Abstract
Coronavirus disease 2019 has markedly varied clinical presentations, with most patients being asymptomatic or having mild symptoms. However, severe acute respiratory disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is common and associated with mortality in patients who require hospitalization. The etiology of susceptibility to severe lung injury remains unclear. Angiotensin II, converted by angiotensin-converting enzyme (ACE) from angiotensin I and metabolized by ACE 2 (ACE2), plays a pivotal role in the pathogenesis of lung injury. ACE2 is identified as an essential receptor for SARS-CoV-2 to enter the cell. The binding of ACE2 and SARS-CoV-2 leads to the exhaustion and down-regulation of ACE2. The interaction and imbalance between ACE and ACE2 result in an unopposed angiotensin II. Considering that the ACE insertion (I)/deletion (D) gene polymorphism contributes to the ACE level variability in general population, in which mean ACE level in DD carriers is approximately twice that in II carriers, we propose a hypothesis of genetic predisposition to severe lung injury in patients with coronavirus disease 2019. It is plausible that the ACE inhibitors and ACE receptor blockers may have the potential to prevent and to treat the acute lung injury after SARS-CoV-2 infection, especially for those with the ACE genotype associated with high ACE level.
The coronavirus disease 2019 (COVID-19) pandemic has resulted in >14 million confirmed cases and 611,823 deaths worldwide as of July 20, 2020 (Worldometer, https://www.worldometers.info/coronavirus). Clinically, COVID-19 has markedly varied presentations, with most patients being asymptomatic or having mild symptoms.1 A small group of patients, however, develop severe acute respiratory syndrome, which is associated with high mortality.2 , 3 Demographic features and certain comorbidities, including male sex, age >65 years, African American race, hypertension, obesity, and diabetes, are associated with adverse outcome.1 , 3 However, many COVID-19 patients without those features also have developed severe lung injury or acute respiratory distress syndrome (ARDS).3 According to a report from New York, 14% hospitalized COVID-19 patients presented with a severe case requiring intensive care due to significant hypoxia.3 However, the susceptibility of developing severe lung injury is not fully understood.
SARS-CoV-2 and the RAS
Like severe acute respiratory syndrome–associated coronavirus (SARS-CoV), which caused an outbreak in 2002, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also uses angiotensin-converting enzyme (ACE) 2 as a binding receptor to enter the cell.4 , 5 The binding and the subsequent cell entry of SARS-CoV lead to exhaustion of ACE2 and the reduced expression of cellular ACE2.6, 7, 8 These observations are mirrored in SARS-CoV-2 infection and have renewed interest in studying the modulation of renin-angiotensin system (RAS) in COVID-19.6 Organ distribution of ACE2, binding of ACE2 with SARS-CoV and SARS-CoV-2, and subsequent modulation of RAS are discussed in detail in other reviews.9, 10, 11
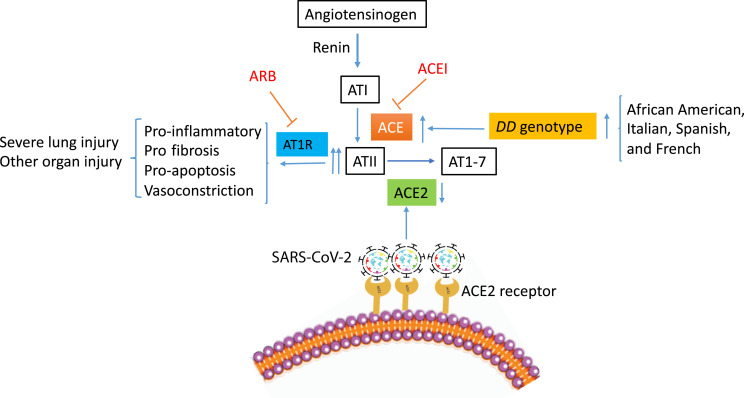
ACE and ACE2 are the two key modulators of RAS.12 ACE converts angiotensin I to angiotensin II (ATII) and degrades the bioactive bradykinin.13 ACE2 converts ATII to AT 1 to 7.14 The interaction between ACE and ACE2 appears to be reciprocal, with one down-regulated while the other is up-regulated15 as the balance between the two is critical in maintaining the physiological homeostasis of RAS. In conditions where tissue or plasma ACE activity is increased or ACE2 expression is decreased, such as in SARS-CoV-2 infection, ATII level becomes unopposed, which may contribute to acute lung injury through various mechanisms, including the following. It increases vascular permeability and causes vasoconstriction.14 Moreover, it induces the apoptosis of endothelial cells and of alveolar epithelial cells.16 , 17 In addition, it promotes fibrosis.18 Last, it boosts the proinflammatory mediators, including IL-6 and IL-8.19 The regulation of RAS is illustrated schematically in Figure 1 .
