Abstract
Life Cycle Assessment (LCA) is a decision-making tool that accounts for multiple impacts across the life cycle of a product or service. This paper presents a conceptual framework to integrate human health impact assessment with risk screening approaches to extend LCA to include near-field chemical sources (e.g., those originating from consumer products and building materials) that have traditionally been excluded from LCA. A new generation of rapid human exposure modeling and high-throughput toxicity testing is transforming chemical risk prioritization and provides an opportunity for integration of screening-level risk assessment (RA) with LCA. The combined LCA and RA approach considers environmental impacts of products alongside risks to human health, which is consistent with regulatory frameworks addressing RA within a sustainability mindset. A case study is presented to juxtapose LCA and risk screening approaches for a chemical used in a consumer product. The case study demonstrates how these new risk screening tools can be used to inform toxicity impact estimates in LCA and highlights needs for future research. The framework provides a basis for developing tools and methods to support decision making on the use of chemicals in products
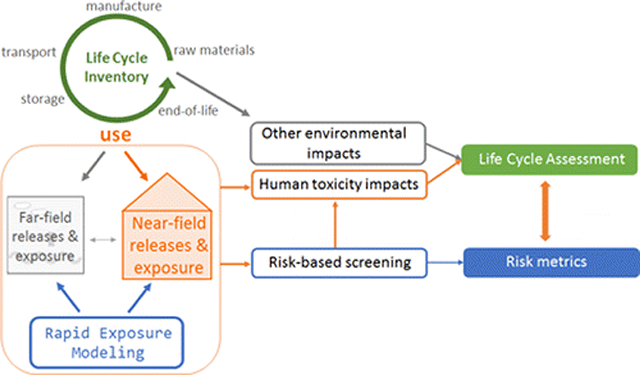
Graphical Abstract
Introduction
Life cycle assessment (LCA) is a multi-impact assessment tool that quantifies the potential environmental burdens and benefits of products and services to avoid making decisions that unknowingly shift the burdens from one life cycle stage to another, from one impact type to another, or, more recently, from one geographic location to another. Based on its comprehensive nature and utility for environmental sustainability, LCA accounts for potential human health impacts, including those resulting from chemical impacts associated with products, alongside other impacts such as energy use, climate change, ozone depletion, and water use.(1, 2) For the human toxicity impact category, practitioners have only recently started assessing the impacts of exposures from near-field (indoor, workplace) chemical sources,(3–14) with life cycle impact assessment (LCIA) methodology historically focusing on emissions to the far-field (or outdoor) environment.(3, 10, 15, 16) The omission of exposures from indoor and workplace sources may underestimate potential chemical impacts when exposures from near-field sources are larger than indirect exposures to far-field releases,(3, 17, 18) which may be the case for many chemicals in consumer products and building materials.(19)
For chemical emissions to outdoor media, impact characterization draws from risk assessment (RA) principles by accounting for a chemical’s release, transport, fate, human exposure, and toxicological effects when arriving at a potential impact metric.(20–24) Efforts to develop assessment methods for the impacts of exposures to indoor and workplace sources primarily focus on extending the human toxicity assessment approaches developed for far-field sources to include near-field media such as indoor air and other exposure routes such as dermal uptake.(3, 11, 16) However, these methods often do not address some of the choices made when the far-field methods were first developed. For example, background exposures were not included for practical reasons, one being insufficient data availability.(22, 23, 25) Aggregate exposure (i.e., the combined exposure to a chemical originating from multiple sources), may be particularly relevant for chemicals with near-field sources, which can often dominate exposure,(3, 17, 18) and can originate from several different sources within the same near-field space (e.g., different products providing exposure to the same chemical).(26, 27) Risk-based screening methods are increasingly addressing chemicals in the near field, resulting in the development of methods to rapidly estimate aggregate exposure to these chemicals.(17, 28–31) This type of risk-based exposure modeling provides an opportunity to include aggregate exposure from near-field chemical sources in LCA.
Although LCIA draws from RA principles, key methodological choices for LCIA differ from risk calculations and yield potential impact scores that are not absolute risks.(22, 23, 32, 33) In the context of this manuscript, the term “risk” refers to an evaluation of the risk or probability of harm associated with exposure to a chemical(34, 35) and is differentiated from the LCA-based impacts of products. One key difference is the consideration of absolute exposures,(9, 36, 37) which leads to the differing product-centric perspective in LCA and chemical-centric perspective in RA. The inclusion of aggregate exposures in LCA would align with both recent chemical legislative actions in the United States indicating that risk estimates are needed in chemical assessments(38) and broader shifts in the environmental decision-making landscape calling for increasing integration of risk and sustainability metrics.(39, 40) Extending LCA to include near-field chemical sources for consumer products and building materials could also benefit from a combined LCA and RA approach where the comprehensive nature of LCA provides a foundation to integrate environmental sustainability metrics with the consideration of chemical risks to human health.
Recommendations for both the integration of RA and LCA and the integration of human health metrics into sustainability-based decisions have been well documented.(9, 39–53) An important commonality between LCA and RA is the use of screening level assessments.(22, 34, 54–58) From the RA-based chemical perspective, screening RAs are being recommended to prioritize the large number of chemicals available in commerce for risks and needs for higher tier assessments.(38, 54, 55, 59) From the LCA-based product perspective, screening techniques are used to narrow multiple product options or to identify hotspots for product improvement. The human toxicity impact category in LCA was designed to provide screening-level assessments,(22, 23) and the recent emergence of high-throughput (HT) methods for risk screening enables new opportunities for integrating these two approaches. For example, the U.S. EPA’s Toxicity Forecaster (ToxCast)(60, 61) and ExpoCast(56) programs support risk-based screening of thousands of chemicals that have previously lacked toxicity or exposure data,(54) which may increase the chemical coverage of LCA. This paper explores adapting HT methods to enhance LCA for sustainable product design by extending human toxicity impacts to account for near-field sources and more risk considerations, such as aggregate exposures, population group differences, and novel sources of toxicity data.(38, 39) To this end, the objectives of this paper are (i) to present a conceptual framework extending human toxicity LCIA to the near-field using new tools for rapid exposure and toxicity assessment; (ii) to evaluate the potential for integrating such tools using a demonstrative case study; and (iii) to identify future research needs for long-term implementation.
Methods
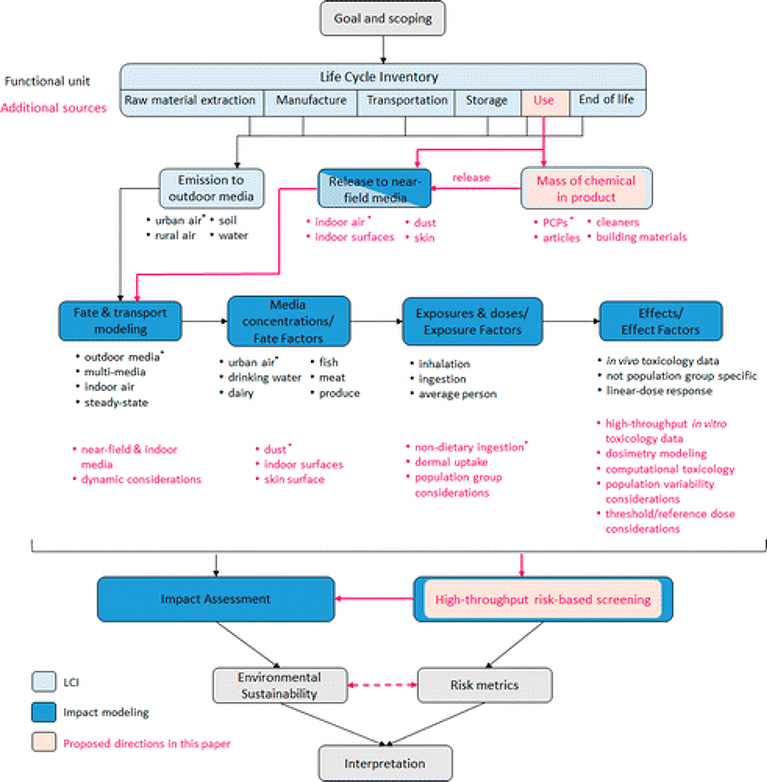
The conceptual framework presents a method to extend LCIA to the near-field by augmenting traditional LCIA methods with RA. The approach calculates potential life cycle impacts alongside a screening RA with aggregate exposures to yield more informative assessments (Figure 1). The framework draws from the parallel knowledge flow between both techniques from inventory (sources), transport, fate, exposure, and toxicological effects such that it can be applied to determine functional-unit-based characterization factors or to multiple chemical-use scenarios to determine aggregate exposures. The latter can be combined with effects to provide risk-based screening information to inform the impact scores. Figure 1 highlights where traditional LCIA methods would be extended to include the near-field and additional risk-based information from the screening step.
Figure 1.
Conceptual framework incorporating human exposures from use-stage near-field sources and high-throughput risk-based data into impact assessment. Black and magenta text indicates current practice and proposed inclusion in LCIA, respectively. Notes: *Exhaustive lists have not been included.
The existing impact assessment methods(1, 20) upon which the framework is built are first briefly described. The ultimate vision is for the source-to-receptor LCIA and RA calculations to be harmonized and streamlined, since both approaches follow the same knowledge flow (Figure 1). The framework is followed by a case study using currently available LCIA tools(62) and the U.S EPA SHEDS-HT (High-throughput Stochastic Human Exposure and Dose Simulation) model designed to support HT screening risk assessments for chemicals used in consumer products.(28) The case study demonstrates aspects of the framework and explores areas where the LCIA and risk screening approaches differ.
Human Toxicity Impact Modeling Overview
As with other impact categories, the impact score (IS) is a product of the life cycle inventory (LCI) describing the emission quantities and compartments (i.e., kg of chemical emitted to a compartment) for a given functional unit (FU) and the corresponding characterization factor (CF) (i.e., potential impact per kg of chemical emitted to a compartment).(1, 63) The FU specifies the product function or service and is the reference unit used to compare across alternatives.(64) The total impact score for a given chemical is the sum of its characterized impact for each compartmental release. The CF is the chemical-specific measure of the toxic impact due to a chemical emission and is expressed, for example, as a human toxicity potential.(1, 65) The CF calculation incorporates fate, transport, and human exposure factors via the intake fraction (iF) (kg intake per kg emitted)(66, 67) and hazard and dose–response via an effect factor (EF) (e.g., effect or disease cases per kg intake)(67, 68) (Figure 1). The iF can differentiate a media-specific CF because the EF is constant for a given chemical (but depends on exposure route, i.e. inhalation or ingestion, and effect). Figure 1 gives examples of outdoor media and exposure pathways included in current LCIA methods. These methods are also used to assess impacts of other substances, such as metals,(67) and are being evaluated for emerging compounds, such as nanomaterials.(14, 69) Additional details of human health impact assessment are available in the Supporting Information (SI).
