Abstract
Auditory neuropathy is the particular form of deafness in humans which cannot be treated by replacement therapy. Human dental pulp stem cells (hDPSCs) are derived from an ectomesenchymal neural crest cell population. Therefore, they possess a promising capacity for neuronal differentiation and repair. miR-124, a key regulator of neuronal development in the inner ear, is expressed at high levels in auditory and vestibular neurons. Here, we evaluated the possible effect of miR-124 in alteration of neural protein markers expression. Using quantitative reverse transcription-PCR (qRT-PCR) analyses and immunofluorescence staining, we studied the expression patterns of neural progenitor markers (Nestin, NOTCH1, and SOX2) and neural markers (β-tubulin III, GATA-3, and peripherin) upon transfection of hDPSCs with miR-124. The qRT-PCR results showed that Nestin was upregulated 6 h post-transfection. In contrast, Nestin expression exhibited a decreasing trend 24 h and 48 h post-transfection. Higher levels of β-tubulin III, 6 h and 16 h post transfection in RNA level as compared with control cells, were determined in transfected DPSCs. However, β-tubulin-III expression decreased 48 h post-transfection. The immunoflourescence results indicated that transfection of hDPSCs with miR-124, only affected Nestin among the studied neural progenitor and neural marker expression in protein level.
Keywords: Sensorineural hearing loss, miR-124, Spiral ganglion neurons, Nestin, DPSCs
Abbreviations: human dental pulp stem cells, hDPSCs; quantitative reverse transcription-PCR, qRT-PCR; spiral ganglion neurons, SGNs; brain derived neurotrophic factor, BDNF; neurotrophin-3, NT3; sonic hedgehog, SHH; basic fibroblast growth factor, bFGF; epidermal growth factor, EGF; bovin serum albumin, BSA; bone morphogenetic protein 4, BMP4
1. Introduction
Sensorineural hearing loss commonly known as irreversible damage to hair cells or auditory nerves. Cochlear implants or hearing aids can improve hearing disability caused by lack of auditory hair cells. However, the effectiveness of an implant greatly depends on appropriate functioning of auditory nerves (Geleoc and Holt, 2014). Auditory neuropathy (i.e., loss or degeneration of spiral ganglion neurons [SGNs]) is considered a serious problem because there is no replacement therapy. In this regard, cell therapy has opened up promising horizons for the treatment and replacement of SGNs (Rivolta, 2010). In recent decades, using a variety of stem cell types, researchers have made progress toward the development of auditory nerve-like neurons and neural progenitor cells (Mahmoudian-Sani et al., 2017, Mohammad-Reza et al., 2017, Rivolta, 2010). An important step toward establishing and developing cell-based therapeutics for auditory system is to select an efficient strategy and appropriate cell population to differentiate into target cells that can then be used for corrective purposes (Rivolta, 2010). Attempts have been made previously to differentiate SGNs using growth factors and neurotrophic agents, including brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), sonic hedgehog (SHH), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF), or alteration of the expression levels of transcription factors, including NGN1 (Chen et al., 2012, Reyes et al., 2008). Also, a number of investigations have examined the possible aspects of miRNAs in inner ear development (Reza and Ghahfarrokhi, 2017). miRNAs have been described as a class of noncoding small RNAs that regulate expression of genes through degradation of mRNA or inhibition of translation after transcription (Mahmoodian sani et al., 2016, Maiorano and Mallamaci, 2010). Increasing evidence suggests that miRNAs play pivotal regulatory roles in eukaryotic gene expression including transcriptional and post-transcriptional levels (Ambasudhan et al., 2011). Among miRNAs, various studies demonstrated high expression levels of miR-124, which is known to induce neurogenesis, in auditory and vestibular neurons (Elkan-Miller et al., 2011a, Maiorano and Mallamaci, 2010, Michael D. Weston et al., 2006). Embryonic stem cells (Gunewardene et al., 2014, Reza and Ghahfarrokhi, 2017, Shi et al., 2007), induced pluripotent stem cells (iPSCs) (Gunewardene et al., 2014, Nishimura et al., 2009), and adult stem cells, including neuronal (Parker et al., 2007, Wei et al., 2008), bone marrow-derived (Kondo et al., 2005), and inner ear stem cells (Chen et al., 2012), obtained from human and animal cell sources have been used for many purposes (Ghasemi-Dehkordi et al., 2015) including in vitro differentiation of auditory neurons. The different tissue types can be used to isolate the stem cells (Alge et al., 2010). In recent years, researches have proposed that dental pulp, a soft part in the pulp cavity of the tooth, may provide a ready source of stem cells (d’Aquino et al., 2009). Human dental pulp stem cells (hDPSCs) are derived from an ectomesenchymal population of neural crest stem cells. Therefore, they possess a promising capacity for neuronal differentiation and repair (Gronthos et al., 2000). As the proportion, availability, and proliferation rate of DPSCs surpass those of bone marrow stromal cells (Kim et al., 2012), DPSCs have been used recently for regenerative medicine purposes (Arthur et al., 2008, Tatullo et al., 2015). DPSCs express pluripotency markers, including SSEA-4, NANOG, and OCT-4 (Tatullo et al., 2015), and they can differentiate into a neuronal lineage (Janebodin et al., 2011, Takeyasu et al., 2006, Tatullo et al., 2015). The moral issues connected with using embryonic stem cells, together with the fact that ectomesenchymal DPSCs can be extracted from adults, make DPSCs very promising for neuro-regeneration purposes. Here, we evaluated the possible roles of miR-124 in differentiation of DPSCs toward SGNs. With this aim in mind, we transfected miR-124 into hDPSCs and then evaluated the expression of neural progenitor markers (Nestin, SOX2, and NOTCH1) and neural markers (GATA-3, β-tubulin III, and peripherin).