Figure 1.
Illustration of the impact severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has on the main metabolic pathways driven by angiotensin-converting enzyme (ACE) and ACE2 in the renin-angiotensin system, proposed mechanisms of ACE DD genotype in the severe lung injury of coronavirus disease 2019, and the potential impact of ACE DD genotype in the high-risk population. The potential therapeutic targets for ACE inhibitors (ACEIs) and angiotensin (AT) 1 receptor blockers (ARBs) are also shown.
Angiotensin II and Acute Lung Injury
Experimental and clinical studies support that the imbalance between ACE and ACE2 and subsequent increased ATII play a significant pathologic role in acute lung injury. In animal model with influenza, the reduction of ACE2 expression appears to be associated with severe lung injury.20 Similarly, binding of SARS-CoV to mouse ACE2 in vivo causes reduced ACE2 expression and greater acute lung injury.8 In a separate mouse model where the lung injury is induced by high-volume ventilation, there appears to be an increased lung injury related to the overproduction of lung ATII.21 In humans, serum ATII level is markedly elevated in patients with ARDS and sepsis,22 , 23 where the microvascular reoxygenation rate and plasma ATII level are inversely associated.23 In a small cohort of COVID-19 patients, plasma ATII levels are markedly elevated compared with healthy controls and are linearly correlated with viral load and the severity of lung injury,24 suggesting a systemic RAS imbalance as a result of ACE2 down-regulation from SARS-CoV-2 infection.
ACE Gene Polymorphism and Acute Lung Injury
ACE gene polymorphism is characterized by the insertion (I) or deletion (D) of a 287-bp Alu repeat sequence in intron 16 of the ACE gene. The insertion/deletion (I/D) polymorphism has been associated with circulating and tissue ACE levels and determines nearly half of the serum ACE level variability in general population,25 where the D allele is associated with higher ACE activity.26 Mean ACE activity levels in DD carriers were approximately twice that in II genotype individuals.25 Therefore, we propose a hypothesis that ACE gene polymorphism may play an important role in patients with COVID-19 who are susceptible to develop severe lung injury or ARDS.
There is an abundance of evidence supporting the relationship of ACE I/D polymorphism and clinical outcome of ARDS. In one study, the 28-day mortality rates are significantly different between the three ACE I/D genotypes (42%, 65%, and 75% for II, ID, and DD, respectively). Patients with the II genotype have a significantly better survival than those with the non-II genotypes.27 In another study, the DD genotype frequency is higher in patients with ARDS and is significantly associated with mortality.28 In a prospective study of ARDS, increased mortality of more than fivefold is found in patients with a homozygous DD genotype compared with the II genotype.29
The relation between ACE gene polymorphism and the disease severity has been investigated in SARS, which caused an outbreak in 2002 that affected >8000 individuals and resulted in 774 deaths worldwide (World Health Organization, https://www.who.int/csr/sars/country/table2004_04_21/en, last accessed July 20, 2020), with mixed results. The frequency of the ACE gene D allele is significantly higher in the hypoxemic group than in the nonhypoxemic group in a small study.30 However, a later study failed to show a significant association of ACE polymorphism with the pulmonary disease severity in the SARS patients.31 To date, there have been few published studies examining the relation of ACE gene polymorphism with acute lung injury of COVID-19. Nevertheless, it is plausible that the severity of acute lung injury of COVID-19 is influenced to some extent by the genotypes of ACE I/D polymorphism. Likely, genetic susceptibility of severe lung injury from SARS-CoV-2 infection is complex and mediated by multiple genes. A recent large genome-wide association study has reported a novel susceptibility locus associated with ABO blood group in COVID-19 patients with severe lung injury.32 Further exploration of genetic susceptibility of severe SARS-CoV-2 infection is warranted.
