Abstract
Background
Invasive fungal infections (IFIs) are life‐threatening opportunistic infections that occur in immunocompromised or critically ill people. Early detection and treatment of IFIs is essential to reduce morbidity and mortality in these populations. (1→3)‐β‐D‐glucan (BDG) is a component of the fungal cell wall that can be detected in the serum of infected individuals. The serum BDG test is a way to quickly detect these infections and initiate treatment before they become life‐threatening. Five different versions of the BDG test are commercially available: Fungitell, Glucatell, Wako, Fungitec‐G, and Dynamiker Fungus.
Objectives
To compare the diagnostic accuracy of commercially available tests for serum BDG to detect selected invasive fungal infections (IFIs) among immunocompromised or critically ill people.
Search methods
We searched MEDLINE (via Ovid) and Embase (via Ovid) up to 26 June 2019. We used SCOPUS to perform a forward and backward citation search of relevant articles. We placed no restriction on language or study design.
Selection criteria
We included all references published on or after 1995, which is when the first commercial BDG assays became available. We considered published, peer‐reviewed studies on the diagnostic test accuracy of BDG for diagnosis of fungal infections in immunocompromised people or people in intensive care that used the European Organization for Research and Treatment of Cancer (EORTC) criteria or equivalent as a reference standard. We considered all study designs (case‐control, prospective consecutive cohort, and retrospective cohort studies). We excluded case studies and studies with fewer than ten participants. We also excluded animal and laboratory studies. We excluded meeting abstracts because they provided insufficient information.
Data collection and analysis
We followed the standard procedures outlined in the Cochrane Handbook for Diagnostic Test Accuracy Reviews. Two review authors independently screened studies, extracted data, and performed a quality assessment for each study. For each study, we created a 2 × 2 matrix and calculated sensitivity and specificity, as well as a 95% confidence interval (CI). We evaluated the quality of included studies using the Quality Assessment of Studies of Diagnostic Accuracy‐Revised (QUADAS‐2). We were unable to perform a meta‐analysis due to considerable variation between studies, with the exception of Candida, so we have provided descriptive statistics such as receiver operating characteristics (ROCs) and forest plots by test brand to show variation in study results.
Main results
We included in the review 49 studies with a total of 6244 participants. About half of these studies (24/49; 49%) were conducted with people who had cancer or hematologic malignancies. Most studies (36/49; 73%) focused on the Fungitell BDG test. This was followed by Glucatell (5 studies; 10%), Wako (3 studies; 6%), Fungitec‐G (3 studies; 6%), and Dynamiker (2 studies; 4%). About three‐quarters of studies (79%) utilized either a prospective or a retrospective consecutive study design; the remainder used a case‐control design.
Based on the manufacturer's recommended cut‐off levels for the Fungitell test, sensitivity ranged from 27% to 100%, and specificity from 0% to 100%. For the Glucatell assay, sensitivity ranged from 50% to 92%, and specificity ranged from 41% to 94%. Limited studies have used the Dynamiker, Wako, and Fungitec‐G assays, but individual sensitivities and specificities ranged from 50% to 88%, and from 60% to 100%, respectively. Results show considerable differences between studies, even by manufacturer, which prevented a formal meta‐analysis. Most studies (32/49; 65%) had no reported high risk of bias in any of the QUADAS‐2 domains. The QUADAS‐2 domains that had higher risk of bias included participant selection and flow and timing.
Authors' conclusions
We noted considerable heterogeneity between studies, and these differences precluded a formal meta‐analysis. Because of wide variation in the results, it is not possible to estimate the diagnostic accuracy of the BDG test in specific settings. Future studies estimating the accuracy of BDG tests should be linked to the way the test is used in clinical practice and should clearly describe the sampling protocol and the relationship of time of testing to time of diagnosis.
Plain language summary
Measurement of β‐D‐glucans to detect invasive fungal infection in immunocompromised people
Why is improving the diagnosis of invasive fungal infections important? Fungal infections occur in people who are unable to fight infection, and these infections can be life‐threatening in this group of people. Fungal infections are difficult to diagnose. Failure to recognize a fungal infection when it is present (a false‐negative test result) leads to delayed treatment and poorer outcomes. An incorrect diagnosis of infection (a false‐positive result) may result in wasted resources and unnecessary investigation and treatment.
What is the aim of this review? The aim of this review is to find out how accurate a blood test is for diagnosis of fungal infections in people who are unable to fight infection. Review authors included 49 studies to answer this question.
What was studied in this review? Five kinds of blood tests were compared. All of these tests use similar biochemical methods to detect the presence of a sugar molecule (β‐D‐glucan) that is a component of the fungal cell wall. This molecule does not normally occur in blood, so its detection indicates that fungi are present. The tests require a blood sample, which is then sent to a laboratory for analysis. Diagnosis of fungal infections is difficult, and the diagnosis is often made only after the disease has advanced. Blood tests can provide an earlier diagnosis, so they would offer an advantage over current methods.
What are the main results of the review? This review included studies of 6244 people who were at risk of getting fungal infections. Study results show that accuracy varied widely across studies. The variation was so great that it was not possible to obtain a reliable estimate of the accuracy of the various tests.
How reliable are results of the studies in this review? In the included studies, the diagnosis of invasive fungal infection was made using criteria developed by the European Organization for Research and Treatment of Cancer (EORTC)*. The EORTC criteria are considered reliable and the studies were generally well conducted, so it is likely that the reference diagnoses were accurate. Accuracy of blood tests for invasive fungal infections varied widely. Some studies found that the blood test was accurate, but others found that the blood test was not very accurate. The reason for this variation is not understood.
*The EORTC criteria provide the reference diagnosis. Results of the blood test are compared to the reference diagnosis.
Who do the results of this review apply to? Most included studies were performed at academic medical centers or public hospitals in the United States, Germany, and Italy. The most common underlying conditions were cancer (47%) and admission to intensive care (33%). A majority of participants were adults. The overall prevalence of invasive fungal infection was 28%.
What are the implications of this review? Accuracy of the diagnosis varied widely across studies. It is not clear whether testing can accurately detect invasive fungal infections. Testing accurately detects disease in some studies, but in others it does not. The reasons for the variation in accuracy are not understood.
How up‐to‐date is this review? The review authors searched for and reviewed studies published up to June 2019.
Summary of findings
Summary of findings 1. Summary of findings.
| Participants/Populations: immunocompromised people at risk for invasive fungal infections | |||||
| Prior testing: none | |||||
| Settings: hospital setting | |||||
| Index test: commercially available serum BDG test | |||||
|
Importance: test needed to accurately detect fungal infections in susceptible people at an early enough stage to facilitate antifungal treatment | |||||
| Reference standard: EORTC/MSG criteria, or by microscopy or autopsy | |||||
| Studies: 49 studies with 6244 participants | |||||
| 1. Test assay | |||||
| Test/Subgroup |
No. of participants (studies) |
Overall sensitivity (range) |
Overall specificity (range) |
Implications | Quality and comments |
| Fungitell | 4316 (36) |
27% to 100% | 0 to 100% | Wide variation in sensitivity and specificity. Summary estimates would not be meaningful | |
| Glucatell | 957 (5) |
50% to 92% | 41% to 94% | Wide variation in sensitivity and specificity. Summary estimates would not be meaningful | |
| Wako | 420 (3) |
50% to 86% | 89% to 100% | Insufficient number of studies for meta‐analysis | |
| Fungitec‐G | 353 (3) |
67% to 88% | 60% to 85% | Too few studies for meta‐analysis | |
| Dynamiker Fungus | 198 (2) |
64% to 81% | 78% to 80% | Too few studies for meta‐analysis | |
| 2. Fungal organism | |||||
| Test/Subgroup |
No. of participants (studies) |
Sensitivity estimate (95% CI) |
Specificity estimate (95% CI) |
Implications | Quality and comments |
| Candida | 1185 (10) |
81% (75% to 86%) |
64% (56% to 72%) |
Results are more homogeneous for Candida testing than for all fungi |
|
BDG: beta‐D‐glucan test; CI: confidence interval; EORTC/MSG: European Organization for Research and Treatment of Cancer Mycoses Study Group.
Background
Target condition being diagnosed
Invasive fungal infection (IFI) is a major cause of morbidity and mortality in immunosuppressed and critically ill people (Lemonovich 2018; Person 2010). Prompt diagnosis is important because early initiation of appropriate antifungal therapy improves patient outcomes (Chamilos 2008; Garey 2006; Morrell 2005; von Eiff 1995). Diagnosis of IFI is challenging because the standard methods of clinical diagnosis (e.g. clinical signs and symptoms, host risk assessment, physical examination, radiography) are not specific to IFI. In addition, traditional microbiological methods often have limited clinical utility because cultures are frequently negative or become positive only in advanced stages of infection (Clancy 2013). Histopathologic examination of infected tissue has been the historic gold standard, but invasive testing may not be feasible in unstable participants or in those with underlying coagulopathy. Although composite definitions for IFI have been developed by the European Organization for Research and Treatment of Cancer Mycoses Study Group (EORTC/MSG), these definitions are best suited for research purposes (De Pauw 2008). The EORTC/MSG diagnostic categories of IFI include proven, probable, and possible disease.
Current strategies for prevention and management of IFI include antifungal prophylaxis, pre‐emptive therapy, empiric treatment, and treatment of established infection (Leroux 2013). Universal prophylaxis is effective and logistically easy, but the medications can have toxic effects, can potentially promote antimicrobial resistance, and are expensive. Empiric therapy based on symptoms or treatment of established IFI potentially delays initiation of potentially life‐saving therapy. In contrast, pre‐emptive therapy is a more selective approach in which people are sequentially monitored and treatment is based on detection of laboratory biomarkers in blood, often before clinical signs or symptoms of an IFI are apparent. Pre‐emptive approaches are designed to identify the highest‐risk people who are most likely to benefit from early antifungal therapy. Examples of fungal biomarkers include circulating fungal DNA and cell wall components such as galactomannan (GM), glucuronoxylomannan, mannan, and (1→3)‐beta‐D‐glucan (BDG). Tests designed to detect these markers may be deployed as part of a pre‐emptive treatment strategy or may be used to facilitate selection of empiric treatment for symptomatic at‐risk people.
Index test(s)
Non‐invasive, non‐culture‐based methods for diagnosing invasive fungal disease have the potential for significant clinical utility (Powers‐Fletcher 2016). BDG is a cell wall polysaccharide found in a wide variety of medically important fungi including Candida species (spp) (Aspergillus spp and Pneumocystis jirovecii; important exceptions are Mucorales, Cryptococcus spp, and the yeast form of Blastomyces (Wright 2011)). Assays designed to detect BDG in human serum have been used both as an adjunct for diagnosis of IFI and for serial surveillance during periods of risk. Commercially available assays include the Fungitell and Glucatell assays (Associates of Cape Code, Falmouth, MA, USA), which are used in America and in Europe, as well as the Fungitec‐G assay (Seikagaku Kogyo Corporation, Tokyo, Japan) and the Wako test (Wako Pure Chemical Industries, Osaka, Japan), both of which are used in Japan. The Dynamiker Fungus assay (Dynamiker Biotechnology Ltd, Tianjin, China) is a new test that was recently developed in China.
These assays are based on the ability of the BDG molecule to induce clot formation in the hemolymph of horseshoe crabs. BDG activates Factor G, which is a serine protease in the horseshoe crab coagulation cascade. Activated Factor G then converts an inactive proclotting enzyme to its active form, which, in turn, cleaves an artificial substrate that can be detected. The assays differ in the substrate used for detection. The Fungitell and Glucatell assays use a chemiluminescent method. The Glucatell test differs from the Fungitell test in that the Glucatell reagent is processed to eliminate Factor C. This makes the Glucatell test more specific for BDG linkages. The Glucatell reagent does not react to other polysaccharides including beta‐glucans with other glycosidic linkages. For the other assays, Dynamiker Fungus uses a spectrophotometric method, the Wako assay is a turbidometric method, and Fungitec‐G is a colorimetric method. Each of these tests uses a different interpretive cut‐off value. In the Fungitell and Glucatell assays, a value of 60 pg/mL or less is negative, a value of 60 to 80 pg/mL is equivocal, and a value of 80 pg/mL or more is positive. For the Fungitec‐G assay, a value greater than 20 pg/mL is considered positive, whereas for Wako, it is 11 pg/mL. The Dynamiker Fungus test considers values above 95 pg/mL as positive. These differences may be due to the fact that the reagents are obtained from different genera of horseshoe crabs (Fungitell reagents are extracted from Limulus polyphemus, whereas Fungitec and Wako reagents are extracted from Tachypleus tridentatus).
Studies vary in the criteria used for BDG positivity. For example, a single positive BDG result may be sufficient to classify a person as "BDG positive" in some studies, whereas other studies may use more stringent criteria such as two consecutive positive tests, or two positive tests within a specified time period. Similarly, studies use different sampling plans, which may affect test performance. Some studies may use a single sample, whereas others may use a prolonged sampling regimen (e.g. twice‐a‐week sampling for several weeks).
Clinical pathway
Presentation
The fungi capable of causing invasive disease in humans are a diverse group of eukaryotic microorganisms including yeasts, molds, and dimorphic fungi. Candida and Aspergillus are the pathogens most commonly diagnosed after solid organ transplantation or critical care (Pappas 2010), and Aspergillus and other filamentous fungi predominate after hematopoietic stem cell transplantation or as a complication of cytotoxic chemotherapy for hematologic malignancy (Kontoyiannis 2010; Neofytos 2009). In addition, Pneumocystisjirovecii remains an important opportunistic pathogen that affects people with AIDS and those receiving cytotoxic or immunosuppressive therapy. Clinical signs and symptoms of IFI vary widely. The clinical presentation of IFI varies widely according to the infecting pathogen, the overall net state of immunosuppression (i.e. the host), and the site and severity of infection. Invasive candidiasis comprises a spectrum of diseases including bloodstream infection and deep‐seated infection (e.g. intra‐abdominal abscess), which may occur independently or concurrently. The filamentous fungi typically present with pulmonary or sino‐cerebral disease. Pneumonia is the most common manifestation of Pneumocystis.
Standard diagnostic practice
In general, the current approach to IFI diagnosis combines a variety of complementary testing modalities. Diagnostic imaging helps clinicians to identify potential sites of infection. Cultures of blood, body fluids, and/or tissue are performed in combination with molecular tests and serum fungal biomarkers in an attempt to detect and identify fungi. Use of targeted imaging may help to guide biopsy sampling of infected tissue for histopathology.
Alternative test(s)
Classical methods of diagnosis include direct stains for fungi (i.e. calcofluor white, cytology, or histopathology) and fungal culture. Despite availability of a variety of test modalities, the clinical utility of this routine testing is often limited. For example, cultures are slow and relatively insensitive. Positive results, however, are useful for definitive organism identification and antifungal susceptibility testing. Cytology and calcofluor white stains applied to body fluid also lack sensitivity. Furthermore, deciphering colonization from invasive disease can be extremely difficult when samples are obtained from non‐sterile body sites such as the respiratory tract. Visualizing fungal elements in tissue remains the diagnostic gold standard for IFI, but invasive testing may not be feasible for critically ill or coagulopathic people. Additionally, biopsy results may be affected by sampling error, and current staining techniques are neither genus‐ nor species‐specific. This level of organism discrimination, however, is essential for selection of optimal antifungal therapy.
Detection of fungal biomarkers including nucleic acid and cell wall components helps support the diagnosis of IFI. Rapid polymerase chain reaction (PCR) techniques targeting fungi have been widely applied in clinical practice (Arvanitis 2014; Avni 2011; Fan 2013; Lu 2011a; Mengoli 2009; Sun 2011). Unfortunately, laboratory‐developed PCR tests lack standardization, and commercial assays are not widely available. The Candida T2 assay (T2 Biosystems, Lexington, MA, USA) is a rapid and accurate test for the detection of Candida DNA directly in whole blood (Tang 2019). Unfortunately, this test targets only the five most common Candida species and requires expensive instrumentation/reagents. Detecting mannan antigen and anti‐mannan antibodies also has potential utility for the diagnosis of invasive candidiasis, but commercial assays are mainly limited to European markets (Mikulska 2010). Last, lateral flow assays for Aspergillus GM have been developed for use with serum and bronchoalveolar lavage samples (Mercier 2019; Verdaguer 2007). A potential benefit of antigens like GM and BDG is that these polysaccharides can be detected non‐invasively in blood at an early stage of infection, whereas release of fungal DNA may be negligible in initial phases of the disease (Monique 2006). Alternatively, limitations of the Aspergillus GM test include limited sensitivity in non‐neutropenic patient populations and potential cross‐reactivity with closely related fungi or other antigenically similar substances (Demiraslan 2017; Verweij 2006; Viscoli 2004).
Rationale
Here we perform an updated review of the BDG literature with a focus on immunocompromised or critically ill people. BDG suffers from many of the same limitations as the Aspergillus GM test. Sensitivity may vary by population and organism type, and false positives are thought to result from cross‐reacting substances in certain medications or materials, or possibly in bacteria (Marty 2006; Tran 2016; Wright 2011). Thus, it is important to understand the diagnostic performance of BDG across a variety of at‐risk populations and testing strategies. Our objective was to provide summary estimates of the diagnostic performance of BDG that could be used to inform future guideline updates and serve as a benchmark for emerging diagnostics tests such as PCR and the Candida T2 assay. Both BDG and the Aspergillus GM test results have been incorporated into the revised EORTC/MSG criteria for probable IFI.
Objectives
Primary objective
To compare the diagnostic accuracy of commercially available tests for serum BDG to detect selected invasive fungal infections (IFIs) among immunocompromised or critically ill people.
Secondary objectives
To assess possible sources of heterogeneity that could affect sensitivity and specificity estimates in this study (see Investigations of heterogeneity).
