Summary
The highly reversible zinc-bromine redox couple has been successfully applied in the zinc-bromine flow batteries; however, non-electroactive pump/pipe/reservoir parts and ion-selective membranes are essential to suppress the bromine diffusion. This work demonstrates a zinc-bromine static (non-flow) battery without these auxiliary parts and utilizing glass fiber separator, which overcomes the high self-discharge rate and low energy efficiency while the advantages of the zinc-bromine chemistry are well preserved. It is achieved by a multifunctional additive, tetrapropylammonium bromide (TPABr), which not only mitigates the bromine cross-diffusion by regulating the fluidic bromine to a condensed solid phase but also provides a favorable interface for zinc electrodeposition toward non-dendritic growth. The proposed zinc-bromine static battery demonstrates a high specific energy of 142 Wh kg−1 with a high energy efficiency up to 94%. By optimizing the porous electrode architecture, the battery shows an ultra-stable cycling life for over 11,000 cycles with controlled self-discharge rate.
Subject Areas: Electrochemical Energy Storage, Energy Systems, Energy Storage
Graphical Abstract

Highlights
-
•
A zinc-bromine static battery with regular sandwich-type configuration
-
•
Self-discharge was suppressed by converting soluble Br2/Br3- species to solid phase
-
•
Zinc dendrite growth was inhibited by the adsorption of TPA+ on the zinc anode
Electrochemical Energy Storage; Energy Systems; Energy Storage
Introduction
Energy storage devices with high energy density, long cycling life, and low cost are eternal goals to meet the ever-increasing demands from portable electronic devices, electric vehicles, and renewable energy sources (Armand and Tarascon, 2008). Conventional lithium-ion batteries have dominated the market for decades owing to their relatively high energy density (150 Wh kg−1) (Dunn et al., 2011; Nitta et al., 2015). However, the inherent safety hazard of the flammable organic electrolyte and high cost limit their large-scale applications such as for stationary energy storage (Tarascon and Armand, 2001; Turcheniuk et al., 2018). Flow batteries with multiple redox couples in aqueous media are one of the most promising technologies for large-scale energy storage (Yang et al., 2011). Among them, zinc-bromine flow batteries are very appealing, owing to their attractive features of long cycling life (Soloveichik, 2015).
A typical Zn-Br2 flow battery is composed of two reservoirs for storage of the ZnBr2 electrolyte, which are connected to the reactor by pumps/pipes (Ke et al., 2018). The basic redox reactions in the battery are summarized as following equations:
At the positive electrode:
| 2 Br- ↔ Br2 + 2 e− (Eo = 1.08 V versus SHE) |
At the negative electrode:
| Zn2+ + 2 e− ↔ Zn (Eo = - 0.76 V versus SHE) |
Overall reaction:
| Zn2+ + 2 Br− ↔ Br2 + Zn (Eo = 1.84 V) |
During the charge process, Br2 is generated at the positive electrode and further complexes with Br− in aqueous media to form highly soluble Br3- ions, while zinc is deposited at the negative electrode simultaneously (Noack et al., 2015). Reverse reactions occur at the respective electrodes during the discharge process, providing a high theoretical energy density of 432 Wh kg−1 (Biswas et al., 2017; Darling et al., 2014). Although the highly soluble Br3- species accelerates the redox kinetics, it reacts with the zinc anode directly by cross-diffusion, leading to self-discharge and low coulombic efficiency (Huskinson et al., 2014; Wang et al., 2017). Another existing issue for zinc-based battery is the dendritic Zn deposition, which potentially triggers an internal short circuit (Li et al., 2018a, 2018b; Yuan et al., 2018). These challenges are alleviated with the multiple approaches of a standard Zn-Br2 flow battery: (1) Bromine complexing agents transform the soluble Br3- species to oily phase (Winsberg et al., 2017; Wu et al., 2019); (2) storage of the oily phase in separated reservoirs allows bromine convection and improves the limiting current density for Zn deposition (Gur, 2018; Soloveichik, 2015); (3) ion-selective membrane further prevents cross-diffusion and short circuits (Hu et al., 2017; Zhang et al., 2019). These approaches are compromised at the expense of cell resistance, energy efficiency (normally <80%), and increased system size and complexity (Biswas et al., 2017; Zhao et al., 2015). They sharply increase the capital costs from $8 per kWh based on ZnBr2 electrolyte and carbon electrode to over $200 per kWh for a battery system, which is not superior at all when compared with the current Li-ion technology (Biswas et al., 2017; Winter et al., 2018). The flowable and corrosive Br2/Br3- species further reduce the reliability of the system, leading to cell failure by parts corrosion instead of by the intrinsic redox chemistry (Evanko et al., 2018).
In typical Zn-Br2 flow batteries, converting the soluble Br2/Br3- species to oily complexation phase by asymmetric quaternary ammonium salts such as methyl ethyl pyrrolidinium bromide (MEPBr) or methyl ethyl morpholinium bromide (MEMBr) does not fully address the cross-diffusion and poor coulombic efficiency (Soloveichik, 2015; Xie et al., 2017). Auxiliary components such as microporous polyolefin or Daramic membrane and the bromine storage system (reservoirs, pumps, pipes) as discussed above are essential to achieve an acceptable performance (Huskinson et al., 2014; Park et al., 2017). Since most of these auxiliary components in Zn-Br2 flow battery are correlated to address the cross-diffusion of the Br2/Br3- species, the most straightforward strategy to simplify the battery system is transforming the soluble species to solid phase. In short, this requires a fundamental change to store Br2/Br3- species in non-flow energy storage systems in a manner that suppresses cross-diffusion and self-discharge by a simple and affordable mechanism, as proposed by Stucky et al. for the aqueous redox-enhanced electrochemical capacitors (Yoo et al., 2017). Efforts had been made recently to realize flowless Zn-Br2 batteries by entrapment of Br3- in the cathodes; however, the coulombic efficiencies are normally lower than 90% and the energy efficiencies are lower than 80% (Chun et al., 2015; Lee et al., 2019).
