Abstract
Background
Paracetamol has been commonly used for the relief of postoperative pain following oral surgery. In this review we investigated the optimal dose of paracetamol and the optimal time for drug administration to provide pain relief, taking into account the side effects of different doses of the drug. This will inform dentists and their patients of the best strategy for pain relief after the surgical removal of wisdom teeth.
Objectives
To assess the beneficial and harmful effects of paracetamol for pain relief after surgical removal of lower wisdom teeth, compared to placebo, at different doses and administered postoperatively.
Search methods
We searched the Cochrane Oral Health Group's Trials Register; the Cochrane Pain, Palliative and Supportive Care Group's Trials Register; CENTRAL; MEDLINE; EMBASE and the Current Controlled Trials Register. Handsearching included several dental journals. We checked the bibliographies of relevant clinical trials and review articles for studies outside the handsearched journals. We wrote to authors of the identified randomised controlled trials (RCTs), to manufacturers of analgesic pharmaceuticals, we searched personal references in an attempt to identify unpublished or ongoing RCTs. No language restriction was applied. The last electronic search was conducted on 24th August 2006.
Selection criteria
Randomised, parallel group, placebo controlled, double blind clinical trials of paracetamol for acute pain, following third molar surgery.
Data collection and analysis
All trials identified were scanned independently and in duplicate by two review authors, any disagreements were resolved by discussion, or if necessary a third review author was consulted. The proportion of patients with at least 50% pain relief was calculated for both paracetamol and placebo. The number of patients experiencing adverse events, and/or the total number of adverse events reported were analysed.
Main results
Twenty‐one trials met the inclusion criteria. A total of 2048 patients were initially enrolled in the trials (1148 received paracetamol, and 892 the placebo) and of these 1968 (96%) were included in the meta‐analysis (1133 received paracetamol, and 835 the placebo). Paracetamol provided a statistically significant benefit when compared with placebo for pain relief and pain intensity at both 4 and 6 hours. Most studies were found to have moderate risk of bias, with poorly reported allocation concealment being the main problem. Risk ratio values for pain relief at 4 hours 2.85 (95% confidence interval (CI) 1.89 to 4.29), and at 6 hours 3.32 (95% CI 1.88 to 5.87). A statistically significant benefit was also found between up to 1000 mg and 1000 mg doses, the higher the dose giving greater benefit for each measure at both time points. There was no statistically significant difference between the number of patients who reported adverse events, overall this being 19% in the paracetamol group and 16% in the placebo group.
Authors' conclusions
Paracetamol is a safe, effective drug for the treatment of postoperative pain following the surgical removal of lower wisdom teeth.
Keywords: Humans; Acetaminophen; Acetaminophen/adverse effects; Acetaminophen/therapeutic use; Analgesics, Non‐Narcotic; Analgesics, Non‐Narcotic/adverse effects; Analgesics, Non‐Narcotic/therapeutic use; Molar, Third; Molar, Third/surgery; Outcome Assessment, Health Care; Pain Measurement; Pain, Postoperative; Pain, Postoperative/drug therapy; Randomized Controlled Trials as Topic; Tooth Extraction; Tooth Extraction/adverse effects
Plain language summary
Paracetamol for pain relief after surgical removal of lower wisdom teeth
The surgical removal of wisdom teeth (third molars) is the most commonly performed surgical procedure undertaken in oral surgery practice. Postoperative complications may include swelling, bruising and limited mouth opening but patients are most often concerned about postoperative pain, which may be severe. Paracetamol is effective in relieving pain with a low incidence of adverse effects. It is one of the most commonly used analgesics and is widely available without prescription around the world. In this review we investigated the optimal dose of paracetamol and the optimal time for drug administration to provide pain relief after the surgical removal of wisdom teeth. The side effects of different doses of the drug were also explored.
Twenty‐one trials (with over 2000 participants) were included. Paracetamol provided a statistically significant benefit when compared with placebo for pain relief at both 4 and 6 hours after taking the drug. It is most effective at 1000 mg dose, and can be taken at six hourly intervals without compromising safety. There was no statistically significant difference between the number of patients who reported adverse events, overall this being 19% in the paracetamol group and 16% in the placebo group. It should be noted that most of the studies were found to have some limitations mainly due to poor reporting of information. However the review concludes that paracetamol is a safe, effective drug for the treatment of postoperative pain following the surgical removal of lower wisdom teeth.
Background
The surgical removal of wisdom teeth is the most commonly performed surgical procedure undertaken in oral surgery practice. Postoperative complications may include swelling, bruising and limited mouth opening but patients are most often concerned about postoperative pain, which may be severe. The pain experienced after oral surgery is a validated and widely used pain model for the clinical evaluation of analgesic efficacy (Cooper 1976). Tissue damage produced during surgery releases chemicals that initiate inflammatory pain by activating and sensitising nerve fibre receptors (Loeser 1999). Chemicals include bradykinin, prostaglandins, serotonin and histamine (Dray 1997).
Paracetamol (acetaminophen) is a nonopioid analgesic possessing antipyretic activity and is effective in relieving pain with a low incidence of adverse effects (Moore 1998). It is one of the most commonly used analgesics and is widely available without prescription around the world. Paracetamol is often grouped with the nonsteroidal anti‐inflammatory drug (NSAID) family, however, it is considered only to have relatively weak anti‐inflammatory activity (Rang 2003). NSAIDs are assumed largely to produce their analgesia as a result of the inhibition of prostaglandin production by the enzyme cyclo‐oxygenase (Malmberg 1992). The mechanism of action has not been fully understood. Among several theories it has been suggested that paracetamol is a selective inhibitor of the newly described COX‐3 enzyme, a cyclo‐oxygenase‐1 variant, in the central nervous system. This inhibition could represent a primary central mechanism by which paracetamol decreases pain and possibly fever (Chandrasekharan 2002). Major evidence has been accumulated showing that paracetamol inhibits cyclo‐oxygenase by reducing the higher oxidative state of the cyclo‐oxygenase enzyme, by reducing oxygen radical co‐substrates (Aronoff 2006). Paracetamol has been shown to be an effective analgesic in the control of postoperative dental pain in a number of clinical trials (Bentley 1987; Kiersch 1994; Mehlisch 1990). Pain intensity following third molar surgery has been suggested to reach its maximum between 3 to 5 hours following surgery (Fisher 1988; Seymour 1985) and therefore this pain model is used to test the efficacy of a single analgesic dose.
A recent systematic review (Barden J 2004) has looked at the efficacy and safety of paracetamol for postoperative pain management and has included the findings of studies involving a wide variety of types of surgery such as gynaecology surgery, abdominal surgery, orthopaedic surgery amongst others including the removal of wisdom teeth. There is some debate as to whether dental pain is different from other pain. It has been suggested that the effect of some analgesics including tramadol were worse for dental pain than for other types of postsurgical pain (Moore 1997).
In this review we investigated the optimal dose of paracetamol and the optimal time for drug administration to provide pain relief, taking into account the side effects of different doses of the drug. This will inform dentists and their patients of the best strategy for best pain relief after the surgical removal of wisdom teeth.
Objectives
To assess the beneficial and harmful effects of paracetamol for pain relief after surgical removal of lower wisdom teeth, compared to placebo, at different doses and administered preoperatively or postoperatively.
Primary
To test the null hypothesis of no difference in the beneficial and harmful effects between paracetamol and placebo for pain relief in patients requiring surgical removal of a lower wisdom tooth or teeth, against the alternative hypothesis of a difference.
Secondary
To test the null hypothesis of no difference in the beneficial and harmful effects between different doses of paracetamol for pain relief in patients requiring surgical removal of a lower wisdom tooth or teeth, against the alternative hypothesis of a difference.
To test the null hypothesis of no difference in the beneficial and harmful effects between different times of administration of paracetamol for pain relief in patients requiring surgical removal of a lower wisdom tooth or teeth, against the alternative hypothesis of a difference.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled double blind clinical trials.
Types of participants
Patients of all health states who required the surgical removal of a lower wisdom tooth and who had at least had a baseline pain intensity of moderate to severe pain. Patients who also required removal of an additional tooth or teeth were included. Surgery was undertaken under local anaesthesia, intravenous sedation or general anaesthesia. Patients taking concurrent analgesia were excluded.
Types of interventions
Efficacy
Paracetamol given as a single dose by mouth in any dose and in any formulation (for example, immediate or slow release) regardless of when the single dose was given (for example, preoperatively or postoperatively).
Side effects
In order to investigate side effects more thoroughly, we included both single and multiple dose studies.
Paracetamol given up to 7 days by mouth in any dose and in any formulation (for example, immediate or slow release) regardless of when the first dose was given (for example, preoperatively or postoperatively).
Types of outcome measures
Pain intensity (visual analogue scale (VAS), categorical verbal rating, verbal numerical scale, global subjective efficacy ratings and other categorical rating scales).
Pain relief (VAS, categorical verbal rating, verbal numerical scale, global subjective efficacy ratings and other categorical rating scales) and derived pain relief outcomes extracted will be total pain relief (TOTPAR), summed pain intensity difference (SPID) over 4 and 6 hours.
Side effects (for example, hepatic and renal) (binary).
Search methods for identification of studies
To identify studies for inclusion or consideration in this review a detailed search strategy was developed for each database searched. These were based on the search strategy developed for MEDLINE but revised appropriately for each database. The search strategy combined a sensitive search strategy for randomised controlled trials (RCTs) revised from phases 1 and 2 of the Cochrane Sensitive Search Strategy for RCTs (as published in Appendix 5b in the Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 (updated September 2006)). The subject search used a combination of controlled vocabulary and free text terms based on the search strategy for searching CENTRAL (seeAppendix 1).
Databases to be searched
The Cochrane Oral Health Group's Trials Register (to 24th August 2006) The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2006, Issue 3) The Cochrane Pain, Palliative and Supportive Care Group's Trials Register (to 24th August 2006) MEDLINE (1966 to 24th August 2006) EMBASE (1980 to 25th August 2006) Current Controlled Trials Register (www.controlled‐trials.com) (to 24th August 2006).
The bibliographies of papers and review articles were checked for studies outside the handsearched journals. Personal references were also searched.
Language
There were no language restrictions and where necessary, translation into the English language of relevant studies was conducted.
Unpublished studies
Authors of RCTs identified were written to in order to obtain further information about the trial and to attempt to identify unpublished or ongoing studies. We also wrote to manufacturers of analgesic pharmaceuticals.
Handsearching
Several journals relevant to this review were handsearched as part of the Cochrane Oral Health Group's ongoing journal handsearching programme. The list of the dental journals handsearched by The Cochrane Collaboration can be found at http://www.ohg.cochrane.org/.
Data collection and analysis
The titles and abstracts (when available) of all reports identified were scanned independently and in duplicate by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained and assessed independently and in duplicate by two review authors to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent quality assessment and data extracted. Studies rejected at this or subsequent stages were recorded in the Characteristics of excluded studies table, and reasons for exclusion were recorded.
Quality assessment
The quality assessment of the included trials was undertaken independently and in duplicate by two review authors based on what is written in the articles. Only double blind trials were included in the review so blinding was not included in the quality assessment.
Two main quality criteria were examined. (1) Allocation concealment, recorded as: (A) Adequate ‐2 points (B) Unclear ‐ 1 point (C) Inadequate ‐ 0 points.
(2) Completeness of follow up (is there a clear explanation for withdrawals and drop outs in each treatment group?) assessed as: (A) Yes ‐ 1 point (B) No ‐ 0 points.
The agreement for the quality criteria between assessors was determined by Kappa statistics. After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. (A) Low risk of bias ‐ 3 points (plausible bias unlikely to seriously alter the results) if all criteria were met. (B) Moderate or high risk of bias ‐ 0 to 2 points. Moderate risk of bias ‐ plausible bias that raises some doubt about the results if one or more criteria are partly met (for example, when authors responded that they had made some attempts to conceal the allocation of patients, to give an explanation for withdrawals, but these attempts were not judged to be ideal, these criteria were categorised as 'partly'). High risk of bias ‐ plausible bias that seriously weakens confidence in the results if one or more criteria were not met as described in the Cochrane Handbook for Systematic Reviews of Interventions 4.2.6.
We also reported whether the authors of included trials have conducted a sample size calculation.
Data extraction
Data were extracted by two review authors independently and in duplicate using specially designed data extraction forms. Any disagreement was discussed and a third review author consulted where necessary. Authors were contacted for clarification of missing information. Data were excluded until further clarification was available if agreement could not be reached. For each trial the following data were recorded.
Year of publication, country of origin, setting and source of study funding.
Details of the participants including demographic characteristics and criteria for inclusion.
Details on the study design (parallel group or cross‐over design).
Details on the type of intervention.
Details of the outcomes reported, including method of assessment and time intervals.
Data synthesis
From the mean total pain relief (TOTPAR) or summed pain intensity difference (SPID) pain indices reported we computed a dichotomous outcome variable for the number of patients with at least 50% pain relief according to the methods outlined in a Cochrane review (Collins 1999). For each of the three objectives we examined the appropriateness of different continuous outcome measurements, and these were meta‐analysed and reported in the final review.
