (39.5) | ||||
| Pneumonia*:olfactory dysfunction† | ||||||
| – Pneumonia | 52 (70.3) | 22 (29.7) | 1.507 | 0.856 | 2.654 | 0.155 |
| – No pneumonia | 138 (61.1) | 88 (38.9) | ||||
*Dependent variable; †independent variable. OR = odds ratio; CI = confidence interval
Table 6.
Gustatory logistic regression and cross-tabulation analysis results
| Parameter | Gustatory dysfunction patients (n (%)) | No gustatory dysfunction (n (%)) | OR | 95% CI for OR | Fisher's exact test value | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Gender*:gustatory dysfunction† | ||||||
| – Female | 147 (65.3) | 78 (34.7) | 1.936 | 1.139 | 3.286 | 0.014 |
| – Male | 37 (49.3) | 38 (50.7) | ||||
| Age*:gustatory dysfunction† | ||||||
| – <50 years old | 123 (62.4) | 74 (37.6) | 1.144 | 0.703 | 1.863 | 0.587 |
| – ≥50 years old | 61 (59.2) | 42 (40.8) | ||||
| Fever*:gustatory dysfunction† | ||||||
| – Fever | 137 (64.7) | 67 (35.3) | 2.132 | 1.299 | 3.499 | 0.003 |
| – No fever | 47 (49) | 49 (51) | ||||
| Headache*:gustatory dysfunction† | ||||||
| – Headache | 82 (61.6) | 51 (38.4) | 1.102 | 0.919 | 1.636 | 0.919 |
| – No headache | 102 (61.1) | 65 (38.9) | ||||
| Pneumonia*:gustatory dysfunction† | ||||||
| – Pneumonia | 48 (64.9) | 26 (35.1) | 1.222 | 0.707 | 2.11 | 0.473 |
| – No pneumonia | 136 (60.2) | 90 (39.8) | ||||
*Dependent variable; †independent variable. OR = odds ratio; CI = confidence interval
Discussion
Olfactory and gustatory disorders have been recognised as early symptoms of the SARS-CoV-2 infection.1–12 Their high frequency in Covid-19 patients makes them fundamental diagnostic markers, potentially enabling the early detection and isolation of suspect cases. Such health control measures will be crucial in the coming months in order to contain any new epidemic clusters.
This study adds to a very small number of other series that have evaluated chemosensitive functions in Covid-19 patients using psychophysical tests.15–18 The objective evaluation of these patients is essential in order to: quantify the exact frequency of chemosensitive disorders, obtain a standardised classification of severity and monitor their recovery over time. The major strength of the present study is the analysis of a large series of patients in an early stage of infection, when olfactory and gustatory disturbances are actually at their maximum severity and have not yet begun to regress. In fact, the studies already published, although objective, mostly evaluate patients two to three weeks after clinical onset. The lack of early objective data is linked to insurmountable logistical problems in evaluating these patients, who are often in home quarantine, and hence there is limited accessibility for the operators who should perform the test. These problems have been overcome by using a remote self-administered psychophysical test that was recently proposed and validated by our research team.19 Moreover, this test guarantees the operator's safety; this risk is another factor limiting the execution of psychophysical tests with infected patients.
The results of this study revealed a very high frequency of chemosensitive disorders in SARS-CoV-2 infected patients. As noted by other authors,1,3,9,11,16–18 in Covid-19, chemosensitive disorders are typically not associated with rhinitis symptoms and nasal obstruction, present only in 9 per cent of the patients included in this study. In 16.2 per cent of patients, the chemosensitive disorder was the first manifestation of the disease, while in 4.6 per cent it was the only manifestation.
The dysfunctions detected were principally complete anosmia and/or ageusia, or severe hyposmia and/or hypogeusia. Such a high rate of severe disorders can be ascribed to the very early stage of the Covid-19 in the patients evaluated, when functional recovery has not yet started.
The association between chemosensitive disorders and female gender has already been reported by other authors, and is hypothetically related to different immune response patterns between men and women.9 In this study, this relationship was detected only for taste, but the series presented an imbalance between males (25 per cent of the pool) and females (75 per cent). Further studies with more homogeneous cases will be necessary to confirm this result.