2. Materials and methods
2.1. Cell culturing and transfection of hDPSCs with miR-124
Oligonucleotide sequences for the miR-124 mimic and scrambled negative control miRNA were obtained from Exiqon (Qiagen, Denmark). DPSCs were donated by the Royan Institute for Biotechnology (Isfahan, Iran). Approximately 15 × 104 DPSCs were cultured in six-well plates with high glucose Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% FBS and 1% Penicillin-Streptomycin. When the cells reached 70–80% confluency (after 24 h), transfection complexes were prepared (Invitrogen, Massachusetts). Briefly, either miRNA mimics or scrambled miRNA were mixed with Lipofectamine 2000 reagent (Invitrogen, MA), and the mixtures was then separately transfected in a final concentration of 24 nM (selected following the literature search) into cells in antibiotic and FBS-free high-glucose DMEM. After 4 h of transfection, the transfection medium was replaced with high-glucose DMEM and 10% FBS containing Penicillin-Streptomycin (1%). The cells were then incubated for an additional 16–48 h.
2.2. RNA isolation, cDNA synthesis, and qRT-PCR
Total RNA was extracted from the samples (6 h, 16 h, 24 h, and 48 h post transfection) using Trizol reagent (Sigma, MI) and quantified using a NanoDrop spectrophotometer (Thermoscientific, MA). The transfection efficiency of the miR-124 mimics was evaluated using BONmiR (Stem Cell Technology Research Center, Tehran, Iran) detection kits, including a BON-miR miRNA 1st-Strand cDNA Synthesis Kit and BON-miR High Specificity miRNA QPCR Core Reagent Kit. U47 was used as an endogenous control gene in the qRT-PCR. Equal amounts of total isolated RNA (1 μg) per sample were reverse transcribed using a cDNA synthesis kit (Yektatajhizazma [YTA], Tehran, Iran) and were transferred into the qRT-PCR reaction. The transcription levels of Nestin, β-tubulin III, GATA-3, and peripherin were evaluated using transcript specific primers and SYBR® Green PCR Master Mix (Yektatajhizazma [YTA], Tehran, Iran). Specific cycling parameters in the qRT-PCR included an initial denaturation step at 95 °C for 3 min, denaturation at 95 °C for 15 s, annealing at 58 °C for Nestin and β-tubulin III, annealing at 60 °C for GATA-3, and annealing at 62 °C for peripherin (all for 20s), followed by an extension step at 72 °C for 25 s. The number of cycles was optimized at 40. The primer sequences used were as follows: Nestin, (forward) CACCCCTCAGCCCTGACCACT and (reverse) CCCTCTATGGCTGTTTCTTTCTCTACCA; β-tubulin III, (forward) CTCAGGGGCCTTTGGACATC and (reverse) CAGGCAGTCGCAGTTTTCAC; GATA-3, (forward) GACGGTCAAGGCAACCACG and (reverse) CCAGGGTAGGGATCCATGAAGC; peripherin, (forward) CACGCTCCTCATTTGGCTCTTC and (reverse) GGCTCTCGCTCTCAGATTCCTC; and 18s rRNA, (forward) GTAACCCGTTGAACCCCATTCGT and (reverse) ACCATCCAATCGGTAGTAGCGACG. The transcription level of 18s rRNA was used as an endogenous control. The 2−ΔΔCt analysis algorithm was used to determine the relative quantification of each sample at the time points analyzed. qRT-PCR reactions were run using a Rotor-Gene 3000 instrument (Corbett Research, Sydney, Australia).
2.3. Immunostaining
To assess the expression of neural progenitor protein markers (Nestin, NOTCH1, and SOX2), both miR-124 transfected and control cells were fixed in 4% paraformaldehyde in PBS at room temperature and washed three times with washing buffer (1% bovin serum albumin (BSA) in PBS containing 10% normal donkey serum and 0.3% Triton X-100 (Sigma, Missouri). Immunostaining was carried out using a standard protocol and a Human/Mouse/Rat Neural Progenitor Cell Marker Antibody Panel, according to the manufacturer's instructions (R&D Systems, Canada). Cell nuclei were stained with DAPI. The cells were exposed to primary antibodies (mouse monoclonal anti-Nestin IgG2A, goat anti-Notch IgG, or goat anti-SOX2 IgG) at a final concentration of 10 μg/ml. After three wash steps, secondary antibodies, including donkey anti-mouse IgG (NorthernLights™) NL493-conjugated antibody or donkey anti-goat NL493-conjugated secondary antibody (R&D Systems, Canada), were applied at a 1:200 dilution rate in PBS, supplemented with 1% BSA. Similarly, immunostaining for GATA-3 (ab199428), peripherin (ab106276), and β-tubulin III (ab78078) (Abcam, Cambridge, U.K.) expression was carried out using the standard protocol of the manufacture. In all the experiments, the technical negative controls excluded the primary antibody.
