Abstract
AIM
To quantitatively evaluate the effect of the combined use of 577-nm subthreshold micropulse macular laser (SML) and multi-point mode pan retinal laser photocoagulation (PRP) on severe non-proliferative diabetic retinopathy (NPDR) with central-involved diabetic macular edema (CIDME) using optical coherence tomography angiography (OCTA).
METHODS
In this observational clinical study, 86 eyes of 86 NPDR patients with CIDME who underwent SML and PRP treatment were included. Images were obtained 1d before laser and post-laser (1d, 1wk, 1, 3, and 6mo) using AngioVue software 2.0. Best corrected visual acuity (BCVA, LogMAR), foveal avascular zone area (FAZ), choriocapillary flow area (ChF), parafoveal vessel density (PVD), capillary density inside disc (CDD), peripapillary capillary density (PCD), macular ganglion cell complex thickness (mGCCT), central macular thickness (CMT), and subfoveal choroidal thickness (ChT) were compared between pre- and post-laser treatment.
RESULTS
BCVA remained stable during 6mo post-laser therapy (pre-laser vs 6mo post-laser: 0.53±0.21 vs 0.5±0.15, P>0.05). PVD, ChF, ChT, CMT, and mGCCT significantly increased 1d post-laser therapy [pre-laser vs 1d post-laser: superficial PVD (%), 40.51±3.42 vs 42.43±4.68; deep PVD (%), 42.66±3.67 vs 44.78±4.52; ChF, 1.72±0.21 vs 1.9±0.12 mm2; ChT, 302.45±69.74 vs 319.38±70.93 µm; CMT, 301.65±110.78 vs 320.86±105.62 µm; mGCCT, 105.71±10.72 vs 115.46±9.64 µm; P<0.05]. However, PVD, ChF and ChT decreased to less than baseline level at 6mo post-laser therapy (pre-laser vs 6mo post-laser: superficial PVD (%), 40.51±3.42 vs 36.32±4.19; deep PVD (%), 42.66±3.67 vs 38.76±3.74; ChF, 1.72±0.21 vs 1.62±0.09 mm2; ChT, 302.45±69.74 vs 289.61±67.55 µm; P<0.05), whereas CMT and mGCCT decreased to baseline level at 6mo post-laser therapy (CMT, 301.65±110.78 vs 297.77±90.23 µm; mGCCT, 105.71±10.72 vs 107.05±11.81 µm; P>0.05). Moreover, FAZ continuously increased while CDD and PCD continuously decreased in 6mo after laser therapy. CMT and ChT had a significant positive correlation with ChF and PVD in most post-laser stages.
CONCLUSION
During a 6-month follow-up period after combined use of SML and PRP therapy, BCVA remained stable and there was a decreased trend in macular edema. Blood flow increased at 1d post-laser therapy and reduced at 6mo post-laser therapy.
Keywords: diabetic macular edema, diabetic retinopathy, subthreshold micropulse laser, optical coherence tomography angiography
INTRODUCTION
Diabetic retinopathy (DR) is the main cause of blindness in the working-age population worldwide[1]–[2]. Traditional 532-nm pan retinal photocoagulation (PRP) therapy has been proven effective and has been recommended in severe non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Regardless of the proven benefit of 532-nm PRP in treating DR, 532-nm laser treatment can cause significant photoreceptor loss, retinal pigment epithelium (RPE), and even thermal damage involving the entire retinal layer. In addition, it cannot be performed in the area very close to the fovea. Nowadays, central-involved diabetic macular edema (CIDME) is commonly treated with intravitreous anti-vascular endothelial growth factor (VEGF) injection. Previous studies[3]–[4] have shown that anti-VEGF agents can reduce CIDME and improve baseline visual acuity. According to the DR statement declared by the American Diabetes Association (ADA) in 2017[5], the best treatment option of CIDME was intravitreous anti-VEGF injection. In the past few decades, bevacizumab was available worldwide and was cheap. However, bevacizumab was identified as an illegal medication due to its off-label use in China. The high cost of anti-VEGF agents such as ranibizumab has been a major problem[6]–[7]. Recently, as laser technology advances, the 577-nm subthreshold micropulse laser (SML) photocoagulation system has led to selective photocoagulation for the RPE with minimal effects on the sensory retina and choroid. According to the international guideline[5], PRP should not be performed until PDR appear. However, in China, DR treatment is more active. According to China's guideline in 2014[8], severe NPDR with diabetic macular edema (DME) can be treated with laser, which is the result of consideration of patients' cost, compliance, expectation, and other factors. Consequently, combined use of 577-nm micropulse and multi-point mode pan retinal laser therapy for DME treatment may be a choice of treatment in severe NPDR with CIDME subjects.
In the past few decades, optical coherence tomography (OCT), a non-invasive real-time cross-sectional imaging technique, has been widely applied in retinal thickness measurement and lesions detection. However, fluorescein fundus angiography (FFA) must be performed when detailed vascular lesions need to be confirmed. FFA has several limitations, for example, it requires intravenous dye injection, which is not suitable for individuals with serious systemic diseases. Severe anaphylaxis may occur in healthy individuals[9]–[10], and it is difficult to locate the lesions layer by layer. Moreover, quantification cannot be performed in FFA. Recently, optical coherence tomography angiography (OCTA) has been introduced. OCTA not only performs imaging of retinal tissue at high resolution but also allows for quantification of the dimensions of the retinal capillary networks. Therefore, the thickness measurement and quantification of subtle vascular changes can be performed simultaneously in a non-invasive manner.