Methods
Criteria for considering studies for this review
Types of studies
Published peer‐reviewed studies that compared the results of BDG tests against a clearly defined reference standard (EORTC criteria or equivalent) for diagnosis of IFI were included in the analysis.
We included the following types of studies.
Retrospective studies in which BDG samples were collected from consecutive people at risk.
Prospective studies in which BDG samples were collected from consecutive people at risk.
Case‐control studies in which controls were people at risk.
We excluded the following types of studies.
Case reports or case series.
Studies reported only as meeting abstracts.
Case‐control studies using healthy controls, due to the high risk of spectrum bias.
Animal studies.
Participants
Study participants included the following categories of immunocompromised people, with results for both the index test and the reference test.
-
Those with cancer, specifically:
patients with hematologic malignancies; those receiving stem cell transplants, chemotherapeutics, or other immunosuppressive drugs; and
patients receiving chemotherapy.
-
Those receiving prolonged immunosuppressive therapy for:
solid organ transplant; or
connective tissue diseases.
-
Individuals with congenital or acquired immune disorders, including:
human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS); or
inherited immune disorders.
People receiving treatment in the intensive care unit (ICU).
There was no restriction on age or comorbidities.
Index tests
We included studies that used any commercially available BDG tests that were approved for clinical use and followed the manufacturer's recommended cut‐off values.
Fungitell (cut‐off: 80 pg/mL).
Glucatell (cut‐off: 80 pg/mL).
Wako (cut‐off: 11 pg/mL).
Fungitec‐G (cut‐off: 20 pg/mL).
Dynamiker Fungus (cut‐off: 95 pg/mL).
Target conditions
The target condition included proven or probable IFI due to Aspergillus or Candida, or other IFIs as defined by EORTC/MSG criteria (De Pauw 2008). It should be noted that EORTC/MSG criteria were developed for people with malignancy and for hematopoietic stem cell transplant recipients; these criteria are not easily generalizable to all risk groups and/or fungal diseases. Therefore, Pneumocystis jirovecii pneumonia (PJP) and Candida studies outside of the cancer population were also included if proven infection was determined by microscopy (Pneumocystis) or by sterile site culture (Candida). People with colonized Candida were considered as non‐cases.
Reference standards
We included studies that used the following reference standards for invasive fungal disease.
Autopsy.
EORTC/MSG criteria from either 2002 or 2008 guidelines (Ascioglu 2002; De Pauw 2008).
Microscopy or sterile site culture for proven PJP or Candida infection, respectively.
The criteria for proven IFI are listed below.
Microscopic analysis of sterile material
Molds: histopathologic, cytopathologic, or direct microscopic examination of a specimen obtained by needle aspiration or biopsy in which hyphae or melanized yeast‐like forms are seen accompanied by evidence of associated tissue damage
Yeast: histopathologic, cytopathologic, or direct microscopic examination of a specimen obtained by needle aspiration or biopsy from a normally sterile site
Culture of sterile material
Molds: recovery of a mold or black yeast by culture obtained by sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with an infectious disease process, excluding bronchoalveolar lavage fluid, a cranial sinus cavity specimen, and urine
Yeast: recovery of a yeast by culture of a sample obtained by a sterile procedure (including a freshly placed drain < 24 hours) from a normally sterile site showing a clinical or radiological abnormality consistent with an infectious disease process
Blood culture
Molds: blood culture that yields a mold in the context of a compatible infectious disease process
Yeast: blood culture that yields yeast or yeast‐like fungi
The criteria for probable IFI include host factors (e.g. receipt of allogeneic stem cell transplant), clinical criteria, and mycologic criteria. As of 2008, the EORTC/MSG mycologic criteria now include biomarker tests such as BDG or GM. This creates a possible source of incorporation bias because the index test (BDG) is sometimes used as part of the reference standard for possible IFI. Therefore, we excluded studies that used BDG as part of the reference standard.
Search methods for identification of studies
Electronic searches
An initial search to identify articles related to the diagnostic accuracy of BDG using the search strategies described in Appendix 1 and Appendix 2 was completed in April 2017. The last update was performed on 26 June 2019.
MEDLINE (R) via Ovid (1946 to June week 3, 2019).
Embase via Ovid (1980 to week 25, 2019).
Because the commercial BDG test was not implemented until 1995, the search was restricted to articles published in 1995 or later. The search was not restricted with respect to language or study design.
We performed an additional electronic search based on the set of potentially relevant studies identified in June 2017 from MEDLINE and Embase. This search was a forward and backward citation search to identify all studies cited by or citing the set of potentially relevant studies. This citation search was performed using SCOPUS on June 6, 2017.
Data collection and analysis
Selection of studies
Two review authors (RLS, SKW) screened the titles and abstracts of all articles to identify potentially relevant studies. Disagreements were resolved by discussion.
Each study in the set of potentially relevant studies was given a full‐text review. An initial abstract form (see Appendix 3) was used to retrieve preliminary information that was used to determine whether the article met the inclusion criteria. Full‐text review was performed independently by two review authors (RLS, SKW). This included information on study design, participant population, sample type, and IFI category (proven, probable, or possible), and whether EORTC/MSG, autopsy, or another method was used as the reference standard. These were reviewed together, and any discrepancies were resolved by discussion between the two review authors. Foreign language articles were assessed by a native speaker with scientific training (but not screened in duplicate) or were translated using Google Translate and reviewed by two review authors (RLS, SKW). The review authors who determined relevance were not blinded to trial authors, publishing journal, or results.
Data extraction and management
Two review authors (SKW, BSW) extracted additional information from the selected studies on the condition (cancer, ICU, organ transplant, etc.), study design (prospective consecutive, retrospective consecutive, or case‐control), sample type (serum, urine, bronchoalveolar lavage fluid, or cerebrospinal fluid), fungal organism (mixed IFI, Candida, Aspergillus, or Pneumocystis jirovecii), and reference standard used (EORTC or study‐specific), using the data abstract form provided in Appendix 4. True‐positive, false‐positive, false‐negative, and true‐negative values were obtained to calculate sensitivity and specificity estimates. Additional information extracted during the full‐text review included use of antifungal agents, sampling protocol, the assay used and the cut‐off value, the number of positive samples needed to constitute a positive test result, and age of the population. All data were recorded, and discrepancies were resolved through discussion or by a third review author (RLS).
Assessment of methodological quality
We assessed study quality using the Quality Assessment of Studies of Diagnostic Accuracy‐Revised (QUADAS‐2) tool (Whiting 2011). Bias was assessed in four domains: participant selection, index test, reference standard, and flow/timing, and applicability was assessed in the first three domains only (participant selection, index test, and reference standard). Both were independently graded as low, high, or unclear quality by two review authors (SKW, BSW), using the interpretations listed in Appendix 5. Discrepancies were then resolved by discussion or were moderated by a third review author (RLS).
Statistical analysis and data synthesis
We transferred data into 2 × 2 matrices to calculate sensitivity and specificity for each study. We used reported values of true positives/negatives and false positives/negatives to calculate sensitivity and specificity. If these values were not reported, we back‐extrapolated using reported sensitivity and specificity values.
Individual study data were presented graphically as forest plots by assay type. Studies were also plotted in receiver operating characteristic (ROC) space. We used the bivariate random‐effects model for meta‐analysis of the pairs of sensitivity and specificity (Reitsma 2005; van Houwelingen 1993). We restricted the analysis to standard cut‐off values recommended by test manufacturers. All statistical analyses were completed using Stata v.14.2 (Stata Corporation, College Station, TX, USA). However, with the exception of studies involving Candida, we were unable to perform a formal meta‐analysis for fungal groups because of high heterogeneity within the data, which prevented estimations of summary accuracy. This diversion from the protocol is explained in the Differences between protocol and review section.
Investigations of heterogeneity
When heterogeneity is present, subgroup analysis can be performed to determine the source. Heterogeneity between studies was supposed to be assessed by meta‐regression performed on pre‐selected covariates. We planned to investigate whether the following covariates or patterns of covariates had contributed to this.
Variation across participant subgroups (people with cancer or in the ICU compared to other groups; pediatric versus adult studies).
Variability in the number of positive results used to define a positive test (single positive result versus two consecutive positive samples).
Differences due to sampling strategies (single sample taken versus multiple samples collected over the length of stay).
Study design factors, including prospective versus retrospective and consecutive versus case‐control.
Test interference (antifungal prophylaxis, pre‐emptive therapy, etc.).
Definition of IFI: using proven and probable IFI (as defined above) as the definition of the target condition, and comparing it only to proven IFI when compared to all other categories and using proven, probable, and possible IFI compared to no IFI.
Because we were unable to perform a formal meta‐analysis, we used ROC plots to visually investigate these potential sources of heterogeneity. This diversion from the protocol is explained in the Differences between protocol and review section.
Sensitivity analyses
We planned to compare pooled sensitivity and specificity estimates for studies that had low overall risk of bias versus those with at least one high risk of bias. However, we were unable to do this because a formal meta‐analysis was not performed. This diversion from the protocol is explained in the Differences between protocol and review section.
Results
Results of the search
Through the literature search in MEDLINE and Embase, we identified 10,354 references. Duplicate references were identified and removed (N = 1671), resulting in 8683 articles. The initial review of titles and abstracts yielded 211 potentially relevant articles (Figure 1).
1.
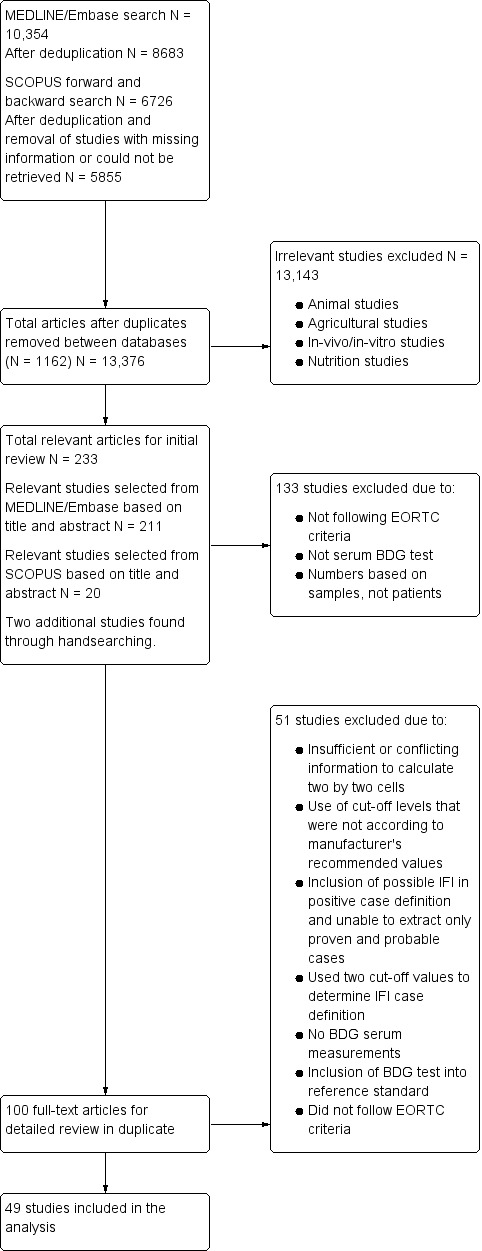
Study flow diagram.
The citation search in SCOPUS (forward and backward search based on potentially relevant references) identified 6726 references. Of these, 747 were duplicates. Also, 124 references had extensive missing information and could not be retrieved, yielding a final number of 5855 references.
We compared results of the citation search (SCOPUS) with results of the initial search (MEDLINE and Embase) and identified 1162 references that had already been included in the initial search. We reviewed titles and abstracts of the remaining 13,376 references. In total, we identified 233 potentially relevant studies (211 from the MEDLINE/Embase searches, 20 from the citation search, and 2 additional articles through handsearching) that were initially reviewed for inclusion using the abstract form in Appendix 3.
After reviewing the 233 potentially relevant studies, we identified 100 studies that met study criteria in which we conducted a full‐text review (Appendix 4). We contacted two study authors to receive clarification on possible IFI results and study design, which we received. At the conclusion of the full‐text review, we identified 49 studies to be included in the systematic review (Table 2; Characteristics of included studies). A flow diagram of the selection process is shown in Figure 1.
1. Characteristics of included studies.
| Study name | Study design | Underlying condition | Fungal type | Test brand | Samples taken | Reference standard |
| Acosta 2011 | Prospective consecutive | ICU | Mixed | Fungitell | Single | EORTC 2008 |
| Alexander 2010 | Prospective consecutive | Organ transplant | Mixed | Fungitell | Multiple | EORTC 2008 |
| Atalay 2014 | Retrospective consecutive | Cancer | Mixed | Fungitell | Single | EORTC 2008 |
| Badiee 2012 | Prospective consecutive | Cancer | Aspergillosis | Glucatell | Multiple | EORTC 2008 |
| Boch 2016 | Prospective consecutive | Cancer | Mixed | Fungitell | Single | EORTC 2008 |
| Ceesay 2015 | Prospective consecutive | Mixed at‐risk | Mixed | Fungitell | Multiple | EORTC 2008 |
| Cornu 2018 | Retrospective case‐control | ICU | Candida | Fungitell | Single | Culture from blood or sterile site |
| Costa 2012 | Retrospective consecutive | Mixed at‐risk | PJP | Fungitell | Single | Microscopy |
| De Vlieger 2011 | Retrospective case‐control | ICU | Aspergillosis | Fungitell | Unknown | EORTC 2008 |
| Del Bono 2011 | Prospective consecutive | ICU | Candida | Fungitell | Single | EORTC 2008 |
| Dichtl 2018 | Retrospective case‐control | Mixed at‐risk | PJP | Wako | Single | PCR |
| Fontana 2012 | Retrospective case‐control | Cancer | Mixed | Fungitell | Multiple | EORTC 2008 |
| Furfaro 2018 | Prospective consecutive | Cancer | Aspergillosis | Fungitell | Multiple | EORTC 2008 |
| Giacobbe 2017 | Retrospective consecutive | ICU | Candida | Fungitell | Single | Culture from blood or sterile site |
| Gupta 2017 | Prospective consecutive | Cancer | Mixed | Fungitell | Single | EORTC 2008 |
| Hachem 2009 | Prospective consecutive | Cancer | Aspergillosis | Fungitell | Multiple | EORTC 2002 |
| Hammarstrom 2015 | Retrospective consecutive | Cancer | Mixed | Glucatell | Multiple | EORTC 2008 |
| Hammarstrom 2018 | Prospective consecutive | Cancer | Mixed | Glucatell | Multiple | EORTC 2008 |
| Hanson 2012 | Prospective consecutive | ICU | Mixed | Fungitell | Multiple | EORTC 2008 |
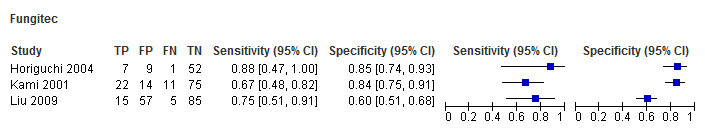
| Horiguchi 2004 | Prospective consecutive | Cancer | Aspergillosis | Fungitec | Unknown | EORTC 2002 |
| Jin 2013 | Prospective consecutive | Cancer | Aspergillosis | Glucatell | Multiple | EORTC 2002 |
| Kami 2001 | Unknown | Cancer | Aspergillosis | Fungitec | Multiple | Study‐specific, comparable to EORTC |
| Kawazu 2004 | Prospective consecutive | Cancer | Aspergillosis | Wako | Multiple | EORTC 2002 |
| Koltze 2015 | Prospective consecutive | Cancer | Mixed | Fungitell | Multiple | EORTC 2008 |
| Koo 2009 | Retrospective consecutive | Cancer | Mixed | Fungitell | Multiple | EORTC 2008 |
| Lahmer 2016a | Retrospective consecutive | ICU | Aspergillosis | Fungitell | Unknown | EORTC 2008 |
| Lahmer 2016b | Retrospective consecutive | ICU | Aspergillosis | Fungitell | Single | EORTC 2008 |
| Leon 2016 | Prospective consecutive | ICU | Candida | Fungitell | Multiple | Culture from blood or sterile site |
| Liu 2009 | Retrospective consecutive | Cancer | Mixed | Fungitec | Single | EORTC 2008 |
| Lo Cascio 2015 | Retrospective consecutive | ICU | Candida | Fungitell | Single | EORTC |
| Mackay 2011 | Prospective consecutive | ICU | Mixed | Fungitell | Single | Study‐specific, comparable to EORTC |
| Martin‐Mazuelos 2015 | Prospective consecutive | ICU | Candida | Fungitell | Multiple | Culture |
| Metan 2012 | Retrospective case‐control | Cancer | Aspergillosis | Fungitell | Multiple | EORTC 2008 |
| Metan 2013 | Retrospective consecutive | Cancer | Aspergillosis | Fungitell | Multiple | EORTC 2008 |
| Mohr 2011 | Prospective consecutive | ICU | Candida | Fungitell | Multiple | Culture |
| Odabasi 2004 | Unknown | Cancer | Mixed | Glucatell | Multiple | EORTC 2002 |
| Persat 2008 | Retrospective case‐control | Cancer | Mixed | Fungitell | Single | EORTC 2002 |
| Pini 2019 | Retrospective case‐control | Mixed | Candida | Fungitell | Single | Culture from blood or sterile site or pathology |
| Posteraro 2011 | Prospective consecutive | ICU | Mixed | Fungitell | Multiple | EORTC 2008 |
| Racil 2010 | Prospective consecutive | Cancer | Mixed | Fungitell | Multiple | EORTC 2002 |
| Rose 2014 | Retrospective consecutive | Mixed at‐risk | Mixed | Fungitell | Single | EORTC 2008 |
| Salerno 2014 | Retrospective consecutive | HIV | PJP | Fungitell | Single | Microscopy/PCR |
| Senn 2008 | Prospective consecutive | Cancer | Mixed | Wako | Multiple | EORTC 2002 |
| Shabaan 2018 | Prospective consecutive | ICU | Candida | Dynamiker | Single | Culture from blood or sterile site |
| Singh 2015 | Prospective consecutive | Organ transplant | Mixed | Fungitell | Multiple | EORTC 2008 |
| Talento 2017 | Prospective consecutive | ICU | Mixed | Fungitell | Multiple | EORTC 2008 |
| Theel 2013 | Prospective consecutive | Mixed at‐risk | Mixed | Fungitell | Single | EORTC 2008 |
| Verduyn Lunel 2009 | Retrospective case‐control | Cancer | Candida | Fungitell | Multiple | Culture from blood or sterile site |
| White 2017 | Retrospective case‐control | Mixed at‐risk | Mixed | Dynamiker | Single | EORTC 2008 |
EORTC: European Organization for Research and Treatment of Cancer; ICU: intensive care unit; PCR: polymerase chain reaction; PJP: Pneumocystis jirovecii pneumonia.
