Abstract
Air pollution containing particulate matter (PM) less than 2.5 μm (PM2.5) plays an essential role in regulating hepatic disease. However, its molecular mechanism is not yet clear, lacking effective therapeutic strategies. In this study, we attempted to investigate the effects and mechanisms of PM2.5 exposure on hepatic injury by the in vitro and in vivo experiments. At first, we found that PM2.5 incubation led to a significant reduction of nuclear factor erythroid-derived 2-related factor 2 (Nrf2), along with markedly reduced expression of different anti-oxidants. Notably, suppressor of IKKε (SIKE), known as a negative regulator of the interferon pathway, was decreased in PM2.5-incubated cells, accompanied with increased activation of TANK-binding kinase 1 (TBK1) and nuclear factor-κB (NF-κB). The in vitro studies showed that Nrf2 positively regulated SIKE expression under the conditions with or without PM2.5. After PM2.5 treatment, Nrf2 knockdown further accelerated SIEK decrease and TBK1/NF-κB activation, and opposite results were observed in cells with Nrf2 over-expression. Subsequently, the gene loss- and gain-function analysis demonstrated that SIKE deficiency further aggravated inflammation and TBK1/NF-κB activation caused by PM2.5, which could be abrogated by SIKE over-expression. Importantly, SIKE-alleviated inflammation was mainly dependent on TBK1 activation. The in vivo studies confirmed that SIKE- and Nrf2-knockout mice showed significantly accelerated hepatic injury after long-term PM2.5 exposure through reducing inflammatory response and oxidative stress. Juglanin (Jug), mainly isolated from Polygonum aviculare, exhibits anti-inflammatory and anti-oxidant effects. We found that Jug could increase Nrf2 activation, and then up-regulated SIKE in cells and liver tissues, mitigating PM2.5-induced liver injury. Together, all these data demonstrated that Nrf2 might positively meditate SIKE to inhibit inflammatory and oxidative damage, ameliorating PM2.5-induced liver injury. Jug could be considered as an effective therapeutic strategy against this disease by improving Nrf2/SIKE signaling pathway.
Keywords: PM2.5, Nrf2/SIKE, TBK1/NF-κB, Inflammation and oxidative stress, Juglanin
Graphical abstract
Highlights
-
•
PM2.5 incubation inhibits Nrf2 and SIKE activation in vitro.
-
•
Nrf2 positively regulates SIKE expression in PM2.5-incubated cells.
-
•
SIKE-regulated inflammatory response requires TBK1 blockage in PM2.5-treated cells.
-
•
Juglanin treatment suppresses inflammation in PM2.5-incubated cells through increasing SIKE expression.
1. Introduction
Particulate matter (PM) is a general term for all particulate matters in the atmosphere, among which the type that has an aerodynamic diameter ≤ 2.5 μm (PM2.5) is the most critical factor that affects human health [1,2]. The adverse effects of PM2.5 are more associated with PM2.5 as a complex other than single or a just few components of PM2.5 particles [3]. The particle sizes, charges and combined effects of individual components of PM2.5 are all important for the adverse health impact of PM2.5 exposure [4]. PM2.5 exposure accounts for about 4.2 million deaths from lung cancer, chronic lung disease, respiratory infections, heart disease and stroke, and is an important ranking risk factor for death [5]. PM2.5 can directly enter the circulatory system, influencing the function of body tissues and organs, including liver [6,7]. Epidemiological study suggests that PM2.5 pollution may be a risk factor for the increase in the incidence of liver injury worldwide, and liver is the main organ for metabolism and detoxification [8,9]. In rodent animals, the whole-body exposure to concentrated ambient PM2.5 results in a nonalcoholic steatohepatitis (NASH)-like phenotype [10]. In the liver of mice with long-term exposure of PM2.5, the disruption of hepatic lipid homeostasis, lobular and portal inflammation, and mild hepatic steatosis have been identified [11]. Recently, we showed that prolonged PM2.5 exposure promoted the risk of nonalcoholic fatty liver disease (NAFLD) through elevating oxidative stress and inflammation [12]. Increasing evidence has demonstrated that oxidative stress, inflammatory response, endoplasmic reticulum (ER) stress and fibrosis are involved in the progression of PM2.5-induced tissue injury [[13], [14], [15]]. Hepatic inflammation, as a pathological condition characterized by the release of pro-inflammatory factors, occurs in most types of chronic liver diseases such as NAFLD and hepatocellular carcinoma (HCC) [16,17]. Therefore, the studies on PM2.5-induced hepatic inflammation are very important in terms of identifying new health risk factors and understanding the pathogenesis of liver diseases. However, the present studies regarding the effects of PM2.5 on liver damage or inflammation are limited, requiring further exploration.
Nrf2 is the master transcriptional regulator of antioxidant gene expression. Upon increased oxidative stress, an adaptor for Nrf2 degradation, known as Kelch-like ECH associated protein 1 (Keap1), is directly meditated by oxidants in the cytoplasm, leading to stabilization and activation of Nrf2 [18]. Nrf2 modulates more than 200 genes encoding cytoprotective phase II detoxification and anti-oxidant enzymes, such as heme oxygenase-1 (HO-1), NAD(P)H dehydrogenase (quinone 1) (NQO-1), glutamate-cysteine ligase subunits (GCLC and GCLM) and glutathione-S-transferase (GST) [19]. Chronic elevation of reactive oxygen species (ROS) is a pivotal factor that results in PM2.5-elicited tissue injury [15,20]. Our previous studies indicated that PM2.5-induced hypothalamus inflammation, renal injury and cardiomyopathy were closely associated with the deregulation of Nrf2 signaling, subsequently regulating inflammatory response both in vivo and in vitro [15,21,22]. Nrf2-regulated signaling pathway was recently suggested to modulate PM-induced hepatic insulin resistance [23]. Moreover, Nrf2 could mediate the expression of numerous anti-inflammatory genes by antioxidant response elements in their promoters to neutralize free radicals, and enhance removal of environmental toxins [24,25]. With the understanding of the mechanistic knowledge of the Keap1-Nrf2 system, a number of Keap1-Nrf2 PPI inhibitors have been developed as promising therapeutics against chronic and inflammatory conditions [[26], [27], [28]]. Unfortunately, the therapeutic potential of the Keap1-Nrf2 on inflammatory hepatic disease induced by air pollution remains unclear.
Suppressor of IKKε (SIKE), as a small coiled-coil domain-containing protein comprised of 207 amino-acid residues, is widely expressed in most tissues, such as the heart, stomach and kidney [29]. SIKE has been identified as an interaction partner for IKKε and a negative modulator of the interferon pathway through negatively regulating TBK1-involved signaling [30]. Additionally, SIKE might be an important negative regulator of pathological cardiac hypertrophy via interacting with TBK1, contributing to cardiac remodeling [31]. Given the critical role of TBK1 in promoting inflammatory response [32], we hypothesized that SIKE/TBK1 might be involved in inflammatory disease. Although the immune response meditated by SIKE has been indicated, its additional biological activities are still far from to be investigated, especially its association with oxidative stress regulated by Nrf2.
Growing studies have demonstrated that a large number of antioxidants exhibit promising activities to reduce oxidative stress and inflammatory response during the progression of acute or chronic hepatic injury [33,34]. Juglanin (Jug), a natural compound belonging to flavonoids, is isolated from crude Polygonum aviculare. Jug exerts anti-inflammatory, anti-fibrotic and anti-cancer effects [[35], [36], [37]]. Jug suppresses IL-1β-induced inflammation in human chondrocytes [35]. In lipopolysaccharide (LPS)-induced acute lung injury, Jug dramatically reduced the inflammation of cell infiltration and NF-κB activation [38]. It also regulates ROS production to inhibit breast cancer progression [36]. More recently, Jug was reported to restrain the Stimulator of interferon genes (STING) signaling, further suppressing inflammation to protect against bleomycin-induced lung injury [39]. However, the biological function of Jug in air pollution-induced hepatic injury has not been reported, and the molecular mechanism remains unknown to a large extent.
According to these previous findings, we hypothesized that Nrf2-and SIKE-associated signaling pathways might be involved in PM2.5-induced hepatic injury through regulating oxidative stress and inflammatory response. On the other, Jug was subjected to animals and cells challenged with PM2.5 to explore whether it could be a promising therapeutic to alleviate air pollution-induced liver disease by targeting Nrf2 and SIKE signaling.
2. Materials and methods
2.1. PM2.5 sampling preparation
The method for PM2.5 sampling preparation was based on previous studies with little modification [40,41]. In brief, to collect exposure mass, quartz filter (8 cm × 10 cm, 2500QATUP, Pallflex Products, Putnam, CT, USA) was used to continuously and weekly gather PM2.5 from Yuquan Road, Beijing, China (January–June 2016) at a flow rate of 166 L/min. Ambient PM2.5 filters were then maintained in −80 °C until administration. Then, the sampling was treated with anhydrous alcohol and dissolved in pyrogen-free water. Subsequently, the extraction was sonicated for 48 h in ultrasonic box and concentrated through vacuum freeze-drying. Next, double-distilled water was added to freeze-dried product, which was centrifuged at 5000 rpm. The water-insoluble fraction was suspended in D-Hank's buffer (Gibco Corporation, USA) and vortexed prior to further analysis. The components of PM2.5 were shown in Supplementary table 1.
2.2. Cell culture and treatment
2.2.1. Cell culture
The HEK-293 cell line was purchased from InvivoGen (InvivoGen Code: hkb-tnfdmyd). Human liver cell line L02 was obtained from Procell Life Science & Technology Co.,Ltd. (Shanghai, China). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml of penicillin, and 100 μg/ml streptomycin at 37 °C and 5% CO2. Juglanin (HPLC purity ≥98%) and Nrf2 activator tBHQ (purity ≥98%) were purchased from Shanghai YuanMu Biological Technology Co. Ltd. (Shanghai, China) and Sigma-Aldrich (USA), respectively.
2.2.2. Cell transfection
The commercial human Nrf2 RNAi products were obtained from Novus Biologicals (H00004780-R01) and used as indicated in product specification. The negative control (siNC) and Nrf2-specific siRNAs (siNrf2) were synthesized and obtained from Shanghai Generay Biotech (Shanghai, China). A cDNA encoding human Nrf2 was generated by PCR from human brain cDNA library (Invitrogen, USA) and subcloned into the eukaryotic expression plasmid pcDNA3-Flag. And the empty vector plasmid (EP) was served as control group. The p-NF-κB-luciferase reporter plasmid was purchased from Beyotime Institute of Biotechnology (Shanghai, China). Human SIKE and p-TBK1 luciferase reporter plasmids based on pcDNA3.1 vector, as well as mammalian expression plasmids encoding HA-tagged SIKE or control plasmid based on pCMV-C-HA plasmid (Beyotime Institute of Biotechnology) were constructed by standard molecular biology techniques. Lipofectamine®3000 (Invitrogen) was used for cell transfection following the manufacturer's protocols.
2.2.3. Recombinant adenovirus generation
To overexpress SIKE, the entire coding region of the human SIKE gene, under control of the cytomegalovirus promoter, was encompassed by replication-defective adenoviral vectors. To knockdown SIKE expression, three human shSIKE constructs were obtained from Santa Cruz (sc-88645). The construct that decreased SIKE levels to the greatest extent was selected for all further analysis. We also constructed constitutively active TBK1 (Ad-TBK1) and shTBK1 (Ad-shTBK1). A similar adenoviral vector encoding the green fluorescent protein (GFP) gene was used as a control, and short hairpin RNA (Ad-shRNA) served as controls. L02 cells were infected with adenovirus in diluted media at a multiplicity of infection of 100 for 24 h.
2.3. Luciferase detection
HEK-293 cells (2 × 105/ml) were plated in 24-well dish and transfected using Lipofectamine 3000 (Invitrogen), with plasmid encoding p-NF-κB, p-TBK1 and SIKE luciferase reporter (firefly luciferase; 100 ng) and pRL-TK (renilla luciferase plasmid; 10 ng) together with siNrf2 or Nrf2 plasmids. Empty pcDNA3.1 vector was used to maintain equal amounts of DNA among wells. Cells were incubated with 0, 25, 50, and 100 μg/ml of PM2.5 after 24 h transfection. Then, the cells were collected and subjected to luciferase activity analysis using a Dual-luciferase Assay (Promega, USA) with a Luminoskan Ascent luminometer (Thermo Fisher Scientific, USA) according to the manufacturer's protocols. Reporter gene activity was analyzed by normalization of the firefly luciferase activity to renilla luciferase activity.
