Abstract
Background
Products sweetened with non‐nutritive sweeteners (NNS) are widely available. Many people with type 1 or type 2 diabetes use NNS as a replacement for nutritive sweeteners to control their carbohydrate and energy intake. Health outcomes associated with NNS use in diabetes are unknown.
Objectives
To assess the effects of non‐nutritive sweeteners in people with diabetes mellitus.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Scopus, the WHO ICTRP, and ClinicalTrials.gov. The date of the last search of all databases (except for Scopus) was May 2019. We last searched Scopus in January 2019. We did not apply any language restrictions.
Selection criteria
We included randomised controlled trials (RCTs) with a duration of four weeks or more comparing any type of NNS with usual diet, no intervention, placebo, water, a different NNS, or a nutritive sweetener in individuals with type 1 or type 2 diabetes. Trials with concomitant behaviour‐changing interventions, such as diet, exercise, or both, were eligible for inclusion, given that the concomitant interventions were the same in the intervention and comparator groups.
Data collection and analysis
Two review authors independently screened abstracts, full texts, and records retrieved from trials registries, assessed the certainty of the evidence, and extracted data. We used a random‐effects model to perform meta‐analysis, and calculated effect estimates as risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, using 95% confidence intervals (CIs). We assessed risk of bias using the Cochrane 'Risk of bias' tool and the certainty of evidence using the GRADE approach.
Main results
We included nine RCTs that randomised a total of 979 people with type 1 or type 2 diabetes. The intervention duration ranged from 4 to 10 months. We judged none of these trials as at low risk of bias for all ’Risk of bias’ domains; most of the included trials did not report the method of randomisation.
Three trials compared the effects of a dietary supplement containing NNS with sugar: glycosylated haemoglobin A1c (HbA1c) was 0.4% higher in the NNS group (95% CI −0.5 to 1.2; P = 0.44; 3 trials; 72 participants; very low‐certainty evidence). The MD in weight change was −0.1 kg (95% CI −2.7 to 2.6; P = 0.96; 3 trials; 72 participants; very low‐certainty evidence). None of the trials with sugar as comparator reported on adverse events.
Five trials compared NNS with placebo. The MD for HbA1c was 0%, 95% CI −0.1 to 0.1; P = 0.99; 4 trials; 360 participants; very low‐certainty evidence. The 95% prediction interval ranged between −0.3% and 0.3%. The comparison of NNS versus placebo showed a MD in body weight of −0.2 kg, 95% CI −1 to 0.6; P = 0.64; 2 trials; 184 participants; very low‐certainty evidence. Three trials reported the numbers of participants experiencing at least one non‐serious adverse event: 36/113 participants (31.9%) in the NNS group versus 42/118 participants (35.6%) in the placebo group (RR 0.78, 95% CI 0.39 to 1.56; P = 0.48; 3 trials; 231 participants; very low‐certainty evidence).
One trial compared NNS with a nutritive low‐calorie sweetener (tagatose). HbA1c was 0.3% higher in the NNS group (95% CI 0.1 to 0.4; P = 0.01; 1 trial; 354 participants; very low‐certainty evidence). This trial did not report body weight data and adverse events.
The included trials did not report data on health‐related quality of life, diabetes complications, all‐cause mortality, or socioeconomic effects.
Authors' conclusions
There is inconclusive evidence of very low certainty regarding the effects of NNS consumption compared with either sugar, placebo, or nutritive low‐calorie sweetener consumption on clinically relevant benefit or harm for HbA1c, body weight, and adverse events in people with type 1 or type 2 diabetes. Data on health‐related quality of life, diabetes complications, all‐cause mortality, and socioeconomic effects are lacking.
Plain language summary
Non‐nutritive sweeteners for diabetes mellitus
Review question
Are non‐nutritive sweeteners beneficial or harmful in people with diabetes?
Background
Non‐nutritive sweeteners are sweetening agents having higher sweetening intensity and lower calorie content per gram compared to caloric sweeteners like sucrose or corn syrups. Both the general population and diabetic people use non‐nutritive sweeteners as a caloric sweetener replacement to control their carbohydrate and energy intake. Most of the non‐nutritive sweeteners approved for human consumption are synthetic (artificial sweeteners); however, increasing numbers of natural non‐caloric sweeteners are becoming available for human consumption. Products sweetened with non‐nutritive sweeteners are widely available on the market: diet beverages, diet yoghourts, desserts, and chewing gums are the most common products containing non‐nutritive sweeteners. Non‐nutritive sweeteners are also available as table‐top sweeteners for use by consumers at home as a sweetening agent for beverages and for cooking and baking.
There is very little information about the health consequences of this intensified non‐nutritive sweeteners consumption in people with diabetes. We wanted to find out whether non‐nutritive sweeteners consumption in people with diabetes has an effect on long‐term average blood sugar levels (glycosylated haemoglobin A1c ‐ HbA1c), body weight, side effects, diabetes complications (such as heart attack, eye or kidney disease), and health‐related quality of life.
Study characteristics
We found nine randomised controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) that allocated people with diabetes to either a group that received a non‐nutritive sweetener or a comparator group. The comparator was usual diet with additional sugar in three studies; placebo (a dummy pill) in five studies; and tagatose (a nutritive low‐calorie sweetener) in one study. The studies included a total of 979 participants; most of the studies were small, with fewer than 100 participants. The length of the studies varied from 4 to 10 months.
This evidence is up‐to‐date as of May 2019.
Key results
Data on health‐related quality of life, diabetes complications, death from any cause, and socioeconomic effects (such as absence from work, visits to general practitioner, medication consumption) were lacking, and data were generally sparse for all comparisons. The available data did not show a clear difference between non‐nutritive sweeteners and sugar, placebo, or the nutritive low‐calorie sweetener tagatose for HbA1c, body weight, and side effects.
Certainty of the evidence
We rated the overall certainty of the evidence as very low, mainly due to the small numbers of included studies and participants and methodological limitations of the included studies.
Summary of findings
Summary of findings 1. Non‐nutritive sweeteners for diabetes mellitus.
| Non‐nutritive sweeteners compared with sucrose, placebo, or a nutritive, low‐calorie sweetener for diabetes mellitus | ||||||
|
Patient: people with diabetes mellitus Settings: outpatients Intervention: non‐nutritive sweeteners (aspartame, rebaudioside A, saccharin, sodium‐cyclamate, sucralose, steviol glycoside) Comparison: sucrose; placebo; nutritive, low‐calorie sweetener (tagatose) | ||||||
| Outcomes/Comparisions | Comparator (sucrose; placebo; nutritive, low‐calorie sweetener) | Non‐nutritive sweeteners (aspartame, rebaudioside A, saccharin, sodium‐cyclamate, sucralose, steviol glycoside) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Health‐related quality of life | Not reported | |||||
| Diabetes complications | Not reported | |||||
| All‐cause mortality | Not reported | |||||
| Non‐serious adverse events (N) | ||||||
| NNS versus sugar | Not reported | |||||
|
NNS versus placebo NNS: aspartame, rebaudioside A, steviol glycoside Follow‐up: 16 to 18 weeks |
356 per 1000 | 278 per 1000 (139 to 555) | RR 0.78 (0.39 to 1.56) | 231 (3) | ⊕⊝⊝⊝a very low | |
| NNS versus nutritive, low‐calorie sweetener | Not reported | |||||
| HbA1c (%) | ||||||
|
NNS versus sugar NNS: aspartame, saccharin, sodium‐cyclamate Follow‐up: 4 to 6 weeks |
The mean HbA1c ranged across control groups from 6.8% to 7.5% | The mean HbA1c in the NNS group was 0.4% higher (0.5% lower to 1.2% higher) | ‐ | 72 (3) | ⊕⊝⊝⊝b very low | |
|
NNS versus placebo NNS: aspartame, rebaudioside A, steviol glycoside Follow‐up: 13 to 16 weeks |
The mean final HbA1c ranged across control groups from 7.3% to 11.4% | The mean HbA1c in the NNS and placebo groups did not differ (MD 0%, −0.1% lower to 0.1% higher) | ‐ | 360 (4) | ⊕⊝⊝⊝c very low | The 95% prediction interval ranged between −0.3% and 0.3% |
|
NNS versus nutritive, low‐calorie sweetener (tagatose) NNS: sucralose Follow‐up: 16 weeks |
The mean HbA1c in the control group was 7.3% | The mean HbA1c in the NNS group was 0.3% higher (0.1% higher to 0.4% higher) | ‐ | 354 (1) | ⊕⊝⊝⊝d very low | |
| Body weight (kg) | ||||||
|
NNS versus sugar NNS: aspartame, saccharin, sodium‐cyclamate Follow‐up: 4 to 6 weeks |
The mean body weight in the control groups was 66.8 kg to 75.9 kg | The mean body weight in the intervention groups was 0.1 kg lower (2.7 kg lower to 2.6 kg higher) | ‐ | 72 (3) | ⊕⊝⊝⊝e very low | |
|
NNS versus placebo NNS: aspartame, rebaudioside A Follow‐up: 12 to 16 weeks |
The mean final body weight ranged across control groups from to 79.4 to 98.4 kg | The mean body weight in the intervention groups was 0.2 kg lower (1 kg lower to 0.6 kg higher) | ‐ | 184 (2) | ⊕⊝⊝⊝f very low | |
| NNS versus nutritive, low‐calorie sweetener | Not reported | |||||
| Socioeconomic effects | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; MD: mean difference; NNS: non‐nutritive sweetener; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level because of inconsistency (no consistent direction of effect) and two levels because of serious imprecision (CI consistent with benefit and harm, small sample size, and small number of studies) ‐ see Appendix 18. bDowngraded by one level because of inconsistency (point estimates varied widely, not all CIs overlapped, no consistent direction of effect); one level because of indirectness (surrogate outcome, insufficient time frame); and one level because of serious imprecision (CI consistent with benefit and harm, small sample size, and small number of studies) ‐ see Appendix 17. cDowngraded by one level because of indirectness (surrogate outcome) and two levels because of serious imprecision (small sample size and small number of studies) ‐ see Appendix 18. dDowngraded by one level because of risk of bias (attrition bias and selective reporting); one level because of indirectness (surrogate outcome); and one level because of imprecision (small number of included studies) ‐ see Appendix 19. eDowngraded by one level because of inconsistency (no consistent direction of effect) and two levels because of serious imprecision (CI consistent with benefit and harm, small sample size, and small number of studies) ‐ see Appendix 17. fDowngraded by one level because of risk of bias (selective reporting) and two levels because of serious imprecision (small sample size and small number of included studies) ‐ see Appendix 18.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder impeding the pancreas from producing enough insulin, body cells from responding properly to the insulin produced, or both. This leads to chronic hyperglycaemia (i.e. elevated plasma glucose levels) and disturbances of carbohydrate, fat, and protein metabolism. In the long term, this condition leads to complications such as retinopathy, nephropathy, neuropathy, and an increased risk for cardiovascular diseases.
Diabetes is one of the most common diseases in the world, and its burden is increasing continuously: the global prevalence of diabetes in adults over 18 years of age was 8.5% in 2014 (WHO 2016). Diabetes was the direct cause of 1.5 million deaths in 2012 (WHO 2016). The global cost of diabetes was about USD 825,000 million per year in 2016 (NCD‐RisC 2016).
A healthy diet, regular physical activity, and pharmacotherapy are key components of diabetes management. For many individuals with diabetes, the most challenging part of the treatment plan is determining what to eat.
Today, nutrition therapy is recommended for all people with type 1 and type 2 diabetes as a component of their overall treatment plan (Evert 2013). The goals of nutrition therapy are to promote and support healthy eating patterns with a variety of nutrient‐dense foods in appropriate portion size to achieve individualised glycaemic, blood pressure, and lipid goals; attain and maintain body weight goals; and delay or prevent complications of diabetes. A further goal is to maintain the pleasure of eating by providing positive messages about food choices and practical tools for day‐to‐day meal planning (Evert 2013).
Description of the intervention
Non‐nutritive sweeteners (NNS) are defined as sweetening agents having higher sweetening intensity and lower calorie content per gram compared to caloric sweeteners like sucrose or corn syrups (Chattopadhyay 2014). Both the general population and individuals with type 1 or type 2 diabetes use NNS as a caloric sweetener replacement to control their carbohydrate and energy intake.
Most of the NNS approved for human consumption are synthetic (artificial sweeteners); however, increasing numbers of natural non‐caloric sweeteners are becoming available for human consumption.
Products sweetened with NNS are widely available on the market: diet beverages, diet yoghourts, desserts, and chewing gums are the most common products with NNS. NNS are also available as table‐top sweeteners for use by consumers at home as a sweetening agent for beverages and for cooking and baking.
With regard to the range of approved artifical sweeteners, there are important differences amongst countries. In the USA, the Food and Drug Administration (FDA) has to date approved six artificial sweeteners for human consumption: acesulfame‐K, aspartame, neotame, saccharin, sucralose, and advantame. Additionally, steviol glycosides, thaumatin, and luo han guo fruit extracts (mogrosides) are approved NNS of natural origin (FDA 2015a). In the European Union, the following 11 NNS are approved for use in foods and drinks by the European Food Safety Authority: acesulfame‐K (E950), advantame (E969), aspartame (E951), aspartame‐acesulfame salt (E962), cyclamate (E952), neohesperidine DC (E959), neotame (E961), saccharin (E954), steviol glycosides (E960), sucralose (E955), and thaumatin (E957) (FSA 2016).
Approved NNS are described in more detail below. Table 2 lists the acceptable daily intake levels defined by the main regulatory bodies (JECFA 2010).
1. Acceptable daily intake levels of non‐nutritive sweeteners as defined by regulatory bodies.
| Sweetener | FDA (mg/kg body weight) (FDA 2015a) | SCF/EFSA (mg/kg body weight) (Mortensen 2006) | JECFA (mg/kg body weight) (JECFA 2010) |
| ACE‐K | 15 | 9 | 15 |
| Advantame | 32.8 | 5 | 5 |
| Aspartame | 50 | 40 | 40 |
| Cyclamate | Not approved | 7 | 11 |
| Luo han guo fruit extracts | Not specified | Not specified | Not specified |
| Neohesperidine DC | Not approved | 5 | Not evaluated |
| Neotame | 0.3 | 2 | 2 |
| Saccharin | 15 | 5 | 5 |
| Sucralose | 5 | 15 | 15 |
| Steviol glycosides | 4 | 4 | 4 |
| Thaumatin | Not approved | Not specified | Not specified |
ACE‐K: acesulfame potassium; DC: dihydrochalcone; EFSA: European Food Safety Authority;FDA: US Food and Drug Administration; JECFA: Joint FAO/WHO Expert Committee on Food Additives; SCF: Scientific Committee on Food (European Commission).
Acesulfame‐K (acesulfame potassium) is a combination of an organic acid and potassium and was first approved for general use as an NNS in 1988. It contains 0 kilocalories (kcal)/g and is 200 times sweeter than sucrose (Chattopadhyay 2014). The estimated daily intake (EDI; i.e. the presumed daily consumption of NNS) ranges from 0.2 to 1.7 mg/kg of body weight (Fitch 2012; Gardner 2012).
Advantame is an N‐substituted derivative of aspartame made from aspartame and vanillin (Otabe 2011). It is approximately 20,000 times sweeter than sucrose (FDA 2015a).
Aspartame is the methyl ester of the dipeptide of the amino acids aspartic acid and the essential amino acid phenylalanine. It was approved for general use in 1981 and is 180 to 200 times sweeter than sucrose (Chattopadhyay 2014). Although it has 4 kcal/g, the intensity of sweet taste means that very small amounts are required to achieve desired sweetness levels. The EDI ranges from 0.2 to 4.1 mg/kg of body weight (Fitch 2012; Gardner 2012).
Cyclamate (cyclamic acid) is used as an NNS in two forms: sodium cyclamate and calcium cyclamate. It is 30 times sweeter than sucrose and contains zero calories (Chattopadhyay 2014). It is used in more than 50 countries (Fitch 2012); however, cyclamate and its salts are currently prohibited from use in the USA (FDA 2015a).
Luo han guo (also known as Siraitia grosvenori) fruit extract is a traditional Chinese herb containing varying levels of mogrosides. Depending on the mogroside content, it is reported to be 100 to 250 times sweeter than sucrose (FDA 2015a).
Neohesperidine dihydrochalcone (DC) is a non‐nutritive sweetener derived from the flavones of citrus fruit. The customary concentration is 400 to 600 times sweeter than sucrose.
Neotame is a dipeptide methyl ester derivate. It has a sweetness factor approximately 7000 to 13,000 times greater than that of sucrose and approximately 30 to 60 times greater than that of aspartame, depending on the food application (Aguilar 2007).
Saccharin is the oldest NNS, first discovered and used in 1879 (FDA 2015b). It is an organic chemical compound (O‐sulfobenzimide) that can be artificially synthesised in various ways. It has no calories and is about 300 times sweeter than sucrose (Chattopadhyay 2014); however, it has an unpleasant bitter or metallic aftertaste. The EDI ranges from 0.1 to 2.0 mg/kg of body weight (Fitch 2012).
Stevia rebaudiana ‐based products are the best‐known NNS of natural origin. Steviol glycosides, extracted from the plant stevia, contain stevioside and rebaudioside A as well as other glycosides (Ceunen 2013). Steviol glycosides are 10 to 15 times sweeter than sucrose. Stevia has been used as a sweetener in some countries (e.g. Japan) for decades, whilst it was approved as a food additive by the European Food Safety Authority in 2011 (EC 2011).The FDA first recognised the use of certain steviol glycosides as a sweetener as generally safe in 2008 (FDA 2008).
Sucralose is an organic chemical compound (trichlorosucrose) that has been approved for general use as a non‐nutritive sweetener since 1999 (Gardner 2012). It is 450 to 650 times sweeter than sucrose and has 0 kcal/g. The quality and intensity of sweet taste is very close to that of sucrose (Chattopadhyay 2014). The EDI ranges from 0.1 to 2.0 mg/kg of body weight (Fitch 2012).
Thaumatin is a mixture of sweet‐tasting polypeptides that can be extracted from the skin surrounding the seeds of the West African katemfe fruit.
Adverse effects of the intervention
Food safety agencies consider consumption of NNS up to the acceptable daily intake to be safe; however, the effects of NNS on glucose metabolism are not clearly understood (Romo‐Romo 2016). Individuals with diabetes may consume NNS for very long periods (i.e. years or even decades) on a daily basis, possibly at an amount exceeding the acceptable daily intake levels (Ilbäck 2003). There has been little research on the negative health outcomes arising as a consequence of consuming such considerable amounts of NNS over long periods, and even less focusing specifically on people with diabetes.
A potentially increased risk for cancer is a starting point for many debates around the safety of NNS (Gallus 2007).
Additionally, some studies indicated that NNS consumption might lead to weight gain instead of the expected weight loss (Mattes 2009), which in people with diabetes could lead to the worsening of glycaemic control, blood pressure, and lipid profile (ADA 2016).
Furthermore, some researchers have also questioned whether NNS (consumed without caloric sweeteners) could enhance the cephalic phase of insulin secretion (the early increase of insulin secretion immediately following gustatory stimulation, prior to the rise of blood glucose) by evoking the recognition of the sweet taste, sight, smell, and expectation of food, and whether in the absence of caloric sweetener intake it could lead to exercise‐induced hypoglycaemia (Ferland 2007; Just 2008).
A systematic review and dose‐response meta‐analysis of prospective studies found a positive association between artificially sweetened soft drink intake and type 2 diabetes risk (Greenwood 2014).
How the intervention might work
The mechanisms by which NNS might influence health outcomes in people with diabetes include improvement in glycaemic control and facilitation of weight management.
One of the key elements in nutrition therapy for type 1 diabetes is carbohydrate‐counting meal planning and adjustments to insulin doses based on carbohydrate intake, in order to maintain blood glucose levels within the normal range. A simple diabetes meal planning approach such as portion control may be an appropriate nutrition strategy for individuals with type 2 diabetes. Use of NNS has the potential to reduce the overall caloric and carbohydrate intake if they substitute for caloric sweeteners, without compensation by intake of additional calories from other food sources (Evert 2013).
If people with diabetes use NNS to replace caloric sweeteners without caloric compensation, then NNS may also be useful in weight management. Since being overweight and obese can worsen glycaemic control and increase cardiometabolic risk, preventing weight gain in individuals with diabetes is considered to be important. Dietary changes can result in modest and sustained weight loss, and they may produce clinically meaningful reductions in glycosylated haemoglobin A1c (HbA1c) and triglycerides (ADA 2016; Pastors 2002).
Why it is important to do this review
One systematic review focusing on the effects of FDA‐approved NNS in individuals with diabetes found that NNS do not appear to affect glycaemic control (Timpe Behnen 2013). However, that systematic review was limited in that it included only studies published in English and only considered NNS available in the USA. New trials have been published since then that could provide additional relevant evidence. Furthermore, it is important to focus on determining the effects of regular NNS use on patient‐important outcomes, such as morbidity, mortality, and adverse effects, which Timpe Behnen 2013 did not address.
Non‐nutritive sweeteners as part of nutrition therapy represent a simple and cheap intervention that might help decrease the need for antidiabetic drugs, insulin, or both, thereby delaying possible complications. Given that diabetes is a major public health problem worldwide, such an intervention might have huge benefits for health systems in terms of reducing burden and costs.
Objectives
To assess the effects of non‐nutritive sweeteners for diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Individuals with type 1 or type 2 diabetes mellitus.
Diagnostic criteria for diabetes mellitus
In order to be consistent with changes in the classification and diagnostic criteria for diabetes mellitus over the years, the diagnosis should be established using the standard criteria valid at the time of trial commencement (e.g. ADA 2003; ADA 2008; WHO 1998). Trials should ideally describe diagnostic criteria. If necessary, we used the study authors' definition of diabetes mellitus. We planned to subject diagnostic criteria to a sensitivity analysis.
Types of interventions
We planned to investigate the following comparisons of intervention versus control/comparator.
Intervention
Any type of NNS, either alone or in combination with another NNS.
NNS plus a behaviour‐changing intervention such as diet, exercise, or both.
Comparisons
Usual diet versus NNS.
No intervention versus NNS.
Placebo versus NNS.
Water versus NNS.
NNS versus a different NNS.
NNS versus NNS of a different dose.
NNS versus a nutritive or low‐calorie sweetener.
Behaviour‐changing intervention such as diet, exercise, or both versus NNS plus behaviour‐changing intervention.
Concomitant interventions had to be similar in the intervention and comparator groups to allow fair comparisons and to isolate the effect of NNS on health outcomes.
Minimum duration of intervention
We considered RCTs in which the intervention had a minimum duration of four weeks.
Minimum duration of follow‐up
Minimum duration of follow‐up was four weeks after start of the intervention. We defined extended follow‐up periods (also called open‐label extension studies) as follow‐up of participants once the original trial as specified in the trial protocol had been terminated.
Summary of specific exclusion criteria
None.
Types of outcome measures
We included outcomes that are measured for as long as follow‐up is carried out at any given time point. We classified the outcome measurement as medium and long term. We defined 'medium term' as at least four weeks to less than six months and 'long term' as six months or more. We used the data at the longest follow‐up available for the meta‐analyses.
Primary outcomes
HbA1c
Body weight
Adverse events
Secondary outcomes
Diabetes complications
All‐cause mortality
Health‐related quality of life
Anthropometric measures other than body weight
Lipid profile
Glucose levels (fasting and postprandial)
Serum insulin
Insulin sensitivity
Socioeconomic effects
We included trials reporting at least one of the listed primary or secondary outcome measures in the publication. Trials not reporting on any of our primary or secondary outcomes were excluded, but we reported some basic information for these trials in the 'Characteristics of studies awaiting classification' table.
Method of outcome measurement
HbA1c: measured in % (mmol/mol).
Body weight: measured in kilograms (kg).
Adverse events: such as hypoglycaemic episodes, abdominal discomfort, flatulence, or diarrhoea measured at any time after participants had been randomised to intervention/comparator groups.
Diabetes complications: defined as diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, and cardiovascular events.
All‐cause mortality: defined as death from any cause and measured at any time after participants were randomised to intervention/comparator groups.
Health‐related quality of life: evaluated by a validated instrument such as Audit of Diabetes‐Dependent Quality of Life (ADDQoL) or 36‐Item Short Form Health Survey (SF‐36).
Anthropometric measures other than body weight (kg): defined as BMI (body mass index; kg/m²), waist circumference (cm), per cent of body fat (%), or waist‐to‐hip ratio.
Lipid profile: analysed by total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, and triglycerides (TG).
Glucose levels: fasting blood glucose levels (mg/dL) and postprandial blood glucose levels (mg/dL).
Serum insulin: measured in microunits/mL.
Insulin sensitivity: analysed by the homeostasis model assessment of insulin resistance (HOMA‐IR).
Socioeconomic effects: such as direct costs defined as admission/readmission rates, average length of hospital stay, visits to general practitioner, visits to the emergency department; medication consumption; indirect costs defined as resources lost due to illness by the participant or their family member or absence from work.
Timing of outcome measurement
With the exception of adverse events and all‐cause mortality (measured at any time after participants were randomised to intervention/comparator groups), we considered outcomes measured after a minimum follow‐up of four weeks.
Search methods for identification of studies
Electronic searches
We searched the following sources from the inception of each database with no restrictions placed on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, crso.cochrane.org) (searched on 23 May 2019).
MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE (R) Daily and Ovid MEDLINE (R); from 1946 to 20 May 2019) (searched on 21 May 2019).
Scopus (www.scopus.com) (searched on 09 January 2019).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched on 23 May 2019).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP, www.who.int/trialsearch) (searched on 28 May 2019).
We did not include Embase in our search as RCTs indexed in Embase are now prospectively added to CENTRAL via a highly sensitive screening process (Cochrane 2018).
Details of the search strategies are shown in Appendix 1. We applied no restrictions on the language of publication when searching the electronic databases or reviewing reference lists of identified trials.
Searching other resources
We attempted to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, (systematic) reviews, meta‐analyses, and health technology assessment reports. In addition, we contacted authors of included trials to identify any additional information on the retrieved trials and to determine if there were further trials that we may have missed.
We did not use abstracts or conference proceedings for data extraction unless full data were available from the trial authors because this information source does not fulfil the CONSORT requirements, which consist of "an evidence‐based, minimum set of recommendations for reporting randomized trials" (CONSORT 2010; Scherer 2018). We planned to list key data of abstracts in an appendix.
We defined grey literature as records detected in ClinicalTrials.gov or WHO ICTRP, and we additionally searched the database of the FDA (www.fda.gov/Food).
Data collection and analysis
Selection of studies
Pairs of review authors (SL, IT, DK) independently screened the abstract, title, or both, of every record retrieved by the literature searches to determine which trials should be assessed further. We performed the screening using Covidence software (Covidence). We obtained the full texts of all potentially relevant records and screened these for eligibility. Any disagreements were resolved through consensus or by recourse to a third review author (SL, IT, DK, or JM). If we could not resolve a disagreement, we categorised the trial as a study awaiting classification and contacted the trial authors for clarification. We have presented a PRISMA flow diagram to describe the process of trial selection (Liberati 2009). All articles excluded after full‐text assessment and the reasons for their exclusion are described in Characteristics of excluded studies tables.
Data extraction and management
Pairs of review authors (SL, IT, DK) independently extracted key participant and intervention characteristics of the trials that met our inclusion criteria. We described interventions using the 'template for intervention description and replication' (TIDieR) checklist (Hoffmann 2014; Hoffmann 2017).
We recorded data on efficacy outcomes and adverse events using standardised data extraction sheets from the Cochrane Metabolic and Endocrine Disorders Group. Any disagreements were resolved by discussion or by consultation with a third review author (SL, IT, DK, or JM) if required. For details see Characteristics of included studies; Table 3; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16; Appendix 17; Appendix 18; Appendix 19.
2. Overview of trial populations.
| Trial ID (trial design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (N) | Randomised (N) | Analysed (primary outcome) (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up |
| Ensor 2015 (parallel RCT) | I: Splenda 1.5 g 3 times a day, dissolved in 125 to 250 mL water | ‐ | ‐ | 253 | 184a | 119 | ‐ | 12 months |
| C: D‐tagatose 15 g 3 times a day, dissolved in 125 to 250 mL water | 241 | 172a | 85 | ‐ | ||||
| 494 | 356 | 204 | 41.3 | |||||
|
Barriocanal 2008 (parallel RCT) |
I: steviol glycoside capsules 250 mg 3 times a day (92% purity) | "Power analysis were also conducted to determine whether the samples were large enough to allow for the detection of a clinically significant change between baseline and post treatment levels within the control and treatment groups. A clinically significant difference was defined based on the range of 'normal' values for each of the parameters considered." | ‐ | ‐ | 8 + 15b | 23b | ‐ | 3 months |
| C: placebo capsules 3 times a day | ‐ | 8 + 15b | 23b | ‐ | ||||
| total: | 53 | 46 | 46 | 86.8 | ||||
|
Maki 2008 (parallel RCT) |
I: rebaudioside A 250 mg 4 times a day in capsules (97% purity) | "The study was designed to provide 90% power (α = 0.05, two‐sided) to detect a 0.5% difference in HbA1c response between treatment groups, assuming a standard deviation of 0.8%." | 175 | 60 | 60 | 58 | 96.7 | 16 weeks |
| C: placebo capsules 4 times a day (microcrystalline cellulose) | 62 | 62 | 58 | 93.5 | ||||
| total: | 122 | 122 | 116 | 95.1 | ||||
|
Grotz 2003 (parallel RCT) |
I: sucralose 667 mg daily in capsules | "The number of subjects was based on achieving at least 90% power to detect a 0.6 treatment group difference in percent HbA1c change from baseline. Post‐study analysis showed that the study provided more than 99.99% power to detect this difference, and more than 90% to detect a difference of 0.3." | ‐ | 67 | 65c | 63 | 94 | 17 weeks |
| C: placebo (cellulose) capsules | 69 | 68c | 65 | 94.2 | ||||
| total: | 136 | 133c | 128 | 94.1 | ||||
|
Colagiuri 1989 (cross‐over RCT) |
I: aspartame 162 mg daily, added to the usual diet | ‐ | ‐ | ‐ | 9 | 9 | ‐ | 6 weeks |
| C: sucrose 45 g daily, added to the usual diet | ‐ | 9 | 9 | ‐ | ||||
| total: | 9 | 9 | 9 | 100 | ||||
|
Cooper 1988 (cross‐over RCT) |
I: saccharin and starch 30 g daily, added to the usual diet | ‐ | ‐ | 17 | 17 | 17 | 100 | 6 weeks |
| C: sucrose 28 g daily, added to the usual diet | 17 | 17 | 17 | 100 | ||||
| total: | 17 | 17 | 17 | 100 | ||||
|
Chantelau 1985 (cross‐over RCT) |
I: sodium‐cyclamate, ad libitum (348 ± 270 mg/day) | ‐ | 10 | 10 | 10 | 10 | 100 | 4 weeks |
| C: sucrose, ad libitum (24 ± 13 g/day) | 10 | 10 | 10 | 100 | ||||
| total: | 10 | 10 | 10 | 100 | ||||
|
Nehrling 1985 (parallel RCT) |
I: aspartame 2.7 g daily, in capsules | ‐ | 63 | 30 | 29 | 29 | 96.7 | 18 weeks |
| C: placebo (cornstarch) 1.8 g daily in capsules | 33 | 33 | 33 | 100 | ||||
| total: | 63 | 62 | 62 | 98.4 | ||||
|
Stern 1976 (parallel RCT) |
I: aspartame 300 mg capsules, 2 capsules 3 times daily added to the usual diet | ‐ | ‐ | ‐ | ‐ | 36 | ‐ | 13 weeks |
| C: matched placebo | ‐ | ‐ | 33 | ‐ | ||||
| total: | 75 | ‐ | 69 | 92 | ||||
| Grand total | All interventions | 437d | 364e | |||||
| All comparators | 432d | 333e | ||||||
| All interventions and comparators | 979d | 661e | ||||||
‐: denotes not reported
C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; RCT: randomised controlled trial.
aWe provided numbers for the intention‐to‐treat analysis. Authors also performed a per‐protocol analysis, with 119 participants in the Splenda and 85 in the tagatose group. bThis trial included participants with type 1 and type 2 diabetes and participants without diabetes. We only reported on participants with type 1 and type 2 diabetes. cFor the two primary outcomes of the trial, the number of participants included in the analyses was reported only for fasting plasma glucose, but not for HbA1c values. dNot all trials described the number of participants randomised to each intervention/comparator group, therefore the numbers do not add up correctly. eThere are cross‐over trials amongst the included trials, therefore the numbers do not add up correctly.
We attempted to find the protocol for each included trial and reported primary, secondary, and other outcomes in comparison with data in publications in Appendix 9 to assess risk of selective outcome reporting.
