Abstract
Background and Purpose
Ample evidence indicates that chronic adolescence stress is associated with an increased risk of developing neuropsychiatric disorders in adulthood. Given the importance of the effective therapeutic ways to overcome adolescent stress-related deficits, the present study investigated the effects of Spirulina platensis (SP), environmental enrichment (EE), and voluntary exercise (EX) and their combination on anxiety or depression-like behaviors, oxidative stress, and alterations of BDNF and 5HT-3 receptors in the prefrontal cortex (PFC) induced by adolescent stress in adult female rats.
Methods
During the adolescent period (PNDs30-40), rats were subjected to restraint stress. Then, the animals were subjected to SP treatment (200 mg/kg/day), EX, EE, and the combined treatments (SP+EX, and SP+EE) for 15 days between PNDs41-55. Subsequently, anxiety or depression-like behaviors, BDNF levels, oxidative stress markers and mRNA expression of BDNF and 5HT3 in the PFC were assessed.
Results
Stressed rats demonstrated enhanced anxiety levels and depression-like behaviors in adulthood. Regarding the oxidative stress markers, stressed rats exhibited significantly higher levels of malondialdehyde, a lipid peroxidation product, higher activities of antioxidant enzymes (glutathione peroxidase and superoxide dismutase) and significantly lower total antioxidant reactivity capacity in the PFC. Additionally, adolescent stress significantly increased 5HT3 receptor mRNA expression and decreased BDNF content and its mRNA expression in the PFC. Treatments with SP, EX, EE, and the combined interventions alleviated these deficits.
Conclusion
Our findings indicate that appropriate interventions during the adolescent period can protect against adolescent stress-induced behavioral, and biochemical defects and oxidative stress damage in adulthood.
Keywords: adolescent stress, anxiety, depression, environmental enrichment, physical activity spirulina, oxidative stress
Introduction
Chronic exposure to stress, whether it occurs during the pre-pubertal period is associated with an increased risk of developing neuropsychiatric disorders in adulthood.1,2 Adolescence is a critical and sensitive period of brain development that is characterized by changes in brain structure and function, particularly in limbic and cortical regions (hippocampus, amygdala and prefrontal cortex).3,4 The prefrontal cortex (PFC) has been implicated in many psychological disorders and is involved in cognitive processes that are influenced by oxidative stress and is a target for the hormones involved in the stress response.5–10
Chronic stress exposure has been shown to modify neuronal function and morphology in corticolimbic structures such as the PFC, hippocampus, and amygdala, three brain regions greatly involved in mood regulation, fear processing, and cognition.11 Studies showed that stress experienced in the adolescence phase is of clinical importance, as it is associated with the development of disorders such as depression, anxiety, and post-traumatic stress disorder in adulthood.12–15 Brain-derived neurotropic factor (BDNF) plays an important role in depressive disorders. Several lines of evidence suggest that exposure to stressors in early life leads to decrease BDNF expression in the PFC and hippocampus and enhances anxiety and depression-like behaviors.16,17 It has been suggested that depression-related behaviors are associated with reducing the expression of BDNF.18,19 It is now well accepted that females are more susceptible to stress effects than the male and the risk of depressive disorders in females is higher than the male.20–23
5-hydroxytryptamine (5-HT3) receptors are ubiquitously expressed in several brain stem nuclei and cortical areas such as the PFC, hippocampus and amygdala,24 and are involved in depression and anxiety-like disorders.25,26 Several lines of studies show that 5-HT3 receptor antagonists modulate serotonergic pathways and show antidepressant and anxiolytic-like effects in various animal models.26–29
Besides behavioral and molecular changes, it has been shown that chronic stress induces oxidative stress, and considerably changes the balance between pro-oxidant and antioxidant factors in the brain.30–32 Oxidative stress has been implicated in the pathogenesis of some neurologic and psychiatric illnesses, including depression and anxiety disorders.33–35 Chronic social stress for 21 days in male rats led to depressive and anxiety-like behaviors and increased oxidative markers likely through nuclear factor-κB translocation and concomitant cytosolic cyclooxygenase 2 up-regulation, associated with decreased superoxide dismutase (SOD) activity and glutathione (GSH) levels in the PFC. In contrast, hippocampus was less susceptible to oxidative damage showing only an increase in protein carbonyl groups and depleted GSH.8 These findings show that PFC is more sensitive to oxidative stress than the hippocampus following chronic isolation stress, which may be important for further investigation regarding anxiety or depressive-like behaviors induced by chronic stress.
There is an increasing demand for findings effective intervention methods for alleviating the detrimental effects of adolescent stress. Plants and medicinal herbs have been used form a long time ago throughout the world as therapeutic agents. Spirulina platensis (SP) is a cyanobacterium, which has attracted attention because of its nutritional value and pharmacological properties.36–38 SP has protective effects against oxidative stress and this effect is related to C-phycocyanin, an active ingredient of Spirulina, which has significant antioxidant properties.39 Phycocyanin attenuated ischemia-reperfusion cardiac dysfunction through its antioxidant and anti-apoptotic actions and modulation of p38 MAPK and ERK1/2.40 Spirulina also has neuroprotective effects in HT22 cells, a mouse hippocampal neuronal cell line, via suppressing oxidative stress and enhancing the BDNF-pCREB signaling pathway.41
A few studies have reported the therapeutic implications of Spirulina for the treatment of neurodegenerative disorders.42–45 It has been reported that Spirulina has a protective effect on hippocampus neural progenitor cells against lipopolysaccharide-induced acute systemic inflammatory.46 In our recent study, we showed that SP has protective effects against adolescent stress-induced deficits in learning and memory, hippocampal BDNF and morphological remolding in adult female rats.47
Physical activity is another alternative intervention for alleviating the detrimental effects of adolescent stress. Studies have shown that exercise improves cognitive deficits48,49 and enhances levels of BDNF in the brain50,51 and reduces the risk of neuropsychiatric diseases.52,53 BDNF plays an important role in mediating the effects of exercise on brain functions.50 Environmental enrichment (EE) is another beneficial intervention for improving anxiety and depression-like behaviors. A large body of research revealed that exposure to EE improves several conditions, including degenerative diseases, epilepsy, traumatic brain injury, anxiety, and depression.54–56 EE increases serotonin concentrations in the PFC and improves the effects of chronic stress by promoting cell survival and differentiation and increasing glucocorticoid receptor expression in the hippocampus.54,57,58
Recently, we reported the beneficial effects of SP, and its combination with voluntary exercise (EX) or environmental enrichment (EE) against adolescent stress-induced deficits in cognitive functions, oxidative stress, hippocampal and amygdala BDNF and morphological remolding in adult female rats.47,59 In continuation of these studies, the present study focused on the effects of chronic stress on the PFC since recent studies have shown that this brain region has greater sensitivity to chronic stress than hippocampus or amygdala.8,60 Thus, using a chronic restraint model, we examined the effects of SP, EX, and EE and their combined interventions on oxidative stress markers and molecular changes (BDNF, and 5-HT3) in the PFC and associated anxiety-/depressive-like behaviors induced by chronic adolescent stress in adult female rats. Findings of the present study can help us to develop new therapeutic ways to reduce the impact of adolescent stress on different aspects of the brain function.
Materials and Methods
Experimental Animals
Wistar female pup rats (30 days old, 60–70g) were obtained from the breeding colony of the Semnan University of Medical Sciences (SUMS), Semnan, Iran. Animals were maintained at 12-h light/dark cycle and in a controlled room temperature (22 ± 2°C) and, housed 5 to 6 per cage given free access to food and water ad libitum. All experiments were approved by the Ethics Committee of Semnan University of Medical Sciences (IR.SEMUMS.REC.1394.208). The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental animals underwent behavioral tests, which are followed by molecular and biochemical measurements (see the below).
Spirulina Platensis
Spirulina platensis (SP) was purchased (product number SPM-12,462,016) from Setendorf Company, Jask, Bandar Abbas, Iran. The SP was administrated at a dose of 200 mg/kg per day by oral gavage for 15 days (PND 41–55). The corresponding control rats were received orally distilled water alone and solutions were refreshed daily. The dose of SP was chosen based on dose-response effects of SP in our previous study.47
Stress Induction
Rats were exposed to restraint stress (2 h/day for 10 days, PND30-40) between 10:00 and 12:00 AM.47,61 During the restraint, each pup rat was restrained in a clear polyethylene cylinder. For achieve to complete immobilization, the cylinder size was adjusted according to the size of the pup rats. A hole in the front of the cylinder was set to allow the animals to breathe freely. This stress protocol increases corticosterone levels as reported in our recent work.47
Voluntary Wheel Running Exercise
Each of the exercising rats was given all day/night access to a cage that was equipped with a running wheel. An electronic system that linked to the running wheel (model 2021.PN) automatically recorded animal movements (Tajhiz Gostar Company, Tehran, Iran). The running wheels were removed from the cages after the 15 days period from PNDs 41–55. No significant differences were found between the amount of running in control and stress groups.
Environmental Enrichment
Rats were randomly assigned to the Plexiglas cages or EE (100 cm × 100 cm × 50 cm) (10 animals per cage), which were equipped with the running wheel, a raised platform and were enriched with a complex of plastic tunnels, steel chains, plastic balls and toys in different size which changed every 5–6 days. EE housing began after exposure to chronic stress before adulthood from PNDs 41–55.
