Abstract
The effects of streptozotocin (STZ) on the brain after intracerebroventricular (ICV) administration in rodents have been suggested to mimic the pathogenesis of sporadic Alzheimer's disease (AD). Oxidative damage, decreased glucose utilization, mitochondrial bioenergetic changes, neuroinflammation and behavioral impairment have been reported in rodents after ICV-STZ administration. However, the molecular mechanisms of STZ effects on brain after ICV administration remain highly controversial. In this study we re-examined several bioenergetic parameters of rat brain mitochondria on day 15 following ICV-STZ treatment. We observed only a moderate but statistically significant decrease in complex I-III activity in brain mitochondria from streptozotocin-treated rats. There were no changes in complex II-III activity or phosphorylation capacity of brain mitochondria after streptozotocin treatment. More importantly, it was observed that ICV-STZ treatment caused variable degrees of body-weight loss in rats, and complex I-III activity was decreased only in those rats showing a significant (more than 10%–35%) loss in body-weights.
Keywords: Alzheimer's disease, Cognitive deficit, Mitochondria, Oxidative damage, Streptozotocin, Neuroscience, Molecular neuroscience, Neurotoxicology, Pathophysiology, Pharmacology
Alzheimer's disease, Cognitive deficit, Mitochondria, Oxidative damage, Streptozotocin, Neuroscience, Molecular neuroscience, Neurotoxicology, Pathophysiology, Pharmacology.
1. Introduction
Alzheimer's disease (AD), a progressive neurodegenerative disorder, is responsible for the majority cases of dementia in the elderly population, and it appears in both familial and sporadic forms (Chakrabarti et al., 2015). More than 95% of AD subjects suffer from the sporadic form of the disease which has an uncertain etiology involving multiple genetic, metabolic and environmental risk factors (Chakrabarti et al., 2015; Reitz et al., 2011). It is generally accepted now that the complex pathogenesis of this disease could not possibly be explained solely by Amyloid Cascade Hypothesis which posits that the toxic actions of misfolded and oligomerized amyloid beta peptide (Aβ42) is the driving force of AD pathogenesis (Iqbal et al., 2014; Sorrentino et al., 2014). This has resulted in the development of several non-transgenic toxin-based animal models in which a gradual development of AD like pathology occurs in the brain along with memory and behavioral deficits. These models are yet to be fully characterized and evaluated, but they show several typical molecular signatures of AD pathogenesis like oxidative damage, neuroinflammation, mitochondrial dysfunction, decreased glucose utilization, altered tau phosphorylation and amyloid beta metabolism in the brain in varying combinations (Grieb, 2016; Nazem et al., 2015). Thus, ICV administration of streptozotocin (STZ) to rodents has been shown in multiple studies to replicate many features of AD pathogenesis at the cellular level both in the cortex and hippocampus along with memory and behavioral deficits, and these changes appear slowly and persist over a long period of time (Chen et al., 2013; Correia et al., 2013; Santos et al., 2012; Erdoğan et al., 2017; Grieb, 2016; Kamat et al., 2016). Although STZ-induced AD model has been suggested as a good tool to understand sporadic AD, the mechanisms of brain-specific toxic actions of STZ remains somewhat conjectural and controversial despite intensive studies (Grieb, 2016; Kamat et al., 2016). Furthermore, the chronological development of different damage mechanisms and their interactions in the brain after administration of ICV-STZ have not been established with certainty, because different groups have reported the brain STZ-effects at different time points after ICV - administration (Chen et al., 2012; Correia et al., 2013; Santos et al., 2012; Du et al., 2015; Gutierre et al., 2014; Zhou et al., 2013). One important variability is the changes in body-weight of the animals after ICV-STZ administration which has been variously reported in the literature as a loss in body-weight or no changes or an initial loss followed by a gain in body-weight (Bloch et al., 2017; Chen et al., 2013; Li et al., 2017; Paidi et al., 2015). However, it is not known if the variability of body-weight changes after ICV-STZ treatment has any effect on the toxic effects STZ in rat brain. Because of the central importance of mitochondrial dysfunction in AD neurodegeneration, we thought it prudent to examine if brain mitochondrial bioenergetic alterations caused by ICV-STZ treatment of rats show variability with the pattern of body-weight loss.
