Abstract
AIM
To assess the effectiveness of the XEN 45 gel stent, either alone or combined with cataract surgery, in advanced stage open angle glaucoma (OAG) patients.
METHODS
Retrospective and single-center study conducted on consecutive OAG patients who underwent a XEN 45 gel stent implantation surgery, between July 2017 and September 2018. The primary efficacy end-point was the mean intraocular pressure (IOP) reduction at the end of the follow-up period. Success was defined as an IOP reduction of at least 20% and an IOP value ≤18 mm Hg without (complete) or with (qualified) hypotensive medication.
RESULTS
Seventy-four patients (80 eyes) were included in the study. In the overall study sample, XEN implant significantly reduced IOP from 21.0 (19.8 to 22.1) mm Hg at baseline to 9.3 (8.2 to 10.4), 10.7 (9.6 to 11.9), 13.4 (12.2 to 14.7), 14.5 (13.6 to 15.4), 14.7 (13.8 to 15.6), and 14.7 (13.9 to 15.4) mm Hg at 1d, 1wk, 1, 3, 6, and 12mo of follow-up, respectively (P<0.0001 each). In the overall study population, at the end of the study the mean IOP reduction was 27.4% (23.3% to 31.5%). Adjusted IOP reduction was similar in XEN and XEN+phacoemulsification groups [30.0 (23.4 to 36.4) mm Hg vs 24.8 (18.4 to 31.2) mm Hg, respectively, P=0.2939]. At the last follow-up visit, 52 (65.0%) eyes were considered success, 29 (36.3%) eyes as complete success and 23 (28.7%) as qualified success. Mean number of hypotensive medications was significantly reduced from 2.8 (2.7 to 3.0) at baseline to 1.1 (0.8 to 1.3), P<0.0001. Kaplan-Meier survival analysis did not find any difference in the success rate between XEN and XEN+PHACO, mean hazard ratio 0.56, 95%CI 0.26 to 1.23; P=0.1469. Needling was performed in 7 (8.8%) eyes at months 1 (n=3); 3 (n=2); 4 (n=1) and 11 (n=1). Eleven (13.8%) eyes presented adverse events.
CONCLUSION
XEN implant, either alone or in combination with phacoemulsification, significantly reduced the IOP and the number of hypotensive medications in patients with OAG in advanced stage.
Keywords: open angle glaucoma, advanced stage, XEN implant, intraocular pressure, minimally-invasive glaucoma surgery
INTRODUCTION
The term minimally-invasive glaucoma surgery (MIGS) comprises different devices, which have become increasingly popular as an effective and safe alternative to the classic glaucoma surgical procedures[1]–[2].
MIGS devices can be divided into: trabecular, suprachoroidal, and subconjunctival[3]–[4]. Among the different MIGS devices currently available in the market, the ab interno XEN gel stent (Allergan plc, Dublin, Ireland) has been the only one that, up to now, allowed subconjunctival filtration[5]–[8].
Different clinical studies have shown good efficacy, in terms of intraocular pressure (IOP) and the amount of hypertensive medications reduction, and a high safety profile of XEN 45 implant either alone or combined with cataract surgery[2],[9]–[18].
Moreover, two systematic reviews evaluated the effects of XEN, either alone or combined with phacoemulsification, on the IOP and hypertensive medication reduction in open angle glaucoma (OAG) patients[19]–[20]. Their results suggested that XEN effectively reduced both IOP and number of hypotensive medications in OAG patients with an acceptable safety profile[19]–[20]. Most of studies have included patients with early to moderate glaucoma who received the XEN 45 stent as an initial surgical therapy. However, because glaucoma is a long-life chronic disease there are many patients with advanced disease or failed previous incisional glaucoma surgeries who might benefit from an XEN 45 stent implantation.
The purpose of this study was to assess the effectiveness of the XEN 45 gel stent, either alone or combined with cataract surgery, in advanced-stage OAG patients. Additionally, the current study also aimed to investigate those variables associated with the success of the surgical procedure.
SUBJECTS AND METHODS
Ethical Approval
The study protocol was approved by the local ethics committee, which waived the need for written informed consent of the participants. This study complied with the guidelines of Good Clinical Practice and adhered to the tenets of the Declaration of Helsinki.
Patients
Retrospective and single-center study conducted on consecutive OAG patients who met inclusion and exclusion criteria and underwent an XEN 45 gel stent implantation surgery, between July 2017 and September 2018, in the Glaucoma Department of the Arruzafa Hospital, Spain. This study included medically uncontrolled patients with advanced OAG, according to Hodapp et al[21], with or without cataract, who had at least a minimum postoperative follow-up period of 12mo.
Subjects with any form of glaucoma other than OAG; progressive retinal or optic nerve disease of any cause, history of major ocular surgery (except phacoemulsification) within the previous 6mo, or lack of attendance to follow-up visits were excluded of the study.