Anxiety and Depression-Like Behavior Tests
Open-Field Test (OFT)
The open-field test was performed to examine anxiety. The apparatus was made of a dark area (72×72×45 cm). The central area (center zone) was defined as the middle 50 × 50 cm area of the field. The test chamber was illuminated at 100 lx. Data were collected over a 20-min period. Animal behavior (total distance, the number of visits and percentage of time spent in the central area) was digitally recorded for subsequent data analysis (Mazerouter Software, Technique Azma Company, Tabriz, Iran).
Elevated Plus Maze (EPM)
Anxiety assessment was conducted in the EPM (Manufacturing of Technique Azma Company, Tabriz, Iran). The apparatus was made of two open arms (50 cm × 10 cm) and two enclosed arms (50 cm × 10 cm, surrounded by 40-cm high wooden walls) raised 50 cm above the floor. All arms were joined in the central neutral area (10 × 10 cm) of the maze so that rats could freely pass from one arm to another. The open arms were bordered by a 0.5-cm high Plexiglas edge to avoid falls. Animal behaviors were recorded by an overhead camera connected to a PC running video tracking software. Rats were moderately located in the neutral area facing one of the open arms and given 5 min to discover the maze. The duration and the number of entries into open and enclosed arms were recorded. To minimize scent trails, the maze was cleaned with a 20% alcohol solution between trials. Increased entry to the open arm/or open arm time indicates anxiety reduction, in contrast, increased entry to the close arm/or close arm time refers to anxiogenic influences.62
Force Swimming Test (FST)
One day before the probe test (pre-test day), rats were placed in a cylindrical swimming tank (50 cm high, 25 cm diameter) was filled with 25°C water for 15 mins. Then, they were removed from water, dried and cleaned with a towel before being returned to their cages. 24 hrs later, animals were put back into the swimming tank for 5 min. All swim sessions were videotaped and behavioral analysis of experimental groups and treatment was accomplished by a blind, observer. On the probe day, immobility behavior (define as floating in water without any movement except that are necessary to hold the animal head above water), swimming behavior (active movement described the goal-directed horizontal movement such as swimming in circles or crossing between quadrants of the cylinder), climbing (upward goal-directed movements of the forepaws along the side of the cylindrical container) and the number of fecal pellets were recorded. An increase in the duration of immobility behavior or decreases in swimming duration and climbing duration represented depression-like behaviors in rats.63
BDNF Measurements
The rats were decapitated, and the whole prefrontal cortex (PFC) was dissected and then immediately frozen at −80°C until used for the preparation of homogenates. The tissues were homogenate in cold lysis buffer and the supernatants, which were obtained after centrifugation at 12,000g for 20 min at −4°C, were used for BDNF assay. The BDNF protein levels were assessed using Rat BDNF ELISA kits (Hangzhou Eastbiopharm Co., LTP) according to the manufacturer’s recommendations. The sensitivity of the assay was 0.01 ng/mL. The level of total protein in supernatants was determined by the Bradford method using bovine serum albumin as a standard.64
Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
The mRNA expression of BDNF protein and 5HT3 receptor was determined by using RT-PCR method. After the behavioral tests on PND 70, the animals were decapitated after anesthesia with ether. Then, the brains were removed and the prefrontal cortex rapidly dissected out on ice and flash frozen at –80°C. RNA was reverse transcribed (RT) into cDNA by using a commercial cDNA synthesis kit (Bioneer Inc., Seoul, South Korea) and stored at –20°C until further use. The primers were synthesized by Shenggong Biotech Limited Company (Shanghai, China). The sequences of the primers are presented in Table 1. Accuracy of the primers was confirmed by direct sequencing of target sequences. PCR was performed using a thermal cycler (ABI 7900HT, 96 wells, SYBR Green) consisting of denaturation at 95°C for 2 min, 45 additional cycles at 94°C for 30 s, and then 45 s of primer annealing at 58°C (for BDNF), 56.5°C (for 5HT3), and 53°C (for GAPDH), followed by a final extension at 72°C for 8 min. All amplifications were performed on Applied Biosystems RT-PCR (ABI 7900HT Fast Real-Time PCR System, US). Gene expression was normalized to the levels of GAPDH as a reference gene in all reactions and gene expression was calculated by using the 2−ΔΔCt method. All primers were designed using AlleleID® version 7.5. Primer Select program (PREMIER Biosoft, USA) and synthesized by Bioneer Inc. (Seoul, South Korea). Accuracy of the primers was confirmed by direct sequencing of target sequences. All tests were conducted in duplicate for each sample.
Table 1.
Primer Sequences Used in Quantitative Reverse Transcriptase Polymerase Chain Reaction
| Primer Name | Sequence (5′ - 3′) | Size (bp) |
|---|---|---|
| BDNF | Forward: GAC CCT GAG TTC CAC CAG G | 165 |
| Reverse: CCA GAG TCC CAT GGG TCC | ||
| 5HT3 | Forward: GGC TCC GCT GCT CTT TGG | 123 |
| Reverse: CGT CCT TCA CCT CCA CAG C | ||
| GAPDH | Forward: CTT CCA GGA GCG AGA TCC C | 267 |
| Reverse: GTG GTT CAC ACC CAT CAC AAA C |
Measurements of Oxidative Stress Markers in the PFC
FRAP and MDA Activity Assay
Following the behavioral tests, animals were decapitated under ether anesthesia and their brains were quickly removed, cleaned with ice-cold saline and stored at –80°C. For ferric reducing antioxidant power (FRAP) and malondialdehyde (MDA) assays, a fraction of tissue was homogenized (1:10 w/v) in cold 1.15% KCl. Homogenates were prepared in a ratio of 100 mg tissue to 1 mL phosphate buffer (50 mmol/l; pH 7.5) containing 1 mM EDTA. The supernatants obtained after centrifugation at 20,000×g for 15 mins at 4°C were used for biochemical analysis. The level of total protein in supernatants was determined by the Bradford method using bovine serum albumin as a standard.64
Enzymes Activity Assay
SOD and GPx activities were measured in the supernatant by using ZellBio kits (Zellbio GmbH, Ulm, Germany). After adding reagents, samples, and standards into the wells, absorbance was measured at 0 and 2 mins with an ELISA microplate reader (AD Touch Reader, apDia, Belgium) at 412 nm. The concentrations of SOD and GPx were expressed as U/mg protein. The sensitivity of the assay for SOD and GPx was 1 U/mL and 5 U/mL, respectively.
Experimental Groups
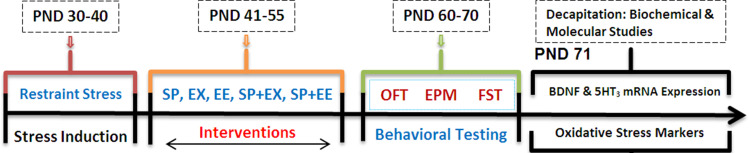
Rats randomly were distributed into 12 following groups (10 animals in each group). Control group: rats received only oral physiological saline using gavage tube; EX group: rats exposed to the running wheel for 15 days; SP group: rats received 200 mg/kg SP per day for 15 days using gavage tube, EE group: rats exposed to the enriched environment; SP + EX group: rats exposed to the running wheel and received SP; SP + EE group: rats exposed to the enriched environment for 15 days and received SP, Stress group: rats exposed to restraint stress, Stress + SP group: rats exposed to stress and then received SP; Stress + EX group: rats received stress and then exposed to the running wheel, Stress + EE group: rats received stress and then exposed to EE; Stress + SP + EX group: rats received stress and then exposed to the running wheel and SP; and Stress + SP + EE group: rats received stress and then exposed to EE and SP. Behavioral assessments began at the PND 61 after the termination of all interventions procedure. One day after the last behavioral test, half of the animals of each group were decapitated, and their brains removed, and stress oxidative markers and BDNF were measured in the PFC. The remaining animals were used for the measurement of mRNA expression of BDNF and 5HT3 in the PFC (Figure 1).
Figure 1.
Timeline of Experiments. Rats were exposed to restraint stress (2 h/day for 10 days, PND 30–40). Then, the animals were subjected to different treatments between PND 41 and 55 of age. Following the interventions, anxiety or depression-like behaviors, BDNF levels, Oxidative stress markers and mRNA expression of BDNF and 5HT3 receptor in prefrontal cortex were examined.
Abbreviation: PND, postnatal day; SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment; OFT, open field test; EPM, elevated plus maze; FST, force swimming test.
Statistical Analysis
Results are expressed as mean ± standard error of the mean (SEM). The significant interactions between treatments on the measured behavioral, biochemical and molecular parameters were determined using an overall two-way analysis of variance (ANOVA) with the fixed factors being treatment (six levels: Saline, SP, EX, EE, SP + EX, and SP + EX) and groups (two levels: control and stress), followed by the Tukey’s post-hoc test for multiple comparisons. The Pearson correlation coefficient was used to examine the association between PFC BDNF levels and anxiety level (open arm time) and depressive behavior (swimming duration) in the experimental groups. The accepted level of significance for all tests was p < 0.05.
