Abstract
This study aims to characterize the potential of three strains of microalgal species (Chlorella sorokiniana KNUA114 and KNUA122; C. vulgaris KNUA104) for use as feedstock, based on their fatty acid compositions. Each strain was molecularly identified using four marker genes (ITS, SSU, rbcL, and tufA) and phylogenetically characterized. C. sorokiniana and C. vulgaris collected from Ulleung Island, South Korea, were homologous with other known species groups. Samples' fatty acid components were measured using GC/MS analysis in growth temperatures of 10 °C, 25 °C, and 35 °C. The growth rate of C. sorokiniana strains was higher than that of C. vulgaris under high-temperature conditions, confirming the potential industrial applicability of the former as feedstock material. Additionally, saturated fatty acid contents and productivities increased as biological resources of the C. sorokiniana strains were higher than those of C. vulgaris under high light intensity and temperature conditions. These results suggest that the fatty acid components of C. sorokiniana strains may potentially be used as biological resources (e.g., feedstock).
Keywords: Bioinformatics, Biotechnology, Genetics, Microbiology, Molecular biology, Plant biology, Biological resource, Chlorella sorokiniana, Chlorella vulgaris, Fatty acid components, Microalgae
Bioinformatics; Biotechnology; Genetics; Microbiology; Molecular biology; Plant biology; Biological resource; Chlorella sorokiniana; Chlorella vulgaris; Fatty acid components; Microalgae
1. Introduction
Microalgae are important primary producers in the process of photosynthesis. Microalgal species have distinct characteristics and are adapted to a wide range of environments (Andersen and Hessen, 1991; Dufossé et al., 2005; Rasal et al., 2019). Previous research confirmed that microalgae could be used as biological resources (Dufossé et al., 2005; Becker, 2007; Vanthoor-Koopmans et al., 2013). Microalgal nutritional compounds, such as vitamins, minerals, fibers, fatty acids, and amino acids, have been investigated to determine the appropriate use for different species (Caporgno and Mathys, 2018). Microalgae do not require supplementation with organic carbon sources during cultivation as they carry out photosynthesis. The higher photosynthetic efficiency and growth rate of microalgae compared to other photosynthetic organisms explain its relatively faster and higher biomass accumulation (Chisti, 2007, 2010; Ötleş and Pire, 2001; Herrero et al., 2006; Spolaore et al., 2006; Randhir et al., 2020). Microalgal biomass contains large amounts of unsaturated fatty acids, lutein, food particles, and other useful materials.
Mass cultivation of microalgae will be required to meet the potential demand for biomass in various industries, leading to the development of the open pond type race way system (Ryu et al., 2019; Banu et al., 2020). This system can effectively produce large amounts of microalgal biomass at a low cost but has a risk of exposure to stress from external environments, such as light and extreme temperatures (Lee et al., 2018; Akizuki et al., 2020; Ma et al., 2020). Microalgae are photosynthetic organisms, but the light intensity and temperature required for optimal growth differ between species (Lee et al., 2018; Ma et al., 2020). Indeed, the range of light intensity and temperature under which microalgal growth is inhibited varies between species (Isdepsky and Borowitzka, 2019; Rayen et al., 2019). Therefore, it is important to select a suitable species for biomass production of microalgae using the open pond type raceway system (Isdepsky and Borowitzka, 2019; Rayen et al., 2019) and one which can adapt to conditions of high light intensity and temperature (Lee et al., 2018; Rayen et al., 2019; Akizuki et al., 2020). Such environmental conditions have been shown to increase the lipid and saturated fatty acid content of microalgae (Han et al., 2016; Hong et al., 2019; Yaisamlee and Sirikhachornkit, 2020), further suggesting the importance of adaptation to these conditions in the microalgal species we aim to select.
Microalgae fatty acid components account for 10%–20% of the organism's dry weight. These compounds are used as biological resources in biofuel and dietary supplements as they contain a varying number of carbon atoms, such as medium-chain (C10–C14), long-chain (C16–C18), and very-long-chain (C20 or more) molecules and derivatives (Ötleş and Pire, 2001; Hu et al., 2008). C16 and C18 fatty acids, in particular, are the main biological resources for biofuel and dietary supplement production (Herrero et al., 2006; Lin and Lin, 2011; Zhou et al., 2011). Methyl ester is the base unit used to produce these fatty acids. Several methods, such as blending, pyrolysis, transesterification, and catalytic digestion, have been employed for the possible application of the abovementioned fatty acid components as biological resources (Ma and Hanna, 1999; Spolaore et al., 2006; Zhou et al., 2011). A previous study has reported that an increasing interest of consumers in biological resources has led to the rise in the demand for high-quality fatty acid ingredients (Ötleş and Pire, 2001; Kumar et al., 2019). This demand in fatty acids for biological resources has led to the rise of conditioning of nutrients, such as nitrate and phosphate to enhance the fatty acid content of microalgae (Anto et al., 2019; Gupta et al., 2019; Huang et al., 2020). Therefore, nutrient deprivation-induced stress affects the production of microalgae biomass and may impair lipid productivity (Yang et al., 2018). Furthermore, the consumption rate of nitrate and phosphate in the culture medium corresponds to the growth of microalgae conditioning (De-Bashan et al., 2005; Xin et al., 2010; Nigam et al., 2011; Wan et al., 2013). However, few studies have investigated the changes in microalgal production under conditions of high light intensity and temperature, or the impact of these growth conditions on fatty acid content and saturated fatty acids and corresponding biomass and lipid productivity. The production of biological resources normally involves two purification processes: supercritical fluid extraction and subcritical water extraction, which are used to extract natural products from various raw materials, including plants, algae, and microalgae (Ötleş and Pire, 2001; Hoydonckx et al., 2004; Herrero et al., 2006). Microbes are employed in these processes to produce linoleic acid (C18:2 ω6) and α-linolenic acid (C18:3 ω3) from oleic acid (C18:1 ω9). Furthermore, microalgae can produce hexadecadienoic (C16:2) and hexadecatrienoic (C16:3) acids from palmitic acid (C16:0) (Liperoti et al., 2009; Zhou et al., 2011). Based on these results, the extraction of fatty acids from microalgae is considerably important for their application as biological resources.
In this study, Korean Chlorella sorokiniana KNUA114 and KNUA122, and Chlorella vulgaris KNUA104 were aseptically isolated from Ulleung Island. Previous observations of these strains suggest that Chlorellaceae is one of the important families of organisms that can be used as biological resources worldwide (Morimura and Tamiya, 1954; Taub and Dollar, 1968; Kay and Barton, 1991; Park et al., 2020). The phylogenetic differences between the isolated C. sorokiniana and C. vulgaris strains were confirmed through morphological and molecular characterization. Furthermore, the value of C. sorokiniana and C. vulgaris strains as a potential biological resource was determined by analysing microalgal growth and fatty acid composition. Based on our findings, the values of C. sorokiniana and C. vulgaris were compared; C. sorokiniana strains were found to be more suitable as fatty acid sources than C. vulgaris due to comparatively faster growth and greater productivity of the former under high light intensity and temperature conditions.