In this work, we demonstrate a zinc-bromine static (non-flow) battery without the auxiliary moving parts and utilizing a glass fiber separator, which overcomes the high self-discharge rate and low energy efficiency while the advantages of the zinc-bromine redox couple are well maintained. This is achieved by an effective complexing agent, tetrapropylammonium bromide (TPABr), reversibly converting the Br2/Br3- species to solid phase in aqueous media. The solid complexation is stored in porous carbons with good interfacial contact, further providing physical confinements to prevent the cross-diffusion and self-discharge. We also witness the zinc electrodeposition is altered from dendritic to non-dendritic growth by the polar TPA+ ions, which prefer to be adsorbed on zinc nucleus, blocking the strong electric field and regulating the ion distribution on the interface (Ding et al., 2013). In such a simplified design, the Zn-Br2 static battery has a similar configuration to the Li-ion batteries. The proposed zinc-bromine static battery demonstrates a high specific energy density of 142 Wh kg−1 at 500 mA g−1 (equivalent to 150 W kg−1) with a high energy efficiency of 94%. Note that the current densities and specific capacities are based on the mass of cathodes only, whereas the power densities and energy densities are calculated with the total mass of the cathode and active materials in electrolytes (ZnBr2 and TPABr). Owing to the highly reversible bromine capture capacity of the TPABr additive, the battery shows an ultra-stable cycling life for over 11,000 cycles with minimum self-discharge rate. Our static battery configuration offers a cost-effective and easy fabrication path for spreading the Zn-Br2 battery to practical application.
Results
Reversible Solid Complexation of Br2 in Aqueous Media
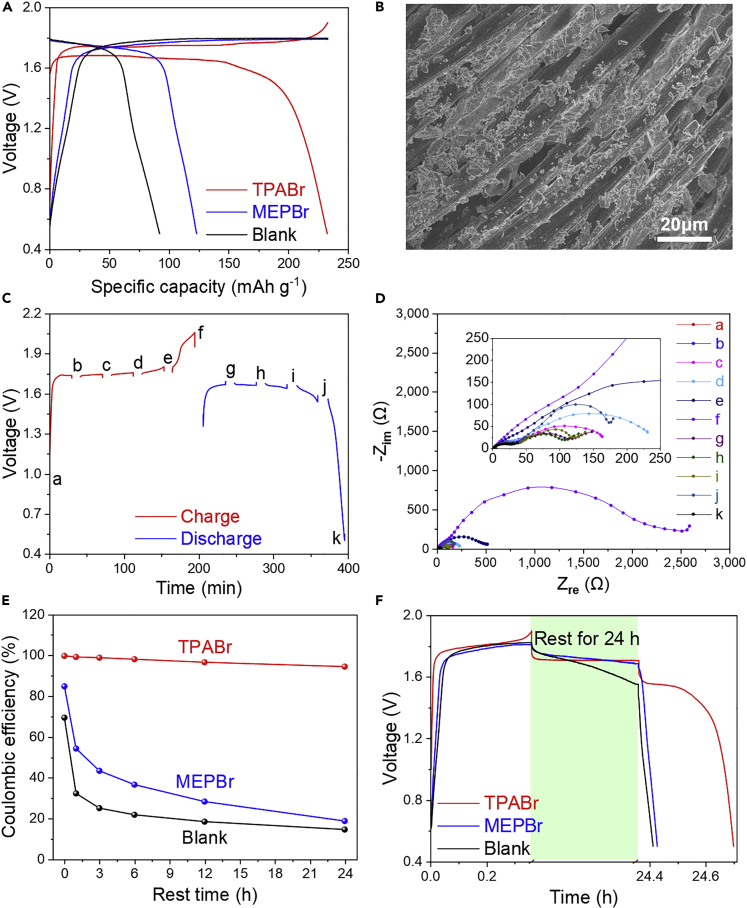
The non-flow zinc-bromine battery with regular porous glass fiber separator is particularly prone to low coulombic efficiency, as shown by the blank electrolyte (Figure 1A). This is due to the serious cross-diffusion of the highly soluble Br2/Br3- species, which results in direct redox reaction with the zinc anode. Although the diffusion is alleviated in flow batteries by the combination of the ion-selective membranes and the bromine complexing agents (such as MEPBr), the zinc-bromine flow batteries are still plagued by self-discharge and low coulombic efficiency (Biswas et al., 2017). Relying solely on the MEPBr additive does not show much improvement for coulombic efficiency in the static battery (Figure 1A). In sharp contrast, the static zinc-bromine battery with the TPABr complexing agent shows high reversibility with decent coulombic efficiency of 99.6%, as shown in Figure 1A. It is much higher than that of the recently reported “flowless” Zn-Br2 batteries, which only show coulombic efficiencies of <90% (Lee et al., 2019). We ascribe it to the strong complexing ability of the TPABr additive, which fully transforms the soluble Br2/Br3- species to solid phase in the electrode. Figure 1B shows the electrochemical generation of a solid complex on the surface of a carbon cloth electrode by passing anodic current in the ZnBr2/TPABr electrolyte.
Figure 1.
Properties of Bromine Complexing Agents
(A) Coulombic efficiencies and capacity retentions of the static batteries with (1) blank (ZnBr2), (2) MEPBr, and (3) TPABr electrolytes at 100 mA g−1 (0.3 mA cm−2).
(B) The image of the carbon cloth electrode after being charged for 1 h at 5 mA cm−2 in TPABr electrolyte.
(C) Br3- solution and its complexation by MEPBr and TPABr, respectively.
(D) Raman spectra of the Br3- solution and the supernatants of the Br3- + MEPBr and the Br3- + TPABr mixtures.
We studied the complexation chemistry of Br3- with TPABr and other quaternary ammonium salts with different chain length to demonstrate the effectiveness of suppressing the cross-diffusion (see Figure S1 for the molecular structure). Figure 1C and Figure S2 visually show the complexing product of the freshly prepared Br3- solution with quaternary ammonium salts (see Methods for detail). Conventional complexing agent of MEPBr for flow batteries is also shown for comparison. The yellow Br3- anion solution is immediately transferred into a colorless supernatant with solid precipitation by the TPABr additive, whereas MEPBr forms an oily liquid with certain amount of Br3- residual in the supernatant, as shown in Figure 1C. The supernatant was further characterized by Raman spectrum (Figure 1D). The symmetric stretching band of Br3- at 165 cm−1 is obvious in the MEPBr mixture (Chen et al., 2010; Yoo et al., 2017), implying insufficient complexation by MEPBr additive. The residual Br3- in the supernatant leads to cross-diffusion, which inevitably decreases the overall columbic efficiency, as shown in Figure 1A. However, the TPABr complexed supernatant is colorless with minor Raman response, verifying that no Br3- remains in the supernatant after complexing. This is consistent with the high coulombic efficiency of the cell with the TPABr complexing agent. This approach is vastly different than the electrostatic attraction mechanism proposed to retain bromine species by the oppositely charged electrodes, which only shows a minor effect on the suppression of self-discharge (Chun et al., 2015). The precipitate discussed here is identical to the TPABr3 solid complex formed electrochemically on the electrodes, as confirmed by Raman spectrum (Figure S3) and XRD pattern (Figure S4).