Study Visits
The protocol included one baseline visit and 6 follow-up visits. Follow-up visits were scheduled at day 1, week 1 (±2d), months 1, 3, and 6 (±2wk) and month 12 (±1mo).
Surgical Technique
All surgical procedures were performed under local anesthesia by two experienced surgeons (Laborda-Guirao T and Hidalgo-Torres A).
XEN implant was placed in the superior nasal quadrant using a standard technique[10]–[18]. After anesthesia and skin disinfection, superior nasal conjunctiva was marked 3 mm from the limbus. Intraoperatively, under the conjunctiva, 0.1 mL of mitomycin-C (MMC) 0.02% was injected using a 30-gauge hypodermic needle and spread with a surgical sponge. Through a 1.8 mm corneal paracentesis incision acetylcholine and a highly cohesive viscoelastic were injected into the anterior chamber. Using an ab interno approach, the pre-loaded injector needle was inserted at the inferotemporal quadrant. For those eyes with a previously failed filtration surgery, who had significant conjunctival fibrosis, XEN implant was placed in the inferior quadrants.
The needle was then directed across the anterior chamber and implanted in the superior-nasal quadrant. The viscoelastic material was aspirated and formation of a bleb was assessed by constant irrigation, into the anterior chamber, of balanced salt solution (BSS). Finally, after administration of intracameral cefuroxime, the clear corneal incisions were hydrated with BSS.
In those patients where cataract surgery was indicated, it was performed a standard phacoemulsification technique and, after the intraocular lens was properly placed in the bag, XEN implantation followed as previously indicated.
In those patients with bleb fibrosis, surgical bleb revision with open conjunctiva would be performed in the operating room. After a conjunctival incision, it will be then dissected and MMC would be used (0.02% for two minutes). Finally, the conjunctiva will be closed. Regarding needling, it was performed with a 30 G and 0.1 mmL of 0.02% MMC was injected.
Study Groups
The study sample was divided in two groups: Group 1 (XEN), eyes underwent XEN implant alone; Group 2 (XEN+PHACO), eyes underwent XEN gel stent implantation combined with phacoemulsification surgery.
Outcomes
The primary efficacy end-point was the mean IOP reduction at the end of the follow-up period. Secondary end-points included mean IOP and the mean number of antiglaucoma medications, and their changes as compared to baseline; proportion of patients classified as success; proportion of patients with a final IOP ≥6 and ≤18 mm Hg; and incidence of adverse events.
Definitions
Success was defined as an IOP reduction of at least 20% and an IOP value ≤18 mm Hg without (Complete success) or with (Qualified success) hypotensive medication. Failure was defined as an IOP >18 mm Hg or a <20% reduction of IOP from baseline at the end of the follow-up period, need for additional glaucoma surgery, or vision threatening complications that led to severe loss of visual acuity (light perception or worse).
Needling or surgical bleb revision, as needed, was indicated in those cases of failure of the procedure due to fibrosis or encapsulation of the bleb that did not respond to massage in the slit lamp and topical hypotensor medications.
Statistical Analysis
A standard statistical analysis was performed using MedCalc Statistical Software version 19.1.5 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2019).
Before the start of the study, it was determined that a sample size of 63 eyes was required to detect a mean IOP difference between baseline and month-12 visit of 3.5 mm Hg, with a type I error of 0.01 and a power of the 90%, assuming a standard deviation of 7 mm Hg.
Additionally, a minimum of 33 eyes per treatment group would be required for detecting a mean IOP change difference of 1.0 mm Hg between groups, assuming a standard deviation of 1.3 mm Hg, at a significance level of 0.05, with a power of 0.90. Data are expressed as number (percentage); mean [standard deviation (SD)]; mean [95% confidence interval (95%CI)]; median (95%CI) or percentages as appropriate.
We examined the distribution of continuous variables with a D'Agostino-Pearson test. A repeated measures ANOVA or a Friedman's two-way analysis test, as appropriate, were used to assess the changes in IOP and in number of antiglaucoma medications. The one-way ANOVA test or the Kruskal-Wallis test, as appropriate, were used to compare differences between groups. Post hoc analysis for pairwise comparisons were done with the Scheffé's method (ANOVA) or the Conover method (Kruskal-Wallis). The Mann-Whitney U test was used in the evaluation of the changes in IOP and in the between XEN and XEN+PHACO treated eyes. Success survival rates were plotted for XEN and XEN+PHACO groups using Kaplan-Meier analysis and were compared using a log-rank test.
A logistic regression model was used to estimate and test factors for their association with success. A backward strategy was adopted, with a statistically significant cut-off for variable screening of 0.05. Categorical variables were compared using a Chi-square test and a Fisher's exact test, as needed.