2.4. Statistical analysis
Statistical analyses of the qRT-PCR data were performed using GraphPad statistical software (GraphPad Software, CA). The relative quantification data were analyzed using the Mann–Whitney test.
3. Results
3.1. The pattern of post-transfection of miR-124 levels
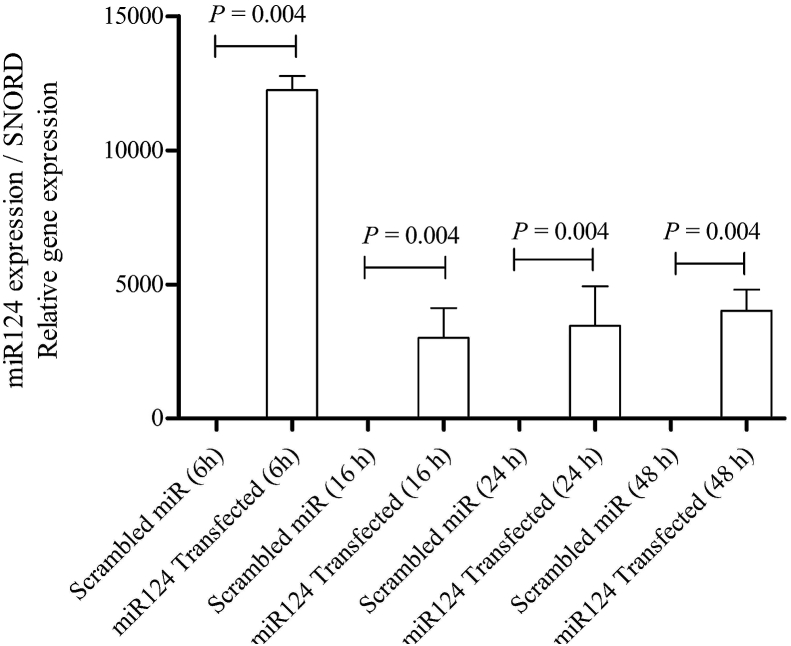
According to the characterization data on the DPSCs provided by the Royan Institute for Biotechnology (Isfahan, Iran), these cells exhibit high expression of STRO-1, CD146, CD73, CD90, and CD105 (data not shown). They are also positive for collagen type-I but negative for collagen type-III and CD45. DPSCs have been shown to have ability to differentiation into adipogenic, osteogenic, and chondrogenic lineages. In the present study, miR-124 was transfected into DPSCs using standard methods. To evaluate the level of miR-124 at different times post-transfection, miR-124 levels were analyzed by qRT-PCR. The results of the qRT-PCR analysis showed that the miR-124 level gradually decreased 16 h, 24 h, and 48 h post-transfection (Fig. 1), possibly due to degradation of miR-124.
Fig. 1.
qRT-PCR analysis of the miR-124 level in DPSCs after transfection (6 h, 16 h, 24 h, and 48 h). As shown by the data, the highest increase in the miR-124 level occurred 6 h post-transfection as compared with control cells (Mann–Whitney, P = 0.004). The data were normalized to expression levels in the control.
3.2. An increased level of miR-124 6 h post-transfection affected the expression of nestin but not that of NOTCH1 or SOX2
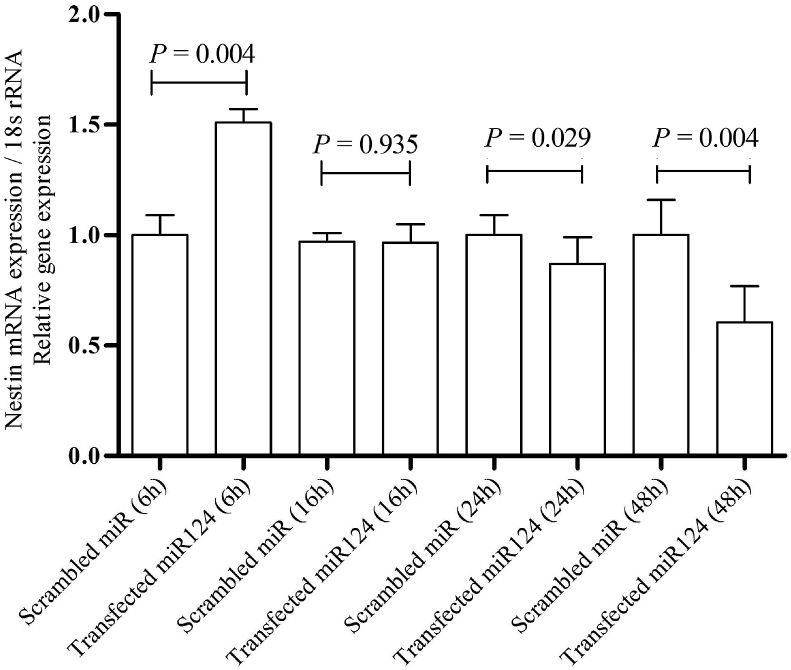
Nestin is an intermediate filament protein, which is a characteristic element of neural stem/progenitor cells. This protein is consistently expressed during differentiation toward neural precursor lineages (Lendahl et al., 1990, Mignone et al., 2004). Six hours post transfection, the qRT-PCR analyses demonstrated an increased level of Nestin mRNA (P = 0.004) in transfected DPSCs under all the conditions tested (Fig. 2). However, Nestin expression exhibited a decreasing trend 24 h and 48 h post-transfection (Fig. 2). Upon normalization with the median expression value, the median fold change in Nestin after 6 h, 16 h, 24 h, and 48 h was 1.51, 0.99, −0.87, and −0.60, respectively (Livak, methods, & 2001, n.d.).
