Abstract
A new cell line derived from dorsal fin of rabbit fish Siganus fuscescens was developed and characterized. The cell line was isolated from the dorsal fin, named as rabbit fish fin (RFF) cell line, and which was sub-cultured for 50 cycles since the development. This cell line was tested for growth in different temperatures and serum concentrations, and the best growing condition was at 20% serum at 28 °C. In cultured RFF cells, amplification of 18S rRNA from genomic DNA and immunostaining of cellular cytokeratin confirmed the proper identity of S. fuscescens fish. After 30th passage of cultures, the cells were exposed to challenge of inflammation, triggered by LPS, and hypoxia, mimicked by CoCl2. Cultured RFF cells showed robust sensitive responses to inflammation and hypoxia in directing the expressions of cytokines and hypoxia inducible factor-1α (HIF-1α). The water extract of aerial part of Scutellaria baicalensis (SBA) has been shown in rabbit fish to prevent inflammation. Here, we extended this notion of testing the efficacy of SBA extract in the developed cultured RFF cells. Application of SBA extract inhibited the expression of LPS-induced inflammatory cytokines, i.e. IL-1β, IL-6, as well as the signaling of NF-κB. The application of CoCl2 in cultured RFF cells triggered the hypoxia-induced cell death and up regulation of HIF-1α. As expected, applied SBA extract in the cultures prevented the hypoxia-induced signaling. Our results show the established RFF cell line may be served as an ideal in vitro model in drug screening relating to inflammation and hypoxia. Additionally, we are supporting the usage of SBA herbal extract in fish aquaculture, which possesses efficacy against inflammation and hypoxia.
Keywords: Cell line, Siganus fuscescens, Anti-inflammation, Anti-hypoxia, Traditional Chinese medicine
Highlights
-
•
A new cell line divided from Siganus fuscescens was established and named as RFF cell.
-
•
The RFF cell exhibited sensitive responses to LPS-induced inflammation and hypoxia environment.
-
•
The aerial part of S. baicalensis extract showed anti-inflammatory and anti-hypoxia effects on RFF cell.
1. Introduction
Siganus fuscescens, known as grey rabbit fish, is wildly cultured and consumed in China, Australia and Indonesia [1,2]. However, this fish is very fragile when facing high temperature and hypoxia condition [3]. Additionally, diseases caused by pathogenic microbes and inflammation are leading to the death of S. fuscescens. These problems of aquaculture usually cause large number of fish's death and economic lost to fishmen. The marine environment of aquaculture farm is very complex and hard to control. The in vivo experiment in fish farm not only time costing but also expensive. Fish cell line is an important model system to study fish biology, e.g. probing the efficacy of targeted drug or feeding [[4], [5], [6], [7]].
Fish skin is the first barrier interacting with outer environment, and therefore which is considered as the biggest immune organ [8]. The skin cell is defending the pathogenic challenge by producing mucus and anti-microbial peptides [9]. In addition, fin cells are highly sensitive to low oxygen, and therefore which is a sensor for survival [10]. Cell line from S. fuscescens fish has not been established, which hinders the drug screening procedure for this fish species. In accord to the need, a cell line deriving from fin of S. fuscescens fish was established and characterized here: the responses of this cultured cells to inflammation and hypoxia were determined.
Roots of Scutellaria baicalensis Georgi. (Scutellariae Radix), a traditional Chinese medicine (TCM), has long history of usage as herbal medicine to treat various types of diseases relating to inflammation. Scutellariae Radix has been reported to possess pharmacological activities, including anti-virus, anti-microbial and anti-inflammation [11]. Chemical and pharmacological analyses have suggested that the flavonoids, i.e. baicalein, baicalin, scutellarin and wogonin, are the major active ingredients responsible for anti-microbial functions [12]. Having identification of active ingredients, we have revealed the aerial parts of S. baicalensis (SBA) contained reasonable amounts of these active flavonoids [13]; however, this aerial part was being disposed during the production of medicinal herbs. To encourage the recycle of wasted materials deriving from S. baicalensis, the SBA extract has been proposed as feeding for fish aquaculture. The intake of SBA by S. fuscescens fishes greatly improved the fish survival, as well as its inflammatory response to microbial [13]. Because of the character of low toxicity and low cost, many TCM with anti-inflammation and anti-hypoxia effects have already been applied in aquaculture feeding [14].
Having the established cell line from rabbit fish fin, named as RFF cell line, we therefore determined the efficacy of SBA in against inflammation and hypoxia. Besides, the signaling, induced by SBA, in NF-κB translocation during inflammation and hypoxia inducible factor-1α (HIF-1α) expression under hypoxia were illustrated here.