ACE Gene Polymorphism and the COVID-19 Pandemic in Various Populations
The racial difference of ACE gene polymorphism is well established. For example, in the United States, African Americans are known to have the highest frequency of the D allele (89%) when compared with Indians (69%) and whites (69%).33 In Europe, populations in Italy, Spain, and France have a high frequency of D allele up to 82% to 87%.34 In contrast, in Asia, the Eastern Asian populations, such as Chinese, Korean, Taiwanese, and Japanese, have a high frequency of ACE gene II allele, which is reportedly higher than the European populations (33% to 51% versus 13% to 27%).35 It is apparent that the racial variance of ACE I/D genotype seems to coincide with the differences of outcomes where the populations with high frequency of D alleles seem to experience higher fatality. For example, African Americans seem to have the disproportionately high fatality rate in the United States.36 , 37 Similarly, patients from Italy, Spain, and France also experience high fatality in Europe. Conversely, the low frequency of ACE D/D and high frequency of II genotype seen in Asian populations seem to be associated with relatively low fatality of COVID-19 in those nations (https://www.worldometers.info/coronavirus/#countries, last accessed August 19, 2020). Although socioeconomic and environmental conditions may play a role, they do not fully explain the severity of acute lung injury in COVID-19. A Scottish study concluded from the observation of influenza that socioeconomic factors do not fully explain ethnic variations in hospitalization for lower respiratory tract infections.38
Potential Benefit of ACE Inhibitors and ATII Receptor Blockers in COVID-19
Animal models have demonstrated promising evidence of an attenuated lung injury by the ACE inhibitors and/or ACE receptor blockers (ARBs) from SARS-CoV infection8 and from ventilation overdrive.8 , 21 Several retrospective studies have been published recently on the impact of ACE inhibitors and ARBs in patients with COVID-19. The use of ACE inhibitors and ARBs was associated with lower risk of all-cause mortality among hospitalized patients with COVID-19.39 Furthermore, the prior use of ACE inhibitors/ARBs was not associated with increased mortality or severe disease in patients with COVID-19.40 In addition, the use of ACE inhibitors in SARS-CoV-2 positive patients with hypertension is associated with nearly 40% lower risk of hospitalization.41 Although limited by the observational nature, these studies offer important support to the ongoing randomized controlled studies where new evidence will be emerging soon for the efficacy of ACE inhibitors and ARBs as treatment for COVID-19 (NCT04367883, NCT04366050, NCT04312009, and NCT04311177; https://clinicaltrials.gov). Although speculative, greater efficacy for those with DD genotype is anticipated because it is associated with higher ACE level than other genotypes should ACE gene polymorphism be tested.
Summary
ACE2 is a binding receptor for SARS-CoV-2 to enter the cell. The down-regulation of ACE2 as a result of SARS-CoV-2 infection is likely the important mechanism of acute lung injury or ARDS related to COVID-19 because of the resultant imbalance between ACE and ACE2 and the overproduction of ATII. The ACE gene polymorphism, which accounts for the differences of the ACE level in general population, may be responsible for the susceptibility to severe lung injury in COVID-19 patients. The absence of ACE D/D genotype in patients with COVID-19 may be protective against developing severe lung injury. Conversely, the presence of ACE D/D allele may be favorable for ACE inhibitors and ARB therapy. In our view, it is highly desirable to test ACE gene polymorphism for COVID-19 patients in the ongoing clinical trials with ACE inhibitors and ARBs. The association, if confirmed, may help to individualize the treatment and to optimize the outcome based on the genotype.
Footnotes
Disclosures: None declared.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A., COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 7.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler C., Cao Z., Wu Y., Faucon E., Mouhat S., Kovacic H., Sabatier J.M. Counter-regulatory “renin-angiotensin” system-based candidate drugs to treat COVID-19 diseases in SARS-CoV-2-infected patients. Infect Disord Drug Targets. 2020 doi: 10.2174/1871526520666200518073329. [Epub ahead of print]. doi: 10.2174/1871526520666200518073329. [DOI] [PubMed] [Google Scholar]
- 11.Magrone T., Magrone M., Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target: a perspective. Endocr Metab Immune Disord Drug Targets. 2020;20:807–811. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 12.Crowley S.D., Gurley S.B., Oliverio M.I., Pazmino A.K., Griffiths R., Flannery P.J., Spurney R.F., Kim H.S., Smithies O., Le T.H., Coffman T.M. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baudin B. New aspects on angiotensin-converting enzyme: from gene to disease. Clin Chem Lab Med. 2002;40:256–265. doi: 10.1515/CCLM.2002.042. [DOI] [PubMed] [Google Scholar]