Life Cycle Inventory
The inclusion of the near-field in LCIA for consumer products (e.g., personal care products (PCPs), cleaners, furniture) and building materials will require a typical LCI (e.g., far-field) to be extended to include near-field sources. The inventory may be in the form of an emission to indoor media (e.g., indoor air) or a mass of chemical contained within a product.(16, 70–73) Any near-field LCIA method should be sufficiently flexible to use both emissions- and mass-based inventory approaches.
To include aggregate exposure estimates, inventories based solely on the FU must be extended to include inventories that identify additional sources of the chemicals from all their anticipated uses. Emerging data sources for risk-based decision making, such as product composition databases,(74) may be leveraged for this purpose to minimize the effort required to build the near-field inventory. For example, the U.S. EPA, through its engagement in computational exposure science, has collected public data on chemical uses(26) and product composition to support estimation of product compositions for rapid exposure modeling.(30) A useful outcome of this work is the Consumer Product Chemical Profile database (CPCPdb),(74) which links chemicals to consumer products with consideration of concentration ranges. This database has been applied to estimate aggregate exposure to chemicals used in consumer products by the U.S. EPA’s SHEDS-HT model.(28)
Fate, Transport, and Exposure
Fate and transport models used in LCIA need to be extended to the indoor environment and its media, such as air, dust, carpet, furniture, and surfaces,(75, 76) while including human exposure estimates(71, 77, 78) accounting for routes and pathways such as dermal uptake and nondietary ingestion (e.g., dust, hand-to-mouth and object-to-mouth contact).(11, 70, 79) While fate and transport models used for the far-field generally assume steady-state conditions,(67, 80) near-field modeling may benefit more from consideration of the time-varying nature of exposure. Acknowledging the frequency and duration of product use will affect both the magnitude of exposure and the magnitude of outdoor releases for some product types, such as PCPs washed down the drain.(70, 81, 82)
Current LCIA exposure methods for the far-field assume average conditions for comparative purposes and do not account for population variability, often because of a lack of site specificity.(25, 63, 83) For chemicals that have exposures that are largely driven by near-field pathways, variability in population exposures could be driven by differences in product use patterns or other indoor activity characteristics.(28, 84) Some population groups may be more sensitive to a specific exposure pathway (e.g., nondietary dust ingestion for children(85, 86)) for a product-chemical combination that if not considered could underestimate impacts when comparing across chemicals. This is why research supporting risk screening, where population variability is often a key consideration,(87) includes the development of models to address population differences.(28) For example, SHEDS-HT simulates population variability of individuals and their activity patterns to report a distribution of near-field exposures. Thus, incorporating this type of model into LCIA would also allow for traditional point estimates in LCA to be extended to include population variabilities and population group considerations.
Effects
LCIA is generally limited to in vivo data, which is not available for many chemicals.(54, 69) The use of HT screening in vitro assays and computational estimates could increase the number of chemicals that can be evaluated in LCIA. Thus, LCIA will likely need to evolve beyond in vivo toxicity data if it is to be applicable for decision support involving new and emerging chemicals.(38) The U.S. National Research Council (NRC)(88) has recommended a transition from in vivo animal testing to in vitro assays(89, 90) to address toxicity gaps on the large numbers of chemicals in commerce. For example, data generated under the Tox21 program (a collaboration between the U.S. National Institutes of Health and U.S. EPA) have resulted in information on the bioactivity of thousands of substances.(91) These data differ from traditional in vivo data because they represent in vitro concentrations rather than an external dose causing an adverse effect. Instead, in vitro toxicity concentrations can be linked to doses using dosimetry modeling to forward-calculate a resulting blood or target tissue concentration from a given exposure.(89, 92) Alternatively, reverse dosimetry can be used to convert a target tissue concentration to an equivalent absorbed dose, which can then be compared with an estimated exposure.(31, 55, 93–95) Other sources of toxicity data include computational (or in silico) models,(96) which do not necessarily require the use of dosimetry modeling and instead may be designed to predict effects based on exposures or absorbed doses.
Consideration of population differences in toxicity may also be needed to avoid underestimation of impacts for sensitive populations,(97, 98) and to better align with regulatory chemical management.(38) As the goal of LCIA is to be comparative, toxicity data used for effect factors are not derived from reference doses that have been adjusted for safety factors,(22, 63, 99) such as sensitive populations.(100) However, the incorporation of population sensitivity will likely be needed to reconcile LCIA with risk information.
Incorporating Risk Screening
The framework includes risk screening by calculating aggregate exposures in tandem to the LCIA calculations. The resulting information can help interpret implications of the dose–response assumptions used in LCIA where a chemical is modeled in the absence of aggregate or cumulative exposure and generally assuming a linear dose–response function beginning at the origin.(22, 63, 101) In RA and risk screening approaches, dose–response may incorporate a dose limit (e.g., reference or threshold dose, or allowable daily limit) under which adverse effects are considered unlikely or unexpected to occur.(39, 102) A linear dose–response in RA traditionally applies to carcinogenic effects while a nonlinear, or threshold-based, dose–response has been traditionally employed for noncarcinogenic effects.(39, 102–104) Thus, the treatment of noncarcinogenic effects in LCA differs from RA.(22) Based on these characteristics, current practice in LCA using a linear dose–response is commonly referred to as the “less is better” approach, while the traditional RA approach considering threshold doses(9, 51, 83, 101) has been referred to as an “only above threshold” approach.(36) This is one area where there is a clear difference between the information provided by RA and LCIA, and the framework proposes an integration of both approaches as a means to yield more informative assessments.(9, 25, 36)
The use of the linear dose–response in LCA was motivated by factors such as the nonspatially specific nature of inventory data and lack of background exposure data (i.e., exposures that the FU source adds to).(22, 25, 99) Additionally, its use can be advantageous as an “only above threshold” approach may not fully characterize the potential impact of a chemical, especially in the absence of information about the effects of chemical mixtures (i.e., cumulative exposure), future chemical sources or stressors contributing to backgrounds, or dose–response behavior at low doses.(39, 22, 63, 99, 101, 105) The linear dose–response also allows for summation of impacts across a population in order to account for higher impacts when more people are exposed to a chemical. A disadvantage of the approach is that it may not adequately account for aggregate chemical exposures. For example, when comparing the impacts of two chemicals, the chemical with the smaller impact score could have an aggregate exposure above an RA-based dose limit, whereas the chemical with the larger impact score could have an aggregate exposure below a dose limit and actually pose a lower risk.
The incorporation of risk screening (Figure 1) draws from recent research using rapid exposure estimates and in vitro bioactivity data to prioritize and screen chemicals for risk.(31, 54–56, 93, 95) The levels of risk can be evaluated by calculating a margin of exposure (i.e., ratio of bioactive dose to estimated dose(31, 34, 55, 106)) or by comparing probabilistic distributions of estimated or modeled aggregate exposures and bioactive doses to determine the degree of overlap.(34, 95, 107) However, the best way to use this additional information in LCIA modeling is not readily apparent without further analysis and hypothesis testing using case studies.
While there are a number of approaches that can be used to structure the risk-based information, one of the most significant choices may be deciding whether to include (a) all anticipated uses of a chemical or (b) all anticipated uses except for the product associated with the FU. Option (a) is similar to exposure estimates used in risk screening but does not give insight regarding the contribution of the product to the aggregated exposure. Option (b) provides an estimate of the background exposure to which the product functional use will contribute.
Option (a) could be used to provide additional screening information to LCIA by identifying chemicals with risks based on dose limits that do not have high CFs or impact scores using standard LCIA linear dose–response methods. Such screening may be useful for two reasons: (1) LCIs could be streamlined by prioritizing chemicals with higher risk or impacts for assessment, and (2) chemicals of higher risk that may typically be excluded from LCIs based on cutoff rules or low CF or impacts could be considered. This would be in alignment with the goals of the human toxicity impact category to screen chemicals for toxicological impacts.(22)
Another method would apply a scaling factor to the impact score as previously suggested by Potting et al.(36) Using option (b), a scaling factor could be applied to differentiate, for example, exposure regimes based on the contribution of the FU product to the aggregate exposure and the resulting proximity of the aggregate exposure to a dose limit. Such an approach would be consistent with the product-centric nature of LCA and is discussed further in the SI. Incorporating these risk-based estimates into LCA will allow not only consideration of the incremental exposures and effects from the use of a chemical in a product or service but also how such exposures may contribute to an individual’s total exposure and effects from a given chemical. The ultimate goal is to provide the practitioner with additional information on the likelihood of adverse effects.
Case Study for LCIA with Risk-Based Screening
A simplified case study is used to gauge the feasibility of integrating selected pieces of the previously discussed tools and data to implement aspects of the conceptual framework. Both aggregate exposure and LCA impact scores were calculated to contrast the differences between FU-based and multisource exposure estimates. All calculations are demonstrative and rely on simplifying assumptions using default model parametrizations. Detailed information is provided in the SI.
Goal, Scope, and Functional Unit
The goal of the LCA was to assess the impact of near-field chemical exposure when considering the potential risk. The scope focused on exposure to one chemical of concern and included release of that chemical during use, manufacturing, and disposal. The selected product-chemical combination was 1,4-dichlorobenzene (p-DCB, CAS: 106–46-7) in a hanging air freshener. The FU was defined as one 160 g hanging air freshener used for 7 weeks in a home.(108, 109) Aggregate exposure due to anticipated consumer uses was limited to p-DCB and did not include other chemicals associated with the product. Although the impacts of these other chemicals may be larger than those of the p-DCB releases (e.g., byproducts from manufacturing(110)), they were excluded because of the demonstrative nature of the case study.