2. Materials and methods
2.1. Culture of fin cells
The isolation of fin cells was followed by reported protocol with minor modification. Two healthy S. fuscescens fishes (approximately 15 g in weight) were collected from an aquaculture farm (Shenzhen, China): the fishes were maintained in an aquarium equipped with seawater recirculation system. The fishes were anesthetized with 2-penoxyethanol (1:10,000) and then washed with diluted bleach (1:100), wiped with 70% ethanol, to remove surface contamination. The fishes were decapitated, and the fins were subsequently removed and placed in HBSS medium with antibiotics (penicillin, 100 U/mL; streptomycin, 100 μg/mL; amphotericin-B 0.01 μg/mL) (Thermo Fisher Scientific, Waltham, MA) for washing. Then, the fin fragments were minced into small pieces (approximately 2 mm2) using surgical scissors. Tissue pieces were put into DMEM (FBS free) (Thermo Fisher Scientific) with antibiotics, then 5 mL collagenase A (0.4 mg/mL; Sigma-Aldrich, St Louis, MO) was added. The tissue mixture was kept in an incubator with orbital shaking at 28 °C at speed of 90 rpm for 60 min. The solution was centrifuged and washed with DMEM to remove collagenase. Then, 5 mL of 0.25% trypsin-EDTA solution were added and maintained for 20 min at 37 °C. Three mL FBS (Thermo Fisher Scientific) was added for trypsin neutralization and centrifuged at 200 X g for 10 min. The cell pellet was resuspended in DMEM and filtered with 180 μm-nylon to remove undigested tissues. Then, centrifuged at 200 X g for 10 min. Cell pellets were suspended and seeded into 25-cm2 flasks and cultured in 3 mL of DMEM (supplemented with 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, amphotericin-B 0.01 μg/mL; pH 7.4) in an incubator at 28 °C with 5% CO2. Afterwards, the medium was replaced with fresh medium by half every 2–3 days. The subculture was carried out at 1:2 split subsequently by trypsinization when primary cell cultures grew to 90–100% of confluence. The culture was designated RFF cell line.
2.2. DNA extraction and PCR analysis
Total genomic DNA were extracted from cultured RFF cells (at passage 30) or fin tissues of S. fuscescens using microElute Genomic DNA kit (Omega Bio-tek, Norcross, GA). The quantitation and quality of isolated DNA was tested by NanoDrop™ 2000 spectrophotometers (Thermo Fisher Scientific). Fragments of S. fuscescens 18S ribosomal RNA (~700 bp) were amplified using primers 3′-CCA TTG GTT CAT TCG GAG TA-5′ and 3′ TTG GAT GGT TTA GTG AGG TC-5’. The PCR products were sequenced, and the obtained sequences were aligned against known S. fuscescens 18S rRNA sequences from NCBI database (GenBank Accession No. AB276986.1).
2.3. Growth curves
The effects of FBS concentration and culture temperature on cell growth were investigated as previously described [15] with modification. RFF cells at a density of 3 × 103 cells per well were seeded into 96-well plates and incubated at different temperatures (28 °C and 37 °C) in DMEM containing different FBS (10–30%). After cultured for 24 h, the non-adherent cells were removed by PBS washing. RFF cells were cultured over 10 days, and the medium was changed every 2 days. Every day, viability assay was applied onto the cells, and the value of OD570nm was used to measure the relative cell number.
2.4. Cell viability assay
MTT assay was employed for revealing cell viability. In brief, cells were seeded in 96-well plate. After the treatment for 1 day, the final concentration of 0.5 mg/mL of MTT (Thermo Fisher Scientific) solution was applied, the production of purple crystal was dissolved in DMSO. The optimized absorbance was set at 570 nm.
2.5. DNA construction and transfection
The vector, pGL4.32 [luc2P/NF-κBRE/Hygro], contains five copies of a NF-κB response element (NF-κB, 5′-GGG AAT TTC CG-3′) that drives transcription of the luciferase reporter geneluc2P (Promega Corporation, Madison, WI), namely as pNF-κB-Luc. The vector, pGL3-basic, contains an interleukin-6 (IL-6) promoter sequence from seabream that drives transcription of the firefly luciferase sequence. The vector pBI-GL (BD Biosciences Clontech, San Jose, CA) contains six hypoxia responsive elements (HRE: 5′- GTG ACT ACG TGC TGC CTA G-3′) having a downstream reporter of firefly luciferase gene; this vector was named as pHRE-Luc. RFF cells (at passage 30) were seeded onto a 24-well cell culture plate at a density of 1 × 105 cells per well. After being cultured for 24 h at 28 °C, RFF cells were transfected with plasmid pNF-κB-Luc or pIL-6-Luc or pHRE-Luc by Lipofectamine™ 3000 transfection kit (Invitrogen, Carlsbad, CA). Briefly, 1 μL of P 3000™ reagent and 0.5 μg plasmid were diluted by 25 μL of Opti-MEM™ medium. Then, the diluted plasmid was added to a tube containing diluted Lipofectamine™ 3000 reagent (0.75 μL in 25 μL of Opti-MEM™ medium) and mixed well. After incubation for 10–15 min at room temperature, 50 μL mixture was added to each well. The cells were treated at 28 °C for 6 h, then the transfecting regent was removed. The cells were pre-treated with various concentrations of drugs for 4 h, then challenged with an inflammatory response by lipopolysaccharide (LPS), or with hypoxia response by applying cobalt (II) chloride (CoCl2), for 24 h. The medium was aspirated, and the cultures were washed by PBS, twice. The cells were lysed by a buffer containing 0.2% Triton X-100, 1 mM dithiothreitol (DTT) and 100 mM potassium phosphate buffer (pH 7.8) at 4 °C. Followed by centrifugation at 13,200 rpm at 10 min, the supernatant was collected and used to perform luciferase assay (Tropix, Bedford, MA). The luminescent reaction was quantified in a Tropix TR717TM Microplate Luminometer, and the activity was expressed as absorbance (up to 595 nm) per mg of protein.