For dichotomous outcomes, the estimate of an intervention was expressed as risk ratios together with 95% confidence intervals. For continuous outcomes, mean differences and 95% confidence intervals were used to summarise the data for each trial.
Clinical heterogeneity was assessed by examining the types of participants, interventions and outcomes in each study. Meta‐analyses were conducted only with studies of similar comparisons reporting the same outcome measures. Risk ratios were used to combine dichotomous data, and mean differences for continuous data, using random‐effects models. The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed by means of Cochran's test for heterogeneity and any heterogeneity investigated. Where both visual analogue scale (VAS) and categorical scales were used to measure pain intensity or pain relief or both, the categorical data were used in the meta‐analysis as this was the most frequently used scale.
Subgroup analyses
Subgroup analyses were planned for studies.
Where patients underwent surgery with local anaesthesia alone, local anaesthesia and intravenous sedation, general anaesthesia alone and general anaesthesia with local anaesthetic.
Where different types of formulation of paracetamol were used: for instance, immediate release versus slow release.
Where different doses of paracetamol were used (1000 mg or more, and less than 1000 mg).
Where time of administration of paracetamol differs: preoperative versus postoperative.
Where TOTPAR was calculated using pain relief measures and pain intensity measures.
The difference between studies comparing up to 1000 mg doses with studies comparing 1000 mg or more, was examined by performing random‐effects metaregression analyses in Stata version 9.0 (Stata Corporation, USA) using the program Metareg.
The results of the metaregressions for comparing the two dose levels, up to 1000 mg and 1000 mg or more are presented in Additional Table 1.
1. Random‐effects metaregression: < 1000 mg vs 1000 mg, Paracetamol vs Placebo.
| Outcome | Number of studies | Slope estimate | 95% CI | Slope interpretation | P value |
| 50% pain relief at 4 hours (using pain relief) | 16 | 0.94 | (0.36 to 1.52) | more pain relief for higher doses | 0.001 |
| 50% pain relife at 6 hours (using pain relief) | 13 | 1.14 | (0.71 to 1.56) | more pain relief for higher doses | <0.001 |
| 50% pain relief at 4 hours (using pain intensity) | 16 | 0.23 | (‐0.84 to 1.30) | more pain relief for higher doses | 0.67 |
| 50% pain relief at 6 hours (using pain intensity) | 14 | 0.43 | (‐0.15 to 1.01) | more pain relief for higher doses | 0.15 |
Results
Description of studies
SeeCharacteristics of included studies and Characteristics of excluded studies tables.
Characteristics of the trial setting and investigators
Of the 67 eligible trials, 46 were excluded as shown in the excluded studies section. Of the 21 included studies, one was conducted in Denmark (Moller 2000), two in Germany (Kubitzek 2003; Lehnert 1990), one in Italy (Dolci 1994), one in Norway (Skoglund 1991), two in Puerto Rico (Olson 2001; Sunshine 1986), one in Thailand (Vattaraphudej 1986), two in the United Kingdom (Seymour 1996; Seymour 2003), and 11 in the United States of America (Cooper 1980; Cooper 1981; Cooper 1988; Cooper 1998; Dionne 1994; Forbes 1984b; Forbes 1989; Forbes 1990; Hersh 2000; Kiersch 1994; Mehlisch 1995). Six trials were conducted at university clinics (Cooper 1998; Hersh 2000; Moller 2000; Olson 2001; Sunshine 1986; Vattaraphudej 1986), five at private practices (Dionne 1994; Forbes 1984b; Forbes 1989; Forbes 1990; Kubitzek 2003), seven did not state a setting (Cooper 1981; Dolci 1994; Kiersch 1994; Mehlisch 1995; Seymour 1996; Seymour 2003; Skoglund 1991). One reported a single site (Cooper 1988), two reported two sites (Forbes 1989; Seymour 2003) and six specifically stated outpatients (Cooper 1980; Cooper 1988; Forbes 1989; Forbes 1990; Hersh 2000; Lehnert 1990). Seventeen trials were sponsored by industry (Cooper 1981; Cooper 1988; Cooper 1998; Dionne 1994; Forbes 1984b; Forbes 1989; Forbes 1990; Hersh 2000; Kiersch 1994; Kubitzek 2003; Lehnert 1990; Mehlisch 1995; Moller 2000; Olson 2001; Seymour 2003; Skoglund 1991; Sunshine 1986), one by a university grant (Vattaraphudej 1986), and it was unclear as whether the remaining three trials (Cooper 1980; Dolci 1994; Seymour 1996) were sponsored, but it is likely that they were from correspondence with some of the authors.
Characteristics of interventions
All included interventions were randomised, parallel group, and double blind. Eleven trials used doses of paracetamol of less than 1000 mg (Cooper 1980; Cooper 1981; Cooper 1988; Dionne 1994; Dolci 1994; Forbes 1984b; Forbes 1989; Forbes 1990; Seymour 1996; Sunshine 1986; Vattaraphudej 1986). Eleven trials used doses of 1000 mg (Cooper 1998; Hersh 2000; Kiersch 1994; Kubitzek 2003; Lehnert 1990; Mehlisch 1995; Moller 2000; Olson 2001; Seymour 1996; Seymour 2003; Skoglund 1991). One study (Seymour 1996) used both doses. Seven trials used paracetamol in tablet form (Dolci 1994; Forbes 1989; Kubitzek 2003; Mehlisch 1995; Moller 2000; Seymour 2003; Skoglund 1991). Seven trials used capsules (Forbes 1984b; Forbes 1989; Forbes 1990; Kiersch 1994; Lehnert 1990; Sunshine 1986; Vattaraphudej 1986). Two trials used caplets (Hersh 2000; Olson 2001) and one trial used effervescent tablets (Moller 2000). Five trials did not state what formulation was used (Cooper 1980; Cooper 1981; Cooper 1988; Cooper 1998; Dionne 1994). All trials used placebos in the same formulation as the intervention.
Characteristics of outcome measures
For all trials it was possible to calculate the number of patients with at least 50% total pain relief (TOTPAR) at either 4 hours, 6 hours or both. Pain intensity was measured in all but one trial (Kubitzek 2003), pain relief was measured in all but two trials (Kubitzek 2003; Seymour 2003). Kubitzek 2003 gave a figure for TOTPAR at six hours, and Seymour 2003 measured pain intensity only. Fifteen trials measured pain intensity at 4 hours using a 4‐point categorical scale of 0 to 3, where 0 was no pain at all and 3 was severe pain. Five trials measured pain intensity using a visual analogue scale (VAS) of 0 to 100 mm where 0 was no pain and 100 was the worst pain imaginable. Twelve trials measured pain intensity at 6 hours using a 4‐point categorical scale, where 0 was no pain and 3 was severe pain, and three trials measured pain intensity at 6 hours using a VAS of 0 to 100 mm, where 0 was no pain and 100 mm was the worst pain imaginable. Sixteen trials measured pain relief at 4 hours using a 5‐point categorical scale of 0 to 4, where 0 was none and 4 was complete pain relief, two trials measured pain relief at 4 hours using a VAS of 0 to 100 mm, in one trial 0 was none and 100 was complete relief, and in the other trial 0 was complete relief and 100 was no relief (these data were reversed for statistical purposes). Twelve trials measured pain relief at 6 hours using a 5‐point categorical scale of 0 to 4, where 0 was none and 4 was complete pain relief, two trials measured pain relief at 6 hours using a VAS of 0 to 100 mm, in one trial 0 was none and 100 was complete relief, and in the other trial 0 was complete relief and 100 was no relief (these data were reversed for statistical purposes). Adverse events and global assessments were recorded in most of the trials. Nineteen trials reported the number of patients with side effects, eight for doses of 1000 mg or more, and 15 for doses of less than 1000 mg. Fifteen trials reported the number of adverse events, seven for doses of 1000 mg or more, and eight for doses of less than 1000 mg. Fourteen trials recorded global assessment using a 5‐point categorical scale of either 0 to 4, or 1 to 5, where 0 or 1 was poor and 4 or 5 was excellent, and four trials used a 4‐point categorical scale of 0 to 3, where 0 was poor and 3 was excellent. (Additional Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17).
2. Number of patients with adverse events (< 1000 mg Paracetamol/Placebo).
| Author | Total: Paracetamol | AEs: Paracetamol | Total: Placebo | AEs: Placebo |
| Cooper 1980 | 37 | 2 | 37 | 6 |
| Cooper 1981 | 37 | 12 | 38 | 4 |
| Dionne 1994 | 27 | 7 | 25 | 5 |
| Dolci 1994 | 80 | 7 | 82 | 8 |
| Forbes 1984b | 43 | 1 | 40 | 2 |
| Forbes 1989 | 26 | 3 | 26 | 2 |
| Forbes 1990 | 41 | 5 | 38 | 0 |
| Gallardo 1990 | 15 | 5 | 11 | 3 |
| Seymour 1996 | 40 | 0 | 39 | 0 |
| Sunshine 1986 | 30 | 1 | 30 | 1 |
| Vattaraphudej 1986 | 16 | 0 | 19 | 0 |
| Totals | 392 | 43 | 385 | 31 |
3. Number of patients with adverse effects (< 1000 mg Paracetamol/No placebo).
| Author | Total: Paracetamol | Total: Adverse events |
| Nystrom 1990 | 45 | 5 |
| Quiding 1981 | 27 | 3 |
| Ragot 1991 | 40 | 1 |
| Reijntes 1987 | 29 | 2 |
| Selcuk 1996 | 52 | 0 |
| Strom 1990 | 33 | 6 |
| Totals | 226 | 17 |
4. Number of patients with adverse events (1000 mg Paracetamol/Placebo).
| Author | Total: Paracetamol | AEs: Paracetamol | Total: Placebo | AEs: Placebo |
| Cooper 1998 | 50 | 25 | 26 | 4 |
| Hersch 2000 | 63 | 12 | 27 | 7 |
| Kiersch 1994 | 91 | 26 | 47 | 13 |
| Kubitzek 2003 | 78 | 4 | 84 | 2 |
| Lehnert 1990 | 49 | 5 | 40 | 4 |
| Mehlisch 1990 | 307 | 32 | 85 | 12 |
| Mehlisch 1995 | 99 | 12 | 40 | 4 |
| Moller 2000 | 120 | 48 | 122 | 56 |
| Olson 2001 | 66 | 10 | 39 | 2 |
| Seymour 1996 | 40 | 0 | 39 | 0 |
| Seymour 2003 | 62 | 24 | 32 | 9 |
| Totals | 1025 | 198 | 581 | 113 |
5. Number of patients with adverse events (1000 mg Paracetamol/No placebo).
| Author | Total: Paracetamol | AEs: Paracetamol |
| Dionne 1983 (1) | 20 | 12 |
| Nystrom 1990 | 46 | 7 |
| Ragot 1991 | 40 | 0 |
| Strom 1990 | 176 | 19 |
| Totals | 282 | 38 |
6. Number of patients with adverse events (All doses of Paracetamol/Placebo).
| Author | Total: Paracetamol | AEs: Paracetamol | Total: Placebo | AEs: Placebo |