2. Materials and Methods
2.1. Sampling and isolation of microalgae
Microalgal bloom samples were collected from on Ulleung Island, South Korea, between April and October 2017. The two collection sites were the Okcheon stream (for C. sorokiniana KNUA114 and KNUA122; 37°28′24.0″N, 130°53′06.8″E; Figure 1A) and the Namseo stream (for C. vulgaris KNUA104; 37°28′01.5″N, 130°50′12.4″E; Figure 1B) (Abou-Shanab et al., 2011). Samples were then transferred to the laboratory and 10-mL aliquots were inoculated into 100 mL of BG-11 medium in a 250-mL flask (Rippka et al., 1979; Bolch and Blackburn, 1996). The flasks were placed in an orbital shaker (VS-202D, Vision Scientific, Bucheon, South Korea) rotating at 160 rpm and incubated at 25 °C with illumination by a fluorescent lamp (400 μmol photons) under light:dark cycle of 16:8 h until algal growth could be observed. Grown algal cultures (100 μL) were streaked onto BG-11 agar plates. Subsequently, single colonies were aseptically transferred onto fresh BG-11 agar plates; this step was repeated until a pure algal culture was obtained (Stanier et al., 1971).
Figure 1.
The collection sites on Ulleung Island, South Korea. (A) Okcheon Stream, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do (C. vulgaris KNUA104 and C. sorokiniana KNUA122; 37°28ʹ24.0ʹʹN, 130°53ʹ06.8ʹʹE) (B) Namseo Stream, Seo-myeon, Ulleung-gun, Gyeongsangbuk-do (C. sorokiniana KNUA114; 37°28ʹ01.5ʹʹN, 130°50ʹ12.4ʹʹE). (C) Light microscope images of C. vulgaris KNUA104, (D) C. sorokiniana KNUA114, and (E) C. sorokiniana KNUA122 (Scale bar = 5 μm). (F) A 250-mL flask containing C. vulgaris KNUA104, (G) C. sorokiniana KNUA114, and (H) C. sorokiniana KNUA122.
2.2. Morphological characterization of microalgal species
Before the morphological analysis (Safi et al., 2014; Krienitz et al., 2004), the isolated algal strains were grown in BG-11 medium for 10 days. A pure culture of algal cells was harvested by centrifuging at 3,000 rpm for 5 min (Centrifuge 5810R, Eppendorf AG, Hamburg, Germany) and cells were washed with distilled water and inspected using a Nikon Eclipse E100 biological microscope (Tokyo, Japan) at 1000× magnification.
2.3. Molecular identification
Before genomic DNA extraction, algal cells were harvested by centrifugation at 3,000 rpm for 5 min (Centrifuge 5810R, Eppendorf AG, Hamburg, Germany) and washed with distilled water. Cells were then mixed with 400 μL DNA extraction buffer (100 mM Tris HCl, 1 M KCl, and 10 mM EDTA), 8 μL RNAse A (50 mg/mL, Elpis-Biotech, Daejeon, South Korea), and sterile glass beads in a 2-mL microfuge tube. The tube was vortexed for 5 min and incubated at 65 °C for 30 min. Cellular debris was removed by centrifuging for 10 min at 13,000 rpm and 4 °C. Subsequently, 400 μL of the supernatant was transferred into a new tube and DNA extraction was performed using a DNA purification kit (Wizard DNA Clean-Up System, Promega, Madison, WI, USA). Marker genes were amplified by PCR using internal transcribed spacer (ITS) region ITS1-F and ITS4-R (Polle et al., 2008; Koetschan et al., 2010); small subunit rRNA gene (SSU) NS1-F and NS8-R (White et al., 1990; Friedl, 1995); large unit ribulose bisphosphate carboxylase (rbcL): rbcL-M379-F, rbcLFP-R, GrbcL-F, and GrbcL-R (Hanyuda et al., 2000; Vieira et al., 2016); and elongation factor tu gene (tufA) tufA-F and tufA-R (Vieira et al., 2016). The PCR primers used in this study are listed in Table S1. Amplified DNA fragments were purified using a PCR purification kit (Spin type-200) (Elpis-Biotech, Daejeon, South Korea), then cloned using the pGEM®-T Easy Vector kit (Promega, Madison, WI, USA) (Weisburg et al., 1991). Cloned DNA samples were sequenced at the Genotech facility in Daejeon, South Korea. Finally, the phylogenetic analysis was performed using ITS-, SSU-, rbcL-, and tufA-specific primers generated according to methods described in Friedl (1995) and Buchheim et al. (2011) and MEGA version 7.0 (Kumar et al., 2008, 2016). The best-fit nucleotide substitution models for ITS (Kimura 2-parameter), SSU (Tamura-Nei), rbcL (General Time Reversible), and tufA (General Time Reversible) were selected using MEGA 7.0, based on the Bayesian information criterion. These models were used to build maximum likelihood phylogenetic trees with 1000 bootstrap replications (Felsenstein, 1985).
2.4. Identification of the optimal temperature and light intensity
The optimal temperature and light intensity conditions for the culture of each microalgal strain were investigated using a PhotoBiobox, a high-throughput system used to analyze photosynthesis (Heo et al., 2015). Each strain was grown from aseptically-transferred, isolated colonies (1.0 × 106 cells/mL, 200 μL), inoculated to 96-well plates containing BG-11 medium and incubated for three days at temperatures ranging from 6°C–28 °C and light intensities ranging from 300–1,000 μmol photons m−2 s−1.
2.5. Laboratory cultivation
A 10-day-old culture of each strain originating from aseptically-transferred, isolated colonies (15 mL) (diluted to OD680 ~0.3, corresponding to the following cell densities: KNUA104, 391.43 ± 16.05 cells × 104 ml−1; KNUA114, 486.32 ± 29.51 cells × 104 ml−1; and KNUA122, 523.41 ± 21.73 cells × 104 ml−1) was inoculated into flasks containing 150 mL of BG-11 medium (performed in triplicates). Flasks were then incubated for 14 days on orbital shakers (VS-202D, Vision Scientific) at 160 rpm in rooms illuminated by fluorescent lamps (300 μmol photons m−2 s−1) under controlled temperature (10 °C, 25 °C, or 35 °C) and a light:dark cycle of 16:8 h. The growth of each strain was subsequently measured as previously described (Hong et al., 2016). Algal density was estimated by measuring the OD680 values with an Optimizer 2120 UV spectrophotometer (Mecasys, South Korea) (Griffiths et al., 2011). The above procedure was scaled up and further performed under the same conditions, and algal cells were inoculated into 2 L of BG-11 medium in a flask (maximum volume of flask; 5 L). The algal density of the scale up culture was estimated by measuring OD680 values.