The analysis of covariance (ANCOVA) was used in the evaluation of the changes in IOP and in the between XEN and XEN+PHACO treated eyes. The model included “Surgical procedure” as a factor and previous surgery, number of hypotensive medications, implant location, and presence of myopia as covariates.
RESULTS
Of the 301 glaucoma surgeries performed between July 2017 and September 2018, 108 eyes underwent XEN or XEN+PHACO surgery. Among them, 74 patients (80 eyes) fulfilled the inclusion and exclusion criteria. Forty eyes underwent XEN and 40 ones underwent XEN+PHACO. Their main baseline clinical and demographic characteristics have been summarized in the Table 1.
Table 1. Baseline clinical and demographic characteristics of the study population.
| Variable | Overall (n=80) | XEN (n=40) | XEN+PHACO (n=40) | Pa |
| Age, y | 0.3780 | |||
| Mean (SD) | 74.0 (10.4) | 74.8 (10.3) | 73.2 (10.5) | |
| 95%CI | 71.7 to 76.3 | 71.5 to 78.0 | 69.9 to 76.6 | |
| Sex | 1.0000 | |||
| Male | 42 (52.5) | 21 (52.5) | 21 (52.5) | |
| Female | 38 (47.5%) | 19 (47.5) | 19 (47.5%) | |
| Myopia, n (%)c | 0.0333b | |||
| No | 62 (77.5) | 27 (67.5) | 35 (87.5) | |
| Yes | 18 (22.5) | 13 (32.5) | 5 (12.5) | |
| Previous surgery, n (%) | <0.0001b | |||
| No | 49 (61.2) | 15 (37.5) | 34 (85.0) | |
| Yes | 31 (38.7) | 25 (62.5) | 6 (15.0) | |
| Implant location, n (%) | 0.0005b | |||
| Superior | 58 (72.5) | 22 (55.0) | 36 (90.0) | |
| Inferior | 22 (27.5) | 18 (45.0) | 4 (10.0) | |
| IOP, mm Hg | 0.0814 | |||
| Mean (SD) | 21.0 (5.2) | 21.8 (5.3) | 20.1 (5.1) | |
| 95%CI | 19.8 to 22.1 | 20.1 to 23.5 | 18.5 to 21.7 | |
| NOAGM, n (%) | 0.1014b | |||
| 1 | 3 (3.7) | 1 (2.5) | 2 (5.0) | |
| 2 | 15 (18.8) | 6 (15.0) | 9 (22.5) | |
| 3 | 56 (70.0) | 28 (70.0) | 28 (70.0) | |
| 4d | 6 (7.5) | 5 (12.5) | 1 (2.5) | |
n: Number of eyes; SD: Standard deviation; CI: Confidence interval; IOP: Intraocular pressure; NOAGM: Number of antiglaucoma medication. aMann-Whitney U test; bChi-squared test with the Cochran-Armitage test; cThe eyes with a myopia with ≥6 diopters were considered as myopic eyes; dThree topical hypotensive medications + systemic carbonic anhydrase inhibitor. Statistical significance (P value) was calculated between XEN alone and combined surgery (XEN+Phacoemulsification, XEN+PHACO).
There were significant differences in the proportion of eyes with high myopia (greater in the XEN+PHACO group); previous filtration surgery (greater in the XEN+PHACO group); and superior-nasal quadrant implantation (greater in the XEN+PHACO group). Among the 31 eyes who underwent a previous glaucoma surgical procedure, 22 eyes had undergone trabeculectomy; 8 eyes undergone a non-penetrating deep sclerectomy; and 1 eye an MIGS procedure.
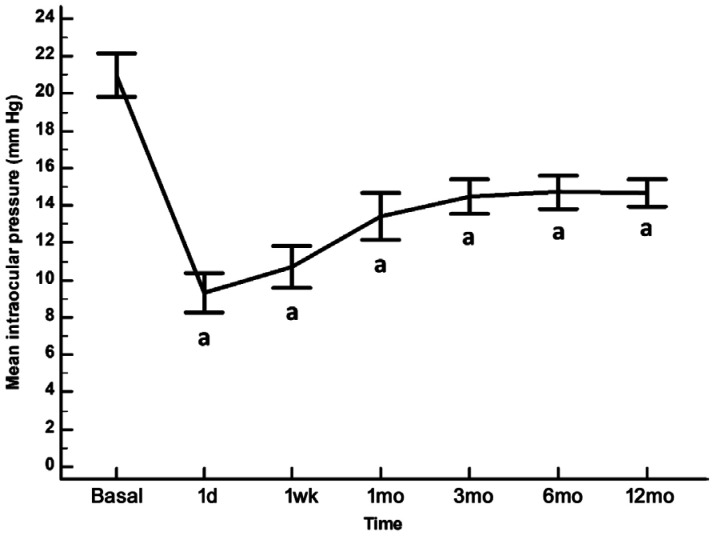
In the overall study sample, XEN implant significantly reduced IOP from 21.0 (19.8 to 22.1) mm Hg at baseline to 9.3 (8.2 to 10.4), 10.7 (9.6 to 11.9), 13.4 (12.2 to 14.7), 14.5 (13.6 to 15.4), 14.7 (13.8 to 15.6), and 14.7 (13.9 to 15.4) mm Hg at 1d, 1wk, 1, 3, 6, and 12mo of follow-up, respectively (P<0.0001 each; Figure 1).
