Abstract
On December 31, 2019, the novel human coronavirus (COVID-19) was identified in Wuhan, China and swiftly spread in all nations and territories around the globe. There is much debate about the major route of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmissions. So, more evidence is required to determine the potential pathway of transmission of SARS-CoV-2 including airborne transmission. Therefore, we examined the potential aerosol transmission of the virus through hospital wards indoor air by confirmed COVID-19 patients on May 7, 2020. In order to capture airborne SARS-CoV-2, the liquid impinger biosampler was used to take fourteen air samples in different wards of the indoor air of the hospital. The specific primer and probe real-time reverse transcriptase-polymerase chain reaction (RT-PCR) were applied to detect viral genomes of the SARS-CoV-2 virus in positive air samples. Accordingly, we found two positive air samples (in the ICU) out of 14 ones taken from different wards with confirmed COVID-19 patients. The results revealed the possibility of airborne transmission of SARS-CoV-2 though more studies are required to determine the role of actual mechanisms such as cough, sneeze, normal breathing and speaking in the emission of airborne size carrier aerosols. Likewise, more quantitative analyses are needed to estimate airborne viability of SARS-CoV-2 in the carrier aerosols.
Keywords: SARS-CoV-2, COVID-19, Indoor air quality, Viral air quality, Hospital
Graphical abstract
Highlights
-
•
To evaluate the possible airborne transmission of the virus in indoor air of hospitals.
-
•
Our finding indicated that two viral RNA positive air samples collected from indoor air
-
•
We suggest airborne transmission should be given attention, especially in indoor.
1. Introduction
In December 31, 2019, the epidemic of novel human coronavirus (COVID-19) was first detected in Wuhan city, Hubei province, China, which has caused pneumonia by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) has reported that the main route to spread of the COVID-19 virus is human-to-human transmission. The WHO confirmed that the global pandemic situation for SARS-CoV-2 with 3.5 million confirmed cases and 250,000 deaths until May 7, 2020. COVID-19 has a very contagious nature and rapidly spreads to all developed and developing countries (WHO, 2020a; WHO, 2020b; Ma et al., 2020; Wang et al., 2020; Sohrabi et al., 2020; Noorimotlagh et al., 2020). In Islamic Republic (IR) of Iran, on February 19, 2020, the Ministry of Health and Medical Education official announcement reported the first death caused by COVID-19 (Takian et al., 2020; Faridi et al., 2020). According to WHO situation report-108 on coronavirus disease (COVID-19) outbreak, on May 7, 2020, Iran was reported to have 103,330 and 6496 total confirmed cases and total deaths, respectively, which was the highest ranking among Eastern Mediterranean countries (WHO, 2020a). The very high prevalence of COVID-19 in the world has triggered rigorous investigations to determine the clinical characteristics and severity of the novel coronavirus disease and its potential transmission route including airborne transmission. Although the previous studies and WHO guidelines reported that the main route of spreading novel coronavirus disease is human to human transmission (prolonged and unprotected exposure), there is a much debate about other routes of transmission such as airborne transmission (Guan et al., 2020; Van Doremalen et al., 2020; Sohrabi et al., 2020; WHO, 2020b; Holshue et al., 2020; Ghinai et al., 2020; Morawska and Cao, 2020; Noorimotlagh et al., 2020). Concerning the transmission and epidemiology of the two zoonotic coronaviruses including Middle East respiratory syndrome (MERS-CoV) and SARS-CoV-1, it is reported that airborne transmission plays a key role in the coronaviruses disease transmission (Morawska and Cao, 2020; Van Doremalen et al., 2020).
Given the limited and inconclusive findings about the airborne transmission of SARS-CoV-2, researchers are encouraged to implement more studies in this area. In order to better understand and manage the pandemic SARS-CoV-2 and protect the health-care personnel and public health, it is very important to determine the potential airborne transmission of SARS-CoV-2 as one of the most important debate between researchers and scientific community of pandemic COVID-19 outbreak. Therefore, we tried to examine the air samples of indoor air quality of hospital wards allotted for patients' treatment with confirmed positive test of COVID-19 to detect potential SARS-CoV-2 transmission.