2.6. Productivity analysis
The dry weights of the microalgal cultures were measured to analyze biomass productivity at the stationary phase. Dry weight was calculated by filtering 10 mL of culture through a pre-weighed GF/C Whatman filter (47 mm, nominal pore size 0.7 μm), which was subsequently dried at 105 °C for 24 h. The dried filters were cooled to room temperature and re-weighed (Zhu and Lee, 1997).
2.7. Lipid extraction and gas chromatography/mass spectrometry (GC/MS) analysis
Each microalgal strain was grown for 10 days and the dilutions of all samples (OD680 = 1.0) were freeze-dried. Thirty milligrams of pulverized, freeze-dried algae were subjected to lipid extraction using a mixture of chloroform and methanol (1:1) as previously described (Hong et al., 2016). Chloroform was evaporated from the extracted mixture using a rotary evaporator. The crude lipid was treated with methanol and a potassium hydroxide solution to facilitate transesterification. To isolate fatty acid methyl esters (FAMEs), hexane was added to the mixture and the reaction was heated at 30 °C for 10 h. Subsequently, the hexane layer was isolated and analyzed using GLC-90 (SUPELCO, Bellefonte, PA, USA) and used as an external standard to determine FAME contents. The fatty acid components present in FAMEs derived from microalgae were analyzed using a 6890N gas chromatograph (Agilent Technology Inc., Santa Clara, CA) equipped with a 5973N mass selective detector (Agilent Technology) and a HP-5MS capillary column [30 m × 0.25 mm (internal diameter) × 0.25 μm (film thickness); Agilent Technology Inc.]. The gas chromatography (GC) oven temperature was maintained at 120 °C for 2 min and subsequently increased to 300 °C at a rate of 5 °C/min and maintained at 300 °C for another 22 min. The injection volume was set to 1 μL, with a split ratio of 20:1. Helium was used as the carrier gas at a constant flow rate of 1 mL/min. The injector and MS source temperatures were set to 250 and 230 °C, respectively. In the electron impact mode, an acceleration voltage of 70 eV was used for sample ionization and the scanning range was increased from 50 to 550 m/z. The Wiley/NBS libraries were used as reference databases (Furuhashi and Weckwerth, 2013).
2.8. Statistical analysis
We compared individual data points using Student's t-test, using a P-value of 0.05 as the statistical significance level. All experiments were performed at least in triplicates and all the results were expressed as mean ± SD.
3. Results and discussion
3.1. Molecular identification and phylogenetic analysis of isolated C. sorokiniana and C. vulgaris strains
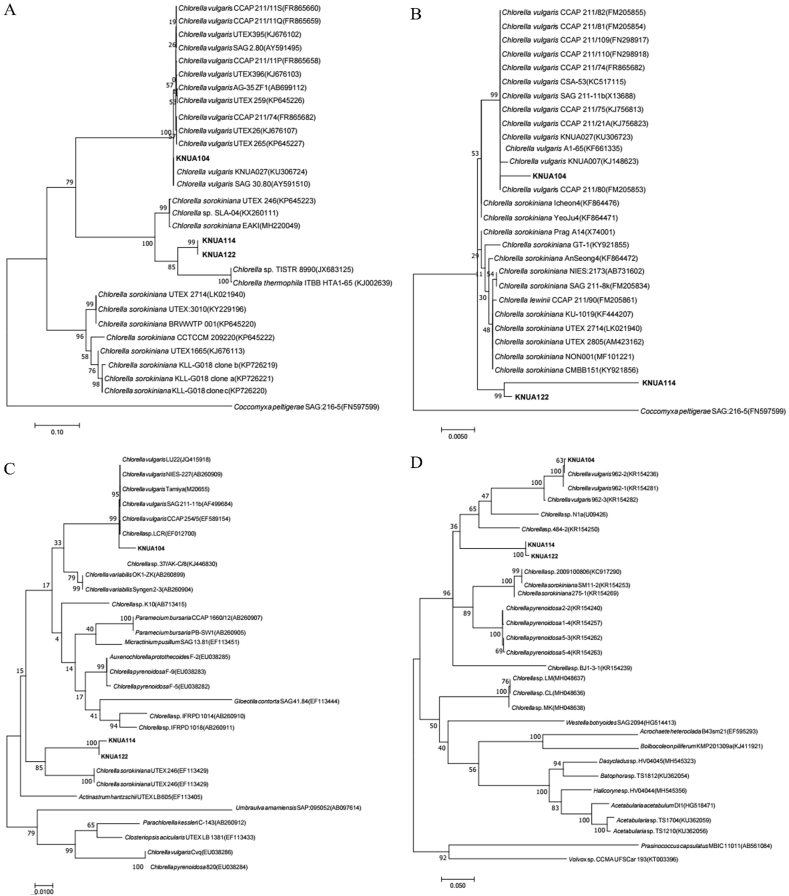
The aseptic isolation of microalgae from a mixed culture with bacteria was performed by spreading or streaking on a BG-11 culture medium (Chang et al., 2013). The C. sorokiniana strains KNUA114 and KNUA122, as well as the C. vulgaris strain KNUA104, all lacked a flagellum, a common feature of species belonging to the genus Chlorellaceae (Figure 1C-E). In previous reports, Chlorellaceae species were described as round (1–4 μm diameter), with green-colored, cup-shaped chloroplasts (Darienko et al., 2010; Safi et al., 2014; Krienitz et al., 2004). Our morphological observations of the strains used in this study are similar to those of other Chlorellaceae members (Figures 1 and 2). The ClustalW analysis by using MEGA 7.0 revealed that the gene sequences of our strains aligned with those of Chlorellaceae and other algal species described in previous studies (Hoshina et al., 2010; Luo et al., 2010). The sequences of four marker genes (ITS, SSU, rbcL, and tufA) were compared between the isolated species and those available in the NCBI database, the results of which are summarized in Table S2. According to the BLAST results, KNUA114 and KNUA122 are homologous and are closely related to the C. sorokiniana strains, while the KNUA104 strain is closely related to other C. vulgaris strains (Figure 2; Table S2).
Figure 2.