Aggregate Exposure and Risk Screening
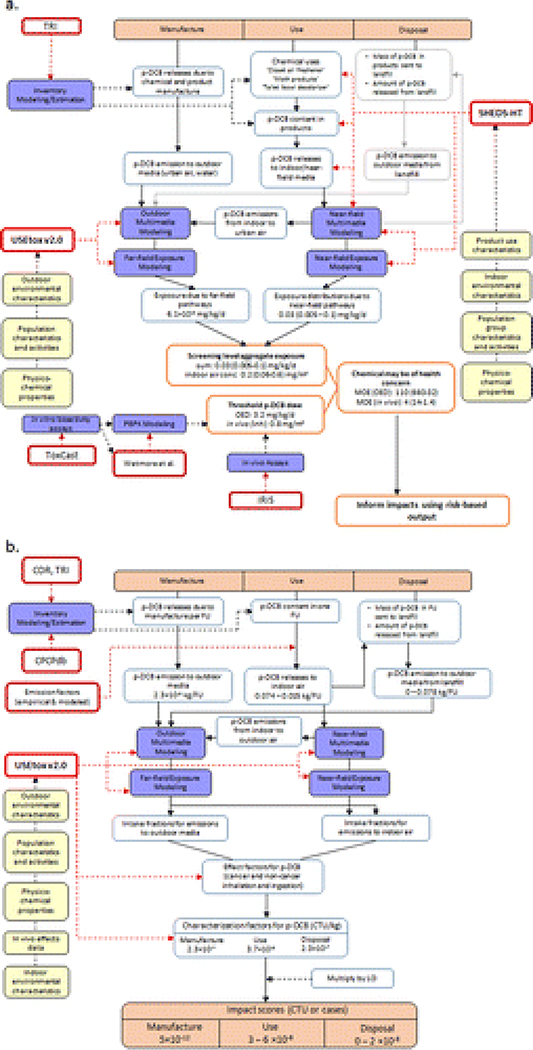
Total exposure aggregated across the considered sources was estimated by summing across exposure routes (inhalation, ingestion, dermal) and near- and far-field exposure pathways (Figure 2a). SHEDS-HT(28) was used to estimate population distributions of exposure (mg/kg/d) in the use-stage for p-DCB when considering all of its associated consumer products. The model uses input from the CPCPdb(74) and other sources(108, 111) to determine the product types in which the chemical is an ingredient and its composition in these products. The product form and usage determine how the chemical is released from the product and how various population groups are exposed via inhalation, dermal, and ingestion routes. Exposure to p-DCB released outdoors was estimated by combining individual intake fractions from USEtox v2.0(62) with the estimated manufacturing and out-the-window releases. The total aggregate exposure estimate was compared to an oral equivalency dose (OED, mg/kg/d) derived from ToxCast(61)in vitro data,(95) consistent with risk screening approaches.(31, 55) The indoor inhalation exposure was also compared to a reference dose derived from in vivo data.(112)Figure 2a summarizes the steps, data sources, and models used to estimate the aggregate exposure to p-DCB.
Figure 2.
Workflow used to calculate a) screening aggregate exposure to p-DCB and b) LCA impact scores for the functional unit (FU) using p-DCB. The diagram also details data sources and models used in the calculations. Blue shaded boxes indicate types of models or data needed for the calculations; red outlined boxes indicate actual data sources or models used, yellow shaded boxes indicate example data types used as input in the models. Gray outlined boxes indicate data streams that were not included in the case study but can be integrated in future work. Notes: The characterization factors and impact scores were aggregated across the inhalation and ingestion exposure routes and cancer and noncancer effects. References: TRI (Toxics Release Inventory),(136) ToxCast,(61) Wetmore et al.,(95) IRIS (Integrated Risk Information System),(112) CDR (Chemical Data Reporting),(137) and CPCPdb.(74)
Life Cycle Impact Assessment
Human health ISs were calculated for p-DCB using the USEtox v2.0 model,(62, 67) which includes CFs for p-DCB. USEtox recently incorporated an indoor inhalation iF model for indoor air emissions that is compatible with current LCIA methods(16) and includes intakes due to a chemical that has been vented outdoors, thereby coupling near-field and far-field exposure pathways into a single metric.(3, 11, 16)Figure 2b summarizes the steps, data sources, and models used to estimate impact scores for the FU.
Results and Discussion
Case Study
For the aggregate exposure calculations, the estimated doses from the manufacturing and use-stage releases to the outdoors were 5 × 10–6 and 6 × 10–5 mg/kg/d, respectively. The mean estimated exposure via near-field exposure pathways in the use-stage was 0.03 (0.005–0.1, 2.5th–97.5th percentiles) mg/kg/d (Table S5). The mean estimated exposure was 2 orders of magnitude larger via the near-field as compared to the far-field pathways. Exposure to the FU product type (air freshener) contributed approximately 20% to the overall mean near-field exposure, meaning the aggregate exposure was dominated by the other consumer products containing p-DCB.
Risk-based screening approaches compare estimated exposures summed across intake routes (e.g., inhalation, dermal, and ingestion) to a bioactive dose (OED in this case). This is typically accomplished by calculating a margin of exposure (MOE), which is the ratio of a dose limit to the estimated dose.(31, 55, 95) The minimum OED for p-DCB from Wetmore et al.(95) is 3.2 mg/kg/d, yielding an estimated MOE of 110 (660–32) (mean, 2.5th–97.5th percentiles). This means the higher range of exposed individuals was estimated to be within an order of magnitude of the bioactive dose. An in vivo reference air concentration (RfC) of 0.8 mg/m3, which has been adjusted to take sensitive populations and uncertainty into account, is also available for comparison to the estimated indoor air concentrations.(112) This yielded a mean MOE of 4 (14–1.4) and was within an order of magnitude for mean and high-end product user estimates. While the MOEs calculated in this case study were based on screening methods, they are consistent with other studies finding that p-DCB may pose a risk to some users.(113, 114)
LCA impact scores were calculated for each life cycle stage to better understand the exposure contributions (and summed over exposure routes and effects, for simplicity) from the near- and far-field. The near-field impact from the use-stage was 3–6 × 10–6 CTU (comparative toxicity units), which was the highest stage-based impact and approximately 105–106 times larger than the corresponding far-field impacts from manufacturing (5 × 10–12 CTU). The disposal-stage only contributed to impacts for the lower indoor emission scenario (2 × 10–8 CTU) and was 2 orders of magnitude smaller than the near-field impact. The difference in impact between the use and manufacturing stages was driven primarily by the large difference in inventory (Tables S7, S8), while the difference between the use and disposal stages was driven by the both the inventory and varying iFs (Tables S7, S8) given that the inventory to the disposal stage is a function of the indoor emissions. The impact scores were dependent on the length of use of the air freshener because it affected both the magnitude of release in the use-stage and the corresponding input to landfill.
Although impact scores were generated without introducing any new tools or concepts into the calculations, the case study allows for comparison of the LCA and risk-based perspectives, which can be meaningful for developing a more thorough interpretation of the impact scores. Both methods estimated that the use-stage had the highest human exposure potential and, therefore, the highest health impact or risk potential for p-DCB; results that have been previously demonstrated empirically.(115) The exposure screening step estimated that some users of the air freshener, when considering all products containing p-DCB, may be exposed to p-DCB within an order of magnitude of screening toxicity doses. The traditional LCIA impact scores did not address background exposure, which was problematic in this example because the background exposure from other products contributed more to the estimated exposure than the FU product. This highlights how aggregate exposure estimates can provide more information if chemical toxicity is a criterion. For example, a product manufacturer may avoid a chemical in a product given the additional information that other sources of this chemical can lead to substantially higher exposures or those near dose limits. As some chemicals may have higher potential for impacts when exposure to all sources are taken into account, this additional information can be used to give a more complete picture of the risks associated with a chemical being assessed for use in a product. The addition of risk-based screening and aggregate exposure to LCA can be further applied to compare different air fresheners (or to other scenarios such as replacing the product with better cleaning(113)) to help determine which scenario is more favorable in terms of human toxicity while the sustainability of the alternatives can be captured through the other life cycle impacts (e.g., climate change, resource depletion, ecotoxicity, etc.).
While uncertainty was not addressed with the simplified calculations, risk-based screening can be performed with consideration of uncertainty and population variability. For example, SHEDS-HT estimates variability distributions of exposure in different population groups, and these distributions can be compared to distributions of dose limits.(34, 95) Ideally, exposure distributions would also take uncertainty into account alongside population variability. In the case of traditional CF development, probabilistic methods for intake fractions can be used to develop CF distributions based on uncertainty and variability, rather than point values. Uncertainty considerations are key inputs to interpretation of any screening-based approach. In the near-term there may be higher certainty associated with comparing or ranking chemicals rather than screening for absolute risk.
Isaacs et al.(28) provided detailed discussion about uncertainty and sensitivity of the SHEDS-HT model. They found the most sensitive parameters to estimated exposures were associated with the consumer product use variables, such as amount of product used, frequency of product use, and product composition, in addition to variables associated with hand-to-mouth exposures. Estimated exposures correlated well with biomonitoring data, and in most cases estimates were larger than biomonitoring values, which is generally favorable in screening methods in order to achieve conservative estimates. Isaacs et al.(28) noted that future research will be focused on improving quantification of the sensitive variables and quantifying and reducing uncertainty. Based on the sensitivity analysis of Isaacs et al.,(28) a major source of uncertainty in this case study was likely the consumer use scenarios of the products containing p-DCB. Thus, a better understanding of product ingredients(26) and product use patterns will ultimately lead to reductions in uncertainty in aggregate exposure estimates. Comparing differing methods to estimate aggregate exposure can also be used to better understand uncertainty by comparing differences in model outputs using different techniques and input data. Other methods to estimate aggregate exposure include using production volumes(31) and exposure heuristics.(29) Additionally, the results of SHEDS-HT and other mechanistic and statistical models are being incorporated into consensus model predictions by EPA for use in risk-based chemical prioritization.(107) When jointly evaluated against exposures inferred from biomarker data using Bayesian approaches, both the predictive power of each model and the associated uncertainty in the predictions can be estimated. Within this evaluation framework, new or revised models can be evaluated for improved predictive ability and contribution to decreased uncertainty.
