Abstract
A series of unexplained pneumonia cases currently were first reported in December 2019 in Wuhan, China. Official names have been announced for the virus responsible, previously known as “2019 novel coronavirus” and the diseases it causes are, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease (COVID-19), respectively. Despite great efforts worldwide to control SARS-CoV-2, the spread of the virus has reached a pandemic. Infection prevention and control of this virus is the primary concern of public health officials and professionals. Currently, several therapeutic options for COVID-19 are proposed and vaccine development has been initiated for prevention purposes. In this review, we will discuss the most recent evidence about the current potential treatment options including anti-inflammatory drugs, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, nucleoside analogs, protease inhibitors, monoclonal antibodies, and convalescent plasma therapy. Some other agents such as vitamin D and melatonin, which were recommended as potential adjuvant treatments for COVID-19 infection are also presented. Moreover, the potential use of convalescent plasma for treatment of COVID-19 infection was described. Furthermore, in the next part of the current review, various vaccination approaches against COVID-19 including whole virus vaccines, recombinant subunit vaccine, DNA vaccines, and mRNA vaccines are discussed.
Keywords: COVID-19, SARS-CoV-2, treatment, vaccine, coronavirus
Introduction
As of December 2019, the seventh member of the human coronavirus family was recognized. Several pneumonia cases of unknown etiology were first reported in December 2019 in Wuhan, China. Subsequent studies were initiated on identification of a causative agent and control of the epidemic.1 This virus is named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its related disease is named COVID-19. Currently, the outbreak has become a major clinical threat to the general population and health-care workers (HCWs) all over the world. This virus has rapidly spread across China and several other countries.2 The genetic analyses on 103 publicly available SARS-CoV-2 genomes in a more recent study has shown the evolution of these viruses into two major types (L and S). These two types of SARS-CoV-2 are defined by means of two different SNPs.3 The expression and distribution of the receptor apparently determine the pathogenic mechanisms of virus as well as probable therapeutic strategies. Angiotensin-converting enzyme 2 (ACE2) is considered as the major determinant of cell tropism and pathogenesis for SARS-CoV-2.4
In a latest report, the expression of ACE2 was not significantly different between men and women, Asian and white people, or subgroups aged older and younger than 60 years. This study also did not support smoking as a predisposing factor in men for infection with SARS-CoV-2.5 The findings of a recent study suggested that elderly patients are more likely to experience disease progression than their younger counterparts.6
Impaired immunity can lead to virus dissemination and destruction of the affected tissues, particularly in organs with high ACE2 expression. The damaged cells can induce pulmonary inflammation mainly mediated by pro-inflammatory macrophages and granulocytes.7 Pulmonary inflammation is the leading cause of life-threatening respiratory condition at the severe stage.8 Therefore, inflammation suppression should be performed at the time of severe lung damage to manage the symptoms. Studies have reported that ALT, LDH, D-dimer and inflammatory markers including hsCRP and ferritin were significantly higher in severe cases than in moderate cases. In addition, serum concentrations of pro-inflammatory cytokines as well as anti-inflammatory cytokines were enhanced in the majority of severe cases and were markedly higher in severe cases than in moderate cases, suggesting cytokine storm might be associated with disease severity.9
The most common symptoms of infection with SARS-CoV-2 which have been reported in various studies include fever, followed by dry cough, fatigue, muscle pain, and dyspnea. Some other symptoms such as expectoration, headaches, hemoptysis, nasal obstruction, runny nose, and other upper respiratory rhinorrhea. Gastrointestinal symptoms (vomiting, and diarrhea) have been reported less frequently.10,11 The majority of the children infected with SARS-CoV-2 indicate mild symptoms, without fever or pneumonia, and will recover within one to two weeks.12 The reasons for mild COVID-19 disease in pediatric patients remains unknown. However, several hypotheses exist including: immune differences between children and adults,13 and the simultaneous presence of other viruses in the lung mucosa of the young children limiting the SARS-CoV2 growth by direct virus-to-virus competition.14 Differences in the ACE2 expression could be another possibility for the mild COVID-19 infections in children.15 Some epidemiological studies have revealed an association between Bacillus Calmette–Guérin (BCG) vaccination and COVID-19 morbidity and mortality. Moreover, the nonspecific beneficial effects of some live vaccines, such as BCG, measles and oral polio vaccines have previously been reported. Therefore, BCG may have the potential to decrease severity of SARS-CoV-2 infection by induction of innate immune memory.15
Contemporary diagnostic tests available for SARS-CoV-2 include RT-PCR, real‐time RT‐PCR, and reverse transcription loop‐mediated isothermal amplification (RT‐LAMP).16,17 Both RT-LAMP and real-time RT-PCR have the same sensitivity.18,19
The first detectible serology marker has been reported, total antibody followed by production of IgM and IgG antibodies, with a median seroconversion time of 11, 12, and 14 days respectively.20 The findings have suggested that antibody testing in addition to RNA tests can provide significant data for diagnosis of SARS-CoV-2 infection.20 Furthermore, technologies based on CRISPR-Cas12 have the potential to provide an in situ diagnostic tool for rapid detection of the SARS-CoV-2 virus. Binding of Cas12a to target DNA can be detected by a reporter molecule providing a fluorescence signal emission. Finally, a visual readout is provided using a paper dipstick.21
Chest CT is a significant tool for COVID-19 diagnosis in clinical practice.22 The most frequent finding reported from chest CT is ground glass opacity (GGO)23,24 and consolidation, interlobular septal thickening in both lungs has been reported as less frequent. In the primary stages of infection, the total number of the peripheral blood leukocytes is normal or decreased, the lymphocyte count is decreased, and in some cases liver enzymes and myoglobin levels indicate increase. Elevated CRP and erythrocyte sedimentation rate (ESR) are frequent.25 Lymphopenia, leukopenia, elevated level of aspartate aminotransferase are the most common laboratory findings.10,11 Primary plasma levels of Interleukin (IL)-1β, IL‐1Rα, IL‐7, IL‐8, IL‐9, IL‐10, basic fibroblast growth factors (FGF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFNγ), IP10, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory proteins (MIP)-1A, MIP1B, platelet-derived growth factor (PDGF), and tumor necrosis factor alpha (TNF-α) has been shown to be higher in patients infected with SARS-CoV-2 than in healthy controls.11
At the time of writing, no vaccine or antiviral drug is available for COVID-19. Infection prevention and control of this virus is the primary concern of public health officials and professionals. Currently, several therapeutic options for COVID-19 are proposed and vaccine development has also been initiated for prevention purposes. In this review, we will discuss the most recent evidence about the current potential treatment options, vaccine development, and future perspectives of COVID-19 in order to help tackle the SARS-CoV-2 pandemic challenges.
Potential Treatment Options of COVID-19
Medications inhibiting viral dissemination and blocking viral replication may decrease the coronavirus‐induced direct cytopathic effects. In addition prevention of inflammatory responses by antibodies or compounds neutralizing cytokines or their cognate receptors may reduce virus-mediated immune pathologies.26 The only treatment currently available for patients with COVID-19 mainly relies on symptomatic treatments as no vaccine has been successfully developed to date. Therefore, effective antiviral therapy and vaccination are under assessment.1 However, according to the World Health Organization (WHO), empirical antibiotics, antiviral agents, and systemic steroids should be used for treatment of severe cases.27 A recent descriptive study reported that most of the studied patients have received antiviral agents such as oseltamivir (75 mg orally twice daily), ganciclovir (0.25 g intravenously twice daily) and lopinavir/ritonavir tablets (400/100 mg every 12 h orally).28 Empiric antimicrobial and antifungal therapy should also be considered for potential bacterial or fungal coinfection.29 The efficacy or adverse effects of intravenous immunoglobulin and systemic steroids being used in some patients are unclear.30 Invasive ventilation may be used for patients with refractory hypoxemia.1 Numerous possible drug candidates have been suggested, including chloroquine, DNA synthesis inhibitors (tenofovir disoproxil and lamivudine), lopinavir/ritonavir (Kaletra R ®), nucleoside analogues, neuraminidase inhibitors, remdesivir, and umifenovir (Arbidol R®) as well as Chinese traditional medicines (ShuFeng JieDu or Lianhua Qing-wen capsules).31,32
Small Molecules
Identification of the key components of the viral infections is a major step for recognizing medications with high target specificity.33 Viral entry to the cell and replication within the cell can be considered as a target for designing the antiviral drugs.33
In addition to several antiviral drugs, chemical small-molecule compounds are also expected to have significant inhibitory properties on various key proteins of viruses including SARS-CoV-2.34 These drugs typically inhibit both viral enzymes such as proteases and components of RNA-dependent RNA polymerase (RdRp).34 Chymotrypsin-like protease (CLPro; 3CLp) and papain-like protease (PLpro) are two main proteases essential for cleavage of viral polypeptides into active components which are required for viral replication and maturation.34 RdRp is an enzyme essential for viral RNA synthesis and catalyzes the replication of RNA.34 The SARS-CoV-2 host cell entry depends on the interaction between ACE2 as the main receptor and viral spike (S) protein.35 Inhibition of this interaction may also represent a potential therapeutic strategy against COVID-19.35 Some studies aim to recognize molecules that inhibit the docking of S protein mediated by receptor-binding domain (RBD) onto cells which express ACE2.35,36 A recent study showed that a clinical-grade soluble recombinant human ACE2 (hrsACE2) protein can decrease SARS-CoV-2 recovery from simian Vero E6 cells. In addition, they have demonstrated that infection of kidney organoids and engineered human capillary organoids can be inhibited by hrsACE2. These findings suggest that hrsACE2 can considerably prevent SARS-CoV-2 infection by blocking its entry at the early stage of infection.37
Umifenovir (Arbidol™; CAS number: 131707−25-0) is a small molecule with an indole core and inhibits viral entry into host cells through inhibition of viral membrane fusion.38,39 It is approved in Russia and China as an antiviral treatment for oral prophylaxis and treatment of influenza infections.40 A recent clinical pilot trial in China reported viral load reduction and decreased mortality rate in COVID-19 patients received arbidol (400 mg; three times; nine days) as compared to the control group.41 Moreover, there are ongoing clinical trials evaluating the potential efficacy of arbidol alone or in combination with other drugs such as chloroquine and lopinavir/ritonavir against SARS-CoV-2 infection (NCT04255017, NCT04260594, NCT04273763, NCT04286503).
There are several other small molecules that are considered for use in treatment of COVID-19 infection. In further sections of this review, the other small-molecule drugs will be discussed in their respective categories.
