Abstract
Given that the novel coronavirus was detected in stool and urine from diagnosed patients, the potential risk of its transmission through the water environment might not be ignored. In the current study, to investigate the spread possibility of COVID-19 via the environmental media, three typical rivers (Yangtze, Han, and Fu River) and watershed cities in Hubei province of China were selected, and a more comprehensive risk assessment analysis method was built with a risk index proposed. Results showed that the risk index in the Yangtze River Basin is about 10−12, compared to 10−10 and 10−8 in the Han and Fu River Basins, and the risk index is gradually reduced from Wuhan city to the surrounding cities. The safety radius and safety time period for the Yangtze, Han, and Fu River are 8 km/14 h, 20 km/30 h and 36 km/36 h, respectively. The linear relationship between the risk potential calculated by the QMRA model and the multiple linear regression proved that the built index model is statistically significant. By comparing the theoretical removal rates for the novel coronavirus, our study proposed an effective method to estimate the potential spread risk of COVID-19 in the typical river basins.
Keywords: COVID-19, Water environment, Risk assessment, QMRA
Graphical abstract
Highlights
-
•
Risk distribution and comparison of watershed cities of COVID-19 were identified.
-
•
Distribution order of risk index was Fu River > Han River > Yangtze River.
-
•
The safety radii of the three rivers were 8 km, 20 km and 36 km, respectively.
-
•
The safety periods of the three rivers were 14 h, 23 h and 36 h, respectively.
-
•
Modified QMRA index model was employed to assess the risk of COVID-19.
1. Introduction
In recent months, a new coronary pneumonia named Coronavirus Disease 2019 (COVID-19) has spread rapidly around the world (Lai et al., 2020). Up to July 25, the number of diagnosed patients in the world has exceeded 15.3 million, and the number is still rising rapidly. As a new member of the coronavirus family, the characters this novel coronavirus is significantly similar to the six known coronavirus genome sequences (Lai et al., 2020; Liaw et al., 2012). In particular, its homology with SARS-CoV exceeds 79% (Chen et al., 2020). Infection from COVID-19 can cause pneumonia, severe acute respiratory syndrome, kidney failure, and final death (Hu et al., 2020).
This new coronavirus has been reported to be more contagious than the SARS virus (Shaylika, 2020). In terms of the route of transmission, similar to SARS, pneumonia infected by the new coronavirus is mainly transmitted through droplets from coughing or sneezing, and might also be transmitted through contact (Cai et al., 2020; Chen, 2020). Of note, recent studies have found that COVID-19 nucleic acid is positive in the stool of some patients with diagnosed COVID-19, and the virus can transfer between liquid and skin (Holshue et al., 2020; Pitol et al., 2017; Zheng et al., 2020). Considering that SARS was able to replicate in human gastrointestinal tract and excreted with urine and stool in the body, resulting in the further survival for days to weeks in water environment field, more attention should be paid to the risk of the fecal mouth transmission for the novel coronavirus, and the risk assessment and analysis of the overall discharge of COVID-19 from a diagnosed patient to the final environment needs urgent evaluation, which still remains unclear up to now (Casanova et al., 2009; Leung et al., 2003; Qu et al., 2020; Wigginton and Boehm, 2020).
As an important vector, rivers could carry the virus and spread it to the downstream (Robins et al., 2019; Skraber et al., 2009), posing a health risk to watershed cities. Due to the flow disturbance, virus that settles to the bottom might be resuspended again and become a potential surface threat (Fauvel et al., 2016a; Rehmann and Soupir, 2009). Moreover, the transverse shear effect of the longitudinal flow of rivers would also enhance the second-stay on the river bank or even immerse into the groundwater, resulting in more serious health risks (Turrell et al., 1996). Still, limited information is known about the transmission and the relevant risk assessment for this new coronavirus at the current stage.