Basic features of included studies
Details of the included studies are presented in Table 3, 'Overall characteristics of included studies'. We included 49 studies with a total of 6244 participants.
2. Summary of included studies.
| Characteristic | n | Percentage |
| Underlying condition | ||
| Cancer HIV/AIDS ICU Mixed at‐risk Organ transplant |
23 1 16 7 2 |
46.9% 2.0% 32.7% 14.3% 4.1% |
| Age of patients | ||
| Adult Neonate Pediatric Mixed Unknown |
26 3 3 5 12 |
53.1% 6.1% 6.1% 10.2% 24.5% |
| Study design | ||
| Prospective consecutive Retrospective consecutive Retrospective case‐control |
26 12 11 |
53.1% 24.5% 22.4% |
| Fungal type | ||
|
Aspergillus only Candida only PJP only Mixed fungal types |
12 10 3 24 |
24.5% 20.4% 6.1% 49.0% |
| Test brand | ||
| Fungitell Glucatell Fungitec‐G Wako Dynamiker Fungus |
36 5 3 3 2 |
73.5% 10.2% 6.1% 6.1% 4.1% |
| Sampling strategy | ||
| Single sample Multiple samples Unknown |
20 26 3 |
40.8% 53.1% 6.1% |
| Reference standard used | ||
| EORTC Proven Candida PJP microscopy/PCR Study‐specific |
36 8 3 2 |
73.5% 16.3% 6.1% 4.1% |
| Low risk of bias | ||
| Participant selection Index test Reference standard Flow and timing |
35 49 41 41 |
71.4% 100.0% 83.7% 83.7% |
EORTC: European Organization for Research and Treatment of Cancer; ICU: intensive care unit; PCR: polymerase chain reaction; PJP: Pneumocystis jirovecii pneumonia.
Participants: nearly half of the studies involved people with hematologic and oncologic diseases (N = 23; 47%), followed by people in the ICU (N = 16) and mixed at‐risk cases (N = 7) (see Table 3). A majority (N = 26; 53%) were focused on adult populations, five had both adult and pediatric cases, and six focused solely on pediatrics (N = 3) or neonates (N = 3).
Study design: of the 49 studies, a little more than half (N = 26; 53%) were prospective in design. Of the 23 retrospective studies, 12 were consecutive, with the remaining employing a case‐control design.
Assay characteristics: a majority (N = 36) used the Fungitell assay, followed by Glucatell (N = 5), Fungitec‐G (N = 3), Wako (N = 3), and Dynamiker Fungus (N = 2).
Sampling: almost all studies reported only estimates based on a single positive test, although two studies did provide results based on two consecutive positive samples. Sampling design varied greatly between studies, ranging from a single sample collection (41%) to multiple samples collected over several weeks. Studies that collected multiple samples reported differing criteria for the classification of a positive BDG result, such as using the first sample collected or the highest BDG value recorded.
Organisms: studies that included all types of organisms were most common (N = 24), although several focused exclusively on Candida (N =10) or Aspergillus (N = 12). Per the selection criteria, all studies either used the EORTC/MSG criteria (N = 36) or followed the criteria used in the diagnosis of proven PJP or candidiasis.
Language: almost all studies (N = 47) were published in English, with the two remaining articles published in Chinese and Japanese.
Excluded studies
From our full‐text review, we identified 51 studies to be excluded (see Characteristics of excluded studies) for the following reasons.
Unable to determine 2 × 2 cell counts for overall sensitivity and specificity estimates (N = 17).
Used cut‐off values that did not follow the manufacturer's recommended level or utilized two cut‐off values to determine a positive test (N = 11).
Included BDG tests as part of the reference standard or did not follow EORTC/MSG guidelines (N = 7).
Did not meet inclusion criteria for the study population or inclusion criteria were unknown (N = 6).
Included probable (PJP or Candida) or possible IFI cases in the IFI definition, which could not be separated (N = 5).
Other reasons (N = 4).
Methodological quality of included studies
Thirty‐two studies had no concerns regarding risk of bias or applicability among the four QUADAS‐2 domains (Figure 2 and Figure 3). Details on bias for individual studies are provided in the Characteristics of included studies table. For studies that had high risk of bias or concerns regarding applicability, this was due mainly to (1) case‐control design (Cornu 2018; De Vlieger 2011; Dichtl 2018; Fontana 2012; Metan 2012; Persat 2008; Pini 2019; Verduyn Lunel 2009; White 2017), and (2) exclusion of possible IFI cases from the findings (Hammarstrom 2015; Hammarstrom 2018; Jin 2013; Theel 2013).
2.
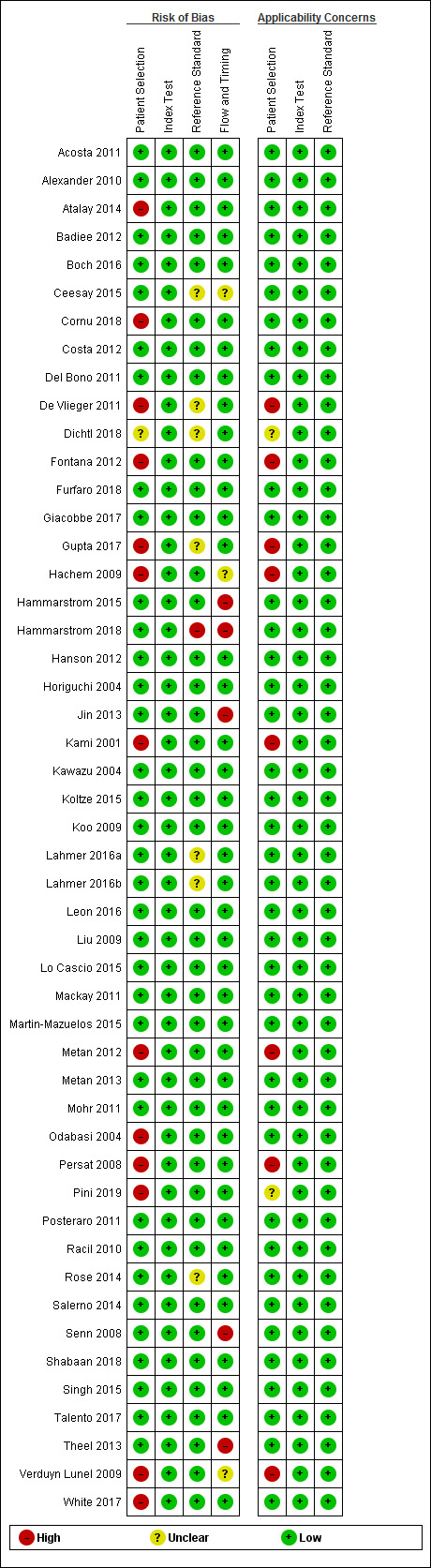
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
3.
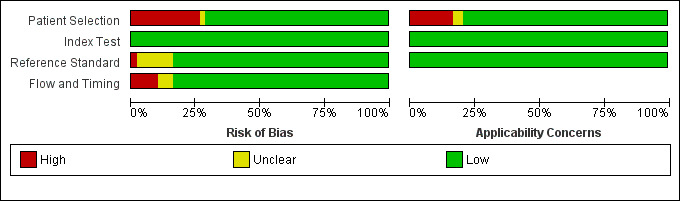
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
Thirty‐six of the studies failed to report the time interval between BDG testing and the reference standard. Only 13 studies provided a time frame; however, we still judged all other studies as low bias if other criteria were met (Figure 2).
Due to study design criteria, all studies pre‐specified cut‐off values or reported values that met the manufacturer's recommendations. BDG is an objective quantitative test that is generally performed without knowledge of the participant's true infection status. Therefore, failure to blind investigators to the reference test poses little risk of bias with respect to interpretation of the BDG test result. Thus, even if the study did not report blinding, we considered both the index test and reference standard domains to still be at low bias (Figure 2; Figure 3).
The reference standard was likely to classify IFIs correctly by using either EORTC/MSG criteria or confirmation by culture or microscopy. The EORTC/MSG criteria were revised in 2008. One of the important changes was that BDG was added as a criterion for IFI. Thus, to avoid incorporation bias, studies had to exclude BDG from the diagnostic criteria. Forty‐two studies reported that they did not incorporate BDG testing in the reference standard, and we excluded two studies that included BDG as part of the reference test. It is unclear in seven studies whether BDG testing had been excluded (Ceesay 2015; De Vlieger 2011; Fontana 2012; Gupta 2017; Lahmer 2016a; Lahmer 2016b; White 2017). We elected to include these studies. Most studies were careful not to incorporate BDG testing, and we assumed that these studies most likely would have done so as well.
Findings
The prevalence of IFI ranged from 4% to 59% among all studies (mean 23%, 95% confidence interval (CI) 18% to 28%). In addition, estimates of sensitivity and specificity varied widely. Due to the high degree of heterogeneity between studies, we did not perform a formal meta‐analysis, with the exception of Candida.
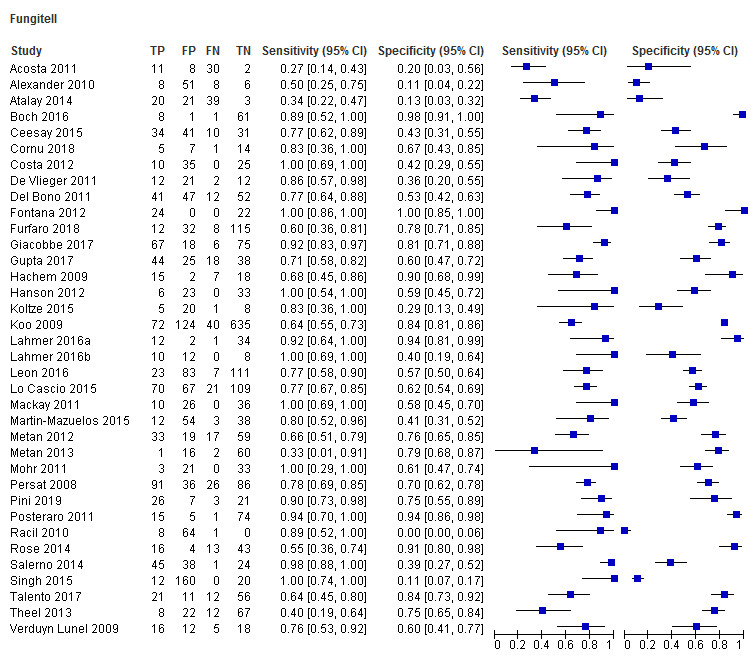
Fungitell (36 studies): sensitivity for individual studies ranged between 27% and 100% and specificity range between 0% and 100% (Figure 4; Figure 5). A large amount of uncertainty was noted in study estimates, as evidenced by wide confidence intervals in the forest plot (Figure 4). Because IFI is relatively rare, many studies had a small number of positive cases. Koo had the largest study, with a study population of 871 (Koo 2009).
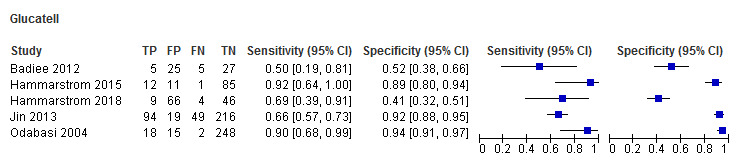
Glucatell (5 studies): study estimates for sensitivity and specificity for Glucatell also ranged widely. Sensitivity ranged from 50% to 92%, and specificity ranged from 41% to 94%, among the 5 studies (Figure 4; Figure 5).
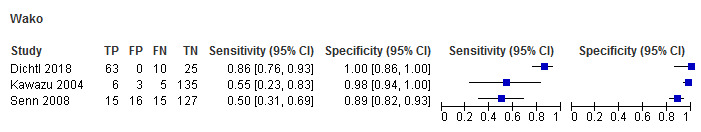
Wako (3 studies): only 3 studies used the Wako assay at the manufacturer's specified cut‐off level. Two studies reported lower sensitivities (55% and 50%, respectively) but higher specificities (98% and 89%) (Figure 4; Figure 5) (Kawazu 2004; Senn 2008). Dichtl 2018 reported fairly high sensitivity (86%) and specificity (100%) among a group of 98 people.
Fungitec‐G (3 studies): estimates for the 3 studies using Fungitec ranged from 67% to 88% for sensitivity and from 60% to 85% for specificity (Figure 4; Figure 5).
Dynamiker (2 studies): only 2 recent studies had published results regarding Dynamiker Fungus (Figure 4; Figure 5). White 2018 reported sensitivity of 81% and specificity of 78%, and Shabaan 2018 reported sensitivity and specificity of 64% and 80%, respectively.
4.
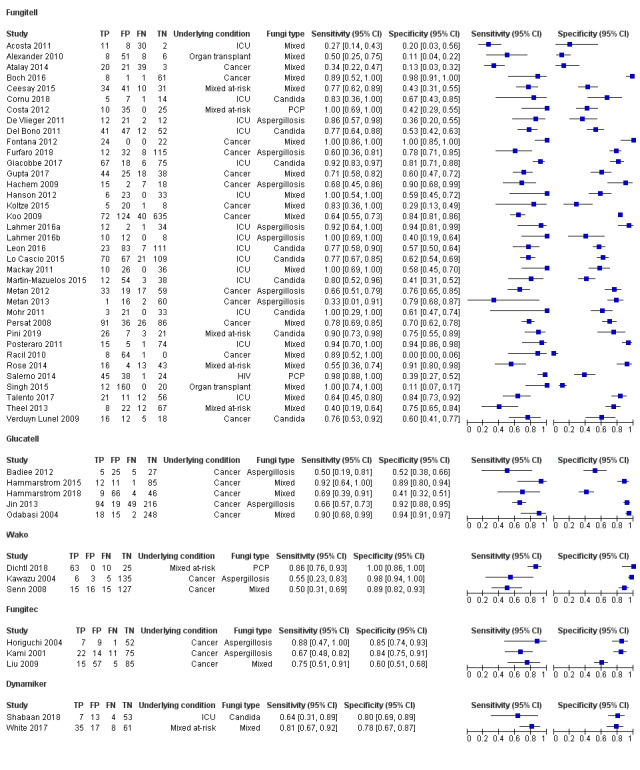
Forest plot of tests: Fungitell, Glucatell, Wako, Fungitec, Dynamiker.
5.
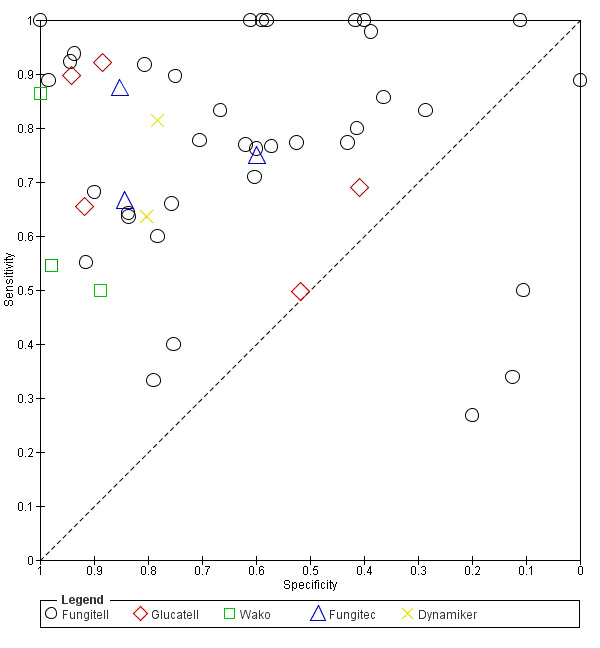
Summary ROC plot of tests: 1 Fungitell, 2 Glucatell, 3 Wako, 4 Fungitec, 5 Dynamiker.
We included 10 studies in the meta‐analysis, from which an estimate for Candida could be obtained. Estimated sensitivity and specificity for these studies was 81.3% (95% CI 75.3% to 86.0%) and 64.1% (95% CI 55.6% to 71.8%), respectively. Almost all (N = 9; 90%) used Fungitell, with 8 of the 10 studies involving people in ICU settings. Forty per cent utilized multiple samples, and the remainder relied on a single test.
Investigations of heterogeneity
Heterogeneity was assessed by ROC plots that examined differences in individual sensitivity and specificity estimates by participant population, fungal organism, reference standard, and single versus multiple testing.