Anti-inflammatory Drugs
Immense immune response is common against viral infections to control the pathogen and this can lead to extensive injury to the host cells. A hyperinflammatory state with extreme release of pro-inflammatory cytokines is the most serious presentation of COVID-19 infection and known as cytokine storm.42 The stimulation of pro-inflammatory cytokines by the virus leads to severe organ injury such as acute respiratory distress syndrome (ARDS).42,43 Initial studies have reported decreased immune cell populations such as lymphocyte subsets (CD4+, CD8+) and natural killer (NK) cells as well as increased cytokines in the course of severe COVID-19 pneumonia.9,11,44,45
Macrophage activation syndrome as well as elevated levels of ferritin, C-reactive protein (CRP), and cytokines like IL-6, IL-8, IL-1β, and TNF-α has been shown in the primary stage of COVID-19 infection.46 Moreover, a significant escalation in inflammatory monocytes mainly CD14 + IL-1β+ cells at the initial stage of recovery was indicated by single cell sequencing of peripheral blood cells.47 The existence of lymphopenia and lower numbers of CD4 and CD8 T cells has been linked to more severe disease.48 In addition, both increased inflammatory CCL6+ Th17 cells and decreased circulating regulatory T cells has been reported in severe cases.48,49 Lymphopenia, spleen atrophy, and pulmonary macrophage infiltration were observed in later stages of infection and autopsy.8 Complement deposition in pulmonary microvasculature due to initiation of alternative complement pathway activation has been reported in COVID-19 infection as well.50
Therefore, primary inhibition of inflammatory pathways may be considered as a major therapeutic option,51 and anti-inflammatory medications can be a supportive treatment for COVID-19. Several anti-inflammatory drugs are currently available including nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, chloroquine/hydroxychloroquine, and immunosuppressants to prevent or diminish the progression of inflammation.52
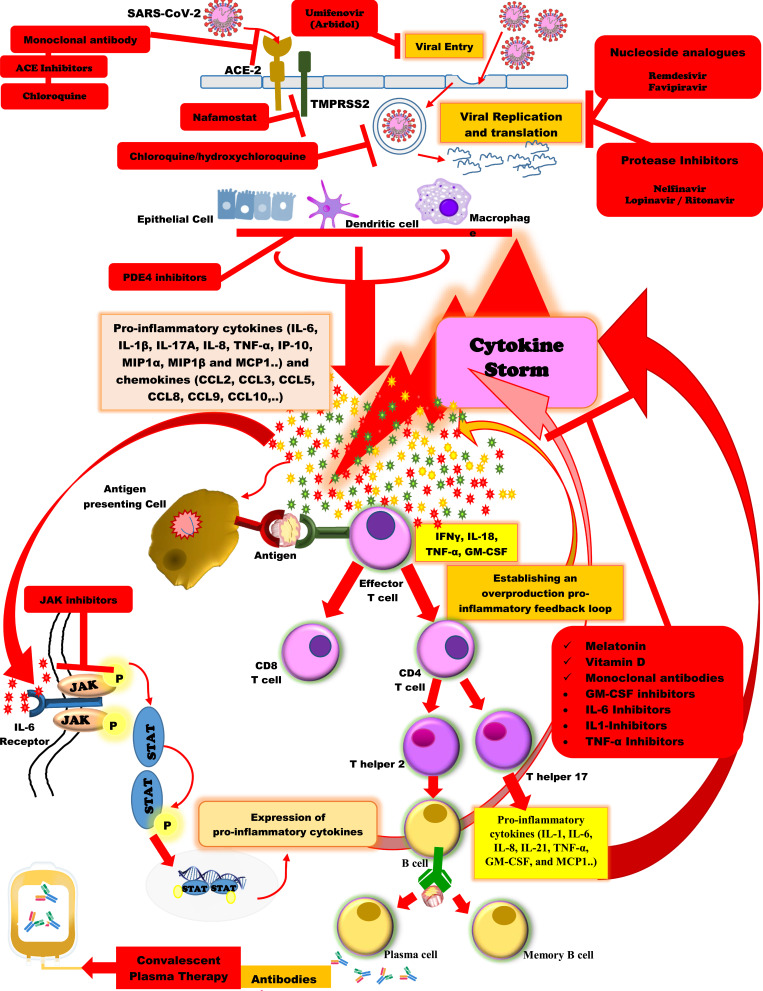
Moreover, particular anti-inflammatory/immunosuppressive agents such as IL-1 inhibitors (anakinra), IL-6 inhibitors (siltuximab), IL-6 receptor antagonist monoclonal antibodies (tocilizumab, sarilumab), TNF-α inhibitors (adalimumab), and the Janus kinase (JAK) inhibitors (fedratinib, baricitinib)52 are being assessed by ongoing clinical trials. However, there is inadequate evidence for their effectiveness and safety against SARS-CoV-2 infection (Figure 1).53,54
Figure 1.
Schematic representation of the immunopathogenesis of SARS-CoV-2 infection and the potential therapeutic options against COVID-19.
However, the dilemma of applying anti-inflammation therapy to COVID-19 patients and using anti-inflammatory/immunosuppressives agents in the presence of an active infection seems to be controversial.55 On the one hand, inflammation control is important to inhibit tissue injury. On the other hand, the anti-inflammation therapy such as corticosteroid may postpone the viral eradication and increase the risk of secondary infection. Although some studies have described the effectiveness of glucocorticoids in the treatment of coronavirus pneumonia (severe acute respiratory syndrome, SARS and Middle East respiratory syndrome, MERS) it remains controversial. Some evidence designated that the advantage of using glucocorticoids is likely outweighed by adverse effects.24 However, several reports have not supported corticosteroid for treatment of COVID-19 lung disease.56 According to insufficient evidence, the temporary WHO guideline does not support using systemic corticosteroids for COVID-19 cases. However, there is evidence proposing that cases on chronic anticytokine management for inflammatory diseases mediated by immune responses are not at a greater risk for worse outcomes from COVID-19 infection.57
JAK Inhibitors
One of the key parts of immunopathology during infection caused by coronaviruses is cytokine and chemokine responses. Increased levels of serum pro-inflammatory cytokines such as IFNγ, TNF-α, IL-2, IL-4, IL-6, IL-7, and IL-10 were described in severe COVID-19 cases and were associated with the severity of disease.58 Numerous cytokines involved in COVID-19 utilize an intracellular signaling pathway mediated by JAKs.59 Baricitinib (brand name Olumiant) is a novel small molecule acting as a JAK inhibitor. It is a food and drug administration (FDA)-approved antirheumatoid arthritis medication which has been suggested as a potential candidate for COVID-19 treatment.60 However, JAK inhibitors can block the production of a variety of inflammatory cytokines including IFN-α, which is essential against viral infection and may not be a suitable option for COVID-19 treatment. There are some recent registered clinical trials of JAK inhibitor ongoing in China (ChiCTR2000030170; ChiCTR2000029580).
Chloroquine and Hydroxychloroquine
Chloroquine is used either for prevention and treatment of malaria or for the treatment of rheumatoid arthritis and lupus erythematosus. It has wide antiviral effects through increasing endosomal pH essential for virus entry.61 Chloroquine can lead to inhibition of uncoating as well as modification of posttranslational newly synthesized proteins in several viruses such as HIV. Initial in vivo studies have proposed that the chloroquine alone or in combination with antiretroviral agents may play a role in COVID-19 treatment.62 It has shown a potent inhibition effect against SARS-CoV through interfering with ACE2.63 Moreover, its in vitro activity against SARS, MERS, HIV, Ebola, Hendra, and Nipah viruses has been shown.63–65 In China, COVID-19 patients were treated with chloroquine to evaluate its effectiveness and safety. Their results indicated that chloroquine prevents the exacerbation of COVID-19.61 An in vitro study has also indicated high effect of remdesivir and chloroquine combination in COVID-19 control.32 Guidelines (version 6) for COVID-19 treatment recommends oral administration of chloroquine phosphate at a dose of 500 mg (300 mg for chloroquine) for adults, twice/day (no more than 10 days).66 Several clinical trials have been registered to evaluate the efficacy and safety of chloroquine in the treatment of COVID-19. A recent clinical trial has reported the short-term efficacy of hydroxychloroquine in the treatment of COVID-19.52 There are studies that reported the superiority of chloroquine phosphate to the control treatment in prevention from progression of pneumonia, improving pulmonary imaging results, viral clearance, and shortening the disease duration.61 The results of an open-label nonrandomized clinical trial showed that hydroxychloroquine treatment significantly decreased the viral load in patients with COVID-19 infection.67 However, these studies have some limitations such as small sample size and debatable methodology.
Some potential adverse effects of chloroquine/hydroxychloroquine include retinopathy, cardiac arrhythmias, and muscle weakness.68 There are also some reports of ototoxicity following treatment with these drugs, which may lead to hearing loss.67 Currently, there is no strong evidence of beneficial potential of chloroquine/hydroxychloroquine for COVID-19 treatment. However, further preclinical and clinical trials are required to prove their effectiveness and safety.
Phosphodiesterase 4 (PDE4) Inhibitors
The PDE4 expression has been established in several inflammatory cells including neutrophils, monocytes, T cells, and eosinophils.69 Cyclic adenosine monophosphate (cAMP) as an intracellular regulator has a key role in modulation of cytokine release through the nuclear factor kB (NF-κΒ) and protein kinase A (PKA) pathway. This leads to inhibition of the main pro-inflammatory cytokines including TNF-α, and the stimulation of the anti-inflammatory IL-10.70 PDE4 is an enzyme account for the hydrolysis of cAMP and reduces its intracellular levels in inflammatory cells.71 Thus, inhibition of PDE4 leads to several anti-inflammatory properties in various cells such as neutrophils, macrophages, monocytes, T cells, and B cells.72
The PDE4 by modification of the intracellular cAMP levels can regulate both pro- and anti-inflammatory cytokine productions.72 Higher expression of PDE4 in cases with inflammatory diseases compared to healthy individuals has been shown previously.73 Inhibitors of PDE4 have been suggested as novel anti-inflammatory treatments used in several inflammatory diseases including rheumatoid arthritis, psoriasis, asthma, and chronic obstructive pulmonary disease (COPD).72–74 They effectively suppress pro-inflammatory cytokines such as IFNγ, TNF-α, IL-2, IL-12, IL-17 and IL-23 in inflammatory cells and also inhibit the reactive oxygen species (ROS) production.72,74,75 IL-17 is a pro-inflammatory cytokine produced by T helper (Th)-17 cells and suggested to have a role in acute pulmonary damage during respiratory viral infections.76 Therefore, IL-17 has been suggested as a target for management of pulmonary damage during COVID-19 infection.77
In addition, in vivo studies on animal models of pulmonary damage injury has shown decreased deposition of pulmonary fibrin and vascular alveolar leakage, as well as improved survival due to the inhibition of PDE4.78,79 The use of PDE4 inhibitors leads to not only upstream suppression of several cytokine signaling pathways, but also regulation of the pro-inflammatory/anti-inflammatory equilibrium. Therefore, it has been speculated that PDE4 inhibitors can be considered as potential therapeutic options against COVID-19 infection.80
Apremilast (Otezla®) is an oral small molecule belongs to the PDE4 inhibitors. Mugheddu et al. recently reported a 45‐year‐old obese male with erythrodermic psoriasis who was under treatment with apremilast, has fully recovered from COVID-19 infection.81 A more recent case report also described a 61-year old male who suffered from moderate psoriasis was receiving apremilast and whose family were diagnosed as being SARS-CoV-2 positive. However, despite his close contact with them, the patient showed no symptoms of COVID-19 infection. The nasopharyngeal swab examination indicated that he was positive for SARS-CoV-2, but the CT results showed no pulmonary changes.82 These reports may suggest anti-inflammatory effects of apremilast against COVID-19 infection. Therefore, it has been suggested that the use of PDE4 inhibitors before the cytokine storm and in the primary stage of COVID-19 pneumonia may signify an appropriate treatment option. Appropriate clinical trials are required in order to explore the potential therapeutic effects of PDE4 inhibitors as well as other therapeutic options.80
Methotrexate
Methotrexate is an immune system suppressant belonging to antimetabolites. A recent study has provided robust reasoning for high-dose methotrexate usage in treatment of inflammatory syndromes mediated by SARS-CoV-2.83 According to the authors, the major mechanism of action of methotrexate is inhibition of folate-dependent methyl transfer enzymes which interferes with the DNA and RNA synthesis leading to inhibition of cell proliferation particularly in dividing cells.83 Furthermore, methotrexate has several anti-inflammatory effects.84 It can affect activated inflammatory T cells which are dividing cells. It also directly binds to high-mobility group box 1 (HMGB1) protein and prevents RAGE–HMGB1 interactions which leads to suppression of cytokine production mediated by macrophages.85 Methotrexate in high dose therapy has been indicated to reduce proliferation of monocytes as well as decrease inflammatory cytokine levels.86 Special attention should be paid to administration of high-dose methotrexate. It is accessible, cost effective with good CNS penetration.55 The use of methotrexate in COVID-19 cases with cytokine storm seems to be an appropriate option. However, this should be evaluated in clinical trials. Management of COVID-19 patients with a combination of recently approved remdesivir with an anti-inflammatory agent will possibly increase treatment efficiency.55,87
Angiotensin-Converting Enzyme Inhibitors (ACEI) or Angiotensin Receptor Blockers (ARBs)
ACE2 as the receptor of SARS-CoV-2 is a significant component of the renin-angiotensin system (RAS). ACEI/ARBs are used primarily for the treatment of individuals with cardiovascular comorbidities.88
On the one hand, overregulation of ACE2 can have a potential role in the increased risk of pulmonary infection.89 As mentioned above, SARS-CoV-2 uses the receptor ACE2 for entry into the target cells.90 It has been reported that both ACEI and ARBs could significantly upregulate mRNA expression of cardiac ACE2.91 Therefore, it has been hypothesized that the increased ACE2 expression may have an association with increased risk of severe course of COVID-19 cases.92
On the other hand, ACE2 may play a protective role in both cardiovascular and pulmonary diseases.89 There is evidence that the ARBs have protective effects against viral spread of SARS-CoV-2.93 It has been observed that individuals using losartan or telmisartan as antihypertensives agents develop fewer attacks of cold and flu-like diseases.94 Both these drugs bind to the AT1 receptors stronger than valsartan.94 They can be administered either orally or in the form of a nasal spray. Improved survival has been observed following treatment with losartan in experimentally infected mice models with H5N1 influenza.95 Utilization of zinc supplement can also facilitate intracellular killing and phagocytosis.96 Therefore, ARBs (telmisartan and losartan) in therapeutic doses along with zinc can be used to control viral replication.93 Since the limited information, rapid clinical trials are required to investigate the use of losartan or telmisartan for inhibition of the viral entry into the host cell.