Previous studies in DME patients have mostly focused on changes of best corrected visual acuity (BCVA) and retina and choroid thickness. Studies in which OCTA characteristics were compared before and after 577-nm laser in severe NPDR with CIDME are limited. In addition, vascular changes of the retina and the choroid were not clear. The purpose of the present study was to quantitatively evaluate the effect of the combined use of 577-nm micropulse and multi-point mode pan retinal laser therapy on severe NPDR with CIDME based on OCTA.
SUBJECTS AND METHODS
Ethical Approval
The study was approved by the institutional review board of Sun Yat-sen Memorial Hospital, Sun Yat-sen University in Guangzhou, China and adhered to the guidelines of the Declaration of Helsinki. The risk of worsening of CIDME after PRP was explained and written informed consent was obtained from all participants.
This study was a retrospective clinical study. A total of 86 eyes of 86 NPDR patients with CIDME were recruited from the ophthalmology department between March 2016 and June 2018. Criteria for inclusion included a diagnosis of severe NPDR with CIDME in patients with type 2 diabetes mellitus (T2DM) who had a record of 577-nm laser treatment. CIDME was defined as DME with an increase of retinal thickness within a radius of 500 µm in the foveal. All the patients were strongly recommended for anti-VEGF treatment, but they chose laser-only treatment due to cost and other problems. The more serious eye was included if the severity was different in both eyes, while a random eye was included if the severity was the same. DR staging was defined according to the classification criteria published in 2017 by the ADA[5]. Based on complete ophthalmic examinations including BCVA, intraocular pressure (IOP), dilated fundus examination with slit-lamp biomicroscopy and indirect ophthalmoscopy, color fundus photographs, OCTA and FFA, the diagnosis of severe NPDR with CIDME was made by two senior retina professors (Xiao JH and Lan YQ). Other data included gender, age, hemoglobin Alc (HbAlc) levels at baseline, the duration of diabetes and other systemic diseases such as hypertension and dyslipidemia were recorded. Patients with severe hypertension and other systemic diseases were excluded. Besides, patients with high levels of myopia (<-3 dioptres or axial length ≥26 mm), glaucoma, and lens opacity that may affect imaging and a history of intraocular surgery or any other retinal diseases were also excluded.
577-nm Pattern Scanning Laser Photocoagulation
Treatment for PRP and DME was performed using yellow 577-nm wavelength (IQ 577, Iridex, USA) with the Super Quad 160 contact lens (Volk Optical., Mentor, OH, USA) and the Area Centralis contact lens (Volk Optical., Mentor, OH, USA), respectively. For PRP treatment, a 3×3 grid multi-point mode was applied once. To create an effective retinal burn (grade 3) during PRP treatment, the spot diameter, duration, and power were adjusted to 200 µm, 100ms, and ∼200 mW, respectively. For CIDME treatment, a test spot of 100 µm in diameter and 200ms in exposure time was initially performed in the continuous-wave mode. When the power was gradually increased and test spots were barely visible, a micropulse mode at 5% duty cycles and quadruple the laser power was transferred. Laser therapy was conducted in a 3×3 pattern mode without spacing over the area of macular edema including the fovea. OCTA was performed at 1d before laser therapy as well as on 1d, 1wk, 1, 3, and 6mo after laser therapy. CIDME retreatment was performed when DME was persistent as determined in OCTA images at least 1mo after laser therapy [central macular thickness (CMT) ≥ baseline][11]–[12].
Image Collection and Analysis
OCTA images were obtained using prototype AngioVue software 2.0 of the RTVue XR Avanti spectral domain OCT device (Optovue, Inc., Fremont, CA, USA; Figures 1 and 2). A split-spectrum amplitude-decorrelation angiography (SSADA) algorithm was used to determine erythrocyte movement in OCTA. Angio Retina 3×3 mm2 mode and HD Angio Disc 4.5×4.5 mm2 mode were performed to assess the foveal avascular zone area (FAZ), choriocapillary flow area (ChF), parafoveal vessel density (PVD), capillary density inside disc (CDD), and peripapillary capillary density (PCD) respectively. The GCC mode and Retina Map were performed to determine macular ganglion cell complex thickness (mGCCT) and CMT. The above parameters were automatically measured by the AngioVue software. In addition, cross line mode was conducted to assess subfoveal choroidal thickness (ChT). ChT was defined as the distance between the outermost edge of the RPE hyper-reflective line and the sclera-choroidal border. The horizontal and vertical sections were manually measured by two independent researchers (Zeng P and Zeng R). The average values of the horizontal and vertical sections were recorded.
Figure 1. OCTA images and B-scan images in an affected right eye.