| Cooper 1980 | 37 | 2 | 37 | 6 |
| Cooper 1981 | 37 | 12 | 38 | 4 |
| Cooper 1998 | 50 | 25 | 26 | 4 |
| Dionne 1994 | 27 | 7 | 25 | 5 |
| Dolci 1994 | 80 | 7 | 82 | 8 |
| Forbes 1984b | 43 | 1 | 40 | 2 |
| Forbes 1989 | 26 | 3 | 26 | 2 |
| Forbes 1990 | 41 | 5 | 38 | 0 |
| Gallardo 1990 | 15 | 5 | 11 | 3 |
| Hersch 2000 | 63 | 12 | 27 | 7 |
| Kiersch 1994 | 91 | 26 | 47 | 13 |
| Kubitzek 2003 | 78 | 4 | 84 | 2 |
| Lehnert 1990 | 49 | 5 | 40 | 4 |
| Mehlisch 1990 | 307 | 32 | 85 | 12 |
| Mehlisch 1995 | 99 | 12 | 40 | 4 |
| Moller 2000 | 120 | 48 | 122 | 56 |
| Olson 2001 | 66 | 10 | 39 | 2 |
| Seymour 1996 | 80 | 0 | 39 | 0 |
| Seymour 2003 | 62 | 24 | 32 | 9 |
| Sunshine 1986 | 30 | 1 | 30 | 1 |
| Vattaraphudej 1986 | 16 | 0 | 19 | 0 |
| Totals | 1417 | 241 | 927 | 144 |
7. Number of patients with adverse events (All doses of Paracetamol/No placebo).
| Author | Totals: Paracetamol | AEs: Paracetamol |
| Dionne 1983 (1) | 20 | 12 |
| Nystrom 1990 | 91 | 12 |
| Quiding 1981 | 27 | 3 |
| Ragot 1991 | 80 | 1 |
| Reintjes 1987 | 29 | 2 |
| Selcuk 1996 | 52 | 0 |
| Strom 1990 | 209 | 25 |
| Totals | 508 | 55 |
8. Total number of adverse events (< 1000 mg Paracetamol/Placebo).
| Author | Total: Paracetamol | AEs: Paracetamol | Total: Placebo | AEs: Placebo |
| Cooper 1980 | 37 | 6 | 38 | 7 |
| Cooper 1981 | 37 | 15 | 37 | 5 |
| Dionne 1994 | 27 | 9 | 25 | 5 |
| Dolci 1994 | 80 | 7 | 82 | 12 |
| Forbes 1984b | 43 | 2 | 40 | 2 |
| Forbes 1990 | 41 | 5 | 38 | 0 |
| Gallardo 1990 | 15 | 5 | 11 | 3 |
| Sunshine 1986 | 30 | 1 | 30 | 2 |
| Totals | 310 | 50 | 301 | 36 |
9. Total number of adverse events (< 1000 mg Paracetamol/No placebo).
| Author | Total: Paracetamol | AEs: Paracetamol |
| Haanaes 1986 | 36 | 11 |
| Nystrom 1988 | 45 | 6 |
| Quiding 1981 | 27 | 4 |
| Ragot 1991 | 40 | 1 |
| Reintjes 1987 | 29 | 2 |
| Selcuk 1996 | 52 | 0 |
| Totals | 229 | 24 |
10. Total number of adverse events (1000 mg Paracetamol/Placebo).
| Author | Totals: Paracetamol | AEs: Paracetamol | Totals: Placebo | AEs: Placebo |
| Breivik 1998 | 119 | 17 | 54 | 10 |
| Cooper 1998 | 50 | 26 | 26 | 5 |
| Hersh 2000 | 63 | 14 | 27 | 8 |
| Kiersch 1994 | 91 | 35 | 47 | 18 |
| Moller 2000 | 120 | 65 | 122 | 83 |
| Olson 2001 | 66 | 10 | 39 | 2 |
| Seymour 2003 | 62 | 36 | 28 | 13 |
| Skoglund 1991 | 67 | 12 | 33 | 10 |
| Totals | 638 | 215 | 376 | 149 |
11. Total number of adverse events (1000 mg Paracetamol/No placebo).
| Author | Totals: Paracetamol | AEs: Paracetamol |
| Dionne 1983 | 20 | 18 |
| Nystrom 1990 | 46 | 10 |
| Ragot 1991 | 40 | 0 |
| Totals | 106 | 28 |
12. Total number of adverse events (All doses of Paracetamol/Placebo).
| Author | Totals: Paracetamol | AEs: Paracetamol | Totals: Placebo | AEs: Placebo |
| Breivik 1998 | 119 | 17 | 54 | 10 |
| Cooper 1980 | 37 | 6 | 38 | 7 |
| Cooper 1981 | 37 | 15 | 37 | 5 |
| Cooper 1998 | 50 | 26 | 26 | 5 |
| Dionne 1994 | 27 | 9 | 25 | 5 |
| Dolci 1994 | 80 | 7 | 82 | 12 |
| Forbes 1984b | 43 | 2 | 40 | 2 |
| Forbes 1990 | 41 | 5 | 38 | 0 |
| Gallardo 1990 | 15 | 5 | 11 | 3 |
| Hersh | 63 | 14 | 27 | 8 |
| Kiersch 1994 | 91 | 35 | 47 | 18 |
| Moller 2000 | 120 | 65 | 122 | 83 |
| Olson 2001 | 66 | 10 | 39 | 2 |
| Seymour 2003 | 62 | 36 | 28 | 13 |
| Skoglund 1991 | 67 | 12 | 33 | 10 |
| Sunshine 1986 | 30 | 1 | 30 | 2 |
| Totals | 948 | 265 | 647 | 185 |
13. Total number of adverse events (All doses of Paracetamol/No placebo).
| Author | Totals: Paracetamol | AEs: Paracetamol |
| Dionne 1983 | 20 | 18 |
| Haanaes 1986 | 36 | 11 |
| Nystrom 1988 | 91 | 16 |
| Quiding 1981 | 27 | 4 |
| Ragot 1991 | 80 | 1 |
| Reijntes 1987 | 29 | 2 |
| Selcuk 1996 | 52 | 0 |
| Totals | 335 | 52 |
14. Global assessment ‐ 5‐point scale(< 1000 mg Paracetamol).
| Author | Total: Paracetamol | Global assessment | Total: Placebo | Global assessment |
| Cooper 1980 | 37 | 0.89 | 38 | 0.89 |
| Cooper 1981 | 37 | 1.92 | 37 | 0.62 |
| Cooper 1988 | 36 | 2.38 | 40 | 2.05 |
| Dionne 1994 | 27 | 2.40 | 25 | 2.00 |
| Dolci 1994 | 72 | 2.10 | 76 | 2.17 |
| Forbes 1984 | 39 | 1.26 | 36 | 0.28 |
| Mean | 248 | 1.83 | 252 | 1.44 |
15. Global assessment ‐ 4‐point scale (< 1000 mg Paracetamol).
| Author | Total: Paracetamol | Global assessment | Total: Placebo | Global assessment |
| Forbes 1989 | 26 | 1.00 | 26 | 0.30 |
| Forbes 1990 | 41 | 1.47 | 38 | 0.56 |
| Sunshine 1986 | 30 | 1.20 | 30 | 0.93 |
| Vattaraphudej 1986 | 16 | 1.6 | 19 | 1.16 |
| Mean | 113 | 1.31 | 113 | 0.70 |
16. Global assessment ‐ 5‐point scale (1000 mg Paracetamol).
| Author | Total: Paracetamol | Global assessment | Total: Placebo | Global assessment |
| Kiersch 1994 | 91 | 1.30 | 47 | 0.60 |
| Kubitzek 2003 | 78 | 1.98 | 84 | 1.45 |
| Mehlisch 1995 | 101 | 1.57 | 40 | 0.45 |
| Olson 2001 | 66 | 2.81 | 39 | 1.93 |
| Seymour 2003 | 62 | 2.50 | 32 | 2.14 |
| Mean | 398 | 1.94 | 242 | 1.29 |
17. Global assessment ‐ 4‐point scale (1000 mg Paracetamol).
| Author | Total: Paracetamol | Global assessment | Total: Placebo | Global assessment |
| Hersh 2000 | 63 | 2.29 | 27 | 0.85 |
| Moller 2000 | 120 | 1.88 | 122 | 1.54 |
| Mean | 183 | 2.02 | 149 | 1.41 |
Risk of bias in included studies
Details of the quality assessment are presented in Additional Table 18; Table 19; Table 20. Seven out of the 21 studies reported adequate concealed allocation, for the remaining studies it was unclear. Over half of the studies (11/21) gave clear explanation of withdrawals or drop outs. Taking these two factors into account only three trials were assessed as being at low risk of bias.
18. Quality assessment.
| Author | Allocation concealment | Follow up | Total (Max‐3) |
| Cooper 1980 | 1 | 0 | 1 |
| Cooper 1981 | 1 | 0 | 1 |
| Cooper 1988 | 1 | 0 | 1 |
| Cooper 1998 | 1 | 1 | 2 |
| Dionne 1994 | 1 | 0 | 1 |
| Dolci 1994 | 1 | 0 | 1 |
| Forbes 1984b | 2 | 0 | 2 |
| Forbes 1989 | 1 | 0 | 1 |
| Forbes 1990 | 2 | 0 | 2 |
| Hersh 2000 | 1 | 1 | 2 |
| Kiersch 1994 | 1 | 1 | 2 |
| Kubitzek 2003 | 1 | 1 | 2 |
| Lehnert 1990 | 1 | 1 | 2 |
| Mehlisch 1995 | 2 | 1 | 3 |
| Moller 2000 | 1 | 1 | 2 |
| Olson 2001 | 2 | 1 | 3 |
| Seymour 1996 | 1 | 1 | 2 |
| Seymour 2003 | 2 | 0 | 2 |
| Skoglund 1991 | 2 | 0 | 2 |
| Sunshine 1986 | 2 | 1 | 3 |
| Vattaraphudej 1986 | 1 | 1 | 2 |
19. Sample size calculation.
| Author | Yes/No |
| Cooper 1980 | No |
| Cooper 1981 | No |
| Cooper 1988 | No |
| Cooper 1998 | No |
| Dionne 1994 | No |
| Dolci 1994 | No |
| Forbes 1984 | No |
| Forbes 1989 | No |
| Forbes 1990 | No |
| Hersh 2000 | No |
| Kiersch 1994 | No |
| Kubitzek 2003 | Yes |
| Lehnert 1990 | No |
| Mehlisch 1995 | No |
| Moller 2000 | Yes |
| Olson 2001 | No |
| Seymour 1996 | No |
| Seymour 2003 | Yes |
| Skoglund 1991 | No |
| Sunshine 1986 | No |
| Vattaraphudej 1986 | No |
20. Agreement between quality assessors.
| Author | Yes/No |
| Cooper 1980 | Yes |
| Cooper 1981 | Yes |
| Cooper 1988 | Yes |
| Cooper 1998 | Yes |
| Dionne 1994 | Yes |
| Dolci 1994 | Yes |
| Forbes 1984 | Yes |
| Forbes 1989 | Yes |
| Forbes 1990 | Yes |
| Hersh 2000 | Yes |
| Kiersch 1994 | Yes |
| Kubitzek 2003 | Yes |
| Lehnert 1990 | Yes |
| Mehlisch 1995 | Yes |
| Moller 2000 | Yes |
| Olson 2001 | Yes |
| Seymour 1996 | Yes |
| Seymour 2003 | Yes |
| Skoglund 1991 | No |
| Sunshine 1986 | Yes |
| Vattaraphudej 1986 | No |
Effects of interventions
Comparison 1: Paracetamol versus placebo using pain relief measurements
(Comparison 1, Outcome 1.1 & Comparison 1, Outcome 1.2) (Analysis 1.1; Analysis 1.2)
1.1. Analysis.
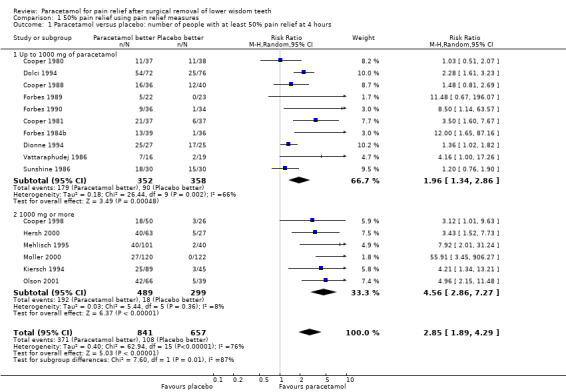
Comparison 1 50% pain relief using pain relief measures, Outcome 1 Paracetamol versus placebo: number of people with at least 50% pain relief at 4 hours.
1.2. Analysis.
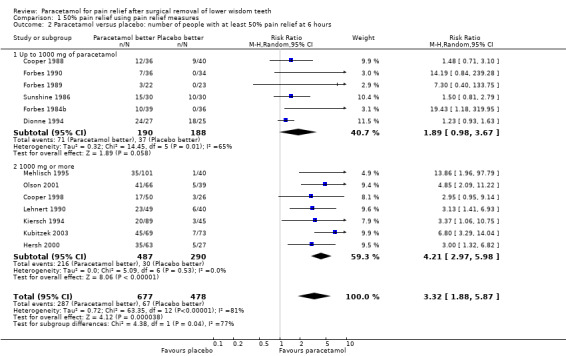
Comparison 1 50% pain relief using pain relief measures, Outcome 2 Paracetamol versus placebo: number of people with at least 50% pain relief at 6 hours.
There are 16 studies providing pain relief measurements for comparing paracetamol versus placebo at 4 hours, 11 at doses up to 1000 mg and 5 at doses of 1000 mg. Overall there was a highly statistically significant benefit with the paracetamol, with risk ratio (RR) values for achieving 50% pain relief for all doses of paracetamol for 4 hours RR 2.85 (95% confidence interval (CI) 1.89 to 4.29), Chi2 = 62.94, degrees of freedom (df) = 15, P < 0.001, I2 = 76%, number needed to treat (to benefit) (NNT) 4 (95% CI 3 to 4). The statistically significant benefit was apparent for both subgroups with RR for up to 1000 mg 1.96 (95% CI 1.34 to 2.86), Chi2 = 26.44, df = 9, P = 0.002, I2 = 66.0%, NNT 4 (95% CI 3 to 5), and RR for 1000 mg 4.56 (95% CI 2.86 to 7.27), Chi2 = 5.44, df = 5, P = 0.36, I2 = 8.2%, NNT 3 (95% CI 3 to 4). Although both had a statistically significant benefit over placebo there was a statistically significant difference between the two subgroups with an enhanced benefit for the higher doses (metaregression P < 0.001, Additional Table 1). This subgroup analysis explained some of the heterogeneity in the overall comparison however, there is still some unexplained heterogeneity between the trials in the up to 1000 mg dose comparison.
