Summary
Adhesive hydrogels containing catechol moieties have many important applications, but the fabrication of effective long-lasting adhesive hydrogels remains a challenge because of oxidative damage. Inspired by the traditional use of quercetin in ancient China, here, we have developed a novel method, based on quercetin-assisted photoradical chemistry, to fabricate a durable adhesive hydrogel, Q-hydrogel. In the presence of light, quercetin generates quinone/semiquinone radicals, which subsequently interact with ammonium persulfate (APS) to produce a large amount of free radicals and initiate polymerization of the hydrogel. As-prepared Q-hydrogel showed good mechanical and adhesive properties, which were attributed to the inherent structural advantages of quercetin. Because of the resistance of quercetin to oxidation, as-prepared Q-hydrogel also showed good adhesive properties even after treatment with oxidizing agents. Capitalizing on its conductivity and adhesive properties, Q-hydrogel was successfully used to produce wearable sensors capable of detecting human motion.
Subject Areas: Polymer Chemistry, Materials Science, Polymers
Graphical Abstract
Highlights
-
•
Quercetin-assisted photoradical chemistry was developed for adhesive hydrogel
-
•
Quercetin composite hydrogel showed durable adhesion capacity
-
•
Quercetin composite hydrogel was used as electronic skin
Polymer Chemistry; Materials Science; Polymers
Introduction
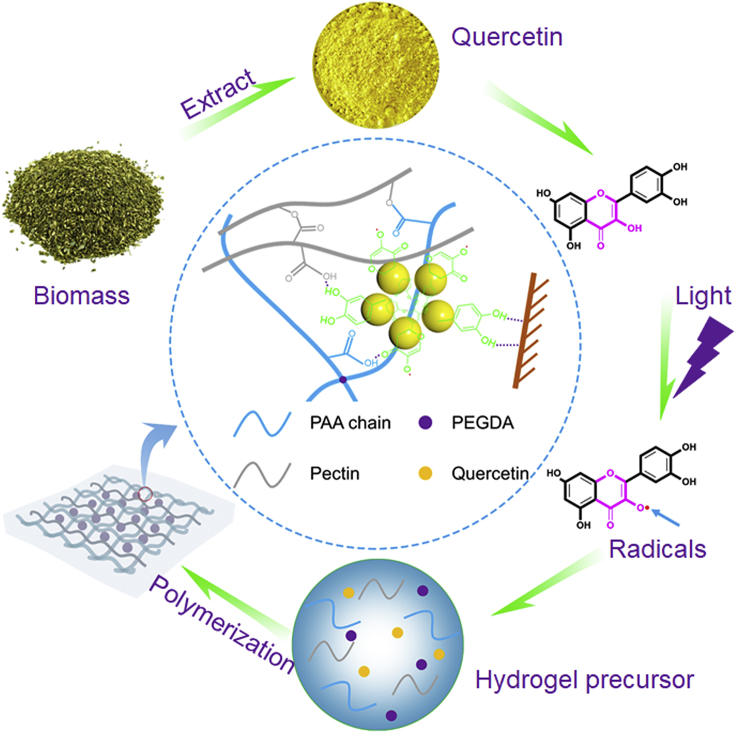
Adhesive hydrogels have huge potential in a variety of areas, including bio-medical care and electronic skins (Gan et al., 2019a, 2019b; Liao et al., 2019; Lei et al., 2017; Ma et al., 2019; Wang et al., 2018; Wirthl et al., 2017; Li et al., 2018; Xu et al., 2019). Different design strategies, including those based on two-layer adhesive surface-dissipative matrices (Chen et al., 2015; Wu et al., 2018; Yang et al., 2016; Zhang et al., 2016), nucleobase tackifying (Gao et al., 2019; Liu et al., 2017, 2019a, 2019b), and well-patterned structures (Jin et al., 2017; Mredha et al., 2018; Zhao et al., 2017) have been used to develop adhesive hydrogels. Recently, mussel-inspired polydopamine-based adhesive hydrogels have been shown to have especially good performance because of their strong cohesive and adsorptive properties (Gao et al., 2013; Han et al., 2017a, 2017b, 2017c; Tang et al., 2019). Building on these studies, many catechol moiety-incorporating compounds have been used to develop adhesive hydrogels (Lee and Konst, 2014; Li et al., 2015; Narkar et al., 2016). Because catechol functional groups can form covalent/non-covalent bonds with different materials (Saiz-Poseu et al., 2019; Wang et al., 2019), these hydrogels can adhere repeatedly well to a variety of surfaces (Gan et al., 2019a, 2019b; Han et al., 2017a, 2017b, 2017c; Lee et al., 2007). Although much progress has been made in this area, the adhesive properties of most hydrogels are destroyed by oxidation (Jing et al., 2018; Maier et al., 2018; Maier and Butler, 2017; Kord Forooshani and Lee, 2017). It is, therefore, important to develop long-lasting adhesive hydrogels that are resistant to oxidation. Quercetin, a flavonoid with catechol functionality that is easily extracted from the flower buds of Sophora japonica L. (He et al., 2018a, 2018b), was widely used to produce royal dyes and herbal medicines in ancient China. Additionally, Schmidt et al. reported that they used catechol moiety-incorporating plant phenols together