2. Materials and methods
2.1. Materials
Streptozotocin, cytochrome c from equine heart, antimycin A, rotenone, bovine serum albumin, hexokinase were purchased from Sigma-Aldrich, St. Louis, USA. Pyruvic acid sodium salt, sodium succinate, ADP-disodum salt, β-NADH disodium salt, ammonium molybdate, sucrose, EDTA, digitonin, etc. were obtained from Sisco Research Laboratory, Mumbai, India. BCA protein assay kit was from Thermo Fisher Scientific, Waltham, USA. All common reagents and buffers were from HiMedia, Mumbai, India.
2.2. Animals
Adult Wistar rats (6–8 months old) of both sexes were randomized and kept as control and experimental groups. The animals were kept in polypropylene cages and fed commercial rat chow with water ad libitum under 12 h day/night cycle at a room temperature of 25° ± 1° as per the guidelines of the Institutional Animal Ethics Committee (IAEC) which has also approved the experimental protocols and treatments of the animals for this study.
2.3. Intracerebroventricular administration of streptozotocin
The animal was anaesthesized with an intra-peritoneal injection of a combination of ketamine (80 mg/kg body-weight) and xylazine (10 mg/kg body-weight) and the head was positioned on the stereotaxic apparatus and secured with ear bars and the tooth bar. After removing the fur from the top of the skull and thorough cleaning with 70% alcohol, a midline sagittal incision was made, the skin retracted, the wound cleaned of blood and the bregma identified. Following the stereotaxic co-ordinates, two holes were drilled on the skull 0.9 mm caudal and 1.5 mm lateral from the bregma on either side of the sagittal suture. The stereotaxic guide cannula was introduced through either hole 4 mm ventral from the brain surface to reach the lateral ventricle. A freshly prepared solution of STZ (3 mg/kg body-weight) in citrate buffer, pH 4.5 was manually injected in a volume of 4 μl through each hole with the help of a Hamilton syringe connected with the stereotaxic guide cannula. The injection was given slowly over a period of 8 min and the cannula left in place for another 5 min before withdrawal. The skin incision was sutured, an antibiotic ointment applied and the animal allowed to recover. The control rats were similarly administered citrate buffer, pH 4.5.
The body-weights of animals were noted regularly pre- and post-ICV-STZ treatment. The animals were sacrificed on day15 following ICV-STZ treatment and brain mitochondria were analyzed for several bioenergetic parameters. The animals were sacrificed in pairs of control and ICV-STZ-treated rats of same sex on a particular day of experimentation. For validation of ICV administration by stereotaxic method, we performed 4 initial experiments where methylene blue was introduced in to lateral ventricles of rats followed by immediate sacrifice of the animals, decapitation, removal of the brain and macroscopic verification of methylene blue in the lateral ventricles.
2.4. Isolation of rat brain mitochondria
The animals were sacrificed by cervical dislocation followed by decapitation. The brains were removed and cerebral cortices cleanly dissected out. Mitochondria were isolated from the cortical tissue by differential centrifugation and digitonin treatment as published earlier (Jana et al., 2011). The final mitochondrial pellet was suspended in isotonic buffer A (145 mM KCl, 50 mM sucrose, 5 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 10 mM phosphate buffer, pH 7.4) or in 50 mM phosphate buffer, pH 7.4 depending on subsequent experimental protocol.
2.5. Measurement of mitochondrial functional parameters
Aliquots of freshly prepared mitochondria suspended in isotonic buffer A were used for the measurement of phosphorylation capacity (inorganic phosphate utilization) in the presence of pyruvate (5 mM), succinate (10 mM) and ADP (0.5 mM) as described in our earlier studies (Bagh et al., 2011).