There are 13 studies providing pain relief measurements for comparing paracetamol versus placebo at 6 hours, 6 doses up to 1000 mg paracetamol, and 7 doses of 1000 mg paracetamol. Overall there was a highly statistically significant benefit with the paracetamol, with RR values for 50% pain relief at 6 hours RR 3.32 (95% CI 1.88 to 5.87), Chi2 = 63.35, df = 12, P < 0.00001, I2 = 81.1%, NNT 3 (95% CI 3 to 4). The statistically significant benefit was apparent in both subgroups with RR for up to 1000 mg 1.89 (95% CI 0.98 to 3.67), Chi2 = 14.45, df = 5, P = 0.01, I2 = 65.4%, NNT 6 (95% CI 4 to 10), and RR for 1000 mg 4.21 (95% CI 2.97 to 5.98), Chi2 = 5.09, df = 6, P = 0.53, I2 = 0%, NNT 3 (95% CI 2 to 3). Although both had a statistically significant benefit over placebo there was a statistically significant difference between the two subgroups with an enhanced benefit for the higher doses (metaregression P < 0.001, Additional Table 1). This subgroup analysis explained some of the heterogeneity in the overall comparison however, there is still some unexplained heterogeneity between the trials in the up to 1000 mg dose comparison.
Comparison 2: Paracetamol versus placebo using pain intensity difference measurements
(Comparison 2, Outcome 2.1 & Comparison 2, Outcome 2.2) (Analysis 2.1; Analysis 2.2)
2.1. Analysis.
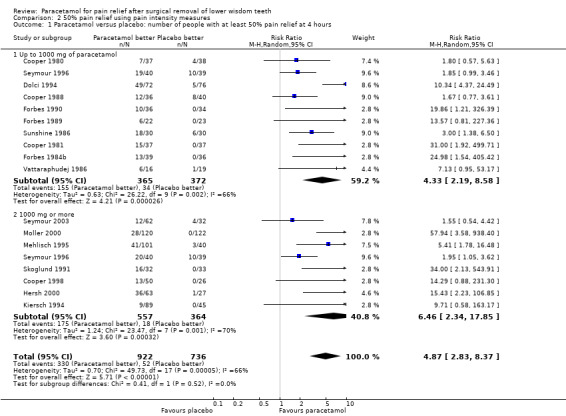
Comparison 2 50% pain relief using pain intensity measures, Outcome 1 Paracetamol versus placebo: number of people with at least 50% pain relief at 4 hours.
2.2. Analysis.
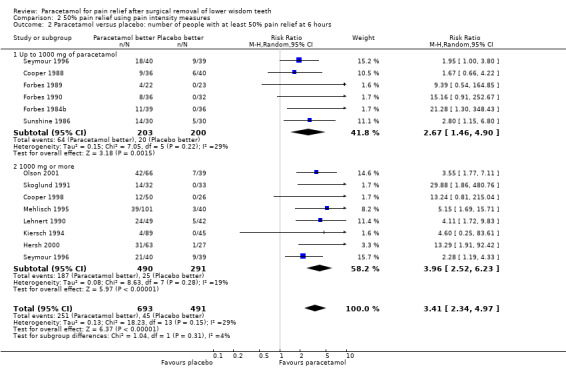
Comparison 2 50% pain relief using pain intensity measures, Outcome 2 Paracetamol versus placebo: number of people with at least 50% pain relief at 6 hours.
There are 18 studies providing pain intensity measurements for comparing paracetamol versus placebo at 4 hours, 10 at doses up to 1000 mg and 8 at doses of 1000 mg. Overall there was a highly statistically significant benefit with paracetamol, with RR values for 50% pain relief at 4 hours RR 4.87 (95% CI 2.83 to 8.37), Chi2 = 49.73, df = 17, P < 0.0001, I2 = 65.8%, NNT 3 (95% CI 3 to 5). The statistically significant benefit was apparent in both subgroups with RR up to 1000 mg 4.33 (95% CI 2.19 to 8.58), Chi2 = 26.22, df = 9, P = 0.002, I2 = 65.7%, NNT 3 (95% CI 3 to 4), and RR for 1000 mg 6.46 (95% CI 2.34 to 17.85), Chi2 = 23.47, df = 7, P = 0.001, I2 = 70.2%, NNT 4 (95% CI 3 to 5). Both had a statistically significant benefit over placebo, but there was no statistically significant difference between the two subgroups (metaregression P = 0.67, Additional Table 1).
There are 14 studies providing pain intensity measurements for comparing paracetamol versus placebo at 6 hours, 6 at doses up to 1000 mg and 8 at doses of 1000 mg. Overall there was a highly statistically significant benefit with paracetamol, with RR values for 50% pain relief RR 3.41 (95% CI 2.34 to 4.97), Chi2 = 18.23, df = 13, P = 0.15, I2 = 28.7%, NNT 4 (95% CI 3 to 4). The statistically significant benefit was apparent in both groups with RR up to 1000 mg 2.67 (95% CI 1.46 to 4.90), Chi2 = 7.05, df = 5, P = 0.22, I2 = 29.1%, NNT 5 (95% CI 3 to 7), and RR for 1000 mg 3.96 (95% CI 2.52 to 6.23), Chi2 = 8.63, df = 7, P = 0.28, I2 = 18.9%, NNT 3 (95% CI 3 to 4). Both had a statistically significant benefit over placebo, but there was no statistically significant difference between the two subgroups (metaregression P = 0.15, Additional Table 1).
Comparison 3: Number of patients with adverse events for paracetamol versus placebo
(Comparison 3, Outcome 3.1) (Analysis 3.1)
3.1. Analysis.
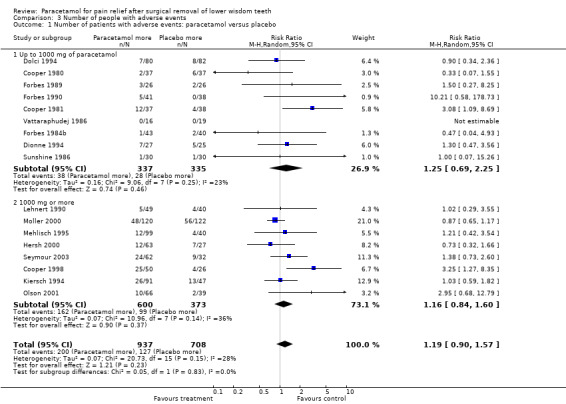
Comparison 3 Number of people with adverse events, Outcome 1 Number of patients with adverse events: paracetamol versus placebo.
There are 17 studies that reported the number of patients with adverse events for paracetamol versus placebo, 9 studies used less than 1000 mg and 8 studies used 1000 mg. There was no statistically significant difference in any group. For all doses of paracetamol the RR for an adverse event RR 1.19 (95% CI 0.90 to 1.57), Chi2 = 20.73, df = 15, P = 0.15, I2 = 27.6%, number needed to treat to harm (NNTH) 33 (95% CI 14.3 to infinity). For doses of less than 1000 mg RR 1.25 (95% CI 0.69 to 2.25), Chi2 = 9.06, df = 7, P = 0.25, I2 = 22.8%, NNTH 33 (95% CI 14.3 to infinity). For 1000 mg paracetamol RR 1.16 (95% CI 0.84 to 1.60), Chi2 = 10.96, df = 7, P = 0.14, I2 = 36.2%, NNTH 33 (95% CI 12.5 to infinity).
Subgroup analyses
Where patients underwent surgery with local anaesthesia alone, local anaesthesia and intravenous sedation, general anaesthesia alone and general anaesthesia with local anaesthetic
When the data were reviewed it was not possible to do a meta‐analysis. Of the 21 included studies, 7 did not state what anaesthesia was used, 7 used combinations of anaesthesia, but were unclear in reporting which patients received which anaesthesia, 4 used local anaesthetic only and 3 used general anaesthetic only.
Where different types of formulation of paracetamol were used (immediate release versus slow release)
Most included studies did not report on the formulation, other than to say whether it was tablets, capsules or caplets. Only one paper indicated that their study used effervescent tablets (Moller 2000) and their results showed that effervescent tablets gave a faster onset of pain relief. Median value for time to onset of analgesia was 20 minutes in the effervescent group and 45 minutes in the tablet group, and time to meaningful pain relief was 45 minutes in the effervescent group and 1 hour in the tablet group. However at the end of a 4‐hour period pain relief was better in the tablet group (4.4) than the effervescent group (3.7).
Where different doses of paracetamol were used (1000 mg or more, and less than 1000 mg)
This meta‐analysis was conducted. 11 studies used doses of 1000 mg or more, and 11 studies used doses of less than 1000 mg (Seymour 1996, used both doses).
NNT for < 1000 mg of paracetamol is 4 (95% CI 3 to 5) at 4 hours and 6 (95% CI 4 to 10) at 6 hours (using pain relief measurements).
NNT for < 1000 mg of paracetamol is 3 (95% CI 3 to 4) at 4 hours and 5 (95% CI 3 to 7) at 6 hours (using intesity measurements).
NNT for 1000 mg of paracetamol is 3 (95% CI 3 to 4) at 4 hours and 3 (95% CI 2 to 3) at 6 hours (using pain relief measurements).
NNT for 1000 mg of paracetamol is 4 (95% CI 3 to 5) at 4 hours, and 3 (95% CI 3 to 4) at 6 hours (using intensity measurements).
Where time of administration of paracetamol differs: preoperative versus postoperative
No included study used a preoperative dose, as the patients did not reach moderate or severe pain before the intervention.
Where total pain relief (TOTPAR) was calculated using pain relief measures and pain intensity measures
This meta‐analysis was undertaken where the relevant data were available. 16 studies had pain relief data and 17 studies had pain intensity data. NNT using pain relief scales for < 1000 mg of paracetamol is 4 (95% CI 3 to 5) at 4 hours, and 6 (95% CI 4 to 10) at 6 hours. NNT using pain intensity scales for < 1000 mg of paracetamol is 3 (95% CI 3 to 4) at 4 hours, and 5 (95% CI 3 to 7) at 6 hours. NNT using pain relief scales for 1000 mg of paracetamol is 3 (95% CI 3 to 4) at 4 hours, and 3 (95% CI 2 to 3) at 6 hours. NNT using pain intensity scales for 1000 mg of paracetamol is 4 (95% CI 3 to 5) at 4 hours, and 3 (95% CI 3 to 4) at 6 hours.
Discussion
The results show paracetamol to be an effective analgesia for use following third molar surgery. The number needed to treat (to benefit) (NNTs) and number needed to treat to harm (NNTHs) support the use of 1000 mg as an optimal dose. It is effective over both 4 and 6 hours. In considering the use of pain relief, or pain intensity difference as a measure of efficacy it was of interest that metaregression showed that pain relief scales showed a statistically significant difference for increased dose, and pain intensity did not. It is acknowledged that this review only considered single dose studies when considering efficacy, multidosed studies may be considered when updating the review. The NNTs and NNTHs found in this review are similar to those recorded by a systematic review (Barden J 2004) where they investigated paracetamol for pain involving various types of surgery. This would confirm yet again the value of the third molar pain model, showing that dental pain is comparable with pain from other sources. The implementation of NICE (National Institute for Health and Clinical Excellence) Guidelines for removal of third molars has led to a decrease in the performance of this surgery, which may have an adverse effect on the number of trials able to use the third molar model. In the United States of America such guidelines have not yet been adopted. It is of interest that in striving to provide evidence based treatment the opportunity for research using the third molar pain model may be adversely affected.
The data available for adverse events show that NNTH for < 1000 mg of paracetamol is 33 (14.3 to infinity), for 1000 mg of paracetamol is 33 (12.5 to infinity) and for all doses 33 (14.3 to infinity), suggesting it is an extremely safe drug. Only one severe adverse event was recorded by any researchers, and that was a severe headache (Olson 2001), two other participants stopped taking paracetamol because of vomiting. However there was a high degree of inconsistency across the trials in the way that adverse events were recorded, raising the concern that only adverse events considered by the researchers to be attributable to paracetamol were recorded, with some trials recording many AEs and some reporting none. The diverse way in which adverse events were recorded led to there being over 20 categories of adverse events. The main categories are shown in Additional Table 21. Of interest are adverse events where placebo scored more highly than paracetamol, which could suggest that paracetamol may possibly have a beneficial effect eg dry socket, but this would require further investigation. As all patients had surgery, and various combinations of local anaesthesia, general anaesthesia, and sedation making it difficult to ascertain which effects are directly related to the intervention. However the results strongly support the use of paracetamol in doses up to 1000 mg as a safe effective analgesia.