Quantitative microbiological risk assessment (QMRA) describes a mathematical relationship between microbial intake and the probability of adverse effects on the human body, based on the characteristics of infectious effects and other data (Haas et al., 2014). QMRA risk assessment has been widely used in the field of water resources and the environment to evaluate a variety of viruses, including adenovirus, rotavirus, and enterovirus in recreational, reclaimed and surface waters in word-wide regions, which give the appropriate resolution for the risk evaluation of COVID-19 (Kundu et al., 2013; McBride et al., 2013; Toze et al., 2010).
In this study, the watershed cities in the three major basins namely the Yangtze River, Han River and Fu River in Hubei province of China were selected to be assessed for the possible waterborne transmission of COVID-19. The QMRA index distribution model was systematically established and modified to conduct the risk assessment and comparative analysis of sewage discharges in each city. Crucial factors such as the dilution, transmission distance, and the inactivation of viruses over time were also analyzed. Through the calculation of risk index, potential suggestions for controlling the risks of COVID-19 are proposed.
2. Methods
2.1. Researching area
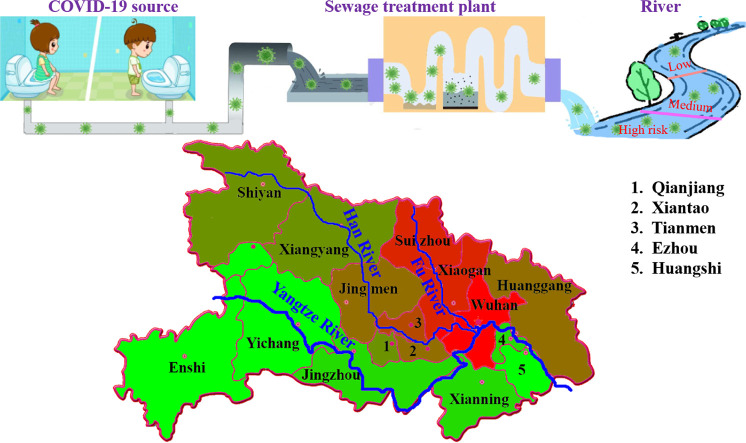
Three rivers (Yangtze River, Han River and Fu River) and the related 19 watershed cities in Hubei Province were selected as the main research objects. The detailed locations are shown in Fig. 1 .
Fig. 1.
Locations of the three selected rivers and watershed cities.
2.2. Data sources
@The number of diagnosed cases (Tables S1 and S2) was obtained from the Health Committee of Hubei Province (http://wjw.hubei.gov.cn/fbjd/xxgkml/sjfb/). The daily flow information for the Yangtze River, Han River and Fu River was obtained from the Hydrology and Water Resources Bureau of Hubei Province (http://sw.hubeiwater.gov.cn/). Daily urban sewage treatment scale is intercepted from the National Bureau of Statistics of China (http://www.stats.gov.cn/tjsj/ndsj/). The information on the residents population and area of cities was obtained from the Hubei Province Bureau of Statistics (http://tjj.hubei.gov.cn/).
2.3. Index model of QMRA
QMRA is used to assess viral risk in the water environment. Research on pathogen biological response mechanisms is based on the hypothesis that pathogens may infect humans, which can be described in Eq. (1).
| (1) |
where P i is the single infection risk index, γ is the constant revealing the infection probability of a single pathogen, and d is the number of ingested pathogens. The γ parameters for other pathogens in the previous studies were listed in Table S3, while the sensitivity and confidence interval analysis were also performed. Since limited information including the related pathological parameters about SARS-CoV-2 virus has been obtained and widely acknowledged so far, the γ parameter in our study was selected as γ = 0.0128 based on the fact that SARS-CoV-2 is a respiratory virus, semi-pathogenic (N50) should be larger than enterovirus to cause infection in the water environment, and the γ parameter should be selected relatively small. For conducting the relative risk comparisons between different cities, the calculation of the risk index for the three river basins was based on the same selected parameters, which might not differ the essentials of the relevant discussion.