Female, 47 years old. A: One day before laser: A1 foveal avascular zone area (FAZ), 1.34 mm2; superficial parafoveal vessel density (sPVD), 38.4%; deep parafoveal vessel density (dPVD), 40.7%, A2 choriocapillary flow area (ChF), 1.71 mm2, A3 capillary density inside disc (CDD), 52.1%; peripapillary capillary density (PCD), 52.3%, A4 and A5 central macular thickness (CMT), 344.72 µm; subfoveal choroidal thickness (ChT), 286.42 µm. B: One day after laser: B1 FAZ, 1.38 mm2; sPVD, 41.5%; dPVD, 42.8%, B2 ChF, 1.64 mm2, B3 CDD, 51.1%; PCD, 51.4%, B4 and B5 CMT, 349.63 µm; ChT, 292.35 µm. C: One week after laser: C1 FAZ, 1.42 mm2; sPVD, 39.2%; dPVD, 41.8%, C2 ChF, 1.75 mm2, C3 CDD, 50.5%; PCD, 50.4%, C4 and C5 CMT, 332.82 µm; ChT, 281.49 µm. D: One month after laser: D1 FAZ, 1.53 mm2; sPVD, 38.7%; dPVD, 41.2%, D2 ChF, 1.46 mm2, D3 CDD, 49.3%; PCD, 50.6%, D4 and D5 CMT, 302.54 µm; ChT, 270.47 µm. E: Two months after laser, the patient had an additional follow-up because of decreased vision. Another SML treatment was performed. E1 FAZ, 1.58 mm2; sPVD, 38.4%; dPVD, 37.9%, E2 ChF, 1.46 mm2, E3 CDD, 49.7%; PCD, 51.2%, E4 and E5 CMT, 397.32 µm; ChT, 272.12 µm. F: Three months after laser: F1 FAZ, 1.60 mm2; sPVD, 37.5%; dPVD, 40.3%, F2 ChF, 1.45 mm2, F3 CDD, 49.5%; PCD, 50.1%, F4 and F5 CMT, 287.63 µm; ChT, 272.43 µm. G: Six months after laser: G1 FAZ, 1.69 mm2; sPVD, 37.2%; dPVD, 39.8%, G2 ChF, 1.44 mm2, G3 CDD, 47.4%; PCD, 48.8%, G4 and G5 CMT, 256.42 µm; ChT, 267.15 µm.
Figure 2. GCC report in the same patient.
Macular ganglion cell complex thickness (mGCCT) was collected automatically in the GCC mode.
Statistical Analysis
Statistical analyses were performed using SPSS 24.0 (SPSS Inc. Chicago, IL, USA). Paired t-tests were applied to compare the above parameters changes (BCVA, FAZ, ChF, superficial and deep PVD, CDD, PCD, mGCCT, CMT, and ChT) between 1d, 1wk, 1, 3, and 6mo after laser therapy and 1d before laser, respectively. Correlations between BCVA and other parameters and correlations between thickness parameters (mGCCT, CMT or ChT) and vessel parameters (FAZ, ChF, superficial and deep PVD, CDD or PCD) in the same post-laser stage were analyzed using Pearson's correlation test. Correlation coefficient was reported. P<0.05 was considered statistically significant.
RESULTS
Patients' Demographics and Clinical Characteristics
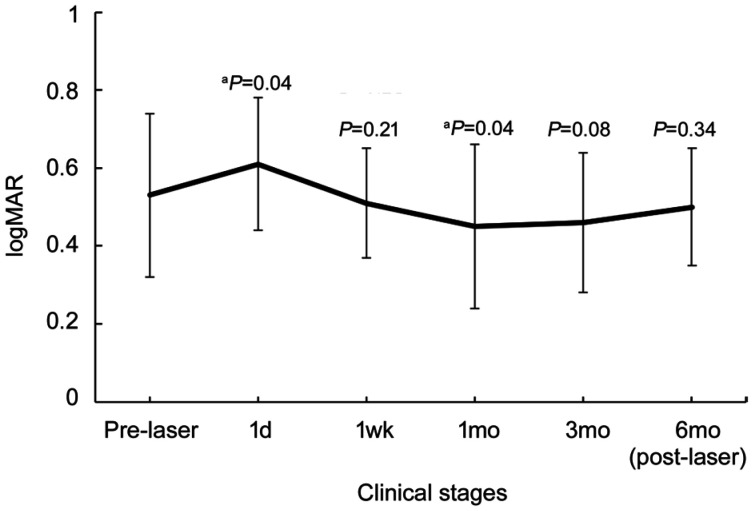
In this study, 86 eyes obtained from 86 patients were included. The patients' demographic characteristics at baseline are presented in Table 1. At baseline, the average IOP was 13.53±3.7 (10.1-18.7) mm Hg and the IOPs of different stages were within normal limits. The BCVA (logMAR) significantly increased 1d post-laser therapy (P=0.04) and decreased to less than baseline in 1mo (P=0.04; Figure 3). The power and number of shots used in 577-nm laser therapy and the number of patients who needed addition SML after the first laser treatment are reported in Table 2. Two patients had obvious decreased vision at two months after laser and referred to our clinic (Figure 1). Two patients (2.33%) still had persistent CIDME during the last follow-up.
Table 1. Patients demographics at baseline.
| Demographic characteristics | Data |
| Eyes, n | 86 |
| Age, y | 58.60±7.84 |
| Sex, female:male | 42:44 |
| Duration of diabetes mellitus, y | 10.43±3.70 |
| Hemoglobin Alc, % | 9.82±3.12 |
| BCVA, logMAR | 0.53±0.21 |
| IOP, mm Hg | 13.53±3.7 |
| Follow-up time (mo) | 7.56±1.12 |
BCVA: Best corrected visual acuity; IOP: Intraocular pressure.
