Abstract
Inebilizumab (Uplizna™; inebilizumab-cdon in the USA) is a humanised anti-CD19 monoclonal antibody being developed by Viela Bio for the treatment of a range of autoimmune diseases associated with CD19-expressing B cells. Inebilizumab targets and depletes CD19-expressing B cells through antibody-dependent cell-mediated cytotoxicity. In June 2020, inebilizumab received its first global approval in the USA for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adult patients who are seropositive for immunoglobulin G autoantibodies against aquaporin-4 (AQP4-IgG). The drug is also undergoing clinical evaluation for kidney transplant desensitization, myasthenia gravis, and IgG4-related disease. This article summarizes the milestones in the development of inebilizumab leading to this first approval for the treatment of AQP4-IgG seropositive NMOSD.
Inebilizumab (Uplizna™): Key points
| A humanized anti-CD19 monoclonal antibody being developed by Viela Bio for the treatment of NMOSD, kidney transplant desensitization, myasthenia gravis and IgG4-related disease |
| Received its first approval on 11 June 2020 in the USA |
| Approved for the treatment of NMOSD in adult patients who are AQP4-IgG seropositive |
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune, demyelinating disease of the CNS now recognized to be distinct from multiple sclerosis [1]. This relatively rare and potentially life-threatening disorder is typically characterized by incomplete recovery from recurrent attacks of optic neuritis and/or transverse myelitis, resulting in accumulating impairment (e.g. blindness and paraplegia) [1–3]. B cells appear to play a prominent role in the immunopathogenesis of NMOSD [4]; ≈ 75–90% of those with the disease have pathogenic immunoglobulin G (IgG) autoantibodies against aquaporin-4 (AQP4), the most abundant water channel in the CNS, detectable in their serum [5].
Inebilizumab (Uplizna™; inebilizumab-cdon in the USA), a humanised, affinity-optimised, afucosylated IgG1 kappa monoclonal antibody that binds to the B-cell surface antigen CD19, is being developed by Viela Bio for the treatment of a range of autoimmune diseases associated with CD19-expressing B cells [6]. Inebilizumab received its first global approval on 11 June 2020 in the USA [7], for the treatment of NMOSD in adult patients who are seropositive for IgG autoantibodies against AQP4. The recommended initial dose is two single 300 mg intravenous infusions given 2 weeks apart [8]. Subsequent doses (starting 6 months from the first infusion) comprise single 300 mg intravenous infusions given every 6 months. Inebilizumab is contraindicated in patients with a history of life-threatening infusion reactions to the drug and in patients with active hepatitis B virus (HBV) infection and active or untreated latent tuberculosis (TB); HBV and TB screening are required prior to the first dose of the drug [8].
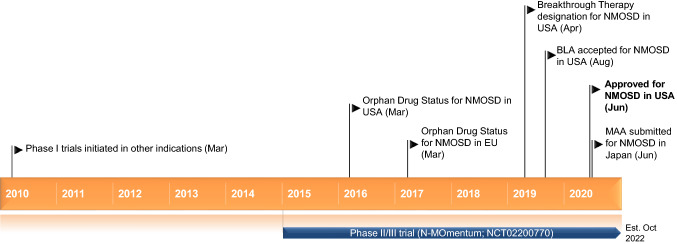
Key milestones in the development of inebilizumab, focusing on its use in the treatment of neuromyelitis optica spectrum disorder (NMOSD). BLA Biologics licence application, MAA marketing authorization application
Inebilizumab is currently undergoing clinical evaluation for kidney transplant desensitization, myasthenia gravis, and IgG4-related disease [6]. Clinical evaluation of the drug for B-cell lymphoma, chronic lymphocytic leukaemia, multiple myeloma, follicular lymphoma, multiple sclerosis and systemic scleroderma has been discontinued.
