Abstract
Oxygen is viewed in medicine as the sole determinant of tissue oxygenation, though carbon dioxide homeostasis is equally important and clinically often ignored. The aims of this study were as follows: (a) to examine the effects of different acute hypoxic conditions on partial pressure of arterial oxygen (), arterial oxygen saturation of hemoglobin (), and regional cerebral saturation of hemoglobin (rSO2); and (b) to evaluate supplemental CO2 as a tool to improve oxygenation in acutely hypoxic individuals. We hypothesized that exposure to gas mixtures with added CO2 would improve oxygenation in hypoxic human subjects. Twenty healthy subjects were exposed to 5‐min intervals of two gas mixtures: hypoxic gas mixture containing 8% oxygen, and a CO2‐enriched mixture containing 8% oxygen plus either 3% or 5% CO2. Ten subjects received the 3% CO2‐enriched mixture, and the remaining 10 subjects received the 5% CO2‐enriched mixture. The order of exposure was randomized. Blood gases, pulse oximetry, end‐tidal CO2, and cerebral oximetry were measured. Compared to the purely hypoxic gas group, was increased in the 3% and 5% CO2‐enriched groups by 14.9 and 9.5 mmHg, respectively. Compared to pure hypoxia, was increased in the 3% and 5% CO2‐enriched groups by 16.8% and 12.9%, respectively. Both CO2‐enriched gas groups had significantly higher end‐exposure rSO2 and recovered to baseline rSO2 within 1 min, compared to the pure hypoxic gas group, which returned to baseline in 5 min. These results suggest that in acutely hypoxic subjects, CO2 supplementation improves blood oxygen saturation and oxygen tension as well as cerebral oxygenation measures.
Keywords: cerebral perfusion, CO2 supplementation, hypoxia, oxygenation
Compared to pure hypoxia, was increased in the 3% and 5% CO2‐enriched groups by 16.8% and 12.9%, respectively. Both CO2‐enriched gas groups had significantly higher end‐exposure rSO2 and recovered to baseline rSO2 within 1 min, compared to the pure hypoxic gas group, which returned to baseline in 5 min. These results suggest that in acutely hypoxic subjects, CO2 supplementation improves blood oxygen saturation and oxygen tension as well as cerebral oxygenation measures.

1. INTRODUCTION
Supplemental oxygen systems have been utilized in aviation and mountaineering for many years, though such solutions address only oxygen supply and not carbon dioxide (capnic) homeostasis. At altitude, decreased atmospheric partial pressure of oxygen (PO2) triggers increases in ventilation, increasing the rates of both oxygen uptake and CO2 elimination (West, 2012). The resulting hypocapnia is generally well‐tolerated in healthy individuals. Hypocapnia is a critical and often overlooked detriment to optimal tissue oxygenation. Hypocapnia may decrease oxygenation by several mechanisms including increases in pulmonary ventilation‐perfusion mismatch, vasoconstriction in many vascular beds, and diminished oxygen unloading from hemoglobin due to the left shift in the oxy‐hemoglobin dissociation curve (Domino, Lu, Eisenstein, & Hlastala, 1993; Gustafsson, Sjoberg, Lewis, & Thorborg, 1993; Naeraa, Petersen, Boye, & Severinghaus, 1966). Hypocapnia concomitantly increases cellular oxygen demand via increased glycolysis, mitochondrial respiration, and sympathoadrenal tone, thereby exacerbating oxygen supply demand mismatch in hypoxic individuals (Laffey & Kavanagh, 2002). Therefore, it is prudent to consider both CO2 and O2 homeostasis in individuals who operate in hypoxic austere environments.