2.4. Western blotting
For nuclear protein extraction in cells, nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Nanjing, China) was used according to the manufacturer's instructions. For extraction of total protein samples, cells and hepatic tissues were homogenized using 10% (wt/vol) hypotonic buffer (pH 8.0, 5 μg/ml soybean trypsin inhibitor, 1 mM EDTA, 4 mM benzamidine, 1 mM Pefabloc SC, 25 mM Tris-HCl, 5 μg/ml leupeptin, 50 μg/ml aprotinin) to yield a homogenate. The final supernatants were collected via centrifugation at 12,000 rpm for 15 min at 4 °C. Bicinchoninic acid (BCA) protein analysis kit (Thermo Fisher Scientific, USA) was used for the measurements of protein concentration according to its instructions. Then, 40 μg protein was subjected to 10–12% SDS-Polyacrylamide-Gel-Electrophoresis (SDS-PAGE) and transferred to polyvinyldene fluoride (PVDF) membranes (Millipore, USA). After blocking in 5% skim milk, all membranes were incubated with primary antibodies (Supplementary Table 2) overnight at 4 °C. Then, the membranes were incubated with corresponding secondary antibodies (Supplementary Table 2) for 1 h at room temperature. The signal was detected with the enhanced chemiluminescence (ECL) Detection system (Thermo Fisher Scientific). Each protein expression was analyzed using Image Lab Software (Version 1.4.2b, National Institutes of Health, USA) and normalized to GAPDH.
2.5. Quantitative real-time PCR (RT-qPCR)
Trizol reagent (Invitrogen, USA) was used to extract the total RNA samples from cells or hepatic tissues according to the manufacturer's introductions. Specifically, 1 μg of total RNA extraction was reverse transcribed with the M-MLV-RT system (Promega, Shanghai, China) following the manufacturer's protocols. The program was performed at 42 °C for 1 h and terminated through deactivation of the enzyme at 70 °C for 10 min. Subsequently, PCR was performed with SYBR Green (Bio-Rad, USA) on an ABI PRISM 7900HT detection system (Applied Biosystems, USA). The primer sequences (Supplementary Table 3) used in this study were produced by Invitrogen Corporation or Generay Biotech (Shanghai, China). The quantification analysis was performed following the 2−ΔΔCt expressions. Relative fold expression of every target gene expression was normalized to GAPDH.
2.6. Cell viability
The MTT Cell Proliferation and Cytotoxicity Assay Kit (Beyotime, Shanghai, China) was used to determine various cells viability according to the manufacturer instructions. The absorbance was finally detected using a microplate reader at 570 nm.
2.7. ROS production in vitro
After various treatments, 1.5 mL of dichloro-dihydro-fluorescein diacetate (DCFH-DA) (10.0 μM, Sigma-Aldrich) was added to cells at 37 °C for 25 min. Then, the samples were analyzed with a fluorescence microscopy (Olympus, Tokyo, Japan).
2.8. Animals and treatments
2.8.1. Ethical approval and animals
All animal experiment protocols were approved by the Institutional Animal Care and Use Committee in Chongqing Key Laboratory of Medicinal Resources in the Three Gorges Reservoir Region, School of Biological and Chemical Engineering, Chongqing University of Education (Chongqing, China). The methods used in this research were in accordance with the Regulations of Experimental Animal Administration issued by the Ministry of Science and Technology of the People's Republic of China. 1) The wild type male mice with C57BL/6 background (6–8 weeks old, weighing 20 ± 2 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). 2) Nrf2 knockout C57BL/6J mice (Nrf2−/−) were purchased from the Jackson Laboratory (Bar Harbor, ME). 3) SIKE knockout C57BL/6 mice (SIKE-/-) were created by Cyagen Biosciences Inc. (Suzhou, China) by CRISPR/Cas-mediated genome engineering. All pups will be genotyped by PCR followed by sequencing analysis. All animals were maintained in a specific pathogen-free (SPF) facility with constant temperature and humidity under a 12 h dark/light cycle, and free access to food and water.
2.8.2. Mice whole body exposure and Juglanin treatment
We established the “real-ambient exposure” system optimizing the inhabited environment, which ensured the availability of food and water for mice and whole-body inhalation PM exposure to mice at the same time. All male mice (6–8 weeks of old, weighing 20 ± 2 g) were exposed to concentrated PM2.5 (150.1 ± 2.5 μg/m3, flow rate of 70 L/min; moderate pollution and equal to air quality rating-4 level [115–150 μg/m3] based on China environmental pollution standards) or filtered air (FA, served as control) for 6 h/day, 5 times a week in a mobile exposure system-HOPE-MED 8052 automatic nose and mouth type inhalation exposure system (Hepu Industry and Trade Co., Ltd., China) according to previous studies [15,21,22]. The wild type mice were simultaneously treated with Juglanin (40 mg/kg) once daily via gavage for 6 h prior to PM2.5 or FA exposure. After PM2.5 challenge for 24 weeks with or without Juglanin administration, all mice were sacrificed for eye blood collection. Body weight of mice was measured each week. Blood pressure of each mouse was weekly measured using a noninvasive blood pressure meter (Surgivet, USA). The hepatic tissue samples were isolated from mice for further analysis.
2.9. Biochemical analysis in vitro and in vivo
Serum levels of tumor necrosis factor-α (TNF-α) (#MTA00B), interleukin-1β (IL-1β) (#MLB00C), IL-6 (#M6000B) and interferon-β (IFN-β) (#MIFNB0) were measured using enzyme-linked immunosorbent assay (ELISA) commercial kits (R&D System, USA) according to the manufacturer's instructions. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and malondialdehyde (MDA) levels in cells or liver samples were measured using commercial kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing) according to the protocols recommended by the manufacturer. Aspartate transaminase (AST) and alanine transaminase (ALT) in hepatic samples were assessed with commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocols.
2.10. Histochemical analysis
The mice liver tissues were fixed with 10% neutral formalin, embedded in paraffin, and sectioned transversely. Thin sections (≤15 μm) were stained with hematoxylin and eosin (H&E) following the standard histopathological processes. All sections were detected by 3 histologists without knowledge of the treatment procedure.
2.11. Immunofluorescence
After each treatment, the cells were washed with PBS, fixed in 4% paraformaldehyde for 30 min at room temperature, blocked with 5% (w/v) bovine serum albumin (BSA, Sigma Aldrich, USA) in PBST, and immunostained with anti-Nrf2 (1:100 dilution; Abcam, USA), anti-SIKE (1:100 dilution; Thermo Fisher Scientific, USA), anti-NF-κB (1:100 dilution; Abcam) and anti-p-TBK1 (1:100 dilution; Cell Signaling Technology, USA) antibody overnight at 4 °C, followed by incubation with a goat anti-mouse Alexa Fluor-488-conjugated secondary antibody and/or goat anti-rabbit Alexa Fluor-594-conjugated secondary antibody (Abcam). After washing with PBS, the cells were stained with DAPI (Beyotime, Shanghai, China) and observed under a fluorescence microscope. Immunofluorescence staining for liver sections was performed using anti-Nrf2 (1:100 dilution; Abcam) and anti-SIKE (1:100 dilution; Thermo Fisher Scientific) as described for cells with little modification. Immunofluorescence images were obtained using a fluorescence microscope. Images were analyzed with Image J software.
2.12. Chromatin immunoprecipitation (ChIP) assay
Cells were stimulated with or without PM2.5. The corresponding control cells were cultured to 80–90% confluency. Then, ChIP analysis was conducted using the Enzymatic Chromatin IP Kit (Millipore) according to the manufacturer's protocols. The antibodies as follows were used to immuno-precipitate crosslinked protein-DNA complexes: rabbit anti-Nrf2 and normal rabbit IgG. The immunoprecipitated DNA was purified for PCR assay with the primers that were specific for the putative binding sites within the promoter of SIKE.
2.13. Statistical analysis
Data were expressed as the means ± standard error (SEM). GraphPad PRISM (version 6.0; GraphPad Software, USA) was used for data analysis. Unpaired Student's t-test was performed to calculate the difference between two groups, and one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was used for calculation between multiple experimental groups. The Pearson single correlation analysis was used to determine the correlation between the expression of cellular p-TBK1 positive cells and nuclear NF-κB positive cells. Values of P < 0.05 were considered statistically significant. Animal feeding and treatment, and histological analysis were performed in a single-blinded fashion.
3. Results
3.1. PM2.5 incubation inhibits Nrf2 and SIKE activation in vitro
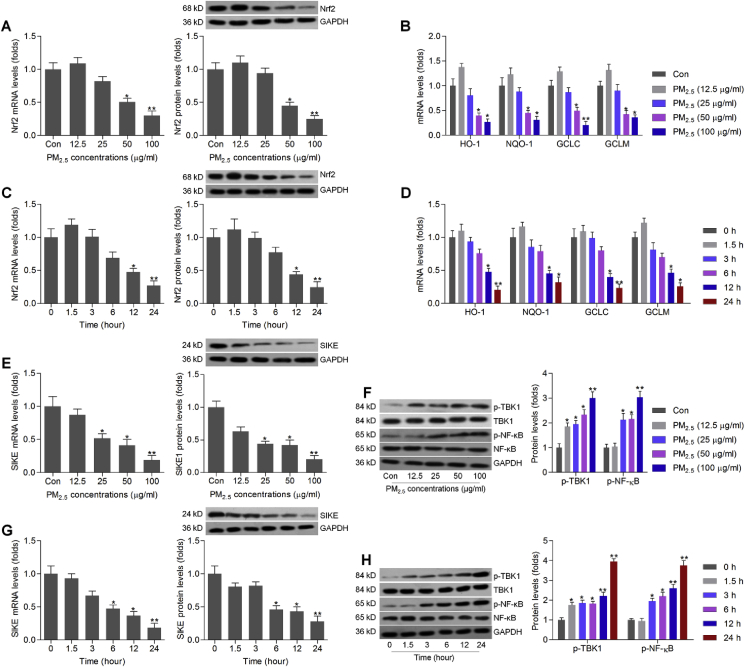
At first, we attempted to explore if Nrf2 signaling could be a potential factor during the pathogenesis of liver-related diseases induced by PM2.5. The effects of PM2.5 on cell viability were measured in L02 cells using CCK-8 analysis. We found that PM2.5 exposure from 25 μg/ml significantly caused the reduction of the cell viability in L02 (Supplementary Fig. 1A). RT-qPCR and western blotting results demonstrated that PM2.5 at lower concentration slightly up-regulated Nrf2 expression though no significant difference was detected. Then, we found that PM2.5 incubation dose-dependently reduced the expression of Nrf2 in human liver cell line L02 (Fig. 1A). Similar results were observed in the expression change of anti-oxidants including HO-1, NQO-1, GCLC and GCLM in PM2.5-exposed L02 cells (Fig. 1B). Furthermore, Nrf-2 expression both from mRNA and protein levels was time-dependently decreased by PM2.5 incubation (Fig. 1C). Also, HO-1, NQO-1, GCLC and GCLM mRNA expression levels were markedly reduced by PM2.5 treatment in a time-dependent manner (Fig. 1D). Subsequently, we found that SIKE expression levels were significantly down-regulated by PM2.5 in a dose- and time-dependent manner. In contrast, p-TBK1 and p-NF-κB protein expression levels were greatly up-regulated in L02 cells in response to PM2.5 (Fig. 1E–H). Therefore, results above demonstrated that PM2.5 exposure reduced Nrf2 and SIKE expression, while promoted the activation of TBK1 and NF-κB in liver cells. Because 25, 50 and 100 μg/ml of PM2.5 showed the significant influence on the cell viability, Nrf2 and SIKE expression change, and thus were selected for the subsequent in vitro experiments.
Fig. 1.