It is worth noting that the voltage profile of the cell with the TPABr electrolyte is significantly different from that of the MEPBr electrolyte, as shown in Figure 2A. Notably, the voltage of the cell with TPABr rises at the end of the charge process, followed by a “knee” point at the beginning of discharge. The large polarization of CMK-3 electrode at the beginning of the discharge is due to blocking of the pores of the electrode by the non-conductive TPABr3 precipitation, which impedes the charge transfer path. Subsequent formation of TPABr3 will aggregate on the electrode surface at the end of charge process, as shown by the SEM image of the charged carbon cloth electrode (Figure 2B). Figure S5 shows the corresponding SEM images of the CMK-3 electrode at different SOC; it is obvious that the surface of the electrode was covered by TPABr3 crystals after it was fully charged (Figure S5F) but it disappeared after discharge (Figure S5G). It is understandable that the MEPBr3 is a flowable oily phase that does not prefer to adsorb on the substrates so a flat voltage is obtained, as evidenced by the clean charged electrode (Figure S6). This phenomenon is supported by the electrochemical impedance spectroscopy (EIS) of the cell at different state of charge (SOC) (Figures 2C and 2D). The semicircles at the high frequency and middle frequency regions are corresponding to the ion migration process and the charge-transfer process, respectively (Orazem and Tribollet, 2017). Obviously, the charge-transfer impedance increases during the charge process with a sudden increase at the end of charge, which results in the rising of the charge potential simultaneously. The high charge transfer resistance is also attributed to the high polarization at the beginning of discharge. But it returns to a normal level with the consumption of the solid bromine phase on the electrode. In contrast, the impedance of the blank cell and MEPBr cell does not show such features (Figure S7).
Figure 2.
Electrochemical Behaviors of the Static Batteries with Different Electrolytes
(A) Voltage profiles at 100 mA g−1 (0.3 mA cm−2).
(B) SEM image of the carbon cloth electrode after being charged for 1 h at 1 mA cm−2 in TPABr electrolyte.
(C and D) EIS spectra of the battery with TPABr at different SOC and the corresponding voltage profile at 100 mA g−1 (0.3 mA cm−2).
(E) Coulombic efficiencies of the batteries versus rest time between charge and discharge processes at 500 mA g−1 (1.5 mA cm−2).
(F) Voltage profiles with 24 h of rest between charge and discharge processes at 500 mA g−1 (1.5 mA cm−2).
Note that the cell has very stable cycling performance with a high coulombic efficiency of 99.6% even when a higher cutoff voltage of 2.2 V is applied (Figure S8). The high coulombic efficiency indicates that the plateau at 2.2 V is attributed to the electrodeposition of TPABr3 on the electrode instead of water splitting. However, the large polarization at high cutoff voltage will definitely decrease the energy efficiency. So, the charge cutoff voltage is set only about 0.1 V higher than the charge voltage plateau. This highlights the importance of the porosity of the electrode to the specific capacity of the cell, which will be discussed in the next section. Figure S9 shows the cyclic voltammogram (CV) curves of the three cells. Different from the other two cells, the cathodic currents of the TPABr cell do not fluctuate with the change of scan rate. Although it is clear that the electrochemical process of the bromine chemistry in solution is controlled by diffusion process, the constant cathodic peak current of the cell with the TPABr additive indicates that the formation of the solid product determines the reaction rate.
Self-discharge is one of the most critical parameters for energy storage systems, determining the performance after intermittent application or storage (Palacin and de Guibert, 2016). Simply transferring the flow-type Zn-Br2 battery to a non-flow battery without using microporous membrane represents a system with serious self-discharge, owing to the fast cross-diffusion of the soluble Br2/Br3- species. We constructed transparent cells to demonstrate the mechanism of self-discharge (Figure S10). All cells were galvanostatically charged at 20 mA. It is obvious that the blank electrolyte and the MEPBr electrolyte changed to yellowish (color of the Br2/Br3- species) only after 10 min of charge, implying a large amount of Br3- had diffused into electrolytes. It results in direct reaction between Br3- in the electrolytes and the zinc anode, namely, severe self-discharge. In sharp contrast, the TPABr electrolyte remained colorless after 2 h of charge with yellow precipitate on the electrode (Figure S10). We thus anticipate superior anti-self-discharge property with the TPABr additive because the formation of TPABr3 solid complexes will greatly suppress the cross-diffusion. We quantified the self-discharge by testing the retained capacity after storage using a charged (activated) Zn-Br2 cell at open-circuit voltage (OCV), as shown in Figure 2E. All cells were charged to the same specific capacity of 180 mAh g−1 (0.54 mAh cm−2) at a current of 500 mA g−1 (1.5 mA cm−2, 21.6 min). The OCV of each cell was recorded during the rest period. Not surprisingly, the blank cell demonstrates a very fast self-discharge rate. Its coulombic efficiency drops from 70% to 32% after only 1 h. Although MEPBr additive can partially complex the Br3- anion as an oily phase as discussed above, its coulombic efficiency still reduces by 30% after 1 h. Only about 20% of the capacity can be delivered after 24 h of storage for these two cells, implying the fast redox with zinc anode caused by the Br3- anion cross-diffusion. On the contrary, the cell with the TPABr additive shows minor self-discharge after 1 h of rest. Remarkably, the coulombic efficiency only drops by 4% after 24 h of storage. Note that, even when utilizing a Nafion membrane with complicated system design, conventional zinc-bromine flow Battery still suffers a self-discharge rate of 10% per day (Lim et al., 1977; Yang et al., 2011). Figure 2F shows the voltage profiles with a rest period of 24 h. The OCV of the two control cells decayed continuously during the rest period, leading to the disappearance of the following discharge voltage plateau. However, the cell with TPABr has a constant OCV, implying the cross-diffusion is greatly suppressed by the TPABr complexing agent.