Heterogeneity could not be explained by the participant population (Figure 6). We restricted this analysis to a single test platform to limit a potential source of variation. We selected the Fungitell assay because it was the most commonly used test platform (36 of 49 studies). In 13 studies involving participants with cancer, sensitivity ranged from 33% to 100% and specificity ranged from 0% to 100%. In 15 studies involving participants who had been admitted to the ICU, sensitivity ranged from 27% to 100% and specificity ranged from 20% to 94%. Finally, in 5 studies with a mixture of participants, sensitivity ranged from 40% to 100% and specificity ranged from 42% to 91%. All participant groups had a wide range of sensitivity and specificity. Considerable overlap could be seen in the ranges of sensitivity and specificity for each group. It was not possible to identify an underlying condition that was associated with higher or lower levels of sensitivity or specificity.
6.
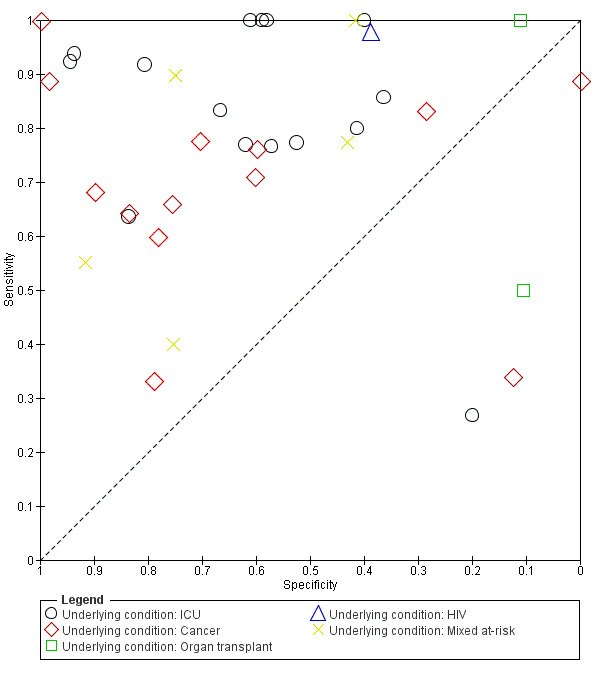
Summary ROC plot of underlying medical conditions for Fungitell studies.
Heterogeneity could not be explained by the reference standard (Figure 7). This analysis was also restricted to studies performed with the Fungitell assay. In 26 tests using EORTC criteria, sensitivity ranged from 27% to 100% and specificity ranged from 0% to 100%. In seven studies testing for Candida, sensitivity ranged from 76% to 100% and specificity ranged from 41% to 81%.
7.
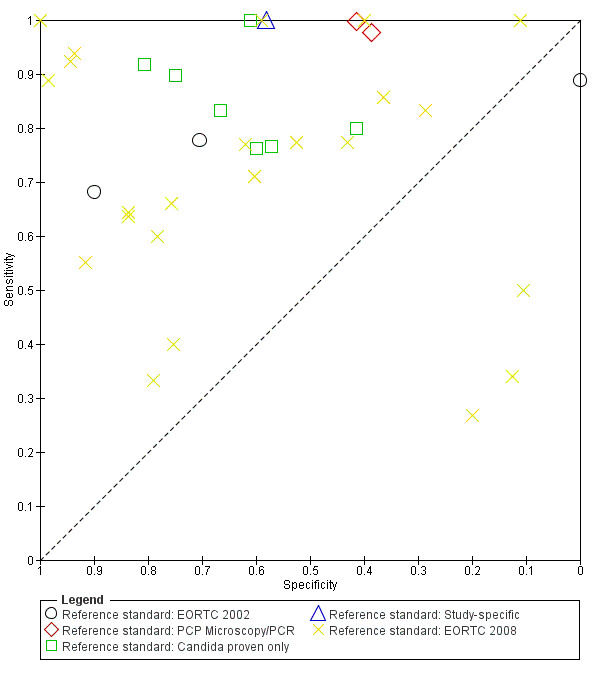
Summary ROC plot of reference standard for Fungitell studies.
In some studies, the BDG test was performed once, and in other studies, BDG testing was performed multiple times (e.g. twice a week). Heterogeneity could not be explained by the number of tests (Figure 8). This analysis was restricted to studies performed with the Fungitell test. In 16 studies that used a single sample, sensitivity ranged from 27% to 100% and specificity ranged from 12% to 98%. In 18 studies that used multiple tests per person, sensitivity ranged from 33% to 100% and specificity ranged from 0% to 100%. Both groups had a wide range of sensitivity and specificity with substantial overlap. It was not possible to identify a sampling policy that was associated with higher or lower levels of sensitivity or specificity.
8.
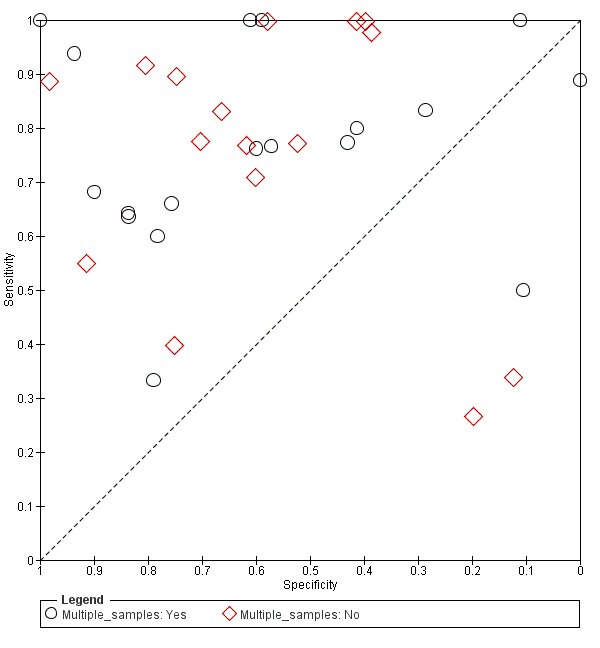
Summary ROC plot of single versus multiple sampling for Fungitell.
Studies that focused on Candida infection did appear to be more homogeneous than those focused on other fungal organisms (Figure 9); therefore, we obtained a summary estimate for these studies.
9.
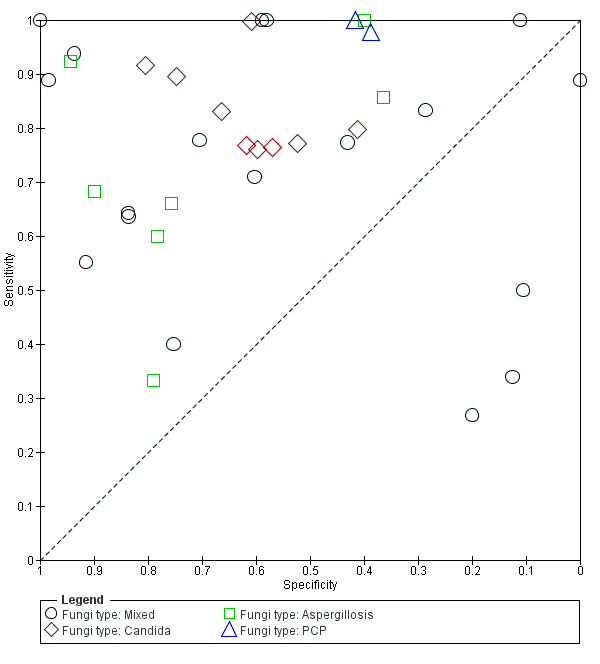
Summary ROC plot of fungal organisms for Fungitell studies.
Discussion
Summary of main results
Literature on the use of (1→3)‐β‐D‐glucan (BDG) for diagnosis of invasive fungal infection (IFI) shows wide variation in diagnostic accuracy. Sensitivity ranged from 27% to 100%, and specificity ranged from 0% to 100%. Because of this variation, we did not perform a formal meta‐analysis, with the exception of Candida studies.
There were many potential sources of heterogeneity. These include study design (case‐control retrospective, prospective), differences in populations (immunodeficient versus critically ill), sampling (single sample, multiple samples, monitoring with two samples per week), assays (Fungitell, Glucatell, etc.), target organisms (all IFI, Candida, Aspergillus, etc), and threshold for positivity (one positive BDG test, two consecutive positive BDG tests). Application of European Organization for Research and Treatment of Cancer (EORTC) criteria is another potential source of heterogeneity. The accuracy with which physicians perform this task may vary, and because the number of physicians in any study is low, differences in classification accuracy are unlikely to average out. We are not aware of any agreement of studies on EORTC criteria. This variation made it difficult to obtain meaningful estimates of sensitivity and specificity. Thus, it is not possible to predict how the BDG test will perform in a particular context.
Going forward, it would be helpful if studies limited variation in these factors. Prospective studies should be preferred over case‐control and retrospective studies. Prospective studies are more closely aligned with the clinical context and allow various sampling policies to be compared in a single study. For example, one could perform twice‐weekly sampling and compare the diagnostic accuracy of the first positive BDG result, two consecutive BDG‐positive results, positive BDG when a person is first symptomatic, etc. It is not clear whether studies on individual organisms are helpful. Several studies focused on infections in a single organism. Although such studies provide useful knowledge regarding test performance, they do not address a clinically relevant question. The clinical question that is addressed by BDG testing is whether a person has an IFI rather than whether a person is infected with a particular organism. It might be better to conduct instrument comparisons in laboratory studies rather than in clinical studies.
We found that the quality of studies was generally good. Risk of bias was generally low. We did exclude a number of case‐control studies that included healthy controls. These study designs produce inflated estimates of sensitivity and specificity due to spectrum bias (White 2019). There is some room for improvement in reporting. Studies should not include BDG as part of the reference test and should explicitly state this. Also, it would be helpful if studies reported results for all four EORTC categories (proven, probable, possible, none). One must aggregate categories to calculate sensitivity and specify; however, to facilitate meta‐analysis, results should be available as individual categories. Studies should report timing of the BDG test relative to the reference test, and whether the reference test was blinded to the BDG test result.
Comparison of our results with other meta‐analyses
Four meta‐analyses on the diagnostic accuracy of BDG have been previously published (He 2015; Karageorgopoulos 2011; Lu 2011b; White 2019). These meta‐analyses studied the use of BDG in similar populations of people (immunocompromised) and included between 13 and 28 studies. Our analysis summarized 49 studies, which reflects the large number of studies conducted in the past five years. Previous reviews have also reported high levels of heterogeneity.
Strengths and weaknesses of the review
This review represents the most up‐to‐date systematic assessment of BDG test performance. The high level of heterogeneity is a significant limitation. Current BDG diagnostic literature remains impacted by variability in study design, heterogenous populations, limited information on baseline use of antifungal therapy or other potential assay interferences, and lack of consistently robust adjudication of potential colonization versus invasive disease. In addition, microscopy for PJP and culture confirmation of Candida are imperfect reference standards that may miss true cases of invasive disease and may impact calculations of test specificity.
There was considerable variation in the prevalence of probable/proven IFI (range 4% to 59%). This could reflect differences in populations or differences in interpretation of the reference standard. Variation in interpretation of the reference standard is a potential source of heterogeneity. We used simple descriptive methods to investigate potential sources of heterogeneity; however, this was largely unsuccessful. Future work might benefit from the application of a latent‐class meta‐analysis, which could potentially address the issue of variable, imperfect reference standards across studies.
Applicability of findings to the review question
We summarized the diagnostic accuracy of several BDG tests. We found significant heterogeneity between study estimates. Given this variability, a summary estimate is unlikely to be applicable at any given location. We were unable to make a meaningful comparison between different commercial tests, and we were unable to determine factors that affect diagnostic accuracy (e.g. population, positivity criteria, sampling).
Authors' conclusions
Implications for practice.
The potential value of BDG testing relies on detecting infection at an early stage. Based on this review, it is unclear whether this occurs. It is also unclear whether a pre‐emptive strategy (supported by BDG testing) leads to earlier diagnosis and better outcomes when compared to prophylaxis or empiric therapy.
Implications for research.
This review was limited by wide variation in outcomes. This, in turn, was driven by wide variation in study designs, positivity criteria, sampling protocols, and tests. It seems it will be necessary to reduce the variation in study design to reduce variation in outcomes. To that end, it would be beneficial if future studies were designed in a way that is most closely aligned with clinical practice, for example, continuous monitoring (e.g. twice weekly) during periods of risk versus testing at a single time point for people with clinical signs or symptoms of invasive fungal infection. Studies could easily compare positivity criteria (one positive sample versus two consecutive positive samples). It is unclear whether additional case‐control and retrospective studies would be informative. Such studies may have been informative in the early development of BDG tests, but they do not reflect the way that BDG tests are used in practice. Timing of the reference test relative to the BDG test result needs to be accurately reported. Studies also need to avoid incorporation bias by insuring that the reference test is blinded from the BDG test result. We are unaware of any study on inter‐rater agreement of the EORTC criteria for IFI. Such a study may be useful.
History
Protocol first published: Issue 5, 2012 Review first published: Issue 7, 2020
Acknowledgements
We thank Robert Schlaberg for contributions to the protocol, Jo Morrison for clinical and editorial advice, Jo Platt for design of the search strategy, and Gail Quinn, Clare Jess, and Tracey Harrison for their contributions to the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
The review authors and the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team are grateful to the following peer reviewers for their time and comments: Michael Darmon, Kathy Godfrey, Zekaver Odabasi, and Luis Ostrosky.
Appendices
Appendix 1. MEDLINE (Ovid) strategies
MEDLINE Ovid
1 BDG*.tw. 2 Fungitel*.tw. 3 Cape Cod.tw. 4 Fungitec*.tw. 5 Seikagaku.tw. 6 Wake Test.tw. 7 (Wako* or Waco*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp beta‐Glucans/ 10 Glucans/ 11 D‐glucan*.tw. 12 9 or 10 or 11 13 exp "Sensitivity and Specificity"/ 14 (sensitivit* or specificit*).tw. 15 predictive value*.tw. 16 diagnosis.fs. 17 analysis.fs. 18 Reagent Kits, Diagnostic/ 19 13 or 14 or 15 or 16 or 17 or 18 20 12 and 19 21 exp Mycoses/ 22 exp Fungi/ 23 fungal.tw. 24 fungus.tw. 25 mycos*.tw. 26 mycot*.tw. 27 aspergill*.tw. 28 candid*.tw. 29 pneumocystis.tw. 30 histoplasmosis.tw. 31 blastomycosis.tw. 32 fusarium.tw. 33 trichosporon.tw. 34 saccharomyces.tw. 35 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36 12 and 35 37 8 or 20 or 36 38 exp animals/ not humans.sh. 39 37 not 38 40 limit 39 to yr="1995 ‐Current"
key:, tw = textword, fs = floating subheading
Appendix 2. Embase (Ovid) search strategy
1 BDG*.tw. 2 Fungitel*.tw. 3 Cape Cod.tw. 4 Fungitec*.tw. 5 Seikagaku.tw. 6 Wake Test.tw. 7 (Wako* or Waco*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp beta‐Glucans/ 10 Glucans/ 11 D‐glucan*.tw. 12 9 or 10 or 11 13 "sensitivity and specificity"/ 14 (sensitivit* or specificit*).tw. 15 predictive value*.tw. 16 di.fs. 17 diagnostic kit/ 18 exp diagnostic procedure/ 19 13 or 14 or 15 or 16 or 17 or 18 20 12 and 19 21 exp mycosis/ 22 exp fungus/ 23 fungal.tw. 24 fungus.tw. 25 mycos*.tw. 26 mycot*.tw. 27 aspergill*.tw. 28 candid*.tw. 29 pneumocystis.tw. 30 histoplasmosis.tw. 31 blastomycosis.tw. 32 fusarium.tw. 33 trichosporon.tw. 34 saccharomyces.tw. 35 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36 12 and 35 37 7 or 20 or 36 38 (exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/ 39 37 not 38 40 limit 39 to yr="1995 ‐ Current"
key:, tw = textword, fs = floating subheading
Appendix 3. Initial abstract form
Reviewer: _____RS _____SW
First author (last name, first initial)________________________________________________________________
Title_________________________________________________________________________________________
Year______________
Study design
_____Prospective consecutive cohort
_____Retrospective consecutive cohort
_____Retrospective case/control (does not include healthy controls)
_____None of the above (specify:_______________________________________________________)
Patient population
_____Cancer patients
_____Prolonged immunosuppressive therapy patients
_____Patients with congenital or acquired immune disorder
_____ICU patients
_____None of the above (specify:_______________________________________________________)
Type of sample
_____Serum
_____Urine
_____BAL
Invasive fungal infection (check all that apply)
_____Proven IFI
_____Probable IFI
_____Possible IFI
_____None of the above (specify:_______________________________________________________)
EORTC/MSG or autopsy used as a reference standard:
_____Yes
_____No (exclude)
_____Other (specify:_______________________________________________________)
Appendix 4. Full abstract form
Reviewer: _____SW _____RS _____BW
Author, date and language of publication: _________________________________________________
1) Able to construct 2 ×2 table? If not, stop here!
| Proven IFI | Probable IFI | Possible IFI | No IFI | Totals | |
| BDG Positive | |||||
| BDG Negative | |||||
| Totals |
a) Prevalence of IFI in the population (if reported): ______________%
b) IFI cases defined as (check all that apply):
____ Proven
____ Probable
____ Possible
____ Proven/Probable
____ Proven/Probable/Possible
2) Study design:
____ Retrospective case‐control
____ Prospective consecutive cohort
____ Other (describe): ___________________________________________________________
a) Controls (check all that apply)
____ At‐risk
____ Other diseases
____ Colonized
____ Healthy
b) Patient population (check all that apply)
Patient age
___ Adult
___ Pediatric
___ Neonate only
___ Mixed (specify age range): _______________________________________
Underlying condition
___ ICU
___ Heme malignancy
___ BMT
___ Solid tumor malignancy
___ SOT (specify organ): _____________________________________________
___ HIV
___ Autoimmune/Connective tissue
___ Mixed at‐risk
a) Invasive fungal infection (IFI)
___ Mixed IFIs
___ Candida only
___ Aspergillus only
___ Pneumocystis (PCP) only
b) Reference standard used to diagnose IFI
___ EORTC
___ Autopsy
___ Study‐specific composite definition
___ PCP only (check all that apply)
___ Microscopy
___ PCR
___ Candida only (check all that apply)
___ Culture
___ Histopathology
___ Other (explain): ______________
c) Assay
___ Glucatell/Funitell
___ Fungitec G
___ Wako‐WB003
___ Difference in titers between Endotoxin Test‐D and Endospecy
___ Other (explain): _______________________________________________________
d) Cut‐off used to define a positive test
___ ≥ 80 pg/mL Glucatell/Fungitell
___ ≥ 20 pg/mL Fungitec G
___ ≥ 11 pg/mL Wako
___ Other (explain): _______________________________________________________
e) Number of positive specimens at cut‐off used to define positive test
___ Single positive
___ Two sequential positives
___ Other (explain): _______________________________________________________
f) Specimen sampling strategy
___ Once
___ Weekly
___ Twice weekly
___ Other (explain): _______________________________________________________
g) Study subject receiving systemic antifungal therapy at the time of testing (include % if known)
___ Antifungal prophylaxis: ______%
___ Empiric therapy: ______%
___ Not reported: ______%
Appendix 5. QUADAS criteria (QUADAS version 2)
Domain 1: patient selection
Risk of bias
1. Was a consecutive or random sample of patients enrolled?
YES if the study specifically states that consecutive patients or a random sample was selected.