Results
Open-Field Test (OFT)
Number of Visits to Center
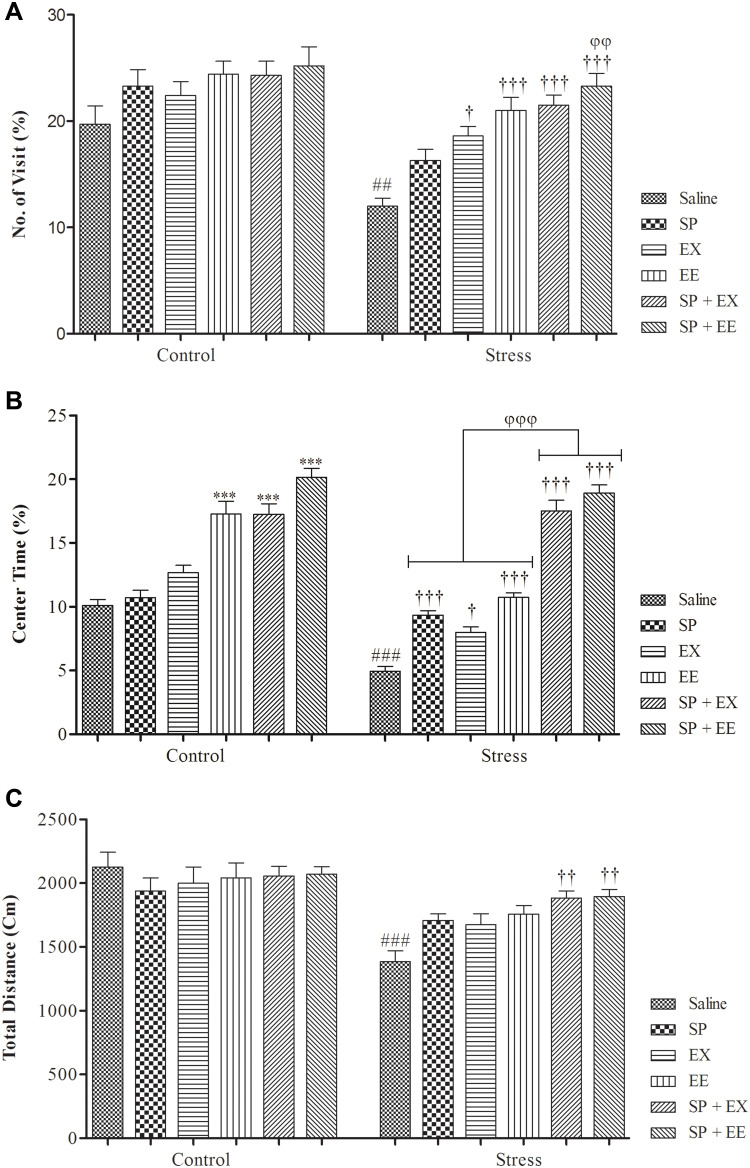
The results of open-field test are shown in Figure 2. A two-way ANOVA on the number of center visits in the OF revealed a significant effect of group (F1, 108 = 35.580, p = 0.0001) and treatment (F5, 108 = 10.928, p = 0.0001), but no significant interaction between two factors (F1, 108 = 1.678, p = 0.146). Post-hoc comparisons indicated the number of center visits in the stress group was significantly lower than the control group (p = 0.003). The number of center visits in the stress group treated with EX (p = 0.021), EE (p = 0.0001) and combined SP + EE (p = 0.0001) and SP + EX (p = 0.0001) was significantly higher than the stress group. The stress group treated with SP + EE exhibited a higher number of center visits than the stress group treated with SP (P = 0.01).
Figure 2.
Anxiety-like behaviors in stressed or non-stressed rats subjected to SP, EX, EE, and the combined treatment. (A) No. of visit; (B) Percent of center time and (C) Total distance. Adolescent stress enhanced anxiety as the stressed rats showed less activity in Open-Field test. Data are expressed as the mean ± S.E.M. In (A–C) ##p = 0.001; ###p = 0.0001 than the corresponding control group; †p<0.01; ††p<0.001; †††p = 0.0001 than the stressed group; ***p = 0.0001 than the control group; φφp<0.001; φφφp<0.0001 SP + EE and SP + EX than the stress group treated with SP, EX and EE.
Abbreviations: SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment.
Center Time
A two-way ANOVA on the time spent in central area showed a significant effect of group (F1, 108 = 72.531, p = 0.0001), and treatment (F5, 108 = 108.930, p= 0.0001), and a significant interaction between two factors (F5, 108 = 9.107, p = 0.0001). Post-hoc comparisons revealed stress groups had less center time than the control group (p = 0.0001). Stress groups treated with SP (p = 0.0001), EX (p= 0.0001), EE (p = 0.0001) and the combined EX+SP (P = 0.0001) and EE + SP (p = 0.0001) spent more time in the center of open field than the stress group alone. Also, stress groups treated with the combined EX+SP or EE + SP spent more time in the center of open field than the stress groups treated with SP, EX and/or EE (all, p = 0.0001). Treatment with EE (p = 0.0001) and the combined SP + EX (p = 0.0001), and SP + EE (p = 0.0001) significantly increased center time in the control group (Figure 2B).
Total Distance
A two-way ANOVA on total distance traveled revealed a significant effect of group (F1, 108 = 40.881, p = 0.0001), no significant effect of treatment (F5, 108 = 2.083, p = 0.073), but a significant interaction between both factors (F1, 108 = 3.005, p = 0.014) (Figure 2C). Between-group comparisons showed that there was a significant difference between stress and control groups (p = 0.0001). Stress groups treated with combined EX+SP (p = 0.005) and EE + SP (p = 0.005) had significantly higher total distance than the stress group.
Elevated Plus Maze (EPM)
Percent of Open Arm Time (OAT)
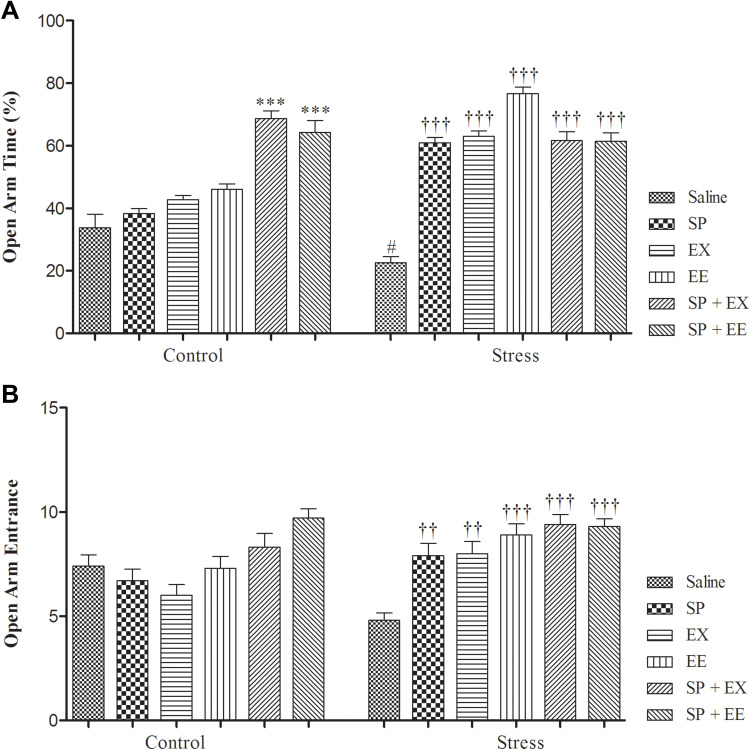
The results of the EPM are shown in Figure 3. A two-way ANOVA for the percent of OAT demonstrated a main effect of group (F1, 108 = 36.627, p = 0.0001), a significant main effect of treatment (F5, 108 = 56.297, p = 0.0001) and significant interaction between group and treatment (F5, 108 = 23.674, p = 0.0001). Between-group comparisons indicated that there was a significant difference between stress and control groups in the percent of OAT (p = 0.047), showing the anxiety in the stress group. The percent of OAT significantly increased in the SP+EX (p = 0.0001) and SP+EE (p = 0.0001) groups than the control group. OAT in the stress groups treated with SP (P = 0.0001), EX (p = 0.0001), EE (p = 0.0001) and the combined EX+SP (p = 0.0001) and EE + SP (p = 0.0001) was significantly higher than the stress group, indicating that SP, EX, EE and the combined treatments could decrease the anxiety levels in the stress groups (Figure 3A). Also, stress groups treated with combined SP + EE or SP + EX had higher OAT than the EE group (both, p = 0.01), showing a synergistic effect between treatments.
Figure 3.
Effects of SP, EX, EE, and the combined treatment on anxiety-like parameters in elevated plus maze. Open arm time (A), and No. of open arm entries (B). Data are expressed as the mean ± S.E.M. In (A and B) #p = 0.01 than the corresponding control group; ††p = 0.001 and †††p = 0.0001 than the stressed group; ***p = 0.0001 than the control group.
Abbreviations: SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment.
Open Arm Entrance (OAE)
For data of OAE in the EPM, a two-way ANOVA revealed a main effect of treatment (F5, 108 = 10.050, p = 0.0001) and a significant interaction between treatment and group (F1, 108 = 6.103, p = 0.0001) but no significant effect of group (F1, 108 = 2.867, p = 0.093). Post-hoc comparisons indicated that the OAE in the stress groups treated with the SP (P = 0.001), EX (p = 0.004), EE (p = 0.0001) and the combined EX+SP (p = 0.0001) and EE+SP (p = 0.0001) was significantly higher than the stress group. No significant difference was found between non-stressed groups for OAE measurements (Figure 3B).