Company Agreements
Originating from Duke University, inebilizumab was initially developed by Cellective Therapeutics Inc., which was acquired by Medimmune Inc. in 2005 [9]. Medimmune Inc. was, in turn, acquired by AstraZeneca PLC in 2007 [10]. In February 2018, AstraZeneca transferred inebilizumab to its spun-out independent biotechnology company, Viela Bio, Inc. [11, 12]; these two companies have subsequently entered into a clinical supply agreement (in February 2018) and a commercial supply agreement (in April 2019), with expiration dates of February 2023 and April 2029, respectively. In 2019, Vielo Bio entered into two partnerships: one with the Jiangsu Hansoh Pharmaceutical Group Company Limited to develop and commercialize inebilizumab for autoimmune diseases and haematological cancers in China [13]; and the other with the Mitsubishi Tanabe Pharma Corporation to develop and commercialize inebilizumab for NMOSD (and other potential indications) in Japan and eight additional Asian countries (South Korea, Taiwan, Singapore, Indonesia, Thailand, Malaysia, the Philippines and Vietnam) [6, 14].
Inebilizumab is covered by Viela Bio-owned and in-licensed pending and issued patents in multiple jurisdictions, including the USA (expiration dates range from 2026–2030).
Scientific Summary
Pharmacodynamics
Inebilizumab targets and depletes CD19-expressing B cells through antibody-dependent cell-mediated cytotoxicity [15]. Inebilizumab depleted CD19-expressing B-cell populations in preclinical models [15, 16] and in phase 1 clinical studies in patients with systemic sclerosis [17] and multiple sclerosis [18].
In the phase II/III N-MOmentum study in patients with NMOSD (NCT02200770) [19, 20], two single 300 mg intravenous infusions of inebilizumab given 2 weeks apart resulted in specific, rapid and durable depletion of peripheral blood B cells [20, 21]. As a surrogate marker for CD19+ B-cell counts, CD20+ B-cell counts were significantly reduced (p < 0.0001 vs placebo) 1 week after the initial infusion of the drug [8, 20, 21]. CD20+ B-cell counts were reduced below the lower limit of normal (LLN) in all inebilizumab-treated patients 4 weeks after the initial infusion; they remained below the LLN in 94% of patients for 28 weeks following the first infusion [8]. T cell counts were unchanged; however, natural killer cells showed a transient decrease 2 weeks after the initial infusion [21]. Peripheral blood CD20- plasmablasts and plasma cells were rapidly depleted (i.e. 1 week after the initial infusion) and remained depleted throughout the 28-week period following the first infusion. Early repletion of B cells was due to the emergence of transitional B cells and naive B cells. There was no relationship between the frequency of NMOSD attacks and CD20+ B-cell depletion in inebilizumab treated subjects [21].
The precise mechanism by which inebilizumab exerts its therapeutic effects in NMOSD is unknown but is presumed to involve binding to CD19 present on pre-B and mature B lymphocytes [8].
Pharmacokinetics
After two single 300 mg intravenous infusions given 2 weeks apart (i.e. on days 1 and 15) in N-MOmentum (NCT02200770), the mean maximum inebilizumab plasma concentration in patients with NMOSD was 108 μg/mL (after the second dose on day 15) and the cumulative area under the plasma concentration-time curve during the 26-week period following the second infusion was 2980 µg day/mL. The estimated inebilizumab central and peripheral volume of distribution is 2.95 and 2.57 L, respectively [8].
Inebilizumab is degraded by proteolytic enzymes that are widely distributed throughout the body [8].The estimated inebilizumab systemic clearance of the first-order elimination pathway is 0.19 L/day. At low plasma concentrations, inebelizumab was likely subject to receptor (CD19)-mediated clearance, which decreased over time, presumably due to B cell depletion by the drug. The mean terminal half-life of inebilizumab is 18 days [8].
The effects of renal or hepatic impairment on the pharmacokinetics of inebilizumab have not been formally studied [8].