2.6. Quantitative real-time PCR
In order to reveal the inflammation and hypoxia response in cultured RFF cells, the expression levels of inflammation cytokines, IL-1β, IL-6, TNF-α, induced by LPS, and HIF -1α, induced by CoCl2, were measured by real-time PCR. Total RNA from cultured cells was isolated by RNAzol reagent (Molecular Research Center, Cincinnati, OH) and then reversed transcribed into cDNAs by using MMLV (Moloney Murine Leukemia Virus) reverse transcriptase according to the manufacturer's instructions (Invitrogen). Real-time PCR was employed here by using FastStart Universal SYBR Green Master (ROX) according to the manufacturer's instructions (Roche Applied Science, Mannheim, Germany). The primers were as followed: 5′-GTG GTA TTC TCC ATG AGC TT-3’ (sense primer, S) and 5′-TTT CAT CAC ACA GGA CAG GTA-3’ (antisense primer, AS) for rabbit fish HIF-1α. For fish inflammatory cytokines the primers were as followed: 5′-ACC ATC TGG CTG CGG GAA C-3’ (S) and 5′-GAA TGA GTC GTG TGG TCT GGA AG-3’ (AS) for fish IL-6; 5′-AGC CAA TCT GGC AAG GAT CA-3’ (S) and 5′-GAT GAT GAA CCA GTT GTT GT-3’ (AS) for fish IL-1 β; 5′-CTT CAC GCT CAA CAA GTC TCA G-3’ (S) and 5′-AAA GCC TGG TCC TGG TTC ACT C-3’ (AS) for fish TNF-α; β-actin was used as an internal control, and its primer sequences were 5′-TTA TGA AGG CTA TGC CCT GCC-3’ (S) and 5′-TGA AGG AGT AGC CAC GCT CTG T-3′ (AS). SYBR green signal was revealed by ABI 7500 Fast Real‐Time PCR system (Applied Biosystems, Foster City, CA). Transcript levels were quantified by using ΔCt value method, where the values of target genes were normalized by β-actin in the same sample for comparison. PCR products were analyzed by gel electrophoresis and melting curve analysis, as to confirm the specific amplification.
2.7. Herbal material preparation
The raw material of SBA (the aerial part of S. baicalensis) was obtained from Hebei Province in 2017, which was authenticated by Dr. Tina Dong, one of the authors. The voucher specimen (SBA#2018-10-20) was deposited at Center for Chinese Medicine at HKUST. The raw material of SBA was smashed into powder then weighed and sonicated with water for 30 min, twice: the volume was 15 times and 10 times, respectively. The extract was frozen dry by Labconco Free Zone Freeze Dry System. The dried extract was dissolved in DMSO to get final concentration of 100 mg/mL. A Waters 2695 series system (Waters, Milford, CT), equipped with a degasser, a binary pump, an auto-sampler, and a thermo-stated column compartment was employed for HPLC analysis of SBA water extract. A Grace prevail select C18 column (particle size 5 μm, 4.60 mm × 250 mm) was used. Acetonitrile (as Solvent A) and 0.1% formic acid (as Solvent B) were utilized as mobile phase, at 1.0 mL/min at room temperature. Gradient elution was as: 0–25 min, 15–21% of solvent A; 25–40 min, 21–28% solvent A; 40–50 min, 28–30% of solvent A; 50–60 min, 30–35% solvent A; 60–65 min, 35-15% of solvent A. A DAD detector at an absorbance of 276 nm was used.
2.8. Immunofluorescent staining
Cells were grown on glass coverslip per-coated by lysine for 24 h. After PBS wash, cells were fixed with 4% paraformaldehyde (PFA) for 15 min. Cells were incubated with 0.2% Triton X-100 in PBS for 10 min and then blocked by 5% BSA for 1 h. Cultures were separately stained with anti-cytokeratin antibody (ab94894, Abcam, Cambridge, UK) or anti-NF-κB p65 antibody (sc-7151, Santa Cruz Biotechnology, Dallas, TX) at 1:100 at 4 °C overnight, followed with the FITC-conjugated anti-rabbit antibodies (AP037F, Sigma-Aldrich). Samples were mounted with ProLong™ Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). Samples were then examined by Leica SP8 Confocal Microscope. The fluorescent intensity was then quantitated by selecting the nuclear and cytoplasmic regions in different fields using the LAX S imaging software.
2.9. Statistical analysis and other assays
Protein concentration was measured by a kit from Bio-Rad (Hercules, CA). Each result was presented as the mean ± SD, calculated from 3 to 5 independent samples, with triplicated. Comparisons of the mean for untreated control cells and treated cells were analyzed using one-way analysis of variance (ANOVA) and Student's t-test. Significant values were represented as *, p < 0.05, **, p < 0.01.
3. Result
3.1. Characterization of established RFF cell line
The cell line RFF was derived from dorsal fin tissue of healthy S. fuscescens (rabbit fish). The culture reached full confluence in 3 days at 28 °C. The cells were sub-cultured at a split ratio of 1 : 2 for every 1–2 days. Fibroblast-like cells and epithelial-like cells were recognized in the first few passages of cultured RFF cells, and thereafter keratinocyte-like cells became predominant, starting from 5 cycles of passage (Fig. 1 A). A strong immunostaining signal in the cells, incubated with anti-cytokeratin antibody, was identified (Fig. 1 B). The RFF cells could be sub-cultured over 50 times without much change in cell morphology and identity. To confirm the origin of RFF cells, a ~700-bp PCR product flanking 18S rRNA was amplified from cultured RFF cells or dorsal fin of S. fuscescens (Supplement Fig. 1). DNA sequencing and comparative analysis showed that the partial 18S rRNA sequence. The sequence amplified from genomic DNA isolated from cultured RFF cells showed ~99% identity with S. fuscescens tissue, and both had ~99% identity to known S. fuscescens 18S rRNA sequence from NCBI database (GenBank Accession No. AB276986.1). Thus, the identity of derived RFF cells was confirmed here.
Fig. 1.