Figure 3. BCVA changes at different clinical stages.
The BCVA (logMAR) significantly increased 1d post-laser therapy and decreased to less than baseline at 1mo after treatment. BCVA increased to baseline level at 6mo post-laser therapy. The P value indicated that the BCVA at certain stage was (not) significantly different from that at baseline. aP<0.05.
Table 2. Laser treatment at different clinical stages.
| Laser treatment parameters | Data |
| PRP mean power, mW | 204.52±.23.70 |
| Mean number of PRP shots, n | 1635.62±160.16 |
| SML mean power, mW | 147.39±10.42 |
| Mean number of SML shots, n | 252.43±37.58 |
| SML1, n (%) | 15 (17.44) |
| Mean number of SML1 shots | 243.55±10.33 |
| SML2, n (%) | 2 (2.33) |
| Mean number of SML2 shots | 243.55±30.28 |
| SML3, n (%) | 13 (15.12) |
| Mean number of SML3 shots | 246.24±32.84 |
| SML6, n (%) | 2 (2.33) |
| Mean number of SML6 shots | 243.55±30.28 |
| Number of P-CIDME at last follow-up, n (%) | 2 (2.33) |
PRP: Pan retinal photocoagulation laser; SML: Subthreshold micropulse macular laser; SML1: SML needed 1mo after laser therapy; SML2: SML needed 2mo after laser therapy; SML3: SML needed 3mo after laser therapy; SML6: SML needed 6mo after laser therapy; P-CIDME: Persistent central-involved diabetic macular edema.
Optical Coherence Tomography Angiography Findings
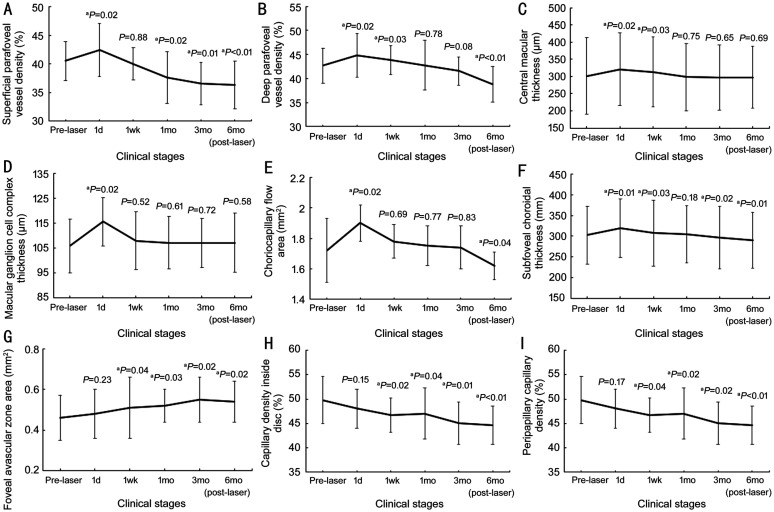
The range of the CMT 1d before laser was 223 to 478 µm. The degree of macular edema is graded as followed: 1) CMT≤250 µm, 3 eyes; 2) 250 µm <CMT≤350 µm, 78 eyes; 3) CMT>350 µm, 5 eyes. OCTA findings showed that superficial and deep PVD, ChF, and ChT were significantly increased at 1d post-laser therapy (superficial PVD, P=0.02; deep PVD, P=0.02; ChF, P=0.02; ChT, P=0.01) and decreased to less than baseline 6mo post-laser (superficial PVD, P<0.01; deep PVD, P<0.01; ChF, P=0.04; ChT, P=0.01; Figure 4A, 4B, 4E, and 4F). In addition, CMT and mGCCT significantly increased at 1d post-laser therapy (CMT, P=0.02; mGCCT, P=0.02), however they decreased to baseline level at 6mo post-laser therapy (CMT, P=0.69; mGCCT, P=0.58; Figure 4C, 4D). Moreover, FAZ continuously increased (Figure 4G) whereas CDD and PCD continuously decreased at 6mo after laser therapy (Figure 4H, 4I). Detailed values corresponding to Figures 3 and 4 were shown in Table 3.
Figure 4. Vascular and thickness changes at different clinical stages.
Superficial and deep parafoveal vessel density (A, B), choriocapillary flow area (E), and subfoveal choroidal thickness (F) were significantly increased at 1d post-laser therapy and decreased continuously to less than baseline level at 6mo post-laser therapy. Central macular thickness (C) and macular ganglion cell complex thickness (D) significantly increased at 1d post-laser therapy and decreased to baseline level at 6mo post-laser therapy. Foveal avascular zone area (G) continuously increased at 6mo after laser therapy. Capillary density inside the disc (H) and peripapillary capillary density (I) continuously decreased at 6mo after laser therapy. The P value indicated that the above parameters at certain stages were (not) significantly different from that at baseline. aP<0.05.
