Abstract
Glaucoma drainage devices have traditionally been reserved for refractory glaucoma. However, there is an increasing body of evidence to suggest the use of these implants at an earlier stage in the surgical management of glaucoma. We describe the mechanics behind their function as well as the various implants available. The implants vary in size, surface area and composition and hence the surgical implantation of these devices are described in detail. The knowledge of such devices and their potential complications is fundamental for the successful management of patients who undergo aqueous-shunt surgery. Careful patient selection and optimal postoperative management is critical to the successful patient outcomes.
Keywords: glaucoma, glaucoma drainage devices, aqueous shunts
INTRODUCTION
The use of glaucoma drainage implants (GDIs; also known as aqueous-shunts, glaucoma drainage devices, setons) are becoming increasingly popular amongst glaucoma specialists[1]. With an ever increasing number of publications on long term outcome data, greater surgical experience as well as concerns about the late complications of augmented trabeculectomy surgery[2], GDIs are now not only being used in the management of refractory end-stage glaucoma but also earlier in the glaucoma paradigm and in some cases as a primary surgical intervention. Over the last quarter of a century there has been great research and evolution in the size, shape and biomaterials of GDIs as well as the surgical technique in a quest to reduce complications and improve their surgical success. In this review we highlight the various GDIs, their indications, the surgical technique and potential complications.
PATHOPHYSIOLOGY
All modern GDI's share a common basic design. This consists of a silicone tube that is inserted into the anterior chamber (or in some cases the vitreous cavity or ciliary sulcus) through a sclera fistula and shunts aqueous humour to an end-plate that is located in the equatorial sub-Tenon's space of the globe, typically centred between (and, in some cases extending under) two adjacent rectus muscles. Typically, GDI's are inserted in the superotemporal quadrant of the globe between the superior and lateral recti due to the greater accessibility to the tissues and the lower risk of postoperative diplopia because of the absence of oblique recti in that quadrant. The subsequent fibrous encapsulation of the plate, which begins as early as 1mo[3], produces a reservoir into which aqueous humour pools. Aqueous then passes through the capsule via a process of passive diffusion and is absorbed by periocular capillaries and lymphatics. The fibrous encapsulation provides the major resistance to aqueous outflow around the plate[4] with the final intraocular pressure (IOP) being determined by the capsular thickness and surface area of encapsulation. A lower final postoperative IOP is typically predicted with a thinner capsule and greater surface area of encapsulation.
GLAUCOMA DRAINAGE IMPLANT TYPES
All GDIs vary in their design with respect to the size, shape and material from which the end-plate is constructed. GDIs can be further sub-divided into “valved” or “non-valved”, depending on whether a flow restriction mechanism limits aqueous humour flow (Figure 1). Commercially available GDIs currently in common use include the Ahmed glaucoma valve (New World Medical, Ranch Cucamongo, California, USA), the Ahmed ClearPath (New World Medical, Ranch Cucamongo, California, USA), the Baerveldt glaucoma implant (Abbott Medical Optics, Santa Ana, California, USA) and the Molteno implant (Molteno Ophthalmic Limited, Dunedin, New Zealand). The idea behind a valved implant is to minimize the risk of post-operative hypotony prior to plate encapsulation, but at the same time allow immediate aqueous outflow and postoperative IOP reduction. Non-valved implants require a temporary restriction of aqueous outflow by internal and/or external tube ligation until encapsulation around the end-plate occurs. This manoeuvre allows resistance to outflow to develop thereby reducing the risk of post-operative hypotony. A summary of the commercially available GDI's in highlighted in Table 1 alongside their advantages and disadvantages.
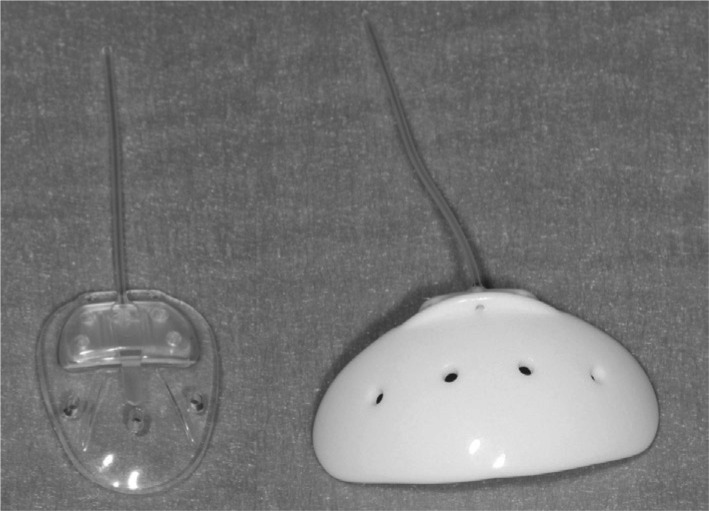
Figure 1. Ahmed valve implant (AVI), Model FP7 (left), Baerveldt glaucoma implant, Model 101-350 (right). Note the fenestrations present on both end-plates and the thin curved profile of the Baerveldt implant.
Table 1. Advantages and disadvantages of commercially available GDIs for anterior chamber use.
| Shunt type | Commercially-available GDI | Plate material | Surface area | Advantages | Disadvantages |
| Non-valved | Baerveldt | Silicone | 250 mm2 | Larger surface area of plate(s) to act as reservoir that may provide increased IOP lowering effect | Delayed functioning until encapsulation of plate occurs More intensive post-operative follow-up required Greater risk of diplopia Greater risk of hypotony |
| 350 mm2 | |||||
| Molteno | Polypropylene | Single plate 130 mm2 | |||
| Double plate 270 mm2 | |||||
| Ahmed Clear Path | Silicone | 250mm2 | |||
| 350mm2 | |||||
| Valved | Ahmed | Polypropylene | 96 mm2 (S3) | Immediate post-operative IOP lowering effect Plate placed in between recti muscles hence lower risk of diplopia Valve minimises risk of post-operative hypotony |
Higher rate of bleb encapsulation Smaller surface area of plate to act as a reservoir that may provide decreased IOP lowering effect Defective valve mechanism can result in hypotony or obstructed outflow |
| 184 mm2 (S2) | |||||
| 364 mm2 (B1) | |||||
| Silicone | 96 mm2 (FP8) | ||||
| 184 mm2 (FP7) | |||||
| 364 mm2 (FX1) |
Ahmed Valve Implant
In 1993, Marteen Ahmed introduced a pressure sensitive valve mechanism known as the Ahmed valve implant (AVI)[7]. The AVI consists of a silicone tube attached to a silicone sheet valve housed within a larger end-plate structure constructed of either polypropylene or silicone. Polypropylene end-plates used in AVI's have been shown to cause a greater inflammatory reaction than their silicone counterparts[8] with later failure rates being higher (13%-18%) with the polypropylene end-plates as compared to 4%-6% with the silicone plate[9]. The valve system consists of two medical-grade silicone membranes, measuring 8 mm in length and 7 mm in width, which creates a Venturi-type chamber. As the inlet of the chamber is wider than the outlet, a pressure gradient between the anterior chamber and sub-Tenon's space is created which enables the valve to open in response to a pressure differential, as described by Bernouilli's equation (flow rate of fluid is inversely proportional to pressure of fluid). The end-plate exerts tension on the silicone membranes to keep them well apposed at lower IOPs, therefore restricting aqueous outflow and postoperative hypotony. An IOP above 8-10 mm Hg forces the silicone membranes apart and thus re-establishing aqueous outflow[10].