- 14.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koka V., Huang X.R., Chung A.C., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimmeler S., Rippmann V., Weiland U., Haendeler J., Zeiher A.M. Angiotensin II induces apoptosis of human endothelial cells: protective effect of nitric oxide. Circ Res. 1997;81:970–976. doi: 10.1161/01.res.81.6.970. [DOI] [PubMed] [Google Scholar]
- 17.Wang R., Alam G., Zagariya A., Gidea C., Pinillos H., Lalude O., Choudhary G., Oezatalay D., Uhal B.D. Apoptosis of lung epithelial cells in response to TNF-alpha requires angiotensin II generation de novo. J Cell Physiol. 2000;185:253–259. doi: 10.1002/1097-4652(200011)185:2<253::AID-JCP10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Marshall R.P., Gohlke P., Chambers R.C., Howell D.C., Bottoms S.E., Unger T., McAnulty R.J., Laurent G.J. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L156–L164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y., Ruiz-Ortega M., Lorenzo O., Ruperez M., Esteban V., Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 20.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerng J.S., Hsu Y.C., Wu H.D., Pan H.Z., Wang H.C., Shun C.T., Yu C.J., Yang P.C. Role of the renin-angiotensin system in ventilator-induced lung injury: an in vivo study in a rat model. Thorax. 2007;62:527–535. doi: 10.1136/thx.2006.061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenz M., Hoffmann B., Bohlender J., Kaczmarczyk G. Angiotensin II formation and endothelin clearance in ARDS patients in supine and prone positions. Intensive Care Med. 2000;26:292–298. doi: 10.1007/s001340051152. [DOI] [PubMed] [Google Scholar]
- 23.Doerschug K.C., Delsing A.S., Schmidt G.A., Ashare A. Renin-angiotensin system activation correlates with microvascular dysfunction in a prospective cohort study of clinical sepsis. Crit Care. 2010;14:R24. doi: 10.1186/cc8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiret L., Rigat B., Visvikis S., Breda C., Corvol P., Cambien F., Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51:197–205. [PMC free article] [PubMed] [Google Scholar]
- 27.Jerng J.S., Yu C.J., Wang H.C., Chen K.Y., Cheng S.L., Yang P.C. Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome. Crit Care Med. 2006;34:1001–1006. doi: 10.1097/01.CCM.0000206107.92476.39. [DOI] [PubMed] [Google Scholar]
- 28.Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J., Humphries S.E., Hill M.R., Laurent G.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 29.Adamzik M., Frey U., Sixt S., Knemeyer L., Beiderlinden M., Peters J., Siffert W. ACE I/D but not AGT (-6)A/G polymorphism is a risk factor for mortality in ARDS. Eur Respir J. 2007;29:482–488. doi: 10.1183/09031936.00046106. [DOI] [PubMed] [Google Scholar]
- 30.Itoyama S., Keicho N., Quy T., Phi N.C., Long H.T., Ha L.D., Ban V.V., Ohashi J., Hijikata M., Matsushita I., Kawana A., Yanai H., Kirikae T., Kuratsuji T., Sasazuki T. ACE1 polymorphism and progression of SARS. Biochem Biophys Res Commun. 2004;323:1124–1129. doi: 10.1016/j.bbrc.2004.08.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan K.C., Tang N.L., Hui D.S., Chung G.T., Wu A.K., Chim S.S., Chiu R.W., Lee N., Choi K.W., Sung Y.M., Chan P.K., Tong Y.K., Lai S.T., Yu W.C., Tsang O., Lo Y.M. Absence of association between angiotensin converting enzyme polymorphism and development of adult respiratory distress syndrome in patients with severe acute respiratory syndrome: a case control study. BMC Infect Dis. 2005;5:26. doi: 10.1186/1471-2334-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., et al. Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. [Epub ahead of print]. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew J., Basheeruddin K., Prabhakar S. Differences in frequency of the deletion polymorphism of the angiotensin-converting enzyme gene in different ethnic groups. Angiology. 2001;52:375–379. doi: 10.1177/000331970105200602. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y.J., Tsai J.C. ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care. 2002;25:1002–1008. doi: 10.2337/diacare.25.6.1002. [DOI] [PubMed] [Google Scholar]
- 35.Saab Y.B., Gard P.R., Overall A.D. The geographic distribution of the ACE II genotype: a novel finding. Genet Res. 2007;89:259–267. doi: 10.1017/S0016672307009019. [DOI] [PubMed] [Google Scholar]
- 36.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 37.Dyer O. Covid-19: black people and other minorities are hardest hit in US. BMJ. 2020;369:m1483. doi: 10.1136/bmj.m1483. [DOI] [PubMed] [Google Scholar]
- 38.Simpson C.R., Steiner M.F., Cezard G., Bansal N., Fischbacher C., Douglas A., Bhopal R., Sheikh A., researchers S. Ethnic variations in morbidity and mortality from lower respiratory tract infections: a retrospective cohort study. J R Soc Med. 2015;108:406–417. doi: 10.1177/0141076815588321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fosbol E.L., Butt J.H., Ostergaard L., Andersson C., Selmer C., Kragholm K., Schou M., Phelps M., Gislason G.H., Gerds T.A., Torp-Pedersen C., Kober L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324:168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khera R., Clark C., Lu Y., Guo Y., Ren S., Truax B., Spatz E.S., Murugiah K., Lin Z., Omer S.B., Vojta D., Krumholz H.M. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with Coronavirus disease-19. medRxiv. 2020 doi: 10.1161/JAHA.120.018086. [Epub] doi: 10.1101/2020.05.17.20104943. [DOI] [PMC free article] [PubMed] [Google Scholar]