Morphology of cultured RFF cells isolated from the fin of rabbit. (A): Morphology of RFF cells after HE stained at passage 30 after culturing. (B): RFF cells were fixed with 4% PFA for 15 min and stained with anti-cytokeratin antibody with 0.2% Triton X-100. Confocal image showed cytokeratin expression in RFF cells. Nucleus was stained with DAPI.
RFF cells at 30th passage was analyzed for cell growth. RFF cells could only grow under the temperature of 28 °C (Fig. 2 ). RFF cells did not survive under 37 °C and ~50% of cells were dead after 24 h of culture. The growth rate of RFF cells was different with various concentrations of FBS (from 10 to 30%) in DMEM at 28 °C, showing maximum growth rate at 20% FBS (Fig. 2A). The growth was at peak after 6 days of culture. Serum at 10% or 30% in the medium could not support the proper growth of RFF cells. Thus, 20% FBS in medium was employed for culture at 28 °C, and which should be used with 6 days of culture.
Fig. 2.
Growth curves of RFF cells at different temperatures and different serum concentrations. RFF cells were cultured under 28 °C or 37 °C in Dulbecco's modified Eagle's medium (DMEM) supplemented with different fetal bovine serum (FBS) concentration (10%, 20%, 30%). MTT assay was applied on these cells every day. The cell number was calculated by MTT assay. Data are shown in percentage of change compared to control group (day 0 of culture), and in Mean ± SD, where n = 4, each with triplicate samples.
3.2. The effect of SBA extract to relieve inflammation
In order to test inflammatory response of cultured RFF cells, LPS was applied here to induce inflammation. Application of LPS (1000 ng/mL) in cultures induced the mRNA expression of pro-inflammatory cytokines, i.e. IL-1β, IL-6, TNF-α, to 2–4 folds (Fig. 3 A): this induction was in a dose-dependent manner. To demonstrate the stimulation of transcriptional rate of NF-κB and IL-6, the luciferase reporter plasmids, i.e. pNF-κB-Luc and pIL-6-Luc, were employed here for cell transfection. In transfected RFF cells, the luciferase activities, driven by pNF-κB-Luc and pIL-6-Luc, were increased ~25 folds and 2.5 folds, respectively, when challenged with LPS (Fig. 3B). In line to mRNA expression, this induction was in a dose-dependent manner.
Fig. 3.
The inflammation response of RFF cells. (A): Expression levels of pro-inflammation cytokines IL1-β, IL-6, TNF-α in cultured RFF cells were measured after treating with different concentrations of LPS for 24 h. Total mRNAs were extracted from RFF cells to perform real-time PCR analysis. (B): Two luciferase reporter plasmids contain NF-κB and IL-6 promoter sequences, named pNF-κB-Luc and pIL-6-Luc, were transfected in cultured RFF. The transfection efficiency was 50–60%, as indicated by vector containing RFP fluorescence protein. The transfected cells were treated with LPS for 24 h. Values are expressed as fold of increase to basal reading (as 1, no LPS added), and in Mean ± SD, where n = 4. Statistical comparison was made with the basal group; *p < 0.05; **p < 0.01.
The developed cell could be used for drug screening. The water extract of aerial part of S. baicalensis (SBA) was proposed as a feeding for rabbit fishes for anti-inflammation [13]. A HPLC chromatogram of the extract of SBA was shown (Supplement Fig. 2A). Besides, the amounts of scutellarin and baicalin were 1.083% and 0.123%, respectively. The aforesaid requirements guaranteed the repeatability of assays thereafter, as quality control parameters. In cultured RFF cells, different concentrations of SBA extract were applied to test the cytotoxicity. Up to 0.5 mg/mL of SBA extract, the cultured REF cells did not show significant cell death (Supplement Fig. 2B). Here, SBA extract was applied for 4 h before the challenged of LPS. The pre-treatment with SBA extract in the cultures, dose-dependently, suppressed the LPS-induced expressions of mRNAs encoding IL-1β, IL-6 and TNF-α (Fig. 4 A). Under treatment of 0.05–0.1 mg/mL of SBA extract, the suppression of cytokine expression by cultured RFF cells was almost reaching maximum. Dexamethasone served as a positive control suppressing the LPS-induced mRNA level to completion (Fig. 4A). The maximal suppression triggered by SBA was as good as that of dexamethasone.
Fig. 4.
SBA water extract suppresses pro-inflammatory responses in cultured RFF cells. RFF cells were separately transfected with pNF-κB-Luc and pIL-6-Luc plasmids, then the cells were per-treated by SBA and dexamethasone (DEX, 40 μM, positive control) for 4 h. LPS (1000 ng/mL) was included to trigger inflammatory response for 24 h. (A) The cell lysates were subjected for mRNA measurements of pro-inflammation cytokines, i.e. IL1-β, IL-6, TNF-α. (B) The pNF-κB-Luc-driven and (C) pIL-6-Luc-driven luciferase assay were assayed. Values are expressed as fold of increase to basal reading (as 1, no LPS added), and in Mean ± SD, where n = 3. Statistical comparison was made with the basal group; *p < 0.05; **p < 0.01.