The AVI, as with other implants, has a hypertensive phase, which is a transient phase of low capsule permeability that begins 3-6wk postoperatively. It is characterised by a gradual rise in IOP which can last for several months. The incidence has been reported to be much higher with AVI's at 40%-80%[11] compared to 20%-30% for non-valved implants[11]–[12]. It has been speculated that encapsulation and bleb fibrosis occur due to the effects of immediate filtration with the AVI as opposed to the delayed filtration induced with non-valved implants. It has been shown that glaucomatous humour is proinflammatory, consisting of various inflammatory factors including prostaglandins, eicosanoids and most importantly tissue growth factor-beta[13]–[14]. When glaucomatous humour encounters conjunctiva and sub-Tenon's it induces a strong inflammatory response, fibrous encapsulation and potentially poor bleb function.
The Ahmed ClearPath (New World Medical, Ranch Cucamongo, California, USA) is a new non-valved implant available in end plate surface areas of 250 mm2 and 350 mm2, made from silicone, with a pre-placed 4.0 polypropelene ligation suture. It has a flexible endplate that conforms to the natural shape of the globe with more anteriorly placed eyelets enabling more efficient suture fixation.
Baerveldt Implant
The Baerveldt implant was pioneered by George Baerveldt in 1992 and consists of a silicone tube connected to a flexible barium impregnated silicone end-plate (Figure 1). The barium allows the end-plate to be identified on facial X-rays. Three models of this implant are commercially available for clinical use. The BG 103-250 and BG 101-350 models consist of end-plates with surface areas of 250 and 350 mm2, respectively. The BG 102-350 model consists of a 350 mm2 end-plate connected to smaller, angles plate (Hoffman Elbow) by a 7 mm silicone tube. The Hoffman elbow is designed for insertion into the pars plana. The unique feature of the Baerveldt implant is the large surface area of the end-plate which can be implanted under the rectus muscle insertions, through a one-quadrant conjunctival incision[15]. Typically, the superotemporal quadrant is the preferred quadrant. The end-plate has fenestrations that allow fibrous bands to grow through the plate serving to reduce the bleb height and secure the implant in place.
Evidence suggests there may be an upper limit to end-plate surface area to achieve IOP lowering, after which additional surface area does not improve pressure control, and may even detrimentally affect surgical outcome. In a prospective randomised controlled study comparing the 350 mm2 and 500 mm2 Baerveldt implant, Lloyd et al[16], showed no significant difference in IOP lowering and surgical success at 18mo, with a lower complication rate in the 350 mm2 group. In another longer-term prospective study, Britt et al[17], showed a greater cumulative probability of success for the 350 mm2 Baerveldt group throughout the 5y of follow-up compared to the 500 mm2 group, which also had a greater trend towards sequelae from hypotony.
Although the role of antifibrotic agents [5-FU and mitomycin-C (MMC)] have been well described in improving the success of trabeculectomy surgery, their use in GDIs remains unproven. A recent Cochrane review of 5 randomised controlled trials (RCT) failed to show any additional benefit in the IOP lowering effect of MMC in GDI's[4].
INDICATIONS
GDI surgery has traditionally been reserved for cases of refractory glaucoma where previous augmented trabeculectomy surgery has failed or is at high risk of failure. Glaucomatous eyes with a history of previous conjunctival incisional surgery (trabeculectomy; extracapsular cataract extraction; pars plana vitrectomy; scleral buckle; corneal graft surgery) or a history of cicatricial conjunctival disease, trauma or aphakia are considered to be at high risk of trabeculectomy failure. Refractory cases of neovascular glaucoma, uveitic glaucoma and iridocorneal endothelial (ICE) syndrome may increase fibroblast proliferation and sub-tenon's encapsulation leading to subsequent trabeculectomy failure. In such cases GDI surgery is preferred.
Drainage implant surgery are commonly used in congenital glaucoma when traditional procedures such as goniotomy or trabeculotomy have failed. The implants are preferable in the management of childhood glaucoma due to iridocorneal dysgenesis, aniridia, aphakia, juvenile rheumatoid arthritis and in Sturge-Weber syndrome. In the latter situation, the risk of expulsive haemorrhage following a marked reduction in IOP is much lower with GDI surgery compared to augmented trabeculectomy. The use of augmented trabeculectomy in childhood glaucoma should be used with caution given the difficulty with postoperative bleb manipulation as well as the risk of progressive bleb thinning with age. A full list of indications is summarised in Table 2.
Table 2. Indications for GDI surgery.
| Previous incisional conjunctival surgery |
| Failed trabeculectomy |
| Complications arising from trabeculectomy in other eye |
| Extracapsular cataract surgery |
| Pars plana vitrectomy |
| Scleral buckling procedures |
| Corneal graft surgery |
| Conjunctival disease |
| Cicatricial inflammatory scarring |
| Long-term antiglaucoma medication use |
| Uveitic glaucoma |
| Neovascular glaucoma |
| Traumatic glaucoma |
| Iridocorneal endothelial syndrome |
| Paediatric glaucoma |
| Congenital paediatric glaucoma |
| Secondary glaucoma |
| Iridocorneal dysgenesis |
| Aphakia |
| Aniridia |
| Juvenile rheumatoid arthritis |
| Uveitis |
| Sturge-Weber Syndrome |
GDI: Glaucoma drainage implant.
SURGICAL TECHNIQUE
As most tube surgery can take anywhere up to 2h, general anaesthesia is often preferred, however some patients prefer peribulbar anaesthesia with sedation. At the time of general anaesthesia a peribulbar block can be administered to displace the globe anteriorly for 1) surgical access, as often the globe can be sunken due to the patient's age and/or the long-term use of prostaglandin analogues and 2) post-operative pain control. Although certain variations in surgical technique are required for implantation of the different implants, basic surgical principles apply to the implantation of all GDI's.
Following the appropriate use of anaesthesia the eye is cleaned and prepared in the usual sterile manner. A lightweight speculum e.g. Khaw speculum should be considered. This neatly hides the drape and eyelashes under the upper and lower fornices without inducing excessive positive pressure and avoids overstretching of the eyelids. A 7-0 silk traction suture on a spatulated needle is passed through the superior peripheral corneal stroma (50% thickness) in the quadrant being exposed. The suture is attached to the drape beneath the eye with artery forceps.