Table 3. BCVA and OCTA parameters at different clinical stages.
| Parameters | Pre-laser | 1d post-laser | 1wk post-laser | 1mo post-laser | 3mo post-laser | 6mo post-laser |
| BCVA | 0.53±0.21 | 0.61±0.17a | 0.51±0.14 | 0.45±0.21a | 0.46±0.18 | 0.5±0.15 |
| SPVD, % | 40.51±3.42 | 42.43±4.68a | 40.02±2.78 | 37.63±4.52a | 36.54±3.68a | 36.32±4.19a |
| DPVD, % | 42.66±3.67 | 44.78±4.52a | 43.81±3.04a | 42.74±5.16 | 41.54±2.94 | 38.76±3.74a |
| CMT, µm | 301.65±110.78 | 320.86±105.62a | 312.73±101.74a | 298.45±97.65 | 296.84±95.42 | 297.77±90.23 |
| mGCCT, µm | 105.71±10.72 | 115.46±9.64a | 107.86±11.54 | 107.03±10.53 | 106.93±9.96 | 107.05±11.81 |
| ChF, mm2 | 1.72±0.21 | 1.9±0.12a | 1.78±0.11 | 1.75±0.13 | 1.74±0.14 | 1.62±0.09a |
| ChT, µm | 302.45±69.74 | 319.38±70.93a | 307.72±79.66a | 304.26±68.75 | 296.44±75.23a | 289.61±67.55a |
| FAZ, mm2 | 0.46±0.11 | 0.48±0.12 | 0.51±0.15a | 0.52±0.08a | 0.55±0.11a | 0.54±0.1a |
| CDD, % | 49.75±4.84 | 48.05±4.02 | 46.67±3.55a | 47.02±5.21a | 45.03±4.32a | 44.63±3.92a |
| PCD, % | 47.19±3.43 | 46.39±4.05 | 46.04±3.72a | 45.67±3.82a | 45.87±3.42a | 44.34±3.87a |
BCVA: Best corrected visual acuity; OCTA: Optical coherence tomography angiography; SPVD: Superficial parafoveal vessel density; DPVD: Deep parafoveal vessel density; CMT: Central macular thickness; mGCCT: Macular ganglion cell complex thickness; ChF: Choriocapillary flow area; ChT: Subfoveal choroidal thickness; FAZ: Foveal avascular zone area; CDD: Capillary density inside disc; PCD: Peripapillary capillary density. aP<0.05, value at certain stage was significantly different from that at baseline.
In most post-laser stages, CMT and ChT had a significant positive correlation with ChF, superficial or deep PVD, respectively (Table 4). CMT and ChT had a significantly positive correlation with FAZ at 1d and 1wk post-laser therapy, whereas a significantly negative correlation was observed with FAZ at 1, 3, and 6mo post-laser therapy. CMT and ChT had a significantly negative correlation with CDD or PCD at 1wk post-laser therapy, whereas a significant positive correlation was observed with CDD or PCD at 1, 3, and 6mo post-laser therapy. No significant correlation was observed between BCVA or mGCCT and other parameters.
Table 4. Correlation analysis between CMT or ChT and vessel parameters.
| Correlation | Vessel parameters |
||||
| 1d | 1wk | 1mo | 3mo | 6mo | |
| CMT | SPVD | SPVD | SPVD | SPVD | SPVD |
| r | 0.52 | 0.43 | 0.47 | 0.39 | 0.56 |
| P | 0.03 | 0.08 | 0.04 | 0.02 | 0.03 |
| DPVD | DPVD | DPVD | DPVD | DPVD | |
| r | 0.57 | 0.62 | 0.53 | 0.44 | 0.61 |
| P | 0.02 | 0.08 | 0.03 | 0.12 | 0.02 |
| ChF | ChF | ChF | ChF | ChF | |
| r | 0.63 | 0.57 | 0.71 | 0.47 | 0.63 |
| P | 0.02 | 0.04 | 0.03 | 0.02 | 0.01 |
| FAZ | FAZ | FAZ | FAZ | FAZ | |
| r | 0.45 | 0.55 | -0.48 | -0.39 | -0.52 |
| P | 0.03 | 0.02 | 0.04 | 0.02 | 0.01 |
| CDD | CDD | CDD | CDD | CDD | |
| r | -0.37 | -0.46 | 0.35 | 0.48 | 0.52 |
| P | 0.12 | 0.03 | 0.01 | 0.03 | 0.02 |
| PCD | PCD | PCD | PCD | PCD | |
| r | -0.43 | -0.67 | 0.46 | 0.54 | 0.62 |
| P | 0.22 | 0.02 | 0.02 | 0.01 | 0.03 |
| ChT | SPVD | SPVD | SPVD | SPVD | SPVD |
| r | 0.42 | 0.48 | 0.52 | 0.53 | 0.42 |
| P | 0.02 | 0.03 | 0.02 | 0.14 | 0.04 |
| DPVD | DPVD | DPVD | DPVD | DPVD | |
| r | 0.52 | 0.42 | 0.63 | 0.54 | 0.51 |
| P | 0.04 | 0.03 | 0.21 | 0.03 | 0.01 |
| ChF | ChF | ChF | ChF | ChF | |
| r | 0.57 | 0.73 | 0.72 | 0.64 | 0.53 |
| P | 0.03 | 0.02 | 0.04 | 0.21 | 0.03 |
| FAZ | FAZ | FAZ | FAZ | FAZ | |
| r | 0.35 | 0.45 | -0.60 | -0.48 | -0.52 |
| P | 0.04 | 0.01 | 0.04 | 0.04 | 0.03 |
| CDD | CDD | CDD | CDD | CDD | |
| r | -0.42 | -0.45 | 0.53 | 0.69 | 0.53 |
| P | 0.04 | 0.02 | 0.03 | 0.04 | 0.02 |
| PCD | PCD | PCD | PCD | PCD | |
| r | -0.51 | -0.72 | 0.61 | 0.44 | 0.51 |
| P | 0.23 | 0.04 | 0.03 | 0.03 | 0.01 |
CMT: Central macular thickness; ChT: Subfoveal choroidal thickness; SPVD: Superficial parafoveal vessel density; DPVD: Deep parafoveal vessel density; ChF: Choriocapillary flow area; FAZ: Foveal avascular zone area; CDD: Capillary density inside disc; PCD: Peripapillary capillary density.