Fig. 2.
qRT-PCR analysis of Nestin mRNA expression in DPSCs post-transfection. As shown by the data, Nestin mRNA expression was upregulated 6 h post-transfection as compared with that of the control (Mann–Whitney, P = 0.004) and downregulated 24 h (Mann–Whitney, P = 0.029) and 48 h (Mann–Whitney, P = 0.004) post-transfection. No statistically significant alteration in Nestin mRNA expression was observed 16 h post-transfection. The data were normalized to expression levels in the control.
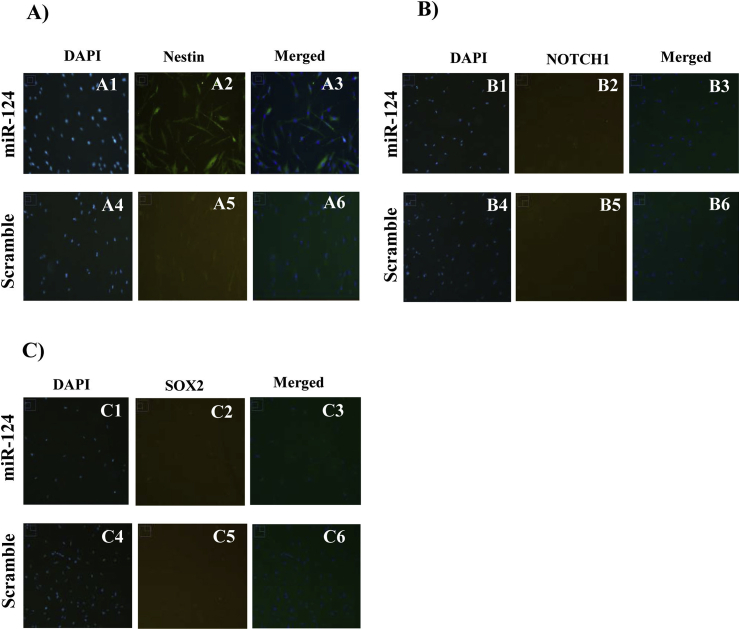
The immunofluorescence analysis revealed that Nestin was upregulated upon hDPSC transfection with miR-124 (Fig. 3A). In addition to Nestin, we examined the protein expression of SOX2 and NOTCH1 in transfected hDPSCs. The SOX2 protein is an important transcription factor belonging to the SRY-related HMG box family that is encoded in neural progenitors and is implicated in neural commitment and self-renewal (Ferri et al., 2004, Neves et al., 2007, Pevny and Nicolis, 2010). NOTCH1 is a member of the Notch family, which plays a role in developmental processes by controlling cell fate decisions (Woo et al., 2009). NOTCH1 is expressed in developing sensory epithelia of the mammalian inner ear (Liu et al., 2012). The immunofluorescence analysis showed that unlike Nestin, there was no significant alteration in NOTCH1 (Fig. 3B) or SOX2 expression post-transfection with miR-124 (Fig. 3C).
Fig. 3.
Immunofluorescence analysis of Nestin (A), NOTCH1 (B), and SOX2 (C) expression in DPSCs 6 h after transfection with miR-124 (A1,2,3; B1,2,3; C1,2,3) versus scrambled miR (A4,5,6; B4,5,6; C4,5,6). As shown by these figures, an increased level of miR-124 6 h post-transfection affected the expression of Nestin but not that of NOTCH1 or SOX2.
3.3. An increased level of miR-124 affected the expression of β-tubulin III but not that of GATA-3 and peripherin
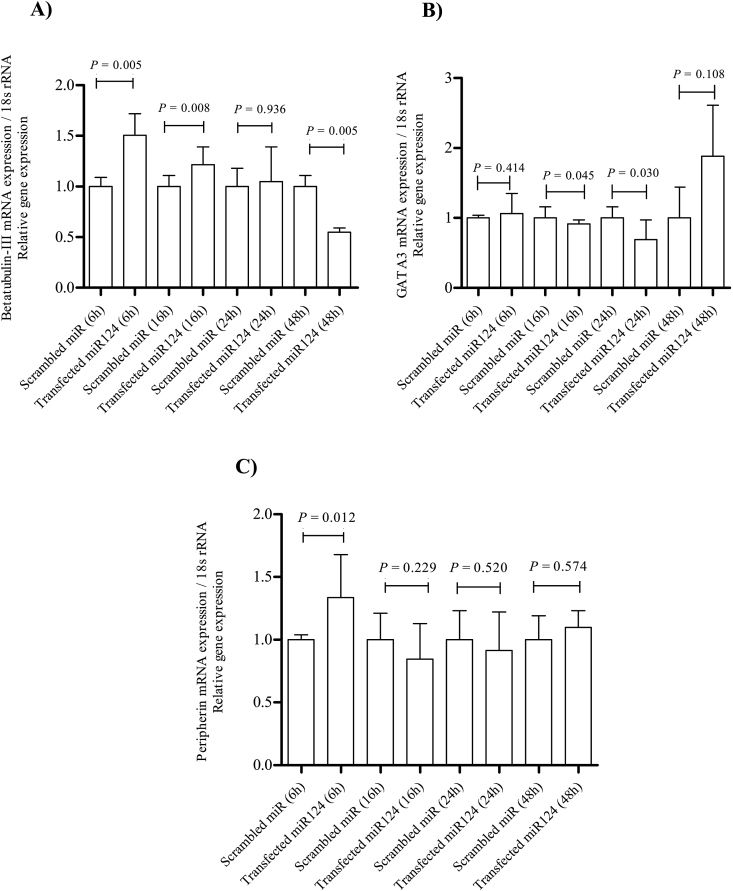
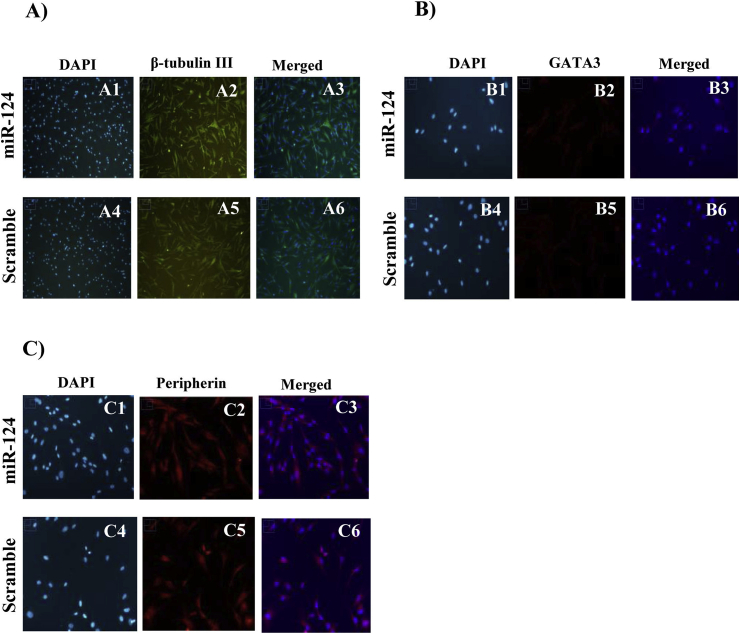