Storing the Solid Br2/Br3- Species in Porous Carbon
Although the mobility of Br2/Br3- is successfully controlled by TPABr complexing agent, exploring a robust conductive porous material to contain the solid phase is essentially important for a static Zn-Br2 battery. Obviously, similar to Li-S battery where pores provide physical confinement for the soluble polysulfides (Chen et al., 2019; Pang et al., 2016), a substrate with large surface area and pore volume is highly desired for the static Zn-Br2 battery. We systematically compared two kinds of popular porous carbon substrates: activated carbon and CMK-3. Brunauer-Emmett-Teller (BET) analysis suggests that the activated carbon has a specific surface area of 879.6 m2 g−1 and pore volume of 0.447 cm3 g−1, whereas the CMK-3 has a specific surface area of 1,456 m2 g−1 and pore volume of 1.624 cm3 g−1, respectively (Figure 3A). Figure 3B shows the voltage profiles of the cells with these two electrodes, at a current density of 100 mA g−1 (0.3 mA cm−2). The carbon loading of the electrodes is both about 3 mg cm−2. Not surprisingly, the CMK-3 cathode delivers a much higher specific capacity than the activated carbon (497 versus 227 mAh g−1, or 1.5 versus 0.7 mAh cm−2). Figure S11 shows the voltage profiles of the symmetric cell using ZnCl2 solution as electrolyte and CMK-3 as both anode and cathode. It is a typical capacitor with the discharge specific capacity of about 43 mAh g−1 (0.1 mAh cm−2), indicating the adsorbing of zinc ions only contributes a little capacity to the static battery. In contrast to the carbon cloth electrode with numerous TPABr3 crystals on the surface (Figure 2B), the morphology of the fresh CMK-3 electrode and the fully charged CMK-3 electrode are identical (Figures 3C and 3D). Although the XRD patterns suggest there is a certain amount of TPABr3 in the fully charged CMK-3 electrode (Figure S12), it indicates that the TPABr3 is stored in the pores of CMK-3. BET analysis of the CMK-3 electrode recovered from the charged cell shows a vast decrease of pore volume (1.624 versus 0.025 cm3 g−1) (Figures S13B and S13C), again proving that the solid bromine phase is stored in the pores. This further indicates the significance of the pore volume in the performance of the Zn-Br2 battery.
Figure 3.
Rate Performances of the Zinc-Bromine Static Batteries with the TPABr Additive
(A) Comparison of the physical characteristics of activated carbon (AC) and CMK-3.
(B) Comparison of the charge and discharge voltage profiles of the batteries with activated carbon (AC) cathode and CMK-3 cathode at 100 mA g−1 (0.3 mA cm−2).
(C) SEM image of the fresh CMK-3 electrode.
(D) SEM image of the fully charged CMK-3 electrode.
(E and F) Rate performance of the battery with CMK-3 cathode and the corresponding charge and discharge voltage profiles. Current densities and specific capacities are based on the mass of cathode.
We witness a superior rate performance of the CMK-3 electrode (Figure 3E) than that of the activated carbon electrode (Figure S14A). The CMK-3 electrode delivers high specific capacities of 426, 398, 369, 326, and 300 mAh g−1 (1.3, 1.2, 1.1, 1.0, and 0.9 mAh cm−2) at the current densities of 500, 1,000, 2,000, 5,000, and 10,000 mA g−1 (1.5, 3.0, 6.0, 15, and 30 mA cm−2), respectively (Figure 3E). The corresponding energy efficiencies are 94%, 93%, 92%, 88%, and 83%, respectively. Remarkably, it delivers a specific capacity of 237 mAh g−1 (0.7 mAh cm−2) with 70% energy efficiency at an ultrahigh current density of 30,000 mA g−1 (90 mA cm−2, equivalent to 127 C). These performances are much better than that of the activated carbon (Figure S14A). The coulombic efficiency of the static Zn-Br2 battery is likely to be independent of the current density, i.e., 99.5% coulombic efficiency is still achieved at slow current of 500 mA g−1 (1.5 mA cm−2). This again implies that the Br2/Br3- species are converted to solid phase and well stored in pores. The CMK-3 cell with MEPBr electrolyte only conveys very limited improvements over the cell with blank electrolyte, as shown in Figure S15. It is worth noting that the CMK-3 has a larger pore size than the activated carbon (3.94 versus 1.02 nm) (Figure 3A). We attribute the decent rate performance to the meso-sized pores of the CMK-3, which facilitate electrolyte penetration compared with micro-sized pores, particularly at high current densities. Figure 3F shows the corresponding voltage profiles of the CMK-3 electrode. Notably, the voltage plateaus are flatter than that of the activated carbon electrode (Figure S14B).