For the assay of respiratory complexes, the mitochondrial pellet suspended in 50 mM phosphate buffer and kept frozen at -20 °C was analyzed within 2–3 days. An aliquot of mitochondrial suspension was diluted suitably in 10 mM phosphate buffer, pH 7.4 followed by 3 cycles of freeze-thawing and then used for the assay. Mitochondrial complex I-III activity was determined spectrophotometrically by monitoring the reduction of ferricytochrome c (0.1 mM) at 550 nm in a reaction mixture containing 50 mM phosphate buffer, pH 7.4. 0.01 mM EDTA, 0.25 mM NADH, 5 mM sodium azide with or without rotenone (10 μM) after addition of an appropriate amount of lysed mitochondrial preparation as adapted from a published procedure (Bagh et al., 2011). The complex II-III activity was similarly assayed by monitoring the pyruvate-supported reduction of ferricytochrome c based on a published procedure (Bagh et al., 2011). Briefly, the reduction of ferricytochrome c (0.6 mg/ml) was monitored at 550 nm in 50 mM phosphate buffer containing succinate (2 mM), EDTA (0.3 mM) and sodium azide (5 mM) in the absence or presence of antimycin A (10 μM). Antimycin A sensitive activity was used to calculate the value of complex II-III.
2.6. Protein estimation
Protein in mitochondrial samples was estimated by using a commercial BCA protein assay kit.
2.7. Statistical analysis
The distribution of data for normality was checked by Shapiro-Wilk test. For data with normal distribution, the comparison among more than two groups was made by repeated measures one-way ANOVA followed by Bonferroni post test. When the values were not normally distributed and experimental values expressed as the percentage of matched controls, comparisons among multiple groups were made by Kruskal-Wallis test followed by Dunn's test, and comparisons between two groups were performed by Wilcoxon Matched-Pairs Signed Rank test to obtain statistical significance. A p value of <0.05 was accepted to indicate statistical significance.
3. Results
3.1. Changes in body-weight after ICV-STZ
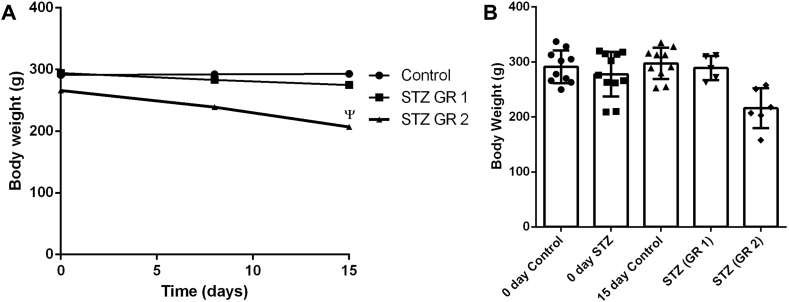
When we analyzed the body-weight changes for 15 days following ICV-STZ treatment, some clear variations were seen. The line diagram in Figure 1A indicates that in some animals moderate to severe loss of body-weights occurred, while in others only minimal changes could be seen. Thus, in Group 1 (GR 1) rats a body-weight loss up to 10% occurred, while Group 2 (GR 2) animals showed weight-loss from above 10% to about 35% (Figure 1A). Figure 1B presents the individual data points (body-weights) in different groups as bar-graphs. We checked the plasma glucose levels by glucose oxidase-peroxidase method using commercial kits in control and ICV-STZ treated rats on day 15, but no significant difference was observed in this parameter among different groups (data not shown).
Figure 1.
Body-weight loss after ICV-STZ administration in rats. The body-weights of animals control (11) and ICV-STZ treated GR 1 (5 animals with body-weight loss up to 10%) and GR 2 (6 animals with body-weight loss more than 10% to nearly 35%) were measured on day 0, day 8 and also on day15. A. Values are presented as the means ± SEM. Statistical comparisons were made by repeated measures one-way Anova followed by Bonferroni Post test. ψ indicates statistical significance p < 0.01, F = 8.888. B. Individual data points are shown in Control, STZ (GR 1) and STZ (GR 2) in the bar-graph (median with range).