with zein protein for preparing a sustainable and high-strength adhesive (Schmidt et al., 2018). Inspired by this, here, we have focused on quercetin, which binds strongly to guest materials and has good antioxidative capacity, to develop an oxidation-resistant, high-performance adhesive hydrogel. This is the first report to describe the use of quercetin-assisted radical chemistry to prepare an adhesive hydrogel (Q-hydrogel) with good antioxidative capacity upon light irradiation in the presence (Figure 1). Upon light irradiation, quercetin generates quinone/semiquinone radicals, which subsequently interact with APS to produce a large amount of free radicals and initiate polymerization of the hydrogel. As-prepared Q-hydrogel had good mechanical properties and, because of the high antioxidative capacity of quercetin, also showed long-lasting adhesion, even after treatment with strong oxidizing agents. As demonstrations of potential applications, Q-hydrogel was incorporated into a textile and used to prepare a wireless wearable sensor capable of detecting human motion.
Figure 1.
Schematic Illustration of Quercetin-Assisted Radical Chemistry Triggered for Preparation of Q-hydrogel upon Light Irradiation
Results
Quercetin-Assisted Radical Chemistry for Preparing Q-hydrogel
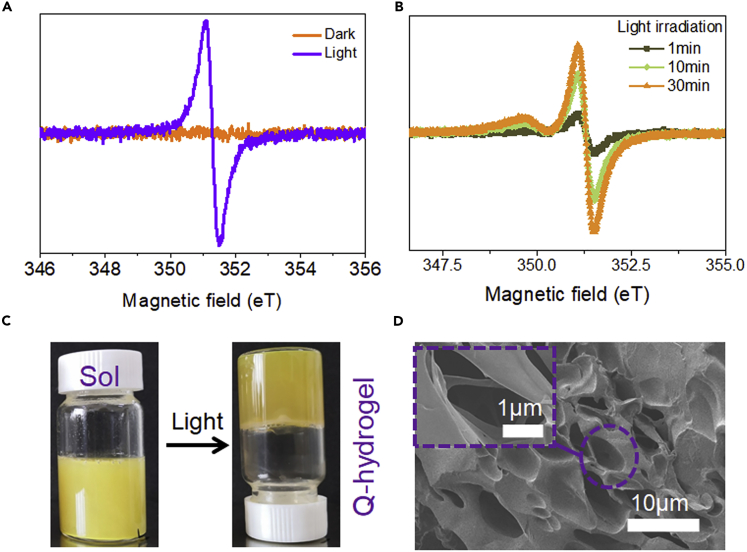
First, we investigated quercetin-assisted radical chemistry. The mechanism of free radical generation was studied using electron spin-resonance spectroscopy (ESR). Quercetin showed maximum absorbance at ∼390 nm (Figure S1) and quercetin-assisted radical chemistry was, therefore, investigated using 375 nm light as the irradiation source. Upon light irradiation, the ESR spectrum of a quercetin/APS showed a signal with a g value of 2.0039, which was attributed to quinone/semiquinone radicals (Figure 2A). The ESR spectrum of the quercetin/APS in the dark showed the same peak, but with much lower intensity, indicating that the quantity of quinone/semiquinone radicals was much smaller (Figure 2A). Pure APS showed a very weak signal in the ESR spectrum upon light irradiation (Figure S2). ESR spectra of the quercetin/APS were also recorded after irradiation for different periods of time. Longer exposure of the quercetin/APS to UV light produced more radicals (Figure 2B). These results demonstrate that APS alone generates few radicals upon light irradiation, whereas a solution of quercetin/APS generates many more radicals under the same conditions, making it possible to polymerize the monomer and cross-linker to form an adhesive hydrogel. Having characterized quercetin-assisted radical chemistry, this was then used to prepare Q-hydrogels. Acrylic acid (AA) monomers, APS, polyethylene glycol diacrylate (PEGDA), and pectin were mixed with suspensions containing different amounts of quercetin (Table S1). Under irradiation with 375 nm light, hydrogels were formed by quercetin-assisted radical chemistry (Figures 2C and 2D). In a control experiment, no hydrogel was formed when the same formulation, but without quercetin, was irradiated under the same conditions (Figure S3). The suspensions containing quercetin also did not form hydrogels without light irradiation (Figure S3). Quercetin-assisted radical chemistry can also initiate the polymerization of other free-radical monomers, such as acrylamide, suggesting that the as-developed quercetin-assisted radical chemistry is generally applicable and can be used to prepare a variety of hydrogels (Figure S4).