Figure 1. Mean IOP in the overall study population.
The vertical bars represent the 95% confidence interval. As compared to baseline, the mean (95%CI) IOP reduction was 11.7 (9.2 to 14.1) mm Hg; 10.2 (7.9 to 12.6) mm Hg; 7.6 (5.0 to 10.1) mm Hg; 6.5 (4.4 to 8.6) mm Hg; 6.2 (4.1 to 8.5) mm Hg; and 6.3 (4.4 to 8.2) mm Hg at 1d, 1wk, 1, 3, 6, and 12mo of follow-up, respectively. aP<0.0001 as compared to baseline (repeated measures ANOVA and the Greenhouse-Geisser correction).
In the overall study population, at the end of the study the mean IOP reduction was 27.4% (23.3% to 31.5%). Unadjusted IOP reduction was significantly greater in the XEN than in the XEN+PHACO group [32.0 (26.1 to 37.9) mm Hg vs 22.8 (17.2 to 28.5) mm Hg, respectively, P=0.0261].
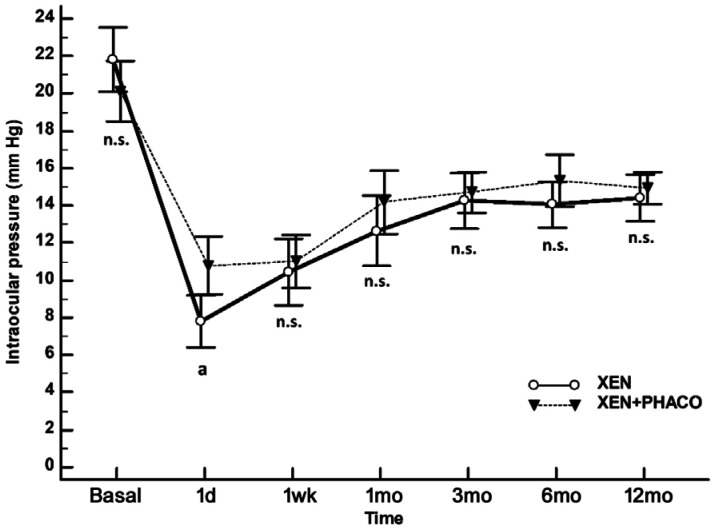
The mean IOP was significantly reduced from 21.8 (20.1 to 23.5) mm Hg and 20.1 (18.5 to 21.7) mm Hg to 14.4 (13.2 to 15.7) mm Hg and 14.9 (14.1 to 15.8) mm Hg in the XEN and XEN+PHACO groups, respectively; P<0.0001 each (Figure 2).
Figure 2. Unadjusted comparison of mean IOP between XEN implant alone and XEN+PHACO.
The vertical bars represent the 95% confidence interval. Statistical significance, at the different time point measurements, was determined using the one-way ANOVA test with the Scheffé's method. As compared to baseline, the mean IOP was significantly reduced, at every time point measured, P<0.001 (repeated measures ANOVA and the Greenhouse-Geisser correction).aP=0.004; n.s.: Not significant.
With the exception of the day 1 (significantly lower in the XEN group), there were no statistically significant differences in any of the measured IOP time points between the XEN and XEN+PHACO groups (Figure 2).
Adjusting for previous surgery, number of hypotensive medications, implant location, and presence of myopia there were no significant differences in the mean IOP changes, at any of the follow-up IOP-time-point measurements, between XEN and XEN+PHACO groups (Table 2).
Table 2. Mean changes in IOP in XEN alone and combined surgery.
