Abstract
Purpose
The aim of the current study was to establish a normative data set for the morphometric parameters of the facial nevre (FN) and cochlear nevre (CN) according to age and sex in the adult population, using 3-dimensional constructive interference in steady state (3D-CISS) sequence on magnetic resonance imaging.
Methods
We retrospectively analyzed 157 ears of 102 adults with no hearing impairment, facial palsy or Ménière's disease. The vertical (VD) and horizontal (HD) diameters as well as cross-sectional areas (CSAs) of the FNs and CNs were measured on the parasagittal oblique image created using the axial 3D-CISS sections passing through the internal acoustic canal. We related the measurement results with side, sex and age.
Results
The mean VD, HD and CSA of the FNs were 1.09 ± 0.18 mm, 0.84 ± 0.17 mm, and 0.75 ± 0.27 mm2, respectively, whereas those of the CNs were 1.34 ± 0.17 mm, 1 ± 0.15 mm and 1.06 ± 0.3 mm2, respectively. There was no statistically significant difference between the morphometric parameters of both the nerves according to side or sex (P > 0.05). However, except for the CSA of the FNs among males, we found a statistically significant decrease in all the morphometric parameters of both the nerves with ageing (P < 0.05).
Conclusion
The normative morphometric data obtained in this study can be beneficial in clinical applications for sensorineural hearing loss, facial palsy and Ménière's disease.
Keywords: Cochlear nerve, Facial nerve, 3 dimensional constructive interference in steady state (3D-CISS)
1. Introduction
The facial nevre (FN) and the vestibulocochlear nerve show an intimate course from the pons to the cerebellopontine angle, and pass close together through the inner acoustic canal (IAC). The vestibulocochlear nerve gives the superior and inferior vestibular nerves within the canal, and is then called the cochlear nerve (CN). FN lies in the anterosuperior, CN in the anteroinferior and the vestibular nerves in the posterior sections of the IAC (Rubinstein et al., 1996; Sheth et al., 2009). Recent studies have demonstrated the clinical relevance of the magnetic resonance imaging (MRI)-detectable morphometric changes in these nerves. MRI-evident enlargement or atrophy of the FN and/or CN have been shown to occur in the course of some diseases such as sensorineural hearing loss, facial palsy and Ménière's disease (Flatz et al., 2018; Giesemann and Hofmann, 2015; Hagino et al., 2011; Henneberger et al., 2017; Peng et al., 2016; Yanagihara et al., 2000). An MRI-based CN measurement is suggested to be a helpful clinical tool for preoperative counselling of the candidates receiving cochlear implants and is recommended to determine the aptness and timing of the operation (Kim et al., 2013; Kutz et al., 2011). It appears that the morphometry of the FN and CN will soon become an important element in the diagnostic as well as prognostic evaluation of numerous diseases.
To date, only a few studies reporting the MRI-based morphometric analysis of the FN and CN have been published in the literature (Jaryszak et al., 2009; Kang et al., 2012; Nakamichi et al., 2013). The reported diameters of the nerves vary across the studies, likely due to the differences in the chosen sequence for imaging and/or the chosen section for performing the measurements in the studies. The aim of the current study was to establish a normative data set for the morphometric parameters of the FN and CN according to age and sex in adult population, using 3-dimensional constructive interference in steady state (3D-CISS) sequence on MRI.
2. Materials and methods
2.1. Patient population and study design
The study was approved by the Ethics Committee of University of Health Sciences, Dışkapı Yıldırım Beyazıt Training and Research Hospital (Approval No: 59/14, Date, 04/02/2019), and the requirement for an informed consent was waived due to the retrospective study design. The clinical and radiological records of the patients who underwent a temporal bone MRI study between 1 Jan 2018 and 1 Jan 2019 at our institution, for tinnitus or with a suspicion of cholesteatoma or middle ear mass were screened. The exclusion criteria were as follows: an audiometric evidence of hearing impairment, a history or the presence of facial palsy and the presence of Ménière's disease. An audiometric evidence of normal hearing was defined as a six-frequency pure-tone average at 250, 500, 1000, 2000, 4000 and 8000 Hz <25 dB HL. within 3 months of temporal MRI study. The study included both ears of 55 of 102 patients, and only a single ear of the remaining 47 patients. Thus, 157 ears of 102 patients were included in the study. There were 54 women and 48 men with a mean age of 43.78 ± 17.46 years (range: 18–76 years).