NO if the study clearly states that the selection of patients was not consecutive or random, or if this can be easily inferred from the design.
UNCLEAR if not reported or cannot be determined.
2. Was a case‐control design avoided?
YES if the study is cross‐sectional, prospective, or retrospective using consecutive patients or a random selection.
NO if the study specifically states that it used a case‐control design, or if this can be easily inferred from the description.
UNCLEAR if not reported or cannot be determined.
3. Did the study avoid inappropriate conclusions?
YES. If the study population was selected from hospitalized patients who are in one of the disease groups included in the study AND if patients were not excluded based on any criteria related to potential diagnosis of IFI by EORTC criteria.
NO. If the study population was selected from hospitalized patients who are not in one of the disease groups included in the study OR if patients were excluded based on any criteria related to potential diagnosis of IFI by EORTC criteria.
UNCLEAR if not reported or cannot be determined.
Could the study have introduced bias? (no criteria provided)
Concerns regarding applicability
Minor concern: study conducted in community or outpatient setting
Major concern: patients on prophylactic therapy at the time of index test
Major concern: patients on antifungal therapy at the time of index test
Major concern: patients who received BDG‐positive antibiotics prior to index test
Major concern: patients who received IVIG prior to the index test
Overall assessment of level of concern regarding applicability (to be determined).
Domain 2: index test
Risk of bias
1. Were the index test results interpreted without knowledge of results of the reference standard?
YES if the study states that the index test was performed prior to assessment of EORTC criteria, or if the study specifically states that interpretation of the index test was blinded to the reference test.
NO if the study clearly states that interpretation of the index test was blinded to the reference test.
UNCLEAR if not reported or cannot be determined.
2. If a threshold was used, was it prespecified?
YES if the threshold was prespecified.
NO if the thresholds were not prespecified.
UNCLEAR if not reported or cannot be determined.
Could the conduct or interpretation of the index test have introduced bias? (to be determined)
Concerns regarding applicability
Major concern: the test is not approved by the FDA or similar government organization.
Major concern: the test is approved but has been modified relative to the package insert.
Major concern: the sampling protocol (sampling frequency, length of sampling) is not clearly described.
Major concern: the sampling protocol (sampling frequency, length of sampling) varies between patients.
Major concern: the sampling protocol differs significantly from other studies included in the sample.
Major concern: the criteria for a positive BDG diagnosis are not clearly specified.
Major concern: the criteria for a positive BDG diagnosis vary.
Major concern: the criteria for a positive BDG diagnosis differ significantly from other studies in the set of included studies.
Overall assessment for concerns regarding applicability (to be determined)
Domain 3: reference standard
Risk of bias
1. Is the reference standard likely to correctly classify the target condition?
YES. The EORTC criteria are used as the reference test.
2. Were the reference standard results interpreted without knowledge of the index test?
Blinding: was interpretation of the reference standard results blinded to results of the index test?
YES if the study clearly states that the index test was not used as part of the reference criteria AND if assessment of the reference criteria was blinded to the index test result.
NO if the study clearly states that the index test was used as part of the reference criteria OR if assessment of the reference criteria was not blinded to the index test result.
UNCLEAR if not reported or cannot be determined.
Incorporation: was the index test not used as part of the criteria for the reference standard?
YES the study clearly states that the index test was not used in the assessment of the reference criteria. This is independent of blinding.
NO the study clearly states that the index test was not excluded from the reference criteria.
UNCLEAR if not reported or cannot be determined..
Could the conduct or interpretation of the index test have introduced bias? (to be determined)
Is there any concern that the target condition as defined by the reference standard does not match the review question?
Major concern: the study does not provide a disaggregated tabulation of each category of reference test diagnosis against each category of index test diagnosis.
Domain 4: timing and flow
Risk of bias
1. Was there an appropriate time interval between index test and reference standard?
YES if the BDG test result for all patients was obtained within 2 weeks of the reference test.
NO if the reference test for all patients was performed more than 4 weeks after the BDG test.
UNCLEAR if not reported or cannot be determined (e.g. if the time interval varies widely).
Explanation: a survey of studies shows kinds of designs:
Cross‐sectional studies in which the index (BDG) and reference test (EORTC) were evaluated at the same time. This design avoids disease progression bias but misses one of the potential benefits of the index test. BDG is attractive because it can be used as a screen to direct therapy prior to the development of EORTC criteria. Thus, one would like to assess the correlation between an early BDG test and the development of IFI (within a reasonable period). Cross‐sectional correlation between BDG and EORTC represents a minimum criterion for usefulness of the test.
Longitudinal studies in which the reference criteria are continuously monitored. This design more closely resembles the intended use of the test but poses risk of disease progression bias or misclassification due to the development of a new independent infection in the intervening period.
Thus, there is a trade‐off between disease progression bias and realistic appraisal of the test. Too short a period prevents progression bias and classification errors but is an unrealistic evaluation of the test as it is intended. Too long a period leads to progression bias or the possibility of a new infection during the interval period. We chose 2 weeks and 4 weeks as cutoffs because it is unlikely that a positive reference test would be due to a new infection that developed during a 2‐week period, and it is possible that a new infection could develop over a 4‐week period. The best cut‐off is uncertain; for that reason, the time period between index and reference tests will be investigated as a possible source of heterogeneity.
2. Did all patients receive a reference standard?
YES if all patients (or a random subset of patients) who received the index test were referred for evaluation by the reference test AND the withdrawal rate was low (less than 1% ‐ a level unlikely to affect results).
NO if a non‐random subset of patients was referred for evaluation by the reference test OR if the withdrawal rate was high (greater than 10%).
UNCLEAR if not reported or cannot be determined.
3. Did all patients receive the same reference standard?
YES EORTC criteria are the only acceptable criteria in this study.
Could patient flow have introduced bias? (to be determined)
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Fungitell | 36 | 4316 |
| 2 Glucatell | 5 | 957 |
| 3 Wako | 3 | 420 |
| 4 Fungitec | 3 | 353 |
| 5 Dynamiker | 2 | 198 |
1. Test.
Fungitell
2. Test.
Glucatell
3. Test.
Wako
4. Test.
Fungitec
5. Test.

Dynamiker
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acosta 2011.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | 847 patients were admitted to the ICU over a 21‐month period. Of these, 51 patients met the study inclusion criteria of having a clinical syndrome compatible with pneumonia and 1 host factor. Two‐thirds (34/51) were male; no information was provided on age range. Thirteen met the criteria for proven or probable IFI | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis or PJP determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Alexander 2010.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | Between August 2004 and March 2006, 79 liver transplant patients were enrolled in the study before transplantation. Six patients were excluded from the analysis. More than half (40/73) were male, with a median age of 52 years. Fourteen patients met the criteria for IFI | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | BDG test was done within 14 days of the reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | BDG tests and equipment were provided by Associates of Cape Cod | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Atalay 2014.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with at least 1 BDG test. Samples were collected twice weekly | ||
| Patient characteristics and setting | Records of all inpatients from August 2009 to August 2011 were reviewed. Forty‐three pediatric and adult patients, most with hematologic or solid tumor malignancies, were selected and had at least 1 BDG test and a diagnosis of proven, probable, or possible IFI. A control group of 40 patients from hematology or oncology wards with no IFI was used for comparison | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Sample taken with 10 days of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Badiee 2012.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly in a prospective study | ||
| Patient characteristics and setting | Between November 2008 and November 2009, all pediatric hematology patients at risk for IFI were enrolled. Sixty‐two patients aged 1 to 14 years (median 9 years old) were analyzed. Ten of these patients had proven or probable IFI | ||
| Index tests | Glucatell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Boch 2016.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | From 2012 to 2015, 99 hematologic patients at high risk for IFI were included. Thirty‐seven of these patients had proven or probable IFI. No information was provided on age or sex of patients | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria without BDG or GM | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ceesay 2015.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | From December 2008 to May 2010, 203 adult patients undergoing HSCT, immunosuppressive therapy, or intensive chemotherapy were included. The median age of patients was 54 years, and 61% were male. During the study period, 40 patients were diagnosed with proven or probable IFI | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC/MSG criteria; unclear whether BDG was excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; BDG was not performed on 26 patients but reason for exclusion unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Cornu 2018.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study of patients with BDG testing | ||
| Patient characteristics and setting | Between February 2012 and February 2014, 47 neonates in the NICU were selected as cases and controls. The median gestational age was 30 weeks, and 70% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis was diagnosed by culture | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Costa 2012.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with BDG collected once or twice per patient | ||
| Patient characteristics and setting | All immunocompromised patients who had undergone bronchoalveolar lavage (BAL) for diagnosis of PJP were screened. Comorbid conditions included AIDS, cancers, organ transplantations, and systemic inflammatory diseases. Sixty‐three patients were included in the analyses. No information was provided on age or sex of patients | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | PJP diagnosed by microscopy | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
De Vlieger 2011.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with 1 BDG test | ||
| Patient characteristics and setting | From July 2005 to December 2006, 110 immunocompromised ICU patients with clinical signs of fungal infection were selected for inclusion and were classified as having proven, probable, or possible IFI. Of these, 14 patients with proven IFI and 33 with no IFI according to the revised EORTC/MSG guidelines were chosen for this study | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2008 EORTC criteria; unclear whether BDG was included | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Del Bono 2011.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | Adult ICU patients admitted between July 2008 and October 2010 who were at risk for invasive candidiasis. A total of 152 patients were included, of whom 53 were diagnosed with proven candidemia. More than half (87/152) were male, and the median age was significantly higher in the proven candidemia group (72 years) compared to the possible (47 years) or no candidemia (52 years) group. | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis as determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | 36 of the 41 patients with proven invasive candidiasis were sampled within 48 hours of the reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Dichtl 2018.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study of patients with BDG testing | ||
| Patient characteristics and setting | 73 patients with confirmed PJP were selected as cases, and 25 controls had clinical signs of PJP but tested negative. Both groups were at risk for IFI, including hematologic malignancies, HIV, and immunosuppressive therapy | ||
| Index tests | Wako test using 11 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | PJP was diagnosed by PCR using respiratory tract specimens | ||
| Flow and timing | All received index test and reference standard; most received BDG test within a week of reference standard, although timing could range from 1 week before to 4 weeks after | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Fontana 2012.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study of patients with at least 1 BDG test. Serum BDG collected weekly | ||
| Patient characteristics and setting | Sera from 46 patients undergoing follow‐up in a hematology clinic between January 2010 and December 2011. Twenty‐four patients had proven or probable IFI. No information on age or sex of patients was provided | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Furfaro 2018.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with BDG testing. Samples were collected twice weekly | ||
| Patient characteristics and setting | Records of all adult neutropenic patients with hematological maligancies with GM and BDG testing from January 2011 to December 2013 were included. The median age was 58 years, and 60% were male. There were no proven cases of invasive aspergillosis and 20 probable cases | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Sample taken within 7 days of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Giacobbe 2017.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with 1 BDG test | ||
| Patient characteristics and setting | Adult ICU patients with candidemia (n = 73) or bacteremia (n = 93) were included. Among candidemia patients, 59% were male and the median age was 64 years | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Candidemia diagnosed by a positive blood culture | ||
| Flow and timing | BDG test was done within 48 hours of the reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Gupta 2017.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | Included 125 pediatric patients with hematologic or solid tumor malignancies with and without IFI. The age of patients ranged from 1 to 15 years, and 58% were male. Two patients had proven IFI, and 60 had probable IFI | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria; unclear if BDG was excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hachem 2009.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice the first week, once every other week after that, as part of a prospective study | ||
| Patient characteristics and setting | Patients with hematologic malignancies or solid tumor malignancies. Patients with proven or probable IA were cases, and those with solid tumor were controls. The age range of patients was 10 to 81 years, and 60% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2002 EORTC criteria | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hammarstrom 2015.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients for whom serum BDG was collected twice weekly | ||
| Patient characteristics and setting | Adult hematology or hematopoietic allogenic stem cell transplantation patients with 2 consecutive BDG tests. Thirteen patients had proven or probable IFI. The median age was 53 years (range 17 to 79 years), and 61% were male | ||
| Index tests | Glucatell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | BDG test was done with 1 week of reference standard. All received index test and reference standard; however, possible IFI cases were excluded from the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Hammarstrom 2018.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once to twice weekly as part of a prospective study. | ||
| Patient characteristics and setting | Between September 2011 and December 2012, 135 adult hematology patients were enrolled in the study. More than half (56%) were male, with a median age of 55 years. Thirteen patients met the criteria for IFI | ||
| Index tests | Glucatell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | All received index test and reference standard; 10 possible IFI cases were excluded from the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Hanson 2012.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | 64 adult ICU patients with a stay of at least 3 days. Included 1 proven and 5 probable cases of invasive candidiasis. The median age of patients was 60 years (range 19 to 82 years), and 69% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Horiguchi 2004.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected as part of a prospective consecutive study | ||
| Patient characteristics and setting | Sixty‐nine adult patients with hematological malignancies were enrolled. Eight met the criteria for proven or probable IFI | ||
| Index tests | Fungitec‐G test using 20 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis as determined by the 2002 EORTC criteria | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Jin 2013.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | Adult patients with hematological malignancies who had been admitted to the hospital between 2005 and 2010. Of the 378 patients in the study, 143 had proven or probable invasive aspergillosis. The age range of patients was between 20 and 76 years, and most were male (78%) | ||
| Index tests | Glucatell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2002 EORTC criteria | ||
| Flow and timing | Unclear time frame between sample and reference standard; possible IA cases were excluded from the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Kami 2001.
| Study characteristics | |||
| Patient Sampling | Both retrospective and prospective BDG samples were collected from patients | ||
| Patient characteristics and setting | All BMT patients were included in this study, as well as some high‐dose chemotherapy patients. Of the 122 patients, 33 had proven invasive aspergillosis. A majority (76%) were male, and patients ranged in age from 17 to 80 years | ||
| Index tests | Fungitec‐G test using 20 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis diagnosed by histologic evidence and positive for Aspergillus in sputum, biopsy, or autopsy | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kawazu 2004.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected weekly as part of a prospective study | ||
| Patient characteristics and setting | Adult hematological patients at high risk for invasive aspergillosis. There were 149 episodes occurring among 96 consecutive patients; of these, 11 were cases of proven or probable invasive aspergillosis. The mean age of patients was 45 years, and 70% were male | ||
| Index tests | Wako test using multiple cut‐offs for positivity, including 2, 3, 5, and 11 pg/mL | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2002 EORTC criteria | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Koltze 2015.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected weekly as part of a prospective study | ||
| Patient characteristics and setting | 34 pediatric patients who had undergone HSCT transplants. Two patients were diagnosed with a proven IFI, and 4 patients had probable invasive aspergillosis. The age of patients ranged from 0 to 16 years, and 56% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Koo 2009.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with at least 1 BDG test | ||
| Patient characteristics and setting | 871 adult patients at risk for IFI who had a BDG test, mostly those with hematologic malignancies. There were 116 proven or probable IFI cases. The median age of patients was 54 years, and 44% were female | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Sample taken within 1 week of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lahmer 2016a.