Figure 2.
Quercetin-Assisted Photoradical Gelation
(A) ESR spectra of quercetin/APS upon light irradiation and in the dark.
(B) ESR spectra of quercetin/APS after light irradiation for different lengths of time.
(C) Q-hydrogel precursor suspension of quercetin/APS in dark environment (left) and after UV irradiation (right).
(D) SEM images of as-formed Q-hydrogel to the condition mentioned in Figure 2C; scale bar, 10μm. Inset: Magnified SEM images of as-formed Q-hydrogel; scale bar, 1 μm.
Mechanical Properties of Q-hydrogels
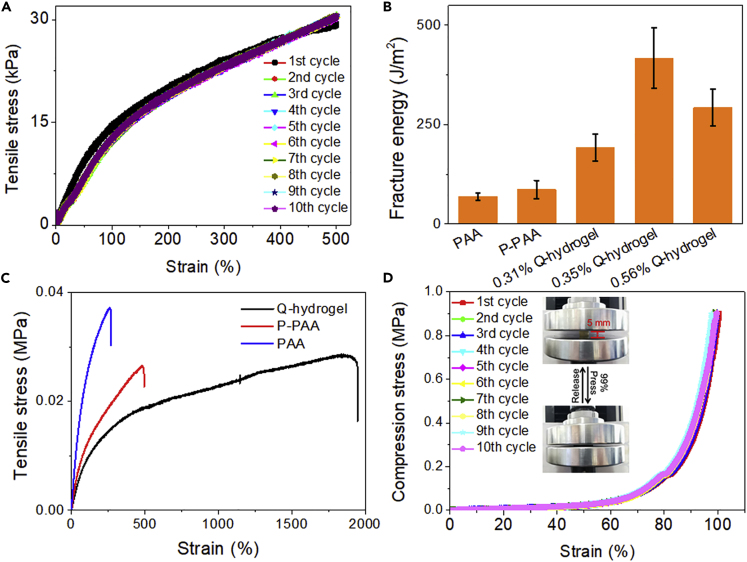
The Q-hydrogels were resilient, stretchable, and tough. Typical tensile stress-strain curves of the hydrogels were investigated and tensile strain was found to reach a maximum value of 1,800% when the amount of quercetin in the hydrogel was 0.35 wt % (Table S1). To understand why 0.35 wt % sample showed the best performance, the polymerization behavior of acrylic acid in the presence of 0.31%, 0.35%, and 0.56% quercetin was studied, since the molecular weight strongly influenced their mechanical properties. Interestingly, GPC trace only showed one main peak for polyacrylic acid formed in the presence of 0.31% and 0.56% quercetin. Mn of polyacrylic acid formed in the presence of 0.31% and 0.56% were ∼160,000 and 420,000, respectively (Figure S5), whereas there were two peaks in GPC trace of polyacrylic acid formed in the presence of 0.35%. Mn of these polyacrylic acid formed in the presence of 0.35% quercetin were ∼180,000 and 17,000, respectively (Figure S5). The difference explained the best performance of the hydrogel triggered by 0.35% quercetin. It is clear that one common strategy to develop a tough hydrogel is to interpenetrate polymer networks with relatively short and long polymer chains. Under deformation, the short-chain networks can be fractured and/or physically de-cross-linked to dissipate mechanical energy, whereas the long-chain networks will maintain the high elasticity of the hydrogels (Zhao, 2014). For further comparison, poly(acrylic acid) hydrogel (PAA hydrogel) and the pectin/poly(acrylic acid) hydrogel (P-PAA hydrogel) were prepared assisted by radical chemistry in the presence of heating (supporting information). Q-hydrogel showed values much higher than the values for PAA hydrogel (∼250%) and P-PAA hydrogel (∼500%) (Figure 3A). The addition of ∼0.35 wt % of quercetin also produced a hydrogel with the highest tensile strain value (Figure 3B). Load-unload tensile stress-strain curves of the Q-hydrogel containing 0.35 wt % were then constructed. The load-unload tensile stress-strain curves showed excellent reproducibility over ten cycles (Figure 3C). The Q-hydrogel was able to withstand high compression to complete deformation without breaking and, after the compressive load was removed, the hydrogel recovered automatically and rapidly to its initial shape, even after ten cycles (Figure 3D). The load-unload compression stress-strain curves indicated that the Q-hydrogel has good recoverability. The good mechanical properties of the hydrogel are attributed to three factors. First, pectin interpenetrates the PAA networks and strengthens the hydrogel. Second, pectin contains a large number of -OH and -COOH groups, which are important active sites of hydrogen bonds in the noncovalent interaction with acrylic acid and quercetin (Servais et al., 2008). Thus, quercetin, PAA, and pectin form noncovalent interactions, which dissipate energy under large deformation and improve the mechanical properties of the hydrogel. Third, quercetin has many hydrophilic hydroxyl groups and can, therefore, form hydrogen bonds within the hydrogel network and thus improve its mechanical properties. Because of its superior mechanical properties, the Q-hydrogel containing 0.35 wt % quercetin was used in all subsequent tests.