Inhibiting the Dendrite Growth
Dendrite growth is the main challenge for batteries with metal anodes (Li et al., 2018a, 2018b; Li and Dai, 2014). Although stable cycling performance of a practical battery relies on a favorable interface between the electrode and electrolyte, the dendritic feature of the zinc metal during plating gives rise to huge problem, particularly for long-term cycling (Wang et al., 2018). The zinc dendrite has a high surface area, which catalyzes the detrimental reaction with electrolyte. It also trends to detach from the anode, leading to fast capacity decay of the zinc-based batteries (Tan et al., 2017). The classic model suggests that the uneven distributions of electric field and zinc ion flow are the main factors for dendrite formation (Wang et al., 2015). During a typical electrodeposition process, zinc atoms nucleate at the active sites to form small protrusions, resulting in a stronger electric field and denser ion distribution above these areas (Parker et al., 2014). The following ions are more facile to deposit along these protrusions, which eventually aggregate as dendrite growth (Haxhimali et al., 2006). The Zn plating behavior in the TPABr electrolyte was revealed by SEM imaging. Zn electrodes were retrieved after plating 2 mAh cm−2 Zn at a current density of 5 mA cm−2 in Zn|Zn symmetric cells. The Zn plated in the blank electrolyte shows a typical needle-shaped morphology with large porosity (Figure 4A). The high-surface-area dendrites exacerbate reaction with the electrolyte, leading Zn pulverization after 100 h (125 cycles) of repeated plating/stripping (Figure 4D). In contrast, the Zn plated in the TPABr electrolyte shows more featureless with compact electrodeposition film (Figures 4C and 4F), implying non-dendritic zinc deposition. This is because the TPA+ cation is a surfactant in nature and prefers to be absorbed on the zinc protrusions, which blocks the strong electric field and regulates the ion distribution on the interface (Ding et al., 2013). The Zn plated with the MEPBr additive has a similar condensed morphology, albeit forming islands instead of extensive film (Figure 4B). Nevertheless, these islands turn into big dendrites after 100 h (125 cycles) of repeated plating/stripping (Figure 4E). This is due to the higher surface activity and polarity of TPA+ than that of MEP+ (Nishiyama et al., 2005; Scott and Willett, 1991), enabling a more powerful absorbability. Repeat stripping/plating measurements were carried out in symmetric Zn|Zn cells to validate the long-term role of the TPABr additive. As shown in Figure 4G, the cell in the blank electrolyte only cycled for 26 h (33 cycles) before the cell failure by short circuit. In sharp contrast, the cell with TPABr additive showed a very stable potential profile for over 2,000 h (2,500 cycles) without any sign of a short circuit (Figure 4G, red). The over 70-fold improvement in cycling life proves that the additive is effective in stabilizing zinc electrodeposition. We further evaluated the stripping/plating reversibility in Zn|Cu cells to demonstrate the degree of the parasitic reaction (Tikekar et al., 2016) (Figure 4H). The coulombic efficiency of the cell in blank electrolyte demonstrated a drastic fluctuation and quickly decreased to 45% before a short circuit occurred at the 192th cycle. However, the cell with TPABr revealed a high coulombic efficiency at 99.9% and stable performance for over 1,500 cycles, demonstrating the favorable interface for Zn electrodeposition. As a result, not only the dendrite-related safety issue is resolved by the TPABr additive but also the electrolyte depletion is greatly suppressed, which avoids electrolyte drying out and will greatly extend the cycling life of the battery.
Figure 4.
Zinc Platting/String Behaviors
(A–C) SEM images of the zinc anodes after once plating in (A) blank, (B) MEPBr, and (C) TPABr electrolytes, respectively.
(D–F) SEM images of the zinc anodes after stripping/plating for 100 h in (D) blank, (E) MEPBr, and (F) TPABr electrolytes, respectively.
(G) Voltage profiles of the Zn|Zn symmetric cells.
(H) Coulombic efficiencies of zinc stripping/plating on Cu foil.
Long-Term Performance of the Zinc-Bromine Static Batteries
The complexing chemistry we explored that converts the Br2/Br3- to solid phase is significantly different from the MEPBr agent for flow Zn-Br2 battery and other approaches that bind Br2/Br3- to the host by interactions (Lee et al., 2019). The soluble Br2/Br3- species in electrolyte are remarkably precipitated as TPABr3 solid complex, whereas free Br2/Br3- is negligible. However, the MEPBr additive can only partially complex with Br2/Br3- species as an oily liquid, which only shows little improvement over the static cell without a complexing agent. The TPABr3 solid phase is stored in the meso-sized pores of CMK-3, guaranteeing charge transfer and further suppressing cross-diffusion in the cell. Moreover, electrodeposition of zinc is favorable with the TPABr additive, which solves the stability issue of the zinc anode. With all these improvements, we are allowed to study the long-term reversibility of the zinc-bromine redox couple in our simplified static cell. In such a device with exactly the same configuration as the Li-ion batteries, regular porous glass fiber is used as the separator without treatment.
Figure 5A shows the long-term cycling stability of the battery with CMK-3 electrode at 5,000 mA g−1 (15 mA cm−2). The initial specific capacity is 324 mAh g−1 (1.0 mAh cm−2), which decreases to 240 mAh g−1 (0.7 mAh cm−2) after 1,000 cycles and 205 mAh g−1 (0.6 mAh cm−2) after 11,000 cycles. The capacity degradation rate is 0.026% per cycle at the first 1,000 cycles, and for the remaining 10,000 cycles it is as low as 0.001% per cycle. The battery demonstrates a high coulombic efficiency of 99.9% and energy efficiencies of 87%–92%, respectively. We notice the energy efficiency increases with the decrease of the current density, as shown in Figure S16. The cell at 500 mA g−1 (1.5 mA cm−2) current density has an energy efficiency of 94%, which is superior to that of the Zn-Br2 flow battery and is comparable with the Li-ion technology (Biswas et al., 2017; Nitta et al., 2015).
Figure 5.
Electrochemical Performances of the Zinc-Bromine Static Batteries with the CMK-3 Electrode
(A) Long-term cycling stability at 5,000 mA g−1 (15 mA cm−2).
(B) Cycling performance of the battery with Cu foil anode at 5,000 mA g−1 (15 mA cm−2).
(C) Cycling performance of the battery with TPABr3 cathode at 1.27 mA cm−2.
(D) Comparison of the Ragone plot of the zinc-bromine static battery with several standard devices: electrochemical capacitors (EC), lead-acid batteries, nickel-metal hydride batteries, lithium-ion batteries, and zinc-bromine flow batteries. Data of these standard devices are cited from the literature (Lin et al., 2015; Shukla et al., 2001).
Benefiting from the high stripping/plating efficiency of zinc in the electrolyte with TPABr additive, the static battery we proposed can even cycle stably without a zinc anode. A piece of Cu foil was used for Zn electrodeposition in a full battery. The discharge cutoff voltage was set at 1.1 V to avoid the plating/stripping of Cu. Remarkably, the battery delivers a comparably high initial specific capacity of 322 mAh g−1 (1.0 mAh cm−2). A specific capacity of 218 mAh g−1 (0.7 mAh cm−2) is maintained after 500 cycles with a high coulombic efficiency of 99.5% and energy efficiencies of 87%–92% (Figure 5B). The corresponding charge and discharge voltage profiles are shown in the Figure S17.
The aqueous zinc-bromine static battery represents a safe battery technology that could bear extensive destruction, such as cutting with scissors. There is no fire ignition or smoking during the destruction; instead, the output voltage is well maintained, as visually demonstrated by the Video S1.