We emailed all authors of included trials to enquire as to whether they would be willing to answer questions regarding their trials. The results of this survey are presented in Appendix 16. We thereafter sought relevant missing information on the trial from the primary trial author(s), if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary trial, we maximised the information yield by collating all available data, and used the most complete data set aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary trial, and trial documents of included trials (such as trial registry information) as secondary references under the study ID of the included trial. Furthermore, we also listed duplicate publications, companion documents, multiple reports of a trial, and trial documents of excluded trials (such as trial registry information) as secondary references under the study ID of the excluded trial.
Data from clinical trial registries
If data from included trials were available as study results in clinical trial registries such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted the data. If there was also a full publication of the trial, we collated and critically appraised all available data. If an included trial was marked as a completed study in a clinical trial registry but no additional information (study results, publication, or both) was available, we added this trial to the 'Characteristics of studies awaiting classification' table.
Assessment of risk of bias in included studies
Pairs of review authors (SL, IT, DK) independently assessed the risk of bias of each included trial. Any disagreements were resolved by consensus or by consultation with a third review author (SL, IT, DK, or JM). In case of disagreement, we consulted the rest of the author team and made a judgement based on consensus. If adequate information was not available from publications, trial protocols, or other sources, we contacted the trial authors to request missing data on the 'Risk of bias' domains.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2019b), to assign assessments of low, high, or unclear risk of bias (for details see Appendix 2; Appendix 3). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions, according to the criteria and associated categorisations therein (Higgins 2019b).
Summary assessment of risk of bias
A 'Risk of bias' graph and 'Risk of bias' summary figure are shown in Figure 2 and Figure 3.
We distinguished between self‐reported and investigator‐assessed outcome measures.
We considered the following self‐reported outcomes.
Body weight
Adverse events
Health‐related quality of life
Glucose levels
We considered the following investigator‐assessed outcomes.
HbA1c
Body weight
Diabetes complications
All‐cause mortality
Anthropometric measures other than body weight
Lipid profile
Glucose levels
Serum insulin
Insulin sensitivity
Socioeconomic effects
Risk of bias for a trial across outcomes
Some 'Risk of bias' domains, such as selection bias (sequence generation and allocation sequence concealment), affect the risk of bias across all outcome measures in a trial. In case of high risk of selection bias, we marked all outcomes investigated in the associated trial as at high risk of bias. Otherwise, we did not perform a summary assessment of the risk of bias across all outcomes for a trial.
Risk of bias for an outcome within a trial and across domains
We assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both trial‐level entries and outcome‐specific entries). We considered low risk of bias to denote a low risk of bias for all key domains; unclear risk to denote an unclear risk of bias for one or more key domains; and high risk to denote a high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains
These are the main summary assessments that we incorporated into our judgements regarding the certainty of evidence in the 'Summary of findings' tables. We defined outcomes as being at low risk of bias when most information came from trials at low risk of bias; unclear risk when most information came from trials at low or unclear risk of bias; and high risk when a sufficient proportion of information came from trials at high risk of bias.
Measures of treatment effect
When at least two included trials were available for a comparison of a given outcome, we tried to express dichotomous data as a risk ratio (RR) or an odds ratio (OR), with 95% confidence intervals (CIs). For continuous outcomes measured on the same scale (e.g. weight loss in kg), we estimated the intervention effect using the mean difference (MD) with 95% CIs. For continuous outcomes measuring the same underlying concept (e.g. health‐related quality of life) but using different measurement scales, we planned to calculate the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, and multiple observations for the same outcome. For more than one available comparison from the same trial eligible for inclusion in the same meta‐analysis, we planned to either combine groups to create a single pair‐wise comparison or appropriately reduce the sample size so that the same participants did not contribute data to the meta‐analysis more than once (splitting the 'shared' group into two or more groups). Whilst the latter approach offers some solution to adjusting the precision of the comparison, it does not account for correlation arising from the same set of participants being in multiple comparisons (Higgins 2019a).
We attempted to re‐analyse cluster‐RCTs that did not appropriately adjust for potential clustering of participants within clusters in their analyses and therefore the variance of the intervention effects was inflated by a design effect. Calculation of a design effect involves estimation of an intracluster correlation coefficient (ICC). We planned to obtain estimates of ICCs through contact with authors or impute them, either using estimates from other included trials that reported ICCs or using external estimates from empirical research (e.g. Bell 2013). We planned to examine the impact of clustering using sensitivity analyses.
Dealing with missing data
If possible, we obtained missing data from the authors of the included trials. We carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat, as‐treated, and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and critically appraised issues concerning missing data and use of imputation methods (e.g. last observation carried forward) if individuals were missing from the reported results.
When change from baseline is the outcome of interest, missing standard deviations (SD) for changes from baseline constitute a special case. If the trial authors did not explicitly present these data, and we could not obtain them from the authors, we calculated the mean change in each group by subtracting the final mean from the baseline mean. When baseline and final SDs were available, we imputed the missing SD using an imputed value for the correlation coefficient (Abrams 2005; Follmann 1992). Here, we planned to use a correlation coefficient of zero (Higgins 2019a, see 16.1.3.2 'Imputing standard deviations for changes from baseline'), and wanted to check in sensitivity analyses whether the overall result of the analysis was robust to the use of different correlation coefficients. We planned to report per outcome which trials with imputed SDs were included. For cross‐over trials with mean difference as the measure of treatment effect, missing SD of the difference was imputed based on correlation coefficient obtained from trials where SD of the difference was given. If there was no such trial, we used the value of 0.5, performing sensitivity analyses for 0 and 0.8 (Higgins 2019a, see 16.4.6.1 'Mean differences').
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1 (Deeks 2019). In view of the low power of this test, we also considered the I² statistic — which quantifies inconsistency across trials —to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). When we found heterogeneity, we attempted to determine the possible reasons for it by examining individual characteristics of the trial and subgroups.
Assessment of reporting biases
If we included 10 or more trials that investigated a given outcome, we would use funnel plots to assess small‐trial effects. There are several possible explanations for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence small‐trial bias), and publication bias (Sterne 2017). We therefore planned to interpret the results carefully (Sterne 2011).
Data synthesis
We planned to undertake (or display) a meta‐analysis only if we judged the participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure a result that was clinically meaningful. Unless good evidence showed homogeneous effects across trials of different methodological quality, we primarily summarised data that are of low risk of bias using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects and planned to present prediction intervals (Borenstein 2017a; Borenstein 2017b; Higgins 2009). A prediction interval needs at least three trials to be calculated and specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). For rare events such as event rates below 1%, we used Peto's odds ratio method, provided that there was no substantial imbalance between intervention and comparator group sizes, and intervention effects were not exceptionally large. In addition, we performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and we planned to carry out subgroup analyses for these, including investigation of interactions (Altman 2003).
Type 1 or type 2 diabetes.
Age groups (children: 0 to 18 years; adults: 19 to 64 years; elderly: 65 years or older).
Length of non‐nutritive sweetener intervention (medium versus long term).
Different types of non‐nutritive sweeteners used.
Different types of sources of non‐nutritive sweeteners (liquid, mixed, solid).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting analysis to the following.
Published trials.
Effect of risk of bias, as specified in the Assessment of risk of bias in included studies section.
Very long or large trials to establish the extent to which they dominated the results.
We used of the following filters, if applicable: diagnostic criteria, imputation used, language of publication (English versus other languages), source of funding (industry versus other), or country (depending on data).
We also tested the robustness of results by repeating the analyses using different statistical models (fixed‐effect and random‐effects models).
Certainty of the evidence
We presented the overall certainty of the evidence for each outcome specified below, according to the GRADE approach, which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (such as directness of results). Two review authors (SL, DK) independently rated the certainty of the evidence for each outcome. We resolved any differences in assessment by discussion or by consultation with a third review author (SL, IT, DK, JM).
We included 'Checklists to aid consistency and reproducibility of GRADE assessments' (Appendix 17; Appendix 18; Appendix 19) to help with standardisation of the 'Summary of findings' tables (Meader 2014). We presented results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding where necessary.
'Summary of findings' table
We presented a summary of the evidence in a 'Summary of findings' table. This provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies; the numbers of participants and trials addressing each important outcome; and a rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019), using the Review Manager 5 table editor (Review Manager 2014). Interventions presented in the 'Summary of findings' table were any type of NNS with or without a behaviour‐changing intervention, and comparators were usual diet, no intervention, placebo, water, or a behaviour‐changing intervention alone.
We reported the following outcomes, listed according to priority.
Health‐related quality of life
Diabetes complications
All‐cause mortality
Adverse events
HbA1c
Body weight (kg)
Socioeconomic effects
Results
Description of studies
For a detailed description of trials, see Table 3, Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search was first run in January 2018, and then updated in May 2019 (see Appendix 1 for details on search strategies). We retrieved 1699 unique records. Most of the references clearly did not meet the inclusion criteria based on title and abstract review and were excluded (Figure 1). We evaluated 94 full texts or records to determine their eligibility for inclusion in the review. Nine RCTs published in 11 records met our inclusion criteria.
1.
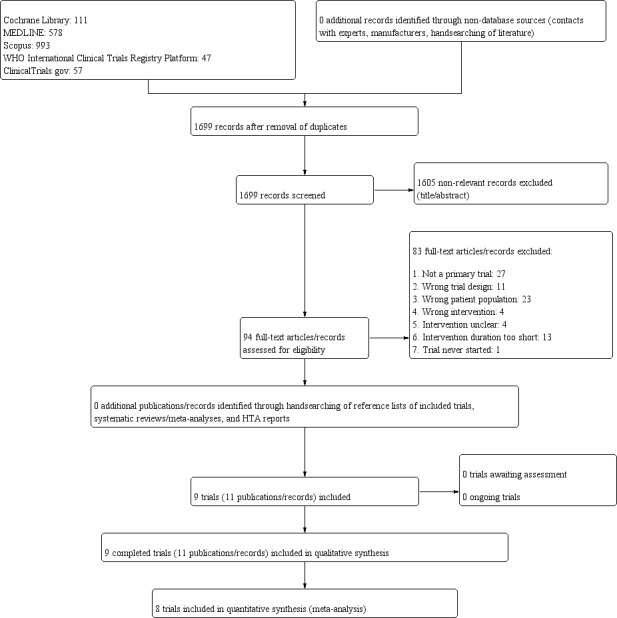
Trial flow diagram.
Ongoing trials
We did not identify ongoing trials matching our in‐ and exclusion criteria.
Included studies
A detailed description of the characteristics of included trials is presented in Characteristics of included studies; Table 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16. The following is an overview of the main results.
Source of data
All included trials were published as full publications, and no additional information was found in trial registries or other trial documents (see Appendix 9). We contacted authors of all included trials by email (Appendix 16). We also contacted the trial authors when important information was needed to make a final decision on the inclusion or exclusion of a study (Appendix 16).
One trial identified in a trial registry was finally excluded based on information received from the authors via email (EUCTR2006‐002395‐18‐DK). In the case of other trial methodological issues that could be resolved through email correspondence with the authors, we used this information to assess the risk of bias (Chantelau 1985).
Comparisons
In five trials NNS were compared to placebo (Barriocanal 2008; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976), whilst in three trials NNS supplementation was compared to a supplement containing sucrose (Chantelau 1985; Colagiuri 1989; Cooper 1988). One trial compared NNS to another type of sweetener (Ensor 2015).
The type of NNS varied widely amongst trials: in two trials a Stevia rebaudiana‐based product was compared to placebo (Barriocanal 2008; Maki 2008); in one trial sucralose was compared to placebo (Grotz 2003); and in two trials aspartame was compared to placebo (Nehrling 1985; Stern 1976). In the trials using a sucrose‐containing comparator, the investigated NNS were: aspartame (Colagiuri 1989), saccharin (Cooper 1988), or cyclamate (Chantelau 1985). In one trial a Stevia rebaudiana‐based product was compared to tagatose (Ensor 2015).
Overview of trial populations
The number of participants initially screened was described in three trials, ranging from 10, in Chantelau 1985, to 175, in Maki 2008.
A total of 661 of 979 randomised participants completed the trials, of these 364 were randomised to the intervention and 333 to the comparator group (see Table 3). The proportion of randomised participants completing the trial ranged between 41.3%, in Ensor 2015, and 100%, in Chantelau 1985; Colagiuri 1989; Cooper 1988. Individual final sample size ranged from 9, in Colagiuri 1989, to 204, in Ensor 2015.
Trial design
Trials were published between the years 1976, Stern 1976, and 2015, Ensor 2015. Dates when trials were performed were not clearly stated in trials.
Six trials were parallel RCTs (Barriocanal 2008; Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976), whilst three trials had a cross‐over design (Chantelau 1985; Colagiuri 1989; Cooper 1988). Five trials with parallel design had placebo as the comparator (Barriocanal 2008; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976), and one used another type of sweetener as comparator (Ensor 2015). The three cross‐over trials compared NNS to sucrose.
Seven trials performed blinding of participants and personnel (Barriocanal 2008, Colagiuri 1989; Cooper 1988; Ensor 2015; Grotz 2003; Nehrling 1985; Stern 1976); one trial clearly stated that participants were not blinded (Chantelau 1985); and the remaining trial reported no information on blinding (Maki 2008). Blinding of outcome assessors was generally not reported.
Six trials had a run‐in period with a duration of either one week (Nehrling 1985; Stern 1976), two weeks (Maki 2008), four weeks (Chantelau 1985; Grotz 2003), or eight weeks (Ensor 2015). In one of these studies participants received placebo capsules two times a day during the run‐in period (Grotz 2003). The duration of the intervention in the included trials varied from four weeks, in Chantelau 1985, to 10 months, in Ensor 2015. Only one trial followed participants after the intervention period (Grotz 2003).
The number of randomised participants varied from nine in a small cross‐over trial, Colagiuri 1989, to 494 in a parallel trial, Ensor 2015. Four trials were multicentre trials (Ensor 2015; Grotz 2003; Maki 2008; Stern 1976), whilst the others were conducted in only one centre.
None of the trials was terminated prematurely.
Settings
All trials were performed in outpatient settings.
Participants
One trial included only individuals with type 1 diabetes (Chantelau 1985); two trials included both individuals with type 1 and 2 diabetes (Barriocanal 2008; Nehrling 1985), whilst all other trials included participants with type 2 diabetes only. Duration of diabetes was reported in two trials for type 1, Barriocanal 2008; Chantelau 1985, and in four trials for type 2 diabetes (Barriocanal 2008; Colagiuri 1989; Grotz 2003; Maki 2008); duration of disease was more than one year, Chantelau 1985, or more than five years, Barriocanal 2008, for individuals with type 1 diabetes, whilst it ranged from more than one year, Barriocanal 2008; Maki 2008, to a mean duration of 10.2 years, Grotz 2003, in those with type 2 diabetes.
All trials included adult males and females. Mean age of participants at baseline was reported in six trials (Barriocanal 2008; Colagiuri 1989; Cooper 1988; Ensor 2015; Grotz 2003, Maki 2008), ranging from 25.4 to 65.6 years. Two studies provided age range of participants (Chantelau 1985; Stern 1976).
Ethnicity was reported in three trials (Ensor 2015; Grotz 2003; Maki 2008): two trials included mainly white people, while the third trial included mainly Asian participants (Ensor 2015). Six of the nine included trials were conducted partly, Ensor 2015, or fully in the USA (Cooper 1988; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976). None of the included trials involved participants from low‐income countries.
Seven trials reported baseline HbA1c levels. Three trials included individuals with HbA1c ≤ 7.5% at screening (type 2 diabetes group in Barriocanal 2008; Colagiuri 1989; Maki 2008). One trial included individuals with a mean baseline HbA1c > 9.5% (Nehrling 1985), whilst three trials included participants with a mean baseline HbA1c between 7.7% and 9.5% (type 1 diabetes group in Barriocanal 2008; Chantelau 1985; Cooper 1988).
Six trials reported BMI at baseline. Individuals with type 1 diabetes in two studies, Barriocanal 2008; Chantelau 1985, and individuals with type 2 diabetes in one study, Ensor 2015, had a normal BMI, whilst other trials including those with type 2 diabetes reported a baseline mean BMI of either 25 kg/m² to 30 kg/m², Barriocanal 2008; Colagiuri 1989; Cooper 1988, or 30 kg/m² to 35 kg/m², Grotz 2003; Maki 2008.
In two trials participants were reported to have comorbidities: hypertension, Barriocanal 2008, or dyslipidaemia, Barriocanal 2008; Maki 2008, both of which were treated with medication.
Major exclusion criteria, mentioned in at least two trials, were comorbidities such as cardiovascular diseases (Barriocanal 2008; Maki 2008), renal failure (Barriocanal 2008; Cooper 1988; Ensor 2015; Maki 2008), or poorly controlled hypertension (Barriocanal 2008; Ensor 2015; Maki 2008); acute illness (Barriocanal 2008; Cooper 1988); or pregnancy (Barriocanal 2008; Ensor 2015; Maki 2008). In five trials no exclusion criteria were mentioned (Chantelau 1985; Colagiuri 1989; Grotz 2003; Nehrling 1985; Stern 1976).
Diagnosis
Only three publications described how diabetes was diagnosed in the trial. In one trial diabetes diagnosis was defined based on the classification of an international workgroup sponsored by the National Diabetes Data Group of the US National Institutes of Health (Colagiuri 1989), whilst in another trial diabetes was established by a fasting plasma glucose > 140 mg/dL, an abnormal oral glucose tolerance test as interpreted by the US Public Health Service criteria, or an unequivocal history of diabetes (Nehrling 1985). In the third trial diagnosis of diabetes was established "according to WHO criteria" (Ensor 2015).
Interventions
In five of the nine trials NNS were provided in capsule form (Barriocanal 2008; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976). In two trials NNS were added to the usual diet (Colagiuri 1989; Cooper 1988), whilst in one trial participants were instructed to consume either NNS or sucrose ad libitum, and the consumed amounts were measured (Chantelau 1985).
Aspartame was administered in three trials (Colagiuri 1989; Nehrling 1985; Stern 1976), in a daily dose ranging from 162 mg, Colagiuri 1989, to 2.7 g, Nehrling 1985. Cyclamate was consumed by participants one trial, ad libitum with a mean daily dose of 348 mg (Chantelau 1985). Saccharin was consumed in one trial, in combination with starch, at 30 g daily (Cooper 1988). Stevia rebaudiana‐based products were consumed in two trials, in the form of 250 mg capsules, administered three times a day, Barriocanal 2008, or four times a day, Maki 2008. Sucralose was the dietary supplement used in two trials, administered in the form of 667 mg capsules, Grotz 2003, or 1500 mg dissolved in water, Ensor 2015.
Outcomes
Three trials specified primary outcomes (Ensor 2015; Grotz 2003; Maki 2008), one of them in the full text of the publication, but not in the abstract (Maki 2008), whilst another trial specified the primary outcome only in the abstract of the publication (Grotz 2003). In the third trial the primary outcome was specified in two trial registries, the publication abstract, and the main text of the publication with some discrepancies between information in the registries and the full‐text publication (adverse events were listed amongst primary outcomes in the publication, but not in the registry entries) (Ensor 2015). Secondary outcomes were explicitly stated in one trial (Ensor 2015). For full details see Appendix 9.
All included trials reported at least one of the primary outcomes of relevance for this review. Eight trials assessed HbA1c (Barriocanal 2008; Chantelau 1985; Colagiuri 1989; Cooper 1988; Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985). Seven trials assessed body weight (Barriocanal 2008; Chantelau 1985; Colagiuri 1989; Cooper 1988; Ensor 2015; Maki 2008; Stern 1976). One trial did not report data on body weight (Barriocanal 2008), whilst another trial reported body weight data as change from baseline to the average of values at weeks 12 and 16 (Maki 2008).
Adverse events were assessed in six trials (Barriocanal 2008; Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976). In two of these trials adverse events were not specified; the authors only stated that "there were no significant differences between the treatment groups in the type, number, or severity of adverse events reported" (Grotz 2003), or that "subjects exhibited no symptoms that could be attributed to the administration" of the NNS or placebo (Stern 1976). Four trials reported data on adverse events (Barriocanal 2008; Ensor 2015; Maki 2008; Nehrling 1985).
None of the included trials investigated all‐cause mortality, diabetes complications, health‐related quality of life, or socioeconomic effects.
Excluded studies
We excluded 83 articles or records after full‐text screening (Figure 1). Excluded references are listed in Characteristics of excluded studies.
We excluded 27 records because they did not describe a primary study (Anonymous 1979; Barbosa Martín 2014; Bastaki 2015; Beringer 1973; Bloomgarden 2011; Chantelau 1986; Corfe 1858; Dinkovski 2017; Gapparov 1996; Healy 2013; Heraud 1976; Macdonald 1970; Mazovetskii 1976; Mehnert 1975; Mehnert 1979; Purdy 1988; Saundby 1887; Skyler 1980; Sloane 1858; Stevens 2013; Stoye 2008; Tuttas 2012; Verspohl 2014; Watal 2014; Williams 1858; Williams 2014; Ylikahri 1980), and a further 11 records due to inappropriate trial design (Farkas 1965; McCann 1956; NCT02813759; Noren 2014; Parimalavalli 2011; Ritu 2016; Schatz 1977; Sharafetdinov 2002; Shigeta 1985; Williams 1857; Wills 1981). We excluded 13 records because the duration of the intervention was shorter than four weeks (ACTRN12618000862246; Baturina 2004; Deschamps 1971; Ferland 2007; Fukuda 2010; Maki 2009; NCT01324921; NCT03680482; PACTR201410000894447; Prols 1973; Pröls 1974; Rogers 1994; Vorster 1987); in four trials the intervention was unclear (IRCT2015091513612N6; Madjd 2017; NCT02412774; Odegaard 2017); whilst in another four trials the intervention was not an NNS (Reyna 2003; Sadeghi 2019; Samanta 1985; Simeonov 2002). One trial described in a registry entry was never started based on information from the authors (EUCTR2006‐002395‐18‐DK).
We excluded 23 records describing trials that did not include participants of relevance for this review (Blackburn 1997; Ferri 2006; Kanders 1988; Knopp 1976; Leon 1989; Maersk 2012; Masic 2017; Morris 1993; NCT02252952; NCT02487537; Peters 2014; Peters 2016; Piernas 2011; Piernas 2013; Reid 1994; Reid 1998; Reid 2010; Rodin 1990; Sørensen 2014; Taljaard 2013; Tsapok 2012; Vazquez Duran 2013; Zöllner 1971).
Risk of bias in included studies
For details on the risk of bias of included trials, see Characteristics of included studies.
For an overview of review authors' judgements about each 'Risk of bias' item for individual trials and across all trials, see Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some trials).
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the particular outcome was not measured in some trials)
Allocation
We judged only one trial as at low risk of selection bias regarding the method of both randomisation and allocation concealment (Nehrling 1985). For another trial we were able to retrieve information on participant selection by contacting the authors; based on this information we judged the method used for generating random sequence to be at low risk of bias, whilst allocation, which was done in an open manner, was judged as at high risk of bias (Chantelau 1985). The remaining seven trials reported only that participants were randomised without providing any further description either on random sequence generation or on allocation concealment (Barriocanal 2008; Colagiuri 1989; Cooper 1988; Ensor 2015; Grotz 2003; Maki 2008; Stern 1976), and were therefore judged as at unclear risk of bias for both domains.
Key prognostic variables (age, gender, BMI, ethnicity, comorbidities including hypertension and cardiovascular disease) were balanced between the intervention groups at baseline, but were not reported in all trials (see Appendix 6; Appendix 7).
Blinding
There was one open‐label trial, which we judged as at high risk of bias for blinding of participants and personnel for the outcome measures body weight and glucose levels (Chantelau 1985). All of the other included trials explicitly reported blinding of participants and personnel (Barriocanal 2008; Colagiuri 1989; Cooper 1988; Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976), which was ensured by using placebo, Barriocanal 2008; Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985; Stern 1976, or by identical packing, Colagiuri 1989, and similar taste of the intervention substance (Cooper 1988). Outcome assessment was less well described across trials, with none of the nine trials providing clear information on blinding of outcome assessors.
Measurements of HbA1c were investigator assessed in all trials where this outcome was measured, and since HbA1c is an objective laboratory measure, we judged performance bias as at low risk even in the trial where participants and personnel were not blinded (Chantelau 1985). For the same reason, we judged detection bias as at low risk in all seven reporting trials.
Where measured, body weight was investigator assessed. Amongst trials reporting body weight, we judged six trials with a double‐blind design as at low risk of performance bias (Barriocanal 2008; Colagiuri 1989; Cooper 1988; Ensor 2015; Maki 2008; Stern 1976), and one trial with a lack of blinding as at high risk of performance bias (Chantelau 1985). As in general there was no information on the blinding of outcome assessors, we judged trials reporting body weight as at unclear risk of detection bias (Barriocanal 2008; Colagiuri 1989; Cooper 1988; Ensor 2015; Maki 2008; Stern 1976). We received additional information from the authors of one study stating that body weight was measured independently by personnel unrelated to the study, therefore we judged detection bias for this study as at low risk (Chantelau 1985).
Adverse events were reported in four trials and were always self‐reported by participants. As participants were adequately blinded in trials reporting adverse events, we judged both performance and detection bias as at low risk in these trials (Barriocanal 2008; Grotz 2003; Nehrling 1985; Stern 1976).
Incomplete outcome data
Six trials reported the numbers of participants randomised to each intervention/comparator group as well as those who finished the trials (Chantelau 1985; Cooper 1988; Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985). The proportion of randomised participants completing these trials per protocol varied from 41.3%, in Ensor 2015, to 100%, in Chantelau 1985; Cooper 1988. The remaining three trials did not report on the number of participants randomised to each intervention/comparator group, only the total number of participants randomised and the number of participants finalising the study (Barriocanal 2008; Colagiuri 1989; Stern 1976). One trial did not clearly report the number of participants analysed (Stern 1976).
Three trials clearly reported the number of participant losses (Grotz 2003; Maki 2008; Nehrling 1985). In one of the trials, the study authors stated that discontinuations did not happen as a consequence of an adverse event, but did not provide further details (Grotz 2003). In one trial reasons for discontinuations included: withdrawal of consent (one), protocol violation (one), personal reasons (one), and adverse events (three) (Maki 2008). In the third trial there was only one dropout, and the reason was an adverse event (Nehrling 1985).
Eight trials reported data for HbA1c. In two trials HbA1c data were reported for all randomised participants (Chantelau 1985; Cooper 1988), whilst in one trial imputed data were balanced in numbers across groups (Maki 2008); we judged these trials reporting on HbA1c to be at low risk of attrition bias. In one trial dropout rates were reported for both groups without a detailed description of reasons (Grotz 2003), whilst in another trial it was unclear whether there were any dropouts (Colagiuri 1989). In a further trial, HbA1c data were reported only for participants completing the trial (Nehrling 1985). We judged these trials as at unclear risk of attrition bias for the outcome HbA1c. In two trials with high dropout rates reasons for attrition were not reported (Barriocanal 2008; Ensor 2015), therefore these trials were judged as at high risk of attrition bias for the outcome HbA1c.
Seven trials collected data for body weight. Three trials reported data on body weight for all randomised participants and were judged to be at low risk of bias (Chantelau 1985; Cooper 1988; Maki 2008). We assessed two trials as at unclear risk of attrition bias either because it was unclear whether there were any dropouts, Colagiuri 1989, or because reasons for attrition were not reported in a trial with low dropout rates, Stern 1976. In one trial with high dropout rates, data on body weight and numbers of and reasons for missing body weight data were not reported (Barriocanal 2008), therefore we judged this trial as at high attrition bias for the outcome body weight. One further trial with high dropout rates mentioned that body weight of participants was measured, but data for body weight (kg) were not provided (Ensor 2015).
Three trials reported adverse events in detail (Barriocanal 2008; Maki 2008; Nehrling 1985). In one trial, adverse events were not reported, but were described to be balanced across groups (Grotz 2003); we judged this trial as at low risk of attrition bias for this outcome. In one study the numbers of and reasons for participant losses due to adverse events were unclear (Stern 1976).
None of the trials performed an intention‐to‐treat analysis.
Selective reporting
We did not find published protocols for any of the included trials. We judged five trials to be at low risk of reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification, because it appeared that all expected outcomes had been reported (Chantelau 1985; Colagiuri 1989; Cooper 1988; Maki 2008; Nehrling 1985). We judged four trials to be at high risk of reporting bias: in one of these trials it was described in the methods that weight and waist circumference were measured, but values were not reported (Barriocanal 2008); in another trial body weight and BMI were measured but data were not reported (Ensor 2015); in a further trial the outcomes HbA1c, fasting glucose, and adverse events were reported incompletely (Grotz 2003); and in the fourth trial body weight and glucose levels were reported in a selective way (Stern 1976). For more details, see Appendix 9; Appendix 10.
Other potential sources of bias
As potential other sources of bias we evaluated the presence of industry sponsorship (Lundh 2017), and for cross‐over studies whether the trial could be biased from carry‐over effects (Higgins 2019a). In one trial investigators declared that they had received products used for supplementation from industry (Barriocanal 2008); in four trials study authors provided a general statement about industry funding (Ensor 2015; Grotz 2003; Maki 2008; Nehrling 1985); and in one study it was unclear if industry funding had been received (Stern 1976); we judged all of these studies to be at unclear risk of bias. One trial had a cross‐over design without a washout period between the two intervention periods (Chantelau 1985), and two trials described both industry funding and cross‐over design without washout period (Colagiuri 1989; Cooper 1988); we judged these trials to be at high risk of bias.
Effects of interventions
See: Table 1
For a summary of the evidence, see Table 1.
Baseline characteristics
For details of baseline characteristics, see Appendix 6; Appendix 7.
Any type of NNS, either alone or in combination with another NNS, versus sugar (i.e. usual diet containing sugar or diet containing sugar with additional sugar as supplement)
We identified three trials comparing the health effects of a NNS with sugar. In two trials NNS were added to the usual diet (Colagiuri 1989; Cooper 1988), whilst in the third study participants were instructed to consume either NNS or sucrose ad libitum, and the consumed amounts were measured (Chantelau 1985). NNS used were aspartame (Colagiuri 1989), saccharin (Cooper 1988), or sodium‐cyclamate (Chantelau 1985). The duration of intervention ranged from four weeks, Chantelau 1985, to six weeks, Colagiuri 1989; Cooper 1988.
Two of the trials involved participants with type 2 diabetes (Colagiuri 1989; Cooper 1988), whilst one trial involved participants with type 1 diabetes (Chantelau 1985).
All three trials had a cross‐over design and were reporting data for the first and second periods together. None of the three trials described a washout period.
Primary outcomes
HbA1c
Three trials compared the effects of NNS as compared to sugar on HbA1c, including overall 72 participants (random‐effects mean difference (MD) 0.4%, 95% confidence interval (CI) −0.5 to 1.2; fixed‐effect MD 0.4%, 95% CI 0.1 to 0.7; P = 0.44; 3 trials; 72 participants; very low‐certainty evidence; Analysis 1.1). There was considerable heterogeneity (I² = 86%), likely caused by the combination of cross‐over study design, low number of participants, short intervention period, and no washout period between interventions. The 95% prediction interval did not provide a meaningful estimate.
1.1. Analysis.