To further demonstrate potential NF-κB and IL-6 suppression mediating by SBA extract, the transfected RFF cells with pNF-κB-Luc or pIL-6-Luc were used here. In transfected RFF cells, the activities of pNF-κB-Luc and pIL-6-Luc were robustly induced by LPS, and which were suppressed markedly by pre-treatment of SBA extract (Fig. 4 B&C). A maximal suppression was revealed at 0.1–0.2 mg/mL of SBA extract. Again, dexamethasone suppressed the LPS induction, robustly. The translocation of p65 subunit of NF-κB in LPS-induced RFF cells was probed here. As expected, application of LPS in cultures induced the nuclear translocation of NF-kB p65 subunit (Fig. 5 A). Around 45% of NF-κB p65 subunit was translocated into nuclear (Fig. 5B). The translocation of NF-κB p65 subunit was inhibited by pre-treatment of SBA extract in a dose-dependent manner. With the application of SBA extract, only about 20%–25% of NF-κB p65 subunit was translocated into nuclear. As a control, dexamethasone suppressed the translocation completely. The translocation rate was about 20% (Fig. 5A&B). These results strongly suggested that SBA extract could relieve inflammation damage by preventing the signaling of NF-κB p65.
Fig. 5.
SBA water extract suppresses the translocation of NF-κB p65 in RFF cells. (A): NF-κB p65 translocation in cultured RFF cells. RFF cell were cultured and pre-treated with different dosage of SBA extract (0.1 and 0.4 mg/mL) and dexamethasone (DEX, 40 μM, positive control) for 4 h, then LPS (1000 ng/mL) was included to trigger inflammatory response for 1 h. Cells were fixed with 4% PFA for 15 min and translocation of NF-κB p65 was determined using a NF-κB p65 antibody with 0.2% Triton X-100, followed with the Alexa Fluor 488 conjugated antibody. Nucleus were stained with DAPI. * indicates the enlarged cells. Representative photo is shown, n = 4. Bar = 25 μm and 10 μm (insert). (B): The translocation of NF-κB were measured by Leica SP8 software (LAX S). Fluorescence intensity (Alexa 488) were counted. Values are expressed as the percentage of nuclear/cytoplasmic NF-κB p65, and in Mean ± SD, where n = 5. Statistical comparison was made by two tails t-test; *p < 0.05; **p < 0.01 vs LPS group, #p < 0.05; #p < 0.01 vs basal group.
3.3. The effect of SBA extract to relieve hypoxia
Hypoxia and high temperature are well-known two lethal causes of fish farming. To mimic hypoxia condition in cultured REF cells, CoCl2 could be used. Increase amount of applied CoCl2 caused the cell to die in a dose-dependent manner (Fig. 6 A). CoCl2 at 100 mM caused ~50% cell death in cultured REF cells. The pre-treatment of SBA extract in cultures prevented the CoCl2-induced cell death in a dose-dependent manner (Fig. 6B). Starting 0.3 mg/mL of SBA extract, the cell protection was fully achieved. Another lethal factor, temperature, was also tested here. At 37 °C, cultured REF cells were dead in accord to time by hours (Fig. 6C). Having 30% of the temperature-induced cell death, application of SBA extract protected the cell in dose-dependent manner (Fig. 6D). At low dose of SBA (0.1 mg/mL), the cell survival could be restored to over 80%.
Fig. 6.
The protection effect of SBA extract on cultured RFF cells. RFF cells were seeding in 96-well and cultured under 28 °C overnight. (A): Different concentrations of CoCl2 was added into cultures to mimic hypoxia condition for 24 h. MTT assay was performed. (B): Different concentrations of SBA was applied in CoCl2 (100 μM)-treated cultures for 24 h. MTT assay was performed. (C): RFF cells were cultured under 28 °C and 37 °C for different time. MTT assay was performed. (D): Different concentration of SBA was added into the cultures for 24 h at 37 °C. MTT assay was performed. Values are expressed in percentage of change compared to control group (no CoCl2, or no SBA, or zero time) in Mean ± SD, where n = 4, each with triplicate samples. Statistical comparison was made with the control group; *p < 0.05; **p < 0.01.
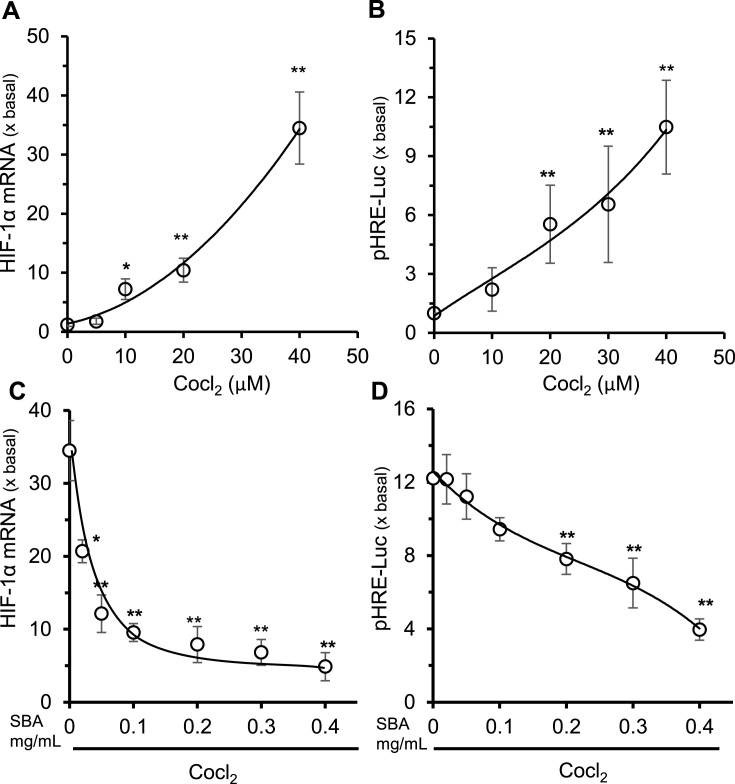
To cope with hypoxia, HIF is the critical one, and which is requiring a binding between an inducible HIFα subunit (HIF-1α, HIF-2α and HIF-3α) and a constitutive HIFβ subunit (HIF-1β, HIF-2β and HIF-3β) [16]. During hypoxia, the activated HIF complex binds to hypoxia response element (HRE) domain, located in the enhancer region of the EPO gene, and therefore the complex promotes the transcription of EPO, as a result to increase the production of red blood cell, as well as oxygen uptake [17]. In RFF cultures, CoCl2 was able to robustly induce the expression of mRNA encoding HIF-1α in a dose-dependent manner (Fig. 7 A). In parallel to mRNA expression, the promoter activity of pHRE-Luc was also induced by CoCl2 in the transfected REF cells (Fig. 7B). In the SBA pre-treated cells, the CoCl2-induced HIF-1α expression was markedly suppressed: the maximal suppression was revealed at ~0.2 mg/mL of SBA extract (Fig. 7C). This suppression was in line by using pHRE-Luc construct in the transfected cells (Fig. 7D), suggesting the anti-hypoxia function of SBA in rabbit fish.