According to the previous study (Haas et al., 2014), the value of d could be calculated from Eq. (2).
| (2) |
Here m is the amounts of water consumed by the exposed population in one time exposure in Table S4 (Fewtrell and Bartram, 2001; Lorna, 2013), and the most unfavorable exposure volume in various daily activities was set at 100 mL. μ is the amounts of virus in the selected water body, which can be calculated from Eq. (3).
| (3) |
In the current study, μ is related to the number of diagnosed patients N, the amounts of viruses discharged into sewage per person per day from diagnosed patients n, the total daily urban sewage volume V, the river discharge volume Q, the estimated treatment degree of the sewage treatment plant θ, and the viral attenuation inactivation rate δ depending on the river flowing time t and distance L.
Here, referring to SARS virus-related research (Hung et al., 2004; Poon et al., 2004), the genome concentration of the SARS virus in patients' stool (n1) and urine (n2) was 107 and 2.5 × 104 cop/L, and increased by an order of magnitude during an outbreak. Given that the volume of stool (v1) and urine (v2) are about 0.5 and 1.5 L/d respectively, the estimated value of n is about 5 × 107 (Eq. (4)). According to the conventional treatment process, the virus removal efficiency θ is equal to 4 log, and δ is calculated via Eq. (5) (Fauvel et al., 2016b).
| (4) |
| (5) |
3. Results and discussion
3.1. Risk assessment of three river basins
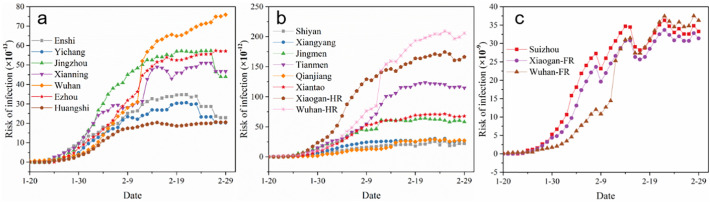
COVID-19 in Hubei Province began to rapidly erupt since January 20 in 2020. Since the rapid increase was observed in the following month, the subsequent 40 days (from January 20 to February 29) were selected as the research period. Fig. 2 shows the risk analysis in the Yangtze River, Han River, and Fu River basins. It can be seen the risk potential of the watershed cities in the Yangtze River basin definitely increased after January 25, which could be related to the rapidly rising number of diagnosed patients. Due to the large flow of the Yangtze River, the virus-laden sewage discharged by sewage treatment plants is greatly diluted, thus the risk indexes of the cities in this basin are generally low. Of note, Wuhan has the highest risk, with a risk index of 7.5 × 10−12, followed by Jingzhou, Ezhou and Xianning in the range of 4 × 10−12–6 × 10−12, while Enshi, Yichang and Huangshi have relatively low risks, with all the risk indexes lower than 4 × 10−12. Compared with the Yangtze River, the overall risk indexes in the Han River basin are about one order higher (Fig. 2b). Because the flow of the Han River is relatively small compared to the Yangtze River, the impact of confirmed patients gave the increasing contribution. Wuhan-Han River section (Wuhan-HR) shows the largest risk index at 2 × 10−10, which is mainly due to its largest numbers of confirmed patients, followed by Xiaogan-Han River section (Xiaogan-HR) and Tianmen with the risk indexes at 1.5 × 10−10 and 1.2 × 10−10, respectively, due to the small amounts of sewage volume and the relatively high numbers of diagnosed patients. Fig. 2c shows the risk analysis of the Fu River Basin. Since the average flow of the river is only about 3 m3/s in this season, and the dilution effect of the sewage discharged into the water body is greatly weakened, the overall risk indexes of the related cities in Fu River Basin increased by three orders compared to the Yangtze River Basin.
Fig. 2.
Risk index in the Yangtze River basin (a), Han River basin (b) and Fu River basin (c) in different periods.