2. Materials and methods
2.1. Site description and air sampling procedure
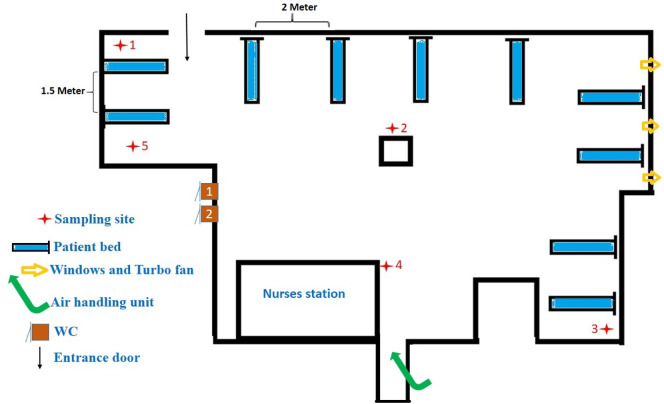
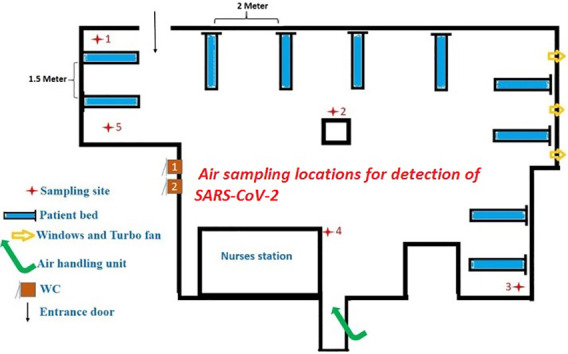
The present study was carried out on May 7, 2020, in Shahid Mustafa Khomeini Hospital wards in Ilam province, East of Iran, assigned for patients' treatment with confirmed COVID-19. Ilam, with 580,000 population, was then reported to have 576 and 54 confirmed COVID-19 cases and deaths, respectively. All of the suspected and confirmed cases of COVID-19 in the other cities were transferred to Shahid Mustafa Khomeini Hospital. The quality of indoor air was checked to see if airborne SARS-CoV-2 existed in the following wards of the hospital complex: intensive care units (ICU) 1 & 2, ICU entrance hall, hospital entrance hall, laboratory ward, CT scan, radiology, men internal ward, woman internal ward and emergency ward. Fig. 1 shows the location of air samples taken from the different points in the indoor air of Hospital.
Fig. 1.
The locations of air samples ( ) and confirmed COVID-19 patients' beds (
) and confirmed COVID-19 patients' beds ( ) for detection of SARS-COV-2.
) for detection of SARS-COV-2.
There are different types of bio-aerosol sampling including passive and active sampling. In the present study, the liquid impinger biosampler (liquid-phase sampler, SKC biosampler) was consumed as a highly efficient technique to capture airborne viruses in bio-aerosol sampling of the COVID-19 virus in the different wards of the hospital. The impinger biosampler was calibrated for a flow rate of 12 L.min−1 at a height of 1.5 m above ground floor to stimulate the breathing zone of people and at least 2 m away from the patient beds (Harbizadeh et al., 2019). The experimental setup compromised from a vacuum pump, connecting tubes, rechargeable battery and the standard impinger biosampler. Fifteen mL of impingement medium, comprise of 13.38 g.L−1 of Dulbecco's Modified Eagle's Medium powder (DMEM), 1.50 g.L−1 Na HCO3, 2 g.L−1 bovine serum albumin with 100 μg.mL−1 streptomycin, 100 U.mL−1 penicillin and 970 mL distilled water was used for 3 h pumping. The collected samples immediately were transferred to a clinical virology laboratory for SARS-COV-2 detection. According to the protocol of Centers for Disease Control and Prevention (CDC) (May 13, CDC, 2020), the sterilization with autoclaves was applied for impinger biosampler and other parts of the experimental setups were disinfected using an ethanol 70% before each bioaerosols sampling in the hospital. The meteorological conditions including temperature (°C), and relative humidity (%) were recorded and monitored using a portable weather station (Kimo). The indoor CO2 concentration was also monitored using a portable Aeroqual series 500 (New Zealand). An aerosol spectrometer (Grimm Aerosol Technik GmbH, Germany) was used to monitor particulate matter in the indoor air samples at each sampling locations (Amarloei et al., 2020; Harbizadeh et al., 2019). The aerosol spectrometer was monitored at various size fractions between 0.25 and 32 μm during 3 min in each sampling location and we extracted the PM1, PM2.5, PM10 and inhalable particle concentrations.