Features and properties of inebilizumab
| Alternative names | 16C4-aFuc; A-fucosylated anti-CD19 antibody; Anti-CD19 MAb-Viela-Bio; MEDI 551; MT-0551; inebilizumab-cdon; inebilizumab; Uplizna™ |
| Class | Antineoplastics; immunotherapies; monoclonal antibodies; eye disorder therapies; skin disorder therapies |
| Mechanism of action | Antibody-dependent cell cytotoxicity |
| Route of administration | Intravenous |
| Pharmacodynamics | Afucosylated, humanized IgGκ monoclonal antibody directed against B-lymphocyte antigen CD19. Depletes CD19-expressing B cells through antibody-dependent cell cytotoxicity |
| Pharmacokinetics | Cmax 108 μg/mL and 26-week AUC 2980 µg day/mL after second of two 300 mg doses 2 weeks apart; central and peripheral Vd 2.95 L and 2.57 L; degraded by widely distributed proteolytic enzymes; systemic clearance 0.19 L/day; mean t½ 18 days |
| Adverse reactions (incidence ≥ 7%) | Urinary tract infection, arthralgia, infusion reaction, nasopharyngitis, headache, and back pain |
| ATC codes | |
| WHO ATC code | L01X-C (monoclonal antibodies); L04A (immunosuppressants); N07 (other nervous system drugs); S01X-A (other ophthalmologicals) |
| EphMRA ATC code | L1G (monoclonal antibody antineoplastics); L4X (other immunosuppressants); N7 (other CNS drugs); S1X1 (other ophthalmologicals, systemic) |
| Chemical name | Immunoglobulin G1, anti-(human CD19 (antigen)) (human monoclonal MEDI-551 heavy chain), disulfide with human monoclonal MEDI-551 kappa-chain, dimer |
| Chemical formula | C6504H10080N1732O2044S44 |
Therapeutic Trials
Treatment with inebilizumab significantly reduced the time to onset of an NMOSD attack in a randomized, double-blind, placebo-controlled, multinational, phase II/III trial (N-MOmentum; NCT02200770) [19, 20]. In the overall intent-to-treat (ITT) population (n = 230), significantly fewer recipients of inebilizumab (n = 174) than placebo (n = 56) experienced an NMOSD attack [12 vs. 39%; hazard ratio 0.272 (95% CI 0.150–0.496; p < 0.0001)]; the number needed to treat (NNT) for 28 weeks to prevent one attack was 3.73 [20]. In this population, inebilizumab also significantly reduced the risk of an NMOSD attack irrespective of race or ethnicity [20], body-mass index [20], or level of pre-existing disability [hazard ratio 0.257 (p = 0.0005) and 0.367 (p = 0.0456) in patients with baseline Expanded Disability Status Scale (EDSS) scores of < 5 and ≥ 5, respectively, on a 0–10 scale (with higher scores indicating greater disability)] [22].
In the subgroup of 213 patients with NMOSD who were AQP4-IgG seropositive, significantly fewer recipients of inebilizumab (n = 161) than placebo (n = 52) experienced an NMOSD attack [11% vs. 42%; hazard ratio 0.227 (95% CI 0.121–0.423; p < 0.0001)]; the NNT was 3.23 [20]. The efficacy of inebilizumab in the AQP4-IgG seronegative population could not be interpreted due to the small number of participants in this cohort (3 attacks occurred in 13 inebilizumab recipients versus 0 attacks in 4 placebo recipients) [20].
At day 197 [the end of the randomized controlled period (RCP) of the trial], significantly fewer inebilizumab than placebo recipients in the overall ITT [16% vs. 34% (odds ratio 0.370; p = 0.0049)] and AQP4-IgG seropositive [16% vs. 35% (odds ratio 0.371; p = 0.007)] populations had disability worsening on the EDSS [20, 22]. Inebilizumab also significantly (p = 0.0023) reduced the risk of disability worsening on the modified Rankin scale [22]. Changes from baseline in low-contrast visual acuity did not differ significantly between inebilizumab and placebo recipients. However, inebilizumab-treated patients were less likely than placebo-treated patients to experience optic neuritis [hazard ratio 0.288 (95% CI 0.120–0.694) in the overall ITT population and 0.222 (95% CI 0.088–0.565) in the AQP4-IgG seropositive population; post hoc analysis] [20].