Fig. 7.
SBA water extract suppresses the hypoxia responses. (A): Expression level of hypoxia related protein HIF-1α. Cultured RFF cells were treated by different concentrations of CoCl2 for 24 h. Total mRNAs were extracted from RFF cells to perform real-time PCR analysis. (B): A luciferase reporter plasmid contains HRE promoter sequence, named pHRE-Luc, was transfected into cultured REF cells: the cells were induced to hypoxia by different concentrations of CoCl2 for 24 h. The pHRE-Luc-driven luciferase activity was measured. (C&D): RFF cells were transfected with pHRE-Luc plasmid then per-treated with different concentrations of SBA extract for 4 h before application of CoCl2 (40 μM) for 24 h. The lysates were subjected for mRNA measurement and luciferase assay. Values are expressed as fold of increase to basal reading (no CoCl2), and in Mean ± SD, where n = 4, each with triplicate samples. Statistical comparison was made with the basal group; *p < 0.05; **p < 0.01.
4. Discussion
In the present study, a cell line derived from the dorsal fin of rabbit fish was established and characterized, which was named as RFF cell line. The RFF cells grew well and could be sub-cultured for over 50th passages so far. The identity of RFF cells was originated from S. fuscescens, as confirmed by genomic sequencing and immunostaining. Cultured RFF cells showed maximal growth rate in medium having 20% FBS: this requirement was similar with other fish cell lines [5,18]. RFF cells could only grow at 28 °C. This temperature restriction was also reported from other established cell lines isolated from marine fish [7,15]. Compared with the zebra fish fin cell line AB.9 (ATCC® CRL-2298™), RFF cells grew under similar temperature but required more serum in the medium, 20% in RFF vs 15% FBS in AB.9 cell. Moreover, the morphology of RFF cell was similar with that of AB.9 cell line [19].
Skin is the first barrier of fish facing various infection factors, and therefore which is highly sensitive to inflammation. When cultured RFF cells were challenged with LPS, the expression of pro-inflammation cytokine was induced, as well as the translocation of NF-kB and the transcription of NF-κB-driven activity. The inflammatory responses of cultured RFF cells, induced by LPS, are similar to other fish cell lines that the expression of inflammatory cytokines, i.e. IL-1β, IL-6, TNF-α, increased robustly [20,21]. This signaling of inflammatory response is a classical mechanism of common inflammation [22]. Moreover, cultured RFF cells showed strong sensitive response to hypoxia. The expression of transcription factor HIF-1α and pHRE-Luc-driven luciferase activity were dose-dependently induced by CoCl2, and this hypoxia response was reported similarly in other marine organisms [23,24]. These results therefore indicated that the developed RFF cell line could be a helpful tool in drug screening for anti-inflammation and/or anti-hypoxia effects.
Herbal products, in particular TCM, provide an abundant resource for drug and/or food development. Several Chinese herbal extracts have been applied in aquaculture feedings to strength fish's immunity and to promote growth [25]. Scutellaria Radix (the dried root of S. baicalensis) has been used as a Chinese medicine for centuries in China and Asia, and this herb is well-known to have multiple pharmacological activities, including anti-inflammation [11] and anti-hypoxia [26]. Recently, the extract of Scutellaria Radix was shown to inhibit the infection of SARS-CoV-2 virus [27]. Indeed, this herb is considered as an expensive herb in the market. In contrast to other herbal extracts, we are using the waste product of Scutellaria Radix during their production line. The stems and leaves of S. baicalensis are being discarded as waste, but those active compounds, e.g. scutellarin and baicalin, are found there predominantly [12]. Scutellarin and baicalin are main functional flavonoids in Scutellaria Radix, as well as in our tested SBA extract. Baicalin was reported to have anti-inflammation effect on hypoxia/reoxygenation and TNF-α induced injury in cultural rat cardiomyocyte [28]. Besides, baicalin protected rat brain microvascular endothelial cells that injured by oxygen-glucose deprivation [29] and attenuated hypoxia-induced pulmonary arteriole remodeling on rat [30]. Scutellarin was reported with similar effect in protecting cardiomyocyte injury from hypoxia [31] and inflammation development in microglial [32]. Scutellarin and baicalin could enhance estrogenic activity and prevent cytotoxicity induced by Aβ (amyloid β-protein) [33]. Thus, the current result demonstrated the efficacy of SBA in cultured RFF cells, i.e. anti-inflammation and anti-hypoxia, could be triggered, at least partly, by scutellarin and baicalin within SBA extract.