Figure 3.
Mechanical Properties of Hydrogel
(A) Tensile loading-unloading curves of Q-hydrogel.
(B) Fracture energy of hydrogels containing different amounts of quercetin.
(C) Typical tensile stress-strain curves of hydrogels.
(D) Recovery of Q-hydrogel after removal of compressive load occurred within 2 min.
Adhesive Properties
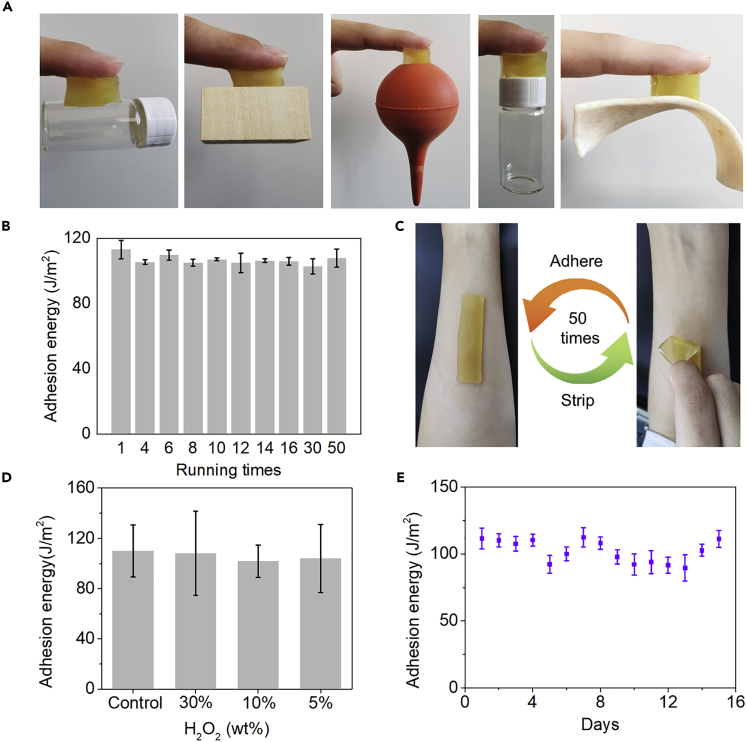
The adhesive properties of Q-hydrogel were systematically investigated. Q-hydrogel can adhere to both hydrophilic and hydrophobic surfaces, such as glass, wood, rubber, polytetrafluoroethylene, and tissue (pork skin) (Figure 4A). The adhesive strength of Q-hydrogel on pork skin was quantified using a tensile adhesion test and found to be ∼110 J/m2 (Figure 4B). Notably, the nice adhesive properties might also be partially contributed by catechol-protein interaction (Schmidt et al., 2018). Q-hydrogel maintained good adhesion to pork skin even after 50 peeling/adhering cycles and showed similar good adhesive properties on the author's skin (Figure 4C). Typically, adhesive hydrogels incorporating catechol moieties lose their adhesive properties in strongly oxidizing environments because of overoxidation of the catechol moieties. The resistance of Q-hydrogel to oxidation was assessed by treating the hydrogel with different concentrations of H2O2 (for 30 min). Surprisingly, Q-hydrogel maintained good adhesion to pork skin after treatment with this strong oxidant (Figure 4D). The continued adhesiveness of Q-hydrogel might be attributable to the way in which quercetin reacts with oxidants (Jing et al., 2018; Maier et al., 2018; Maier and Butler, 2017). Specifically, although part of the catechol structure was oxidized by H2O2, which reduced the adhesive strength, chemical oxidation opened the lactone ring of quercetin to generate a series of carboxylic acid and hydroxyl moieties and enhanced the adhesive properties, instead (Scheme S1) (Nimse and Pal, 2015; Osman et al., 2008). Generally, the enhanced adhesive strength might compromise the reduced one. That is the reason for the minor decrease for adhesive performance of Q-hydrogel after oxidation. Encouraged by this result, we further investigated the long-term adhesive properties of Q-hydrogel and found that its adhesive strength was not reduced after storing for 15 days (Figure 4E).