21. List of adverse events.
| Adverse events | Paracetamol | Placebo |
| Nausea | 21 | 11 |
| Vomiting | 11 | 3 |
| Nausea and/or vomiting, stomach cramps, abdominal pain | 3 | 3 |
| Headache | 47 | 31 |
| Drowsiness, sleepiness, somnolence | 36 | 13 |
| Dizziness, fainting, syncope | 9 | 4 |
| Bleeding | 11 | 7 |
| Chills, flushes, fever, flu‐like symptoms | 5 | 0 |
| Paraesthesia | 4 | 2 |
| Jawache | 1 | 0 |
| Swelling | 1 | 6 |
| Cellulitis | 1 | 0 |
| Dry socket | 11 | 12 |
| Surgical complications | 6 | 13 |
| CNS | 5 | 6 |
| GI | 12 | 2 |
| Body as a whole | 8 | 3 |
| Respiratory | 2 | 0 |
| Psychiatric | 0 | 1 |
| Other, hiccups, hearing/vestibular, miosis, | 5 | 1 |
The efficacy of paracetamol decreases with times, and the recommended interval between doses is 8 hours, which would suggest there may be some benefit in a slow release formulation. None of the studies in this trial used a slow release formulation, but a trial (Coulthard 2001) compared sustained release and standard release formulations of paracetamol and found that the sustained release was statistically significantly more effective at 6 and 8 hours, with no loss of efficacy at 4 hours. Safety for both formulations was comparable, making sustained release paracetamol a safe and effective choice. The methodology used in the included trials was generally good. This resulted in a large number of participants being included in this meta‐analysis, while using only double blind randomised trials. The included trials gave a strong, consistent result. Many of the trials were done by researchers with extensive experience in the field of pain research, whose methods have been refined with experience. A large proportion of the trials were done in the United States, and were mostly funded by pharmaceutical companies. This seems to be reflected in the methodology. However, quality assessment showed, there were only three trials with a low risk of bias, and 18 with moderate/high risk. This was mainly the result of unreported allocation concealment methods. In speaking to some of the authors it is highly likely that the allocation concealment was good in all the trials, but that the details were not well reported. Most trials were sponsored by pharmaceutical companies who supplied paracetamol and placebo in identical appearance. The reporting of withdrawals and drop outs was sporadic, and even when numbers were cited it was not always clear to which treatment group the participant had been originally allocated.
Mean global assessments (Additional Table 14; Table 15; Table 16; Table 17) all showed higher scores for paracetamol than placebo. It is of interest that despite achieving 50% pain relief participants did not record 50% on a global assessment scale. This again raises the question of the value of the instruments used to measure the efficacy of an intervention. None of the trials relied on global assessments as their only measure of efficacy, but this information could be of value to other researchers. It raises interesting questions concerning patient's expectations and the difficulties associated with quantifying such a subjective experience.
A lot of valuable information was gathered, incidental to the main findings, in most of the trials. So though the topic was concerned with the use of paracetamol for pain, information collected in many of the trials shed valuable light on subjects such as side effects, measuring instruments, and methodology. Further appraisal of the multidisciplinary approach to research, a broader view of data collection, and a more accurate reporting of data already collected could be extremely valuable in the future. It would allow research to be more widely used in various meta‐analyses. Data from areas seemingly unrelated to the original null hypothesis, eg comparison of pain relief and pain intensity as a measuring tool, adverse event reporting, the significance of global assessments etc. could be more readily available. If the third molar trial population does decrease it would be advantageous to collect as much data as possible from any trial being undertaken.
Authors' conclusions
Implications for practice.
Paracetamol (acetaminophen) is an effective drug to use for postoperative pain following oral surgery, and the reporting of adverse events shows it to be a safe drug (number needed to treat (to benefit) (NNT) is 3 for 1000 mg of paracetamol at 6 hours, number needed to treat to harm (NNTH) 33). It is most effective at 1000 mg dose, and can be taken at six hourly intervals without compromising safety. It could be considered more readily by dentist and patients both as a first choice analgesic, or to be taken alternately with doses of other analgesics such as nonsteroidal anti‐inflammatory drugs (NSAIDS).
Implications for research.
There is a large body of research in this area, and further research, other than as a comparison seems unnecessary. However, in one trial (Moller 2000) it was found that an effervescent formulation appeared to have a faster onset of pain relief, which would be beneficial to patients who are looking for a rapid onset of relief. It may be helpful to undertake some research to confirm these findings. The use of pain relief and pain intensity difference as a measure of pain relief may be another area for further investigation. It is valuable to have NNT/NNTH as a baseline for comparison with other analgesics. Maximizing the third molar pain model population by multidisciplinary research is another area of interest highlighted by this review.
What's new
| Date | Event | Description |
|---|---|---|
| 4 February 2014 | Review declared as stable | This review is no longer being updated as it has been superseded by reviews conducted by the Cochrane Pain, Palliative and Supportive Care Group (PaPaS). |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 6 March 2012 | Amended | Additional tables linked to text. |
| 31 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to thank Sylvia Bickley (Cochrane Oral Health Group) for her assistance with literature searching and Luisa Fernandez Mauleffinch (Cochrane Oral Health Group) for her help with the preparation of this review. We would also like to thank the following referees, who reviewed this work at various stages: Barry Elliott Cole, Mike Hill, Kimito Yamashiro and Lasse Skoglund. We are grateful to Stephen Cooper, Donald Mehlisch, Philip Moller, Alberto Umile, for providing information on their trials, and to Prisana Pripatnanont for translation and information.
Appendices
Appendix 1. CENTRAL search strategy
#1. MOLAR THIRD single term (MeSH) #2. (wisdom next tooth) #3. (wisdom next teeth) #4. (third near molar*) #5. (#1 or #2 or #3 or #4) #6. TOOTH EXTRACTION single term (MeSH) #7. (extract* near tooth) #8. (extract* near teeth) #9. (extract* near (third next molar*)) #10. (extract* near (third near molar*)) #11. (remov* near tooth) #12. (remov* near teeth) #13. (surgical* near remov*) #14. (surgery near remov*) #15. (surgical* near extract*) #16. (surgery near extract*) #17. (#6 or #7 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) #18. (#5 and #17)
Data and analyses
Comparison 1. 50% pain relief using pain relief measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol versus placebo: number of people with at least 50% pain relief at 4 hours | 16 | 1498 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [1.89, 4.29] |
| 1.1 Up to 1000 mg of paracetamol | 10 | 710 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.34, 2.86] |
| 1.2 1000 mg or more | 6 | 788 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [2.86, 7.27] |
| 2 Paracetamol versus placebo: number of people with at least 50% pain relief at 6 hours | 13 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 3.32 [1.88, 5.87] |
| 2.1 Up to 1000 mg of paracetamol | 6 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.98, 3.67] |
| 2.2 1000 mg or more | 7 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [2.97, 5.98] |
Comparison 2. 50% pain relief using pain intensity measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paracetamol versus placebo: number of people with at least 50% pain relief at 4 hours | 17 | 1658 | Risk Ratio (M‐H, Random, 95% CI) | 4.87 [2.83, 8.37] |
| 1.1 Up to 1000 mg of paracetamol | 10 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [2.19, 8.58] |
| 1.2 1000 mg or more | 8 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 6.46 [2.34, 17.85] |
| 2 Paracetamol versus placebo: number of people with at least 50% pain relief at 6 hours | 13 | 1184 | Risk Ratio (M‐H, Random, 95% CI) | 3.41 [2.34, 4.97] |
| 2.1 Up to 1000 mg of paracetamol | 6 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.46, 4.90] |
| 2.2 1000 mg or more | 8 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 3.96 [2.52, 6.23] |
Comparison 3. Number of people with adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with adverse events: paracetamol versus placebo | 17 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.90, 1.57] |
| 1.1 Up to 1000 mg of paracetamol | 9 | 672 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.69, 2.25] |
| 1.2 1000 mg or more | 8 | 973 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.84, 1.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cooper 1980.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 298 participants randomised to 6 groups, withdrawals unclear (51 from all groups) Number randomised to intervention: male 13, female 24, mean age 22.5 Number randomised to placebo: male 11, female 27, mean age 23.5 Number of third molars removed: mean for intervention and placebo 1.9 Baseline pain intensity: mean for intervention 2.41 (moderate 22, severe 15), mean for placebo 2.42 (moderate 22, severe 16) Setting ‐ outpatients (USA) | |
| Interventions | Paracetamol 500 mg versus placebo Formulation not stated Anaesthesia: not stated | |
| Outcomes | PI at 4 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical scale 0‐4 (poor ‐ excellent) Adverse events table | |
| Notes | Sponsored unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cooper 1981.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 248 participants randomised to 5 groups, withdrawals unclear (48 from all groups) Number randomised to intervention: male 15, female 22, mean age 22.2 Number randomised to placebo: male 13, female 24, mean age 23.7 Number of third molars removed: not stated Baseline pain intensity: mean for intervention 2.2 (moderate 29, severe 9), mean for placebo 2.3 (moderate 26, severe 11) Setting not stated (USA) | |
| Interventions | Paracetamol 650 mg versus placebo Formulation: not stated Anaesthesia: LA or GA | |
| Outcomes | PI at 4 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical scale 0‐4 (poor ‐ excellent) Total number of adverse events and number of people with adverse events reported | |
| Notes | Sponsored by Adria Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cooper 1988.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 165 participants randomised to 4 groups, withdrawals unclear (22 from all groups) Number randomised to intervention: male 7, female 29, mean age 24.6 Number randomised to placebo: male 11, female 29, mean age 24.7 Number of third molars removed: mean for intervention 1.4, mean for placebo 1.5 Baseline pain intensity: mean for intervention 2.4 (moderate 21, severe 15), mean for placebo 2.4 (moderate 25, severe 15) Setting ‐ outpatients ‐ single site (USA) | |
| Interventions | Paracetamol 600 mg versus placebo Formulation: not stated Anaesthesia: LA or LA and SED | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical scale 0‐4 (poor ‐ excellent) Total number of adverse events | |
| Notes | Sponsored by Parke‐Davis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cooper 1998.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 177 participants randomised to 4 groups, no withdrawals Number randomised to intervention: male 23, female 27, mean age 23.6 Number randomised to placebo: male 12, female 14, mean age 22.7 Number of third molars removed: not stated Baseline pain intensity: mean for intervention: categorical 2.3, VAS 60.3, mean for placebo: categorical 2.2, VAS 62.8 Setting ‐ Georgetown University Hospital (USA) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: not stated Anaesthesia: LA or LA and SED | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe), VAS scale 0‐100 mm (none ‐ worst pain imaginable) PR at 4 hours and 6 hours: categorical scale 0‐4 (no relief ‐ complete relief) Global assessment: not stated Adverse effects table | |
| Notes | Sponsored by Whitehall‐Robins | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dionne 1994.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 135 participants randomised to 5 groups, withdrawals unclear (11 from all groups) Number randomised to intervention: male 14, female 13, mean age 29.6 Number randomised to placebo: male 15, female 10, mean age 28.2 Number of third molars removed: not stated Baseline pain intensity for intervention and placebo: not stated Setting: private dental practice (USA) | |
| Interventions | Paracetamol 650 mg versus placebo Formulation: not stated Anaesthesia: LA, or LA and SED, or GA | |
| Outcomes | PI at 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical scale 1‐5 (poor ‐ excellent) Adverse effects table | |
| Notes | Sponsored by Upjohn | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dolci 1994.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 338 participants enrolled in 4 groups, withdrawals unclear (40 from all groups) Number randomised to intervention: male 28, female 44, mean age 27.9, age range 18‐49 Number randomised to placebo: male 28, female 48, mean age 27.2, age range 18‐45 Number of third molars removed: not stated Baseline pain intensity: range for intervention and placebo given together as average 21.4 (2.08 ‐ 2.19) Setting not stated (Italy) | |
| Interventions | Paracetamol 500 mg versus placebo Formulation: tablets Anaesthesia: not stated | |
| Outcomes | PI at 4 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical 0‐4 (negative ‐ very good) Adverse effects table | |