A 3-clock hour, fornix-based conjunctiva and sub-Tenon's flap is created, usually in the superotemporal quadrant, using blunt Westcott scissors. Should the conjunctiva and sub-Tenon's layer be non-mobile due to previous surgery, hydrodissection by slow injection of balanced salt solution can be used. The flap is lifted using Moorfields forceps allowing for blunt dissection between the Tenon's and episcleral bed. Horizontal and vertical relaxing incisions are made of approximately 6 mm, to provide surgical exposure beyond the equator of the globe. Further posterior dissection of the sub-Tenon's space is carried out beyond the equator of the globe (Figure 2A). Light cautery may be needed to preserve haemostasis and visibility. A muscle hook is the used to identify and isolate the superior and lateral recti on either side of the surgical site. Surgical spears can be used to clean the muscles by removing Tenon's attachments from the muscle edges. GDIs with larger anterior-posterior plate dimensions, such as the AGI, should be avoided from implantation in the superonasal quadrant as it may come within 1 mm of the optic nerve[18].
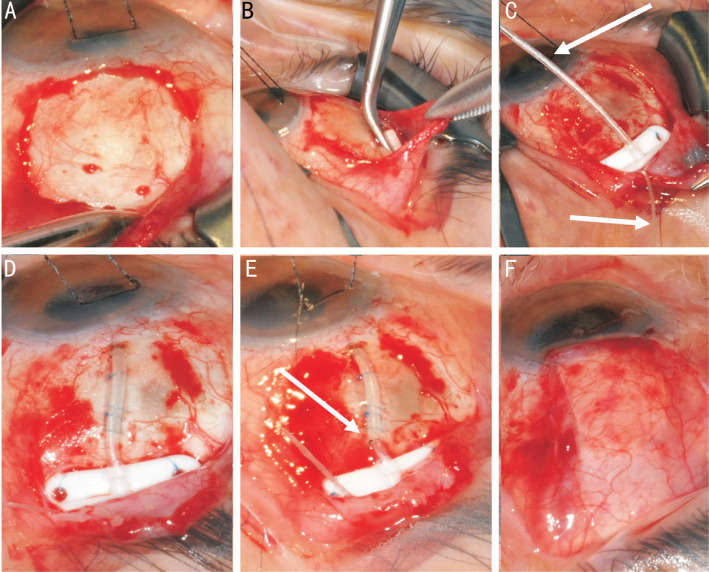
Figure 2. Stages of Baerveldt tube insertion.
A: Subtenons pocket is fashioned in the superotemporal quadrant; B: Mitomycin C can be applied using a PVA corneal shield in the subtenons pocket; C: The place is secured to sclera through the eyelets of the plate (note the supramid suture within the tube, and coming out the end plate); D: The tube is inserted into the anterior chamber fixed with two mattress sutures; E: An external ligating suture is secured; F: The patch graft is sutured over the tube and the conjunctiva and subtenons closed.
Should antimetabolites be considered, a corneal sponge can be soaked in 0.4 mg/mL of MMC, with the excess slightly soaked with a dry spear (Figure 2B). Using disposable Moorfields forceps to hold the conjunctiva and disposable McPherson forceps to hold the sponge, glide the sponge into the sub-Tenon's space behind the equator for 3min. Do not remove the forces as there is a risk the sponge could be lost posteriorly. Following this, rinse the area with 20 mL of balanced salt solution.
In the case of non-valved implants, it is necessary to irrigate the balanced salt solution through the tube using a 27-gauge Rycroft cannula, prior to insertion into the anterior chamber, to ensure the tube is patent. This is also imperative in valved implants to ensure the valve opens and functions properly. In the non-valved implant an internal ligating (e.g. 3-0 supramid suture S. Jackson Inc. Alexandria, Virginia, USA) is then fed through the tube from the posterior plate entrance to restrict aqueous flow. Due to minuscule variations in internal tube diameter between GDI's, as well as similar variations in suture diameter, the internal ligating suture may not feed all the way down the tube.
The external plate is then tucked posteriorly into the sub-Tenon's space. For AGIs this is achieved without the involvement of recti muscle. Winged non-valved implants e.g. Baerveldt implant do however required implantation under the recti muscles. The Baerveldt implant is placed on the field of surgery and held obliquely with Moorfields forceps. Using a muscle hook the superior rectus is isolated and muscle hook is tilted to lift the edge of the muscle. The superior wing of the Baerveldt implant is then fed in between the sclera and the superior rectus, until three quarters of the wing is sitting under the superior rectus. Using a similar manoeuvre the inferior wing is then placed under the lateral rectus. To ensure the wings of the implant are placed under their respective recti muscles, use the Moorfields forceps to pull the implant forward at the ridge of the implant. The implant should remain behind the muscles insertions.
Callipers are used to confirm the anterior edge of the implant is 9-10 mm posterior to the limbus. Using 9-0 prolene, tightly secure the implant to the sclera, passing the needle partial thickness through the sclera and then the suture through the eyelets on either side of the ridge. The suture knots should be rotated into the suture hole eyelets (Figure 2C). There should be very little or no movement of the body of the plate when pulled forwards and backwards using Moorfields forceps at the ridge of the plate.
The tube is now ready to be shortened for entry into the anterior chamber. The corneal traction suture is loosened off and the globe centred into primary position. The tube is laid against the sclera and limbus at the proposed angle it is to be inserted into the anterior chamber. Westcott scissors are used to cut the tube, bevel up, to permit its extension 1-2 mm into the anterior chamber. Beware not to pull the tube when cutting it, as this falsely extends the tube, and hence the tube will be cut too short. Cauterise the sclera 1-2 mm posterior to the limbus where the tube is to be inserted. Most frequently a 23-gauge needle is used as this allows the tube to comfortably enter the anterior chamber with minimal peri-tube aqueous leakage. The 23-gauge needle is leurlocked onto a 2-mL syringe and using large artery forceps it is bent to a 40-45 degree angle, roughly one third of the way down the shaft of the needle. The longer the needle to the bend point, the less control the surgeon will have when entering the anterior chamber with the needle. Using countertraction from the corneal traction suture, advance the needle through the cauterised sclera 1-2 mm posterior to the limbus and into the anterior chamber, slightly above and parallel to the iris plane. Use toothed forceps to hold the scleral tunnel, and feed the tube into the anterior chamber using straight tying forceps. The angle at which the needle enters the anterior chamber is critical, as it dictates the position of the tube. Ideally the tube should be away from the cornea to reduce the risk of corneal decompensation, but also not touching iris to prevent chronic anterior uveitis. The bevel of the tube should point upwards to prevent iris incarceration. If the tube position is deemed to be incorrect then remove the tube and close the scleral tunnel using a 10-0 nylon cross suture. If there is high peripheral anterior synaechiae, a surgical iridectomy may need to be performed into which the tube is then fed. Alternatively the tube can be placed in the sulcus plane and left long to be visualised in the pupillary margin. Once the tube is positioned correctly check for peri-tube limbal leakage. A long scleral tunnel using a paediatric micro-vitreoretinal blade (MVR) between 7 and 4 mm posterior to the limbus can be constructed, with the tube inserted through. This can provide an extra ‘layer’ of barrier tissue to lower the risk of erosion. The tube is then secured to the sclera with 2 small scleral mattress sutures using 9-0 prolene (Figure 2D). It is important that these sutures do not compress the tube, but merely prevent lateral movement of the tube—a presumed risk factor for melting of the patch graft.