Few prior studies have examined hypocapnia as a contributor to hypoxia. Mosso's, 1898 experiments were the first to administer supplemental CO2 to hypoxic subjects in hypobaric chambers and in field studies in the Alps (Mosso, 1898). Over the next several decades, four more studies demonstrated a relationship between normocapnia and optimal oxygenation at altitude with a primary interest in improving oxygenation at high altitude (Childs, Hamlin, & Henderson, 1935; Douglas & Haldane, 1909; Douglas, Haldane, Henderson, & Schneider, 1913; Henderson, 1938). In 1988, TC Harvey demonstrated in an uncontrolled study that administration of supplemental CO2 improved acute mountain sickness symptoms in mountaineers (Harvey et al., 1988). Studies by Bärtsch and Maher cast doubt on these findings, as neither found beneficial effects of supplemental CO2 (Bartsch, Baumgartner, Waber, Maggiorini, & Oelz, 1990; Maher et al., 1975). Two recent studies have examined supplemental inspired CO2 as a tool to increase oxygenation and performance. In 2003, Imray et al. studied subjects at 150 m and at 3459 m breathing 3% CO2, 35% oxygen plus 3% CO2, and 35% oxygen, and measured effects on cerebral and peripheral oxygenation after breathing the gases for 5 min. At sea level and at altitude, they observed markedly increased arterial blood oxygen and cerebral oxygenation in subjects breathing the mixture of CO2 and oxygen (Imray et al., 2003). In 2007, Van Dorp et al. demonstrated that subjects with mild to moderate hypoxia performed better on vigilance and task performance tests following inhalation of gas mixtures with added CO2 (Van Dorp et al., 2007). Ashkanian et al. found that inhalation of 5% CO2 increased cerebral blood flow and cerebral oxygenation in normoxic individuals (Ashkanian, Borghammer, Gjedde, Ostergaard, & Vafaee, 2008). Physiologically the maintenance of normal oxygen and normal CO2 levels are critical to optimal tissue oxygen delivery.
We hypothesized that breathing a gas mixture of 8% oxygen supplemented with CO2 (3% and 5%) would improve oxygenation in hypoxic subjects when compared with breathing a gas mixture of 8% oxygen. The aims of this study were to examine the effects of inhaled hypoxic gas enriched with CO2 on partial pressure of arterial oxygen (), oxygen saturation of hemoglobin (), and regional cerebral saturation of hemoglobin (rSO2).
2. METHODS
The study protocol was approved by the Mayo Clinic Institutional Review Board. Volunteer subjects were selected from a healthy volunteer subject pool at Mayo Clinic for inclusion in the study. To avoid hypoxic collapse (loss of consciousness), the exposure time was limited to 5 min. Exclusion criteria included age <18 years and any history of obstructive or restrictive pulmonary disease, cardiovascular disease, epilepsy, diabetes mellitus, chronic headaches, neurological disorders, and hematological disorders. A negative urine pregnancy test was required for female subjects. Informed consent was obtained from all subjects prior to enrollment.
2.1. Equipment and procedures
Protocols were performed at the Aerospace Medicine and Vestibular Research Laboratory at the Mayo Clinic in Arizona (altitude 500 m [1,640 ft]; ambient pressure 716 mmHg). Subjects donned a Gentex military helmet and aviator tight‐fit facemask throughout the experimental protocols. Prior to initiation of the protocol, the mask hose was occluded at the inlet and the subjects were asked to inhale, effectively demonstrating a tight seal to ensure the absence of significant gas leaks. End‐tidal CO2 was recorded at the exhalation port of the aviator mask through a capnographic device (LifeSense® Tabletop Capnography, Nonin Medical, Inc.). Cerebral oximetry (rSO2) was recorded via Nonin Equanox 7600. The Equanox dual emitter and dual detector sensor (8000CA) was attached to the left forehead to measure rSO2.
Three different hypoxic conditions were induced by provision of premixed gas mixtures through the aviator mask. The acute hypoxic condition was simulated by use of a gas mixture containing 8% oxygen, balance nitrogen. The two supplemental CO2 conditions were induced by use of gas mixtures containing 8% oxygen, either 3% or 5% CO2, and balance nitrogen. Normoxia was achieved by providing ambient air through the mask. This study did not record measures of ventilation.
All baseline data were recorded under normoxic conditions prior to gas administration. For initial gas mixture exposure, subjects were randomized to either pure hypoxia or hypoxia with added CO2 for 5 min. This was followed by a 20‐min normoxia washout period, then administration of the alternative protocol. Ten subjects received 3% CO2 and 8% oxygen, balance nitrogen mixes. The remaining 10 subjects received 5% CO2 and 8% oxygen, balance nitrogen gas. Data were recorded at 1‐min intervals during each 5‐min gas exposure. Additionally, arterial blood gas analysis (ABGA) was performed at the end of each 5‐min gas exposure. The arterial blood was obtained by radial artery puncture by standard technique and immediately analyzed on an automated blood gas analysis machine (Radiometer ABL80 with cooximetry).