DISCUSSION
In the past few decades, the effects of laser therapy have been frequently assessed using OCT. In several studies, CMT, ChT, and mGCCT were three major parameters that were assessed before and after laser therapy. Previous studies have demonstrated that traditional 532-nm PRP alone may persistently cause increased CMT and mGCCT last for more than 12mo after laser therapy. ChT began to decrease at 1mo post-laser therapy[13]–[14]. In our study, yellow (577-nm) wavelength was performed, and our results may be different from the previous studies. CMT, mGCCT, and ChT significantly increased at 1d post-laser therapy, however a reduction in ChT values was observed at 6mo post-laser therapy. No obvious changes in CMT or mGCCT were observed at 6mo after laser therapy when compared to baseline values. Similar to 532-nm laser, 577-nm laser therapy also caused immediate thickening of the CMT and mGCCT after completion of PRP. It may contribute to PRP-induced inflammation and disruption of the blood retina barrier. However, unlike the 532-nm laser, thickening of the CMT and mGCCT was transient, which may be related to the specificity of the yellow wavelength in RPE. In PRP, traditional 532-nm laser therapy is performed weekly for 3 or 4 times, however when using the 577-nm laser, the PRP treatment process can be shortened to one time. The yellow wavelength is better absorbed by melanin and less scatter is produced when compared to green (532-nm) and other yellow (561-nm to 568-nm) wavelengths. A study performed by Han et al[15] may provide evidence for the transient thickening of the CMT and mGCCT. Compared to the traditional laser, TUNEL-positive cells and inflammatory cells in mouse neural retinas were significantly decreased on selective retina therapy (SRT) when using a microsecond-pulsed laser. Additionally, negligible glial activation was observed in SRT. Therefore, less collateral thermal injury was observed to the neural retina and less inflammatory responses may be produced when using the 577-nm laser. Although the 577-nm laser caused less damage, it can still thicken the CMT and mGCCT. PRP alone with a 577-nm laser also caused persistently increased CMT and mGCCT[16]–[17]. The combined foveal micro-pulse treatment counteracted the thickening of CMT and mGCCT induced by PRP. In our study, the rapid reduced CMT after additional SML in some cases may provide evidence for the above hypothesis. The reason for rapid reduced CMT may mainly contribute to the resolution of DME. Additionally, because PRP were not performed in an additional SML treatment. Although CMT and mGCCT at 6mo after laser therapy was not significantly thinner when compared to the baseline, we cannot deny the effect of the foveal micro-pulse treatment on DME.
Most previous studies[16],[18] have focused on thickness changes when using the 577-nm laser, however, the vascular changes were not very clear. OCTA, a non-invasive high-resolution imaging machine, has provided us with the chances of observing the detailed vascular changes. To the best of our knowledge, this was the first study to quantitatively evaluate vascular changes before and after combined use 577-nm micropulse and multi-point mode pan retinal laser on NPDR with CIDME using OCTA. As is mentioned above, all the included eyes were CIDME and the initial CMT of most eyes were about 350 µm. That is, most eyes were severe DME. Consistent with the trend of ChT, superficial and deep PVD, and ChF significantly increased at 1d post-laser therapy and decreased to less than baseline level at 6mo post-laser therapy, we hypothesized that 577-nm micropulse laser treatment in the fovea mainly simultaneously affected both the retinal and choroidal vessel network. Ciliochoroidal effusion may be an explanation for increased ChF and ChT. At the first day after laser therapy, the inflammatory response was activated. Capillaries were dilated and leukocytes were aggregated, which may be the main reason of the higher vessel density and increased ChT. As the laser lesions became evident, the capillaries began to shrink. According to Cole et al[19], 532-nm laser therapy in the macula caused deep and superficial lesions. Deep lesions damaged more layers of the neural retina when compared to the superficial ones, therefore, decreased signal can be seen in the deep lesions at the level of choriocapillaris in OCTA images whereas both increased and decreased lesions can be observed in the superficial ones at the same level. In our subjects, a slight decrease in signal may be observed more frequently at 6mo post-laser therapy at the level of choriocapillaris in OCTA images and this may be the major reason of reduced ChF. Considering the specificity in RPE produced by the 577-nm laser effect, the damage of more neural retina layers may not be a reason for a frequently decreased signal. Slightly decreased OCTA signal at the level of choriocapillaris may be caused by both low OCT signal and low flow[20]. It is likely that increasingly diffused melanin content near RPE after laser therapy contributed to the low signal. In addition, an increase of oxygen lowered the level of VEGF, which caused the low flow of the retina and the choroid. Increased FAZ area may be a subsequent change[21]–[23]. Recent studies[24]–[27] as well as our recently published study[28] suggested that a lower PVD and ChF accompanied by an increased FAZ area were observed in patients with more serious DR when compared to no DR or mild DR subjects. Moreover, we noticed that CDD and PCD continuously decreased at later stages, suggesting possible ischemia of the optic nerve head. In line with previous studies on 532-nm and 577-nm laser therapy[16],[29], BCVA remained stable over 6mo after therapy. Only a small number of patients (2/86) still had persistent CIDME at the last follow-up. Therapy using 577-nm laser treatment has several advantages. The cost of 577-nm laser is lower when compared to that of anti-VEGF drugs. Moreover, unlike the traditional 532-nm laser, a yellow wavelength can be repeatedly performed on a macular area. Many patients in the clinic refused to receive anti-VEGF treatment when they knew the high cost. We should at least recommend a laser treatment for them, especially for the ones with low compliance. Our study has several limitations. Firstly, the sample size was small. Another limitation is that the follow-up time was relatively short. Visual function examinations such as visual field test was not performed. Further prospective studies with more patients will help to better understand the effect of 577-nm laser therapy.