3.2. Distribution and analysis of urban risks in river basins
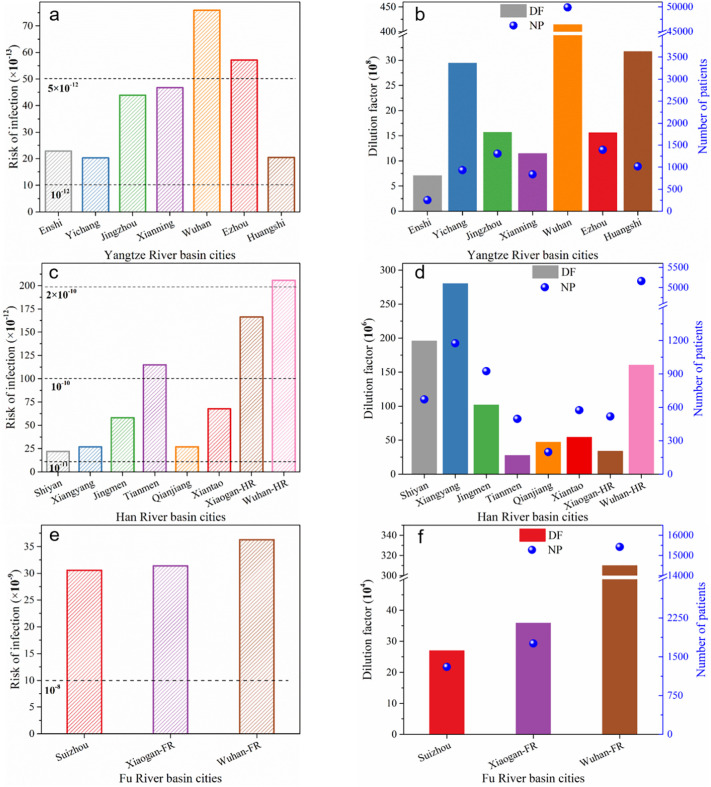
The urban risk distribution, and the information about the dilution (equal to V × Q) and the number of diagnosed patients are listed in Figs. 3 and S1. As illustrated in Fig. 3a, the risk distribution of cities in the Yangtze River Basin is centered on Wuhan, which is mainly attributed to the number of diagnosed patients decreasing from Wuhan to both side cities (Fig. 3b). It can be seen, due to the larger volume of sewage in Yichang and Huangshi, the risk indexes of them are relatively lower. The risk distribution of the Hanjiang River Basin has gradually increased from Shiyan to Wuhan, except for Jingmen and Tianmen (Fig. 3c). Although the number of diagnosed patients is not large in Jingmen and Tianmen, the overall risk indexes are relatively higher due to the lower sewage volume (Fig. 3d). However, there is no obvious pattern of risk distribution in the Fu River Basin (Fig. 3e), the limited variation was observed for the risk assessment among the three selected cities, due to the difference of ratios of patients number to sewage amount is small (Fig. 3f).
Fig. 3.
Cities risk, dilution and diagnosed patients in the Yangtze River Basin (a), (b), Han River Basin (c), (d), and Fu River Basin (e), (f).
3.3. Risk varies with distance L and time t
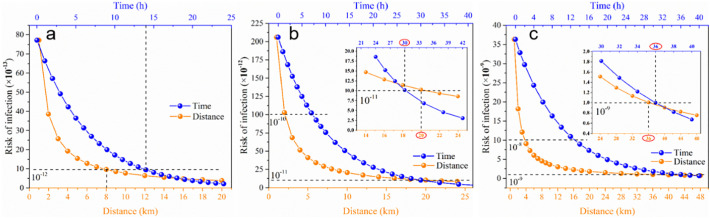
When sewage is discharged into the river, the virus will inactivate with the river transportation distance L and time t, and the corresponding risk index will gradually change. Variations in Wuhan's risk indexes for the three rivers with L and t are shown in Fig. 4 . It could be deduced that the risk decreased rapidly with the increasing distance of water transport, and the safety radius from sewage outlets in the Yangtze River, Han River, and Fu River are 8 km, 20 km, and 36 km, respectively. However, due to the long safety distance of the Han River and Fu River, downstream cities may have the possibility of overlapping risks. As for the trend of risk over time, similar to distance, the risk can be reduced by an order of magnitude by about 14 h in the Yangtze River Basin, compared to 30 h in Han River Basin and 36 h in Fu River Basin. Therefore, the Han River and the Fu River may also have a trend of overlapping risks under time period consideration.