Forced Swim Test
Swimming Time
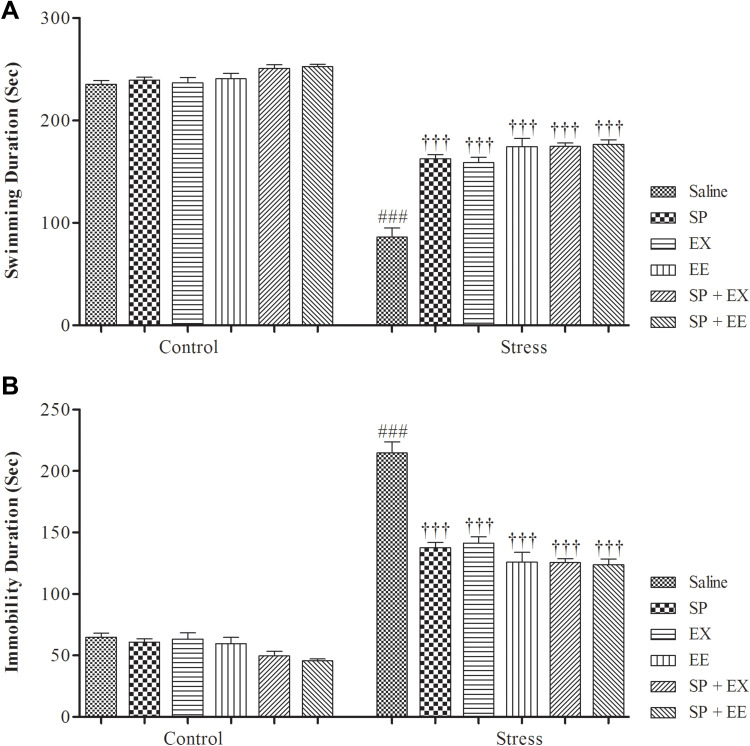
A two-way ANOVA revealed a significant effect of group (F1, 108 = 895.333, p = 0.0001), a significant effect of treatment (F5, 108 = 31.294, p = 0.0001) and a significant interaction between both factors (F5, 108 = 18.330, p = 0.0001) (Figure 4A). Between-group comparisons showed a significant difference between the stress and control groups (p = 0.0001). Swimming time in the stress group treated with SP (p = 0.0001), EX (p = 0.0001), EE (p = 0.0001), combined EX+SP (p = 0.0001) and EE+SP (p = 0.0001) was significantly higher than the stress group.
Figure 4.
Depression-like behaviors in stressed or non-stressed rats subjected to SP, EX, EE, and the combined treatment in Force Swimming Test. (A) Swimming duration (s); and (B) Immobility duration (s). Adolescent stress enhanced depression-like behaviors as the stressed rats showed increased immobility time than control group. Data are expressed as the mean ± S.E.M. In (A and B) ###p = 0.0001 than the corresponding control group; †††p = 0.0001 than the stressed group.
Abbreviations: SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment.
Immobility Time
A two-way ANOVA revealed a significant effect of group (F1, 108 = 890.232, p = 0.0001), a significant effect of treatment (F5, 108 = 31.418, p = 0.0001), and a significant interaction between both factors (F5, 108 = 18.459, p = 0.0001) (Figure 4B). Furthermore, between-group comparisons showed a significant difference between the stress and control groups (P = 0.0001). Immobility time in the stress group treated with SP (p = 0.0001), EX (p = 0.0001), EE (p = 0.0001), combined EX+SP (p = 0.0001) and EE+SP (p = 0.0001) was significantly higher than the stress group, indicating that SP, EX, EE and the combined treatments decreased depression‑like behaviour in stressed rats.
BDNF Levels
Prefrontal Cortex BDNF
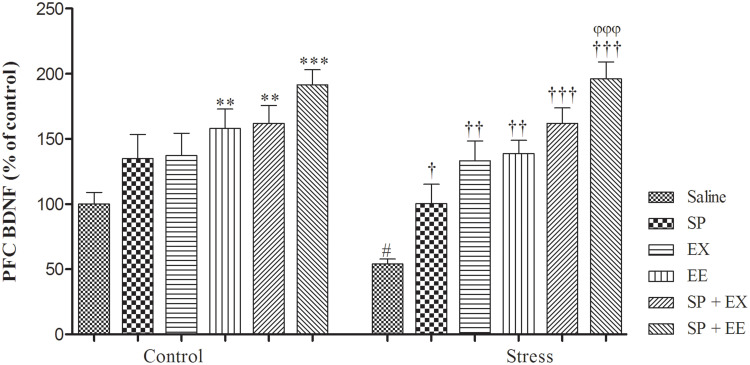
A two-way ANOVA on BDNF levels (Figure 5) revealed significant effects of stress (F1, 48 = 4.550, p = 0.038) and treatment (F5, 48 = 17.776, p = 0.0001) and no significant interaction between two factors (F5, 48 = 1.172, p = 0.337). Between-group comparisons indicated that the BDNF levels of the stress group were significantly lower than the control group (p = 0.033). BDNF levels in the stress group exposed to EX (p = 0.006), EE (p = 0.002), the combined SP + EX (p = 0.0001) and SP + EE (p = 0.0001) were significantly higher than the stress group, showing that these treatments increased BDNF levels in the PFC of the stress group. In addition, the levels of PFC BDNF in the stress group with SP + EE was significantly higher than the stress group treated with the SP (p = 0.0001).
Figure 5.
Prefrontal cortex BDNF levels in stressed or non-stressed rats subjected to SP, EX, EE and combined treatment. Chronic stress reduced prefrontal cortex BDNF, and alone treatment EX and EE and combined treatment of SP + EE and SP + EX restored this deficit. Data are expressed as the mean±S.E.M. #p = 0.01 than the corresponding control group; †p = 0.01, ††p = 0.001 †††p = 0.0001 than the stressed group; φφφp<0.0001 SP + EE than the stress group treated with SP, EX, EE and SP+EX. **p = 0.001; ***p = 0.0001 than the control group.
Abbreviations: SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment.
We found a significant positive correlation in individual animals of the control group, between PFC BDNF levels and open arm time (r = 0.963, p <0.01) or swimming duration (r = 0.80, p <0.05). Chronic stress disrupted these positive associations, which recovered by all treatments in the stress groups (data not shown).
BDNF and 5HT3 mRNA Expression in the PFC
BDNF mRNA Expression
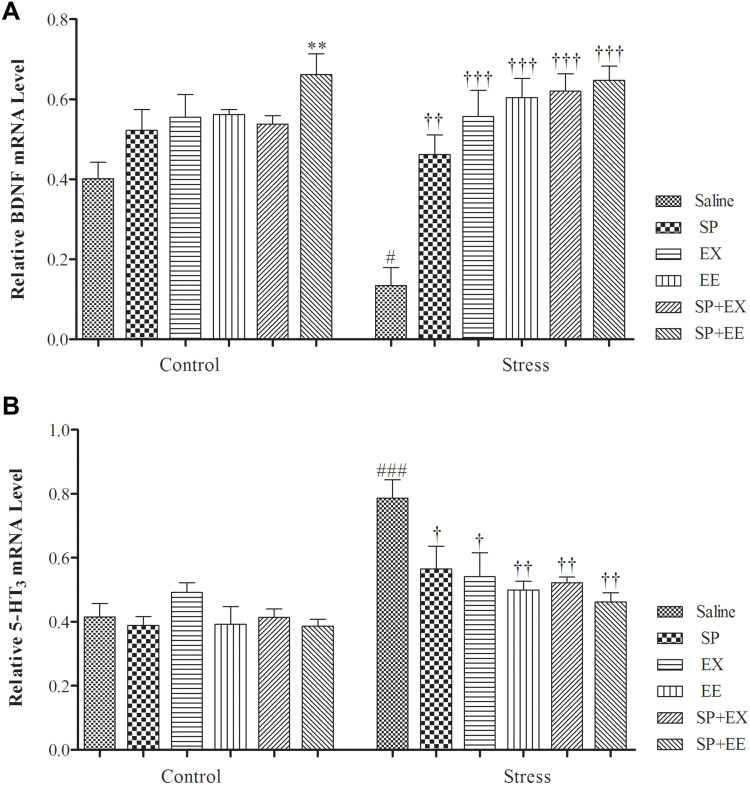
A two-way ANOVA on PFC BDNF mRNA expression (Figure 6A) revealed significant effects of group (F1, 36 = 3.808, p = 0.048), of treatment (F5, 36 = 13.290, p = 0.0001) and a significant interaction between two factors (F5, 36 = 2.858, p = 0.028). Paired comparisons showed that PFC BDNF mRNA expression in the restraint group was significantly lower than the control group (P = 0.032). BDNF mRNA expression in the stress group exposed to SP (p = 0.003), EX (p = 0.0001), EE (p = 0.0001), the combined SP + EX (p = 0.0001) and SP + EE (p = 0.0001) was significantly higher than the stress group, showing that these treatments increased BDNF mRNA expression in the PFC of the stress group.
Figure 6.