In conclusion, during a 6mo follow-up period after combined use of 577-nm micropulse and multi-point mode pan retinal laser therapy, BCVA remained stable and there was a decreased trend in macular edema. Increased blood flow at 1d post-laser therapy and reduced blood flow at 6mo post-laser therapy were consistent with the trending changes in thickness of the retina and the choroid, respectively. However, further assessment of the visual function after laser should be performed.
Acknowledgments
Foundations: Supported by the Natural Science Foundation of Guangdong Province (No.2015A030313019); the Sun Yat-sen Clinical Research Cultivation Project (No.SYS-C-201705).
Conflicts of Interest: Li ZJ, None; Xiao JH, None; Zeng P, None; Zeng R, None; Gao X, None; Zhang YC, None; Lan YQ, None.
REFERENCES
- 1.Inagaki K, Ohkoshi K, Ohde S, Deshpande GA, Ebihara N, Murakami A. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561-577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol. 2015;59(1):21–28. doi: 10.1007/s10384-014-0361-1. [DOI] [PubMed] [Google Scholar]
- 2.Abramoff MD, Fort PE, Han IC, Jayasundera KT, Sohn EH, Gardner TW. Approach for a clinically useful comprehensive classification of vascular and neural aspects of diabetic retinal disease. Invest Ophthalmol Vis Sci. 2018;59(1):519. doi: 10.1167/iovs.17-21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraz DA, Vasquez LM, Preti RC, Motta A, Sophie R, Bittencourt MG, Sepah YJ, Monteiro MLR, Nguyen QD, Takahashi WY. A randomized controlled trial of panretinal photocoagulation with and without intravitreal ranibizumab in treatment-naive eyes with non–high-risk proliferative diabetic retinopathy. Retina. 2015;35(2):280–287. doi: 10.1097/IAE.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 4.Yan PS, Qian C, Wang WZ, Dong Y, Wan GM, Chen Y. Clinical effects and safety of treating diabetic macular edema with intravitreal injection of ranibizumab combined with retinal photocoagulation. Ther Clin Risk Manag. 2016:527. doi: 10.2147/TCRM.S99224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SU, Maturi RK. Therapeutic options in refractory diabetic macular oedema. Drugs. 2017;77(5):481–492. doi: 10.1007/s40265-017-0704-6. [DOI] [PubMed] [Google Scholar]
- 7.Hutton DW, Stein JD, Bressler NM, Jampol LM, Browning D, Glassman AR, Diabetic Retinopathy Clinical Research Network Cost-effectiveness of intravitreous ranibizumab compared with panretinal photocoagulation for proliferative diabetic retinopathy: secondary analysis from a diabetic retinopathy clinical research network randomized clinical trial. JAMA Ophthalmol. 2017;135(6):576–584. doi: 10.1001/jamaophthalmol.2017.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retina Group of Chinese Medical Association. Guidebook of Diabetic Retinopathy (2014) Chin J Ophthalmol. 2014;50(11):851–865. [Google Scholar]
- 9.Kwiterovich KA, Maguire MG, Murphy RP, Schachat AP, Bressler NM, Bressler SB, Fine SL. Frequency of adverse systemic reactions after fluorescein angiography. Ophthalmology. 1991;98(7):1139–1142. doi: 10.1016/s0161-6420(91)32165-1. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Saez MP, Ordoqui E, Tornero P, Baeza A, Sainza T, Zubeldia JM, Baeza ML, Baeza ML. Fluorescein-induced allergic reaction. Ann Allergy Asthma Immunol. 1998;81(5):428–430. doi: 10.1016/S1081-1206(10)63140-7. [DOI] [PubMed] [Google Scholar]
- 11.Vujosevic S, Gatti V, Muraca A, Brambilla M, Villani E, Nucci P, Rossetti L, de Cilla' S. Optical coherence tomography angiography changes after subthreshold micropulse yellow laser in diabetic macular edema. Retina. 2018:1. doi: 10.1097/IAE.0000000000002383. [DOI] [PubMed] [Google Scholar]
- 12.Jorge EC, Jorge EN, Botelho M, Farat JG, Virgili G, El Dib R. Monotherapy laser photocoagulation for diabetic macular oedema. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD010859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demirok G, Kocamaz MF, Topalak Y, Altay Y, Tabakci B, Şengün A. Changes in the macular ganglion cell complex thickness and central macular thickness after argon laser panretinal photocoagulation. Semin Ophthalmol. 2017;32(6):759–763. doi: 10.1080/08820538.2016.1177098. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZW, Meng XM, Wu ZF, Zou WJ, Zhang J, Zhu DY, Chen TT, Zhang Q. Changes in choroidal thickness after panretinal photocoagulation for diabetic retinopathy: a 12-week longitudinal study. Invest Ophthalmol Vis Sci. 2015;56(4):2631. doi: 10.1167/iovs.14-16226. [DOI] [PubMed] [Google Scholar]