As measured cumulatively from baseline up to the end of the RCP, the mean number of active MRI lesions [1.6 vs 2.3 (rate ratio 0.566; p = 0.0034) in the overall ITT population and 1.7 vs 2.3 (rate ratio 0.568; p = 0.0042) in the AQP4-IgG seropositive population] and NMOSD-related inpatient hospitalizations [1.0 vs 1.4 (rate ratio 0.286; p = 0.01) in the overall ITT population and 1.0 vs 1.4 (rate ratio 0.258; p = 0.012) in the AQP4-IgG seropositive population] were significantly lower in inebilizumab recipients than placebo recipients [20].
In an interim analysis of the ongoing, open-label extension period (OLP) of the trial [23, 24], 85% of the 216 patients who enrolled in the OLP (of whom 165 and 51 were originally randomized to inebilizumab and placebo) were free of NMOSD attack after 1 year of open-label inebilizumab treatment; the annualized attack rate was 0.113 [24]. Compared with the last RCP visit, disability assessed using the EDSS was improved at 1 year, regardless of treatment allocation in the RCP [24].
In N-MOmentum, adults with a diagnosis of NMOSD, an EDSS score of ≤ 8, and a history of ≥ 1 attack in the year before screening (or ≥ 2 attacks in the 2 years before screening), were randomized to receive intravenous inebilizumab 300 mg or placebo on days 1 and 15 of the 197-day (28-week) RCP. The primary outcome measure was time (in days) from day 1 to the onset of an NMOSD attack (as determined by a blinded, independent, three-member adjudication committee), on or before day 197 [20]. Patients who experienced an adjudicated relapse during the RCP, or who completed the RCP without a relapse, were eligible to receive inebilizumab for up to 1 year in an OLP. Those originally allocated to inebilizumab in the RCP received a 300 mg dose of the drug on day 1 of the OLP to maintain B-cell depletion (as well as placebo on day 15 to maintain masking); those originally assigned to placebo received 300 mg doses of inebilizumab on days 1 and 15 to establish B-cell depletion. Subsequently, all participants received inebilizumab 300 mg every 26 weeks to maintain B-cell depletion [20].
Key clinical trials of inebilizumab (Viela Bio)
| Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
| Inebilizumab, placebo | Neuromyelitis optica spectrum disorder | II/III | Completed | Multinational |
EudraCT2014-000253-36 N-MOmentum CD-IA-MEDI-551-1155 |
| Inebilizumab, VIB4920 | Kidney transplant desensitization | II | Active (not recruiting) | USA |
VIB0551.P2.S1 |
Adverse Events
Intravenous inebilizumab therapy was generally well tolerated over the 6.5-month RCP of the N-MOmentum trial in patients with NMOSD (NCT02200770) [20]. Results for safety endpoints in the AQP4-IgG seropositive population (n = 213) were similar to those in the overall ITT population (n = 230). The most common (incidence > 5%) treatment-emergent adverse events (TEAEs) in patients treated with inebilizumab 300 mg on days 1 and 15 of the RCP were urinary tract infection (11% vs 9% with placebo), arthralgia (10% vs 4%), infusion-related reaction (9% vs 11%), nasopharyngitis (7% vs 11%), headache (7% vs 7%) and back pain (7% vs 4%). All infusion-related reactions were mild or moderate (grade 1–2) in severity [20].
Ten serious adverse events (arthralgia, atypical pneumonia, third-degree burns, acute cholangitis, acute cholecystitis, diarrhoea, abnormal hepatic function, myelitis, urinary tract infection and blurred vision) occurred in eight inebilizumab-treated patients (5%); no serious adverse event was reported in more than one inebilizumab recipient [20]. TEAEs leading to dose interruption occurred in three patients receiving inebilizumab (2%) compared with none receiving placebo. TEAEs leading to treatment discontinuation occurred in two patients receiving inebilizumab (1%) compared with none receiving placebo. No deaths occurred during the RCP [20].
Longer-term, no new safety concerns were identified during treatment with inebilizumab for an average of 2 (range 0.2–4.4) years across both the RCP and OLP of the N-MOmentum trial [24]. The most common TEAEs in the combined RCP and OLP safety analysis included urinary tract infection (22.2%), nasopharyngitis (16.4%), infusion-related reaction (12.4%) and back pain (12.4%). Serious adverse events occurred in 16% of patients [24]. Two deaths occurred during the OLP, one due to NMOSD and the other to new presumed inflammatory brain lesions of undetermined aetiology [20, 24].