5. Conclusion
A fin cell line from S. fuscescens (rabbit fish), designated as RFF, was established and characterized. The cells exhibited sensitive responses to inflammation and hypoxia. Therefore, RFF cell line can be an excellent in vitro system in drug/feeding screening. The water extract of aerial part of S. baicalensis protected the survival of cultured RFF cells in dose-dependent manners in fighting against inflammation, hypoxia and high temperature. For an economic reason, the aerial part of S. baicalensis is the waste product of Scutellaria Radix production.
Credit author statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work is supported by Shenzhen Science and Technology Innovation Committee (ZDSYS201707281432317; JCYJ20170413173747440; JCYJ20180306174 903174), China Postdoctoral Science Foundation (2019M653087), Zhongshan Municipal Bureau of Science and Technology (ZSST20SC03); Guangzhou Science and Technology Committee Research Grant (GZSTI16SC02; GZSTI17SC02); Hong Kong RGC Theme-based Research Scheme (T13-605/18-W); Hong Kong Innovation Technology Fund (UIM/340, UIM/385, ITS/500/18FP; TCPD/17–9); TUYF19SC02, PD18SC01 and HMRF18SC06.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fsi.2020.07.050.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplement Figure 1. Gene identify of isolated RFF cell (A): PCR products, primer flanking the 18S rRNA, were identified at 1.5% agarose gel electrophoresis. The genomic DNA from isolated RFF cells (Cell) and S. fuscescens fin (Tissue) showed PCR fragment at ~700 bp. Blank contained no genomic DNA only. Markers in bp are shown. (B): Sequences of PCR products (primer sequences removed) from cell and tissue of S. fuscescens in comparation with NCBI database (GenBank Accession No. AB276986.1).
Supplement Figure 2. HPLC fingerprint and cytotoxicity of SBA extract. (A): Fingerprint of the water extract of SBA. Twenty μL of 100 mg/mL of SBA water extract was subjected to HPLC-DAD analysis, and the chemical fingerprint was revealed at the wavelength 276 nm. The identification of scutellarin and baicalin were shown here (upper panel). Fingerprint of standard chemical markers (scutellarin and baicalin at 10 mg/mL) was performed by same chromatographic condition (lower panel). Representative figures are shown, n = 3. (B): Cytotoxicity assay of SBA extract. The different concentrations of SBA water extract were applied onto cultured RFF cells for 24 h, and MTT assay was performed. Values are shown in percentage of viability, as compared to control group (no extract), in Mean ± SD, where n = 4, each with triplicate samples. Statistical comparison was made with the control group; *p < 0.05; **p < 0.01.
References
- 1.Castellana B., Marin-Juez R., Planas J.V. Transcriptional regulation of the gilthead seabream (Sparus aurata) interleukin-6 gene promoter. Fish Shellfish Immunol. 2013;35:71–78. doi: 10.1016/j.fsi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Duray M.N. Aquaculture Department, Southeast Asian Fisheries Development Center; 1998. Biology and Culture of Siganids; pp. 1–63. [Google Scholar]
- 3.Abdel-Tawwab M., Monier M.N., Hoseinifar S.H. Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019;45:997–1013. doi: 10.1007/s10695-019-00614-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen S.L., Sha Z.X., Ye H.Q. Establishment of a pluripotent embryonic cell line from sea perch (Lateolabrax japonicus) embryos. Aquaculture. 2003;218:141–151. [Google Scholar]
- 5.Ma C., Fan L., Ganassin R. Production of zebrafish germ-line chimeras from embryo cell cultures. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98:2461–2466. doi: 10.1073/pnas.041449398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hightower L.E., Renfro J.L. Recent applications of fish cell culture to biomedical research. J. Exp. Zool. 1988;248:290–302. doi: 10.1002/jez.1402480307. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero M., Castaño A., Gonzalez A. Characterization of RTG-2 fish cell line by random amplified polymorphic DNA. Ecotoxicol. Environ. Saf. 1998;40:56–64. doi: 10.1006/eesa.1998.1642. [DOI] [PubMed] [Google Scholar]
- 8.Salmon J., Armstrong C., Ansel J. The skin as an immune organ. West. J. Med. 1994;160:146–152. [PMC free article] [PubMed] [Google Scholar]
- 9.Ángeles Esteban M. An overview of the immunological defenses in fish skin. ISRN Immunol. 2012:1–29. 2012. [Google Scholar]
- 10.Zhang Z., Wells M.C., Boswell M.G. Identification of robust hypoxia biomarker candidates from fin of medaka (Oryzias latipes) Comp. Biochem. Physiol. C : Toxicol. Pharmacol. 2012;155:11–17. doi: 10.1016/j.cbpc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong G., Wang H., Kong X. Flavonoids are identified from the extract of Scutellariae Radix to suppress inflammatory-induced angiogenic responses in cultured RAW 264.7 macrophages. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-35817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G., Rajesh N., Wang X. Identification of flavonoids in the stems and leaves of scutellaria baicalensis Georgi. J. Chromatogr. B. 2011;879:1023–1028. doi: 10.1016/j.jchromb.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y.T., Chan G.K.L., Wang H.Y. The anti-bacterial effects of aerial parts of Scutellaria baicalensis: potential application as an additive in aquaculture feedings. Aquaculture. 2020;526(15):735418. [Google Scholar]