2.2. SARS-CoV-2 virus detection in air samples using real time reverse transcription–polymerase chain reaction (real time RT-PCR) assay
After air sampling, the samples were transferred to the laboratory in an insulated box with cool packs (with 4 °C). The specific primer and probe real-time reverse transcriptase- polymerase chain reaction (RT-PCR) targeting ORF1ab and N genes (Nucleoprotein gene) were applied to detection of viral genomes of the SARS-CoV-2 virus in the air samples of the hospital indoor air samples. Before RT-PCR, the viral RNA was extracted from the air sample impingement medium, using a GeneAll Ribospin™ (GeneAll, Seoul, KOREA) and stored in −20 degrees C at the time of testing process. PCR amplification was carried out by Sansure Biotech Inc. kit (Changsha, China) according to manufacturer instruction. The results were considered negative when the cycle's threshold values reached higher than 40 cycles.
We added 30 μL Master Mix into each well, and then added 20 μL of the extracted viral RNA to the well containing reagent mix. PCR tubes were placed within the Mic Real-Time PCR System (Bio Molecular Systems, Australia). The RT-PCR test was completed in around 124 min and we checked the fluorescent curves to analyze results for S-shape amplification curves and CT values. Positive results with a CT value of less than 40 indicated the presence of SARS-CoV-2 RNA. The Cycle threshold (CT) values were around 38 and 35 for ORF1ab and nucleoprotein gene, respectively.
The LoD of the kit is 200 copies/mL. Controls Materials 2019-nCoV-PCR-Negative Control: A “no template” (negative) control was used to monitor whether there was any contamination for the rRT-PCR process in each detection run.
Controls Materials 2019-nCoV-PCR-Positive Control: A positive template control was used to monitor whether the rRT-PCR process worked properly in each detection run. An internal control in extraction step was applied by Wuhan CoV E-gene kit (TIB Molbiol, Berlin, Germany), to ensure the extraction and PCR amplification process.
2.3. Quality controls for air sampling and the virus detection
All equipment and materials used for this study were disposable, sterile and RNAse/ DNAse free.
As mentioned above, positive, negative, and internal controls were used as process quality control/or/assurance protocol. Routinely, before each sampling project, we collected samples from process ingredients and raw materials and a blank air sample using Impinger/Bubble in different places or under closed laminar flow cabinet after sterilization to make sure the procedures were not contaminated.
3. Results and discussion
The novel human coronavirus (COVID-19) has recently emerged and rapidly spread through all developed and developing countries and caused a pandemic with great health burden worldwide. In the present study, we examined the hospital indoor viral air quality to determine the potential of airborne transmission of SARS-CoV-2. In this regards, Table 1 and Table 2 provide precise information about environmental conditions of the hospital wards and the particulate matter concentrations with different aerodynamic diameters during the bioaerosols sampling, respectively. Table 3 demonstrates the characteristics of the air samplings (Bioaerosols) for SARS-CoV-2 detection in the indoor air of hospital wards. As depicted in Table 3, there were 183 patients confirmed with COVID-19 with different conditions from severe and critical to mild circumstances. Of the total 14 air samples taken from different points in the hospital wards, two air samples were positive in terms of SARS-CoV-2presence. Our results are opposed to the results of a study by Faridi et al. (2020), who did not report any positive samples at Imam Khomeini Hospital complex in Tehran. Additionally, our results are also in contrast with the recent published report by WHO (https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations) and Ong et al. (2020) who reported that airborne transmission is not the transmission route for SARS-CoV-2 to drive the pandemic. Ong et al. (2020) suggest that environment, due to the significant contamination caused by respiratory droplets and fecal shedding of patients with confirmed COVID-19, is the potential medium of transmission (Ong et al., 2020). In contrast to the above-mentioned reports, the results of the present research supported the findings