Prefrontal cortex BDNF & 5HT3 receptor mRNA expression in stressed or non-stressed rats subjected to SP, EX, EE and combined treatment. BDNF mRNA expression in the restraint group was significantly lower than control group (A). Stressed rats showed increased in 5HT3 receptor mRNA expression than control group (B). Data are expressed as the mean±S.E.M. In (A and B) #p = 0.01; ###p = 0.0001 than the corresponding control group; p<0.001; †p = 0.01, ††p = 0.0001, †††p = 0.0001 than the stressed group; **p = 0.001 than the control group.
Abbreviations: SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment.
5HT3 mRNA Expression
A two-way ANOVA on PFC 5HT3 mRNA expression (Figure 6B) revealed significant effects of stress (F1, 40 = 30.170, p = 0.0001), of treatment (F5, 40 = 3.682, p = 0.008) and of interaction between two factors (F5, 40 = 3.144, p = 0.017). Paired comparisons showed that 5HT3 mRNA expression in the restraint group was significantly higher than the control group (P = 0.0001). 5HT3 mRNA expression in the stress group exposed to SP (p = 0.037), EX (p = 0.024), EE (p = 0.004), the combined SP + EX (p = 0.005) and SP + EE (p = 0.0001) was significantly lower than the stress group, indicating that these treatments decreased 5HT3 receptor expression in the PFC of the stress group.
Oxidative Stress Markers
FRAP Levels
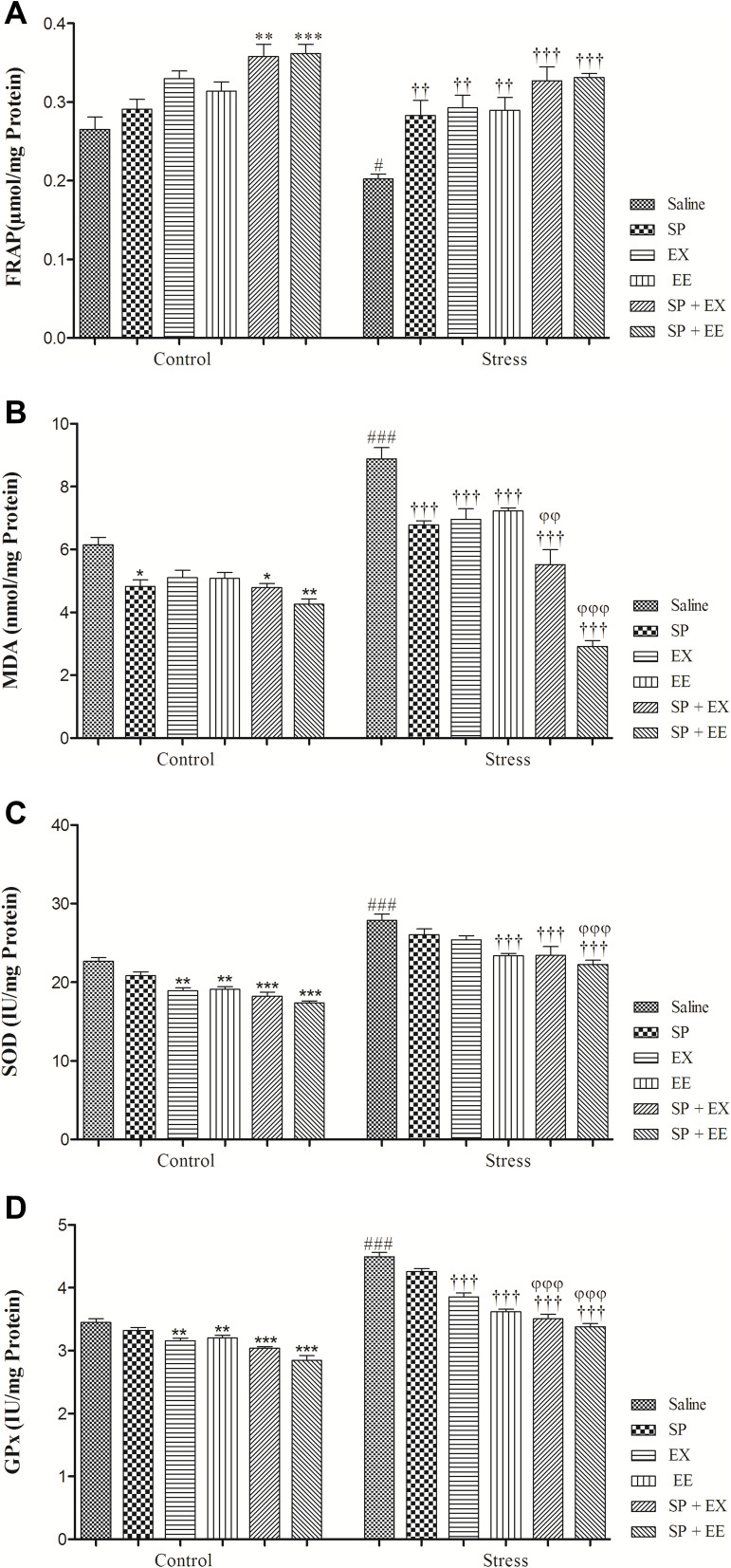
The result of FRAP level is illustrated in Figure 7A. A two-way ANOVA on FRAP level revealed significant effects of stress (F1, 48 = 16.304, p = 0.0001), of treatment (F5, 48 = 18.025, p = 0.0001) and no interaction between two factors (F5, 48 = 0.837, p = 0.530). Between-group comparisons indicated that the FRAP levels of the stress group were significantly lower than the control group (p = 0.042). FRAP levels in the stress group exposed to SP (p = 0.007), EX (p = 0.001), EE (p = 0.003), the combined SP + EX (p = 0.0001) and SP + EX (p = 0.0001) were significantly higher than the stress group, indicating that these treatments increased FRAP in the PFC of the stress group.
Figure 7.
Oxidative stress markers and antioxidant enzymes activity in stressed or non-stressed rats subjected to SP, EX, EE and combined treatment. FARP in the stress group was significantly lower (A) and MDA was significantly higher than control group (B). Stressed rats showed increased SOD and GPx activities in prefrontal cortex (C, D). Data are expressed as the mean±S.E.M. In (A–D) #p = 0.01; ###p = 0.0001 than the corresponding control group; ††p = 0.001; †††p = 0.0001 than the stressed group; *p = 0.01; **p = 0.001; ***p = 0.0001 than the control group; φφp = 0.001; φφφp = 0.0001 SP + EX and SP + EE than the stress group treated with SP, EX and EE.
Abbreviations:SP, Spirulina platensis; EX, voluntary exercise; EE, enriched environment.
MDA Levels
The result of MDA level is illustrated in Figure 7B. A two-way ANOVA on MDA level revealed significant effects of stress (F1, 48 = 84.902, p = 0.0001), of treatment (F5, 48 = 52.568, p = 0.0001) and a significant interaction between two factors (F5, 48 = 16.919, p = 0.0001). Between-group comparisons showed that the MDA levels of the stress group were significantly higher than the control group (p = 0.0001). MAD levels in the stress group exposed to SP (p = 0.0001), EX (p = 0.0001), EE (p = 0.0001), the combined SP + EX (p = 0.0001) and SP + EE (p = 0.0001) were significantly lower than the stress group, showing that these treatments decreased MDA in the PFC of the stress group.
Activity of Antioxidant Enzymes
SOD Activity
The result of SOD activity is illustrated in Figure 7C. A two-way ANOVA on SOD level revealed significant effects of stress (F1, 48 = 237.909, p = 0.0001), of treatment (F5, 48 = 23.009, p = 0.0001) and no significant interaction between two factors (F5, 48 = 0.781, p = 0.568). Between-group comparisons showed that the SOD levels of the stress group were significantly higher than the control group (p = 0.0001). SOD activity in the stress group exposed to EE (p = 0.0001), the combined SP + EX (p = 0.0001) and SP + EE (p = 0.0001) was significantly lower than the stress group, showing that these treatments decreased SOD activity in the PFC of the stress group. Also, SOD activity in the stress group exposed to SP + EE (p = 0.0001) was significantly lower than the stress group received SP (p = 0.0001).
GPx Activity
The result of GPx activity is illustrated in Figure 7D. A two-way ANOVA on GPx levels revealed significant effects of stress (F1, 48 = 468.676, p = 0.0001), of treatment (F5, 48 = 68.859, p = 0.0001) and a significant interaction between two factors (F4, 36 = 11.268, p = 0.0001). Between-group comparisons showed that the GPx levels of the stress group were significantly higher than the control group (p = 0.0001). GPx activity in the stress group exposed to EX (p = 0.0001), EE (p = 0.0001), the combined SP + EX (p = 0.0001) and SP + EE (p = 0.0001) were significantly lower than the stress group, indicating that these treatments decreased GPx activity in the PFC of the stress group. GPx activity in the stress group exposed to the combined SP + EX (all, p = 0.01) or SP + EE (all, p = 0.0001) was significantly lower than the stress groups treated with the SP, EX, and EE.
Discussion
Findings of the present study demonstrate that adolescent stress enhances anxiety or depression–like symptoms in adulthood. Adolescent stress increases oxidative stress and 5HT-3 receptor expression and reduces the expression of the BDNF gene and its content in the PFC. Treatment with Spirulina platensis, exposure to wheel-running activity or enriched environment, and the combined interventions alleviated behavioral, biochemical and molecular deficits induced by adolescent stress, providing important information regarding effective and new therapeutic procedures against adolescent stress-induced behavioral, biochemical and molecular abnormalities in adulthood.