2.2. MRI protocol and morphometric analysis
Temporal bone MRI was performed on a 1.5 T unit (Magnetom Aera, Siemens, Erlangen, Germany), with a 20-channel head coil. Morphometric analyses of the FN and CN were performed on the 3D-CISS images. For this sequence, an axially oriented 3D slab was placed to include both the inner ears, and the images were obtained using the following parameters: TR = 1200 ms, TE = 269 ms, flip angle = 150°, FOV = 170 mm, slice thickness = 0.6 mm and voxel size = 0.3 × 0.3 × 0.6 mm.
The measurements were performed on the picture archiving and communication system (ExtremePacs, Ankara, Turkey). First, the FN and CN were identified on axial images (Fig. 1). Then, a parasagittal oblique image perpendicular to the course of the IAC, was created with the use of the multiplanar reformation tools. The vertical (VD) and horizontal (HD) diameters of both the nerves were measured at the point nearest to the fundus of the IAC, where the CN and FN could individually be identified (Fig. 2, Fig. 3). The cross-sectional areas (CSA) of the nerves were calculated using the following formula: CSA = π (V/2) (H/2).
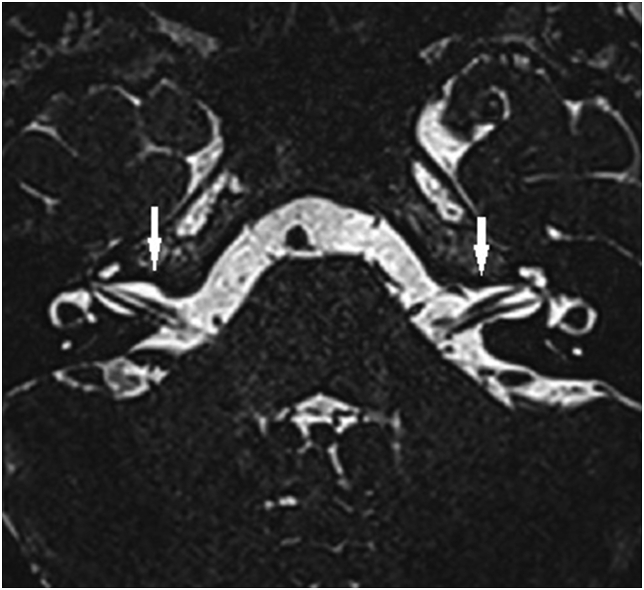
Fig. 1.
Axial three dimensional constructive interference in steady state (3D-CISS) image of the normal-hearing ears of a 24-year-old man at the level of the inner acoustic canals. The courses of both facial and vestibulocochlear nerves in the cerebellopontine angle cisterns and inner acoustic canals are clearly seen (white arrows).
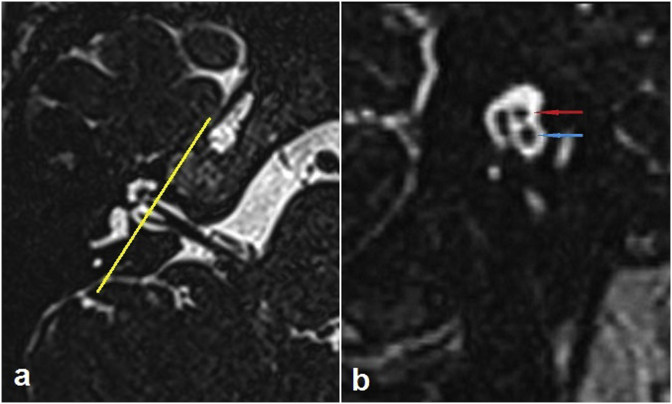
Fig. 2.
Three dimensional constructive interference in steady state (3D-CISS) images of the normal-hearing right ear of a 40-year-old woman. (a) Axial section at the level of the inner acoustic canal. The yellow line which is drawn perpendicular to the course of the inner acoustic canal represents the plane in which the parasagittal oblique image is created. (b) Parasagittal oblique section created with the use of multiplanar reformation tools. The red and blue arrows show the facial and cochlear nerves, respectively.
Fig. 3.