Comparison 1: NNS versus sugar, Outcome 1: HbA1c (%)
Due to the short, Chantelau 1985, or missing, Colagiuri 1989; Cooper 1988, run‐in periods and short intervention duration lasting only four, Chantelau 1985, to six weeks, Colagiuri 1989; Cooper 1988, carry‐over effects and effects of the consumption before the study start might have had a considerable impact on results.
Body weight (kg)
Three trials reported weight change (MD −0.1 kg, 95% CI −2.7 to 2.6; P = 0.96; 3 trials; 72 participants; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: NNS versus sugar, Outcome 2: Body weight (kg)
Adverse events
None of the trials reported on non‐serious or serious adverse events.
Secondary outcomes
Diabetes complications
None of the trials reported on diabetes complications.
All‐cause mortality
None of the trials reported on all‐cause mortality.
Health‐related quality of life
None of the trials reported on health‐related quality of life.
Anthropometric measures other than body weight (kg)
None of the trials reported on anthropometric measures other than body weight.
Lipid profile
Three trials reported total cholesterol (MD −0.8 mg/dL, 95% CI −11.1 to 9.6; P = 0.88; 3 trials; 72 participants; Analysis 1.3). Three trials reported HDL cholesterol (MD −1.1 mg/dL, 95% CI −5.6 to 3.4; P = 0.64; 3 trials; 72 participants; Analysis 1.4). One trial reported LDL cholesterol (MD 1.2 mg/dL, 95% CI −15.6 to 18; 1 trial; 34 participants; Analysis 1.5). Three trials reported triglycerides (MD −1.5 mg/dL, 95% CI −15 to 11.9; P = 0.82; 3 trials; 72 participants; Analysis 1.6).
1.3. Analysis.