Molecular phylogenetic analysis according to the Maximum Likelihood (ML) tree. Numbers at the nodes indicate the bootstrap probabilities (>50%) of the ML analyses (1000 replicates). (A) Internal transcribed spacer (ITS) region, (B) small subunit rRNA gene (SSU), (C) large unit ribulose bisphosphate carboxylase (rbcL), and (D) elongation factor tu gene (tufA). The green, blue, and red boxes indicate C. vulgaris KNUA104, C. sorokiniana KNUA114, and C. sorokiniana KNUA122, respectively.
3.2. Analysis of the growth patterns under high light intensity and temperature conditions
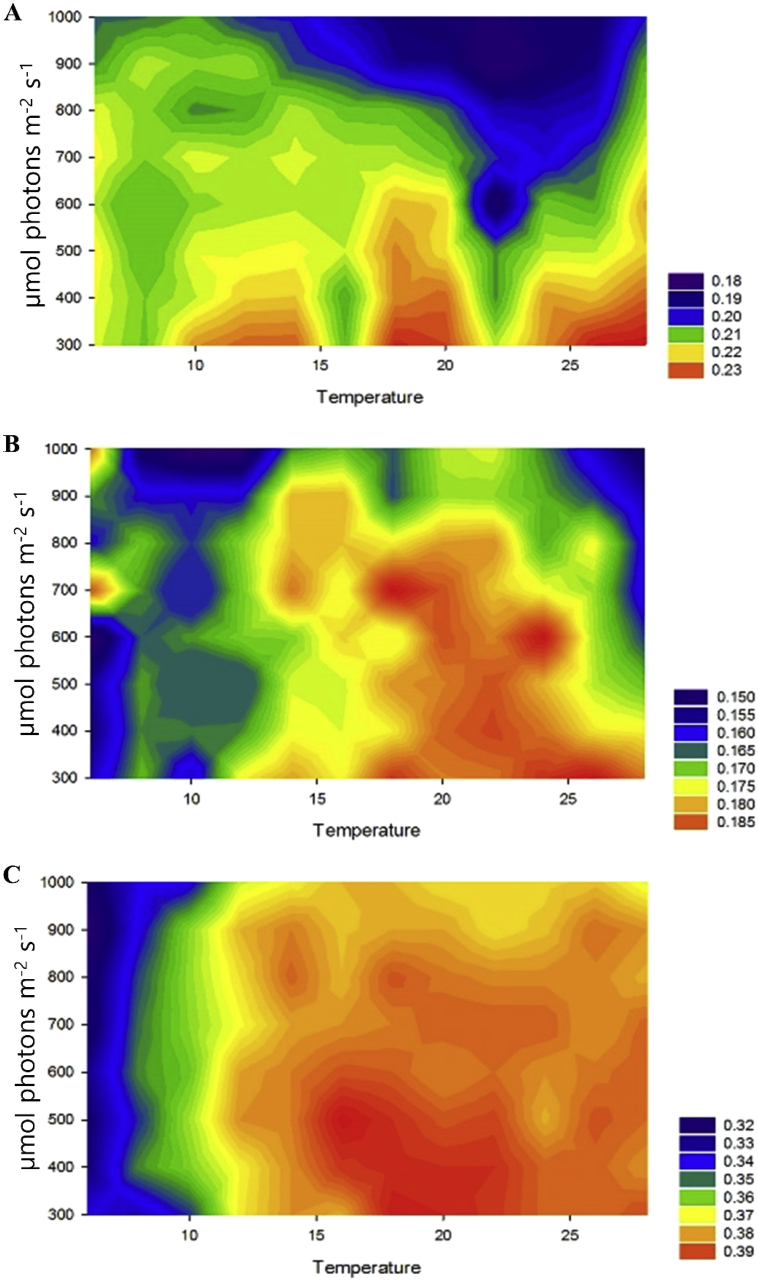
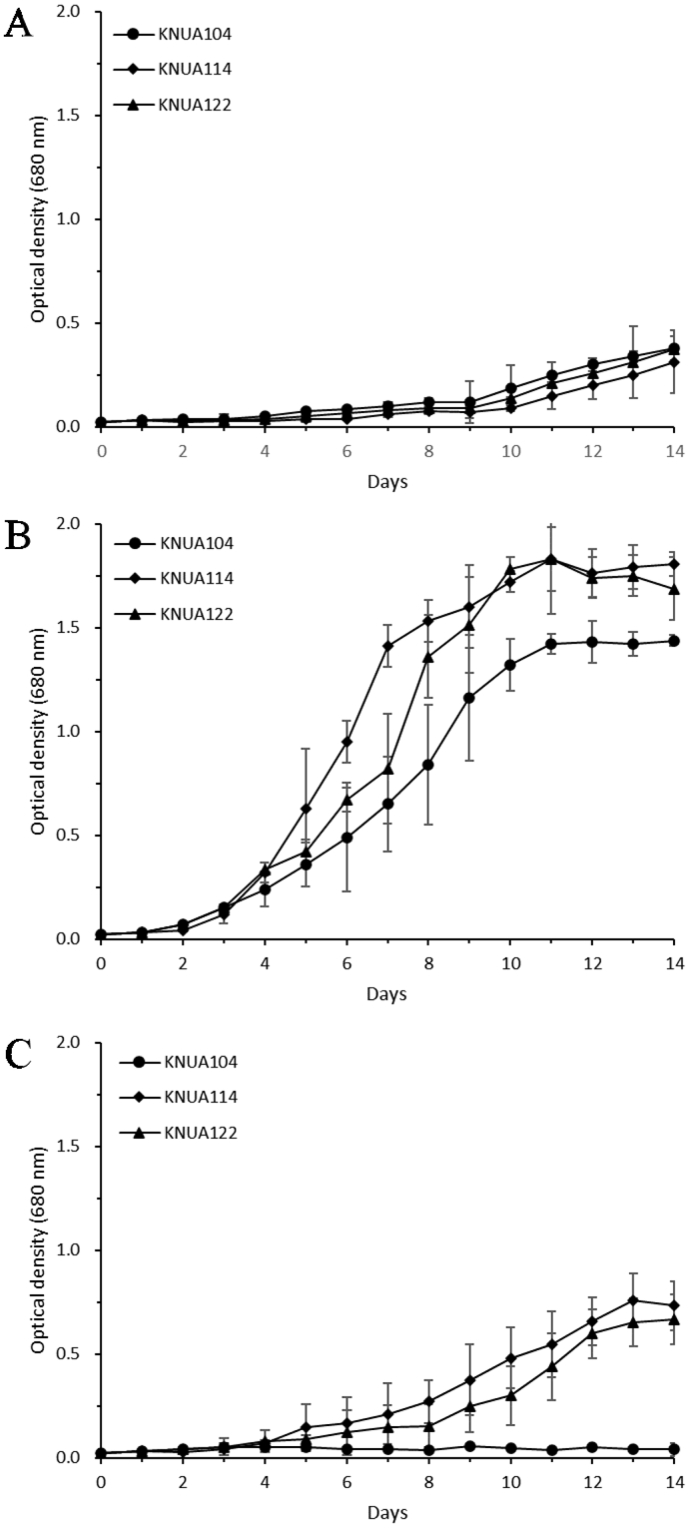
In previous research, Chlorellaceae have been shown to grow optimally under high light intensity rather than low-light (Mandal and Mallick, 2012; An et al., 2016). Our PhotoBiobox analysis revealed that C. sorokiniana KNUA114 and KNUA122 strains were red-colored under high light intensity, unlike C. vulgaris KNUA104. This suggests that the former strains had a higher photosynthetic efficiency than C. vulgaris KNUA104 (Figure 3). Moreover, C. sorokiniana KNUA114 and KNUA122 showed better growth patterns than C. vulgaris KNUA104 under various temperature conditions, particularly at high temperatures (Figure 4), reaching the stationary phase within 10 days of inoculation (Figure 4B). Furthermore, C. sorokiniana KNUA114 and KNUA122 strains grew better at high temperatures than at low temperatures, whereas C. vulgaris KNUA104 did not grow well at high temperatures (Figure 4C). Our results revealed that the C. sorokiniana KNUA114 and KNUA122 strains had higher photosynthetic efficiencies and growth rates than C. vulgaris KNUA104 strain under high light intensity and high-temperature conditions.