Collectively, the role of ACEIs and/or ARBs in facilitating SARS-CoV-2 entry and virus replication cannot be ignored. On the contrary, beneficial effect of ACEIs and/or ARBs in clinical outcomes after infection still remains unknown. Therefore, it seems that we are facing a double-edged sword depending on the disease phase. At the early stage of disease, increased expression of ACE2 may enhance infectivity and the use of ACEI/ARBs would be a potential risk factor, but once infection is established, ACE2 downregulation may lead to COVID-19 progression. Accordingly, upregulation of ACE2 in the acute phase of the disease may turn out to be advantageous.89
There are several observational studies performed in different countries such as U S,97 U,98 China,99–101 and Italy102 which were studied on confirmed COVID-19. None of these studies reported association between ACE inhibitors or ARBs and severity of COVID-19 infection. Moreover, there is no study described either benefits or harms of ACE inhibitors or ARBs as treatment for COVID-19 patients.
Nucleoside Analogs
Generally, nucleoside analogs are synthetic nucleosides that mimic their physiological counterparts. They inhibit the cellular nucleotide synthesis pathways and or viral replication through impairment DNA/RNA synthesis. Nucleoside analogs can target the RNA-dependent RNA polymerase that is necessary for viral RNA replication.103
Favipiravir (T-705, Avigan, or favilavir) is a guanine analog with activity against various RNA viruses such as influenza, Ebola, yellow fever, and enterovirus.104 It is a small molecule with a plasma protein binding of about 60%. Therefore, it may appear in breast milk.105
Favipiravir has shown effective antiviral activity in Vero E6 cells and has been suggested as a possible candidate for treatment of COVID-19.32 Favipiravir in combination with other antiviral agents such as IFN-α or baloxavir marboxil was used against SARS-CoV-2.106 An experimental treatment with favipiravir indicated its superior therapeutic effects against COVID-19 compared to lopinavir/ritonavir, concerning improvement of disease and viral clearance.105
Ribavirin is another nucleotide guanine analog (guanosine analog) used for treatment against hepatitis C virus (HCV) and respiratory syncytial virus (RSV) infection as well as against SARS-CoV and MERS-CoV infections.107 In vitro studies have indicated synergistic effects of ribavirin with both leukocytic IFN-β and IFN-α against SARS-CoV.108,109 It may significantly impact the treatment of COVID-19 infection due to its availability and low cost.110 Ribavirin has shown adverse reactions including anemia and altered liver function.107,111 Therefore, special dosage consideration should be given.108,109
Remdesivir (GS-5734) (CAS number: 1809249−37-3) is a novel small-molecule adenine nucleotide analog that was originally developed to treat Ebola virus.112 It is considered as a prodrug that is metabolized into its pharmacologically active form recognized as GS-441524.113 It can block replication of different coronaviruses such as SARS-CoV and MERS-CoV.112 Remdesivir has antiviral activity by inhibition of RdRp which cause interference with the RNA synthesis of virus. It is effective in the primary stage of infection and leads to a reduction of viral RNA levels.114 In vitro and in vivo studies have reported that the antiviral activity of remdesivir and IFN-β combination is superior compared to that of lopinavir/ritonavir-IFN-β against MERS-CoV. Remdesivir has also shown promising results in the treatment of SARS-CoV-2 infection.115 Currently there are several ongoing clinical trials on remdesivir in COVID-19 patients in numerous countries including France (NCT04314817, NCT04315948), USA (NCT04302766), Republic of Korea and Singapore (NCT04280705), and China (NCT04252664, NCT04257656).
Protease Inhibitors (PIs)
PIs are a class of antiviral medications that are mainly used for treatment of HIV and HCV. They selectively bind to viral proteases and block proteolytic cleavage of protein precursors, which leads to inhibition of infectivity and replication. Both lopinavir (ABT-378) and ritonavir (ABT-538) are protease inhibitors, used against HIV.116,117 The lopinavir/ritonavir combination is extensively used in the HIV management. Favorable therapeutic effects have been reported for lopinavir/ritonavir (Kaletra™) with ribavirin against SARS-CoV and MERS-CoV.117,118 This combination has been also suggested for COVID-19.119 In a previous study, following confirmed in vitro susceptibility of SARS-CoV to lopinavir/ritonavir, the clinical progress of SARS-CoV positive patients who were treated with a combination of ribavirin and lopinavir/ritonavir were compared with those treated with ribavirin only. The adverse outcome in the group who were treated with lopinavir/ritonavir was significantly lower than that of the other group.117 Furthermore, there is a case report of a patient with MERS-CoV infection who was successfully treated with triple combination of lopinavir/ritonavir, ribavirin and IFN-α and respiratory viral clearance achieved within six days.118
Nelfinavir is also an antiretroviral drug that selectively inhibits the HIV protease.120 Its inhibitory effect has been shown for SARS-CoV suggesting its potential therapeutic effect for COVID-19.121 A previous study suggested that cytopathic effect of SARS-CoV infection could be inhibited by nelfinavir. The use of quantitative RT-PCR indicated that production of virions from Vero cells has been reduced by nelfinavir.120
In a recent trial, oral lopinavir/ritonavir (400 mg/100 mg) twice daily for 14 days was prescribed for adults with severe COVID-19 infection. The results were compared to those who received standard treatment. The primary data from this trial indicated no advantage of using lopinavir/ritonavir treatment beyond standard care (ChiCTR2000029308).122 Determination of lopinavir/ritonavir effectiveness in COVID-19 patients needs to be further investigated. There are some clinical trials being conducted to assess the efficacy of lopinavir/ritonavir in combination with remdesivir and IFN-β-1a in COVID-19 cases in Hong Kong (NCT04276688), Republic of Korea (NCT04307693), China (NCT04255017, NCT04261907, NCT04286503), and in Europe (NCT04315948).
Type I IFNs
Immune intervention approaches to increase the immunity may help combat against viral infections. IFNs are the most broadly prescribed drugs with strong antiviral activities. IFN is a significant cytokine and represents one of the first lines of host defense against both DNA and RNA viruses.123 IFNs are classified into three groups, type I, II and III IFNs. Type I IFNs include structurally similar cytokines and comprise 13–14 subtypes of IFN-α together with IFN-β, IFN-ε, IFN-κ, IFN-ω, IFN-δ, IFN-ζ, and IFN-τ. In contrast, type II IFNs consist of IFN-γ.124
Type I IFNs possess a wide range of biological activities and are the first cytokines produced during viral infections. They induce a wide range of proteins implicated in impairment of viral replication in targeted cells. Experiences from SARS-CoV have demonstrated the better antiviral activity of IFN-β compared to IFN-α.125 A previous study evaluated antiviral effect of recombinant IFN-α and IFN-β against two clinical isolates of SARS-CoV (FFM-1 and Hong Kong) which were replicated in Caco2 and Vero cells. Their findings indicated that IFN-β was more effective than IFN-α in Caco2 cells. Moreover, in Vero cells, IFN-β was the most potent inhibitor of SARS-CoV and its selectivity index for SARS-CoV strain FFM-1 was 50 and 25 times higher than that of IFN-α and IFN-γ, respectively.126
Nasal drops of IFN can maintain high levels of IFN-α in the nasal mucosa which may prevent the virus passing through the nasal mucosa.123 A recent trial on the protective effect of recombinant human IFN-α (rhIFN-α) nasal drops against COVID-19 in HCWs reported a positive preventive effect of rhIFN-α nasal drops in susceptible healthy individuals against COVID-19 infection.123
Moreover, a recent trial (phase I and II/not yet recruiting) has been registered to evaluate the adverse effects of rintatolimod and IFN-α-2b in management of cancer cases with mild or moderate COVID-19 infection (NCT04379518).
A recent study suggested that treatment with IFN-α2b considerably decreases viral clearance from the upper respiratory tract as well as decreases circulating levels of the inflammatory markers including IL-6 and CRP in COVID-19 patients.127 According to WHO, IFN-α2b can be considered as a possible antiviral for the management of COVID-19.128 Heberon® Alpha R is a human recombinant IFN-α2b which has indicated antiviral activities.129 A multicenter prospective observational study in Cuba enrolled confirmed cases of COVID-19, suggested the therapeutic effects of IFNα-2b for COVID-19. It also showed that using Heberon® Alpha R may potentially improve the rates of recovery as well as case fatalities.130
Melatonin as a Potential Adjuvant Treatment
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone with a variety of functions in health and has been effectively used to treat sleep disorders, atherosclerosis, respiratory diseases, and viral infections. The efficacy of melatonin has been recognized in experimental animal models and in human studies. Melatonin may have helpful adjuvant utility in treatment of COVID-19 induced pneumonia.131 Excessive inflammation as a significant feature in COVID-19 patients can depress immune system and trigger cytokine storm. Due to anti-oxidative, anti-inflammatory, and immune enhancing properties, melatonin may have indirect antiviral effects.132 A meta-analysis of randomized controlled trials revealed the association between melatonin supplementation and reduced level of TNF-α and IL-6.133 Therefore, while there is no direct evidence of melatonin administration in COVID-19 patients, both anti-inflammatory role of melatonin and its safety profile may indicate its potential beneficial effect in COVID-19 patients.134
Monoclonal Antibodies
Neutralizing monoclonal antibodies are an important part of protective immunity against several viral infections.135 Some antigenic sites on surface proteins of a virus can be targeted by neutralizing monoclonal antibodies.136 Therefore, this strategy can be considered as a potential option for prophylactic and therapeutic purposes. Moreover, effective monoclonal antibodies can be produced by immunization of humanized mice.135 Neutralizing antibodies against coronaviruses mainly target S protein that facilitate virus entry into host cells through binding to the human ACE2 protein.136 Effectiveness of monoclonal antibodies have been established in animal models for both MERS-CoV and SARS-CoV.135 The efficiency of a high-affinity anti-S1 human monoclonal antibody (80R) against SARS-CoV infection through inhibition of syncytia formation between cells expressing the S protein and those expressing the SARS-CoV receptor ACE2 was described previously.137
According to the former experience in the treatment of viral infections including SARS, MERS, influenza, and Ebola, mortality rate can be reduced by administration of stimulant plasma.138,139 In passive immunization, antibodies specific for a certain epitope of pathogen can lead to a decreased viral proliferation and disease severity.140 Due to the similarities between the SARS-CoV and SARS-CoV-2, some studies have repurposed the monoclonal antibodies against SARS-CoV for COVID-19 patients.139
Currently, RBD on the S protein is the main target of neutralizing monoclonal antibodies against SARS-CoV-2.141,142 The findings of recent studies show that B38, H4, as well as 47D11 are novel antibodies that have indicated promising results in neutralizing the SARS-CoV-2 infection.143,144 The 47D11 antibody targets an epitope in the SARS2-S-S1B domain (residues 338–506) and neutralizes SARS-CoV and SARS-CoV-2 through an unknown mechanism.143 A recent study has isolated monoclonal antibodies from the B cells of COVID-19 recovered cases as well as from individuals infected with the SARS-CoV in 2003.135 A recent study on four human monoclonal antibodies (B38, B5, H2, and H4) isolated from a convalescent COVID-19 patient showed binding between studied antibodies and SARS-CoV-2 RBD. A competition assay to evaluate the ability of each antibody for inhibition of binding between RBD and ACE indicated that B38 and H4 antibodies completely blocked the binding of ACE-2 to RBD.144 These findings show that monoclonal antibodies have a potential therapeutic efficacy against COVID-19 infection. Several trials are evaluating the efficacy of various monoclonal antibodies for the treatment of hospitalized patients with COVID-19 (Table 1).