An inferotemporal paracentesis is then constructed using a 15 degree or MVR blade. The IOP is raised within the eye using BSS solution, to between 20-30 mm Hg which is estimated by digital palpation. In non-valved tubes, the ridge of the plate is then pulled forward using Moorfields forceps to ensure there is posterior aqueous flow onto the plate, to ensure the tube is functioning.
In cases of non-valved tubes, variations exist on how the tube is externally ligated to prevent early postoperative hypotony. A 7-0 vicryl ligature can be used to completely externally ligate the tube close to the implant flange. This ligature will usually rupture at 4-6wk postoperatively, allowing the plate to develop some fibrosis in the meantime. Partial aqueous flow can be achieved in the meantime by constructing a small 2 mm Sherwood slit, on the anterior aspect of the tube, 5 mm posterior to the limbus. This is done using a 15 degree blade. Another technique used to externally ligate the tube is to partially externally ligate the tube. Once the eye pressure is between 20 and 30 mm Hg the flow rate on the plate is identified. A 10-0 nylon is then tied around the tube close to the implant flange and tightened until the flow rate is roughly halved, at which point it is locked (Figure 2E). This allows partial flow onto the plate from the beginning, controlling IOP, as well as reducing the risk of hypotony. In cases where the risk of hypotony is higher e.g. myopic eyes or uveitic eyes, the 10-0 nylon can be further tightened, allowing for minimal aqueous flow onto the plate. The benefit of this technique is that the 10-0 nylon can undergo argon suture lysis (ASL) earlier postoperatively, giving the surgeon greater options for postoperative IOP control. With any form of external tube ligation it is important not to tie the suture so tight that the tube is castrated.
A connective tissue graft, such as donor sclera, processed pericardium (Tutoplast) or corneal tissue (Figure 3), is used to cover the anterior portion of the tube. The purpose is to prevent extrusion of the tube through the conjunctiva. The graft is cut to the appropriate shape and size, approx 4×6 mm2 and sutured to the sclera using 10-0 nylon. In cases of Tutoplast a double layer maybe necessary as the material is thinner than donor sclera. Care should be taken to bevel or thin the edges of the limbal aspect of the patch graft to minimise postoperative dellen formation.
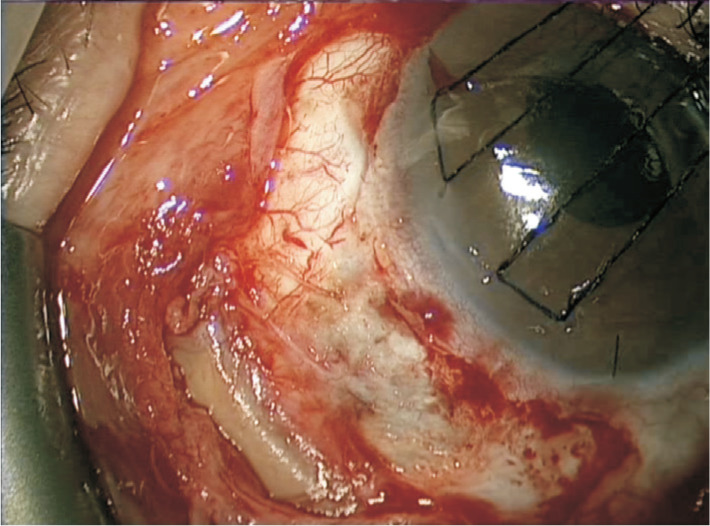
Figure 3. Encircling band in a patient undergoing Baerveldt tube surgery. The fibrous capsule was dissected and the buckle was locally excised prior to insertion of the plate. Note the retracted conjunctiva making closure challenging.
The supramid suture is trimmed and sutured to the sclera next to the patch graft using a 10-0 nylon suture. The benefit of tying it down with a dark non-absorbable suture is the ability to locate the supramid should it need to be removed in the future.
The conjunctival and sub-Tenon's layers are then sutured back to their original position using 10-0 nylon sutures. An anterior mattress suture in front of the patch graft is important to prevent conjunctival retraction. Conjunctival mattress sutures or a running continuous suture using 10-0 vicryl can be used to close the initial conjunctival relaxing incisions (Figure 2E). Instead of conjunctival sutures a fibrin sealant (Tisseel glue) can be used to close the conjunctiva, however in cases where a Sherwood slit is constructed, the glue may obstruct aqueous outflow.
Subconjunctival injection of antibiotics and steroids is recommended. In cases where a general anaesthetic is the sole anaesthetic, a small sub-Tenon's lignocaine injection can be given above the patch graft during conjunctival closure to provide effective postoperative pain relief. The eye is then patched and shielded overnight.
SPECIAL SITUATIONS
Pars Plana Insertion
It is possible to insert the tube into the vitreous cavity through a pars plana incision in aphakic (or possibly psedudophakic) eyes in which a vitrectomy has been performed. Situations where this may arise include a very shallow anterior chamber, aphakia, vitreous in the anterior chamber and penetrating keratoplasty (PKP). The pars plana insertion has the advantage of keeping the tube away from the cornea, especially after PKP, reducing the risk of corneal decompensation, graft failure and epithelial downgrowth. Pars plana insertion can also be combined with cases in which a vitrectomy is required such as a diabetic retinopathy with secondary glaucoma.
A Hoffman elbow has been designed for pars plana insertion, in which the angulated cannula of the Hoffman elbow is inserted through the MVR sclerostomy following vitrectomy and fluid-gas exchange.
Pre-existing Scleral Buckle
The presence of a scleral buckle presents an extremely challenging situation in cases of refractory glaucoma unresponsive to medical therapy. Although cyclodiode can be used, the effects are unpredictable and often temporary. The presence of the scleral buckle can make the placement of the plate quite challenging (Figure 3). Previous conjunctival dissection, increases the risk of intraoperative bleeding as well as postoperative scarring, potentially reducing the efficacy of the GDI. Postoperative conjunctival retraction and symblepharon formation is also more common.
An anteriorly placed scleral buckle may allow dissection of the fibrous capsule around the buckle and placement of the plate posteriorly. The ‘wings’ of the plate may need to be excised to ensure the plate fits in the sub-Tenon's pocket. It is important to secure the plate to the sclera or fibrous capsule surrounding the buckle, to prevent potential plate migration from subsequent scarring. The tube is then fed over the buckle. Be wary to feed the tube under the buckle as often the sclera is necrosed and thinned in this area, making the eye vulnerable to perforation. If the plate is unable to fit posterior to the buckle, the tube can be castrated and fed directly into the fibrous capsule which itself acts as resistance to aqueous outflow. In some case the buckle can be excised, in conjunction with the vitreoretinal surgeons, although the patient should be warned about the 10%-15% risk of retinal re-detachment. The presence of silicone oil in the anterior chamber also provides its own challenges. The tube should be kept longer than usual to minimise silicone oil escape through the tube, which may block it or cause sub-conjunctival silicone oil exposure. An inferior quadrant tube implant can also be considered in these cases. Ideally the silicone oil should be removed prior to tube surgery although this again needs to be discussed with the vitreoretinal surgeons.