Figure 3.
Growth pattern analysis using a PhotoBiobox and measurement of optical density. Growth rate matrix for (A) C. vulgaris KNUA104, (B) C. sorokiniana KNUA114, and (C) C. sorokiniana KNUA122 at temperatures ranging from 6°C–30 °C and light intensities from 300–1,000 μmol photons m−2 s−1.
Figure 4.
Growth curve of C. sorokiniana KNUA114, C. sorokiniana KNUA122, and C. vulgaris KNUA104 at (A) 10 °C, (B) 25 °C, and (C) 35 °C under a light intensity of 300 μmol photons m−2 s−1.
Light intensity and temperature are known to influence microalgal growth and, indirectly, biomass productivity (Renaud et al., 2002; Ras et al., 2013; Xu et al., 2019). Furthermore, each microalgal species has a unique optimum for light intensity and temperature, hence selecting a microalgae species suitable for the biomass production environment is one of the strategies to increase biomass productivity (Yoo et al., 2010; Álvarez-Díaz et al., 2017; Aketo et al., 2020). Growth results showed the characteristics of C. sorokiniana (KNUA114 and KNUA122 strains) and C. vulgaris (KNUA104) for light intensity and temperature. Under light intensity conditions, the C. sorokiniana KNUA114 and KNUA122 strains showed a wide range of tolerance throughout the tested light intensity. Moreover, the growth of C. sorokiniana KNUA114 and KNUA122 strains was not inhibited under 600 μmol photons m−2 s−1, while that of C. vulgaris KNUA104 was significantly inhibited. High light intensity inhibits microalgal growth, but the wide range of growth investigated (300–1000 μmol photons m−2 s−1) suggests high applicability for biomass production of C. sorokiniana KNUA114 and KNUA122 strains. Furthermore, the C. sorokiniana KNUA114 and KNUA122 strains showed higher growth rates and productivity than C. vulgaris KNUA104 at 25 and 35 °C. Therefore, we conclude that the C. sorokiniana KNUA114 and KNUA122 strains, which showed tolerance to a wide range of light intensity, high growth rate, and high lipid productivity among the tested microalgae strains, are more suitable biological sources than C. vulgaris KNUA104.
3.3. Fatty acid components of C. sorokiniana KNUA114 and KNUA122, and C. vulgaris KNUA104
Strains were harvested during the stationary phase and analyzed for fatty acid productivity and chain composition using an SPV reaction and GC/MS, and we found that C. sorokiniana KNUA114 and KNUA122 strains had higher fatty acid contents under high-temperature conditions. Furthermore, the fatty acid components of C. sorokiniana KNUA114 and KNUA122 strains were incomparable with those of the C. vulgaris KNUA104 strain, as the growth of the latter was inhibited at high temperatures (Figure 4C, Table 1). Previous research has described saturated fatty acids (SFAs) as biological resources for biofuel production (Chang et al., 2013; Yong et al., 2019). These fatty acid components are commonly found in microalgae (Hong et al., 2016), which may be used as a potential source for these valuable compounds (Ötleş and Pire, 2001; Alberts, 2003; Herrero et al., 2006; Spolaore et al., 2006; Knothe, 2008; Mohammady, 2011). The fatty acid composition of microalgae grown in this study varied with the temperature conditions used; under higher temperatures, SFA concentration was higher. In particular, 40%–50% of fatty acids of C. sorokiniana KNUA114 and KNUA122 were composed of SFAs when grown at 35 °C (46.78 and 44.40%, respectively). Based on these results, the positive influence of temperature on fatty acid composition in microalgal biomass was confirmed, as well as an increase in lipid productivity and SFAs concentration. Similar trends have also been reported in previous research (Pasquet et al., 2014; Wei et al., 2015), highlighting the potential use of C. sorokiniana KNUA114 and KNUA122 as a biological resource which can adapt to extreme temperature stress.
Table 1.
Fatty acid productivity and composition of C. sorokiniana KNUA122 and KNUA114, and C. vulgaris KNUA104, under different cultivation temperatures.
| Strain | KNUA104 | KNUA114 | KNUA122 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 10 | 25 | 10 | 25 | 35 | 10 | 25 | 35 | |
| Total lipid contents (%) | 15.92 | 19.31 | 21.31 | 23.21 | 23.28 | 20.87 | 22.46 | 23.12 | |
| Fatty acid composition (%) | C16:0 | 20.66 | 24.63 | 20.02 | 26.54 | 43.25 | 20.04 | 25.5 | 40.98 |
| C16:1 | 0.90 | 0.10 | 1.16 | 2.30 | 3.48 | 1.09 | 1.53 | 3.75 | |
| C16:2 | 6.34 | 6.16 | 12.20 | 9.97 | 6.47 | 13.43 | 9.57 | 7.27 | |
| C16:3 | 9.34 | 8.90 | 14.69 | 13.88 | 6.85 | 13.92 | 12.58 | 7.17 | |
| C18:0 | 0.10 | 1.56 | 0.21 | 0.65 | 2.64 | 0.41 | 0.79 | 2.55 | |
| C18:1 ω9 | 8.12 | 9.97 | 1.28 | 2.30 | 5.13 | 2.18 | 3.23 | 5.09 | |
| C18:2 ω6 | 22.42 | 21.48 | 22.46 | 21.45 | 17.87 | 22.76 | 21.15 | 18.02 | |
| C18:3 ω3 | 31.64 | 27.10 | 27.51 | 22.17 | 13.42 | 25.17 | 24.66 | 14.3 | |
| SFAa | 21.24 | 26.29 | 20.70 | 27.93 | 46.78 | 21.45 | 27.28 | 44.40 | |
| UFAb | 78.76 | 73.71 | 79.30 | 72.07 | 53.22 | 78.55 | 72.72 | 55.60 | |
| MUFAc | 9.02 | 10.07 | 2.44 | 4.60 | 8.61 | 3.27 | 4.76 | 8.84 | |
| PUFAd | 69.74 | 63.64 | 76.86 | 67.47 | 44.61 | 75.28 | 67.96 | 46.76 | |
| Productivity (g/L) | Biomass | 0.48 | 0.71 | 0.52 | 0.78 | 0.81 | 0.51 | 0.75 | 0.78 |
| Lipid | 0.08 | 0.14 | 0.11 | 0.18 | 0.19 | 0.11 | 0.17 | 0.18 | |
Percentage of saturated fatty acids.