PM2.5incubation inhibits Nrf2 and SIKE activation in vitro. (A) RT-qPCR and western blot results of Nrf2, and (B) RT-qPCR analysis of HO-1, NQO-1, GCLC and GCLM in L02 cells incubated with 24 h of PM2.5 (0–100 μg/ml as indicated). (C) RT-qPCR and western blot results of Nrf2, and (D) RT-qPCR results of HO-1, NQO-1, GCLC and GCLM in L02 cells exposed to PM2.5 (100 μg/ml) for the shown time. (E) RT-qPCR and western blot analysis of SIKE, and (F) western blot results for p-TBK1 and p-NF-κB/p65 in L02 cells treated with different concentrations of PM2.5 as indicated for 24 h. (G) RT-qPCR and western blot analysis of SIKE, and (H) western blot results for p-TBK1 and p-NF-κB in L02 cells cultured with PM2.5 (100 μg/ml) for the indicated time. Data are expressed as means ± SEM (n = 3 independent observations). *P < 0.05 and **P < 0.01 versus the Con group without any treatments.
3.2. Nrf2 positively regulates SIKE expression in PM2.5-incubated cells
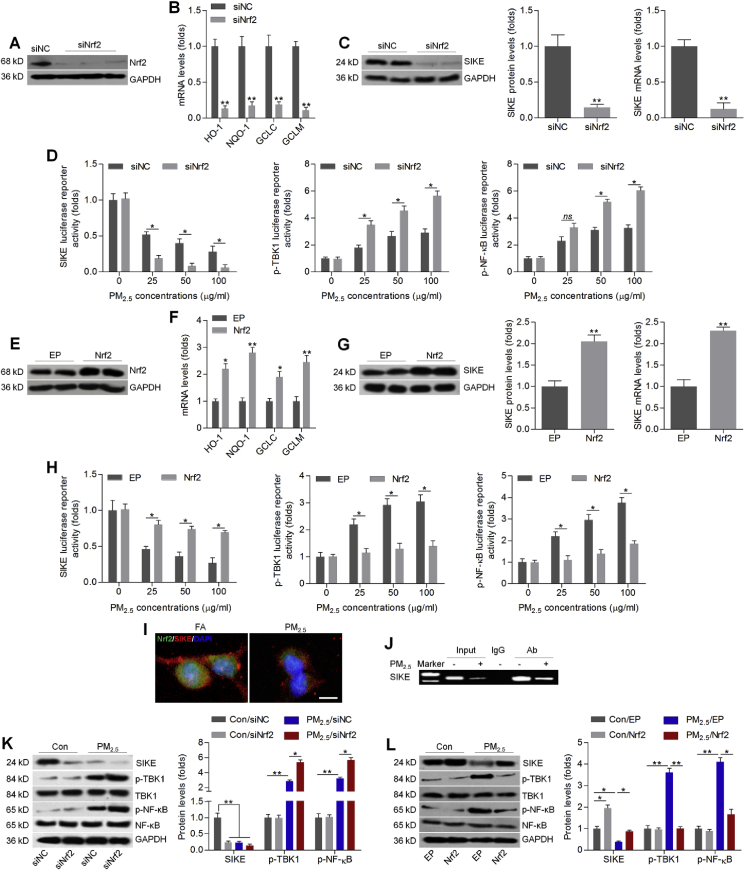
Subsequently, Nrf2 was inhibited or over-expressed to further explore its regulatory effect on SIKE and TBK1/NF-κB signaling. As shown in Fig. 2A, Nrf2 was successfully inhibited by transfection with its specific siRNA. As expected, HO-1, NQO-1, GCLC and GCLM expression levels were markedly reduced in L02 cells with Nrf2 knockdown (Fig. 2B). Notably, western blot and RT-qPCR results showed that SIKE protein and mRNA expression levels were greatly decreased in L02 cells transfected with siNrf2 (Fig. 2C). HEK-293 cells have been widely used for studying gene function due to its relatively higher transfection efficiency [42]. To further study the relation between SIKE signaling and Nrf2 activation with greater depth, we transfected HEK-293 cells with luciferase reporter vectors and/or siNrf2, followed by treatment with 0, 25, 50 and 100 μg/ml of PM2.5 for another 24 h. As shown in Fig. 2D, knockdown of Nrf2 significantly down-regulated SIKE, while promoted p-TBK1 and p-NF-κB activation reporter gene expression, compared with normal cells. Then, Nrf2 was over-expressed in L02 cells by transfection with its specific plasmids, which were along with greatly increased expression of HO-1, NQO-1, GCLC and GCLM (Fig. 2E and F). Of note, SIKE expression both from protein and mRNA levels were highly elevated in L02 cells with Nrf2 over-expression (Fig. 2G). Luciferase reporter analysis also demonstrated that Nrf2 over-expression abrogated the regulatory effects of PM2.5 on SIKE, p-TBK1 and p-NF-κB activation in HEK-293 cells (Fig. 2H). Results above demonstrated that Nrf2 might positively regulate SIKE expression, whereas inhibit TBK1 and NF-κB activation. Immunofluorescence staining further confirmed that PM2.5 stimulation obviously reduced Nrf2 and SIKE expression levels, as evidenced by the weaker fluorescence (Fig. 2I). Furthermore, ChIP assay detected the binding of Nrf2 on the SIKE promoter region in L02 cells with or without PM2.5 exposure (Fig. 2J). Subsequently, western blot analysis confirmed that Nrf2 knockdown markedly reduced SIKE expression under normal condition. PM2.5-induced increases in p-TBK1 and p-NF-κB were significantly further elevated by Nrf2 knockdown (Fig. 2K). In contrast, SIKE expression was up-regulated when Nrf2 was over-expressed in L02 cells in the absence of PM2.5. In response to PM2.5, promoting Nrf2 greatly rescued SIKE expression in L02 cells, whereas effectively down-regulated p-TBK1 and p-NF-κB (Fig. 2L). Therefore, results above indicated that Nrf2 might positively regulate SIKE, contributing to the blockage of TBK1 and NF-κB.
Fig. 2.
Nrf2 positively regulates SIKE expression in PM2.5-incubated cells. (A) L02 cells were transfected with Nrf2 specific siRNA for 24 h, and then were collected for transfection efficiency determination using western blotting analysis. (B) RT-qPCR analysis of HO-1, NQO-1, GCLC and GCLM in L02 cells transfected with siNrf2 for 24 h. (C) Western blot (left panel) and RT-qPCR (right panel) analysis of SIKE in L02 cells with siNrf2 transfection for 24 h. (D) Luciferase reporter analysis with HEK-293 cells that were co-transfected with the indicated reporter plasmids plus siNC or siNrf2 and then left untreated or treated with PM2.5 (0, 25, 50 or 100 μg/ml) for 24 h. (E) L02 cells were transfected with empty plasmid (EP) of Nrf2 plasmids (Nrf2) for 24 h. Cells were then collected for western blotting analysis to measure Nrf2 expression levels. (F) RT-qPCR analysis of HO-1, NQO-1, GCLC and GCLM in L02 cells transfected with Nrf2 plasmids for 24 h. (G) Western blot (left panel) and RT-qPCR (right panel) analysis of SIKE in L02 cells transfected with 24 h of Nrf2 plasmids. (H) Luciferase reporter analysis with HEK-293 cells transfected with the indicated reporter plasmids plus EP or Nrf2 plasmids and then left untreated or treated with increasing concentrations of PM2.5 for 24 h. (I) L02 cells were treated with PM2.5 for 24 h, followed by immunofluorescence staining of Nrf2 (green fluorescence) and SIKE (red fluorescence). Scale bar was 25 μm. (J) Binding of Nrf2 to SIKE by ChIP assay. L02 cells were treated with or without PM2.5 (100 μg/ml) for 24 h. Cross-linked chromatin was immunoprecipitated with an antibody to Nrf2, in the absence of antibody (input), or an isotype-matched control (IgG). Isolated DNA was purified and analyzed by PCR. (K) L02 cells were transfected with siNrf2 for 24 h, and were then incubated with 100 μg/ml of PM2.5 for another 24 h. Subsequently, all cells were collected for western blot analysis of SIKE, p-TBK1 and p-NF-κB. (L) L02 cells were transfected with Nrf2 plasmids for 24 h, followed by PM2.5 (100 μg/ml) incubation for another 24 h. Then, western blot analysis was used to determine SIKE, p-TBK1 and p-NF-κB protein levels. Data are expressed as means ± SEM (n = 3 independent observations). *P < 0.05 and **P < 0.01; ns, no significant difference.
3.3. Effects of SIKE on TBK1/NF-κB signaling pathway in PM2.5-treated cells
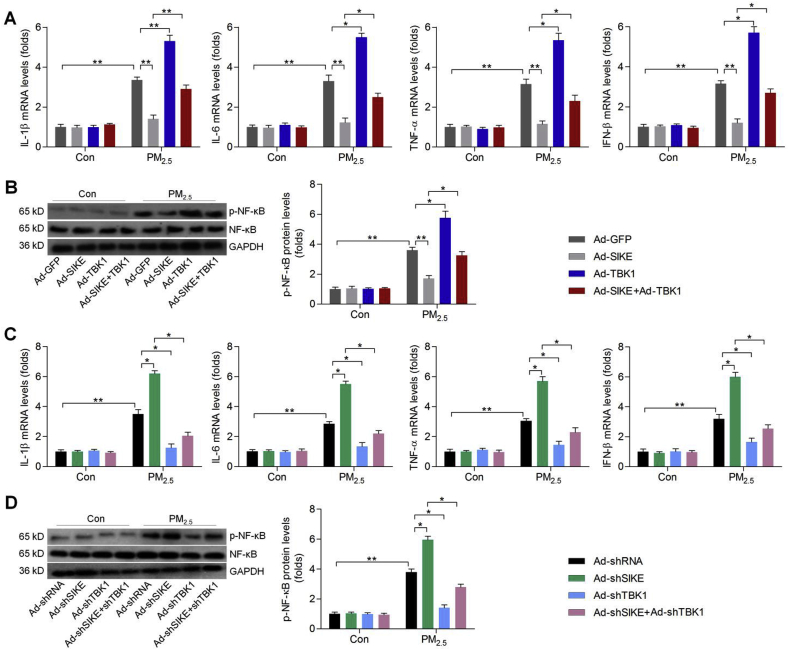
TBK1/NF-κB signaling pathway contributed to inflammatory response [32,43,44]. To further explore the underlying molecular mechanism, the gain- and loss-of-function studies were conducted in L02 cells through infection with either Ad-shSIKE to suppress SIKE expression or Ad-SIKE to over-express SIKE (Fig. 3A). The cells were subsequently treated with PM2.5 for 24 h. Results showed that SIKE deletion markedly promoted the expression of inflammatory factors including IL-1β, IL-6, TNF-α and IFN-β induced by PM2.5, while SIKE over-expression effectively reduced the expression of these factors (Fig. 3B). Then, western blotting showed that SIKE knockdown further accelerated the activation of TBK1 and NF-κB in PM2.5-incubated L02 cells, whereas over-expressing SIEK significantly reduced the phosphorylation of TBK1 and NF-κB (Fig. 3C). NF-κB nuclear expression is critical for the release of pro-inflammatory regulators, and was thus investigated in L02 cells. Western blotting results showed that nuclear NF-κB expression levels were markedly elevated by PM2.5 stimulation, while being further promoted in cells with SIKE knockdown. As expected, SIKE over-expression significantly reduced NF-κB nuclear translocation (Fig. 3D). Immunofluorescence staining indicated that NF-κB nuclear translocation stimulated by PM2.5 was further enhanced by SIKE deficiency, along with obviously up-regulated expression of p-TBK1. In contrast, improving SIKE expression restrained NF-κB nuclear translocation in PM2.5-treated cells, and decreased p-TBK1 expression levels were detected, as evidenced by the weaker fluorescence (Fig. 3E). Western blot analysis further suggested that the inhibitory effects of SIKE on TBK1 phosphorylation were dose-dependent (Supplementary Fig. 1B). Analysis of all groups as a single cohort found a significant correlation between the intensity of p-TBK1 positive cells in cells with the intensity of NF-κB positive cells in nuclear (Supplementary Fig. 1C). These data implicated SIKE as a negative regulator of hepatic injury through suppressing the activation of TBK1/NF-κB signaling.
Fig. 3.