In the LCIA calculations, uncertainty was likely driven by the indoor intake fraction as this life cycle stage had the highest exposure potential. Uncertainty in the use-stage emission is likely lower as we calculated a range based on measured and modeled data. Uncertainty in CFs due to outdoor release from the USEtox model has been well characterized to be within a factor of 100–1000.(20) The uncertainty in the indoor iF may be lower than the outdoor iF, as a comparison of iFs estimated from three different indoor fate and exposure models(8, 75, 77, 78) were within 1–2 orders of magnitude with each other.(31)
The case study identified potential obstacles for integrating LCA and aggregate exposure techniques. For example, the inventory modeling did not support development of full product near-field LCIs because of the lack of information regarding other chemical components in the product. Although several of the models, tools, data, and concepts needed for both screening and assessment are overlapping (Figure 2), their use was not harmonized or integrated here, leading to inconsistencies in their application. For example, SHEDS-HT estimates an indoor air concentration (see the SI) instead of using an iF approach where the iF is combined with an emission rate to yield an intake rate. While the SHEDS-HT and iF approaches estimate exposure based on mass-balance principles, they are applied in differing manners. As such, methods to automate and harmonize the use of these tools will be necessary to minimize resource needs for an integrated approach. In theory, the tools identified in this work can be integrated such that the same inventory data, models, and inputs will support them all. An advantage of the LCIA method is that the near- and far-field exposure pathways for release to indoor air are coupled, avoiding the need to calculate a separate inventory to the far-field during the use-stage.(11) This type of coupling can lead to improved aggregate exposure modeling methods that yield estimates of chemical fate and transport after a chemical has been released from a product.(11, 70)
Challenges and Recommendations
The case study presented here was an initial demonstration of the integration of high-throughput aggregate exposure modeling techniques(28) into LCIA, specifically for near-field sources. The framework presents several research opportunities and challenges to be addressed in order to achieve its long-term implementation.
Methodologically, the relationship between near- and far-field modeling in LCIA and the interpretation of these modeling approaches, should be carefully established. First, it is necessary to define the level of consistency that should be maintained regarding modeling choices for the two exposure categories. The near-field exposure models should be detailed enough to capture relevant exposure pathways associated with product-chemical combinations. As with any model development, there is a challenge to understand how to balance the desired level of detail within CFs with the appropriate level of uncertainty to support the intended decision. These issues should be explored further and may ultimately yield chemical and product combinations for which simplifying assumptions may be valid and yield acceptable levels of uncertainty. It is likely that CFs will be developed using product archetypes, for example, there will be a different CF for a given chemical used in a household cleaner than when it is used in a personal care product or building material.(11) Additionally, near-field models used in LCIA should reconcile far- and near-field models to account for near-field releases that result in far-field exposure pathways,(3, 16, 70, 82, 116) and Fantke et al.(11) have recently proposed a modeling framework for this. The use of multisource or background ambient conditions should also be investigated and considered for far-field CF development. It is well-known that near- and far-field pathways for several persistent organic pollutants can both be important contributors to overall exposure.(85, 117–121) This presents a challenge because it will require combining both types of exposures if taking multiple sources into account and will increase the data requirements for the accompanying risk screening step.
Regarding data needs, the estimation of inventory in both the near- and far-field will continue to be a challenge to both LCA and risk-based screening.(19, 39, 122, 123) For example, the collection and modeling of chemical composition of consumer products(30) and building materials will be a continuing need for incorporating near-field impacts in LCA. Although these challenges are generally recognized, it is worth emphasizing the need for continuously enhancing and extending methods for data collection and computationally based estimation if both approaches are to address the growing number of chemicals in commerce.(30, 124) Thus, a key research area will be to develop rapid techniques for near- and far-field inventory estimations. Furthermore, a key to developing the inventory will be ensuring the FU-based inventory and aggregate or multisource inventory are developed consistently.
Techniques to combine chemical usage, source, and inventory information for rapid aggregate exposure estimation(30) will be key inputs to incorporating risk-based screening into LCIA. Approaches to incorporate and interpret aggregate or background exposure with regard to calculating CFs and impact scores will require further evaluation. Regardless of the approach, the risk-based screening step will need to be performed periodically because the aggregate exposure will vary over time based on the activities of the exposed population and changes to source scenarios. Additionally, the incorporation of a risk-based screening step relying on exposure to a single chemical at a given point in time does not take effects of chemical mixtures (i.e., cumulative exposures) or future exposure scenarios into account.(101) Ideally, LCA and risk assessment will be based on cumulative exposures.(39, 101, 125) Research on this topic is ongoing,(125) for example by linking cumulative exposures to adverse outcome pathways,(126) and methods in LCA will need to be updated as advances are made.
The incorporation of population variability and population group considerations in exposure calculations should also be explored. As more emphasis is placed on topics like children’s health,(97) it will be important for LCA to provide this type of information. For LCA, this represents a shift from the traditional generic nature of the assessment to a more situation-specific understanding of the impacts. Methods to adopt such concerns are already being developed through the introduction of spatial differentiation in far-field impact assessment.(127) However, these efforts may need to be extended to nonspatial determinants of exposure that integrate human choice and lifestyle differences into impact models.
The case study demonstrated that developing a viable framework to include near-field exposure modeling, HT exposure tools, and next-generation toxicology is challenging given the unique and overlapping data, models, and operational structures that present substantial complexity.(128) These conceptual differences present an operational challenge to the framework and will require harmonization across disciplines to properly identify linkages for integration, especially given the comprehensive nature of LCA and its need for data from several different disciplines. The interdisciplinary nature of the framework poses its own challenge because the roles of the associated research communities in supporting the framework will need to be clearly established. For example, it may not be practical for LCA practitioners to perform risk screening, requiring LCA, exposure, and toxicology researchers to collaborate on common “calculators” to be used across all fields.
The consideration and quantification of uncertainty in LCA(19, 129) and exposure/risk assessment(39, 130) poses the final and perhaps greatest challenge given its importance to decision making. Although it has been argued that uncertainty may not be a basis for exclusion of impacts in LCA studies,(131, 132) decision makers may be more confident applying LCA if the uncertainty has been properly characterized. Uncertainty in LCA can occur during the inventory; fate, transport and exposure modeling; and effects characterization(20, 39) and include both model and parameter/data uncertainty. For computationally based HT methods, there is an understanding that uncertainty will be larger than in higher tier methods because of the many required assumptions and data estimations.(30, 31, 34) Fortunately, there are approaches to address uncertainty within each of these fields. Rosenbaum et al.(20) reported uncertainty for CFs based on differences across outputs of models used to develop the USEtox(62) model. This method could also be applied to the near-field.(31) HT aggregate exposure models are being developed and compared to biomonitoring data that provide a source for model evaluation and uncertainty quantification.(17, 28, 29) Uncertainty in hazard (e.g., toxicity) and dose–response relationships remains a challenge in both RA and LCA and is becoming more complex with the addition of novel data streams such as in vitro data.(20, 39) As progress is made in toxicology, especially given the growing amounts and reliability of in vitro(133) and in silico data, the framework should be updated with any resulting approaches. Ultimately, methods to quantify uncertainty should continue to be developed, given the continued uncertainties of exposure and toxicity models and data in the foreseeable future.
Although several challenges to the framework have been identified, the effort required to overcome these challenges should not outweigh the value of the framework. The framework provides a basis for the future development of software tools to combine LCA methods with near-field human exposure models and to allow for assessments across a wider range of chemicals and products, including emerging chemicals of interest. The assessments generated by these tools will allow for human toxicity impacts in LCA to be more consistent with typical regulatory decision making rooted in RA.(38, 39) Similarly this work will also support better consideration of life cycle stages(134, 135) and other chemical impacts in RA. Given the growing importance of sustainability, practitioners and decision-makers who develop or apply LCA, chemical alternatives assessment,(35) and RA methodologies should find much value in the proposed framework.