Figure 4.
Adhesive Properties of Q-hydrogel
(A) Q-hydrogel sticking to different surfaces.
(B) Adhesive strength of Q-hydrogel on pork skin after 50 cycles of adhesion-peeling.
(C) Repeated adhesion-peeling of Q-hydrogel on author's skin. No residue or irritation of the skin was found after 50 cycles of adhesion-peeling.
(D) Adhesion energy of Q-hydrogel/pork skin after treatment of Q-hydrogel with different concentrations of H2O2 (error bar means the standard deviation).
(E) Adhesion energy of Q-hydrogel/porcine skin after different storage times. The hydrogel was stored in a sealed environment at room temperature (21°C).
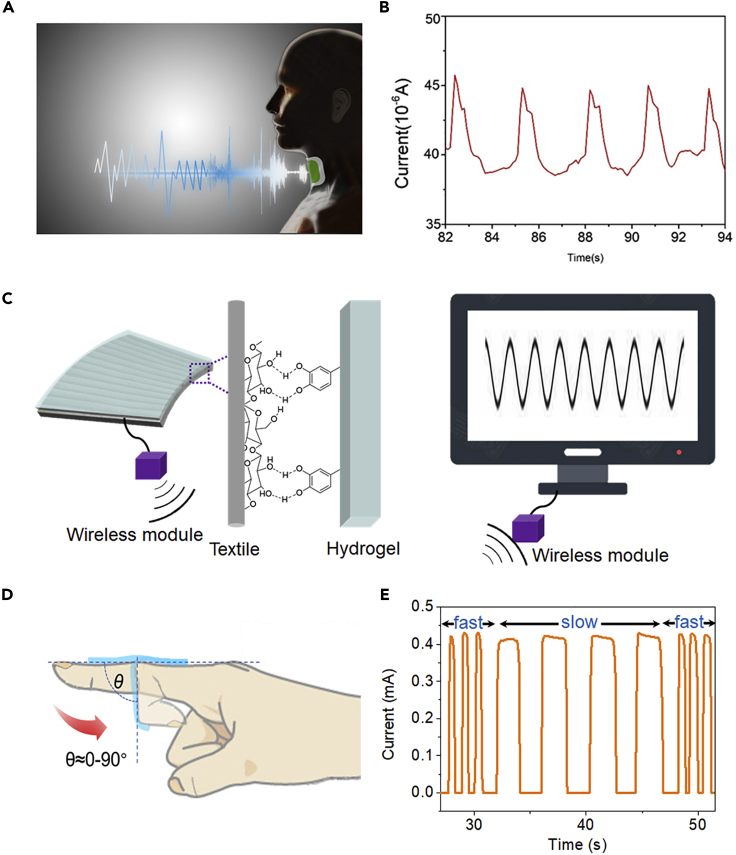
Wearable Sensors for Monitoring Human Motion
Polyacrylic acid network in Q-hydrogel provided proton carriers and enabled it conductive (Moharram et al., 1998; Rhim et al., 2005). Therefore, the possibility of using Q-hydrogel in a wearable electronic device or as an electronic skin was investigated next. The electrical characteristics of Q-hydrogel were measured using a CHI 660e electrochemical workstation as described in Supplemental Note 3. Q-hydrogel shows good conductivity and, in preliminary experiments, its sensitivity to touch and bending were investigated (Figure S6). Q-hydrogel showed a good response to mechanical stimulation at different speeds (Figures S7 and S8). Q-hydrogel was next used to sense swallowing motion by spreading the sensor over the Adam's apple of a male volunteer (Figure 5A). In the initial state, the sensor was taut and the current was small. Swallowing caused an upward movement of the Adam's apple, which released the tension in the sensor, leading to a larger current. Marked variations in the strength of the current were thus caused by swallowing (Figure 5B). Encouraged by these results, which show that Q-hydrogel is very sensitive to distortion, the adhesive hydrogel was next used to prepare a wearable wireless conductive textile (Figure S9). Q-hydrogel, which was prepared in situ in the fabric, adhered very well to the textile because of the presence of quercetin moieties. After intensive twisting, the conductivity of the textile did not noticeably change, showing that the hydrogel was stably incorporated into the textile (Figure S10). As-prepared textile was then coupled to a home-made setup to fabricate a wireless wearable sensor, which was used to sense bending motion (Figures 5C and S11). As-obtained textile was attached to the index finger to detect the bending motion of the second joint (0°–90°) (Figure 5D and Video S1). The strength of the current showed a regular variation with the angle of the finger joint (Figure 5E). The changes in current intensity could, in the future, be used to record swallowing, or other motions, of patients.