Previous Penetrating Keratoplasty
Tube surgery following PKP also remains a difficult management issue. Often these patients can have an occluded angle with high encircling peripheral anterior synaechiae with an obscured view at the graft host junction, thus making tube insertion and positioning challenging. In such cases a surgical iridectomy can be performed prior to tube insertion, into which the tube can then be implanted. An alternative is to implant the tube into the ciliary sulcus, leaving it visible in the pupillary margin.
In glaucomatous cases in which PKP surgery is predicted, prior tube surgery may be performed to reduce the risk of post-PKP IOP spikes and thus preserving the corneal graft viability. As well as this during the ‘open-sky’ PKP surgery the corneal surgeons can alter the tube position to reduce the risk of endothelial rubbing and corneal graft failure. Only 55% of corneal grafts have reported to survive at 3y following a GDI into the anterior chamber[19].
POSTOPERATIVE MANAGEMENT AND INTERVENTIONS
The patients should be checked a few hours following surgery to ensure the anterior chamber is formed and the IOP satisfactory. They are commenced on preservative free topical steroids and antibiotics, every 2h and 4 times a day respectively. An unsatisfactory IOP can be managed with restarting anti-glaucoma medication. Tube manipulation e.g. ASL should be performed no earlier than 3wk postoperatively, until plate encapsulation and some degree of resistance to aqueous outflow has developed. Topical antibiotics are continued for 4wk, but topical steroids are kept longer and tapered slowly over 3-4mo (Figure 4). Follow-up examinations should be focused on anterior chamber depth and tube position, IOP, bleb formation over the plate and conjunctival healing. In non-valved implants if a nylon external ligation suture has been used, ASL can be performed using a Blumenthal suturelysis lens at 50-100 mm spot size, 300 mW power for 0.05s. If a vicryl external ligating suture has been used, depending on its size, the tube should be become patent between 3 and 6wk postoperatively. Patients should be warned about the potential sudden drop in IOP and risk of hypotony. Should there be a hypertensive phase as previously described following AVI, topical anti-glaucoma medication can be used until the IOP settled which take up to 6mo following surgery.
Figure 4. Shallow diffuse bleb following a Baerveldt tube implant in juvenile uveitic glaucoma. Note the corneal patch graft allows you to see the tube underneath and improves the cosmesis of the patient.
The internal supramid stent suture can be removed 3mo following non-valved GDI surgery to further lower the IOP or to reduce the anti-glaucoma medication. This is required in up to 50% of all cases. It can be performed under topical anaesthesia in a surgical theatre setting. During removal note should be taken to ensure the tube remains in the anterior chamber. A small volume of 0.1 mL viscoelastic into the anterior chamber can be administered prior to removal to prevent a sudden drop in IOP. This is especially true on aphakic eyes, where there is a tendency for vitreous to migrate and block the tube during supramid stent removal. In cases with a higher risk of hypotony (uveitic glaucoma, high-flow glaucoma, myopia) partial supramid removal should be considered.
COMPLICATIONS
Prevention and Management
With the increasing use of GDI's in the management of refractory glaucoma, understanding their potential complications is essential in managing these patients. Aqueous shunts are associated with similar intraoperative and postoperative complications as occur after trabeculectomy. Additionally, there are complications associated with the implantation of the hardware.
Hypotony
Intraoperative techniques are deployed during non-valved GDI surgery to reduce the risk of early postoperative hypotony, as described previously. However, despite these techniques hypotony remains one of the most common early complications following GDI surgery. Even AVI's are at risk for hypotony-related complications reported to be 8%-13%[20]. This can occur due to peri-tube leakage or a malfunctioning valve system leading to over drainage. Should the anterior chamber shallow, or the eye become compromised, injection of 0.1-0.15 mL of viscoelastic into the anterior chamber can help temporise the situation until scarring occurs to resolve the hypotony. However, despite medical management, should clinically significant early hypotony persist, a revision of the AVI with internal and/or external ligation sutures may be required.
In non-valved tubes, such as the Baerveldt tube, hypotony can also be caused by peri-tube aqueous leakage, Sherwood slits that are too large as well as the failure to adequately externally ligate the tube, which may show a large bleb over the plate. Most of these cases will settle without intervention as a fibrous capsule form around the plate. Other rarer causes of early hypotony include ciliary body shutdown and cyclodialysis cleft, which in both cases the plate would appear flat. Following ASL of the nylon external ligation suture hypotony can occur due to over drainage. Patients who develop hypotony-related complications such a shallowing of the anterior chamber and choroidal fold (Figure 5) should be managed with prompt cycloplegia and intracameral viscoelastic. However, if the hypotony has not resolved within 7-10d, repeat external ligation of the tube may need to be performed by reflecting back the conjunctiva from the patch graft or ligating the tube within the anterior chamber (Figure 6). The shallowing of the anterior chamber can lead to corneal-tube touch and subsequent corneal oedema and scarring. Even in the absence of corneal-tube touch in hypotony, corneal damage may still occur as blinking may indent the cornea against the tube.
Figure 5. An internal anterior chamber 8-0 prolene suture around a Baerveldt 350-mm2 tube to manage postoperative hypotony.
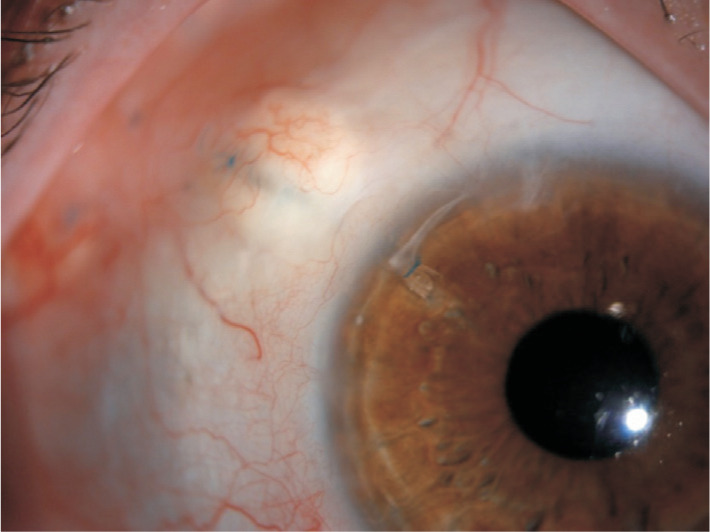
Figure 6. Hypotony with significant choroidal folds following Baerveldt implant surgery in Sturge-Webber syndrome.
Elevated Intraocular Pressure
Elevated IOP in the early postoperative period may be due to obstruction of the tube by fibrin, blood, iris, fibrovascular membranes, vitreous or silicone oil. A blockage should be considered if the tube remains non-functional after the external ligation suture has been released and there is no bleb over the plate. Often blockages due to fibrin or blood will resolve within a few days, although intracameral tissue plasminogen activator can be successfully used. Nd:YAG can be used to clear iris plugs from the internal ostium. A blockage due to vitreous suggests the presence of vitreous in the anterior chamber. This will require a mechanical sweeping of the vitreous strand of the tube, through the paracentesis and an anterior vitrectomy with triamcinolone-assisted visualisation. Beware of aphakic or aniridia patients, as they can often get plugging of the tube during supramid removal. The sudden decompression of the eye and opening of the tube can suck the vitreous into the lumen. This should always be checked and viscoelastic can be left in the anterior chamber to tamponade the vitreous membrane. In cases where a blockage at the external ostium is proposed, transcameral irrigation up the ostium of the tube can be considered.