Fig. 4.
The risk index of the Yangtze River Basin (a), Han River Basin (b) and Fu River Basin (c) cities varies with L and t.
3.4. Risk fitting of the QMRA index model
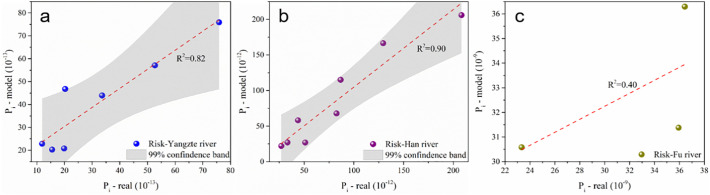
According to epidemiology theory, the spread risk of airborne viruses is closely related to the proportion of infected and population density (Tang et al., 2020; Zhang et al., 2018). To better verify the QMRA index model, the real infection index was calculated through multiple linear regression analysis (Table S5), by combining infected proportion and population density (Table S6). As illustrated in Fig. 5 , R2 of the Yangtze River and the Han River Basins are 0.84 and 0.90, respectively. However, due to the only three cities in the Fu River Basin, the fitting indexes are poorly correlated with R2 = 0.40. In general, the acceptable linear relationship indicates that the QMRA index model was statistically significant.
Fig. 5.
Linear fitting of risk between index model of QMRA and real index infection in Yangtze River Basin (a), Han River Basin (b) and Fu River Basin (c).
3.5. Risk comparisons and control approaches in Wuhan
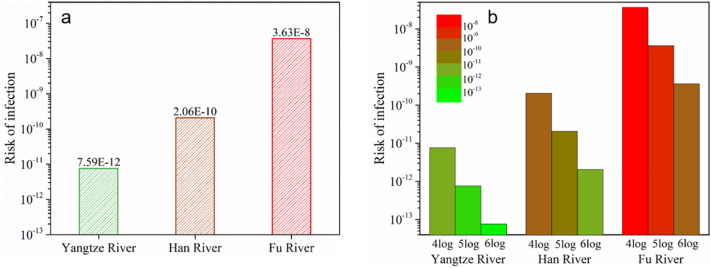
Given that Wuhan city is the area with the worst epidemic situation, and where the three rivers meet, the risk indexes of the three rivers are compared in Fig. 6a. Due to the influence of river flow, risk indexes of the Yangtze River, Han River, and Fu River are 7.59 × 10−12, 2.06 × 10−10, and 3.63 × 10−8, respectively. Furthermore, Fig. 6b shows the effect of the virus removal rate in sewage treatment processes on the index in Wuhan. Obviously, when the removal rates are 4 log, 5 log, and 6 log, the risk indexes of these three rivers show an order of magnitude reduction. Based on the above analysis, wastewater treatment plants in cities of the small flow River Basins and high-risk index areas, should strengthen the disinfection process during the epidemic period to control risks. Similarly, daily household disinfection of the toilet also plays a vital role in reducing the risk.
Fig. 6.
Risk comparisons of the three rivers in Wuhan (a), and the relationship between the virus removal rate in sewage plants and infection risk (b).