Effects of SIKE on TBK1/NF-κB signaling pathway in PM2.5-treated cells. (A) L02 cells were infected with Ad-shRNA, Ad-shSIKE, Ad-GFP or Ad-SIKE for 24 h. Then, all cells were harvested for western blotting of SIKE expression levels. L02 cells were infected with Ad-shRNA, Ad-shSIKE, Ad-GFP or Ad-SIKE for 24 h, and were then subjected to PM2.5 (100 μg/ml) exposure for another 24 h. Subsequently, (B) RT-qPCR was used for IL-1β, IL-6, TNF-α and IFN-β mRNA levels. (C) Western blot analysis was performed for p-TBK1 and p-NF-κB protein expression levels in whole cells. (D) Western blot analysis of NF-κB in nuclear of cells as treated. (E) Immunofluorescence staining for NF-κB (green fluorescence) and p-TBK1 (red fluorescence) in cells. Scale bar was 25 μm. Data are expressed as means ± SEM (n = 3 independent observations). *P < 0.05 and **P < 0.01.
3.4. SIKE-regulated inflammatory response requires TBK1 blockage in PM2.5-treated cells
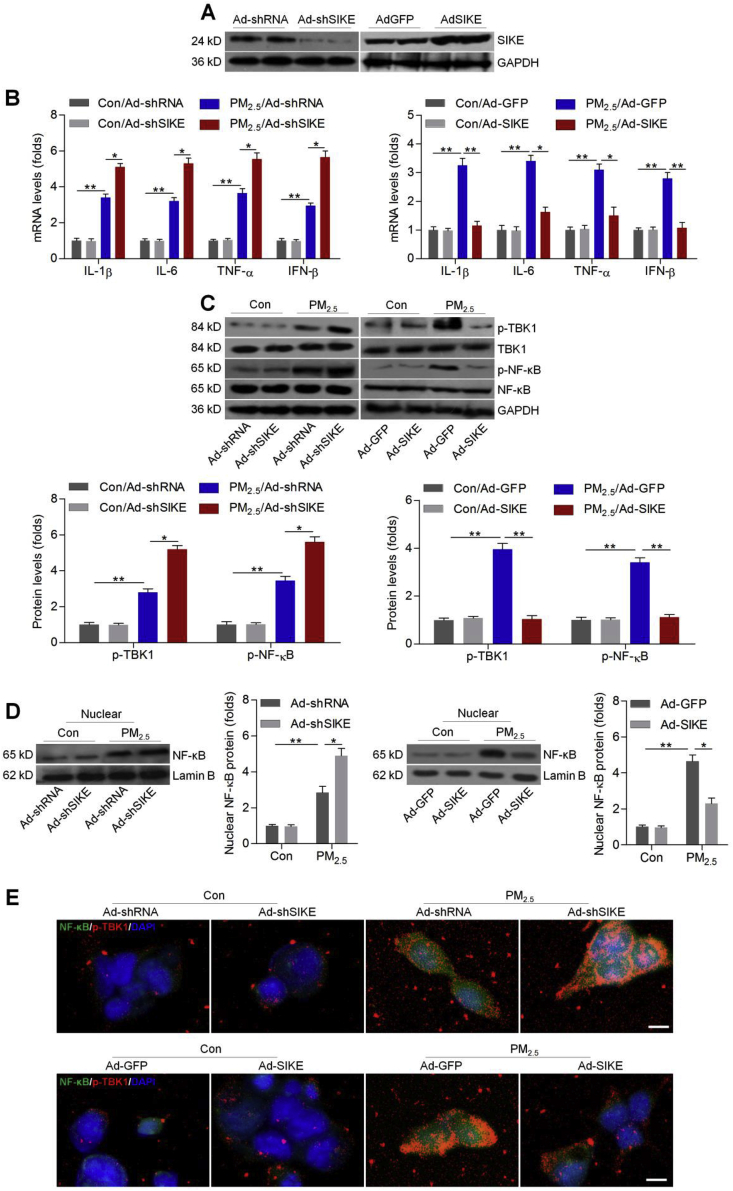
To further explore if SIKE-regulated inflammatory response was TBK1-dependent, we constructed adenoviral vectors to knock down TBK1 (Ad-shTBK1) or over-express TBK1 (Ad-TBK1) in L02 cells (Supplementary Fig. 2). Under basal conditions, alterations in either SIKE or TBK1 expression had no significant effect on the expression of inflammatory factors. When L02 cells were exposed to PM2.5, however, co-infection with Ad-TBK1 counteracted Ad-SIKE-reduced expression of IL-1β, IL-6, TNF-α and IFN-β, as well as p-NF-κB (Fig. 4A and B). Conversely, Ad-shSIKE-promoted expression of pro-inflammatory factors and p-NF-κB were markedly abolished by Ad-shTBK1 (Fig. 4C and D). Thus, TBK1 was indispensable for SIKE-regulated inflammation.
Fig. 4.
SIKE-regulated inflammatory response requires TBK1 blockage in PM2.5-treated cells. L02 cells were infected with Ad-GFP, Ad-SIKE, Ad-TBK1 or the combination of Ad-SIKE and Ad-TBK1 for 24 h, followed by PM2.5 (100 μg/ml) exposure for another 24 h. Then, (A) RT-qPCR was used to determine IL-1β, IL-6, TNF-α and IFN-β mRNA levels. (B) Western blot analysis was conducted for cellular p-NF-κB. L02 cells were infected with Ad-shRNA, Ad-shSIKE, Ad-shTBK1 or the combination of Ad-shSIKE and Ad-shTBK1 for 24 h, followed by PM2.5 (100 μg/ml) incubation for another 24 h. Next, all cells were harvested for further experiments. (C) RT-qPCR analysis of IL-1β, IL-6, TNF-α and IFN-β in cells. (D) Western blot analysis for p-NF-κB protein expression levels. Data are expressed as means ± SEM (n = 3 independent observations). *P < 0.05 and **P < 0.01.
3.5. Juglanin improves Nrf2 activation to inhibit oxidative stress in PM2.5-incubated cells
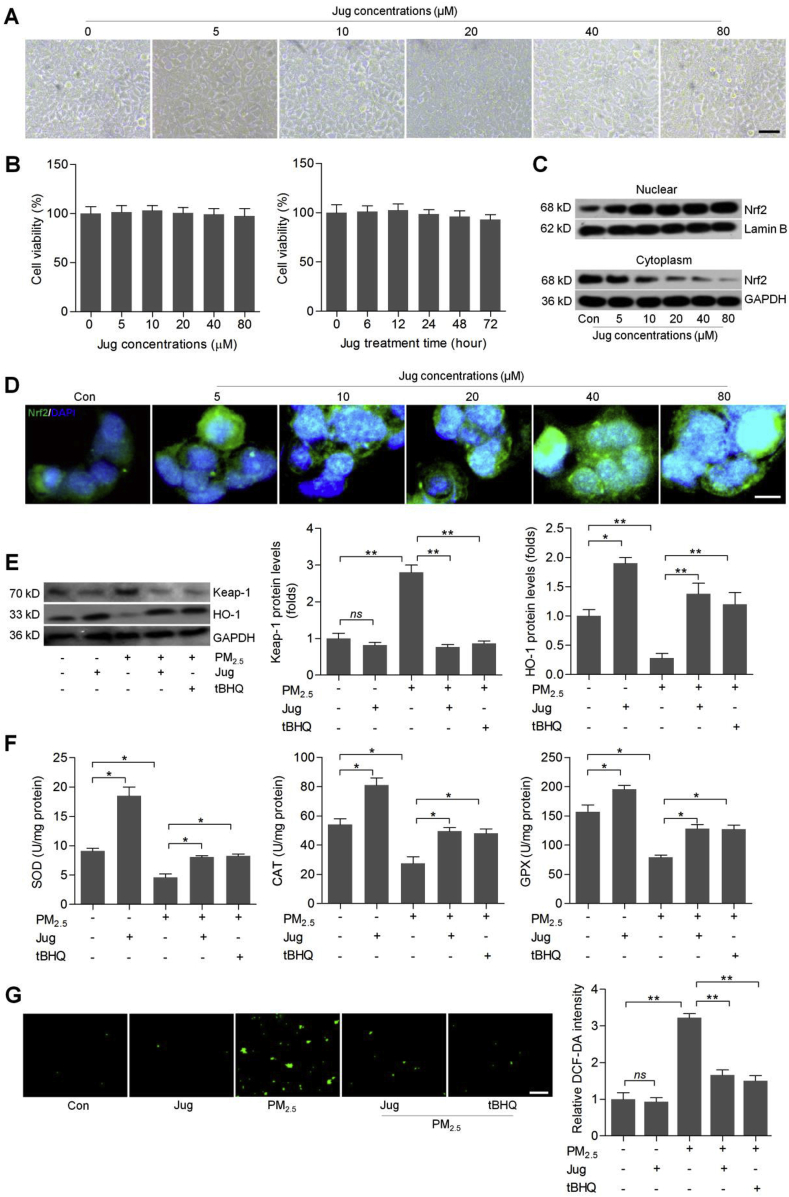
Juglanin was then chosen to further explore if pharmacological intervention could alleviate PM2.5-induced ROS production and inflammation by improving Nrf2/SIKE signaling pathway. As shown in Fig. 5A and B, Jug treatments at different concentrations had no significant cytotoxicity to L02 cells. In addition, 40 μM of Jug treatment showed no cytotoxic effects on L02 cells. CCK-8 confirmed that the viability of cells treated with Jug alone was similar to that of the control group (Supplementary Fig. 3A). As presented in Supplementary Fig. 3B, the cellular LDH release was not markedly different between the control group and the group treated with Jug alone. Western blotting and immunofluorescence staining confirmed that Jug treatments markedly induced Nrf2 nuclear translocation in a dose-dependent manner (Fig. 5C and D). In contrast, Keap-1, as an essential suppressor of Nrf2, was down-regulated by Jug in PM2.5-exposed cells, which was similar with the effects of tBHQ, known as a typical Nrf2 activator. In addition, HO-1 expression levels were slightly elevated by Jug under normal conditions. Also, PM2.5-decreased HO-1 expression was markedly restored by Jug co-treatment (Fig. 5E). Furthermore, in cells without PM2.5 stimulation, SOD, CAT and GPX activities were markedly improved by Jug treatment. Also, PM2.5-reduced SOD, CAT and GPX levels were highly rescued in L02 cells co-treated with Jug (Fig. 5F). Finally, DCF-DA staining showed that Jug treatment could evidently reduce ROS accumulation in PM2.5-stimulated L02 cells (Fig. 5G). Therefore, these data demonstrated that Jug exhibited anti-oxidative effects to decrease PM2.5-caused ROS accumulation mainly through activating Nrf2.
Fig. 5.
Juglanin improves Nrf2 activation to inhibit oxidative stress in PM2.5-incubated cells. (A) L02 cells were treated with different concentrations of Jug (0–80 μM) for 24 h. Then, morphology of cells was observed. (B) Left, L02 cells were incubated with Jug at the indicated concentrations for 24 h, and were then collected for cell viability calculation using western blotting analysis. Right, L02 cells were treated with 40 μM of Jug for the shown time, followed by MTT analysis to assess the cell viability. (C) Western blotting results for nuclear and cytoplasm Nrf2 expression levels in L02 cells incubated with increasing concentrations of Jug for 24 h. (D) Immunofluorescence staining of Nrf2 in Jug-treated L02 cells for 24 h. Scale bar was 25 μm. (E–G) L02 cells were treated with PM2.5 (100 μg/ml) for 24 h together with Jug (40 μM) or t-BHQ (10 μM). Then, all cells were collected for further analysis as follows. (E) Western blot analysis of Keap-1 and HO-1. (F) Assessments of SOD, CAT and GPX activities in cells. (G) DCF-DA staining of L02 cells. Data are expressed as means ± SEM. *P < 0.05 and **P < 0.01; ns, no significant difference.