Figure 5.
Performance of Wearable Q-hydrogel Sensor
(A) Schematic illustration of sensing man's throat during cyclic swallowing using Q-hydrogel.
(B) Current response curve of a sensor attached to a man's throat during cyclic swallowing.
(C) Schematic illustration of wireless setup developed for sensing motion.
(D) Illustration of cyclic bending and recovery of wireless wearable sensor on finger.
(E) Current response curve of sensor upon periodical bending motion with different speeds.
Discussion
In summary, inspired by the traditional uses of quercetin, we have developed an efficient adhesive Q-hydrogel using quercetin-assisted radical chemistry. As-prepared Q-hydrogel has a number of advantages. First, Q-hydrogel shows long-lasting adhesiveness, which is attributed to the resistance of quercetin to oxidation. Second, the hydrogel adheres well to textiles, has good conductive properties. and is easily prepared under mild conditions. Q-hydrogel could thus be prepared in situ within textiles to produce wearable sensors. Since many naturally occurring phenolic compounds contain catechol moieties, our work may inspire others to use these natural phenolic compounds to construct other durable adhesive hydrogels.
Limitation of the Study
We have developed a durable adhesive hydrogel using quercetin. However, the gelation of hydrogel required about 30-min UV irradiation. This long irradiation time and short wavelength might hinder its future applications in vivo. However, the long irradiation time might be shortened by adding N,N,N′,N'-tetramethylethylenediamine as catalyst. Additionally, sodium periodate should be used for further evaluating the susceptibility of the Q-hydrogel to be oxidized.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Zhijun Chen (Chenzhijun@nefu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31800494 and 31870553) and the Young Elite Scientists Sponsorship Program by CAST (2018QNRC001).
Author Contributions
Z.C. and S.L. participated in the conception and design of the research. D.X. carried out the experiments and characterizations. Z.C., D.X., and M.J. prepared the figures and drafted the manuscript. X.Z., J.L., N.N., and S.L. revised the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101342.
Contributor Information
Zhijun Chen, Email: chenzhijun@nefu.edu.cn.
Shujun Li, Email: lishujun@nefu.edu.cn.
Supplemental Information
References
- Chen Q., Zhu L., Chen H., Yan H., Huang L., Yang J., Zheng J. A novel design strategy for fully physically linked double network hydrogels with tough, fatigue resistant, and self-healing properties. Adv. Funct. Mater. 2015;25:1598–1607. [Google Scholar]
- Gan D., Xu T., Xing W., Ge X., Fang L., Wang K., Ren F., Lu X. Mussel-inspired contact-active antibacterial hydrogel with high cell affinity, toughness, and recoverability. Adv. Funct. Mater. 2019;29:1805964. [Google Scholar]
- Gan D., Xing W., Jiang L., Fang J., Zhao C., Ren F., Fang L., Wang K., Lu X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-09351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Sun Y., Zhou J., Xu R., Duan H. Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl. Mater. Interfaces. 2013;5:425–432. doi: 10.1021/am302500v. [DOI] [PubMed] [Google Scholar]
- Gao Y., Jia F., Gao G. Transparent and conductive amino acid-tackified hydrogels as wearable strain sensors. Chem. Eng. J. 2019;375:121915. [Google Scholar]
- Han L., Lu X., Liu K., Wang K., Fang L., Weng L., Zhang H., Tang Y., Ren F., Zhao C. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano. 2017;11:2561–2574. doi: 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- Han L., Lu X., Wang M., Gan D., Deng W., Wang K., Fang L., Liu K., Chan C., Tang Y. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small. 2017;13:1601916. doi: 10.1002/smll.201601916. [DOI] [PubMed] [Google Scholar]
- Han L., Yan L., Wang K., Fang L., Zhang H., Tang Y., Ding Y., Weng L., Xu J., Weng J. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017;9:e372. [Google Scholar]