4. Conclusions
By establishing a novel synthetic model, the preliminary analysis of the risk assessment for COVID-19 in three river basins was carried out. Due to the huge differences in river flows, the values of risk index followed as PiYangtze < PiHan < PiFu. In the same watershed, the amounts of sewage and diagnosed patients would play circular roles in the transmission of COVID-19. The risk distribution in the Yangtze River and Han River basins generally took Wuhan as the center and decreased toward the surrounding cities, while there was no obvious pattern in Fu River basins. The safety radius in the Yangtze River Basin was obtained as 8 km with 14 h safety time, while it was 20 km and 36 km for the Han River and Fu River with 30 h and 36 h safety time period, respectively. Through multiple linear regression analysis, the QMRA index model was proved to be statistically reasonable. Our study demonstrated that higher treatment standard in sewage plants and disinfection of sanitary appliances in households could significantly reduce the risk related to COVID-19. Further attention should be paid to the risk of groundwater virus diffusion caused by pipeline leakage during sewage pipeline transportation. Still, due to the limited research on the risk of SARS-CoV-2 virus transmission in the water environment at this stage, and the survival of the virus could be affected by many environmental and human factors, some uncertainty and limitations might exist concerning the risk assessment at the current stage, which needs further investigation in the future.
CRediT authorship contribution statement
Bo Yang: Writing - original draft, Conceptualization, Formal analysis. Wei Li: Data curation, Software. Jingquan Wang: Data curation, Investigation. Zixin Tian: Resources, Methodology. Xin Cheng: Conceptualization, Resources. Yongli Zhang: Writing - review & editing. Rui Qiu: Methodology, Software. Shuhua Hou: Validation. Hongguang Guo: Writing - review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Special Funds for Prevention and Control of COVID-19 of Sichuan University (Grant No. 2020scunCoV20009), Innovation Spark Project in Sichuan University (Grant No. 2082604401254).
Editor: Jay Gan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141353.
Appendix A. Supplementary data
Supplementary material
References
- Cai J., Sun W., Huang J., Gamber M., Wu J., He G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020;26(6):1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.J., Liu W.Y., Zhang Q., Xu K., Ye G.M., Wu W.C., Sun Z.Y., Liu F., Wu K.L., Zhong B., Mei Y., Zhang W.X., Chen Y., Li Y.R., Shi M., Lan K., Liu Y.L. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerging Microbes & Infections. 2020;9(1):313–319. doi: 10.1080/22221751.2020.1725399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel B., Cauchie H.-M., Gantzer C., Ogorzaly L. Contribution of hydrological data to the understanding of the spatio-temporal dynamics of F-specific RNA bacteriophages in river water during rainfall-runoff events. Water Res. 2016;94(7):328–340. doi: 10.1016/j.watres.2016.02.057. [DOI] [PubMed] [Google Scholar]
- Fauvel B., Gantzer C., Cauchie H.-M., Ogorzaly L. In situ dynamics of F-specific RNA bacteriophages in a small river: new way to assess viral propagation in water quality studies. Food and Environmental Virology. 2016;9(1):89–102. doi: 10.1007/s12560-016-9266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell L., Bartram J. In: Water Quality: Guidelines, Standards, and Health: Assessment of Risk and Risk Management for Water-related Infectious Disease. Fewtrell Lorna, Bartram Jamie., editors. IWA Pub; 2001. [Google Scholar]
- Haas C.N., Rose J.B., Gerba C.P. John Wiley & Sons Inc; 2014. Quantitative Microbial Risk Assessment. [Google Scholar]