Comparison 1: NNS versus sugar, Outcome 3: Total cholesterol (mg/dL)
1.4. Analysis.

Comparison 1: NNS versus sugar, Outcome 4: HDL cholesterol (mg/dL)
1.5. Analysis.

Comparison 1: NNS versus sugar, Outcome 5: LDL cholesterol (mg/dL)
1.6. Analysis.

Comparison 1: NNS versus sugar, Outcome 6: Triglycerides (mg/dL)
Glucose levels (fasting and postprandial)
Two trials reported fasting blood glucose levels (MD −5.0 mg/dL, 95% CI −28.3 to 18.3; P = 0.67; 2 trials; 52 participants; Analysis 1.7). One trial reported postprandial blood glucose levels (MD 11.9 mg/dL, 95% CI −20.7 to 44.5; 1 trial; 20 participants; Analysis 1.8).
1.7. Analysis.

Comparison 1: NNS versus sugar, Outcome 7: Fasting blood glucose levels (mg/dL)
1.8. Analysis.

Comparison 1: NNS versus sugar, Outcome 8: Postprandial blood glucose levels (mg/dL)
Serum insulin
One trial reported serum insulin levels (MD 0.8 microunits/mL, 95% CI −2.8 to 4.4; 1 trial; 34 participants; Analysis 1.9).
1.9. Analysis.

Comparison 1: NNS versus sugar, Outcome 9: Serum insulin (microunits/mL)
Insulin sensitivity
None of the trials reported on insulin sensitivity.
Socioeconomic effects
None of the trials reported on socioeconomic effects.
Any type of NNS, either alone or in combination with another NNS, versus no intervention
We identified no trials comparing NNS with no intervention.
Any type of NNS, either alone or in combination with another NNS, versus placebo
We identified five trials comparing the health effects of an NNS with placebo. In all of these trials both NNS and placebo were added as a dietary supplement (in the form of capsules) to the usual diet. Two trials added Stevia rebaudiana‐based products, one in the form of steviol glycoside capsules (250 mg three times a day for 3 months) (Barriocanal 2008), and the other as rebaudioside A (250 mg capsules four times a day for 16 weeks) (Maki 2008). The capsules differed slightly in the purity of stevia content (92% purity in the first study and 97% in the second study). One study compared the effects of sucralose (667 mg daily in capsules for 13 weeks) with placebo (Grotz 2003), whilst two trials investigated aspartame as the intervention (Nehrling 1985; Stern 1976), with an intervention duration of 18 and 13 weeks and a daily dose of 2.7 g and 1.8 g, respectively.
For this comparison, three trials evaluated participants with type 2 diabetes (Grotz 2003; Maki 2008; Stern 1976), one study both participants with type 1 and type 2 diabetes (Nehrling 1985); and one study both participants with type 1 and type 2 diabetes, however these were analysed as separate study groups (Barriocanal 2008).
Primary outcomes
HbA1c
Of the four trials comparing NNS with placebo, two trials provided data as final value scores and two as change‐from‐baseline scores. NNS had no substantial effect on HbA1c (MD 0%, 95% CI −0.1 to 0.1; P = 0.99; 4 trials; 360 participants; very low‐certainty evidence; Analysis 2.1; Figure 4). The 95% prediction interval ranged between −0.3% and 0.3%.
2.1. Analysis.

Comparison 2: NNS versus placebo, Outcome 1: HbA1c (%)
4.

Forest plot of comparison: 2 NNS versus placebo, outcome: 2.1 HbA1c (%).
Body weight (kg)
Two trials reported data on body weight: one of them reported data as change from baseline to the average of values at weeks 12 and 16, with baseline defined as the average of values at weeks −2 and 0 (Maki 2008), whilst the other reported mean (standard error) values for baseline and week 13 (Stern 1976). Comparing NNS with placebo showed an MD in body weight of −0.2 kg, 95% CI −1 to 0.6; P = 0.64; 2 trials; 184 participants; Analysis 2.2; Figure 5.
2.2. Analysis.

Comparison 2: NNS versus placebo, Outcome 2: Body weight (kg)
5.

Forest plot of comparison: 2 NNS versus placebo, outcome: 2.2 Body weight (kg).
Adverse events
Three trials reported the numbers of participants experiencing at least one non‐serious adverse event, with a total of 36/113 participants (31.9%) in the NNS group versus 42/118 participants (35.6%) in the placebo group having a non‐serious adverse event (risk ratio (RR) 0.78, 95% CI 0.39 to 1.56; P = 0.48; 3 trials; 231 participants; very low‐certainty evidence; Analysis 2.3; Figure 6). The 95% prediction interval did not provide a meaningful estimate.
2.3. Analysis.

Comparison 2: NNS versus placebo, Outcome 3: Adverse events (n/N)
6.

Forest plot of comparison: 2 NNS versus placebo, outcome: 2.3 Adverse events (n/N).
Two further trials only narratively reported on adverse events. One of these trials provided the incidence of experienced symptoms, reporting that "mild gastrointestinal complaints were the most common discomforts observed" (Stern 1976). The other trial only mentioned that "there were no significant differences between the treatment groups in type, number, or severity of adverse events reported" (Grotz 2003).
Two trials reported on serious adverse events (Appendix 12): no serious adverse events occurred in one study (Barriocanal 2008), and 4/60 and 3/62 serious adverse events occurred in the NNS and placebo groups, respectively, in the other trial (Maki 2008).
Secondary outcomes
Diabetes complications
None of the trials reported on diabetes complications.
All‐cause mortality
None of the trials reported on all‐cause mortality.
Health‐related quality of life
None of the trials reported on health‐related quality of life.
Anthropometric measures other than body weight (kg)
Barriocanal 2008 reported on BMI (MD −0.4 kg/m², 95% CI −3 to 2.2; P = 0.76; 1 trial; 46 participants; Analysis 2.4).
2.4. Analysis.

Comparison 2: NNS versus placebo, Outcome 4: BMI (kg/m²)
Lipid profile
Three trials reported total cholesterol, two of them with final value scores and one as change‐from‐baseline scores (MD 2 mg/dL, 95% CI −4.8 to 8.8; P = 0.57; 3 trials; 228 participants; Analysis 2.5). Two trials reported HDL cholesterol, one of them providing data as final value scores and one as change‐from‐baseline scores (MD −0.4 mg/dL, 95% CI −2.2 to 1.4; P = 0.67; 2 trials; 168 participants; Analysis 2.6). Two trials reported LDL cholesterol, one of them with providing data as final value scores and one as change‐from‐baseline scores (3.1 mg/dL, 95% CI −2.9 to 9.1; P = 0.31; 2 trials; 168 participants; Analysis 2.7). Two trials reported triglycerides, both of them providing data as final value scores (MD 18.5 mg/dL, 95% CI −6.8 to 43.7; P = 0.15; 2 trials; 106 participants; Analysis 2.8).
2.5. Analysis.

Comparison 2: NNS versus placebo, Outcome 5: Total cholesterol (mg/dL)
2.6. Analysis.

Comparison 2: NNS versus placebo, Outcome 6: HDL cholesterol (mg/dL)
2.7. Analysis.

Comparison 2: NNS versus placebo, Outcome 7: LDL cholesterol (mg/dL)
2.8. Analysis.

Comparison 2: NNS versus placebo, Outcome 8: Triglycerides (mg/dL)
Glucose levels (fasting and postprandial)
Five trials reported fasting blood glucose levels, four of them providing data as final value scores and one as change‐from‐baseline scores (MD 2.2 mg/dL, 95% CI −11.6 to 16.1; P = 0.75; 5 trials; 384 participants; Analysis 2.9). One trial reported postprandial blood glucose levels (MD −1.1 mg/dL, 95% CI −55.1 to 53.1; P = 0.97; 1 trial; 62 participants; Analysis 2.10).
2.9. Analysis.

Comparison 2: NNS versus placebo, Outcome 9: Fasting blood glucose levels (mg/dL)
2.10. Analysis.

Comparison 2: NNS versus placebo, Outcome 10: Postprandial blood glucose levels (mg/dL)
Serum insulin
Two trials reported serum insulin levels, one reporting data as final value scores and one as change‐from‐baseline scores (MD −2.5 microunits/mL, 95% CI −5.4 to 0.4; 2 trials; 152 participants; Analysis 2.11).
2.11. Analysis.

Comparison 2: NNS versus placebo, Outcome 11: Serum insulin (microunits/mL)
Insulin sensitivity
None of the trials reported on insulin sensitivity.
Socioeconomic effects
None of the trials reported on socioeconomic effects.
Any type of NNS, either alone or in combination with another NNS, versus water
We identified no trials comparing NNS with water.
Any type of NNS, either alone or in combination with another NNS, versus NNS of a different dose
We identified no trials comparing NNS with a different dose of NNS.
Any type of NNS, either alone or in combination with another NNS, versus a nutritive (low‐calorie) sweetener
One trial compared the effects of NNS alone (sucralose 1.5 g, three times a day, dissolved in 125 mL to 250 mL of water) with a nutritive, low‐calorie sweetener (tagatose 15 g, three times a day, dissolved in 125 mL to 250 mL of water) (Ensor 2015), with an intervention duration of 10 months. The trial included participants with type 2 diabetes.
Primary outcomes
HbA1c
One trial reported HbA1c (MD 0.3%, 95% CI 0.1 to 0.4; P = 0.01; 1 trial; 354 participants; very low‐certainty evidence; Analysis 3.1 in favour of the nutritive (low‐calorie) sweetener).
3.1. Analysis.

Comparison 3: NNS versus another type of sweetener, Outcome 1: HbA1c (%)
Body weight (kg)
One trial measured body weight, but data were not reported. It was only stated that "there was no observed effect of D‐tagatose on changes on body weight" compared to the NNS group.
Adverse events
The trial stated that adverse events were assessed, but the number of participants and types of adverse events were not reported.
Secondary outcomes
Diabetes complications
One trial did not report data on diabetes complications.
All‐cause mortality
One trial did not report data on all‐cause mortality.
Health‐related quality of life
One trial did not report data on health‐related quality of life.
Anthropometric measures other than body weight (kg)
One trial assessed BMI narratively, stating that there were no significant differences between the sucralose and the tagatose groups.
Lipid profile
Ensor 2015 reported on total cholesterol (MD 1 mg/dL, 95% CI −5.1 to 7.1; P = 0.75; 1 trial; 354 participants; Analysis 3.2); HDL cholesterol (MD 1.3 mg/dL, 95% CI −0.3 to 2.8; P = 0.11; 1 trial; 354 participants; Analysis 3.3); LDL cholesterol (MD 3 mg/dL, 95% CI −2.5 to 8.5; P = 0.29; 1 trial; 354 participants; Analysis 3.4); and triglycerides (MD −22 mg/dL, 95% CI −44.9 to 0.9; P = 0.06; 1 trial; 354 participants; Analysis 3.5).
3.2. Analysis.

Comparison 3: NNS versus another type of sweetener, Outcome 2: Total cholesterol (mg/dL)
3.3. Analysis.

Comparison 3: NNS versus another type of sweetener, Outcome 3: HDL cholesterol (mg/dL)
3.4. Analysis.

Comparison 3: NNS versus another type of sweetener, Outcome 4: LDL cholesterol (mg/dL)
3.5. Analysis.

Comparison 3: NNS versus another type of sweetener, Outcome 5: Triglycerides (mg/dL)
Glucose levels (fasting and postprandial)
One trial reported fasting blood glucose levels (MD 6.50 mg/dL, 95% CI −0.79 to 13.79; P = 0.08; 1 trial; 354 participants; Analysis 3.6).
3.6. Analysis.

Comparison 3: NNS versus another type of sweetener, Outcome 6: Fasting glucose (mg/dL)
Serum insulin
One trial reported serum insulin concentrations narratively, stating that "there was no detectable consistent change in serum insulin concentrations (Ensor 2015)".
Insulin sensitivity
One trial did not report data on insulin sensitivity.
Socioeconomic effects
One trial did not report data on socioeconomic effects.
Any type of NNS, either alone or in combination with another NNS, plus a behaviour‐changing intervention such as diet, exercise, or both versus any of the comparators (usual diet, no intervention, placebo, water, a different NNS, NNS of a different dose, another type of sweetener)
We identified no trials comparing NNS combined with a behaviour‐changing intervention versus a comparator of interest.
Subgroup analyses
We did not perform subgroup analyses because there were not enough trials to estimate effects in various subgroups.
Sensitivity analyses
We could not perform a sensitivity analysis for published trials versus unpublished trials because all available data originated from published trials. Sensitivity analyses for risk of bias were not meaningful because of the low number of studies investigating the same comparisons and outcomes and due to the diversity in follow‐up periods. We could not perform sensitivity analysis excluding large trials because none of the included trials had more than 1000 participants randomised to each intervention group. There were also no long‐term trials with a follow‐up period of six months or more, therefore a sensitivity analysis for long‐term trials was not possible. Diagnostic criteria were described in only three trials (Colagiuri 1989; Ensor 2015; Nehrling 1985), which had different comparison groups, therefore a sensitivity analysis for diagnostic criteria was not feasible. All included trials were published in English, and there were only two trials either declaring no commercial funding, Stern 1976, or providing no statement about funding in the manuscript, Chantelau 1985, therefore sensitivity analyses according to language of publication or excluding trials funded by a pharmaceutical company were not meaningful.
It was not feasible to combine results from cross‐over trials and trials with parallel design, as cross‐over trials were available only for the comparison NNS versus sugar, and trials with a parallel design were only available for the comparisons NNS versus placebo and NNS versus another type of sweetener. We performed sensitivity analyses to investigate the impact of assumed correlation coefficients for the imputation of the standard deviation of difference in cross‐over trials with mean difference as the measure of treatment effect (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9), and concluded that the assumed correlation coefficient has no relevant effect on the overall effect estimate.
4.1. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 1: HbA1c (%)
4.2. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 2: Body weight (kg)
4.3. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 3: Total cholesterol (mg/dL)
4.4. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 4: HDL cholesterol (mg/dL)
4.5. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 5: LDL cholesterol (mg/dL)
4.6. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 6: Triglycerides (mg/dL)
4.7. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 7: Fasting blood glucose levels (mg/dL)
4.8. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 8: Postprandial blood glucose levels (mg/dL)
4.9. Analysis.