| Variable | XEN | XEN+PHACO | Difference | Pa |
| MCIOP 1d, mm Hg | 0.1515 | |||
| Mean (SE) | 12.9 (1.1) | 10.4 (1.1) | 2.5 (1.8) | |
| 95%CI | 10.7 to 15.2 | 8.1 to 12.7 | -1.0 to 6.1 | |
| MCIOP 1wk, mm Hg | 0.7507 | |||
| Mean (SE) | 10.5 (1.1) | 9.9 (1.1) | 0.6 (1.8) | |
| 95%CI | 8.3 to 12.8 | 7.7 to 12.2 | -2.9 to 4.0 | |
| MCIOP 1mo, mm Hg | 0.3749 | |||
| Mean (SE) | 8.4 (1.2) | 6.7 (1.2) | 1.7 (1.9) | |
| 95%CI | 5.9 to 10.9 | 4.2 to 9.2 | -2.1 to 5.6 | |
| MCIOP 3mo, mm Hg | 0.8023 | |||
| Mean (SE) | 6.7 (1.0) | 6.3 (1.0) | 0.4 (1.6) | |
| 95%CI | 4.6 to 8.8 | 4.2 to 8.4 | -2.8 to 3.6 | |
| MCIOP 6mo, mm Hg | 0.3141 | |||
| Mean (SE) | 7.1 (1.1) | 5.4 (1.1) | 1.7 (1.6) | |
| 95%CI | 5.0 to 9.2) | 3.3 to 7.6 | -1.6 to 4.9 | |
| MCIOP 12mo, mm Hg | 0.5219 | |||
| Mean (SE) | 6.8 (0.9) | 5.9 (0.9) | 0.9 (1.4) | |
| 95%CI | 4.9 to 8.6 | 4.0 to 7.7 | -1.9 to 3.7 |
MCIOP: Mean change in intraocular pressure; SE: Standard error; CI: Confidence interval. aAnalysis of covariance (ANCOVA). The model included “Surgical procedure” as a factor and previous surgery, number of hypotensive medications, implant location, and presence of myopia as covariates.
Once adjusted for previous surgery, number of hypotensive medications, implant location, and presence of myopia ANCOVA analysis did not find significant differences in IOP reduction between XEN and XEN+PHACO groups [30.0 (23.4 to 36.4) mm Hg vs 24.8 (18.4 to 31.2) mm Hg, respectively, P=0.2939].
Mean number of hypotensive medications was significantly reduced from 2.8 (2.7 to 3.0) at baseline to 1.1 (0.8 to 1.3), P<0.0001. There was not significant difference in the mean reduction of hypotensive medications between XEN [1.8 (1.4 to 2.2)] and XEN+PHACO [1.7 (1.3 to 2.1)], P=0.6394.
At the last follow-up visit, 52 (65.0%) eyes were considered success, 29 (36.3%) eyes as complete success and 23 (28.7%) as qualified success. Success rate was 72.5% (29/40 eyes) and 57.5% (23/40 eyes) in the XEN and XEN+PHACO groups, respectively; P=0.1622.
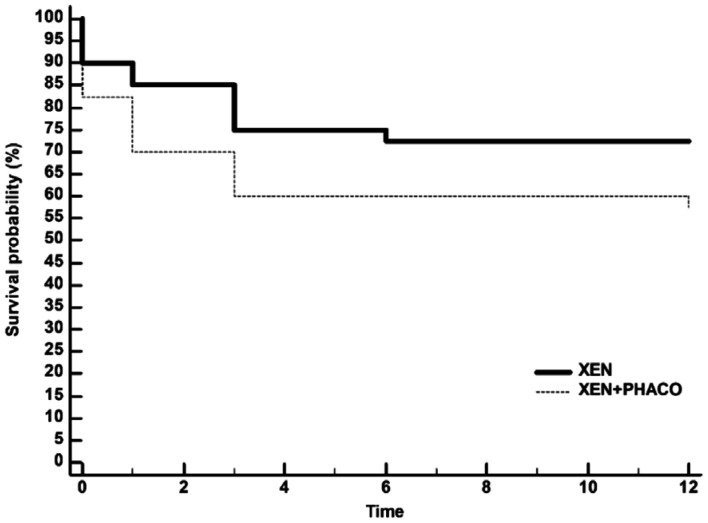
Kaplan-Meier survival analysis did not find any difference in the success rate between XEN and XEN+PHACO, mean hazard ratio 0.56, 95%CI 0.26 to 1.23; P=0.1469 (Figure 3).
Figure 3. Kaplan-Meier survival curves for failure in eyes treated with XEN alone and XEN+PHACO.
Failure occurred in 11 (27.5%) XEN-treated eyes and 17 (42.5%) XEN+PHACO-treated eyes. Mean hazard ratio: 0.56, 95%CI: 0.26 to 1.23, P=0.1469.
At month 12, 73 (91.2%) eyes had an IOP ≥6 and ≤18 mm Hg, 35 (43.8%) of them without treatment. Logistic regression analysis found none factor significantly associated with success (Table 3).