3.2. Effects of ICV-STZ administration on brain respiratory chain complex activities
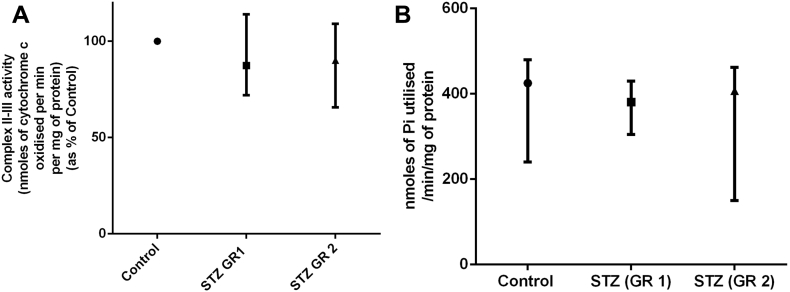
The changes in mitochondrial respiratory chain complex activities in rat brain cortex after ICV-STZ administration are presented in Figure 2 and Figure 3A and the values with respect to control shown as the medians with range. The changes in mitochondrial complex I-III activity was first compared between the control and ICV-STZ treated groups (Figure 2A). Our results show that on day 15 after ICV-STZ treatment the brain cortical mitochondria showed a statistically significant decrease (nearly 25%) in complex I-III activity compared to that in control (Figure 2A). However, when the STZ-effects were compared with respect to control rats in GR 1 (less than 10% body-weight loss after ICV-STZ) and GR 2 (more than 10%–35% weight loss after ICV-STZ) separately, an interesting phenomenon was observed. The decrease in complex I-III activity after STZ-treatment was mild and not statistically significant in GR 1, but in GR 2 ICV-STZ caused a very significant lowering of complex I-III activity (Figure 2B). The individual data points (complex I-III activity as percentage of control) are presented as bar-graphs in Figure 2C. When Spearman's correlation analysis was done between percentage loss in complex I-III activity and percentage loss in body-weights in ICV-STZ treated group, no strong association (Spearman's co-efficient ρ = 0.354) or statistical significance (p = 0.286) could be found (data not shown). When brain cortical mitochondrial complex II-III activities of control and STZ- treated rats (GR 1 and GR 2) were compared, no statistical difference was observed among the groups (Figure 3A).
Figure 2.
Mitochondrial complex I-III activity in brain cortex after ICV-STZ administration in rats. Mitochondria were isolated from rat brain cortical tissue on day 15 after ICV-STZ treatment followed by the measurement of complex I-III activity as described in Materials and Methods. Animals of same sex were sacrificed on particular dates in pairs of control and experimental, and complex I-III activity of the experimental animal, normalized to per mg protein, was expressed as percentage of the corresponding control rat (taken as100%). Values were expressed as the median with range (min, max); A. Control (11 animals) and STZ-treated (11 animals); statistical comparisons made by Wilcoxon Matched-Pairs Signed Rank test. ∗p < 0.01 vs Control. B. Control (11 animals) and STZ-treated rats (STZ (GR 1) 5 animals and STZ (GR 2) 6 animals). Statistical comparisons performed with Kruskal-Wallis test followed by Dunn's test; statistically significant, ∗p < 0.01, F = 7.652. C. Individual data points are shown for Control, STZ (GR 1) and STZ (GR 2) animals in the bar-graph (median with range).
Figure 3.
Mitochondrial complex II-III activity and phosphorylation capacity in brain of control and ICV-STZ treated rats. Mitochondria were isolated from brain cortical tissue of rats on day 15 after ICV-STZ treatment, sacrificed in pairs of control and treated animals on a given date, followed by the measurement of complex II-III activity (A) and phosphorylation capacity (B) as described in the text. Values are expressed as median with range (min, max) from three groups: Control (11 animals), STZ-treated rats (STZ (GR 1) 5 animals and STZ (GR 2) 6 animals). Statistical comparisons were made with Kruskal-Wallis test followed by Dunn's test.
3.3. Brain mitochondrial phosphorylation capacity after ICV-STZ treatment
When isolated cortical mitochondria were incubated in vitro with pyruvate, succinate and ADP (state 3 respiration) from control and STZ- treated GR 1 and GR 2 animals, the mitochondrial phosphorylation capacity (an indirect estimate of mitochondrial ATP production) was not statistically different among the three groups (Figure 3B).