Comparison 4: Sensitivity analysis: NNS versus sugar, Outcome 9: Serum insulin (microunits/mL)
Assessment of reporting bias
We did not use funnel plots due to the limited number of trials (N = 3 for the comparison NNS versus sugar, N = 5 for the comparison NNS versus placebo, and only one trial for the comparison NNS versus another type of sweetener).
Discussion
Summary of main results
This Cochrane Review investigated the health effects of NNS compared with any other type of dietary intervention in people with type 1 or type 2 diabetes. We included nine trials with a total of 979 randomised participants. We judged all trials to have unclear or high risk of bias in one or more ’Risk of bias’ domains. We found no evidence of benefit or harm on patient‐important outcomes. Evidence on the use of NNS showed neither clear benefit nor harm for HbA1c, body weight, and adverse events for the comparisons NNS versus sugar and NNS versus placebo (very low‐certainty evidence). For the comparison NNS versus a nutritive, low‐calorie sweetener (tagatose), there was a small benefit for HbA1c in favour of the nutritive, low‐calorie sweetener, based on very low‐certainty evidence and which we judged as clinically irrelevant.
Overall completeness and applicability of evidence
The evidence for health benefits or harms related to NNS consumption in diabetes mellitus as compared to a diet without NNS, a diet containing sugar, or a diet containing a nutritive, low‐calorie sweetener, is incomplete. We included nine completed trials involving adult participants with either type 1 or type 2 diabetes.
For the comparison NNS versus sugar, there were only three small cross‐over trials available, which contributed data for meta‐analyses for HbA1c (three trials), body weight (three trials), total cholesterol (three trials), HDL cholesterol (three trials), triglycerides (three trials), and fasting blood glucose levels (two trials). For the outcomes LDL cholesterol, postprandial blood glucose levels, and serum insulin, data were available from only one trial. Consequently, there remains a paucity of evidence regarding the effects of these interventions in diabetes on medium‐ or longer‐term health.
For the comparison NNS versus placebo, five trials were available, all with a parallel study design. Three of these trials were small, with fewer than 100 participants, whilst the other two trials included between 100 and 200 participants. These trials contributed data for meta‐analyses for HbA1c (four trials), body weight (two trials), total cholesterol (three trials), HDL cholesterol (two trials), LDL cholesterol (two trials), triglycerides (two trials), fasting blood glucose levels (five trials), serum insulin levels (two trials), and adverse events (three trials). For the outcomes BMI and postprandial blood glucose levels, data were available from only one trial.
For the comparison NNS versus a nutritive sweetener, only one trial was available, which provided data on the outcomes HbA1c, lipid profile, and fasting glucose.
There were no data from included trials with regard to health‐related quality of life, diabetes complications, all‐cause mortality, or socioeconomic effects.
Our ability to draw firm conclusions was further limited by notable variations in the characteristics of the interventions assessed (i.e. the different types of NNS used in different trials) and participants included in the trials (i.e. participants with type 1 or type 2 diabetes, with or without different comorbidities). Whilst we chose to combine trials with type 1 and type 2 diabetes participants in one comparison, and attempted to explore variation through subgroup analyses, our ability to do this was limited because of the low number of trials in total. Furthermore, the types of NNS used in the included trials varied widely amongst trials, but due to the low number of included trials we were also not able to conduct a subgroup analysis by type of NNS.
With regard to applicability, eight of the nine included trials were conducted in upper‐middle‐ or high‐income countries. This likely limits the generalisability of the findings to other countries, particularly low‐resource settings. Furthermore, in most of the included trials diagnostic criteria for diabetes were not specified, which may limit the interpretation of data.
Quality of the evidence
For all outcomes evaluated using GRADE, we judged the evidence to be of very low‐certainty for all three comparisons (NNS versus sugar, placebo, or a nutritive, low‐calorie sweetener). The evidence was downgraded primarily due to design limitations (risk of bias) and imprecision (small sample sizes and low number of included studies).
Potential biases in the review process
The search for trials in this area was performed using a broad search strategy, by searching in both electronic databases and trials registries, without applying restrictions, such as based on language. It is unlikely that trials that have been conducted and published have been missed; however, unpublished trials, or ongoing trials not registered in clinical trials registries could be missing. Should such trials be identified, we will include them in future updates of the review.
We aimed to reduce bias wherever possible by having at least two review authors work independently on trial selection, data extraction, and 'Risk of bias' and GRADE assessments.
We were not able to explore the potential for publication bias using funnel plots, since there were no outcomes of interest with 10 or more trials included in meta‐analyses.
Agreements and disagreements with other studies or reviews
In our search for additional trials we checked other systematic reviews and meta‐analyses. Most of these assessed the use of NNS compared to another dietary intervention in healthy or general populations (Azad 2017; Toews 2019), whilst the number of systematic reviews including participants with diabetes was limited.
One systematic review collecting evidence on the health effects of NNS in diabetes included not only medium‐ and long‐term outcomes, but also short‐term trials with an intervention duration of four weeks or less (Timpe Behnen 2013). After narratively summarising their findings, the authors of this systematic review concluded that "nonnutritive sweeteners do not appear to affect glycemic control in patients with diabetes". It should be noted that this systematic review included only studies published in English and considered only NNS available in the USA. To our knowledge, our review is the first systematic review attempting to address patient‐important outcomes, such as health‐related quality of life or socioeconomic effects.
Authors' conclusions
Implications for practice.
There is no firm evidence as to whether non‐nutritive sweeteners compared with any other type of dietary intervention (including sugar, placebo, or nutritive, low‐calorie sweeteners) have substantial effects on health outcomes. Data on patient‐important outcomes such as adverse events, diabetes complications, health‐related quality of life, and socioeconomic effects are scarce or lacking.
Implications for research.
It remains to be determined whether there are any substantial beneficial or harmful effects of consuming non‐nutritive sweeteners in people with type 1 or type 2 diabetes mellitus. There is a need for further long‐term randomised controlled trials conducted with rigorous methodology, with large sample size that are investigating patient‐relevant endpoints (especially adverse events, diabetes complications, health‐related quality of life, and socioeconomic effects).
History
Protocol first published: Issue 11, 2017 Review first published: Issue 5, 2020
Notes
We have based parts of the Methods, as well as Appendix 1 and Appendix 3, of this Cochrane Review protocol on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
The Information Specialist for the Cochrane Metabolic and Endocrine Disorders Group (CMED), Maria‐Inti Metzendorf, reviewed the MEDLINE search strategy and adapted it to CENTRAL, ClinicalTrials.gov, and the WHO ICTRP.
SL received research funding from the Alexander von Humboldt Foundation.
The review authors and the CMED editorial base are grateful to the peer reviewer Isolde Sommer, Department for Evidence‐based Medicine and Evaluation, Danube University Krems, Austria, for her time and comments.
Appendices
Appendix 1. Search strategies
| MEDLINE (Ovid SP) |
| 1. Non‐Nutritive Sweeteners/ 2. ((non nutritive or nonnutritive) adj3 sweetener*).mp. 3. ((high intensity or intense or high potency) adj3 sweetener*).mp. 4. ((non calori* or noncalori* or low calori* or lowcalori*) adj3 sweetener*).mp. 5. ((non sugar or nonsugar or artificial or natural) adj3 sweetener*).mp. 6. sugar substitute*.mp. 7. Aspartame/ 8. (aspartam* or NutraSweet).mp. 9. Saccharin/ 10. saccharin*.mp. 11. (trichlorosucrose or sucralose or Splenda).mp. 12. Stevia/ 13. (stevi* or sweetleaf* or rebiana* or rebaudioside*).mp. 14. Cyclamates/ 15. (cyclamate* or cyclamic acid).mp. 16. (acesulfam* or acetosulfam*).mp. 17. advantame.mp. 18. "luo han guo" or siraita or mogroside*.mp. 19. neohesperi*.mp. 20. neotame.mp. 21. thaumatin.mp. 22. or/1‐21 23. exp Diabetes Mellitus/ 24. diabet*.mp. 25. (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D).mp. 26. or/23‐25 27. 22 and 26 28. ..dedup 27 |
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
| 1. MESH DESCRIPTOR Non‐Nutritive Sweeteners 2. ((non nutritive or nonnutritive) ADJ3 sweetener*):TI,AB,KY 3. ((high intensity or intense or high potency) ADJ3 sweetener*):TI,AB,KY 4. ((non calori* or noncalori* or low calori* or lowcalori*) ADJ3 sweetener*):TI,AB,KY 5. ((non sugar or nonsugar or artificial or natural) ADJ3 sweetener*):TI,AB,KY 6. sugar substitute*:TI,AB,KY 7. MESH DESCRIPTOR Aspartame 8. (aspartam* or NutraSweet):TI,AB,KY 9. MESH DESCRIPTOR Saccharin 10. saccharin*:TI,AB,KY 11. (trichlorosucrose or sucralose or Splenda):TI,AB,KY 12. MESH DESCRIPTOR Stevia 13. (stevi* or sweetleaf* or rebiana* or rebaudioside*):TI,AB,KY 14. MESH DESCRIPTOR Cyclamates 15. (cyclamate* or cyclamic acid):TI,AB,KY 16. (acesulfam* or acetosulfam*):TI,AB,KY 17. advantame:TI,AB,KY 18. "luo han guo" or siraita or mogroside*:TI,AB,KY 19. neohesperi*:TI,AB,KY 20. neotame:TI,AB,KY 21. thaumatin:TI,AB,KY 22. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 23. MESH DESCRIPTOR Diabetes Mellitus EXPLODE ALL TREES 24. diabet*:TI,AB,KY 25. (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D):TI,AB,KY 26. #22 OR #23 OR #24 27. #22 AND #26 |
| ICTRP Search Portal (Standard search) |
| diabet* AND aspartam* OR diabet* AND saccharin* OR diabet* AND trichlorosucrose* OR diabet* AND sucralose* OR diabet* AND stevi* OR diabet* AND sweetleaf* OR diabet* AND rebiana* OR diabet* AND rebaudioside* OR diabet* AND cylcamate* OR diabet* AND cyclamic* OR diabet* AND acesulfam* OR diabet* AND acetosulfam* OR diabet* AND advantam* OR diabet* AND luo han guo OR diabet* AND siraita OR diabet* AND mogroside* OR diabet* AND neohesperi* OR diabet* AND neotame* OR diabet* AND thaumatin* OR diabet* AND sweetener* OR diabet* AND sugar substitute* |
| ClinicalTrials.gov (Expert search) |
| (sweeteners OR sweetener OR "sugar substitute" OR "sugar substitutes" OR aspartame OR NutraSweet OR saccharin OR trichlorosucrose OR sucralose OR Splenda OR stevia OR steviol OR stevioside OR sweetleaf OR rebiana OR rebaudioside OR cyclamate OR cyclamates OR "cyclamic acid" OR acesulfam OR acesulfame OR acetosulfam OR acetosulfame OR advantame OR "luo han guo" OR mogroside OR siraita OR neohesperidin OR neotame OR thaumatin) AND (diabetes OR diabetic OR IDDM OR NIDDM OR MODY OR T1DM OR T2DM OR T1D OR T2D) |
| Scopus (www.scopus.com) |
| TITLE‐ABS‐KEY ( "non nutritive sweetener*" OR "nonnutritive sweetener*" OR "high intensity sweetener*" OR "intense sweetener*" OR "high potency sweetener*" OR "non calori* sweetener*" OR "noncalori* sweetener*" OR "low calori* sweetener*" OR "lowcalori* sweetener*" OR "non sugar sweetener*" OR "nonsugar sweetener*" OR "artificial sweetener*" OR "natural sweetener*" OR "sugar substitute*" OR aspartam* OR nutrasweet OR saccharin* OR trichlorosucrose OR sucralose OR splenda OR stevi* OR sweetleaf* OR rebiana* OR rebaudioside* OR cyclamate* OR "cyclamic acid*" OR acesulfam* OR acetosulfam* OR advantame OR "luo han guo" OR mogroside OR siraita OR neohesperi*din OR neotame OR thaumatin ) AND TITLE‐ABS‐KEY ( diabetes OR diabetic OR iddm OR niddm OR mody OR t1dm OR t2dm OR t1d OR t2d ) |
Appendix 2. Assessment of risk of bias
| 'Risk of bias' domains |
|
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included trial, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment) We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgement for selection bias (Corbett 2014). Chance imbalances may also affect judgements on the risk of attrition bias. In the case of unadjusted analyses, we distinguished between trials that we rated as being at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials we judged as being at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We will reclassify judgements of unclear, low, or high risk of selection bias as specified in Appendix 3. Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed, or adjudicated outcome measures (see below).
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed, or adjudicated outcome measures (see below).
Incomplete outcome data (attrition bias due to amount, nature, or handling of incomplete outcome data) For each included trial and/or each outcome, we described the completeness of data, including attrition and exclusions from the analyses. We stated whether the trial reported attrition and exclusions, and report the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator groups). We also noted if the trial reported the reasons for attrition or exclusion and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
Selective reporting (reporting bias due to selective outcome reporting) We assessed outcome reporting bias by integrating the results of Appendix 9 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mathieu 2009), with those of Appendix 10 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for trials reporting unadjusted analyses: comparison of results obtained using method details alone with results using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | 'Risk of bias' judgementusing methods reporting | Information gained from study characteristics data | Risk of bias using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly randomised, or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias, compared with using methods reporting alone. bImbalance identified that appears likely to be due to chance. cDetails for the remaining important prognostic variables are not reported. | |||
Appendix 4. Descriptions of participants
| Trial ID | ||
| Ensor 2015 | Inclusion criteria |
|
| Exclusion criteria |
|
|
| Diagnostic criteria | According to WHO criteria | |
| Barriocanal 2008 | Inclusion criteria | For Group 1
For Group 2
|
| Exclusion criteria |
|
|
| Diagnostic criteria | — | |
| Maki 2008 | Inclusion criteria |
|
| Exclusion criteria |
|
|
| Diagnostic criteria | — | |
| Grotz 2003 | Inclusion criteria |
|
| Exclusion criteria | — | |
| Diagnostic criteria | — | |
| Colagiuri 1989 | Inclusion criteria |
|
| Exclusion criteria | — | |
| Diagnostic criteria | Based on the National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance (National Diabetes Data Group 1979). | |
| Cooper 1988 | Inclusion criteria | Type 2 diabetes mellitus outpatients |
| Exclusion criteria |
|
|
| Diagnostic criteria | — | |
| Chantelau 1985 | Inclusion criteria |
|
| Exclusion criteria | — | |
| Diagnostic criteria | — | |
| Nehrling 1985 | Inclusion criteria |
|
| Exclusion criteria | — | |
| Diagnostic criteria | Diagnosis of diabetes had been established by a fasting plasma glucose > 140 mg/dL, an abnormal oral glucose tolerance test as interpreted by the US Public Health Service criteria, or an unequivocal history of diabetes; insulin‐dependent diabetes mellitus: individuals who, by history, developed ketosis or ketoacidosis when adequate exogenous insulin was not provided; non‐insulin‐dependent diabetes mellitus: individuals who are not on insulin and are not ketotic or who, if on insulin, have no history of ketoacidosis | |
| Stern 1976 | Inclusion criteria |
|
| Exclusion criteria | — | |
| Diagnostic criteria | — | |
| —: denotes not reported BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; DPP: dipeptidyl peptidase; GLP: glucagon‐like peptide; HbA1c: glycosylated haemoglobin A1c; WHO: World Health Organization. | ||
Appendix 5. Description of interventions*
| Trial ID | Ensor 2015 |
| Brief name | Sucralose (Splenda) or tagatose dissolved in water 3 times a day |
| Recipient | Participants with type 2 diabetes |
| Why | "D‐tagatose provides glycemic and lipoprotein control through a mechanism of action unlike any agent that is currently available on the market in the United States." |
| What (materials) | Sucralose (in form of Splenda) 1.5 g or tagatose 15 g, 3 times a day, dissolved in 125 to 250 mL of water |
| What (procedures) | "after the 8 week lead‐in period, fasting (minimum of 8 hours) subjects returned to the study sites and underwent medical history review followed by baseline tests" "the treatment period consisted of 12 monthly visits, the first (...) of which was used to gather the baseline data for efficacy and safety parameters and also included the first distribution of test and placebo treatments" "subsequent visits occurred monthly" |
| Who provided | "subjects continued on a weight‐maintaining diet plus exercise under physician's recommendation" |
| How (mode of delivery; individual or group) | "visits occurred monthly and were of two types: (1) supply visits and (2) supply and procedures visits"; supply refers to the "distribution of test and placebo treatments" |
| Where | At the "study sites" |
| When and how much | Study products had to be taken 3 times a day for 10 months. |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | "Three populations were evaluated: the intention‐to‐treat (ITT) population consisted of all randomized subjects who received at least one dose of their randomized treatment (...), the per protocol (PP) population consisted of all ITT subjects who had at least 80% compliance with medication for 75% of the dosing time points and had no major protocol violations (...), the safety population consisted of all randomized subjects who received at least one dose of their randomized treatment and had at least one post‐treatment visit evaluating safety." "Investigators were to withdraw subjects from study treatment (and therefore the evaluable population for assessment of efficacy as measured by HbA1c) after additional antidiabetic medication has been prescribed. However, subjects were advised to continue the rest of the trial procedures for the assessment of safety parameters." |
| Extent of intervention fidelity | "Analysis population", sucralose vs tagatose: ITT, 72.7% vs 71.4%; PP, 47.0% vs 35.3%; safety, 81.8% vs 76.8%. "The ITT population was approximately evenly divided between males and females (...) with approximately equivalent distributions in the D‐tagatose and placebo" (that means sucralose) groups |
| Trial ID | Barriocanal 2008 |
| Brief name | Steviol glycoside or placebo capsules 3 times a day |
| Recipient | Participants with type 1 diabetes, type 2 diabetes, and healthy controls |
| Why | "According to the Joint FAO/WHO Expert Committee on Food Additives (JECFA 2004), consumption of Stevia has been generally regarded as safe. However, JECFA requested additional information in order to change the temporary accepted daily intake (ADI) of 0‐2 mg/kg/day for steviol glycoside, including the potential effects of low doses on blood glucose and blood pressure." |
| What (materials) | Steviol glycoside capsules 250 mg 3 times a day or matching placebo. "Steviol glycoside was provided by Steviafarma Industrial S.A., Maringa, Brazil. Purity of steviol glycosides (measured three times) was ≥ 92%" |
| What (procedures) | "Volunteers attended the investigation centre every 2 weeks during the 3‐months study period for determination of capillary blood glucose, BP and weight. At these visits, volunteers were asked about adverse events and the capsules were counted to check for compliance" |
| Who provided | — |
| How (mode of delivery; individual or group) | There were face‐to‐face visits at regular intervals: "Volunteers attended the investigation centre every 2 weeks..." |
| Where | At "the investigation centre" |
| When and how much | Capsules had to be taken 3 times a week for 3 months. |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | "the capsules were counted to check for compliance" |
| Extent of intervention fidelity | "All volunteers who completed the study followed the prescribed treatment schedule throughout the 3‐month period, and degree of compliance was similar in both groups (steviol glycoside and placebo)" |
| Trial ID | Maki 2008 |
| Brief name | Rebaudioside A or placebo capsules 4 times a day |
| Recipient | Men and women with type 2 diabetes |
| Why | "The Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) specifically requested additional studies involving repeated exposure to dietary and therapeutic doses of steviol glycosides in people with diabetes to help define an acceptable intake of steviol glycosides (...). The present study, conducted as part of a clinical program designed to address the question raised by the JECFA" |
| What (materials) | "rebaudioside A (97% purity; rebiana, the common name for rebaudioside A) in 250 mg capsules provided by Cargill, Incorporated, Wayzata, MN" or placebo (microcrystalline cellulose). "Subjects took four capsules each day: two 250 mg capsules (rebaudioside A or placebo) with the first meal of the day and two 250 mg capsules (rebaudioside A or placebo) with the evening meal to achieve a daily dosage of 1000 mg" |
| What (procedures) | "Subjects visited the clinic four times at four‐week intervals during the 16‐week treatment period for laboratory assessments. Study coordinators contacted the subjects between the clinic visits at four‐week intervals beginning two weeks after randomization, to reinforce study instructions and answer questions" "Compliance was assessed by capsule count and subject interview" |
| Who provided | "Study coordinators contacted the subjects...." |
| How (mode of delivery; individual or group) | Face‐to‐face visits were held every 4 weeks; between these visits another way of contact (no details provided) was established every 4 weeks. |
| Where | "Subjects visited the clinic..." |
| When and how much | Four 250 mg capsules were to be taken each day, for 16 weeks. |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | "To be eligible for randomization, subjects were required to be at least 80% compliant with taking placebo capsules (microcrystalline cellulose) during the lead‐in period." "Compliance was assessed by capsule count and subject interview" |
| Extent of intervention fidelity | "Mean study product compliance in the rebaudioside A and placebo groups was 96.3% and 100%, respectively (p = 0.207)" |
| Trial ID | Grotz 2003 |
| Brief name | Sucralose or placebo capsules |
| Recipient | Men and women with type 2 diabetes |
| Why | "Consumption of sucralose is expected in those with diabetes, who often use non‐nutritive sweeteners to reduce their intake of refined sugars (Toeller 1993). Moreover, mean sucralose consumption may be more in this population..." |
| What (materials) | "Subjects received two capsules per day of either placebo or sucralose (McNeil Specialty Products Company, New Brunswick, NJ), to be taken at breakfast and dinnertime for the next 13 weeks. The daily sucralose does was 667 mg." |
| What (procedures) | "Test material compliance was checked by pill count and by qualitative measurement of sucralose in urine samples collected once every 2 weeks beginning 2 weeks before the test phase. During the test phase, subjects were seen at least once every 2 weeks for HbA1c, fasting plasma glucose, and fasting serum C‐peptide assessment. Additionally, any adverse events or changes in medications, including antidiabetic ones, were recorded" |
| Who provided | — |
| How (mode of delivery; individual or group) | Face‐to‐face meetings were held every 2 weeks during the intervention period. |
| Where | At "five US medical centers" |
| When and how much | 2 capsules were to be taken each day, for 13 weeks. |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | The "4‐week placebo‐blind run‐in period was designed to help distance from the actual test phase of the study any nontreatment effects that might occur with test phase initiation, such as possible changes in dietary behaviors. Baseline blood glucose homeostasis measures were taken at the end of the 4‐week placebo run‐in..." "Test material compliance was checked by pill count and by qualitative measurement of sucralose in urine samples collected once every 2 weeks beginning 2 weeks before the test phase" |
| Extent of intervention fidelity | "More than 96% of subjects in both groups were considered compliant based on capsule counts and the results of the qualitative assays for sucralose in collected urine samples" |
| Trial ID | Colagiuri 1989 |
| Brief name | Aspartame or sucrose added to the usual diet |
| Recipient | Men and women with type 2 diabetes |
| Why | "The use of sweetening agents by diabetic individuals is common. A survey of our diabetic clinic population showed that 65% regularly use these products. (...) Medium‐term studies that have examined the addition of sucrose to the diet of noninsulin‐dependent diabetes mellitus (NIDDM) subjects for periods of 2‐6 wk have produced conflicting results (Coulston 1985; Peterson 1986; Bantle 1986; Coulston 1987)" |
| What (materials) | "Subjects were randomly allocated to one of two groups. 1) Sucrose (45 g) was added to the usual diet. The three main meals were supplemented with 10 g sucrose, and 5 g sucrose was added to the midmorning, mid afternoon, and supper tea or coffee. 2) Aspartame (162 mg) was added to the usual diet. ... Each of the three main meals was supplemented with 36 mg aspartame, and 18 mg aspartame was added to the between‐meal beverages". "The sucrose and aspartame were packed in plain sachets labelled A or B according to a code. Each sachet contained 5 g sucrose or 18 mg of aspartame (Equal®, Searle Laboratories, Crows Nest, New South Wales, Australia) bulked to 0.5 g with lactose" |
| What (procedures) | "Subjects remained in each group for 6 wk and then transferred to the comparative treatment group for a further 6 wk. The subject's ability to comply with the study requirements was assessed regularly throughout both dietary periods." |
| Who provided | — |
| How (mode of delivery; individual or group) | — |
| Where | — |
| When and how much | In the sucrose group the 3 main meals were supplemented with 10 g sucrose, and 5 g sucrose was added to the mid‐morning, mid‐afternoon, and supper tea or coffee. In the aspartame group each of the 3 main meals was supplemented with 36 mg aspartame, and 18 mg aspartame was added to the between‐meal beverages. |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| Trial ID | Cooper 1988 |
| Brief name | Saccharin and starch or sucrose |
| Recipient | Men and women with type 2 diabetes |
| Why | "The effects of using moderate amounts of sucrose as a sweetener for non‐insulin‐dependent diabetic patients who are consuming their usual 'diabetic diets' at home are unknown." |
| What (materials) | "The usual diet of each patient was supplemented daily with either 28 g sucrose (sucrose diet) or saccharin and starch (saccharin diet). The saccharin and starch supplements were equivalent to about 28 g sucrose in sweetness and energy, respectively." "The supplements were divided amongst each of three main meals and in case of sucrose, an evening supper. The usual foods to which the supplements were added were hot beverages, fruit juice, milk, cereals, and stewed fruit. The test meals consisted of a standard breakfast (cereal, whole milk, wholemeal bread, polyunsaturated margarine, and tea, coffee or water) to which either 8 g sucrose or 1 saccharin tablet plus 10 g corn flour were added. The test meals provided 1.5 MJ (15% protein, 33% fat, 52% carbohydrate, 3.3 g fibre). The sucrose supplement was the sole source of sucrose in the test meal and it represented 8.2% of total meal energy." |
| What (procedures) | "Patients were visited weekly for delivery of supplements, weight recording, and encouragement of compliance. At the beginning and end of each dietary period they visited hospital on two consecutive mornings for metabolic assessment with the test meals given in random order. For test meals, patients were fasted overnight and rested throughout the experimental procedure. ... All meal studies commenced between 0830 and 1000 h. The time taken for meal consumption was kept constant for each patient and ranged between 8 and 15 min." |
| Who provided | — |
| How (mode of delivery; individual or group) | Face‐to‐face contact was established every week: "Patients were visited weekly for delivery of supplements..." "At the beginning and end of each dietary period they visited hospital on two consecutive mornings for metabolic assessment..." |
| Where | "Patients were visited" in their homes and "At the beginning and end of each dietary period they visited hospital" |
| When and how much | 3 times a day, for 6 weeks |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | "Since adherence to usual diet was an important condition of this study, it was strongly emphasized that there should be no change in usual eating pattern, other than by the addition of the supplements. Food records were kept throughout the study" |
| Extent of intervention fidelity | "no variation in eating patterns was detected" |
| Trial ID | Chantelau 1985 |
| Brief name | Sodium‐cyclamate or sucrose |
| Recipient | Men and women with type 1 diabetes |
| Why | "the potential effects of a long‐term use of sucrose in Type 1 diabetes are unknown" |
| What (materials) | "During the sucrose‐period, sucrose and sucrose‐sweetened foods were allowed ad libidum. The patients were provided with a brochure listing the carbohydrate and sugar content of sucrose‐containing foods. The intake of sucrose‐sweetened soft drinks, however, was discouraged. During the cyclamate period, sodium cyclamate was allowed ad libidum within the limitations set up by the World Health Organisation (JECFA 1982), i.e. not more than 11 mg/kg body weight per day." "During the cyclamate period, the patients were given packages of cyclamate tablets and liquids." |
| What (procedures) | "After a 4‐week run‐in‐period, patients were assigned to use either sucrose or sodium‐cyclamate as sweetener in random order for 4 weeks each. They were then asked to change over to sodium‐cyclamate or sucrose, respectively, for another 4‐week period." "For study purposes, patients were asked to note the frequency and the amount of sucrose intake as assessed in common measures, such as 'one teaspoon of sugar', 'one sugar cube', 'one Mars bar', 'one piece of Black Forest cake' etc." "The consumption of sodium cyclamate was assessed at the end of the study period by counting the tablets and measuring the liquids that were left." "To obtain a more detailed assessment of nutrient intake, a 3‐day dietary monitoring period was carried out within each of the three observation periods." |
| Who provided | — |
| How (mode of delivery; individual or group) | Face‐to‐face meetings were established every second week during the intervention period. |
| Where | "Bi‐weekly, all patients were followed up in our outpatient clinic..." |
| When and how much | Ad libitum for 4 weeks |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | "To obtain a more detailed assessment of nutrient intake, a 3‐day dietary monitoring period was carried out within each of the three observation periods." |
| Extent of intervention fidelity | "The evaluation of the 3‐day dietary monitoring revealed that nutrient intake was comparable between the three observation periods", i.e. the run‐in period, the cyclamate period, and the sucrose period, "with regard to the consumption of carbohydrates, protein and fat." |
| Trial ID | Nehrling 1985 |
| Brief name | Aspartame or placebo capsules |
| Recipient | Adult participants with type 1 or type 2 diabetes |
| Why | "Recently, several anecdotal and undocumented reports in the lay press have suggested a number of adverse reactions associated with use of aspartame. These include headaches and other neurologic symptoms. The use of a placebo‐controlled, double‐blind experimental design allowed us to evaluate the significance of these reports" |
| What (materials) | "Aspartame was given in the form of capsules, each containing 0.3 g aspartame. Three capsules were taken with each meal, for a total of 9 capsules per day (2.7 g aspartame). The placebo consisted of identical capsules filled with 0.2 g corn starch." "Capsules were provided in coded bottles, which contained either aspartame or placebo according to a randomization table" |
| What (procedures) | "Capsules ... were assigned to subjects in sequential order. Separate sequences were used for IDDM and NIDDM, to insure equal numbers in each group." "Each subject had two baseline visits 1 wk apart.... Subjects started taking aspartame or placebo capsules after the second set of baseline blood samples were drawn. During the study, each subject kept a log of capsules taken. Subjects were asked to make up missed or forgotten capsules with the next meal, or as soon as they remembered." "Return visits were scheduled after 3, 6, 9, 12, 15, 17, and 18 wk taking the capsules. At each visit, the logs of capsules ingested were collected, the bottles of capsules collected and remaining capsules counted, and a new bottle of capsules disbursed." |
| Who provided | — |
| How (mode of delivery; individual or group) | Face‐to‐face visits were held regularly: "Return visits were scheduled after 3, 6, 9, 12, 15, 17, and 18 wk taking the capsules." |
| Where | "Subjects were recruited from clinics of the University of Illinois Hospital" |
| When and how much | 3 times a day, for 18 weeks |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | "At each visit, the logs of capsules ingested were collected, the bottles of capsules collected and remaining capsules counted, and a new bottle of capsules disbursed." |
| Extent of intervention fidelity | "There were no serious instances of noncompliance, although a number of subjects forgot occasional doses and later made up the missing doses." |
| Trial ID | Stern 1976 |
| Brief name | Aspartame or placebo capsules |
| Recipient | Men and women with type 2 diabetes |
| Why | "In those persons with metabolic disorders that require limited use of sugar, such as diabetes mellitus, aspartame would be of special value." |
| What (materials) | "1.8 g aspartame daily for 90 days" "Those receiving aspartame received 0.6 g three times daily for a total of 1.8 g daily" "They were instructed to continue their usual diet and to take two capsules of the assigned study preparation three times daily with meals." |
| What (procedures) | — |
| Who provided | — |
| How (mode of delivery; individual or group) | — |
| Where | — |
| When and how much | Capsules had to be consumed 3 times daily with meals. |
| Tailoring | NA |
| Modification of intervention throughout the trial | NA |
| Strategies to improve or maintain intervention fidelity | — |
| Extent of intervention fidelity | — |
| *This table is based on the TIDieR checklist (Hoffmann 2014). —: denotes not reported BP: blood pressure; DB: diet beverage; IDDM: insulin‐dependent diabetes mellitus; ITT: intention‐to‐treat; JECFA: Joint FAO/WHO Expert Committee on Food Additives; NA: not applicable; NIDDM: non‐insulin‐dependent diabetes mellitus; PP: per protocol; TIDieR: template for intervention description and replication; wk: week. | |
Appendix 6. Baseline characteristics (I)
| Trial ID | Intervention(s) and comparator(s) | Duration of intervention/duration of follow‐upa | Description of participants | Trial period | Country | Setting | Ethnic groups (%) | Duration of diabetes (mean years (SD)) |
| Ensor 2015 | I: sucralose | 10 months/10 months | Men and women with type 2 diabetes | ‐ | India, USA | Outpatients | Asian: 72 Caucasian (understood to be white): 12 Latino: 11 Black: 5 |
‐ |
| C: D‐tagatose | ‐ | |||||||
| Barriocanal 2008 | I: steviol glycoside | 3 months/3 months | Men and women with type 1 diabetes, type 2 diabetes, and healthy participants | 2005 to 2006b | Paraguay | Outpatients | ‐ | Type 1 diabetes patients: > 5 years; type 2 diabetes patients: > 1 year and < 10 years |
| C: placebo | ‐ | Type 1 diabetes patients: > 5 years; type 2 diabetes patients: > 1 year and < 10 years | ||||||
| Maki 2008 | I: rebaudioside A | 16 weeks/16 weeks | Men and women with type 2 diabetes | 2006 to 2007b | USA | Outpatients | Non‐Hispanic white: 68 African‐American: 22 Hispanic: 8 Other: 2 | > 1 year |
| C: placebo | Non‐Hispanic white: 73 African‐American: 19 Hispanic: 6 Other: 2 | > 1 year | ||||||
| Grotz 2003 | I: sucralose | 13 weeks/17 weeks | Men and women with type 2 diabetes | ‐ | USA | Outpatients | White: 76 Black: 13 Asian: 5 Hispanic: 6 Other: 0 | 9.3 (6.9) |
| C: placebo | White: 83 Black: 6 Asian: 3 Hispanic: 7 Other: 1 | 10.17 (7.7) | ||||||
| Colagiuri 1989 | I: aspartame | 6 weeks/6 weeks | Men and women with type 2 diabetes | ‐ | Australia | Outpatients | ‐ | 8.6 (5.0) |
| C: sucrose | ‐ | 8.6 (5.0) | ||||||
| Cooper 1988 | I: saccharin and starch | 6 weeks/6 weeks | Men and women with type 2 diabetes | ‐ | Australia | Outpatients | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ||||||
| Chantelau 1985 | I: sodium‐cyclamate | 4 weeks/4 weeks | Men and women with type 1 diabetes | 1985 | Germany | Outpatients | ‐ | > 1 year |
| C: sucrose | ‐ | > 1 year | ||||||
| Nehrling 1985 | I: aspartame | 18 weeks/18 weeks | Adult participants with type 1 or type 2 diabetes | ‐ | USA | Outpatients | ‐ | ‐ |
| C: placebo (cornstarch) | ‐ | ‐ | ||||||
| Stern 1976 | I: aspartame | 13 weeks/13 weeks | Men and women with type 2 diabetes | ‐ | USA | Outpatients | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ||||||
| ‐: denotes not reported aFollow‐up under randomised conditions until end of trial (= duration of intervention + follow‐up postintervention or identical to duration of intervention). bDates were not clearly stated. C: comparator; I: intervention; SD: standard deviation. | ||||||||
Appendix 7. Baseline characteristics (II)
| Trial ID | Intervention(s) and comparator(s) | Sex (female %) | Age (mean/range years (SD)) | HbA1c (%) | BMI (mean kg/m² (SD)) | Co‐medications/Co‐interventions (% of participants) | Comorbidities (% of participants) |
| Ensor 2015 | I: sucralose | ‐ | 52/22 to 74 | ‐ | ≤ 25 | ‐ | ‐ |
| C: D‐tagatose | ‐ | ≤ 25 | ‐ | ‐ | |||
| Barriocanal 2008 | I: steviol glycoside | 52.3 | 25.4; 58.2a,b |
7.1 (1.6) 6.8 (1.2)a |
23.2 (3.3) 28.7 (3.4)a |
Antihypertensive medication (‐) | Hypertension (‐) |
| C: placebo | ‐ | ‐ | 8.2 (1.4) 6.8 (1.6)a |
22.4 (1.0) 30.1 (3.3)a |
Antihypertensive medication (‐) | Hypertension (‐) | |
| Maki 2008 | I: rebaudioside A | 46.7 | 59.1 (9.3) | 6.7 (0.9) | 33.7 (4.6) | Insulin (11.7) Sulphonylurea (33.3) Metformin (73.3) Thiazolidinedione (28.3) Antihypertensive medication (56.7) Dyslipidaemia medication (66.7) | Hypertension (56.7) Dyslipidaemia (66.7) |
| C: placebo | 51.6 | 61.5 (8.7) | 6.7 (0.8) | 33.6 (4.7) | Insulin (9.7) Sulphonylurea (41.9) Metformin (71) Thiazolidinedione (45.2) Antihypertensive medication (71) Dyslipidaemia medication (62.9) | Hypertension (71) Dyslipidaemia (62.9) |
|
| Grotz 2003 | I: sucralose | 25 | 57.2 (8.4) | ‐ | 31.6 (5.6) | Insulin (46) Oral hypoglycaemic agent (54) |
‐ |
| C: placebo | 33 | 58.0 (8.7) | ‐ | 31.6 (7.6) | Insulin (48) Oral hypoglycaemic agent (52) |
‐ | |
| Colagiuri 1989 | I: aspartame | 11.1 | 65.9 (2.1) | 7.2 (1.1) | 26.4 (2.1) | Sulphonylurea (66.6) | ‐ |
| C: sucrose | 11.1 | 65.9 (2.1) | 7.2 (1.1) | 26.4 (2.1) | Sulphonylurea (66.6) | ‐ | |
| Cooper 1988 | I: saccharin and starch | 64.7 | 62.2 (14.0) | 8.1 (7.3 ‐ 17.8) | 26.0 (3.0) | ‐ | ‐ |
| C: sucrose | 64.7 | 62.2 (14.0) | 8.1 (7.3 ‐ 17.8) | 26.0 (3.0) | ‐ | ‐ | |
| Chantelau 1985 | I: sodium‐cyclamate | 80 | 25 to 43 | 7.55 (0.42) | < 25 | Insulin (100) | ‐ |
| C: sucrose | 80 | 25 to 43 | 7.55 (0.42) | < 25 | Insulin (100) | ‐ | |
| Nehrling 1985 | I: aspartame | ‐ | ‐ | 12.0 (3.2) | ‐ | ‐ | ‐ |
| C: placebo (cornstarch) | ‐ | ‐ | 10.7 (2.3) | ‐ | ‐ | ‐ | |
| Stern 1976 | I: aspartame | 82.6 | 21 to 70b | ‐ | ‐ | Oral hypoglycaemic agent (‐) | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | Oral hypoglycaemic agent (‐) | ‐ | |
| ‐: denotes not reported aData are reported first for participants with type 1 diabetes (group 1), then for those with type 2 diabetes (group 2). bData are available for the whole trial population only. BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; s.c.: subcutaneous; SD: standard deviation. | |||||||
Appendix 8. Trial endpoints and timing of outcome measurement
| Trial ID | Review's primary and secondary outcomes | Timing of outcome measurement |
| Ensor 2015 | HbA1c | 2, 4, 6, 8, 10 months |
| Body weight (kg) | 2, 4, 6, 8, 10 months | |
| Adverse events | 10 months | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | 2, 4, 6, 8, 10 months | |
| Lipid profile | 2, 4, 6, 8, 10 months | |
| Glucose levels (fasting) | 2, 4, 6, 8, 10 months | |
| Serum insulin | 2, 4, 6, 8, 10 months | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Barriocanal 2008 | HbA1c | 3 months |
| Body weight (kg) | 4, 6, 8, 10 weeks and 3 months | |
| Adverse events | 3 months | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | 3 months | |
| Lipid profile | 3 months | |
| Glucose levels (fasting) | 3 months | |
| Serum insulin | 3 months | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Maki 2008 | HbA1c | 4, 8, 12, 16 weeks |
| Body weight (kg) | 12, 16 weeks | |
| Adverse events | 16 weeks | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | 12, 16 weeks | |
| Glucose levels (fasting) | 4, 8, 12, 16 weeks | |