Table 3. Logistic regression analysis of 80 eyes included in the study to evaluate the potential factors associated with the surgical success.
| Variable | Success |
P | |
| OR | 95%CI | ||
| Age, 1-year increment | 1.00 | 0.96 to 1.05 | 0.8976 |
| Sex, man | 1.17 | 0.47 to 2.93 | 0.7426 |
| Myopia, yes | 1.10 | 0.71 to 1.50 | 0.8663 |
| Location, superior | 0.82 | 0.29 to 2.34 | 0.7135 |
| Previous surgery, yes | 2.57 | 0.93 to 7.09 | 0.0680 |
| Procedure, XEN alone | 1.95 | 0.77 to 4.95 | 0.1621 |
| Baseline IOP, per mm Hg increment | 1.33 | 1.13 to 1.55 | 0.0004 |
| Baseline hypotensive medication, per 1-treatment increment | 1.29 | 0.61 to 2.70 | 0.5060 |
OR: Odds ratio; CI: Confidence interval; IOP: Intraocular pressure.
Needling was performed in 7 (8.8%) eyes at months 1 (n=3), 3 (n=2), 4 (n=1) and 11 (n=1). Regarding surgical bleb revision, none of the eyes required that treatment.
As regards the safety profile, 11 (13.8%) eyes presented adverse events. Five (6.3%) eyes choroidal detachment, flattening of anterior chamber (n=2), iris blockage (n=1), hypotony maculopathy (n=1), diplopia (n=1), ocular hypertension secondary to corticosteroids (n=1). All the complications were resolved with medical or surgical (flattening of anterior chamber, iris blockage, and diplopia) therapy without sequelae.
DISCUSSION
According to the results of this retrospective 12-month follow-up study, XEN implant, either alone or in combination with phacoemulsification, was able to significantly reduced both IOP and number of hypotensive medications in a cohort of patients with advanced OAG.
The results of the current study did not significantly differ from the currently available scientific evidence[9]–[20]. Nevertheless, it should be highlighted that our study was conducted in OAG patients in advanced stage.
In the overall study sample, the mean IOP reduction was in line with that reported by Pérez-Torregrosa et al[9] (29.3%), Mansouri et al[10] (31%), or Ibáñez-Muñoz et al[16] (28.4%). In a prospective and noncomparative study conducted in medically uncontrolled severe-stage OAG patients, Kalina et al[22] found a mean IOP reduction of the 33% (25% to 41%). This IOP reduction was similar to that observed in our study [difference 5.6% (-2.0% to 13.2%), P=0.1445].
However, it is much lower than that reported by de Gregorio et al[13] (41.8%) or Ozal et al[23] (53.6%). Additionally, this study found a success rate (IOP reduction ≥20% and an IOP value ≤18 mm Hg) of the 65%. This figure seems to be lower than that reported by other authors[13],[16],[22],[24]–[25]. de Gregorio et al[13] informed of a success rate (postoperative IOP ≥6 and ≤17 mm Hg, with or without glaucoma medications) of the 97.5% after 1-year of follow-up. Ibáñez-Muñoz et al[16] reported a success (IOP reduction ≥ 20%) rate in 22 eyes with OAG in advanced stage of the 84.6%. Grover et al[24] observed, in an open label and multicenter clinical study, a success (IOP reduction ≥ 20%) rate of the 75.4%. Schlenker et al[25] found a 75% survival rate at about 10mo for complete success at an IOP ≤21 mm Hg.
Such differences may be explained by the different baseline characteristics of the study populations, as well as by the different success criteria used in these studies. Kalina et al[22], using success criteria similar to ours (IOP reduction of ≥20% and an IOP <18 mm Hg with and without medication or any secondary glaucoma intervention) observed a success rate of the 90.6%.
Nevertheless, our results are similar to those reported by other authors[10]–[12],[14],[18]. In this study 31 (38.7%) eyes had undergone previous filtration surgery. Interestingly, the mean IOP reduction was greater in the eyes with a previous surgery than in those without [32.3% (19.6%) vs 24.3% (17.4%)], although this difference was not statistically significant (P=0.0619). In a retrospective study, Karimi et al[15] reported that XEN appears to be effective in previous failed filtration surgery.
A retrospective study evaluated the effectiveness of the XEN implant in patients without and with prior glaucoma intervention (including trabeculectomy, micro-bypass stent implantation, cyclophotocoagulation, laser procedures, and phacoemulsification)[26]. The results of this study found that, at month 12, success (IOP reduction of ≥20% and IOP <18 mm Hg without and with medication) was achieved by the 72% of the eyes undergone a previous glaucoma intervention[26]. This result is similar to that observed in our study, where the 77.4% (24/31) of the eyes with a previous glaucoma surgery were considered success.
In the current study, in 22 (27.5%) eyes, due to its characteristics, the XEN implant was placed in the inferior quadrants. There were no significant differences between eyes with XEN in superior quadrants or in the inferior ones in either success rate (63.8% vs 68.2%, respectively, P=0.7652), mean IOP reduction (25.4% vs 32.8, respectively, P=0.1083), or mean reduction in the number of hypotensive medications (1.8 vs 1.8, respectively, P=0.9624). These results might open the door to the use of XEN in eyes that have undergone prior filtration surgeries and do not have the possibility to perform the implantation in the conjunctival superior quadrants.