β-tubulin III is a microtubule member of the tubulin family of neural-specific markers (Caccamo et al., 1989, Sullivan and Cleveland, 1986). The results revealed a temporal alteration in the expression of the neuron-specific marker β-tubulin III at the mRNA level. DPSCs transfected with miR-124 mimics had higher levels of this marker 6 h (P = 0.005) and 16 h (P = 0.008) post-transfection as compared with those in control cells. However, the expression of β-tubulin III decreased 48 h (P = 0.005) post-transfection (Fig. 4A). Upon normalization with the median expression value, the median β-tubulin III expression fold change 6 h, 16 h, 24 h, and 48 h post-transfection was 1.50, 1.21, 1, and −0.5 respectively. However, the immunofluorescence analysis revealed no significant alteration in β-tubulin III expression upon hDPSC transfection with miR-124 6 h post-transfection (Fig. 5A). GATA-3, as a transcription factor, has an essential role in auditory development (Lawoko-Kerali et al., 2004) and peripherin (type III intermediate neurofilament), known as a marker for mature type II primary auditory neurons (Hafidi, 1998, Lawoko-Kerali et al., 2004). The results of qRT-PCR and immunofluorescence analyses revealed no significant qualitative alteration in mRNA (Fig. 4B and C) and protein (Fig. 5B and C) expression levels of GATA-3 and peripherin in the miR-124 transfected samples.
Fig. 4.
qRT-PCR analyses of β-tubulin III, GATA-3, and peripherin mRNA expression in DPSCs post-transfection. The data show increased β-tubulin III mRNA expression 6 h (Mann–Whitney, P = 0.005) and 16 h (Mann–Whitney, P = 0.008) post-transfection as compared with that in the control (A). There was no statistically significant alteration in GATA-3 (B) and peripherin (C) mRNA expression in the scrambled control versus miR-124 transfected cells in any post-transfection hours.
Fig. 5.
Immunofluorescence analyses of β-tubulin III (A), GATA-3 (B), and peripherin (C) expression in DPSCs 6 h after transfection with miR-124 (A1,2,3; B1,2,3; C1,2,3) versus scrambled miR (A4,5,6; B4,5,6; C4,5,6). As shown by the figures, the immunofluorescence analysis revealed no significant alteration in the expression of β-tubulin III, GATA3 and Peripherin upon hDPSC transfection with miR-124 6 h post-transfection (however the results of qRT-PCR revealed upregulation of the neuron-specific marker β-tubulin III at the mRNA level).