3.6. Juglanin treatment suppresses inflammation in PM2.5-incubated cells through increasing SIKE expression
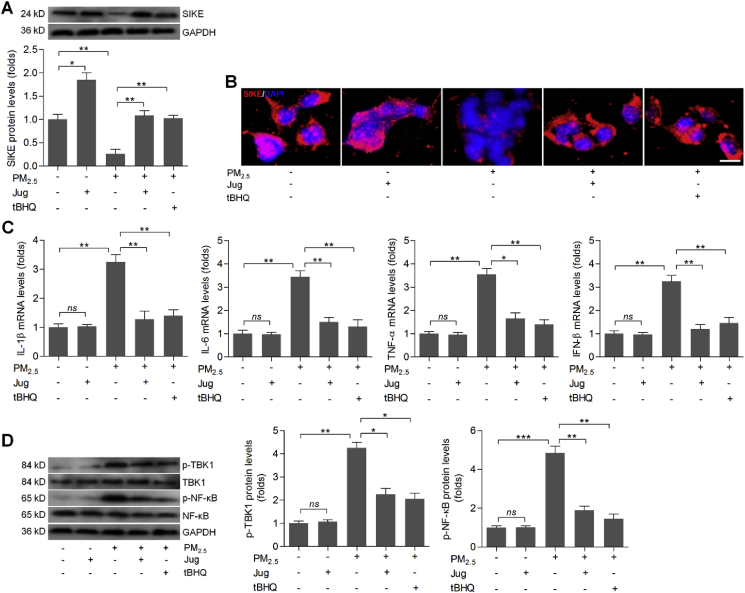
In this regard, we found that Jug treatment caused slight up-regulation of SIEK in L02 cells without PM2.5 stimulation. As expected, PM2.5-induced cells had lower SIKE expression compared with the control group, which was, however, markedly improved by Jug co-treatment (Fig. 6A). Immunofluorescence staining confirmed that Jug administration could rescue SIKE expression in PM2.5-stimulated cells, as evidenced by the stronger fluorescence than that of the PM2.5-alone group (Fig. 6B). Then, RT-qPCR analysis showed that Jug incubation markedly decreased the mRNA levels of IL-1β, IL-6, TNF-α and IFN-β in L02 cells with PM2.5 stimulation (Fig. 6C). Consistently, PM2.5-promoted p-TBK1 and p-NF-κB were significantly abolished by Jug co-incubation (Fig. 6D). Therefore, results above illustrated that Jug had anti-inflammatory effects to alleviate PM2.5-induced injury in vitro, which might be associated with the improvement of SIEK signaling.
Fig. 6.
Juglanin treatment suppresses inflammation in PM2.5-incubated cells through increasing SIKE expression. L02 cells were treated with PM2.5 (100 μg/ml) for 24 h together with Jug (40 μM) or t-BHQ (10 μM). Then, all cells were harvested for the following studies. (A) Western blot analysis of SIKE in cells. (B) Immunofluorescence staining of SIKE in cells. Scale bar was 25 μm. (C) RT-qPCR analysis of IL-1β, IL-6, TNF-α and IFN-β in cells. (D) Western blot analysis for p-TBK1 and p-NF-κB protein expression levels. Data are expressed as means ± SEM (n = 3 independent observations). *P < 0.05, **P < 0.01 and ***P < 0.001; ns, no significant difference.
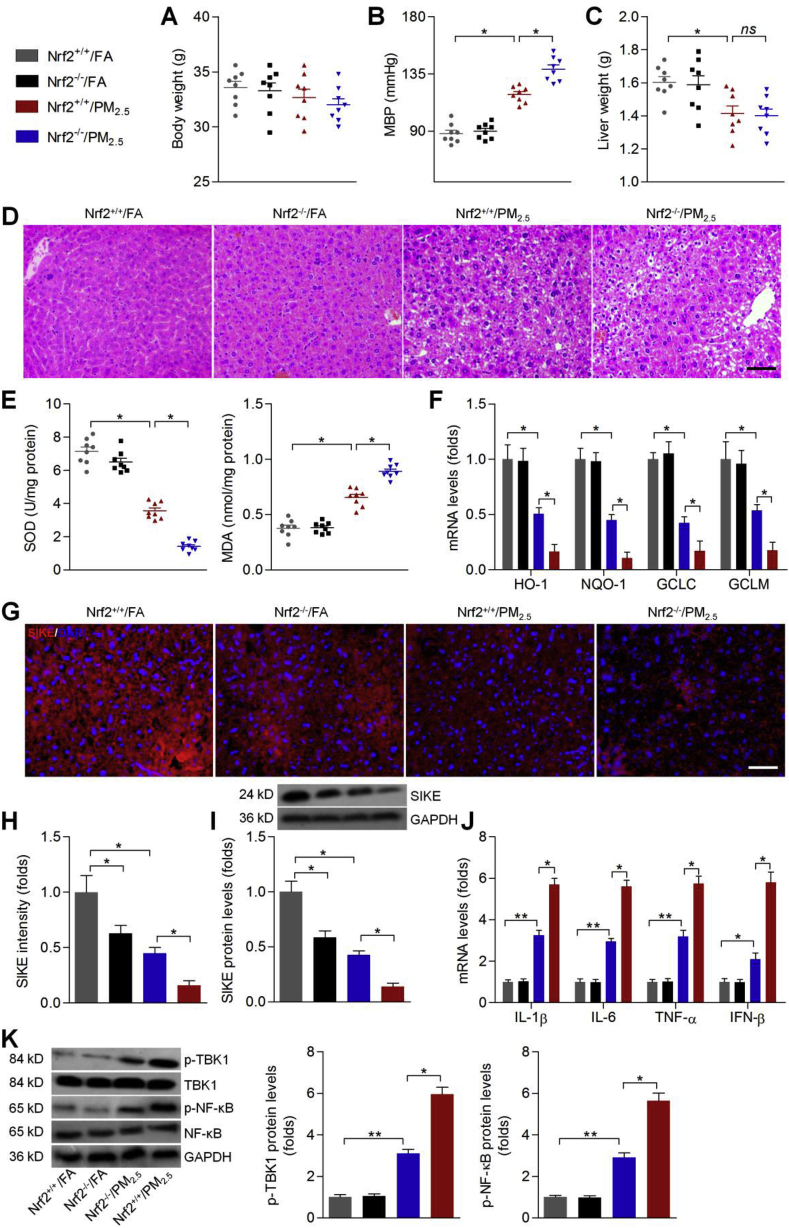
3.7. Effects of SIKE expression on PM2.5-induced hepatic injury in mice
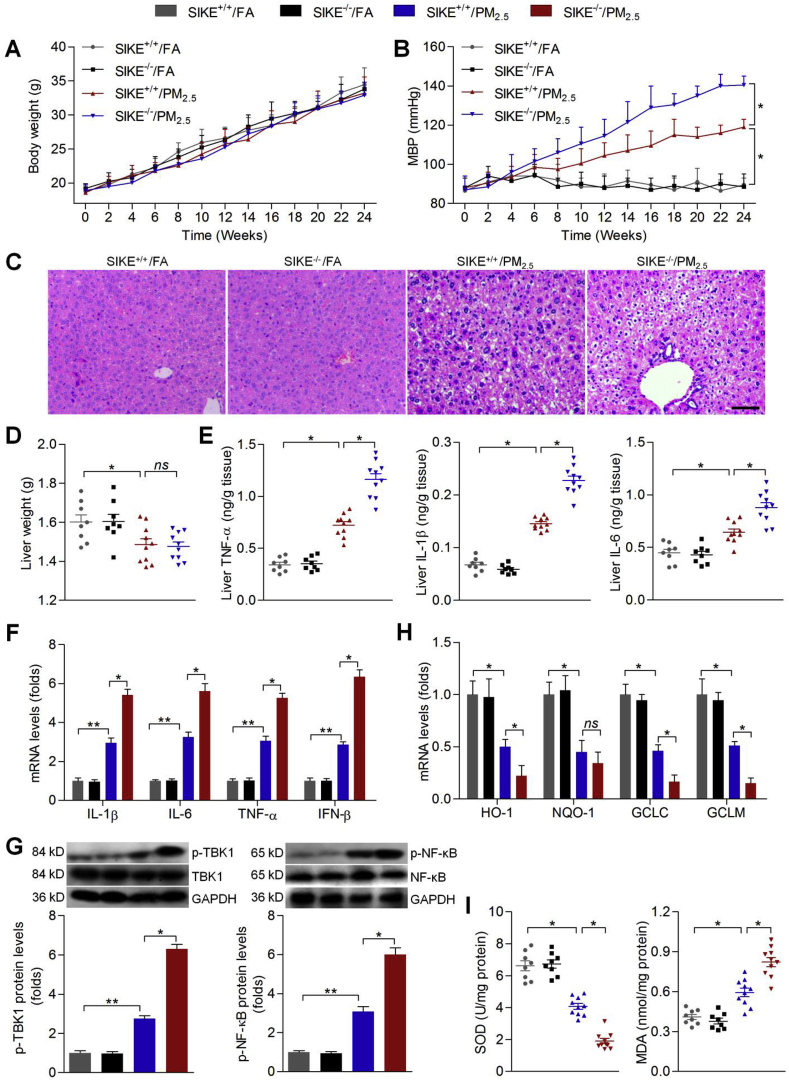
The in vitro studies showed that Nrf2/SIKE signaling might have protective effects against PM2.5-induced oxidative and inflammatory damage by improving anti-oxidants and suppressing TBK1/NF-κB signaling. To further elucidate in vivo effect of PM2.5 exposure, as well as underlying molecular mechanisms, male C57BL/6 mice were exposed to concentrate ambient PM2.5 for 24 weeks. To evaluate the intracellular impact of PM2.5 exposure, we performed transmission electron microscopy (TEM) analysis of the cell ultrastructure with the liver tissue samples from the mice exposed to PM2.5 or FA. The liver of the mice exposed to PM2.5 displayed ultrastructure damage when compared with those from the mice exposed to FA (Supplementary Fig. 4A). Our preliminary experiment showed that PM2.5 long-term exposure caused decreases in SIKE in liver tissues of wild type mice (Supplementary Fig. 4B). To further confirm these findings, the in vivo studies were performed using SIKE+/+ or SIKE-/- mice with or without PM2.5 challenge. SIKE was not detectable in liver of SIKE-/- mice (Supplementary Fig. 4C). As shown in Fig. 7A, long-term PM2.5 exposure showed no significant effects on the change of body weight in both wild type and SIKE-knockout mice. Nevertheless, PM2.5 exposure led to a significant increase in MBP compared with the FA group; however, SIKE deletion could further promote MBP in PM2.5-challenged mice (Fig. 7B). H&E staining showed that PM2.5 exposure resulted in histological changes in liver sections, which were further accelerated by SIKE deletion (Fig. 7C). Hepatic function in mice was then assessed. As expected, PM2.5-challenged mice had higher AST and ALT in liver than that of the FA mice, which were, however, further aggravated by SIKE deficiency (Supplementary Fig. 5A). In addition, PM2.5 caused a reduction in liver weight of mice compared with the FA mice. SIKE deletion showed no significant effects on the change of liver weight in PM2.5-exposed mice (Fig. 7D). Subsequently, in response to PM2.5, significantly increased IL-1β, IL-6 and TNF-α were detected in liver samples, which were further promoted in mice with SIKE deficiency (Fig. 7E). RT-qPCR analysis further confirmed that compared to the PM2.5-single group, SIKE absence led to higher expression of IL-1β, IL-6, TNF-α and IFN-β in hepatic tissues of PM2.5-treated mice, accompanied with markedly up-regulated activation of TBK1 and NF-κB (Fig. 7F and G). Subsequently, we unexpectedly found that PM2.5-inhibited expression of anti-oxidants such as HO-1, GCLC and GCLM were further down-regulated in mice with SIKE deletion (Fig. 7H). Moreover, after PM2.5 challenge, SIKE-/- mice showed lower SOD activity and higher MDA levels in hepatic samples (Fig. 7I). These data demonstrated that SIKE deletion could further accelerate PM2.5-induced hepatic injury and dysfunction by promoting inflammatory response and oxidative stress.
Fig. 7.
Effects of SIKE expression on PM2.5-induced hepatic injury in mice. (A) Body weight change of mice (n = 8 per group). (B) MBP results were shown (n = 8 per group). (C) H&E staining of liver sections (n = 5 per group). Scale bar was 50 μm. (D) Liver weight (n = 8 per group). (E) ELISA results for IL-1β, IL-6 and TNF-α in liver samples (n = 8 per group). (F) RT-qPCR of hepatic IL-1β, IL-6, TNF-α and IFN-β mRNA expression levels (n = 5 per group). (G) Western blot analysis of p-TBK1 and p-NF-κB in liver samples (n = 5 per group). (H) RT-qPCR analysis of HO-1, NQO-1, GCLC and GCLM in liver samples (n = 5 per group). (I) Hepatic SOD activity and MDA levels (n = 8 per group). Data are expressed as means ± SEM. *P < 0.05 and **P < 0.01.