It was recommended to avoid the use of XEN implant in eyes with high myopia[27]. The current study included 18 (22.5%) eyes with high myopia. We did not find significant differences between eyes with or without high myopia in IOP reduction [30.5% (18.6%) vs 26.5% (18.6%)], respectively, P=0.4211]; success rate [66.7% vs 64.5%, respectively, P=0.8896]; mean reduction in the number of hypotensive medications [2.1 (1.2) vs 1.7 (1.2), respectively, P=0.1570]; or postoperative complications [16.7% vs 11.3%, respectively, P=0.8248].
Although was not originally planned as a primary outcome in the study protocol and it was a post-hoc analysis, as far as we know, this is the first study reporting the efficacy and safety of XEN in a cohort of patients with high myopia. The results of the current study suggested that XEN can be safely and effectively used in glaucomatous eyes with high myopia. However, further investigations with a larger number of patients recruited would be needed.
As expected, and reported in previously published studies[2],[9]–[20],[22]–[26], XEN implant significantly reduced the number of hypotensive medications.
The current study did not find any baseline factor significantly associated with success. As regards to the safety profile, the incidence and type of complications was similar to that previously published[2],[9]–[20],[22]–[26]. It should be noted the incidence of choroidal detachment (6.3%), it was observed in 2 eyes with high myopia and 3 ones without high myopia. Needling was performed in 7 (8.8%) eyes, but none of the eyes required surgical bleb revision.
This study has some limitation that should be taken into account when interpreting its results. The first one is its retrospective design. Selection bias and potential confounders are inherent to retrospective studies. Nevertheless, in order to minimize these issues, inclusion and exclusion criteria have been selected carefully and the sample size was adequate. Another limitation is the fact that some of the post-hoc analysis (IOP reduction difference between high myopic eyes, XEN location; and previous filtration surgery) may be underpowered for detecting the observed difference. According to the results of this study, the power for detecting mean IOP reduction differences between eyes with or without high-myopia, superior and inferior XEN location; and eyes with and without previous filtration surgery were 13%, 37%, and 46%, respectively. The third limitation is the 1-year follow-up period. Because glaucoma is a long-life chronic disease, 1-year follow-up may represent a relatively short period in a lifetime of the disease.
In conclusion, Based on the results of this study, XEN implant, either alone or in combination with phacoemulsification, significantly reduced the IOP and the number of hypotensive medications in patients with OAG in advanced stage. This study also suggested that XEN may be effective in eyes with previously failure filtration surgery and eyes with high myopia. Finally, in eyes with fibrosis in the superior conjunctival quadrants, XEN can be placed in the inferior ones with good effectiveness and safety results. Further studies are needed, particularly prospective, randomized, and multicenter clinical trials to confirm these results and to identify potential predictive factors associated with good clinical outcomes.
Acknowledgments
Foundation: Medical writing and Editorial assistant services have been provided by Ciencia y Deporte S.L. and covered by a Grant from Allergan. Support for this assistance was funded by Allergan S.A.
Conflicts of Interest: Laborda-Guirao T has received a Grant from Allergan during the conduct of the study; Cubero-Parra JM, None; Hidalgo-Torres A, None.
REFERENCES
- 1.Bar-David L, Blumenthal EZ. Evolution of glaucoma surgery in the last 25 years. Rambam Maimonides Med J. 2018;9(3):e0024. doi: 10.5041/RMMJ.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcos Parra MT, Salinas López JA, López Grau NS, Ceausescu AM, Pérez Santonja JJ. XEN implant device versus trabeculectomy, either alone or in combination with phacoemulsification, in open-angle glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1741–1750. doi: 10.1007/s00417-019-04341-y. [DOI] [PubMed] [Google Scholar]
- 3.Caprioli J, Kim JH, Friedman DS, Kiang T, Moster MR, Parrish RK, 2nd, Rorer EM, Samuelson T, Tarver ME, Singh K, Eydelman MB. Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American glaucoma society and the food and drug administration, Washington, DC, February 26, 2014. Ophthalmology. 2015;122(9):1795–1801. doi: 10.1016/j.ophtha.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansari E. An update on implants for minimally invasive glaucoma surgery (MIGS) Ophthalmol Ther. 2017;6(2):233–241. doi: 10.1007/s40123-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary A, Salinas L, Guidotti J, Mermoud A, Mansouri K. XEN Gel Implant: a new surgical approach in glaucoma. Expert Rev Med Devices. 2018;15(1):47–59. doi: 10.1080/17434440.2018.1419060. [DOI] [PubMed] [Google Scholar]