4. Discussion
Hearing loss is a frequent form of sensory deficit and represents a severe social and health problem in humans (Ciorba et al., 2012). Furthermore, loss of auditory nerves can influence the efficacy of cochlear prostheses (Géléoc and Holt, 2014, Müller and Barr-Gillespie, 2015). The present study is the first to reveal that temporal increases in the level of miR-124 mimic in DPSCs affected the expression of some neural progenitors and neural markers. The importance of miR-124 in neurogenesis, particularly in SGNs development in the inner ear has been well documented (Elkan-Miller et al., 2011a). This study aimed to investigate aspects of DPSC differentiation toward SGNs upon transfection with miR-124. The main reason underlying the selection of DPSCs in the present study is the neural crest origin of these cells. Thus, they offer excellent potential as regards differentiation toward neural progenitor cells and neurons. However, the potential of DPSC differentiation toward auditory neurons has not been studied. Attempts have been made in recent years to generate SGNs using various protocols and cell sources (Chen et al., 2012, Shi and Edge, 2013). The strategies and approaches included co-culturing, application of growth and neurotrophic factors, genetic manipulation, and induction of transcription factors inside stem cells to increase the expression of neuron-related markers (Chen et al., 2009, Chen et al., 2012, Reyes et al., 2008, Rivolta et al., 2006, Shi et al., 2007). Previous research also demonstrated that neural progenitors derived from human embryonic stem cells differentiated into auditory neurons. Shi et al. (2007) demonstrated BMP4 treatment can lead to the upregulation of NGN1, Brn3, TrkB, and TrkC, pointing to sensory neuron generation. Using human fetal auditory stem cells, Chen et al. evaluated the function and expression of stem cell markers (NGN1, Neurofilament 200, Parvalbumin, and β-tubulin III) in differentiated cells (Chen et al., 2009). In a murine model, embryonic stem cells expressing NGN1 were grown in medium containing BDNF and Glial cell-derived neurotrophic factor (GDNF). The results revealed that 50–75% of embryonic stem cells expressed markers of early neurons and that the greater part of the cells were glutamatergic phenotype (Reyes et al., 2008). In another promising study, researchers generated active neurosensory progenitors using media containing EGF, bFGF, and small peptide Y27632 (Needham et al., 2014). Another strategy employed for the generation of SGNs was co-culturing of iPSCs with mouse cochlea (Nishimura et al., 2009). Gunewardene et al. (2014) established an in vitro neural induction protocol for differentiation of human iPSCs toward neurosensory cells. Their results showed that this protocol was capable of generating functionally active neurosensory progenitors. Using various differentiation protocols, Chen et al. acquired two types of otic progenitors and electrophysiologically evaluated the functional properties of the resulting cells. They demonstrated that these otic progenitors differentiated into auditory neurons following transplantation into a murine model of neuropathic deafness (Chen et al., 2012). Other researchers demonstrated using SHH and retinoic acid aided the induction of glutamatergic sensory neuron markers, pointing to the differentiation of stem cells toward sensory cells (Kondo et al., 2005) In a murine model, Jiang et al. transfected inner ear neural stem cells with miR-124 and observed alterations in the expression of TrkB and CDC42, thereby demonstrating a significant role for miR-124 in neuronal differentiation (Jiang et al., 2016). The results of the present study proposed that miR-124 promotes upregulation of Nestin and β-tubulin III in DPSCs but that it does not promote SGN differentiation or affect the phenotype. Similarly, a study conducted by Alonso et al. (Duran Alonso et al., 2012) showed that growth factors induced the differentiation of bone marrow-derived MSCs into Nestin- and SOX2-expressing neural progenitors (Duran Alonso et al., 2012). The results of the present study showed that increased levels of miR-124 did not lead to profound changes, as compared with those induced by growth factors. In this study, miR-124 influenced only the expression of Nestin, a neural progenitor marker. It is likely that the lack of significant effects induced by miR-124 is due to the concentration (24 nM) used. Future studies should examine the effects of a range of concentrations of this miRNA. Although previous research has established the roles of various miRNAs in the progression of different cell processes, including development and differentiation (Elkan-Miller et al., 2011b, Friedman et al., 2009, Patel and Hu, 2012, Wang et al., 2010), miRNAs do not seem to be as effective as other factors (e.g., transcription factors and growth/neurotrophic factors) in increasing differentiation markers or inducing the morphology of interest in target cells. Thus, miRNAs should be used with other factors. The role of intracellular microenvironment required by miRNAs should not be forgotten either. As compared with the requirements of other factors, miRNAs may require further specific conditions to affect cells. On the other hand, miRNAs are more cost-effective than growth factors. Nevertheless, their unexpected outcomes should not be underestimated. Despite the well-established potential of DPSCs in neurogenesis, it is possible that the differentiate capacity of these cells into auditory neuron lines may be inadequate using a strategy involving miR-124.
5. Conclusion
The results indicated that transfection of DPSC with miR-124 mimic altered the expression of some neural progenitor and neural markers of SGNs in RNA or protein level. However, miR-124 did not significantly affect the expression of specific markers for SGNs.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
Authors sincerely appreciate the cooperation of the staff of Cellular and Molecular Research Center of Basic Health Sciences Institute of Shahrekord University of Medical Sciences. This research project was a part of a Ph.D. thesis and was supported by deputy of research and technology of Shahrekord University of Medical Sciences (grant number 2178).