Table 1.
Monoclonal Antibodies Under Trials for Evaluation of Efficacy and Safety as Treatment Options of COVID-19 Infection
| Identifier Number | Brief Aim of Trial | Study Phase | Study Sponsor/Country | Interventions |
|---|---|---|---|---|
| NCT04425629 | To assess the safety, tolerability, and efficacy of anti-spike monoclonal antibodies for the treatment of ambulatory patients with COVID-19 | Phase 1 Phase 2 |
Regeneron Pharmaceuticals/France | Drug: REGN10933+REGN10987 Drug: placebo |
| NCT04346277 | Using IC14 to block CD14-mediated cellular activation in patients early in the development of ARDS. | - | Implicit Bioscience/Italy | Biological: IC14 (A recombinant chimeric monoclonal antibody against CD14) |
| NCT04391309 | Assess IC14 in reducing the severity of respiratory disease in hospitalized COVID-19 patients | Phase 2 | Implicit Bioscience | Biological: IC14 Other: placebo |
| NCT04409509 | To evaluate CSL312 in COVID 19 | Phase 2 | CSL Behring/United States | Biological: garadacimab, factor XIIa antagonist monoclonal antibody Drug: placebo |
| NCT04351152 | To assess whether the use of lenzilumab + current standard of care (SOC) can alleviate the cytokine release syndrome (CRS) and inhibit development to respiratory failure and/or death in high risk COVID-19 patients | Phase 3 | Humanigen, Inc./US | Biological: lenzilumab (anti-human GM-CS monoclonal antibody) Drug: standard of care |
| NCT04341116 | To assess the safety and efficacy of intravenous infusion of TJ003234 in cases with severe COVID-19 under supportive care, and to assess its effect on cytokines levels. | Phase 1 Phase 2 |
I-Mab Biopharma Co. Ltd/US | Drug: TJ003234 (Anti-GM-CSF monoclonal antibody) Drug: placebo |
| NCT04432298 | To evaluate the efficacy and safety of intravenous pamrevlumab as compared to placebo in hospitalized cases of acute COVID-19. | Phase 2 | FibroGen | Drug: pamrevlumab (human recombinant monoclonal antibody against connective tissue growth factor (CTGF) Drug: placebo |
| NCT04324021 | To assess the efficacy and safety of emapalumab or anakinra, vs standard of care (SoC) | Phase 2 Phase 3 |
Swedish Orphan Biovitrum/Italy | Biological: emapalumab (anti-interferon-gamma (IFNγ) monoclonal antibody) Biological: anakinra (recombinant form of a human receptor antagonist (IL-1ra)) |
| NCT04429529 | To assess safety of TY027 as a treatment for COVID-19 | Phase 1 | Tychan Pte Ltd/Singapore | Biological: TY027 Other: 0.9% saline |
| NCT04386239 | To assess the efficacy and safety of sarilumab in COVID-19 patients | Early Phase 1 | ASST Fatebenefratelli Sacco | Drug: sarilumab (human monoclonal antibody against the interleukin-6 receptor) prefilled syringe |
| NCT04357808 | To assess the efficacy and safety of sarilumab for mild to moderate COVID-19 infection | Phase 2 | Maria del Rosario Garcia de Vicuña Pinedo/Spain | Drug: sarilumab Other: standard of care |
| NCT04324073 | To assess the therapeutic effect and tolerance of sarilumab in patients with moderate, severe pneumonia or critical COVID-19. | Phase 2 Phase 3 |
Assistance Publique - Hôpitaux de Paris/France | Drug: sarilumab |
| NCT04351243 | To assess the efficacy and safety of gimsilumab in subjects with acute respiratory distress syndrome secondary to COVID-19. | Phase 2 | Kinevant Sciences GmbH/US | Drug: gimsilumab (anti-GM-CSF human monoclonal antibody) Drug: placebo |
| NCT04343651 | To assess the efficacy and safety of leronlimab for mild to moderate COVID-19 infection | Phase 2 | CytoDyn, Inc./US | Drug: leronlimab (humanized immunoglobulin (IgG4 monoclonal antibody against CC chemokine receptor 5 (CCR5; CD195) Drug: placebos (700 mg) |
| NCT04347239 | To assess the efficacy and safety of leronlimab for patients with severe COVID-19 infection | Phase 2 | CytoDyn, Inc./US | Drug: leronlimab (700 mg) Drug: placebos |
| NCT04305106 | To assess the efficacy and safety of bevacizumab for severe and critical COVID-19 patients. | NA | Qilu Hospital of Shandong University/China | Drug: bevacizumab (humanized anti-VEGF monoclonal antibody) |
| NCT04413838 | To assess the efficiency and security of nivolumab therapy in obese individuals with COVID-19 infection | Hospices Civils de Lyon/France | Drug: nivolumab (fully human IgG4 (S228P) monoclonal antibody) Other: routine standard of care |
|
| NCT04435184 | To assess the efficacy and safety of crizanlizumab in hospitalized patients with COVID-19 infection. | Phase 2 | Johns Hopkins University/US | Drug: crizanlizumab (anti-P-selectin monoclonal antibody) Other: 0.9% saline |
| NCT04377750 | To assess the efficacy and safety of tocilizumab in the treatment of severe COVID-19 cases with suspected pulmonary hyperinflammation | Phase 3 | Hadassah Medical Organization/Israel | Drug: tocilizumab (humanized monoclonal antibody against the IL-6 receptor) |
| NCT04331808 | To assess the therapeutic effect and tolerance of tocizilumab in patients with moderate, severe pneumonia or critical pneumonia associated with COVID-19 infection | Phase 2 | Assistance Publique - Hôpitaux de Paris/France | Drug: tocilizumab |
| NCT04330638 | To compare the efficacy of combinations of anti-IL drugs to best standard of care in treatment of patients with severe COVID-19 | Phase 3 | University Hospital, Ghent/Belgium | Other: usual care Drug: anakinra Drug: siltuximab Drug: tocilizumab |
| NCT04341584 | To compare the efficacy of anakinra in patients with Covid-19 infection | Phase 2 | Assistance Publique - Hôpitaux de Paris/France | Drug: anakinra |
| NCT04397497 | To assess the efficacy and safety of mavrilimumab in hospitalized patients with severe COVID- 19 infection and a hyper-inflammatory status. | Phase 2 | Ospedale San Raffaele/Italy | Drug: mavrilimumab (human monoclonal antibody against GM-CSF-R) Drug: placebo |
| NCT04390464 | To assess the potential treatment effect for COVID-19 disease (repurposed drugs) | Phase 4 | Cambridge University Hospitals NHS Foundation Trust/UK | Drug: ravulizumab (humanized monoclonal antibody against C5) Drug: baricitinib Other: standard of care |
| NCT04369469 | To evaluate the efficacy and safety of intravenous ravulizumab compared to best supportive care in patients with COVID-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome | Phase 3 | Alexion Pharmaceuticals | Biological: ravulizumab Other: best supportive care |
| NCT04322773 | To assess the effectiveness of IL-6 receptor inhibitors in the tratment of patients with severe COVID-19 infection. | Phase 2 | Marius Henriksen/Denmark | Drug: tocilizumab(RoActemra) iv (humanized monoclonal antibody against the IL-6 receptor) Drug: tocilizumab(RoActemra) sc Drug: sarilumab (Kevzara) sc (humanized monoclonal antibodyagainst the IL-6 receptor) Other: standard medical care |
| NCT04365153 | To assess whether early treatment with canakinumab inhibits progressive heart and respiratory failure in patients with COVID-19 infection | Phase 2 | The Cleveland Clinic/US | Drug: canakinumab (human monoclonal anti-IL-1β antibody) injection 600 mg Drug: canakinumab Injection 300 mg Drug: placebos |
GM-CSF Inhibitors
GM-CSF is a cytokine with an important role in regulation of inflammation. In inflammatory conditions, the concentration of circulating GM-CSF is increased. Interaction between ligand and GM-CSF receptor-α can stimulate pro-inflammatory pathways and can lead to elevated production of pro-inflammatory cytokines such as TNF, IL1, IL6, IL-12, and IL23.145 It has been shown that GM-CSF+T cells are greatly associated with severity of COVID-19 infection.146 Therefore, clinical trials are initiated, evaluating whether the humanized monoclonal antibodies such as lenzilumab (class IgG1 κ) and mavrilimumab (human isoform lgG4), TJ 003234 (IgG1) that target GM-CSF can alleviate the cytokine release syndrome (CRS) and inhibit respiratory failure and/or death in COVID-19 patients147 (Table 1).
Lenzilumab is a recombinant monoclonal antibody (class IgG1 κ) with the potential to target GM-CSF.148 In a recent clinical study lenzilumab was used to treat hospitalized patients with severe COVID-19 pneumonia. Their results indicated clinical improvement in 92% of the patients (11 out of 12). In addition, cytokine analysis revealed a decrease in inflammatory myeloid cells following treatment with lenzilumab. Therefore, according to the results, they suggested that GM-CSF neutralization with lenzilumab was accompanied with improvement of clinical outcomes, oxygen requirement, and cytokine storm. Moreover, it was well tolerated with no adverse effects.148
In contrast, a case report study has described that despite the use of lenzilumab and hydroxychloroquine for treatment of a male with COVID-19 infection, clinical progression of symptoms continued. His subsequent treatment with tocilizumab showed significant clinical improvement within 48 hours. Consequently, they suggested the superiority of tocilizumab over lenzilumab in the management of CRS.149 Therefore, clinical trials should be conducted to assess the effect of lenzilumab on treatment and clinical outcome in patients with COVID-19 infection (Table 1).
IL-6 Inhibitors
It has been established that IL-6 has a crucial role in COVID-19 induced cytokine storms.150 IL-6 is a pro-inflammatory cytokine and is secreted by several cells including monocytes and lymphocytes. Increased serum levels of IL-6 has been correlated with the severe COVID-19 infection and its poor prognosis.151 IL-6 has several biological functions such as modulation of the host immune response and regulation of immune cell proliferation and differentiation and can lead to activation of Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway.152,153 Prompt synthesis of IL-6 plays a crucial role in host defense during infection; however, its excessive production can lead to disease pathology.154
Inhibition of IL-6 is a therapeutic approach in numerous diseases such as rheumatoid arthritis with beneficial effects.155–157 Currently, IL-6 inhibitory agents are being evaluated for patients suffering from COVID-19.158 Therefore, several FDA approved anti-IL-6 agents such as tocilizumab, siltuximab, and sarilumab are currently being repurposed for treatment of COVID-19 infection.154
Siltuximab (Sylvant) is an FDA approved chimeric monoclonal antibody that binds to IL-6 and is used for the management of neoplastic diseases. Its treatment effects in COVID-19 patients with serious respiratory complications is under evaluation in a clinical trial (NCT04322188).
Tocilizumab is a humanized monoclonal antibody against the IL-6 receptor (IL‐6R) which can bind to both membrane-bound and soluble IL-6Rs and block IL-6-mediated signaling.159 It is an immunosuppressive drug and is used for treatment of rheumatoid arthritis. A retrospective observational study discussed the treatment response of tocilizumab therapy in COVID‐19 cases.160 It has been suggested that tocilizumab is an effective treatment choice in critically ill COVID‐19 patients with increased levels of IL‐6.160,161 However, a recent study reported a potential association between administration of tocilizumab and increased secondary bacterial infections in critically ill COVID-19 cases.162 These controversial results highlight the urgent need for evaluation of immunosuppressive agents in randomized controlled trials to recognize their therapeutic role in COVID-19 infection.
Sarilumab (Kevzara) is also a fully human, monoclonal antibody and IL-6 receptor antagonist. It is FDA approved and is under evaluation of efficacy and safety for hospitalized patients with COVID-19 in a recent clinical trial (NCT04315298).
There are some other clinical trials evaluating therapeutic efficacy of anti-IL-6 agents together with other drugs such as hydroxychloroquine, azithromycin, and antivirals such as remdesivir enrolling diverse groups of the COVID-19 patients (Table 1).