Percentage of unsaturated fatty acids.
Percentage of monounsaturated fatty acids.
Percentage of polyunsaturated fatty acids.
Our findings suggests that C. sorokiniana KNUA114 and KNUA122 produce fatty acid components, such as palmitic (C16:0), hexadecenoic (C16:1), hexadecadienoic (C16:2), hexadecatrienoic (C16:3), stearic (C18:0), oleic (C18:1 ω9), linoleic (C18:2 ω6), and α-linolenic acid (C18:3 ω3) more efficiently than C. vulgaris KNUA104, particularly at under high light intensity and temperature conditions. Taken together, our findings suggest that C. sorokiniana KNUA114 and KNUA122 strains can be used as a potential source of fatty acids, due to their autotrophic ability and versatility in growing under various environmental stress conditions, as opposed to the C. vulgaris KNUA104 strain.
4. Conclusion
In this study, we investigated three strains of two species belonging to the Chlorella genus, collected on Ulleung Island, South Korea. We demonstrated that the C. sorokiniana KNUA114 and KNUA122 strains could be cultured more efficiently than the C. vulgaris KNUA104 strain with a wide range of light intensity and temperature conditions. Our findings demonstrate that C. sorokiniana KNUA114 and KNUA122 strains generated higher yields of fatty acid components compared to C. vulgaris KNUA104 under high light intensity and temperature conditions, suggesting their potential use as biological raw materials. Future research should focus on screening for the genes encoding the various fatty acids in C. sorokiniana KNUA114 and KNUA122 strains to further evaluate the usefulness of these strains as biological sources of fatty acids. We assume that C. sorokiniana KNUA114 and KNUA122 strains were investigated as a potential feedstock for fatty acid components compared with C. vulgaris KNUA104 strain and the ability of these strains to autotrophically produce fatty acids that can be used as biological resources was demonstrated in this study.
Declarations
Author contribution statement
Hyun-Sik Yun: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Young-Saeng Kim: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ho-Sung Yoon: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1A6A1A05011910 and 2018R1D1A3B07049385), South Korea. We are also grateful for the financial support of the Next-Generation BioGreen 21 Program (grant no. PJ01366701 and grant no. PJ013240), South Korea.
Competing interest statement
The authors declare no conflicts of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Ji-Won Hong (National Marine Biodiversity Institute of Korea) for helpful discussions and assisting with the Materials and Methods.
Contributor Information
Young-Saeng Kim, Email: kyslhh1228@hanmail.net.
Ho-Sung Yoon, Email: hsy@knu.ac.kr.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abou-Shanab R.A., Hwang J.H., Cho Y., Min B., Jeon B.H. Characterization of microalgal species isolated from fresh water bodies as a potential source for biodiesel production. Appl. Energy. 2011;88:3300–3306. [Google Scholar]
- Aketo T., Hoshikawa Y., Nojima D., Yabu Y., Maeda Y., Yoshino T., Takano H., Tanaka T. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J. Biosci. Bioeng. 2020;129:565–572. doi: 10.1016/j.jbiosc.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Akizuki S., Kishi M., Cuevas-Rodríguez G., Toda T. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2020;171:115445. doi: 10.1016/j.watres.2019.115445. [DOI] [PubMed] [Google Scholar]
- Alberts B. Molecular Biology of the Cell. Ann. Bot. 2003;91:401. fourth ed. [Google Scholar]
- Álvarez-Díaz P., Ruiz J., Arbib Z., Barragán J., Garrido-Pérez M., Perales J. Freshwater microalgae selection for simultaneous wastewater nutrient removal and lipid production. Algal Res. 2017;24:477–485. [Google Scholar]
- An B.K., Kim K.E., Jeon J.Y., Lee K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. SpringerPlus. 2016;5:718. doi: 10.1186/s40064-016-2373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen T., Hessen D.O. Carbon, nitrogen, and phosphorus content of freshwater zooplankton. Limnol. Oceanogr. 1991;36:807–814. [Google Scholar]
- Anto S., Pugazhendhi A., Mathimani T. Lipid enhancement through nutrient starvation in Chlorella sp. and its fatty acid profiling for appropriate bioenergy feedstock. Biocatal. Agric. Biotechnol. 2019;20:101179. [Google Scholar]
- Banu J.R., Kannah R.Y., Kavitha S., Ashikvivek A., Bhosale R.R., Kumar G. Cost effective biomethanation via surfactant coupled ultrasonic liquefaction of mixed microalgal biomass harvested from open raceway pond. Bioresour. Technol. 2020;304:123021. doi: 10.1016/j.biortech.2020.123021. [DOI] [PubMed] [Google Scholar]
- Becker E. Micro-algae as a source of protein. Biotechnol. Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Bolch C.J., Blackburn S.I. Isolation and purification of Australian isolates of the toxic cyanobacteriumMicrocystis aeruginosa Kütz. J. Appl. Phycol. 1996;8:5–13. [Google Scholar]
- Buchheim M.A., Keller A., Koetschan C., Förster F., Merget B., Wolf M. Internal transcribed spacer 2 (nu ITS2 rRNA) sequence-structure phylogenetics: towards an automated reconstruction of the green algal tree of life. PloS One. 2011;6 doi: 10.1371/journal.pone.0016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporgno M.P., Mathys A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018;5:58. doi: 10.3389/fnut.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Hong J.W., Chae H., Kim H.S., Park K.M., Lee K.I., Yoon H.S. Natural production of alkane by an easily harvested freshwater cyanobacterium, Phormidium autumnale KNUA026. ALGAE. 2013;28:93–99. [Google Scholar]
- Chisti Y. Biodiesel from microalgae. Biotechnol. Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Chisti Y. Fuels from microalgae. Biofuels. 2010;1:233–235. [Google Scholar]
- Darienko T., Gustavs L., Mudimu O., Menendez C.R., Schumann R., Karsten U., Friedl T., Proeschold T. Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta) Eur. J. Phycol. 2010;45:79–95. [Google Scholar]