Suprachoroidal haemorrhage is one of the most devastating complications following GDI surgery. It is characterised by a shallow anterior chamber, decreased vision and the sudden onset of severe pain, in the presence of raised IOP. It can occur following the sudden decompression of the globe during supramid removal, but is more likely to occur in a hypotonous eye with choroidal effusions. Preventing hypotony is the most effective treatment in preventing suprachoroidal haemorrhage. Mild to moderate suprachoroidal haemorrhages can be observed with a good visual prognosis. Larger kissing choroidals may require drainage when the blood clots have lysed and liquefied.
In aqueous misdirection the IOP can be elevated and is characterised by a shallow anterior chamber in the presence of patent iridectomy and a normal posterior segment examination. It is more common in patients with axial hypermetropia and is most likely to occur during supramid removal when the sudden decompression causes shallowing of the anterior chamber, anterior rotation of ciliary body and posterior flow of aqueous behind the vitreous base. Treatment includes cycloplegia to posteriorly rotate the ciliary body, as well as topical anti-glaucoma medication. In pseudophakic patients a Nd:YAG hyaloidotomy can be performed preferably through a pre-exisiting iridectomy. Should this not resolve the aqueous misdirection, cyclodiode laser can be used to posteriorly rotate the ciliary body and in more extreme cases a pars plana vitrectomy may be required. Although the visual prognosis is good, patients with a pre-existing PKP are at higher risk of graft failure following aqueous misdirection.
Late IOP elevation is most likely due to be from a thickened fibrous capsule leading to partial aqueous outflow. Although bleb needling and revisions of the bleb can be considered, their success remains sub-optimal.
Motility Disturbances
Transient diplopia is not uncommon following GDI surgery, but generally resolves as the postoperative periocular oedema improves. Motility disturbances may be due to extraocular muscle restriction by the plate, stretching of the extraocular muscles by the fibrous capsule, surgical trauma and scarring of the muscles. In the TVT study, the Baerveldt 100-350 group experienced a 5% rate of diplopia and a 9.9% incidence of strabismus at 3y, with risk factors being a larger plate size, older age and previous extraocular muscle surgery[2],[21]. GDI placement in the superonasal quadrant is typically avoided due to the restriction of the superior oblique muscle, resulting in a pseudo-Brown's syndrome.
Treatment of persistent diplopia and strabismus associated with GDI surgery consists of prism spectacles, decompression of the fibrous capsule, extraocular muscle surgery and in more severe cases explantation of the implant.
Infection
As with any intraocular surgery, infection is a rare but serious complication. In the TVT study the incidence was 1% in the tube group compared to 3% in the trabeculectomy group[2],[21]. The presence of a foreign material can only not cause endophthalmitis, but can lead to a panophthalmitis and orbital cellulitis. The most common cause is conjunctival erosion of the tube, causing bacteria to migrate along the tube and into the eye, as well as around the plate. Haemophilus influenza and Streptococcus are the most commonly identified organisms[22]. In such cases of infection removal of the implant may be necessary to completely sterilise the implant site. Endophthalmitis should be treated urgently with an intravitreal tap and antibiotics.
Tube Migration, Extrusion and Erosion
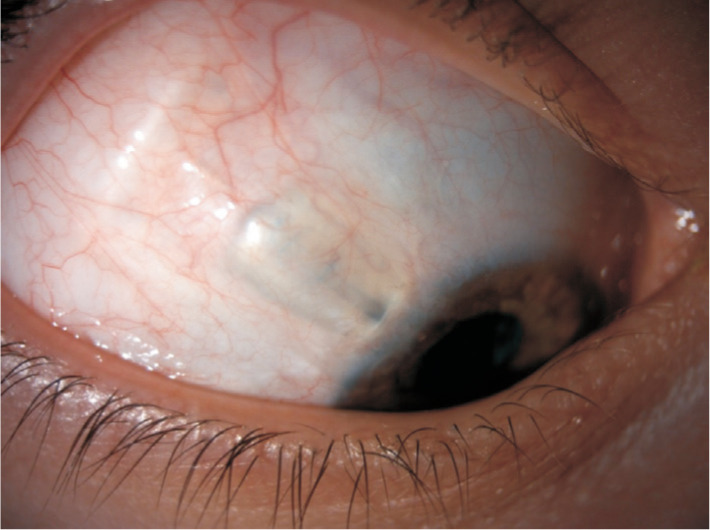
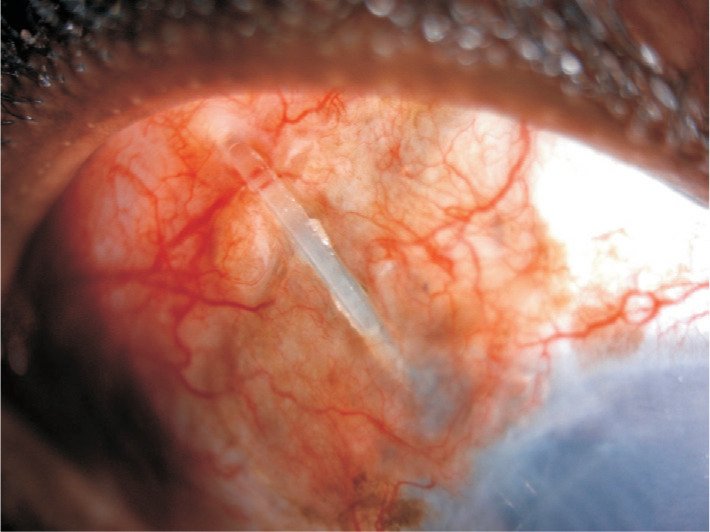
The continuous mechanical rubbing of the upper eye lid against the elevated conjunctiva overlying the tube is thought to be the strongest risk factor in tube erosion. In the TVT study at 5y postoperatively, 5% of tubes eroded through the overlying conjunctiva[2]. No differences exist in the incidence of exposure amongst the Ahmed, Baerveldt and Molteno implants. The earliest sign of impending tube erosion is loss of the conjunctival vascular architecture over the tube[23]. Once a tube has eroded though the conjunctival tissue, primary surgical intervention is warranted to prevent endophthalmitis or plate extrusion (Figure 7). A repeat patch graft should be sutured over the exposed tube. If there is sufficient mobile healthy conjunctiva around the tube this can be undermined and extended to cover the new patch graft. However, often this is not the case given the complex nature of these glaucomatous eyes. In such cases a large rotational conjunctival pedicle flap from the superior bulbar conjunctiva may cover the new patch graft. Another option is to extend the tube, with a tube extender, and place it into healthier tissue which maybe inferiorly. In more severe cases the tube may need to be castrated with the sclerostomy closed and covered with amniotic membrane. Risk factors for recurrent erosion include black race, diabetes mellitus, a greater number of pre-tube antiglaucoma medications and concomitant procedures at the time of tube surgery[24]. Tube migration can occur if the plate is not tightly secured to the sclera. The tube can retract from the anterior chamber. This is especially true of paediatric patients undergoing tube surgery. As the eye grows the tube can retract and hence it should initially be left slightly longer with in the anterior chamber to account for this.