- 14.Abarike E.D., Jian J., Tang J. Influence of traditional Chinese medicine and Bacillus species (TCMBS) on growth, immune response and disease resistance in Nile tilapia, Onilot reochromis icus. Aquacult. Res. 2018;49:2366–2375. [Google Scholar]
- 15.Tong S.L., Li H., Miao H.Z. The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounder Paralichthys olivaceus. Aquaculture. 1997;156:327–333. [Google Scholar]
- 16.Yan L., Hu Q., Mak M.S.H. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep30014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu E.Y., Zheng Z.X., Zheng B.Z. Tectorigenin, an isoflavone aglycone from the rhizome of Belamcanda chinensis, induces neuronal expression of erythropoietin via accumulation of hypoxia-inducible factor-1 alpha. Phytother Res. 2019:1–9. doi: 10.1002/ptr.6599. [DOI] [PubMed] [Google Scholar]
- 18.Le Y., Li Y., Jin Y. Establishment and characterization of a brain cell line from sea perch, Lateolabrax japonicus. Vitro Cell Dev. Biol. Anim. 2017;53:834–840. doi: 10.1007/s11626-017-0185-7. [DOI] [PubMed] [Google Scholar]
- 19.Badakov R., Jaźwińska A. Efficient transfection of primary zebrafish fibroblasts by nucleofection. Cytotechnology. 2006;51:105–110. doi: 10.1007/s10616-006-9018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q., Li C., Yu Z. Molecular and immune response characterizations of IL-6 in large yellow croaker (Larimichthys crocea) Fish Shellfish Immunol. 2016;50:263–273. doi: 10.1016/j.fsi.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Fierro-Castro C., Barrioluengo L., López-Fierro P. Fish cell cultures as in vitro models of inflammatory responses elicited by immunostimulants. Expression of regulatory genes of the innate immune response. Fish Shellfish Immunol. 2013;35:979–987. doi: 10.1016/j.fsi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelster B., Egg M. Hypoxia-inducible transcription factors in fish: expression, function and interconnection with the circadian clock. J. Exp. Biol. 2018;221:jeb163709. doi: 10.1242/jeb.163709. [DOI] [PubMed] [Google Scholar]
- 24.Richards J.G. Fish Physiology. vol. 27. Academic Press; 2009. Metabolic and molecular responses of fish to hypoxia; pp. 443–485. [Google Scholar]
- 25.Choi W., Mo W., Wu S. Effects of traditional Chinese medicines (TCM) on the immune response of grass carp (Ctenopharyngodon idellus) Aquacult. Int. 2014;22:361–377. [Google Scholar]
- 26.Hussain I., Waheed S., Ahmad K.A. Scutellaria baicalensis targets the hypoxia‐inducible factor-1α and enhances cisplatin efficacy in ovarian cancer. J. Cell. Biochem. 2018;119:7515–7524. doi: 10.1002/jcb.27063. [DOI] [PubMed] [Google Scholar]
- 27.Liu H., Ye F., Sun Q. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. 2020:1–18. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L., Wu X.d., Davey A.K. The anti-inflammatory effect of baicalin on hypoxia/reoxygenation and TNF-α induced injury in cultural rat cardiomyocytes. Phytother Res. 2010;24:429–437. doi: 10.1002/ptr.3003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P., Hou J., Fu J. Baicalin protects rat brain microvascular endothelial cells injured by oxygen-glucose deprivation via anti-inflammation. Brain Res. Bull. 2013;97:8–15. doi: 10.1016/j.brainresbull.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Yan S., Wang Y., Liu P. Baicalin attenuates hypoxia-induced pulmonary arterial hypertension to improve hypoxic cor pulmonale by reducing the activity of the p38 MAPK signaling pathway and MMP-9. Evid. base Compl. Alternative Med. 2016:1–9. doi: 10.1155/2016/2546402. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., Yu J., Wu J. Scutellarin protects cardiomyocyte ischemia–reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 2016;157:200–207. doi: 10.1016/j.lfs.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Wang S., Wang H., Guo H. Neuroprotection of scutellarin is mediated by inhibition of microglial inflammatory activation. Neuroscience. 2011;185:150–160. doi: 10.1016/j.neuroscience.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J.T.T., Choi R.C.Y., Chu G.K.Y. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. J. Agric. Food Chem. 2007;55:2438–2445. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Gene identify of isolated RFF cell (A): PCR products, primer flanking the 18S rRNA, were identified at 1.5% agarose gel electrophoresis. The genomic DNA from isolated RFF cells (Cell) and S. fuscescens fin (Tissue) showed PCR fragment at ~700 bp. Blank contained no genomic DNA only. Markers in bp are shown. (B): Sequences of PCR products (primer sequences removed) from cell and tissue of S. fuscescens in comparation with NCBI database (GenBank Accession No. AB276986.1).
Supplement Figure 2. HPLC fingerprint and cytotoxicity of SBA extract. (A): Fingerprint of the water extract of SBA. Twenty μL of 100 mg/mL of SBA water extract was subjected to HPLC-DAD analysis, and the chemical fingerprint was revealed at the wavelength 276 nm. The identification of scutellarin and baicalin were shown here (upper panel). Fingerprint of standard chemical markers (scutellarin and baicalin at 10 mg/mL) was performed by same chromatographic condition (lower panel). Representative figures are shown, n = 3. (B): Cytotoxicity assay of SBA extract. The different concentrations of SBA water extract were applied onto cultured RFF cells for 24 h, and MTT assay was performed. Values are shown in percentage of viability, as compared to control group (no extract), in Mean ± SD, where n = 4, each with triplicate samples. Statistical comparison was made with the control group; *p < 0.05; **p < 0.01.