| Serum insulin | 12, 16 weeks | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Grotz 2003 | HbA1c | 2, 4, 6, 8, 10, 12, 13, 15, 17 weeks |
| Body weight (kg) | ‐ | |
| Adverse events | 17 weeks | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | ‐ | |
| Glucose levels (fasting) | 2, 4, 6, 8, 10, 12, 13, 15, 17 weeks | |
| Serum insulin | ‐ | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Colagiuri 1989 | HbA1c | 6 weeks |
| Body weight (kg) | 6 weeks | |
| Adverse events | ‐ | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | 6 weeks | |
| Glucose levels (fasting) | 6 weeks | |
| Serum insulin | ‐ | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Cooper 1988 | HbA1c | 6 weeks |
| Body weight (kg) | 6 weeks | |
| Adverse events | ‐ | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | 6 weeks | |
| Glucose levels (fasting) | 6 weeks | |
| Serum insulin | 6 weeks | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Chantelau 1985 | HbA1c | 4 weeks |
| Body weight (kg) | 4 weeks | |
| Adverse events | ‐ | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | 4 weeks | |
| Glucose levels (postprandial) | 4 weeks | |
| Serum insulin | ‐ | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Nehrling 1985 | HbA1c | 9, 17, 18 weeks |
| Body weight (kg) | ‐ | |
| Adverse events | 18 weeks | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | ‐ | |
| Glucose levels (fasting and postprandial) | 9, 17, 18 weeks | |
| Serum insulin | ‐ | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| Stern 1976 | HbA1c | ‐ |
| Body weight (kg) | 13 weeks | |
| Adverse events | 13 weeks | |
| Diabetes complications | ‐ | |
| All‐cause mortality | ‐ | |
| Health‐related quality of life | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | 13 weeks | |
| Glucose levels (fasting) | 4, 8, 13 weeks | |
| Serum insulin | ‐ | |
| Insulin sensitivity | ‐ | |
| Socioeconomic effects | ‐ | |
| ‐: denotes not reported HbA1c: glycosylated haemoglobin A1c | ||
Appendix 9. Matrix of trial endpoints (publications and trial documents)
| Trial ID | Endpoints |
| Ensor 2015 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:NCT00955747 Primary outcome measure: HbA1c Secondary outcome measure(s): ‐ Other outcome measures: ‐ | |
|
Source:CTRI/2009/091/000536 Primary outcome measure: HbA1c Secondary outcome measure(s): body weight, lipid profile, glucose levels (fasting), serum insulin Other outcome measures: ‐ | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: HbA1c, adverse events Secondary outcome measure(s): body weight, anthropometric measures other than body weight (kg), lipid profile (total‐C, HDL, LDL, TG), glucose levels (fasting), serum insulin Other outcome measures: ‐ | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: HbA1c, adverse events Secondary outcome measure(s): anthropometric measures other than body weight (kg), lipid profile (total‐C, HDL, LDL, TG), glucose levels (fasting), serum insulin Other outcome measures: ‐ | |
| Barriocanal 2008 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, body weight (IA), adverse events, anthropometric measures other than body weight (kg), lipid profile (total‐C, HDL, LDL, TG), glucose levels (f/pp?) (IA), serum insulin | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, adverse events, glucose levels | |
| Maki 2008 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: HbA1c Secondary outcome measure(s): ‐ Other outcome measures: body weight (kg), adverse events, lipid profile (total‐C, HDL, LDL, TG), fasting glucose levels, serum insulin | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, body weight (kg), adverse events, lipid profile, fasting glucose levels, serum insulin | |
| Grotz 2003 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, fasting glucose levels (IA), adverse events | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: HbA1c, fasting glucose levels (IA) Secondary outcome measure(s): ‐ Other outcome measures: adverse events | |
| Colagiuri 1989 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, body weight (IA), lipid profile (total‐C, HDL, TG), fasting glucose levels (IA) | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c; lipid profile; fasting glucose levels; serum insulin | |
| Cooper 1988 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, body weight (IA), lipid profile (total‐C, HDL, LDL, TG), fasting glucose levels (IA), serum insulin | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: lipid profile (TG), fasting glucose levels (IA), serum insulin | |
| Chantelau 1985 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, body weight (IA), lipid profile, postprandial glucose levels (IA, SR) | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, body weight, lipid profile, glucose levels (IA, SR) | |
| Nehrling 1985 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, adverse events, fasting glucose levels (IA), postprandial glucose levels (IA) | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: HbA1c, adverse events, fasting glucose levels, postprandial glucose levels | |
| Stern 1976 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: body weight (unit?), adverse events, lipid profile (total‐C, TG), fasting glucose levels | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measure: ‐ Secondary outcome measure(s): ‐ Other outcome measures: adverse events | |
| ‐ denotes not reported
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents, or multiple reports of a primary trial). cPrimary and secondary outcomes refer to verbatim specifications in publication/records. Unspecified outcome measures refer to all outcomes not described as primary or secondary outcome measures. f/pp?: not clear whether fasting or postprandial; FDA/EMA: US Food and Drug Administration/European Medicines Agency; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HOMA: homeostatic model assessment; IA: investigator‐assessed; LDL: low‐density lipoprotein; NT: no trial documents available; SR: self‐reported; TG: triglycerides; total‐C: total cholesterol. |
Appendix 10. High risk of outcome reporting bias according to Outcome Reporting Bias In Trials (ORBIT) classification
| Trial ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Ensor 2015 | Body weight | Yes | No | No | No |
| Anthropometric measures other than body weight (kg) | Yes | No | No | No | |
| Barriocanal 2008 | Body weight | Yes | No | No | No |
| Anthropometric measures other than body weight (kg) | Yes | No | No | No | |
| Maki 2008 | NA | ||||
| Grotz 2003 | HbA1c | Yes | No | No | No |
| Adverse events | Yes | No | No | No | |
| Colagiuri 1989 | NA | ||||
| Cooper 1988 | NA | ||||
| Chantelau 1985 | NA | ||||
| Nehrling 1985 | NA | ||||
| Stern 1976 | Glucose levels, fasting | No | No | Yes | No |
|
aClear that outcome was measured and analysed; trial report stated that outcome was analysed but reported only that result was not significant (Classification 'A', table 2, Kirkham 2010).
bClear that outcome was measured and analysed; trial report stated that outcome was analysed but reported no results ( Classification 'D', table 2, Kirkham 2010).
cClear that outcome was measured but was not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (Classification 'E', table 2, Kirkham 2010).
dUnclear whether outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010). HbA1c: glycosylated haemoglobin A1c; NA: not applicable. | |||||
Appendix 11. Definition of endpoint measurementa
| Study ID | Endpoints | Definition |
| Ensor 2015 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Body weight | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | BMI | |
| Lipid profile | Blood lipids (total cholesterol, HDL and LDL cholesterol, triglyceride levels; IO) | |
| Glucose levels (fasting and postprandial) | Fasting blood glucose (IO) | |
| Insulin sensitivity/serum insulin | Insulin (IO) | |
| All hypoglycaemic events | Reported episodes of hypoglycaemia (SO) | |
| Severe/serious hypoglycaemia | ‐ | |
| Nocturnal hypoglycaemia | ‐ | |
| Severe/serious adverse events (specify) | Incidence of SAEs | |
| Barriocanal 2008 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Weight (IO) | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | Waist circumference, BMI (IO) | |
| Lipid profile | Total cholesterol, HDL and LDL cholesterol, triglycerides (IO) | |
| Glucose levels (fasting and postprandial) | Glucose (IO) | |
| Insulin sensitivity/serum insulin | Insulin (IO) | |
| All hypoglycaemic events | ND | |
| Severe/serious hypoglycaemia | ND | |
| Nocturnal hypoglycaemia | ND | |
| Severe/serious adverse events (specify) | ND | |
| Maki 2008 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Body weight (IO) | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | Fasting lipids (total cholesterol, LDL cholesterol, HDL cholesterol, non‐HDL cholesterol, triglycerides) (IO) | |
| Glucose levels (fasting and postprandial) | Fasting glucose (IO) | |
| Insulin sensitivity/serum insulin | Fasting insulin (IO) | |
| All hypoglycaemic events | Frequency of hypoglycaemic episodes | |
| Severe/serious hypoglycaemia | Severe hypoglycaemic episode: "required assistance from another person to actively administer carbohydrate, glucagon, or other resuscitative actions" | |
| Nocturnal hypoglycaemia | ND | |
| Severe/serious adverse events (specify) | ND | |
| Grotz 2003 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | ‐ | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | ‐ | |
| Glucose levels (fasting and postprandial) | Fasting plasma glucose (IO) | |
| Insulin sensitivity/serum insulin | ‐ | |
| All hypoglycaemic events | ND | |
| Severe/serious hypoglycaemia | ND | |
| Nocturnal hypoglycaemia | ND | |
| Severe/serious adverse events (specify) | ND | |
| Colagiuri 1989 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Body weight (IO) | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | Serum lipids (total and HDL cholesterol and triglycerides) (IO) | |
| Glucose levels (fasting and postprandial) | Fasting concentrations of plasma glucose (IO) | |
| Insulin sensitivity/serum insulin | Serum insulin (IO) | |
| All hypoglycaemic events | ‐ | |
| Severe/serious hypoglycaemia | ‐ | |
| Nocturnal hypoglycaemia | ‐ | |
| Severe/serious adverse events (specify) | ‐ | |
| Cooper 1988 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Weight (IO) | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | Fasting triglycerides, fasting total cholesterol, fasting LDL cholesterol, fasting HDL cholesterol (IO) | |
| Glucose levels (fasting and postprandial) | Fasting blood glucose (IO) | |
| Insulin sensitivity/serum insulin | Fasting plasma insulin (IO) | |
| All hypoglycaemic events | ‐ | |
| Severe/serious hypoglycaemia | ‐ | |
| Nocturnal hypoglycaemia | ‐ | |
| Severe/serious adverse events (specify) | ‐ | |
| Chantelau 1985 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Body weight (IO) | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | Total cholesterol, HDL cholesterol, triglycerides (IO) | |
| Glucose levels (fasting and postprandial) | Daily blood glucose readings ("self‐monitoring using battery powered reflectance meters or reagent strips only") (SO), random postprandial plasma glucose (IO) | |
| Insulin sensitivity/serum insulin | ‐ | |
| All hypoglycaemic events | ‐ | |
| Severe/serious hypoglycaemia | ‐ | |
| Nocturnal hypoglycaemia | ‐ | |
| Severe/serious adverse events (specify) | ‐ | |
| Nehrling 1985 | All‐cause mortality | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | HbA1c (IO) | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | ‐ | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | ‐ | |
| Glucose levels (fasting and postprandial) | Fasting plasma glucose, 2‐hour postprandial plasma glucose (IO) | |
| Insulin sensitivity/serum insulin | ‐ | |
| All hypoglycaemic events | ND | |
| Severe/serious hypoglycaemia | ND | |
| Nocturnal hypoglycaemia | ND | |
| Severe/serious adverse events (specify) | ND | |
| Stern 1976 | ‐ | ‐ |
| Diabetes‐related complications | ‐ | |
| HbA1c | ‐ | |
| Health‐related quality of life | ‐ | |
| Body weight (kg) | Weight (IO) | |
| Socioeconomic effects | ‐ | |
| Anthropometric measures other than body weight (kg) | ‐ | |
| Lipid profile | Cholesterol, triglycerides (IO) | |
| Glucose levels (fasting and postprandial) | Fasting glucose (IO) | |
| Insulin sensitivity/serum insulin | ‐ | |
| All hypoglycaemic events | ND | |
| Severe/serious hypoglycaemia | ND | |
| Nocturnal hypoglycaemia | ND | |
| Severe/serious adverse events (specify) | ND | |
| ‐: denotes not reported aIn addition to definition of endpoint measurement, description of who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement). BMI: body mass index; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; ND: not defined; SAEs: serious adverse events. | ||
Appendix 12. Adverse events (I)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths (% of participants) | Participants with at least 1 adverse event (N) | Participants with at least 1 adverse event (%) | Participants with at least 1 severe/serious adverse event (N) | Participants with at least 1 severe/serious adverse event (%) |
| Ensor 2015 | I: sucralose | 207 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: D‐tagatose | 185 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Barriocanal 2008 | I: steviol glycoside | 23 | 0 | 0 | 3 | 13.0 | 0 | 0 |
| C: placebo | 23 | 0 | 0 | 5 | 21.7 | 0 | 0 | |
| Maki 2008 | I: rebaudioside A | 60 | 0 | 0 | 27 | 45.0 | 4 | 6.7 |
| C: placebo | 62 | 0 | 0 | 23 | 37.1 | 3 | 4.8 | |
| Grotz 2003 | I: sucralose | 67 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 69 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Colagiuri 1989 | I: aspartame | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cooper 1988 | I: saccharin and starch | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chantelau 1985 | I: sodium‐cyclamate | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nehrling 1985 | I: aspartame | 30 | 0 | 0 | 6 | 20 | ‐ | ‐ |
| C: placebo (cornstarch) | 33 | 0 | 0 | 14 | 42.4 | ‐ | ‐ | |
| Stern 1976 | I: aspartame | 36 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 33 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| ‐: denotes not reported C: comparator; I: intervention; N: number of participants. | ||||||||
Appendix 13. Adverse events (II)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) | Participants with at least 1 hospitalisation (N) | Participants with at least 1 hospitalisation (%) | Participants with at least 1 outpatient treatment (N) | Participants with at least 1 outpatient treatment (%) |
| Ensor 2015 | I: sucralose | 207 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: D‐tagatose | 185 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Barriocanal 2008 | I: steviol glycoside | 23 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 23 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Maki 2008 | I: rebaudioside A | 60 | 2 | 3.3 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 62 | 1 | 1.6 | ‐ | ‐ | ‐ | ‐ | |
| Grotz 2003 | I: sucralose | 67 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 69 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Colagiuri 1989 | I: aspartame | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cooper 1988 | I: saccharin and starch | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chantelau 1985 | I: sodium‐cyclamate | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nehrling 1985 | I: aspartame | 30 | 1 | 3.3 | ‐ | ‐ | ‐ | ‐ |
| C: placebo (cornstarch) | 33 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Stern 1976 | I: aspartame | 36 | 1 | 2.8 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 33 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| ‐: denotes not reported C: comparator; I: intervention; N: number of participants. | ||||||||
Appendix 14. Adverse events (III)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least 1 specific adverse event (N) | Participants with at least 1 specific adverse event (%) |
| Ensor 2015 | I: sucralose | 207 | (1) Hypoglycaemia (2) Pancreatitis (3) GI disturbances |
(1) 0 (2) 0 (3) ‐ |
(1) 0 (2) 0 (3) ‐ |
| C: D‐tagatose | 185 | (1) Hypoglycaemia (2) Pancreatitis (3) GI disturbances |
(1) 0 (2) 0 (3) ‐ |
(1) 0 (2) 0 (3) ‐ |
|
| Barriocanal 2008 | I: steviol glycoside | 23 | (1) Abdominal fullnessa (2) Headachea (3) Dizzinessa (4) Nauseaa (5) Astheniaa | (1) ‐ (2) ‐ (3) ‐ (4) ‐ (5) ‐ | (1) ‐ (2) ‐ (3) ‐ (4) ‐ (5) ‐ |
| C: placebo | 23 | ‐ | (1) (2) |
(1) (2) |
|
| Maki 2008 | I: rebaudioside A | 60 | (1) Influenza‐like symptoms (2) Gastroenteritis (3) Gastrointestinal haemorrhage (4) Cyst (5) Hypoglycaemic episodes | (1) 1 (2) 1 (3) 1 (4) 1 (5) ‐ | (1) 1.7 (2) 1.7 (3) 1.7 (4) 1.7 (5) ‐ |
| C: placebo | 62 | (1) Gastroenteritis (2) Fracture (3) Bronchitis (4) Hypoglycaemic episodes | (1) 1 (2) 1 (3) 1 (4) ‐ | (1) 1.6 (2) 1.6 (3) 1.6 (4) ‐ | |
| Grotz 2003 | I: sucralose | 67 | ‐ | ‐ | ‐ |
| C: placebo | 69 | ‐ | ‐ | ‐ | |
| Colagiuri 1989 | I: aspartame | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | |
| Cooper 1988 | I: saccharin and starch | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | |
| Chantelau 1985 | I: sodium‐cyclamate | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | |
| Nehrling 1985 | I: aspartame | 30 | (1) Headaches (2) Constipation (3) Itching (4) Sinus congestion (5) Gastroenteritis (6) Severe diarrhoea | (1) 1 (2) 2 (3) 1 (4) 1 (5) 1 (6) 1 | (1) 3.3 (2) 6.7 (3) 3.3 (4) 3.3 (5) 3.3 (6) 3.3 |
| C: placebo (cornstarch) | 33 | (1) Eczema (2) Dizziness (3) Eye twitching (4) Blurred vision (5) Foot pain (6) Nausea (7) Musculoskeletal pain (8) Rash (9) Itching (10) Ketoacidosis (11) Diarrhoea (12) Loose stools (13) Less frequent stools (14) Constipation (15) General malaise (16) Dry skin (17) Gastroenteritis | (1) 1 (2) 1 (3) 1 (4) 1 (5) 1 (6) 3 (7) 1 (8) 2 (9) 2 (10) 1 (11) 1 (12) 1 (13) 1 (14) 1 (15) 1 (16) 1 (17) 1 | (1) 3.0 (2) 3.0 (3) 3.0 (4) 3.0 (5) 3.0 (6) 9.1 (7) 3.0 (8) 6.1 (9) 6.1 (10) 3.0 (11) 3.0 (12) 3.0 (13) 3.0 (14) 3.0 (15) 3.0 (16) 3.0 (17) 3.0 | |
| Stern 1976 | I: aspartame | 36 | (1) Nausea (2) Constipation (3) Diarrhoea (4) Loss of appetite (5) Nervousness (6) Reticulum cell sarcoma | (1) 1 (2) 2 (3) 3 (4) 1 (5) 2 (6) 1 | (1) 2.8 (2) 5.6 (3) 8.3 (4) 2.8 (5) 5.6 (6) 2.8 |
| C: placebo | 33 | (1) Cramps (2) Nausea (3) Constipation (4) Loss of appetite (5) Nervousness | (1) 2 (2) 1 (3) 5 (4) 1 (5) 2 | (1) 6.1 (2) 3.0 (3) 15.2 (4) 3.0 (5) 6.1 | |
| ‐: denotes not reported aNot specified whether participants in the intervention or the control group experienced this specific adverse event. C: comparator; GI: gastrointestinal; I: intervention; N: number of participants. | |||||
Appendix 15. Adverse events (IV)
| Trial ID | Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with at least 1 hypoglycaemic episode (N) | Participants with at least 1 hypoglycaemic episode (%) | Participants with at least 1 nocturnal hypoglycaemic episode (N) | Participants with at least 1 nocturnal hypoglycaemic episode (% participants) | Participants with at least 1 severe/serious hypoglycaemic episode (N) | Participants with at least 1 severe/serious hypoglycaemic episode (%) |
| Ensor 2015 | I: sucralose | 207 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: D‐tagatose | 185 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Barriocanal 2008 | I: steviol glycoside | 23 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 23 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maki 2008 | I: rebaudioside A | 60 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 62 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Grotz 2003 | I: sucralose | 67 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 69 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Colagiuri 1989 | I: aspartame | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cooper 1988 | I: saccharin and starch | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chantelau 1985 | I: sodium‐cyclamate | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: sucrose | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nehrling 1985 | I: aspartame | 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo (cornstarch) | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Stern 1976 | I: aspartame | 36 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐: denotes not reported C: comparator; I: intervention; N: number of participants. | ||||||||
Appendix 16. Survey of trial investigators providing information on included trials
| Trial ID | Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Madjd 2017 | 31 July 2018; 10 January 2019 |
No answer | NA | NA |
| IRCT2015091513612N6 | 3 August 2018 | No answer | NA | NA |
| Ensor 2015 | 17 June 2019 | No answer | NA | NA |
| Barriocanal 2008 | 1 August 2018 | No answer | NA | NA |
| Maki 2008 | 3 August 2018 | No answer | NA | NA |
| EUCTR2006‐002395‐18‐DK | 30 July 2018 | 31 July 2018 | NA | 31 July 2018: the author informed us "that the study have never been executed" |
| Grotz 2003 | 3 August 2018 | No answer | NA | NA |
| Colagiuri 1989 | 3 August 2018 | No answer | NA | NA |
| Cooper 1988 | 3 August 2018 | No answer | NA | NA |
| Chantelau 1985 | 3 August 2018 | 3 August 2018 | 10 August 2018 Questions regarding method and outcomes reported |
11 August 2018: authors clarified methodological issues (participants were recruited until N = 10 was achieved; randomisation of what to start with (sucrose versus cyclamate) was done openly by flipping a coin; body weight was measured independently by personnel unrelated to the study; there were no dropouts); raw data are no longer available. They wrote that "separate statistical analysis – although not reported ‐ for sucrose first versus cyclamate first was done, but did not reveal any significant difference". |
| Nehrling 1985 | No email address available | NA | NA | NA |
| Stern 1976 | No email address available | NA | NA | NA |
| NA: not applicable. | ||||
Appendix 17. Checklist to aid consistency and reproducibility of GRADE assessments (for comparison NNS versus sugar)
| Item | Health‐related quality of life | Diabetes complications | All‐cause mortality | Adverse events | HbA1c | Body weight (kg) | Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | NR | NR | NR | NR | Unclear | Unclear | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | ||||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Unclear | ||||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Unclear | ||||||
| Was an objective outcome used? | Yes | Yes | ||||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | ||||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | ||||||
| No other biases reported (i.e. no potential of other bias)? | Unclear | Unclear | ||||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | ||||||
| Inconsistencyb | Point estimates did not vary widely? | No (↓) | Yes | |||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap at least 1 point estimate; no: at least 1 outlier: where the confidence intervals of some of the studies do not overlap with those of most included studies)? | No (↓) | Substantial | ||||||
| Was the direction of effect consistent? | No (↓) | No (↓) | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I²): low (I² < 40%), moderate (I² 40% to 60%), high (I² > 60%)? | High (↓) | Low | ||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Statistically significant (↓) | Not statistically significant | ||||||
| Indirectness | Were the populations in the included studies applicable to the decision context? | Applicable | Applicable | |||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | ||||||
| Was the included outcome not a surrogate outcome? | No (↓) | Yes | ||||||
| Was the outcome time frame sufficient? | Insufficient (↓) | Sufficient | ||||||
| Were the conclusions based on direct comparisons? | Yes | Yes | ||||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | No (↓) | |||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | ||||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | ||||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | NA | NA | ||||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | |||||
| Was grey literature searched? | Yes | Yes | ||||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | ||||||
| There was no industry influence on studies included in the review? | Unclear | Unclear | ||||||
| There was no evidence of funnel plot asymmetry? | NA | NA | ||||||
| There was no discrepancy in findings between published and unpublished trials? | NA | NA | ||||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry, and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the certainty of the evidence (GRADE) as shown in the footnotes of the 'Summary of findings' table(s). HbA1c: glycosylated haemoglobin; NA: not applicable; NR: not reported. | ||||||||
Appendix 18. Checklist to aid consistency and reproducibility of GRADE assessments (for comparison NNS versus placebo)
| Item | Health‐related quality of life | Diabetes complications | All‐cause mortality | Adverse events | HbA1c | Body weight (kg) | Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | NR | NR | NR | Unclear | Unclear | Unclear | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | |||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Unclear | |||||
| Was an objective outcome used? | Yes | Yes | Yes | |||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | |||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | Unclear | |||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | |||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | |||||
| Inconsistencyb | Point estimates did not vary widely? | Yes | Yes | Yes | ||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap at least 1 point estimate; no: at least 1 outlier: where the confidence intervals of some of the studies do not overlap with those of most included studies)? | Substantial | Substantial | Substantial | |||||
| Was the direction of effect consistent? | No (↓) | Yes | No (↓) | |||||
| What was the magnitude of statistical heterogeneity (as measured by I²): low (I² < 40%), moderate (I² 40% to 60%), high (I² > 60%)? | Moderate | Low | Low | |||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Not statistically significant | Not statistically significant | |||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Applicable | Applicable | Applicable | ||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | |||||
| Was the included outcome not a surrogate outcome? | Yes | No (↓) | Yes | |||||
| Was the outcome time frame sufficient? | Sufficient | Sufficient | Sufficient | |||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | |||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | No (↓) | No (↓) | ||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Intermediate | Low (↓) | |||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | |||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | NA | NA | |||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | ||||
| Was grey literature searched? | Yes | Yes | Yes | |||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | |||||
| There was no industry influence on studies included in the review? | No (↓) | No (↓) | No (↓) | |||||
| There was no evidence of funnel plot asymmetry? | NA | NA | NA | |||||
| There was no discrepancy in findings between published and unpublished trials? | NA | NA | NA | |||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry, and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the certainty of the evidence (GRADE) as shown in the footnotes of the 'Summary of findings' table(s). HbA1c: glycosylated haemoglobin; NA: not applicable; NR: not reported. | ||||||||
Appendix 19. Checklist to aid consistency and reproducibility of GRADE assessments (for comparison NNS versus a nutritive sweetener)
| Item | Health‐related quality of life | Diabetes complications | All‐cause mortality | Adverse events | HbA1c | Body weight (kg) | Socioeconomic effects | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | NR | NR | NR | NR | Unclear | Unclear | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | ||||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | ||||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Unclear | ||||||
| Was an objective outcome used? | Yes | Yes | ||||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | No (↓) | No (↓) | ||||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | No (↓) | No (↓) | ||||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | ||||||
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | ||||||
| Inconsistencyb | Point estimates did not vary widely? | NA | NA | |||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap at least 1 point estimate; no: at least 1 outlier: where the confidence intervals of some of the studies do not overlap with those of most included studies)? | NA | NA | ||||||
| Was the direction of effect consistent? | NA | NA | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I²): low (I² < 40%), moderate (I² 40% to 60%), high (I² > 60%)? | NA | NA | ||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | NA | NA | ||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Applicable | Applicable | |||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | ||||||
| Was the included outcome not a surrogate outcome? | No (↓) | Yes | ||||||
| Was the outcome time frame sufficient? | Sufficient | Sufficient | ||||||
| Were the conclusions based on direct comparisons? | Yes | Yes | ||||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | NA | NA | |||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | High | High | ||||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | ||||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | NA | NA | ||||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | |||||
| Was grey literature searched? | Yes | Yes | ||||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | ||||||
| There was no industry influence on studies included in the review? | Unclear | Unclear | ||||||
| There was no evidence of funnel plot asymmetry? | NA | NA | ||||||
| There was no discrepancy in findings between published and unpublished trials? | NA | NA | ||||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry, and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the certainty of the evidence (GRADE) as shown in the footnotes of the 'Summary of findings' table(s). HbA1c: glycosylated haemoglobin; NA: not applicable; NR: not reported. | ||||||||
Data and analyses
Comparison 1. NNS versus sugar.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 HbA1c (%) | 3 | 72 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.54, 1.24] |
| 1.2 Body weight (kg) | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.72, 2.59] |
| 1.3 Total cholesterol (mg/dL) | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐11.10, 9.56] |
| 1.4 HDL cholesterol (mg/dL) | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐5.59, 3.42] |
| 1.5 LDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Triglycerides (mg/dL) | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐14.96, 11.91] |
| 1.7 Fasting blood glucose levels (mg/dL) | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐5.02 [‐28.31, 18.26] |
| 1.8 Postprandial blood glucose levels (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.9 Serum insulin (microunits/mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 2. NNS versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 HbA1c (%) | 4 | 360 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.13, 0.13] |
| 2.1.1 Studies with final value scores | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.51, 0.28] |
| 2.1.2 Studies with change‐from‐baseline scores | 2 | 252 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.13, 0.17] |
| 2.2 Body weight (kg) | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.01, 0.63] |
| 2.2.1 Studies with final value scores | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐6.82, 8.62] |
| 2.2.2 Studies with change‐from‐baseline scores | 1 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.02, 0.62] |
| 2.3 Adverse events (n/N) | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.39, 1.56] |
| 2.4 BMI (kg/m²) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.5 Total cholesterol (mg/dL) | 3 | 228 | Mean Difference (IV, Random, 95% CI) | 1.99 [‐4.82, 8.80] |
| 2.5.1 Studies with final value scores | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 3.75 [‐10.83, 18.32] |
| 2.5.2 Studies with change‐from‐baseline scores | 1 | 122 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐6.20, 9.20] |
| 2.6 HDL cholesterol (mg/dL) | 2 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐2.17, 1.39] |
| 2.6.1 Studies with final value score | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐4.99, 4.35] |
| 2.6.2 Studies with change‐from‐baseline scores | 1 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.32, 1.52] |
| 2.7 LDL cholesterol (mg/dL) | 2 | 168 | Mean Difference (IV, Random, 95% CI) | 3.09 [‐2.90, 9.08] |
| 2.7.1 Studies with final values | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 1.04 [‐11.45, 13.53] |
| 2.7.2 Studies with change‐from‐baseline scores | 1 | 122 | Mean Difference (IV, Random, 95% CI) | 3.70 [‐3.13, 10.53] |
| 2.8 Triglycerides (mg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 Studies with final value scores | 2 | 106 | Mean Difference (IV, Random, 95% CI) | 18.47 [‐6.78, 43.72] |
| 2.9 Fasting blood glucose levels (mg/dL) | 5 | 384 | Mean Difference (IV, Random, 95% CI) | 2.24 [‐11.60, 16.07] |
| 2.9.1 Studies with final value scores | 4 | 251 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐23.65, 25.79] |
| 2.9.2 Studies with change‐from‐baseline scores | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 3.96 [‐7.30, 15.22] |
| 2.10 Postprandial blood glucose levels (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.10.1 Studies with final value scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.11 Serum insulin (microunits/mL) | 2 | 152 | Mean Difference (IV, Random, 95% CI) | ‐2.51 [‐5.39, 0.37] |
| 2.11.1 Studies with final value scores | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐11.13, 3.73] |
| 2.11.2 Studies with change‐from‐baseline scores | 1 | 122 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐5.42, 0.82] |
Comparison 3. NNS versus another type of sweetener.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 HbA1c (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Total cholesterol (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 HDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4 LDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 Triglycerides (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Fasting glucose (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Sensitivity analysis: NNS versus sugar.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 HbA1c (%) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Correlation coefficient: 0 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.52, 1.24] |
| 4.1.2 Correlation coefficient: 0.8 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.57, 1.26] |
| 4.2 Body weight (kg) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Correlation coefficient: 0 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐3.81, 3.68] |
| 4.2.2 Correlation coefficient: 0.8 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.75, 1.61] |
| 4.3 Total cholesterol (mg/dL) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.3.1 Correlation coefficient: 0 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐15.35, 13.62] |
| 4.3.2 Correlation coefficient: 0.8 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐10.21, 7.46] |
| 4.4 HDL cholesterol (mg/dL) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.4.1 Correlation coefficient: 0 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐7.40, 5.21] |
| 4.4.2 Correlation coefficient: 0.8 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐3.98, 1.86] |
| 4.5 LDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5.1 Correlation coefficient: 0 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5.2 Correlation coefficient: 0.8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6 Triglycerides (mg/dL) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.6.1 Correlation coefficient: 0 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐19.91, 16.84] |
| 4.6.2 Correlation coefficient: 0.8 | 3 | 72 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐10.75, 7.77] |
| 4.7 Fasting blood glucose levels (mg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.7.1 Correlation coefficient: 0 | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐5.01 [‐37.78, 27.75] |
| 4.7.2 Correlation coefficient: 0.8 | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐5.05 [‐19.99, 9.88] |
| 4.8 Postprandial blood glucose levels (mg/dL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8.1 Correlation coefficient: 0 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8.2 Correlation coefficient: 0.8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9 Serum insulin (microunits/mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9.1 Correlation coefficient: 0 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9.2 Correlation coefficient: 0.8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barriocanal 2008.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria For group 1:
For group 2:
For group 3:
Exclusion criteria
Diagnostic criteria: — Setting: outpatients Age group: adults and elderly people Sex: females and males Country where trial was performed: Paraguay |
|
| Interventions |
Intervention(s): steviol glycoside capsules (250 mg 3 times a day; purity of steviol glycosides was 92%) Comparator(s): matching placebo Duration of intervention: 3 months Duration of follow‐up: 3 months Run‐in period: none Number of study centres: not reported (presumably 1) |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, body weight (kg), adverse events, anthropometric measures other than body weight (kg), lipid profile (total‐C, HDL, LDL, TG), glucose levels (fasting), serum insulin | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding: Steviafarma Industrial S.A., Maringa, Brazil and non‐commercial funding: Ministry of Agriculture of Paraguay and the Banco Interamericano de Desarrollo (Interamerican Development Bank) Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The aim of this study was to investigate the effect of steviol glycosides consumption in humans (both diabetics ‐ Type 1 and Type 2 ‐ and non‐diabetics with normal/low‐normal blood pressure) in order to comply with the first part (the pharmacological effects of steviol glycosides in humans) of the Annex 2 of the 63rd meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Volunteers were randomly assigned to receive either steviol glycoside capsules 250 mg t.d.i. or matching placebo" Comment: no information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Volunteers were randomly assigned to receive either steviol glycoside capsules 250 mg t.d.i. or matching placebo" Comment: no information about allocation concealment |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "matching placebo" was used Comment: self‐reported outcome |
| Blinding of participants and personnel (performance bias) anthropometric measures other than body weight | Low risk |
Quote from publication: "matching placebo" was used Comment: investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) body weight | Low risk |
Quote from publication: "matching placebo" was used Comment: investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "matching placebo" was used Comment: investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "matching placebo" was used Comment: investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) insulin sensitivity/serum insulin | Low risk |
Quote from publication: "matching placebo" was used Comment: investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "matching placebo" was used Comment: investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) adverse events | Low risk | Comment: participants (i.e. outcome assessors) were blinded; self‐reported outcome |
| Blinding of outcome assessment (detection bias) anthropometric measures other than body weight | Unclear risk | Comment: no information about the blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) body weight | Unclear risk | Comment: no information about the blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: no information about the blinding of outcome assessors; the outcome measurement is unlikely to have been influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: no information about the blinding of outcome assessors; the outcome measurement is unlikely to have been influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) insulin sensitivity/serum insulin | Low risk | Comment: no information about the blinding of outcome assessors; the outcome measurement is unlikely to have been influenced by potential lack of blinding |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: no information about the blinding of outcome assessors; the outcome measurement is unlikely to have been influenced by potential lack of blinding |
| Incomplete outcome data (attrition bias) adverse events | Low risk |
Quote from publication: "No drop‐outs were due to side effects" Comment: no missing data for adverse events |
| Incomplete outcome data (attrition bias) anthropometric measures other than body weight | High risk |
Quote from publication: "Eighty‐six volunteers (45 women, 41 men) were enrolled in the study and 76 completed it." "The study group consisted of 76 subjects (30 with Type 2 diabetes, 16 with Type 1 diabetes and 30 without diabetes" (Group 1: type 1 diabetes, Group 2: type 2 diabetes). "Ten volunteers (4 in Group 1, 3 in Group 2 and 3 in Group 3) decided to discontinue the study for no specific reason, but no due to side effects" Comment: in total 20 participants with type 1 diabetes were randomised and 16 were analysed; in total 33 participants with type 2 diabetes were randomised and 30 analysed; reasons for attrition and balance of missing data across groups were not reported |
| Incomplete outcome data (attrition bias) body weight | High risk |