Parasagittal oblique section through the point nearest to the fundus of the IAC where the cochlear (blue ar) and facial (red ar) nerves can be individually identified. The vertical and horizontal diameter measurements of the nerves are shown as green lines.
Two experienced radiologists performed the measurements independently, with no knowledge of the clinical data of the participants. In the cases of discrepancy in the measurements of the nerves, a common re-examination was performed, and the final decisions were made by consensus.
2.3. Statistical analysis
The normality of distribution of continuous variables was tested using Shapiro-Wilk test. The Mann-WhitneyU test was performed to compare non-normal data between the left and right sides. Kruskal-Wallis and Dunn's multiple comparison tests were used to compare non-normal numerical data among the age groups. We tested the relation of age with each parameter of FN and CN using Pearson's correlation. The descriptive statistics were expressed as mean ± standard deviations (mean ± SD). Statistical analysis was performed with the SPSS for Windows, version 24.0, and a P value < 0.05 was accepted as statistically significant.
3. Results
The mean VD, CD and CSA values of CNs and FNs of the current sample of 157 ears are demonstrated in Table 1. Among the 55 patients in whom both the inner ears were evaluated, no statistically significant differences were recorded between the two sides in terms of all the three morphometric parameters of both the nerves (Table 2).
Table 1.
Facial and cochlear nerve sizes of the study population.
| Facial nerve [mean ± SD (range)] | Cochlear nerve [mean ± SD (range)] | |
|---|---|---|
| Vertical diameter (mm) | 1.09 ± 0.18 (0.60–1.50) | 1.34 ± 0.17 (0.90–1.70) |
| Horizontal diameter (mm) | 0.84 ± 0.17 (0.40–1.20) | 1 ± 0.15 (0.60–1.40) |
| Cross-sectional area (mm2) | 0.75 ± 0.27 (0.11–1.41) | 1.06 ± 0.3 (0.33–1.93) |
SD standard deviation.
Table 2.
Facial and cochlear nerve sizes according to side.
| Right (55 ears) (mean ± SD) | Left (55 ears) (mean ± SD) | P | |
|---|---|---|---|
| Facial nerve | |||
| Vertical diameter (mm) | 1.08 ± 0.2 | 1.1 ± 0.17 | 0.631 |
| Horizontal diameter (mm) | 0.83 ± 0.18 | 0.84 ± 0.15 | 0.735 |
| Cross-sectional area (mm2) | 0.75 ± 0.29 | 0.75 ± 0.26 | 0.965 |
| Cochlear nerve | |||
| Vertical diameter (mm) | 1.34 ± 0.18 | 1.35 ± 0.17 | 0.713 |
| Horizontal diameter (mm) | 0.99 ± 0.16 | 1.01 ± 0.15 | 0.593 |
| Cross-sectional area (mm2) | 1.05 ± 0.32 | 1.06 ± 0.28 | 0.730 |
SD standard deviation.
A non-normal distribution of all the parameters of the two nerves (VD, CD and CSA) was recorded using the Shapiro-Wilk test. The results of the morphometric analyses of the FNs and CNs according to sex and age are summarized in Table 3, Table 4, respectively. No statistically significant differences in the morphometric parameters of both the nerves were evident among the genders (P > 0.05). However, except for the CSA of FNs among the males, we found a statistically significant decrease in all the morphometric parameters of both the nerves with ageing (P < 0.05). The differences of FN diameters in females between the ones younger than 29 and those older than 60 years were found to be statistically significant (P values for VD and HD differences were 0.013 and 0.044, respectively). Likewise, the differences in the FN diameters in males under 29 and over 70 years of age were statistically significant (P values for both VD and HD differences were 0.02). Furthermore, all morphometric parameter of CNs showed statistically significant differences between the patients under 29 and over 60 years of age, in both the genders (P < 0.05) (To compare the nerve sizes among the age groups, firstly, the Kruskal-Wallis test was performed to investigate if any of the groups showed significant difference. In case, the P value from Kruskal-Wallis test was significant, post hoc test was performed to determine the subgroup differences. The P values given in Table 3, Table 4 belong to Kruskal-Wallis test). There was a negative weak correlation between age and FN-VD and FN-HD, and a negative moderate correlation between age and FN-CSA, CN-VD, CN-HD and CN-CSA (Table 5).
Table 3.