3.8. Effects of Nrf2 activation on PM2.5-induced hepatic injury in mice
Here, mice with or without Nrf2 expression were included to further confirm our hypothesis. At first, western blot results demonstrated that Nrf2 was not detectable in hepatic tissues of Nrf2−/− mice (Supplementary Fig. 6). Consistently, PM2.5 long-term exposure showed no significant effects on the change of body weight either in Nrf2+/+ or Nrf2−/− mice (Fig. 8A). Also, MBP was clearly increased in PM2.5-challenged mice, which were further enhanced in mice without Nrf2 expression (Fig. 8B). PM2.5 caused a significant decrease in the liver weight of mice, and Nrf2 knockout showed no significant effects on the change of liver weight (Fig. 8C). PM2.5-induced histological alterations were further enhanced in mice with Nrf2 knockout, accompanied with markedly promoted hepatic ALT and AST levels (Fig. 8D and Supplementary Fig. 5B). Thus, Nrf2 was critically involved in the maintaining of hepatic function in mice when stimulated by PM2.5. We then found that PM2.5-reduced hepatic SOD activity was further decreased in Nrf2−/− mice, while MDA levels in liver were greatly up-regulated (Fig. 8E). Compared with the PM2.5-treated mice, Nrf2−/− mice showed lower HO-1, NQO-1, GCLC and GCLM expression levels in hepatic tissues (Fig. 8F). Subsequently, we found that Nrf2−/− mice without PM2.5 exposure had lower SIKE expression levels than that of the wild type group, which were in line with the results observed in vitro. However, after long-term PM2.5 challenge, Nrf2−/− mice showed further reduced expression of hepatic SIKE compared to the PM2.5-alone group (Fig. 8G–I). RT-qPCR analysis confirmed that after PM2.5 exposure for 24 weeks, Nrf2 deletion markedly enhanced the expression of pro-inflammatory factors, including IL-1β, IL-6, TNF-α and IFN-β, accompanied with accelerated activation of TBK1 and NF-κB (Fig. 8J and K). Therefore, these in vivo findings elucidated that Nrf2 could regulate SIKE expression to protect against PM2.5-inudced liver damage.
Fig. 8.
Effects of Nrf2 activation on PM2.5-induced hepatic injury in mice. (A) Body weight of mice was recorded (n = 8 per group). (B) MBP results for mice from each group (n = 8 per group). (C) Liver weight of mice (n = 8 per group). (D) H&E staining of hepatic sections (n = 5 per group). Scale bar was 50 μm. (E) SOD and MDA measurements in liver samples (n = 8 per group). (F) RT-qPCR analysis of HO-1, NQO-1, GCLC and GCLM in liver tissues. (G) Immunofluorescence staining of SIKE in hepatic sections (n = 5 per group). Scale bar was 50 μm. (H) Quantification of SIKE fluorescence intensity. (I) Western blot results for SIKE in liver samples (n = 5 per group). (J) RT-qPCR results for hepatic IL-1β, IL-6, TNF-α and IFN-β mRNA expression levels (n = 5 per group). (K) Western blot analysis of liver p-TBK1 and p-NF-κB (n = 5 per group). Data are expressed as means ± SEM. *P < 0.05 and **P < 0.01; ns, no significant difference.
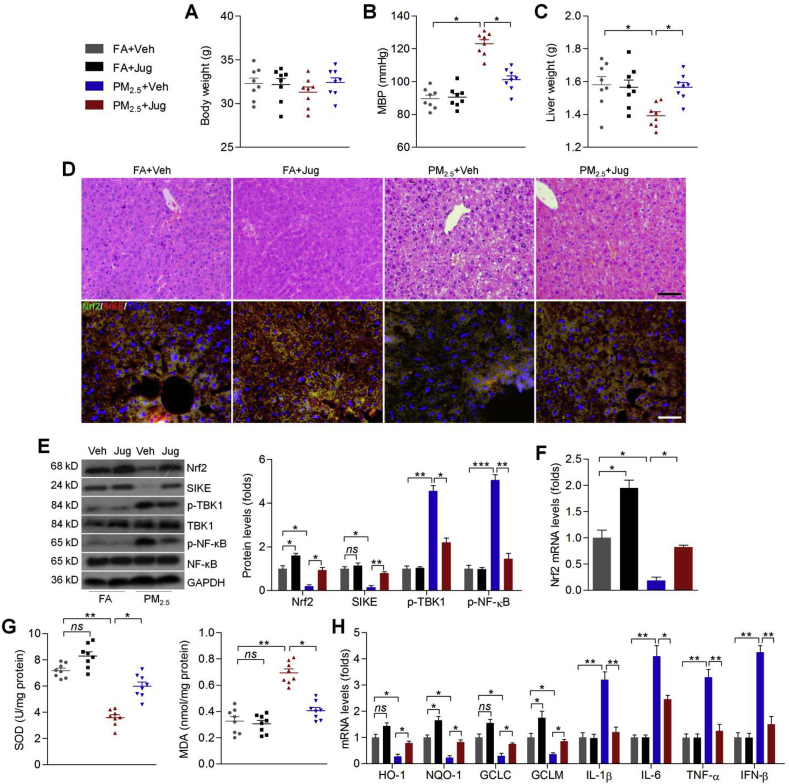
3.9. Juglanin ameliorates PM2.5-induced hepatic injury through improving Nrf2/SIKE signaling pathway in mice
Here, we attempted to further explore the potential of Jug to improve PM2.5-inudced hepatic injury in vivo. Consistently, there was no significant difference in the change of body weight from each group of mice (Fig. 9A). PM2.5-induced MBP was effectively reduced by Jug co-treatment (Fig. 9B). PM-2.5-reduced liver weight was rescued in Jug-treated mice (Fig. 9C). Jug administration also improved PM2.5-induced hepatic dysfunction, as evidenced by the markedly reduced hepatic ALT and AST levels (Supplementary Fig. 5C). H&E results confirmed that Jug supplementation alleviated the histological impairments in hepatic samples of mice challenged with PM2.5. Immunofluorescence staining showed that Nrf2 and SIKE expression were slightly increased by Jug administration, which were both evidently reduced in liver of PM2.5-challenged mice. However, Jug co-treatment clearly improved Nrf2 and SIKE expression in PM2.5-treated mice (Fig. 9D). Western blot analysis further demonstrated that PM2.5-decreased Nrf2 and SIKE could be restored by Jug co-treatment, while p-TBK1 and p-NF-κB expression levels stimulated by PM2.5 were effectively repressed in mice with Jug intervention (Fig. 9E). As shown in Fig. 9F, PM2.5 exposure markedly reduced Nrf2 expression from mRNA levels, which were rescued in mice co-treated with Jug. Compared with the PM2.5 group, hepatic SOD activities were markedly rescued by Jug. In contrast, PM2.5-enhanced MDA levels were markedly abrogated by Jug administration (Fig. 9G). Finally, RT-qPCR analysis showed that PM2.5-induced oxidative stress and inflammatory response were effectively diminished by Jug co-treatment, as evidenced by the rescued expression of HO-1, NQO-1, GCLC and GCLM, and the reduced mRNA levels of IL-1β, IL-6, TNF-α and IFN-β (Fig. 9H). Taken together, these data suggested that Jug could alleviate PM2.5-induced liver injury by improving Nrf2/SIKE signaling to suppress oxidative stress and inflammation.
Fig. 9.
Juglanin ameliorates PM2.5-induced hepatic injury through improving Nrf2/SIKE signaling pathway in mice. (A) Body weight change of mice (n = 8 per group). (B) MBP results for mice (n = 8 per group). (C) Liver weight of mice (n = 8 per group). (D) Up panel, H&E staining for liver samples; down panel, immunofluorescence staining of Nrf2 (green) and SIKE (red) in liver sections (n = 5 per group). Scale bar was 50 μm. (E) Western blot analysis for liver Nrf2, SIKE, p-TBK1 and p-NF-κB (n = 5 per group). (F) RT-qPCR results for hepatic Nrf2 mRNA expression levels (n = 5 per group). (G) SOD and MDA in liver tissues were measured (n = 8 per group). (H) RT-qPCR results for hepatic IL-1β, IL-6, TNF-α, IFN-β, HO-1, NQO-1, GCLC and GCLM (n = 5 per group). Data are expressed as means ± SEM. *P < 0.05 and **P < 0.01; ns, no significant difference. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
According to the statistics of World Health Organization (WHO), the outdoors pollution amongst the top 8 environmental risks to health. Especially in China and India, adding to the challenge are the side effects of rapid economic development: air pollution, contaminated water, and encroaching urbanization, all of which threaten health of people [45,46]. Toxicological studies have indicated that the toxicity of PM2.5 is a result of the combined effect of particles and the adsorbed toxic pollutants [3,4]. The harmful effect triggered by PM includes the oxidative stress mechanism, which can regulate the pathogenesis of inflammation in various organs [15,22,47,48]. The signaling pathways through which PM2.5 exposure promotes hepatic dysfunction and impairments of hepatic lipid/glucose metabolism have been widely revealed [[8], [9], [10],23]. However, the pronounced effect of PM2.5 exposure on the meditation of hepatic pathways associated with oxidative stress and inflammation has not been fully characterized.
The composition of PM2.5 is very complex, and the organic carbon particles and insoluble heavy metals may find their way to the systemic circulatory system and extrapulmonary organs from their deposition sites [49,50]. Therefore, these toxic compositions may have extensive and rapid adverse reactions in the liver. In this study, the top of ambient PM2.5 among all chemical components was Sulfur, and Sulfur was the principle component and associated with PM2.5-induced disease [51,52]. PM2.5 concentration at 150.1 ± 2.5 μg/m3 that's equal to air quality rating-4 level in China standard was used for in vivo experiments to mimic the real-world ambient PM2.5 for individuals. Thus, the exposure concentrations of PM2.5 in the exposure chamber were much higher than the WHO Air Quality Guideline in 2005 goals with PM2.5 at 25 mg/m3 (~6.0-fold of the WHO goal for PM2.5). Moreover, we clearly indicated that ambient PM2.5 exposure induced oxidative stress and inflammatory response in liver of mice through restraining Nrf2 and SIKE activation, leading to hepatic injury consequently. There was an interaction between oxidative stress and inflammation caused by PM2.5 exposure. A critical finding in this study was that Nrf2 might positively modulate SIKE expression with or without PM2.5 stimulation. Nrf2 deficiency promoted SIKE reduction, subsequently enhancing TBK1/NF-κB activation and associated inflammatory response. However, Nrf2 over-expression increased SIKE expression and decreased the release of inflammatory factors, along with the inactivation of TBK1/NF-κB. Moreover, the in vitro studies showed that SIKE negatively regulated NF-κB activation to control the pro-inflammatory response in PM2.5-incubated liver cells, which was largely dependent on TBK1 inactivation. The in vivo experiments using loss-of-function approaches confirmed that Nrf2/SIKE signaling exhibited protective effects against PM2.5-induced hepatic injury by mitigating oxidative stress and inflammation. The pharmacological intervention analysis showed that Jug treatment could activate Nrf2 signaling, and then improve SIKE, subsequently inhibiting oxidative and inflammatory damage induced by PM2.5. Collectively, all these findings demonstrated that Nrf2 may positively regulate SIKE expression to suppress oxidative stress and inflammation, ameliorating PM2.5-triggered hepatic damage. Jug, as a potential Nrf2 activator, could attenuate hepatic oxidative stress and inflammatory response induced by PM2.5 through improving SIKE.
Nrf2 is the predominant manager of the cellular defense system. Nrf2 mediates the expression of genes that encode antioxidant proteins, detoxifying enzymes, metabolic alteration enzymes and stress response proteins, most of which play critical roles in regulating cellular defense system, particularly in oxidative stress modulation [[26], [27], [28],53]. Through controlling such transcriptional network, Nrf2 is capable of coordinating a fine-tuned response to multiple stress conditions and detrimental assaults, maintaining the cellular microenvironmental homeostasis [54]. The Nrf2-Keap1 complex is a promising therapeutic target for inflammatory disease. Direct disruption of the Keap1-Nrf2 system could activate the protein levels of Nrf2 and thereafter display a cell protection molecular mechanism against oxidative attack when endogenous stress defense mechanisms are imbalanced [55]. Therefore, improving Nrf2 activity is effective for providing cytoprotection against different chronic and inflammatory conditions, including PM2.5-induced tissue damage [15,22,24,25]. Nrf2 is involved in the signaling pathway that results in PM-elicited spermatogenesis dysfunction [56]. In lung cells, Nrf2 protects against diverse PM components-triggered mitochondrial oxidative damage [57]. Our group also showed that Nrf2 could alleviate PM2.5-induced cardiac injury, hypothalamus dysfunction and renal damage [15,21,22]. Therapeutic strategy to improve Nrf2 activation has been suggested to effectively alleviate PM2.5-caused pulmonary injury in mice by suppressing oxidative stress and inflammatory response [58,59]. In our study, we confirmed that PM2.5 incubation led to the reduction of Nrf2, suppressing the expression of anti-oxidant factors, such as HO-1, NQO-1, GCLC, GCLM and SOD, which are involved in the cellular defense system [60]. After PM2.5 challenge, Nrf2−/− mice exhibited accelerated oxidative damage in liver tissues with lower expression of anti-oxidants. These data strongly demonstrated that improving Nrf2 activation might be effective to protect cells and animals from PM2.5-induced injury through improving the anti-oxidative signaling.