Figure 7. Tube erosion following Baerveldt implant surgery in a young African-Caribbean male with uveitic glaucoma and a decompensated cornea.
Corneal-Related Complications
Corneal endothelial loss, decompensation, persistent corneal oedema and graft failure have all been reported following GDI surgery. In patients without pre-existing corneal graft surgery or corneal pathology, the rate of corneal decompensation has been reported as low as 7% in the 1-year TVT study[20] and as high as 22% in the Baerveldt group in the ABC study[25]. However, in patients with corneal graft surgery the incidence of corneal decompensation has been reported to be as high as 70% at 18mo following anterior chamber GDI surgery[26]. In this study, GDI surgery was shown to be an independent risk factor for graft failure and as such patients should be appropriately consented for the risk.
The aetiology of corneal oedema as well as corneal decompensation following GDI surgery appears to be multifactorial. Endothelial cell loss can occur because of refractory preoperative elevated IOP, intraoperative tube manipulation as well as postoperative hypotony and mechanical trauma from tube touch. The insertion of the tube in the anterior chamber may induce a breakdown in the blood-aqueous barrier and trigger an immunologic graft rejection.
Tube-corneal touch is the only modifiable risk factor. The tube portion of the GDI should be placed far from the corneal endothelium, towards the iris plane. In cases of persistent corneal edema, the tube can be shortened or re-sited elsewhere in the anterior chamber. Pars plana or ciliary sulcus placement may be indicated in cases at high risk of corneal endothelial cell damage.
OUTCOMES
Recently published prospective randomised clinical studies on GDI surgery have provided surgeons with a greater insight into their long-term safety and efficacy. These include four major studies; the TVT study compared the Baerveldt 101-350 with augmented trabeculectomy in eyes with prior surgery[2],[20], whereas the Primary Tube Versus Trabeculectomy (PTVT) study compared primary Baerveldt 101-350 with augmented trabeculectomy in eyes with no prior glaucoma surgery. Both the Ahmed Baerveldt Comparison (ABC) study[25],[27] and the Ahmed Versus Baerveldt (AVB) study[28] compared the Ahmed valve FP7 and the Baerveldt 101-350. The results of each study are summarised in Table 3 and reinforce that both the AVI and the Baerveldt implant are effective treatments for refractory and high-risk glaucoma.
Table 3. Summary of major randomised clinical trials involves long-term outcomes of GDIs.
| Clinical trial | No. of patients | Treatment groups | Follow-up | Major findings |
| Tube vs Trabeculectomy (TVT) study[21] | 212 | 350-mm2 Baerveldt glaucoma implant, augmented trabeculectomy with mitomycin C | 5y | Baerveldt implant had a lower cumulative probability of failure (29.8% Baerveldt vs 46.9% trabeculectomy) Similar mean IOP (14.4 mm Hg Baerveldt vs 12.6 mm Hg trabeculectomy) and mean number of antiglaucoma medications (1.4 Baerveldt vs 1.2 trabeculectomy) between both surgical procedures Trabeculectomy had a higher rate of reoperation (9% Baerveldt vs 29% trabeculectomy) |
| Primary Tube vs Trabeculectomy (PTVT) study[29] | 276 | 350-mm2 Baerveldt glaucoma implant, augmented trabeculectomy with mitomycin C | 1y | Trabeculectomy had a lower cumulative probability of failure (7.9% trabeculectomy vs 17.3% Baerveldt) Similar mean IOP (12.4 mm Hg trabeculectomy vs 13.8 mm Hg Baerveldt) but lower mean number of antiglaucoma medications in trabeculectomy group (0.9 trabeculectomy vs 2.1 Baerveldt) Serious complications requiring reoperation or loss of 2 or more Snellen line greater in trabeculectomy group (7% vs 1%) |
| Ahmed Baerveldt Comparison (ABC) study[25],[27] | Ahmed glaucoma implant valve FP7, 350-mm2 Baerveldt glaucoma implant | 5y | Mean IOP was lower with Baerveldt implant (12.7 mm Hg Baerveldt vs 14.7 mm Hg Ahmed) Use of antiglaucoma medication was similar between implants (1.8 Baerveldt vs 2.2 Ahmed) No significant difference in cumulative probability of failure between implants (39.4% Baerveldt vs 44.7% Ahmed) Baerveldt implant had a greater number of failures due to hypotony, explantation or loss of light perception (17% Baerveldt vs 8% Ahmed) |
|
| Ahmed vs Baerveldt (AVB) study[28] | 238 | Ahmed glaucoma implant valve FP7, 350-mm2 Baerveldt glaucoma implant | 5y | Mean IOP was lower with Baerveldt implant (13.6 mm Hg Baerveldt vs 16.6 mm Hg Ahmed) Use of antiglaucoma medication was lower with the Baerveldt implant (1.2 Baerveldt vs 1.8 Ahmed) Baerveldt implant has a lower cumulative probability of failure (40% Baerveldt vs 53% Ahmed) No significant difference in late complications between the implants (69% Baerveldt vs 63% Ahmed) Hypotony resulting in failure was higher in the Baerveldt implant (4% Baerveldt vs 0 Ahmed) |
GDI: Glaucoma drainage implant.
In the TVT study, 212 patients had undergone either prior cataract surgery, prior trabeculectomy, or both[20]. At five years the Baerveldt 101-350 group had higher success rates compared with augmented trabeculectomy with MMC, although both procedures resulted in similar IOP reduction and number of antiglaucoma medication. Early postoperative complications were reported to be higher in the trabeculectomy group, however rates of late postoperative complications and reoperations were similar.
The results of the TVT study has prompted the conduction of another prospective multicentre randomised controlled study. PTVT study[29] was designed to compare the safety and efficacy of tube surgery (Baerveldt 101-350) with augmented trabeculectomy with MMC as a primary glaucoma surgical procedure. At 12mo the Baerveldt 101-350 had a greater cumulative probability of failure compared to the trabeculectomy with MMC group (17.3% vs 7.9%). The trabeculectomy group had a greater mean drop in IOP at 12mo and number of medications as well as a greater complete success rate compared to the tube group. However, the trabeculectomy group had a higher number of postoperative complications.
In the ABC study, a total number of 276 patients were randomised to AVI (model FP7) or Baerveldt 101-350[25],[27]. There was no statistical difference in the final IOP and number of antiglaucoma medication at five years, although the Baerveldt group did have a mean IOP 2 mm Hg lower than AGI group, as well as a lower mean number of medications. The cumulative probability of failure was 44.7% in the AGI group and 39.4% in the Baerveldt group at 5y (P=0.65). Twice the number of patients failed in the AGI group due to uncontrolled IOP or reoperation whereas there was a greater incidence of persistent hypotony and loss of light perception vision in the Baerveldt group. Findings from the five-year outcomes of the AVB study[28] were similar to the ABC study. The cumulative failure rate was 53% in the AGI group and 40% in the Baerveldt group. The Baerveldt group had a mean IOP 3 mm Hg lower than the AGI group, with a lower mean number of antiglaucoma medication at 5y. Hypotony resulted in failure in 4% in the Baerveldt group compared to none in the AVI group.