- 15.Han JW, Choi J, Kim YS, Kim J, Brinkmann R, Lyu J, Park TK. Comparison of the neuroinflammatory responses to selective retina therapy and continuous-wave laser photocoagulation in mouse eyes. Graefes Arch Clin Exp Ophthalmol. 2018;256(2):341–353. doi: 10.1007/s00417-017-3883-7. [DOI] [PubMed] [Google Scholar]
- 16.Shin JS, Lee YH. Changes in macular retinal layers and peripapillary nerve fiber layer thickness after 577-nm pattern scanning laser in patients with diabetic retinopathy. Korean J Ophthalmol. 2017;31(6):497. doi: 10.3341/kjo.2016.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JJ, Im JC, Shin JP, Kim IT, Park DH. One-year follow-up of macular ganglion cell layer and peripapillary retinal nerve fibre layer thickness changes after panretinal photocoagulation. Br J Ophthalmol. 2014;98(2):213–217. doi: 10.1136/bjophthalmol-2013-304349. [DOI] [PubMed] [Google Scholar]
- 18.Latalska M, Prokopiuk A, Wróbel-Dudzińska D, Mackiewicz J. Subthreshold micropulse yellow 577 nm laser therapy of diabetic macular oedema in rural and urban patients of south-eastern Poland. Ann Agric Environ Med. 2017;24(1):96–99. doi: 10.5604/12321966.1233899. [DOI] [PubMed] [Google Scholar]
- 19.Cole ED, Novais EA, Louzada RN, Moult EM, Lee BK, Witkin AJ, Waheed NK, Duker JS, Baumal CR. Visualization of changes in the choriocapillaris, choroidal vessels, and retinal morphology after focal laser photocoagulation using OCT angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT356. doi: 10.1167/iovs.15-18473. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201. doi: 10.1038/srep42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JR, Choi W, Hong HK, Kim Y, Jun Park S, Hwang Y, Kim P, Joon Woo S, Hyung Park K, Oh WY. Imaging laser-induced choroidal neovascularization in the rodent retina using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT331. doi: 10.1167/iovs.15-18946. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto M, Matsuura T, Ogata N. Effects of panretinal photocoagulation on choroidal thickness and choroidal blood flow in patients with severe nonproliferative diabetic retinopathy. Retina. 2016;36(4):805–811. doi: 10.1097/IAE.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 23.Li ZJ, Zhang YC, Liao YR, Zeng R, Zeng P, Lan YQ. Comparison of efficacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in type-1 and threshold retinopathy of prematurity (ROP) BMC Ophthalmol. 2018;18:19. doi: 10.1186/s12886-018-0685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonett JM, Scarinci F, Picconi F, Giorno P, de Geronimo D, di Renzo A, Varano M, Frontoni S, Parravano M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017;95(8):e751–e755. doi: 10.1111/aos.13404. [DOI] [PubMed] [Google Scholar]
- 25.Sambhav K, Abu-Amero KK, Chalam KV. Deep capillary macular perfusion indices obtained with OCT angiography correlate with degree of nonproliferative diabetic retinopathy. Eur J Ophthalmol. 2017;27(6):716–729. doi: 10.5301/ejo.5000948. [DOI] [PubMed] [Google Scholar]
- 26.Papakostas TD, Kiss S. Optical coherence tomography angiography to evaluate ischemia in diabetic eyes. JAMA Ophthalmol. 2018;136(8):936. doi: 10.1001/jamaophthalmol.2018.2256. [DOI] [PubMed] [Google Scholar]
- 27.Cao D, Yang DW, Huang ZN, Zeng YK, Wang J, Hu YY, Zhang L. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018;55(5):469–477. doi: 10.1007/s00592-018-1115-1. [DOI] [PubMed] [Google Scholar]
- 28.Li ZJ, Alzogool M, Xiao JH, Zhang S, Zeng P, Lan YQ. Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol. 2018;55(10):1075–1082. doi: 10.1007/s00592-018-1202-3. [DOI] [PubMed] [Google Scholar]
- 29.Kato F, Nozaki M, Kato A, Hasegawa N, Morita H, Yoshida M, Ogura Y. Evaluation of navigated laser photocoagulation (navilas 577+) for the treatment of refractory diabetic macular edema. J Ophthalmol. 2018;2018:1–7. doi: 10.1155/2018/3978514. [DOI] [PMC free article] [PubMed] [Google Scholar]