IL1-Inhibitors
IL-1 is one of the key mediators of innate immune system and inflammation.163 IL-1β can stimulate pro-inflammatory signaling in the respiratory tract.164
Anakinra (Kineret) is a recombinant, nonglycosylated human IL-1 receptor antagonist (IL-1Ra).165 Anakinra has a very favorable safety profile and is mainly used for management of hyperinflammatory disorders such as Still’s disease, as well as in the treatment of cytokine storm syndromes.166 It also has been used against some severe viral infections including H1N1, EBV, and Ebola.167 A recent case report described a critical COVID-19 case who were successfully treated with anakinra.168 In addition, the use of anakinra in patients hospitalized with severe COVID-19 was evaluated in a prospective cohort study. Their findings indicated that anakinra has resulted in decreased necessity for invasive mechanical ventilation and reduced mortality.169 However, verification of its efficacy, dosing, and outcome measures will need controlled trials. As of now, there are some ongoing clinical trials using anakinra against COVID-19 infection (Table 1).
TNF-α Inhibitors
TNF-α known as a major pro-inflammatory cytokine produced by stimulated macrophages, NK cells, T cells, and B cells.170 TNFα is implicated in primary inflammatory events and activates a series of several inflammatory molecules, including other cytokines, and chemokines, such as IL-8, CCL2, CCL3, and CCL4. TNF-α has an important role in the initiation and maintenance of CRS.171 Therefore, TNF-α can also be considered as a potential therapeutic target against COVID-19.
Adalimumab is a recombinant human IgG1 monoclonal antibody against human TNF-α which has been approved for management of rheumatoid arthritis.150 Currently, a trial has been registered in the Chinese Clinical Trial Registry (ChiCTR2000030089) to assess its efficacy and safety in patients with severe COVID-19 infection.
Convalescent Plasma
Most of the cases that recovered from COVID-19 infection show humoral and cellular immune responses against SARS-CoV-2. Therefore, some research groups have measured SARS-CoV-2 neutralizing antibody titers in the convalescent plasma.135 Recently, convalescent plasma has been extensively suggested for COVID-19. However, the effect of convalescent plasma cannot be differentiated from the effects of patient comorbidities, disease severity, or effect of other treatments.172 It has previously been used for treatment of some diseases including polio, measles, mumps as well as outbreaks of respiratory infections similar to COVID-19 such as H1N1 influenza, SARS, and MERS.173–175 According to FDA, antibody-rich plasma from COVID-19 survivors might be effective against the infection.176
A previous study reported on three HCWs infected with SARS-CoV who were successfully treated with convalescent plasma transfusion. A reduction of viral load was observed and all cases survived.173 Another study reported reduced viral load in majority of the patients with Ebola virus disease immediately after initiation of brincidofovir and convalescent plasma therapy.177 Moreover, potential efficiency of convalescent plasma therapy for clinical improvements of patients infected with MERS-CoV was revealed previously.174 The promising use of convalescent plasma therapy for influenza A (H5N1) infection was also described previously. The nonresponder patients to oseltamivir treatment alone fully recovered by using a combination of oseltamivir with convalescent plasma therapy. The viral loads decreased within eight hours of transfusion.175 Patients recovered from COVID-19 with a high neutralizing antibody titer are considered to be the eligible donors. Based on the preliminary results of a trial, convalescent plasma therapy can be a promising option for severe COVID-19 patients. They have reported that one dose of convalescent plasma with a high concentration of neutralizing antibodies can lead to a viral load reduction and tends to improve clinical outcomes.178 However, randomized clinical studies are required to investigate the optimal dose and treatment time point.178
In the largest current study, the safety of convalescent plasma was explored in 5000 hospitalized critically ill COVID-19 patients.179 They have reported less than 1% severe adverse effects and 14.9% mortality rate in seven days, which is consistent with the deadly nature of COVID-19. Therefore, they have suggested that transfusion of convalescent plasma is harmless in hospitalized COVID-19 cases.179 Another recent study reported the outcomes of convalescent plasma transfusion in patients with severe COVID-19 infection. They have reported its useful therapeutic effects, especially in nonintubated patients.180 There are some limitations for using convalescent plasma therapy such as the difficulties in collection and possibility of contamination with infectious agents, transfusion-associated circulatory overload (TACO), adverse reactions to transfusion, and variation of neutralizing antibody responses.135
Vitamin D
Vitamin D is a fat-soluble vitamin required for regulation of calcium and phosphate levels.181 SARS-CoV-2 uses ACE2 as a functional receptor for entry into target cells.182 In addition, downmodulation of ACE2 in the pulmonary tissues due to the infection with SARS-CoV-2 leads to severe acute respiratory failure.183 Downmodulation of alveolar ACE2 result in decreased angiotensin II level which causes its increased level and increased alveolar permeability and lung injury.184 Vitamin D3 is able to increase the ACE2 expression in several tissues including lung microvascular endothelial cells.185,186
Elevated number of cell-bound ACE2s may function as a double-edged sword. On the one hand an increased number of ACE2 means an increased number of receptors for SARS-Cov-2 to enter the host cell. On the other hand these excessive ACE2s can competitively bind with the virus and neutralize it. Moreover, excessive ACE2 can protect the lung from injury through negative regulation of the RAS.187,188 However, vitamin D3 also plays an important role in innate and adaptive immune responses.184 It has immunomodulatory effects such as downregulation of pro-inflammatory cytokines.189–192 It has an important role in inhibiting the cytokine storm.193 Vitamin D deficiency has been correlated with greater susceptibility to viral infections.194 Therefore, it has been hypothesized that supplementation with vitamin D may reduce the severity of COVID-19 infection.195 It has been recognized that a number of immune cells express the vitamin D receptor (VDR). Several immune cells including macrophages, monocytes, dendritic cells, T cells, and B cells can convert 25-hydroxyvitamin D into its biologically active form 1,25-dihydroxyvitamin D (1,25-(OH)2D). Binding of 1,25(OH)2D to VDR promotes the expression of numerous genes, including cytokine-related genes.196
Both pro-inflammatory and anti-inflammatory cytokines are produced by innate immune system in COVID-19 cases.11 The production of Th1 cytokines including IFN-γ and TNF-α can be inhibited by vitamin D.197 Furthermore, vitamin D supplementation can lead to decreased expression of macrophage mediated pro-inflammatory cytokines and elevated anti-inflammatory cytokines.198 The protective effect of sufficient vitamin D levels against RSV and influenza infections has been indicated previously.199 Decrease of serum 25-hydroxy levels with age200 may be attributed to higher case fatality rates (CFRs) in older COVID-19 patients.201 In addition, vitamin D deficiency is more frequent in countries with high geographic latitudes.195 Lack of memory B cells and atypical innate immune response can result in over activation of adaptive immune system in older individuals, which is followed by cytokine storm as seen in COVID-19 cases.48 A recent study reported the highest age-specific CFR due to COVID-19 in Spain, France, and Italy where vitamin D deficiency is more frequent than other countries (mean 25OHD concentration <0.25 ng/L).202 Therefore, supplementation with vitamin D may play a role in prevention of infection as well as improve the disease outcomes. Further studies are required to explore the whether 25-hydroxyvitamin D (25(OH)D) levels are associated with prevention and treatment of COVID-19. There are several clinical trials registered in clincaltrial.gov that are investigating whether vitamin D levels affect outcomes in COVID-19 infection and whether vitamin D deficiency is associated with increased risk of COVID-19 infection (for instance: NCT04394390, NCT04411446, NCT04385940, NCT04334005, NCT04386044, NCT04363840).
Vaccination
Vaccination may offer the best choice for COVID-19 control. Epitopes, mRNA, and S protein-RBD structure-based vaccines have been broadly suggested.203,204 Precise recognition of virus surface proteins and the host receptors is essential for understanding of cross-species transmission as well as for the establishment of animal models for vaccine development. Since the S protein is the major inducer of neutralizing antibodies, most of the vaccines being developed for coronaviruses target this protein. Various vaccines have been evaluated based on S protein including full-length and recombinant S protein vaccines, viral vector-based vaccine, DNA-based vaccine, and RBD protein-based vaccines.205,206
There are several challenges in developing an effective vaccine for coronavirus infections including: lack of appropriate animal models that resemble human infection, clinical trials in humans are expensive to perform.207 Moreover, due to the great diversity in antigenic variants, antibody-mediated protective immunity against coronaviruses does not persist for very long. Human ACE2 transgenic mouse and rhesus monkey models have been used for vaccine development,206 and some clinical trials are in progress for SARS-CoV-2 vaccines.208,209
Whole Virus Vaccines
Classic methods for viral vaccinations involve taking a live-attenuated or inactive whole virus. Whole virus vaccines have been shown to be inherently immunogenic and also have ability of toll-like receptors (TLRs) stimulation. A live influenza vaccine that expresses SARS-CoV-2 proteins has been developed by researchers at the University of Hong Kong.210 Moreover, Codagenix Inc. has established a “codon deoptimization” technology to attenuate viruses and is currently exploring the SARS-CoV-2 vaccine strategies.211
Recombinant Subunit Vaccine
Generally, subunit-based vaccines are safer with fewer side effects than the other types of vaccines. They stimulate the immune system without introducing infectious viruses. Studies have reported increased T cell immune responses and production of high titer neutralizing antibodies in vivo due to subunit vaccines.212,213 The S protein contains three receptor-binding S1 heads and a trimeric S2 stalk. A recombinant subunit vaccine-based on the trimeric S protein is under preclinical test.204 Recently, The University of Queensland is developing subunit-based vaccines using the “molecular clamp”. A molecular clamp is a polypeptide used to stabilize the shape of proteins in experimental vaccines to improve recognition of the correct antigen, resulting stronger immune responses.214,215 The RBD of S protein plays a key role in interaction with the ACE2 as well as in the induction of neutralizing antibody against the virus. Therefore, RBD is being used in development of SARS-CoV-2 subunit vaccines.216
DNA Vaccines
DNA vaccines as the third generation vaccines represent a novel approach. They contain DNA encoding particular antigens from a pathogen. The DNA is directly injected into the body and induces adaptive immune responses.217 Currently, different methods such as electroporation as a vaccine delivery system and addition of adjuvant to enhance the immune responses have been established to improve the efficiency of vaccines.218 Inovio Pharmaceuticals has announced its collaboration with Beijing Advaccine Biotechnology to start a preclinical trials for COVID-19 DNA Vaccine INO-4800 development.219
mRNA Vaccines
During the last two decades, mRNA vaccines have been developed for prophylactic and therapeutic purposes. Preclinical and clinical trials have revealed that mRNA-based vaccines elicit a safe and long-lasting immune response both in animal models and in humans. mRNA-based vaccines consists of mRNAs encoding a disease-specific antigen being translated by the host machinery. These types of vaccines are unable to integrate into the host genome and they can also produce multimeric antigens. Therefore, mRNA vaccines are superior to conventional vaccines in terms of safety and immune responses.220,221 A phase 1 clinical trial has been started for mRNA-1273, an mRNA vaccine, encoding a stable form of the SARS-CoV-2 protein of spike protein. In silico design of vaccine immunogens would allow its rapid production and efficacy evaluation.211
Other Vaccine Approaches
A COVID-19 vaccine is being developed using Hyleukin-7 platform technology (IL-7 fused to hyFc) to enhance the immune responses. Hyleukin-7 is designed to hybridize IgD and IgG4 to generate longer-lasting and more effective Fc fusion proteins. Improved vaccine efficacy in influenza A virus infection model has been reported by Genexine Inc. Fc-fused IL-7 has resulted the accumulation of pulmonary T cells as well as increased plasmacytoid dendritic cells.222
Bacillus Calmette–Guérin (BCG) Vaccine
In spite of global strict containment measures to combat the COVID-19 pandemic, increasing number of cases continue, especially in the US and Europe.223 There is a great variance in fatality rates across the world.224 For instance, studies from East Asian countries have reported lower incidence and case fatality rate due to COVID-19 infection.225
Mycobacterium bovis BCG vaccine is one of the most widely used vaccines worldwide. The main use of this vaccine is for vaccination against disseminated forms of tuberculosis in children. After introduction of BCG, studies revealed the decreased mortality independent of tuberculosis among vaccinated children.226 Some clinical evidence supports a role of BCG vaccination in nontargeted protective effects against viral infections. Several studies have demonstrated the effect of BCG vaccination on the reduction of acute lower respiratory tract infections.227,228 A study in Guinea-Bissau showed that BCG reduced the incidence of respiratory syncytial virus infection, which may support this hypothesis.229 Moreover, ecological studies have reported lower mortality due to COVID-19 infection in countries with mandatory BCG vaccination policies than other countries.230–232 However, there are several biases that should be considered. For instance, genetic diversity and polymorphism in population structure across regions, dissimilarities in detection and reports of infected cases, and different intervention policies used in different locations.223 Due to the controversial reports about the protective role of BCG vaccination against COVID-19 infection, a recent epidemiological study was conducted on a global scale using linear and nonlinear statistical methods. This study suggested a significant association between COVID-19 distribution and mortality rate with BCG vaccination.224 Furthermore, in another recent study used linear mixed models analysis indicated that mandated BCG vaccination policies significantly affect the mortality rate of COVID-19 infection.233 The association between BCG immunization and decreased spread and severity of the COVID-19 pandemic has also been reported in some other studies.234–236
Moreover, effectiveness of BCG as nonspecific immunotherapy against virus-mediated clinical conditions has been previously indicated.237 Two recent studies have suggested an association between the BCG vaccination and reduced morbidity and mortality due to COVID-19.232,234 Moreover, an unpublished study compared COVID-19 fatality rates between countries with high disease burden and those with BCG revaccination policies. They have found significant differences in the COVID-19 fatality rates between the two groups of countries. The finding supports the possible protective role of BCG vaccination against COVID-19.234 Based on promising research it has been concluded that the BCG vaccination has beneficial off-target effects. However, there are some controversial studies about the effectiveness of BCG vaccination in induction of heterologous immunity. A randomized clinical multicenter trial reported that BCG vaccination has no effect on hospitalization rate of Danish children up to the age of 15 months.238 Furthermore, it appears that protective effect of BCG vaccine disappears with time. A previous study established no cross protection between BCG and the SARS-CoV.239,240
There are some potential confounders that may affect interpretation of correlation between BCG vaccination and COVID-19 related mortality.230–232 These include the possible underreporting as well as lower competence for SARS-COV-2 testing in low income countries.230–232 Therefore, randomized controlled trials are required to investigate whether BCG vaccination can induce trained immunity and protect against COVID-19. It is rational to suggest that these should first be initiated in high risk populations including HCWs as well as older individuals. Currently, trials have just begun in some countries such as the Netherlands, Australia, Greece, Britain, Germany, Denmark, and the US to investigate its potential protective effect against SARS-CoV-2 infection.223 There are some ongoing trials to explore the possible effects of vaccination in hospital staff.241
However, strategies for immunity induction for protection purposes against SARS-CoV-2 infection may not be limited to BCG. For instance, VPM1002 vaccine (Vakzine Projekt Management) which is a novel genetically modified BCG vaccine and is being considered for clinical trials to assess its effectiveness against SARS-CoV-2 infections223 (Table 2).