Laboratory testing showed reduced immunoglobulin levels, decreased neutrophil counts and (consistent with the B cell-depleting mechanism of action of the drug) lower lymphocyte counts in patients treated with inebilizumab [8]. At the end of the RCP, the total immunoglobulin level was decreased by ≈ 8% from baseline in patients receiving inebilizumab, whereas it was increased by 6% from baseline in patients receiving placebo. Relative to baseline, there were mean decreases of ≈ 4% and 32% in IgG and immunoglobulin M (IgM) levels, respectively, in patients receiving inebilizumab as compared with mean increases of 6% and ≈ 13%, respectively, in patients receiving placebo. In the combined RCP and OLP safety analysis, 6.6% and 31% of inebilizumab recipients had IgG and IgM levels below the LLN at 1 year; 13% and 42% had IgG and IgM levels below the LLN at 2 years [8].
Mild neutropenia (absolute neutrophil count 1.0–1.5 × 109/L) was observed in 6.9% of patients treated with inebilizumab versus 1.9% of patients treated with placebo [8], while moderate/grade 3 neutropenia (absolute neutrophil count 0.5–1.0 × 109/L) was observed in 1.9% of patients receiving inebilizumab as compared with none receiving placebo [8, 20]. At the end of the RCP, 12% of inebilizumab recipients versus 4.2% of placebo recipients had a neutrophil count below the LLN; 5.3% versus 4.2% had a lymphocyte count below the LLN [8].
Treatment-emergent anti-inebilizumab antibodies, which were detected in 3% and 7% of inebilizumab- and placebo-treated patients, respectively, had no effect on the pharmacokinetics or pharmacodynamics of the drug [20].
There was no decrease in anti-tetanus toxoid IgG levels (measured to evaluate the effect of inebilizumab on vaccine- generated antibody titres) at the end of the RCP [20].
Ongoing Clinical Trials
The OLP in the phase II/III N-MOmentum trial in patients with NMOSD (NCT02200770) is ongoing [24]. An open-label, randomized, phase II study (NCT04174677) designed to assess the efficacy of inebilizumab, alone or in combination with VIB4920, in highly sensitized candidates awaiting a first or second kidney transplantation from a deceased donor is also ongoing, although recruitment has been voluntarily paused due to the COVID-19 pandemic [25]. Initiated in December 2019, this study plans to enrol 36 patients aged 18–65 years at two research sites in the USA; the estimated completion date is April 2022.
The manufacturer of inebilizumab plans to initiate two new trials evaluating the efficacy of the drug in other potential indications in mid-2020: a pivotal phase III study in patients with myasthenia gravis and a phase IIb study in patients with IgG4-related disease. No further details of either of these studies are currently available [6, 25].
The activity and safety of inebilizumab in anti-NMDA receptor encephalitis is to be assessed in an investigator-initiated (University of Utah), randomized, double-blind, placebo-controlled, phase IIb study (ExTinguish; NCT04372615). This study, which is not yet recruiting, plans to enrol 116 patients and has an estimated start date of January 2021 and an estimated completion date of January 2027.
Current Status
Inebilizumab received its first global approval on 11 June 2020 in the USA for the treatment of AQP4-IgG seropositive adults with NMOSD [7].
Compliance with Ethical Standards
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Footnotes
Enhanced material for this AdisInsight Report can be found at 10.6084/m9.figshare.12660503.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
References
- 1.Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc. 2017;92(4):663–679. doi: 10.1016/j.mayocp.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mealy MA, Kessler RA, Rimler Z, et al. Mortality in neuromyelitis optica is strongly associated with African ancestry. Neurol Neuroimmunol Neuroinflamm. 2018;2018:e468. doi: 10.1212/NXI.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett JL, O’Connor KC, Bar-Or A, et al. B lymphocytes in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2015;2:e104. doi: 10.1212/NXI.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid SHM, Whittam D, Mutch K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 2017;264(10):2088–2094. doi: 10.1007/s00415-017-8596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viela Bio. Viela Bio reports fourth quarter and full year 2019 financial results and business highlights [media release] 25 Mar 2020. https://vielabio.com.