2.2. Statistical analysis
Data analysis followed collection using descriptive statistics, Student's t tests, and repeated‐measures ANOVA. All results are presented as means ± SD.
3. RESULTS
A sample of 20 subjects (men:women, 10:10) was enrolled. Subject demographics showed values of: age (35 ± 9.2 years, range 22–51), height (172.4 ± 9.1 cm), weight (76.6 ± 15.5 kg), and BMI (25.7 ± 4.7). There was no significant difference in age or BMI range or mean values by sex. Subjects were healthy, denied use of any medications, and were all nonsmokers. All 20 subjects completed the entire experimental protocol, including recordings at baseline, during exposure to the different hypoxic gas mixtures, and during the two post‐exposure normoxia periods.
Baseline mean rSO2 values ranged from 63%–82.3%, with 73.3 ± 4.9%. There was no significant difference between rSO2 baseline values before the different exposures. Experimental rSO2 values are shown in Figure 1. The purely hypoxic gas exposure yielded the lowest rSO2 values (rSO2 = 60.7 ± 6.9%). The 3% added CO2 group was found to have rSO2 values higher than their relative pure hypoxic gas exposure values after 4 min, persisting with significant elevation after 5 min of exposure (rSO2 = 66.5 ± 6.7%) and after 1 min of recovery in normoxic conditions. By the second minute of recovery, there was no significant difference in rSO2 between pure hypoxic gas and 3% CO2‐enriched gas groups. The 5% CO2‐enriched gas group had rSO2 values significantly higher than their relative purely hypoxic gas values after 2 min of exposure, persisting with significant elevation after 5 min of exposure (rSO2 = 72.6 ± 4.3%) and for the first 5 min of recovery in normoxic conditions. By 10 min of recovery, there was no significant difference between rSO2 in the purely hypoxic gas values compared to the 5% CO2‐enriched gas values.
FIGURE 1.
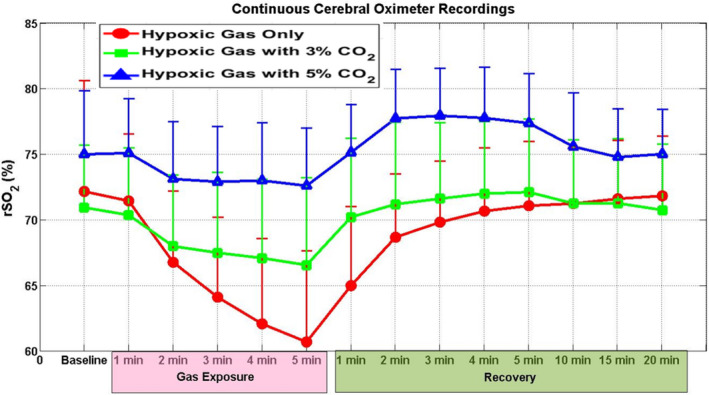
Continuous cerebral oximetry (means ± SD) by exposure time. Note that both capnic exposures (3% and 5%) demonstrate significantly improved rSO2 compared to hypoxic conditions alone. This improvement is more pronounced and longer lasting in the 5% group, persisting until 10 min into normoxic recovery conditions
End‐tidal CO2 measured at 1‐min intervals over 5 min of exposure demonstrated consistent values across the three groups, with steady state reached by 2 min into the exposure. The 5% CO2‐enriched gas group had the highest , followed by the 3% CO2‐enriched gas group, and the pure hypoxia group with the lowest. As expected, each group's correlated with the percentage of supplemental inspired CO2. A comparison of is demonstrated in Figure 2 below.
FIGURE 2.