- 8.de Gregorio A, Pedrotti E, Stevan G, Bertoncello A, Morselli S. XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial data and potential role in clinical practice. Clin Ophthalmol. 2018;12:773–782. doi: 10.2147/OPTH.S146919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, Gargallo-Benedicto A, Osorio-Alayo V, Barreiro-Rego A, Duch-Samper A. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol. 2016;91(9):415–421. doi: 10.1016/j.oftal.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Mansouri K, Guidotti J, Rao HL, Ouabas A, D'Alessandro E, Roy S, Mermoud A. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN gel implant surgery: 1-year results. J Glaucoma. 2018;27(2):140–147. doi: 10.1097/IJG.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 11.Widder RA, Dietlein TS, Dinslage S, Kühnrich P, Rennings C, Rössler G. The XEN45 Gel Stent as a minimally invasive procedure in glaucoma surgery: success rates, risk profile, and rates of re-surgery after 261 surgeries. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):765–771. doi: 10.1007/s00417-018-3899-7. [DOI] [PubMed] [Google Scholar]
- 12.Hohberger B, Welge-Lüßen UC, Lämmer R. MIGS: therapeutic success of combined Xen Gel Stent implantation with cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):621–625. doi: 10.1007/s00417-017-3895-3. [DOI] [PubMed] [Google Scholar]
- 13.de Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38(3):1129–1134. doi: 10.1007/s10792-017-0571-x. [DOI] [PubMed] [Google Scholar]
- 14.Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246. doi: 10.1155/2017/5457246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimi A, Lindfield D, Turnbull A, Dimitriou C, Bhatia B, Radwan M, Gouws P, Hanifudin A, Amerasinghe N, Jacob A. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye (Lond) 2019;33(3):469–477. doi: 10.1038/s41433-018-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibáñez-Muñoz A, Soto-Biforcos VS, Rodríguez-Vicente L, Ortega-Renedo I, Chacón-González M, Rúa-Galisteo O, Arrieta-Los Santos A, Lizuain-Abadía ME, Del Río Mayor JL. XEN implant in primary and secondary open-angle glaucoma: a 12-month retrospective study. Eur J Ophthalmol. 2019:1120672119845226. doi: 10.1177/1120672119845226. [DOI] [PubMed] [Google Scholar]
- 17.Reitsamer H, Sng C, Vera V, Lenzhofer M, Barton K, Stalmans I, Apex Study Group Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):983–996. doi: 10.1007/s00417-019-04251-z. [DOI] [PubMed] [Google Scholar]
- 18.Smith M, Charles R, Abdel-Hay A, Shah B, Byles D, Lim LA, Rossiter J, Kuo CH, Chapman P, Robertson S. 1-year outcomes of the Xen45 glaucoma implant. Eye (Lond) 2019;33(5):761–766. doi: 10.1038/s41433-018-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatzara A, Chronopoulou I, Theodossiadis G, Theodossiadis P, Chatziralli I. XEN implant for glaucoma treatment: a review of the literature. Semin Ophthalmol. 2019;34(2):93–97. doi: 10.1080/08820538.2019.1581820. [DOI] [PubMed] [Google Scholar]
- 20.Buffault J, Baudouin C, Labbé A. XEN® Gel Stent for management of chronic open angle glaucoma: a review of the literature. J Fr Ophtalmol. 2019;42(2):e37–e46. doi: 10.1016/j.jfo.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Hodapp E, Parrish R, Anderson DR. Clinical decisions in glaucoma. Mo CV Mosby. 1993;52-61 [Google Scholar]
- 22.Kalina AG, Kalina PH, Brown MM. XEN® gel stent in medically refractory open-angle glaucoma: results and observations after one year of use in the United States. Ophthalmol Ther. 2019;8(3):435–446. doi: 10.1007/s40123-019-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozal SA, Kaplaner O, Basar BB, Guclu H, Ozal E. An innovation in glaucoma surgery: XEN45 gel stent implantation. Arq Bras Oftalmol. 2017;80(6):382–385. doi: 10.5935/0004-2749.20170093. [DOI] [PubMed] [Google Scholar]
- 24.Grover DS, Flynn WJ, Bashford KP, Lewis RA, Duh YJ, Nangia RS, Niksch B. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. doi: 10.1016/j.ajo.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, Reitsamer H, Hengerer FH, Ahmed IIK. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579–1588. doi: 10.1016/j.ophtha.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Hengerer FH, Auffarth G, Conrad-Hengerer I. Comparison of minimally invasive XEN45 gel stent implantation in glaucoma patients without and with prior interventional therapies. Ophthalmol Ther. 2019;8(3):447–459. doi: 10.1007/s40123-019-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huth A, Viestenz A. High myopia in vitrectomized eyes: Contraindication for minimally invasive glaucoma surgery implant? Ophthalmologe. 2020;117(5):461–466. doi: 10.1007/s00347-019-00950-4. [DOI] [PubMed] [Google Scholar]