4. Discussion
Many studies have confirmed significant memory deficits with increased production of reactive oxygen species (ROS), accumulation of oxidative damage markers like malonaldehyde and protein carbonyls, depletion of reduced glutathione and decreased activities of antioxidant enzymes in the brain within 2–3 weeks of STZ administration by ICV (Santos et al., 2012; Gutierre et al., 2014; Ishrat et al., 2006; Sharma and Gupta, 2001; Zhou et al., 2013). Neurodegenerative changes with neuronal loss have also been reported as early effects of ICV-STZ administration (Santos et al., 2012; Kraska et al., 2012). Although mitochondrial dysfunction is considered as a hallmark signature of AD pathogenesis, ICV-STZ induced brain mitochondrial damage has been investigated to a lesser extent. Nevertheless, the limited studies indicate multiple bioenergetic deficits in rat brain hippocampus and cortex after ICV-STZ treatment which include decreased oxygen uptake, diminished respiratory complex activities, decreased respiratory control ratio, decreased ATP synthesis and a loss in mitochondrial membrane potential (Correia et al., 2013; Du et al., 2015; Li et al., 2017; Paidi et al., 2015). These results from different studies are not exactly comparable because the toxic effects of STZ on the brain after ICV-administration develop slowly over the weeks, and these studies have analyzed mitochondrial defects at different time points after STZ treatment. In the current study we could observe only moderate decrease in complex I-III activity without any change in complex II-III activity or ATP synthesis on day 15 after ICV-STZ treatment. More importantly, we observed that the decrease in mitochondrial complex I-III activity in brain cortical tissue following ICV-STZ treatment occurred with statistical significance only in those rats showing a conspicuous loss of body-weights (GR 2). However, overall (GR 1 and GR 2 taken together) there was no strong statistical association between the degree of body-weight loss and the extent of decrease in brain complex I-III activity in ICV-STZ treated rats as determined by using Spearman's correlation co-efficient. Apparently, this may look paradoxical, but it tends to suggest that the two groups (GR 1 and GR 2) have differential sensitivity to STZ in terms of body-weight loss and loss of complex I-III activity. However, additional experiments would be necessary to explore this possibility and also to establish if loss of mitochondrial complex I-III activity has a causal link with body-weight loss.
It is interesting that there are variable reports of loss of body-weight in rats following ICV-STZ treatment which has not been examined thoroughly in the context of toxic effects of STZ in the brain (Bloch et al., 2017; Chen et al., 2013; Li et al., 2017; Paidi et al., 2015; Pathan et al., 2006). In our study in ICV-STZ group, the animals showed a variable loss of body-weights, and in some of those rats showing very significant body-weight loss, we noticed a dramatic change in feeding behavior with animals refusing to take food or water. This observation was consistent with another study published earlier (Paidi et al., 2015). Further, when given systemically STZ acts as a diabetogenic agent and destroys β cells of the pancreas after gaining entry in to the cells through GLUT2 transporters (Grieb, 2016; Šalković-Petrišić and Riederer, 2010-). In brain GLUT2 transporters are present in certain areas including hypothalamus where these are located in neurons, endothelial cells as well as special type of ependymal cells called tanycytes (Elizondo-Vega et al., 2015; García et al., 2003; Thorens, 2015). GLUT2 transporters in hypothalamus possibly act as glucose sensors and control feeding behavior, thermoregulation as well as glucose homeostasis in peripheral organs (Elizondo-Vega et al., 2015; Thorens, 2015). Thus, it is plausible that damage to the GLUT2 containing hypothalamic cells by STZ after ICV administration leads to altered feeding behavior and loss of body-weight in rats, but how this happens in some rats but not in others in the current study remains an open question. However, it will be interesting to probe if other toxic effects of ICV-STZ administration on brain reported in the literature such as oxidative stress, decreased cerebral glucose utilization and neuroinflammation also differ in GR 1 and GR 2 rats. The mechanisms of the multiple toxic effects of STZ on brain after ICV administration are largely unknown, but the damage to GLUT2 containing cells in the brain by STZ could play an important role in this process (Grieb, 2016; Knezovic et al., 2017; Šalković-Petrišić and Riederer, 2010).
It is also interesting to note in the context of our results that moderate inhibition of complex I-III activity has been shown to increase the neuronal AMP content and activation of AMP dependent protein kinase (AMPK) which causes improved bioenergetic performance of mitochondria and reprogramming of neuronal metabolism (Du et al., 2015). This may partially explain our failure to demonstrate any loss of complex II-III activity or ATP synthesis by mitochondria after ICV-STZ treatment. However, the unaltered mitochondrial phosphorylation capacity in the presence of a decreased complex I-III activity in ICV-STZ treated rats may also arise from the fact that we measured mitochondrial phosphorylation capacity in the presence of pyruvate and succinate which would by-pass the complex I-III and continue mitochondrial respiration and ATP synthesis through complex II-III. Overall this brief study has indicated several complex issues with regard to STZ effects on brain mitochondria, which should be examined more thoroughly in order to understand the suitability of this toxin in developing a model for sporadic AD.