of a recent preprint paper published by Santarpia et al. (2020). Santarpia et al. (2020) reported the presence of the viral RNA in the air samples of eleven samples (nine samples in the Nebraska Biocontaminant Unit and two samples in the National Quarantine Unit) isolation rooms and in the hallway spaces at University of Nebraska Medical Center. The mean concentration of 2.86 copies/L of air in air samples in the room's samples was positive; this in fact revealed the capability of aerosol transportation in the rooms. Finally, authors suggest that due to the low concentration of recovered virus in the samples, it is difficult to find the infectious virus in the samples (Santarpia et al., 2020). Their study suffered from a minor limitation: the distance of air samplers from the patient beds was not considered which could affect the interpretation of the reported results (Santarpia et al., 2020). In another experimental study, Van Doremalen et al. (2020) reported about the possibility of aerosol transmission of SARS-CoV-2 in their experiments using a laboratory nebulized (Van Doremalen et al., 2020). The authors applied a Collison nebulizer to generate aerosol and demonstrated that the viable SARS-CoV-2 virus could be capable in the aerosol for 3 h after nebulization (Van Doremalen et al., 2020). The Collison nebulizer is widely applied to generate fine aerosols from liquid a supply and the type and viscosity of nebulized fluid could affect the size distribution of generated aerosols (May, 1973). These results and the rapid spreading of the virus in the world reinforce that the possibility of air transmission of SARS-CoV-2 virus, as well. However, the main routes of preventing and limiting the exposure to COVID-19 recommended by WHO are hand washing and observing social distance (WHO, 2020a; Morawska and Cao, 2020). These measures could not prevent people infection through inhalation of small droplets and aerosols emission by a COVID-19 patients (in many cases they are asymptomatic) that can travel distance of meters or 10 of meters in the air and transmit the viable virus of SARS-CoV-2. On the other hand, the virus can be viable in the aerosols about 3 h (Morawska and Cao, 2020; Van Doremalen et al., 2020). Moreover, cough mechanism could generate large carrier droplets in the air and considering the size distribution of droplets, larger droplets with viral content deposited close to the emission point and in contrast smaller droplets can be transmitted several meters further in the indoor air (potential aerosol transmission) (Faridi et al., 2020; Morawska and Cao, 2020; Hadei et al., 2020). In another similar study, about aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak, Liu et al. (2020) confirmed that the resuspension process of the virus aerosol, which deposit on protective apparel or floor surface, could be a potential transmission route. Hence, application of efficient sanitization procedures is critical to minimize aerosol transmission of SARS-CoV-2. In another study, Gheysarzadeh et al. (2020) reported about five nurses infected with SARS-CoV-2 during patient care in Shahid Mustafa Khomeini Hospital which is a good agreement with the results of the present experiment.
Table 1.
The environmental conditions of the confirmed COVID-19 patient rooms in the hospital wards.
| Air sample no. | Hospital ward | Status of door and window | Temperature (°C) | Relative humidity (%) | CO2 concentration (ppm) | Ventilation system |
|---|---|---|---|---|---|---|
| 1 | ICU | 1 D/ 3 W | 24 | 45 | 362 | Mechanical |
| 2 | ICU | 1 D/ 3 W | 24 | 40 | 362 | Mechanical |
| 3 | ICU | 1 D/ 3 W | 25 | 45 | 390 | Mechanical |
| 4 | ICU | 1 D/ 3 W | 24 | 45 | 385 | Mechanical |
| 5 | ICU | 1 D/ 0 W | 24 | 45 | 372 | Mechanical |
| 6 | ICU 2 | 1 D/ 0 W | 25 | 50 | 377 | Not applicable |
| 7 | ICU entrance hall | 1 D/ 0 W | 26 | 40 | 357 | Air conditioner + natural |
| 8 | Hospital entrance hall | 1 D/ 0 W | 27 | 40 | 341 | Natural |
| 9 | Laboratory ward | 1 D/ 2 W | 25 | 45 | 358 | Natural |
| 10 | CT scan | 1 D/ 0 W | 25 | 45 | 375 | Natural |
| 11 | Radiology | 1 D/ 0 W | 25 | 45 | 383 | Natural |
| 12 | Men internal ward | 1 D/ 0 W | 26 | 50 | 377 | Natural |
| 13 | Woman internal ward | 1 D/ 0 W | 26 | 50 | 360 | Natural |
| 14 | Emergency ward | 1 D/ 0 W | 27 | 50 | 354 | Natural |
Not applicable: no hospitalized patients.
Table 2.