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Alge D.L., Zhou D., Adams L.L., Wyss B.K., Shadday M.D., Woods E.J. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regenerat. Med. 2010;4(1):73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambasudhan R., Talantova M., Coleman R., Yuan X., Zhu S., Lipton S.A., Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9(2):113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur A., Rychkov G., Shi S., Koblar S.A., Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells (Dayton) 2008;26(7):1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- Caccamo D.V., Herman M.M., Frankfurter A., Katsetos C.D., Collins V.P., Rubinstein L.J. An immunohistochemical study of neuropeptides and neuronal cytoskeletal proteins in the neuroepithelial component of a spontaneous murine ovarian teratoma. Primitive neuroepithelium displays immunoreactivity for neuropeptides and neuron-associated beta-tu. Am. J. Pathol. 1989;135(5):801–813. http://www.ncbi.nlm.nih.gov/pubmed/2817080 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Chen W., Johnson S.L., Marcotti W., Andrews P.W., Moore H.D., Rivolta M.N. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cell. 2009;27(5):1196–1204. doi: 10.1002/stem.62. [DOI] [PubMed] [Google Scholar]
- Chen W., Jongkamonwiwat N., Abbas L., Eshtan S.J., Johnson S.L., Kuhn S. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490(7419):278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba A., Bianchini C., Pelucchi S., Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin. Interv. Aging. 2012;7:159. doi: 10.2147/CIA.S26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Aquino R., De Rosa A., Laino G., Caruso F., Guida L., Rullo R. Human dental pulp stem cells: from biology to clinical applications. J. Exp. Zool. B Mol. Dev. Evol. 2009;312B(5):408–415. doi: 10.1002/jez.b.21263. [DOI] [PubMed] [Google Scholar]
- Duran Alonso M.B., Feijoo-Redondo A., Conde de Felipe M., Carnicero E., Garcia A.S., Garcia-Sancho J. Generation of inner ear sensory cells from bone marrow-derived human mesenchymal stem cells. Regen. Med. 2012;7(6):769–783. doi: 10.2217/rme.12.65. [DOI] [PubMed] [Google Scholar]
- Elkan-Miller T., Ulitsky I., Hertzano R., Rudnicki A., Dror A.A., Lenz D.R. Integration of transcriptomics, proteomics, and microRNA analyses reveals novel microRNA regulation of targets in the mammalian inner ear. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkan-Miller T., Ulitsky I., Hertzano R., Rudnicki A., Dror A.A., Lenz D.R. Integration of transcriptomics, proteomics, and MicroRNA analyses reveals novel MicroRNA regulation of targets in the mammalian inner ear. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A.L.M., Cavallaro M., Braida D., Di Cristofano A., Canta A., Vezzani A. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development (Camb.) 2004;131(15):3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Friedman L.M., Dror A.A., Mor E., Tenne T., Toren G., Satoh T. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc. Natl. Acad. Sci. U. S. A. 2009;106(19):7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc G.S.G., Holt J.R. Sound strategies for hearing restoration. Science. 2014;344(6184) doi: 10.1126/science.1241062. 1241062–1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc G.S.G., Holt J.R. Sound strategies for hearing restoration. Science (New York, N.Y.) 2014;344(6184):1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi-Dehkordi P., Allahbakhshian-Farsani M., Abdian N., Mirzaeian A., Saffari-Chaleshtori J., Heybati F. Comparison between the cultures of human induced pluripotent stem cells (hiPSCs) on feeder-and serum-free system (Matrigel matrix), MEF and HDF feeder cell lines. J. Cell. Commun. Signal. 2015;9(3):233–246. doi: 10.1007/s12079-015-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunewardene N., Bergen N. Van, Crombie D., Needham K., Dottori M., Nayagam B.A. Directing human induced pluripotent stem cells into a neurosensory lineage for auditory neuron replacement. BioResearch Open Access. 2014;3(4):162–175. doi: 10.1089/biores.2014.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidi A. Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain Res. 1998;805(1–2):181–190. doi: 10.1016/S0006-8993(98)00448-X. [DOI] [PubMed] [Google Scholar]
- Janebodin K., Horst O.V., Ieronimakis N., Balasundaram G., Reesukumal K., Pratumvinit B., Reyes M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Du J., Zhang X., Zhou W., Zong L., Dong C. miR-124 promotes the neuronal differentiation of mouse inner ear neural stem cells. Int. J. Mol. Med. 2016;38(5):1367–1376. doi: 10.3892/ijmm.2016.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.-C., Bae H., Kwon I.-K., Lee E.-J., Park J.-H., Khademhosseini A., Hwang Y.-S. Osteoblastic/cementoblastic and neural differentiation of dental stem cells and their applications to tissue engineering and regenerative medicine. Tissue Eng. B Rev. 2012;18(3):235–244. doi: 10.1089/ten.TEB.2011.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Johnson S.A., Yoder M.C., Romand R., Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102(13):4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko-Kerali G., Rivolta M.N., Lawlor P., Cacciabue-Rivolta D.I., Langton-Hewer C., van Doorninck J.H., Holley M.C. GATA3 and NeuroD distinguish auditory and vestibular neurons during development of the mammalian inner ear. Mech. Dev. 2004;121(3):287–299. doi: 10.1016/j.mod.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lendahl U., Zimmerman L.B., McKay R.D. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Liu Z., Owen T., Fang J., Zuo J. Overactivation of Notch1 signaling induces ectopic hair cells in the mouse inner ear in an age-dependent manner. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0034123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K., methods T.S. Elsevier; 2001. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2− ΔΔCT Method.https://www.sciencedirect.com/science/article/pii/S1046202301912629 undefined. (n.d.) Retrieved from. [DOI] [PubMed] [Google Scholar]