- De-Bashan L.E., Antoun H., Bashan Y. Cultivation factors and population size control the uptake of nitrogen by the microalgae Chlorella vulgaris when interacting with the microalgae growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2005;54:197–203. doi: 10.1016/j.femsec.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Dufossé L., Galaup P., Yaron A., Arad S.M., Blanc P., Murthy K.N.C., Ravishankar G.A. Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005;16:389–406. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Friedl T. Inferring taxonomic positions and testing genus level assignments in coccoid green lichen algae: a phylogenetic analysis of 18S ribosomal RNA sequences from Dictyochloropsis reticulata and from members of the genus Myrmecia (Chlorophyta, Trebouxiophyceae cl. nov.) J. Phycol. 1995;31:632–639. [Google Scholar]
- Furuhashi T., Weckwerth W. The handbook of plant metabolomics. 2013. Introduction to Lipid (FAME) Analysis in algae using gas chromatography–mass spectrometry; pp. 215–225. [Google Scholar]
- Griffiths M.J., Garcin C., Hille R.P., Harrison S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods. 2011;85:119–123. doi: 10.1016/j.mimet.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Gupta N., Khare P., Singh D. Nitrogen-dependent metabolic regulation of lipid production in microalga Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019;174:706–713. doi: 10.1016/j.ecoenv.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Han F., Pei H., Hu W., Han L., Zhang S., Ma G. Effect of high-temperature stress on microalgae at the end of the logarithmic phase for the efficient production of lipid. Environ. Technol. 2016;37:2649–2657. doi: 10.1080/09593330.2016.1158867. [DOI] [PubMed] [Google Scholar]
- Hanyuda T., Arai S., Ueda K. Variability in the rbcL introns of caulerpalean algae (Chlorophyta, Ulvophyceae) J. Plant Res. 2000;113:403–413. [Google Scholar]
- Heo J., Cho D.H., Ramanan R., Oh H.M., Kim H.S. PhotoBiobox: a tablet sized, low-cost, high throughput photobioreactor for microalgal screening and culture optimization for growth, lipid content and CO2 sequestration. Biochem. Eng. J. 2015;103:193–197. [Google Scholar]
- Herrero M., Cifuentes A., Ibañez E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae: a review. Food Chem. 2006;98:136–148. [Google Scholar]
- Hong J.W., Jo S., Kim O.H., Jeong M.R., Kim H., Park K.M., Yoon H.S. Characterization of a Korean domestic cyanobacterium Limnothrix sp. KNUA012 for biofuel feedstock. J. Life Sci. 2016;26:460–467. [Google Scholar]
- Hong M.E., Yu B.S., Patel A.K., Choi H.I., Song S., Sung Y.J., Chang W.S., Sim S.J. Enhanced biomass and lipid production of Neochloris oleoabundans under high light conditions by anisotropic nature of light-splitting CaCO3 crystal. Bioresour. Technol. 2019;287:121483. doi: 10.1016/j.biortech.2019.121483. [DOI] [PubMed] [Google Scholar]
- Hoshina R., Iwataki M., Imamura N. Chlorella variabilis and Micractinium reisseri sp. nov.(Chlorellaceae, Trebouxiophyceae): redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010;58:188–201. [Google Scholar]
- Hoydonckx H.E., De Vos D.E., Chavan S.A., Jacobs P.A. Esterification and transesterification of renewable chemicals. Top. Catal. 2004;27:83–96. [Google Scholar]
- Hu Q., Sommerfeld M., Jarvis E., Ghirardi M., Posewitz M., Seibert M., Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- Huang B., Mimouni V., Lukomska E., Morant-Manceau A., Bougaran G. Carbon partitioning and lipid remodeling during phosphorus and nitrogen starvation in the marine microalga Diacronema lutheri (Haptophyta) J. Phycol. 2020;57:558. doi: 10.1111/jpy.12995. [DOI] [PubMed] [Google Scholar]
- Isdepsky A., Borowitzka M.A. In-pond strain selection of euryhaline Tetraselmis sp. strains for reliable long-term outdoor culture as potential sources of biofuel and other products. J. Appl. Phycol. 2019;31:3359–3370. [Google Scholar]
- Kay R.A., Barton L.L. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991;30:555–573. doi: 10.1080/10408399109527556. [DOI] [PubMed] [Google Scholar]
- Knothe G. “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuel. 2008;22:1358–1364. [Google Scholar]
- Koetschan C., Förster F., Keller A., Schleicher T., Ruderisch B., Schwarz R., Müller T., Wolf M., Schultz J. The ITS2 Database III—sequences and structures for phylogeny. Nucleic Acids Res. 2010;38:D275–D279. doi: 10.1093/nar/gkp966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienitz L., Hegewald E.H., Hepperle D., Huss V.A.R., Rohr T., Wolf M. Phylogenetic relationship of Chlorella and Parachlorella gen. Nov. (Chlorophyta, Trebouxiophyceae) Phycologia. 2004;43:529–542. [Google Scholar]
- Kumar S., Nei M., Dudley J., Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings Bioinf. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B.R., Deviram G., Mathimani T., Duc P.A., Pugazhendhi A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019;17:583–588. [Google Scholar]
- Lee K.K., Lim P.E., Poong S.W., Wong C.Y., Phang S.M., Beardall J. Growth and photosynthesis of Chlorella strains from polar, temperate and tropical freshwater environments under temperature stress. J. Oceanol. Limnol. 2018;36:1266–1279. [Google Scholar]
- Lin Q., Lin J. Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour. Technol. 2011;102:1615–1621. doi: 10.1016/j.biortech.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Liperoti R., Landi F., Fusco O., Bernabei R., Onder G. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence. Curr. Pharmaceut. Des. 2009;15:4165–4172. doi: 10.2174/138161209789909683. [DOI] [PubMed] [Google Scholar]
- Luo W., Pröschold T., Bock C., Krienitz L. Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae) Plant Biol. 2010;12:545–553. doi: 10.1111/j.1438-8677.2009.00221.x. [DOI] [PubMed] [Google Scholar]
- Ma F., Hanna M.A. Biodiesel production: a review. Bioresour. Technol. 1999;70:1–15. [Google Scholar]
- Ma D., Li Y., Fu H. Effect of high temperature on the balance between photosynthetic light absorption and energy utilization in Chlorella pyrenoidosa (Chlorophyceae) J. Oceanol. Limnol. 2020;38:186–194. [Google Scholar]