Interestingly, no significant correlation was found between olfactory disorders and headaches, and no other symptoms related to central nervous system involvement were reported by the patients. This finding, together with the tendency for spontaneous remission of the dysfunction within a few weeks,9,14,16–19 would suggest a pathogenesis linked to inflammatory damage in the olfactory epithelium. However, the ability of SARS-CoV-2 to invade the central nervous system causing neuronal damage cannot be excluded.20
Some authors have proposed an association between the presence of chemosensitive disorders and mild Covid-19.21,22 In our case history, there was no significant association between olfactory or gustatory disorders and the early relief of lung involvement. In considering these results, it should be clarified that not all the patients routinely underwent a computed tomography scan of the chest. Moreover, the time of pulmonary deterioration is generally longer than that observed in this study. Prospective objective studies will be necessary to clarify the prognostic value of chemosensitive disorders.8
-
•
The proposed and validated psychophysical test is effective for fast, objective evaluation of early-stage coronavirus disease 2019 (Covid-19) patients
-
•
Chemosensitive disorders are frequent, early symptoms of the coronavirus infection
-
•
In a significant number of cases, chemosensitive disorders are the first or only manifestation of Covid-19
-
•
This study establishes a solid objective base for prospective evaluation of functional recovery and the prognostic value of chemosensitive disorders
Conclusion
The psychophysical test proposed and validated by Vaira et al.19 has proven to be an effective tool for the fast and objective evaluation of patients in the early stages of Covid-19. Based on our results, chemosensitive disorders have been confirmed to be frequent and early symptoms of the coronavirus infection, and in a significant number of cases they are the first or only manifestation of Covid-19. This study establishes a solid objective base for the prospective evaluation of the functional recovery and the prognostic value of chemosensitive disorders.
Competing interests
None declared
References
- 1.Vaira LA, Salzano G, Deiana G, De Riu G. Ageusia and anosmia: common findings in COVID-19 patients. Laryngoscope 2020;130:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C et al. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis 2020. Epub 2020 Apr 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020;323:2089–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology 2020;58:295–8 [DOI] [PubMed] [Google Scholar]
- 5.Russel B, Moss C, Rigg A, Hopkins C, Papa S, Van Hemelrijck M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: what does the current evidence say? Ecancermedicalscience 2020;14:ed98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaira LA, Salzano G, Deiana G, De Riu G. Response to isolated anosmia and ageusia in COVID-19 with spontaneous recovery; an ignored entity. Laryngoscope 2020. Epub 2020 Jun 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaira LA, Salzano G, Deiana G, Salzano FA, De Riu G. In response to: In reference to anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 2020. Epub 2020 Jun 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins C, Vaira LA, De Riu G. Self-reported olfactory loss in COVID-19: is it really a favorable prognostic factor? Int Forum Allergy Rhinol 2020;10:926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020. Epub 2020 Apr 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechien JR, Hopkins C, Saussez S. Sniffing out the evidence; it's now time for public health bodies recognize the link between COVID-19 and smell and taste disturbance. Rhinology 2020. Epub 2020 Apr 30 [DOI] [PubMed] [Google Scholar]
- 11.Vaira LA, Salzano G, De Riu G. The importance of olfactory and gustatory disorders as early symptoms of coronavirus disease (COVID-19). Br J Oral Maxillofac Surg 2020;58:615–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaira LA, Salzano G, Deiana G, De Riu G. In response to anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 2020. Epub 2020 Jul 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karimi-Galougahi M, Raad N, Mikaniki N. Anosmia and the need for COVID-19 screening during the pandemic. Otolaryngol Head Neck Surg 2020. Epub 2020 May 5 [DOI] [PubMed] [Google Scholar]
- 14.Sedaghat AR, Gengler I, Speth MM. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg 2020. Epub 2020 May 5 [DOI] [PubMed] [Google Scholar]
- 15.Moein ST, Hahemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol 2020. Epub 2020 Apr 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 2020;42:1252–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020;42:1560–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020. Epub 2020 May 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck 2020;42:1570–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaira LA, Salzano G, Fois AF, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol 2020. Epub 2020 Apr 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan CH, Faraji FF, Divya PP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol 2020;10:821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan CH, Faraji F, DeConde AS. Reply to “Self-reported olfactory loss in COVID-19: is it really a favorable prognostic factor?”. Int Forum Allergy Rhinol 2020;10:927–928 [DOI] [PMC free article] [PubMed] [Google Scholar]