We note the solubility of the electrolyte and the amount of electrolyte would affect the practical energy density. However, we here prove that using TPABr3 as the active material in the cathode (the complexed solid form of bromine during charge) could potentially solve the solubility dependency issue of the TPABr + ZnBr2 electrolyte. As shown in Figure 5C, the battery demonstrates a high specific capacity of 90 mAh g−1, or 6.3 mAh cm−2, based on the total mass of the cathode (weight of TPABr3, CMK-3, super P, and PVDF), together with a high coulombic efficiency of 99.5% and energy efficiency of 90% at a current density of 1.27 mA/cm2. It is worth noting that the areal capacity of this battery is superior to state-of-the-art lithium ion batteries (6.3 versus 4 mAh cm−2) and is much higher than the capacity delivered by the TPABr + ZnBr2 electrolyte (2.7 mAh cm−2).
The Ragone plot in Figure 5D shows the comparison of the zinc-bromine static battery with several other standard devices (Lin et al., 2015; Shukla et al., 2001). The power density and energy density of the zinc-bromine static battery is based on the total mass of the cathode (CMK-3, super P, and PVDF) and the active materials in electrolyte (ZnBr2 and TPABr). The zinc-bromine static battery delivers a high energy density of 142 Wh kg−1 at a power density of 150 W kg−1. Impressively, even at an ultrahigh power density of 13 kW kg−1 (exceeding the maximum power density of electrochemical capacitors), it still retains a high energy density of 99 Wh kg−1. The power performance is much superior to other zinc-based batteries involving metal oxide intercalation electrodes (Kundu et al., 2016; Pan et al., 2016). Table 1 summarizes the key parameters of the energy storage devices involving Zn-Br2 redox couple or based on bromine chemistry (Biswas et al., 2017; Huskinson et al., 2014; Lai et al., 2013; Yoo et al., 2017; Yu et al., 2020). These batteries, mainly run with the “flowing electrolyte” concept and are plugged with high cell polarization and complicated system design. They are challenged by the low energy efficiencies and high capital costs, which are key parameters for large-scale energy storage. In contrast, the zinc-bromine static battery delivers a higher energy density, power density, energy efficiency, and longer cycling life. Overall, these results demonstrate that the zinc-bromine redox couple in a simple cell configuration as the Li-ion batteries can deliver stable long-term cycling performance with high energy as well as power densities.
Table 1.
Comparison of Different Energy Storage Devices Involving Zn-Br2 Redox Couple or Based on Bromine Chemistry
| Battery Type | Maximum Energy Density (Wh kg−1) | Maximum Power Density (W kg−1) | Coulombic Efficiency (%) | Energy Efficiency (%) | Cycling Life | Reference |
|---|---|---|---|---|---|---|
| Zn-Br2 (flow) | 135 | NA | 95 | 75 | 1,000 | (Biswas et al., 2017) |
| Zn-Br2 (single) | NA | NA | 92 | 82 | 70 | (Lai et al., 2013) |
| Zn-Br2 (supercapattery) | 90 | 3,100 | 99 | NA | 7,000 | (Yu et al., 2020) |
| EV-Br2 hybrid supercapacitor | 64 | 3,000 | 97 | 84 | 7,000 | (Yoo et al., 2017) |
| AQDS-Br2 flow battery | 50 | NA | 95 | NA | 15 | (Huskinson et al., 2014) |
| Zn-Br2 (static) | 142 | 13,000 | 99.9 | 94 | 11,000 | This work |
For comparison, all energy densities were converted using the total weight of the positive, negative electrodes and the active material mass.
Discussion
The zinc-bromine chemistry is promising for large-scale energy storage, as demonstrated by the commercialized Zn-Br2 flow battery in the past decades. However, the complicated system and the resulted high capital costs of the Zn-Br2 flow battery made it not superior to the current Li-ion technology. We proposed a revolutionary battery configuration to demonstrate the advantages of the zinc-bromine redox couple. In such a simplified module, all the auxiliary components such as the microporous polyolefin or Daramic membrane and the electrolyte flowing system for flow batteries are eliminated. Instead, the Zn-Br2 static battery has a regular sandwich-type configuration with the readily available glass fiber as the separator, which is as simple as a typical Li-ion battery. This is enabled by regulating the active Br2/Br3- species in the battery from a highly soluble phase to solid phase by a multifunctional additive. The TPABr additive in electrolyte converts the soluble Br2/Br3- species by reversible complexation. By the combination with a highly porous CMK-3 electrode, the cross-diffusion and self-discharge are fundamentally resolved. The aforementioned auxiliary components are thus excluded while all the advantages of the Zn-Br2 redox couple are well maintained. Although the long-term cycling of the zinc metal-based batteries is challenged by the dendritic growth on Zinc anode, it is simultaneously overcome by the multifunctional TPABr additive, which shows favorable interface for zinc electrodeposition toward non-dendrite growth. The Zn-Br2 static battery we proposed demonstrates a high specific energy of 142 Wh kg−1 at 500 mA g−1 (equivalent to 150 W kg−1) with a high energy efficiency of 94%. In particular, the battery shows an ultra-stable cycling life for over 11,000 cycles with minimum self-discharge rate.
Given the fact that all materials in the battery are readily available and inexpensive, the static battery is anticipated to have a dramatic cutoff of the capital costs compared with the flow batteries. Moreover, the Zn-Br2 static battery has a similar configuration as the Li-ion batteries but all of the components are not sensitive to moisture and oxygen. Dry rooms or other special environments are not required during cell assembly, further simplifying the scale-up production. Our static battery configuration offers a cost-effective, easy fabrication path for spreading the Zn-Br2 battery to practical application.
Limitations of the Study
The zinc-bromine static battery we proposed here still suffers from little rate of self-discharge (4% per 24 h), which reflects the conversion of the soluble Br2/Br3- species to the solid TPABr3 phase is not complete. Further in-depth study about the complexing chemistry and its kinetics might afford a more efficient bromine complexing agent. In addition, the performance of the static cells was studied by using Swagelok cells instead of pouch cells. Thus, design of a pouch cell with Ah capacity is essential to evaluate the practical potential of this technology.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xiao Liang (xliang@hnu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets/code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the financial support provided by National Key Research and Development Program, China (No. 2019YFA0210600), the State Grid Shanghai Municipal Electric Power Company (No. 520970190004), and the Innovative Research Groups of Hunan Province (No. 2019JJ10001).
Author Contributions
L.G. and X.L. designed this study. L.G. prepared the materials and carried out the electrochemical experiments. L.G. conducted the Raman analyses and SEM studies. Z.L. conducted the XRD measurements. Y.Z. conducted the BET analyses. L.G. carried out data processing and prepared the figures. L.G. and X.L. wrote the manuscript. All authors contributed to the scientific discussion.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101348.