- He T., Niu N., Chen Z., Li S., Liu S., Li J. Novel quercetin aggregation-induced emission luminogen (AIEgen) with excited-state intramolecular proton transfer for in vivo bioimaging. Adv. Funct. Mater. 2018;28:1706196. [Google Scholar]
- He T., Wang H., Chen Z., Liu S., Li J., Li S. Natural quercetin AIEgen composite film with antibacterial and antioxidant properties for in situ sensing of Al3+ residues in food, detecting food spoilage, and extending food storage times. ACS Appl. Bio Mater. 2018;1:636–642. doi: 10.1021/acsabm.8b00128. [DOI] [PubMed] [Google Scholar]
- Jin Y., Liu C., Chai W., Compaan A., Huang Y. Self-supporting nanoclay as internal scaffold material for direct printing of soft hydrogel composite structures in air. ACS Appl. Mater. Interfaces. 2017;9:17456–17465. doi: 10.1021/acsami.7b03613. [DOI] [PubMed] [Google Scholar]
- Jing X., Mi H.Y., Lin Y.J., Enriquez E., Peng X.F., Turng L.S. Highly stretchable and biocompatible strain sensors based on mussel-inspired super-adhesive self-healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces. 2018;10:20897–20909. doi: 10.1021/acsami.8b06475. [DOI] [PubMed] [Google Scholar]
- Kord Forooshani P., Lee B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. A Polym. Chem. 2017;55:9–33. doi: 10.1002/pola.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.P., Konst S. Novel hydrogel actuator inspired by reversible mussel adhesive protein chemistry. Adv. Mater. 2014;26:3415–3419. doi: 10.1002/adma.201306137. [DOI] [PubMed] [Google Scholar]
- Lee H., Lee B.P., Messersmith P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448:338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- Lei Z., Wang Q., Sun S., Zhu W., Wu P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017;29:1700321. doi: 10.1002/adma.201700321. [DOI] [PubMed] [Google Scholar]
- Li L., Yan B., Yang J., Chen L., Zeng H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015;27:1294–1299. doi: 10.1002/adma.201405166. [DOI] [PubMed] [Google Scholar]
- Li L., Lu F., Wang C., Zhang F., Liang W., Kuga S., Dong Z., Zhao Y., Huang Y., Wu M. Flexible double-cross-linked cellulose-based hydrogel and aerogel membrane for supercapacitor separator. J. Mater. Chem. A. 2018;6:24468–24478. [Google Scholar]
- Liao H., Guo X., Wan P., Yu G. Conductive MXene nanocomposite Organohydrogel for flexible, healable, low-temperature tolerant strain sensors. Adv. Funct. Mater. 2019;29:1904507. [Google Scholar]
- Liu X., Zhang Q., Gao G. Bioinspired adhesive hydrogels tackified by nucleobases. Adv. Funct. Mater. 2017;27:1703132. [Google Scholar]
- Liu X., Zhang Q., Duan L., Gao G. Bioinspired nucleobase-driven nonswellable adhesive and tough gel with excellent underwater adhesion. ACS Appl. Mater. Interfaces. 2019;11:6644–6651. doi: 10.1021/acsami.8b21686. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang Q., Duan L., Gao G. Tough adhesion of nucleobase-tackifed gels in diverse solvents. Adv. Funct. Mater. 2019;29:1900450. [Google Scholar]
- Ma Z., Shi W., Yan K., Pan L., Yu G. Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem. Sci. 2019;10:6232–6244. doi: 10.1039/c9sc02033k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier G.P., Butler A. Siderophores and mussel foot proteins: the role of catechol, cations, and metal coordination in surface adhesion. J. Biol. Inorg. Chem. 2017;22:739–749. doi: 10.1007/s00775-017-1451-6. [DOI] [PubMed] [Google Scholar]
- Maier G.P., Bernt C.M., Butler A. Catechol oxidation: considerations in the design of wet adhesive materials. Biomater. Sci. 2018;6:332–339. doi: 10.1039/c7bm00884h. [DOI] [PubMed] [Google Scholar]
- Moharram M.A., Soliman M.A., El-Gendy H.M. Electrical conductivity of poly (acrylic acid)–polyacrylamide complexes. J. Appl. Polym. Sci. 1998;68:2049–2055. [Google Scholar]
- Mredha M.T.I., Guo Y.Z., Nonoyama T., Nakajima T., Kurokawa T., Gong J.P. A facile method to fabricate anisotropic hydrogels with perfectly aligned hierarchical fibrous structures. Adv. Mater. 2018;30:1704937. doi: 10.1002/adma.201704937. [DOI] [PubMed] [Google Scholar]