Supplementary Material
Acknowledgment
This research was part of the Chemical Safety for Sustainability National Research Program in the U.S. Environmental Protection Agency’s (EPA) Office of Research and Development. This research was supported in part by an appointment of S. Csiszar to the Postdoctoral Research Program at the National Risk Management Research Laboratory, Office of Research and Development, U.S. EPA administered by the Oak Ridge Institute for Science and Education through Interagency Agreement No. DW-89–92298301 between the U.S. Department of Energy and the U.S. EPA. Expert support from the U.S. EPA’s Office of Air and Radiation, Indoor Environments Division is appreciated. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
References
- 1.Bare J TRACI 2.0: the tool for the reduction and assessment of chemical and other environmental impacts 2.0 Clean Technol. Environ. Policy 2011, 13 (5) 687– 696DOI: 10.1007/s10098-010-0338-9 [DOI] [Google Scholar]
- 2.Bare JC; Gloria TP Environmental impact assessment taxonomy providing comprehensive coverage of midpoints, endpoints, damages, and areas of protection J. Cleaner Prod. 2008, 16 (10) 1021– 1035DOI: 10.1016/j.jclepro.2007.06.001 [DOI] [Google Scholar]
- 3.Hellweg S; Demou E; Bruzzi R; Meijer A; Rosenbaum R; Huijbregts M; McKone T Integrating Human Indoor Air Pollutant Exposure within Life Cycle Impact Assessment Environ. Sci. Technol. 2009, 43 (6) 1670– 1679DOI: 10.1021/es8018176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demou E; Hellweg S; Hunerbühler K An occupational chemical priority list for future life cycle assessments J. Cleaner Prod. 2011, 19 (12) 1339– 1346DOI: 10.1016/j.jclepro.2011.03.011 [DOI] [Google Scholar]
- 5.Hellweg S; Demou E; Scheringer M; McKone TE; Hungerbuhler K Confronting workplace exposure to chemicals with LCA: examples of trichloroethylene and perchloroethylene in metal degreasing and dry cleaning Environ. Sci. Technol. 2005, 39 (19) 7741– 8DOI: 10.1021/es047944z [DOI] [PubMed] [Google Scholar]
- 6.Kijko G; Margni M; Partovi-Nia V; Doudrich G; Jolliet O Impact of Occupational Exposure to Chemicals in Life Cycle Assessment: A Novel Characterization Model Based on Measured Concentrations and Labor Hours Environ. Sci. Technol. 2015, 49 (14) 8741– 8750DOI: 10.1021/acs.est.5b00078 [DOI] [PubMed] [Google Scholar]
- 7.Demou E; Hellweg S; Wilson MP; Hammond SK; McKone TE Evaluating Indoor Exposure Modeling Alternatives for LCA: A Case Study in the Vehicle Repair Industry Environ. Sci. Technol. 2009, 43 (15) 5804– 5810DOI: 10.1021/es803551y [DOI] [PubMed] [Google Scholar]
- 8.Wenger Y; Li D; Jolliet O Indoor intake fraction considering surface sorption of air organic compounds for life cycle assessment Int. J. Life Cycle Assess. 2012, 17 (7) 919– 931DOI: 10.1007/s11367-012-0420-0 [DOI] [Google Scholar]
- 9.Walser T; Juraske R; Demou E; Hellweg S Indoor Exposure to Toluene from Printed Matter Matters: Complementary Views from Life Cycle Assessment and Risk Assessment Environ. Sci. Technol. 2014, 48 (1) 689– 697DOI: 10.1021/es403804z [DOI] [PubMed] [Google Scholar]
- 10.Skaar C; Jørgensen R Integrating human health impact from indoor emissions into an LCA: a case study evaluating the significance of the use stage Int. J. Life Cycle Assess. 2013, 18 (3) 636– 646DOI: 10.1007/s11367-012-0506-8 [DOI] [Google Scholar]
- 11.Fantke P; Ernstoff AS; Huang L; Csiszar SA; Jolliet O Coupled near-field and far-field exposure assessment framework for chemicals in consumer products Environ. Int. 2016, 94, 508– 518DOI: 10.1016/j.envint.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 12.Collinge W; Landis AE; Jones AK; Schaefer LA; Bilec MM Indoor environmental quality in a dynamic life cycle assessment framework for whole buildings: Focus on human health chemical impacts Build. Environ. 2013, 62, 182– 190DOI: 10.1016/j.buildenv.2013.01.015 [DOI] [Google Scholar]
- 13.Park HS; Ji C; Hong T Methodology for assessing human health impacts due to pollutants emitted from building materials Build. Environ. 2016, 95, 133– 144DOI: 10.1016/j.buildenv.2015.09.001 [DOI] [Google Scholar]
- 14.Walser T; Meyer D; Fransman W; Buist H; Kuijpers E; Brouwer D Life-cycle assessment framework for indoor emissions of synthetic nanoparticles J. Nanopart. Res. 2015, 17 (6) 1– 18DOI: 10.1007/s11051-015-3053-y [DOI] [Google Scholar]
- 15.Chaudhary A; Hellweg S Including Indoor Offgassed Emissions in the Life Cycle Inventories of Wood Products Environ. Sci. Technol. 2014, 48 (24) 14607– 14614DOI: 10.1021/es5045024 [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum RK; Meijer A; Demou E; Hellweg S; Jolliet O; Lam NL; Margni M; McKone TE Indoor Air Pollutant Exposure for Life Cycle Assessment: Regional Health Impact Factors for Households Environ. Sci. Technol. 2015, 49 (21) 12823– 12831DOI: 10.1021/acs.est.5b00890 [DOI] [PubMed] [Google Scholar]
- 17.Wambaugh J; Setzer RW; Reif DM; Gangwal S; Mitchell-Blackwood J; Arnot JA; Jolliet O; Frame A; Rabinowitz J; Knudsen TB; Judson RS; Egeghy P; Vallero DA; Cohen Hubal EA High-Throughput Models for Exposure-Based Chemical Prioritization in the ExpoCast Project Environ. Sci. Technol. 2013, 47, 8479– 8488DOI: 10.1021/es400482g [DOI] [PubMed] [Google Scholar]
- 18.Wormuth M; Demou E; Scheringer M; Hungerbuhler K Assessments of direct human exposure: the approach of EU risk assessments compared to scenario-based risk assessment Risk Anal. 2007, 27 (4) 979– 90DOI: 10.1111/j.1539-6924.2007.00936.x [DOI] [PubMed] [Google Scholar]
- 19.Finnveden G; Hauschild MZ; Ekvall T; Guinée J; Heijungs R; Hellweg S; Koehler A; Pennington D; Suh S Recent developments in Life Cycle Assessment J. Environ. Manage. 2009, 91 (1) 1– 21DOI: 10.1016/j.jenvman.2009.06.018 [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum R; Bachmann T; Huijbregts M; Jolliet O; Juraske R; Koehler A; Larsen H; MacLeod M; Margni M; McKone T; Payet J; Schuhmacher M; van de Meent D; Hauschild M USEtox - The UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity Int. J. Life Cycle Assess. 2008, 13, 532– 546DOI: 10.1007/s11367-008-0038-4 [DOI] [Google Scholar]
- 21.Jolliet O; Müller-Wenk R; Bare J; Brent A; Goedkoop M; Heijungs R; Itsubo N; Peña C; Potting J; Pennington D; Rebitzer G; Schenck R; Stewart M; Udo de Haes H; Weidema B The LCIA midpoint-damage framework of the UNEP-SETAC Life Cycle Initiative Int. J. Life Cycle Assess. 2004, 9 (6) 394– 404DOI: 10.1007/BF02979083 [DOI] [Google Scholar]
- 22.Pennington D; Crettaz P; Tauxe A; Rhomberg L; Brand K; Jolliet O Assessing human health response in life cycle assessment using ED10s and DALYs: part 2--Noncancer effects Risk Anal. 2002, 22 (5) 947– 63DOI: 10.1111/1539-6924.00263 [DOI] [PubMed] [Google Scholar]
- 23.Hertwich EG; Mateles SF; Pease WS; McKone TE Human toxicity potentials for life-cycle assessment and toxics release inventory risk screening Environ. Toxicol. Chem. 2001, 20 (4) 928– 39DOI: 10.1002/etc.5620200431 [DOI] [PubMed] [Google Scholar]
- 24.Huijbregts MAJ; Thissen U; Guinée JB; Jager T; Kalf D; van de Meent D; Ragas AMJ; Wegener Sleeswijk A; Reijnders L Priority assessment of toxic substances in life cycle assessment. Part I: Calculation of toxicity potentials for 181 substances with the nested multi-media fate, exposure and effects model USES–LCA Chemosphere 2000, 41 (4) 541– 573DOI: 10.1016/S0045-6535(00)00030-8 [DOI] [PubMed] [Google Scholar]
- 25.Bare JC Risk Assessment and Life-Cycle Impact Assessment (LCIA) for Human Health Cancerous and Noncancerous Emissions: Integrated and Complementary with Consistency within the USEPA Hum. Ecol. Risk Assess. 2006, 12, 493– 509DOI: 10.1080/10807030600561683 [DOI] [Google Scholar]
- 26.Dionisio KL; Frame AM; Goldsmith M-R; Wambaugh JF; Liddell A; Cathey T; Smith D; Vail J; Ernstoff AS; Fantke P; Jolliet O; Judson RS Exploring consumer exposure pathways and patterns of use for chemicals in the environment Toxicol. Rep. 2015, 2, 228– 237DOI: 10.1016/j.toxrep.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodson RE; Nishioka M; Standley LJ; Perovich LJ; Brody JG; Rudel RA Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products Environ. Health Perspect. 2012, 120 (7) 935– 943DOI: 10.1289/ehp.1104052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaacs KK; Glen WG; Egeghy P; Goldsmith MR; Smith L; Vallero D; Brooks R; Grulke CM; Özkaynak H SHEDS-HT: An Integrated Probabilistic Exposure Model for Prioritizing Exposures to Chemicals with Near-Field and Dietary Sources Environ. Sci. Technol. 2014, 48 (21) 12750– 12759DOI: 10.1021/es502513w [DOI] [PubMed] [Google Scholar]