The particulate matter concentrations with different aerodynamic diameter during the air sampling in the hospital wards.
| Sample no. | Hospital ward | PM1 (μg/m3) | PM2.5 (μg/m3) | PM10 (μg/m3) | Inhalable particle (μg/m3) |
|---|---|---|---|---|---|
| 1 | ICU | 8.15 | 12.48 | 22.93 | 24.95 |
| 2 | ICU | 9.03 | 13.30 | 28.07 | 37.03 |
| 3 | ICU | 9.63 | 13.93 | 24.97 | 29.17 |
| 4 | ICU | 8.9 | 12.9 | 26.77 | 30.87 |
| 5 | ICU | 9.27 | 13.00 | 23.13 | 25.08 |
| 6 | ICU | 10.57 | 16.23 | 41.37 | 56.87 |
| 7 | ICU entrance hall | 11.93 | 17.40 | 37.17 | 43.07 |
| 8 | Hospital entrance hall | 12.33 | 18.23 | 37.8 | 48.7 |
Table 3.
location of air sampling points in Shahid Mustafa Khomeini hospital wards for detection of SARS-CoV-2.
| Air sample no. | Hospital ward | Number of health-care worker | No. of patients | Status of COVID-19 patients | Presence of SARS-CoV-2 in air sample |
|---|---|---|---|---|---|
| 1 | ICU | 6 people in the hospital in each shift | 10 | Severea, criticala | Positive |
| 2 | ICU | 6 people in the hospital in each shift | 10 | Severe, critical | Negative |
| 3 | ICU | 6 people in the hospital in each shift | 10 | Severe, critical | Positive |
| 4 | ICU | 6 people in the hospital in each shift | 10 | Severe, critical | Negative |
| 5 | ICU | 6 people in the hospital in each shift | 10 | Severe, critical | Negative |
| 6 | ICU 2 | Empty | 0 | Empty | Negative |
| 7 | ICU Entrance hall | Empty | Not applicabled | Empty | Negative |
| 8 | Hospital entrance hall | Not applicable | Not applicable | Not applicable | Negative |
| 9 | Laboratory ward | 2 people in the hospital in each shift | About 30 per day | Suspected, confirmed | Negative |
| 10 | CT scan | 1 people in the hospital in each shift | About 20 per day | Suspected, confirmed | Negative |
| 11 | Radiology | 1 people in the hospital in each shift | About 15 per day | Suspectedb, confirmed | Negative |
| 12 | Men internal ward | 6 people in the hospital in each shift | 25 | Confirmed and mildc | Negative |
| 13 | Woman internal ward | 6 people in the hospital in each shift | 25 | Confirmed and mild | Negative |
| 14 | Emergency ward | 6 people in the hospital in each shift | 18 | Suspected | Negative |
Not applicable: no hospitalized patients.
Patients under intubation.
No intubation and no oxygen mask.
Patients under oxygen mask.
Not applicable.
Thus, according to the results of above-mentioned studies, hospital indoor air could be a potential source for transmission of viable SARS-CoV-2 virus as we identified two positive cases out of fourteen air samples in the indoors of hospital air (Liu et al., 2020; Hadei et al., 2020; Morawska and Cao, 2020). Although, different routes with unknown mode of generation of airborne SARS-CoV-2 such as coughing, speaking, sneezing and normal breathing are involved in the carrier aerosols from a patient's respiratory systems (Faridi et al., 2020). We suggest that more research is required to determine the mode of aerosols generation that contain airborne SARS-CoV-2 from different routes of emissions (coughing, speaking, sneezing and normal breathing) from patients' respiratory systems. Besides, the results of this study are qualitative (based on the presence and absence of the virus in the air samples), therefore, the quantitative determination of number of viable and total viruses in the aerosol containing airborne SARS-CoV-2 as well as examination in all wards of hospital are extremely important. Finally, due to the potential of airborne transmission of virus in the indoor hospital air, it is very important to take high levels of personal protective equipment (PPE) (Using facemask) for health care workers in the hospitals.
4. Conclusion
To summarize, the rapid global spread of the COVID-19 and its increasing trend in infecting people indicate that there are several pathways to the virus transmission such as airborne pathway in addition to person-to-person transmission. Therefore, we examined the potential aerosol transmission of virus in indoor air of hospital wards with confirmed COVID-19 patients. Accordingly, we found two positive out of 14 air samples, which were taken from different wards of the hospital air. Due to the potential of airborne (aerosol) transmission of virus in the indoor air of hospital and based on national and international evidence, it is very important to take the highest levels of PPE precautions to ensure health security especially for healthcare personnel at hospitals.