Our findings indicated that adolescent restraint stress is accompanied with anxiogenic and depression-like behaviors in adulthood. In the EPM, rats submitted to chronic adolescent stress spent less time in open arms and had fewer open arm entries than the control group;, and in the open-field test, center time and center distance were significantly reduced in the saline-stress group than the saline-control group. Data from both tasks showed increased anxiety following chronic adolescent stress. Stressed rats exhibited increased immobility time and reduced swimming duration in the FST, showing depression-like behavior. These findings are in agreement with other studies demonstrating stress-induced anxiety or depression-like behaviors in different lifespan.14,21,55,65 More likely, these mood disorders are associated with increased glucocorticoids and their neurotoxic effects on hippocampus, amygdala, and PFC.21,66 We recently reported that the same stress protocol of the present study increased serum corticosterone levels, which remained at a high level even in the adulthood, showing that adolescent stress increased basal corticosterone levels.47 Moreover, adolescent stress increased levels of corticosterone when challenged in either juvenility or adulthood.67 We found that all treatments recovered anxiety and depression-like symptoms in the stress group.
We found that chronic adolescent stress reduces BDNF levels and its mRNA expression on the PFC, suggesting that downregulation of BDNF is a possible mechanism that mediates the effect of adolescent stress on anxiety or depression-like behaviors. The BDNF-TrkB pathway is important in the regulation of mood and emotional behaviors.18 For example, intra-hippocampal infusions of BDNF could improve behavioral responses in rodent depression models.68 It has been reported that exposure to stressors in early life reduces BDNF expression in the PFC and hippocampus and enhances anxiety and depression-like behaviors.16,17 We found that treatment with Spirulina platensis, voluntary exercise and enriched environment and combined treatments during the adolescent phase prevented stress-induced depressive or anxious behaviors in adulthood. The stress group treated with the Spirulina platensis and exercise showed even higher open arm time in the EPM than the corresponding control group. The reason for this effect is not clear, and needs further work. The combined treatments of Spirulina platensis with voluntary exercise or enriched environment increased open arm time in the control group, suggesting their anti-anxiety effects. The applied treatments also normalized decreased mRNA expression of BDNF and its content in the PFC. The combined treatment of Spirulina platensis and enriched environment significantly increased PFC BDNF than Spirulina platensis alone, suggesting an existence of synergistic effects between these treatments on BDNF levels. Although the mechanisms underlying the synergistic effects between these treatments are not clear, but it is likely that the beneficial effects of these treatments mediate via common downstream pathways. It seems that normalizing the BDNF pathway is an important mechanism that mediates the protective and beneficial effects of Spirulina platensis against adolescent stress-induced mood disorders. In line with these findings, we recently reported that the combined treatment of Spirulina platensis with either environmental enrichment or physical exercise significantly increased hippocampal BDNF levels and recovered stress-induced cognitive functions.47 SP is rich in β-carotene, which possesses many important biological activities. It has been demonstrated that β-carotene and fermented Spirulina maxima extract increased the phosphorylation of both p-ERK and p-CREB and sequentially up-regulated the expression of BDNF in the hippocampus and improved memory performance in mice.69 In addition, it has been shown that non-protein components of Spirulina platensis stimulate BDNF gene transcription through the heme oxygenase-1 induction in glial cells.70 These findings highlight the role of BDNF on the beneficial effects of Spirulina platensis on brain functions. Both exercise or enriched environment increase hippocampal BDNF in adolescent male and female rats.71–73 BDNF mediates beneficial effects of exercise or enriched environment on brain functions and plasticity in a variety of conditions.50,74-77
Serotonin system plays an important role in anxiety or depression behaviors.78 Our findings indicated that adolescent stress increased mRNA expression of 5HT3 receptor in the PFC, reflecting the potential involvement of 5HT3 receptors located in the PFC in depressive or anxious disorders. It has been accepted that early life stress induces the persistent changes in 5-HT receptors and serotonin transporter mRNA expression in key brain regions such as PFC and amygdala.79 5-HT3 receptors are widely expressed in brain stem nuclei cortical areas such as the amygdala, hippocampus and cortex, and are involved in the regulation of mood and behavioral activities.80 5-HT3 antagonists exhibit protective and therapeutic effects in a variety of psychiatric disorders such as depression as antagonism of 5HT3 receptors (z6 and ondansetron) has been shown to facilitate serotonin neurotransmission and normalize HPA-axis hyperactivity in animal models of depression.25,81 These findings indicate that 5HT-3 receptor antagonists are potential therapeutic candidates for stress-related depressive disorders. We observed that all treatments recovered the increased expression of 5HT-3 receptor in the PFC, suggesting that normalizing the 5HT-3 receptor expression may be another mechanism that the Spirulina platensis, voluntary exercise and enriched environment and combined treatments during adolescent phase period prevented stress-induced depressive or anxious behaviors in adulthood.
Our results showed that adolescent stress induces oxidative stress in the PFC as evidenced by a decrease in the total antioxidant power, increased in MDA production, and enhanced the activity of antioxidant enzymes GPx and SOD. Oxidative stress has been known as an imbalance between oxidant and antioxidant systems. Elevated formation of reactive oxygen species (ROS) and reduction in antioxidant enzymes can increase oxidative stress.82 Brain cells are at particular risk of being damaged by free radicals because the brain has a high oxygen turnover, and is rich in polyunsaturated fatty acids, which are potential targets for lipid peroxidation.83 Antioxidant defense enzymes including SOD and GPx maintain cell against oxidative damage.84 Given the inhibitory effects of glucocorticoids on brain SOD and GPx activities, it is reasonable to assume that the increased levels of these enzymes in the PFC following chronic stress are a compensatory response to stress.85 In fact, increased PFC GPx and SOD activities after stress suggest a chronic stress-induced increased in ROS production and a compensatory adaption of the tested free-radical scavenging enzymes. Increased lipid peroxidation was correlated with increased MDA in the cortex, cerebellum, midbrain, and hippocampus in the rat brain.86 Lipid peroxidation is a result of an imbalance between pro-oxidant and antioxidant systems and increases under stress conditions.87 Treatment with Spirulina platensis, voluntary exercise and enriched environment especially their combination significantly increased the total antioxidant reactivity capacity, and decreased the levels of lipid peroxidation marker MDA and the activities of antioxidant enzyme activities SOD and GPx. Several studies have showed that antioxidant properties of Spirulina platensis in different animal models.37,38,88 Spirulina platensis by itself did not modulate the SOD and GPx activities, but enhanced the effects of EX or EE on the activities of these enzymes in the present study. Antioxidant activity of Spirulina platensis largely related to phycocyanin, an active protein of Spirulina, which has significant antioxidant and radical scavenging properties, offering protection against oxidative stress.89,90 A recent study has shown that Spirulina platensis at a dose of 300 mg/kg attenuated sodium arsenite-induced oxidative stress, testicular damage, and sperm abnormalities in male rats.91
Previous studies have shown that rats housed in EE demonstrate reduced oxidative biomarker than rats housed in standard laboratory conditions.92 EE also prevents oxidative stress in Zebrafish submitted to unpredictable chronic stress.93 Previous studies have demonstrated the anti-oxidative stress effects of exercise. For example, voluntary physical activity has been shown to mitigate anxiety and depression-like behaviors and oxidative stress in a rat model of fetal alcohol spectrum disorders,94 and to protect the heart from oxidative stress in diabetic rats.95 As aforementioned, findings of the present study demonstrated similar anti-oxidative stress of Spirulina platensis at a dose of 200 mg/kg, voluntary exercise and enriched environment in rats submitted to chronic adolescent stress. Although the underlying mechanisms of anti-oxidative stress properties of these treatments are not known, they may interact with HPA axis, and thus, reduce oxidative stress. This assumption is supported by our recent findings demonstrating that the same treatments reduce corticosterone levels in rats submitted to adolescent chronic stress.47 Taken together, similar to previous studies indicating beneficial effects of EX and EE against the long-term effects of adolescent stress, the present findings demonstrate that Spirulina platensis alone and combined with EE and EX reversed enhanced anxiety levels and depressive behaviors and also concurrently decreased oxidative stress, and 5-HT3 receptor, and increased BDNF content in the PFC of adult female rats that have been exposed to stress when juvenile. Further studies are needed to determine the role of oxidative stress, increased BDNF and decreased 5-HT3 receptors in the beneficial effects of Spirulina platensis against – chronic stress-induced anxiety and depressive behaviors.
As aforementioned, the SP was administrated by an oral tube in the stress and corresponding control groups, but the stressed and related control groups exposed to EX or EE did not have an oral tube. This should be considered as a limitation of the study, which may affect the accurate comparisons of the stress groups exposed to EE or EX with stress groups received SP. Another limitation of the present study is lack of sex hormones measurement during the estrus cycle. Since all animals were in the same age at the beginning of the study and maintained in similar conditions, it seems that the estrus cycle in the experimental groups was largely synchronized.