- 29.Wambaugh JF; Wang A; Dionisio KL; Frame A; Egeghy P; Judson R; Setzer RW High throughput heuristics for prioritizing human exposure to environmental chemicals Environ. Sci. Technol. 2014, 48 (21) 12760– 7DOI: 10.1021/es503583j [DOI] [PubMed] [Google Scholar]
- 30.Egeghy PP; Sheldon LS; Isaacs KK; Özkaynak H; Goldsmith M-R; Wambaugh JF; Judson RS; Buckley TJ Computational Exposure Science: An Emerging Discipline to Support 21st-Century Risk Assessment Environ. Health Perspect. 2016, 124 (6) 697– 702DOI: 10.1289/ehp.1509748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin HM; Ernstoff A; Arnot JA; Wetmore BA; Csiszar SA; Fantke P; Zhang XM; McKone TE; Jolliet O; Bennett DH Risk-Based High-Throughput Chemical Screening and Prioritization using Exposure Models and in Vitro Bioactivity Assays Environ. Sci. Technol. 2015, 49 (11) 6760– 6771DOI: 10.1021/acs.est.5b00498 [DOI] [PubMed] [Google Scholar]
- 32.Harder R; Holmquist H; Molander S; Svanström M; Peters GM Review of Environmental Assessment Case Studies Blending Elements of Risk Assessment and Life Cycle Assessment Environ. Sci. Technol. 2015, 49 (22) 13083– 13093DOI: 10.1021/acs.est.5b03302 [DOI] [PubMed] [Google Scholar]
- 33.Burke TA; Doull J; McKone T; Paustenbach DJ; Scheuplein R; Udo de Haes HA; Young JS Human Health Impact Assessment in Life Cycle Assessment: Analysis by an Expert Panel; ILSI Health and Environmental Sciences Institute: Washington, DC, 1996. [Google Scholar]
- 34.Embry MR; Bachman AN; Bell DR; Boobis AR; Cohen SM; Dellarco M; Dewhurst IC; Doerrer NG; Hines RN; Moretto A; Pastoor TP; Phillips RD; Rowlands JC; Tanir JY; Wolf DC; Doe JE Risk assessment in the 21st century: roadmap and matrix Crit. Rev. Toxicol. 2014, 44 (sup3) 6– 16DOI: 10.3109/10408444.2014.931924 [DOI] [PubMed] [Google Scholar]
- 35.National Research Council, A Framework to Guide Selection of Chemical Alternatives; The National Academies Press: Washington, DC, 2014. [PubMed] [Google Scholar]
- 36.Potting J; Hauschild M; Wenzel H Less is better” and “only above threshold”: Two incompatible paradigms for human toxicity in life cycle assessment? Int. J. Life Cycle Assess. 1999, 4 (1) 16– 24DOI: 10.1007/BF02979391 [DOI] [Google Scholar]
- 37.Udo de Haes HA; Sleeswijk AW; Heijungs R Similarities, Differences and Synergisms Between HERA and LCA—An Analysis at Three Levels Hum. Ecol. Risk Assess. 2006, 12 (3) 431– 449DOI: 10.1080/10807030600561659 [DOI] [Google Scholar]
- 38.Frank R Lautenberg Chemical Safety for the 21st Century Act, H.R. 2576, 2016, 114th Congress, Washington, DC, USA. [Google Scholar]
- 39.National Research Council, Science and Decisions: Advancing Risk Assessment; The National Academies Press: Washington, DC, 2009. [PubMed] [Google Scholar]
- 40.National Research Council, Sustainability and the US EPA: Committee on Incorporating Sustainability in the US Environmental Protection Agency; The National Academies Press: Washington, DC, 2011. [Google Scholar]
- 41.Hertwich EG; McKone TE Pollutant-specific scale of multimedia models and its implications for the potential dose Environ. Sci. Technol. 2001, 35 (1) 142– 8DOI: 10.1021/es9911061 [DOI] [PubMed] [Google Scholar]
- 42.Bare JC; Norris GA; Pennington DW; McKone T Traci: The Tool for the Reduction and Assessment of Chemical and Other Environmental Impacts J. Ind. Ecol. 2002, 6 (3–4) 49– 78DOI: 10.1162/108819802766269539 [DOI] [Google Scholar]
- 43.Hertwich E; McKone T; Pease W Parameter uncertainty and variability in evaluative fate and exposure models Risk Anal. 1999, 19, 1193– 1204DOI: 10.1111/j.1539-6924.1999.tb01138.x [DOI] [PubMed] [Google Scholar]
- 44.Shatkin JA Informing Environmental Decision Making by Combining Life Cycle Assessment and Risk Analysis J. Ind. Ecol. 2008, 12 (3) 278– 281DOI: 10.1111/j.1530-9290.2008.00031.x [DOI] [Google Scholar]
- 45.Lim SR; Lam CW; Schoenung JM Priority screening of toxic chemicals and industry sectors in the U.S. toxics release inventory: a comparison of the life cycle impact-based and risk-based assessment tools developed by U.S. EPA J. Environ. Manage. 2011, 92 (9) 2235– 40DOI: 10.1016/j.jenvman.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 46.Askham C; Gade AL; Hanssen OJ Linking chemical risk information with life cycle assessment in product development J. Cleaner Prod. 2013, 51, 196– 204DOI: 10.1016/j.jclepro.2013.01.006 [DOI] [Google Scholar]
- 47.Meyer D; Upadhyayula VK The use of life cycle tools to support decision making for sustainable nanotechnologies Clean Technol. Environ. Policy 2014, 16 (4) 757– 772DOI: 10.1007/s10098-013-0686-3 [DOI] [Google Scholar]
- 48.Kobayashi Y; Peters GM; Khan SJ Towards More Holistic Environmental Impact Assessment: Hybridisation of Life Cycle Assessment and Quantitative Risk Assessment Procedia CIRP 2015, 29, 378– 383DOI: 10.1016/j.procir.2015.01.064 [DOI] [Google Scholar]
- 49.Sexton K; Linder SH Integrated Assessment of Risk and Sustainability in the Context of Regulatory Decision Making Environ. Sci. Technol. 2014, 48 (3) 1409– 1418DOI: 10.1021/es4043066 [DOI] [PubMed] [Google Scholar]
- 50.Cowell SJ; Fairman R; Lofstedt RE Use of risk assessment and life cycle assessment in decision making: a common policy research agenda Risk Anal. 2002, 22 (5) 879– 94DOI: 10.1111/1539-6924.00258 [DOI] [PubMed] [Google Scholar]
- 51.Barberio G; Scalbi S; Buttol P; Masoni P; Righi S Combining life cycle assessment and qualitative risk assessment: The case study of alumina nanofluid production Sci. Total Environ. 2014, 496, 122– 131DOI: 10.1016/j.scitotenv.2014.06.135 [DOI] [PubMed] [Google Scholar]
- 52.Shatkin JA Nano LCRA: An Adaptive Screening-Level Life Cycle Risk-Assessment Framework for Nanotechnology In Nanotechnology: Health and Environmental Risks, 2nd ed.; Shatkin JA, Ed.; CRC Press: Boca Raton, FL, 2012; pp 149– 170. [Google Scholar]
- 53.Organisation for Economic Co-operation and Development (OECD), Guidance Manual Towards the Integration of Risk Assessment into Life Cycle Assessment of Nano-Enabled Applications; Paris, France, 2015. [Google Scholar]
- 54.Judson R; Houck K; Martin M; Knudsen T; Thomas RS; Sipes N; Shah I; Wambaugh J; Crofton K In Vitro and Modelling Approaches to Risk Assessment from the US Environmental Protection Agency ToxCast Programme Basic Clin. Pharmacol. Toxicol. 2014, 115 (1) 69– 76DOI: 10.1111/bcpt.12239 [DOI] [PubMed] [Google Scholar]
- 55.Thomas RS; Philbert MA; Auerbach SS; Wetmore BA; Devito MJ; Cote I; Rowlands JC; Whelan MP; Hays SM; Andersen ME; Meek ME; Reiter LW; Lambert JC; Clewell HJ 3rd; Stephens ML; Zhao QJ; Wesselkamper SC; Flowers L; Carney EW; Pastoor TP; Petersen DD; Yauk CL; Nong A Incorporating new technologies into toxicity testing and risk assessment: moving from 21st century vision to a data-driven framework Toxicol. Sci. 2013, 136 (1) 4– 18DOI: 10.1093/toxsci/kft178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen Hubal EA; Richard A; Aylward L; Edwards S; Gallagher J; Goldsmith MR; Isukapalli S; Tornero-Velez R; Weber E; Kavlock R Advancing exposure characterization for chemical evaluation and risk assessment J. Toxicol. Environ. Health, Part B 2010, 13 (2–4) 299– 313DOI: 10.1080/10937404.2010.483947 [DOI] [PubMed] [Google Scholar]
- 57.Todd JA; Curran MA Streamlined Life-Cycle Assessment: A Final Report from the SETAC North America Streamlined LCA Workgroup; Society of Environmental Toxicology and Chemistry (SETAC) and SETAC Foundation for Environmental Education: 1999. [Google Scholar]
- 58.Hochschorner E; Finnveden G Evaluation of two simplified Life Cycle assessment methods Int. J. Life Cycle Assess. 2003, 8 (3) 119– 128DOI: 10.1007/BF02978456 [DOI] [Google Scholar]
- 59.Judson RS; Kavlock RJ; Setzer RW; Hubal EA; Martin MT; Knudsen TB; Houck KA; Thomas RS; Wetmore BA; Dix DJ Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment Chem. Res. Toxicol. 2011, 24 (4) 451– 462DOI: 10.1021/tx100428e [DOI] [PubMed] [Google Scholar]
- 60.Kavlock R; Chandler K; Houck K; Hunter S; Judson R; Kleinstreuer N; Knudsen T; Martin M; Padilla S; Reif D; Richard A; Rotroff D; Sipes N; Dix D Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management Chem. Res. Toxicol. 2012, 25 (7) 1287– 302DOI: 10.1021/tx3000939 [DOI] [PubMed] [Google Scholar]
- 61.Dix DJ; Houck KA; Martin MT; Richard AM; Setzer RW; Kavlock RJ The ToxCast program for prioritizing toxicity testing of environmental chemicals Toxicol. Sci. 2006, 95 (1) 5– 12DOI: 10.1093/toxsci/kfl103 [ Crossref], [ PubMed], [DOI] [PubMed] [Google Scholar]
- 62.USEtox USEtox Model version 2.0: UNEP/SETAC scientific consensus model for characterizing human and ecotoxicological impacts of chemical emissions in life cycle impact assessments, 2015. http://www.usetox.org/ (accessed Sept 30, 2016).