Table 2.
The Clinical Trials to Evaluate Whether Vaccination has any Effect on Prevention and/or Clinical Progression of COVID-19 Infection
| Identifier Number | Aim/Study Population | Phase | Sponser/Country | Intervention/Treatment |
|---|---|---|---|---|
| NCT04435379 | To assess whether vaccination of elderly with VPM1002 could decrease hospital admissions and/or severe respiratory infectious diseases | Phase 3 | Vakzine Projekt Management GmbH/Germany | Biological: VPM1002 Biological: placebo |
| NCT04387409 | To assess whether vaccination of health-care professionals with VPM1002 could decrease the number of days absent from work due to respiratory disease | Phase 3 | Vakzine Projekt Management GmbH/Germany | Biological: VPM1002 Biological: placebo |
| NCT04348370 | To assess the efficacy of BCG vaccine for HCWs | Phase 4 | Texas A&M University/United States | Biological: BCG vaccine Biological: placebo |
| NCT04373291 | To assess protective effect BCG vaccine in HCWs against COVID-19 | Phase 3 | Bandim Health Project | Biological: BCG-Denmark Biological: saline |
| NCT04384549 | To assess efficacy of BCG vaccine in the prevention of COVID-19 in HCWs | Phase 3 | Assistance Publique - Hôpitaux de Paris/France | Biological: BCG vaccine Biological: placebo |
| NCT04414267 | To validate whether vaccination of individuals susceptible to COVID-19 with BCG vaccine can reduce their disease susceptibility (using both clinical and immunological criteria) | Phase 4 | Hellenic Institute for the Study of Sepsis/Greece | Biological: BCG vaccine Biological: placebo |
| NCT04369794 | To assess the effect of former (the titer of anti-BCG IFN-γ) or recent BCG exposure (boost with intradermal vaccine) on clinical progression of disease | Phase 4 | University of Campinas/Brazil | Biological: BCG vaccine Biological: placebo |
| NCT04327206 | To assess the effect BCG vaccination in protection of HCWs following against COVID-19 | Phase 3 | Murdoch Childrens Research Institute/Australia | Biological: BCG vaccine Drug: placebo 0.9% NaCl |
| NCT04347876 | To assess whether previous BCG vaccination may modify the prognosis in COVID-19 positive cases | Observational | Assiut University/Egypt | Diagnostic test: tuberculin test |
| NCT04417335 | To assess the whether BCG vaccine can decrease hospital admission and improve the clinical course of COVID-19 infection in elderly individuals (≥60 years old). | Phase 4 | Radboud University/Netherlands | Biological: BCG vaccine Biological: placebo |
| NCT04350931 | To assess whether previous BCG vaccination can be used as immune-prophylaxis among Egyptian HCWs against COVID-19 infection | Phase 3 | Ain Shams University/Egypt | Biological: intradermal injection of BCG vaccine Other: placebo |
| NCT04379336 | To assess whether BCG vaccine can decrease morbidity and mortality in HCWs exposed to SARS-CoV-2 | Phase 3 | TASK Applied Science/South Africa | Biological: BCG Other: placebo |
| NCT04362124 | To assess if BCG vaccination in HCWs can decrease the severity of COVID-19 Infection | Phase 3 | Universidad de Antioquia/Colombia | Biological: BCG vaccine Biological: placebo |
| NCT04328441 | To reduce absenteeism among HCWs with direct patient contacts during the epidemic phase of COVID-19. Secondary objective: to reduce hospital admission, ICU admission or death in HCW with direct patient contacts during the epidemic phase of COVID-19. | Phase 3 | UMC Utrecht/Netherlands | Biological: BCG vaccine Biological: placebo |
Abbreviations: HCWs, health-care workers; BCG, Bacillus Calmette–Guérin.
A previous study has reviewed the molecular mechanism of nonspecific effects of BCG vaccine against viral infections.242 The epigenetic reprogramming of monocytes known as trained immunity is recommended as a potential reason for nonspecific effects of BCG. In a previous study, autophagy was recognized as a key component of trained immunity.243
Conclusion
The knowledge about this novel virus is still limited and the treatment options or vaccination are under assessment. SARS‐CoV and MERS-CoV outbreaks have provided comprehensive insights into how to fight the SARS-CoV-2 pandemic. As of now, no vaccine or antiviral drug is available for COVID-19. What we can do currently is take measures to reduce the spread of COVID-19. Based on former experience in management of MERS and SARS infections, the WHO recommends infection control interventions to decrease the risk of transmission of infections. Implementation of control measures should be directed at limiting the possible sources of infection to prevent the risk of disease transmission in the absence of effective treatments. In addition, the asymptomatic spreaders also need to be focused.
Acknowledgments
This research has been supported by Tehran University of Medical Sciences, Tehran, Iran.
Abbreviations
ARDS, acute respiratory distress syndrome; ACE2, angiotensin-converting enzyme 2; ACEI, angiotensin-converting enzyme inhibitors, ARB, angiotensin receptor blockers; BCG, Bacillus Calmette–Guérin; CFRs, case fatality rates; COVID-19, coronavirus disease COVID-19; CLPro; 3CLp, chymotrypsin-like protease; CRP, C-reactive protein; cAMP, cyclic adenosine monophosphate; CRS, cytokine release syndrome; FGF, fibroblast growth factors; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; GGO, ground glass opacity; HCWs, healthcare workers; HCV, hepatitis C virus; HMGB1, high-mobility group box 1; IFNγ, interferon gamma; JAK, Janus kinase; JAK-STAT, Janus kinase-signal transducer and activator of transcription; MIP, macrophage inflammatory proteins; MERS, Middle East respiratory syndrome; MCP-1, monocyte chemoattractant protein-1; NK, natural killer cells; NSAID, non-steroidal anti-inflammatory drugs; PLpro, papain-like protease; PDE4, phosphodiesterase 4; PDGF, platelet-derived growth factor; PIs, protease Inhibitors; RBD, receptor-binding domain; RAS, renin-angiotensin system; RSV, respiratory syncytial virus; RdRp, RNA-dependent RNA polymerase; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; The Food and Drug Administration; TLRs, toll-like receptors; TACO, transfusion-associated circulatory overload; TNF-α, tumor necrosis factor alpha.
Disclosure
The author declares no conflicts of interest.
References
- 1.Sun PLX, Xu C, Sun W, Pan B, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92(6):548–551. doi: 10.1002/jmv.25722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qun LMM, Xuhua G, Peng W, Xiaoye Wang MPH. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. MedRxiv. 2020. [Google Scholar]
- 6.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80(6):e14–e18. doi: 10.1016/j.jinf.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickbakhsh S, Mair C, Matthews L, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A. 2019;116(52):27142–27150. doi: 10.1073/pnas.1911083116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlen M, Karlsson MJ, Zhong W, et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science. 2019;366(6472):6472. doi: 10.1126/science.aax9198 [DOI] [PubMed] [Google Scholar]
- 16.Bhadra S, Jiang YS, Kumar MR, Johnson RF, Hensley LE, Ellington AD. Real-time sequence-validated loop-mediated isothermal amplification assays for detection of Middle East respiratory syndrome coronavirus (MERS-CoV). PloS One. 2015;10(4):e0123126. doi: 10.1371/journal.pone.0123126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JF, Choi GK, Tsang AK, et al. Development and evaluation of novel real-time reverse transcription-PCR assays with locked nucleic acid probes targeting leader sequences of human-pathogenic coronaviruses. J Clin Microbiol. 2015;53(8):2722–2726. doi: 10.1128/JCM.01224-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang P, Wang H, Cao Z, et al. A Rapid and Specific Assay for the Detection of MERS-CoV. Front Microbiol. 2018;9:1101. doi: 10.3389/fmicb.2018.01101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Baek YH, Kim YH, Choi YK, Song MS, Ahn JY. One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for Detecting MERS-CoV. Front Microbiol. 2016;7:2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myhrvold C, Freije CA, Gootenberg JS, et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science. 2018;360(6387):444–448. doi: 10.1126/science.aas8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020;1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanne JP. Chest CT Findings in 2019 Novel Coronavirus (2019-Ncov) Infections from Wuhan, China: Key Points for the Radiologist. Radiological Society of North America; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng S-Q, Peng H-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9(2):575. doi: 10.3390/jcm9020575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zheng X, Tong Q, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobaiqy M, Qashqary M, Al-Dahery S, et al. Therapeutic management of patients with COVID-19: a systematic review. Infect Prevent Pract. 2020;2(3):100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai CC, Wang CY, Hsueh PR. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020. doi: 10.1016/j.jmii.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai -C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020 [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Cao R, Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019 nCoV) in vitro [published online February 4, 2020]. Cell Res. 2020;30;269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Savi C, Hughes DL, Kvaerno L. Quest for a COVID-19 Cure by repurposing small-molecule drugs: mechanism of action, clinical development, synthesis at scale, and outlook for supply. Org Process Res Dev. 2020;24(6):940–976. doi: 10.1021/acs.oprd.0c00233 [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Zhou Q, Li Y, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Central Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandra S, Bart P, Vasilios P, Christos P, Mihael P. Direct ACE2- Spike RBD Binding Disruption with Small Molecules: A Strategy for COVID-19 Treatment. 2020. [Google Scholar]
- 36.Sachin P, Jeremy H, Pedro JB, et al. Drug repurposing for covid-19: discovery of potential small-molecule inhibitors of spike Protein-ACE2 receptor interaction through virtual screening and consensus scoring. 2020.