- 7.US Food & Drug Administration. FDA approves new therapy for rare disease affecting optic nerve, spinal cord [media release] 11 June 2020. https://www.fda.gov.
- 8.Viela Bio. UpliznaTM (inebilizumab-cdon): US prescribing information. 2020. https://www.accessdata.fda.gov. Accessed 1 July 2020.
- 9.MedImmune Inc. MedImmune completes cellective acquisition [media release] 17 Oct 2005. http://www.medimmune.com.
- 10.AstraZeneca. AstraZeneca successfully completes acquisition of MedImmune [media release] 19 June 2007. http://www.astrazeneca.com.
- 11.Astra Zeneca. MedImmune to create stand-alone company for early-stage inflammation and autoimmunity biologics [media release] 28 Feb 2018. https://www.astrazeneca.com.
- 12.Viela Bio. Viela Bio spins out of MedImmune; launches with up to $250 Million Series A financing from investor consortium led by Boyu Capital, 6 Dimensions Capital, and Hillhouse Capital [media release] 28 Feb 2018. http://www.vielabio.com.
- 13.Viela Bio. Viela Bio enters strategic collaboration with Hansoh Pharma to develop and commercialize inebilizumab for autoimmune diseases and hematologic cancers in China [media release] 28 May 2019. http://www.vielabio.com.
- 14.Mitsubishi Tanabe Pharma Corporation. Mitsubishi Tanabe Pharma enters into a licensing agreement with Viela Bio for inebilizumab, a treatment agent for neuromyelitis optica spectrum disorder, in Japan and other Asian regions [media release] 9 Oct 2020. http://www.mt-pharma.co.jp.
- 15.Chen D, Gallagher S, Monson NL, et al. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J Clin Med. 2016;5(12):107. doi: 10.3390/jcm5120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst R, Wang Y, Gallagher S, et al. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther. 2010;335(1):213–222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 17.Schiopu E, Chatterjee S, Hsu V, et al. Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study. Arthritis Res Ther. 2016;18(131):1–14. doi: 10.1186/s13075-016-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agius MA, Klodowska-Duda G, Maciejowski M, et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler. 2019;25(2):235–245. doi: 10.1177/1352458517740641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cree BA, Bennett JL, Sheehan M, et al. Placebo-controlled study in neuromyelitis optica—ethical and design considerations. Mult Scler. 2016;22(7):862–872. doi: 10.1177/1352458515620934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 21.Bennett J, Aktas O, Rees W, et al. Characterization of B cell subset changes following treatment of neuromyelitis optica spectrum disorder with inebilizumab: results from the N-MOmentum study randomized control period [abstract no. 1097] Mult Scler J. 2019;25(Suppl 2):590–592. [Google Scholar]
- 22.Marignier R, Bennett JL, Kim HJ, et al. Inebilizumab prevents neuromyelitis optica spectrum disorder disability: outcomes from the N-MOmentum randomised, placebo-controlled, double-masked trial [abstract no. P1604] Mult Scler J. 2019;25(Suppl 2):896–898. [Google Scholar]
- 23.Cree B, Bennett J, Kim HJ, et al. The N-MOmentum study—a randomised, placebo-controlled, double-blind trial of Inebilizumab for neuromyelitis optica spectrum disorder: randomised controlled period and open-label extension results [abstract no. 139] Mult Scler J. 2019;25(Suppl 2):42–44. [Google Scholar]
- 24.Cree BAC, Bennett JL, Kim HJ, et al. Long-term efficacy and safety of inebilizumab for neuromyelitis optica spectrum disorder in the randomized, double-blind N-MOmentum study and extension [abstract no. 3998]. Neurology. 2020;94(15 Suppl).
- 25.Viela Bio. Viela Bio reports first quarter 2020 financial results and program highlights [media release] 13 May 2020. http://www.vielabio.com.