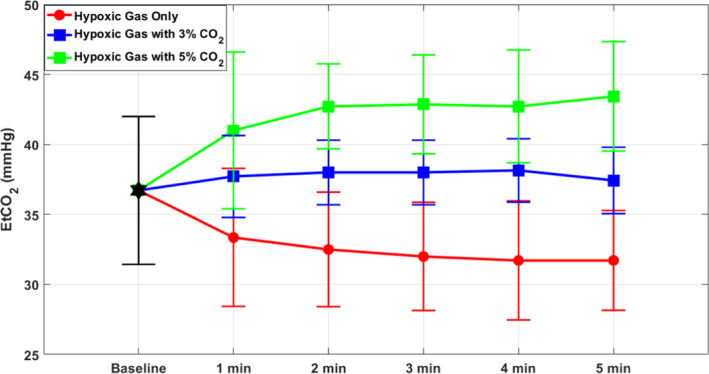
End‐tidal CO2 () measured at 1‐min intervals during gas exposure (means ± SD). correlates with concentration of CO2 in the inhaled gas mixture. Note that each group reaches steady state at 1 min
Figure 3 illustrates and measured at the end of a 5‐min gas mixture exposure. in the 3% CO2‐enriched gas group increased to 50.4 mmHg from 35.5 mmHg hypoxic gas baseline. in the 5% CO2‐enriched gas increased to 46.8 mmHg from 37.3 mmHg hypoxic gas baseline. values were elevated in the CO2‐enriched gas compared to pure hypoxic gas values (pure hypoxic gas mix = 35.5 ± 2.7%, 3% CO2‐enriched gas = 50.4 ± 4.4%, df = 18, p < .001; pure hypoxic = 37.3 ± 12.3%, 5% CO2‐enriched gas = 46.8 ± 7.2%, df = 13, p = .05).
FIGURE 3.
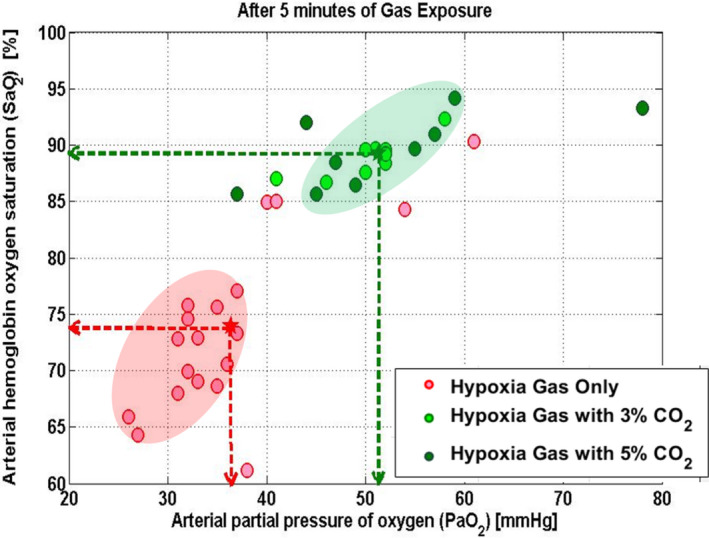
Arterial hemoglobin oxygen saturation () and arterial partial pressure of oxygen () after 5 min of gas exposure measured by arterial blood gas analysis. Note that both 3% and 5% CO2‐enriched gas groups demonstrate significantly higher and compared to purely hypoxic conditions
improvements paralleled changes in . in the 3% CO2‐enriched gas group increased to 88.9% from 72.1% in the hypoxic gas. in the 5% CO2‐enriched gas increased to 88.8% from 75.9% hypoxic gas. Overall, was elevated in both the 3% and 5% CO2‐enriched gas groups compared to hypoxic gas (pure hypoxia = 72.12 ± 6.3%, 3% CO2‐enriched gas = 88.9 ± 1.6, df = 18, p < 0.001; pure hypoxic gas mix = 75.9 ± 9.0%, 5% CO2‐enriched gas = 88.8 ± 3.8%, df = 13, p = 0.002).
4. DISCUSSION
The administration of 3% or 5% CO2‐enriched hypoxic gas mixtures (8% inhaled oxygen) improved , , and rSO2 of hypoxic individuals in the laboratory setting compared to purely hypoxic stress (Figure 3).