- 37.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e907. doi: 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leneva IA, Russell RJ, Boriskin YS, Hay AJ. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 39.Blaising J, Lévy PL, Polyak SJ, Stanifer M, Boulant S, Pécheur EI. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antiviral Res. 2013;100(1):215–219. doi: 10.1016/j.antiviral.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 40.Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. [Google Scholar]
- 45.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen W, Su W, Tang H, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discovery. 2020;6(1):31. doi: 10.1038/s41421-020-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translat Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingraham NE, Lotfi-Emran S, Thielen BK, et al. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8(6):544–546. doi: 10.1016/S2213-2600(20)30226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quartuccio L, Sonaglia A, McGonagle D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. e325-e331. doi: 10.1016/S2665-9913(20)30127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safavi F, Nath A. Silencing of immune activation with methotrexate in patients with COVID-19. J Neurol Sci. 2020;116942. doi: 10.1016/j.jns.2020.116942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haberman R, Axelrad J, Chen A, et al. Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med. 2020;383(1):85–88. doi: 10.1056/NEJMc2009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT Signaling to Control Cytokine Release Syndrome in COVID-19. Trends Pharmacol Sci. 2020. doi: 10.1016/j.tips.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–862. doi: 10.1038/nrd.2017.201 [DOI] [PubMed] [Google Scholar]
- 60.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- 62.Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. 2008;77(2):150–152. doi: 10.1016/j.antiviral.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012 [DOI] [PubMed] [Google Scholar]
- 67.Gautret P, Lagier JC, Parola P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents;2020. 105949. doi: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Page CP. Phosphodiesterase inhibitors for the treatment of asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2014;165(3):152–164. doi: 10.1159/000368800 [DOI] [PubMed] [Google Scholar]
- 70.Raker VK, Becker C, Steinbrink K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front Immunol. 2016;7:123. doi: 10.3389/fimmu.2016.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azevedo MF, Faucz FR, Bimpaki E, et al. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev. 2014;35(2):195–233. doi: 10.1210/er.2013-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H, Zuo J, Tang W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front Pharmacol. 2018;9:1048. doi: 10.3389/fphar.2018.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28(7):753–763. doi: 10.1016/j.cellsig.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 74.Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi: 10.1016/j.bcp.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 75.Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–855. doi: 10.1111/j.1476-5381.2009.00559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crowe CR, Chen K, Pociask DA, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Megna M, Napolitano M, Fabbrocini G. May IL-17 have a role in COVID-19 infection? Med Hypotheses. 2020;140:109749. doi: 10.1016/j.mehy.2020.109749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Méhats C, Franco-Montoya ML, Boucherat O, et al. Effects of phosphodiesterase 4 inhibition on alveolarization and hyperoxia toxicity in newborn rats. PloS One. 2008;3(10):e3445. doi: 10.1371/journal.pone.0003445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sisson TH, Christensen PJ, Muraki Y, et al. Phosphodiesterase 4 inhibition reduces lung fibrosis following targeted type II alveolar epithelial cell injury. Physiol Rep. 2018;6(12):e13753. doi: 10.14814/phy2.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dalamaga M, Karampela I, Mantzoros CS. Commentary: phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism. 2020;109:154282. doi: 10.1016/j.metabol.2020.154282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mugheddu C, Pizzatti L, Sanna S, Atzori L, Rongioletti F. CID-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: safety and compatibility of apremilast with critical intensive care management. J Eur Acad Dermatol Venereol. 2020. doi: 10.1111/jdv.16625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olisova OY, Anpilogova EM, Svistunova DA. Apremilast as a potential treatment option for COVID-19: no symptoms of infection in a psoriatic patient. Dermatol Ther. 2020;e13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frohman EM, Villemarette-Pittman NR, Cruz RA, et al. Part II. High-dose methotrexate with leucovorin rescue for severe COVID-19: an immune stabilization strategy for SARS-CoV-2 induced ‘PANIC’ attack. J Neurol Sci. 2020;116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cribbs AP, Kennedy A, Penn H, et al. Methotrexate Restores Regulatory T Cell Function Through Demethylation of the FoxP3 Upstream Enhancer in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(5):1182–1192. doi: 10.1002/art.39031 [DOI] [PubMed] [Google Scholar]
- 85.Kuroiwa Y, Takakusagi Y, Kusayanagi T, et al. Identification and characterization of the direct interaction between methotrexate (MTX) and high-mobility group box 1 (HMGB1) protein. PloS One. 2013;8(5):e63073. doi: 10.1371/journal.pone.0063073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology. 2003;42(10):1189–1196. doi: 10.1093/rheumatology/keg323 [DOI] [PubMed] [Google Scholar]
- 87.Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-Converting Enzyme Inhibitors in Hypertension: to Use or Not to Use? J Am Coll Cardiol. 2018;71(13):1474–1482. doi: 10.1016/j.jacc.2018.01.058 [DOI] [PubMed] [Google Scholar]
- 89.Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus Disease 2019 (COVID-19): do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? J Am Heart Assoc. 2020;9(7):e016509. doi: 10.1161/JAHA.120.016509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffmann M, Kleine-Weber H, Krüger N, Mueller MA, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Biorxiv. 2020. [Google Scholar]
- 91.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- 92.Sommerstein R, Gräni C, Cullen MR, Basu S. Preventing a COVID-19 pandemic: ACE inhibitors as a potential risk factor for fatal COVID-19. BMJ. 2020;368:m810.32111649 [Google Scholar]
- 93.Phadke M, Saunik S. COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev Res. 2020. doi: 10.1002/ddr.21666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phadke M, Saunik S. Rapid response: use of angiotensin receptor blockers such as Telmisartan, Losartsan in nCoV Wuhan Corona Virus infections—Novel mode of treatment. Response to the emerging novel coronavirus outbreak. BMJ. 2020;368:m406. doi: 10.1136/bmj.l6968 [DOI] [PubMed] [Google Scholar]
- 95.Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020;9:72. doi: 10.12688/f1000research.22211.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao H, Dai W, Zhao L, Min J, Wang F. The Role of Zinc and Zinc Homeostasis in Macrophage Function. J Immunol Res. 2018;2018:6872621. doi: 10.1155/2018/6872621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 Testing, Hospital Admission, and Intensive Care Among 2,026,227 United States Veterans Aged 54–75 Years. medRxiv. 2020. [Google Scholar]
- 98.Bean D, Kraljevic Z, Searle T, et al. ACE-inhibitors and Angiotensin-2 Receptor Blockers are not associated with severe SARS- COVID19 infection in a multi-site UK acute Hospital Trust. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng Y, Ling Y, Bai T, et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y, Huang F, Xu J, et al. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020. [Google Scholar]
- 101.Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia, Italy. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leyssen P, Balzarini J, De Clercq E, Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol. 2005;79(3):1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Clercq E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem Asian J. 2019;14(22):3962–3968. doi: 10.1002/asia.201900841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study. Engineering. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahn DG, Shin HJ, Kim MH, et al. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J Jr. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326(4):905–908. doi: 10.1016/j.bbrc.2004.11.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int j Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):396. doi: 10.1126/scitranslmed.aal3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Agostini ML, Andres EL, Sims AC, et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2):2. doi: 10.1128/mBio.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin Y, Yang H, Ji W, et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12(4):4. doi: 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med j. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 117.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther. 2016;21(5):455–459. doi: 10.3851/IMP3002 [DOI] [PubMed] [Google Scholar]
- 119.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamamoto N, Yang R, Yoshinaka Y, et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318(3):719–725. doi: 10.1016/j.bbrc.2004.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamamoto N, Matsuyama S, Hoshino T, Yamamoto N. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. bioRxiv. 2020. [Google Scholar]
- 122.Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meng Z, Wang T, Li C, et al. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 124.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604 [DOI] [PubMed] [Google Scholar]
- 125.Scagnolari C, Vicenzi E, Bellomi F, et al. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9(6):1003–1011. [PubMed] [Google Scholar]
- 126.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet. 2003;362(9380):293–294. doi: 10.1016/S0140-6736(03)13973-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b Treatment for COVID-19. Front Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Health O. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-Ncov) Infection is Suspected: Interim Guidance, 28 January 2020. Geneva: World Health Organization; 2020:2020. [Google Scholar]
- 129.Nodarse-Cuní HL-SP. Cuban interferon alpha-2b. Thirty years as an effective and safe drug. Biotecnol Apl. 2020;34(1):1211–1217. [Google Scholar]
- 130.Pereda R, Gonzalez D, Rivero H, et al. Therapeutic effectiveness of interferon-alpha2b against COVID-19: the Cuban experience. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 131.Reiter RJ, Ma Q, Sharma R. Treatment of Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 2020;3(1):43–57. doi: 10.32794/mr11250047 [DOI] [Google Scholar]
- 132.Zhang R, Wang X, Ni L, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zarezadeh M, Khorshidi M, Emami M, et al. Melatonin supplementation and pro-inflammatory mediators: a systematic review and meta-analysis of clinical trials. Eur J Nutr. 2019. doi: 10.1007/s00394-019-02123-0 [DOI] [PubMed] [Google Scholar]
- 134.Zhang R, Wang X, Ni L, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marovich M, Mascola JR, Cohen MS. Monoclonal Antibodies for Prevention and Treatment of COVID-19. Jama. 2020. doi: 10.1001/jama.2020.10245 [DOI] [PubMed] [Google Scholar]
- 136.Prabakaran P, Zhu Z, Xiao X, et al. Potent human monoclonal antibodies against SARS CoV, Nipah and Hendra viruses. Expert Opin Biol Ther. 2009;9(3):355–368. doi: 10.1517/14712590902763755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sui J, Li W, Murakami A, et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci U S A. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cutino-Moguel MT, Eades C, Rezvani K, Armstrong-James D. Immunotherapy for infectious diseases in haematological immunocompromise. Br J Haematol. 2017;177(3):348–356. doi: 10.1111/bjh.14595 [DOI] [PubMed] [Google Scholar]
- 139.van den Brink EN, Ter Meulen J, Cox F, et al. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J Virol. 2005;79(3):1635–1644. doi: 10.1128/JVI.79.3.1635-1644.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279(5):3197–3201. doi: 10.1074/jbc.C300520200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ju B, Zhang Q, Ge X, et al. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 142.Pinto D, Park YJ, Beltramello M, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- 143.Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):2251. doi: 10.1038/s41467-020-16256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274–1278. doi: 10.1126/science.abc2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamilton JA. GM-CSF-Dependent Inflammatory Pathways. Front Immunol. 2019;10:2055. doi: 10.3389/fimmu.2019.02055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23(8):403–408. doi: 10.1016/S1471-4906(02)02260-3 [DOI] [PubMed] [Google Scholar]
- 147.De Luca G, Cavalli G, Campochiaro C, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020. doi: 10.1016/S2665-9913(20)30170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Temesgen Z, Assi M, Vergidis P, et al. First Clinical Use of Lenzilumab to Neutralize GM-CSF in Patients with Severe and Critical COVID-19 Pneumonia. medRxiv. 2020. [Google Scholar]