In summary, the present study demonstrates stressed rats during the adolescent period showed anxiety and depressive-like symptoms, and higher levels of malondialdehyde, higher activities of antioxidant enzymes glutathione peroxidase and superoxide dismutase, and lower total antioxidant reactivity capacity in the PFC in adults. Moreover, adolescent stress significantly increased 5-HT3 receptor mRNA expression, and decreased BDNF content and its mRNA expression in the PFC. Treatments with Spirulina platensis, EX and EE, and the combined interventions alleviated these deficits. Some treatments even reduced anxiety level and oxidative stress markers and enhanced BDNF content in control rats. Thus, findings of the present study provide important evidences for the development of new pharmacological and therapeutic ways to prevent the effects of early stress experience on behavioral deficits in adults.
Acknowledgments
This work was supported by grants from the Semnan University of Medical Sciences (Semnan, Iran) and Iran National Science Foundation (95836886). In addition, Mr. Nasroallah Moradi-kor carried out this work in partial project fulfillment of the requirements to obtain the Ph.D. degree in Neuroscience.
Abbreviations
SP, Spirulina platensis; EE, environmental enrichment (EE); EX, voluntary exercise (EX); PFC, prefrontal cortex; PND, post-natal day; OFT, open field test; EPM, elevated plus maze; RT-PCR, reverse transcriptase polymerase chain reaction; FST, force swimming test; FRAP, ferric reducing antioxidant power; MDA, malondialdehyde; SOD, superoxidase dismutase; GPx, glutathione peroxidase; OAT, open arm time; OAE, open arm entry; BDNF, brain derived neurotropic factor.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Brydges NM. Pre-pubertal stress and brain development in rodents. Curr Opin Behav Sci. 2016;7:8–14. doi: 10.1016/j.cobeha.2015.08.003 [DOI] [Google Scholar]
- 2.Lähdepuro A, Savolainen K, Lahti-Pulkkinen M, et al. The impact of early life stress on anxiety symptoms in late adulthood. Sci Rep. 2019;9(1):4395. doi: 10.1038/s41598-019-40698-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuhrmann D, Knoll LJ, Blakemore S-J. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19(10):558–566. doi: 10.1016/j.tics.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 5.George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. Depression. 1994;2(2):59–72. doi: 10.1002/depr.3050020202 [DOI] [Google Scholar]
- 6.Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8(199). doi: 10.3389/fnbeh.2014.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Morais H, de Souza CP, da Silva LM, et al. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res. 2014;258:52–64. doi: 10.1016/j.bbr.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 8.Zlatković J, Todorović N, Bošković M, Pajović SB, Demajo M, Filipović D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem. 2014;393(1):43–57. doi: 10.1007/s11010-014-2045-z [DOI] [PubMed] [Google Scholar]
- 9.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten AFT, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2014;1:89–99. doi: 10.1016/j.ynstr.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GW, Ban M, Craig TK, Harris TO, Herbert J, Uher R. Serotonin transporter length polymorphism, childhood maltreatment, and chronic depression: a specific gene–environment interaction. Depress Anxiety. 2013;30(1):5–13. doi: 10.1002/da.21982 [DOI] [PubMed] [Google Scholar]
- 14.Brydges NM, Jin R, Seckl J, Holmes MC, Drake AJ, Hall J. Juvenile stress enhances anxiety and alters corticosteroid receptor expression in adulthood. Brain Behav. 2014;4(1):4–13. doi: 10.1002/brb3.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. J R Soc Med. 2012;105(4):151–156. doi: 10.1258/jrsm.2011.110222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai M, Zhu X, Zhang Y, et al. Abnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early life. PLoS One. 2012;7(10):e46921. doi: 10.1371/journal.pone.0046921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 19.Badowska-Szalewska E, Spodnik E, Klejbor I, Morys J. Effects of chronic forced swim stress on hippocampal brain-derived neutrophic factor (BDNF) and its receptor (TrkB) immunoreactive cells in juvenile and aged rats. Acta Neurobiol Exp. 2010;70:370–381. [DOI] [PubMed] [Google Scholar]
- 20.Sotiropoulos I, Silva J, Kimura T, et al. Female hippocampus vulnerability to environmental stress, a precipitating factor in tau aggregation pathology. J Alzheimers Dis. 2015;43(3):763–774. doi: 10.3233/JAD-140693 [DOI] [PubMed] [Google Scholar]
- 21.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 22.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424. doi: 10.1037/0033-2909.115.3.424 [DOI] [PubMed] [Google Scholar]
- 23.Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther. 2000;38(6):619–628. doi: 10.1016/S0005-7967(99)00098-4 [DOI] [PubMed] [Google Scholar]
- 24.Chameau P, van Hooft JA. Serotonin 5-HT 3 receptors in the central nervous system. Cell Tissue Res. 2006;326(2):573–581. doi: 10.1007/s00441-006-0255-8 [DOI] [PubMed] [Google Scholar]
- 25.Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10(1):28. doi: 10.1186/s13041-017-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bétry C, Etiévant A, Oosterhof C, Ebert B, Sanchez C, Haddjeri N. Role of 5-HT3 receptors in the antidepressant response. Pharmaceuticals. 2011;4(4):603–629. doi: 10.3390/ph4040603 [DOI] [Google Scholar]
- 27.Wolf H. Preclinical and clinical pharmacology of the 5-HT3 receptor antagonists. Scand J Rheumatol. 2000;29(113):37–45. doi: 10.1080/030097400446625 [DOI] [PubMed] [Google Scholar]
- 28.Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol. 1997;325(2–3):129–135. doi: 10.1016/S0014-2999(97)00115-5 [DOI] [PubMed] [Google Scholar]
- 29.Bhatt S, Mahesh R, Devadoss T, Jindal AK. Antidepressant-like effect of novel 5-HT3 receptor antagonist Nn-butyl-3-ethoxyquinoxalin-2-carboxamide (6p): an approach using rodent behavioral antidepressant tests. Indian J Pharmacol. 2013;45(4):348. doi: 10.4103/0253-7613.115014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontella FU, Siqueira IR, Vasconcellos APS, Tabajara AS, Netto CA, Dalmaz C. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005;30(1):105–111. doi: 10.1007/s11064-004-9691-6 [DOI] [PubMed] [Google Scholar]
- 31.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667(1–3):222–229. doi: 10.1016/j.ejphar.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Kumar RS, Narayanan SN, Nayak S. Ascorbic acid protects against restraint stress-induced memory deficits in wistar rats. Clinics. 2009;64(12):1211–1217. doi: 10.1590/S1807-59322009001200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–205. doi: 10.1124/jpet.116.237503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012:1–11. doi: 10.1155/2012/428010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Li W, Jiang Z-G, Ghanbari H. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14(12):24438–24475. doi: 10.3390/ijms141224438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Daim MM, Abuzead SMM, Halawa SM, Mukhopadhyay P. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8(9):e72991. doi: 10.1371/journal.pone.0072991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan Z, Bhadouria P, Bisen P. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6(5):373–379. doi: 10.2174/138920105774370607 [DOI] [PubMed] [Google Scholar]
- 38.Nasirian F, Dadkhah M, Moradi-kor N, Obeidavi Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab Syndr Obes. 2018;11:375. doi: 10.2147/DMSO.S172104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4(3):207–216. doi: 10.2174/1389203033487216 [DOI] [PubMed] [Google Scholar]
- 40.Khan M, Varadharaj S, Ganesan LP, et al. C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am J Physiol Heart Circ Physiol. 2006;290(5):H2136–H2145. doi: 10.1152/ajpheart.01072.2005 [DOI] [PubMed] [Google Scholar]
- 41.Choi W, Kang D, Heo S-J, Lee H. Enhancement of the neuroprotective effect of fermented Spirulina maxima associated with antioxidant activities by ultrasonic extraction. Appl Sci. 2018;8(12):2469. doi: 10.3390/app8122469 [DOI] [Google Scholar]
- 42.Koppula S, Kumar H, More SV, Kim BW, Kim IS, Choi D-K. Recent advances on the neuroprotective potential of antioxidants in experimental models of parkinson’s disease. Int J Mol Sci. 2012;13(8):10608–10629. doi: 10.3390/ijms130810608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chattopadhyaya I, Gupta S, Mohammed A, Mushtaq N, Chauhan S, Ghosh S. Neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced parkinsonism in rats. BMC Complement Altern Med. 2015;15(1):296. doi: 10.1186/s12906-015-0815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thaakur S, Sravanthi R. Neuroprotective effect of Spirulina in cerebral ischemia–reperfusion injury in rats. J Neural Transm. 2010;117(9):1083–1091. doi: 10.1007/s00702-010-0440-5 [DOI] [PubMed] [Google Scholar]