Facial nerve sizes according to sex and age.
| Age range (years) | Female (81 ears) |
Male (76 ears) |
||||||
|---|---|---|---|---|---|---|---|---|
| N | VD (mm) | HD (mm) | CSA (mm2) | N | VD (mm) | HD (mm) | CSA (mm2) | |
| 18–19 | 5 | 1.23 ± 0.11 | 0.94 ± 0.1 | 0.99 ± 0.38 | 4 | 1.25 ± 0.06 | 0.95 ± 0.06 | 0.93 ± 0.1 |
| 20–29 | 20 | 1.2 ± 0.15 | 0.93 ± 0.15 | 0.91 ± 0.18 | 17 | 1.17 ± 0.12 | 0.91 ± 0.16 | 0.84 ± 0.23 |
| 30–39 | 10 | 1.08 ± 0.18 | 0.79 ± 0.15 | 0.88 ± 0.24 | 13 | 1.13 ± 0.23 | 0.88 ± 0.17 | 0.84 ± 0.33 |
| 40–49 | 12 | 1.06 ± 0.09 | 0.86 ± 0.13 | 0.68 ± 0.23 | 17 | 1.11 ± 0.21 | 0.86 ± 0.21 | 0.83 ± 0.4 |
| 50–59 | 13 | 1.03 ± 0.09 | 0.82 ± 0.09 | 0.66 ± 0.11 | 7 | 1.07 ± 0.2 | 0.84 ± 0.21 | 0.76 ± 0.28 |
| 60–69 | 13 | 0.96 ± 0.15 | 0.7 ± 0.14 | 0.54 ± 0.2 | 12 | 1.02 ± 0.11 | 0.71 ± 0.11 | 0.6 ± 0.17 |
| 70–79 | 8 | 0.95 ± 0.18 | 0.73 ± 0.15 | 0.55 ± 0.2 | 6 | 0.92 ± 0.23 | 0.73 ± 0.15 | 0.54 ± 0.27 |
| Total | 81 | 1.08 ± 0.18 | 0.83 ± 0.16 | 0.73 ± 0.26 | 76 | 1.1 ± 0.19 | 0.84 ± 0.18 | 0.77 ± 0.29 |
| P | 0.001* | 0.001* | 0.001* | 0.032* | 0.012* | 0.056 | ||
HD horizontal diameter, VD vertical diameter, CSA cross-sectional area, * statistical significance.
Data are presented as mean ± standard deviation.
There were no statistically significant differences between males and females in terms of any morphometric measurements.
Table 4.
Cochlear nerve sizes according to sex and age.
| Age range (years) | Female (81 ears) |
Male (76 ears) |
||||||
|---|---|---|---|---|---|---|---|---|
| N | VD (mm) | HD (mm) | CSA (mm2) | N | VD (mm) | HD (mm) | CSA (mm2) | |
| 18–19 | 5 | 1.44 ± 0.11 | 1.07 ± 0.08 | 1.21 ± 0.17 | 4 | 1.48 ± 0.05 | 1.15 ± 0.06 | 1.33 ± 0.1 |
| 20–29 | 20 | 1.42 ± 0.13 | 1.12 ± 0.08 | 1.24 ± 0.11 | 17 | 1.43 ± 0.21 | 1.06 ± 0.19 | 1.22 ± 0.41 |
| 30–39 | 10 | 1.42 ± 0.15 | 1.05 ± 0.12 | 1.16 ± 0.27 | 13 | 1.42 ± 0.04 | 1.04 ± 0.07 | 1.16 ± 0.12 |
| 40–49 | 12 | 1.33 ± 0.19 | 0.98 ± 0.21 | 1.04 ± 0.36 | 17 | 1.36 ± 0.19 | 1 ± 0.2 | 1.1 ± 0.38 |
| 50–59 | 13 | 1.3 ± 0.1 | 0.96 ± 0.12 | 0.98 ± 0.19 | 7 | 1.3 ± 0.15 | 0.96 ± 0.05 | 0.97 ± 0.17 |
| 60–69 | 13 | 1.21 ± 0.1 | 0.87 ± 0.09 | 0.8 ± 0.18 | 12 | 1.26 ± 0.19 | 0.93 ± 0.21 | 0.83 ± 0.29 |
| 70–79 | 8 | 1.22 ± 0.18 | 0.93 ± 0.14 | 0.89 ± 0.24 | 6 | 1.17 ± 0.19 | 0.95 ± 0.08 | 0.87 ± 0.22 |
| Total | 81 | 1.33 ± 0.17 | 0.99 ± 0.14 | 1.03 ± 0.27 | 76 | 1.36 ± 0.18 | 1.01 ± 0.16 | 1.08 ± 0.32 |
| P | 0.001* | 0.001* | 0.001* | 0.004* | 0.033* | 0.003* | ||
HD horizontal diameter, VD vertical diameter, CSA cross-sectional area, * statistical significance.