| Study characteristics | |||
| Patient Sampling | Retrospective study; sampling strategy uncertain | ||
| Patient characteristics and setting | 49 immunosuppressed patients who were in the ICU between December 2014 and December 2015. Of these, 13 had probable invasive aspergillosis. The mean age of patients was 59 years (range 18 to 84 years), and 57% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis as determined by the 2008 EORTC criteria; unclear whether BDG was excluded | ||
| Flow and timing | BDG sampled within 6 days (± 2 days) of the reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lahmer 2016b.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with 1 BDG test | ||
| Patient characteristics and setting | 30 adult hematologic patients in ICU for septic shock. Of these, 10 had proven or probable invasive aspergillosis. The mean age of patients was 59 years, and about half (56%) were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2008 EORTC criteria; unclear whether BDG was excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Leon 2016.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | 233 ICU patients with a hospital stay longer than 7 days. Included 31 cases of invasive candidiasis, 154 colonized, and 48 with neither. The mean age of patients was 66.7 years, and two‐thirds (67%) were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity; 2 sequential positives required | ||
| Target condition and reference standard(s) | Invasive candidiasis diagnosed by a positive blood culture | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Liu 2009.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with 1 BDG test | ||
| Patient characteristics and setting | 162 hematologic or BMT patients ranging in age from 6 to 85 years. Twenty had proven or probable IFI | ||
| Index tests | Fungitec‐G using 20 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI as determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unknown time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lo Cascio 2015.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with 1 BDG test | ||
| Patient characteristics and setting | 267 ICU patients with high risk factors for invasive candidiasis. Included 91 patients with proven or probable IFIs. The median age was 61.5 years, and 34% were female | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Proven invasive candidiasis determined by the 2008 EORTC criteria as positive blood culture; unclear whether BDG was included for probable invasive candidiasis | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mackay 2011.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | 72 ICU neonates with clinically suspected late‐onset sepsis. Of these, 19 had proven or probable IFI. The median gestational age was 31 weeks, and 63% were male | ||
| Index tests | Fungitell test using 60 and 80 pg/mL as cut‐offs for positivity | ||
| Target condition and reference standard(s) | Mixed IFI diagnosed by a positive culture from a sterile site | ||
| Flow and timing | Unclear on time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Martin‐Mazuelos 2015.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | 107 ICU patients with a stay of at least 7 days. There were 15 cases of invasive candidiasis. The mean age of patients was 62.7 years, and 66% were male. | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis diagnosed by a positive culture | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Metan 2012.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients with at least 1 BDG test. The number of samples collected varied from 1 to 23 per patient | ||
| Patient characteristics and setting | 128 hematologic patients with proven or probable IFI selected, as well as patients with no IFI; possible IFI cases were excluded. The median age was 47 years (range 17 to 77 years), and 57% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Sample taken within 10 days of reference standard; all patients selected for study received index test and reference standard for this analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Metan 2013.
| Study characteristics | |||
| Patient Sampling | Retrospective study of patients for whom serum BDG was collected twice weekly | ||
| Patient characteristics and setting | 84 patients who underwent an autologous HSCT between April 2009 and December 2010. There were 3 cases of probable invasive aspergillosis. The median age was 47 years (range 19 to 71 years), and 70% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive aspergillosis as determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | BDG sampled within 10 days of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | Original article excluded from the analysis 5 possible cases, which we included | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mohr 2011.
| Study characteristics | |||
| Patient Sampling | Serum BDG samples taken twice weekly as part of a prospective sequential study | ||
| Patient characteristics and setting | 57 ICU patients with a stay of 5 days or longer. Most were male (70%), and the median age was 39 years (range 18 to 76 years). There were 3 proven cases of invasive candidiasis | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis diagnosed by a positive culture from a sterile site | ||
| Flow and timing | Sample taken the same day of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Odabasi 2004.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly | ||
| Patient characteristics and setting | 283 adult hematologic patients, of whom 20 had proven or probable IFI. No information was provided on age or sex of patients | ||
| Index tests | Glucatell test using 60 pg/mL as cut‐off for positivity; actual measurements were also reported | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2002 EORTC criteria | ||
| Flow and timing | Test within 10 days of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | Grant from the Associates of Cape Cod was provided | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Persat 2008.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study of patients with 1 BDG test | ||
| Patient characteristics and setting | 117 hematologic or ICU patients with proven or probable IFI, as well as 122 at‐risk controls. No information on age or sex of patients was provided | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2002 EORTC criteria | ||
| Flow and timing | BDG sample that was closest to the reference standard was used, but no time frame was specified; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Pini 2019.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study of patients with BDG testing | ||
| Patient characteristics and setting | 29 patients with confirmed Candida were selected as cases, and 28 controls were at risk. Cases and controls were selected from adult non‐neutropenic patients hospitalized between November 2011 and January 2015; most (77%) were from the ICU | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis was diagnosed by either culture or histopathology | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Posteraro 2011.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once or more often per patient as part of a prospective study | ||
| Patient characteristics and setting | 95 adult ICU patients with sepsis and a stay of 5 or more days. Of these, 16 had proven IFI. The median age of patients was 69 years (range 18 to 93 years), and 68% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Test within 24 to 72 hours of reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Racil 2010.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly in a prospective study | ||
| Patient characteristics and setting | 91 patients with hematologic malignancies; patients could be enrolled more than once. A total of 104 patient cycles were included, of which 3 were proven and 9 were probable IFIs. No information on age or sex of patients was provided | ||
| Index tests | Fungitell test using 60 and 80 pg/mL as cut‐offs for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rose 2014.
| Study characteristics | |||
| Patient Sampling | Retrospective study of consecutive patients with 1 BDG test | ||
| Patient characteristics and setting | 132 patients who were seen for suspected fungal pneumonia were included. Of these, 34 had proven or probable IFI. A little more than half (55%) were male and of Caucasian race (55%). Most had either a hematologic malignancy (44%) or an allogenic stem cell transplantation (30%) | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria; unclear if BDG was included | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Salerno 2014.
| Study characteristics | |||
| Patient Sampling | Retrospective study of consecutive patients with 1 BDG test | ||
| Patient characteristics and setting | 108 adult HIV patients with serum samples analyzed for BDG. Of these, 46 had proven PJP. The mean age of PJP cases was 42.9 years; the mean age of non‐cases was 45.0 years; about two‐thirds (69%) of patients were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Pneumocystis jirovecii pneumonia (PJP) diagnosed by microbiological confirmation | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Senn 2008.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly in the absence of fever, and daily if fever was present, as part of a prospective study | ||
| Patient characteristics and setting | 95 adult hematologic patients hospitalized for myeloablative chemotherapy between 2002 and 2006. There were 30 cases of proven or probable IFI. The average age of patients was 57 years (range 19 to 77 years), and 61% were male | ||
| Index tests | Wako test using 11 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI as determined by the 2002 EORTC criteria | ||
| Flow and timing | Median time interval between sample and reference standard was 7.5 days, with a range of 0 to 51 days; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Shabaan 2018.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once within 24 hours of prospective study enrollment | ||
| Patient characteristics and setting | 77 ICU neonates at high risk for IFI. There were 11 definite and 25 suspected cases of IFI. Two‐thirds (68%) were male | ||
| Index tests | Dynamiker Fungus using 95 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Invasive candidiasis diagnosed by a positive blood culture | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Singh 2015.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | 199 adult liver transplant recipients at high risk for IFI. Of these, 12 were diagnosed with proven IFIs. The median age of patients was 58 years (range 19 to 75 years), and 70% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI as determined by the 2008 EORTC criteria, with BDG excluded | ||
| Flow and timing | Unknown time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Talento 2017.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected twice weekly as part of a prospective study | ||
| Patient characteristics and setting | 100 adult ICU patients with a hospital stay of 7 days or longer. There were 33 proven and probable cases of IFI. The mean age at enrollment was 64.4 years (range 20 to 85 years), and 67% were male | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Theel 2013.
| Study characteristics | |||
| Patient Sampling | Serum BDG collected once per patient as part of a prospective study | ||
| Patient characteristics and setting | 123 immunocompromised patients at risk for IFI. There were 4 cases of proven IFI and 16 probable cases. No information was provided on age or sex of patients | ||
| Index tests | Fungitell test using 80 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria with BDG excluded | ||
| Flow and timing | Unclear time frame between sample and reference standard; 14 possible cases were excluded from the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Verduyn Lunel 2009.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study for which serum BDG was collected twice weekly | ||
| Patient characteristics and setting | Records of hematology/oncology patients admitted to hospital intensive cytotoxic chemotherapy or to undergo HSCT between January 1999 and December 2005. Of these, 21 patients had proven invasive candidiasis. Thirty hematology controls were selected as a comparison group. Almost two‐thirds (32/51) were male, with age range of 3 to 65 years | ||
| Index tests | Fungitell test with < 60 pg/mL considered negative | ||
| Target condition and reference standard(s) | Invasive candidiasis isolated in blood culture or from sterile site | ||
| Flow and timing | Unclear time frame between sample and reference standard, but median was 3 days (range ‐104 to 190 days); all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
White 2017.
| Study characteristics | |||
| Patient Sampling | Retrospective case‐control study of patients with BDG testing | ||
| Patient characteristics and setting | Cases and controls were both at risk for invasive fungal conditions. Patients had the following underlying conditions: HIV, organ transplant, and hematology malignancies | ||
| Index tests | Dynamiker Fungus test using 95 pg/mL as cut‐off for positivity | ||
| Target condition and reference standard(s) | Mixed IFI determined by the 2008 EORTC criteria; unclear if BDG was excluded. PJP diagnosed by PCR and/or radiologic evidence | ||
| Flow and timing | Unclear time frame between sample and reference standard; all received index test and reference standard and were included in the analysis | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
BAL: bronchoalveolar lavage; BDG: beta‐D‐glucan test; BMT: bone marrow transplant; EORTC: European Organization for Research and Treatment of Cancer; IA: inflammatory arthritis; IFI: Invasive fungal infection; GM: galactomannan; HSCT: hematopoietic stem cell transplantation; ICU: Intensive care unit; NICU: neonatal intensive care unit; PJP: Pneumocystis jirovecii pneumonia; PCR: polymerase chain reaction.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acosta 2012 | Unable to determine cell counts |
| Akamatsu 2007 | Assay cut‐off did not follow manufacturer's recommendations |
| Azoulay 2016 | Possible IFI included in case definition; unable to separate |
| Badiee 2016 | Assay cut‐off did not follow manufacturer's recommendations |
| Bellanger 2011 | Study utilizes 2 cut‐off values for determining positivity of BDG |
| Bhaskaran 2017 | Only bronchoalveolar lavage fluid results given |
| Boluk 2016 | Included BDG test as part of reference standard |
| Brasier 2015 | Unable to determine cell counts |
| Calitri 2017 | Unable to determine cell counts |
| Dobias 2018 | Study population did not match inclusion guidelines |
| Donato 2017 | Study population did not match inclusion guidelines |
| Ellis 2008 | Possible IFI included in case definition; unable to separate |
| Goudijl 2013 | Assay cut‐off did not follow manufacturer's recommendations |
| Guitard 2016 | Unable to determine cell counts |
| Han 2015 | Unable to determine cell counts |
| Hartl 2018 | Possible IFI included in case definition; unable to separate |
| Heyland 2011 | Did not follow EORTC guidelines; questionable patient population |
| Hoenigl 2014 | Only bronchoalveolar lavage fluid results given |
| Ji 2008 | Unable to determine cell counts |
| Kato 2010 | Included BDG as part of reference standard |
| Kishimoto 2019 | Study population did not match inclusion guidelines |
| Kumar 2018 | Study population did not match inclusion guidelines |
| Lahmer 2016c | Unable to determine cell counts |
| Lahmer 2017 | Study population did not match inclusion guidelines |
| Leon 2009 | Assay cut‐off did not follow manufacturer's recommendations |
| Leon 2012 | Unable to determine cell counts |
| Levesque 2015 | Unable to determine cell counts |
| Levesque 2017 | Unable to determine cell counts |
| Matsumara 2011 | Study utilizes 2 cut‐off values for determining positivity of BDG |
| McKeating 2018 | Unable to determine patient population |
| Metan 2016 | Unable to determine cell counts |
| Montagna 2012 | Unable to determine cell counts |
| Mutschlechner 2015 | Unable to determine cell counts |
| Oz 2014 | Possible IFI included in case definition; unable to separate |
| Pazos 2005 | Assay cut‐off did not follow manufacturer's recommendations |
| Pazos 2006 | Assay cut‐off did not follow manufacturer's recommendations |
| Picardi 2019 | Not a diagnostic accuracy study |
| Presterl 2009 | Assay cut‐off did not follow manufacturer's recommendations |
| Ramos 2017 | Cannot separate confirmed Candida cases from probable Candida cases |
| Rhein 2014 | Only cerebrospinal fluid results given |
| Sax 2011 | Did not follow EORTC guidelines |
| Shi 2015 | Did not follow EORTC guidelines |
| Su 2017 | Did not follow EORTC guidelines |
| Sulahian 2014 | Unable to determine cell counts |
| Tasaka 2007 | Unable to determine cell counts |
| Watanabe 2009 | Assay cut‐off did not follow manufacturer's recommendations |
| White 2018 | Unable to determine cell counts |
| Wood 2013 | Cannot separate confirmed PJP cases from probable cases |
| Yang 2012 | Did not follow EORTC guidelines |
| Yu 2010 | Unable to determine cell counts |
| Zheng 2017 | Assay cut‐off did not follow manufacturer's recommendations |
BDG: beta‐D‐glucan test; EORTC: European Organization for Research and Treatment of Cancer; IFI: invasive fungal infection; PJP: Pneumocystis jirovecii pneumonia.
Differences between protocol and review
We made the following changes to the information published in the protocol of this review (Schmidt 2012).
QUADAS‐2 was used in place of QUADAS‐1. No comparison was made with GM due to lack of data and time constraints.
We intended to perform a meta‐analysis of all BDG tests but did not do so due to the high level of heterogeneity between studies.
We did not perform a formal analysis of heterogeneity using meta‐regression because we did not perform a meta‐analysis.
We excluded studies that used BDG as part of the reference standard because these studies had high risk of incorporation bias.
We did not include studies from which we could not extract data for a 2 × 2 table.
We included data from the Dynamiker test, which became commercially available after the protocol was published.
We did not explore the impact of diagnostic thresholds (cut‐off values) because almost all studies used the manufacturer's recommended cut‐off and because the level of heterogeneity between studies was high.
Contributions of authors
Kimberly Hanson: study design, writing of the manuscript. Robert Schmidt: study design, evaluation of study eligibility, data extraction, quality assessment, statistical analysis, writing of the manuscript. Brandon Walker: data extraction, quality assessment. Sandra White: evaluation of study eligibility, data extraction, quality assessment, statistical analysis, writing of the manuscript.
Sources of support
Internal sources
-
Salary Support, USA
All investigators received salaries from ARUP Laboratories (BSW) or the University of Utah (SKW, RLS, KEH). RLS and KEH serve as medical directors at ARUP Laboratories. ARUP is wholly owned by the University of Utah and performs clinical laboratory tests including BDG testing.
External sources
No sources of support supplied
Declarations of interest
Kimberly Hanson: none known. Robert Schmidt: none known. Brandon Walker: none known. Sandra White: none known.