- Mahmoodian sani M.R., Hashemzadeh-Chaleshtori M., Saidijam M., Jami M.-S., Ghasemi-Dehkordi P. MicroRNA-183 family in inner ear: hair cell development and deafness. J. Audiol. Otol. 2016;20(3):131. doi: 10.7874/jao.2016.20.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudian-Sani M.-R., Hashemzadeh-Chaleshtori M., Jami M.-S., Saidijam M. In vitro differentiation of human bone marrow mesenchymal stem cells to hair cells using growth factors. Int. Tinnitus J. 2017;21(2):179–184. doi: 10.5935/0946-5448.20170030. [DOI] [PubMed] [Google Scholar]
- Maiorano N.A., Mallamaci A. The pro-differentiating role of miR-124: indicating the road to become a neuron. RNA Biol. 2010;7(5):528–533. doi: 10.4161/rna.7.5.12262. [DOI] [PubMed] [Google Scholar]
- Weston Michael D., Pierce Marsha L., Rocha-Sanchez Sonia, Kirk W., Beisel G.A.S. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111(1):95–104. doi: 10.1016/J.BRAINRES.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Mignone J.L., Kukekov V., Chiang A.-S., Steindler D., Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004;469(3):311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Mohammad-Reza Mahmoudian-sani, Mehri-Ghahfarrokhi Ameneh, Hashemzadeh-Chaleshtori Morteza, Masoud Saidijam M.-S.J. Comparison of three types of mesenchymal stem cells (bone marrow, adipose tissue, and umbilical cord-derived) as potential sources for inner ear regeneration. Int. Tinnitus J. 2017;21(2):121–126. doi: 10.5935/0946-5448.20170023. [DOI] [PubMed] [Google Scholar]
- Müller U., Barr-Gillespie P.G. New treatment options for hearing loss. Nat. Rev. Drug Discov. 2015;14(5):346–365. doi: 10.1038/nrd4533. [DOI] [PubMed] [Google Scholar]
- Needham K., Hyakumura T., Gunewardene N., Dottori M., Nayagam B.A. Electrophysiological properties of neurosensory progenitors derived from human embryonic stem cells. Stem Cell Res. 2014;12(1):241–249. doi: 10.1016/j.scr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Neves J., Kamaid A., Alsina B., Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J. Comp. Neurol. 2007;503(4):487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakagawa T., Ono K., Ogita H., Sakamoto T., Yamamoto N. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20(14):1250–1254. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- Parker M.A., Corliss D.A., Gray B., Anderson J.K., Bobbin R.P., Snyder E.Y., Cotanche D.A. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear. Res. 2007;232(1):29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Hu B.H. MicroRNAs in inner ear biology and pathogenesis. Hear. Res. 2012;287(1–2):6–14. doi: 10.1016/J.HEARES.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L.H., Nicolis S.K. Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 2010;42(3):421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Reyes J.H., O'Shea K.S., Wys N.L., Velkey J.M., Prieskorn D.M., Wesolowski K. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J. Neurosci.: Off. J. Soc. Neurosci. 2008;28(48):12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza M., Ghahfarrokhi A.M. MicroRNAs : effective elements in ear-related diseases and hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2017;0(0):0. doi: 10.1007/s00405-017-4470-6. [DOI] [PubMed] [Google Scholar]
- Rivolta M.N. Stem cells and cell lines from the human auditory organ: applications, hurdles and bottlenecks in the development of regenerative therapies for deafness. Drug Discov. Today. 2010;15(7–8):283–286. doi: 10.1016/j.drudis.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Rivolta M.N., Li H., Heller S. Embryonic Stem Cell Protocols. Humana Press; New Jersey: 2006. Generation of inner ear cell types from embryonic stem cells; pp. 71–92. [DOI] [PubMed] [Google Scholar]
- Shi F., Corrales C.E., Liberman M.C., Edge A.S.B. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 2007;26(11):3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- Shi F., Edge A.S.B. Prospects for replacement of auditory neurons by stem cells. Hear. Res. 2013;297:106–112. doi: 10.1016/j.heares.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.F., Cleveland D.W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc. Natl. Acad. Sci. U. S. A. 1986;83(12):4327–4331. doi: 10.1073/pnas.83.12.4327. http://www.ncbi.nlm.nih.gov/pubmed/3459176 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyasu M., Nozaki T., M.D Differentiation of dental pulp stem cells into a neural lineage. Pediatr. Dent. J. 2006;16(2):154–162. doi: 10.1016/S0917-2394(06)70081-7. [DOI] [Google Scholar]
- Tatullo M., Marrelli M., Shakesheff K.M., White L.J. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J. Tissue Eng. Regenerat. Med. 2015;9(11):1205–1216. doi: 10.1002/term.1899. [DOI] [PubMed] [Google Scholar]
- Wang X.-R., Zhang X.-M., Zhen J., Zhang P.-X., Xu G., Jiang H. MicroRNA expression in the embryonic mouse inner ear. Neuroreport. 2010;21(9):611–617. doi: 10.1097/WNR.0b013e328338864b. [DOI] [PubMed] [Google Scholar]
- Wei D., Levic S., Nie L., Gao W., Petit C., Jones E.G., Yamoah E.N. Cells of adult brain germinal zone have properties akin to hair cells and can be used to replace inner ear sensory cells after damage. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105(52):21000–21005. doi: 10.1073/pnas.0808044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.-M., Kim J., Han H.-W., Chae J.-I., Son M.-Y., Cho S. Notch signaling is required for maintaining stem-cell features of neuroprogenitor cells derived from human embryonic stem cells. BMC Neurosci. 2009;10:97. doi: 10.1186/1471-2202-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]