Data are presented as mean ± standard deviation.
There were no statistically significant differences between males and females in terms of any morphometric measurements.
Table 5.
Correlation between nevre size and age.
| FN-VD | FN-HD | FN-CSA | CN-VD | CN-HD | CN-CSA | ||
|---|---|---|---|---|---|---|---|
| Age | r | −0.359∗ | −0.376∗ | −0.403∗ | −0.432∗ | −0.411∗ | −0.459∗ |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| n | 157 | 157 | 157 | 157 | 157 | 157 | |
FN facial nevre, CN cochlear nevre, HD horizontal diameter, VD vertical diameter, CSA cross-sectional area.
r: Spearman rank correlation coefficient, n: Sample size.
* Significant at 0.05 level.
4. Discussion
We performed morphometric analyses of the FNs and CNs in normal-hearing adults and obtained a normative data set to be utilized in clinical practice. We showed that the sizes of FNs and CNs are symmetrical in normal-hearing adults, and they do not significantly differ between the two genders. Moreover, we showed that the FNs and CNs significantly decrease in size with ageing.
The rapid advances in imaging technologies over the last few years have made it possible to obtain images of relatively small anatomical structures with high resolutions. The 3D-CISS is a gradient-echo MRI sequence, of which the image contrast is determined by the T2/T1 ratio of the tissue. Since an excellent contrast between the cerebrospinal fluid and other structures is achieved by 3D-CISS, it is widely used in the assessment of the structures within the cerebellopontine angle and IAC (Hingwala et al., 2011). The CISS sequence can be performed in MRI devices with both 1.5T and 3T field strengths. 3T MRI has the advantages of higher signal-to-noise ratio and better spatial resolution compared to the 1.5T MRI (Soher et al., 2007). While the use of 3T MRI was previously limited to research fields, it is now being increasingly used in clinical settings as well. Most of the previous studies on the morphometry of the FNs and CNs were performed using 3T MRI (Giesemann and Hofmann, 2015; Kang et al., 2012; Nakamichi et al., 2013). To the best of our knowledge, there is only one study in the current literature comparing the diagnostic value of 3D-CISS at 3T and 1.5T MRI in imaging the IAC structures (Garcia et al., 2012). In this prospective study, where the neurovascular compression was evaluated, the compression could not be well defined in 1.5T MRI due to blurring and insufficiency of contour sharpness in 6 of 47 cases, whereas in all the 47 cases, neurovascular compression was clearly identified in 3T MRI. On the other hand, in their morphometric study on FNs and CNs which was conducted on 1.5T MRI, Jaryszak et al. (2009) reported no case, where the morphometric measurements could not be performed due to lack of nerve contour sharpness. Likewise, in our study which was also carried out on 1.5T MRI, we could clearly visualize the FNs and CNs in all the cases.
Using 3D-CISS sequence, Henneberger et al. (1017) quantitatively evaluated the facial-vestibulocochlear nerve complexes of patients with Ménière's disease and showed that there occurs a significant enlargement in the FNs, CNs and vestibular nerves of both the sides of these patients, compared to those of the control group. Apart from Ménière's disease, MRI-detectable morphometric changes in the FNs and/or CNs are shown in other disorders as well, including sensorineural hearing loss and facial palsy (Giesemann and Hofmann, 2015; Hagino et al., 2011; Peng et al., 2016; Yanagihara et al., 2000). Peng et al. (2016) showed a significant size reduction of the CNs in patients with auditory neuropathy spectrum disorder. FN swelling and 12–32% dilatation has been reported in patients with facial palsy (Hagino et al., 2011). These findings suggest that the morphometry of the FNs and CNs may soon be an indispensable part of the clinical applications in these disorders.