New
References
References to studies included in this review
Acosta 2011 {published data only}
- Acosta J, Catalan M, Palacio-Perez-Medel A, Lora D, Montejo JC, Cuetara MS, et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1→3)-β-D-glucan chromogenic assay in serum samples. Clinical Microbiology and Infection 2011;17:1053-60. [DOI] [PubMed] [Google Scholar]
Alexander 2010 {published data only}
- Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB. The (1,3) β-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. Journal of Clinical Microbiology 2010;48(11):4083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atalay 2014 {published data only}
- Atalay A, Sav H, Koc AN, Yildiz O, Demir G, Eser B, et al. Assessment of β-D-(1→3)-glucan assay for diagnosis of invasive fungal infections. Acta Medica Mediterranea 2014;30:685-90. [Google Scholar]
Badiee 2012 {published data only}
- Badiee P, Alborzi A, Karimi M, Pourabbas B, Haddadi P, Mardaneh J, et al. Diagnostic potential of nested PCR, galactomannan EIA, and beta-D-glucan for invasive aspergillosis in pediatric patients. Journal of Infection in Developing Countries 2012;6(4):352-7. [DOI] [PubMed] [Google Scholar]
Boch 2016 {published data only}
- Boch T, Spiess B, Cornely OA, Vehreschild JJ, Rath PM, Steinmann J, et al. Diagnosis of invasive fungal infections in haematological patients by combined use of galactomannan, 1,3-β-D-glucan, Aspergillus PCR, multifungal DNA-microarray, and Aspergillus azole resistance PCRs in blood and bronchoalveolar lavage samples: results of a prospective muticentre study. Clinical Microbiology and Infection 2016;22:862-8. [DOI] [PubMed] [Google Scholar]
Ceesay 2015 {published data only}
- Ceesay MM, Desai SR, Berry L, Cleverley J, Kibbler CC, Pomplun S, et al. A comprehensive diagnostic approach using galactomannan, targeted β-D-glucan, baseline computerized tomography and biopsy yields a significant burden of invasive fungal disease in at risk haematology patients. British Journal of Haematology 2015;168:219-29. [DOI] [PubMed] [Google Scholar]
Cornu 2018 {published data only}
- Cornu M, Goudjil S, Kongolo G, Leke A, Poulain D, Chouaki T, et al. Evaluation of the (1,3)-β-D-glucan assay for the diagnosis of neonatal invasive yeast infections. Medical Mycology 2018;56:78-87. [DOI] [PubMed] [Google Scholar]
Costa 2012 {published data only}
- Costa JM, Botterel F, Cabaret O, Foulet F, Cordonnier C, Bretagne S. Association between circulating DNA, serum (1→3)-β-D-glucan, and pulmonary fungal burden in Pneumocystis pneumonia. Clinical Infectious Diseases 2012;55(2):e5-8. [DOI] [PubMed] [Google Scholar]
Del Bono 2011 {published data only}
- Del Bono V, Delfino E, Furfaro E, Mikulska M, Nicco E, Bruzzi P, et al. Clinical performance of the (1,3)-β-D-glucan assay in early diagnosis of nosocomial candida bloodstream infections. Clinical and Vaccine Immunology 2011;18(12):2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vlieger 2011 {published data only}
- De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W, Van Wijngaerden E. Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: an autopsy-based study. Journal of Clinical Microbiology 2011;49(11):3783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dichtl 2018 {published data only}
- Dichtl K, Seybold U, Wagener J. Evaluation of a turbidimetric β-D-glucan test for detection of Pneumocystis jirovecii pneumonia. Journal of Clinical Microbiology 2018;56(7):e00286-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontana 2012 {published data only}
- Fontana C, Gaziano R, Favaro M, Casalinuovo IA, Pistoia ES, Di Francesco P. (1-3)-β-D-glucan vs galactomannan antigen in diagnosing invasive fungal infections (IFIs). Open Microbiology Journal 2012;6:70-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furfaro 2018 {published data only}
- Furfaro E, Giacobbe DR, Del Bono V, Signori A, Guolo F, Minetto P, et al. Performance of serum (1,3)-β-D-glucan screening for the diagnosis of invasive aspergillosis in neutropenic patients with haematological malignancies. Mycoses 2018;61:650-5. [DOI] [PubMed] [Google Scholar]
Giacobbe 2017 {published data only}
- Giacobbe DR, Mikulska M, Tumbarello M, Furfaro E, Spadaro M, Losito AR, et al. Combined use of serum (1,3)-β-D-glucan and procalcitonin for the early differential diagnosis between candidaemia and bacteraemia in intensive care units. Critical Care (London, England) 2017;21:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2017 {published data only}
- Gupta P, Ahmad A, Khare V, Kumar A, Banerjee G, Verma N, et al. Comparative evaluation of pan-fungal real-time PCR, galactomannan and (1-3)-β-D-glucan assay for invasive fungal infection in paediatric cancer patients. Mycoses 2017;60:234-40. [DOI] [PubMed] [Google Scholar]
Hachem 2009 {published data only}
- Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. Utility of galactomannan enzyme immunoassay and (1,3) β-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillusfumigatus infection in hematologic malignancy patients. Journal of Clinical Microbiology 2009;47(1):129-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammarstrom 2015 {published data only}
- Hammarstrom H, Kondori N, Friman V, Wenneras C. How to interpret serum levels of beta-glucan for the diagnosis of invasive fungal infections in adult high-risk hematology patients: optimal cut-off levels and confounding factors. European Journal of Clinical Microbiology & Infectious Diseases 2015;34:917-25. [DOI] [PubMed] [Google Scholar]
Hammarstrom 2018 {published data only}
- Hammarstrom H, Aspelund AS, Christensson B, Heuβel CP, Isaksson J, Kondori N, et al. Prospective evaluation of a combination of fungal biomarkers for the diagnosis of invasive fungal disease in high-risk haematology patients. Mycoses 2018;61:623-32. [DOI] [PubMed] [Google Scholar]
Hanson 2012 {published data only}
- Hanson KE, Pfeiffer CD, Lease ED, Balch AH, Zaas AK, Perfect JR, et al. β-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PloS One 2012;7(8):e42282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horiguchi 2004 {published data only}
- Horiguchi Y. The performance of (1,3)-beta-D-glucan and Aspergillus galactomannan measurement for early diagnosis of invasive aspergillosis in patients with hematological disease. Journal of the Japanese Association for Infectious Diseases 2004;78:566-73. [DOI] [PubMed] [Google Scholar]
Jin 2013 {published data only}
- Jin X, Chen Y, Yu N, Zuo X, Song S, Yin X, et al. Detection of galactomannan and (1→3)-β-D-gluan for early diagnosis of invasive aspergillosis in hematological cancer patients. International Journal of Pharmacology 2013;9(1):86-91. [Google Scholar]
Kami 2001 {published data only}
- Kami M, Fukui T, Ogawa S, Kazuyama Y, Machida U, Tanaka Y, et al. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clinical Infectious Diseases 2001;33:1504-12. [DOI] [PubMed] [Google Scholar]
Kawazu 2004 {published data only}
- Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, et al. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-β-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. Journal of Clinical Microbiology 2004;42(6):2733-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koltze 2015 {published data only}
- Koltze A, Rath P, Schöning S, Steinmann J, Wichelhaus TA, Bader P, et al. β-D-glucan screening for detection of invasive fungal disease in children undergoing allogeneic hematopoietic stem cell transplantation. Journal of Clinical Microbiology 2015;53:2605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koo 2009 {published data only}
- Koo S, Bryar JM, Page JH, Baden LR, Marty FM. Diagnostic performance of the (1→3)-β-D-glucan assay for invasive fungal disease. Clinical Infectious Diseases 2009;49:1650-9. [DOI] [PubMed] [Google Scholar]
Lahmer 2016a {published data only}
- Lahmer T, Neuenhahn M, Held J, Rasch S, Schmid RM, Huber W. Comparison of 1,3-β-D-glucan with galactomannan in serum and bronchoalveolar fluid for the detection of Aspergillus species in immunosuppressed mechanical ventilated critically ill patients. Journal of Critical Care 2016;36:259-64. [DOI] [PubMed] [Google Scholar]
Lahmer 2016b {published data only}
- Lahmer T, Rasch S, Schnappauf C, Beitz A, Schmid RM, Huber W. Comparison of serum galactomannan and 1,3-beta-D-glucan determination for early detection of invasive pulmonary aspergillosis in critically ill patients with hematological malignancies and septic shock. Mycopathologia 2016;181:505-11. [DOI] [PubMed] [Google Scholar]
Leon 2016 {published data only}
- Leon C, Ruiz-Santana S, Saavedra P, Castro C, Loza A, Zakariya I, et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Critical Care (London, England) 2016;20:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu F, Wu T, Cai P, Liu Y, Lu Y, Zhou JR, et al. Diagnostic value of plasma (1,3)-β-D glucan assay for invasive fungal infections in patients with hematological disorders. Journal of Experimental Hematology 2009;17(4):1043-6. [PubMed] [Google Scholar]
Lo Cascio 2015 {published data only}
- Lo Cascio G, Koncan R, Stringari G, Russo A, Azzini A, Ugolini A, et al. Interference of confounding factors on the use of (1,3)-beta-D-glucan in the diagnosis of invasive candidiasis in the intensive care unit. European Journal of Clinical Microbiology & Infectious Diseases 2015;34:357-65. [DOI] [PubMed] [Google Scholar]
Mackay 2011 {published data only}
- Mackay CA, Ballot DE, Perovic O. Serum 1,3-β-D-glucan assay in the diagnosis of invasive fungal disease in neonates. Pediatric Reports 2011;3:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Mazuelos 2015 {published data only}
- Martin-Mazuelos E, Loza A, Castro C, Macias D, Zakariya I, Saavedra P, et al. β-D-glucan and Candida albicans germ tube antibody in ICU patients with invasive candidiasis. Intensive Care Medicine 2015;41:1424-32. [DOI] [PubMed] [Google Scholar]
Metan 2012 {published data only}
- Metan G, Koç AN, Atalay A, Kaynar LG, Ozturk A, Alp E, et al. What should be the optimal cut-off of serum 1,3-β-D-glucan for the detection of invasive pulmonary aspergillosis in patients with haematological malignancies? Scandinavian Journal of Infectious Diseases 2012;44:330-6. [DOI] [PubMed] [Google Scholar]
Metan 2013 {published data only}
- Metan G, Koc AN, Kaynar LG, Atalay A, Ozturk A, Eser B, et al. What is the role of the (1→3)-β-D-glucan assay in the screening of patients undergoing autologous haematopoietic stem-cell transplantation? Mycoses 2013;56:34-8. [DOI] [PubMed] [Google Scholar]
Mohr 2011 {published data only}
- Mohr JF, Sims C, Paetznick V, Rodriguez J, Finkelman MA, Rex JH, et al. Prospective survey of (1→3)-β-D-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. Journal of Clinical Microbiology 2011;49(1):58-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odabasi 2004 {published data only}
- Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, et al. β-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clinical Infectious Diseases 2004;39:199-205. [DOI] [PubMed] [Google Scholar]
Persat 2008 {published data only}
- Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A. Contribution of the (1→3)-β-D-glucan assay for diagnosis of invasive fungal infections. Journal of Clinical Microbiology 2008;46(3):1009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pini 2019 {published data only}
- Pini P, Colombari B, Marchi E, Castagnoli A, Venturelli C, Sarti M, et al. Performance of Candida albicans germ tube antibodies (CAGTA) and its association with (1→3)-β-D-glucan (BDG) for diagnosis of invasive candidiasis (IC). Diagnostic Microbiology and Infectious Disease 2019;93:39-43. [DOI] [PubMed] [Google Scholar]
Posteraro 2011 {published data only}
- Posteraro B, De Pascale G, Tumbarello M, Torelli R, Pennisi MA, Bello G, et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-β-D-glucan assay, Candida score, and colonization index. Critical Care 2011;15:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Racil 2010 {published data only}
- Racil Z, Kocmanova I, Lengerova M, Weinbergerova B, Buresova L, Toskova M, et al. Difficulties in using 1,3-β-D-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies - high frequency of false-positive results and their analysis. Journal of Medical Microbiology 2010;59:1016-22. [DOI] [PubMed] [Google Scholar]
Rose 2014 {published data only}
- Rose SR, Vallabhajosyula S, Velez MG, Fedorko DP, VanRaden MJ, Gea-Banacloche JC, et al. The utility of bronchoalveolar lavage beta-D-glucan testing for the diagnosis of invasive fungal infections. Journal of Infection 2014;69:278-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salerno 2014 {published data only}
- Salerno D, Mushatt D, Myers L, Zhuang Y, la Rua N, Calderon EJ, et al. Serum and BAL beta-D-glucan for the diagnosis of Pneumocystis pneumonia in HIV-positive patients. Respiratory Medicine 2014;108(11):1688-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senn 2008 {published data only}
- Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, et al. 1,3-β-D-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clinical Infectious Diseases 2008;46:878-85. [DOI] [PubMed] [Google Scholar]
Shabaan 2018 {published data only}
- Shabaan AE, Elbaz LM, El-Emshaty WM, Shouman B. Role of serum (1,3)-β-D-glucan assay in early diagnosis of invasive fungal infections in a neonatal intensive care unit. Jornal de Pediatria 2018;94(5):559-65. [DOI] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh N, Winston DJ, Limaye AP, Pelletier S, Safdar N, Morris MI, et al. Performance characteristics of galactomannan and β-D-glucan in high-risk liver transplant recipients. Transplantation 2015;99(12):2543-50. [DOI] [PubMed] [Google Scholar]
Talento 2017 {published data only}
- Talento AF, Dunne K, Joyce EA, Palmer M, Johnson E, White PL, et al. A prospective study of fungal biomarkers to improve management of invasive fungal diseases in a mixed specialty critical care unit. Journal of Critical Care 2017;40:119-27. [DOI] [PubMed] [Google Scholar]
Theel 2013 {published data only}
- Theel ES, Jespersen DJ, Iqbal S, Bestrom JE, Rollins LO, Misner LJ, et al. Detection of (1,3)-β-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia 2013;175:33-41. [DOI] [PubMed] [Google Scholar]
Verduyn Lunel 2009 {published data only}
- Verduyn Lunel FM, Mennink-Kersten MASH, Ruegebrink D, Lee HAL, Donnelly JP, Blijlevens NMA, et al. Value of Candida serum markers in patients with invasive candidiasis after myeloablative chemotherapy. Diagnostic Microbiology and Infectious Disease 2009;64:408-15. [DOI] [PubMed] [Google Scholar]
White 2017 {published data only}
- White PL, Price JS, Posso RB, Barnes RA. An evaluation of the performance of the Dynamiker® Fungus (1-3)-β-D-glucan assay to assist in the diagnosis of invasive aspergillosis, invasive candidiasis and Pneumocystis pneumonia. Medical Mycology 2017;55:843-50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acosta 2012 {published data only}
- Acosta J, Catalan M, Palacio PM, Montejo JC, De La Cruz-Bertolo J, Moragues MD, et al. Prospective study in critically ill non-neutropenic patients: diagnostic potential of (1,3)-β-D-glucan assay and circulating galactomannan for the diagnosis of invasive fungal disease. European Journal of Clinical Microbiology & Infectious Diseases 2012;31:721-31. [DOI] [PubMed] [Google Scholar]
Akamatsu 2007 {published data only}
- Akamatsu N, Sugawara Y, Kaneko J, Tamura S, Makuuchi M. Pre-emptive treatment of fungal infection based on plasma (1→3)-β-D-glucan levels after liver transplantation. Infection 2007;35(5):346-51. [DOI] [PubMed] [Google Scholar]
Azoulay 2016 {published data only}
- Azoulay E, Guigue N, Darmon M, Mokart D, Lemiale V, Kouatchet A, et al. (1,3)-β-D-glucan assay for diagnosing invasive fungal infections in critically ill patients with hematological malignancies. Oncotarget 2016;7(16):21484-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badiee 2016 {published data only}
- Badiee P, Hashemizadeh Z, Ramzi M, Karimi M, Mohammadi R. Non-invasive methods to diagnose fungal infections in pediatric patients with hematologic disorders. Jundishapur Journal of Microbiology 2016;9(11):e41573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellanger 2011 {published data only}
- Bellanger AP, Grenouillet F, Henon T, Skana F, LeGrand F, DeConinck E, et al. Retrospective assessment of β-D-(1,3)-glucan for presumptive diagnosis of fungal infections. APMIS 2011;119:280-6. [DOI] [PubMed] [Google Scholar]
Bhaskaran 2017 {published data only}
- Bhaskaran A, Kabbani D, Singer LG, Prochnow T, Bhimji A, Rotstein C, et al. (1,3)-β-D-glucan in bronchoalveolar lavage of lung transplant recipients for the diagnosis of invasive pulmonary aspergillosis. Medical Mycology 2017;55:173-9. [DOI] [PubMed] [Google Scholar]
Boluk 2016 {published data only}
- Boluk G, Kazak E, Ozkalemkas F, Ener B, Akalin H, Agca H, et al. Comparison of galactomannan, beta-D-glucan, and Aspergillus DNA in sera of high-risk adult patients with hematological malignancies for the diagnosis of invasive aspergillosis. Turkish Journal of Medical Sciences 2016;46:335-42. [DOI] [PubMed] [Google Scholar]
Brasier 2015 {published data only}
- Brasier AR, Zhao Y, Spratt HM, Wiktorowicz JE, Ju H, Wheat LJ, et al. Improved detection of invasive pulmonary aspergillosis arising during leukemia treatment using a panel of host response proteins and fungal antigens. PloS One 2015;10(11):e0143165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calitri 2017 {published data only}
- Calitri C, Caviglia I, Cangemi G, Furfaro E, Bandettini R, Fioredda F, et al. Performance of 1,3-beta-D-glucan for diagnosing invasive fungal diseases in children. Mycoses 2017;60(12):789-95. [DOI] [PubMed] [Google Scholar]
Dobias 2018 {published data only}
- Dobias R, Jaworska P, Tomaskova H, Kanova M, Lyskova P, Vrba Z, et al. Diagnostic value of serum galactomannan, (1-3)-beta-D-glucan, and Aspergillus fumigatus-specific IgA and IgG assays for invasive pulmonary aspergillosis in non-neutropenic patients. Mycoses 2018;61(8):576-86. [DOI] [PubMed] [Google Scholar]
Donato 2017 {published data only}
- Donato L, Gonzalez T, Canales M, Legarraga P, Garcia P, Rabagliati R. The 1,3-beta-d-glucan in critical adult patients as diagnostic tool for invasive Candida spp. infection, performance evaluation [Evaluación del rendimiento de 1,3-β-d-glucano como apoyodiagnóstico de infecciones invasoras por Candida spp.en pacientes críticos adultos]. Revista Chilena de Infectologia 2017;34(4):340-6. [DOI] [PubMed] [Google Scholar]
Ellis 2008 {published data only}
- Ellis M, al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, et al. Assessment of the clinical utility of serial β-D-glucan concentrations in patients with persistent neutropenic fever. Journal of Medical Microbiology 2008;57:287-95. [DOI] [PubMed] [Google Scholar]
Goudijl 2013 {published data only}
- Goudjil S, Kongolo G, Dusol L, Imestouren F, Cornu M, Leke A, et al. (1→3)-β-D-glucan levels in candidiasis infections in the critically ill neonate. Journal of Maternal-Fetal & Neonatal Medicine 2013;26(1):44-8. [DOI] [PubMed] [Google Scholar]
Guitard 2016 {published data only}
- Guitard J, Tabone MD, Senghor Y, Cros C, Moissenet D, Markowicz K, et al. Detection of β-D-glucan for the diagnosis of invasive fungal infection in children with hematological malignancy. Journal of Infection 2016;73:607-15. [DOI] [PubMed] [Google Scholar]