| Notes | Sponsored ‐ unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Forbes 1984b.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 191 participants randomised to 4 groups, withdrawals unclear (43 from all groups, 164 used in reporting of adverse events) Number randomised to intervention: male 19, female 20, mean age 21.95 Number of third molars removed: mean 2.44 Number randomised to placebo: male 21, female 15, mean age 15‐32 Number of third molars removed: mean 2.78 Baseline pain intensity: mean for intervention 2.46 (moderate 21, severe 18), mean for placebo 2.47 (moderate 19, severe 17) Setting: private dental practice (USA) | |
| Interventions | Paracetamol 650 mg versus placebo Formulation: capsules Anaesthesia: GA | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical scale 0‐4 (poor ‐ excellent) Adverse effects: reported as total number of adverse events, and number of patients with adverse events | |
| Notes | Sponsored by McNeil Consumer Products | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Forbes 1989.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 107 participants randomised to 5 groups, withdrawals unclear (19 from all groups, 98 participants used in reporting of adverse events) Number randomised to intervention: male 9, female 13, mean age 20.59, age range 17‐31 Number randomised to placebo: male 12, female 11, mean age 23.74, age range 16‐39 Number of third molars removed: mean for intervention 2.59, mean for placebo 2.09 Baseline pain intensity: mean for intervention 2.45, mean for placebo 2.39 Setting ‐ 2 sites, private dental practice, outpatients (USA) | |
| Interventions | Paracetamol 600 mg versus placebo Formulation ‐ 1 tablet & 1 capsule Anaesthesia: LA and GA | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none ‐ complete) Global assessment : categorical 0‐4 (poor ‐ excellent) Adverse effects reported by number of patients with adverse events | |
| Notes | Sponsored by Boots Company Ltd., G.H. Besselaar Associates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Forbes 1990.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 206 participants randomised to 6 groups Number randomised to intervention: male 20, female 16, (5 withdrawals), mean age 22.5, age range 16‐46 Number randomised to placebo: male 18, female 16, (4 withdrawals), mean age 23.65, age range 16‐45 Number of third molars removed: mean for intervention 2.58, mean for placebo 2.35 Baseline pain intensity: mean for intervention 2.39 (moderate 22, severe 14), mean for placebo 2.32 (moderate 23, severe 11) Setting ‐ private dental practice outpatients (USA) | |
| Interventions | Paracetamol 600 mg versus placebo Formulation: capsules Anaesthesia: LA and GA | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none ‐ complete) Global assessment: categorical 0‐4 (poor ‐ excellent) Adverse effects table | |
| Notes | Sponsored by Syntex Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hersh 2000.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 210 participants randomised to 4 groups, no withdrawals Number randomised to intervention: male 20, female 43, mean age 23.3 Number of third molars removed/patient: (1/1), (2/6), (3/5), (4/51) Number randomised to placebo: male 9, female 8, mean age 23.7 Number of wisdom teeth removed per patient: (1/1) (2/5) (3/3) (4/18) Baseline pain intensity: mean for intervention 2.3 (moderate 47, severe 16), mean for placebo 2.2 (moderate 22, severe 5) Setting ‐ University of Pennsylvania School of Dental Medicine ‐ outpatients (USA) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: caplets Anaesthesia: LA or LA and SED | |
| Outcomes | PI at 4 hours and 6 hours: categorical 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical 0‐4 (no relief ‐ complete relief) Global assessment: categorical 0‐4 (poor ‐ excellent) Adverse effects by total number of adverse events, and number of patients with adverse events | |
| Notes | Sponsored by Whitehall‐Robins | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kiersch 1994.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 232 participants enrolled in 3 groups Number randomised to intervention 91, withdrawals 4 (2 before and 2 after randomisation): male 72, female 17, mean age 23.1, age range 15‐39 Number of third molars removed/patient: (1/0), (2/0), (3/31), (4/54) Number randomised to placebo 47, withdrawals 2: male 35, female 10, mean age 24.7, age range 15‐39 Number of third molars removed/patient: (1/0), (2/0), (3/19), (4/26) Baseline pain intensity: mean for intervention and placebo categorical 2.12, VAS 58.35 Setting not stated (USA) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: capsules Anaesthesia: not stated | |
| Outcomes | PI at 4 hours and 6 hours: categorical 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical 0‐4 (none ‐ complete), VAS 0‐100 mm (no pain ‐ worst pain I can imagine) Global assessment: categorical 0‐4 (poor ‐ excellent) Adverse effects reported by total number of adverse events, and by number of patients with adverse events | |
| Notes | Sponsored by Syntex Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kubitzek 2003.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 245 participants randomised to 3 groups, no withdrawals Number randomised to intervention 78 Number randomised to placebo 84: male:female, 40% male over both groups Number of third molars removed: 1 or 2 for each patient Baseline pain intensity: moderate to severe 65‐76% in both groups Setting: dental practice (Germany) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: tablets Anaesthesia: LA | |
| Outcomes | PI: not stated PR given as TOTPAR at 6 hours Global assessment: categorical 1‐5 (poor ‐ excellent) Adverse effects not stated | |
| Notes | Sponsored by Novartis Consumer Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lehnert 1990.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 150 participants randomised to 3 groups, 50 to each Number randomised to intervention, 1 withdrawal: male 23, female 26, mean age 25.3, age range 17‐52 Number randomised to placebo, 2 withdrawals: male 24, female 18, mean age 25.1, age range 18‐53 Number of hird molars removed: not stated Baseline pain intensity: mean for intervention 2.55 (moderate 22, severe 27), mean for placebo 2.5 (moderate 21, severe 21) Setting: outpatients (Germany) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: capsules Anaesthesia: not stated | |
| Outcomes | PI at 6 hours, categorical scale 0‐3 (no pain ‐ severe) PR at 6 hours, categorical scale 0‐3 (none ‐ complete) Global assessment: categorical scale 0‐3 (poor ‐ excellent) Adverse effects by number of patients | |
| Notes | Sponsored by GH Besselar Associates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mehlisch 1995.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 240 participants randomised to 3 groups, 1 withdrawal from the acetaminophen group Number randomised to intervention (1 withdrawal): male 30, female 71, mean age 25.3, age range 15‐60 Number of third molars removed/patient: (1/0, 2/95, 3/3, 4/3) Number randomised to placebo: male 19, female 21, mean age 24.2, age range 15‐48 Number of third molars removed/patient: (1/0, 2/39, 3/0, 4/1) Baseline pain intensity: mean for intervention 2.21 (moderate 80, severe 21), mean for placebo 2.20 (moderate 32, severe 8) Setting not stated (USA) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: 2 tablets Anaesthesia: not stated | |
| Outcomes | PI at 4 hours and 6 hours, categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours, categorical scale 0‐4 (none ‐ complete) Global assessment: categorical scale 0‐4 (poor ‐ excellent) Adverse events reported by number of patients | |
| Notes | Sponsored by Biomedical Research Group; and Merck Research Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Moller 2000.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 242 participants randomised to 4 groups, no withdrawals Number randomised to Intervention A: male 27, female 33, mean age 24.5 Number randomised to Intervention B: male 26, female 34, mean age 26.2 Number randomised to Placebo A: male 21, female 41, mean age 25.0 Number randomised to Placebo B: male 24, female 36, mean age 24.6 Number of third molars removed per patient, in both groups: 1 Mean baseline intensity Intervention A: categorical 2.00 (moderate 60) VAS 49.4 Mean baseline intensity Intervention B: categorical 2.00 (moderate 60) VAS 47.3 Mean baseline intensity Placebo A: categorical 2.00 (moderate 61, severe 1) VAS: 50.5 Mean baseline intensity Placebo B: categorical 2.00 (moderate 61) VAS: 47.6 Setting: Department of Oral and Maxillofacial Surgery, Royal Dental College, Aarhus (Denmark) | |
| Interventions | Intervention A: Paracetamol 1000 mg versus Placebo A Formulation: effervescent tablets Intervention B: Paracetamol 1000 mg versus Placebo B Formulation: tablets Anaesthesia: not stated | |
| Outcomes | PI at 4 hours: categorical scale 0‐3 (none ‐ severe) VAS scale 0‐100 mm (no pain ‐ worst possible pain) PR at 4 hours: categorical 0‐4 (none ‐ complete) Global assessment: categorical 0‐3 (poor ‐ excellent) Adverse effects reported as total number of adverse events, and number of patients with adverse events | |
| Notes | Sponsored by Bristol Myers Squibb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Olson 2001.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 239 participants randomised to 4 groups, no withdrawals Number randomised to intervention: male 22, female 44, mean age 22.2 Number randomised to placebo: male 11, female 28, mean age 23.9 Number of third molars removed/patient: intervention ‐ (1/1), (2/64), (3/1), (4/0), placebo ‐ (1/1), (2/38), (3/0), (4/0) Baseline pain intensity: mean for intervention 2.86 (moderate 9, severe 57) mean for placebo 2.9 (moderate 4, severe 35) Setting: University of Puerto Rico School of Dentistry (Puerto Rico) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: caplets Anaesthesia: LA | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none ‐ complete relief) Global assessment: categorical scale 0‐4 (poor ‐ excellent) Adverse effects table | |
| Notes | Sponsored by Whitehall Robins | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Seymour 1996.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 206 participants randomised to 5 groups Number randomised to intervention A, 1 withdrawal: male 12, female 28, mean age 23.8 Number randomised to intervention B, 1 withdrawal: male 12, female 28 mean age 27.7, 1 withdrawal Number randomised to placebo, 2 withdrawals: male 15, female 24, mean age 24.6 Number of third molars removed/patient: not stated Baseline pain intensity: mean for intervention A, VAS 54.9, mean for intervention B, VAS 54.2, mean for placebo VAS 56.5 Setting: not stated (UK) | |
| Interventions | Intervention A: paracetamol 500 mg versus placebo Intervention B: paracetamol 1000 mg versus placebo Formulation: not stated Anaesthesia: GA | |
| Outcomes | PI at 6 hours: VAS scale 0‐100 mm (no pain ‐ unbearable pain) Global assessment: categorical 0 ‐3 (very good ‐ very poor) but categories 1 & 2, and 4 & 5 not reported separately so unable to include data in tables Adverse effects ‐ none reported by any participants in any group | |
| Notes | Sponsored ‐ unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Seymour 2003.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 167 randomised to 3 groups, withdrawals unclear (14 from all groups) Number randomised to intervention: male 19, female 43, mean age 25.0 Number randomised to placebo: male 11, female 21, mean age 25.1 Number of third molars removed/patient: intervention ‐ (1/2), (2/14), (3/12), (4/34), placebo ‐ (1/3), (2/5), (3/9), (4/15) Baseline pain intensity: mean for intervention 50.6, mean for placebo 54.1 Setting not clear (2 sites, Cardiff and Hexham, UK) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: tablets Anaesthesia: GA | |
| Outcomes | PI at 4 hours: VAS scale 0‐100 mm (none ‐ worst pain imaginable) PR: not stated Global assessment: categorical scale 1‐5 (very poor ‐ very good) Adverse effects table | |
| Notes | Sponsored by Reckitt Benckiser Healthcare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Skoglund 1991.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 147 participants randomised to 4 groups, withdrawals unclear (8 from all groups) Number randomised to intervention: male 16, female 16, mean age 24.7 Number randomised to placebo: male 16, female 17, mean age 24.4 Number of third molars removed: not stated Baseline pain intensity: mean for intervention approx 55, mean for placebo, approx 45 Setting not stated (Norway) | |
| Interventions | Paracetamol 1000 mg versus placebo Formulation: tablets Anaesthesia: LA | |
| Outcomes | PI at 4 hours and 6 hours: VAS scale 0‐100 mm (none ‐ pain cannot be worse) PR at 4 hours and 6 hours: VAS scale 0‐100 mm (complete relief ‐ no relief) reversed for statistical analysis Global assessment: not stated Adverse effects table | |
| Notes | Sponsored by Apothekernes Laboratorium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sunshine 1986.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 182 participants randomised to 6 groups, no withdrawals (only patients with moderate to severe pain were randomised) Number randomised to intervention: male 6, female 24, mean age 21.9 Number randomised to placebo: male 14, female 16, mean age 23 Number of third molars removed: not stated Baseline pain intensity: mean for intervention 2.00, mean for placebo 2.00 Setting: University of Puerto Rico School of Dentistry Clinic | |
| Interventions | Paracetamol 650 mg versus placebo Formulation: capsules Anaesthesia: LA | |
| Outcomes | PI at 4 hours and 6 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours and 6 hours: categorical scale 0‐4 (none 0% ‐ complete 100%) Global assessment: categorical 0‐3 (poor ‐ excellent) Overall improvement 1‐7 (very much worse ‐ very much better) Adverse effects reported by number of patients with adverse events | |
| Notes | Sponsored by Upjohn | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Vattaraphudej 1986.