- Holshue M., DeBolt C., Lindquist S., Lofy K., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S., Kim L., Tong S., Lu X., Lindstrom S., Pillai S. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., Ma H., Chen W., Lin Y., Zheng Y., Wang J., Hu Z., Yi Y., Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.N., Cheng V.C.C., Wu A.K.L., Tang B.S.F., Chan K.H., Chu C.M., Wong M.M.L., Hui W.T., Poon L.L.M., Tse D.M.W., Chan K.S., Woo P.C.Y., Lau S.K.P., Peiris J.S.M., Yuen K.Y. Viral loads in clinical specimens and SARS manifestations. (Research) Emerg. Infect. Dis. 2004;10(9) doi: 10.3201/eid1009.040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu A., McBride G., Wuertz S. Adenovirus-associated health risks for recreational activities in a multi-use coastal watershed based on site-specific quantitative microbial risk assessment. Water Res. 2013;47(16):6309–6325. doi: 10.1016/j.watres.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K.S., Chan H.L.Y., Wu A.K.L., Lee N., Yuen K.Y., Sung J.J.Y. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw C.W.J., Lim A.S.E., Koh W.H.V., Loh J.P., Kan C., Chan K.W., Ting P.J., Ng S.H., Chew S.W.J., Dua A., Tan C.H., Gao Q.H.C., Ho H.P.V., Lee V., Tan B.H. Epidemiology of the four human coronaviruses 229E, HKU1, NL63 and OC43 detected over 30 months in the Singapore military. Int. J. Infect. Dis. 2012;16(S1):e135. [Google Scholar]
- Lorna F. Water quality: guidelines, standards and health: assessment of risk and risk management for water-related infectious disease. Water Intelligence Online. 2013;12 [Google Scholar]
- McBride G.B., Stott R., Miller W., Bambic D., Wuertz S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Res. 2013;47(14):5282–5297. doi: 10.1016/j.watres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Pitol A.K., Bischel H.N., Kohn T., Julian T.R. Virus transfer at the skin-liquid interface. Environ. Sci. Technol. 2017;51(24):14417–14425. doi: 10.1021/acs.est.7b04949. [DOI] [PubMed] [Google Scholar]
- Poon L.L., Chan K.H., Wong O.K., Cheung T.K., Ng I., Zheng B., Seto W.H., Yuen K.Y., Guan Y., Peiris J.S. Detection of SARS coronavirus in patients with severe acute respiratory syndrome by conventional and real-time quantitative reverse transcription-PCR assays. Clin. Chem. 2004;50(1):67–72. doi: 10.1373/clinchem.2003.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ. Sci. Technol. 2020;54(7):3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Rehmann C.R., Soupir M.L. Importance of interactions between the water column and the sediment for microbial concentrations in streams. Water Res. 2009;43(18):4579–4589. doi: 10.1016/j.watres.2009.06.049. [DOI] [PubMed] [Google Scholar]
- Robins P.E., Farkas K., Cooper D., Malham S.K., Jones D.L. Viral dispersal in the coastal zone: a method to quantify water quality risk. Environ. Int. 2019;126:430–442. doi: 10.1016/j.envint.2019.02.042. [DOI] [PubMed] [Google Scholar]
- Shaylika C. Comprehensive review of coronavirus disease 2019 (COVID-19) Biom. J. 2020 doi: 10.1016/j.bj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skraber S., Schijven J., Italiaander R., de Roda Husman A.M. Accumulation of enteric bacteriophage in fresh water sediments. J. Water Health. 2009;7(3):372–379. doi: 10.2166/wh.2009.098. [DOI] [PubMed] [Google Scholar]
- Tang B., Bragazzi N.L., Li Q., Tang S., Xiao Y., Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infect Dis Model. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toze S., Bekele E., Page D., Sidhu J., Shackleton M. Use of static quantitative microbial risk assessment to determine pathogen risks in an unconfined carbonate aquifer used for managed aquifer recharge. Water Res. 2010;44(4):1038–1049. doi: 10.1016/j.watres.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Turrell W.R., Brown J., Simpson J.H. Salt intrusion and secondary flow in a shallow, well-mixed estuary. Estuar. Coast. Shelf Sci. 1996;42(2):153–169. [Google Scholar]
- Wigginton K.R., Boehm A.B. Environmental engineers and scientists have important roles to play in stemming outbreaks and pandemics caused by enveloped viruses. Environ. Sci. Technol. 2020;54(7):3736–3739. doi: 10.1021/acs.est.0c01476. [DOI] [PubMed] [Google Scholar]
- Zhang N., Huang H., Su B., Ma X., Li Y. A human behavior integrated hierarchical model of airborne disease transmission in a large city. Build. Environ. 2018;127:211–220. doi: 10.1016/j.buildenv.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material