Han 2015 {published data only}
- Han S, Su X, Zhao R, Fang K. The effect of albumin on (1,3)-β-D-glucan for diagnosis of invasive fungal infection. Chinese Critical Care Medicine 2015;27(8):672-6. [DOI] [PubMed] [Google Scholar]
Hartl 2018 {published data only}
- Hartl B, Zeller I, Manhart A, Selitsch B, Lass-Florl C, Willinger B. A retrospective assessment of four antigen assays for the detection of invasive candidiasis among high-risk hospitalized patients. Mycopathologia 2018;183(3):513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heyland 2011 {published data only}
- Heyland D, Jiang X, Day AG, Laverdiere M. Serum β-D-glucan of critically ill patients with suspected ventilator-associated pneumonia: preliminary observations. Journal of Critical Care 2011;26:536.e1-536.e9. [DOI] [PubMed] [Google Scholar]
Hoenigl 2014 {published data only}
- Hoenigl M, Prattes J, Spiess B, Wagner J, Prueller F, Raggam RB, et al. Performance of galactomannan, beta-D-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. Journal of Clinical Microbiology 2014;52(6):2039-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2008 {published data only}
- Ji Y, Huang X. Investigation of the cutoff value of a serum 1,3-β-D-glucan assay for early diagnosis of invasive fungal infection in Chinese hematopoietic stem cell transplant recipients. Chinese Journal of Hematology 2008;29(6):405-7. [PubMed] [Google Scholar]
Kato 2010 {published data only}
- Kato K, Onoda S, Asano J, Fukaya S, Yoshida S. Evaluation of the clinical cutoff level of serum (1→3)-β-D-glucan in patients with connective tissue diseases complicated by deep fungal infections. Modern Rheumatology / The Japan Rheumatism Association 2010;20:366-9. [DOI] [PubMed] [Google Scholar]
Kishimoto 2019 {published data only}
- Kishimoto K, Kasai M, Kawamura N, Ito Y, Yoshida M, Hasegawa D, et al. Clinical features in proven and probable invasive fungal disease in children and adolescents at a pediatric referral center: a 5-year experience. World Journal of Pediatrics 2019;15:270-5. [DOI] [PubMed] [Google Scholar]
Kumar 2018 {published data only}
- Kumar M, Mugunthan M. Beta-D-glucan and Aspergillus galactomannan assays in the diagnosis of invasive fungal infections. Medical Journal Armed Forces India 2019;75(4):357-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lahmer 2016c {published data only}
- Lahmer T, Held J, Rasch S, Schnappauf C, Beitz A, Schmid RM, et al. Usage of 1,3-β-D-glucan for early detection of invasive mycoses and outcome parameter in immunocompromised critically ill patients. Mycopathologia 2016;181:815-21. [DOI] [PubMed] [Google Scholar]
Lahmer 2017 {published data only}
- Lahmer T, da Costa CP, Held J, Rasch S, Ehmer U, Schmid RM, et al. Usefulness of 1,3 beta-D-glucan detection in non-HIV immunocompromised mechanical ventilated critically ill patients with ARDS and suspected Pneumocystis jirovecii pneumonia. Mycopathologia 2017;182(7-8):701-8. [DOI] [PubMed] [Google Scholar]
Leon 2009 {published data only}
- Leon C, Ruiz-Santana S, Saavedra P, Galvan B, Blanco A, Castro C, et al. Usefulness of the "Candida score" for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Critical Care Medicine 2009;37(5):1624-33. [DOI] [PubMed] [Google Scholar]
Leon 2012 {published data only}
- Leon C, Ruiz-Santana S, Saavedra P, Castro C, Ubeda A, Loza A, et al. Value of β-D-glucan and Candida albicans germ tube antibody for discriminating between Candida colonization and invasive candidiasis in patients with severe abdominal conditions. Intensive Care Medicine 2012;38:1315-25. [DOI] [PubMed] [Google Scholar]
Levesque 2015 {published data only}
- Levesque E, El Anbassi S, Sitterle E, Foulet F, Merle JC, Botterel F. Contribution of (1,3)-beta-D-glucan to diagnosis of invasive candidiasis after liver transplantation. Journal of Clinical Microbiology 2015;53(3):771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levesque 2017 {published data only}
- Levesque E, Rizk F, Noorah Z, Ait-Ammar N, Cordonnier JC, El Anbassi S, et al. Detection of (1,3)-β-D-glucan for the diagnosis of invasive fungal infection in liver transplant recipients. International Journal of Molecular Sciences 2017;18:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsumara 2011 {published data only}
- Matsumara Y, Ito Y, Iinuma I, Yasuma K, Yamamoto M, Matsushima A, et al. Quantitative real-time PCR and the (1→3)-β-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clinical Microbiology and Infection 2012;18:591-7. [DOI] [PubMed] [Google Scholar]
McKeating 2018 {published data only}
- McKeating C, White PL, Posso R, Palmer M, Johnson E, McMullan R. Diagnostic accuracy of fungal PCR and beta-d-glucan for detection of candidaemia: a preliminary evaluation. Journal of Clinical Pathology 2018;71(5):420-4. [DOI] [PubMed] [Google Scholar]
Metan 2016 {published data only}
- Metan G, Elmali F. The influence of the control group characteristics for the diagnostic performance of 1,3-beta-D-glucan in invasive aspergillosis. Journal de Mycologie Medicale 2016;26(4):408-10. [DOI] [PubMed] [Google Scholar]
Montagna 2012 {published data only}
- Montagna MT, De Giglio O, Napoli C, Lovero G, Caggiano G, Delia M, et al. Invasive fungal infections in patients with hematologic malignancies (Aurora project): lights and shadows during 18-months surveillance. International Journal of Molecular Sciences 2012;13:774-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutschlechner 2015 {published data only}
- Mutschlechner W, Risslegger B, Willinger B, Hoenigl M, Bucher B, Eschertzhuber S, et al. Bronchoalveolar lavage fluid (1,3)-β-D-glucan for the diagnosis of invasive fungal infections in solid organ transplantation: a prospective multicenter study. Transplantation 2015;99(9):e140. [DOI] [PubMed] [Google Scholar]
Oz 2014 {published data only}
- Oz HTH, Koc AN, Atalay MA, Eser B, Yildiz O, Kaynar LG. The diagnostic value of the galactomannan and (1,3)-beta-D-glucan in diagnosis of invasive aspergillosis. Nobel Medicus 2014;10:44-9. [Google Scholar]
Pazos 2005 {published data only}
- Pazos C, Ponton J, Del Palacio A. Contribution of (1→3)-β-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. Journal of Clinical Microbiology 2005;43(1):299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pazos 2006 {published data only}
- Pazos C, Moragues MD, Quindos G, Ponton J, Palacio A. Diagnostic potential of (1→3)-β-D-glucan and anti-Candida albicans germ tube antibodies for the diagnosis and therapeutic monitoring of invasive candidiasis in neutropenic adult patients. Revista Iberoamericana de Micologia 2006;23:209-15. [DOI] [PubMed] [Google Scholar]
Picardi 2019 {published data only}
- Picardi M, Della Pepa R, Giordano C, Pugliese N, Mortaruolo C, Trastulli F, et al. (1-3)-beta-D-glucan serum increase and small-airway-invasive radiological findings as early signs of pulmonary aspergillosis in high-risk hematologic patients in the posaconazole era: preliminary observations. Annals of Hematology 2019;98(2):527-31. [DOI] [PubMed] [Google Scholar]
Presterl 2009 {published data only}
- Presterl E, Parschalk B, Bauer E, Lassnigg A, Hajdu S, Graninger W. Invasive fungal infections and (1,3)-β-D-glucan serum concentrations in long-term intensive care patients. Journal of Infectious Diseases 2009;13:707-12. [DOI] [PubMed] [Google Scholar]
Ramos 2017 {published data only}
- Ramos JT, Villar S, Bouza E, Bergon-Sendin E, Rivilla AP, Collados CT, et al. Performance of a quantitative PCR-based assay and beta-D-glucan detection for diagnosis of invasive candidiasis in very-low-birth-weight preterm neonatal patients (CANDINEO Study). Journal of Clinical Microbiology 2017;55(9):2752-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhein 2014 {published data only}
- Rhein J, Bahr NC, Morawski BM, Schutz C, Zhang Y, Finkelman M, et al. Detection of high cerebrospinal fluid levels of (1→3)-β-D-glucan in cryptococcal meningitis. Open Forum Infectious Diseases 2014;1(3):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sax 2011 {published data only}
- Sax PE, Komarow L, Finkelman MA, Grant PM, Andersen J, Scully E, et al. Blood (1→3)-β-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clinical Infectious Diseases 2011;53(2):197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2015 {published data only}
- Shi X, Fan L, Liu D, Li L, Min L. Early serological diagnosis of invasive aspergillosis in renal transplant recipients. Journal Southern Medical University 2015;35(11):1659-61. [PubMed] [Google Scholar]
Su 2017 {published data only}
- Su KC, Chou KT, Hsiao YH, Tseng CM, Su VY, Lee YC, et al. Measuring (1,3)-β-D-glucan in tracheal aspirate, bronchoalveolar lavage fluid, and serum for detection of suspected Candida pneumonia in immunocompromised and critically ill patients: a prospective observational study. BMC Infectious Diseases 2017;17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sulahian 2014 {published data only}
- Sulahian A, Porcher R, Bergeron A, Touratier S, Raffoux E, Menotti J, et al. Use and limits of (1-3)-β-D-glucan assay (Fungitell), compared to galactomannan determination (Platelia Aspergillus), for diagnosis of invasive aspergillosis. Journal of Clinical Microbiology 2014;52(7):2328-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tasaka 2007 {published data only}
- Tasaka S, Hasegawa N, Kobayashi S, Yamada W, Nishimura T, Takeuchi T, et al. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest 2007;131(4):1173-80. [DOI] [PubMed] [Google Scholar]
Watanabe 2009 {published data only}
- Watanabe T, Yasuoka A, Tanuma J, Yazaki H, Honda H, Tsukada K, et al. Serum (1→3)-β-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clinical Infectious Diseases 2009;49:1128-31. [DOI] [PubMed] [Google Scholar]
White 2018 {published data only}
- White PL, Posso RB, Gorton RL, Price JS, Wey E, Barnes RA. An evaluation of the performance of the Dynamiker Fungus (1-3)-beta-D-glucan assay to assist in the diagnosis of Pneumocystis pneumonia. Medical Mycology 2018;56(6):778-81. [DOI] [PubMed] [Google Scholar]
Wood 2013 {published data only}
- Wood BR, Komarow L, Zolopa AR, Finkelman MA, Powderly WG, Sax PE. Test performance of blood beta-glucan for Pneumocystis jirovecii pneumonia in patients with AIDS and respiratory symptoms. AIDS 2013;27:967-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang GH, Sheng ZY. The value of assay of (1,3)-β-D-glucan in bronchoalveolar lavage fluid for detection of invasive pulmonary fungal infection in critically ill patients in intensive care unit. Chinese Critical Care Medicine 2012;24(2):90-5. [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu J, Li R, Gao L, Lu Q, Wang X. Utility of galactomannan enzyme immunoassay and (1,3)-β-D-glucan assay in invasive fungal infection. National Medical Journal of China 2010;90:371-4. [PubMed] [Google Scholar]
Zheng 2017 {published data only}
- Zheng F, Zha H, Yang D, Deng J, Zhang Z. Diagnostic values and limitations of (1,3)-β-D-glucans and galactomannan assays for invasive fungal infection in patients admitted to pediatric intensive care unit. Mycopathologia 2017;182:331-8. [DOI] [PubMed] [Google Scholar]
Additional references
Arvanitis 2014
- Arvanitis M, Ziakas PD, Zacharioudakis IM, Zervou FN, Caliendo AM, Mylonakis E. PCR in diagnosis of invasive aspergillosis: a meta-analysis of diagnostic performance. Journal of Clinical Microbiology 2014;52(10):3731-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ascioglu 2002
- Ascioglu S, Rex JH, Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical Infectious Diseases 2002;34:7-14. [DOI] [PubMed] [Google Scholar]
Avni 2011
- Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. Journal of Clinical Microbiology 2011;49(2):665-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamilos 2008
- Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clinical Infectious Diseases 2008;47(4):503-9. [DOI] [PubMed] [Google Scholar]
Clancy 2013
- Clancy CJ, Nguyen MH. Finding the "missing 50%" of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clinical Infectious Diseases 2013;56(9):1284-92. [DOI] [PubMed] [Google Scholar]
De Pauw 2008
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 2008;46:1813-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demiraslan 2017
- Demiraslan H, Atalay MA, Eren E, Demir K, Kaynar L, Koc AN, et al. Assessing the risk of false positive serum galactomannan among patients receiving piperacillin/tazobactam for febrile neutropenia. Medical Mycology 2017;55(5):535-40. [DOI] [PubMed] [Google Scholar]
Fan 2013
- Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PLoS One 2013;8(9):e73099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garey 2006
- Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clinical Infectious Diseases 2006;43(1):25-31. [DOI] [PubMed] [Google Scholar]
He 2015
- He S, Hang JP, Zhang L, Wang F, Zhang DC, Gong FH. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-D-glucan for invasive fungal infection: focus on cutoff levels. Journal of Microbiology, Immunology and Infection 2015;48:351-61. [DOI] [PubMed] [Google Scholar]
Karageorgopoulos 2011
- Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. Beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clinical Infectious Diseases 2011;52(6):750-70. [DOI] [PubMed] [Google Scholar]
Karageorgopoulos 2013
- Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clinical Microbiology and Infection 2013;19:39-49. [DOI] [PubMed] [Google Scholar]
Kontoyiannis 2010
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clinical Infectious Diseases 2010;50(8):1091-100. [DOI] [PubMed] [Google Scholar]
Lemonovich 2018
- Lemonovich TL. Mold infections in solid organ transplant recipients. Infectious Disease Clinics of North America 2018;32(3):687-701. [DOI] [PubMed] [Google Scholar]
Leroux 2013
- Leroux S, Ullmann AJ. Management and diagnostic guidelines for fungal diseases in infectious diseases and clinical microbiology: critical appraisal. Clinical Microbiology and Infection 2013;19(12):1115-21. [DOI] [PubMed] [Google Scholar]
Lu 2011a
- Lu Y, Ling G, Qiang C, Ming Q, Wu C, Wang K, et al. PCR diagnosis of Pneumocystis pneumonia: a bivariate meta-analysis. Journal of Clinical Microbiology 2011;49(12):4361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2011b
- Lu Y, Chen YQ, Guo YL, Qin SM, Wu C, Wang K. Diagnosis of invasive fungal disease using serum (1→3)-β-D-glucan: a bivariate meta-analysis. Internal Medicine 2011;50:2783-91. [DOI] [PubMed] [Google Scholar]
Marty 2006
- Marty FM, Lowry CM, Lempitski SJ, Kubiak DW, Finkelman MA, Baden LR. Reactivity of (1→3)-β-D-glucan assay with commonly used intravenous antimicrobials. Antimicrobial Agents and Chemotherapy 2006;50(10):3450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mengoli 2009
- Mengoli C, Cruciani M, Barnes RA, Loeffler J, Donnelly JP. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infectious Diseases 2009;9(2):89-96. [DOI] [PubMed] [Google Scholar]
Mercier 2019
- Mercier T, Dunbar A, Kort E, Schauwvlieghe A, Reynders M, Guldentops E, et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: a comparative multicenter study. Medical Mycology 2020;58(4):444-52. [DOI] [PubMed] [Google Scholar]
Mikulska 2010
- Mikulska M, Calandra T, Sanguinetti M, Poulain D, Viscoli C, Third European Conference on Infections in Leukemia Group. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Critical Care (London, England) 2010;14(6):R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monique 2006
- Monique AS, Miennink-Kersten H, Ruegebrink D, Wasei N, Willem JG, Verweij PE. In vitro release by Aspergillus fumigatus of galactofuranose antigens, 1,3-β-d-glucan, and DNA: surrogate markers used for diagnosis of invasive aspergillosis. Journal of Clinical Microbiology 2006;44(5):1711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrell 2005
- Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrobial Agents and Chemotherapy 2005;49(9):3640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neofytos 2009
- Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clinical Infectious Diseases 2009;48(3):265-73. [DOI] [PubMed] [Google Scholar]
Pappas 2010
- Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clinical Infectious Diseases 2010;50(8):1101-11. [DOI] [PubMed] [Google Scholar]
Person 2010
- Person AK, Kontoyiannis DP, Alexander BD. Fungal infections in transplant and oncology patients. Infectious Disease Clinics of North America 2010;24(2):439-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powers‐Fletcher 2016
- Powers-Fletcher MV, Hanson KE. Nonculture diagnostics in fungal disease. Infectious Disease Clinics of North America 2016;30(1):37-49. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. [DOI] [PubMed] [Google Scholar]
Schmidt 2012
- Schmidt RL, Schlaberg R, Hanson KE. (1→3)β-D-glucan testing for the detection of invasive fungal infections in immunocompromised patients.. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD009833. [DOI: 10.1002/14651858.CD009833] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2011
- Sun W, Wang K, Gao W, Su X, Qian Q, Lu X, et al. Evaluation of PCR on bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis: a bivariate metaanalysis and systematic review. PLoS One 2011;6(12):e28467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2019
- Tang DL, Chen X, Zhu CG, Li ZW, Xia Y, Guo XG. Pooled analysis of T2 Candida for rapid diagnosis of candidiasis. BMC Infectious Diseases 2019;19(1):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2016
- Trang T, Beal SG. Application of the 1,3-β-D-glucan (Fungitell) assay in the diagnosis of invasive fungal infections. Archives of Pathology and Laboratory Medicine 2016;140(2):181-5. [DOI] [PubMed] [Google Scholar]
van Houwelingen 1993
- Houwelingen HC, Zwinderman KH, Stijnen T. A bivariate approach to meta-analysis. Statistics in Medicine 1993;12(4):2273-84. [DOI] [PubMed] [Google Scholar]
Verdaguer 2007
- Verdaguer V, Walsh TJ, Hope W, Cortez KJ. Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert Review of Molecular Diagnostics 2007;7(1):21-32. [DOI] [PubMed] [Google Scholar]
Verweij 2006
- Verweij PE, Mennink-Kersten MASH. Issues with galactomannan testing. Medical Mycology 2006;44(Suppl 1):S179-S183. [DOI] [PubMed] [Google Scholar]
Viscoli 2004
- Viscoli C, Machetti M, Cappellano P, Bucci B, Bruzzi P, Van Lint MT, et al. False-positive galactomannan patelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clinical Infectious Diseases 2004;38(6):913-6. [DOI] [PubMed] [Google Scholar]
von Eiff 1995
- Eiff M, Roos N, Schulten R, Hesse M, Zühlsdorf M, de Loo J. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 1995;62(6):341-7. [DOI] [PubMed] [Google Scholar]
White 2019
- White SK, Walker BS, Hanson KE, Schmidt RL. The diagnostic accuracy of beta-D-glucan (Fungitell®) testing among patients with hematological malignancies or solid organ tumors: a systematic review and meta-analysis. American Journal of Clinical Pathology 2019;151(3):275-85. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Ruties AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al, QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
Wright 2011
- Wright WF, Overman SB, Ribes JA. (1-3)-β-D-glucan assay: a review of its laboratory and clinical application. Laboratory Medicine 2011;42(11):679-85. [Google Scholar]