SIKE was initially identified as an IKKε-associated protein through yeast two-hybrid screening, and it is ubiquitously expressed in various tissues [29,30]. The profound involvement of IKKε in inflammation and immunity demonstrates the potential of SIKE in regulating the progression of immune diseases [61]. Recently, SIEK was found to negatively regulate pathological cardiac remodeling through suppressing protein kinase B (AKT) activation by regulating TBK1. Over-expressing TBK1 was sufficient to abrogate the protective effects of SIKE on the pathological cardiac hypertrophy [31]. Furthermore, TBK1 activation has been linked to inflammatory diseases, such as steatohepatitis [62]. TBK1 plays a modulatory role in the cascade that results in NF-κB activation and expression of inflammatory factors [32]. Suppressing TBK1 activity could ameliorate fatty liver progression through inhibiting inflammation [63]. Therefore, improving SIKE to suppress TBK1 might be effective for inflammation inhibition. In our study, we for the first time demonstrated that PM2.5 incubation led to SIKE reduction through a dose- and time-dependent manner in liver cells, whereas inflammatory signaling TBK1/NF-κB was markedly activated. The in vivo experiments validated the down-regulation of SIKE in liver of PM2.5-challenged mice. These findings demonstrated the potential of SIKE in controlling liver damage caused by air pollution. In addition, gain- and loss-function approaches indicated that SIKE negatively modulated the inflammatory response in PM2.5-treated cells and/or liver tissues. In the absence of SIKE, PM2.5-caused expression of IL-1β, IL-6, TNF-α and IFN-β was further accelerated, along with exacerbated activation of NF-κB. However, the “rescue” studies in cells showed that SIKE over-expression led to the striking inhibition of NF-κB activity, repressing inflammation consequently. Consistent with previous studies, the in vitro studies using the recombinant adenoviral vector infection analysis demonstrated that SIKE deficiency led to TBK1 over-activation, subsequently promoting NF-κB activation and inflammation. In contrast, restoring SIKE in liver cells markedly reduced TBK1 phosphorylation, and then alleviated inflammation. Importantly, we found that in PM2.5-incubated liver cells, SIKE-restrained inflammatory response and NF-κB activity was largely dependent on TBK1 activation. These in vitro analysis further demonstrated that SIKE regulated the development of hepatic injury caused by air pollution mainly through its suppression to TBK1 signaling.
An important and novel finding here was that Nrf2 exhibited a positive role in meditating SIKE expression. The in vitro studies using L02 cells showed that Nrf2 knockdown markedly reduced SIKE in liver cells, while promoting Nrf2 rescued SIKE expression either under normal condition or PM2.5 stresses. Luciferase reporter analysis confirmed that Nrf2 positively regulated SIKE expression in HEK-293 cells stimulated by PM2.5. Furthermore, Nrf2 exhibited an inhibitory role in TBK1/NF-κB activation, restricting inflammatory response eventually, which might be associated with the improvement of SIKE signal. The in vivo analysis indicated that after long-term PM2.5 exposure, SIKE-knockout mice had further decreased expression of anti-oxidants in liver samples, demonstrating that SIKE might be involved in the progression of oxidative stress induced by stresses. Therefore, although we illustrated the regulatory effect of Nrf2 on SIKE, further studies are still required to explore how Nrf2 regulates SIKE, as well as the potential correlation between the two signaling pathways.
Juglanin, as natural compound extracted from Polygonum aviculare, exhibits various biological activities, such as anti-inflammation, anti-cancer and anti-apoptotic cell death [35,36,38,64]. Given the results that oxidative stress and inflammation played critical roles in regulating PM2.5-induced hepatic injury via Nrf2/SIKE signaling, we then attempted to find chemoprevention targeting Nrf2 to subsequently ameliorate this disease. We first showed that Jug boosted Nrf2 protein expression levels and its nuclear translocation, as well as the down-streaming signals such as HO-1. We then found that Nrf2 activation by Jug markedly increased the anti-oxidant capacity of L02 cells, as proved by the up-regulated antioxidant enzymes SOD, CAT and GPX. At the same time, Nrf2 suppressor Keap-1 was decreased in PM2.5-stimulated cells. All these results regulated by Jug decreased cellular ROS in response to PM2.5. As expected, with the improvement of Nrf2, SIKE expression levels were increased in Jug-treated cells in the absence or presence of PM2.5. Consistently, PM2.5-caused inflammation and activation of TBK1/NF-κB were effectively repressed in cells co-treated with Jug. The in vivo studies using a PM2.5-induced chronic hepatic injury model supported the protective effects of Jug against PM2.5-elicited liver damage. Further assessments showed that Jug significantly attenuated PM2.5-induced oxidative stress and inflammatory conditions by improving Nrf2/SIKE signaling and reducing TBK1/NF-κB activity.
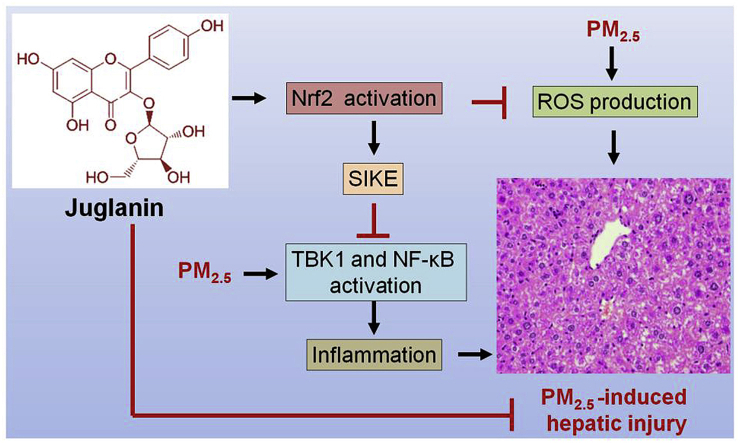
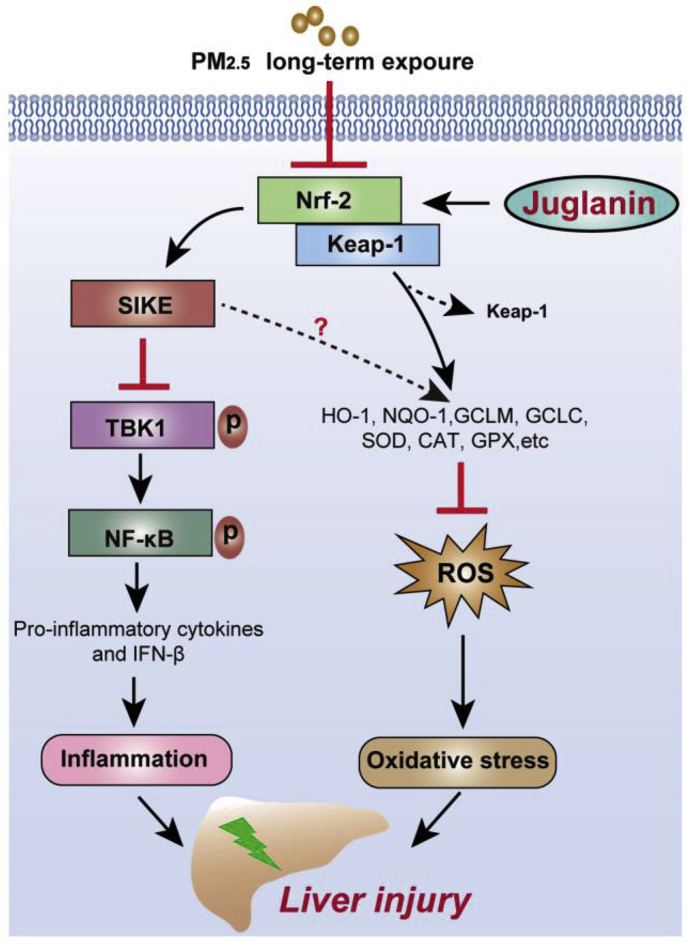
In summary, as exhibited in Fig. 10, we identified that Nrf2 could improve SIKE signaling to suppress oxidative stress and inflammatory response induced by PM2.5. Additionally, SIKE-inhibited activation of NF-κB and inflammatory response was dependent on the blockage of TBK1. Despite more studies are still warranted, the application of Nrf2/SIKE-induced hepatic protection was verified in mice with long-term PM2.5 exposure. Pharmacological intervention analysis further confirmed that Jug, as a potential activator of Nrf2, suppressed oxidative stress, improved SIKE expression and then inhibited TBK1 to suppress inflammatory response, alleviating air pollution-induced chronic hepatic inflammation. Therefore, targeting Nrf2/SIKE and/or its association with TBK1 may represent promising treatments for developing effective strategies against PM2.5-induced pathological hepatic injury.
Fig. 10.
Proposed mechanism of Nrf2/SIKE-mediated hepatic injury induced by long-term PM2.5exposure. After PM2.5 long-term challenge, down-regulated activation of Nrf2 repressed SIKE expression, contributing to oxidative stress and inflammatory response through reducing anti-oxidants and facilitating TBK1/NF-κB signaling. These effects led to hepatic injury consequently. However, Juglanin treatment showed protective effects against PM2.5-induced liver injury by improving Nrf2/SIKE signaling pathway.
Declaration of competing interest
The authors see no conflict of interest.
Acknowledgments
This work was supported by (1) National Natural Science Foundation of China (NSFC Grant No.: 81703527); (2) Chongqing Research Program of Basic Research and Frontier Technology (Grant No.: cstc2018jcyjA3686, cstc2018jcyjAX0784, cstc2018jcyjA1472, cstc2018jcyjAX0811, cstc2018jcyjA3533 & KJZD-M201801601); (3) School-level Research Program of Chongqing University of Education (Grant No.: KY201710B & 17GZKP01); (4) Advanced Programs of Post-doctor of Chongqing (Grant No.: 2017LY39); (5) Science and Technology Research Program of Chongqing Education Commission of China (Grant No.: KJQN201901608, KJQN201901615 & KJ1601402); (6) Children's Research Institute of National Center for Schooling Development Programme and Chongqing University of Education (Grant No.: CSDP19FSO1108) and (7) Chongqing Professional Talents Plan for Innovation and Entrepreneurship Demonstration Team (CQYC201903258).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101645.
Contributor Information
Jun Tan, Email: tanjun@cque.edu.cn.
Bochu Wang, Email: wangbc2000@126.com.