SUMMARY
The use of GDI's in the management of both primary glaucoma and refractory glaucoma is becoming significantly more common. With an increasing number of published randomised controlled trials, the safety and efficacy of GDI's is now well established, gaining confidence amongst glaucoma surgeons. There are many different types of GDI's all of which have evolved in size, material and design over time. Nevertheless, the principles of tube surgery have remained the same. The awareness of potential complications specific to tube surgery, careful patient selection and risk stratification are all paramount to the surgical success following GDI surgery.
Acknowledgments
Conflicts of Interest: Agrawal P, None; Bhardwaj P, None.
REFERENCES
- 1.Vinod K, Gedde SJ, Feuer WJ, Panarelli JF, Chang TC, Chen PP, Parrish RK., 2nd Practice preferences for glaucoma surgery: a survey of the American glaucoma society. J Glaucoma. 2017;26(8):687–693. doi: 10.1097/IJG.0000000000000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study Group Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814.e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikbov MM, Khusnitdinov II. The results of the use of Ahmed valve in refractory glaucoma surgery. J Curr Glaucoma Pract. 2015;9(3):86–91. doi: 10.5005/jp-journals-10008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foo VHX, Htoon HM, Welsbie DS, Perera SA. Aqueous shunts with mitomycin C versus aqueous shunts alone for glaucoma. Cochrane Database Syst Rev. 2019;4:CD011875. doi: 10.1002/14651858.CD011875.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006;17(2):181–189. doi: 10.1097/01.icu.0000193080.55240.7e. [DOI] [PubMed] [Google Scholar]
- 6.Gedde SJ, Parrish RK, 2nd, Budenz DL, Heuer DK. Update on aqueous shunts. Exp Eye Res. 2011;93(3):284–290. doi: 10.1016/j.exer.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Coleman AL, Hill R, Wilson MR, Choplin N, Kotas-Neumann R, Tam M, Bacharach J, Panek WC. Initial clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1995;120(1):23–31. doi: 10.1016/s0002-9394(14)73755-9. [DOI] [PubMed] [Google Scholar]
- 8.Lubiński W, Krzystolik K, Gosławski W, Kuprjanowicz L, Mularczyk M. Comparison of polypropylene and silicone Ahmed® glaucoma valves in the treatment of neovascular glaucoma: a 2-year follow-up. Adv Clin Exp Med. 2018;27(1):15–20. doi: 10.17219/acem/66806. [DOI] [PubMed] [Google Scholar]
- 9.Ishida K, Netland PA, Costa VP, Shiroma L, Khan B, Ahmed II. Comparison of polypropylene and silicone Ahmed Glaucoma Valves. Ophthalmology. 2006;113(8):1320–1326. doi: 10.1016/j.ophtha.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Lim KS. Control and optimisation of fluid flow in glaucoma drainage device surgery. Eye (Lond) 2018;32(2):230–234. doi: 10.1038/eye.2017.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayyala RS, Zurakowski D, Monshizadeh R, Hong CH, Richards D, Layden WE, Hutchinson BT, Bellows AR. Comparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucoma. Ophthalmic Surg Lasers. 2002;33(2):94–101. [PubMed] [Google Scholar]
- 12.Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol. 2003;136(6):1001–1008. doi: 10.1016/s0002-9394(03)00630-5. [DOI] [PubMed] [Google Scholar]
- 13.Epstein E. Fibrosis response to aqueous. Its relationship to glaucoma in black patients. Br J Ophthalmol. 1959;43:641–647. doi: 10.1136/bjo.43.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu DY, Lovicu FJ. Myofibroblast transdifferentiation: the dark force in ocular wound healing and fibrosis. Prog Retin Eye Res. 2017;60:44–65. doi: 10.1016/j.preteyeres.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd MA, Baerveldt G, Heuer DK, Minckler DS, Martone JF. Initial clinical experience with the baerveldt implant in complicated glaucomas. Ophthalmology. 1994;101(4):640–650. doi: 10.1016/s0161-6420(94)31283-8. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd MA, Baerveldt G, Fellenbaum PS, Sidoti PA, Minckler DS, Martone JF, LaBree L, Heuer DK. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994;101(8):1456–1463. discussion 1463–1464. doi: 10.1016/s0161-6420(94)31152-3. [DOI] [PubMed] [Google Scholar]
- 17.Britt MT, LaBree LD, Lloyd MA, Minckler DS, Heuer DK, Baerveldt G, Varma R. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology. 1999;106(12):2312–2318. doi: 10.1016/S0161-6420(99)90532-8. [DOI] [PubMed] [Google Scholar]
- 18.Leen MM, Witkop GS, George DP. Anatomic considerations in the implantation of the Ahmed glaucoma valve. Arch Ophthalmol. 1996;114(2):223–224. doi: 10.1001/archopht.1996.01100130217023. [DOI] [PubMed] [Google Scholar]
- 19.Kwon YH, Taylor JM, Hong S, Honkanen RA, Zimmerman MB, Alward WL, Sutphin JE. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001;108(2):272–278. doi: 10.1016/s0161-6420(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 20.Patel S, Pasquale LR. Glaucoma drainage devices: a review of the past, present, and future. Semin Ophthalmol. 2010;25(5-6):265–270. doi: 10.3109/08820538.2010.518840. [DOI] [PubMed] [Google Scholar]
- 21.Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube versus Trabeculectomy Study Group Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e2. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89(4):454–458. doi: 10.1136/bjo.2004.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(6):1089–1098. doi: 10.1016/j.ophtha.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Huddleston SM, Feldman RM, Budenz DL, Bell NP, Lee DA, Chuang AZ, Mankiewicz KA, Koval MS, Truong E, Moraczewski A. Aqueous shunt exposure: a retrospective review of repair outcome. J Glaucoma. 2013;22(6):433–438. doi: 10.1097/IJG.0b013e3181f3e5b4. [DOI] [PubMed] [Google Scholar]
- 25.Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM, Ahmed Baerveldt Comparison Study Group Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118(3):443–452. doi: 10.1016/j.ophtha.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarenga LS, Mannis MJ, Brandt JD, Lee WB, Schwab IR, Lim MC. The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol. 2004;138(2):200–205. doi: 10.1016/j.ajo.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 27.Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM, Ahmed Baerveldt Comparison Study Group Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–316. doi: 10.1016/j.ophtha.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christakis PG, Kalenak JW, Tsai JC, Zurakowski D, Kammer JA, Harasymowycz PJ, Mura JJ, Cantor LB, Ahmed II. The Ahmed versus baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093–2102. doi: 10.1016/j.ophtha.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Gedde SJ, Feuer WJ, Shi W, Lim KS, Barton K, Goyal S, Ahmed IIK, Brandt J, Primary Tube Versus Trabeculectomy Study Group Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology. 2018;125(5):650–663. doi: 10.1016/j.ophtha.2018.02.003. [DOI] [PubMed] [Google Scholar]