Cerebral oximetry profiles varied markedly between the three exposures (Figure 1). Both CO2‐enriched gas groups demonstrated rSO2 profiles that were more favorable than purely hypoxic gas, with the 5% CO2‐enriched gas group demonstrating the highest overall end‐exposure rSO2. Both CO2‐enriched gas groups returned to baseline within 2 min of normoxic recovery, whereas the hypoxia only group required 5 min to return to baseline. Interestingly, both CO2 supplemented groups exceeded baseline rSO2 during the recovery phase, whereas the purely hypoxic gas group did not. This suggests that, compared to hypoxic gas alone, gas with added CO2 improves cerebral oxygenation during the hypoxic exposure (Imray et al., 2003) and leads to a much more rapid recovery when normoxia is restored. However, it is important to note that it is unclear if this increase in cerebral oxygenation results in metabolic or functional changes. Pre‐exposure rSO2 baselines of the three groups also showed variability, though all matched their post‐exposure baselines. We believe that this variability was a result of smaller samples in the CO2‐enriched gas groups, and we emphasize that the hypoxia only group mean approximately equals the mean of the combined 3% and 5% CO2‐enriched gas groups, as would be statistically expected.
The response dynamics of cerebral tissue oximetry may be explained by the more complex biological nature of CO2 alterations. While changes in oxygen are mediated in the body exclusively by hemoglobin binding (and to a minimal extent plasma and myoglobin), CO2 is bound to hemoglobin, bound to proteins, and converted to bicarbonate. Hence, changes in CO2 will depend on more than just the rapid interaction with hemoglobin (as is the case with oxygen), but rather also depend on alterations in bicarbonate, plasma protein binding, and tissue binding, and by consequence require a longer time to compensate (Farhi & Rahn, 1955). The maintenance of capnic homeostasis by supplementing inhaled CO2 therefore appears to afford the cerebral tissue a more rapid recovery mechanism.
Our results expand upon the findings of Ashkanian and Imray, which reported improved with CO2 supplementation in normoxic individuals (Ashkanian et al., 2008; Imray et al., 2003). We found the same effect, though we tested this in acutely normobaric hypoxic individuals rather than normoxic or hypobaric hypoxic individuals. Harvey et al. found higher in hypoxic individuals following supplemental CO2 inhalation, a finding that was mirrored in our data (Harvey et al., 1988). Our findings of improved rSO2 with CO2‐enriched gas exposure may explain the findings of improvement in cognitive function of Van Dorp et al. (2007).
Our results support the argument that oxygenation requires maintenance of normal oxygen and CO2 levels. The discussion of oxygenation tends to center on oxygen alone, while little attention is given to the equally important component of carbon dioxide homeostasis (Swenson, 2019). We hypothesize that supplemental CO2 may improve oxygenation by several mechanisms. Foremost, hypocapnia impairs respiratory efficiency by increasing small airway resistance (Croxton, Lande, & Hirshman, 1995) and reducing lung compliance (Cutillo, Omboni, Perondi, & Tana, 1974), thereby increasing ventilation‐perfusion mismatch, as has been demonstrated in hyperventilated dogs (Domino, Swenson, et al., 1993). Hypocapnia induces systemic vasoconstriction in many vascular beds including the brain (Gustafsson et al., 1993), thereby impairing blood flow to tissues. Hypocapnia also increases intrapulmonary shunting due to hypoxic vasoconstriction (Domino, Lu, et al., 1993) and decreases oxygen offloading at the tissues due to a left shift in the oxygen‐hemoglobin dissociation curve (Naeraa et al., 1966). Supplemental inspired CO2 may ameliorate these deleterious effects of hypocapnia, thereby improving V/Q mismatch, increasing effective ventilatory surface area, and improving oxygenation. Our results demonstrate the previously discussed physiologic effects of supplemental inhaled CO2, ultimately leading to improved oxygenation with supplemental inhaled CO2. Taken as a whole, these results suggest that supplemental inhaled CO2 with or without supplemental added O2 may be of value as a tool to improve oxygenation in individuals operating in hypoxic environments.
4.1. Limitations
The degree of hypoxia was of significant severity such that we had to limit the duration of exposure to avoid inherent hypoxic collapse. As a result, the reported data represent the state of the individual subjects after 5 min and are not representative of a true steady state from a respiratory physiology perspective.
The absence of objective measures of ventilation is a limitation of this work and as such it is difficult to differentiate the direct effect of CO2 on gas exchange as opposed to the effect of CO2 as a respiratory stimulant. Clinically, the experimenters did not perceive any significant difference in breathing patterns among subjects.