- 45.Pabon MM, Jernberg JN, Morganti J, et al. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS One. 2012;7(9):e45256. doi: 10.1371/journal.pone.0045256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachstetter AD, Jernberg J, Schlunk A, et al. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PLoS One. 2010;5(5):e10496. doi: 10.1371/journal.pone.0010496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. Horm Behav. 2019;112:20–31. doi: 10.1016/j.yhbeh.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 48.Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci. 2016;73(5):975–983. doi: 10.1007/s00018-015-2102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grace L, Hescham S, Kellaway LA, Bugarith K, Russell VA. Effect of exercise on learning and memory in a rat model of developmental stress. Metab Brain Dis. 2009;24(4):643. doi: 10.1007/s11011-009-9162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaynman S, Ying Z, Gomez‐Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- 51.Vanzella C, Neves JD, Vizuete AF, et al. Treadmill running prevents age-related memory deficit and alters neurotrophic factors and oxidative damage in the hippocampus of wistar rats. Behav Brain Res. 2017;334:78–85. doi: 10.1016/j.bbr.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 52.Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N, Homberg J. Agomelatine, venlafaxine, and running exercise effectively prevent anxiety-and depression-like behaviors and memory impairment in restraint stressed rats. PLoS One. 2017;12(11):e0187671. doi: 10.1371/journal.pone.0187671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapmanee S, Charoenphandhu J, Charoenphandhu N. Beneficial effects of fluoxetine, reboxetine, venlafaxine, and voluntary running exercise in stressed male rats with anxiety-and depression-like behaviors. Behav Brain Res. 2013;250:316–325. doi: 10.1016/j.bbr.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 54.Brenes JC, Rodríguez O, Fornaguera J. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav. 2008;89(1):85–93. doi: 10.1016/j.pbb.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 55.Ilin Y, Richter-Levin G, Baune B. Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS One. 2009;4(1):e4329. doi: 10.1371/journal.pone.0004329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jankowsky JL, Melnikova T, Fadale DJ, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25(21):5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampedro-Piquero P, Begega A, Arias J. Increase of glucocorticoid receptor expression after environmental enrichment: relations to spatial memory, exploration and anxiety-related behaviors. Physiol Behav. 2014;129:118–129. doi: 10.1016/j.physbeh.2014.02.048 [DOI] [PubMed] [Google Scholar]
- 58.Veena J, Srikumar B, Mahati K, Bhagya V, Raju T, Shankaranarayana Rao B. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009;87(4):831–843. doi: 10.1002/jnr.21907 [DOI] [PubMed] [Google Scholar]
- 59.Moradi-Kor N, Ghanbari A, Rashidipour H, et al. Therapeutic effects of spirulina platensis against adolescent stress-induced oxidative stress, brain-derived neurotrophic factor alterations and morphological remodeling in the amygdala of adult female rats. J Exp Pharmacol. 2020;12:75. doi: 10.2147/JEP.S237378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maluach AM, Misquitta KA, Prevot TD, et al. Increased neuronal DNA/RNA oxidation in the frontal cortex of mice subjected to unpredictable chronic mild stress. Chron Stress. 2017;1:2470547017724744. doi: 10.1177/2470547017724744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vyas A, Mitra R, Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7 [DOI] [PubMed] [Google Scholar]
- 63.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94(2):147–160. doi: 10.1007/BF00176837 [DOI] [PubMed] [Google Scholar]
- 64.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 65.Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1):39–47. doi: 10.1016/j.psyneuen.2011.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gameiro GH, da Silva Andrade A, de Castro M, Pereira LF, Tambeli CH, de Arruda Veiga MCF. The effects of restraint stress on nociceptive responses induced by formalin injected in rat’s TMJ. Pharmacol Biochem Behav. 2005;82(2):338–344. doi: 10.1016/j.pbb.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Jacobson-Pick S, Richter-Levin G. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav Brain Res. 2010;214(2):268–276. doi: 10.1016/j.bbr.2010.05.036 [DOI] [PubMed] [Google Scholar]
- 68.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037(1–2):204–208. doi: 10.1016/j.brainres.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 69.Choi WY, Kang DH, Lee HY. Effect of fermented spirulina maxima extract on cognitive-enhancing activities in mice with scopolamine-induced dementia. Evid Based Complement Alternat Med. 2018;2018:1–9. doi: 10.1155/2018/7218504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morita K, Itoh M, Nishibori N, Her S, Lee M-S. Spirulina non-protein components induce BDNF gene transcription via HO-1 activity in C6 glioma cells. Appl Biochem Biotechnol. 2015;175(2):892–901. doi: 10.1007/s12010-014-1300-9 [DOI] [PubMed] [Google Scholar]
- 71.McCreary JK, Erickson ZT, Hao Y, Ilnytskyy Y, Kovalchuk I, Metz GAS. Environmental intervention as a therapy for adverse programming by ancestral stress. Sci Rep. 2016;6(1):37814. doi: 10.1038/srep37814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmadalipour A, Sadeghzadeh J, Vafaei AA, Bandegi AR, Mohammadkhani R, Rashidy-Pour A. Effects of environmental enrichment on behavioral deficits and alterations in hippocampal BDNF induced by prenatal exposure to morphine in juvenile rats. Neuroscience. 2015;305:372–383. doi: 10.1016/j.neuroscience.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 73.Uysal N, Kiray M, Sisman A, et al. Effects of voluntary and involuntary exercise on cognitive functions, and VEGF and BDNF levels in adolescent rats. Biotech Histochem. 2015;90(1):55–68. doi: 10.3109/10520295.2014.946968 [DOI] [PubMed] [Google Scholar]
- 74.Patten AR, Sickmann H, Hryciw BN, et al. Long-term exercise is needed to enhance synaptic plasticity in the hippocampus. Learn Mem. 2013;20(11):642–647. doi: 10.1101/lm.030635.113 [DOI] [PubMed] [Google Scholar]
- 75.Chourbaji S, Hortnagl H, Molteni R, Riva MA, Gass P, Hellweg R. The impact of environmental enrichment on sex-specific neurochemical circuitries - effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience. 2012;220:267–276. doi: 10.1016/j.neuroscience.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Luikart BW, Birnbaum S, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Charney DS, Woods SW, Goodman WK, Heninger G. Serotonin function in anxiety. Psychopharmacology. 1987;92(1):14–24. doi: 10.1007/BF00215473 [DOI] [PubMed] [Google Scholar]
- 79.Bravo JA, Dinan TG, Cryan JF. Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain. Front Mol Neurosci. 2014;7:24. doi: 10.3389/fnmol.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajkumar R, Mahesh R. Review: the auspicious role of the 5-HT3 receptor in depression: a probable neuronal target? J Psychopharmacol. 2010;24(4):455–469. doi: 10.1177/0269881109348161 [DOI] [PubMed] [Google Scholar]
- 81.Gupta D, Radhakrishnan M, Kurhe Y. 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: modulation of hypothalamic–pituitary–adrenocortical and brain serotonergic system. Pharmacol Biochem Behav. 2014;124:129–136. doi: 10.1016/j.pbb.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 82.Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2(2):188–194. doi: 10.1016/S1369-5274(99)80033-2 [DOI] [PubMed] [Google Scholar]
- 83.Metodiewa D, Koska C. Reactive oxygen species and reactive nitrogen species: relevance to cyto(neuro)toxic events and neurologic disorders. An overview. Neurotox Res. 1999;1(3):197–233. doi: 10.1007/BF03033290 [DOI] [PubMed] [Google Scholar]
- 84.Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003;243(1/2):147–152. doi: 10.1023/a:1021620414979 [DOI] [PubMed] [Google Scholar]
- 85.McIntosh LJ, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res. 1998;791(1–2):209–214. doi: 10.1016/S0006-8993(98)00115-2 [DOI] [PubMed] [Google Scholar]
- 86.Liu J, Wang X, Shigenaga MK, Yeo HC, Mori A, Ames BN. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996;10(13):1532–1538. doi: 10.1096/fasebj.10.13.8940299 [DOI] [PubMed] [Google Scholar]
- 87.Vijayavel K, Anbuselvam C, Balasubramanian MP. Naphthalene-induced hematological disturbances and oxidative stress in an estuarine edible crab, Scylla serrata. Environ Toxicol. 2005;20(4):464–466. doi: 10.1002/tox.20133 [DOI] [PubMed] [Google Scholar]
- 88.Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013;2(2):164–173. doi: 10.1016/j.algal.2013.01.004 [DOI] [Google Scholar]
- 89.Asghari A, Fazilati M, Latifi AM, Salavati H, Choopani A. A review on antioxidant properties of Spirulina. J Appl Biotechnol Rep. 2016;3(1):345–351. [Google Scholar]
- 90.Finamore A, Palmery M, Bensehaila S, Peluso I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly Spirulina. Oxid Med Cell Longev. 2017;2017:3247528. doi: 10.1155/2017/3247528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bashandy SA, El Awdan SA, Ebaid H, Alhazza IM. Antioxidant potential of Spirulina platensis mitigates oxidative stress and reprotoxicity induced by sodium arsenite in male rats. Oxid Med Cell Longev. 2016;2016:7174351. doi: 10.1155/2016/7174351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marmol F, Rodriguez CA, Sanchez J, Chamizo VD. Anti-oxidative effects produced by environmental enrichment in the hippocampus and cerebral cortex of male and female rats. Brain Res. 2015;1613:120–129. doi: 10.1016/j.brainres.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 93.Marcon M, Mocelin R, Sachett A, Siebel AM, Herrmann AP, Piato A. Enriched environment prevents oxidative stress in zebrafish submitted to unpredictable chronic stress. PeerJ. 2018;6:e5136. doi: 10.7717/peerj.5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: protective effects of voluntary physical exercise. Neuropharmacology. 2012;62(4):1607–1618. doi: 10.1016/j.neuropharm.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 95.Ghorbanzadeh V, Mohammadi M, Mohaddes G, Dariushnejad H, Chodari L, Mohammadi S. Protective effect of crocin and voluntary exercise against oxidative stress in the heart of high-fat diet-induced type 2 diabetic rats. Physiol Int. 2016;103(4):459–468. doi: 10.1556/2060.103.2016.4.6 [DOI] [PubMed] [Google Scholar]