Supplemental Information
References
- Armand M., Tarascon J.M. Building better batteries. Nature. 2008;451:652–657. doi: 10.1038/451652a. [DOI] [PubMed] [Google Scholar]
- Biswas S., Senju A., Mohr R., Hodson T., Karthikeyan N., Knehr K.W., Hsieh A.G., Yang X.F., Koel B.E., Steingart D.A. Minimal architecture zinc-bromine battery for low cost electrochemical energy storage. Energy Environ. Sci. 2017;10:114–120. [Google Scholar]
- Chen X., Hou T.Z., Persson K.A., Zhang Q. Combining theory and experiment in lithium-sulfur batteries: current progress and future perspectives. Mater. Today. 2019;22:142–158. [Google Scholar]
- Chen X., Rickard M.A., Hull J.W., Jr., Zheng C., Leugers A., Simoncic P. Raman spectroscopic investigation of tetraethylammonium polybromides. Inorg. Chem. 2010;49:8684–8689. doi: 10.1021/ic100869r. [DOI] [PubMed] [Google Scholar]
- Chun S.E., Evanko B., Wang X.F., Vonlanthen D., Ji X.L., Stucky G.D., Boettcher S.W. Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nat. Commun. 2015;6:7818. doi: 10.1038/ncomms8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling R.M., Gallagher K.G., Kowalski J.A., Ha S., Brushett F.R. Pathways to low-cost electrochemical energy storage: a comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 2014;7:3459–3477. [Google Scholar]
- Ding F., Xu W., Graff G.L., Zhang J., Sushko M.L., Chen X.L., Shao Y.Y., Engelhard M.H., Nie Z.M., Xiao J. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013;135:4450–4456. doi: 10.1021/ja312241y. [DOI] [PubMed] [Google Scholar]
- Dunn B., Kamath H., Tarascon J.M. Electrical energy storage for the grid: a battery of choices. Science. 2011;334:928–935. doi: 10.1126/science.1212741. [DOI] [PubMed] [Google Scholar]
- Evanko B., Yoo S.J., Lipton J., Chun S.E., Moskovits M., Ji X.L., Boettcher S.W., Stucky G.D. Stackable bipolar pouch cells with corrosion-resistant current collectors enable high-power aqueous electrochemical energy storage. Energy Environ. Sci. 2018;11:2865–2875. [Google Scholar]
- Gur T.M. Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018;11:2696–2767. [Google Scholar]
- Haxhimali T., Karma A., Gonzales F., Rappaz M. Orientation selection in dendritic evolution. Nat. Mater. 2006;5:660–664. doi: 10.1038/nmat1693. [DOI] [PubMed] [Google Scholar]
- Hu B., DeBruler C., Rhodes Z., Liu T.L. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage. J. Am. Chem. Soc. 2017;139:1207–1214. doi: 10.1021/jacs.6b10984. [DOI] [PubMed] [Google Scholar]
- Huskinson B., Marshak M.P., Suh C., Er S., Gerhardt M.R., Galvin C.J., Chen X.D., Aspuru-Guzik A., Gordon R.G., Aziz M.J. A metal-free organic-inorganic aqueous flow battery. Nature. 2014;505:195. doi: 10.1038/nature12909. [DOI] [PubMed] [Google Scholar]
- Ke X.Y., Prahl J.M., Alexander J.I.D., Wainright J.S., Zawodzinski T.A., Savinell R.F. Rechargeable redox flow batteries: flow fields, stacks and design considerations. Chem. Soc. Rev. 2018;47:8721–8743. doi: 10.1039/c8cs00072g. [DOI] [PubMed] [Google Scholar]
- Kundu D., Adams B.D., Duffort V., Vajargah S.H., Nazar L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy. 2016;1:8. [Google Scholar]
- Lai Q.Z., Zhang H.M., Li X.F., Zhang L.Q., Cheng Y.H. A novel single flow zinc-bromine battery with improved energy density. J. Power Sources. 2013;235:1–4. [Google Scholar]
- Lee J.H., Byun Y., Jeong G.H., Choi C., Kwen J., Kim R., Kim I.H., Kim S.O., Kim H.T.( High-energy efficiency membraneless flowless Zn-Br battery: utilizing the electrochemical-chemical growth of polybromides. Adv. Mater. 2019;31:1904524. doi: 10.1002/adma.201904524. [DOI] [PubMed] [Google Scholar]
- Li G.X., Liu Z., Huang Q.Q., Gao Y., Regula M., Wang D.W., Chen L.Q., Wang D.H. Stable metal battery anodes enabled by polyethylenimine sponge hosts by way of electrokinetic effects. Nat. Energy. 2018;3:1076–1083. [Google Scholar]
- Li L., Basu S., Wang Y.P., Chen Z.Z., Hundekar P., Wang B.W., Shi J., Shi Y.F., Narayanan S., Koratkar N. Self-heating-induced healing of lithium dendrites. Science. 2018;359:1513–1516. doi: 10.1126/science.aap8787. [DOI] [PubMed] [Google Scholar]
- Li Y.G., Dai H.J. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014;43:5257–5275. doi: 10.1039/c4cs00015c. [DOI] [PubMed] [Google Scholar]
- Lim H., Lackner A., Knechtli R. Zinc-bromine secondary battery. J. Electrochem. Soc. 1977;124:1154–1157. [Google Scholar]
- Lin T.Q., Chen I.W., Liu F.X., Yang C.Y., Bi H., Xu F.F., Huang F.Q. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science. 2015;350:1508–1513. doi: 10.1126/science.aab3798. [DOI] [PubMed] [Google Scholar]
- Nishiyama N., Zheng T., Yamane Y., Egashira Y., Ueyama K. Microporous carbons prepared from cationic surfactant-resorcinol/formaldehyde composites. Carbon. 2005;43:269–274. [Google Scholar]
- Nitta N., Wu F.X., Lee J.T., Yushin G. Li-ion battery materials: present and future. Mater. Today. 2015;18:252–264. [Google Scholar]
- Noack J., Roznyatovskaya N., Herr T., Fischer P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 2015;54:9775–9808. doi: 10.1002/anie.201410823. [DOI] [PubMed] [Google Scholar]
- Orazem M.E., Tribollet B. John Wiley & Sons; 2017. Electrochemical Impedance Spectroscopy. [Google Scholar]
- Palacin M.R., de Guibert A. Why do batteries fail? Science. 2016;351:7. doi: 10.1126/science.1253292. [DOI] [PubMed] [Google Scholar]