- 63.Pennington DW; Margni M; Payet J; Jolliet O Risk and Regulatory Hazard-Based Toxicological Effect Indicators in Life-Cycle Assessment (LCA) Hum. Ecol. Risk Assess. 2006, 12 (3) 450– 475DOI: 10.1080/10807030600561667 [DOI] [Google Scholar]
- 64.International Organization for Standardization, ISO 14044: Environmental management- Life cycle assessment - Requirements and guidelines. In International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- 65.McKone T; Hertwich E The human toxicity potential and a Strategy for Evaluating Model Performance in Life Cycle Impact Assessment Int. J. Life Cycle Assess. 2001, 6 (2) 106– 109DOI: 10.1007/BF02977846 [DOI] [Google Scholar]
- 66.Bennett DH; McKone TE; Evans JS; Nazaroff WW; Margni MD; Jolliet O; Smith KR Defining intake fraction Environ. Sci. Technol. 2002, 36 (9) 206A– 211ADOI: 10.1021/es0222770 [ ACS Full Text ACS Full Text], [DOI] [PubMed] [Google Scholar]
- 67.Rosenbaum R; Huijbregts M; Henderson A; Margni M; McKone TE; van de Meent D; Hauschild M; Shaked S; Li DS; Gold LS; Jolliet O USEtox human exposure and toxicity factors for comparative assessment of toxic emissions in life cycle analysis: sensitivity to key chemical properties Int. J. Life Cycle Assess. 2011, 16, 710– 727DOI: 10.1007/s11367-011-0316-4 [DOI] [Google Scholar]
- 68.Huijbregts MA; Rombouts LJ; Ragas AM; van de Meent D Human-toxicological effect and damage factors of carcinogenic and noncarcinogenic chemicals for life cycle impact assessment Integr. Environ. Assess. Manage. 2005, 1 (3) 181– 244DOI: 10.1897/2004-007R.1 [DOI] [PubMed] [Google Scholar]
- 69.Gilbertson LM; Wender BA; Zimmerman JB; Eckelman MJ Coordinating modeling and experimental research of engineered nanomaterials to improve life cycle assessment studies Environ. Sci.: Nano 2015, 2 (6) 669– 682DOI: 10.1039/C5EN00097A [DOI] [Google Scholar]
- 70.Jolliet O; Ernstoff AS; Csiszar SA; Fantke P Defining Product Intake Fraction to Quantify and Compare Exposure to Consumer Products Environ. Sci. Technol. 2015, 49 (15) 8924– 8931DOI: 10.1021/acs.est.5b01083 [DOI] [PubMed] [Google Scholar]
- 71.Little JC; Weschler CJ; Nazaroff WW; Liu Z; Cohen Hubal EA Rapid methods to estimate potential exposure to semivolatile organic compounds in the indoor environment Environ. Sci. Technol. 2012, 46 (20) 11171– 8DOI: 10.1021/es301088a [DOI] [PubMed] [Google Scholar]
- 72.Huang L; Jolliet O A parsimonious model for the release of volatile organic compounds (VOCs) encapsulated in products Atmos. Environ. 2016, 127, 223– 235DOI: 10.1016/j.atmosenv.2015.12.001 [DOI] [Google Scholar]
- 73.Liang Y; Xu Y Emission of Phthalates and Phthalate Alternatives from Vinyl Flooring and Crib Mattress Covers: The Influence of Temperature Environ. Sci. Technol. 2014, 48 (24) 14228– 14237DOI: 10.1021/es504801x [DOI] [PubMed] [Google Scholar]
- 74.Goldsmith MR; Grulke CM; Brooks RD; Transue TR; Tan YM; Frame A; Egeghy PP; Edwards R; Chang DT; Tornero-Velez R; Isaacs K; Wang A; Johnson J; Holm K; Reich M; Mitchell J; Vallero DA; Phillips L; Phillips M; Wambaugh JF; Judson RS; Buckley TJ; Dary CC Development of a consumer product ingredient database for chemical exposure screening and prioritization Food Chem. Toxicol. 2014, 65, 269– 79DOI: 10.1016/j.fct.2013.12.029 [DOI] [PubMed] [Google Scholar]
- 75.Bennett DH; Furtaw EJ Jr. Fugacity-based indoor residential pesticide fate model Environ. Sci. Technol. 2004, 38 (7) 2142– 52DOI: 10.1021/es034287m [DOI] [PubMed] [Google Scholar]
- 76.Zhang X; Diamond ML; Ibarra C; Harrad S Multimedia modeling of polybrominated diphenyl ether emissions and fate indoors Environ. Sci. Technol. 2009, 43 (8) 2845– 50DOI: 10.1021/es802172a [DOI] [PubMed] [Google Scholar]
- 77.Zhang X; Arnot JA; Wania F Model for screening-level assessment of near-field human exposure to neutral organic chemicals released indoors Environ. Sci. Technol. 2014, 48 (20) 12312– 9DOI: 10.1021/es502718k [DOI] [PubMed] [Google Scholar]
- 78.Shin HM; McKone TE; Bennett DH Intake fraction for the indoor environment: a tool for prioritizing indoor chemical sources Environ. Sci. Technol. 2012, 46 (18) 10063– 72DOI: 10.1021/es3018286 [DOI] [PubMed] [Google Scholar]
- 79.Huang L; Ernstoff A; Fantke P; Csiszar SA; Jolliet O A review of models for near-field exposure pathways of chemicals in consumer products Sci. Total Environ. 2016, DOI: 10.1016/j.scitotenv.2016.06.118 [DOI] [PubMed] [Google Scholar]
- 80.van Zelm R; Huijbregts MAJ; van de Meent D USES-LCA 2.0—a global nested multi-media fate, exposure, and effects model Int. J. Life Cycle Assess. 2009, 14 (3) 282– 284DOI: 10.1007/s11367-009-0066-8 [DOI] [Google Scholar]
- 81.Csiszar SA; Ernstoff AS; Fantke P; Meyer DE; Jolliet O High-throughput exposure modeling to support prioritization of chemicals in personal care products Chemosphere 2016, 163, 490– 498DOI: 10.1016/j.chemosphere.2016.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ernstoff AS; Fantke P; Csiszar SA; Henderson AD; Chung S; Jolliet O Multi-pathway exposure modelling of chemicals in cosmetics with application to shampoo Environ. Int. 2016, 92–93, 87– 86DOI: 10.1016/j.envint.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 83.Sleeswijk A General prevention and risk minimization in LCA A combined approach Environ. Sci. Pollut. Res. 2003, 10 (1) 69– 77DOI: 10.1065/espr2001.09.090 [DOI] [PubMed] [Google Scholar]
- 84.Jayjock MA; Chaisson CF; Franklin CA; Arnold S; Price PS Using publicly available information to create exposure and risk-based ranking of chemicals used in the workplace and consumer products J. Exposure Sci. Environ. Epidemiol. 2009, 19 (5) 515– 24DOI: 10.1038/jes.2008.43 [DOI] [PubMed] [Google Scholar]
- 85.Jones-Otazo HA; Clarke JP; Diamond ML; Archbold JA; Ferguson G; Harner T; Richardson GM; Ryan JJ; Wilford B Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs Environ. Sci. Technol. 2005, 39 (14) 5121– 30DOI: 10.1021/es048267b [DOI] [PubMed] [Google Scholar]
- 86.Roberts JW; Wallace LA; Camann DE; Dickey P; Gilbert SG; Lewis RG; Takaro TK Monitoring and Reducing Exposure of Infants to Pollutants in House Dust In Reviews of Environmental Contamination and Toxicology Vol 201; Whitacre MD, Ed.; Springer US: Boston, MA, 2009; pp 1– 39. [DOI] [PubMed] [Google Scholar]
- 87.U.S. Environmental Protection Agency, Guidelines for Exposure Assessment; EPA/600/Z-92/001; Risk Assessment Forum, U.S. EPA: Washington, DC, 1992. [Google Scholar]
- 88.National Research Council, Toxicity Testing in the 21st Century: A Vision and a Strategy; The National Academy of Sciences: Washington, DC, 2007. [Google Scholar]
- 89.Andersen ME; Krewski D Toxicity testing in the 21st century: bringing the vision to life Toxicol. Sci. 2008, 107 (2) 324– 330DOI: 10.1093/toxsci/kfn255 [ Crossref], [ PubMed], [DOI] [PubMed] [Google Scholar]
- 90.Krewski D; Acosta D Jr.; Andersen M; Anderson H; Bailar JC 3rd; Boekelheide K; Brent R; Charnley G; Cheung VG; Green S Jr.; Kelsey KT; Kerkvliet NI; Li AA; McCray L; Meyer O; Patterson RD; Pennie W; Scala RA; Solomon GM; Stephens M; Yager J; Zeise L Toxicity testing in the 21st century: a vision and a strategy J. Toxicol. Environ. Health, Part B 2010, 13 (2–4) 51– 138DOI: 10.1080/10937404.2010.483176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kavlock RJ; Austin CP; Tice RR Toxicity testing in the 21st century: implications for human health risk assessment Risk Anal. 2009, 29 (4) 485– 7discussion 492–7.DOI: 10.1111/j.1539-6924.2008.01168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andersen ME Toxicokinetic modeling and its applications in chemical risk assessment Toxicol. Lett. 2003, 138 (1–2) 9– 27DOI: 10.1016/S0378-4274(02)00375-2 [DOI] [PubMed] [Google Scholar]
- 93.Wetmore BA; Wambaugh JF; Ferguson SS; Sochaski MA; Rotroff DM; Freeman K; Clewell HJ; Dix DJ; Andersen ME; Houck KA; Allen B; Judson RS; Singh R; Kavlock RJ; Richard AM; Thomas RS Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment Toxicol. Sci. 2012, 125 (1) 157– 174DOI: 10.1093/toxsci/kfr254 [DOI] [PubMed] [Google Scholar]
- 94.Rotroff DM; Wetmore BA; Dix DJ; Ferguson SS; Clewell HJ; Houck KA; Lecluyse EL; Andersen ME; Judson RS; Smith CM; Sochaski MA; Kavlock RJ; Boellmann F; Martin MT; Reif DM; Wambaugh JF; Thomas RS Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening Toxicol. Sci. 2010, 117 (2) 348– 58DOI: 10.1093/toxsci/kfq220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wetmore BA; Wambaugh JF; Allen B; Ferguson SS; Sochaski MA; Setzer RW; Houck KA; Strope CL; Cantwell K; Judson RS; LeCluyse E; Clewell HJ; Thomas RS; Andersen ME Incorporating High-Throughput Exposure Predictions With Dosimetry-Adjusted In Vitro Bioactivity to Inform Chemical Toxicity Testing Toxicol. Sci. 2015, 148 (1) 121– 36DOI: 10.1093/toxsci/kfv171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kavlock RJ; Ankley G; Blancato J; Breen M; Conolly R; Dix D; Houck K; Hubal E; Judson R; Rabinowitz J; Richard A; Setzer RW; Shah I; Villeneuve D; Weber E Computational toxicology--a state of the science mini review Toxicol. Sci. 2007, 103 (1) 14– 27DOI: 10.1093/toxsci/kfm297 [ Crossref], [ PubMed], [DOI] [PubMed] [Google Scholar]
- 97.U.S. Environmental Protection Agency, A framework for assessing health risks of environmental exposures to children; EPA/600/R-05/093F; National Center for Environmental Assessment, U.S. EPA: Washington, DC, 2006. [Google Scholar]
- 98.Wetmore BA; Allen B; Clewell HJ 3rd; Parker T; Wambaugh JF; Almond LM; Sochaski MA; Thomas RS Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing Toxicol. Sci. 2014, 142 (1) 210– 24DOI: 10.1093/toxsci/kfu169 [DOI] [PubMed] [Google Scholar]
- 99.Krewitt W; Pennington DW; Olsen SI; Crettaz P; Jolliet O Indicators for human toxicity in Life Cycle Impact Assessment. In Life-cycle impact assessment: striving toward best practice; Udo de Haes HA; Finnveden G; Goedkoop M; Hauschild M; Hertwich EG; Hofstetter P; Jolliet O; Klopffer W; Krewitt W; Lindeijer E; Muller-Wenk R; Olsen SI; Potting J; Steen B, Eds.; Society of Environmental Toxicology and Chemistry (SETAC): Pensacola, FL, 2002. [Google Scholar]
- 100.U.S. Environmental Protection Agency, A Review of the Reference Dose and Reference Concentration Processes; Risk Assessment Forum: Washington, DC, 2002. [Google Scholar]
- 101.Bare JC; Udo de Haes HA; Pennington DW Life cycle impact assessment sophistication Int. J. Life Cycle Assess. 1999, 4 (5) 299– 306DOI: 10.1007/BF02979184 [DOI] [Google Scholar]
- 102.Environmental Protection Agency US, A review of the reference dose and reference concentration processes; EPA/630/P-02/002F; Risk Assessment Forum, U.S. EPA: Washington, DC, 2002. [Google Scholar]
- 103.International Programme on Chemical Safety, WHO Human Health Risk Assessment Toolkit: Chemical Hazards; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- 104.Chiu WA; Slob W A Unified Probabilistic Framework for Dose-Response Assessment of Human Health Effects Environ. Health Perspect. 2015, 123 (12) 1241– 54DOI: 10.1289/ehp.1409385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKone ET; Kyle DA; Jolliet O; Irving Olsen S; Hauschild M Dose-Response Modeling for Life Cycle Impact Assessment - Findings of the Portland Review Workshop Int. J. Life Cycle Assess. 2006, 11 (2) 137– 140DOI: 10.1065/lca2006.02.005 [DOI] [Google Scholar]
- 106.Pastoor TP; Bachman AN; Bell DR; Cohen SM; Dellarco M; Dewhurst IC; Doe JE; Doerrer NG; Embry MR; Hines RN; Moretto A; Phillips RD; Rowlands JC; Tanir JY; Wolf DC; Boobis ARA 21st century roadmap for human health risk assessment Crit. Rev. Toxicol. 2014, 44 (sup3) 1– 5DOI: 10.3109/10408444.2014.931923 [DOI] [PubMed] [Google Scholar]
- 107.Environmental Protection Agency US, Exposure SAP White Paper New High-throughput Methods to Estimate Chemical Exposure; EPA-HQ-OPP-2014–0331-0005; Scientific Advisory Panel Meeting, July 2014, 2014. https://www.regulations.gov/document?D=EPA-HQ-OPP-2014-0331-0005 (accessed Sept 30, 2016). [Google Scholar]
- 108.Guerrero PA; Corsi RL Emissions of p-dichlorobenzene and naphthalene from consumer products J. Air Waste Manage. Assoc. 2012, 62 (9) 1075– 84DOI: 10.1080/10962247.2012.694399 [DOI] [PubMed] [Google Scholar]
- 109.Shinohara N; Ono K; Gamo M P-dichlorobenzene emission rates from moth repellents and leakage rates from cloth storage cases Indoor Air 2008, 18 (1) 63– 71DOI: 10.1111/j.1600-0668.2007.00508.x [DOI] [PubMed] [Google Scholar]
- 110.Liu W; Zheng M; Wang D; Xing Y; Zhao X; Ma X; Qian Y Formation of PCDD/Fs and PCBs in the process of production of 1,4-dichlorobenzene Chemosphere 2004, 57 (10) 1317– 1323DOI: 10.1016/j.chemosphere.2004.09.024 [DOI] [PubMed] [Google Scholar]
- 111.U.S. NIH, Household Products Database: Health & SafetyInformation on Household Products. U.S. National Institutes of Health, Health & Human Services, National Library of Medicine, Bethesda, MD, 2015. https://householdproducts.nlm.nih.gov/ (accessed Sept 30, 2016). [Google Scholar]
- 112.U.S. Environmental Protection Agency, Integrated Risk Information System (IRIS), Chemical Assessment Summary: 1,4-Dichlorobenzene; CASRN 106–46-7; National Center for Environmental Assessment, U.S. EPA: Washington, DC, 1994. [Google Scholar]
- 113.European Chemicals Agency (ECHA), Annex XV Restriction Report, Proposal for a Restriction: 1,4-Dichlorobenzene; 2012.