In order to determine the presence of thinning or thickening of the FNs and CNs, reference data of normal values of their morphometric parameters are needed. There is a limited number of studies reporting the normative MR-based morphometric data on FNs and CNs in the literature. The neural sizes presented by these studies show variation within a relatively wide range. The reported mean VDs of the CNs vary between 1.10 ± 0.21 mm and 1.4 ± 0.21 mm (Jaryszak et al., 2009; Kang et al., 2012; Nakamichi et al., 2013). And for the FN, the reported mean VDs range between 0.95 ± 0.21 mm and 1.18 ± 0.17 mm (Kang et al., 2012; Nakamichi et al., 2013). The variations in the reported neural diameters is most likely due to the differences between the studies in terms of the chosen imaging sequence and/or the selected nerve section on which the measurements were performed. Giesemann and Hofmann (2015) measured the VD of the CN at the area of cochlear aperture and reported a mean value of 0.65 mm, whereas Kang et al. (2012) found the VD of CN as 1.10 ± 0.21 mm in the middle of the IAC. Likewise, measuring the FN VD at the nearest point to the IAC fundus, Nakamichi et al. (2013) reported a mean value of 1.18 ± 0.17 mm, whereas this value was reported as 0.95 ± 0.21 mm in the study conducted by Kang et al. (2012) in which the measurements were done in the middle of the IAC. In our study, we chose to perform the morphometric measurements on the nearest section to the IAC fundus, as we observed that this was the best section on which the CN and FN could individually be identified. We found the mean VDs of CNs and FNs as 1.34 ± 0.17 mm and 1.09 ± 0.18 mm, respectively. The results we recorded in our study are very close to those of Nakamichi et al. (2013), probably because the same anatomical section was selected for morphometric measurements in both studies.
In their study, including both adults and children, Kang et al. (2012) recorded a significant size difference in the FNs among children under and over the age of 5 years. Moreover, they showed that the size of the CNs among children, and the sizes of the FNs and CNs among adults do not differ among age groups. However, we found that the sizes of FNs and CNs significantly reduce with ageing, in both male and female adults. Furthermore, unlike the study of Kang et al. (2012), we recorded a non-normal data distribution in our study. This may be due to some extreme samples in the study population or the likelihood of the presence of some participants of different ethnic origin.
Among the results of the previous studies, there is inconsistency regarding the relationship of the sizes of FNs and CNs with gender. Nakamichi et al. (2013) reported a tendency towards larger sizes of FNs and CNS in men, whereas Kang et al. (2012) found no sex-related size differences in both the nerves. In consistence with the findings of Kang et al. (2012), we showed that the sizes of the FNs and CNs do not differ with sex.
The major limitation of the current study is that it was carried out only in adult population. The other limitation is that the measurements were performed on a relatively smaller sample size. Further studies with large samples, including children, is needed to establish a comprehensive data set of FN and CN morphometry.
5. Conclusion
Recent research data imply that MRI-based morphometry of FN and CN will soon be an indispensable part of clinical practice in several disorders. The normative morphometric data obtained in this study can be beneficial in clinical applications of sensorineural hearing loss, facial palsy and Ménière's disease.
Conflicts of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Meltem Özdemir, Email: meltemkaan99@gmail.com.
Rasime Pelin Kavak, Email: drrpelindemir6@hotmail.com.