Quote from publication: "Eighty‐six volunteers (45 women, 41 men) were enrolled in the study and 76 completed it." "The study group consisted of 76 subjects (30 with Type 2 diabetes, 16 with Type 1 diabetes and 30 without diabetes" (Group 1: type 1 diabetes, Group 2: type 2 diabetes). "Ten volunteers (4 in Group 1, 3 in Group 2 and 3 in Group 3) decided to discontinue the study for no specific reason, but no due to side effects" Comment: in total 20 participants with type 1 diabetes were randomised and 16 were analysed; in total 33 participants with type 2 diabetes were randomised and 30 analysed; reasons for attrition and balance of missing data across groups were not reported |
| Incomplete outcome data (attrition bias) glucose levels | High risk |
Quote from publication: "Eighty‐six volunteers (45 women, 41 men) were enrolled in the study and 76 completed it." "The study group consisted of 76 subjects (30 with Type 2 diabetes, 16 with Type 1 diabetes and 30 without diabetes" (Group 1: type 1 diabetes, Group 2: type 2 diabetes). "Ten volunteers (4 in Group 1, 3 in Group 2 and 3 in Group 3) decided to discontinue the study for no specific reason, but no due to side effects" Comment: in total 20 participants with type 1 diabetes were randomised and 16 were analysed; in total 33 participants with type 2 diabetes were randomised and 30 analysed; reasons for attrition and balance of missing data across groups were not reported |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "Eighty‐six volunteers (45 women, 41 men) were enrolled in the study and 76 completed it." "The study group consisted of 76 subjects (30 with Type 2 diabetes, 16 with Type 1 diabetes and 30 without diabetes" (Group 1: type 1 diabetes, Group 2: type 2 diabetes). "Ten volunteers (4 in Group 1, 3 in Group 2 and 3 in Group 3) decided to discontinue the study for no specific reason, but no due to side effects" Comment: in total 20 participants with type 1 diabetes were randomised and 16 were analysed; in total 33 participants with type 2 diabetes were randomised and 30 analysed; reasons for attrition and balance of missing data across groups were not reported |
| Incomplete outcome data (attrition bias) insulin sensitivity/serum insulin | High risk |
Quote from publication: "Eighty‐six volunteers (45 women, 41 men) were enrolled in the study and 76 completed it." "The study group consisted of 76 subjects (30 with Type 2 diabetes, 16 with Type 1 diabetes and 30 without diabetes" (Group 1: type 1 diabetes, Group 2: type 2 diabetes). "Ten volunteers (4 in Group 1, 3 in Group 2 and 3 in Group 3) decided to discontinue the study for no specific reason, but no due to side effects" Comment: in total 20 participants with type 1 diabetes were randomised and 16 were analysed; in total 33 participants with type 2 diabetes were randomised and 30 analysed; reasons for attrition and balance of missing data across groups were not reported |
| Incomplete outcome data (attrition bias) lipid profile | High risk |
Quote from publication: "Eighty‐six volunteers (45 women, 41 men) were enrolled in the study and 76 completed it." "The study group consisted of 76 subjects (30 with Type 2 diabetes, 16 with Type 1 diabetes and 30 without diabetes" (Group 1: type 1 diabetes, Group 2: type 2 diabetes). "Ten volunteers (4 in Group 1, 3 in Group 2 and 3 in Group 3) decided to discontinue the study for no specific reason, but no due to side effects" Comment: in total 20 participants with type 1 diabetes were randomised and 16 were analysed; in total 33 participants with type 2 diabetes were randomised and 30 analysed; reasons for attrition and balance of missing data across groups were not reported |
| Selective reporting (reporting bias) | High risk | Comment: described in the methods that weight and waist circumference were measured, but values were not reported |
| Other bias | Unclear risk | Comment: steviol glycoside capsules were supplied by the industry |
Chantelau 1985.
| Study characteristics | ||
| Methods | Study design: cross‐over randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria: — Diagnostic criteria: — Setting: outpatients Age group: adults Sex: females and males Country where trial was performed: Germany |
|
| Interventions |
Intervention(s): sodium‐cyclamate 348 ± 270 mg/day Comparator(s): sucrose 24 ± 13 g/day Duration of intervention: 4 weeks Duration of follow‐up: 4 weeks Run‐in period: 4 weeks Number of study centres: 1 |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, body weight, lipid profile (total‐C, HDL, TG), glucose levels (postprandial) | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: — Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "we have studied the metabolic effects of sucrose included in the diet of Type 1 diabetic outpatients treated with continuous subcutaneous insulin infusion" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "patients were assigned to use either sucrose or sodium‐cyclamate as sweetener in random order" Comment: based on information from the authors random sequence was generated by tossing a coin |
| Allocation concealment (selection bias) | High risk |
Quote from publication: "They were then asked to change over to sodium‐cyclamate or sucrose, respectively, for another 4‐week period." Comment: based on information from the authors allocation to treatment groups was done "openly" |
| Blinding of participants and personnel (performance bias) body weight | High risk |
Quote from publication: "During the sucrose‐period, sucrose and sucrose‐sweetened foods were allowed ad libidum." "During the cyclamate period, sodium cyclamate was allowed ad libidum within the limitations set up by the World Health Organisation." Comment: participants were not blinded; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) glucose levels | High risk |
Quote from publication: "During the sucrose‐period, sucrose and sucrose‐sweetened foods were allowed ad libidum." "During the cyclamate period, sodium cyclamate was allowed ad libidum within the limitations set up by the World Health Organisation." Comment: participants were not blinded; postprandial plasma glucose; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "During the sucrose‐period, sucrose and sucrose‐sweetened foods were allowed ad libidum." "During the cyclamate period, sodium cyclamate was allowed ad libidum within the limitations set up by the World Health Organisation." Comment: participants were not blinded; investigator‐assessed outcome measure; outcome unlikely to have been influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "During the sucrose‐period, sucrose and sucrose‐sweetened foods were allowed ad libidum." "During the cyclamate period, sodium cyclamate was allowed ad libidum within the limitations set up by the World Health Organisation." Comment: participants were not blinded; total cholesterol, HDL‐cholesterol, triglycerides were assessed by the investigators; outcome unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) body weight | Low risk | Comment: the publication does not address blinding of outcome assessors; based on information from the authors, body weight was measured independently by personnel unrelated to the study; exact equipment used for measurement; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Incomplete outcome data (attrition bias) anthropometric measures other than body weight | Low risk |
Quote from publication: "Ten Type 1 diabetic subjects, eight women and two men (...) volunteered to participate in the study" Comment: data available for all included participants |
| Incomplete outcome data (attrition bias) glucose levels | Low risk |
Quote from publication: "Ten Type 1 diabetic subjects, eight women and two men (...) volunteered to participate in the study" Comment: data available for all included participants |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Ten Type 1 diabetic subjects, eight women and two men (...) volunteered to participate in the study" Comment: data available for all included participants |
| Incomplete outcome data (attrition bias) lipid profile | Low risk |
Quote from publication: "Ten Type 1 diabetic subjects, eight women and two men (...) volunteered to participate in the study" Comment: data available for all included participants |
| Selective reporting (reporting bias) | Low risk | Comment: low risk of bias according to ORBIT |
| Other bias | Unclear risk | Comment: cross‐over design without washout period between interventions |
Colagiuri 1989.
| Study characteristics | ||
| Methods | Study design: cross‐over randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria: — Diagnostic criteria: criteria for type 2 diabetes mellitus: based on the National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance Setting: outpatients Age group: adults and elderly people (median: 66 ± 5 years) Sex: females and males Country where trial was performed: Australia |
|
| Interventions |
Intervention(s): aspartame 162 mg daily, added to the usual diet Comparator(s): sucrose 45 g daily, added to the usual diet Duration of intervention: 6 weeks Duration of follow‐up: 6 weeks for both interventions Run‐in period: not reported Number of study centres: 1 |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, body weight (kg), lipid profile (total‐C, HDL, TG), glucose levels (fasting) | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding; aspartame (Equal) was supplied by Searle Laboratories, Division of Searle Australia Propriety Limited, Crows Nest, New South Wales, Australia Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The aim of this study was to compare the metabolic effects of the daily addition of sucrose or an equivalent sweetening amount of aspartame to the usual diet of subjects with well‐controlled NIDDM. The purpose was twofold: to further examine the issue of a possible deleterious effect of sucrose in the diabetic diet and to ascertain whether an alternative sweetener has any particular advantage" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Subjects were randomly allocated to one of two groups" Comment: unclear how sequence was determined |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Subjects were randomly allocated to one of two groups" Comment: insufficient information to judge allocation concealment |
| Blinding of participants and personnel (performance bias) body weight | Low risk |
Quote from publication: "The sucrose and aspartame were packed in plain sachets labelled A or B according to a code. Each sachet contained 5 g sucrose or 18 mg aspartame (...) bulked to 0.5 g with lactose." Comment: appropriate packing of sweeteners to ensure blinding; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "The sucrose and aspartame were packed in plain sachets labelled A or B according to a code. Each sachet contained 5 g sucrose or 18 mg aspartame (...) bulked to 0.5 g with lactose." Comment: appropriate packing of sweeteners to ensure blinding; fasting glucose; investigator‐assessed |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "The sucrose and aspartame were packed in plain sachets labelled A or B according to a code. Each sachet contained 5 g sucrose or 18 mg aspartame (...) bulked to 0.5 g with lactose." Comment: appropriate packing of sweeteners to ensure blinding; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) insulin sensitivity/serum insulin | Low risk |
Quote from publication: "The sucrose and aspartame were packed in plain sachets labelled A or B according to a code. Each sachet contained 5 g sucrose or 18 mg aspartame (...) bulked to 0.5 g with lactose." Comment: appropriate packing of sweeteners to ensure blinding; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "The sucrose and aspartame were packed in plain sachets labelled A or B according to a code. Each sachet contained 5 g sucrose or 18 mg aspartame (...) bulked to 0.5 g with lactose." Comment: appropriate packing of sweeteners to ensure blinding; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) body weight | Unclear risk | Comment: no information about the blinding of outcome assessors; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) insulin sensitivity/serum insulin | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome measure |
| Incomplete outcome data (attrition bias) body weight | Unclear risk |
Quote from publication: "Nine subjects (eight males, one female) who satisfied the criteria for NIDDM were studied." Comment: the number of participants randomised is not clearly described |
| Incomplete outcome data (attrition bias) glucose levels | Unclear risk |
Quote from publication: "Nine subjects (eight males, one female) who satisfied the criteria for NIDDM were studied." Comment: the number of participants randomised is not clearly described |
| Incomplete outcome data (attrition bias) HbA1c | Unclear risk |
Quote from publication: "Nine subjects (eight males, one female) who satisfied the criteria for NIDDM were studied." Comment: the number of participants randomised is not clearly described |
| Incomplete outcome data (attrition bias) lipid profile | Unclear risk |
Quote from publication: "Nine subjects (eight males, one female) who satisfied the criteria for NIDDM were studied." Comment: the number of participants randomised is not clearly described |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is unavailable, but it seems that the published report includes all expected outcomes (ORBIT classification) |
| Other bias | Unclear risk | Comment: cross‐over design without washout period; aspartame was supplied by the industry |
Cooper 1988.
| Study characteristics | ||
| Methods | Study design: cross‐over randomised controlled trial | |
| Participants |
Inclusion criteria: type 2 diabetes mellitus outpatients Exclusion criteria:
Diagnostic criteria: — Setting: outpatients Age group: adults and elderly people Sex: females and males Country where trial was performed: Australia |
|
| Interventions |
Intervention(s): saccharin and starch 30 g daily Comparator(s): sucrose 28 g daily Duration of intervention: 6 weeks Duration of follow‐up: 6 weeks each dietary sequence Run‐in period: none Number of study centres: 1 |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, body weight (kg), lipid profile (total‐C, HDL, LDL, TG), glucose levels (fasting), serum insulin | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding: grant from the Australian Sugar Industry in co‐operation with CSR and Millaquin Sugar Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The aim of this study was to compare both the short‐ and medium‐term metabolic effects of sucrose supplementation with those of saccharin and starch supplementation in non‐insulin‐dependent diabetic outpatients" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence (11 sucrose diet first and 6 saccharin diet first)" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence" Comment: insufficient information about the allocation concealment |
| Blinding of participants and personnel (performance bias) body weight | Low risk |
Quote from publication: "The usual diet of each patient was supplemented daily with either 28 g sucrose (sucrose diet) or saccharin and starch (saccharin diet). The saccharin and starch supplements were equivalent to about 28 g sucrose in sweetness and energy, respectively." Comment: placebos were described to be similar in taste (sweetness); investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "The usual diet of each patient was supplemented daily with either 28 g sucrose (sucrose diet) or saccharin and starch (saccharin diet). The saccharin and starch supplements were equivalent to about 28 g sucrose in sweetness and energy, respectively." Comment: placebos were described to be similar in taste (sweetness); fasting glucose; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "The usual diet of each patient was supplemented daily with either 28 g sucrose (sucrose diet) or saccharin and starch (saccharin diet). The saccharin and starch supplements were equivalent to about 28 g sucrose in sweetness and energy, respectively." Comment: placebos were described to be similar in taste (sweetness); investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) insulin sensitivity/serum insulin | Low risk |
Quote from publication: "The usual diet of each patient was supplemented daily with either 28 g sucrose (sucrose diet) or saccharin and starch (saccharin diet). The saccharin and starch supplements were equivalent to about 28 g sucrose in sweetness and energy, respectively." Comment: placebos were described to be similar in taste (sweetness); investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "The usual diet of each patient was supplemented daily with either 28 g sucrose (sucrose diet) or saccharin and starch (saccharin diet). The saccharin and starch supplements were equivalent to about 28 g sucrose in sweetness and energy, respectively." Comment: placebos were described to be similar in taste (sweetness); investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) body weight | Unclear risk | Comment: the blinding of outcome assessors was not addressed; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) insulin sensitivity/serum insulin | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Incomplete outcome data (attrition bias) body weight | Low risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence (11 sucrose diet first and 6 saccharin diet first)" Comment: no missing outcome data; results for all 17 randomised participants were reported |
| Incomplete outcome data (attrition bias) glucose levels | Low risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence (11 sucrose diet first and 6 saccharin diet first)" Comment: no missing outcome data; results for all 17 randomised participants were reported |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence (11 sucrose diet first and 6 saccharin diet first)" Comment: no missing outcome data; results for all 17 randomised participants were reported |
| Incomplete outcome data (attrition bias) insulin sensitivity/serum insulin | Low risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence (11 sucrose diet first and 6 saccharin diet first)" Comment: no missing outcome data; results for all 17 randomised participants were reported |
| Incomplete outcome data (attrition bias) lipid profile | Low risk |
Quote from publication: "patients were randomly allocated to each 6‐week dietary sequence (11 sucrose diet first and 6 saccharin diet first)" Comment: no missing outcome data; results for all 17 randomised participants were reported |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is unavailable, but the publication seems to include all expected outcomes (ORBIT classification) |
| Other bias | Unclear risk | Comment: cross‐over without washout period; industry funding |
Ensor 2015.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: "according to WHO criteria" Setting: outpatients Age group: adults Sex: females and males Country where trial was performed: India, USA |
|
| Interventions |
Intervention(s): Splenda 1.5 g, 3 times a day, dissolved in 125 to 250 mL of water Comparator(s): tagatose 15 g, 3 times a day, dissolved in 125 to 250 mL of water Duration of intervention: 10 months Duration of follow‐up: 10 months Run‐in period: 8 weeks Number of study centres: multicentre study (number of centres not provided) |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, lipid profile (total‐C, HDL, TG), glucose levels (fasting) | |
| Identification |
Trial identifier:NCT00955747; CTRI/2009/091/000536 Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding: Biospherics subsidiary of Spherix Inc; non‐commercial funding: grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences, US National Institutes of Health Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote: "The primary objective of this Phase 3 clinical trial was to evaluate the placebo‐controlled effect of D‐tagatose on glycemic control and safety in subjects with type 2 diabetes over the course of a 10‐month treatment. The secondary objectives of this clinical trial were to evaluate the placebo‐controlled effects of D‐tagatose on fasting blood glucose, insulin, lipid profiles, and changes in BMI." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "A total of 494 subjects were randomized into the study" "There were 494 subjects randomized, 185 subjects in the US and 309 subjects in India", "Randomization was stratified according to screening HbA1c values (<7.5% and ≥7.5%) to achieve a balanced distribution of subjects across two arms (treatment and placebo)" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "This was a Phase 3, multicenter, randomized, double‐blind, placebo‐controlled, parallel group study" Comment: not clear whether allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) anthropometric measures other than body weight | Low risk |
Quote from publication: "The placebo amounts were chosen to match sweetness for blinding. The powder packets were the same size and bore the same labeling with the exception of the designation 'Substance A' or 'Substance B'" Comment: placebos were described to be similar in sweetness and packaging; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) body weight | Low risk |
Quote from publication: "The placebo amounts were chosen to match sweetness for blinding. The powder packets were the same size and bore the same labeling with the exception of the designation 'Substance A' or 'Substance B'" Comment: placebos were described to be similar in sweetness and packaging; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "The placebo amounts were chosen to match sweetness for blinding. The powder packets were the same size and bore the same labeling with the exception of the designation 'Substance A' or 'Substance B'" Comment: placebos were described to be similar in sweetness and packaging; fasting glucose; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "The placebo amounts were chosen to match sweetness for blinding. The powder packets were the same size and bore the same labeling with the exception of the designation 'Substance A' or 'Substance B'" Comment: placebos were described to be similar in sweetness and packaging; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "The placebo amounts were chosen to match sweetness for blinding. The powder packets were the same size and bore the same labeling with the exception of the designation 'Substance A' or 'Substance B'" Comment: placebos were described to be similar in sweetness and packaging; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) anthropometric measures other than body weight | Unclear risk | Comment: the blinding of outcome assessors was not addressed; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) body weight | Unclear risk | Comment: the blinding of outcome assessors was not addressed; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: the outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Incomplete outcome data (attrition bias) anthropometric measures other than body weight | High risk |
Quote from publication: "A total of 494 subjects were randomized into the study (...) Of these, 480 were treated, 248 with placebo and 232 with D‐tagatose" "The ITT population was approximately evenly divided between males and females (...) with approximately equivalent distributions in the D‐tagatose and placebo groups." "Three analysis populations were evaluated: (1) The Intent‐to‐Treat (ITT) population, (2) the Per Protocol (PP) population, and (3) the Safety population." Comment: in total 494 participants were randomised, out of these 356 (72.1%) were analysed in the ITT population, 204 (41.3%) in the PP population, and 392 (79.4%) in the safety population; reasons for attrition were not reported |
| Incomplete outcome data (attrition bias) body weight | High risk |
Quote from publication: "A total of 494 subjects were randomized into the study (...) Of these, 480 were treated, 248 with placebo and 232 with D‐tagatose" "The ITT population was approximately evenly divided between males and females (...) with approximately equivalent distributions in the D‐tagatose and placebo groups." "Three analysis populations were evaluated: (1) The Intent‐to‐Treat (ITT) population, (2) the Per Protocol (PP) population, and (3) the Safety population." Comment: data for body weight (kg) not provided |
| Incomplete outcome data (attrition bias) glucose levels | High risk |
Quote from publication: "A total of 494 subjects were randomized into the study (...) Of these, 480 were treated, 248 with placebo and 232 with D‐tagatose" "The ITT population was approximately evenly divided between males and females (...) with approximately equivalent distributions in the D‐tagatose and placebo groups." "Three analysis populations were evaluated: (1) The Intent‐to‐Treat (ITT) population, (2) the Per Protocol (PP) population, and (3) the Safety population." Comment: in total 494 participants were randomised, out of these 356 (72.1%) were analysed in the ITT population, 204 (41.3%) in the PP population, and 392 (79.4%) in the safety population; reasons for attrition were not reported |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "A total of 494 subjects were randomized into the study (...) Of these, 480 were treated, 248 with placebo and 232 with D‐tagatose" "The ITT population was approximately evenly divided between males and females (...) with approximately equivalent distributions in the D‐tagatose and placebo groups." "Three analysis populations were evaluated: (1) The Intent‐to‐Treat (ITT) population, (2) the Per Protocol (PP) population, and (3) the Safety population." Comment: in total 494 participants were randomised, out of these 356 (72.1%) were analysed in the ITT population, 204 (41.3%) in the PP population, and 392 (79.4%) in the safety population; reasons for attrition were not reported |
| Incomplete outcome data (attrition bias) lipid profile | Unclear risk |
Quote from publication: "A total of 494 subjects were randomized into the study (...) Of these, 480 were treated, 248 with placebo and 232 with D‐tagatose" "The ITT population was approximately evenly divided between males and females (...) with approximately equivalent distributions in the D‐tagatose and placebo groups." "Three analysis populations were evaluated: (1) The Intent‐to‐Treat (ITT) population, (2) the Per Protocol (PP) population, and (3) the Safety population." Comment: in total 494 participants were randomised, out of these 356 (72.1%) were analysed in the ITT population, 204 (41.3%) in the PP population, and 392 (79.4%) in the safety population; reasons for attrition were not reported |
| Selective reporting (reporting bias) | High risk | Comment: body weight and BMI were both measured, but it is only reported that no significant differences were observed between intervention and control groups. For serum insulin concentration, it is only stated that "there was no detectable consistent change in serum insulin concentrations in this trial". |
| Other bias | Unclear risk | Comment: the study was supported in part by a commercial grant |
Grotz 2003.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria: — Diagnostic criteria: — Setting: outpatients Age group: adults Sex: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): sucralose, 667 mg daily in capsules Comparator(s): placebo (cellulose) capsules Duration of intervention: 13 weeks Duration of follow‐up: 17 weeks (13 weeks intervention and 4 weeks follow‐up) Run‐in period: 4 weeks; all participants received placebo capsules 2 times a day Number of study centres: 5 |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, glucose levels (fasting), adverse events | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding: McNeil Specialty Products Company and Tate Lyle Speciality Sweeteners Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To investigate the effect of 3‐months’ daily administration of high doses of sucralose, a non‐nutritive sweetener, on glycemic control in subjects with type 2 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "The study had a double‐blind, randomized, parallel‐group design" Comment: no information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "The study had a double‐blind, randomized, parallel‐group design" Comment: insufficient information to judge whether intervention allocation could have been foreseen in advance |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "subjects were randomized to treatment groups, the identity of which was unknown to either the study subjects or the investigators" Comment: it is stated that blinding of participants and key personnel was ensured; self‐reported outcome |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "subjects were randomized to treatment groups, the identity of which was unknown to either the study subjects or the investigators" Comment: it is stated that blinding of participants and key personnel was ensured; fasting glucose; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "subjects were randomized to treatment groups, the identity of which was unknown to either the study subjects or the investigators" Comment: it is stated that blinding of participants and key personnel was ensured; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) adverse events | Low risk |
Quote from publication: "subjects were randomized to treatment groups, the identity of which was unknown to either the study subjects or the investigators" Comment: it is stated that blinding of participants (i.e. assessors of adverse events) was ensured; self‐reported outcome |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Incomplete outcome data (attrition bias) adverse events | Low risk |
Quote from publication: "There were no significant differences between the treatment groups in the type, number, or severity of adverse events reported. No subjects discontinued from the study because of an adverse event, and no adverse events were documented as being probably or definitely related to treatment" Comment: adverse events are not listed in the manuscript, but they were described to be balanced in numbers across intervention groups |
| Incomplete outcome data (attrition bias) glucose levels | Unclear risk |
Quote from publication: "A total of 136 subjects entered the test phase of the study. Of theses, 67 were randomized to receive sucralose and 69 to receive placebo. Eight subjects (4 each in the sucralose and placebo groups) discontinued after randomization to the test phase, none as a consequence of an adverse event. Therefore, 128 subjects completed the study" and were analysed Comment: missing outcome data are balanced in numbers across intervention groups; dropout rates are low (6.0% in the sucralose and 5.8% in the placebo group); reason for attrition is not provided |
| Incomplete outcome data (attrition bias) HbA1c | Unclear risk | Comment: missing outcome data are balanced in numbers across intervention groups; dropout rates are low (6.0% in the sucralose and 5.8% in the placebo group); reason for attrition is not provided |
| Selective reporting (reporting bias) | High risk | Comment: there are outcomes of interest (HbA1c, fasting glucose, adverse events) which were reported incompletely (ORBIT classification) |
| Other bias | Unclear risk | Comment: commercial funding |
Maki 2008.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria:
Diagnostic criteria: — Setting: outpatients Age group: adults Sex: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): rebaudioside A 1000 mg daily in capsules (4 x 250 mg capsules; 97% purity) Comparator(s): placebo capsules (microcrystalline cellulose) Duration of intervention: 16 weeks Duration of follow‐up: 16 weeks Run‐in period: 2 weeks Number of study centres: 6 |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, body weight (kg), adverse events, lipid profile (total‐C, HDL, LDL, TG), glucose levels (fasting), serum insulin | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding: from Cargill Inc, Food Ingredients and Systems North America to the last author for consulting services Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The present study was designed to provide data on the effects, if any, of steviol glycosides on glucose homeostasis in individuals with type 2 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Subjects were randomly assigned to receive placebo or rebaudioside A" Comment: the method used to generate the allocation sequence was not described |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Subjects were randomly assigned to receive placebo or rebaudioside A" Comment: no information about the allocation concealment provided |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: double‐blind procedure |
| Blinding of participants and personnel (performance bias) body weight | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: double‐blind procedure |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: double‐blind procedure |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: double‐blind procedure |
| Blinding of participants and personnel (performance bias) insulin sensitivity/serum insulin | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: double‐blind procedure |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: double‐blind procedure |
| Blinding of outcome assessment (detection bias) adverse events | Low risk |
Quote from publication: "This was a randomized, double‐blind, placebo‐controlled clinical trial" Comment: participants (i.e. outcome assessors) were blinded; self‐reported outcome |
| Blinding of outcome assessment (detection bias) body weight | Unclear risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; method of assessment not reported |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) insulin sensitivity/serum insulin | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Incomplete outcome data (attrition bias) adverse events | Low risk |
Quote from publication: "122 persons with previously diagnosed type 2 diabetes mellitus were randomly assigned to receive either rebaudioside A 1000 mg/d (N = 60) or placebo (N = 62) for 16 weeks", "The reasons for discontinuation included (...) adverse events [rebaudioside A, N = 2 (gastrointestinal haemorrhage and hyperglycemia) and placebo, N = 1 (bronchitis)", "A total of 50 subjects reported at least one adverse event during the study..." Comment: both discontinuation of the study due to an adverse event and adverse events not leading to discontinuation were properly described |
| Incomplete outcome data (attrition bias) body weight | Low risk |
Quote from publication: "122 persons with previously diagnosed type 2 diabetes mellitus were randomly assigned to receive either rebaudioside A 1000 mg/d (N = 60) or placebo (N = 62) for 16 weeks" Comment: body weight was described for all the 122 participants randomised |
| Incomplete outcome data (attrition bias) glucose levels | Low risk |
Quote from publication: "122 persons with previously diagnosed type 2 diabetes mellitus were randomly assigned to receive either rebaudioside A 1000 mg/d (N = 60) or placebo (N = 62) for 16 weeks" Comment: fasting glucose levels were described for all the 122 participants randomised |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "122 persons with previously diagnosed type 2 diabetes mellitus were randomly assigned to receive either rebaudioside A 1000 mg/d (N = 60) or placebo (N = 62) for 16 weeks" "Glycosylated hemoglobin data were imputed by last‐observation carried forward for four subjects in the rebaudioside A group and two in the placebo group). The results did not differ materially when the data were analyzed with and without the imputed data points (data not shown)." Comment: imputed data balanced in numbers across intervention groups; imputed data not presented; missing data: 3.3% |
| Incomplete outcome data (attrition bias) insulin sensitivity/serum insulin | Low risk |
Quote from publication: "122 persons with previously diagnosed type 2 diabetes mellitus were randomly assigned to receive either rebaudioside A 1000 mg/d (N = 60) or placebo (N = 62) for 16 weeks" Comment: serum insulin levels were described for all the 122 participants randomised |
| Incomplete outcome data (attrition bias) lipid profile | Low risk |
Quote from publication: "122 persons with previously diagnosed type 2 diabetes mellitus were randomly assigned to receive either rebaudioside A 1000 mg/d (N = 60) or placebo (N = 62) for 16 weeks" Comment: total‐C, HDL, LDL, and TG levels were described for all the 122 participants randomised |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is unavailable, but based on ORBIT classification all expected outcomes seem to have been included in the publication |
| Other bias | Unclear risk | Comment: the last author received commercial funding |
Nehrling 1985.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria: — Diagnostic criteria: diagnosis of diabetes had been established by a fasting plasma glucose > 140 mg/dL, an abnormal oral glucose tolerance test as interpreted by the US Public Health Service criteria, or an unequivocal history of diabetes; insulin‐dependent diabetes mellitus: participants who, by history, developed ketosis or ketoacidosis when adequate exogenous insulin was not provided; non‐insulin‐dependent diabetes mellitus: individuals who are not on insulin and are not ketotic or who, if on insulin, have no history of ketoacidosis Setting: presumably outpatients Age group: adults Sex: not reported, but probably females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): aspartame 2.7 g daily, in capsules Comparator(s): placebo capsules containing cornstarch, 1.8 g daily Duration of intervention: 18 weeks Duration of follow‐up: 18 weeks Run‐in period: 1 week Number of study centres: 1 |
|
| Outcomes | Reported outcomes in full text of publication: HbA1c, adverse events, glucose levels (fasting and postprandial) | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: commercial funding: GD Searle & Co., Skokie, Illinois, and non‐commercial funding: University of Illinois Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "... subjects having either insulin‐dependent or non‐insulin‐dependent diabetes completed a randomised, double‐blind study comparing effects of aspartame or a placebo on blood glucose control" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Capsules were provided in coded bottles, which contained either aspartame or placebo according to a randomization table, and were assigned to subjects in sequential order" Comment: random number tables are an adequate method to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Capsules were provided in coded bottles, which contained either aspartame or placebo according to a randomization table, and were assigned to subjects in sequential order" Comment: the method used ensured that intervention allocation could not have been foreseen in advance or changed after assignment |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "identical appearing placebo capsules" were used Comment: self‐reported outcome |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "identical appearing placebo capsules" were used Comment: fasting and postprandial glucose levels; investigator‐assessed outcome |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "identical appearing placebo capsules" were used Comment: investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) adverse events | Low risk |
Quote from publication: "identical appearing placebo capsules" were used Comment: outcome assessors (i.e. participants) were blinded; self‐reported outcome |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: the outcome measurement is unlikely to have been influenced by lack of blinding; investigator‐assessed outcome |
| Incomplete outcome data (attrition bias) adverse events | Low risk |
Quote from publication: "Of the 63 subjects, 62 completed the study." Comment: the only dropout was because of an adverse event (gastrointestinal symptoms). Types and numbers of adverse reactions are also clearly stated for participants who completed the study. |
| Incomplete outcome data (attrition bias) glucose levels | Unclear risk | Comment: only 1 participant dropped out during the study from the aspartame group; dropout rate: 1.6%; the table containing fasting plasma glucose results does not report on sample sizes, i.e. it is unclear whether the data shown are for the 62 participants who completed the study |
| Incomplete outcome data (attrition bias) HbA1c | Unclear risk | Comment: only 1 participant dropped out during the study from the aspartame group; dropout rate: 1.6%; the table containing HbA1c results does not report on sample sizes, i.e. it is unclear whether the data shown are for the 62 participants who completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is unavailable, but based on ORBIT classification all expected outcomes seem to have been included in the publication |
| Other bias | Unclear risk | Comment: the study was supported in part by a commercial grant |
Stern 1976.
| Study characteristics | ||
| Methods | Study design: parallel randomised controlled trial | |
| Participants |
Inclusion criteria:
Exclusion criteria: — Diagnostic criteria: — Setting: outpatients Age group: adults Sex: females and males Country where trial was performed: USA |
|
| Interventions |
Intervention(s): aspartame 1.8 g daily, in the form of 2 capsules 3 times daily added to the usual diet Comparator(s): matched placebo Duration of intervention: 13 weeks Duration of follow‐up: 13 weeks Run‐in period: 1 week Number of study centres: 2 |
|
| Outcomes | Reported outcomes in full text of publication: body weight (unit unclear), adverse events, lipid profile (total‐C, TG), glucose levels (fasting) | |
| Identification |
Trial identifier: — Trial terminated early: no |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding: grant‐in‐aid from G.D. Searle & Co. (for presentation of study results at a scientific meeting) Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The present study was designed to determine whether non‐insulin‐dependent diabetic subjects could consume 1.8 g aspartame daily for 90 days (a) without signs or symptoms of intolerance occurring, (b) without fasting plasma phenylalanine levels exceeding 4 mg/100 ml, and/or (c) without deterioration in diabetic control" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "randomly assigned volunteers" Comment: no information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "randomly assigned volunteers" Comment: no information on allocation concealment |