| Methods | Randomised, parallel group, double blind study | |
| Participants | 83 enrolled to 4 groups, withdrawals unclear (16 from all groups) Number randomised to intervention: male 8, female 8 Number randomised to placebo: male 10, female 9 Number of third molars removed: mean for intervention 1.25, mean for placebo 1.32 Baseline pain intensity: mean for intervention 2.37, mean for placebo 2.26 Setting: Dept of Oral Surgery, Khon Kaen University, Thailand | |
| Interventions | Paracetamol 650 mg versus placebo Formulation: capsules Anaesthesia not stated | |
| Outcomes | PI at 4 hours: categorical scale 0‐3 (none ‐ severe) PR at 4 hours: categorical scale 0‐4 (no relief ‐ total relief) Global assessment: categorical scale 0‐3 (poor ‐ excellent) Adverse effects, none reported | |
| Notes | Sponsored by Khon Kaen University, Thailand, Dr Sompong Thongpradith, Merck, and Russel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
GA ‐ general anaesthetic, LA ‐ local anaesthetic, PI ‐ pain intensity, PR ‐ pain relief, SED ‐ sedation, TOTPAR ‐ total pain relief, VAS ‐ visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adame 1979 | Title not in journal quoted, unable to find paper. |
| Barden J 2004 | Meta‐analysis, dental papers included individually where appropriate. |
| Becker 1990 | Not a third molar study. |
| Bentley 1987 | Unable to extract data for third molars only, written to authors. |
| Breivik 1998 | Dose given at 3 hours postoperatively regardless of baseline pain, unable to get 4‐hour data. Study used for side effects only. |
| Cooper 1986 | Unable to extract third molar data, written to authors. |
| Cooper 1989 | Unable to extract third molar data, written to authors. |
| Cooper 1991 | Unclear if third molars only, written to authors. |
| Dionne 1983 (1) | Not single dose, administered preoperatively. |
| Dionne 1983 (2) | Not placebo controlled, not single dose, administered preoperatively. |
| Dionne 1986 | Not placebo controlled. |
| Dolci 1993 | The data for the participants in this study are duplicated in Dolci 1994. |
| Edwards 2002 | This was a meta‐analysis. Need to identify source of data to clarify whether third molar studies, and exclude duplication. Written to authors. |
| Forbes 1982 | Unable to extract third molar data, written to author.s |
| Forbes 1984a | Not third molar study. |
| Gallardo 1990 | Not third molar study (periodontal surgery). |
| Gustafsson 1983 | Patients given either paracetamol preoperatively and placebo postoperatively or vice versa, unable to extract relevant data. |
| Haanaes 1986 | Not placebo controlled. Study used for side effects only. |
| Irvine 1982 | Not placebo controlled. |
| Laska 1983 | Not placebo controlled. |
| Lecointre 1991 | Not placebo controlled. |
| Liashek 1987 | Multiple doses, unable to extract single dose data. |
| Macleod 2002 | Not placebo controlled. |
| Medve 2001 | Only 8‐hour SPID and TOTPAR available, need 4‐hour and/or 6‐hour to include in review, written to authors. |
| Mehlisch 1984 | Unable to extract third molar data, written to authors. |
| Mehlisch 1990 | Unable to extract third molar data. |
| Moore 1986 | Multiple doses given, unable to extract single dose data. |
| Nystrom 1988 | Not placebo controlled. |
| Petersen 1983 | Unable to locate complete article. |
| Quiding 1981 | Not placebo controlled. |
| Quiding 1982 (1) | Not placebo controlled. |
| Quiding 1982 (2) | Not placebo controlled. |
| Quiding 1984 | No placebo used. |
| Ragot 1991 | Not placebo controlled. |
| Reijntjes 1987 | Not placebo controlled. |
| Rodrigo 1987 | Mixed parallel and cross‐over trial, multiple doses, unable to extract relevant data. |
| Rodrigo 1989 | Not placebo controlled. |
| Rosen 1985 | Not placebo controlled, multiple doses used, unable to extract single dose data. |
| Sakata 1989 | Unable to obtain study. |
| Selcuk 1996 | Not placebo controlled. |
| Seymour 1981 | Cross‐over trial, baseline pain not stated, unable to extract relevant data. |
| Seymour 1983 | Acetaminophen administered intravenously. |
| Skjelbred 1979 | Multiple doses, unable to extract single dose data. |
| Strom 1990 | Not placebo controlled. |
| Van Aken 2004 | Propacetamol administered intravenously. |
| Winter 1983 | Unable to extract third molar data, written to authors. |
SPID ‐ summed pain intensity difference, TOTPAR ‐ total pain relief
Contributions of authors
Conceiving, designing and co‐ordinating the review (Kiaran Weil (KW), Paul Coulthard (PC)). Developing search strategy and undertaking searches (Zahid Afzal (ZA), Arjen van Wijk (AJW), KW). Screening search results and retrieved papers against inclusion criteria (ZA, KW), Marco Esposito (ME), Lee Hooper (LH), PC). Appraising quality (KW, ZA). Extracting data from papers (KW, LH, Helen Worthington (HW)). Writing to authors for additional information (KW). Data management for the review and entering data into RevMan (KW). Analysis and interpretation of data (KW, LH, HW). Writing the review (KW). Providing general advice on the review (ME, LH, PC, HW). Performing previous work that was the foundation of current study (PC).
Sources of support
Internal sources
The University of Manchester, UK.
The University of Amsterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cooper 1980 {published data only}
- Cooper SA, Precheur H, Rauch D, Rosenheck A, Ladov M, Engel J. Evaluation of oxycodone and acetaminophen in treatment of postoperative dental pain. Oral Surgery, Oral Medicine, and Oral Pathology 1980;50(6):496‐501. [DOI] [PubMed] [Google Scholar]
Cooper 1981 {published data only}
- Cooper SA, Breen JF, Giuliani RL. The relative efficacy of indoprofen with opiod‐analgesic combinations. Journal of Oral Surgery 1981;39(1):21‐5. [PubMed] [Google Scholar]
Cooper 1988 {published data only}
- Cooper SA, Firestein A, Cohn P. Double‐blind comparison of meclofenamate sodium with acetaminophen, acetaminophen with codeine and placebo for relief of postsurgical dental pain. The Journal of Clinical Dentistry 1988;1(2):31‐4. [PubMed] [Google Scholar]
Cooper 1998 {published data only}
- Cooper SA, Reynolds DC, Reynolds B, Hersh EV. Analgesic efficacy and safety of (R)‐ ketoprofen in postoperative dental pain. Journal of Clinical Pharmacology 1998;38(2 Suppl):11S‐18S. [DOI] [PubMed] [Google Scholar]
Dionne 1994 {published data only}
- Dionne RA, Snyder J, Hargreaves KM. Analgesic efficacy of flurbiprofen in comparison with acetaminophen, acetaminophen plus codeine, and placebo after impacted third molar removal. Journal of Oral and Maxillofacial Surgery 1994;52(9):919‐24. [DOI] [PubMed] [Google Scholar]
Dolci 1994 {published data only}
- Dolci G, Ripari M, Pacifici L, Umile A. Evaluation of piroxicam‐beta‐cyclodextrin, piroxicam, paracetamol and placebo in post‐operative oral surgery pain. International Journal of Clinical Pharmacology Research 1994;14(5‐6):185‐91. [PubMed] [Google Scholar]
Forbes 1984b {published data only}
- Forbes JA, Barkaszi BA, Ragland RN, Hankle JJ. Analgesic effect of acetaminophen, phenyltoloxamine and their combination in postoperative oral surgery pain. Pharmacotherapy 1984;4(4):221‐6. [DOI] [PubMed] [Google Scholar]
Forbes 1989 {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Yorio CC, Selinger LR, Rosenmertz SK, et al. Evaluation of flurbiprofen, acetaminophen, an acetaminophen‐codeine combination, and placebo in postoperative oral surgery pain. Pharmacotherapy 1989;9(5):322‐30. [DOI] [PubMed] [Google Scholar]
Forbes 1990 {published data only}
- Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen‐codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6(Pt 2))):94S‐105S. [PubMed] [Google Scholar]
Hersh 2000 {published data only}
- Hersh EV, Levin LM, Cooper SA, Doyle G, Waksman J, Wedell D, et al. Ibuprofen liquigel for oral surgery pain. Clinical Therapeutics 2000;22(11):1306‐18. [DOI] [PubMed] [Google Scholar]
Kiersch 1994 {published data only}
- Kiersch TA, Halladay SC, Hormel PC. A single‐dose, double‐blind comparison of naproxen sodium, acetaminophen, and placebo in postoperative dental pain. Clinical Therapeutics 1994;16(3):394‐404. [PubMed] [Google Scholar]
Kubitzek 2003 {published data only}
- Kubitzek F, Ziegler G, Gold MS, Liu JM, Ionescu E. Analgesic efficacy of low‐dose diclofenac versus paracetamol and placebo in postoperative dental pain. Journal of Orofacial Pain 2003;17(3):237‐44. [PubMed] [Google Scholar]
Lehnert 1990 {published data only}
- Lehnert S, Reuther J, Wahl G, Barthel K. [The efficacy of paracetamol (Tylenol) and acetyl salicylic acid (Aspirin) in treating postoperative pain]. Deutsche Zahnarztliche Zeitschrift 1990;45(1):23‐6. [PubMed] [Google Scholar]
Mehlisch 1995 {published data only}
- Mehlisch DR, Jasper RD, Brown P, Korn SH, McCarroll K, Murakami AA. Comparative study of ibuprofen lysine and acetaminophen in patients with postoperative dental pain. Clinical Therapeutics 1995;17(5):852‐60. [DOI] [PubMed] [Google Scholar]
Moller 2000 {published data only}
- Moller PL, Norholt SE, Ganry HE, Insuasty JH, Vincent FG, Skoglund LA, et al. Time of onset of analgesia and analgesic efficacy of effervescent acetaminophen 1000 mg compared to tablet acetominophen 1000 mg in postoperative dental pain: a single‐dose, double‐blind, randomized, placebo‐controlled study. Journal of Clinical Pharmacology 2000;40(4):370‐8. [DOI] [PubMed] [Google Scholar]
Olson 2001 {published data only}
- Olson NZ, Otero AM, Marrero I, Tirado S, Cooper S, Doyle G, et al. Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain. Journal of Clinical Pharmacology 2001;41(11):1238‐47. [DOI] [PubMed] [Google Scholar]
Seymour 1996 {published data only}
- Seymour RA, Kelly PJ, Hawkesford JE. The efficacy of ketoprofen and paracetamol (acetaminopen) in postoperative pain after third molar surgery. British Journal of Clinical Pharmacology 1996;41(6):581‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 2003 {published data only}
- Seymour RA, Hawkesford JE, Sykes J, Stillings M, Hill CM. An investigation into the comparative efficacy of soluble aspirin and solid paracetamol in postoperative pain after third molar surgery. British Dental Journal 2003;194(3):153‐7. [DOI] [PubMed] [Google Scholar]
Skoglund 1991 {published data only}
- Skoglund LA, Skjelbred P, Fyllingen G. Analgesic efficacy of acetaminophen 1000 mg, acetaminophen 2000 mg, and the combination of acetaminophen 1000 mg and codeine phosphate 60 mg versus placebo in acute postoperative pain. Pharmacotherapy 1991;11(5):364‐9. [PubMed] [Google Scholar]
Sunshine 1986 {published data only}
- Sunshine A, Marrero I, Olson N, McCormick N, Laska EM. Comparative study of flurbiprofen, zomepirac sodium, acetaminophen plus codeine, and acetaminophen for the relief of postsurgical dental pain. The American Journal of Medicine 1986;80(3A):50‐4. [DOI] [PubMed] [Google Scholar]
Vattaraphudej 1986 {published data only}
- Vattaraphudej T, Thongnoppakao P, Koukongviriyapan V. [Comparison of the efficacy of analgesics in pain after oral surgery]. The Journal of the Dental Association of Thailand 1986;36(6):198‐206. [PubMed] [Google Scholar]
References to studies excluded from this review
Adame 1979 {published data only}
- Adame C, et al. Postoperative development of inflammation after extraction of impacted third molar as a model for clinical assessment of anti‐inflammatory drugs. I. Preliminary report. Boletin de Estudios Medicos y Biologicos 1979;30(8):308‐9. [Google Scholar]
Barden J 2004 {published data only}
- Barden J, Edwards J, Moore A, McQuay H. Single dose oral paracetamol (acetaminophen) for postoperative pain. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004602] [DOI] [PubMed] [Google Scholar]
Becker 1990 {published data only}
- Becker J, Beckmann J, Bertelt C, Gundert‐Remy U, Rohmel J, Ohlendorf D. [Double blind biometric study on postoperative effects of analgesics]. Deutsche Zahnarztliche Zeitschrift 1990;45(1):36‐8. [PubMed] [Google Scholar]
Bentley 1987 {published data only}
- Bentley KC, Head TW. The additive analgesic efficacy of acetaminophen, 1000 mg, and codeine, 60 mg, in dental pain. Clinical Pharmacology and Therapeutics 1987;42(6):634‐40. [DOI] [PubMed] [Google Scholar]
Breivik 1998 {published data only}
- Breivik EK, Bjornsson GA. Variation in surgical trauma and baseline pain intensity: effects on assay sensitivity of an analgesic trial. European Journal of Oral Sciences 1998;106(4):844‐52. [DOI] [PubMed] [Google Scholar]
Cooper 1986 {published data only}
- Cooper SA, Erlichman MC, Mardirossian G. Double‐blind comparison of an acetaminophen‐codeine‐caffeine combination in oral surgery pain. Anesthesia Progress 1986;33(3):139‐42. [PMC free article] [PubMed] [Google Scholar]
Cooper 1989 {published data only}
- Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double‐blind, placebo‐controlled study. Journal of Clinical Pharmacology 1989;29(11):1026‐30. [DOI] [PubMed] [Google Scholar]