- Mandal S., Mallick N. Biodiesel production by the green microalga Scenedesmus obliquus in a recirculatory aquaculture system. Appl. Environ. Microbiol. 2012;78:5929–5934. doi: 10.1128/AEM.00610-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammady N.G.E. Characterization of the fatty acid composition of Nannochloropsis salina as a determinant of biodiesel properties. Z. Naturforsch. 2011;66:328–332. doi: 10.1515/znc-2011-7-802. [DOI] [PubMed] [Google Scholar]
- Morimura Y., Tamiya N. Preliminary experiments in the use of Chlorella as human food. Food Technol. 1954;8:179–182. [Google Scholar]
- Nigam S., Rai M.P., Sharma R. Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am. J. Biochem. Biotechnol. 2011;7:124–129. [Google Scholar]
- Ötleş S., Pire R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001;84:1708–1714. [PubMed] [Google Scholar]
- Park J., Kumar A.N., Cayetano R.D.A., Kim S.H. Assessment of Chlorella sp. as a potential feedstock for biological methane production. Bioresour. Technol. 2020;305:123075. doi: 10.1016/j.biortech.2020.123075. [DOI] [PubMed] [Google Scholar]
- Pasquet V., Ulmann L., Mimouni V., Guihéneuf F., Jacquette B., Morant-Manceau A., Tremblin G. Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J. Appl. Phycol. 2014;26:2265–2271. [Google Scholar]
- Polle J.E., Struwe L., Jin E. Identification and characterization of a new strain of the unicellular green alga Dunaliella salina (Teod.) from Korea. J. Microbiol. Biotechnol. 2008;18:821–827. [PubMed] [Google Scholar]
- Randhir A., Laird D.W., Maker G., Trengove R., Moheimani N.R. Microalgae: a potential sustainable commercial source of sterols. Algal Res. 2020;46:101772. [Google Scholar]
- Ras M., Steyer J.P., Bernard O. Temperature effect on microalgae: a crucial factor for outdoor production. Rev. Environ. Sci. Biotechnol. 2013;12:153–164. [Google Scholar]
- Rasal V.M., Yadre S.G., Shukla S.P., Ravi P., Mishra M.K., Munilkumar S., Pal A.K., Lakra W., Dasgupta S. Microalgae distribution and diversity in the Narmada river basin around Chutka, Madhya Pradesh, India. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:1488–1501. [Google Scholar]
- Rayen F., Behnam T., Dominique P. Optimization of a raceway pond system for wastewater treatment: a review. Crit. Rev. Biotechnol. 2019;39:422–435. doi: 10.1080/07388551.2019.1571007. [DOI] [PubMed] [Google Scholar]
- Renaud S.M., Thinh L.V., Lambrinidis G., Parry D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture. 2002;211:195–214. [Google Scholar]
- Rippka R., Deruelles J., Waterbury J.B., Herdman M., Stanier R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiol. 1979;111:1–61. [Google Scholar]
- Ryu K.H., Lee J.Y., Heo S., Lee J.H. Improved microalgae production by using a heat supplied open raceway pond. Ind. Eng. Chem. Res. 2019;58:9099–9108. [Google Scholar]
- Safi C., Zebib B., Merah O., Pontalier P.Y., Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew. Sustain. Energy Rev. 2014;35:265–278. [Google Scholar]
- Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- Stanier R.Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol. Rev. 1971;35:171. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub F.B., Dollar A.M. The nutritional inadequacy of Chlorella and Chlamydomonas as food for Daphnia pulex. Limnol. Oceanogr. 1968;13:607–617. [Google Scholar]
- Vanthoor-Koopmans M., Wijffels R.H., Barbosa M.J., Eppink M.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013;135:142–149. doi: 10.1016/j.biortech.2012.10.135. [DOI] [PubMed] [Google Scholar]
- Vieira H.H., Bagatini I.L., Guinart C.M., Vieira A.A.H. tufA gene as molecular marker for freshwater Chlorophyceae. ALGAE. 2016;31:155–165. [Google Scholar]
- Wan C., Bai F.W., Zhao X.Q. Effects of nitrogen concentration and media replacement on cell growth and lipid production of oleaginous marine microalga Nannochloropsis oceanica DUT01. Biochem. Eng. J. 2013;78:32–38. [Google Scholar]
- Wei L., Huang X., Huang Z. Temperature effects on lipid properties of microalgae Tetraselmis subcordiformis and Nannochloropsis oculata as biofuel resources. Chin. J. Oceanol. Limnol. 2015;33:99–106. [Google Scholar]
- Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S., Taylor J. Vol. 18. Academic Press; New York: 1990. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protocols: a Guide to Methods and Applications; pp. 315–322. [Google Scholar]
- Xin L., Hong-Ying H., Ke G., Ying-Xue S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010;101:5494–5500. doi: 10.1016/j.biortech.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Xu K., Zou X., Wen H., Xue Y., Qu Y., Li Y. Effects of multi-temperature regimes on cultivation of microalgae in municipal wastewater to simultaneously remove nutrients and produce biomass. Appl. Microbiol. Biotechnol. 2019;103:8255–8265. doi: 10.1007/s00253-019-10051-6. [DOI] [PubMed] [Google Scholar]
- Yaisamlee C., Sirikhachornkit A. Characterization of Chlamydomonas very high light-tolerant mutants for enhanced lipid production. J. Oleo Sci. 2020;69:359–368. doi: 10.5650/jos.ess19270. [DOI] [PubMed] [Google Scholar]
- Yang L., Chen J., Qin S., Zeng M., Jiang Y., Hu L., Xiao P., Hao W., Hu Z., Lei A. Growth and lipid accumulation by different nutrients in the microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels. 2018;11:40. doi: 10.1186/s13068-018-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W.K., Lim P.E., Vello V., Sim K.S., Majid N.A., Mustafa E.M., Sulaiman N.M.N., Liew K.E., Chen B.J.T., Phang S.M. Metabolic and physiological regulation of Chlorella sp.(Trebouxiophyceae, Chlorophyta) under nitrogen deprivation. J. Oceanol. Limnol. 2019;37:186–198. [Google Scholar]
- Yoo C., Jun S.Y., Lee J.Y., Ahn C.Y., Oh H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010;101:S71–S74. doi: 10.1016/j.biortech.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Zhou X.R., Green A.G., Singh S.P. Caenorhabditis elegans Δ12-desaturase FAT-2 is a bifunctional desaturase able to desaturate a diverse range of fatty acid substrates at the Δ12 and Δ15 positions. J. Biol. Chem. 2011;286:43644–43650. doi: 10.1074/jbc.M111.266114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.J., Lee Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997;9:189–194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.