| Blinding of participants and personnel (performance bias) adverse events | Low risk |
Quote from publication: "The study design was double blind with the subjects randomly assigned to receive aspartame or matching placebo capsules." Comment: it is stated that placebos were similar; self‐reported outcome measure |
| Blinding of participants and personnel (performance bias) body weight | Low risk |
Quote from publication: "The study design was double blind with the subjects randomly assigned to receive aspartame or matching placebo capsules." Comment: it is stated that placebos were similar; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) glucose levels | Low risk |
Quote from publication: "The study design was double blind with the subjects randomly assigned to receive aspartame or matching placebo capsules." Comment: it is stated that placebos were similar; fasting glucose levels; investigator‐assessed outcome measure |
| Blinding of participants and personnel (performance bias) lipid profile | Low risk |
Quote from publication: "The study design was double blind with the subjects randomly assigned to receive aspartame or matching placebo capsules." Comment: it is stated that placebos were similar; investigator‐assessed outcome measure |
| Blinding of outcome assessment (detection bias) adverse events | Low risk | Comment: outcome assessors (i.e. participants) were blinded; self‐reported outcome |
| Blinding of outcome assessment (detection bias) body weight | Unclear risk | Comment: not reported; investigator‐assessed outcome |
| Blinding of outcome assessment (detection bias) glucose levels | Low risk | Comment: this outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed |
| Blinding of outcome assessment (detection bias) lipid profile | Low risk | Comment: this outcome is unlikely to have been influenced by lack of blinding; investigator‐assessed |
| Incomplete outcome data (attrition bias) adverse events | Unclear risk |
Quote from publication: "Sixty‐nine subjects completed the study." "Six other participants were lost to follow‐up or were discontinued for medical reasons not related to the study" Comment: dropout rate: 8%; reasons for attrition and whether they were balanced across groups was not described |
| Incomplete outcome data (attrition bias) body weight | Unclear risk |
Quote from publication: "Sixty‐nine subjects completed the study." "Six other participants were lost to follow‐up or were discontinued for medical reasons not related to the study" Comment: dropout rate: 8%; reasons for attrition and whether they were balanced across groups was not described; unit for body weight is missing, therefore results are incomplete |
| Incomplete outcome data (attrition bias) glucose levels | High risk | Comment: glucose levels were measured in both centres, but data are reported for only 1 study centre; missing data: 62.3% |
| Incomplete outcome data (attrition bias) lipid profile | Unclear risk |
Quote from publication: "Sixty‐nine subjects completed the study." "Six other participants were lost to follow‐up or were discontinued for medical reasons not related to the study" Comment: dropout rate: 8%; reasons for attrition and whether they were balanced across groups was not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: data for body weight were reported incompletely (without unit), data for glucose levels were reported only for 1 of the 2 study centres |
| Other bias | Unclear risk | Comment: funding of the study is unclear |
—: denotes not reported
BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; HbA1c: glycosylated haemoglobin A1c; HDL: high‐density lipoprotein; HOMA: homeostatic model assessment; IA: investigator‐assessed; JECFA: Joint FAO/WHO Expert Committee on Food Additives; LDL: low‐density lipoprotein; ORBIT: Outcome Reporting Bias In Trials; SR: self‐reported; total‐C: total cholesterol; TG: triglycerides; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12618000862246 | Duration of intervention < 4 weeks |
| Anonymous 1979 | Not a primary study (narrative overview) |
| Barbosa Martín 2014 | Not a primary study (narrative overview) |
| Bastaki 2015 | Not a primary study (narrative overview) |
| Baturina 2004 | Duration of intervention < 4 weeks |
| Beringer 1973 | Not a primary study (narrative overview) |
| Blackburn 1997 | Participants were non‐diabetic |
| Bloomgarden 2011 | Not a primary study (narrative overview) |
| Chantelau 1986 | Not a primary study (narrative overview) |
| Corfe 1858 | Not a primary study (narrative overview) |
| Deschamps 1971 | Duration of intervention < 4 weeks |
| Dinkovski 2017 | Not a primary study (narrative overview) |
| EUCTR2006‐002395‐18‐DK | Trial was never started (based on information from authors: "the study have never been executed"). |
| Farkas 1965 | Not a randomised controlled trial |
| Ferland 2007 | Duration of intervention < 4 weeks |
| Ferri 2006 | Participants were non‐diabetic. |
| Fukuda 2010 | Duration of intervention < 4 weeks |
| Gapparov 1996 | Not a primary study (narrative overview) |
| Healy 2013 | Not a primary study (narrative overview) |
| Heraud 1976 | Not a primary study (narrative overview) |
| IRCT2015091513612N6 | Intervention unclear ("8 candies with no sugar, 6 biscuits, and 5 sugar bars, daily") |
| Kanders 1988 | Participants were non‐diabetic |
| Knopp 1976 | Participants were non‐diabetic |
| Leon 1989 | Participants were non‐diabetic |
| Macdonald 1970 | Not a primary study (narrative overview) |
| Madjd 2017 | Intervention unclear ("subjects were instructed to continue to drink DBs (250 mL) once daily after lunch (main meal) 5 times a week") |
| Maersk 2012 | Participants were non‐diabetic |
| Maki 2009 | Duration of intervention < 4 weeks |
| Masic 2017 | Participants were non‐diabetic |
| Mazovetskii 1976 | Not a primary study (narrative overview) |
| McCann 1956 | Not a randomised controlled trial |
| Mehnert 1975 | Not a primary study (narrative overview) |
| Mehnert 1979 | Not a primary study (narrative overview) |
| Morris 1993 | Participants were non‐diabetic |
| NCT01324921 | Duration of intervention < 4 weeks |
| NCT02252952 | Participants were non‐diabetic |
| NCT02412774 | Intervention unclear ("diet beverages after the main meal") |
| NCT02487537 | Participants were non‐diabetic |
| NCT02813759 | Not a randomised clinical trial |
| NCT03680482 | Duration of intervention < 4 weeks |
| Noren 2014 | Not a randomised controlled trial |
| Odegaard 2017 | Intervention unclear ("diet beverage (DB) of choice ") |
| PACTR201410000894447 | Duration of intervention < 4 weeks |
| Parimalavalli 2011 | Not a randomised clinical trial |
| Peters 2014 | Participants were non‐diabetic |
| Peters 2016 | Participants were non‐diabetic |
| Piernas 2011 | Participants were non‐diabetic |
| Piernas 2013 | Participants were non‐diabetic |
| Prols 1973 | Duration of intervention < 4 weeks |
| Pröls 1974 | Duration of intervention < 4 weeks |
| Purdy 1988 | Not a primary study (narrative overview) |
| Reid 1994 | Participants were non‐diabetic |
| Reid 1998 | Participants were non‐diabetic |
| Reid 2010 | Participants were non‐diabetic |
| Reyna 2003 | Concomitant interventions were not similar: one group received a diet based on the American Diabetic Association's nutrition recommendations, and the other group received a modified, low‐calorie diet containing a fat replacer (beta‐glucans derived from oats) and the sweeteners, sucralose and fructose |
| Ritu 2016 | Not a randomised controlled trial |
| Rodin 1990 | Participants were non‐diabetic |
| Rogers 1994 | Duration of intervention < 4 weeks |
| Sadeghi 2019 | Wrong intervention (not an NNS) |
| Samanta 1985 | Effects of an intervention with either 20 g glucose, 20 g sucrose, or 26 g honey |
| Saundby 1887 | Not a primary study (narrative overview) |
| Schatz 1977 | Not a randomised controlled trial |
| Sharafetdinov 2002 | Not a randomised controlled trial |
| Shigeta 1985 | Not a randomised controlled trial |
| Simeonov 2002 | Effect of consuming 200 mL Aronia melanocarpa juice (with artificial sweeteners, but also containing flavonoids, vitamins, minerals, trace elements) compared to no intervention |
| Skyler 1980 | Not a primary study (narrative overview) |
| Sloane 1858 | Not a primary study (narrative overview) |
| Stevens 2013 | Not a primary study (narrative overview) |
| Stoye 2008 | Not a primary study (narrative overview) |
| Sørensen 2014 | Participants were non‐diabetic |
| Taljaard 2013 | Participants were non‐diabetic |
| Tsapok 2012 | Participants were non‐diabetic |
| Tuttas 2012 | Not a primary study (narrative overview) |
| Vazquez Duran 2013 | Participants were non‐diabetic |
| Verspohl 2014 | Not a primary study (narrative overview) |
| Vorster 1987 | Duration of intervention < 4 weeks |
| Watal 2014 | Not a primary study (narrative overview) |
| Williams 1857 | Not a randomised controlled trial |
| Williams 1858 | Not a primary study (narrative overview) |
| Williams 2014 | Not a primary study (narrative overview) |
| Wills 1981 | Not a randomised controlled trial |
| Ylikahri 1980 | Not a primary study (narrative overview) |
| Zöllner 1971 | Participants were non‐diabetic |
ADA: American Dietetic Association; NNS: non‐nutritive sweetener.
Differences between protocol and review
We deleted the criteria "the trial does not address this outcome" from the description of unclear risk of bias for "blinding of participants and study personnel" and "blinding of outcome assessment"; in cases where it was not explicitly stated that the trial was blinded, we assumed it was not blinded.
Interventions described as "diet beverages", "diet sodas", or "diet soft drinks" were included only when the sweeteners used in the products were sufficiently described to ascertain that they were non‐nutritive sweeteners (NNS). We contacted the study authors for additional information on the types of sweeteners used/allowed in their study. We excluded studies that did not specify the type of sweetener.
"NNS versus a nutritive or low‐calorie sweetener" was added to the list of comparisons. We modified the comparator "usual diet" to "sugar (i.e. usual diet containing sugar or diet containing sugar with additional sugar as supplement)".
Contributions of authors
The protocol drafting and search strategy development tasks correspond to the protocol version of this Cochrane Review; the other tasks correspond to activities for the review version.
Szimonetta Lohner (SL): protocol drafting, search strategy development, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates.
Daniela Kuellenberg de Gaudry (DK): protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates.
Ingrid Toews (IT): protocol drafting, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates.
Tamas Ferenci (TF): data analysis, data interpretation, review drafting, and future review updates.
Joerg J Meerpohl (JM): protocol drafting, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates.
Harriet Sommer (HS): protocol drafting.
All review authors (SL, DK, IT, TF, JM) read and approved the final review draft.
Sources of support
Internal sources
None, Other
External sources
-
World Health Organization (WHO), Switzerland
Jorg Merpohl received financial support from the WHO to conduct a systematic review on health effects of non‐sugar sweeteners in healthy adults and children.
-
Alexander von Humboldt Foundation, Germany
Szimonetta Lohner was financially supported as a part of a Humboldt Research Fellowship.
Declarations of interest
SL: was financially supported by the Alexander von Humboldt Foundation.
DK: none known.
IT: none known.
TF: none known.
JM: we have received financial support from the World Health Organization (WHO) to conduct a systematic review on health effects of non‐sugar sweeteners in healthy adults and children.
HS: none known.
New
References
References to studies included in this review
Barriocanal 2008 {published data only}
- Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N, et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in type 1 and type 2 diabetics. Regulatory Toxicology and Pharmacology : RTP 2008;51(1):37-41. [DOI] [PubMed] [Google Scholar]
Chantelau 1985 {published data only}
- Chantelau EA, Gosseringer G, Sonnenberg GE, Berger M. Moderate intake of sucrose does not impair metabolic control in pump-treated diabetic out-patients. Diabetologia 1985;28(4):204-7. [DOI] [PubMed] [Google Scholar]
Colagiuri 1989 {published data only}
- Colagiuri S, Miller JJ, Edwards RA. Metabolic effects of adding sucrose and aspartame to the diet of subjects with noninsulin-dependent diabetes mellitus. American Journal of Clinical Nutrition 1989;50(3):474-8. [DOI] [PubMed] [Google Scholar]
Cooper 1988 {published data only}
- Cooper PL, Wahlqvist ML, Simpson RW. Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: short- and medium-term metabolic effects. Diabetic Medicine 1988;5(7):676-80. [DOI] [PubMed] [Google Scholar]
Ensor 2015 {published data only}
- CTRI/2009/091/000536. Effects of Naturlose (Tagatose) on blood sugar control and safety of Naturlose over one year in subjects with type 2 diabetes under diet control and exercise. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2009/091/000536 (first received 17 April 2007; last updated 21 May 2019).
- Ensor M, Banfield AM, Smith RR, Williams J, Lodder RA. Safety and efficacy of D-tagatose in glycemic control in subjects with type 2 diabetes. Journal of Endocrinology, Diabetes and Obesity 2015;3:1. [PMC free article] [PubMed] [Google Scholar]
- NCT00955747. Naturlose (D-Tagatose) efficacy evaluation trial (NEET). clinicaltrials.gov/ct2/show/study/NCT00955747 (first received 10 August 2009; last updated 15 November 2014).
Grotz 2003 {published data only}
- Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. Journal of the American Dietetic Association 2003;103(12):1607-12. [DOI] [PubMed] [Google Scholar]
Maki 2008 {published data only}
- Maki KC, Curry LL, Reeves MS, Toth PD, McKenney JM, Farmer MV, et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food and Chemical Toxicology 2008;46 Suppl 7:47-53. [DOI] [PubMed] [Google Scholar]
Nehrling 1985 {published data only}
- Nehrling JK, Kobe P, McLane MP, Olson RE, Kamath S, Horwitz DL. Aspartame use by persons with diabetes. Diabetes Care 1985;8(5):415-7. [DOI] [PubMed] [Google Scholar]
Stern 1976 {published data only}
- Stern SB, Bleicher SJ, Flores A, Gombos G, Recitas D, Shu J. Administration of aspartame in non-insulin-dependent diabetics. Journal of Toxicology & Environmental Health 1976;2(2):429-39. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12618000862246 {published data only}
- ACTRN12618000862246. Do low-calorie sweeteners influence intestinal glucose absorption in patients with type 2 diabetes? www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375119 (first received 22 May 2018).
Anonymous 1979 {published data only}
- No authors listed. Saccharin substitutes for diabetics. Geriatrics 1979;34(10):15. [PubMed] [Google Scholar]
Barbosa Martín 2014 {published data only}
- Barbosa-Martín E, Sabido-Cortés D, Aranda-Gozález I, Betancur-Ancona D. Use of stevia rebaudiana extract as a sweetener of chocolates for people with diabetes. In: Stevia rebaudiana: Chemical Composition, Uses and Health Promoting Aspects. Nova Science Publishers, 2014:147-58. [Google Scholar]
Bastaki 2015 {published data only}
- Bastaki S. Pharmacotherapy of nonnutritive sweeteners in diabetes mellitus. International Journal of Diabetes and Metabolism 2015;23(1):11-2. [Google Scholar]
Baturina 2004 {published data only}
- Baturina BA, Sharafetdinov XX, Meshcheriakova BA, Plotnikova OA, Sokolov AI, Gapparov MM. Effect of food additive Neotame (N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine-1-methyl) on glucose level in blood of patients with diabetes mellitus type 2. Voprosy Pitaniia 2004;73(6):18-20. [PubMed] [Google Scholar]
Beringer 1973 {published data only}
- Beringer A. Are sweetening substances dangerous for diabetics? Wiener Medizinische Wochenschrift 1973;123(4):41-8. [PubMed] [Google Scholar]
Blackburn 1997 {published data only}
- Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. American Journal of Clinical Nutrition 1997;65(2):409-18. [DOI] [PubMed] [Google Scholar]
Bloomgarden 2011 {published data only}
- Bloomgarden ZT. Nonnutritive sweeteners, fructose, and other aspects of diet. Diabetes Care 2011;34(8):1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chantelau 1986 {published data only}
- Chantelau E. Sugar substitutes in the diet therapy of type I diabetes mellitus. Deutsche Medizinische Wochenschrift 1986;111(31-32):1220-2. [PubMed] [Google Scholar]
Corfe 1858 {published data only}
- Corfe G. Diabetes treated by saccharine food. BMJ 1858;s4-1(58):102-3. [Google Scholar]
Deschamps 1971 {published data only}
- Deschamps I, Tichet J, Lestradet H. Influence of cyclamate on blood sugar in normal and diabetic children. Diabete 1971;19(1):21-3. [PubMed] [Google Scholar]
Dinkovski 2017 {published data only}
- Dinkovski N. Natural sweetener is suitable for diabetics. www.foodmanufacture.co.uk/Article/2017/05/01/Natural-sweetener-is-suitable-for-diabetics (accessed 25 January 2018).
EUCTR2006‐002395‐18‐DK {published data only}
- EUCTR2006-002395-18-DK. Intervention studier med steviol til belysning af dosis respons forhold samt langtidseffekt hos personer med type 2 diabetes. www.clinicaltrialsregister.eu/ctr-search/trial/2006-002395-18/DK (accessed 3 August 2018).
Farkas 1965 {published data only}
- Farkas CS, Forbes CE. Do non-caloric sweeteners aid patients with diabetes to adhere to their diets? Journal of the American Dietetic Association 1965;46:482-4. [PubMed] [Google Scholar]
Ferland 2007 {published data only}
- Ferland A, Brassard P, Poirier P. Is aspartame really safer in reducing the risk of hypoglycemia during exercise in patients with type 2 diabetes? Diabetes Care 2007;30(7):e59. [DOI] [PubMed] [Google Scholar]
Ferri 2006 {published data only}
- Ferri LA, Alves-Do-Prado W, Yamada SS, Gazola S, Batista MR, Bazotte RB. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytotherapy Research 2006;20(9):732-6. [DOI] [PubMed] [Google Scholar]
Fukuda 2010 {published data only}
- Fukuda M, Terata T, Tsuda K, Sugawara M, Kitatani N, Seino Y. Aspartame-acesulfame K-containing low-energy erythritol sweetener markedly suppresses postprandial hyperglycemia in mild and borderline diabetics. Food Science and Technology Research 2010;16(5):457-66. [Google Scholar]
Gapparov 1996 {published data only}
- Gapparov MM. Sugar substitutes in specialized child nutrition products for the prevention and treatment of diabetes mellitus. Voprosy Pitaniia 1996;5:68-70. [PubMed] [Google Scholar]
Healy 2013 {published data only}
- Healy AM. Artificial sweeteners and high-fructose corn syrup: effects on diabetes and weight. Integrative Medicine Alert 2013;16(10):114-9. [Google Scholar]
Heraud 1976 {published data only}
- Heraud G, Roux E. Chemical sweeteners in current dietetics applied to diabetic patients. Ouest Medical 1976;29(7):503-6. [Google Scholar]
IRCT2015091513612N6 {published data only}IRCT2015091513612N6
- IRCT2015091513612N6. Comparison of glycemic control in patients with type 2 diabetes on regular diabetic diet or artificial sweeteners. apps.who.int/trialsearch/Trial3.aspx?trialid=IRCT2015091513612N6 (accessed 14 January 2019).
Kanders 1988 {published data only}
- Kanders BS, Lavin PT, Kowalchuk MB, Greenberg I, Blackburn GL. An evaluation of the effect of aspartame on weight loss. Appetite 1988;11 Suppl 1:73-84. [PubMed] [Google Scholar]
Knopp 1976 {published data only}
- Knopp RH, Brandt K, Arky RA. Effects of aspartame in young persons during weight reduction. Journal of Toxicology and Environmental Health 1976;2(2):417-28. [DOI] [PubMed] [Google Scholar]
Leon 1989 {published data only}
- Leon AS, Hunninghake DB, Bell C, Rassin DK, Tephly TR. Safety of long-term large doses of aspartame. Archives of Internal Medicine 1989;149(10):2318-24. [PubMed] [Google Scholar]
Macdonald 1970 {published data only}
- Macdonald I. The therapeutic potential of artificial sweeteners. Practitioner 1970;204(220):268-70. [PubMed] [Google Scholar]
Madjd 2017 {published data only}
- Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Beneficial effects of replacing diet beverages with water on type 2 diabetic obese women following a hypo-energetic diet: a randomized, 24-week clinical trial. Diabetes, Obesity & Metabolism 2017;19(1):125-32. [DOI] [PubMed] [Google Scholar]
Maersk 2012 {published data only}
- Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. American Journal of Clinical Nutrition 2012;95(2):283-9. [DOI] [PubMed] [Google Scholar]
Maki 2009 {published data only}
- Maki KC, Curry LL, McKenney JM, Farmer MV, Reeves MS, Dicklin MR, et al. Glycemic and blood pressure responses to acute doses of rebaudioside A, a steviol glycoside, in subjects with normal glucose tolerance or type 2 diabetes mellitus. FASEB Journal. Conference: Experimental Biology 2009;23(1 Suppl):351.6. [Google Scholar]
Masic 2017 {published data only}
- Masic U, Harrold JA, Christiansen P, Cuthbertson DJ, Hardman CA, Robinson E, et al. Effects of non-nutritive sweetened beverages on appetite during active weight loss (SWITCH): protocol for a randomized, controlled trial assessing the effects of non-nutritive sweetened beverages compared to water during a 12-week weight loss period and a follow up weight maintenance period. Contemporary Clinical Trials 2017;53:80-8. [DOI] [PubMed] [Google Scholar]
Mazovetskii 1976 {published data only}
- Mazovetskii AG. Sugar substitutes in the treatment of diabetes mellitus. Sovetskaia Meditsina 1976;6:93-6. [PubMed] [Google Scholar]
McCann 1956 {published data only}
- McCann MB, Trulson MF, Stulb SC. Non-caloric sweeteners and weight reduction. Journal of the American Dietetic Association 1956;32(4):327-30. [PubMed] [Google Scholar]
Mehnert 1975 {published data only}
- Mehnert H, Dietze G, Haslbeck M. Sugar and sugar substitutes in dietary treatment of disorders of carbohydrate metabolism. Nutrition and Metabolism 1975;18(Suppl 1):171-90. [PubMed] [Google Scholar]
Mehnert 1979 {published data only}
- Mehnert H, Foerster H. Oral administration of fructose as sugar substitute in the diet of diabetes mellitus patients. Aktuelle Ernahrungsmedizin Klinik und Praxis 1979;4(4):178-93. [Google Scholar]
Morris 1993 {published data only}
- Morris DH, Cuneo P, Stuart MJ, Mance MJ, Bell KJ, Puleo E, et al. High-intensity sweetener, energy and nutrient intakes of overweight women and men participating in a weight-loss program. Nutrition Research (New York, NY) 1993;13(2):123-32. [Google Scholar]
NCT01324921 {published data only}
- NCT01324921. Effect of nutritional products on metabolic parameters in subjects with type 2 diabetics. clinicaltrials.gov/show/NCT01324921 (first received 29 March 2011).
NCT02252952 {published data only}
- NCT02252952. The effect of sugar sweetened and diet beverages consumed as part of a weight-maintenance diet on fat storage. clinicaltrials.gov/ct2/show/NCT02252952 (first received 30 September 2014).
NCT02412774 {published data only}
- NCT02412774. Effects of replacing diet beverages with water on weight loss and plasma glucose control in type 2 diabetes. clinicaltrials.gov/ct2/show/NCT02412774 (first received 9 April 2015).
NCT02487537 {published data only}
- NCT02487537. Immediate and long-term induction of incretin release by artificial sweeteners 2 (ILIAS-2). clinicaltrials.gov/show/NCT02487537 (first received 1 July 2015).
NCT02813759 {published data only}
- NCT02813759. Sucralose in subjects with diabetes mellitus insulin requesting (SDMIR). clinicaltrials.gov/ct2/show/record/NCT02813759 (first received 27 June 2016).
NCT03680482 {published data only}
- NCT03680482. To compare the effects of non-nutritive sweeteners intake in subjects with T2DM. clinicaltrials.gov/ct2/show/NCT03680482 (first received 21 September 2018).
Noren 2014 {published data only}
- Noren E, Forssell H. Very low calorie diet without aspartame in obese subjects: improved metabolic control after 4 weeks treatment. Nutrition Journal 2014;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odegaard 2017 {published data only}
- Odegaard A, Hirahatake K. The effect of diet beverage intake on measures of diabetes control: a pilot study. Circulation 2017 March 7;135(Suppl 1):AP293. [Google Scholar]
PACTR201410000894447 {published data only}
- PACTR201410000894447. Efficacy and cost of stevia rebaudiana bertoni extract as adjunctive therapy in Sahrawi patients with type 2 diabetes. pactr.samrc.ac.za/Search.aspx (accessed 27 January 2018).
Parimalavalli 2011 {published data only}
- Parimalavalli R, Radhaisri S. Glycaemic index of stevia product and its efficacy on blood glucose level in type 2 diabetes. Indian Journal of Science and Technology 2011;4(3):318-21. [Google Scholar]
Peters 2014 {published data only}
- Peters JC, Wyatt HR, Foster GD, Pan Z, Wojtanowski AC, Vander Veur SS, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring, MD) 2014;22(6):1415-21. [DOI] [PubMed] [Google Scholar]
Peters 2016 {published data only}
- Peters JC, Beck J, Cardel M, Wyatt HR, Foster GD, Pan Z, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity (Silver Spring, MD) 2016;24(2):297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piernas 2011 {published data only}
- Piernas C, Tate DF, Popkin BM. Does diet beverage intake affect consumption patterns? Results from the choice RCT study. Obesity (Silver Spring, MD) 2011;19:S70. [Google Scholar]
Piernas 2013 {published data only}
- Piernas C, Tate DF, Wang X, Popkin BM. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. American Journal of Clinical Nutrition 2013;97(3):604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prols 1973 {published data only}
- Prols H, Haslbeck M, Mehnert H. Investigations into the action of high doses of saccharin on the metabolism in diabetics. Deutsche Medizinische Wochenschrift 1973;98(41):1901-4. [DOI] [PubMed] [Google Scholar]
Pröls 1974 {published data only}
- Pröls H, Wittmann P, Haslbeck M, Mehnert H. Investigations on the effect of high sodium cyclamate doses on the metabolism of diabetics. Munchener Medizinische Wochenschrift (1950) 1974;116(43):1885-8. [Google Scholar]
Purdy 1988 {published data only}
- Purdy CW. The use of saccharin in diabetes. JAMA 1988;259(8):1260. [Google Scholar]
Reid 1994 {published data only}
- Reid M, Hammersley R. The effects of sucrose on everyday eating in normal weight men and women. Appetite 1994;22(3):221-31. [DOI] [PubMed] [Google Scholar]
Reid 1998 {published data only}
- Reid M, Hammersley R. The effects of sugar on subsequent eating and mood in obese and non-obese women. Psychology, Health & Medicine 1998;3(3):299-313. [Google Scholar]
Reid 2010 {published data only}
- Reid M, Hammersley R, Duffy M. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite 2010;55(1):130-6. [DOI] [PubMed] [Google Scholar]
Reyna 2003 {published data only}
- Reyna NY, Cano C, Bermudez VJ, Medina MT, Souki AJ, Ambard M, et al. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. American Journal of Therapeutics 2003;10(6):438-43. [DOI] [PubMed] [Google Scholar]
Ritu 2016 {published data only}
- Ritu M, Nandini J. Nutritional composition of Stevia rebaudiana, a sweet herb, and its hypoglycaemic and hypolipidaemic effect on patients with non-insulin dependent diabetes mellitus. Journal of the Science of Food & Agriculture 2016;96(12):4231-4. [DOI] [PubMed] [Google Scholar]
Rodin 1990 {published data only}
- Rodin J. Comparative effects of fructose, aspartame, glucose, and water preloads on calorie and macronutrient intake. American Journal of Clinical Nutrition 1990;51(3):428-35. [DOI] [PubMed] [Google Scholar]
Rogers 1994 {published data only}
- Rogers PJ, Blundell JE. Reanalysis of the effects of phenylalanine, alanine, and aspartame on food intake in human subjects. Physiology & Behavior 1994;56(2):247-50. [DOI] [PubMed] [Google Scholar]
Sadeghi 2019 {published data only}
- Sadeghi F, Salehi S, Kohanmoo A, Akhlaghi M. Effect of natural honey on glycemic control and anthropometric measures of patients with type 2 diabetes: a randomized controlled crossover trial. International Journal of Preventive Medicine 2019;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samanta 1985 {published data only}
- Samanta A, Burden AC, Jones GR. Plasma glucose responses to glucose, sucrose, and honey in patients with diabetes mellitus: an analysis of glycaemic and peak incremental indices. Diabetic Medicine 1985;2(5):371-3. [DOI] [PubMed] [Google Scholar]
Saundby 1887 {published data only}
- Saundby R. Jambul in diabetes; saccharine in diabetes. Lancet 1887;130(3347):834. [Google Scholar]
Schatz 1977 {published data only}
- Schatz H, Winkler G, Pfeiffer EF. Sweeteners and sugar exchange foods in the diet of juvenile diabetics. Munchener Medizinische Wochenschrift 1977;119(7):213-4. [PubMed] [Google Scholar]
Sharafetdinov 2002 {published data only}
- Sharafetdinov KK, Meshcheriakova VA, Plotnikova OA, Gapparov MG. Comparative study of postprandial glycaemia in type 2 diabetic patients after consumption of mono- and disaccharides and sweeteners. Voprosy Pitaniia 2002;71(2):22-6. [PubMed] [Google Scholar]
Shigeta 1985 {published data only}
- Shigeta H, Yoshida T, Nakai M, Mori H, Kano Y, Nishioka H, et al. Effects of aspartame on diabetic rats and diabetic patients. Journal of Nutritional Science and Vitaminology 1985;31(5):533-40. [DOI] [PubMed] [Google Scholar]
Simeonov 2002 {published data only}
- Simeonov SB, Botushanov NP, Karahanian EB, Pavlova MB, Husianitis HK, Troev DM. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Medica (Plovdiv) 2002;44(3):20-3. [PubMed] [Google Scholar]
Skyler 1980 {published data only}
- Skyler JS, Miller NE. The use of sweeteners by diabetic patients. Practical Cardiology 1980;6(10):119-29. [Google Scholar]
Sloane 1858 {published data only}
- Sloane J. Leicester infirmary: observations on the saccharine treatment of diabetes mellitus. British Medical Journal 1858;s4-1(74):425-7. [Google Scholar]
Stevens 2013 {published data only}
- Stevens HC. Diabetes and diet beverage study has serious limitations. American Journal of Clinical Nutrition 2013;98(1):248-9. [DOI] [PubMed] [Google Scholar]
Stoye 2008 {published data only}
- Stoye U, Schmutz E, Krebs S, Koch S. Expert advice: Stevia. Zeitschrift fur Phytotherapie 2008;29(3):137. [Google Scholar]
Sørensen 2014 {published data only}
- Sørensen LB, Vasilaras TH, Astrup A, Raben A. Sucrose compared with artificial sweeteners: a clinical intervention study of effects on energy intake, appetite, and energy expenditure after 10 wk of supplementation in overweight subjects. American Journal of Clinical Nutrition 2014;100(1):36-45. [DOI] [PubMed] [Google Scholar]
Taljaard 2013 {published data only}
- Taljaard C, Covic NM, Graan AE, Kruger HS, Smuts CM, Baumgartner J, et al. Effects of a multi-micronutrient-fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: a randomised, double-blind, controlled intervention. British Journal of Nutrition 2013;110(12):2271-84. [DOI] [PubMed] [Google Scholar]
Tsapok 2012 {published data only}
- Tsapok PI, Imbriakov KV, Chuchkova MR. Sugar substitute products impact on oral fluid biochemical properties. Stomatologiia 2012;91(2):23-5. [PubMed] [Google Scholar]
Tuttas 2012 {published data only}
- Tuttas K, Kirch W. Steviol glycosides as sweetener in diabetes? Deutsche Medizinische Wochenschrift 2012;137(15):806. [DOI] [PubMed] [Google Scholar]
Vazquez Duran 2013 {published data only}
- Vazquez Duran M, Castillo Martinez L, Orea Tejada A, Tellez Olvera DA, Delgado Perez LG, Marquez Zepeda B, et al. Effect of decreasing the consumption of sweetened caloric and non-caloric beverages on weight, body composition and blood pressure in young adults. European Journal of Preventive Cardiology 2013;20(1 Suppl 1):S120. [Google Scholar]
Verspohl 2014 {published data only}
- Verspohl EJ. Type 2 diabetes mellitus: importance of sugar and sugar substitutes. Medizinische Monatsschrift fur Pharmazeuten 2014;37(5):191-2. [PubMed] [Google Scholar]
Vorster 1987 {published data only}
- Vorster HH, Tonder E, Kotze JP, Walker AR. Effects of graded sucrose additions on taste preference, acceptability, glycemic index, and insulin response to butter beans. American Journal of Clinical Nutrition 1987;45(3):575-9. [DOI] [PubMed] [Google Scholar]
Watal 2014 {published data only}
- Watal G, Dhar P, Srivastava SK, Sharma B. Herbal medicine as an alternative medicine for treating diabetes: the global burden. Evidence-based Complementary and Alternative Medicine 2014;2014:Article ID 596071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1857 {published data only}
- Williams T, Lond MD. On the effects of saccharine diet in diabetes mellitus. British Medical Journal 1857;s4-1(51):1041-2. [Google Scholar]
Williams 1858 {published data only}
- Williams T. Saccharine diet in diabetes. British Medical Journal 1858;s4-1(53):18. [Google Scholar]
Williams 2014 {published data only}
- Williams O. Botanicals in diabetes treatment: a look at stevia. drugtopics.modernmedicine.com/drug-topics/content/tags/diabetes/botanicals-diabetes-treatment-look-stevia?page=full (accessed 27 January 2018).
Wills 1981 {published data only}
- Wills JH, Serrone DM, Coulston F. A 7-month study of ingestion of sodium cyclamate by human volunteers. Regulatory Toxicology and Pharmacology : RTP 1981;1(2):163-76. [Google Scholar]
Ylikahri 1980 {published data only}
- Ylikahri R, Pelkonen R. Artificial sweeteners in the diabetic diet. Duodecim 1980;96(9):659-62. [PubMed] [Google Scholar]
Zöllner 1971 {published data only}
- Zöllner N, Pieper M. Concluding report of a 3-year clinical study on cyclamate. Arzneimittel-Forschung 1971;21(3):431-2. [PubMed] [Google Scholar]
Additional references
Abrams 2005
- Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823-44. [DOI] [PubMed] [Google Scholar]
ADA 2003
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-20. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes - 2008. Diabetes Care 2008;31(Suppl 1):S12-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
ADA 2016
- American Diabetes Association. Obesity management for the treatment of type 2 diabetes. Diabetes Care 2016;39(Suppl 1):S47–51. [DOI] [PubMed] [Google Scholar]
Aguilar 2007
- Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Dekant W, et al. Neotame as a sweetener and flavour enhancer. Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. EFSA Journal 2007;581:1-43. [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azad 2017
- Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ : Canadian Medical Association Journal 2017;189(28):E929-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bantle 1986
- Bantle JP, Laine DC, Thomas JW. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA 1986;256:3241-6. [PubMed] [Google Scholar]
Bell 2013
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psycho-Oncology 2013;22:1738-47. [DOI] [PubMed] [Google Scholar]
Borenstein 2017a
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5-18. [DOI] [PubMed] [Google Scholar]
Borenstein 2017b
- Borenstein M. Prediction intervals. www.meta-analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera-Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120-6. [DOI] [PubMed] [Google Scholar]
Ceunen 2013
- Ceunen S, Geuns JM. Steviol glycosides: chemical diversity, metabolism, and function. Journal of Natural Products 2013;76(6):1201-28. [DOI] [PubMed] [Google Scholar]
Chattopadhyay 2014
- Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners - a review. Journal of Food Science and Technology 2014;51(4):611-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2018
- Cochrane. CENTRAL creation details. www.cochranelibrary.com/central/central-creation (accessed 21 August 2018).
CONSORT 2010
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79-85. [DOI] [PubMed] [Google Scholar]
Coulston 1985
- Coulston AM, Hollenbeck CB, Donner CC, Williams R, Chiou YA, Reaven GM. Metabolic effects of added dietary sucrose in individuals with noninsulin-dependent diabetes mellitus (NIDDM). Metabolism 1985;34:962-6. [DOI] [PubMed] [Google Scholar]
Coulston 1987
- Coulston AM, Hollenbeck CB, Swislocki AL, Chen YD, Reaven GM. Deleterious metabolic effects of high-carbohydrate, sucrose-containing diets in patients with non-insulin-dependent diabetes mellitus. American Journal of Medicine 1987;82:213-20. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 19 October 2017. Melbourne, Australia: Veritas Health Innovation.Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
EC 2011
- European Commission. Commission regulation (EU) No 1131/2011 of 11 November 2011 amending annex II to regulation (EC) No 1333/2008 of the European Parliament and of the council with regard to steviol glycosides. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:295:0205:0211:EN:PDF (accessed 19 October 2017).
Evert 2013
- Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al, American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36(11):3821-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2008
- US Food and Drug Administration. Agency response letter GRAS notice no. GRN 000252. www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154988.htm (accessed 19 October 2017).
FDA 2015a
- US Food and Drug Administration. High-intensity sweeteners. www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397716.htm (accessed 26 January 2017).
FDA 2015b
- US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm (accessed 19 October 2017).
Fitch 2012
- Fitch C, Keim KS, Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. Journal of the Academy of Nutrition and Dietetics 2012;112(5):739-58. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769-73. [DOI] [PubMed] [Google Scholar]
FSA 2016
- Food Standards Agency. Current EU approved additives and their E numbers. www.food.gov.uk/science/additives/enumberlist#toc-4 (accessed 26 January 2017).
Gallus 2007
- Gallus S, Scotti L, Negri E, Talamini R, Franceschi S, Montella M, et al. Artificial sweeteners and cancer risk in a network of case–control studies. Annals of Oncology 2007;18:40-4. [DOI] [PubMed] [Google Scholar]
Gardner 2012
- Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, et al, American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young, American Diabetes Association. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012;35(8):1798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenwood 2014
- Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. British Journal of Nutrition 2014;112(5):725-34. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hoffmann 2017
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:j2998. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilbäck 2003
- Ilbäck NG, Alzin M, Jahrl S, Enghardt-Barbieri H, Busk L. Estimated intake of the artificial sweeteners acesulfame-K, aspartame, cyclamate and saccharin in a group of Swedish diabetics. Food Additives and Contaminants 2003;20(2):99-114. [DOI] [PubMed] [Google Scholar]
JECFA 1982
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants. World Health Organization; 1982 April, WHO Technical Report Series No.: 683.
JECFA 2004
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Compendium of food additive specifications: Addendum 12. In: WHO Technical Report Series. 63rd Meeting 2004 June 8–17; Geneva. Geneva, Switzerland: World Health Organization, 2004.
JECFA 2010
- World Health Organization. Evaluations of the Joint FAO/WHO expert committee on food additives. apps.who.int/food-additives-contaminants-jecfa-database/search.aspx?fcc=1 (accessed 26 January 2017).
Jones 2015
- Jones CW, Keil LG, Holland WC, Caughey MC, Platts-Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Medicine 2015;13:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Just 2008
- Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008;51(3):622-7. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta-analyses of studies that evaluate interventions: explanation and elaboration. PLOS Medicine 2009;6(7):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. [DOI] [PubMed] [Google Scholar]
Mattes 2009
- Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. American Journal of Clinical Nutrition 2009;89(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortensen 2006
- Mortensen A. Sweeteners permitted in the European Union: safety aspects. Scandinavian Journal of Food and Nutrition 2006;50(3):104-16. [Google Scholar]
National Diabetes Data Group 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28(12):1039-57. [DOI] [PubMed] [Google Scholar]
NCD‐RisC 2016
- NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otabe 2011
- Otabe A, Fujieda T, Masuyama T, Ubukata K, Lee C. Advantame - an overview of the toxicity data. Food and Chemical Toxicology 2011;49(Suppl 1):S2-7. [DOI] [PubMed] [Google Scholar]
Pastors 2002
- Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002;25(3):608-13. [DOI] [PubMed] [Google Scholar]
Peterson 1986
- Peterson DB, Lambert J, Gerring S, Darling P, Carter RD, Jelfs R, et al. Sucrose in the diet of diabetic patients - just another carbohydrate? Diabetologia 1986;29:216-20. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Romo‐Romo 2016
- Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PLOS ONE 2016;18(11):e0161264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherer 2018
- Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.MR000005.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Timpe Behnen 2013
- Timpe Behnen EM, Ferguson MC, Carlson A. Do sugar substitutes have any impact on glycemic control in patients with diabetes? Journal of Pharmacy Technology 2013;29:61-5. [Google Scholar]
Toeller 1993
- Toeller M. Diet and diabetes. Diabetes/Metabolism Reviews 1993;9:93-108. [DOI] [PubMed] [Google Scholar]
Toews 2019
- Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019;364:k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539-53. [DOI] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Diabetes. Fact Sheet (Reviewed November 2016). www.who.int/mediacentre/factsheets/fs312/en (accessed 5 January 2017).
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