- Narkar A.R., Barker B., Clisch M., Jiang J., Lee B.P. pH responsive and oxidation resistant wet adhesive based on reversible catechol-boronate complexation. Chem. Mater. 2016;28:5432–5439. doi: 10.1021/acs.chemmater.6b01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. [Google Scholar]
- Osman A., Makris D.P., Kefalas P. Investigation on biocatalytic properties of a peroxidase-active homogenate from onion solid wastes: an insight into quercetin oxidation mechanism. Process. Biochem. 2008;43:861–867. [Google Scholar]
- Rhim J.W., Hwang H.S., Kim D.S., Park H.B., Lee C.H., Lee Y.M., Moon G.Y., Nam S.Y. Aging effect of poly (vinyl alcohol) membranes crosslinked with poly (acrylic acid-co-maleic acid) Macromol. Res. 2005;13:135–140. [Google Scholar]
- Saiz-Poseu J., Mancebo-Aracil J., Nador F., Busqué F., Ruiz-Molina D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2019;58:696–714. doi: 10.1002/anie.201801063. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Hamaker B.R., Wilker J.J. High strength adhesives from catechol cross-linking of zein protein and plant phenolics. Adv. Sustain. Syst. 2018;2:1700159. [Google Scholar]
- Servais A.B., Valenzuela C.D., Kienzle A., Ysasi A.B., Wagner W.L., Tsuda A., Ackermann M., Mentzer S.J. Functional mechanics of a pectin-based pleural sealant after lung injury. Tissue Eng. A. 2008;24:695–702. doi: 10.1089/ten.tea.2017.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P., Han L., Li P., Jia Z., Wang K., Zhang H., Tan H., Guo T., Lu X. Mussel-inspired electroactive and antioxidative scaffolds with incorporation of polydopamine-reduced graphene oxide for enhancing skin wound healing. ACS Appl. Mater. Interfaces. 2019;11:7703–7714. doi: 10.1021/acsami.8b18931. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhou H., Chen W., Li Q., Yan B., Jin X., Ma A., Liu H., Zhao W. Dually synergetic network hydrogels with integrated mechanical stretchability, thermal responsiveness, and electrical conductivity for strain sensors and temperature alertors. ACS Appl. Mater. Interfaces. 2018;10:14045–14054. doi: 10.1021/acsami.8b02060. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang X., Yang K., Fu Y., Xu T., Li S., Zhang D., Wang L., Lee C. A novel double-crosslinking-double-network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv. Funct. Mater. 2019:1904156. [Google Scholar]
- Wirthl D., Pichler R., Drack M., Kettlguber G., Moser R., Gerstmayr R., Hartmann F., Bradt E., Kaltseis R., Siket C.M. Instant tough bonding of hydrogels for soft machines and electronics. Sci. Adv. 2017;3:e1700053. doi: 10.1126/sciadv.1700053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Cao Y., Su H., Wang C. Tough gel electrolyte using double polymer network design for the safe, stable cycling of lithium metal anode. Angew. Chem. Int. Ed. 2018;57:1361–1365. doi: 10.1002/anie.201709774. [DOI] [PubMed] [Google Scholar]
- Xu J., Jin R., Ren X., Gao G. Cartilage-inspired hydrogel strain sensors with ultrahigh toughness, good self-recovery and stable anti-swelling properties. J. Mater. Chem. A. 2019;7:25441–25448. [Google Scholar]
- Yang Y., Wang X., Yang F., Shen H., Wu D. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels. Adv. Mater. 2016;28:7178–7184. doi: 10.1002/adma.201601742. [DOI] [PubMed] [Google Scholar]
- Zhang H.J., Sun T.L., Zhang A.K., Ikura Y., Nakajima T., Nonoyama T., Kurokawa T., Ito O., Ishitobi H., Gong J. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016;28:4884–4890. doi: 10.1002/adma.201600466. [DOI] [PubMed] [Google Scholar]
- Zhao X. Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft Matter. 2014;10:672–687. doi: 10.1039/C3SM52272E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Fang R., Rong Q., Liu M. Bioinspired nanocomposite hydrogels with highly ordered structures. Adv. Mater. 2017;29:1703045. doi: 10.1002/adma.201703045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.