- 149.Megan M, Jared N, Jacquelyn H, et al. Case Report: use of Lenzilumab and Tocilizumab for the Treatment of Coronavirus Disease 2019. Immunotherapy;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Picchianti Diamanti A, Rosado MM, Pioli C, Sesti G, Laganà B. Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: the Fragile Balance between Infections and Autoimmunity. Int J Mol Sci. 2020;21(9):3330. doi: 10.3390/ijms21093330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/j.immuni.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 153.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17(6):395–412. doi: 10.1038/nrd.2018.45 [DOI] [PubMed] [Google Scholar]
- 154.Kato S, Kurzrock R. Repurposing Interleukin-6 Inhibitors to Combat COVID-19. J Immunother Precision Oncol. 2020;3(2):52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2010;2(5):247–256. doi: 10.1177/1759720X10378372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van Rhee F, Casper C, Voorhees PM, et al. Long-term safety of siltuximab in patients with idiopathic multicentric Castleman disease: a prespecified, open-label, extension analysis of two trials. Lancet Haematol. 2020;7(3):e209–e217. e209-e217. doi: 10.1016/S2352-3026(19)30257-1 [DOI] [PubMed] [Google Scholar]
- 157.Van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti–interleukin-6 monoclonal antibody, for Castleman’s disease. J clin oncol. 2010;28(23):3701–3708. doi: 10.1200/JCO.2009.27.2377 [DOI] [PubMed] [Google Scholar]
- 158.Ascierto P, Fox B, Urba W. SITC statement on anti-IL-6/IL-6R for COVID-19. Society Immunother Cancer. 2020. [Google Scholar]
- 159.Calabrese C, Rajendram P, Sacha G, Calabrese L. Practical aspects of targeting IL-6 in COVID-19 disease. Cleve Clin J Med. 2020. doi: 10.3949/ccjm.87a.ccc018 [DOI] [PubMed] [Google Scholar]
- 160.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: A single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18(1):1–5. doi: 10.1186/s12967-020-02339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kimmig LM, Wu D, Gold M, et al. IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8–27. doi: 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 Is Responsible for Acute Lung Immunopathology but Increases Survival of Respiratory Influenza Virus Infection. J Virol. 2005;79(10):6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. doi: 10.1182/blood.2018894618 [DOI] [PubMed] [Google Scholar]
- 167.van der Ven AJ, Netea MG, van der Meer JW, de Mast Q. Ebola Virus Disease has Features of Hemophagocytic Lymphohistiocytosis Syndrome. Fron Med. 2015;2:4. doi: 10.3389/fmed.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Filocamo G, Mangioni D, Tagliabue P, et al. Use of anakinra in severe COVID-19: a case report. Int j Infect Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. e393-e400. doi: 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184 [DOI] [PubMed] [Google Scholar]
- 171.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu P, Hao X, Lau EHY, et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveillance. 2020;25:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919–922. doi: 10.1093/jac/dki346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. SpringerPlus. 2015;4(1):709. doi: 10.1186/s40064-015-1490-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou B, Zhong N, Guan Y. Treatment with Convalescent Plasma for Influenza A (H5N1) Infection. N Eng J Med. 2007;357(14):1450–1451. doi: 10.1056/NEJMc070359 [DOI] [PubMed] [Google Scholar]
- 176.Tanne JH. Covid-19: FDA Approves Use of Convalescent Plasma to Treat Critically Ill Patients. British Medical Journal Publishing Group; 2020. [DOI] [PubMed] [Google Scholar]
- 177.Florescu DF, Kalil AC, Hewlett AL, et al. Administration of Brincidofovir and Convalescent Plasma in a Patient With Ebola Virus Disease. Clin Infect Dis. 2015;61(6):969–973. doi: 10.1093/cid/civ395 [DOI] [PubMed] [Google Scholar]
- 178.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Nat Acad Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Joyner M, Wright RS, Fairweather D, et al. Early Safety Indicators of COVID-19 Convalescent Plasma in 5,000 Patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu STH, Lin H-M, Baine I, et al. Convalescent plasma treatment of severe COVID-19: A matched control study. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 181.Pilz S, März W, Cashman KD, et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front Endocrinol (Lausanne). 2018;9:373. doi: 10.3389/fendo.2018.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jalil R, Behroz MP, Mohammad A. Vitamin D3 Administration to Patients with Confirmed COVID-19. Iran J Public Health. 2020;49(Supple):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lin M, Gao P, Zhao T, et al. Calcitriol regulates angiotensin-converting enzyme and angiotensin converting-enzyme 2 in diabetic kidney disease. Mol Biol Rep. 2016;43(5):397–406. doi: 10.1007/s11033-016-3971-5 [DOI] [PubMed] [Google Scholar]
- 186.Cui C, Xu P, Li G, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26:101295. doi: 10.1016/j.redox.2019.101295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu L, Yuan K, Phuong HT, Park BM, Kim SH. Angiotensin-(1–5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides. 2016;86:33–41. doi: 10.1016/j.peptides.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 189.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. doi: 10.3390/nu7064240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1):1. doi: 10.1002/rmv.1909 [DOI] [PubMed] [Google Scholar]
- 192.Arboleda JF, Fernandez GJ, Urcuqui-Inchima S. Vitamin D-mediated attenuation of miR-155 in human macrophages infected with dengue virus: implications for the cytokine response. Infect Genetics Evolution. 2019;69:12–21. doi: 10.1016/j.meegid.2018.12.033 [DOI] [PubMed] [Google Scholar]
- 193.Khare D, Godbole NM, Pawar SD, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52(4):1405–1415. doi: 10.1007/s00394-012-0449-7 [DOI] [PubMed] [Google Scholar]
- 194.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Razdan K, Singh K, Singh D. Vitamin D Levels and COVID-19 Susceptibility: is there any Correlation? Med Drug Discovery. 2020;7:100051. doi: 10.1016/j.medidd.2020.100051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Takeuti FAC, Guimaraes FSF, Guimaraes PSF. Applications of vitamin D in sepsis prevention. Discov Med. 2018;25(140):291–297. [PubMed] [Google Scholar]
- 197.Sharifi A, Vahedi H, Nedjat S, Rafiei H, Hosseinzadeh-Attar MJ. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS. 2019;127(10):681–687. doi: 10.1111/apm.12982 [DOI] [PubMed] [Google Scholar]
- 198.Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12(1):1. doi: 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Grant WB. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27(9):853. doi: 10.1097/INF.0b013e3181817bc1 [DOI] [PubMed] [Google Scholar]
- 200.Vásárhelyi B, Sátori A, Olajos F, Szabó A, Beko G. [Low vitamin D levels among patients at Semmelweis University: retrospective analysis during a one-year period]. Orv Hetil. 2011;152(32):1272–1277. doi: 10.1556/OH.2011.29187 [DOI] [PubMed] [Google Scholar]
- 201.Novel, C.P.E.R.E. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 202.Zhang, Y.P. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. medRxiv. 2020. [Google Scholar]
- 203.Abdelmageed MI, Abdelmoneim AH, Mustafa MI, et al. Design of multi epitope-based peptide vaccine against E protein of human 2019-nCoV: an immunoinformatics approach. Biorxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xie L, Sun C, Luo C, et al. SARS-CoV-2 and SARS-CoV spike-RBD structure and receptor binding comparison and potential implications on neutralizing antibody and vaccine development. Biorxiv. 2020. [Google Scholar]
- 205.Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. doi: 10.3390/v12020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.China MoNDotPsRo. The Military Successfully Developed a Recombinant SARS-CoV-2 Vaccine. 2020.
- 209.Trial AMVeC. 2020. Available online: https://www.modernatx.com/modernaswork-potential-vaccine-against-covid-19 (accessed on 23 March 2020).
- 210.China coronavirus: hong Kong researchers have already developed vaccine but need time to test it, expert reveals. South China Morning Post. https://wwwscmpcom/news/hong-kong/health-environment/article/3047956/china-coronavirus-hongkong-researchers-have Accessed 28February 2020 2020.
- 211.Chen W-H, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bisht H, Roberts A, Vogel L, Subbarao K, Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334(2):160–165. doi: 10.1016/j.virol.2005.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Okba NM, Raj VS, Haagmans BL. Middle East respiratory syndrome coronavirus vaccines: current status and novel approaches. Curr Opin Virol. 2017;23:49–58. doi: 10.1016/j.coviro.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mukherjee R. Global efforts on vaccines for COVID-19: since, sooner or later, we all will catch the coronavirus. J Biosci. 2020;45(1):1. doi: 10.1007/s12038-020-00040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Australia’s been asked to make a coronavirus vaccine at ‘unprecedented speed’. Science Alert 2020 https://wwwsciencealertcom/australian-scientists-asked-to-make-coronavirusvaccine-at-unprecedented-speed Accessed 28February 2020 2020.
- 216.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang Z-Y, Kong W-P, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23(3):421–429. doi: 10.1016/j.coi.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Inovio Accelerates Timeline for COVID-19 DNA Vaccine INO-4800 Available from http://irinoviocom/news-andmedia/news/press-release-details/2020/Inovio-Accelerates-imeline-for-COVID-19-DNA-Vaccine-INO-4800/defaultaspx Accessed 03March 2020 2020.
- 220.Li J, Zhang C, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261. doi: 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Seo YB, Im SJ, Namkoong H, et al. Crucial roles of interleukin-7 in the development of T follicular helper cells and in the induction of humoral immunity. J Virol. 2014;88(16):8998–9009. doi: 10.1128/JVI.00534-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.O’Neill LA, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–337. doi: 10.1038/s41577-020-0337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ventura L, Vitali M, Spica VR. BCG vaccination and socioeconomic variables vs Covid-19 global features: clearing up a controversial issue. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 225.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Moorlag S, Arts RJ, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. [DOI] [PubMed] [Google Scholar]
- 227.Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med. 2018;379(2):138–149. doi: 10.1056/NEJMoa1714021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wardhana DE, Mandang V, Mandang V, Jim E, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43(3):185–190. [PubMed] [Google Scholar]
- 229.Stensballe LG, Nante E, Jensen IP, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls: community based case–control study. Vaccine. 2005;23(10):1251–1257. doi: 10.1016/j.vaccine.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 230.Gursel M, Gursel I. Is global BCG vaccination coverage relevant to the progression of SARS-CoV-2 pandemic? Med Hypotheses. 2020;109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shet A, Ray D, Malavige N, Santosham M, Bar-Zeev N. Differential COVID-19-attributable mortality and BCG vaccine use in countries. MedRxiv. 2020. [Google Scholar]
- 232.Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020. [Google Scholar]
- 233.Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Medrxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dayal D, Gupta S. Connecting BCG Vaccination and COVID-19: additional Data. MedRxiv. 2020. [Google Scholar]
- 235.Klinger D, Blass I, Rappoport N, Linial M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: A multivariable analysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Green CM, Fanucchi S, Fok ET, et al. COVID-19: A model correlating BCG vaccination to protection from mortality implicates trained immunity. MedRxiv. 2020. [Google Scholar]
- 237.Salem A, Nofal A, Hosny D. Treatment of common and plane warts in children with topical viable bacillus Calmette‐Guerin. Pediatr Dermatol. 2013;30(1):60–63. doi: 10.1111/j.1525-1470.2012.01848.x [DOI] [PubMed] [Google Scholar]
- 238.Stensballe LG, Ravn H, Birk NM, et al. BCG Vaccination at birth and rate of hospitalization for infection until 15 months of age in danish children: a randomized clinical multicenter trial. J Pediatric Infect Dis Soc. 2019;8(3):213–220. doi: 10.1093/jpids/piy029 [DOI] [PubMed] [Google Scholar]
- 239.de Castro MJ, Pardo-Seco J, Martinón-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60(11):1611–1619. doi: 10.1093/cid/civ144 [DOI] [PubMed] [Google Scholar]
- 240.Yu Y, Jin H, Chen Z, et al. Children’s vaccines do not induce cross reactivity against SARS-CoV. J Clin Pathol. 2007;60(2):208–211. doi: 10.1136/jcp.2006.038893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mogica JAP, Nava V, Torres J. COVID-19 Related Mortality and The BCG Vaccine. medRxiv. 2020. [Google Scholar]
- 242.Moorlag S, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 243.Buffen K, Oosting M, Quintin J, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10(10):e1004485. doi: 10.1371/journal.ppat.1004485 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- China coronavirus: hong Kong researchers have already developed vaccine but need time to test it, expert reveals. South China Morning Post. https://wwwscmpcom/news/hong-kong/health-environment/article/3047956/china-coronavirus-hongkong-researchers-have Accessed 28February 2020 2020.
- Australia’s been asked to make a coronavirus vaccine at ‘unprecedented speed’. Science Alert 2020 https://wwwsciencealertcom/australian-scientists-asked-to-make-coronavirusvaccine-at-unprecedented-speed Accessed 28February 2020 2020.
- Inovio Accelerates Timeline for COVID-19 DNA Vaccine INO-4800 Available from http://irinoviocom/news-andmedia/news/press-release-details/2020/Inovio-Accelerates-imeline-for-COVID-19-DNA-Vaccine-INO-4800/defaultaspx Accessed 03March 2020 2020.