Cooper 1991 {published data only}
- Cooper SA, Kupperman A. The analgesic efficacy of flurbiprofen compared to acetaminophen with codeine. The Journal of Clinical Dentistry 1991;2(3):70‐4. [PubMed] [Google Scholar]
Dionne 1983 (1) {published data only}
- Dionne RA, Campbell RA, Cooper SA, Hall DL, Buckingham B. Suppression of postoperative pain by preoperative administration of ibuprofen in comparison to placebo, acetaminophen, and acetaminophen plus codeine. Journal of Clinical Pharmacology 1983;23(1):37‐43. [DOI] [PubMed] [Google Scholar]
Dionne 1983 (2) {published data only}
- Dionne RA, Sisk AL, Fox PC, Wirdzek PR, Gracely RH, Dubner R. Suppression of postoperative pain by preoperative adminsitration of flurbiprofen in comparison to acetominophen and oxycodone plus acetominophen. Current Therapeutic Research 1983;34(1):15‐29. [Google Scholar]
Dionne 1986 {published data only}
- Dionne RA. Suppression of dental pain by the preoperative administration of flurbiprofen. The American Journal of Medicine 1986;80(3A):41‐9. [DOI] [PubMed] [Google Scholar]
Dolci 1993 {published data only}
- Dolci G, Ripari M, Pacifici L, Umile A. [Analgesic efficacy and the tolerance for piroxicam‐beta‐cyclodextrin compared to piroxicam, paracetamol and placebo in the treatment of postextraction dental pain]. Minerva Stomatologica 1993;42(5):235‐41. [PubMed] [Google Scholar]
Edwards 2002 {published data only}
- Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta‐analysis of single‐dose oral tramadol plus acetaminophen in acute postoperative pain. Journal of Pain and Symptom Management 2002;23(2):121‐30. [DOI] [PubMed] [Google Scholar]
Forbes 1982 {published data only}
- Forbes JA, Beaver WT, White EH, White RW, Neilson GB, Shackleford RW. Diflunisal. A new oral analgesic with an unusually long duration of action. JAMA 1982;248(17):2139‐42. [DOI] [PubMed] [Google Scholar]
Forbes 1984a {published data only}
- Forbes JA, Kolodny AL, Chachich BM, Beaver WT. Nalbuphine, acetaminophen, and their combination in postoperative pain. Clinical Pharamacology and Therapeutics 1984;35(6):843‐51. [DOI] [PubMed] [Google Scholar]
Gallardo 1990 {published data only}
- Gallardo F, Rossi E. Analgesic efficacy of flurbiprofen as compared to acetaminophen and placebo after periodontal surgery. Journal of Periodontology 1990;61(4):224‐7. [DOI] [PubMed] [Google Scholar]
Gustafsson 1983 {published data only}
- Gustafsson I, Nystrom E, Quiding H. Effect of preoperative paracetamol on pain after oral surgery. European Journal of Clinical Pharmacology 1983;24(1):63‐5. [DOI] [PubMed] [Google Scholar]
Haanaes 1986 {published data only}
- Haanaes HR, Benterud UJ, Skoglund LA. RF 46‐790 versus paracetamol: effect on post‐operative pain. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1986;24(11):598‐601. [PubMed] [Google Scholar]
Irvine 1982 {published data only}
- Irvine GH, Lutterloch MJ, Bowerman JE. Comparison of diflunisal and paracetamol in the management of pain following wisdom teeth removal. British Dental Journal 1982;152(1):18‐20. [DOI] [PubMed] [Google Scholar]
Laska 1983 {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498‐509. [DOI] [PubMed] [Google Scholar]
Lecointre 1991 {published data only}
- Lecointre C. [Efficacy and tolerance of tiaprofenic acid for extraction complications. Results of a randomized double‐blind study, tiaprofenic acid versus paracetamol]. L'Information Dentaire 1991;73(35):3063‐6. [PubMed] [Google Scholar]
Liashek 1987 {published data only}
- Liashek P Jr, Desjardins PJ, Triplett RG. Effect of pretreatment with acetaminophen‐propoxyphene for oral surgery pain. Journal of Oral and Maxillofacial Surgery 1987;45(2):99‐103. [DOI] [PubMed] [Google Scholar]
Macleod 2002 {published data only}
- Macleod AG, Ashford B, Voltz M, Williams B, Cramond T, Gorta L, et al. Paracetamol versus paracetamol‐codeine in the treatment of post‐operative dental pain: a randomized, double‐blind, prospective trial. Australian Dental Journal 2002;47(2):147‐51. [DOI] [PubMed] [Google Scholar]
Medve 2001 {published data only}
- Medve RA, Wang J, Karim R. Tramadol and acetaminophen tablets for dental pain. Anesthesia Progress 2001;48(3):79‐81. [PMC free article] [PubMed] [Google Scholar]
Mehlisch 1984 {published data only}
- Mehlisch DR, Frakes LA. A controlled comparative evaluation of acetaminophen and aspirin in the treatment of postoperative pain. Clinical Therapeutics 1984;7(1):89‐97. [PubMed] [Google Scholar]
Mehlisch 1990 {published data only}
- Mehlisch DR, Sollecito WA, Helfrick JF, Leibold DG, Markowitz R, Schow CE Jr, et al. Multicenter clinical trial of ibuprofen and acetaminophen in the treatment of postoperative dental pain. Journal of the American Dental Association 1990;121(2):257‐63. [DOI] [PubMed] [Google Scholar]
Moore 1986 {published data only}
- Moore PA, Werther JR, Seldin EB, Stevens CM. Analgesic regimens for third molar surgery: pharmacologic and behavioral considerations. Journal of the American Dental Association 1986;113(5):739‐44. [DOI] [PubMed] [Google Scholar]
Nystrom 1988 {published data only}
- Nystrom E, Gustafsson I, Quiding H. The pain intensity at analgesic intake, and the efficacy of diflunisal in single doses and effervescent acetaminophen in single and repeated doses. Pharmacotherapy 1988;8(3):201‐9. [DOI] [PubMed] [Google Scholar]
Petersen 1983 {published data only}
- Petersen JK. A double‐blind cross‐over study of the analgesic and anti‐inflammatory effects of dexamethasone, and paracetamol following surgical removal of lower impacted third molars. International Journal of Oral Surgery 1983;12(4):266. [Google Scholar]
Quiding 1981 {published data only}
- Quiding H, Oksala E, Happonen RP, Lehtimaki K, Ojala T. The visual analog scale in multiple‐dose evaluations of analgesics. The Journal of Clinical Pharmacology 1981;21(10):424‐9. [DOI] [PubMed] [Google Scholar]
Quiding 1982 (1) {published data only}
- Quiding H, Oikarinen V, Huitfeldt B, Koskimo M, Leikomaa H, Nyman C. An analgesic study with repeated doses of phenazone, phenazone plus dextropropoxyphene, and paracetamol, using a visual analogue scale. International Journal of Oral Surgery 1982;11(5):304‐9. [DOI] [PubMed] [Google Scholar]
Quiding 1982 (2) {published data only}
- Quiding H, Persson G, Ahlstrom U, Bangens S, Hellem S, Johansson G, et al. Paracetamol plus supplementary doses of codeine, an analgesic study of repeated doses. European Journal of Clinical Pharmacology 1982;23(4):315‐9. [DOI] [PubMed] [Google Scholar]
Quiding 1984 {published data only}
- Quiding H, Oikarinen V, Sane J, Sjoblad AM. Analgesic efficacy after single and repeated doses of codeine and acetaminophen. Journal of Clinical Pharmacology 1984;24(1):27‐34. [DOI] [PubMed] [Google Scholar]
Ragot 1991 {published data only}
- Ragot JP. [Comparison of analgesic activity of mefenamic acid and paracetamol in treatment of pain after extraction of impacted lower 3d molar]. L'Information Dentaire 1991;73(21):1659‐64. [PubMed] [Google Scholar]
Reijntjes 1987 {published data only}
- Reijntjes RJ, Boering G, Wesseling H, Rijn LG. Suprofen versus paracetamol after oral surgery. International Journal of Oral and Maxillofacial Surgery 1987;16(1):45‐9. [DOI] [PubMed] [Google Scholar]
Rodrigo 1987 {published data only}
- Rodrigo MR, Rosenquist JB, Cheung LK. Paracetamol and diflunisal for pain relief following third molar surgery in Hong Kong Chinese. International Journal of Oral and Maxillofacial Surgery 1987;16(5):566‐71. [DOI] [PubMed] [Google Scholar]
Rodrigo 1989 {published data only}
- Rodrigo C, Chau M, Rosenquist J. A comparison of paracetamol and diflunisal for pain control following 3rd molar surgery. International Journal of Oral and Maxillofacial Surgery 1989;18(3):130‐2. [DOI] [PubMed] [Google Scholar]
Rosen 1985 {published data only}
- Rosen M, Absi EG, Webster JA. Suprofen compared to dextropropoxyphene hydrochloride and paracetamol (Cosalgesic) after extraction of wisdom teeth under general anaesthesia. Anaesthesia 1985;40(7):639‐41. [DOI] [PubMed] [Google Scholar]
Sakata 1989 {published data only}
- Sakata LA, Rocha B, et al. Effects of benzydamine after surgical removal of impacted teeth [Efeitos da benzidamina após a extraçäo de dentes inclusos]. Revista da Associacao Paulista de Cirurgioes Dentistas 1989;43(4):167‐70. [Google Scholar]
Selcuk 1996 {published data only}
- Selcuk E, Gomel M, Bellibas SE, Kose T, Tuglular I. Comparison of the analgesic effects of diflunisal and paracetamol in the treatment of postoperative dental pain. International Journal of Clinical Pharmacology Research 1996;16(2‐3):57‐65. [PubMed] [Google Scholar]
Seymour 1981 {published data only}
- Seymour RA, Rawlins MD. Pharmacokinetics of parenteral paracetamol and its analgesic effects in post‐operative dental pain. European Journal of Clinical Pharmacology 1981;20(3):215‐8. [DOI] [PubMed] [Google Scholar]
Seymour 1983 {published data only}
- Seymour RA. Analgesic efficacy and plasma concentration of three analgesics in pain after lower third molar removal. SAAD Digest 1983;5(7):172‐88. [PubMed] [Google Scholar]
Skjelbred 1979 {published data only}
- Skjelbred P, Lokken P. Paracetamol versus placebo: effects on post‐operative course. European Journal of Clinical Pharmacology 1979;15(1):27‐33. [DOI] [PubMed] [Google Scholar]
Strom 1990 {published data only}
- Strom C, Forsberg O, Quiding H, Engevall S, Larsson O. Analgesic efficacy of acetaminophen sustained release. Journal of Clinical Pharmacology 1990;30(7):654‐9. [DOI] [PubMed] [Google Scholar]
Van Aken 2004 {published data only}
- Aken H, Thys L, Veekman L, Buerkle H. Assessing analgesia in single and repeated administrations of propacetamol for postoperative pain: comparison with morphine after dental surgery. Anesthesia and Analgesia 2004;98(1):159‐65. [DOI] [PubMed] [Google Scholar]
Winter 1983 {published data only}
- Winter L, Appleby F, Ciccone PE, Pigeon JG. A double‐blind, comparative evaluation of acetaminophen, caffeine, and the combination of acetaminophen and caffeine in outpatients with postoperative oral surgery pain. Current Therapeutic Research 1983;33(1):115‐22. [Google Scholar]
Additional references
Aronoff 2006
- Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clinical Pharmacology and Therapeutics 2006;79(1):9‐19. [DOI] [PubMed] [Google Scholar]
Chandrasekharan 2002
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;99(21):13926‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1999
- Collins SL, Moore RA, McQuay HJ, Wiffen PJ, Edwards JE. Single dose oral ibuprofen and diclofenac for postoperative pain. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001548] [DOI] [PubMed] [Google Scholar]
Cooper 1976
- Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clinical Pharmacology and Therapeutics 1976;20(2):241‐50. [DOI] [PubMed] [Google Scholar]
Coulthard 2001
- Coulthard P, Hill CM, Frame JW, Barry H, Ridge BD, Bacon TH. Pain control with paracetamol from a sustained release formulation and a standard release formulation after third molar surgery: a randomised controlled trial. British Dental Journal 2001;191(6):319‐24. [DOI] [PubMed] [Google Scholar]
Dray 1997
- Dray A. Kinins and their receptors in hyperalgesia. Canadian Journal of Physiology and Pharmacology 1997;75(6):704‐12. [PubMed] [Google Scholar]
Fisher 1988
- Fisher SE, Frame JW, Rout PG, McEntegart DJ. Factors affecting the onset and severity of pain following the surgical removal of unilateral impacted mandibular third molar teeth. British Dental Journal 1988;164(11):351‐4. [DOI] [PubMed] [Google Scholar]
Loeser 1999
- Loeser JD, Melzack R. Pain: an overview. Lancet 1999;353(9164):1607‐9. [DOI] [PubMed] [Google Scholar]
Malmberg 1992
- Malmberg AB, Yaksh TL. Antinociceptive effects of spinal non‐steroidal anti‐inflammatory agents on the formalin test in the rat. Journal of Pharmacology and Experimental Therapeutics 1992;263:136‐46. [PubMed] [Google Scholar]
Moore 1997
- Moore RA, McQuay HJ. Single‐patient data meta‐analysis of 3453 postoperative patients: oral tramadol versus placebo, codeine and combination analgesics. Pain 1997;69(3):287‐94. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore A, Collins S, Carroll D, McQuay H, Edwards J. Single dose paracetamol (acetaminophen), with and without codeine, for postoperative pain. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001547] [DOI] [PubMed] [Google Scholar]
Rang 2003
- Rang HP, Dale MM, Ritter JM, Moore PK. Anti‐inflammatory and immunosuppresant drugs. Pharmacology. 5th Edition. Churchill Livingstone, 2003:244‐61. [Google Scholar]
Seymour 1985
- Seymour RA, Meechan JG, Blair GS. An investigation into post‐operative pain after third molar surgery under local analgesia. The British Journal of Oral and Maxillofacial Surgery 1985;23(6):410‐8. [DOI] [PubMed] [Google Scholar]