- Pan H.L., Shao Y.Y., Yan P.F., Cheng Y.W., Han K.S., Nie Z.M., Wang C.M., Yang J.H., Li X.L., Bhattacharya P. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy. 2016;1:7. [Google Scholar]
- Pang Q., Liang X., Kwok C.Y., Nazar L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy. 2016;1:11. [Google Scholar]
- Park M., Ryu J., Wang W., Cho J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2017;2:18. [Google Scholar]
- Parker J.F., Chervin C.N., Nelson E.S., Rolison D.R., Long J.W. Wiring zinc in three dimensions re-writes battery performance-dendrite-free cycling. Energy Environ. Sci. 2014;7:1117–1124. [Google Scholar]
- Scott B., Willett R.D. A copper(II) bromide dimer system exhibiting piezochromic and thermochromic properties: the crystal structure and electronic spectroscopy of the two room-temperature phases of bis(tetrapropylammonium) hexabromodicuprate(II) J. Am. Chem. Soc. 1991;113:5253–5258. [Google Scholar]
- Shukla A.K., Arico A.S., Antonucci V. An appraisal of electric automobile power sources. Renew. Sust. Energ. Rev. 2001;5:137–155. [Google Scholar]
- Soloveichik G.L. Flow batteries: current status and trends. Chem. Rev. 2015;115:11533–11558. doi: 10.1021/cr500720t. [DOI] [PubMed] [Google Scholar]
- Tan P., Chen B., Xu H.R., Zhang H.C., Cai W.Z., Ni M., Liu M.L., Shao Z.P. Flexible Zn- and Li-air batteries: recent advances, challenges, and future perspectives. Energy Environ. Sci. 2017;10:2056–2080. [Google Scholar]
- Tarascon J.M., Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414:359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- Tikekar M.D., Choudhury S., Tu Z.Y., Archer L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy. 2016;1:1–7. [Google Scholar]
- Turcheniuk K., Bondarev D., Singhal V., Yushin G. Ten years left to redesign lithium-ion batteries. Nature. 2018;559:467–470. doi: 10.1038/d41586-018-05752-3. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Lai Q.Z., Xu P.C., Zheng D.Y., Li X.F., Zhang H.M. Cage-like porous carbon with superhigh activity and Br-2-complex-entrapping capability for bromine-based flow batteries. Adv. Mater. 2017;29:6. doi: 10.1002/adma.201605815. [DOI] [PubMed] [Google Scholar]
- Wang F., Borodin O., Gao T., Fan X.L., Sun W., Han F.D., Faraone A., Dura J.A., Xu K., Wang C.S. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018;17:543. doi: 10.1038/s41563-018-0063-z. [DOI] [PubMed] [Google Scholar]
- Wang K.L., Pei P.C., Ma Z., Chen H.C., Xu H.C., Chen D.F., Wang X.Z. Dendrite growth in the recharging process of zinc-air batteries. J. Mater. Chem. A. 2015;3:22648–22655. [Google Scholar]
- Winsberg J., Hagemann T., Janoschka T., Hager M.D., Schubert U.S. Redox-flow batteries: from metals to organic redox-active materials. Angew. Chem. Int. Ed. 2017;56:686–711. doi: 10.1002/anie.201604925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M., Barnett B., Xu K. Before Li ion batteries. Chem. Rev. 2018;118:11433–11456. doi: 10.1021/acs.chemrev.8b00422. [DOI] [PubMed] [Google Scholar]
- Wu Y.T., Huang P.W., Howe J.D., Yan Y., Martinez J., Marianchuk A., Zhang Y.M., Chen H., Liu N. In operando visualization of the electrochemical formation of liquid polybromide microdroplets. Angew. Chem. Int. Ed. 2019;58:15228–15234. doi: 10.1002/anie.201906980. [DOI] [PubMed] [Google Scholar]
- Xie C.X., Duan Y.Q., Xu W.B., Zhang H.M., Li X.F. A low-cost neutral zinc-iron flow battery with high energy density for stationary energy storage. Angew. Chem. Int. Ed. 2017;56:14953–14957. doi: 10.1002/anie.201708664. [DOI] [PubMed] [Google Scholar]
- Yang Z.G., Zhang J.L., Kintner-Meyer M.C.W., Lu X.C., Choi D.W., Lemmon J.P., Liu J. Electrochemical energy storage for green grid. Chem. Rev. 2011;111:3577–3613. doi: 10.1021/cr100290v. [DOI] [PubMed] [Google Scholar]
- Yoo S.J., Evanko B., Wang X., Romelczyk M., Taylor A., Ji X., Boettcher S.W., Stucky G.D. Fundamentally addressing bromine storage through reversible solid-state confinement in porous carbon electrodes: design of a high-performance dual-redox electrochemical capacitor. J. Am. Chem. Soc. 2017;139:9985–9993. doi: 10.1021/jacs.7b04603. [DOI] [PubMed] [Google Scholar]
- Yu F., Zhang C., Wang F., Gu Y., Zhang P., Waclawik E., Du A., Ostrikov K., Wang H. A zinc bromine “supercapattery” system combining triple functions of capacitive, pseudocapacitive and battery-type charge storage. Mater. Horiz. 2020;7:495–503. [Google Scholar]
- Yuan Z.Z., Liu X.Q., Xu W.B., Duan Y.Q., Zhang H.M., Li X.F. Negatively charged nanoporous membrane for a dendrite-free alkaline zinc-based flow battery with long cycle life. Nat. Commun. 2018;9:11. doi: 10.1038/s41467-018-06209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y., Chen J.X., Peng S., Peng S.Y., Zhang Z.Z., Tong Y.X., Miller P.W., Yan X.P. Emerging porous materials in confined spaces: from chromatographic applications to flow chemistry. Chem. Soc. Rev. 2019;48:2566–2595. doi: 10.1039/c8cs00657a. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Ding Y., Li Y.T., Peng L.L., Byon H.R., Goodenough J.B., Yu G.H. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015;44:7968–7996. doi: 10.1039/c5cs00289c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets/code.