References
- Flatz W.H., Henneberger A., Reiser M.F., Gürkov R., Ertl-Wagner B. In vivo morphometric analysis of human cranial nerves using magnetic resonance imaging in Ménière's disease ears and normal hearing ears. J Vis Exp. Feb. 2018;21:132. doi: 10.3791/57091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Naraghi R., Zumbrunn T., Rösch J., Hastreiter P., Dörfler A. High-resolution 3D-constructive interference in steady-state MR imaging and 3D time-of-flight MRangiography in neurovascular compression: a comparison between 3T and 1.5T. AJNR Am J Neuroradiol. 2012;33(7):1251–1256. doi: 10.3174/ajnr.A2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesemann A., Hofmann E. Some remarks on imaging of the inner ear: options and limitations. Clin. Neuroradiol. 2015;25(Suppl. 2):197–203. doi: 10.1007/s00062-015-0422-y. [DOI] [PubMed] [Google Scholar]
- Hagino K., Tsunoda A., Tsunoda R., Kishimoto S. Measurement of the facial nerve caliber in facial palsy: implications for facial nerve decompression. Otol. Neurotol. 2011;32(4):686–689. doi: 10.1097/MAO.0b013e318210b8e2. [DOI] [PubMed] [Google Scholar]
- Henneberger A., Ertl-Wagner B., Reiser M., Gürkov R., Flatz W. Morphometric evaluation of facial and vestibulocochlear nerves using magnetic resonance imaging: comparison of Ménière's disease ears with normal hearing ears. Eur. Arch. Oto-Rhino-Laryngol. 2017;274(8):3029–3039. doi: 10.1007/s00405-017-4616-6. [DOI] [PubMed] [Google Scholar]
- Hingwala D., Chatterjee S., Kesavadas C., Thomas B., Kapilamoorthy T.R. Applications of 3D CISS sequence for problem solving in neuroimaging. Indian J. Radiol. Imaging. 2011;21(2):90–97. doi: 10.4103/0971-3026.82283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaryszak E.M., Patel N.A., Camp M., Mancuso A.A., Antonelli P.J. Cochlear nerve diameter in normal hearing ears using high-resolution magnetic resonance imaging. The Laryngoscope. 2009;119(10):2042–2045. doi: 10.1002/lary.20516. [DOI] [PubMed] [Google Scholar]
- Kang W.S., Hyun S.M., Lim H.K., Shim B.S., Cho J.H., Lee K.S. Normative diameters and effects of aging on the cochlear and facial nerves in normal-hearing Korean ears using 3.0-tesla magnetic resonance imaging. The Laryngoscope. 2012;122(5):1109–1114. doi: 10.1002/lary.23184. [DOI] [PubMed] [Google Scholar]
- Kim B.G., Chung H.J., Park J.J., Park S., Kim S.H., Choi J.Y. Correlation of cochlear nerve size and auditory performance after cochlear implantation in postlingually deaf patients. JAMA Otolaryngol Head Neck Surg. 2013;139:604–609. doi: 10.1001/jamaoto.2013.3195. [DOI] [PubMed] [Google Scholar]
- Kutz J.W., Jr., Lee K.H., Isaacson B., Booth T.N., Sweeney M.H., Roland P.S. Cochlear implantation in children with cochlear nerve absence or deficiency. Otol. Neurotol. 2011;32:956–961. doi: 10.1097/MAO.0b013e31821f473b. [DOI] [PubMed] [Google Scholar]
- Nakamichi R., Yamazaki M., Ikeda M., Isoda H., Kawai H., Sone M., Nakashima T., Naganawa S. Establishing normal diameter range of the cochlear and facial nerves with 3D-CISS at 3T. Magn. Reson. Med. Sci. 2013;12(4):241–247. doi: 10.2463/mrms.2013-0004. [DOI] [PubMed] [Google Scholar]
- Peng L., Xiao Y., Liu L., Mao Z., Chen Q., Zhou L., Liao B., Liu A., Wang X. Evaluation of cochlear nerve diameter and cross-sectional area in ANSD patients by 3.0-Tesla MRI. Acta Otolaryngol. 2016;136(8):792–799. doi: 10.3109/00016489.2016.1159329. [DOI] [PubMed] [Google Scholar]
- Rubinstein D., Sandberg E.J., Cajade-Law A.G. Anatomy of the facial and vestibulocochlear nerves in the internal auditory canal. AJNR Am J Neuroradiol. 1996;17(6):1099–1105. [PMC free article] [PubMed] [Google Scholar]
- Sheth S., Branstetter B.F., Escott E.J. Appearance of normal cranial nerves on steady-state free precession MR images. Radiographics. 2009;29(4):1045–1055. doi: 10.1148/rg.294085743. [DOI] [PubMed] [Google Scholar]
- Soher B.J., Dale B.M., Merkle E.M. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging. Clin N Am. 2007;15(3):277–290. doi: 10.1016/j.mric.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Yanagihara N., Honda N., Hato N., Murakami S. Edematous swelling of the facial nerve in Bell's palsy. Acta Otolaryngol. 2000;120(5):667–671. doi: 10.1080/000164800750000522. [DOI] [PubMed] [Google Scholar]