- 114.Chin JY; Godwin C; Jia C; Robins T; Lewis T; Parker E; Max P; Batterman S Concentrations and risks of p-dichlorobenzene in indoor and outdoor air Indoor Air 2013, 23 (1) 40– 9DOI: 10.1111/j.1600-0668.2012.00796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.U.S. Department of Health and Human Services, Toxicological Profile for Dichlorobenzenes; Agency for Toxic Substances and Disease Registry: Atlanta, GA, 2006. [PubMed] [Google Scholar]
- 116.Csiszar SA; Ernstoff AS; Fantke P; Jolliet O Stochastic modeling of near-field exposure to parabens in personal care products J. Exposure Sci. Environ. Epidemiol. 2016, DOI: 10.1038/jes.2015.85 [ Crossref], [ PubMed], [DOI] [PubMed] [Google Scholar]
- 117.Harrad S; Diamond M New directions: Exposure to polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs): Current and future scenarios Atmos. Environ. 2006, 40 (6) 1187– 1188DOI: 10.1016/j.atmosenv.2005.10.006 [DOI] [Google Scholar]
- 118.Ampleman MD; Martinez A; DeWall J; Rawn DF; Hornbuckle KC; Thorne PS Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements Environ. Sci. Technol. 2015, 49 (2) 1156– 64DOI: 10.1021/es5048039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harrad S; Hazrati S; Ibarra C Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: Implications for human exposure Environ. Sci. Technol. 2006, 40 (15) 4633– 4638DOI: 10.1021/es0609147 [DOI] [PubMed] [Google Scholar]
- 120.Allen JG; McClean MD; Stapleton HM; Nelson JW; Webster TF Personal exposure to Polybrominated Diphenyl Ethers (PBDEs) in residential indoor air Environ. Sci. Technol. 2007, 41 (13) 4574– 4579DOI: 10.1021/es0703170 [DOI] [PubMed] [Google Scholar]
- 121.Johnson PI; Stapleton HM; Sjodin A; Meeker JD Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum Environ. Sci. Technol. 2010, 44 (14) 5627– 5632DOI: 10.1021/es100697q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Egeghy P; Judson R; Gangwal S; Mosher S; Smith D; Vail J; Hubal EAC The exposure data landscape for manufactured chemicals Sci. Total Environ. 2012, 414, 159– 166DOI: 10.1016/j.scitotenv.2011.10.046 [DOI] [PubMed] [Google Scholar]
- 123.Breivik K; Arnot JA; Brown TN; McLachlan MS; Wania F Screening organic chemicals in commerce for emissions in the context of environmental and human exposure J. Environ. Monit. 2012, 14 (8) 2028– 37DOI: 10.1039/c2em30259d [DOI] [PubMed] [Google Scholar]
- 124.Cashman SA; Meyer DE; Ingwersen WW; Edelen A; Abraham J; Barrett W; Gonzalez MA; Randall P; Ruiz-Mercado GJ; Smith RL Mining Available Data from the United States Environmental Protection Agency to Support Rapid Life Cycle Inventory Modeling of Chemical Manufacturing Environ. Sci. Technol. 2016, 50, 9013DOI: 10.1021/acs.est.6b02160 [DOI] [PubMed] [Google Scholar]
- 125.MacDonell MM; Haroun LA; Teuschler LK; Rice GE; Hertzberg RC; Butler JP; Chang Y-S; Clark SL; Johns AP; Perry CS; Garcia SS; Jacobi JH; Scofield MA Cumulative Risk Assessment Toolbox: Methods and Approaches for the Practitioner J. Toxicol. 2013, 2013, 1– 36DOI: 10.1155/2013/310904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teeguarden JG; Tan Y-M; Edwards SW; Leonard JA; Anderson KA; Corley RA; Kile ML; Simonich SM; Stone D; Tanguay RL; Waters KM; Harper SL; Williams DE Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework Environ. Sci. Technol. 2016, 50 (9) 4579– 4586DOI: 10.1021/acs.est.5b05311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wegener Sleeswijk A; Heijungs R GLOBOX: A spatially differentiated global fate, intake and effect model for toxicity assessment in LCA Sci. Total Environ. 2010, 408 (14) 2817– 32DOI: 10.1016/j.scitotenv.2010.02.044 [DOI] [PubMed] [Google Scholar]
- 128.Ingwersen W; Hawkins T; Transue T; Meyer D; Moore G; Kahn E; Arbuckle P; Paulsen H; Norris G A new data architecture for advancing life cycle assessment Int. J. Life Cycle Assess. 2015, 20 (4) 520– 526DOI: 10.1007/s11367-015-0850-6 [DOI] [Google Scholar]
- 129.van Zelm R; Huijbregts MA J. Quantifying the Trade-off between Parameter and Model Structure Uncertainty in Life Cycle Impact Assessment Environ. Sci. Technol. 2013, 47 (16) 9274– 9280DOI: 10.1021/es305107s [DOI] [PubMed] [Google Scholar]
- 130.National Research Council, Exposure Science in the 21st Century: A Vision and a Strategy; The National Academies Press: Washington, DC, 2012. [PubMed] [Google Scholar]
- 131.Gavankar S; Suh S; Keller AF Life cycle assessment at nanoscale: review and recommendations Int. J. Life Cycle Assess. 2012, 17 (3) 295– 303DOI: 10.1007/s11367-011-0368-5 [DOI] [Google Scholar]
- 132.Wender BA; Foley RW; Prado-Lopez V; Ravikumar D; Eisenberg DA; Hottle TA; Sadowski J; Flanagan WP; Fisher A; Laurin L; Bates ME; Linkov I; Seager TP; Fraser MP; Guston DH Illustrating Anticipatory Life Cycle Assessment for Emerging Photovoltaic Technologies Environ. Sci. Technol. 2014, 48 (18) 10531– 10538DOI: 10.1021/es5016923 [DOI] [PubMed] [Google Scholar]
- 133.Edwards SW; Tan YM; Villeneuve DL; Meek ME; McQueen CA Adverse Outcome Pathways-Organizing Toxicological Information to Improve Decision Making J. Pharmacol. Exp. Ther. 2016, 356 (1) 170– 81DOI: 10.1124/jpet.115.228239 [DOI] [PubMed] [Google Scholar]
- 134.Georgopoulos PG; Brinkerhoff CJ; Isukapalli S; Dellarco M; Landrigan PJ; Lioy PJ A tiered framework for risk-relevant characterization and ranking of chemical exposures: applications to the National Children’s Study (NCS) Risk Anal. 2014, 34 (7) 1299– 316DOI: 10.1111/risa.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Egeghy PP; Vallero DA; Cohen Hubal EA Exposure-based prioritization of chemicals for risk assessment Environ. Sci. Policy 2011, 14 (8) 950– 964DOI: 10.1016/j.envsci.2011.07.010 [DOI] [Google Scholar]
- 136.U.S. Environmental Protection Agency, Toxics Release Inventory(TRI) Program; U.S. EPA:Washington, DC, 2016. https://www.epa.gov/toxics-release-inventory-tri-program (accessed Sept 30, 2016). [Google Scholar]
- 137.U.S. Environmental Protection Agency, 2012 Chemical DataReporting Results; U.S. EPA: Washington, DC, 2014. https://www.epa.gov/chemical-data-reporting/2012-chemical-data-reporting-results (accessed Sept 30, 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.