Minxuan Xu, Email: minxuanxu@foxmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Xu M.X., Dai X.L., Kuang Q., Zhu L.C., Hu L.F., Lou D.S.…Wang B.C. Dysfunctional Rhbdf2 of proopiomelanocortin mitigates ambient particulate matter exposure-induced neurological injury and neuron loss by antagonizing oxidative stress and inflammatory reaction. J. Hazard Mater. 2020:123158. doi: 10.1016/j.jhazmat.2020.123158. [DOI] [PubMed] [Google Scholar]
- 2.Lu F., Xu D.Q., Cheng Y.B. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015;136:196–204. doi: 10.1016/j.envres.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Brook R.D., Franklin B., Cascio W. Airpollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 4.Wan G.H., Rajagopalan Sanjay, Sun Q.H. Real-world exposure of airborne particulate matter triggers oxidative stress in an animal model. Int. J. Physiol. Pathophysiol. Pharmacol. 2010;2:64–68. [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu Y.N., Wang G.H., Zhou F. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol. Environ. Saf. 2019;167:178–187. doi: 10.1016/j.ecoenv.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Laing S., Wang G.H., Briazova T. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am. J. Physiol. Cell Physiol. 2010;299:C736–C749. doi: 10.1152/ajpcell.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez R., Zheng Z., Fan Z.J. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am. J. Transl. Res. 2013;5:224–234. [PMC free article] [PubMed] [Google Scholar]
- 8.Li R., Wang Y.X., Chen R.C. Ambient fine particulate matter disrupts hepatic circadian oscillation and lipid metabolism in a mouse model. Environ. Pollut. 2020;262:114179. doi: 10.1016/j.envpol.2020.114179. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M.J., Zhao H., Deng J.M. Role of the CLOCK protein in liver detoxification. Br. J. Pharmacol. 2019;176:4639–4652. doi: 10.1111/bph.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z., Xu X.H., Zhang X.B. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J. Hepatol. 2013;58:148–154. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Z., Zhang X.B., Wang J.M. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J. Hepatol. 2015;63:1397–1404. doi: 10.1016/j.jhep.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M.X., Ge C.X., Qin Y.T. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic. Biol. Med. 2019;130:542–556. doi: 10.1016/j.freeradbiomed.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Tang M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total Environ. 2020;710:136397. doi: 10.1016/j.scitotenv.2019.136397. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Xue L.J., Chen Q. Cardiorespiratory responses to fine particles during ambient PM pollution waves: findings from a randomized crossover trial in young healthy adults. Environ. Int. 2020;139:105590. doi: 10.1016/j.envint.2020.105590. [DOI] [PubMed] [Google Scholar]
- 15.Ge C.X., Hu L.F., Lou D.S. Nrf2 deficiency aggravates PM-induced cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation via RIPK3-regulated mitochondrial disorder. Aging (Albany NY) 2020;12:4836–4865. doi: 10.18632/aging.102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan S.L., Wong L.L., Chan K.C.A. Development of a novel inflammation-based index for hepatocellular carcinoma. Liver Canc. 2020;9:167–181. doi: 10.1159/000504252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J.W., Liu X., Zhang T.T. Hepatocyte TMEM16A deletion retards NAFLD progression by ameliorating hepatic glucose metabolic disorder. Adv. Sci. (Weinh) 2020;7:1903657. doi: 10.1002/advs.201903657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 19.Lu M.C., Ji J.A., Jiang Z.Y. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med. Res. Rev. 2016;36:924–963. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- 20.Jiang J.J., Li Y., Liang S. Combined exposure of fine particulate matter and high-fat diet aggravate the cardiac fibrosis in C57BL/6J mice. J. Hazard Mater. 2020;391:122203. doi: 10.1016/j.jhazmat.2020.122203. [DOI] [PubMed] [Google Scholar]
- 21.Xu M.X., Zhu Y.F., Chang H.F. Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic. Biol. Med. 2016;99:259–272. doi: 10.1016/j.freeradbiomed.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Ge C.X., Xu M.X., Qin Y.T. iRhom2 loss alleviates renal injury in long-term PM2.5-exposed mice by suppression of inflammation and oxidative stress. Redox Biol. 2018;19:147–157. doi: 10.1016/j.redox.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J.X., Zhang W., Lu Z.B. Airborne PM-induced hepatic insulin resistance by Nrf2/JNK-mediated signaling pathway. Int. J. Environ. Res. Publ. Health. 2017;14(7):787. doi: 10.3390/ijerph14070787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz S., Pergola P.E., Zager R.A. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesh Y.V., Negi G., Sharma S.S. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013;1:394–397. doi: 10.1016/j.redox.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q.M., Gao Y., Ci X.X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. Longevity. 2019;2019:7090534. doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon H.Y., Kang N.I., Lee H.K. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem. Pharmacol. 2008;75:2214–2223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C.S., Zhuang C.L., Zhu K.K. Identification of a novel small-molecule Keap1-Nrf2 PPI inhibitor with cytoprotective effects on LPS-induced cardiomyopathy. J. Enzym. Inhib. Med. Chem. 2018;33:833–841. doi: 10.1080/14756366.2018.1461856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machek M.L., Sonnenschein H.A., Graham S.K.I. Predicting and validating a model of suppressor of IKKepsilon through biophysical characterization. Protein Sci. 2019;28:1423–1436. doi: 10.1002/pro.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Yan C.Z., Liu Ji. SIKE of black carp is a substrate of TBK1 and suppresses TBK1-mediated antiviral signaling. Dev. Comp. Immunol. 2019;90:157–164. doi: 10.1016/j.dci.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Deng K.Q., Wang A.B., Ji Y.X. Suppressor of IKKϵ is an essential negative regulator of pathological cardiac hypertrophy. Nat. Commun. 2016;7:11432. doi: 10.1038/ncomms11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balka K.R., Louis C., Saunders T.L. TBK1 and IKKε act redundantly to mediate STING-induced NF-κB responses in myeloid cells. Cell Rep. 2020;31:107492. doi: 10.1016/j.celrep.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 33.Martins R.M., Pinto R.A., Soeiro T.J. Addition of berberine to preservation solution in an animal model of ex vivo liver transplant preserves mitochondrial function and bioenergetics from the damage induced by ischemia/reperfusion. Int. J. Mol. Sci. 2018;19(1):284. doi: 10.3390/ijms19010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E.S., Kwon M.H., Kim H.M. Curcumin analog CUR5-8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metab. Clin. Exp. 2020;103:154015. doi: 10.1016/j.metabol.2019.154015. [DOI] [PubMed] [Google Scholar]
- 35.Chen X.X., Zhang C.Y., Wang X. Juglanin inhibits IL-1β-induced inflammation in human chondrocytes. Artif. Cells Nanomed. Biotechnol. 2019;47:3614–3620. doi: 10.1080/21691401.2019.1657877. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F.X., Xu R.S. Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson's disease and cell culture via inactivating TLR4/NF-κB pathway. Biomed. Pharmacother. 2018;97:1011–1019. doi: 10.1016/j.biopha.2017.08.132. [DOI] [PubMed] [Google Scholar]
- 37.Chen L., Xiong Y.Q., Xu J. Juglanin inhibits lung cancer by regulation of apoptosis, ROS and autophagy induction. Oncotarget. 2017;8:93878–93898. doi: 10.18632/oncotarget.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Z.W., Yuan Y.F. Juglanin suppresses fibrosis and inflammation response caused by LPS in acute lung injury. Int. J. Mol. Med. 2018;41:3353–3365. doi: 10.3892/ijmm.2018.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun S.C., Han R., Hou S.S. Juglanin alleviates bleomycin-induced lung injury by suppressing inflammation and fibrosis via targeting sting signaling. Biomed. Pharmacother. 2020;127:110119. doi: 10.1016/j.biopha.2020.110119. [DOI] [PubMed] [Google Scholar]
- 40.Ying Z.K., Xu X.H., Bai Y. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ. Health Perspect. 2014;122:79–86. doi: 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogino K., Zhang R., Takahashi H. Allergic airway inflammation by nasal inoculation of particulate matter (PM2.5) in NC/Nga mice. PloS One. 2014;9 doi: 10.1371/journal.pone.0092710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W.S., Wang Y.J., Qu C.B. The RNA genome of hepatitis E virus robustly triggers an antiviral interferon response. Hepatology. 2018;67:2096–2112. doi: 10.1002/hep.29702. [DOI] [PubMed] [Google Scholar]
- 43.Chen B., Li C., Yao J. Zebrafish NIK mediates IFN induction by regulating activation of IRF3 and NF-κB. J. Immunol. 2020;204:1881–1891. doi: 10.4049/jimmunol.1900561. [DOI] [PubMed] [Google Scholar]
- 44.Xu W., Mo J., Ocak U. Activation of Melanocortin 1 Receptor attenuates early brain injury in a rat model of subarachnoid hemorrhage via the suppression of neuroinflammation through AMPK/TBK1/NF-κB pathway in rats. Neurotherapeutics. 2020;17:294–308. doi: 10.1007/s13311-019-00772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q.L., Qiu M.Z., Lai K.F. Cough and environmental air pollution in China. Pulm. Pharmacol. Therapeut. 2015;35:132–136. doi: 10.1016/j.pupt.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Bagcchi S.J. Air pollution on the agenda in India. Lancet Respir. Med. 2015;3:272. doi: 10.1016/S2213-2600(15)00088-0. [DOI] [PubMed] [Google Scholar]
- 47.Dai P.Y., Shen D., Shen J.K. The roles of Nrf2 and autophagy in modulating inflammation mediated by TLR4 - NF-κB in A549 cell exposed to layer house particulate matter 2.5 (PM) Chemosphere. 2019;235:1134–1145. doi: 10.1016/j.chemosphere.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Chu C., Zhang H.Y., Cui S.J. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard Mater. 2019;369:180–190. doi: 10.1016/j.jhazmat.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Takenaka S., Karg E., Roth C. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001;109:547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kreyling W.G., Semmler M., Erbe F. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health Part A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 51.Jiménez E., Linares C., Rodríguez L.F. Short-term impact of particulate matter (PM2.5) on daily mortality among the over-75 age group in Madrid (Spain) Sci. Total Environ. 2009;407:5486–5492. doi: 10.1016/j.scitotenv.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Han X.K., Guo Q.J., Strauss Harald. Multiple Sulfur isotope constraints on sources and formation processes of sulfate in Beijing PM aerosol. Environ. Sci. Technol. 2017;51:7794–7803. doi: 10.1021/acs.est.7b00280. [DOI] [PubMed] [Google Scholar]
- 53.Hseu Y.C., Chang C.T., Gowrisankar Y.V. Zerumbone exhibits antiphotoaging and dermatoprotective properties in Ultraviolet A-irradiated human skin fibroblast cells via the activation of Nrf2/ARE defensive pathway. Oxid. Med. Cell. Longevity. 2019;2019:4098674. doi: 10.1155/2019/4098674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roos N.J., Duthaler U., Bouitbir J. The uricosuric benzbromarone disturbs the mitochondrial redox homeostasis and activates the NRF2 signaling pathway in HepG2 cells. Free Radic. Biol. Med. 2020;152:216–226. doi: 10.1016/j.freeradbiomed.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Lin Y.S., Zheng X.H., Chen J.F. Bruguiera gymnorrhiza protective effect of (L.) Lam. fruit on dextran sulfate sodium-induced ulcerative colitis in mice: role of Keap1/Nrf2 pathway and gut microbiota. Front. Pharmacol. 2019;10:1602. doi: 10.3389/fphar.2019.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi X.X., Fu L.J. Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des. Dev. Ther. 2019;13:2811–2824. doi: 10.2147/DDDT.S198444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardo M., Xu F.F., Shemesh M. Nrf2 protects against diverse PM components-induced mitochondrial oxidative damage in lung cells. Sci. Total Environ. 2019;669:303–313. doi: 10.1016/j.scitotenv.2019.01.436. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Xue L., Li B.Y. Therapeutic potential of bixin in PM2.5 particles-induced lung injury in an Nrf2-dependent manner. Free Radic. Biol. Med. 2018;126:166–176. doi: 10.1016/j.freeradbiomed.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Wang A.S., Xu Y., Zhang Z.W. Sulforaphane protects MLE-12 lung epithelial cells against oxidative damage caused by ambient air particulate matter. Food Funct. 2017;8:4555–4562. doi: 10.1039/c7fo00969k. [DOI] [PubMed] [Google Scholar]
- 60.Cui Y.T., Li Y., Huang N. Structure based modification of chalcone analogue activates Nrf2 in the human retinal pigment epithelial cell line ARPE-19. Free Radic. Biol. Med. 2020;148:52–59. doi: 10.1016/j.freeradbiomed.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 61.Piao W.J., Song C., Chen H.Y. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J. Leukoc. Biol. 2009;86:863–875. doi: 10.1189/jlb.0309189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho C.S., Park H.W., Ho A. Lipotoxicity induces hepatic protein inclusions through TANK binding kinase 1-mediated p62/sequestosome 1 phosphorylation. Hepatology. 2018;68:1331–1346. doi: 10.1002/hep.29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X.X., Rao H.Y., Zhao J.M. STING expression in monocyte-derived macrophages is associated with the progression of liver inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Lab. Invest. 2020;100:542–552. doi: 10.1038/s41374-019-0342-6. [DOI] [PubMed] [Google Scholar]
- 64.Zhou G.Y., Yi Y.X., Jin L.X. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed. Pharmacother. 2016;81:318–328. doi: 10.1016/j.biopha.2016.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.