Declarations
Author contribution statement
Jit Poddar, Sukhpal Singh, Pardeep Kumar: Performed the experiments.
Sharadendu Bali: Contributed reagents, materials, analysis tools or data.
Sumeet Gupta: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sasanka Chakrabarti: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Chancellor and management of Maharishi Markandeshwar (Deemed to be) University, Mullana, Ambala, India. Jit Poddar was supported by the Indian Council of Medical Research, Govt. of India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the Chancellor of Maharishi Markandeshwar (Deemed to be) University, Mullana, Ambala, India for administrative help and encouragement to conduct this study.
References
- Bagh M.B., Thakurta I.G., Biswas M., Behera P., Chakrabarti S. Age-related oxidative decline of mitochondrial functions in rat brain is prevented by long term oral antioxidant supplementation. Biogerontology. 2011;12:119–131. doi: 10.1007/s10522-010-9301-8. [DOI] [PubMed] [Google Scholar]
- Bloch K., Gil-Ad I., Vanichkin A., Hornfeld S.H., Koroukhov N., Taler M., Vardi P., Weizman A. Intracerebroventricular streptozotocin induces obesity and dementia in lewis rats. J. Alzheimers Dis. 2017;60:121–136. doi: 10.3233/JAD-161289. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Khemka V.K., Banerjee A., Chatterjee G., Ganguly A., Biswas A. Metabolic risk factors of sporadic Alzheimer's Disease: implications in the pathology, pathogenesis and treatment. Aging Dis. 2015;6:282–289. doi: 10.14336/AD.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tian Z., Liang Z., Sun S., Dai C.-L., Lee M.H., LaFerla F.M., Grundke-Iqbal I., Iqbal K., Liu F., Gong C.-X. Brain gene expression of a sporadic (icv-STZ Mouse) and a familial mouse model (3xTg-AD Mouse) of Alzheimer's disease. PloS One. 2012;7 doi: 10.1371/journal.pone.0051432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liang Z., Blanchard J., Dai C.-L., Sun S., Lee M.H., Grundke-Iqbal I., Iqbal K., Liu F., Gong C.-X. A non-transgenic mouse model (icv-STZ Mouse) of Alzheimer’s Disease: similarities to and differences from the transgenic model (3xTg-AD Mouse) Mol. Neurobiol. 2013;47:711–725. doi: 10.1007/s12035-012-8375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia S.C., Santos R.X., Santos M.S., Casadesus G., LaManna J.C., Perry G., Smith M.A., Moreira P.I. Mitochondrial abnormalities in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. Curr. Alzheimer Res. 2013;10:406–419. doi: 10.2174/1567205011310040006. [DOI] [PubMed] [Google Scholar]
- Du L.-L., Chai D.-M., Zhao L.-N., Li X.-H., Zhang F.-C., Zhang H.-B., Liu L.-B., Wu K., Liu R., Jian-Wang J.-Z., Zhou X.-W. AMPK activation ameliorates Alzheimer's disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer's disease model in rats. J. Alzheimers Dis. 2015;43:775–784. doi: 10.3233/JAD-140564. [DOI] [PubMed] [Google Scholar]
- Elizondo-Vega R., Cortes-Campos C., Barahona M.J., Oyarce K.A., Carril C.A. The role of tanycytes in hypothalamic glucosensing. J. Cell Mol. Med. 2015;19:1471–1482. doi: 10.1111/jcmm.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdoğan M.E., Aydın S., Yanar K., Mengi M., Kansu A.D., Cebe T., Belce A., Çelikten M., Çakatay M. The effects of lipoic acid on redox status in brain regions and systemic circulation in streptozotocin-induced sporadic Alzheimer's disease model. Metab. Brain Dis. 2017;32(4):1017–1031. doi: 10.1007/s11011-017-9983-6. [DOI] [PubMed] [Google Scholar]
- García M. de los A., Millán C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zúñiga F, Vera J.C., Oñate S.A., Nualart F. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J. Neurochem. 2003;86:709–724. doi: 10.1046/j.1471-4159.2003.01892.x. [DOI] [PubMed] [Google Scholar]
- Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer's disease: in search of a relevant mechanism. Mol. Neurobiol. 2016;53:1741–1752. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierre J.M., Carvalho F.B., Schetinger M.R.C., Marisco P., Agostinho P., Rodrigues M., Rubin M.A., Schmatz R., Da Silva C.R., De P Cognato G., Farias J.G., Signor C., Morsch V.M., Mazzanti C.M., Bogo M., Bonan C.D., Spanevello R. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer's type. Life Sci. 2014;96:7–17. doi: 10.1016/j.lfs.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C.-X. Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem. Pharmacol. 2014;88:631–639. doi: 10.1016/j.bcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T., Khan M.B., Hoda M.N., Yousuf S., Ahmad M., Ansari M.A., Ahmad A.S., Islam F. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav. Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Jana S., Sinha M., Chanda D., Roy T., Banerjee K., Munshi S., Patro B.S., Chakrabarti S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine : implications in dopamine cytotoxicity and pathogenesis of Parkinson's disease. Biochim. Biophys. Acta. 2011;1812:663–673. doi: 10.1016/j.bbadis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Kamat P.,K., Kalani A., Rai S., Tota S.K., Kumar A., Ahmad A.S. Streptozotocin intracerebroventricular-induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer's disease (sAD)-like pathology. Mol. Neurobiol. 2016;53:4548–4562. doi: 10.1007/s12035-015-9384-y. [DOI] [PubMed] [Google Scholar]
- Knezovic A., Loncar A., Homolak J., Smailovic U., Osmanovic Barilar J., Ganoci L., Bozina N., Riederer P., Salkovic-Petrisic M. Rat brain glucose transporter-2, insulin receptor and glial expression are acute targets of intracerebroventricular streptozotocin: risk factors for sporadic Alzheimer's disease? J. Neural. Transm. 2017;124:695–708. doi: 10.1007/s00702-017-1727-6. [DOI] [PubMed] [Google Scholar]
- Kraska A., Santin M.D., Dorieux O., Joseph-Mathurin N., Bourrin E., Petit F., Jan C., Chaigneau M., Hantraye P., Lestage P., Dhenain M. In vivo cross-sectional characterization of cerebral alterations induced by intracerebroventricular administration of streptozotocin. PloS One. 2012;7 doi: 10.1371/journal.pone.0046196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Qin L., Lu H.-L., Li P.-J., Song Y.-J., Yang R.-L. Methylene blue improves streptozotocin-induced memory deficit by restoring mitochondrial function in rats. Brain. 2017;Res1657:208–214. doi: 10.1016/j.brainres.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Nazem A., Sankowski R., Bacher M., Al-Abed Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflammation. 2015;12:74. doi: 10.1186/s12974-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidi R.,K., Nthenge-Ngumbau D.N., Singh R., Kankanala T., Mehta H., Mohanakumar K.P. Mitochondrial deficits accompany cognitive decline following single bilateral intracerebroventricular streptozotocin. Curr. Alzheimer Dis. 2015;12:785–795. doi: 10.2174/1567205012666150710112618. [DOI] [PubMed] [Google Scholar]
- Pathan Asif R., Viswanad Bhoomi, Sonkusare Swapnil K., Ramarao Poduri. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006;79:2209–2216. doi: 10.1016/j.lfs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šalković-Petrišić Melita, Riederer Peter. Brain glucose transporter protein 2 and sporadic Alzheimer’s disease. Translat. Neurosci. 2010;1:200–206. [Google Scholar]
- Santos T.O., Mazucanti C.H.Y., Xavier G.F., Torrão A.S. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol. Behav. 2012;107:401–413. doi: 10.1016/j.physbeh.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Sharma M., Gupta Y.K. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–1029. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- Sorrentino P., Iuliano A., Polverino A., Jacini F., Sorrentino G. The dark sides of amyloid in Alzheimer's disease pathogenesis. FEBS Lett. 2014;588:641–652. doi: 10.1016/j.febslet.2013.12.038. [DOI] [PubMed] [Google Scholar]
- Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–232. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- Zhou I., Yu G., Chi L., Zhu J., Zhang W., Zhang Y., Zhang L. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013;38:136–145. doi: 10.1016/j.neuro.2013.07.007. [DOI] [PubMed] [Google Scholar]