One additional potential limitation of this study design is subtle mask leak, which is a difficult variable to entirely eliminate, though it was addressed to the best of our abilities by an initial test to ascertain proper mask fit. We assessed for excessive ventilatory variation among individuals by measuring at 1‐min intervals during gas exposure, which we found to be markedly consistent between subjects within gas mixture groups. Across all subjects, end‐tidal CO2 reached steady state within 1 min of exposure and remained constant for the remainder of the exposure, as is demonstrated in Figure 2. We interpret this data to indicate limited intersubject variability in minute ventilation and appropriate mask fit. We did not measure ventilation in this study, hence quantification of differences in ventilation beyond the measured cannot be provided. An important effect of adding CO2 to respired gas is the increase in ventilation due to the respiratory stimulant effect of CO2, which also will increase oxygenation to a certain extent (Imray et al., 2000). Additionally, one PaCO2 level and three levels were not readable due to technical errors.
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTIONS
J.S. and G.P. conceived and designed research, wrote IRB and protocol, performed the experiments, and edited the manuscript. R.D. wrote the manuscript with exception of methods and results. G.P. performed the statistical analysis and wrote the methods and results sections of the manuscript. M.C. conceived and designed research, wrote IRB and protocol, and performed the experiments.
ACKNOWLEDGMENT
We gratefully acknowledge the valuable assistance and expertise of the Pulmonary Medicine staff at Mayo Clinic with the numerous arterial blood gas measurements.
Stepanek J, Dunn RA, Pradhan GN, Cevette MJ. Supplemental CO2 improves oxygen saturation, oxygen tension, and cerebral oxygenation in acutely hypoxic healthy subjects. Physiol Rep. 2020;8:e14513 10.14814/phy2.14513
Funding information
This study was funded entirely by Mayo Clinic institutional funding.
Jan Stepanek and Ryan A. Dunn are co‐first authors.
REFERENCES
- Ashkanian, M. , Borghammer, P. , Gjedde, A. , Ostergaard, L. , & Vafaee, M. (2008). Improvement of brain tissue oxygenation by inhalation of carbogen. Neuroscience, 156, 932–938. 10.1016/j.neuroscience.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Bartsch, P. , Baumgartner, R. W. , Waber, U. , Maggiorini, M. , & Oelz, O. (1990). Comparison of carbon‐dioxide‐enriched, oxygen‐enriched, and normal air in treatment of acute mountain sickness. Lancet, 336, 772–775. 10.1016/0140-6736(90)93240-P [DOI] [PubMed] [Google Scholar]
- Childs, S. B. , Hamlin, H. , & Henderson, Y. (1935). Possible value of inhalation of carbon dioxide in climbing great altitude. Nature, 135, 457–458. 10.1038/135457a0 [DOI] [Google Scholar]
- Croxton, T. L. , Lande, B. , & Hirshman, C. A. (1995). Role of intracellular pH in relaxation of porcine tracheal smooth muscle by respiratory gases. American Journal of Physiology, 268, L207–213. 10.1152/ajplung.1995.268.2.L207 [DOI] [PubMed] [Google Scholar]
- Cutillo, A. , Omboni, E. , Perondi, R. , & Tana, F. (1974). Effect of hypocapnia on pulmonary mechanics in normal subjects and in patients with chronic obstructive lung disease. American Review of Respiratory Disease, 110, 25–33. [DOI] [PubMed] [Google Scholar]
- Domino, K. B. , Lu, Y. , Eisenstein, B. L. , & Hlastala, M. P. (1993). Hypocapnia worsens arterial blood oxygenation and increases VA/Q heterogeneity in canine pulmonary edema. Anesthesiology, 78, 91–99. 10.1097/00000542-199301000-00014 [DOI] [PubMed] [Google Scholar]