CRediT authorship contribution statement
Azra Kenarkoohi: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Supervision, Funding acquisition. Zahra Noorimotlagh: Methodology, Validation, Resources, Writing - original draft, Writing - review & editing. Shahab Falahi: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition. Ali Amarloei: Conceptualization, Methodology, Validation, Formal analysis, Resources, Supervision, Funding acquisition. Seyyed Abbas Mirzaee: Conceptualization, Methodology, Validation, Resources, Writing - original draft, Writing - review & editing, Project administration. Iraj Pakzad: Methodology, Validation, Resources, Supervision. Elham Bastani: Methodology, Validation, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The financially support of the present work by Ilam University of Medical Sciences (grant No. 99123/10) is greatly appreciated. We would also like to thank health-care personnel of Shahid Mustafa Khomeini Hospital Complex, Ilam province, Iran.
References
- (CDC), C. F. D. C. A. P . Interim Recommendations for U.S. Households With Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases; 2020. Cleaning and disinfection for households. May 13. [Google Scholar]
- (WHO), W. H. O . 2020. Coronavirus Disease (COVID-19) Situation Report– 108, Data as Received by WHO From National Authorities by 10:00 CEST, 7 May 2020. [Google Scholar]
- (WHO), W. H. O . vol. 3. 2020. WHO Characterizes COVID-19 as a Pandemic. [Google Scholar]
- Amarloei A., Fazlzadeh M., Jafari A.J., Zarei A., Mazloomi S. Particulate matters and bioaerosols during Middle East dust storms events in Ilam, Iran. Microchem. J. 2020;152 [Google Scholar]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., Jandaghi N.Z.S., Sadeghniiat K., Nabizadeh R., Yunesian M. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysarzadeh A., Sadeghifard N., Safari M., Balavandi F., Falahi S., Kenarkoohi A., Tavan H. Report of five nurses infected with severe acute respiratory syndrome coronavirus 2 during patient care: case series. New Microbes and New Infections. 2020;36 doi: 10.1016/j.nmni.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I., Mcpherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., Fricchione M.J., Chugh R.K., Walblay K.A., Ahmed N.S., Stoecker W.C., Hasan N.F., Burdsall D.P., Reese H.E., Wallace M., Wang C., Moeller D., Korpics J., Novosad S.A., Benowitz I., Jacobs M.W., Dasari V.S., Patel M.T., Kauerauf J., Charles E.M., Ezike N.O., Chu V., Midgley C.M., Rolfes M.A., Gerber S.I., Lu X., Lindstrom S., Verani J.R., Layden J.E., Brister S., Goldesberry K., Hoferka S., Jovanov D., Nims D., Saathoff-Huber L., Hoskin Snelling C., Adil H., Ali R., Andreychak E., Bemis K., Frias M., Quartey-Kumapley P., Baskerville K., Murphy E., Murskyj E., Noffsinger Z., Vercillo J., Elliott A., Onwuta U.S., Burck D., Abedi G., Burke R.M., Fagan R., Farrar J., Fry A.M., Hall A.J., Haynes A., Hoff C., Kamili S., Killerby M.E., Kim L., Kujawski S.A., Kuhar D.T., Lynch B., Malapati L., Marlow M., Murray J.R., Rha B., Sakthivel S.K.K., Smith-Jeffcoat S.E., Soda E., Wang L., Whitaker B.L., Uyeki T.M. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadei M., Hopke P.K., Jonidi A., Shahsavani A. A letter about the airborne transmission of SARS-CoV-2 based on the current evidence. Aerosol Air Qual. Res. 2020;20:911–914. [Google Scholar]
- Harbizadeh A., Mirzaee S.A., Khosravi A.D., Shoushtari F.S., Goodarzi H., Alavi N., Ankali K.A., Rad H.D., Maleki H., Goudarzi G. Indoor and outdoor airborne bacterial air quality in day-care centers (DCCs) in greater Ahvaz, Iran. Atmos. Environ. 2019;216 [Google Scholar]
- Holshue M.L., Debolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10) doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 2020 doi: 10.1101/2020.03.08.982637. [DOI] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May K.R. The collison nebulizer: description, performance and application. J. Aerosol Sci. 1973;4:235–243. [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorimotlagh Z., Karami C., Mirzaee S.A., Kaffashian M., Mami S., Azizi M. Immune and bioinformatics identification of T cell and B cell epitopes in the protein structure of SARS-CoV-2: a systematic review. Int. Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxIV. 2020 doi: 10.1101/2020.03.23.20039446. [DOI] [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) International Journal of Surgery. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takian A., Raoofi A., Kazempour-Ardebili S. COVID-19 battle during the toughest sanctions against Iran. Lancet (London, England) 2020;395:1035. doi: 10.1016/S0140-6736(20)30668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]