- Domino, K. B. , Swenson, E. R. , Polissar, N. L. , Lu, Y. , Eisenstein, B. L. , & Hlastala, M. P. (1993). Effect of inspired CO2 on ventilation and perfusion heterogeneity in hyperventilated dogs. Journal of Applied Physiology, 75(3), 1306–1314. 10.1152/jappl.1993.75.3.1306 [DOI] [PubMed] [Google Scholar]
- Douglas, C. G. , & Haldane, J. S. (1909). The causes of periodic or Cheyne‐Stokes breathing. Journal of Physiology, 38, 401–419. 10.1113/jphysiol.1909.sp001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, C. , Haldane, J. , Henderson, Y. , & Schneider, E. (1913). Physiological observations made on Pike's peak. Philosophical Transactions of the Royal Society B: Biological Sciences, 203, 185–318. [Google Scholar]
- Farhi, L. E. , & Rahn, H. (1955). Gas stores of the body and the unsteady state. Journal of Applied Physiology, 7, 472–484. 10.1152/jappl.1955.7.5.472 [DOI] [PubMed] [Google Scholar]
- Gustafsson, U. , Sjoberg, F. , Lewis, D. H. , & Thorborg, P. (1993). The effect of hypocapnia on skeletal muscle microcirculatory blood flow, oxygenation and pH. International Journal of Microcirculation: Clinical and Experimental, 12, 131–141. [PubMed] [Google Scholar]
- Harvey, T. C. , Raichle, M. E. , Winterborn, M. H. , Jensen, J. , Lassen, N. A. , Richardson, N. V. , & Bradwell, A. R. (1988). Effect of carbon dioxide in acute mountain sickness: A rediscovery. Lancet, 2, 639–641. 10.1016/s0140-6736(88)90465-5 [DOI] [PubMed] [Google Scholar]
- Henderson, Y. (1938). Adventures in respiration. London, UK: Bailliere, Tindall and Cox. [Google Scholar]
- Imray, C. H. E. , Brearey, S. , Clarke, T. , Hale, D. , Morgan, J. , Walsh, S. , … Birmingham Medical Research Expeditionary Society . (2000). Cerebral oxygenation at high altitude and the response to carbon dioxide, hyperventilation and oxygen. Clinical Science, 98, 159–164. 10.1042/cs0980159 [DOI] [PubMed] [Google Scholar]
- Imray, C. H. , Walsh, S. , Clarke, T. , Tiivas, C. , Hoar, H. , Harvey, T. C. , … Birmingham Medical Research Expeditionary Society . (2003). Effects of breathing air containing 3% carbon dioxide, 35% oxygen or a mixture of 3% carbon dioxide/35% oxygen on cerebral and peripheral oxygenation at 150 m and 3459 m. Clinical Science, 104, 203–210. 10.1042/cs1040203 [DOI] [PubMed] [Google Scholar]
- Laffey, J. G. , & Kavanagh, B. P. (2002). Hypocapnia. New England Journal of Medicine, 347, 43–53. 10.1056/NEJMra012457 [DOI] [PubMed] [Google Scholar]
- Maher, J. T. , Cymerman, A. , Reeves, J. T. , Cruz, J. C. , Denniston, J. C. , & Grover, R. F. (1975). Acute mountain sickness: Increased severity in eucapnic hypoxia. Aviation, Space and Environmental Medicine, 46, 826–829. [PubMed] [Google Scholar]
- Mosso, A. (1898). Life of man on the High Alps. London, UK: Fisher Unwin. [Google Scholar]
- Naeraa, N. , Petersen, E. S. , Boye, E. , & Severinghaus, J. W. (1966). pH and molecular CO2 components of the Bohr effect in human blood. Scandinavian Journal of Clinical and Laboratory Investigation, 18, 96–102. 10.3109/00365516609065612 [DOI] [PubMed] [Google Scholar]
- Swenson, E. R. (2019). The unappreciated role of carbon dioxide in ventilation/perfusion matching. Anesthesiology, 131, 226–228. 10.1097/ALN.0000000000002791 [DOI] [PubMed] [Google Scholar]
- Van Dorp, E. , Los, M. , Dirven, P. , Sarton, E. , Valk, P. , Teppema, L. , … Dahan, A. (2007). Inspired carbon dioxide during hypoxia: Effects on task performance and cerebral oxygen saturation. Aviation, Space and Environmental Medicine, 78, 666–672. [PubMed] [Google Scholar]
- West, J. B. (2012). High‐altitude medicine. American Journal of Respiratory and Critical Care Medicine, 186, 1229–1237. 10.1164/rccm.201207-1323CI [DOI] [PubMed] [Google Scholar]


